Published online Jun 27, 2025. doi: 10.4240/wjgs.v17.i6.106777
Revised: April 3, 2025
Accepted: May 12, 2025
Published online: June 27, 2025
Processing time: 80 Days and 20.8 Hours
Ferroptosis is a newly recognized form of regulated cell death characterized by iron-dependent accumulation of lipid reactive oxygen species. It has been ex
To investigate the role of mitochondrial alanyl-tRNA synthetase 2 (AARS2) in ferroptosis and its epigenetic regulation of acyl-CoA synthetase long-chain family member 4 (ACSL4) through histone lactylation during IIR injury.
We established a mouse model to mimic IIR and conducted AARS2 knockdown as treatment. The expression of AARS2 in intestinal tissues was measured by western blot. The integrity of intestinal tissues was detected by hematoxylin and eosin staining, serum fatty acid-binding protein, protein levels of ZO-1 and occluding. An in vitro hypoxia-reperfusion (H/R) cell model was established, and cell viability was measured by CCK-8. The in vitro and in vivo ferroptosis was determined by the accumulation of Fe2+ and malondialdehyde (MDA). The epi
We observed a notable elevated AARS2 level in intestinal tissue of mice in IIR model group, which was reversed by shAARS2 treatment. Knockdown of AARS2 repressed alleviated intestinal barrier disruption and repressed the accumulation of ferroptosis biomarker Fe2+ and MDA during IIR. The in vitro results showed that shAARS2 alleviated impaired cell viability caused by H/R, as well as repressed ferroptosis. Knockdown of AARS2 notably downregulated the RNA and protein expression of ACSL4. Mechanistically, knockdown of AARS2 downregulated the enrichment of H3K18 La modification on AARS2, as well as suppressed its promoter activity. Overexpression of AARS2 could abolish the protective effects of shACSL4 in vitro.
The elevation of AARS2 during IIR led to cell ferroptosis via epigenetically upregulating the expression of ACSL4. Our findings presented AARS2 as a promising therapeutic target for IIR.
Core Tip: This investigation reveals mitochondrial alanyl-tRNA synthetase 2 (AARS2) as a pivotal regulator of ferroptotic cell death in intestinal ischemia-reperfusion (I/R) injury. Our findings demonstrate that AARS2 augments histone H3K18 lactylation modification, which subsequently drives the transcriptional upregulation of acyl-CoA synthetase long-chain family member 4 through chromatin remodeling. This molecular cascade potentiates the accumulation of lipid peroxidation products and exacerbates intestinal epithelial damage. Pharmacological inhibition of AARS2 effectively suppresses ferroptosis progression and maintains mucosal barrier integrity, establishing its clinical relevance for managing I/R-associated tissue injury.
- Citation: Dong W, Huang SX, Qin ML, Pan Z. Mitochondrial alanyl-tRNA synthetase 2 mediates histone lactylation to promote ferroptosis in intestinal ischemia-reperfusion injury. World J Gastrointest Surg 2025; 17(6): 106777
- URL: https://www.wjgnet.com/1948-9366/full/v17/i6/106777.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i6.106777
The pathophysiological cascade of ischemia-reperfusion (I/R) injury initiates with transient blood flow cessation, culminating in tissue dysfunction and potential organ system collapse. Within gastrointestinal pathologies, intestinal I/R (IIR) represents a critical vascular emergency frequently encountered in surgical contexts including strangulated abdominal hernias, intestinal malrotation complications, and transplant-related ischemia. This condition also emerges as a secondary crisis in systemic disorders ranging from septicemia-induced circulatory collapse to right ventricular failure syndromes[1,2]. The ischemic phase induces structural compromise through enhanced vascular leakage and breakdown of epithelial tight junctions, whereas reperfusion exacerbates tissue injury via free radical generation and neutrophil-mediated inflammation[3]. When mucosal repair mechanisms become overwhelmed, bacterial translocation through compromised barriers may instigate lethal systemic inflammatory responses[4]. The molecular underpinnings of IIR involve intricate cross-talk between oxidative stress pathways, inflammatory cascades, and programmed cell death modalities[5]. Current therapeutic paradigms emphasize reperfusion-phase interventions, with research priorities focusing on neutralizing oxidative stress mediators, modulating dysregulated immune activation, and developing cytoprotective strategies to preserve enterocyte viability[6].
Since the discovery of lactate in 1780, it has often been mistakenly regarded as a metabolic waste product associated with hypoxia, producing various harmful effects under anaerobic conditions[7]. The lactate shuttle hypothesis describes the role of lactate in substrate transfer for oxidation and gluconeogenesis, as well as in cellular signal transduction[8,9]. Increasing evidence highlights lactate as a crucial regulator in coordinating systemic metabolism[10]. Notably, lactate is now recognized not merely as a byproduct of anaerobic metabolism but as a signaling molecule capable of inducing histone lysine lactylation, an epigenetic modification that regulates gene expression[11,12]. Similar to acetylation and succinylation, lactylation has emerged as a critical post-translational modification influencing transcriptional regulation in diverse pathological contexts, including cancer, inflammation, and metabolic disorders[13].
Mitochondrial alanyl-tRNA synthetase 2 (AARS2), traditionally known for catalyzing alanyl-tRNA formation during mitochondrial protein synthesis, has recently been identified as a novel lactate-modifying enzyme that induces lysine alanylation and disrupts lactate homeostasis[14]. Intriguingly, AARS2-mediated lactylation has been implicated in mitochondrial dysfunction and oxidative stress in cardiac ischemia models, while its deficiency exacerbates metabolic dysregulation in hepatic injury[15]. However, its role in IIR injury remains unexplored. Given the centrality of lactate metabolism and epigenetic reprogramming in I/R pathophysiology, we hypothesize that AARS2 may orchestrate in
As a critical regulator of phospholipid biosynthesis and eicosanoid metabolism, acyl-CoA synthetase long-chain family member 4 (ACSL4) has emerged as a molecular nexus coordinating iron-dependent lipid peroxidation cascades (a hallmark of ferroptosis) with innate immune activation. Recent proteomic studies suggest that ACSL4 undergoes la
In this study, we aim to: (1) Elucidate the functional role of AARS2 in IIR injury using in vivo and in vitro models; (2) Characterize its regulatory effects on ACSL4-mediated lactylation modifications; and (3) Evaluate the therapeutic potential of targeting the AARS2/ACSL4 axis to mitigate intestinal barrier dysfunction. Our findings will provide novel insights into lactylation-driven epigenetic mechanisms in I/R injury and identify potential therapeutic targets for this critical condition.
Eight-to-ten-week-old male C57BL/6J mice, obtained from GemPharmatech (Chengdu, China), were housed in the animal center. Anesthesia was administered via intraperitoneal injection of 100–150 μL of 2.5% 2,2,2-Tribromoethanol (Sigma, United States). Abdominal laparotomy was performed to expose the superior mesenteric artery (SMA), as described in previous studies. To create intestinal ischemia, warm sterile saline (37 °C) was applied to the SMA, and the vessels were occluded using atraumatic microvascular clips for 30 minutes. Ischemia was confirmed by observing a color change in the intestines from red-pink to wine-red. Following this, the clamps were carefully removed to permit reperfusion for 6 hours, after which blood samples were collected and intestinal tissues harvested (n = 6).
The murine intestinal epithelial cell line Caco-2 was utilized to establish an in vitro model of IIR injury using the oxygen–glucose deprivation/reperfusion (H/R) method. The H/R duration (6 hours hypoxia/2 hours reoxygenation) was selected based on standardized protocols for epithelial cell injury modeling, differing from in vivo ischemia times due to distinct metabolic rates in vitro. In this procedure, Caco-2 cells were incubated in a hypoxic chamber (95% N2, 5% CO2) with glucose-free medium for 6 hours, followed by transfer to a normoxic chamber (95% O2, 5% CO2) with glucose-containing medium for 2 hours.
The murine intestinal epithelial Caco-2 cells were employed to configure a hypoxia-reoxygenation (H/R) system simulating IIR pathophysiology. Following initial optimization of exposure duration through pilot metabolic flux analyses, we implemented a 6-hour hypoxic phase (maintained in an anaerobic workstation with 95% N2/5% CO2 atmosphere) using substrate-depleted culture medium, succeeded by a 2-hour reoxygenation period under hyperoxic conditions (95% O2/5% CO2) with nutrient restoration. This protocol refinement accounts for the accelerated metabolic kinetics of in vitro systems compared to physiological ischemic timelines, ensuring reproducible modeling of epithelial barrier dysfunction while maintaining cellular viability thresholds.
Intestinal fatty acid-binding protein (FABP, a specific marker of epithelial injury), interleukin (IL)-6, and tumor necrosis factor-alpha (TNF-α) levels were quantified using a sandwich immunoassay platform (ELISA). Serum samples were centrifuged at 10000 × g to remove particulates, followed by standard curve construction and analyte detection following standardized incubation protocols (pre-coated plates at 37 °C for 1 hour). Absorbance at 450 nm was measured using a microplate reader (BioTek Synergy H1) with triplicate technical replicates for data normalization.
Proximal ileum specimens were immersion-fixed in neutral-buffered 4% paraformaldehyde (Sangon Biotech, China) for 48 hours, paraffin-embedded, and sectioned at 5 μm thickness. Modified hematoxylin (8 minutes) and eosin (30 sec differentiation) staining was performed for microscopic analysis. Mucosal injury was evaluated by two blinded pathologists using a digital slide scanning system (Hamamatsu NanoZoomer S60), with semi-quantitative grading (0-5 scale) based on Chiu's modified criteria incorporating villus structural integrity (%), epithelial denudation, and crypt architectural distortion.
Tissue lysates were prepared using ice-cold RIPA buffer (Solarbio, China) containing 1 mM PMSF protease inhibitor, homogenized via pulsed ultrasonication (3 × 5-sec bursts at 30% amplitude), and centrifuged at 12000 × g (4 °C, 15 minutes). Proteins were resolved using a discontinuous SDS-PAGE system (10% stacking/12% resolving gels) under constant voltage (80V for 30 minutes, 120V for 60 minutes), then transferred to PVDF membranes (0.45 μm, Millipore) via wet electrophoretic transfer (300 mA, 90 minutes). After blocking with 5% non-fat milk-TBST, membranes were probed with: Rabbit anti-AARS2 (Proteintech 22696-1-AP, 1:1000, 4 °C overnight); Mouse anti-ACSL4 (Proteintech 81196-1-RR, 1:1000); Human-validated anti-GPX4 (Proteintech 67763-1-Ig); Goat polyclonal anti-β-actin (Abcam ab115777, 1:1000). HRP-conjugated streptavidin secondary antibody (Proteintech SA00001-2, 1:2000) was applied for 1 hour at room temperature with agitation. Chemiluminescent signals were captured using a Tanon 5200 imaging system and quantified via densitometric analysis (Image Lab 6.0 software).
RNA isolation was performed employing a guanidinium thiocyanate-phenol-based extraction system (TRIzol™ Reagent, Takara Bio, Shiga, Japan), with RNA integrity verified through spectrophotometric analysis (A260/A280 ratio > 1.8). Reverse transcription was carried out using PrimeScript™ RT Master Mix (Takara) prior to quantitative amplification. Real-time PCR analysis was implemented with SYBR Green fluorescent detection chemistry (GoTaq® qPCR Master Mix, Promega, WI, United States) on a QuantStudio 5 thermocycler (Applied Biosystems), utilizing the following cycling parameters: Initial denaturation at 95 °C for 3 minutes, followed by 40 cycles of 95 °C for 15 sec and 60 °C for 30 sec. Gene-specific primers were designed using Primer-BLAST (NCBI) and synthesized by Sangon Biotech (China). The primers for the genes of interest are as follows: AARS2 (Forward: 5'-ATGCTGGACCTGATCAAGGT-3', Reverse: 5'-TCCAGGTCCAGTTCTTCAGC-3'), ACSL4 (Forward: 5'-GCTGGCTTTGGAATGTCTGT-3', Reverse: 5'-CAGCCACACAG TTGCTGAAC-3'), ZO-1 (Forward: 5'-CCTCCATCTCGTCGGTATCC-3', Reverse: 5'-GCGTTCTTCATCCACAGCAC-3'), Occludin (Forward: 5'-CAGCAGCCATGTACCTGAAG-3', Reverse: 5'-GTCATCCACAGGCGAAGTCT-3'), and β-actin (Forward: 5'-GGCTGTATTCCCCTCCATCG-3', Reverse: 5'-CCAGTTGGTAACAATGCCATGT-3'). Primer validation included three-level quality control: Melting curve profiling showing single-peak dissociation patterns; Agarose gel electrophoresis (2%) confirming predicted amplicon sizes; Sequencing verification of representative PCR products (BGI, Shenzhen).
The oxidative degradation of membrane lipids was evaluated through measurement of malondialdehyde (MDA), a terminal byproduct of polyunsaturated fatty acid peroxidation. Cellular homogenates prepared in ice-cold hypotonic buffer (containing 1% protease inhibitor cocktail, pH 7.4) were centrifuged at 13500 × g (4 °C, 5 minutes) to obtain clarified lysates. MDA quantification was performed via thiobarbituric acid reactive substances (TBARS) assay (Sigma-Aldrich, MAK085) with optimized reaction conditions: Lysate supernatants were mixed with 0.67% TBA in 20% acetic acid (v/v), heated at 95 °C for 60 minutes, and cooled to room temperature. The resultant MDA-TBA chromophore was quantified spectrophotometrically at 532 nm (reference 600 nm) using a microplate reader (Molecular Devices SpectraMax i3x), with concentrations calculated against a tetramethoxypropane-derived standard curve.
Labile iron pool levels were determined via a ferrozine-based colorimetric assay (Abcam, ab83366). Briefly, cell pellets were lysed in iron assay buffer supplemented with 1 mM ascorbate to prevent redox cycling, followed by centrifugation (12000 × g, 10 minutes, 4 °C). The chromogenic reaction (30 minutes incubation at 37 °C) generated a stable Fe2+-ferrozine complex detectable at 550 nm, with iron content normalized to total protein concentration determined by BCA assay.
Chromatin-protein interactions were interrogated using the EZ-Magna Chromatin immunoprecipitation (ChIP)® Kit (Millipore, 17-10086) with optimized crosslinking parameters. Cellular DNA-protein complexes were stabilized via formaldehyde-mediated covalent crosslinks (1% final concentration, 10-min fixation at 37 °C), followed by quenching with 125 mM glycine. Chromatin fragmentation was achieved through controlled ultrasonication (Branson Sonifier 450, 30% amplitude, 10 cycles of 15-sec pulses/45-sec cooling) to generate 200-500 bp fragments, which were immunoprecipitated using H3K18 La-specific antibodies (Millipore) or IgG as a control. Negative control primers targeting the GAPDH promoter were selected based on: (1) Pre-experimental verification showing no significant H3K18 La enrichment in this region (Supplementary Figure 1); and (2) Established protocols using housekeeping gene promoters as non-target controls. Primers targeting the ACSL4 promoter region (-582 to -329 bp) were designed using Primer-BLAST (NCBI) and synthesized by Sangon Biotech (China). The primer sequences were as follows: ACSL4 promoter (Forward): 5'-CTGAGCTACCTGAATTGCG-3'; ACSL4 promoter (Reverse): 5'-TGCATCGATTAGGCCTACAG-3'. Negative control primers for GAPDH promoter region: GAPDH (Forward): 5'-TACTAGCGGTTTTACGGGCG-3'; GAPDH (Reverse): 5'-TCGAACAGGAGGAGCAGAGAGCGA-3'. The precipitated chromatin DNA was subsequently analyzed through qPCR assays.
The ACSL4 promoter region was amplified from genomic DNA, cloned into the pGL3-Basic vector, and verified by sequencing. HEK293T cells were transfected with shRNA and ACSL4 promoter constructs using Lipofectamine 2000. Dual-luciferase reporter assays (Promega, Madison, WI, United States) were performed, and the results were expressed as the ratio of firefly luciferase activity to Renilla luciferase activity.
Analyses utilized GraphPad Prism 8.0 with data expressed as mean ± SD. Normality was confirmed via Shapiro-Wilk testing. Parametric comparisons employed: Unpaired two-tailed t-tests (inter-group); One-way ANOVA with Tukey's post hoc (multi-group). Nonparametric datasets used Wilcoxon signed-rank (paired) or Mann-Whitney U tests. Tukey's post hoc testing controlled family-wise error rates during multi-group comparisons. Significance thresholds: aP < 0.05, bP < 0.01, cP < 0.001; NS = not significant.
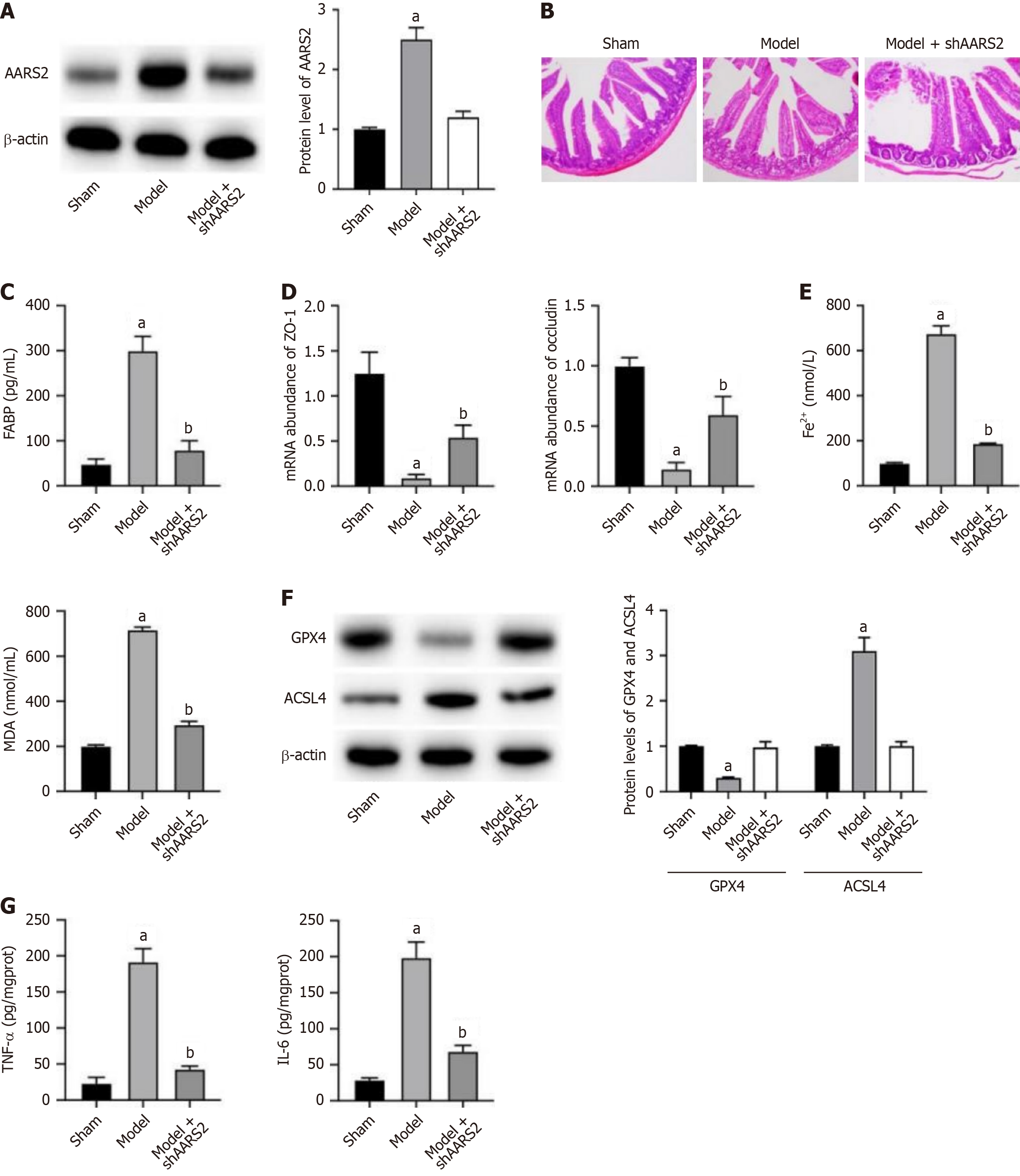
To investigate the role of AARS2 in IIR, we established a mouse model and performed knockdown of AARS2 as treatment. We observed a notable elevated AARS2 level in intestinal tissue of mice in model group, which was reversed by shAARS2 treatment (Figure 1A). The results from intestinal tissue hematoxylin and eosin staining (Figure 1B) and serum FABP examination indicated that intestinal barrier disruption was alleviated under shAARS2 treatment (Figure 1C). Furthermore, the mRNA abundance of tight junction proteins ZO-1 and occludin showed similar results with tissue histological analysis, indicating recovered intestinal epithelial barrier upon shAARS2 treatment (Figure 1D). Moreover, there was a significant elevation in levels of Fe2+ and MDA (Figure 1E) in model group, along with decreased GPX4 and increased ACSL4 (Figure 1F), suggesting induction of ferroptosis. The depletion of AARS2 alleviated ferroptosis in intestinal tissues (Figure 1E and F). The elevated levels of inflammatory cytokines, including TNF-α and IL-6, was also repressed by knockdown of AARS2 (Figure 1G).
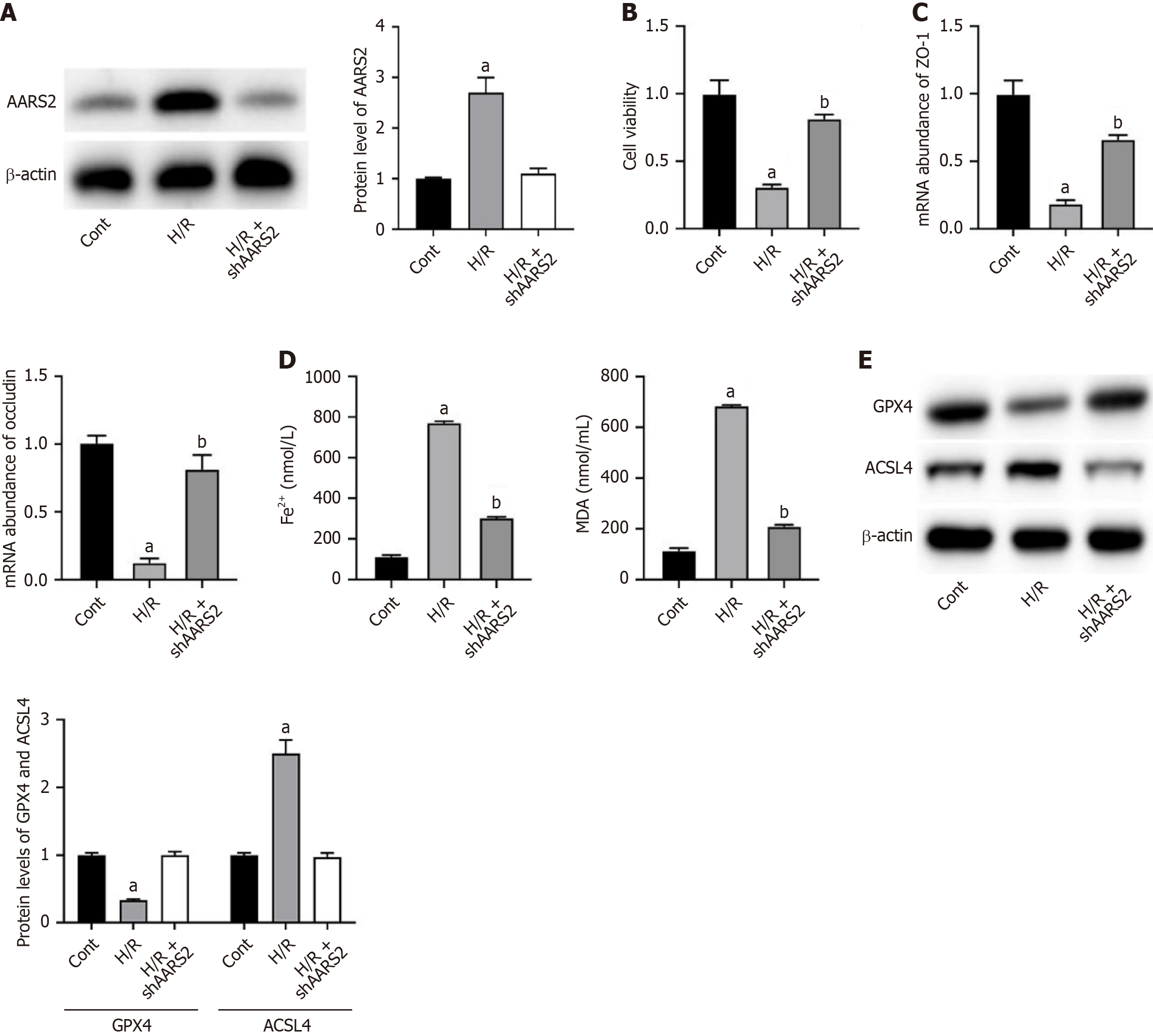
We nest verified the effects of AARS2 in an H/R cell model. As shown in Figure 2A and B, H/R induced AARS2 expression (Figure 2A) in Caco-2 cells and suppressed cell viability (Figure 2B), which was downregulated by shAARS2. Similar with the in vivo results, H/R downregulated mRNA abundance of ZO-1 and occluding, which was recovered by shAARS2 (Figure 2C). Furthermore, H/R induced accumulation of Fe2+ and MDA (Figure 2D), decreased GPX4 and increased ACSL4 (Figure 2E). The knockdown of AARS2 suppressed ferroptosis (Figure 2D and E).
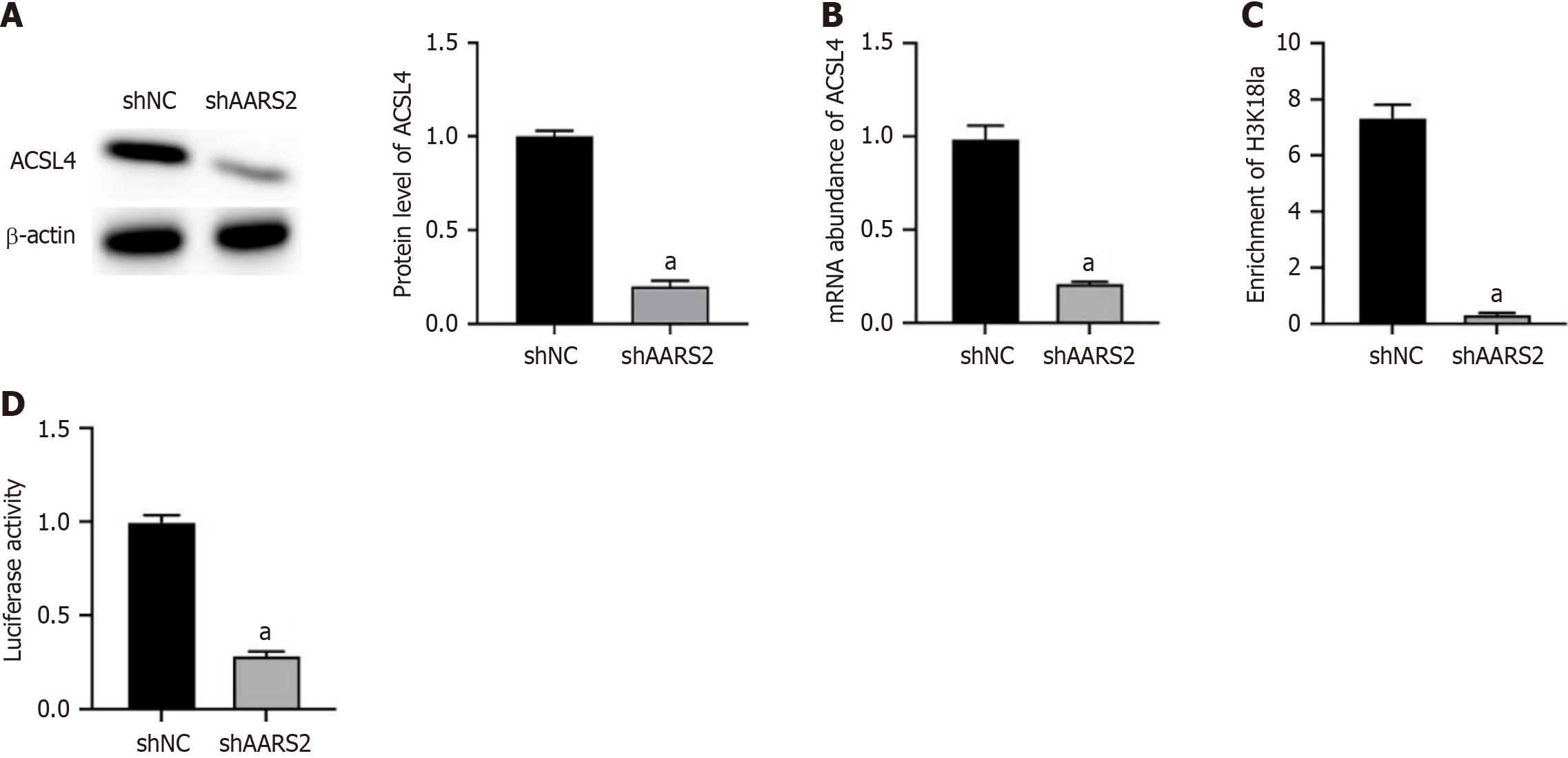
Subsequently, we determined the potential molecule mechanisms underlying ARRS2-regulated ferroptosis. As ACSL4 is a critical regulator during ferroptosis, we observed that knockdown of AARS2 notably downregulated the RNA and protein expression of ACSL4 (Figure 3A and B). Moreover, knockdown of AARS2 downregulated the enrichment of H3K18 La modification on AARS2 (Figure 3C), as well as suppressed its promoter activity (Figure 3D). Negative control experiments confirmed no significant H3K18 La enrichment at the GAPDH promoter (Supplementary Figure 1).
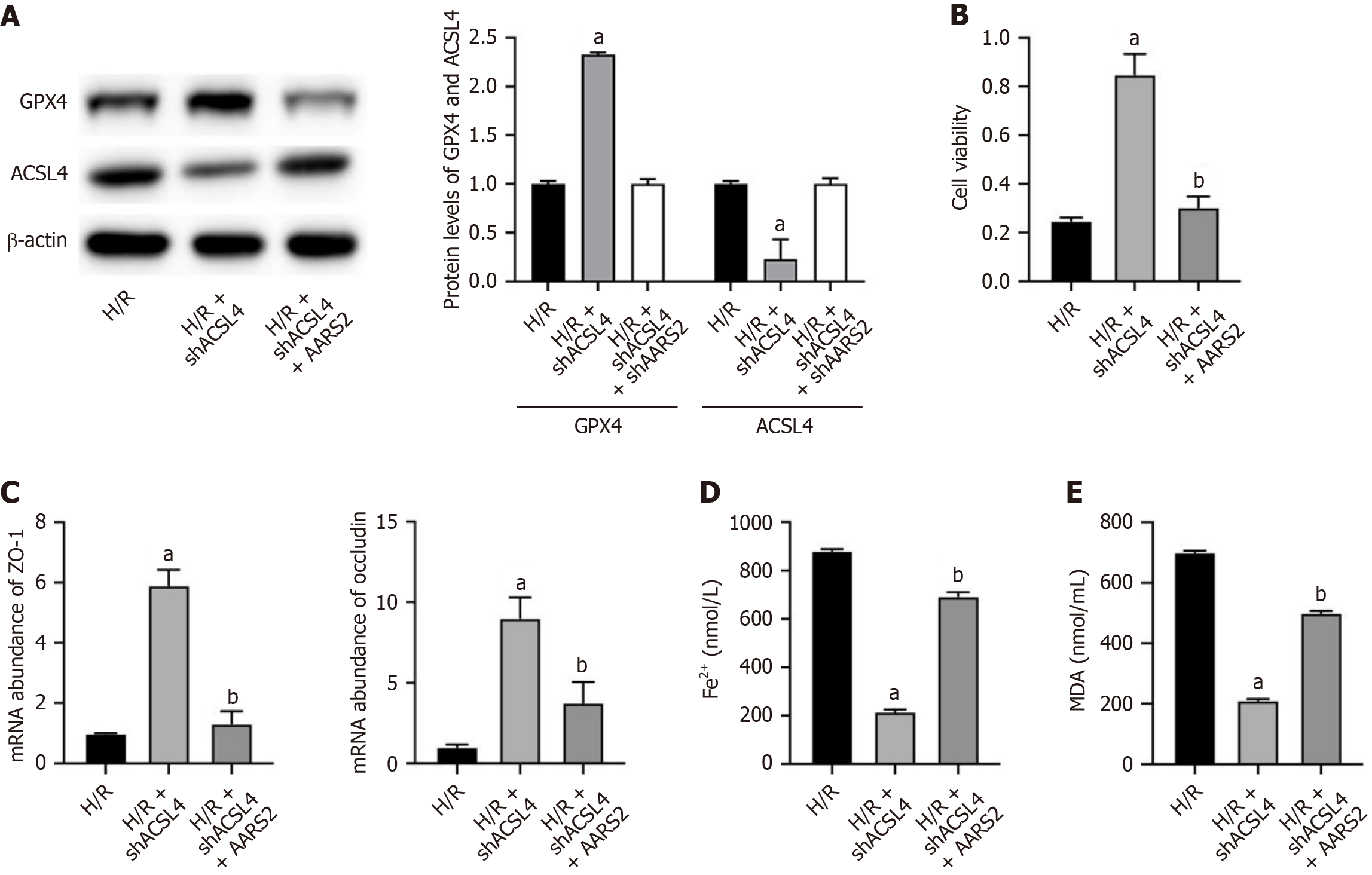
Knockdown of ACSL4 in H/R cell model led to downregulated protein expression of ACSL4 (Figure 4A) and enhance cell viability (Figure 4B), which was recovered by overexpression of AARS2. The knockdown of ACSL4 also recovered the expression of ZO-1 and occluding in H/R cell model, whereas overexpression of AARS2 abolished this effect (Figure 4C). Furthermore, the repressed ferroptosis by shACSL4 was also reversed by overexpression of AARS2 (Figure 4D and E).
Emerging evidence since the seminal discovery of ferroptosis by Stockwell's team has established this iron-dependent cell death pathway as a pivotal contributor to diverse pathological conditions[16,17]. The ferroptotic cascade requires four cardinal elements: Redox-active iron pools, oxygen-derived free radicals, polyunsaturated fatty acid substrates, and compromised antioxidant defenses[18]. Central to this process is ACSL4, which governs ferroptotic susceptibility through its enzymatic regulation of phospholipid remodeling[19,20]. Genetic ablation of ACSL4 confers cellular protection against membrane lipid peroxidation cascades and subsequent ferroptotic demise[21]. Despite these advances, the upstream regulatory networks controlling ACSL4 dynamics in I/R-associated ferroptosis remain poorly characterized.
Our experimental data reveal ischemia-phase upregulation of ACSL4 expression patterns, with subsequent exacerbation of reperfusion-triggered ferroptotic damage. Through shRNA-mediated ACSL4 knockdown in Caco-2 enterocytes, we established that targeted suppression of this enzyme during hypoxic preconditioning significantly attenuated ferroptosis biomarkers. This therapeutic strategy preserved cellular integrity by intercepting the lipid peroxidation cascade at its metabolic origin. Furthermore, our mechanistic exploration identified novel upstream modulators of ACSL4 transcriptional regulation under ischemic stress.
While our study establishes AARS2 as a ferroptosis driver in murine IIR models, critical questions remain regarding its clinical applicability. Human tissue expression profiling (GTEx database) reveals ubiquitous AARS2 expression across organs, with particularly high levels in metabolically active tissues like the intestine and liver[21]. Notably, single-cell RNA sequencing of human intestinal biopsies shows AARS2 upregulation in epithelial cells from inflammatory bowel disease patients[22], suggesting conserved roles in human mucosal injury. However, interspecies differences warrant caution: Murine AARS2 shares 89% amino acid identity with humans, but its lactylation substrate specificity may vary[23].
Histone lactylation is an essential epigenetic regulatory mechanism involved in various cellular processes and has recently emerged as a promising therapeutic target for pathological conditions[24,25]. Its role in I/R injury is increasingly gaining attention[26,27]. For instance, HSPA12A functions as a critical modulator of glycolytic flux-dependent post-translational modifications. This molecular chaperone impedes HMGB1 lactylation through suppression of Warburg effect activation in hepatocytes, effectively blocking damage-associated molecular pattern release. The consequent attenuation of monocyte recruitment and NLRP3 inflammasome activation establishes HSPA12A as a gatekeeper of sterile inflammation[28]. Similarly, in brain ischemic stroke, astrocytic low-density lipoprotein receptor-related protein 1 suppresses glucose uptake, glycolysis, and lactate production, leading to decreased lactylation of ARF1. This reduction promotes astrocyte-to-neuron mitochondria transfer, demonstrating a protective effect against stroke[29].
The therapeutic potential of AARS2 modulation must be balanced against its pleiotropic functions. In cancer, AARS2-mediated lactylation of cGAS facilitates immune evasion by suppressing STING pathway activation[30], while in metabolic disorders, hepatic AARS2 promotes steatosis via lactylation of PPARγ coactivators[31]. Such tissue-specific effects highlight the risk of off-target consequences when systemically inhibiting AARS2. Notably, AARS2 global knockout mice exhibit embryonic lethality[32], emphasizing its essential roles in development. Future strategies may require tissue-selective delivery (e.g., gut-targeted nanoparticles) or time-limited inhibition during acute IIR phases.
The AARS1/2 synthetase complex functions as a lactate-responsive molecular switch, converting extracellular L-lactate flux into epigenetic signals via catalytic activation of lysine lactylation cascades–a process essential for cellular metabolic memory[33,34]. AARS2 plays a particularly important role in regulating lactylation modification in diseases, especially in cancer, inflammation, and metabolism-related diseases. In diseases, the lactylation modification regulated by AARS2 affects the function of various proteins, including the cGAS protein involved in antiviral innate immune responses. Under conditions of elevated lactate levels, AARS2 can lactylate cGAS, leading to its inactivation and thereby inhibiting the antiviral innate immune response. This finding explains why many severe diseases, including cancer, exhibit elevated lactate levels, as high levels of lactate can create an immunosuppressive microenvironment that contributes to disease progression[35]. Additionally, the role of AARS2 under hypoxic conditions has also garnered attention. Studies have found that the accumulation of AARS2 under hypoxia can rapidly trigger lactylation modifications, particularly targeting the mitochondrial pyruvate dehydrogenase E1α subunit and carnitine palmitoyltransferase 2, effectively inhibiting oxidative phosphorylation and reducing the production of reactive oxygen species and oxidative damage[36]. In our findings, the I/R-induced upregulation of AARS2 led to accumulated lactylation in ACSL4, which resulted in ferroptosis of intestinal epithelial cells during IIR. In vitro study revealed that AARS2 regulates the H3K18 La modification on ACSL4 promoter region and activated its transfection and expression. Besides, overexpression of AARS2 recovered the anti-ferroptosis effects of shACSL4, which validated the AARS2-ACSL4 regulatory axis during IIR.
Targeting the AARS2-ACSL4 axis presents both opportunities and hurdles. First, the deep catalytic pocket of AARS2’s lactylation domain complicates small-molecule inhibitor design. Second, lactate metabolism is essential for cellular homeostasis—broad-spectrum lactylation inhibition may disrupt physiological processes like wound healing. Third, compensatory mechanisms (e.g., other synthetases like AARS1) could limit therapeutic efficacy. Alternative approaches could include: (1) Substrate competition: Lactate analogs that block AARS2-lactate binding; (2) Epigenetic editing: CRISPR-dCas9 systems to erase H3K18 La at ACSL4 promoter; and (3) Combination therapy: Co-targeting AARS2 and ferroptosis inhibitors (e.g., liproxstatin-1). Preclinical validation in human intestinal organoid models and non-human primates will be critical next steps.
Our study revealed the role of AARS2 in exacerbating IIR progression. The elevation of AARS2 during IIR led to cell ferroptosis via epigenetically upregulating the expression of ACSL4. Our findings presented AARS2 as a promising therapeutic target for IIR.
| 1. | Gonzalez LM, Moeser AJ, Blikslager AT. Animal models of ischemia-reperfusion-induced intestinal injury: progress and promise for translational research. Am J Physiol Gastrointest Liver Physiol. 2015;308:G63-G75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 183] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 2. | Hou J, Ness SS, Tschudi J, O'Farrell M, Veddegjerde R, Martinsen ØG, Tønnessen TI, Strand-Amundsen R. Assessment of Intestinal Ischemia-Reperfusion Injury Using Diffuse Reflectance VIS-NIR Spectroscopy and Histology. Sensors (Basel). 2022;22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 3. | Akbari G. Emerging roles of microRNAs in intestinal ischemia/reperfusion-induced injury: a review. J Physiol Biochem. 2020;76:525-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 4. | Tan C, Norden PR, Yu W, Liu T, Ujiie N, Lee SK, Yan X, Dyakiv Y, Aoto K, Ortega S, De Plaen IG, Sampath V, Kume T. Endothelial FOXC1 and FOXC2 promote intestinal regeneration after ischemia-reperfusion injury. EMBO Rep. 2023;24:e56030. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 5. | Alicehajic A, Duivenvoorden AAM, Lenaerts K. Unveiling the molecular complexity of intestinal ischemia-reperfusion injury through omics technologies. Proteomics. 2024;24:e2300160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 6. | Wang Z, Sun R, Wang G, Chen Z, Li Y, Zhao Y, Liu D, Zhao H, Zhang F, Yao J, Tian X. SIRT3-mediated deacetylation of PRDX3 alleviates mitochondrial oxidative damage and apoptosis induced by intestinal ischemia/reperfusion injury. Redox Biol. 2020;28:101343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 162] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 7. | Ding P, Ma Z, Fan Y, Feng Y, Shao C, Pan M, Zhang Y, Huang D, Han J, Hu Y, Yan X. Emerging role of ubiquitination/deubiquitination modification of PD-1/PD-L1 in cancer immunotherapy. Genes Dis. 2023;10:848-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 8. | Fang Y, Xu X, Ding J, Yang L, Doan MT, Karmaus PWF, Snyder NW, Zhao Y, Li JL, Li X. Histone crotonylation promotes mesoendodermal commitment of human embryonic stem cells. Cell Stem Cell. 2021;28:748-763.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 90] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 9. | Hou JY, Zhou L, Li JL, Wang DP, Cao JM. Emerging roles of non-histone protein crotonylation in biomedicine. Cell Biosci. 2021;11:101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 10. | Chen AN, Luo Y, Yang YH, Fu JT, Geng XM, Shi JP, Yang J. Lactylation, a Novel Metabolic Reprogramming Code: Current Status and Prospects. Front Immunol. 2021;12:688910. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 136] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 11. | Chen L, Huang L, Gu Y, Cang W, Sun P, Xiang Y. Lactate-Lactylation Hands between Metabolic Reprogramming and Immunosuppression. Int J Mol Sci. 2022;23:11943. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 158] [Reference Citation Analysis (0)] |
| 12. | Fan H, Yang F, Xiao Z, Luo H, Chen H, Chen Z, Liu Q, Xiao Y. Lactylation: novel epigenetic regulatory and therapeutic opportunities. Am J Physiol Endocrinol Metab. 2023;324:E330-E338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 54] [Reference Citation Analysis (0)] |
| 13. | Lin J, Liu G, Chen L, Kwok HF, Lin Y. Targeting lactate-related cell cycle activities for cancer therapy. Semin Cancer Biol. 2022;86:1231-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 48] [Reference Citation Analysis (0)] |
| 14. | Liu L, Gao J, Liu X, Zhang F, Hu B, Zhang H, Wang Z, Tang H, Shi JH, Zhang S. AARS2 as a novel biomarker for prognosis and its molecular characterization in pan-cancer. Cancer Med. 2023;12:21531-21544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Mao Y, Zhang J, Zhou Q, He X, Zheng Z, Wei Y, Zhou K, Lin Y, Yu H, Zhang H, Zhou Y, Lin P, Wu B, Yuan Y, Zhao J, Xu W, Zhao S. Hypoxia induces mitochondrial protein lactylation to limit oxidative phosphorylation. Cell Res. 2024;34:13-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 113] [Article Influence: 113.0] [Reference Citation Analysis (0)] |
| 16. | Yu H, Guo P, Xie X, Wang Y, Chen G. Ferroptosis, a new form of cell death, and its relationships with tumourous diseases. J Cell Mol Med. 2017;21:648-657. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 260] [Cited by in RCA: 512] [Article Influence: 56.9] [Reference Citation Analysis (0)] |
| 17. | Do Van B, Gouel F, Jonneaux A, Timmerman K, Gelé P, Pétrault M, Bastide M, Laloux C, Moreau C, Bordet R, Devos D, Devedjian JC. Ferroptosis, a newly characterized form of cell death in Parkinson's disease that is regulated by PKC. Neurobiol Dis. 2016;94:169-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 516] [Article Influence: 57.3] [Reference Citation Analysis (0)] |
| 18. | Magtanong L, Ko PJ, Dixon SJ. Emerging roles for lipids in non-apoptotic cell death. Cell Death Differ. 2016;23:1099-1109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 203] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 19. | Jiang X, Stockwell BR, Conrad M. Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol Cell Biol. 2021;22:266-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2184] [Cited by in RCA: 4181] [Article Influence: 1045.3] [Reference Citation Analysis (0)] |
| 20. | Zeng F, Nijiati S, Tang L, Ye J, Zhou Z, Chen X. Ferroptosis Detection: From Approaches to Applications. Angew Chem Int Ed Engl. 2023;62:e202300379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 116] [Article Influence: 58.0] [Reference Citation Analysis (0)] |
| 21. | Doll S, Proneth B, Tyurina YY, Panzilius E, Kobayashi S, Ingold I, Irmler M, Beckers J, Aichler M, Walch A, Prokisch H, Trümbach D, Mao G, Qu F, Bayir H, Füllekrug J, Scheel CH, Wurst W, Schick JA, Kagan VE, Angeli JP, Conrad M. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat Chem Biol. 2017;13:91-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1074] [Cited by in RCA: 2695] [Article Influence: 299.4] [Reference Citation Analysis (0)] |
| 22. | Zhu Z, Hou Q, Wang B, Li C, Liu L, Gong W, Chai J, Guo H. A novel mitochondria-related gene signature for controlling colon cancer cell mitochondrial respiration and proliferation. Hum Cell. 2022;35:1126-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Zhou Y, Chen B, Li L, Pan H, Liu B, Li T, Wang R, Ma X, Wang B, Cao Y. Novel alanyl-tRNA synthetase 2 (AARS2) homozygous mutation in a consanguineous Chinese family with premature ovarian insufficiency. Fertil Steril. 2019;112:569-576.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Zhao X, Han J, Zhu L, Xiao Y, Wang C, Hong F, Jiang P, Guan MX. Overexpression of human mitochondrial alanyl-tRNA synthetase suppresses biochemical defects of the mt-tRNA(Ala) mutation in cybrids. Int J Biol Sci. 2018;14:1437-1444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Yu J, Chai P, Xie M, Ge S, Ruan J, Fan X, Jia R. Histone lactylation drives oncogenesis by facilitating m(6)A reader protein YTHDF2 expression in ocular melanoma. Genome Biol. 2021;22:85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 508] [Article Influence: 127.0] [Reference Citation Analysis (0)] |
| 26. | Lv X, Lv Y, Dai X. Lactate, histone lactylation and cancer hallmarks. Expert Rev Mol Med. 2023;25:e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 75] [Reference Citation Analysis (0)] |
| 27. | Wang N, Wang W, Wang X, Mang G, Chen J, Yan X, Tong Z, Yang Q, Wang M, Chen L, Sun P, Yang Y, Cui J, Yang M, Zhang Y, Wang D, Wu J, Zhang M, Yu B. Histone Lactylation Boosts Reparative Gene Activation Post-Myocardial Infarction. Circ Res. 2022;131:893-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 221] [Article Influence: 73.7] [Reference Citation Analysis (0)] |
| 28. | Rho H, Terry AR, Chronis C, Hay N. Hexokinase 2-mediated gene expression via histone lactylation is required for hepatic stellate cell activation and liver fibrosis. Cell Metab. 2023;35:1406-1423.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 153] [Article Influence: 76.5] [Reference Citation Analysis (0)] |
| 29. | Du S, Zhang X, Jia Y, Peng P, Kong Q, Jiang S, Li Y, Li C, Ding Z, Liu L. Hepatocyte HSPA12A inhibits macrophage chemotaxis and activation to attenuate liver ischemia/reperfusion injury via suppressing glycolysis-mediated HMGB1 lactylation and secretion of hepatocytes. Theranostics. 2023;13:3856-3871. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 61] [Reference Citation Analysis (0)] |
| 30. | Zhou J, Zhang L, Peng J, Zhang X, Zhang F, Wu Y, Huang A, Du F, Liao Y, He Y, Xie Y, Gu L, Kuang C, Ou W, Xie M, Tu T, Pang J, Zhang D, Guo K, Feng Y, Yin S, Cao Y, Li T, Jiang Y. Astrocytic LRP1 enables mitochondria transfer to neurons and mitigates brain ischemic stroke by suppressing ARF1 lactylation. Cell Metab. 2024;36:2054-2068.e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 70] [Article Influence: 70.0] [Reference Citation Analysis (0)] |
| 31. | Zhang X, Li J, Zhang Y, Gao M, Peng T, Tian T. AARS2-Related Leukodystrophy: a Case Report and Literature Review. Cerebellum. 2023;22:59-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 32. | Wang S, Lv H, Zhou F. AARS1 and AARS2, the L-lactate sensors and universal lactyltransferases. Sci Bull (Beijing). 2024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 33. | Wang JY, Chen SF, Zhang HQ, Wang MY, Zhu JH, Zhang X. A homozygous mutation of alanyl-transfer RNA synthetase 2 in a patient of adult-onset leukodystrophy: A case report and literature review. Brain Behav. 2019;9:e01313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 34. | Wang D, Yu M, Zhang W, Wang Z, Yuan Y. AARS2 Compound Heterozygous Variants in a Case of Adult-Onset Leukoencephalopathy With Axonal Spheroids and Pigmented Glia. J Neuropathol Exp Neurol. 2018;77:997-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 35. | Li H, Liu C, Li R, Zhou L, Ran Y, Yang Q, Huang H, Lu H, Song H, Yang B, Ru H, Lin S, Zhang L. AARS1 and AARS2 sense L-lactate to regulate cGAS as global lysine lactyltransferases. Nature. 2024;634:1229-1237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 74] [Article Influence: 74.0] [Reference Citation Analysis (0)] |
| 36. | Liu H, Ge B. Lactylation as a post-translational regulator of cGAS and immunity. Mol Cell. 2024;84:4483-4485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |