Published online Jun 27, 2025. doi: 10.4240/wjgs.v17.i6.106264
Revised: April 4, 2025
Accepted: May 9, 2025
Published online: June 27, 2025
Processing time: 84 Days and 3.2 Hours
Colonoscopic polypectomy is a crucial procedure for the prevention and treatment of colorectal cancer, with its success and safety largely dependent on the quality of bowel preparation. Currently, polyethylene glycol electrolyte solution remains the standard method for bowel preparation, but its use may cause patient discomfort and incomplete cleansing.
To evaluate impact of enhanced and conventional bowel preparation protocols on the outcomes of colonoscopic polypectomy.
This retrospective cohort study collected data from 130 patients who underwent colonoscopic polypectomy between March 2023 and June 2024. Patients were divided into the conventional bowel preparation group (n = 65) and enhanced bowel preparation group (n = 65). Primary outcome measures included Boston Bowel Preparation Scale (BBPS) scores, procedure-related parameters, complication rates, and prognosis. Statistical analysis was performed using SPSS version 25.0, with P < 0.05 indicating statistical significance.
The enhanced group demonstrated significant advantages over the conventional group, with higher BBPS total scores (4.2 ± 0.7 vs 3.1 ± 0.8, P < 0.001), higher one-time complete resection rates (95.4% vs 83.1%, P = 0.01), shorter operative times (23.1 ± 4.8 vs 25.4 ± 5.2 min, P = 0.03), and lesser intraoperative blood loss (18.2 ± 4.5 vs 20.3 ± 5.1 mL, P = 0.04). Total complication rates were significantly lower (5.9% vs 16.9%, P = 0.05), particularly for bleeding (1.5% vs 16.9%, P = 0.01) and infection (1.5% vs 7.7%, P = 0.04). The enhanced group also showed lower 6-month recurrence rates (3.1% vs 10.8%, P = 0.05) and higher patient satisfaction (87.7% vs 76.9%, P = 0.04) than did the conventional group.
The enhanced bowel preparation protocol demonstrates significant advantages, particularly in improving surgical outcomes, reducing complications, and increasing patient satisfaction, underscoring its importance of its application during colonoscopic polypectomy.
Core Tip: This study evaluates the impact of enhanced vs conventional bowel preparation protocols on colonoscopic polypectomy outcomes, highlighting the superior efficacy of the enhanced approach. The findings reveal significant improvements in bowel preparation quality, one-time complete resection rates, operative efficiency, and patient satisfaction, alongside reduced complication rates and lower 6-month recurrence rates. These results underscore the clinical importance of adopting enhanced bowel preparation protocols to optimize surgical outcomes and enhance patient care in colonoscopic polypectomy.
- Citation: Ma YP, Zheng XY, Shen XF, Ling YT, Qian MP, Ni MJ. Impact of enhanced bowel preparation on complications and prognosis following colonoscopic polypectomy. World J Gastrointest Surg 2025; 17(6): 106264
- URL: https://www.wjgnet.com/1948-9366/full/v17/i6/106264.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i6.106264
Colonoscopic polypectomy plays a crucial role in the prevention and treatment of colorectal cancer by identifying and removing precancerous polyps. The effectiveness and safety of this procedure are fundamentally dependent on the quality of bowel preparation, which significantly influences colonic mucosa visualization and subsequent polyp detection and removal[1-4].
Inadequate bowel preparation induces several critical challenges during colonoscopic polypectomy. Poor visualization due to inadequate cleansing can result in missed polyps and incomplete resection, potentially triggering the development of colorectal cancer. Furthermore, suboptimal preparation increases procedural complications, including bleeding, perforation, and infection, while also necessitating repeat procedures that exhaust healthcare resources and decrease patient well-being[5-7].
Currently, polyethylene glycol (PEG) electrolyte solutions has been the standard approach for bowel preparation. These non-absorbable, osmotically balanced solutions function by inducing hypotonic diarrhea to cleanse the bowel[8-11]. Despite its widespread use, however, PEG-based preparations have been associated with patient discomfort and occasionally incomplete cleansing. Recent attention has focused on enhancing traditional protocols by adding sodium phosphate (NaP), an osmotic laxative that promotes bowel evacuation by drawing water, into the intestinal lumen[12-15]. Studies have found that the combination of PEG and NaP could be a promising approach for improving bowel cleansing efficacy and reducing procedure-related complications[16,17]. However, there remains a significant gap in our understanding of the comparative effectiveness of combined PEG and NaP protocols vs. conventional PEG-only preparations in colonoscopic polypectomy.
The primary objective of this investigation was to evaluate the impact of these different preparation methods on procedure-related complications and outcomes. The secondary objectives included assessing polyp removal completeness, patient recovery parameters, and recurrence rates. We hypothesized that the enhanced preparation protocol would demonstrate superior bowel cleansing, improved polyp removal rates, reduced complications, and better postoperative outcomes compared to the conventional preparation.
The current research seeks to contribute meaningful evidence for optimizing bowel preparation protocols in colonoscopic polypectomy. Our findings provide valuable guidance for clinical practice, potentially improving procedure safety, effectiveness, and patient outcomes following gastrointestinal endoscopy. By identifying more effective pre
This retrospective cohort study collected clinical data from patients who underwent colonoscopic polypectomy at XXXX between March 2023 and June 2024. The study protocol was approved by the hospital ethics committee (approval number pending). Given the retrospective nature of the current study, informed consent was waived.
Based on the inclusion and exclusion criteria, clinical data from 130 patients were included. Patients were divided into two groups according to their preoperative bowel preparation methods: A conventional bowel preparation group (65 cases) and an enhanced bowel preparation group (65 cases). The inclusion criteria were as follows: Age 18-75 years; colonic polyps confirmed via colonoscopy or imaging; complete clinical data including preoperative examinations, surgical records, and follow-up data; and a follow-up period ≥ 6 months. The exclusion criteria were as follows: Severe cardiac, hepatic, or renal dysfunction; history of inflammatory bowel disease; contraindications to colonoscopy or bowel preparation; incomplete clinical data; and loss to follow-up.
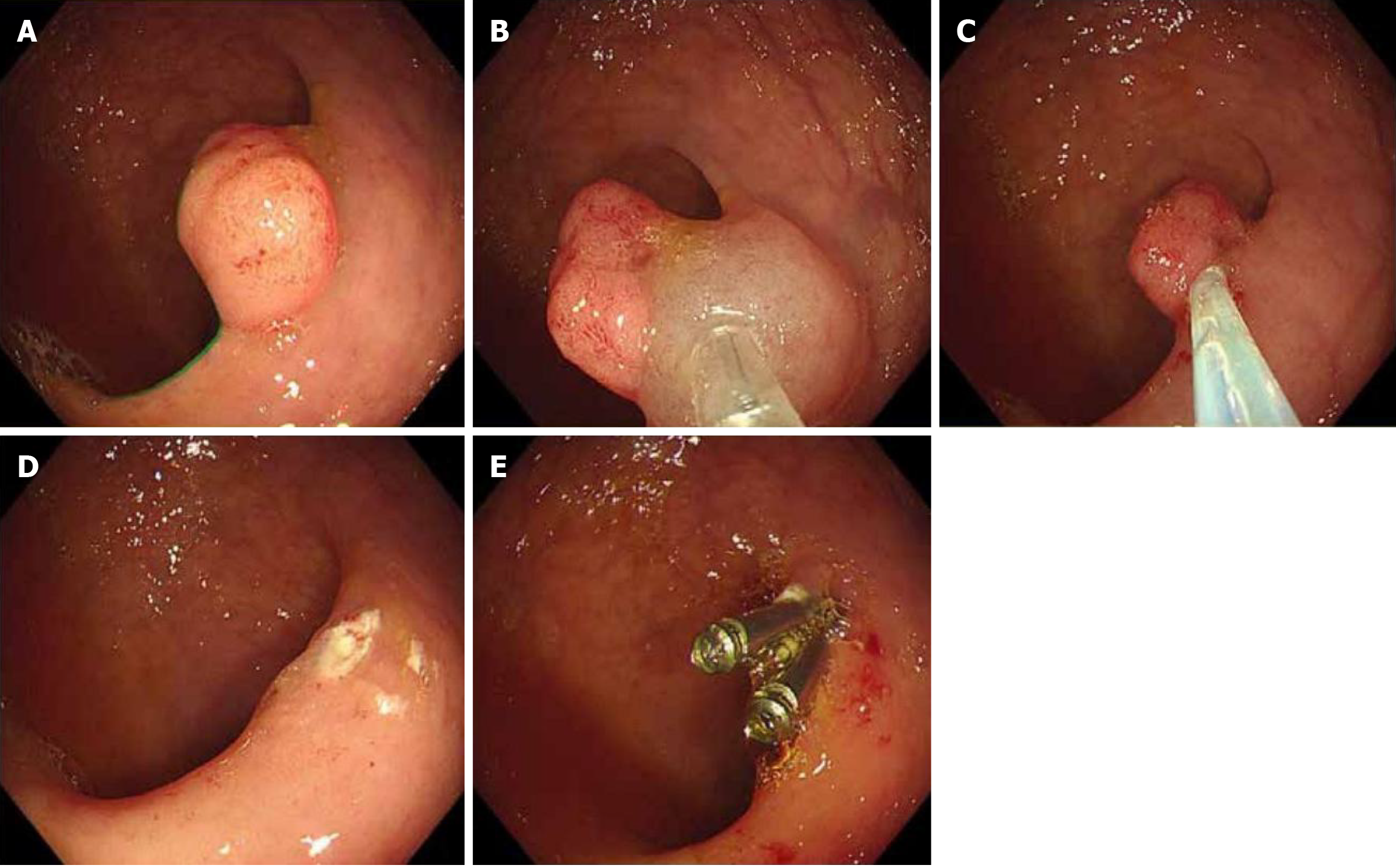
Two main techniques were used to perform colonoscopic polypectomy based on the polyp characteristics (Figure 1). For elevated (types Ip and Isp) and small (< 2 cm) is lesions, cold or hot snare polypectomy is preferred. During this procedure, maintaining an adequate stalk length or distance from the intestinal wall is crucial. The endoscopist must ensure complete lesion capture while carefully avoiding normal mucosal entrapment. Gentle shaking of the snare helps verify proper tissue capture before resection. For more complex cases, endoscopic mucosal resection is performed. This technique begins by thoroughly evaluating the extent and morphology of the lesions. The procedure involves submucosal injections at the lesion margins using a colonoscopic injection needle. The injection solution, comprising glycerol fructose, epinephrine, and methylene blue, creates a protective cushion. Multiple injections may be necessary to achieve complete mucosal elevation. After removing the injection needle, a snare is inserted to capture the elevated lesion along with a margin of the surrounding normal mucosa. The resection is completed using a high-frequency generator, followed by careful inspection of the resection site for bleeding[18].
Hemostasis management is an essential component of the procedure. Minor bleeding can be controlled by spraying epinephrine–saline solution at the resection site. When additional hemostasis is needed, titanium clips provide an effective alternative. All resected specimens are carefully processed and sent for pathological examination to ensure accurate diagnosis and staging. Postoperative care follows a structured protocol to minimize complications. Patients are closely monitored for potential complications, particularly bleeding and perforation. A strict dietary progression is implemented, beginning with nil by mouth for 6 h post-procedure. On the first postoperative day, patients are started on a warm liquid diet, such as rice soup and noodle soup. From days 2 through 7, patients progress to a liquid and semi-liquid diet, avoiding spicy, irritating, and high-fiber foods that could irritate the healing mucosa[19,20].
Follow-up care is tailored to the specific procedure and findings. For cases involving piecemeal resection, a follow-up colonoscopy is scheduled within 1 month to check for residual tumors. This is particularly important for large colorectal polyps, which carry a higher risk of malignant transformation. When the pathology results indicate malignant trans
The included patients were divided into two distinct bowel preparation groups: Conventional and enhanced. The conventional group received standard written materials and verbal instructions regarding bowel preparation, whereas the enhanced group participated in interactive education sessions with additional psychological support and guidance, including opportunities to ask questions and address concerns. On the examination day, both groups followed identical check-in procedures at the endoscopy center where initial assessment of bowel preparation adequacy was performed before scheduling the colonoscopy. The basic bowel preparation protocol, comprising a low-residue diet for 3 days before the procedure, followed by clear liquids the day before examination and nil by mouth after 18:00, remained consistent across both groups. All patients were instructed to take PEG electrolyte solution between 19:00 and 20:00 with 2000 mL water and had the option to use simethicone to reduce intestinal gas. The primary distinction between the groups was the educational approach and level of psychological support provided, with the enhanced group receiving more comprehensive guidance to potentially improve preparation compliance and quality.
This study employed comprehensive observation indicators to evaluate the effectiveness of different bowel preparation methods. The primary outcome measure included bowel cleansing quality assessed using the Boston Bowel Preparation Scale (BBPS), which scored the cleanliness of three colonic segments (the rectum-sigmoid, transverse-descending, and ascending-cecum segments) on a scale of 0-3, with total scores ranging from 0-9. Procedure-related parameters were carefully documented, including operative time, one-time complete resection rate, and intraoperative blood loss. Complications, encompassing both immediate and delayed bleeding, perforation, infection, and postoperative pain scores measured using the visual analog scale (VAS), were systematically recorded. This study also tracked several recovery indicators, including length of hospital stay, time to first liquid diet, and patient satisfaction scores. Long-term outcomes were assessed through pathological examination of resected specimens over a 6-month follow-up period, during which polyp recurrence rates and late complications were monitored. Additionally, patient compliance with the preparation protocol and any adverse reactions to the bowel preparation regimens were documented to provide a comprehensive assessment of the safety and efficacy of the two preparation methods.
Statistical analysis was performed using SPSS version 25.0 (IBM Corp., Armonk, NY, United States). All data were tested for normal distribution using the Kolmogorov-Smirnov test. Continuous variables, including BBPS scores, operative time, intraoperative blood loss, length of hospital stay, and VAS pain scores, were expressed as mean ± SD for normally distributed data or median (interquartile range) for non-normally distributed data. Comparisons between the conventional and enhanced bowel preparation groups were performed using the independent student’s t-test for normally distributed data or Mann-Whitney U test for non-normally distributed data. Categorical variables, including one-time complete resection rates, complication rates (bleeding, perforation, and infection), patient satisfaction levels, and polyp recurrence rates, were presented as frequencies and percentages (n, %). Comparisons between groups were conducted using the χ2 test or Fisher’s exact test when appropriate. The correlation between bowel preparation quality and procedure-related outcomes was analyzed using Spearman’s rank correlation coefficient. Multiple logistic regression analysis was performed to identify independent risk factors for complications and incomplete resection. For the 6-month follow-up data, Kaplan-Meier curves were constructed to analyze recurrence-free survival, with the log-rank test being used to compare differences between groups. A P value of < 0.05 indicated statistical significance. All statistical tests were two-sided, and 95% confidence intervals were calculated where appropriate.
This study compared two groups (conventional and enhanced bowel preparation) with 65 patients each. Both groups had similar demographic and clinical characteristics (Table 1), with a comparable mean age (55.2 ± 10.3 vs 54.8 ± 10.5 years, P = 0.82), similar gender distribution (58.5% and 56.9% of those in the conventional and enhanced groups were males, respectively), and similar body mass index measurements (24.1 ± 3.2 vs 23.9 ± 3.1 kg/m2, P = 0.78). Both groups also showed comparable comorbidity profiles, with hypertension being the most common (23.1% vs 21.5%, P = 0.89), followed by diabetes (12.3% vs 10.8%, P = 0.81) and cardiovascular disease (7.7% vs 6.2%, P = 0.76). Regarding polyp characteristics, the number (1.3 ± 0.6 vs 1.4 ± 0.5, P = 0.42) and mean size (8.2 ± 2.1 vs 8.0 ± 2.0 mm, P = 0.68) of the polyps were similar in both groups. The distribution of polyp types was also comparable, with adenomatous polyps being the most prevalent (61.5% vs 64.6%, P = 0.72), followed by hyperplastic and serrated types. No significant differences in other clinical factors, including previous colonoscopy history (15.4% vs 16.9%, P = 0.85), family history of colorectal cancer (12.3% vs 10.8%, P = 0.81), anticoagulant use (7.7% vs 6.2%, P = 0.76), and history of inflammatory bowel disease (3.1% vs 1.5%, P = 0.50), were observed between the groups.
| Characteristic | Conventional bowel preparation group (n = 65) | Enhanced bowel preparation group (n = 65) | P value |
| Age (years) | 55.2 ± 10.3 | 54.8 ± 10.5 | 0.82 |
| Gender, n (%) | |||
| Male | 38 (58.5) | 37 (56.9) | 0.85 |
| Female | 27 (41.5) | 28 (43.1) | |
| BMI (kg/m2) | 24.1 ± 3.2 | 23.9 ± 3.1 | 0.78 |
| Comorbidities, n (%) | |||
| Hypertension | 15 (23.1) | 14 (21.5) | 0.89 |
| Diabetes | 8 (12.3) | 7 (10.8) | 0.81 |
| Cardiovascular disease | 5 (7.7) | 4 (6.2) | 0.76 |
| Polyp characteristics | |||
| Number of polyps | 1.3 ± 0.6 | 1.4 ± 0.5 | 0.42 |
| Mean polyp size (mm) | 8.2 ± 2.1 | 8.0 ± 2.0 | 0.68 |
| Polyp type, n (%) | |||
| Adenomatous | 40 (61.5) | 42 (64.6) | 0.72 |
| Hyperplastic | 20 (30.8) | 19 (29.2) | |
| Serrated | 5 (7.7) | 4 (6.2) | |
| Previous colonoscopy, n (%) | 10 (15.4) | 11 (16.9) | 0.85 |
| Family history of colorectal cancer, n (%) | 8 (12.3) | 7 (10.8) | 0.81 |
| Current use of anticoagulants, n (%) | 5 (7.7) | 4 (6.2) | 0.76 |
| History of inflammatory bowel disease, n (%) | 2 (3.1) | 1 (1.5) | 0.50 |
The enhanced bowel preparation group demonstrated significantly superior outcomes compared to the conventional group across multiple indicators (Table 2). The total BBPS score was significantly higher in the enhanced group (4.2 ± 0.7) than in the conventional group (3.1 ± 0.8, P < 0.001). Segment-specific BBPS scores also showed significant improvements in the enhanced group: Rectum-sigmoid (1.6 ± 0.3 vs 1.2 ± 0.4, P < 0.001), transverse-descending (1.4 ± 0.4 vs 1.0 ± 0.3, P < 0.001), and ascending-cecum (1.2 ± 0.3 vs 0.9 ± 0.3, P < 0.001). The adequacy of bowel preparation rate was significantly higher in the enhanced group [92.3% (60/65)] compared to the conventional group [85.0% (55/65), P = 0.04].
| Indicator | Conventional bowel preparation group (n = 65) | Enhanced bowel preparation group (n = 65) | P value |
| Boston bowel preparation scale score | |||
| Total score | 3.1 ± 0.8 | 4.2 ± 0.7 | < 0.001 |
| Rectum-sigmoid | 1.2 ± 0.4 | 1.6 ± 0.3 | < 0.001 |
| Transverse-descending | 1.0 ± 0.3 | 1.4 ± 0.4 | < 0.001 |
| Ascending-cecum | 0.9 ± 0.3 | 1.2 ± 0.3 | < 0.001 |
| Adequacy of the bowel preparation rate | 85.0% (55/65) | 92.3% (60/65) | 0.04 |
| Cecal intubation rate | 90.8% (59/65) | 95.4% (62/65) | 0.21 |
| Withdrawal time (min) | 6.2 ± 1.2 | 8.1 ± 1.4 | < 0.001 |
| Adenoma detection rate | 30.8% (20/65) | 38.5% (25/65) | 0.23 |
| Sessile serrated lesion detection rate | 5.0% (3/65) | 7.7% (5/65) | 0.42 |
| Frequency of recommending an appropriate screening or surveillance interval | 86.2% (56/65) | 93.8% (61/65) | 0.12 |
Although the cecal intubation rate was numerically higher in the enhanced group [95.4% (62/65) vs 90.8% (59/65)], this difference did not reach statistical significance (P = 0.21). Withdrawal time was significantly longer in the enhanced group (8.1 ± 1.4 minutes) compared to the conventional group (6.2 ± 1.2 min, P < 0.001). The adenoma detection rate (ADR) and sessile serrated lesion detection rate (SSLDR) were numerically higher in the enhanced group [ADR: 38.5% (25/65) vs 30.8% (20/65), P = 0.23; SSLDR: 7.7% (5/65) vs 5.0% (3/65), P = 0.42], but these differences were not statistically significant. Finally, the frequency of recommending an appropriate screening or surveillance interval was higher in the enhanced group [93.8% (61/65)] compared to the conventional group [86.2% (56/65)], though this difference was not statistically significant (P = 0.12).
A comparison of procedural and clinical outcomes between the two groups revealed several significant advantages in the enhanced bowel preparation group (Table 3). In particular, the enhanced group demonstrated shorter operative times (23.1 ± 4.8 vs 25.4 ± 5.2 minutes, P = 0.03), higher one-time complete resection rates (95.4% vs 83.1%, P = 0.01), and lesser intraoperative blood loss (18.2 ± 4.5 vs 20.3 ± 5.1 mL, P = 0.04). Notably, postoperative recovery parameters also favored the enhanced group, who showed significantly shorter hospital stays (2.1 ± 0.5 vs 3.4 ± 0.8 days, P < 0.001), shorter time to first liquid diet (10.2 ± 1.8 vs 12.3 ± 2.1 hours, P = 0.02), and lower postoperative pain scores (2.3 ± 0.7 vs 3.8 ± 0.9, P < 0.001). Although polyp detection parameters remained similar between the groups (number of polyps: 1.4 ± 0.5 vs 1.3 ± 0.6, P = 0.42; mean polyp size: 8.0 ± 2.0 vs 8.2 ± 2.1 mm, P = 0.68), the enhanced group showed a lower need for repeat colonoscopy due to inadequate preparation (3.1% vs 10.8%, P = 0.05) and higher patient satisfaction rates (87.7% vs 76.9%, P = 0.04). The enhanced group also tended to have less frequent use of additional sedation (10.8% vs 20.0%, P = 0.12), although this difference did not reach statistical significance.
| Parameter | Conventional bowel preparation group (n = 65) | Enhanced bowel preparation group (n = 65) | P value |
| Operative time (min) | 25.4 ± 5.2 | 23.1 ± 4.8 | 0.03 |
| One-time complete resection rate | 83.1% (54/65) | 95.4% (62/65) | 0.01 |
| Intraoperative blood loss (mL) | 20.3 ± 5.1 | 18.2 ± 4.5 | 0.04 |
| Number of polyps detected | 1.3 ± 0.6 | 1.4 ± 0.5 | 0.42 |
| Mean polyp size (mm) | 8.2 ± 2.1 | 8.0 ± 2.0 | 0.68 |
| Adenoma detection rate | 30.8% (20/65) | 38.5% (25/65) | 0.23 |
| Sessile serrated lesion detection rate | 5.0% (3/65) | 7.7% (5/65) | 0.42 |
| Frequency of recommending an appropriate screening or surveillance interval | 86.2% (56/65) | 93.8% (61/65) | 0.12 |
| Postoperative hospital stay (days) | 3.4 ± 0.8 | 2.1 ± 0.5 | < 0.001 |
| Time to first liquid diet (hours) | 12.3 ± 2.1 | 10.2 ± 1.8 | 0.02 |
| Postoperative pain score | 3.8 ± 0.9 | 2.3 ± 0.7 | < 0.001 |
| Frequency of use of additional sedation during the procedure | 20.0% (13/65) | 10.8% (7/65) | 0.12 |
| Frequency of need for repeat colonoscopy due to inadequate preparation | 10.8% (7/65) | 3.1% (2/65) | 0.05 |
| Frequency of patient satisfaction with procedure | 76.9% (50/65) | 87.7% (57/65) | 0.04 |
Analysis of complications revealed significantly lower adverse events in the enhanced bowel preparation group than in the conventional group (Table 4). In particular, the enhanced group showed a notably lower total complication rate (5.9% vs 16.9%, P = 0.05) and significantly lower rates of bleeding (1.5% vs 16.9%, P = 0.01), infection (1.5% vs 7.7%, P = 0.04), and abdominal pain (6.2% vs 15.4%, P = 0.04) than did the conventional group. Albeit not statistically significant, the enhanced group also demonstrated favorable trends in other complications, including lower rates of perforation (0.0% vs 3.1%, P = 0.10), nausea and vomiting (4.6% vs 12.3%, P = 0.12), and abdominal distension (3.1% vs 10.8%, P = 0.07). Additionally, the enhanced group showed reduced need for additional sedation (3.1% vs 7.7%, P = 0.21) and significantly decreased patient dissatisfaction with the preparation process (4.6% vs 13.8%, P = 0.05) compared to the conventional group, suggesting that the enhanced bowel preparation protocol had better overall tolerability and safety profiles.
| Complication | Conventional bowel preparation group (n = 65) | Enhanced bowel preparation group (n = 65) | P value |
| Total complication rate | 16.9% (11/65) | 5.9% (4/65) | 0.05 |
| Bleeding | 11 (16.9) | 1 (1.5) | 0.01 |
| Perforation | 2 (3.1) | 0 (0.0) | 0.10 |
| Infection | 5 (7.7) | 1 (1.5) | 0.04 |
| Nausea and vomiting | 8 (12.3) | 3 (4.6) | 0.12 |
| Abdominal pain | 10 (15.4) | 4 (6.2) | 0.04 |
| Abdominal distension | 7 (10.8) | 2 (3.1) | 0.07 |
| Need for additional sedation | 5 (7.7) | 2 (3.1) | 0.21 |
| Patient dissatisfaction with the preparation | 9 (13.8) | 3 (4.6) | 0.05 |
The enhanced group exhibited a shorter postoperative hospital stay (2.1 ± 0.5 vs 3.4 ± 0.8 days, P < 0.001), reduced time to the first liquid diet (10.2 ± 1.8 vs 12.3 ± 2.1 hours, P = 0.02), and lower postoperative pain scores (2.3 ± 0.7 vs 3.8 ± 0.9, P < 0.001) (Table 5). Additionally, the time to the first bowel movement was shorter (18.3 ± 3.1 vs 24.5 ± 4.2 hours, P < 0.001), and postoperative fatigue scores were lower (2.8 ± 0.6 vs 4.2 ± 0.8, P < 0.001). Patient satisfaction with recovery was higher in the enhanced group (89.2% vs 70.8%, P = 0.01), and rates of postoperative nausea and vomiting were significantly reduced (4.6% vs 15.4%, P = 0.04). Although not statistically significant, favorable trends were observed for postoperative dizziness (3.1% vs 10.8%, P = 0.07) and urinary retention (1.5% vs 7.7%, P = 0.05), suggesting that the enhanced protocol's potential to optimize recovery and improve patient outcomes.
| Indicator | Conventional bowel preparation group (n = 65) | Enhanced bowel preparation group (n = 65) | P value |
| Postoperative hospital stay (days) | 3.4 ± 0.8 | 2.1 ± 0.5 | < 0.001 |
| Time to first liquid diet (h) | 12.3 ± 2.1 | 10.2 ± 1.8 | 0.02 |
| Postoperative pain score | 3.8 ± 0.9 | 2.3 ± 0.7 | < 0.001 |
| Time to the first bowel movement (h) | 24.5 ± 4.2 | 18.3 ± 3.1 | < 0.001 |
| Postoperative fatigue score | 4.2 ± 0.8 | 2.8 ± 0.6 | < 0.001 |
| Patient satisfaction with recovery | 70.8% (46/65) | 89.2% (58/65) | 0.01 |
| Postoperative nausea and vomiting | 15.4% (10/65) | 4.6% (3/65) | 0.04 |
| Postoperative dizziness | 10.8% (7/65) | 3.1% (2/65) | 0.07 |
| Postoperative urinary retention | 7.7% (5/65) | 1.5% (1/65) | 0.05 |
The enhanced bowel preparation group showed favorable trends in long-term outcomes compared to the conventional group (Table 6). The 6-month recurrence rate was significantly lower in the enhanced group [3.1% (2/65)] than in the conventional group [10.8% (7/65), P = 0.05]. Tumor marker levels were also reduced in the enhanced group, with significantly lower CA19-9 (28.1 ± 8.5 vs 35.2 ± 10.3, P = 0.02) and CEA levels (3.9 ± 0.9 vs 4.8 ± 1.2, P = 0.04). Although not statistically significant, the enhanced group demonstrated numerically lower rates of pathological confirmation of malignancy [6.2% (4/65) vs 7.7% (5/65), P = 0.76], lymph node metastasis [7.7% (5/65) vs 12.3% (8/65), P = 0.32], and late complications [0.0% (0/65) vs 3.1% (2/65), P = 0.10]. Postoperative infection rates were also lower in the enhanced group [3.1% (2/65) vs 7.7% (5/65), P = 0.10]. Patient satisfaction with pathological results was higher in the enhanced group [87.7% (57/65) vs 80.0% (52/65)], though this difference was not statistically significant (P = 0.12).
| Indicator | Conventional bowel preparation group (n = 65) | Enhanced bowel preparation group (n = 65) | P value |
| Pathological confirmation of malignancy | 5 (7.7) | 4 (6.2) | 0.76 |
| 6-month recurrence rate | 10.8% (7/65) | 3.1% (2/65) | 0.05 |
| Late complications | 2 (3.1) | 0 (0.0) | 0.10 |
| Tumor marker levels (CA19-9) | 35.2 ± 10.3 | 28.1 ± 8.5 | 0.02 |
| Tumor marker levels (CEA) | 4.8 ± 1.2 | 3.9 ± 0.9 | 0.04 |
| Lymph node metastasis rate | 12.3% (8/65) | 7.7% (5/65) | 0.32 |
| Postoperative infection rate | 7.7% (5/65) | 3.1% (2/65) | 0.10 |
| Patient satisfaction with the pathological results | 80.0% (52/65) | 87.7% (57/65) | 0.12 |
The enhanced bowel preparation group demonstrated significantly better compliance and tolerability outcomes compared to the conventional group. The overall compliance rate was notably higher in the enhanced group than in the conventional group (89.2% vs 70.8%, P = 0.01), with improved adherence to the medication schedule (84.6% vs 68.0%, P = 0.02) and higher completion rates of the full bowel preparation course (86.2% vs 65.0%, P = 0.003). The enhanced group also experienced fewer adverse effects than did the conventional group, with decreased rates of adverse drug reactions (4.6% vs 12.3%, P = 0.04), reduced frequency of nausea and vomiting (4.6% vs 15.4%, P = 0.04), and a tendency toward less abdominal distension (3.1% vs 10.8%, P = 0.07). Patient satisfaction was significantly higher in the enhanced group than in the conventional group (87.7% vs 72.3%, P = 0.02), with lower rates of dissatisfaction with the preparation process (4.6% vs 13.8%, P = 0.05). Although not significant, the enhanced group showed a trend toward reduced need for additional sedation (3.1% vs 7.7%, P = 0.21), suggesting better overall tolerability of the enhanced preparation protocol (Table 7).
| Indicator | Conventional bowel preparation group (n = 65) | Enhanced bowel preparation group (n = 65) | P value |
| Overall compliance rate | 70.8% (46/65) | 89.2% (58/65) | 0.01 |
| Adherence to the medication schedule | 68.0% (44/65) | 84.6% (55/65) | 0.02 |
| Completion of the full bowel preparation course | 65.0% (42/65) | 86.2% (56/65) | 0.003 |
| Frequency of adverse drug reactions | 12.3% (8/65) | 4.6% (3/65) | 0.04 |
| Patient satisfaction with medication | 72.3% (47/65) | 87.7% (57/65) | 0.02 |
| Frequency of additional sedation required | 7.7% (5/65) | 3.1% (2/65) | 0.21 |
| Patient dissatisfaction with the preparation process | 13.8% (9/65) | 4.6% (3/65) | 0.05 |
| Frequency of nausea and vomiting | 15.4% (10/65) | 4.6% (3/65) | 0.04 |
| Frequency of abdominal distension | 10.8% (7/65) | 3.1% (2/65) | 0.07 |
The current study provides compelling evidence for the superiority of an enhanced bowel preparation protocol over conventional methods in colonoscopic polypectomy. The identification and removal of precancerous polyps through colonoscopic polypectomy have been a cornerstone in the prevention and treatment of colorectal cancer. A fundamental determinant to the success of this approach lies in the quality of bowel preparation, which directly impacts the endo
Suboptimal bowel preparation creates significant obstacles to successful colonoscopic polypectomy. Limited visibility from inadequate cleansing not only hampers the detection of polyps but also increases the risk of incomplete resection, which may promote the development of colorectal cancer. The consequences of poor preparation extend beyond missed lesions to encompass elevated risk for procedural complications, such as bleeding, perforation, and infection[24-26]. Moreover, inadequate preparation often necessitates repeat procedures, creating unnecessary strain on healthcare systems and causing increased patient discomfort and inconvenience.
The significance of adequate bowel preparation in colonoscopic polypectomy extends far beyond immediate procedural success, particularly considering its crucial role in the prevention of colorectal cancer. The enhanced bowel preparation protocol demonstrated promising results with tendencies toward higher ADR (38.5% vs 30.8%, P = 0.23) and significantly improved one-time complete resection rates (95.4% vs 83.1%, P = 0.01). This improvement is particularly crucial given that adenomas represent the primary precursor lesions for colorectal cancer; hence, missing these lesions during colonoscopy can lead to interval colorectal cancer. The distribution of polyp types (adenomatous: 64.6% vs 61.5%, hyperplastic: 29.2% vs 30.8%, serrated: 6.2% vs 7.7%) provides further evidence regarding the effectiveness of the protocol, suggesting enhanced visualization across all polyp subtypes, which is particularly beneficial for traditionally challenging-to-detect flat or sessile adenomas. Moreover, the enhanced bowel preparation protocol promoted long-term cancer prevention benefits as evidenced by the lower 6-month recurrence rates (3.1% vs 10.8%, P = 0.05) and better tumor marker profiles (CA19-9: 28.1 ± 8.5 vs 35.2 ± 10.3, P = 0.02; CEA: 3.9 ± 0.9 vs 4.8 ± 1.2, P = 0.04) than with the conventional bowel preparation protocol. The enhanced protocol also induced significant improvements in quality metrics, including higher BBPPS scores and longer withdrawal times (8.1 ± 1.4 vs 6.2 ± 1.2 min, P < 0.001) than with the conventional protocol. These findings have broader implications for screening programs, suggesting potential improvements in program adherence and more appropriate surveillance intervals. The technical aspects of the procedure were also enhanced, with decreased bleeding rates (1.5% vs 16.9%, P = 0.01) and improved complete resection rates. Moving forward, further research is needed to assess the long-term impact of the enhance protocol on colorectal cancer incidence, cost-effectiveness, and effectiveness in high-risk populations. Based on these findings, our practical recommendations include implementing enhanced bowel preparation protocols for all screening colonoscopies, developing comprehensive patient education programs, and standardizing preparation protocols across practice settings.
The enhanced protocol promoted significantly better postoperative outcomes than did the conventional, including shorter hospital stays (2.1 ± 0.5 vs 3.4 ± 0.8 days, P < 0.001) and lower postoperative pain scores (2.3 ± 0.7 vs 3.8 ± 0.9, P < 0.001). Patient satisfaction was also notably higher with the enhanced protocol than with the conventional protocol (87.7% vs 76.9%, P = 0.04), with less need for repeat procedures due to inadequate preparation (3.1% vs 10.8%, P = 0.05). These findings suggest that the benefits of enhanced bowel preparation extend beyond the immediate procedural setting.
The study revealed better long-term outcomes in the enhanced group than in the conventional group, with lower 6-month recurrence rates (3.1% vs 10.8%, P = 0.05) and better tumor marker profiles (CA19-9: 28.1 ± 8.5 vs 35.2 ± 10.3, P = 0.02; CEA: 3.9 ± 0.9 vs 4.8 ± 1.2, P = 0.04). These findings suggest that better initial bowel preparation may have implications for long-term disease control and surveillance.
A key finding of the current study was the significantly higher compliance rate in the enhanced group than in the conventional group (89.2% vs 70.8%, P = 0.01), with better adherence to medication schedules and completion rates of the preparation course. This improved compliance likely contributed to the better outcomes observed across multiple parameters.
These findings have important implications for clinical practice. The enhanced bowel preparation protocol not only improves immediate procedural outcomes but also appears to benefit long-term patient outcomes and healthcare resource utilization. The reduction in complications and the need for repeat procedures could translate into significant cost savings and improved healthcare efficiency.
However, several questions remain for future research, including the cost-effectiveness of the enhanced protocol, its applicability in different patient populations, and the potential for further optimization of the preparation regimen. Additionally, longer term follow-up studies would be valuable to assess the durability of the observed benefits.
Based on the results of our study, we recommend the implementation of the enhanced bowel preparation protocol in clinical practice using the following strategies. First, healthcare institutions can establish standardized multimedia educational platforms, including short videos, illustrated manuals, and interactive applications, allowing the patients to visually understand the importance and the specific steps of bowel preparation. These educational resources can be integrated into outpatient appointment processes, where they are provided by nurses during colonoscopy scheduling. Second, dedicated bowel preparation coordinators, who are responsible for patient education, follow-up, and psychological support, can be added to the existing nursing workflows. This can be achieved by reallocating existing nursing resources or training specialist nurses, without significantly increasing personnel costs. Third, a tiered psychologic support system can be established, wherein standard psychologic guidance materials are provided for routine patients whereas one-on-one psychologic counseling or telephone guidance are arranged for highly anxious patients or those with a history of poor bowel preparation. Fourth, existing hospital information systems or mobile healthcare platforms can be utilized to implement automatic reminders that send personalized guidance and encouragement 24-48 hours prior to the examination. Finally, healthcare institutions can establish quality improvement teams to regularly evaluate the efficacy of protocol implementation and to continuously optimize processes based on patient feedback. These measures can be gradually implemented within existing medical frameworks to improve patient compliance and examination quality while avoiding excessive pressure on medical resources.
The present study has several limitations. First, we acknowledge the potential selection bias and confounding factors due in this retrospective cohort study, although we aimed to control for these through statistical approaches. Second, the single-center study design might limit the generalizability of our finding, because the patient characteristics and practice patterns might vary across healthcare institutions. Third, the sample size was relatively small, which might have impacted the statistical power of the analyses including certain secondary outcomes, particularly for the detection of low-frequency events. Furthermore, the 6-month follow-up period might have been insufficient for the comprehensive evaluation of the long-term outcomes, such as distant recurrence and survival.
The enhanced bowel preparation protocol exhibited significant clinical advantages across multiple metrics, including the polyp detection rate, procedural outcomes, patient satisfaction, and resource utilization. Based on these findings, we strongly recommend the adoption of this protocol in routine clinical practice for colonoscopic polypectomy. Implementation strategies should include standardized patient education, dedicated coordination personnel, psychologic support systems, and regular quality assessment. These evidence-based improvements should offer substantial benefits to both patients and healthcare systems in efforts on colorectal cancer prevention.
| 1. | Jastaniah A, AlBusaidi N, Bandegi P, Grushka J. Intussusception after colonoscopic polypectomy: a rare complication. BMJ Case Rep. 2023;16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 2. | Ji JH, Kim HW, Park J, Park SJ, Cheon JH, Kim TI, Park JJ. Risk factors for post-polypectomy bleeding in patients with end-stage renal disease undergoing colonoscopic polypectomy. Surg Endosc. 2024;38:846-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Liu Y, Zhang L, Zhao M. Effects of Music Therapy on Negative Emotions and Physiological Parameters in Patients Undergoing Colonoscopic Polypectomy: A Retrospective Study. Noise Health. 2024;26:14-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Oktan MA, Meral CE, Arslan A, Kaya Y, Hazer B, Tuncalı B. A CASE OF PNEUMOTHORAX AFTER COLONOSCOPIC POLYPECTOMY: A CASE REPORT AND REVIEW OF THE LITERATURE. Gastroenterol Nurs. 2024;47:217-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Chen HY, Tu MH, Chen MY. Using a Mobile Health App (ColonClean) to Enhance the Effectiveness of Bowel Preparation: Development and Usability Study. JMIR Hum Factors. 2025;12:e58479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Yin H, Wang Y, Wang H, Li T, Xu X, Li F, Huang L. Derivation and validation of a prediction model for inadequate bowel preparation in Chinese outpatients. Sci Rep. 2025;15:1430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 7. | Zhou Y, Ji H, Zhang S, Zhang X, Zhang J, Wang Y, Wang H, Zhang Y, Du S. Effects of Different Bowel Preparation Regimens and Age Factors on the Gut Microbiota: A Prospective Randomized Controlled Study. J Gastroenterol Hepatol. 2025;40:599-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Kang HS, Na SY, Yoon JY, Jung Y, Seo GS, Cha JM. Efficacy, tolerability, and safety of oral sulfate tablet versus 2 L-polyethylene glycol/ascorbate for bowel preparation in older patients: prospective, multicenter, investigator single-blinded, randomized study. J Gastroenterol. 2024;59:402-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 9. | Li JS, He ZX, Li ZS, Bai Y. Concerns regarding oral sulfate tablet versus polyethylene glycol/ascorbate for bowel preparation in older patients. J Gastroenterol. 2024;59:526-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Panigrahi MK, Gupta S, Rath MM, Prakash JH, Anirvan P, Chaudhary M, Rai A, Nayak HK, R U AG, Padhy BM. Same-day yoga-based Laghu Shankhaprakshalana versus standard polyethylene glycol for rescue colonoscopy in inadequate bowel preparation-Feasibility and cost-effectiveness. Indian J Gastroenterol. 2024;43:1059-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Sara B, Ghinwa H, Layla M, Mahmoud H, Ali K, Remy M. Split doses versus whole dose bowel preparation using polyethylene glycol for colonoscopy: A multicentric prospective Lebanese randomized trial between 2021 and 2023. Health Sci Rep. 2024;7:e2047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Li T, Huang F, Diaz-Dussan D, Zhao J, Srinivas S, Narain R, Tian W, Hao X. Preparation and Characterization of Thermoresponsive PEG-Based Injectable Hydrogels and Their Application for 3D Cell Culture. Biomacromolecules. 2020;21:1254-1263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 13. | Lin Y, He D, Hu H, Yi P, Liu X, Huang J, Wu S, Li G. Preparation and Properties of Polydimethylsiloxane (PDMS)/Polyethylene Glycol (PEG)-Based Amphiphilic Polyurethane Elastomers. ACS Appl Bio Mater. 2019;2:4377-4384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Vafaei S, Boddu VK, Jala S, Bezawada PK, Hattori N, Higashi S, Sugiura T, Manseki K. Preparation of Nanostructured Sn/Ti Oxide Hybrid Films with Terpineol/PEG-Based Nanofluids: Perovskite Solar Cell Applications. Materials (Basel). 2023;16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 15. | Zhang C, Zhang Z, Qi Y. Preparation, Structure, and Properties of Polystyrene-Microsphere-Reinforced PEG-Based Hydrogels. Polymers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Hung SY, Chen HC, Ke TW, Chen JH, Hsiao KH, Wang HM, Chiang HC, Chang SC, Chen YC, Hsieh MH, Tsai YY, Hsieh YW, Chen WT. Noninferiority clinical trial comparing the bowel cleansing efficacy of sodium phosphate tablets (Quiklean(®)) with a polyethylene glycol/bisacodyl kit. World J Gastroenterol. 2021;27:428-441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (3)] |
| 17. | Yang D, Tao K, Chen G, Zhang L, He Q, Xu H. Randomized Controlled Trial of Polyethylene Glycol versus Oral Sodium Phosphate for Bowel Preparation in Unsedated Colonoscopy. Gastroenterol Res Pract. 2020;2020:6457079. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Cheng CL, Tang JH, Hsieh YH, Kuo YL, Fang KC, Tseng CW, Su IC, Chang CC, Tsui YN, Lee BP, Zou KY, Lee YS, Leung FW. Comparing Right-Sided Colon Adenoma and Serrated Polyp Miss Rates With Water Exchange and CO 2 Insufflation: A Randomized Controlled Trial. Am J Gastroenterol. 2024;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Bosch A, Albisetti M, Goldenberg NA, Van Ommen HC, Rizzi M. Results of a multinational survey on the diagnostic and management practices of catheter-related arterial thrombosis in children and neonates: communication from the ISTH SSC Subcommittee on Pediatric and Neonatal Thrombosis and Hemostasis. J Thromb Haemost. 2025;23:1107-1116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 20. | Nouh AK, Haj Mohamad H, Toubah AM, Jaber AA, Alkaram SS, Shaheen M, Hashmi UUR. Successful Management of Severe Dengue With Gastrointestinal Bleeding: A Case Report Highlighting Endoscopic Hemostasis. Cureus. 2024;16:e74142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 21. | Likhtshteyn M, Marzouk E, Arroyo-Mercado FM, Chawla G, Rosengarten S, Lerer R, Ojeda-Martinez H, Thor S. Human immunodeficiency virus patients with low CD4 counts are more likely to have precancerous polyps identified during index colonoscopy. World J Gastrointest Endosc. 2023;15:545-552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 22. | Sadri S, Aghajani A, Soleimani H, Ghorbani Kalkhajeh S, Nazari H, Brouki Milan P, Peyravian N, Pezeshkian Z, Malekzadeh Kebria M, Shirazi F, Shams E, Naderi Noukabadi F, Nazemalhosseini-Mojarad E, Salehi Z. Exploring the Role of the TGF-β Signaling Pathway in Colorectal Precancerous Polyps Biochemical Genetics. Biochem Genet. 2025;63:1116-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 23. | Zhao M, Jacobs JP. Fecal matters: Microbial signatures distinguish clinically relevant subtypes of precancerous colorectal polyps. Cell Host Microbe. 2023;31:692-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 24. | Baile-Maxía S, Mangas-Sanjuán C, Ladabaum U, Sánchez-Ardila C, Sala-Miquel N, Hassan C, Rutter MD, Bretthauer M, Zapater P, Jover R. Risk factors for metachronous colorectal cancer or advanced lesions after endoscopic resection of serrated polyps: a systematic review and meta-analysis. Gastrointest Endosc. 2024;100:605-615.e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 25. | Jung YS, Park JH, Park CH. Impact of proton pump inhibitors on the risk of small bowel or colorectal bleeding: A systematic review and meta-analysis. United European Gastroenterol J. 2023;11:861-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 26. | Wu S, Wu Y, Hu X, Wu F, Zhao J, Pan F, Liu X, Li Y, Ao Y, Zhuang P, Jiao J, Zheng W, Zhang Y. Fruit but not vegetable consumption is beneficial for low prevalence of colorectal polyps in a high-risk population: findings from a Chinese Lanxi Pre-colorectal Cancer Cohort study. Eur J Nutr. 2024;63:1759-1769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |