INTRODUCTION
Unlike pancreatic head cancer, pancreatic neck or body cancer is commonly diagnosed at the locally advanced stage after the celiac axis branches, among them the common hepatic artery (CHA), having already been involved by the neoplastic process. In terms of current recommendations, celiac axis (CA) and superior mesenteric artery (SMA) invasion presents a contraindication to pancreatectomy for ductal adenocarcinoma[1,2]. However, in certain instances, excision of the CA together with its branches allows a curative procedure to be performed and obviates arterial reconstruction, incurring a high risk of serious complications. In 1953, while carrying out a distal pancreatectomy along with a gastrectomy for locally advanced gastric carcinoma, Appleby pioneered taking advantage of the chance of the re-establishment of the blood supply to the liver after CA and CHA resection by way of the constantly existent pancreaticoduodenal arcade from the SMA basin[3]. In 1976, Nimura applied Appleby’s technique to treat locally advanced pancreatic body-tail carcinoma[4] and in 1991, Nagino et al[5] and Hishinuma et al[6] succeeded in preserving the stomach in the absence of its invasion from pancreatic tumor by means of sparing the gastroduodenal and right gastroepiploic arteries (GDA and RGEA), thereby modifying Appleby’s operation. By the year 2003, two dozen such operative interventions had been accomplished[5,7-15], at most, but refinements in diagnosis and surgical technique have progressively promoted their growing in number[5,11,15-24]. The modified Appleby procedure case reviews in the literature tend to say nothing about the pattern of the celiac-mesenteric arterial vasculature encountered, in as much as, when dealing with variant arteries and the classical arterial architecture, this sort of surgery can be successfully performed without vascular reconstruction. We give an account of two cases of the modified Appleby operation for pancreatic body borderline resectable cancer, in one of which (as yet not described) a R0 distal resection was accompanied by excision, not only of the CA and the CHA, but left gastric arteries as well in the context of aberrant arteries Michels, type VIIIb.
CASE REPORT
Case 1
On the 12th October, 2011, a 64-year-old woman presented with complaints of constant severe upper abdominal pain relievable with Tramadol administration (four times daily), fatigue and a weight loss of 4 kg in a month. According to the past history, back pain had started 5 mo earlier and had been extending into the lower abdomen. The patient had endured the pain for quite a long time and it was not until October that she sought medical attention and was examined. Computed tomography (CT) and magnetic resonance imaging (MRI) revealed cancer of the pancreatic body and tail with affected CA branches. No evidence of dissemination was observed. Performance status was Eastern Cooperative Oncology Group score of 2, Karnofsky index 70%. On admission, the patient was in a moderately grave condition due to the pain experienced and was asthenic with skin pallor. Pulse was 72/min, regular and good volume. Blood pressure was 110/70 mmHg. Breath sounds were vesicular. The abdomen was soft with tenderness in the epigastrium through the thinned anterior wall of which a solid mass was palpable.
Instrumentally derived findings: Abdominal ultrasound (US) showed evidence of pancreatic body tumor extending to the CA, CHA, splenic artery (SA), superior mesenteric vein (SMV) and confluence of the portal vein (PV). The GDA showed close tumor contact over 1 cm, suspicious for ingrowth. Extrinsic compression of the SMV, PV confluence and CHA was documented.
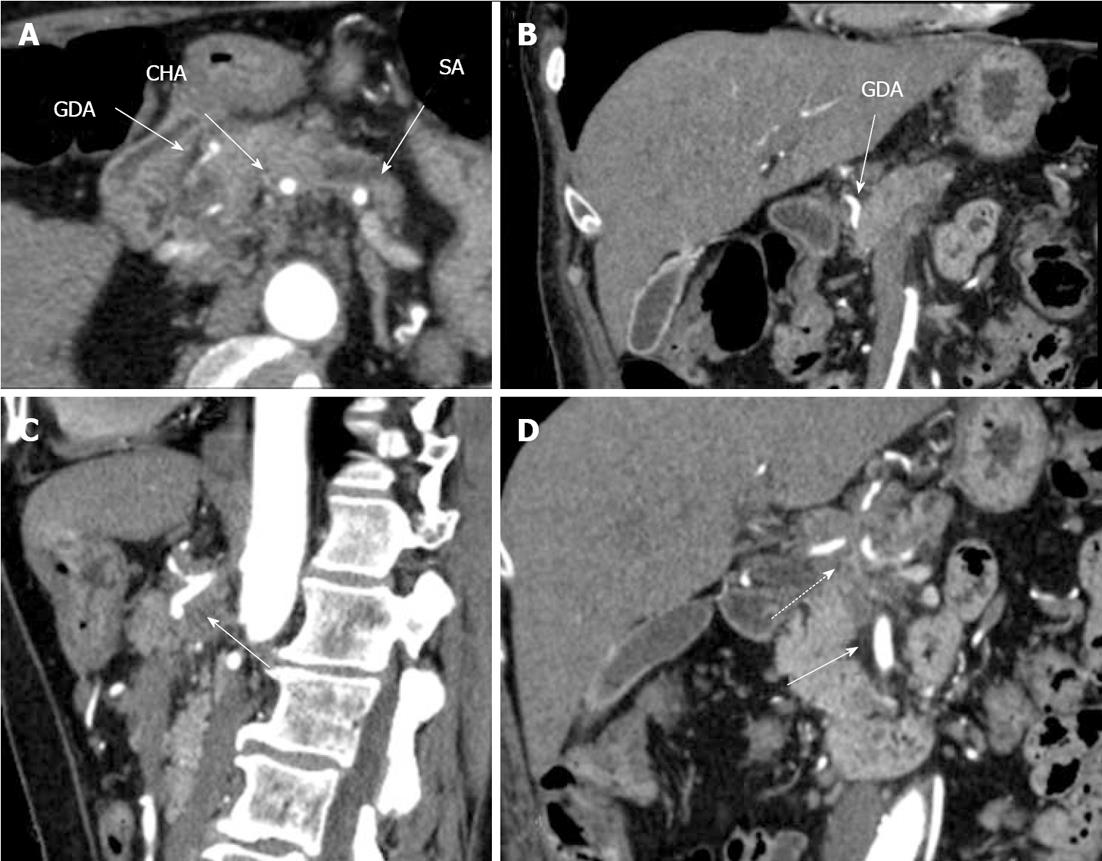
The CT showed that the liver structure appeared unchanged with no focal lesions. A space occupying mass 25 mm in diameter was visualized in the neck and body of the pancreas. Pancreatic hypertension and atrophy of the pancreas’ tail were noted. The duct of Wirsung was shown to be dilated up to 7 mm in the tail portion with a blunt cutoff (interrupted duct sign) at the level of the body of the gland, where tiny 2-3 mm cystic entities were discernable. The SMA was apparently traveling just along the left contour of the growth. The GDA was found to be circumferentially encased by the tumor over a length of 1 cm. An accessory renal artery was visible on the right. The lymph nodes in the pancreatic head and paraaortic regions and along the course of the SMA measured up to 17, 10 and 7-9 mm respectively. The conclusion was adenocarcinoma of the pancreatic neck and body with involvement of the CA, CHA and, probably, GDA and confluence of the PV (Figure 1).
Figure 1 Preoperative computed tomography.
Arterial phase. A: Axial image. The common hepatic (CHA) and splenic (SA) arteries present circumferential adjacency to pancreatic body ductal adenocarcinoma. The gastroduodenal artery (GDA) appears to be completely encircled by tumor; B: Frontal view. Computed tomography (CT) evidences circumferential infiltration of GDA; C: The celiac artery (CA) along with CHA springing from it, are completely circumscribed by tumor (arrow); D. All three CA branches (dashed arrow) show circumferential tumor contact. The superior mesenteric artery is unaffected (arrow).
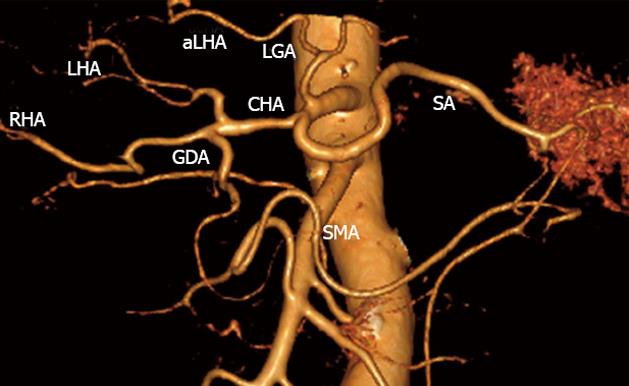
A perivaterian diverticulum, simple left kidney cysts, a splenic cyst, lung emphysema and aortal, coronary and iliac atherosclerosis were identified, classical celiaco-mesenterial arterial anatomy (Michels, type 1) (Figure 2).
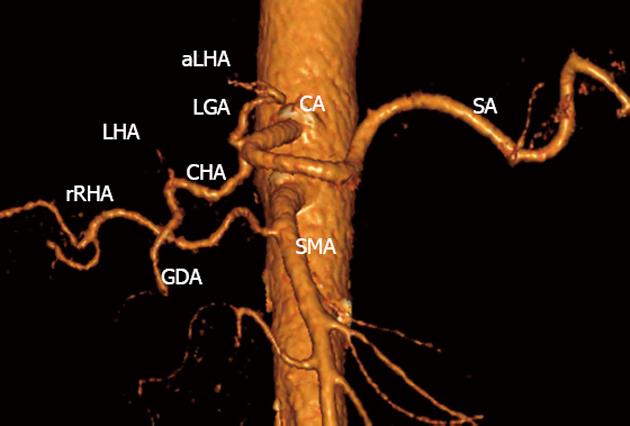
Figure 2 Three-dimensional computed tomography-angiography before surgery.
Classical arterial architecture (Michels, type I). Tumor-induced common hepatic (CHA) stenosis is noted. Anatomical variation of type I is observed: CHA trifurcation in the absence of proper hepatic artery. RHA: Right hepatic artery; LHA: Left hepatic artery; GDA: Gastroduodenal artery; LGA: Left gastric artery; SMA: Superior mesenteric artery; SA: Splenic artery; aLHA: Accessory left hepatic artery.
Esophagogastroduodenoscopy (EGDS) showed focal gastritis and Paquet’s stage 1 upper third esophageal varices.
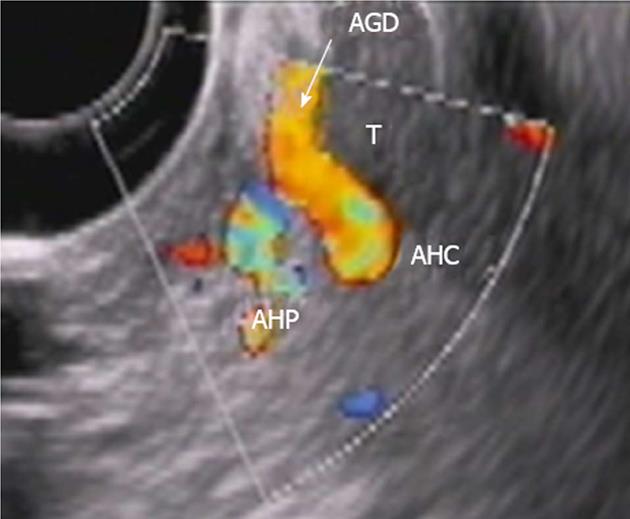
Endoscopic ultrasound (EUS) showed a tumor of the pancreatic body, presumably adenocarcinoma, with neoplastic process spreading to the CHA, distal third of the CA and confluence of the PV. The new growth was seen to be intimately in contact with the left wall of the GDA over a run of 1 cm. Regional lymph node enlargement, most likely related to their being metastatic, was defined (Figure 3).
Figure 3 Endoscopic ultrasound.
Tumor (T) abutment to gastroduodenal artery (AGD and arrow) without its encasement. HAC: Common hepatic, HAP: Proper hepatic artery.
Abdominal MRI showed a tumor of the neck-body of the pancreas, atrophy of the parenchyma of the pancreas’ tail and pancreatic hypertension in the tail portion of the gland. Infiltration of the retroperitoneal fat with extension encircling the CA, SMA and PV confluence was depicted.
Preoperative concept was of a 64-year-old female diagnosed with a T4NxM0 ductal adenocarcinoma of the pancreatic body with invasion of the CA branches and PV and no evidence for distant metastases. She was deemed to have borderline resectable disease because of suspected tumor encroachment on the GDA. Distal pancreatectomy with resection of the CA and PV was planned. The final extent of the procedure was intended to be decided after intraoperative exploration.
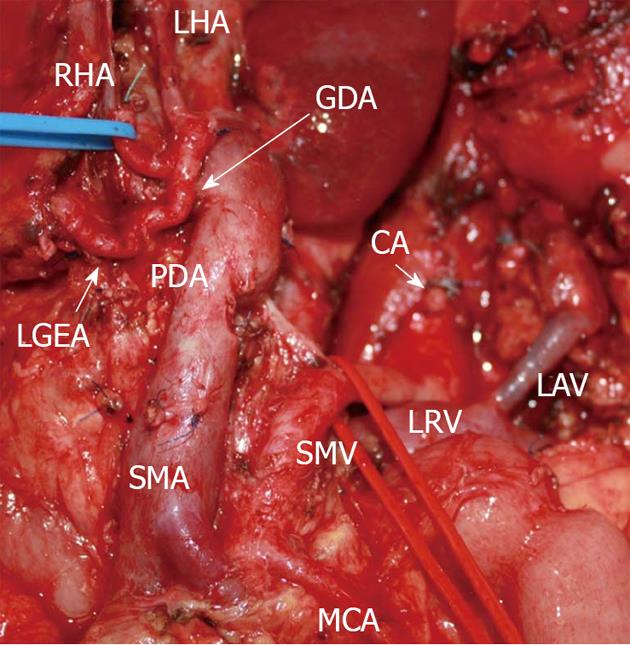
The operation was carried out on the 5th December, 2012. At surgery, no distant metastases were found. A whitish solid tumor taking up the whole of the pancreatic body and growing into the CA trifurcation and CHA with adherence to the left 180o of the uninvolved GDA was discovered. On duplex ultrasound with a clamp across the CHA there was a sufficient arterial blood flow in the liver and the hepatic arterial pulsation was present as before. The lesion was judged resectable. A corpocaudal pancreatectomy with resection of the CA and its branches was completed (Figure 4). At that, the RGEA was not sacrificed, which provided the gastric blood supply, with the stomach and liver’s color remaining unaltered throughout the operative time period.
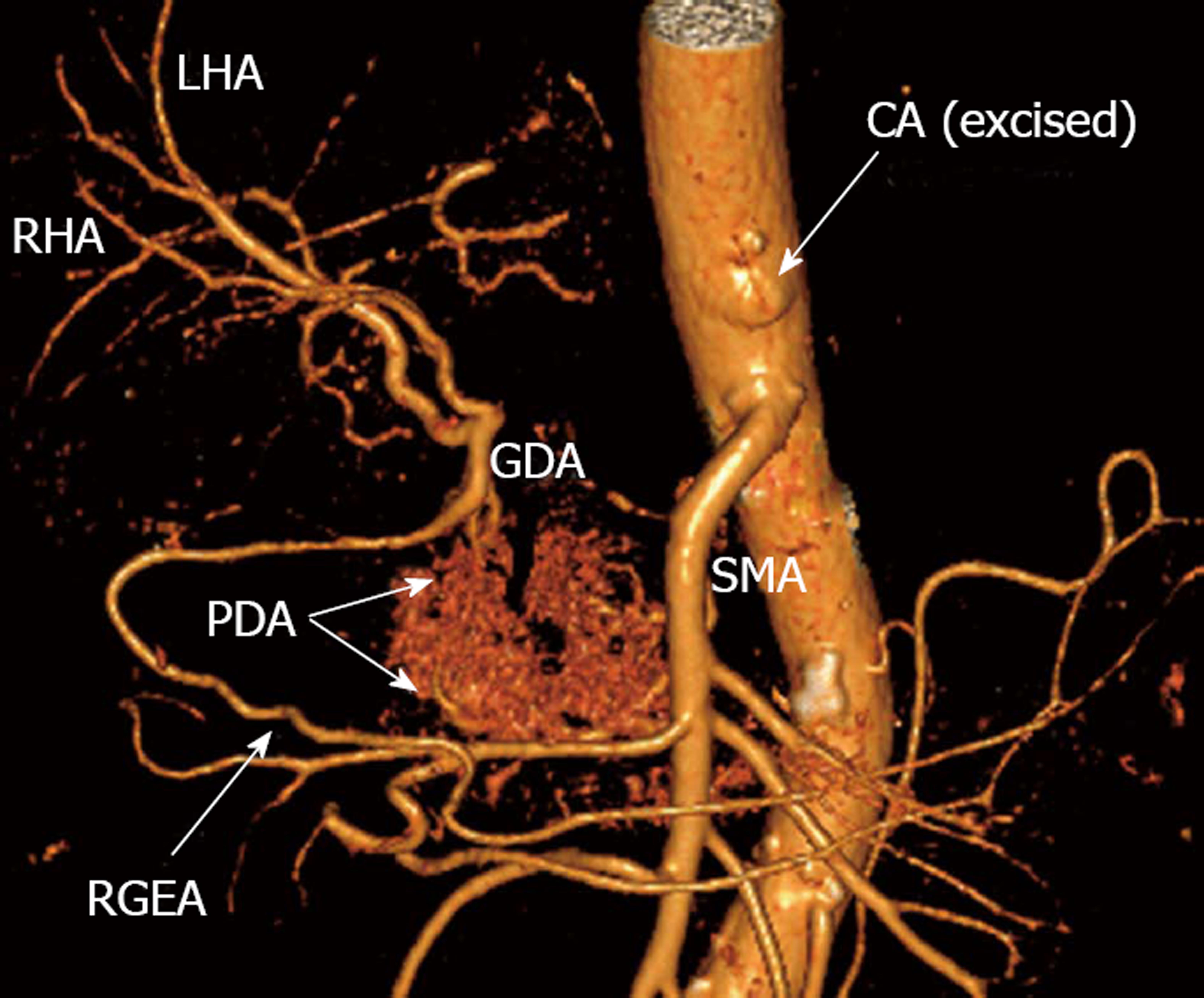
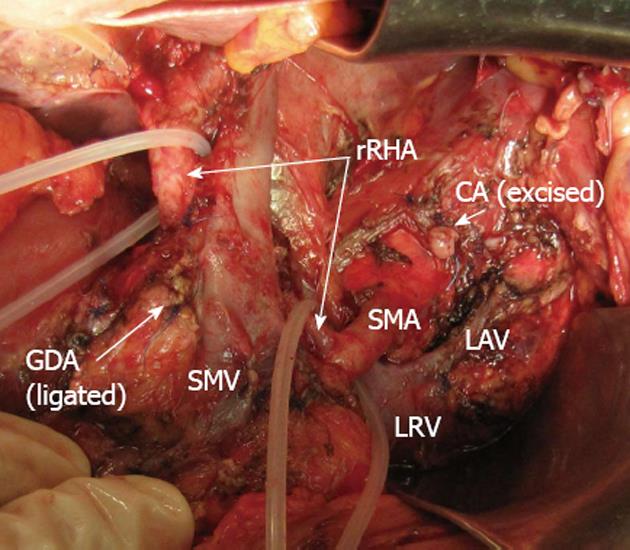
Figure 4 Photograph.
View of operating field after distal pancreatectomy with excision of celiac artery (CA) and its branches. Liver and stomach are fed with blood from the superior mesenteric artery (SMA) via pancreaticoduodenal arcade (PDA) and, thereafter, through the gastroduodenal artery (GDA). Full-blown right gastro-epiploic artery is found. Superior mesenteric vein (SMV) was resected at the site of confluence with splenic vein. LAV: Left adrenal vein; MCA: Medial colic; RHA: Right hepatic; LHA: Left hepatic arteries; LRV: Left renal vein; LGEA: Left gastro-epiploic artery.
Post surgery, the patient developed a retroperitoneal pancreatic fluid collection in the projection of the cut edge of the gland with an amylase of 60 000 (pancreatic fistula Class B), which was drained on postoperative day 5. Nine days after the surgery, EGDS recognized areas of gastric mucosa ischemia of mixed (portal and arterial) genesis, ischemic gastropathy. Recurrent hydrothorax was repeatedly addressed with pleural tapping (G3, Dindo-Clavien). After management with antibiotics, antisecretory and anti-ulcer therapy and treatment for diabetes mellitus, the patient’s condition stabilized, body temperature returned to normal and complete pain abolition was achieved. The glycemic profile was stable with a blood sugar level of 7-9 mmol/L under insulin therapy and the patient was discharged to receive adjuvant gemcitabine chemotherapy.
Microscopic examination and pathological diagnosis showed a moderately-differentiated pancreatic body-tail adenocarcinoma (pT3N1b, G2) with CA branches and PV involvement. The patient had a R1 distal resection owing to the vascular specimen margin from the sites contacting with the SMV and GDA (Figure 5). The 6 month follow-up CT yielded no evidence for disease recurrence and CT angiography displayed an ample blood flow in the liver and stomach (Figure 6).
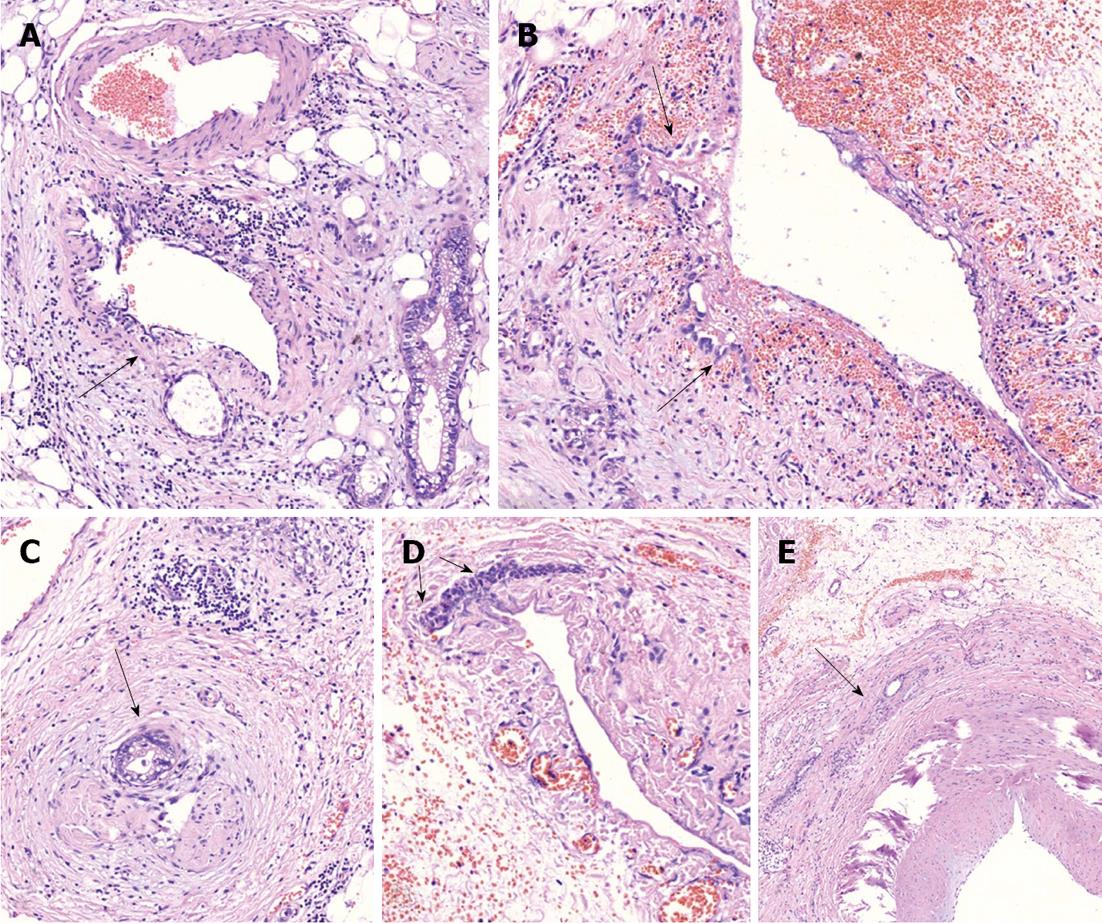
Figure 5 On microscopic examination.
A: Perivascular tumor growth (complexes of malignant cells in adventitia of small artery of peripancreatic fat (arrow), HE, × 200; B: Tumor incursion into vein wall (arrow), HE, x 200; C. invasion of the nerve by the tumor (arrow), HE, x 50; D: Vein wall involvement (complexes of malignant cells in media of 2-mm diameter vein (arrows), HE, × 50; E: Tumor complexes in the common hepatic artery adventitia (arrow), HE, × 50.
Figure 6 Three-dimensional computed tomography-angiography following distal pancreatectomy with excision of celiac artery and its branches.
Blood supply to liver and stomach is delivered from superior mesenteric artery (SMA) via pancreaticoduodenal arcade (PDA) and then through gastroduodenal artery (GDA). There is robust right gastro-epiploic artery (RGEA) appearing in its entirety. RHA: Right hepatic artery; LHA: Left hepatic artery; CA: Celiac axis; GDA: Gastroduodenal artery.
Case 2
In May, 2010, a 65-year-old woman consulted a doctor about pain in her right upper abdominal quadrant. On examination, a diagnosis of chronic pancreatitis was made and she was given conservative therapy which was of no benefit. In November 2010, abdominal CT invited by the pain worsening was undertaken and revealed a mass in the pancreatic body.
She entered the Moscow Herzen Institute of Oncology. CT detected an up to 5.6 cm pancreatic body tumor spreading to the CA branches and superior mesenteric arteries. On fine-needle aspiration biopsy, a well-differentiated adenocarcinoma was identified. The neoplasm was considered not to be amenable to resection. 8 courses of a palliative combination chemotherapeutic regimen consisting of 200 mg of eloxatin + 3600 mg of gemzar were instituted.
In July, 2011, the follow-up CT showed no drastic evolution in the disease course with persistent infiltration of the CA, its branches, superior mesenteric and splenic veins and no distant metastases (Figure 7). On CT angiography, variant arterial architecture Michels, type VIIIb, with a replaced right hepatic artery (rRHA) coming from the SMA and an accessory left hepatic artery (LHA) given off by the left gastric artery (LGA) was determined (Figure 8).
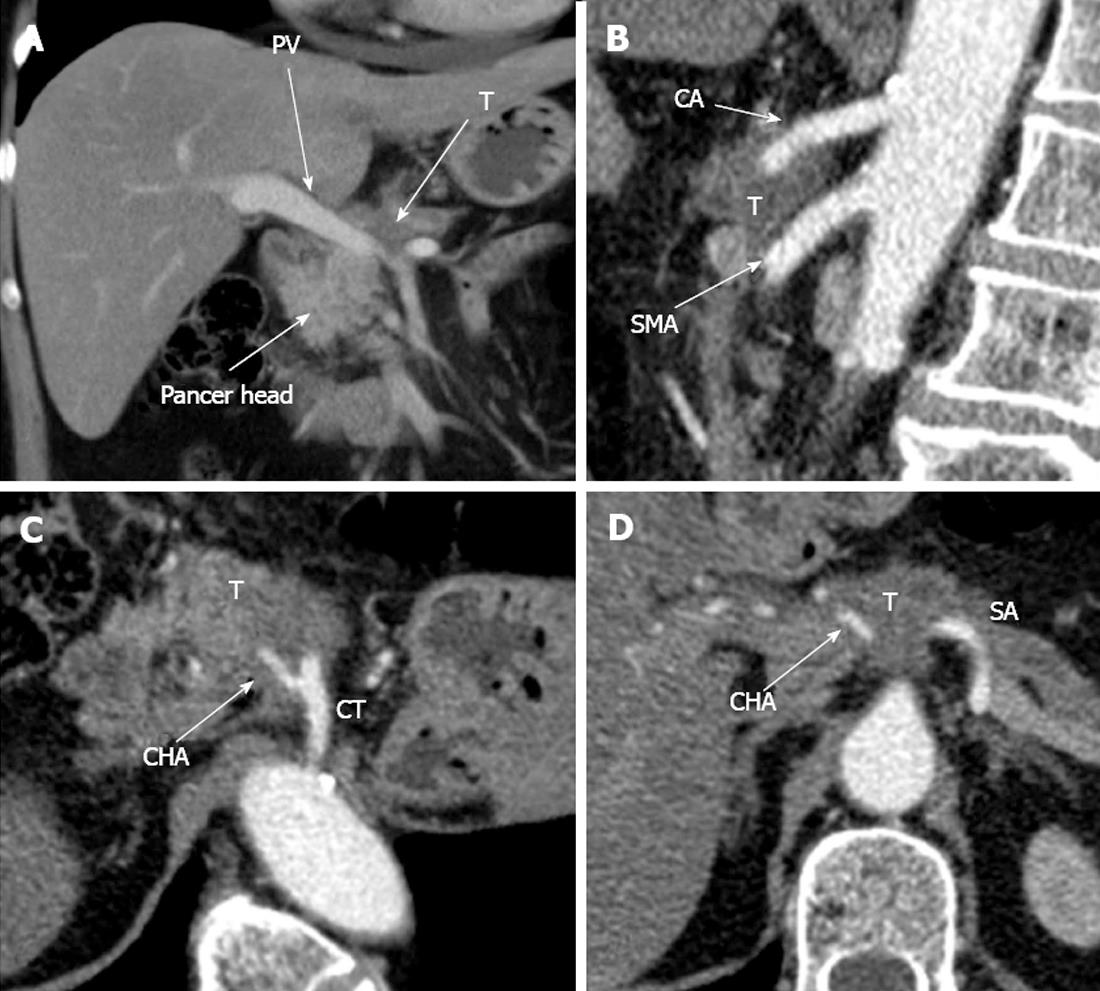
Figure 7 Computed tomography prior to operation.
A: Axial view. Venous phase. Hypovascular tumor of pancreatic neck (T) is shown to abut portal vein (PV) trunk. Pancreatic head is demonstrated to be intact; B: Sagittal view. Arterial phase. Circumferential encasement of celiac artery (CA) by hypovascular tumor of pancreatic body and the latter’s adherence to anterior aspect of superior mesenteric artery (SMA); C, D: Axial image. Arterial phase. Circumferential contiguity of tumor to CА along with common hepatic (CHA) and splenic (SA), both arising from the former, is visualized. CT: Celiac trunk.
Figure 8 Three-dimensional computed tomography angiography before surgery.
Variant arterial anatomy: replaced right hepatic artery (rRHA) originating from superior mesenteric artery (SMA), accessory left hepatic (aLHA) - from left gastric (LGA) (Michels, type VIIIb). CA: Celiac artery; LHA: Left hepatic artery; SA: Splenic artery; GDA: Gastroduodenal artery; CHA: Common hepatic.
Reasoning from the absence of interval neoplastic progression, we opted for an attempt at a radical procedure. Preoperative diagnosis was a ductal pancreatic body adenocarcinoma, cT4NxM0. Upon abdominal inspection, a pancreatic body solid whitish knobby tumor, up to 3-4 cm in diameter, involving the splenic and CHAs, proximal segment of the CA and LHA with peritumoral fibrosis and contraction in the center was disclosed. On table US demonstrated the blood flow in the left hepatic lobe to be sustained subsequent to briefly clamping the LHA, which encouraged us to undertake a subtotal pancreatectomy with CA excision and resection of the common and left hepatic arteries. As we did so, the GDA was also resected and ligated, that is to say, the pancreaticoduodenal arcade was unlocked (Figure 9). She was discharged on postoperative day 12 after uneventful postoperative recovery to be continued on neoadjuvant gemcitabine chemotherapy.
Figure 9 Photograph.
View of operating field after distal pancreatectomy with excision of celiac artery (CA), left gastric, common hepatic and left hepatic arteries. Superior mesenteric artery (SMA)-derived blood feeding of right hepatic lobe carried via the replaced right hepatic artery (rRHA). Blood supply to stomach is routed from SMA via pancreaticoduodenal arcades and then through gastroduodenal artery (GDA) with the latter’s proximal segment being resected and ligated. CA: Celiac artery; LAV: Left adrenal vein; LRV: Left renal vein; SMV: Superior mesenteric vein.
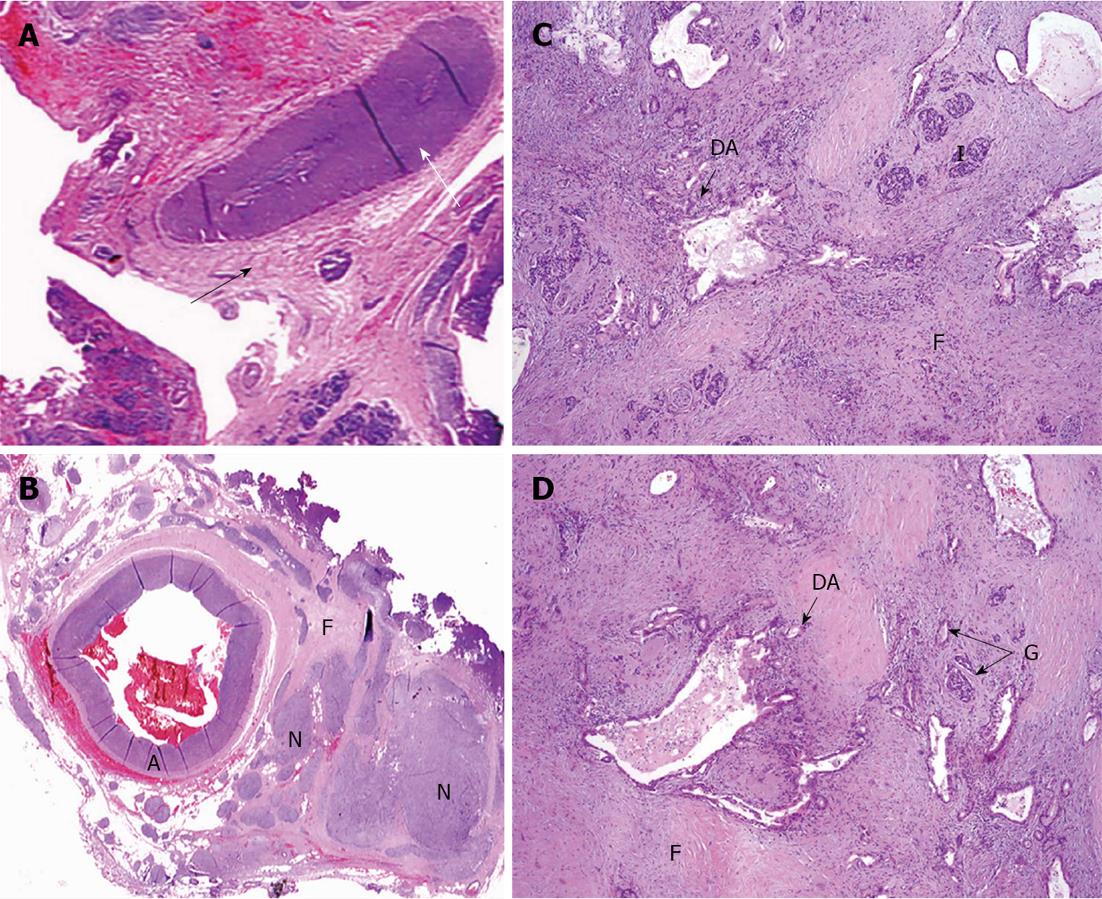
Conclusion on histological examination was a well-differentiated adenocarcinoma of the pancreatic body, pT2N0M0, G1. Tumor structures, measuring up to 1.2 cm in their greatest dimension, were found throughout an immense fibrotic bulk harboring remnants of pancreatic tissue composed of ductules and atrophic islets (Figure 10). The shortest margin-to-margin distance between the tumor and the specimen was 3 mm and a R0 resection was achieved. No features of post chemotherapeutic changes were identified.
Figure 10 Under microscope.
A: Common hepatic (CHA) section obtained from close to the point of its transection (white arrow) amid fibrotic zone (black arrow) along pancreas margin. No evidence of tumor growth (× 5); B: Celiac plexus and trunk area of diffuse fibrosis (F) (× 5); C: Pancreatic tissue with apparent diffuse fibrosis (F), groups of islets left (I) and that of glandular formations of ductal adenocarcinoma of pancreas (DA) (× 50); D: Structures of DA throughout fibrotic tissue (F) containing remnants of pancreatic tissue (atrophic islets and ductules) (HE, × 5). A: Artery; N: Nerve plexus with large ganglion (G).
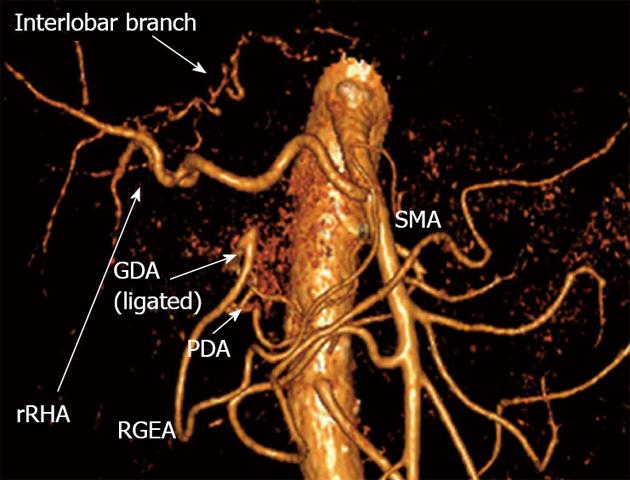
The 12-mo follow-up CT evidenced no disease recurrence, the patient feels well and goes on working as a doctor. There is an adequate arterial blood supply to the liver and stomach on CT angiography. Sufficient arterial nutrition of the left hepatic lobe is afforded by the engagement of the interlobar artery having an extraparenchymal hilar course (Figures 11 and 12).
Figure 11 Three-dimensional computed tomography angiography subsequent to distal pancreatectomy with excision of celiac artery, left gastric, common hepatic and left hepatic arteries.
Blood supply to right hepatic lobe is provided by superior mesenteric artery (SMA) through the replaced right hepatic artery (rRHA) and that to left hepatic lobe - via interlobar collateral anastomosing with rRHA. Stomach is supplied from SMA via pancreaticoduodenal artery (PDA) and, thereafter, through gastroduodenal artery (GDA) and right gastro-epiploic artery (RGEA).
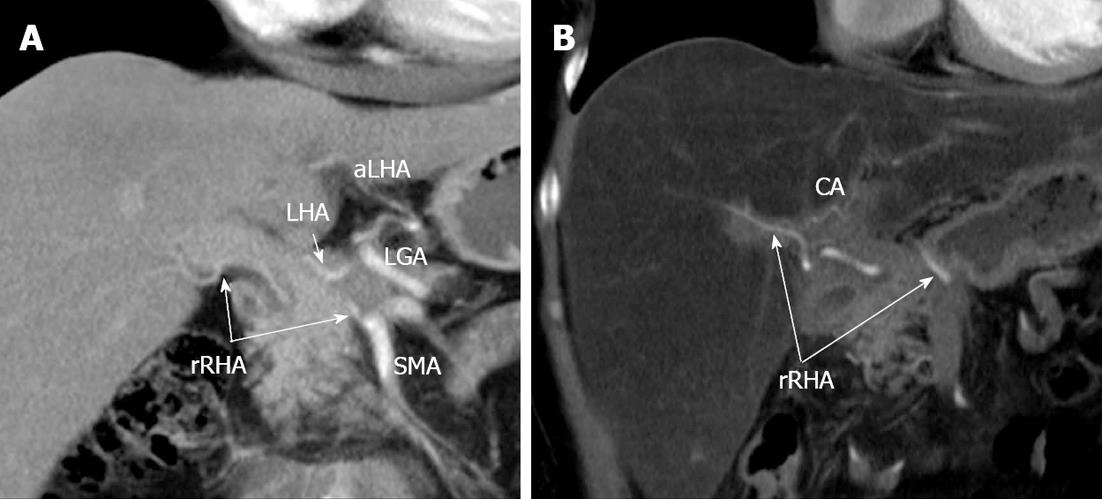
Figure 12 Coronal image.
Arterial phase. A: Previous to surgery. Aberrant arterial vasculature (Michels, type VIIIb): replaced right hepatic artery (rRHA) stemming from superior mesenteric artery (SMA), the left gastric artery (LGA) giving rise to accessorial left hepatic artery (aLHA). No interlobar collateral is detectable; B: Distal pancreatectomy with excision of celiac artery (CA), LGA, common hepatic (CHA) and left hepatic (LHA) arteries. Increased blood flow via rRHA is displayed and extraparenchymal hilar interlobar collateral transmitting blood supply to left hepatic lobe became visible.
DISCUSSION
The observations under review might be dually instructive. In the first place, they illustrate the feasibility of the maintenance of the blood flow in the left hepatic lobe after the modified Appleby operation with CHA and LHA resection in the presence of the RHA departing from the SMA. Secondly, both cases pinpoint key bothersome aspects of preoperative determination of pancreatic cancer resectability.
The modified Appleby technique has rightly gained acceptance as an effective approach to the management of pancreatic body cancer in cases of CHA involvement by tumor. We have performed 11 modified Appleby procedures, at 10 of which the classical arterial anatomy, identical to that encountered in Case 1, was found. An aberrant arterial pattern was present only in Case 2. Theoretically, when dealing with most variants in the celiac-mesenteric arterial architecture, this operation is quite safe with regard to the re-establishment of the arterial blood supply to the liver and stomach. Yet, cases with the replaced LHA (Michels, types IV and VIIIa) or the CHA (Michels, type X) arising from the LGA[25], as well as the described above situation, when the tumor involvement requires resection of either of the hepatic arteries (more often the LHA), present a real challenge secondary to CA excision. With all the patterns mentioned above, except variation Michels, type X, in the instance of which Appleby’s operation is not feasible without recourse to vascular repair, it is vital to know the sources of the collateral blood supply to the liver to avoid unnecessary arterioplasty.
Investigations of collateral circulation during temporary balloon occlusion of either of the hepatic arteries have first of all been spurred by both the needs of hepatopancreatobiliary surgery and advancements in interventional radiology for hepatopancreatobiliary diseases. The development of collateral blood flow with one of the hepatic arteries being occluded was shown to be a possibility and to depend heavily on the site of vascular obstruction[26,27]. Hepatic interlobar arterial collaterals were exhaustively analyzed in autopsied specimens and corrosion casts[26-30], as well as with radiological studies[31-37] called into being by the evolution of hepatic surgery, transplantation, interventional radiology, endovascular chemotherapy and embolization. Angiography demonstrated the interlobar branch-relayed collateral blood flow between the hepatic arteries[31-37] to be readily noted at the occlusion of either of the hepatic arteries[32,33,37], which was demonstrated by computerized tomographic angiography in our Case 2 (Figure 11). The interlobar arterial collaterals may be responsible for the poor distribution of a chemotherapeutic agent at its selective intraarterial infusion[32]. Injuries to these collateral pathways, participating in the blood supply of the hilar bile ducts, may induce ischemia of the biliary tract after liver resections and biliary surgery[28,29]. The majority of investigators are in agreement that the interlobar collateral is extraparenchymal, passes cranial to the bifurcation of the PVs in the hepatic hilum in close proximity to the bile ducts[32,33,37-40] and makes the crucial contribution of the blood supply to the biliary tract, as well as one of the hepatic lobes in the event of liver major route interruption[29,36,37,41]. So far it has not been clear whether there are transparenchymal branches to connect the hepatic lobes[31,33,36,42].
Case 2 demonstrates that LHA excision at a distal pancreatectomy with resection of CA and its branches is permissible by virtue of the fact that an arterial blood supply keeps coming to the left hepatic lobe thanks to the availability of the interlobar collateral. In this case, the arterial blood supply to the left hepatic lobe and segment 1 kept being furnished through the interlobar collateral, originating from the rRHA and running in the liver hilum (Figure 12). The evidence for the functioning of the interlobar collateral emerges quite frequently on angiography at chemoembolization of the hepatic arteries or for control of external hemorrhage and/or hemobilia (Figures 13 and 14).
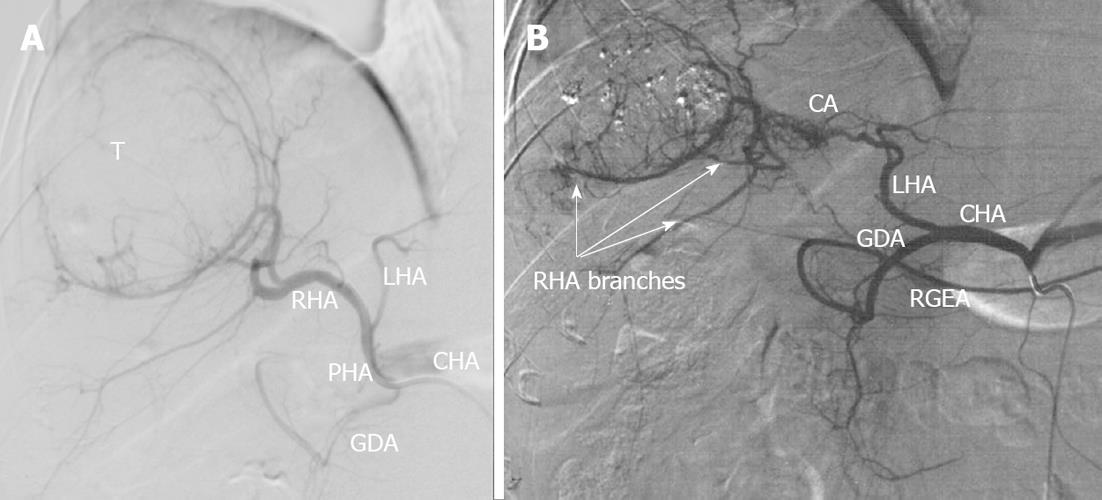
Figure 13 Selective celiacography in a 37-year-old man with firearm (machine gun shots) liver wounds, false aneurysms of left hepatic lobe and hemobilia.
A: Classical arterial architecture (Michels, type 1). There is communicating interlobar artery (CA) connecting right and left hepatic arteries (RHA and LHA). Turbulent blood flow is seen in areas of pulsative hematomas (H); B: Control of hemorrhage was achieved with RHA occlusion but arterial branches of right hepatic lobe keep being filled owing to CA-conveyed blood transit from the left hepatic artery (LHA). GDA: Gastroduodenal artery; PHA: Proper hepatic artery; CHA: Common hepatic artery; RGEA: Right gastroepiploic artery; T: Tumor.
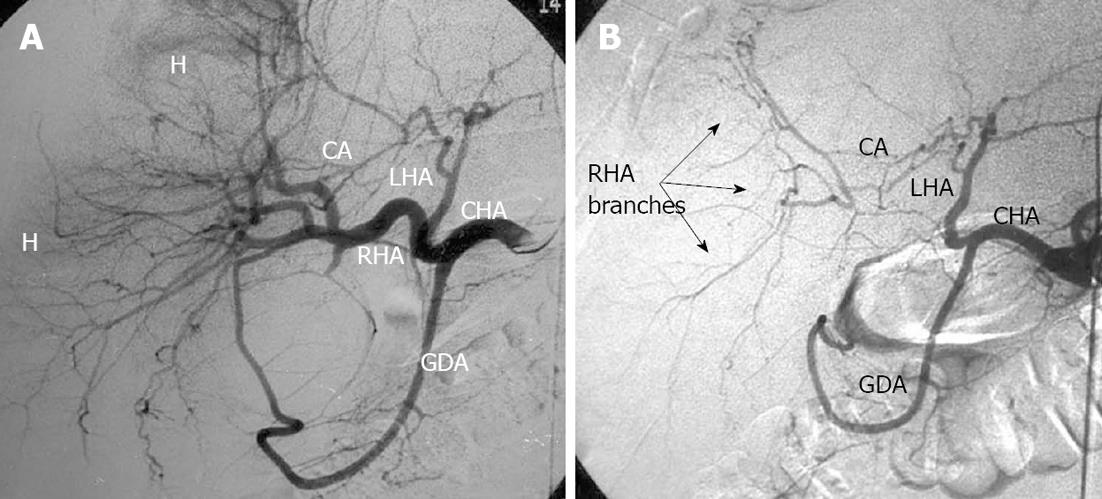
Figure 14 Selective celiacography in a 64-year-old man with hepatocellular cancer.
A: Before and after chemoembolization through the right hepatic artery; B: Arterial branches of right hepatic lobe keep being filled owing to communicating interlobar artery (CA)-conveyed blood transit from the left hepatic artery (LHA). GDA: Gastroduodenal artery; CHA: Common hepatic artery; RHA: Right hepatic artery; H: Hematoma.
Pancreatic cancer remains one of the most aggressive neoplastic processes and the ways of its management are in the development stage[43,44]. Despite impressive progress attained in the diagnosis and treatment of otherwise sited malignances, the resectability and 5 year survival rates for pancreatic cancer are still very poor with those for pancreatic body and tail of 10% and 10%[45-47] in North America and the Western Europe and of 34% and 18% in Japan respectively[48]. Compared to pancreatic head cancer, typically manifesting itself in jaundice, and for lack of specific symptoms, carcinoma of the pancreatic body is generally recognized at more advanced stages, presenting with rather a sizable tumor, distant metastases and back pain.
The pancreatic body is a fairly modest size across and tumor advancement to the retroperitoneal organs, nerve plexuses, SA, CHA and CA does not take long, which potentially causes the neoplasm to be interpreted as unresectable in compliance with the adopted Jakarta International Community Center classification[1]. Just the same, not only does radical removal of pancreatic body carcinoma with the use of Appleby’s technique improve patient life quality, it also significantly prolongs survival, which was attested to, not only in cases of arterial resection necessitating no reconstruction[5,11,13-21], but in those resorting to repair as well[16,23-25].
Completeness (radicality) of resection is one of the dominant independent prognostic factors for pancreatic cancer[46-53]. A curative resection of tumor is the only means of treatment that will hold out a hope of long-term survival for pancreatic cancer patients, although only 10%-15% of them would be eligible for a radical procedure[54]. Preoperative resectability estimation is an outstanding problem of great concern resulting primarily from the intricacies of the involvement evaluation of the major peripancreatic arteries in so far as their actual invasion is regarded as a contraindication to a curative procedure[55-57]. Since CT is invariably rated the gold standard for the diagnosis of pancreatic cancer, it is of critical importance that patients with a resectable tumor should not be denied surgery on account of a false-positive CT finding (i.e., when a neoplasm is misinterpreted as nonresectable at CT). On the other hand, what counts for no less is the prevention of an unneeded cumbersome procedure, fraught with devastating morbidity, for an unresectable lesion, keeping in mind that a R2 resection is associated with poor survival.
In the historical series of studies conducted by different authors prior to 2000, from 15% to 70% pancreatic cancer cases thought of as resectable from CT data turned out to be nonresectable at operation[58-61]. As of now, the sensitivity and specificity of the assessment of vascular involvement, even with a > 90o circular contact and marked vascular deformity (D or E according to Phoa), are reported at 60% and 90% correspondingly[62,63], which indicates that the accuracy of pancreatic cancer resectability appraisal is an elusive troublesome question. Based on the findings reported by various researchers, Li et al[64] defined the following CT criteria for major vascular invasion with an exhibited sensitivity of the method of 79% and a specificity of 99%: embedment of the arterial trunk in tumor, encasement by tumor > 180o or > 50% of the vessel circumference coupled with either irregularity of the wall contour or arterial narrowing. Loyer et al[65] established that with type A (a fat plane between tumor and vessel) or type B (a normal pancreatic parenchyma separating the tumor from the vessel), the accuracy of the resectability prediction was 95% and Phoa et al[62] showed type D (a vascular wall concavity against the tumor to be consistent with a 88% risk for invasion and a 7% predicted resectability) and type E (complete vascular encirclement by tumor) to correlate with a 0% resectability, depending on tumor surface irregularity and vascular deformity. Nevertheless, there is presently no consensus of opinion as to the modality of choice for the assessment of pancreatic cancer extension previous to surgery, since studies that would offer sufficient accuracy are lacking[66,67].
Both our cases demonstrate the unresolved problem of pancreatic cancer resectability determination to be currently pressing, among other reasons, as a consequence of CT being employed for this purpose in the majority of clinics (and quite routinely as a single option). The basic guide to the accuracy of resectability estimation is the ability of a diagnostic technique to identify the presence or absence of invasion of the major peripancreatic arteries. In Case 1, circumferential encasement of the GDA was found on CT and in Case 2, CHA, LHA and CA bifurcation was recognized. At surgery and subsequently under the microscope, the finding in Case 1 proved to be a mere close tumor-artery contact free of ingrowth (which ensured a R1-resection level). In observation 2, the bulk of the tissue misdiagnosed as tumor at CT turned out to be fibrosis with no features of post-therapeutic changes, which in great part enabled a R0 resection level. From strict considerations relying on the belief that the larger is the tumor-vessel contact area, the higher is the likelihood of vascular invasion[68], both cases might have been judged unresectable, as concluded from the CT interpretation[69]. Nonetheless, in both cases, the tumor was found to be resectable, which suggests that it is desirable that CT evidence-based conclusion in favor of unresectability should be confirmed with further clarifying adjunct modalities, such as EUS.
We feel that the salient features of the reported cases might be of equal interest to hepatopancreatobiliary surgeons as well as diagnostic radiologists engaged in this challenging line.