Published online Jun 27, 2025. doi: 10.4240/wjgs.v17.i6.106637
Revised: April 4, 2025
Accepted: April 27, 2025
Published online: June 27, 2025
Processing time: 88 Days and 6 Hours
Endoscopic papillectomy (EP) via endoscopic retrograde cholangiopancreatography has emerged as a less invasive alternative to surgery for duodenal papillary adenomas (DPAs), which is traditionally associated with notable postoperative risks.
To compare quality of life (QoL) and outcomes between DPA patients undergoing EP vs surgical resection, and to assess the influencing factors of QoL and complications.
We conducted a retrospective, single-center analysis involving patients treated for DPA at the Drum Tower Hospital of Nanjing University Medical School from 2011 to 2023. The participants completed post-discharge telephone surveys using the 12-item short form survey to assess mental (MCS) and physical component summary (PCS) scores, with norm-based scoring where ≥ 50 denotes normal. Multivariate regression analysis adjusted for confounding variables was used to compare QoL scores.
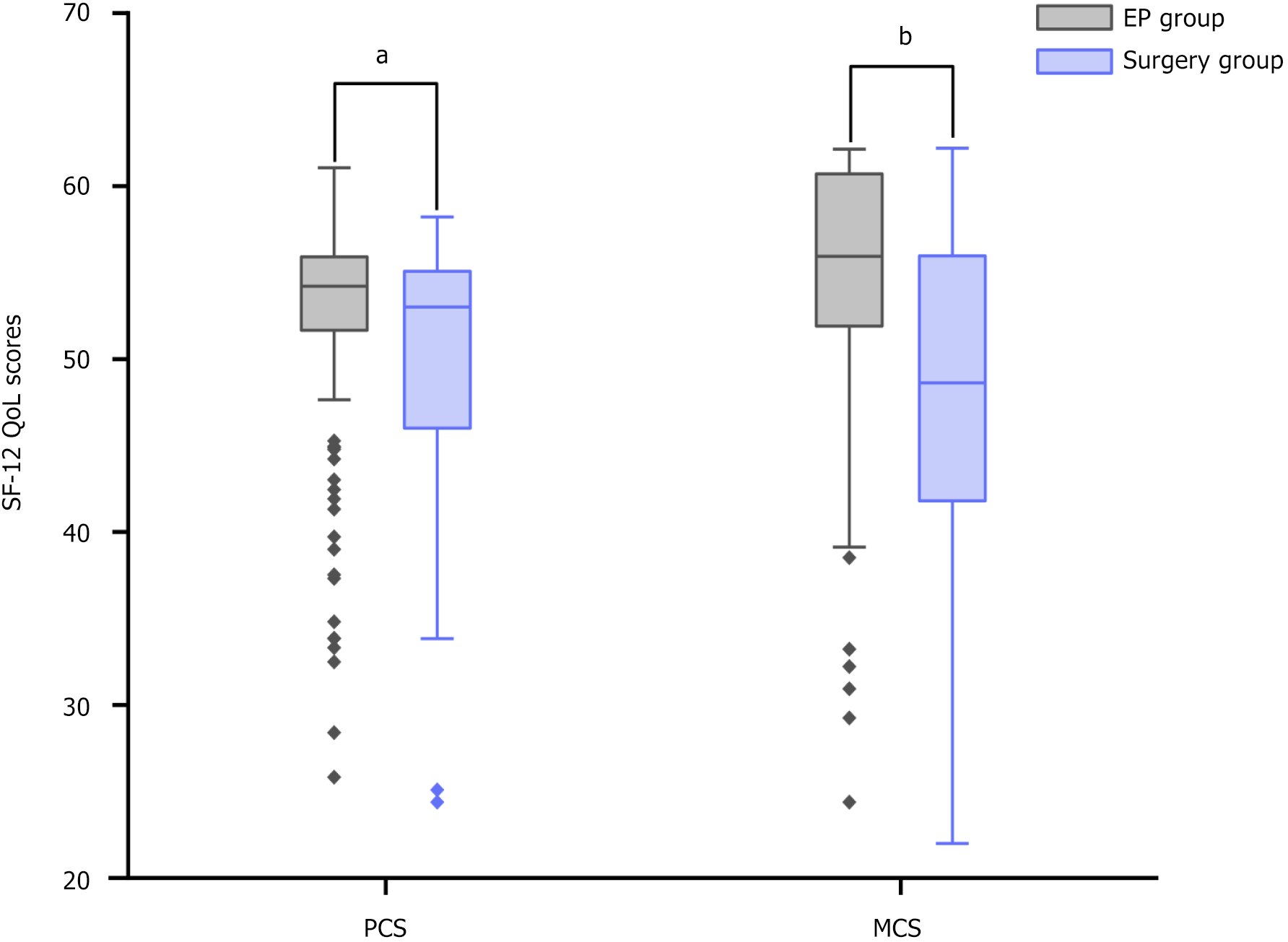
Compared with EP patients, surgically treated patients had significantly lower PCS [median: 53.0, interquartile range (IQR): 46.0-55.1 vs 54.2, IQR: 51.7-55.9, P = 0.008] and MCS scores (median: 48.6, IQR: 41.8-56.0 vs 55.9, IQR: 51.7-60.7, P < 0.001). These disparities persisted even after adjustments for demographic and medical factors. Long-term follow-up of the EP group revealed that abdominal pain and poor sleep were factors negatively impacting PCS scores, whereas postoperative pancreatitis and hypertension were associated with lower MCS scores.
EP has emerged as a QoL-preserving alternative for patients with DPA, conditional upon ensuring equivalent efficacy and safety. QoL outcomes should be considered when choosing interventions for this patient population.
Core Tip: The detection of duodenal papillary adenomas has increased due to advancements in endoscopic techniques, although the prevalence of this condition remains low. Traditionally, surgical resection is the primary treatment, but it has significant drawbacks, such as trauma and high complication rates. Endoscopic removal methods have gained popularity because they are less invasive and better preserve postoperative quality of life. Our study uniquely compared postoperative quality of life and long-term outcomes between patients who received endoscopic and surgical treatments, identifying key factors that affect quality of life after treatment. This research supports endoscopic therapy as a patient-centered approach for managing these adenomas, highlighting the balance between curative intent and quality of life preservation.
- Citation: Wang FL, Tang XX, Wu R, Gao YJ, Liu YR, Wang L, Zou XP, Zhang B. Quality of life and outcomes in patients undergoing endoscopic papillectomy vs surgical treatment for duodenal papillary adenomas. World J Gastrointest Surg 2025; 17(6): 106637
- URL: https://www.wjgnet.com/1948-9366/full/v17/i6/106637.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i6.106637
Duodenal papillary adenomas (DPAs), rare benign tumors that favor women aged 60-80 years[1,2], are categorized into sporadic (40%) and familial adenomatous polyposis-linked (60%) subtypes[3]. Recent endoscopic advancements have improved detection, with duodenoscopy, endoscopic retrograde cholangiopancreatography (ERCP), and histopathology being pivotal for diagnosis[4,5]. Historically, surgical resection, although effective, has posed significant risks, prompting the rise of endoscopic papillectomy (EP) as a less invasive alternative treatment[5-8]. EP, which offers targeted removal and reduced complication rates, is associated with an increased risk of recurrence, notably in patients with familial adenomatous polyposis and incomplete resections[9-11]. Recently, a novel modified EP for duodenal major papilla adenoma disclosed by our group demonstrated a relatively high en bloc resection rate and thus decreased the recurrence rate caused by incomplete resection[12,13]. While previous studies have focused on recurrence and complications, there are limited data on the quality of life (QoL) differences between EP and surgical resection, a crucial factor influencing treatment choice. Post-procedural pain, dietary constraints, emotional health, and functional impacts significantly affect QoL, underscoring the need for studies comparing QoL outcomes between endoscopic and surgical duodenal adenoma treatments[14-16].
The current study focused on three principal objectives that can collectively advance our understanding of DPA management and its impact on patient well-being. First, we aimed to evaluate and compare the physical and psychological components of QoL in patients who received either endoscopic or surgical treatment for DPAs. Second, we aimed to pinpoint the specific factors that correlate with reduced QoL following adenoma treatment. Finally, we aimed to clarify the incidence and nature of complications associated with various treatment modalities for DPAs. Our study will enrich the field of DPA management by synthesizing patient feedback, clinical outcomes, and complication rates, suggesting a holistic perspective for gauging treatment efficacy and highlighting patient welfare in medical choices.
This retrospective single-center study conducted at Drum Tower Hospital of Nanjing University Medical School aimed to investigate the QoL and associated factors in patients with DPAs who underwent EP or surgical resection between 2011 and 2023. All included patients provided written informed consent for data analysis in the framework of scientific studies. This study was approved by the Ethics Committee of Nanjing Drum Tower Hospital, the Affiliated Hospital of Medical School, Nanjing University (No. 2024-351-01, ethical approval on June 28, 2024). The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki. We used a modified Chinese version 2.0 of the 12-item short-form health survey to assess the quality of physical and mental health[17]. This scale is a shortened version of the 36-item short-form health survey, covering 8 health domains through 12 questions: Physical functioning, role limitations due to physical health problems, bodily pain, general health perception, vitality, social functioning, role limitations due to emotional problems, and mental health. The scale generates two scores: The physical component summary (PCS) and the mental component summary (MCS), reflecting an individual’s physical and mental health status, respectively. Scores typically range from 0 to 100, with 50 representing the average; higher scores indicate better health states. This tool is widely used in clinical research and public health fields. Its application in patients undergoing pancreaticoduodenectomy and its ability to capture physical and mental health make it effective for assessing the impact of interventions on patients’ QoL. Data collection involved a telephone survey, with up to three attempts made to contact nonresponsive patients, and ethical considerations were observed.
Inclusion criteria: (1) Age over 18 years; (2) Complete medical history and endoscopic and surgical resection data; and (3) Patients who successfully underwent endoscopic resection of DPA or surgical operation and received telephone follow-up.
Exclusion criteria: (1) Non-original anatomical structure (such as after gastroenterostomy, Billroth II, or Roux-en-Y procedures); (2) Inability to cooperate with follow-up surveys or presence of cognitive impairment; (3) Accidental death due to other diseases or accidents; (4) Missing critical extracted values; and (5) Refusal to accept telephone follow-up or inability to contact due to reasons such as the phone number being invalid, wrong number, or the phone being turned off.
Among the 250 patients initially enrolled in the EP group, 155 were ultimately included after accounting for exclusions due to contact issues, refusal, death, and various medical reasons. Similarly, 43 of the 81 patients who underwent surgical resection met the similar exclusion criteria. Among them, 36 patients underwent pancreaticoduodenectomy, and 7 underwent surgical local resection of the papillary adenoma. The study also collected demographic data, recurrence rates, and details on post-relapse treatments, alongside information on lifestyle factors (smoking and alcohol consumption), common comorbidities, and adenoma-related complications.
Additionally, the factors that influence prognosis, including adenoma size, surgical approach, ductal invasion, and adenoma pathology, were explored. Notably, subjective experiences of postoperative abdominal pain, the use of pain medication, changes in appetite, sleep, and weight were also documented to provide a comprehensive understanding of the QoL of patients post-treatment.
All patients underwent routine endoscopic ultrasound (EUS) before EP to assess tumor infiltration depth, lymph node metastasis, and involvement of other organs.
The basic EP procedure was described in our previous report[13]. Based on intraoperative findings and combined with imaging examinations, DPAs were resected using either traditional endoscopic snare papillectomy (ESP) or modified ESP. For patients found to have bile duct extension during EUS, radiofrequency ablation of the bile duct was utilized. If any residual tissue was suspected, argon plasma coagulation was employed to ablate it. To prevent potential postoperative complications, such as stenosis or obstruction, pancreatic and/or biliary duct stents may be placed. Hemostatic clips were strategically deployed to manage or prevent bleeding.
Surgical resection is indicated for adenomas with significant extension into the common bile duct and/or pancreatic duct, for adenomas that are too large to be locally excised, or when biopsy reveals invasive malignant tumors. The surgical management of patients in the study was meticulously planned and executed by surgeons with specialized expertise. Prior to surgery, several factors, including preoperative imaging, laboratory parameters, patient preference, and surgeon experience, were meticulously considered when tailoring the surgical strategy to each patient’s unique circumstances. Surgical options included pancreaticoduodenectomy (Whipple procedure), which involves removal of the head of the pancreas, the first part of the small intestine (duodenum), the gallbladder, and a portion of the bile duct, or local excision of duodenal papillary tumors, a less invasive approach that involves the removal of the tumor with clear margins while preserving as much of the surrounding tissue as possible.
For descriptive analyses, the PCS and MCS scores are presented as the median and interquartile range (IQR). Continuous measurements are presented as the mean ± SD if they are normally distributed or median and IQR if they are not normally distributed, and categorical variables are summarized as counts and percentages. We used SPSS (version 25.0) for all the analyses.
A total of 198 patients were enrolled in this study, 155 of whom were in the EP group (101 males and 54 females) and 43 of whom were in the surgery group (23 males and 20 females). Patients in the EP group were slightly younger, with a mean age of 56.9 ± 10.4 years, than those in the surgery group, who had a mean age of 60.1 ± 8.68 years (P = 0.051). Patients who underwent surgery had a significantly lower body mass index (BMI) than those treated endoscopically (P < 0.001). Sex distribution, presence of comorbidities, smoking habits, and alcohol consumption did not significantly differ between the two groups. Patients in the surgery group had higher alkaline phosphatase (ALP) and gamma-glutamyl transferase (GGT) (P = 0.003 and 0.001, respectively) levels but lower hemoglobin (Hb) levels (P < 0.001) than those in the EP group. We consider that the higher ALP and GGT levels in DPA patients undergoing surgical treatment may be due to their tumors being more aggressive and larger compared to those in the endoscopic group. These larger tumors can compress the bile ducts or pancreatic ducts, lead to impaired bile and pancreatic juice drainage and dilation of the bile ducts and pancreatic ducts, and cause biliary obstruction and intrahepatic bile stasis, thereby resulting in elevated levels of ALP and GGT. Additionally, the infiltration and aggressiveness of the tumor can lead to decreased appetite, and impaired digestive and absorption functions, thereby affecting nutritional status and resulting in a lower BMI and anemia. The carcinoembryonic antigen level, total bilirubin level, and white blood cell count did not significantly differ between the two groups (Table 1).
| Variable | EP (n = 155) | Surgery (n = 43) | P value |
| Age, years | 0.051 | ||
| mean ± SD | 56.9 ± 10.4 | 60.1 ± 8.68 | |
| Range | 28-81 | 39-75 | |
| ≤ 39 | 7 (4.5) | 1 (2.3) | |
| 40-49 | 29 (18.7) | 3 (7.0) | |
| 50-59 | 60 (38.7) | 17 (39.5) | |
| 60-69 | 43 (27.7) | 16 (37.2) | |
| ≥ 70 | 16 (10.3) | 6 (14.0) | |
| Sex | 0.162 | ||
| Female | 54 (38.4) | 20 (46.5) | |
| Male | 101 (65.2) | 23 (53.5) | |
| BMI (kg/m2) | 0.001 | ||
| Normal/low (≤ 23.9) | 74 (47.7) | 33 (76.7) | |
| Overweight (24-27.9) | 66 (42.6) | 9 (20.9) | |
| Obese (≥ 28) | 15 (9.7) | 1 (2.3) | |
| Comorbidities | |||
| History of hypertension | 63 (40.6) | 17 (39.5) | 0.896 |
| History of diabetes | 26 (16.8) | 8 (18.6) | 0.778 |
| History of hyperlipidemia | 36 (23.2) | 4 (9.3) | 0.053 |
| History of heart disease | 27 (17.4) | 3 (7.0) | 0.195 |
| History of liver disease | 38 (24.5) | 13 (30.2) | 0.448 |
| History of renal disease | 31 (20.0) | 10 (23.3) | 0.641 |
| History of other tumors | 20 (12.9) | 5 (11.6) | 0.824 |
| History of stroke | 4 (2.6) | 1 (2.3) | 1.000 |
| Current smoking | 26 (16.8) | 4 (9.3) | 0.227 |
| Current alcohol consumption | 11 (7.1) | 2 (4.7) | 0.822 |
| CEA (ng/mL), median (IQR) | 1.0 (0.5-1.6) | 1.1 (0.6-1.9) | 0.299 |
| CA-199 (U/mL), median (IQR) | 9.5 (5.8-12.5) | 15.0 (9.6-26.6) | < 0.001 |
| TBil (μmol/L), median (IQR) | 10.5 (7.1-13.7) | 11.5 (9.0-15.8) | 0.138 |
| DBil (μmol/L), median (IQR) | 2.4 (1.8-3.5) | 3.1 (2.1-4.9) | 0.020 |
| ALT (U/L), median (IQR) | 16.5 (12.6-22.4) | 18.8 (11.7-56.0) | 0.090 |
| ALP (U/L), median (IQR) | 66.7 (55.8-85.0) | 74.4 (64.2-144.3) | 0.003 |
| GGT (U/L), median (IQR) | 20.9 (15.9-30.7) | 25.4 (19.2-459.6) | 0.001 |
| Hb (g/L), median (IQR) | 137.0 (126.0-150.0) | 128.0 (117.0-136.0) | < 0.001 |
| WBC (× 109/L), median (IQR) | 5.1 (4.4-6.3) | 5.2 (4.1-6.6) | 0.942 |
| PLT (× 109/L), median (IQR) | 186.0 (157.0-215.0) | 201.0 (177.0-234.0) | 0.029 |
The median length of tumors in the surgery group was 30.0 mm, with a range from 18.0 mm to 40.0 mm. In contrast, tumors in the EP group had a significantly smaller median length of 14.0 mm, ranging from 10.0 mm to 17.0 mm. Similarly, the median width of the tumors in the surgery group was 15.0 mm, with a range from 13.0 mm to 30.0 mm. In the EP group, the median tumor width was 10.0 mm, ranging from 7.0 mm to 14.0 mm. Bile duct invasion was significantly more common in the surgery group, at 27.9%, than in the EP group, at only 9.0% (P = 0.001). The tumors in the surgical group also presented a significantly higher pathological grade (P < 0.001). Both common bile duct dilatation (41.9% vs 16.1%, P < 0.001) and pancreatic duct dilatation (23.3% vs 0%, P < 0.001) were significantly more common in the surgery group. Despite the differences noted above, pancreatic ductal invasion, vascular invasion, nerve invasion, and lymph node metastasis did not significantly differ between the two groups (Table 2). It should be noted that the dif
| Variable | EP (n = 155) | Surgery (n = 43) | P value |
| Tumor size, median (IQR) (mm) | |||
| Length | 14.0 (10.0-17.0) | 30.0 (18.0-40.0) | < 0.001 |
| Width | 10.0 (7.0-14.0) | 15.0 (13.0-30.0) | < 0.001 |
| Bile duct invasion | 14 (9.0) | 12 (27.9) | 0.001 |
| Pancreatic duct invasion | 3 (1.9) | 1 (2.3) | 1.000 |
| Neurological invasion | 0 (0.0) | 1 (2.3) | 0.217 |
| Vascular invasion | 0 (0.0) | 1 (2.3) | 0.217 |
| Lymphatic metastasis | 0 (0.0) | 0 (0.0) | |
| Duodenal diverticulum | 8 (5.2) | 0 (0.0) | 0.279 |
| Preoperative dilatation of the common bile duct | 25 (16.1) | 18 (41.9) | < 0.001 |
| Preoperative dilatation of the pancreatic duct | 0 (0.0) | 10 (23.3) | < 0.001 |
| Pathology | < 0.001 | ||
| No dysplasia | 32 (20.6) | 0 (0.0) | |
| Adenoma/LGD | 98 (63.2) | 14 (32.6) | |
| Adenoma/HGD | 6 (3.9) | 5 (11.6) | |
| Adenoma/LGD with focal HGD | 14 (9.0) | 10 (23.3) | |
| Adenoma/HGD with focal adenocarcinoma | 5 (3.2) | 10 (23.3) | |
| Adenocarcinoma | 0 (0.0) | 4 (9.3) |
Although the overall occurrence of postoperative abdominal pain did not significantly differ between the groups, postoperative abdominal pain was more frequent in the surgical group (P = 0.001). Moreover, the nature and location of the pain also notably differed between the groups. Right upper quadrant pain was more common in the EP group, which may be related to bile duct stones, pancreatitis, and postoperative intestinal dysfunction, whereas left upper quadrant pain was more common in the surgical group. Abdominal pain in different locations after surgical and endoscopic treatments in patients with DPA may indicate different postoperative inflammation patterns or pathophysiological mechanisms. We speculate that left upper quadrant abdominal pain after surgical treatment might be related to the extensive surgical trauma, severe inflammatory response, and postoperative complications such as intra-abdominal adhesions and infections. In contrast, endoscopic treatment involves relatively minor and localized trauma, with postoperative inflammatory responses mainly concentrated at the site of the local lesion. Hidden pain was common in both groups, but abdominal colic and distension were more frequently observed in the surgical group. Patients who underwent surgery lost more weight postoperatively than patients in the EP group (P = 0.047). Notably, immediate postoperative complications, such as bleeding, pancreatitis, cholangitis, or perforation, did not significantly differ between the groups. However, delayed postoperative bleeding was more common in the EP group (P < 0.001). Surgical treatment, particularly pancreaticoduodenectomy, carried a greater risk of specific complications, such as pancreatic fistulae, bile fistulae, and abdominal and incisional infections. Among the 43 patients who underwent open surgery, 10 (23.3%) developed a postoperative pancreatic fistula, 2 (4.7%) developed a postoperative bile fistula, 1 (2.3%) developed a postoperative chylous fistula, 15 (34.9%) developed a postoperative abdominal infection, and 2 (4.7%) developed a postoperative incisional infection. Among the 155 patients treated with endoscopy, 133 were reviewed regularly, and local tumor recurrence occurred in 8 (5.2%) patients, 6 of whom underwent additional endoscopic treatment. Among the 43 patients who underwent surgical treatment, 41 were reviewed regularly; 1 (2.3%) experienced recurrence after undergoing local excision of a duodenal papillary tumor and subsequently underwent endoscopic treatment, and the remaining patients did not experience any recurrence. Postoperative recurrence did not significantly differ between the two groups (Table 3).
| Variable | EP (n = 155) | Surgery (n = 43) | P value |
| Quality of life | |||
| PCS score, median (IQR) | 54.2 (51.7-55.9) | 53.0 (46.0-55.1) | 0.008 |
| ≥ 50 | 129 (83.2) | 29 (67.4) | 0.023 |
| MCS score, median (IQR) | 55.9 (51.7-60.7) | 48.6 (41.8-56.0) | < 0.001 |
| ≥ 50 | 124 (80.0) | 20 (46.5) | < 0.001 |
| Follow-up time, months, median (IQR) | 37.0 (25.0-54.0) | 46.0 (25.0-65.0) | 0.451 |
| Range | 13-131 | 5-91 | 0.113 |
| ≤ 24 | 38 (24.5) | 10 (23.3) | |
| 25-60 | 85 (54.8) | 19 (44.2) | |
| 61-120 | 26 (16.8) | 14 (32.5) | |
| > 120 | 6 (3.9) | 0 (0.0) | |
| Follow-up pancreatitis | 8 (5.2) | 0 (0.0) | 0.279 |
| Follow-up cholangitis | 6 (3.9) | 2 (4.7) | 1.000 |
| Follow-up bile duct stones | 12 (7.7) | 1 (2.3) | 0.357 |
| Abdominal pain | 32 (20.6) | 14 (32.6) | 0.102 |
| Frequency of abdominal pain | 0.001 | ||
| Infrequent | 25 (16.1) | 9 (20.9) | |
| Sometimes | 7 (4.5) | 5 (11.6) | |
| Region of abdominal pain | < 0.001 | ||
| Back | 1 (0.6) | 0 (0.0) | |
| Epigastric region | 14 (9.0) | 10 (23.3) | |
| Right upper abdomen | 9 (5.8) | 0 (0.0) | |
| Right lower abdomen | 1 (0.6) | 0 (0.0) | |
| Left upper abdomen | 7 (4.5) | 3 (7.0) | |
| Left lower abdomen | 0 (0.0) | 1 (2.3) | |
| Abdominal pain classification | < 0.001 | ||
| Sting | 2 (1.3) | 0 (0.0) | |
| Colic | 2 (1.3) | 1 (2.3) | |
| Hidden anguish | 22 (14.2) | 10 (23.3) | |
| Distending pain | 6 (3.9) | 3 (7.0) | |
| Appetite decline | 10 (6.5) | 7 (16.3) | 0.084 |
| Weight loss | 15 (9.7) | 9 (20.9) | 0.047 |
| Poor sleep | 26 (16.8) | 9 (20.9) | 0.527 |
| Bleeding | |||
| Postoperative bleeding | 9 (5.8) | 1 (2.3) | 0.597 |
| Delayed bleeding | 11 (7.1) | 0 (0.0) | < 0.001 |
| Pancreatitis | 17 (11.0) | 1 (2.3) | 0.149 |
| Cholangitis | 2 (1.3) | 0 (0.0) | 1.000 |
| Perforation | 4 (2.6) | 0 (0.0) | 0.579 |
| Papillary stricture | 2 (1.3) | 0 (0.0) | 1.000 |
| Pancreatic fistula | 0 (0.0) | 10 (23.3) | < 0.001 |
| Biliary fistula | 0 (0.0) | 2 (4.7) | 0.046 |
| Chylous fistula | 0 (0.0) | 1 (2.3) | |
| Abdominal infection | 0 (0.0) | 15 (34.9) | < 0.001 |
| Infection of incisional wound | 0 (0.0) | 2 (4.7) | 0.046 |
| Regular review | 133 (85.8) | 41 (95.3) | 0.090 |
| Recurrence | 8 (5.2) | 1 (2.3) | 0.176 |
| Post-relapse treatment | |||
| Untreated | 2 (25.0) | 0 (0.0) | |
| EMR | 4 (50.0) | 1 (100.0) | |
| EMR with submucosal injection | 2 (25.0) | 0 (0.0) |
The univariate analysis results indicate that patients who underwent surgical treatment experienced a significant decrease in both PCS (P = 0.008) and MCS (P < 0.001) scores postoperatively compared with those who received endoscopic treatment (Figure 1 and Table 3). To delve deeper into these findings and account for potential confounding variables, four models were developed (detailed in Supplementary Tables 1-4). These models aimed to adjust for various factors that might influence the observed differences in PCS and MCS scores, such as age, sex, BMI, tumor characteristics, laboratory values, and postoperative symptoms or complications. By controlling for these variables, we sought to isolate the direct effect of the treatment method (surgery vs EP) on postoperative QoL measures.
As shown in Supplementary Table 1, after adjusting for age, sex, BMI, and follow-up time, both PCS [coefficient: -2.66, 95% confidence interval (CI): -5.015 to -0.304, P = 0.027] and MCS scores (coefficient: -5.695, 95%CI: -8.216 to -3.174, P < 0.001) remained significantly lower in the surgical group than in the EP group. Age was negatively correlated with both PCS (coefficient: -0.10, 95%CI: -0.192 to -0.005, P = 0.039) and MCS scores (coefficient: -0.11, 95%CI: -0.213 to -0.013, P = 0.027), indicating that older patients had lower QoL scores regardless of the treatment method. Sex was also found to influence MCS score, with women having lower scores (coefficient: -3.56, 95%CI: -5.665 to -1.448, P = 0.001), potentially due to greater anxiety levels. BMI and follow-up duration did not significantly impact QoL after adjustment.
As shown in Supplementary Table 2, after adjustment for tumor characteristics (size, bile duct invasion, duct dilatations, and pathology), there remained a persistent significant reduction in MCS score but not PCS score for the surgical group compared with the EP group (coefficient: -5.39, 95%CI: -8.975 to -1.799, P = 0.003). Bile duct invasion significantly impacted MCS score (coefficient: -4.42, 95%CI: -7.591 to -1.247, P = 0.007), highlighting the role of tumor invasiveness in mental health outcomes.
After adjustment for baseline laboratory values (carcinoma antigen-199, direct bilirubin, ALP, GGT, Hb, and platelets), as shown in Supplementary Table 3, the mode of treatment still significantly affected both PCS (coefficient: -3.60, 95%CI:
Adjustment for postoperative symptoms and complications (abdominal pain, weight loss, bleeding, pancreatic fistula, and incision infection) in Supplementary Table 4 confirmed a reduced QoL after surgery compared with EP. Abdominal pain and postoperative bleeding were detrimental to both PCS and MCS, whereas pancreatic fistula and incision infection specifically affected MCS.
The sensitivity analysis presented in Table 4 serves to validate and refine the initial findings on the impact of treatment modality (EP vs surgical resection) on the QoL of patients with DPAs. Patients who underwent surgical treatment had significantly lower PCS (coefficient: -3.12, 95%CI: -5.400 to -0.838, P = 0.008) and MCS scores (coefficient: -6.13, 95%CI: -8.633 to -3.619, P < 0.001) than those treated endoscopically in the unadjusted model. The association persisted when demographic characteristics, such as age, sex, and BMI, were accounted for, but the effect sizes slightly changed. PCS (coefficient: -2.66, 95%CI: -5.015 to -0.304, P = 0.027) and MCS scores (coefficient: -5.695, 95%CI: -8.216 to -3.174, P < 0.001) continued to be lower in the surgical group than in the control group, suggesting that demographic differences alone do not account for the observed QoL discrepancies. After further adjustment for adenoma pathological characteristics and laboratory indices, PCS score did not significantly differ between surgically treated and endoscopically treated patients. This finding suggests that some of the variation in PCS could be explained by these factors. However, the significant difference in MCS persisted, indicating a more consistent detrimental effect of surgery on mental health aspects of QoL, even after adjusting for these additional factors. Finally, when all demographic data, adenoma characteristics, laboratory indices, and postoperative complications were incorporated, the analysis still revealed a significant reduction in both PCS (coefficient: -3.99, 95%CI: -7.780 to -0.199, P = 0.039) and MCS scores (coefficient: -5.98, 95%CI: -10.218 to -1.748, P = 0.006) for surgically treated patients compared with endoscopically treated patients. The persistence of these findings in the fully adjusted model underscores the robustness of the association between surgical treatment and reduced QoL. For patients, a 5-point decrease in the MCS score can manifest as increased feelings of anxiety and depression, along with difficulties in daily functioning. Patients may experience heightened emotional distress, reduced ability to cope with stress, and challenges in maintaining social relationships and work performance. Understanding these implications is crucial for clinicians to provide comprehensive support and tailored interventions.
| Variable | PCS | MCS | ||||||||||
| B | SE | β | t value | P value | 95%CI | B | SE | β | t value | P value | 95%CI | |
| Group | -3.12 | 1.15 | -0.19 | -2.70 | 0.008 | -5.400 to | -6.13 | 1.27 | -0.33 | -4.82 | < 0.001 | -8.633 to |
| Model 1 | -2.66 | 1.19 | -0.16 | -2.23 | 0.027 | -5.015 to | -5.695 | 1.28 | -0.30 | -4.46 | < 0.001 | -8.216 to |
| Model 1 + model 2 | -2.68 | 1.69 | -0.16 | -1.59 | 0.114 | -6.031 to 0.655 | -5.34 | 1.79 | -0.28 | -2.98 | 0.003 | -8.873 to |
| Model 1 + model 2 + model 3 | -2.84 | 1.81 | -0.17 | -1.57 | 0.118 | -6.405 to 0.725 | -5.20 | 1.92 | -0.28 | -2.71 | 0.007 | -8.985 to |
| Model 1 + model 2 + model 3 + model 4 | -3.99 | 1.92 | -0.25 | -2.08 | 0.039 | -7.780 to | -5.98 | 2.15 | -0.33 | -2.79 | 0.006 | -10.218 to |
The results in Table 5, derived from both univariate and multivariate analyses, highlight several factors that significantly influence both PCS and MCS scores in patients who have undergone EP. According to the univariate analysis, coexistent hypertension (P = 0.016), a history of heart disease/stroke (P = 0.005), abdominal pain (P < 0.001), decreased appetite (P = 0.033), poor sleep (P = 0.001), weight loss (P = 0.024), and longer follow-up times (P = 0.005) were found to be associated with lower PCS scores. Female sex (P = 0.02), coexistent hyperlipidemia (P = 0.007), coexistent hypertension (P = 0.007), a history of liver disease (P = 0.048), poor sleep (P = 0.047), smoking (P = 0.029), and post-ERCP pancreatitis (PEP) (P = 0.024) were found to be associated with lower MCS scores. The multivariate analysis revealed that abdominal pain (P < 0.001) and poor sleep (P = 0.002) were independently associated with lower PCS scores, whereas PEP (P = 0.034) and coexistent hypertension (P = 0.007) were contributing factors to lower MCS scores. These results emphasize the importance of comprehensive care for patients undergoing EP, focusing not only on the technical aspects of the procedure but also on the management of comorbidities and postoperative symptoms such as pain and sleep disturbance. Additionally, it highlights the need for tailored interventions addressing specific factors, such as hypertension, and postoperative complications, such as pancreatitis, to optimize long-term QoL outcomes.
| Variable | PCS median (IQR) | Univariate analysis | Multivariate analysis | Adjusted R2 | MCS median (IQR) | Univariate analysis | Multivariate analysis | Adjusted R2 | ||||
| P value | Coefficient | 95%CI | P value | P value | Coefficient | 95%CI | P value | |||||
| Sex | 0.81 | 21.00% | 0.02 | -2.15 | -4.474 to 0.184 | 0.071 | 12.40% | |||||
| Female | 54.3 (51.5-55.9) | 55.8 (47.8-59.0) | ||||||||||
| Male | 54.2 (51.7-55.9) | 56.0 (53.5-60.7) | ||||||||||
| Coexistent hyperlipidemia | 0.74 | 0.007 | -2.05 | -4.595 to 0.497 | 0.114 | |||||||
| Yes | 54.1 (50.9-55.9) | 54.9 (47.6-57.6) | ||||||||||
| No | 54.2 (51.7-55.9) | 56.0 (53.3-60.7) | ||||||||||
| Coexistent hypertension | 0.016 | -0.9 | -2.660 to 0.871 | 0.318 | 0.007 | -3.02 | -5.187 to | 0.007 | ||||
| Yes | 53.4 (50.8-55.3) | 55.6 (49.4-60.7) | ||||||||||
| No | 54.9 (52.7-55.9) | 57.0 (54.0-60.7) | ||||||||||
| History of heart disease/stroke | 0.005 | -2.33 | -4.688 to 0.021 | 0.052 | 0.64 | |||||||
| Yes | 51.5 (44.0-55.3) | 55.9 (49.0-60.3) | ||||||||||
| No | 54.3 (52.3-55.9) | 56.0 (53.2-60.7) | ||||||||||
| History of liver disease | 0.52 | 0.048 | -1.58 | -4.688 to 0.021 | 0.22 | |||||||
| Yes | 54.5 (52.0-55.9) | 55.8 (49.8-57.0) | ||||||||||
| No | 54.2 (51.3-55.9) | 56.0 (53.3-60.7) | ||||||||||
| Follow-up time (months) | 0.005 | 0.5 | -0.648 to 1.646 | 0.391 | 0.136 | |||||||
| ≤ 24 | 52.8 (50.2-55.3) | 55.9 (53.5-60.6) | ||||||||||
| 25-60 | 55.2 (52.1-56.2) | 55.9 (50.1-58.3) | ||||||||||
| 61-120 | 53.6 (52.6-55.3) | 58.9 (50.9-60.8) | ||||||||||
| > 120 | 53.0 (50.8-55.3) | 57.7 (52.3-60.7) | ||||||||||
| Abdominal pain | < 0.001 | -4.31 | -6.500 to | < 0.001 | 0.08 | |||||||
| Yes | 51.0 (44.9-53.1) | 54.1 (46.5-57.3) | ||||||||||
| No | 55.3 (53.1-55.9) | 56.0 (53.3-60.7) | ||||||||||
| Appetite decline | 0.033 | 0.58 | -2.993 to 4.146 | 0.75 | 0.07 | |||||||
| Yes | 50.9 (46.3-53.8) | 52.4 (41.7-56.0) | ||||||||||
| No | 54.3 (51.7-55.9) | 56.0 (52.7-60.7) | ||||||||||
| Poor sleep | 0.001 | -3.8 | -6.232 to | 0.002 | 0.047 | -0.72 | -3.592 to 2.158 | 0.623 | ||||
| Yes | 51.7 (43.3-55.3) | 55.4 (46.5-59.4) | ||||||||||
| No | 54.5 (52.1-55.9) | 56.0 (53.2-60.7) | ||||||||||
| Weight loss | 0.024 | -1.13 | -4.153 to 1.897 | 0.462 | 0.66 | |||||||
| Yes | 52.6 (46.6-53.5) | 56.3 (49.1-60.5) | ||||||||||
| No | 54.6 (51.7-55.9) | 56.0 (52.6-60.7) | ||||||||||
| Smoking | 0.32 | 0.029 | 1.73 | -1.266 to 4.728 | 0.256 | |||||||
| Yes | 55.1 (53.6-55.9) | 56.8 (53.3-60.7) | ||||||||||
| No | 54.2 (51.3-55.9) | 55.9 (50.9-59.9) | ||||||||||
| PEP | 0.62 | 0.024 | -3.76 | -7.235 to | 0.034 | |||||||
| Yes | 55.2 (51.2-56.6) | 53.6 (44.8-56.3) | ||||||||||
| No | 54.2 (51.7-55.9) | 56.0 (53.0-60.7) | ||||||||||
The widespread use of diagnostic tools, such as esophagogastroduodenoscopy, ultrasonography, and ERCP, has led to increased detection rates of papillary adenomas[4]. Indeed, the proximity of the DPA to both the bile duct and the pancreatic duct highlights the necessity for careful and thorough management upon detection[18,19]. Endoscopic resection of major papilla adenomas in the duodenum has evolved into a minimally invasive yet effective first-line therapy, offering a safer alternative to traditional surgical interventions. However, its recurrence and residual rates as well as the difficulty of resection en bloc in the duodenum cannot be ignored[20,21].
Current studies on DPAs have focused on the indications for endoscopic resection, the efficacy of endoscopic and surgical treatments, recurrence, and the occurrence of complications[1,22]. The effects of different treatment modalities on postoperative QoL in patients with DPA remain relatively understudied. This gap in knowledge is particularly significant because QoL is a fundamental aspect of patient-centered care, encompassing not only physical health but also psychological well-being, social functioning, and overall life satisfaction. The present study comprehensively evaluated the impact of different treatment modalities, specifically EP vs surgical resection, on the postoperative QoL of patients with DPA. Our study employed the 12-item short-form health survey, a validated instrument that assesses health status across mental and physical domains[17], to assess the physical and mental aspects of postoperative QoL and revealed that surgical treatment had a significantly negative impact on both physical and mental QoL compared with EP, even after adjusting for various confounding factors. The negative influence of surgery can be attributed partly to complications, such as abdominal pain, and postoperative issues, such as pancreatic and biliary fistulae, which can severely affect patient well-being.
The findings of Hwang et al[14] highlight an important comparison in the management of early-stage ampullary carcinoma, suggesting that long-term clinical outcomes between patients who undergo EP alone and those who need additional surgery do not significantly differ. This finding underscores the potential of EP as a viable and less invasive initial treatment option for select cases of early-stage disease. However, the heightened risks associated with radical surgery, such as pancreaticoduodenectomy, must be acknowledged[23]. The procedure, although necessary in certain cases, is associated with a considerable risk profile. Complications such as pancreatic, intestinal, biliary, and celiac fistulae, as well as infections, bleeding, and delayed gastric emptying, can initiate a cascade of interrelated issues[24]. These complications are not only distressing for patients but can also escalate rapidly, leading to life-threatening conditions such as septic shock, massive hemorrhage, and even death. Previous research has shown that although patients who undergo pancreaticoduodenectomy may experience improvements in their QoL over time, their QoL typically does not fully recover to the levels observed in the general healthy population, and gastrointestinal dysfunction persists in long-term survivors and is an independent predictor of low QoL[25]. This discrepancy underscores the lasting impact of such invasive procedures on the physical and psychosocial well-being of patients.
After controlling for relevant confounders in the four models described above, our study revealed that older age at surgery is associated with poorer physical functioning and lower mental QoL for women than for men. Notably, abdominal pain, which might be caused by dyspepsia, delayed gastric emptying, or visceral nociceptive hypersensitivity, is a factor that significantly affects the PCS and MCS of patients. In the long-term follow-up of patients receiving endoscopic treatment, multifactorial regression revealed that poor sleep was a significant factor affecting PCS, whereas PEP and hypertension were significant factors affecting MCS. We speculate that the mechanism by which PEP and poor sleep affect MCS may be mediated by abdominal pain. However, hypertension had an independent negative effect on patients in the EP group that was not mediated by the treatment modality.
Our study provides a basis for personalized treatment decisions. For elderly and frail patients, our findings suggest that EP may help maintain a higher QoL, although this must be balanced against the potential risk of tumor recurrence. In cases with larger invasive tumors, surgery may be necessary to ensure complete tumor resection, despite the associated physiological burden and potential impact on QoL. Understanding these implications is crucial for clinicians to provide comprehensive support and tailored interventions, balancing the need for effective tumor control with the preservation of patient QoL.
Our study, as a pioneering effort to compare the impact of endoscopic and surgical treatments on postoperative QoL in patients with DPAs, has several acknowledged limitations. Despite the multivariate analysis efforts, the two groups (endoscopic vs surgical treatment) were not completely balanced in terms of baseline characteristics, which could introduce bias. We attempted propensity score matching analysis to eliminate baseline differences, which reduced the sample size. Second, the relatively small number of patients included, particularly those who underwent surgical treatment, limited the ability to conduct detailed subgroup analyses, such as comparisons of different surgical approaches and the impact of EP vs surgical local resection on QoL. Third, the follow-up period may not have been sufficiently long to capture the full spectrum of QoL changes over time, potentially overestimating the negative impact of surgery as QoL may improve further with additional recovery time after surgery[25]. Fourth, the low response rate may introduce potential selection bias, with the assumption that patients who did not respond may have either experienced satisfactory recovery and thus deemed the survey unnecessary or, conversely, were too unwell or unwilling to revisit their medical experiences. This potential selection bias could lead to a decrease in sample representativeness and imbalance between the groups, which may affect the internal validity of the study and limit the generalizability of the research findings. Finally, without baseline QoL measurements, accurately assessing the net change in QoL resulting from the treatments is challenging. Therefore, a prospective study design with a larger, more diverse patient cohort, systematic and prolonged follow-up, and a focus on obtaining preoperative QoL data would strengthen the evidence base. Additionally, engaging patients more effectively throughout the follow-up period, potentially through incentives or alternative modes of communication, could also improve response rates and data completeness.
Our long-term follow-up findings suggest that endoscopic treatment for DPAs may have a more favorable impact than surgical intervention on the physical QoL of patients. Our study contributes to the growing body of evidence supporting endoscopic therapy as a patient-centered approach for managing DPAs, emphasizing the importance of balancing curative intent with QoL preservation. Although these findings are compelling, they also indicate a need for larger scale, controlled studies with longer follow-up periods to confirm and refine our understanding of the comparative QoL outcomes between endoscopic and surgical treatments.
| 1. | Hautefeuille V, Williet N, Turpin A, Napoleon B, Dupré A, Huguet F, Bignon AL, Camus M, Chevaux JB, Coriat R, Cros J, Edeline J, Koch S, Neuzillet C, Perkins G, Regimbeau JM, Sefrioui D, Vitellius C, Vullierme MP, Bouché O, Gaujoux S; Thésaurus National de Cancérologie Digestive (TNCD); Société Nationale Française de Gastroentérologie (SNFGE); Fédération Francophone de Cancérologie Digestive (FFCD); Fédération Nationale des Centres de Lutte Contre le Cancer (UNICANCER); Groupe Coopérateur multidisciplinaire en Oncologie (GERCOR); Société Française de Chirurgie Digestive (SFCD); Société Française d'Endoscopie Digestive (SFED); Association de Chirurgie Hépato-Bilio-Pancréatique et Transplantation (ACHBT); Société́ Française de Pathologie (SFP); Société Française de Radiothérapie Oncologique (SFRO); Réseau National de Référence des Tumeurs Rares du Péritoine (RENAPE); Société Nationale Française de Colo-Proctologie (SNFCP); Association Française pour l’Étude du Foie (AFEF); Société Française de Radiologie (SFR). Ampullary tumors: French Intergroup Clinical Practice Guidelines for diagnosis, treatments and follow-up (TNCD, SNFGE, FFCD, UNICANCER, GERCOR, SFCD, SFED, ACHBT, AFC, SFRO, RENAPE, SNFCP, AFEF, SFP, SFR). Dig Liver Dis. 2024;56:1452-1460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 2. | Seifert E, Schulte F, Stolte M. Adenoma and carcinoma of the duodenum and papilla of Vater: a clinicopathologic study. Am J Gastroenterol. 1992;87:37-42. [PubMed] |
| 3. | Panzeri F, Crippa S, Castelli P, Aleotti F, Pucci A, Partelli S, Zamboni G, Falconi M. Management of ampullary neoplasms: A tailored approach between endoscopy and surgery. World J Gastroenterol. 2015;21:7970-7987. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 49] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 4. | Sanford Z, Adkins J, Knight C, Lahiry S. Continuing Advancements in Diagnosis and Management of Ampullary Adenoma. Am Surg. 2017;83:e302-e304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 5. | Chini P, Draganov PV. Diagnosis and management of ampullary adenoma: The expanding role of endoscopy. World J Gastrointest Endosc. 2011;3:241-247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 56] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 6. | Meneghetti AT, Safadi B, Stewart L, Way LW. Local resection of ampullary tumors. J Gastrointest Surg. 2005;9:1300-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Chapman BC, Gleisner A, Ibrahim-Zada I, Overbey DM, Paniccia A, Meguid C, Brauer B, Gajdos C, McCarter MD, Schulick RD, Edil BH. Laparoscopic pancreaticoduodenectomy: changing the management of ampullary neoplasms. Surg Endosc. 2018;32:915-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | Lee CHA, Shingler G, Mowbray NG, Al-Sarireh B, Evans P, Smith M, Usatoff V, Pilgrim C. Surgical outcomes for duodenal adenoma and adenocarcinoma: a multicentre study in Australia and the United Kingdom. ANZ J Surg. 2018;88:E157-E161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Cheng CL, Sherman S, Fogel EL, McHenry L, Watkins JL, Fukushima T, Howard TJ, Lazzell-Pannell L, Lehman GA. Endoscopic snare papillectomy for tumors of the duodenal papillae. Gastrointest Endosc. 2004;60:757-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 151] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 10. | Han S, Turkeltaub JA, Jonas D, Attwell AR, Duloy AM, Edmundowicz SA, Hammad HT, Wagh MS, Wani S, Shah RJ. The timing of recurrence after endoscopic papillectomy. Surg Endosc. 2024;38:688-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Vanbiervliet G, Strijker M, Arvanitakis M, Aelvoet A, Arnelo U, Beyna T, Busch O, Deprez PH, Kunovsky L, Larghi A, Manes G, Moss A, Napoleon B, Nayar M, Pérez-Cuadrado-Robles E, Seewald S, Barthet M, van Hooft JE. Endoscopic management of ampullary tumors: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2021;53:429-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 82] [Article Influence: 20.5] [Reference Citation Analysis (1)] |
| 12. | Lv Y, Wang P, Chen J, Zhao L, Chen L, Zhuang Y, Wang L, Zou X. Indicative value of pathological classification of duodenal papillary adenomas in clinical diagnosis and treatment. Surg Endosc. 2022;36:5183-5197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 13. | Wang P, Jiang C, Wang Y, Zhou L, Zhang S, Ding X, Lv Y, Wang L, Zou X. Outcome of a novel modified endoscopic papillectomy for duodenal major papilla adenoma. Surg Endosc. 2020;34:5160-5167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Hwang JS, So H, Oh D, Song TJ, Park DH, Seo DW, Lee SK, Kim MH, Hong SM, Yang J, Lee SS. Long-term outcomes of endoscopic papillectomy for early-stage cancer in duodenal ampullary adenoma: Comparison to surgical treatment. J Gastroenterol Hepatol. 2021;36:2315-2323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Fritzsche JA, Klein A, Beekman MJ, van Hooft JE, Sidhu M, Schoeman S, Fockens P, Bourke MJ, Voermans RP. Endoscopic papillectomy; a retrospective international multicenter cohort study with long-term follow-up. Surg Endosc. 2021;35:6259-6267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Choi SJ, Lee HS, Kim J, Choe JW, Lee JM, Hyun JJ, Yoon JH, Kim HJ, Kim JS, Choi HS. Clinical outcomes of endoscopic papillectomy of ampullary adenoma: A multi-center study. World J Gastroenterol. 2022;28:1845-1859. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 17. | Fong DYT, Wong JYH, Choi EPH, Lam KF, Kwok C. The English and Chinese language versions of the Short Form 12-item Health Survey are equivalent. Health Qual Life Outcomes. 2021;19:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Singh AD, Burke CA, Draganov PV, Bapaye J, Nishimura M, Ngamruengphong S, Kushnir V, Sharma N, Kaul V, Singh A, Bapaye A, Banerjee D, Bayudan A, De Leon MR, Singh RR, Mony S, Gandhi A, Hollander T, Bittner K, Beauvais J, Lyu R, Liska D, Stevens T, Walsh M, Bhatt A. Incidence and risk factors for recurrence of ampullary adenomas after endoscopic papillectomy: Comparative analysis of familial adenomatous polyposis and sporadic ampullary adenomas in an international multicenter cohort. Dig Endosc. 2024;36:834-842. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 6] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 19. | Wang Y, Khizar H, Zhou H, Jin H, Lou Q, Zhang X, Yang J. Endoscopic treatment for early duodenal papillary carcinoma: long-term outcomes. J Gastroenterol Hepatol. 2024;39:1367-1373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 20. | Dioscoridi L, Donnarumma D, Forti E, Pugliese F, Cintolo M, Bonato G, Bravo M, Palermo A, Mutignani M. Recurrence rate and management after endoscopic papillectomy in a tertiary referral center. Dig Liver Dis. 2024;56:2143-2148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Woo SM, Real MJ, Will BM, Kim EJ, Chou J, Alsaiari AA, Nakshabandi A, Chalhoub WM, Haddad NG. Clinical outcomes: endoscopic resection of duodenal ampullary lesions. Transl Gastroenterol Hepatol. 2023;8:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 22. | Kawashima H, Ishikawa T, Yamao K, Mizutani Y, Iida T, Uetsuki K, Yamamura T, Furukawa K, Nakamura M. Current status of and future issues related to endoscopic papillectomy. Nagoya J Med Sci. 2023;85:648-658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 23. | Saito R, Kawaida H, Amemiya H, Nakata Y, Izumo W, Furuya M, Maruyama S, Takiguchi K, Shoda K, Ashizawa N, Nakayama Y, Shiraishi K, Furuya S, Akaike H, Kawaguchi Y, Ichikawa D. Clinical significance of postoperative complications after pancreatic surgery in time-to-complication and length of postoperative hospital stay: a retrospective study. Langenbecks Arch Surg. 2024;409:173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 24. | de Bakker JK, Suurmeijer JA, Toennaer JGJ, Bonsing BA, Busch OR, van Eijck CH, de Hingh IH, de Meijer VE, Molenaar IQ, van Santvoort HC, Stommel MW, Festen S, van der Harst E, Patijn G, Lips DJ, Den Dulk M, Bosscha K, Besselink MG, Kazemier G; Dutch Pancreatic Cancer Group. Surgical Outcome After Pancreatoduodenectomy for Duodenal Adenocarcinoma Compared with Other Periampullary Cancers: A Nationwide Audit Study. Ann Surg Oncol. 2023;30:2448-2455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 25. | Allen CJ, Yakoub D, Macedo FI, Dosch AR, Brosch J, Dudeja V, Ayala R, Merchant NB. Long-term Quality of Life and Gastrointestinal Functional Outcomes After Pancreaticoduodenectomy. Ann Surg. 2018;268:657-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |