Published online Aug 27, 2025. doi: 10.4240/wjgs.v17.i8.109069
Revised: June 2, 2025
Accepted: June 18, 2025
Published online: August 27, 2025
Processing time: 118 Days and 9.5 Hours
Sigmoid colon cancer faces challenges due to anatomical diversity, including variable inferior mesenteric artery (IMA) branching and tumor localization com
To comprehensively evaluate the impact of three-dimensional (3D) visualization technology on enhancing surgical precision and safety, as well as optimizing perioperative outcomes in laparoscopic sigmoid cancer resection.
A prospective cohort of 106 patients (January 2023 to December 2024) undergoing laparoscopic sigmoid cancer resection was divided into the 3D (n = 55) group and the control (n = 51) group. The 3D group underwent preoperative enhanced computed tomography reconstruction (3D Slicer 5.2.2 & Mimics 19.0). 3D recon
The 3D group demonstrated a significantly shorter operative time (172.91 ± 20.69 minutes vs 190.29 ± 32.29 minutes; P = 0.002), reduced blood loss (31.5 ± 11.8 mL vs 44.1 ± 23.4 mL, P = 0.001), earlier postoperative flatus (2.23 ± 0.54 days vs 2.53 ± 0.61 days; P = 0.013), shorter hospital length of stay (13.47 ± 1.74 days vs 16.20 ± 7.71 days; P = 0.013), shorter postoperative length of stay (8.6 ± 2.6 days vs 10.5 ± 4.9 days; P = 0.014), and earlier postoperative exhaust time (2.23 ± 0.54 days vs 2.53 ± 0.61 days; P = 0.013). Furthermore, the 3D group exhibited a higher mean number of lymph nodes harvested (16.91 ± 5.74 vs 14.45 ± 5.66; P = 0.030).
The 3D visualization technology effectively addresses sigmoid colon anatomical complexity through surgical navigation, improving procedural safety and efficiency.
Core Tip: This prospective study evaluated the role of three-dimensional (3D) reconstruction technology in laparoscopic sigmoid colon cancer surgery. Preoperative 3D models generated via contrast-enhanced computed tomography and 3D Slicer/Mimics software guided tumor localization, vascular assessment, and lymph node dissection. Compared with the control group, the 3D group showed shorter operative time, less blood loss, earlier postoperative recovery, and higher lymph node yield, demonstrating that 3D visualization enhances surgical precision and safety in managing anatomical complexities of laparoscopic sigmoid colon cancer.
- Citation: Zhao ZX, Yao RD, Hu ZJ, Chen CQ, Zhu S, Yao Y. Navigating anatomical complexity in laparoscopic sigmoid cancer surgery: A three-dimension reconstruction protocol for intraoperative safety and efficiency. World J Gastrointest Surg 2025; 17(8): 109069
- URL: https://www.wjgnet.com/1948-9366/full/v17/i8/109069.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i8.109069
Colorectal cancer (CRC) ranks among the most prevalent malignancies of the digestive tract, with significant global variations in its anatomical distribution[1,2]. Epidemiologically, the rectum represents the most common primary tumor site (35%-50% of CRC cases), followed by the sigmoid colon (20%-30%), while ascending and descending colon cancers collectively account for 15%-25% of cases[3,4]. In recent years, the incidence of malignant tumors of the sigmoid colon, a significant component of CRC, has been on the rise[5]. Due to its special location and complex anatomical characteristics, clinical treatment encounters numerous challenges, which significantly affect people's health and quality of life[6]. Therefore, it is extremely urgent to conduct in-depth research on sigmoid colon malignancies, which is of crucial significance for improving treatment outcomes and patient prognosis.
Therapeutic strategies for sigmoid colon cancer adhere to a multimodal paradigm, with radical surgical resection remaining the cornerstone of curative-intent treatment[7,8]. Over the past decade, minimally invasive techniques—particularly laparoscopic and robotic-assisted approaches—have revolutionized perioperative management by achieving equivalent oncological outcomes to open surgery while conferring advantages in intraoperative blood loss and postoperative recovery[9,10]. This anatomical complexity is compounded by significant inter-patient variations in sigmoid colon morphology, including differences in mesenteric fixation, length, and classification of critical vascular structures, and factors that directly influence intraoperative decision-making.
Firstly, the selection of surgical approaches for sigmoid colon tumors is anatomically guided, depending on tumor location within the sigmoid colon (proximal, mid, or distal segments)[11,12]. Left hemicolectomy is indicated for tumors located in the proximal sigmoid colon, with lymphadenectomy extending to the territories of the left colic artery (LCA) and inferior mesenteric artery (IMA; D3-level dissection), guided by tumor staging and lymphatic drainage patterns[11]. For distal sigmoid tumors approaching the rectosigmoid junction, sigmoidorectal resection with partial mesorectal excision (low anterior resection) is recommended[13]. Secondly, the surgical strategy is influenced by the anatomical length of the sigmoid colon. In cases of shorter sigmoid colon, extended left hemicolectomy is often required to achieve tension-free anastomosis. Thirdly, the vascular architecture of the sigmoid colon, predominantly supplied by the IMA, demonstrates considerable anatomical variability in branching patterns and vascular territory distribution[14,15]. Moreover, the surgical strategy concerning IMA ligation level (high vs low) remains a subject of persistent controversy, particularly regarding its impact on lymphatic clearance efficacy and perfusion of the anastomotic site[16,17]. In summary, factors such as the tumor location, sigmoid colon length, IMA classification, mesentery length, presence of adhesions, and the choice between high or low ligation, all significantly influence the selection of surgical protocols for sigmoid colon cancer.
The significant anatomical variations encountered in sigmoid colon cancer surgery necessitate the development of sophisticated preoperative visualization strategies. While conventional cross-sectional imaging modalities [computed tomography (CT)/magnetic resonance imaging] provide essential diagnostic information, their inherent two-dimensional limitations impede precise spatial characterization of tumor localization, vascular anatomical variants, and critical visceral adjacency – a deficiency that frequently culminates in intraoperative navigational uncertainty[18,19]. Emerging evidence has validated the clinical value of computer-assisted three-dimensional (3D) reconstruction technology in enhancing surgical planning precision, intraoperative anatomical variation detection, and real-time navigation accuracy, ultimately contributing to optimized postoperative clinical outcomes[15,19,20]. However, the clinical application of 3D visualization in sigmoid colon cancer management remains constrained by insufficient system validation and procedural standardization, mandating urgent further investigations. Building on this premise, our study implemented preoperative contrast-enhanced CT-based 3D reconstruction into sigmoid cancer surgical procedures. This innovative protocol not only generated patient-specific surgical roadmaps but also provided augmented reality guidance for intraoperative navigation, with preliminary assessment of its efficacy in overcoming anatomical complexity challenges inherent to minimally invasive approaches.
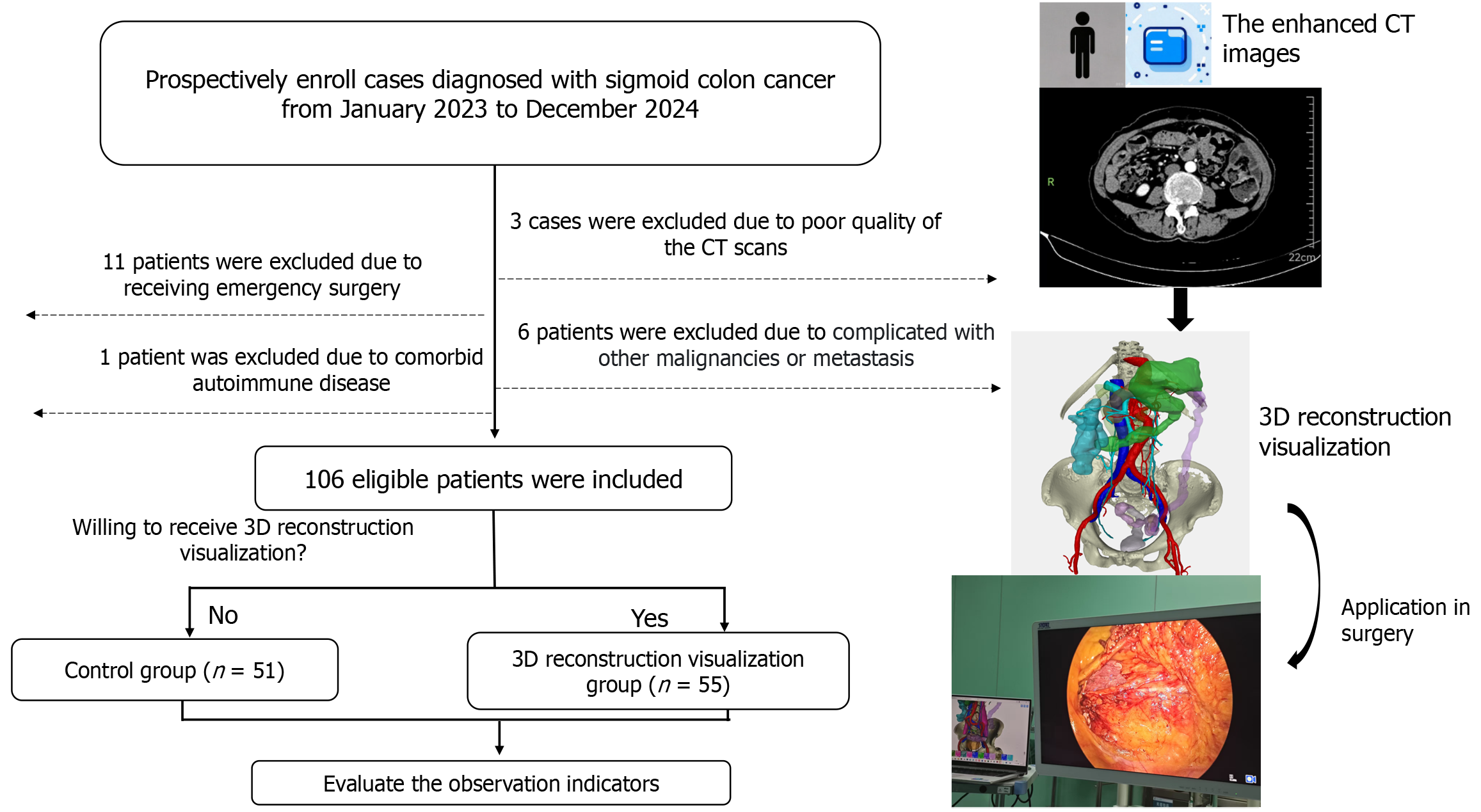
This prospective cohort study was conducted at the Department of General Surgery, Fuyang City People's Hospital from January 2023 to December 2024. Inclusion criteria were as follows: (1) Age ≥ 18 years; (2) Confirmed sigmoid colon adenocarcinoma (colonoscopy and pathological examination); (3) Scheduled for elective laparoscopic sigmoid colectomy; (4) Preoperative double-phase abdominal contrast-enhanced CT; and (5) Written informed consent. Exclusion criteria were: (1) Metastatic disease (M1); (2) Synchronous malignancies; (3) Uncompensated cardiopulmonary dysfunction; (4) Cognitive/psychiatric impairment precluding treatment cooperation; (5) Complicated with immune system diseases; (6) Poor quality of the CT scans; (7) Receiving emergency surgery due to bleeding or obstruction; and (8) Conversion from laparoscopy to open surgery. Patients were assigned to the 3D reconstruction group or control group based on their informed consent preference for 3D-assisted surgery.
This study was approved by the Ethics Committee of Fuyang City People's Hospital (Ethics Approval No.[2024] 203) and all procedures followed the Declaration of Helsinki principles. The study design and flow chart are shown in Figure 1.
Preoperatively, we carried out 3D reconstruction and visualization of the patient's surgical-relevant vessels and organs using enhanced CT scan data. Specifically, the CT scans in DICOM format were imported into 3D modeling software (3D Slicer 5.2.2 and Mimics 19.0) for 3D reconstruction. The 3D reconstruction and visualization encompassed the colon and tumor, the main feeding arteries, namely the IMA, LCA, sigmoid artery (SA), and superior rectal artery (SRA), as well as the veins that drain the sigmoid colon, such as the inferior mesenteric vein (IMV). It also included lymph nodes with suspicious signs of metastasis, adjacent hazardous vessels like the arc of Riolan (marginal arterial arcade), iliac artery/vein, and abdominal aorta/vein, and important anatomical landmarks, for example, the pancreas and ureter. The reconstruction process for each patient took approximately 2 hours. The 3D images can be rotated and sliced successively in any direction.
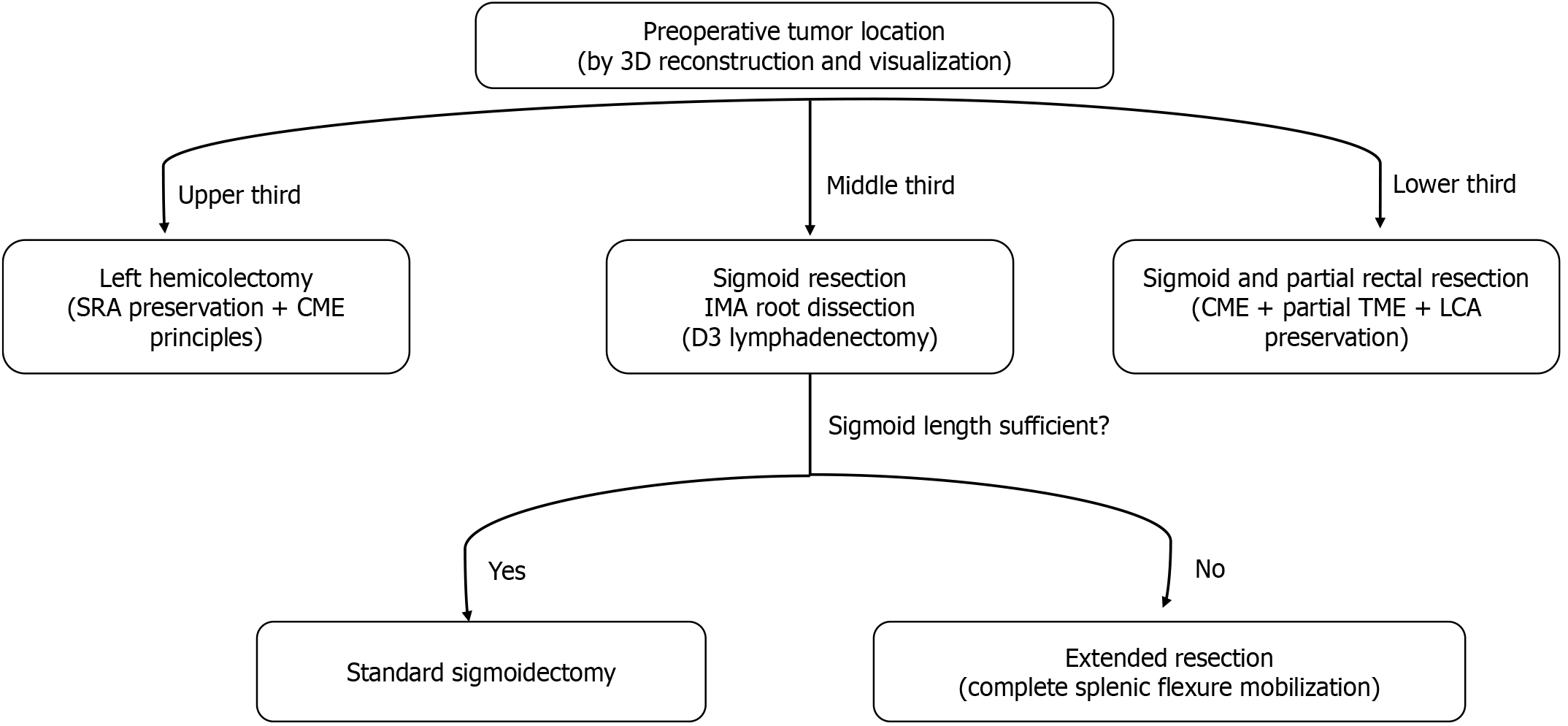
In the control group, the surgical plan was determined or changed based on the preoperative contrast-enhanced CT images, the results of colonoscopy, and the intraoperative exploration findings. Additionally, decisions regarding whether to preserve the SRA or LCA were made according to the location of the tumor and the course of blood vessels. In the 3D visualization-assisted group, an appropriate surgical approach was determined in advance according to the tumor location (Figure 2). The definitions of tumor location are as follows: The upper sigmoid colon refers to the segment from the junction of the sigmoid colon and the descending colon to the proximal 1/3 of the sigmoid colon. The middle sigmoid colon refers to the middle 1/3 region of the sigmoid colon. The lower sigmoid colon refers to the distal 1/3 near the rectosigmoid junction[21]. Moreover, with the aid of 3D visualization, surgeons preoperatively classified the following IMA branching patterns: (1) Type I, where the LCA arises independently from the IMA; (2) Type II, where the LCA and SA both branch from a common trunk of the IMA; (3) Type III, where the LCA, SA, and SRA all branch at the same location; and (4) Type IV, where the LCA is absent, leaving only the SA and SRA. Additionally, prior to the operation, efforts were made to clarify the spatial structural relationships among the LCA, IMA, and IMV, as well as the course of the marginal artery. This was crucial to avoid accidental injury during the operation. During surgery in the 3D visualization-assisted group, the trocar was placed at an appropriate position. The 3D reconstruction visual images then played a guiding role in the surgical procedures. They also assisted the surgeon in identifying key anatomical landmarks, such as the ureter, gonadal vessels and lower margin of the pancreas, and helped the surgeon reach the correct anatomical plane.
The basic characteristics of the two groups were assessed by the evaluation of seven factors: Gender, age, height, weight, body mass index, clinical stage, tumor location, surgical approach and IMA types. We subsequently analyzed additional factors, including the operating time, intraoperative blood loss, postoperative exhaust time, number of lymph nodes dissected, and the occurrence of lymph vessel and nerve invasion. We further considered tumor invasion depth (T stage), presence of lymph node metastasis (N stage), hospital length of stay, postoperative length of stay, and the incidence of postoperative complications, including anastomotic leakage, intestinal obstruction, and wound infection. Postoperative length of stay was defined as the time from surgery to discharge, whereas hospital length of stay refers to the time from admission to discharge.
Using GraphPad Prism 8 software for statistical analysis, measurement data were analyzed using the Student’s t-test or Mann–Whitney U test, while enumeration data were analyzed using the χ2 or Fisher's exact tests. Statistical significance was set at P < 0.05.
From January 2023 to December 2024, 127 patients diagnosed with sigmoid colon cancer were included (Figure 1). Of these patients, 11 were excluded as they underwent emergency surgery due to obstruction or perforation. Six patients were excluded as they had concurrent liver metastases or other malignant tumors. Three patients were excluded due to the substandard quality of their enhanced CT scans. One patient was excluded as they had an autoimmune disease. In total, 106 patients with sigmoid colon malignancies were included in the study. Among them, 55 patients were willing to receive 3D reconstruction-visualized assisted surgical treatment, while 51 patients received surgical treatment without 3D reconstruction assistance (control group). The baseline data of the two groups are shown in Table 1. Statistical analysis revealed no significant differences between the two groups in terms of these baseline parameters.
| Characteristics | Observation group (n = 55) | Control group (n = 51) | t value/χ2 | P value |
| Age (years) | 69.27 ± 12.38 | 66.31 ± 14.20 | 1.134 | 0.259 |
| Gender | 0.019 | 0.891 | ||
| Male | 32 | 29 | ||
| Female | 23 | 22 | ||
| Height (cm) | 162.96 ± 8.09 | 163.06 ± 8.99 | 0.057 | 0.955 |
| Weight (kg) | 61.85 ± 12.67 | 61.95 ± 11.01 | 0.041 | 0.967 |
| BMI (kg/m2) | 23.20 ± 3.94 | 23.192 ± 3.06 | 0.008 | 0.993 |
| Clinical stage | 3.931 | 0.140 | ||
| I | 13 | 21 | ||
| II | 26 | 17 | ||
| III | 16 | 13 | ||
| Tumor location | 0.489 | 0.783 | ||
| Upper | 5 | 6 | ||
| Middle | 17 | 13 | ||
| Lower | 33 | 32 | ||
| IMA types | 0.642 | 0.887 | ||
| I | 27 | 25 | ||
| II | 10 | 12 | ||
| III | 15 | 12 | ||
| IV | 3 | 2 | ||
| Surgical approach | 0.617 | 0.735 | ||
| Left hemicolectomy | 8 | 10 | ||
| Sigmoid colectomy | 19 | 15 | ||
| Low anterior resection | 28 | 26 |
The 3D reconstruction visualization technology ensures the smooth progress of laparoscopic sigmoid colon cancer surgery through precise preoperative planning and intraoperative augmented reality navigation. Its core applications are as follows.
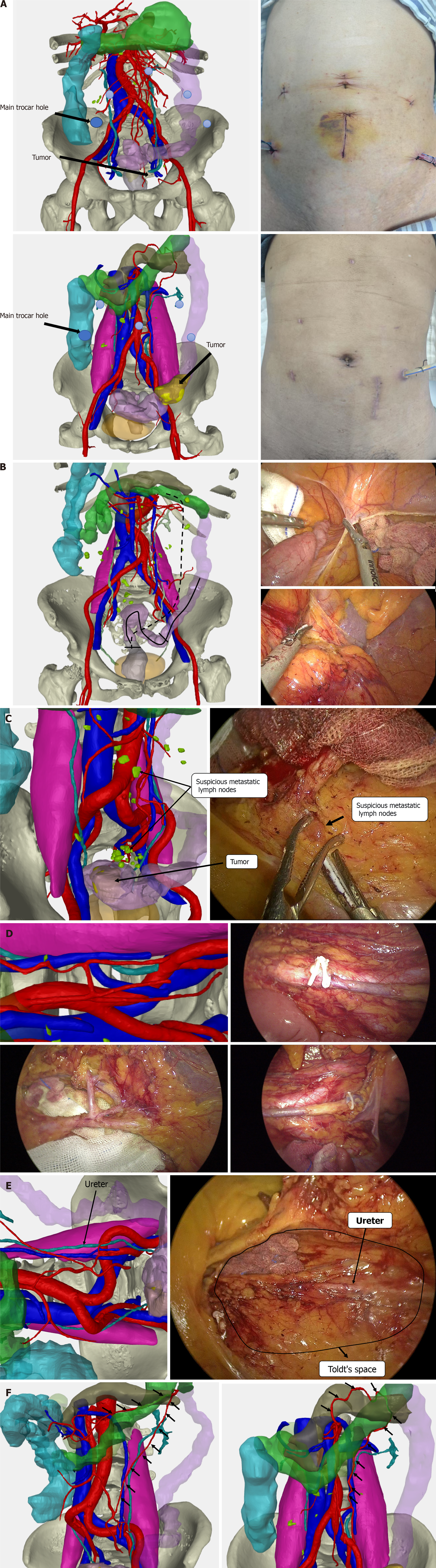
Tumor localization and optimization of trocar placement: Based on the precise location of the tumor in the sigmoid colon (upper, middle, or lower segment), the 3D model guides the positioning of trocar puncture holes (five-hole method or modified layout) in laparoscopic surgery. This helps avoid important blood vessels or organs and prevents insufficient distance during mobilization of the splenic flexure (Figure 3A).
Hierarchical decision-making on the approach and surgical method: Through 3D imaging, the position of the tumor and the length of the sigmoid colon are judged to determine the surgical approach, and options include left hemicolectomy, sigmoid colectomy, or anterior resection of the rectum (Figure 2).
Prediction of splenic flexure mobilization: By measuring the virtual sigmoid colon length and estimating the ana
Classification of the IMA and lymph node dissection: Preoperative 3D vascular imaging clarifies the anatomical classification of the IMA. Combined with the status of regional lymph node metastasis, an individualized dissection strategy is formulated (Figure 3C). If possible, the LCA or SRA is preserved to ensure the blood supply of the anastomotic site (Figure 3D).
Navigation of the anatomical path in Toldt's space: 3D reconstruction identifies anatomical landmarks (such as the duodenojejunal flexure and the ureter), guiding surgeons to enter the correct anatomical plane (Toldt's space), reducing collateral damage and intraoperative bleeding (Figure 3E).
Visualized protection of the marginal arch: 3D imaging clearly shows the marginal artery, preventing accidental damage during the operation and thus avoiding intestinal and anastomotic ischemia and necrosis (Figure 3F).
The perioperative characteristics of sigmoid colon cancer patients are summarized in Table 2. The observation group demonstrated significant reductions in operative time (172.91 ± 20.69 minutes vs 190.29 ± 32.29 minutes; P = 0.002), intraoperative blood loss (31.45 ± 11.82 mL vs 44.12 ± 23.36 mL; P = 0.001), and time to first postoperative flatus (2.23 ± 0.54 days vs 2.53 ± 0.61 days; P = 0.013) compared to the control group. Additionally, the observation group exhibited a higher mean number of lymph nodes harvested (16.91 ± 5.74 vs 14.45 ± 5.66; P = 0.030). No significant intergroup differences were observed in pathological parameters including lymph vessel invasion, nerve invasion, depth of tumor invasion, lymph node metastasis, or postoperative complications such as anastomotic leakage, wound infection, and intestinal obstruction. Notably, the observation group showed shorter hospitalization durations, with both hospital length of stay (13.47 ± 1.74 days vs 16.20 ± 7.71 days; P = 0.013) and postoperative length of stay (8.6 ± 2.6 days vs 10.5 ± 4.9 days; P = 0.014) being significantly reduced compared to controls.
| Characteristics | Observation group (n = 55) | Control group (n = 51) | t value/χ2 | P value |
| T | 0.416 | 0.677 | ||
| T1/T2 | 37 | 37 | ||
| T3/T4 | 18 | 14 | ||
| N | 0.237 | 0.813 | ||
| N0 | 39 | 38 | ||
| N+ | 16 | 13 | ||
| Lymph vessel invasion | 0.173 | 0.678 | ||
| Yes | 16 | 13 | ||
| No | 39 | 38 | ||
| Nerve invasion | 0.043 | 0.835 | ||
| Yes | 15 | 13 | ||
| No | 40 | 38 | ||
| Number of lymph node dissections | 16.91 ± 5.74 | 14.45 ± 5.66 | 2.197 | 0.030 |
| Operating time (minutes) | 172.91 ± 20.69 | 190.29 ± 32.29 | 3.272 | 0.002 |
| Intraoperative blood loss (mL) | 31.45 ± 11.82 | 44.12 ± 23.36 | 3.525 | 0.001 |
| Postoperative exhaust time (day) | 2.23 ± 0.54 | 2.53 ± 0.61 | 2.256 | 0.013 |
| Postoperative length of stay (days) | ||||
| mean ± SD | 8.6 ± 2.6 | 10.5 ± 4.9 | 2.754 | 0.014 |
| Median (IQR) | 8.00 (8.00, 10.00) | 9.00 (7.00, 12.00) | 0.201 | |
| Hospital length of stay (days) | ||||
| mean ± SD | 13.47 ± 1.74 | 16.20 ± 7.71 | 2.256 | 0.013 |
| Median (IQR) | 14.00 (12.00, 15.00) | 35.1 (31.4, 38.6) | 0.169 | |
| Intestinal obstruction | 1.000 | |||
| Yes | 2 | 1 | ||
| No | 53 | 50 | ||
| Anastomotic leakage | 0.497 | |||
| Yes | 0 | 2 | ||
| No | 55 | 59 | ||
| Wound infection | 0.486 | |||
| Yes | 0 | 1 | ||
| No | 54 | 50 | ||
Due to its variable anatomical configurations (including the course of the intestinal canal, the attachment points of the mesentery, and the spatial relationships with adjacent organs), surgical treatment of tumors in the sigmoid colon poses significant challenges[22,23]. The anatomical location of the tumor (at the descending-sigmoid junction, the middle segment, or the recto-sigmoid junction) directly determines the choice of surgical approach: Left hemicolectomy is suitable for proximal tumors, while lesions in the recto-sigmoid junction area require anterior resection of the rectum combined with partial mesenteric resection[24]. The goal of radical tumor resection requires two key elements: (1) Sufficient resection of the intestine (ensuring that the proximal and distal resection margins are ≥ 5 cm); and (2) Systematic lymph node dissection (lymph nodes at the root of the IMA, intermediate group, and around the intestine). Determining whether to mobilize the splenic flexure of the colon to achieve a tension-free anastomosis is also one of the practical challenges faced by clinicians[25]. Moreover, if the tumor is located in the lower part of the sigmoid colon, the LCA can be preserved; if the tumor is in the upper part of the sigmoid colon, the SRA can be retained[26,27]. This can increase the blood supply to the anastomotic site, reduce the incidence of anastomotic leakage, and promote the rapid recovery of the gastrointestinal tract after surgery. Thorough lymph node dissection is of vital importance for the prognosis of patients with sigmoid colon cancer, especially for the root of the IMA[28,29]. Given the numerous anatomical variations of the IMA and its branches, unidentified variations can complicate blood vessel skeletonization, prolong the operation, and even cause damage to vital vessels[15,30]. Protecting the marginal artery during surgery is also crucial, as accidental damage can significantly increase the operation time and the incidence of postoperative complications. These above-mentioned clinical problems result in huge challenges for surgeons and patients.
In order to solve these problems, in the present study, we preoperatively established patient-specific 3D visualization models using contrast-enhanced CT reconstruction technology for sigmoid colon cancer patients. These reconstructed 3D models precisely delineated anatomical structures, providing critical guidance for surgical planning. The 3D re
This investigation has several limitations that warrant acknowledgment. First, the cohort size (n = 106) and single-center design inherently restrict the generalizability of findings. Despite the attainment of statistical significance in key endpoints, the limitations stemming from a small sample size and single-center design impede the widespread acceptance of the study's generalizability. Furthermore, the non-randomized control design introduces inherent selection bias. Second, technical difficulties have restricted the widespread implementation of the research. Conducting 3D reconstruction using CT images requires close collaboration among surgeons, radiologists, and 3D reconstruction technicians. Low-quality images and inexperienced technicians pose severe challenges to the entire reconstruction process. Third, the absence of longitudinal oncologic outcomes (5-year disease-free survival/overall survival) precludes assessment of the technology's impact on cancer control efficacy.
Looking ahead, integrating artificial intelligence (AI) powered automated segmentation algorithms into 3D re
Considering the aforementioned limitations, further in-depth research is imperative. First and foremost, subsequent multicenter randomized controlled trials (phase II/III) should be conducted to more thoroughly validate the research findings. This large-scale, well-controlled study design will enhance the generalizability and reliability of the results, providing a more solid foundation for clinical application. Secondly, aiming to reduce reconstruction time and boost the accuracy of 3D reconstruction images, exploring the application of machine learning algorithms or integrated AI in the reconstruction process is a highly promising research avenue. These advanced technologies can automate complex tasks, such as vascular segmentation, leading to more efficient and precise reconstructions. In the future, to drive the clinical translation of 3D reconstruction images forward, integrating 3D images into laparoscopic or robotic systems shows great potential. This integration can offer real-time, intuitive visual guidance for surgeons during operations, potentially improving surgical outcomes and reducing complications. Finally, it remains to be investigated whether 3D re
This study demonstrated that preoperative 3D visualization significantly enhanced the precision and safety of laparoscopic sigmoid colon cancer surgery. By integrating patient-specific anatomical modeling with real-time intraoperative guidance, the technology addresses the challenges posed by the region’s complex vascular variability and spatial relationships.
| 1. | Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4235] [Cited by in RCA: 11431] [Article Influence: 3810.3] [Reference Citation Analysis (4)] |
| 2. | Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 9945] [Article Influence: 4972.5] [Reference Citation Analysis (2)] |
| 3. | Morgan E, Arnold M, Gini A, Lorenzoni V, Cabasag CJ, Laversanne M, Vignat J, Ferlay J, Murphy N, Bray F. Global burden of colorectal cancer in 2020 and 2040: incidence and mortality estimates from GLOBOCAN. Gut. 2023;72:338-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 511] [Cited by in RCA: 922] [Article Influence: 461.0] [Reference Citation Analysis (1)] |
| 4. | Wong MCS, Huang J, Lok V, Wang J, Fung F, Ding H, Zheng ZJ. Differences in Incidence and Mortality Trends of Colorectal Cancer Worldwide Based on Sex, Age, and Anatomic Location. Clin Gastroenterol Hepatol. 2021;19:955-966.e61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 235] [Article Influence: 58.8] [Reference Citation Analysis (0)] |
| 5. | Patel SG, Dominitz JA. Screening for Colorectal Cancer. Ann Intern Med. 2024;177:ITC49-ITC64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 6. | Jiang F, Ji M, Jin F, Liu J, Liu X. Clinical application of two-port laparoscopic surgery in sigmoid colon and upper rectal cancer resection. Front Oncol. 2023;13:1248280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 7. | Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. 2014;383:1490-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1965] [Cited by in RCA: 2293] [Article Influence: 208.5] [Reference Citation Analysis (1)] |
| 8. | Lordick F, Carneiro F, Cascinu S, Fleitas T, Haustermans K, Piessen G, Vogel A, Smyth EC; ESMO Guidelines Committee. Gastric cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33:1005-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 686] [Article Influence: 228.7] [Reference Citation Analysis (0)] |
| 9. | Holzmacher JL, Luka S, Aziz M, Amdur RL, Agarwal S, Obias V. The Use of Robotic and Laparoscopic Surgical Stapling Devices During Minimally Invasive Colon and Rectal Surgery: A Comparison. J Laparoendosc Adv Surg Tech A. 2017;27:151-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 10. | Luo D, Liu Y, Zhu H, Li X, Gao W, Li X, Zhu S, Yu X. The MicroHand S robotic-assisted versus Da Vinci robotic-assisted radical resection for patients with sigmoid colon cancer: a single-center retrospective study. Surg Endosc. 2020;34:3368-3374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Samalavicius NE, Klimasauskiene V, Dulskas A. Open Left Hemicolectomy for Proximal Sigmoid Cancer. Dis Colon Rectum. 2022;65:e14-e15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Chin CC, Yeh CY, Tang R, Changchien CR, Huang WS, Wang JY. The oncologic benefit of high ligation of the inferior mesenteric artery in the surgical treatment of rectal or sigmoid colon cancer. Int J Colorectal Dis. 2008;23:783-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 80] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 13. | Efetov SK, Zubayraeva AA, Serednyakova DV, Mozharov RN, Saltovets RR, Koziy AY. Vascular-oriented D3 lymph node dissection with left colic artery preservation for distal sigmoid colon cancer: a variety of techniques. Tech Coloproctol. 2024;28:119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Murono K, Kawai K, Kazama S, Ishihara S, Yamaguchi H, Sunami E, Kitayama J, Watanabe T. Anatomy of the inferior mesenteric artery evaluated using 3-dimensional CT angiography. Dis Colon Rectum. 2015;58:214-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 15. | Zhao ZX, Hu ZJ, Yao RD, Su XY, Zhu S, Sun J, Yao Y. Three-dimensional printing for preoperative rehearsal and intraoperative navigation during laparoscopic rectal cancer surgery with left colic artery preservation. World J Gastrointest Surg. 2024;16:3104-3113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 16. | Nakamura Y, Yamaura T, Kinjo Y, Harada K, Kawase M, Kawabata Y, Kanto S, Ogo Y, Kuroda N. Level of Inferior Mesenteric Artery Ligation in Sigmoid Colon and Rectal Cancer Surgery: Analysis of Apical Lymph Node Metastasis and Recurrence. Dig Surg. 2023;40:167-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 17. | Zeng J, Su G. High ligation of the inferior mesenteric artery during sigmoid colon and rectal cancer surgery increases the risk of anastomotic leakage: a meta-analysis. World J Surg Oncol. 2018;16:157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 94] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 18. | Liu Y, Meng S, Wei M, Dai F, Xiao L, Mu X, Tang J. Reconstruction of three-dimensional models for complex female pelvic tumors. Int J Gynaecol Obstet. 2022;157:747-749. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 19. | Miyamoto R, Tadano S, Sano N, Inagawa S, Adachi S, Yamamoto M. The impact of three-dimensional reconstruction on laparoscopic-assisted surgery for right-sided colon cancer. Wideochir Inne Tech Maloinwazyjne. 2017;12:251-256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Fletcher J, Ilangovan R, Hanna G, Miskovic D, Lung P. The impact of three-dimensional reconstruction and standardised CT interpretation (AMIGO) on the anatomical understanding of mesenteric vascular anatomy for planning complete mesocolic excision surgery: A randomised crossover study. Colorectal Dis. 2022;24:388-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 21. | Morarasu S, O'Brien L, Clancy C, Dietrich D, Maurer CA, Frasson M, Garcia-Granero E, Martin ST. A systematic review and meta-analysis comparing surgical and oncological outcomes of upper rectal, rectosigmoid and sigmoid tumours. Eur J Surg Oncol. 2021;47:2421-2428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 22. | Wang Y, Wang Y, Zou L, Deng L, Wu T, Liu L, Jiang J, An T. Does the level of inferior mesenteric artery ligation affect short-term and long-term outcomes of patients with sigmoid colon cancer or rectal cancer? A single-center retrospective study. World J Surg Oncol. 2022;20:274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 23. | Planellas P, Marinello F, Elorza G, Golda T, Farrés R, Espín-Basany E, Enríquez-Navascués JM, Kreisler E, Cornejo L, Codina-Cazador A. Extended Versus Standard Complete Mesocolon Excision in Sigmoid Colon Cancer: A Multicenter Randomized Controlled Trial. Ann Surg. 2022;275:271-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 24. | Quan J, Liu J, Zhou S, Mei S, Qiu W, Wan Y, Wang X, Tang J. Surgical outcomes of left hemicolon sparing resection versus extensive resection in treating synchronous colorectal cancer involving the right-sided colon and sigmoid colon or rectum. World J Surg Oncol. 2023;21:131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 25. | Reddy SH, Gupta V, Yadav TD, Singh G, Sahni D. Lengthening of left colon after rectal resection: What all is adequate? A prospective cohort study. Int J Surg. 2016;31:27-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Li B, Wang J, Yang S, Shen J, Li Q, Zhu Q, Cui W. Left colic artery diameter is an important factor affecting anastomotic blood supply in sigmoid colon cancer or rectal cancer surgery: a pilot study. World J Surg Oncol. 2022;20:313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 27. | Zhang JW, Sun Y. Preservation versus non-preservation of left colic artery during laparoscopic radical operation for sigmoid colon cancer. Asian J Surg. 2023;46:5014-5015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 28. | Crocetti D, Cavallaro G, Tarallo MR, Chiappini A, Polistena A, Sapienza P, Fiori E, De Toma G. Preservation of left colic artery with lymph node dissection of IMA root during laparoscopic surgery for rectosigmoid cancer. Results of a retrospective analysis. Clin Ter. 2019;170:e124-e128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 29. | Park SS, Park B, Park EY, Park SC, Kim MJ, Sohn DK, Oh JH. Outcomes of high versus low ligation of the inferior mesenteric artery with lymph node dissection for distal sigmoid colon or rectal cancer. Surg Today. 2020;50:560-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 30. | Zhou J, Chen J, Wang M, Chen F, Zhang K, Cong R, Fan X, Yang J, He B. A study on spinal level, length, and branch type of the inferior mesenteric artery and the position relationship between the inferior mesenteric artery, left colic artery, and inferior mesenteric vein. BMC Med Imaging. 2022;22:38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |