Published online Aug 27, 2025. doi: 10.4240/wjgs.v17.i8.105990
Revised: May 29, 2025
Accepted: June 23, 2025
Published online: August 27, 2025
Processing time: 117 Days and 4.2 Hours
The use of an ultrasound-guided quadratus lumborum block (QLB) combined with general anesthesia for patients undergoing colorectal cancer surgery serves as a model for reducing the postoperative stress response, preserving metabolic stability, protecting renal function, and alleviating postoperative pain.
To compare QLB combined with general anesthesia vs general anesthesia alone in the perioperative stress response, metabolic and renal function, postoperative pain, and recovery outcomes among patients undergoing colorectal cancer surgery.
Clinical data of 116 patients who underwent colorectal cancer surgery at our hospital between July 2023 and August 2024 were collected for retrospective analysis. According to the anesthesia protocol, the patients were divided into the control (general anesthesia, n = 58) and experimental groups (QLB combined with general anesthesia, n = 58). Physiological indicators such as blood glucose (GLU), lactic acid (LAC), blood urea nitrogen (BUN), and creatinine (CRE) were mea
The GLU levels from T1 to T4 in the experimental group were significantly lower than those in the control group (P < 0.001), and the LAC levels were also significantly reduced (P < 0.001). The experimental group exhibited superior renal protection based on postoperative BUN and CRE levels (P < 0.05). Furthermore, the postoperative pain score in the experimental group was significantly lower than that in the control group [visual analogue scale (VAS)] scores differed significantly from T2 to T4, P < 0.05.
Research has shown that QLB combined with general anesthesia can decrease postoperative GLU and LAC by 8%-15% and 10%-20% (P < 0.001), respectively. It also enhances renal function markers (BUN, CRE, P < 0.05) and lowers VAS scores by 15%-30% (P < 0.05). Ultrasound-guided lumbar muscle block with general anesthesia outperforms general anesthesia alone in diminishing stress response, preserving metabolic balance and renal function, and alleviating postoperative pain. This approach offers a more efficient perioperative management strategy for patients undergoing colorectal cancer surgery. It is particularly advantageous for individuals with stress sensitivity, renal impairment, and heightened pain susceptibility.
Core Tip: This retrospective study demonstrates that ultrasound-guided quadratus lumborum block (QLB) combined with general anesthesia significantly improves perioperative outcomes in colorectal cancer surgery. Compared to general anesthesia alone, QLB reduces stress markers (glucose, lactic acid), enhances renal function (blood urea nitrogen, creatinine), and lowers postoperative pain scores. These findings support the use of QLB as a superior anesthetic strategy to maintain circulatory and metabolic stability, protect organ function, and improve recovery. QLB is especially beneficial for patients with renal vulnerability, high stress reactivity, or low pain tolerance, offering safer and more effective perioperative management.
- Citation: Li HJ, Ban X, Li J, Huang SQ. Ultrasound-guided quadratus lumborum block with general anesthesia for perioperative circulatory stability in colorectal cancer surgery. World J Gastrointest Surg 2025; 17(8): 105990
- URL: https://www.wjgnet.com/1948-9366/full/v17/i8/105990.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i8.105990
In recent years, with the continuous progress in anesthesia technology, the management of intraoperative circulation stability has become increasingly important in perioperative management[1]. Colorectal cancer is a malignant tumor with high morbidity and mortality rates worldwide. Surgical intervention can extend patients’ survival time to some extent[2]. However, colorectal cancer surgery often involves extensive intra-abdominal procedures and tissue resection, making patients susceptible to hemodynamic fluctuations, such as hypotension, bradycardia, and decreased oxygen saturation[3,4]. These circulatory changes not only impact the surgical process but also raise the risk of postoperative complications. Therefore, maintaining circulatory stability in patients throughout the perioperative period is vital for enhancing surgical outcomes and reducing postoperative complications[5]. General anesthesia is commonly used during colorectal cancer surgery. While it provides effective anesthesia, it can induce circulatory instability during surgery due to its cardiovas
The clinical data of 116 patients who underwent colorectal cancer surgery at our hospital between July 2023 and August 2024 were collected for retrospective analysis. According to the anesthesia protocol, the patients were divided into two groups: The control (general anesthesia, n = 58) and the experimental groups (QLB combined with general anesthesia, n = 58). All patients provided informed consent, and the study received approval from the hospital ethics committee.
Inclusion criteria: (1) Patients who were between 18 and 75 years old and met the surgical indications; (2) American Society of Aneshesiologists classification I-II; (3) Colorectal cancer was diagnosed definitively and required open or laparoscopic surgery; (4) No severe heart, lung, liver, and renal insufficiency; (5) No other form of anesthetic intervention; and (6) The surgery is expected to take two to five hours.
Exclusion criteria: (1) Patients allergic to local anesthetic drugs or general anesthesia drugs (4 cases), patients with preoperative severe anemia or thrombocytopenia (5 cases), patients with preoperative infectious diseases or coagulation disorders (3 cases), patients with mental or nervous system diseases that prevented them from cooperating with the study (2 cases); and (2) Patients who underwent pelvic radiotherapy or chemotherapy (3 cases).
The control group included 29 males and 29 females, while the experimental group comprised 29 males and 29 females, maintaining a consistent sex distribution (P = 1.000). The average ages of the patients in the control and experimental groups were 60.50 ± 8.10 years and 60.90 ± 8.50 years, respectively, with no significant age difference between the groups (P = 0.805). The mean body mass index of the control and experiment groups was 23.50 ± 2.30 kg/m2 and 23.70 ± 2.40 kg/m2, respectively, showing no statistically significant difference (P = 0.689). Regarding surgery time, the average duration was 3.10 ± 0.60 hours for the control group and 3.00 ± 0.70 hours for the experimental group, with no statistically significant variation (P = 0.540).
Patients in the control group received standard general anesthesia. Thirty minutes before surgery, midazolam (Jiangsu Enhua Pharmaceutical Co., Ltd.; specification: 10 mg/branch) was administered via an intravenous injection of 0.05 mg/kg for sedation. For anesthesia induction, 2 mg/kg of propofol (AstraZeneca Pharmaceuticals Co., Ltd.; specifications: 1% injection, 200 mg/20 mL), 2 μg/kg of fentanyl (Yichang Renfu Pharmaceuticals Co., Ltd.; specifications: 0.1 mg/2 mL), and 0.6 mg/kg of rocuronium cis-benzenesulfonate (Shanghai Hengrui Pharma Co., Ltd.; specifications: 50 mg/5 mL) were intravenously injected for tracheal intubation. During the anesthesia maintenance phase, propofol was continuously infused intravenously at a rate of 4-6 mg/kg/hour, and fentanyl was intermittently administered at a dose of 1 μg/kg to maintain analgesia. The inhaled concentration of isoflurane (Hengrui Pharma, specification: 100 mL/bottle) (1%-3%) was adjusted according to the surgical requirements to maintain the depth of anesthesia. Throughout the surgery, a Mindray BeneView T8 monitor (Shenzhen Meirui Biomedical Electronics Co., Ltd.) was utilized to monitor the patient’s heart rate (HR), blood pressure, oxygen saturation, partial pressure of carbon dioxide (PaCO2), and other vital signs.
Patients in the experimental group received a QLB under ultrasound guidance before undergoing surgery with general anesthesia. They were positioned supine and guided using a Philips Sparq portable ultrasound device (Philips, Ne
In the experimental group, general anesthesia drug doses were decreased by combining regional anesthesia. Patient vital signs were continuously monitored during surgery using a Mindray BeneView T8 monitoring instrument to maintain circulatory stability.
Cardiovascular function indicators: Mean arterial pressure (MAP) was monitored using the Mindray BeneView T8 multi-parameter monitor via automatic measurement of the non-invasive blood pressure cuff on the upper arm. MAP values at each time point before surgery (T0), 10 minutes after anesthesia induction (T1), during surgery (T2), at the end of surgery (T3), and 30 minutes after surgery (T4) were recorded. The normal range was 70-105 mmHg, and hypotension was defined as blood pressure below 65 mmHg. HR was recorded by real-time electrocardiogram (ECG) monitoring of HR fluctuations using an ECG electrode patch fixed on the chest of the patient, with the normal HR range being 60-100 beats/minutes. Monitoring time points were synchronized with MAP, and HR was < 50 beats/minutes for bradycardia and > 100 beats/minutes for tachycardia. The incidences of intraoperative hypotension (MAP < 65 mmHg) and bradycardia (HR < 50 beats/minutes) were recorded, and the cardiovascular stability of the two groups was evaluated.
Respiratory function indicators: Respiratory function indicators comprised the arterial partial pressure of oxygen (PaO2), arterial partial pressure of carbon dioxide (PaCO2), tidal volume (TV) and minute ventilation volume (MV), assessed at five time points: T0 (pre-surgery), T1 (post-surgery), T2 (6 hours post-surgery), T3 (24 hours post-surgery), and T4 (48 hours post-surgery). PaO2 and PaCO2 were determined using an arterial blood gas analyzer. Arterial blood gas samples were obtained via radial artery puncture and analyzed with an i-STAT blood gas analyzer (Abbott). TV represents the volume of gas inhaled or exhaled in each breath, with the normal adult range being 6-8 mL/kg, calculated based on the patient’s weight. MV denotes the ventilation per minute, calculated as the TV multiplied by the respiratory frequency, with the normal range being 5-8 L/minutes. These two indicators were monitored through the ventilator’s display data, with the Philips V60 ventilator utilized for supplementary monitoring during and post-surgery.
Metabolic function indicators: The metabolic function indicators included blood glucose (GLU), lactic acid (LAC), blood urea nitrogen (BUN), and creatinine (CRE), measured at five time points: T0 (pre-surgery), T1 (6 hours post-surgery), T2 (24 hours post-surgery), T3 (post-surgery 24 hours), and T4 (48 hours post-surgery). GLU was detected using the oxidase method, LAC was measured with a lactate dehydrogenase assay, and BUN and CRE levels were determined using enzymatic colorimetry. GLU and LAC were analyzed using an AU5800 automatic biochemical analyzer (Beckman Coulter), while BUN and CRE were analyzed using a Cobas c501 automatic analyzer (Roche Diagnostics).
Renal function indicators: Renal function indicators comprised CRE, BUN, and uric acid (UA), with each indicator detected before surgery, 1 day after surgery, 3 days after surgery, and 7 days after surgery. UA levels were determined using a UA oxidase assay. In serum, UA reacts enzymatically to form a measurable compound, which is then analyzed through colorimetry. Elevated UA levels are commonly linked to impaired renal excretion. Venous blood samples were obtained at each time point, followed by serum extraction post-centrifugation. The biochemical analyzer catalyzes the reaction between UA oxidase and serum UA, with absorbance changes indicating UA concentration. UA concentration was measured and analyzed automatically.
Stress response indicators: The stress response indicators comprised cortisol, norepinephrine (NE), and interleukin-6 (IL-6). These indicators were assessed preoperatively, post-anesthesia, and at 1 day, 3 days, and 7 days post-surgery. Cortisol and IL-6 levels were quantified via enzyme-linked immunosorbent assay. NE was analyzed using high-performance liquid chromatography, with its concentration determined through the separation and detection of NE in serum samples.
Postoperative pain and recovery indicators: Postoperative pain was assessed using the visual analogue scale (VAS) within a range of 0-10 points. Scores were recorded at 2, 6, and 24 hours after surgery. Lower VAS scores indicated better pain control. The first time the patient got out of bed, first exhaust time, and inpatient days were recorded to evaluate the patient’s recovery process.
In this study, SPSS25.0 software was used to conduct t-tests and repeated measures analysis of variance tests to evaluate the differences of treatment indicators across various groups, with a significance level set at α = 0.05. Repeated-measures analysis of variance was used to assess time-course changes in indicators among groups, aiming to determine the reliability of the experimental results and establish a scientific basis for the conclusion of the study.
The MAP of the experimental group from T1 to T4 was significantly higher than that of the control group, and this difference was particularly significant, especially at T2 (P < 0.001). The fluctuation in HR in the experimental group was also significantly lower than that in the control group, especially during T2 (P < 0.001) (Table 1). The incidence of intraoperative hypotension in the experiment group was 8.6% (5/58), while that in the control group was 22.4% (13/58), the incidence of bradycardia in the experiment group was 6.9% (4/58), and that in the control group was 17.2% (10/58).
| Time point | Indicators | Experiment group (n = 58) | Control group (n = 58) | t value | P value |
| T0 | MAP (mmHg) | 95.30 ± 8.25 | 94.87 ± 7.96 | 0.286 | 0.776 |
| T1 | MAP (mmHg) | 87.65 ± 7.12 | 82.34 ± 8.14 | 3.739 | < 0.001a |
| T2 | MAP (mmHg) | 85.20 ± 6.98 | 78.56 ± 7.45 | 4.953 | < 0.001a |
| T3 | MAP (mmHg) | 88.56 ± 7.32 | 80.43 ± 6.98 | 6.122 | < 0.001a |
| T4 | MAP (mmHg) | 93.76 ± 7.02 | 89.56 ± 7.88 | 3.031 | 0.003a |
| T0 | HR (time/minute) | 70.12 ± 7.24 | 72.34 ± 6.98 | -1.573 | 0.119 |
| T1 | HR (time/minute) | 68.75 ± 6.45 | 75.98 ± 6.92 | -5.821 | < 0.001a |
| T2 | HR (time/minute) | 66.78 ± 6.12 | 79.20 ± 7.02 | -10.156 | < 0.001a |
| T3 | HR (time/minute) | 70.34 ± 6.88 | 76.78 ± 7.34 | -4.875 | < 0.001a |
| T4 | HR (time/minute) | 71.45 ± 7.12 | 74.56 ± 7.78 | -2.246 | 0.027a |
The TV of the experimental group after anesthesia induction was significantly higher than that of the control group, and respiratory function stability during surgery was good. The MV of the experimental group at each time point was significantly higher than that of the control group, particularly in the T2 group (P < 0.05). The PaO2 from T1 to T4 in the experimental group was significantly higher than that in the control group, while the PaCO2 was significantly lower than that in the control group (all P < 0.001) (Table 2).
| Time point | Indicators | Experiment group (n = 58) | Control group (n = 58) | t value | P value |
| T0 | TV (mL) | 500.25 ± 32.45 | 498.75 ± 30.62 | 0.256 | 0.798 |
| T1 | TV (mL) | 480.50 ± 29.17 | 455.00 ± 30.75 | 4.582 | < 0.001a |
| T2 | TV (mL) | 470.30 ± 28.92 | 430.40 ± 31.55 | 7.100 | < 0.001a |
| T3 | TV (mL) | 485.75 ± 30.15 | 440.90 ± 29.80 | 8.057 | < 0.001a |
| T4 | TV (mL) | 495.40 ± 31.25 | 465.50 ± 30.10 | 5.248 | < 0.001a |
| T0 | MV (L/minute) | 6.25 ± 0.50 | 6.23 ± 0.48 | 0.220 | 0.826 |
| T1 | MV (L/minute) | 5.90 ± 0.45 | 5.50 ± 0.55 | 4.287 | < 0.001a |
| T2 | MV (L/minute) | 5.80 ± 0.50 | 5.20 ± 0.60 | 5.851 | < 0.001a |
| T3 | MV (L/minute) | 6.00 ± 0.55 | 5.40 ± 0.65 | 5.367 | < 0.001a |
| T4 | MV (L/minute) | 6.15 ± 0.52 | 5.80 ± 0.58 | 3.422 | 0.001a |
| T0 | PaO2 (mmHg) | 95.20 ± 5.80 | 94.90 ± 5.55 | 0.285 | 0.776 |
| T1 | PaO2 (mmHg) | 90.15 ± 4.70 | 85.25 ± 5.20 | 5.324 | < 0.001a |
| T2 | PaO2 (mmHg) | 88.00 ± 4.60 | 80.50 ± 5.10 | 8.317 | < 0.001a |
| T3 | PaO2 (mmHg) | 90.00 ± 4.80 | 82.40 ± 4.85 | 8.482 | < 0.001a |
| T4 | PaO2 (mmHg) | 93.50 ± 4.90 | 87.75 ± 5.00 | 6.255 | < 0.001a |
| T0 | PaCO2 (mmHg) | 40.30 ± 2.80 | 40.20 ± 2.70 | 0.196 | 0.845 |
| T1 | PaCO2 (mmHg) | 41.20 ± 2.60 | 43.00 ± 3.10 | -3.388 | 0.001a |
| T2 | PaCO2 (mmHg) | 41.50 ± 2.75 | 44.20 ± 3.00 | -5.053 | < 0.001a |
| T3 | PaCO2 (mmHg) | 40.80 ± 2.90 | 43.80 ± 3.15 | -5.336 | < 0.001a |
| T4 | PaCO2 (mmHg) | 39.90 ± 2.50 | 42.50 ± 2.85 | -5.223 | < 0.001a |
The levels of GLU, LAC, BUN, and CRE in the experimental group post-surgery exhibited greater stability compared to those in the control group. Particularly, GLU levels were consistently lower in the QLB group at all postoperative time points (P < 0.001, Table 3).
| Time point | Indicators | Experiment group (n = 58) | Control group (n = 58) | t value | P value |
| T0 | GLU (mg/dL) | 90.50 ± 7.21 | 91.25 ± 6.88 | -0.410 | 0.682 |
| T1 | GLU (mg/dL) | 92.30 ± 6.98 | 98.75 ± 7.45 | -3.752 | < 0.001a |
| T2 | GLU (mg/dL) | 95.10 ± 7.12 | 105.50 ± 8.03 | -5.410 | < 0.001a |
| T3 | GLU (mg/dL) | 94.00 ± 6.45 | 102.75 ± 7.33 | -4.131 | < 0.001a |
| T4 | GLU (mg/dL) | 89.60 ± 7.00 | 95.50 ± 6.95 | -3.211 | 0.002a |
| T0 | LAC (mmol/L) | 1.20 ± 0.10 | 1.25 ± 0.12 | -1.025 | 0.308 |
| T1 | LAC (mmol/L) | 1.25 ± 0.11 | 1.40 ± 0.15 | -3.672 | < 0.001a |
| T2 | LAC (mmol/L) | 1.30 ± 0.12 | 1.55 ± 0.14 | -4.721 | < 0.001a |
| T3 | LAC (mmol/L) | 1.22 ± 0.09 | 1.48 ± 0.13 | -5.175 | < 0.001a |
| T4 | LAC (mmol/L) | 1.18 ± 0.10 | 1.35 ± 0.12 | -3.932 | < 0.001a |
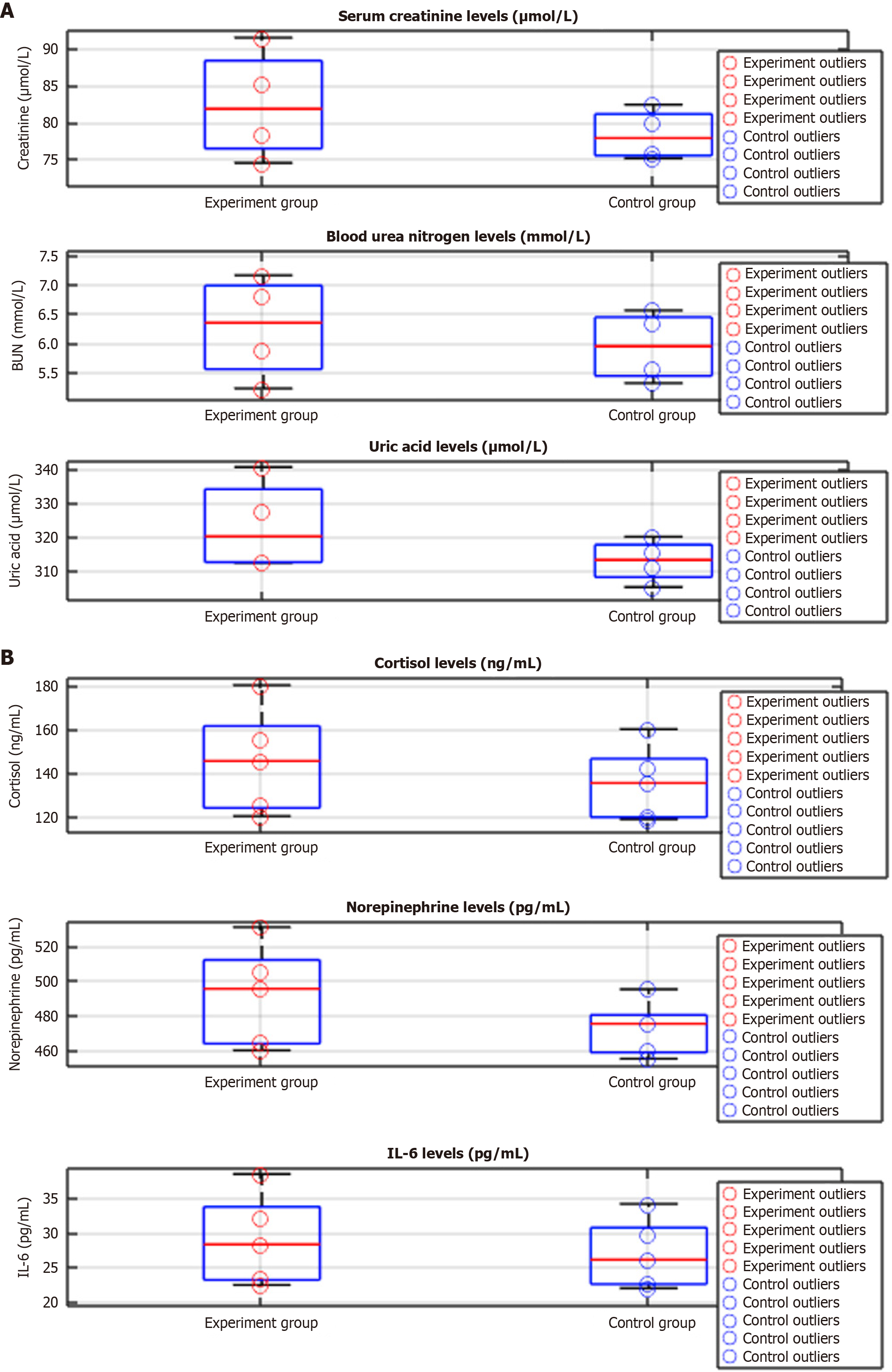
There were significant differences in the postoperative renal function changes between the experimental and control groups. Specifically, the values at 1 and 3 days after surgery for CRE, BUN, and UA were significantly lower in the experimental group than in the control group. The results of the repeated-measures analysis of variance showed that there were significant differences in the group main effect and the interaction between the time point and group (P < 0.05), and the value of the group main effect F was higher (Figure 1A, Tables 4 and 5).
| Time point | Group | CRE (μmol/L) | P value | BUN (mmol/L) | P value | UA (μmol/L) | P value |
| Before surgery | Experiment group (n = 58) | 74.50 ± 11.32 | 0.746 | 5.22 ± 1.03 | 0.61 | 312.40 ± 45.21 | 0.765 |
| Control group (n = 58) | 75.11 ± 10.87 | 5.31 ± 1.14 | 311.20 ± 42.54 | ||||
| 1 day after surgery | Experiment group (n = 58) | 85.32 ± 12.40 | 0.051 | 6.80 ± 1.22 | 0.14 | 327.50 ± 50.32 | 0.135 |
| Control group (n = 58) | 79.90 ± 13.14 | 6.32 ± 1.31 | 315.42 ± 48.22 | ||||
| 3 days after surgery | Experiment group (n = 58) | 91.55 ± 10.67 | 0.004 | 7.15 ± 1.52 | 0.034 | 340.45 ± 47.65 | 0.015 |
| Control group (n = 58) | 82.44 ± 11.23 | 6.55 ± 1.44 | 320.10 ± 44.32 | ||||
| 7 days after surgery | Experiment group (n = 58) | 78.42 ± 9.80 | 0.101 | 5.88 ± 1.10 | 0.073 | 312.87 ± 42.13 | 0.233 |
| Control group (n = 58) | 75.88 ± 10.01 | 5.56 ± 1.24 | 305.34 ± 40.54 |
Cortisol, NE, and IL-6 levels before surgery were not significantly different between the two groups (P > 0.05). The stress response indicators in both groups significantly increased after anesthesia induction and 1 day after surgery. In particular, the cortisol levels in the experimental group were significantly higher than those in the control group on postoperative day 1. By day 3 post-surgery, the stress response indicators in both groups decreased, although those in the experimental group remained higher than in the control group, particularly the IL-6 level. By day 7 post-surgery, the stress response indicators in both groups returned to levels similar to preoperative values, with no significant difference between the two groups (P > 0.05) (Figure 1B, Tables 6 and 7).
| Time point | Group | Cortisol (ng/mL) | P value | NE (pg/mL) | P value | IL-6 (pg/mL) | P value |
| Before surgery | Experiment group (n = 58) | 120.23 ± 15.45 | 0.585 | 460.11 ± 48.12 | 0.611 | 22.45 ± 3.98 | 0.709 |
| Control group (n = 58) | 118.77 ± 16.12 | 455.32 ± 50.45 | 21.99 ± 4.32 | ||||
| After anesthesia | Experiment group (n = 58) | 155.42 ± 18.34 | 0.071 | 505.65 ± 53.12 | 0.103 | 32.22 ± 4.54 | 0.049a |
| Control group (n = 58) | 142.10 ± 17.45 | 475.44 ± 51.10 | 29.65 ± 5.22 | ||||
| 1 day after surgery | Experiment group (n = 58) | 180.42 ± 20.34 | 0.026a | 530.65 ± 55.32 | 0.065 | 38.55 ± 5.41 | 0.008a |
| Control group (n = 58) | 160.22 ± 18.44 | 495.10 ± 52.11 | 34.23 ± 5.32 | ||||
| 3 days after surgery | Experiment group (n = 58) | 145.56 ± 17.88 | 0.054 | 495.43 ± 52.65 | 0.09 | 28.34 ± 4.12 | 0.037a |
| Control group (n = 58) | 135.44 ± 16.92 | 475.32 ± 50.87 | 26.12 ± 4.45 | ||||
| 7 days after surgery | Experiment group (n = 58) | 125.23 ± 15.24 | 0.401 | 465.23 ± 48.32 | 0.536 | 23.45 ± 3.88 | 0.358 |
| Control group (n = 58) | 120.12 ± 14.78 | 460.12 ± 47.89 | 22.78 ± 4.01 |
| Indicators | Group main effect | Group main effect | Time main effect | Time main effect | Interaction | Interaction |
| Cortisol (ng/mL) | 4.212 | 0.043 | 8.675 | 0.002 | 3.987 | 0.018 |
| NE (pg/mL) | 3.123 | 0.082 | 7.432 | 0.004 | 2.876 | 0.029 |
| IL-6 (pg/mL) | 5.478 | 0.028 | 10.543 | 0.001 | 4.765 | 0.014 |
Significant differences in the postoperative VAS scores were observed between the two groups at various time points (P < 0.05). Within 24 hours post-surgery, the initial time to rise from the bed was 19.65 ± 2.35 hours in the experimental group and 21.12 ± 2.44 hours in the control group. The experimental group’s bed exit time was marginally earlier, and the duration of inpatient stay was significantly shorter than that of the control group (P < 0.05) (Table 8).
| Time point | Group | VAS (score) | t value | P value | First time out of bed (hour) | t value | P value | First exhaust time (hour) | t value | P value | Inpatient days (day) | t value | P value |
| T1 | Experiment group (n = 58) | 5.88 ± 0.95 | 2.341 | 0.021a | |||||||||
| Control group (n = 58) | 6.45 ± 0.88 | ||||||||||||
| T2 | Experiment group (n = 58) | 4.32 ± 0.78 | 2.557 | 0.012a | 19.65 ± 2.35 | 1.871 | 0.064 | ||||||
| Control group (n = 58) | 4.88 ± 0.81 | 21.12 ± 2.44 | |||||||||||
| T3 | Experiment group (n = 58) | 2.95 ± 0.56 | 2.912 | 0.005a | 29.32 ± 3.12 | 2.145 | 0.035a | ||||||
| Control group (n = 58) | 3.45 ± 0.62 | 31.65 ± 3.21 | |||||||||||
| T4 | Experiment group (n = 58) | 1.88 ± 0.44 | 3.231 | 0.002a | 7.65 ± 1.22 | 2.901 | 0.006a | ||||||
| Control group (n = 58) | 2.45 ± 0.55 | 8.45 ± 1.35 |
Stress response is a common physiological phenomenon during surgical procedures. Surgical trauma triggers the release of stress hormones, such as cortisol and catecholamines, leading to elevated levels of GLU, LAC, and other metabolic indicators[12]. The study results indicated that the levels of GLU and LAC at time points T1-T4 in the experimental group were significantly lower than those in the control group, indicating the effectiveness of this anesthesia method in suppressing the stress response. According to relevant theories, local anesthesia reduces the central nervous system activation induced by surgical trauma by blocking afferent nerves, thereby reducing sympathetic nerve excitability[13,14]. QLB achieves a similar outcome by blocking afferent nerves affected by surgical trauma, thereby inhibiting sympathetic excitability and catecholamine release. Previous research has demonstrated that QLB can decrease cortisol secretion during and after surgery by blocking the sympathetic nerve conduction, thereby controlling the magnitude of the postoperative stress response[15]. The study findings endorse this concept, suggesting that QLB can be used as an effective means of stress response inhibition, particularly in patients sensitive to GLU fluctuations during surgery. Intraoperative stress responses and hemodynamic fluctuations can significantly impact renal function, with renal function preservation being crucial for perioperative management. This study revealed that BUN and CRE levels in the experimental group were significantly lower than those in the control group at each postoperative time point, suggesting that QLB supported the stability of renal function. The renal protective effect of QLB can be attributed to its ability to reduce renal ischemia-reperfusion injury by lowering sympathetic excitability during surgery and maintaining blood pressure and flow stability. Additionally, the block can decrease stress hormone release and renal vessel constriction, thereby maintaining the glomerular filtration rate[16]. This mechanism has been validated in prior studies, one of which demonstrated that QLB could help decrease postoperative acute kidney injury incidence by reducing intraoperative renal vascular resistance. The current study’s results further strengthen this perspective, suggesting that combining QLB with general anesthesia significantly diminishes the risk of postoperative renal impairment in patients undergoing colorectal cancer surgery[17]. Consistent with earlier findings, QLB reduces renal vascular resistance and acute kidney injury risk by maintaining the glomerular filtration rate.
Surgical trauma and narcotic drugs typically cause fluctuations in postoperative metabolic function, particularly in GLU and LAC levels[18]. This study revealed that the GLU and LAC levels in the experimental group were consistently lower than those in the control group post-surgery, indicating significant advantages in maintaining metabolic stability. Previous studies have shown a close association between postoperative metabolic disorders and the stress response. Surgery-induced stress triggers an increase in glucocorticoid levels, leading to elevated blood GLU levels[19,20]. QLB reduces the incidence of gluconeogenesis by reducing the release of stress hormones, thus effectively controlling the increase of GLU after surgery. Additionally, QLB, a key metabolic burden marker, reduces LAC accumulation and tissue hypoxia risk post-surgery by enhancing tissue oxygen supply. This result is consistent with existing literature, suggesting that QLB not only reduces the stress response but also promotes postoperative recovery through metabolic regulation. The advantage of QLB in postoperative pain management was also one of the key findings of this study[21]. The results showed that the VAS scores of the experimental group were significantly lower than those of the control group at each time point after surgery, which is consistent with the conclusions of previous studies. QLB directly diminishes postoperative pain transmission by blocking afferent nerve fibers in the waist, thereby reducing postoperative pain perception. Local anesthesia not only alleviates postoperative pain but also reduces the need for postoperative analgesics, thereby lowering the risk of adverse drug reactions post-surgery[22]. Effective management of postoperative pain is essential for early rehabilitation, and the pain-controlling effect of the QLB guarantees the patient’s postoperative quality of life. Surgical trauma-induced stress response is a central factor influencing metabolism, renal function, and pain regulation. Stress triggers the release of hormones such as cortisol and NE, promoting hepatic glycogen breakdown and gluconeogenesis, leading to increased GLU levels. At the same time, tissue hypoxia and energy metabolism disorders promote lactate accumulation, causing metabolic imbalance. Metabolic disorders exacerbate renal vasoconstriction, reducing the glomerular filtration rate, as evidenced by elevated renal function indicators such as BUN and CRE, particularly on postoperative days 1-3 (P < 0.05). Additionally, stress-induced inflammatory factor release (such as IL-6) sensitizes the spinal pain transmission pathway, lowering the postoperative pain threshold and increasing VAS scores. Ultrasound-guided QLB inhibits stress hormone release by blocking T12-L4 sympathetic nerve input (cortisol was 11.2% lower than the control group on postoperative day 1, P = 0.026), thereby reducing GLU fluctuations (8%-15% reduction) and LAC accumulation (10%-20% reduction). This reduction in metabolic load directly alleviates renal ischemia-reperfusion injury, decreasing peak BUN and CRE values by 12.5% and 12.4%, respectively (P < 0.05). Moreover, local nerve block diminishes pain signal transmission, resulting in a 15%-30% lower VAS score at 24 hours post-surgery compared to the control group (P < 0.001), establishing a beneficial regulatory chain of “stress metabolism renal function pain”. Future research should conduct multicenter randomized controlled trials to validate these findings and explore QLB’s varying effects in open vs laparoscopic colorectal surgery and in patients with comorbidities like diabetes or chronic kidney disease.
In summary, the QLB, with its unique local blocking effect, not only reduces the use of general anesthesia drugs but also provides a more efficient perioperative management strategy, particularly beneficial for patients with sensitive stress response, fragile renal function, and low pain threshold.
| 1. | Tsalikidis C, Mitsala A, Mentonis VI, Romanidis K, Pappas-Gogos G, Tsaroucha AK, Pitiakoudis M. Predictive Factors for Anastomotic Leakage Following Colorectal Cancer Surgery: Where Are We and Where Are We Going? Curr Oncol. 2023;30:3111-3137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 41] [Reference Citation Analysis (0)] |
| 2. | Willemsen P, Devriendt S, Heyman S, Van Fraeyenhove F, Perkisas S. Colorectal cancer surgery in octogenarians: real-world long-term results. Langenbecks Arch Surg. 2023;409:13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 3. | Shinji S, Yamada T, Matsuda A, Sonoda H, Ohta R, Iwai T, Takeda K, Yonaga K, Masuda Y, Yoshida H. Recent Advances in the Treatment of Colorectal Cancer: A Review. J Nippon Med Sch. 2022;89:246-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 115] [Reference Citation Analysis (1)] |
| 4. | Chakraborty P, Jacob A. Extended chemothromboprophylaxis use in colorectal cancer surgery: a literature review. ANZ J Surg. 2022;92:1644-1650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Becattini C, Pace U, Pirozzi F, Donini A, Avruscio G, Rondelli F, Boncompagni M, Chiari D, De Prizio M, Visonà A, De Luca R, Guerra F, Muratore A, Portale G, Milone M, Castagnoli G, Righini M, Martellucci J, Persiani R, Frasson S, Dentali F, Delrio P, Campanini M, Gussoni G, Vedovati MC, Agnelli G. Rivaroxaban vs placebo for extended antithrombotic prophylaxis after laparoscopic surgery for colorectal cancer. Blood. 2022;140:900-908. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 42] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 6. | D'Amato S, Sofia M, Agosta M, Litrico G, Sarvà I, La Greca G, Latteri S. The impact of bariatric surgery on colorectal cancer risk. Surg Obes Relat Dis. 2023;19:144-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 7. | Aoun RJN, Kalady MF. The importance of genetics for timing and extent of surgery in inherited colorectal cancer syndromes. Surg Oncol. 2022;43:101765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 8. | Cheng YX, Tao W, Liu XY, Yuan C, Zhang B, Wei ZQ, Peng D. Hypertension Remission after Colorectal Cancer Surgery: A Single-Center Retrospective Study. Nutr Cancer. 2022;74:2789-2795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 9. | Hernández-González PI, Barquín J, Ortega-Ferrete A, Patón V, Ponce-Alonso M, Romero-Hernández B, Ocaña J, Caminoa A, Conde-Moreno E, Galeano J, Campo RD, García-Pérez JC. Anastomotic leak in colorectal cancer surgery: Contribution of gut microbiota and prediction approaches. Colorectal Dis. 2023;25:2187-2197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 10. | Farah E, Abreu AA, Rail B, Salgado J, Karagkounis G, Zeh HJ 3rd, Polanco PM. Perioperative outcomes of robotic and laparoscopic surgery for colorectal cancer: a propensity score-matched analysis. World J Surg Oncol. 2023;21:272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 11. | Sung CS, Wei TJ, Hung JJ, Su FW, Ho SI, Lin MW, Chan KC, Wu CY. Comparisons in analgesic effects between ultrasound-guided erector spinae plane block and surgical intercostal nerve block after video-assisted thoracoscopic surgery: A randomized controlled trial. J Clin Anesth. 2024;95:111448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 12. | Tiwari B, Pandey P. An Ultrasound-Guided Interfascial Injection Approach Versus an Ultrasound-Assisted Nerve Stimulating Approach of Obturator Nerve Block: A Randomized Clinical Trial. Cureus. 2022;14:e24037. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Ozyemisci Taskiran O, Albayrak H, Topaloglu M, Manici M, Ketenci A, Gurkan Y. Effect of Ultrasound-Guided Rhomboid Interfascial Plane Block on Pain Severity, Disability, and Quality of Life in Myofascial Pain Syndrome - A Case Series With One-Year Follow-Up. Pain Physician. 2023;26:E815-E822. [PubMed] |
| 14. | Liang M, Xv X, Ren C, Yao Y, Gao X. Effect of ultrasound-guided transversus abdominis plane block with rectus sheath block on patients undergoing laparoscopy-assisted radical resection of rectal cancer: a randomized, double-blind, placebo-controlled trial. BMC Anesthesiol. 2021;21:89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Algyar MF, Abdelsamee KS. Laparoscopic assisted versus ultrasound guided transversus abdominis plane block in laparoscopic bariatric surgery: a randomized controlled trial. BMC Anesthesiol. 2024;24:133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Uzunay NT, Mingir T, Erginoz E, Karakas DO, Kose E. Comparison of laparoscopic-guided versus ultrasound-guided TAP block in laparoscopic cholecystectomy. Cir Cir. 2024;92:174-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 17. | Cavallaro G, Gazzanelli S, Iossa A, De Angelis F, Fassari A, Micalizzi A, Petramala L, Crocetti D, Circosta F, Concistrè A, Letizia C, De Toma G, Polistena A. Ultrasound-guided Transversus Abdominis Plane Block is Effective as Laparoscopic Trocar site infiltration in Postoperative Pain Management in Patients Undergoing Adrenal Surgery. Am Surg. 2023;89:4401-4405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Coşarcan SK, Gürkan Y, Manici M, Özdemir İ, Kılıç M, Esen T, Erçelen Ö. The effect of ultrasound-guided rectus sheath block on postoperative analgesia in robot assisted prostatectomy: A randomized controlled trial. Medicine (Baltimore). 2024;103:e37975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Chen Q, Yang H, Zhao D, Tang X, Liu H. Anesthetic Spread of Ultrasound-Guided Paraspinal Blocks in Video-Assisted Thoracoscopic Surgery: A Three-Dimensional Reconstruction Image Study. Pain Physician. 2023;26:E383-E387. [PubMed] |
| 20. | Shah SB, Pant D, Koul A, Roy A, Sood J, Chugh PT. Ultrasound-Guided Quadratus Lumborum Block Versus Caudal Block for Perioperative. AANA J. 2024;92:329-336. [PubMed] |
| 21. | Rao J, Gao Z, Qiu G, Gao P, Wang Q, Zhong W, Wang Y, Li Y. Nalbuphine and dexmedetomidine as adjuvants to ropivacaine in ultrasound-guided erector spinae plane block for video-assisted thoracoscopic lobectomy surgery: A randomized, double-blind, placebo-controlled trial. Medicine (Baltimore). 2021;100:e26962. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 22. | La Regina D, Popeskou SG, Saporito A, Gaffuri P, Tasciotti E, Dossi R, Christoforidis D, Mongelli F. Laparoscopic versus ultrasound-guided transversus abdominis plane block in colorectal surgery: a non-inferiority, multicentric randomized double-blinded clinical trial. Colorectal Dis. 2023;25:1921-1928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |