Published online May 27, 2025. doi: 10.4240/wjgs.v17.i5.106000
Revised: March 19, 2025
Accepted: March 20, 2025
Published online: May 27, 2025
Processing time: 99 Days and 18.6 Hours
The development of slow transit constipation (STC) is associated with intestinal barrier damage. Huangqi decoction (HQD) is effective in treating STC, but me
To investigate whether HQD alleviates STC by downregulating the nuclear factor κB (NF-κB) signaling pathway and restoring intestinal barrier function.
KM mice were divided into control, model, and HQD treatment groups. Fresh colonic tissues were collected for single-cell RNA sequencing and spatial tra
HQD improved intestinal motility, restored mucosal epithelium function and morphology. Single-cell RNA sequencing and spatial transcriptome sequencing data showed a reduction in goblet cells, decreased mucin 2 secretion, and activated apoptotic pathways in STC mice. The population of intestinal stem cells was reduced, and proliferation along with Wnt/β-catenin pathways were inhibited. STC also altered the distribution of intestinal cell states, increasing immune-associated Enterocyte_C3. Aberrant NF-κB pathway activation was noted across various cell types. After HQD treatment, NF-κB pathway activity was down-regulated, while cell proliferation pathways were up-regulated, alongside an increase in Enterocyte_C1 related to material transport. Immunocytochemical, Western blot, and immunohistochemistry analyses confirmed NF-κB pathway activation in goblet cells of STC mice, with HQD inhibiting this aberrant activation.
STC involves intestinal mucosal barrier damage. HQD may treat STC by suppressing NF-κB signaling in epithelial cells, restoring intestinal epithelial cell function, and promoting mucosal barrier repair.
Core Tip: Huangqi decoction (HQD) alleviates slow transit constipation (STC) by suppressing the nuclear factor κB pathway and restoring intestinal barrier integrity. Employing single-cell RNA sequencing and spatial transcriptomics sequencing, we demonstrated HQD reverses STC-induced goblet cell depletion, enhances mucin 2 secretion, and reactivates Wnt/β-catenin-mediated intestinal stem cell proliferation. HQD downregulated nuclear factor κB signaling across cell types, notably inhibiting hyperactivation in goblet cells while promoting Enterocyte_C1 differentiation. These findings reveal HQD’s dual mechanism: Repairing mucosal barrier dysfunction via epithelial regulation and modulating enterocyte states, positioning it as a therapeutic strategy targeting mucosal repair in STC.
- Citation: Chen HX, Xiao GZ, Yang CX, Zheng YH, Lei MY, Xu H, Ren DL, Huang L, He QL, Lin HC. Huangqi decoction ameliorated intestinal barrier dysfunction via regulating NF-κB signaling pathway in slow transit constipation model mice. World J Gastrointest Surg 2025; 17(5): 106000
- URL: https://www.wjgnet.com/1948-9366/full/v17/i5/106000.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i5.106000
Chronic constipation represents a widespread gastrointestinal condition, with a prevalence rate of 16%[1]. Slow transit constipation (STC) represents one of the significant subtypes of chronic constipation. STC is marked by significantly prolonged intestinal transit duration, leading to increased retention of intestinal contents in the colon. This disruption affects the balance of gut microbiota and metabolic products, resulting in damage to the intestinal mucosal barrier and the onset of inflammatory processes. These alterations contribute to excessive absorption of water and electrolytes, reduced mucus secretion, and ultimately lead to the desiccation and hardening of the stool, making it difficult to pass. Research has indicated that the pathogenesis of STC is remarkably complex and may be related to abnormalities in interstitial cells of Cajal, dysfunctions in the nervous system, the imbalance of intestinal flora, and impairment of the intestinal barrier[2-4]. Recently, the significance of the intestinal mucosal barrier in both the development and therapeutic management of STC has garnered increasing attention.
The intestinal mucosal barrier is composed of various elements, including the microbial barrier, mucus barrier, intestinal epithelial cell barrier, and immune barrier, which collectively perform essential functions such as material exchange, transport, and immune defense[5]. In a healthy state, the mucosal barrier exists in a dynamic equilibrium. In animal models of STC induced by loperamide or diphenoxylate, significant pathological changes in the intestinal mucosa are observed, characterized by atrophy of intestinal mucosal glands, decreased numbers of goblet cells, diminished mucus production, and mucosal inflammation[6-8]. Additionally, alterations in the expression of proteins such as claudin-1, occludin, aquaporins (AQPs), and inflammatory mediators are observed[9,10]. This suggests that both morphological and functional changes occur in intestinal epithelial cells. Treatment with various pharmacological agents for STC results in a significant restoration of the histological characteristics of the intestinal mucosal barrier, along with elevated expression levels of junctional molecules such as E-cadherin, ZO-1, occludin, and claudin-1[9-11]. In summary, the intestinal mucosal barrier is critically involved in the onset and advancement of chronic constipation.
In traditional Chinese medicine (TCM), the treatment of constipation is based on theories of Qi, blood, and yin-yang. Regarding STC, deficiency syndromes are predominant. Among these, patients with Qi deficiency-type constipation primarily follow the treatment principle of tonifying Qi and moistening the intestines[12]. Huangqi decoction (HQD), originating from the classical text Prescriptions of the Bureau of Taiping People’s Welfare Pharmacy consists of key ingredients including Astragalus membranaceus (Fisch) Bge (Huangqi), Citri Reticulatae Pericarpium (Chenpi), Mel (Fengmi), and Semen Cannabis (Huomaren). This formulation, along with its modifications, is frequently employed in the treatment of Qi deficiency-type constipation and functional constipation, yielding notable clinical efficacy. According to research by Chinese scholars, HQD is traditionally used to treat symptoms such as constipation, secret congestion, asthenia in the elderly[13]. Additionally, studies have found that HQD and its modified formulations exhibit good efficacy in treating qi-deficiency constipation and functional constipation[14-16]. In our previous research, astragaloside IV, one of the main active components of HQD, was shown to significantly alleviate symptoms in STC mice[17,18]. Furthermore, a meta-analysis indicated that traditional Chinese medicinal preparations or formulas primarily containing Huangqi are safe and effective for treating qi-deficiency functional constipation[19]. However, despite the notable efficacy of HQD as a classical formulation in treating STC, its molecular mechanisms remain inadequately studied, particularly its role in regulating intestinal barrier function, which has yet to be fully elucidated.
The aim of this research is to evaluate the potential of HQD in alleviating constipation through the modulation of the intestinal mucosal barrier. We established a loperamide-induced STC mouse model to investigate the therapeutic efficacy of HQD. Employing multi-omics methodologies and in vitro experiments, including single-cell RNA sequencing (scRNA-seq) and spatial transcriptome sequencing (ST-seq), we analyzed the changes in intestinal mucosal cells and elucidated the mechanisms by which HQD targets the intestinal mucosal barrier to treat STC.
HQD is composed of Astragalus membranaceus (Fisch) Bge (Huangqi), 40 g; Citri Reticulatae Pericarpium (Chenpi), 9 g; Mel (Fengmi), 15 g; and Semen Cannabis (Huomaren), 15 g. Huangqi, Chenpi, and Huomaren were procured from Chinese Medicine Pharmacy of Shuguang Hospital, Shanghai University of TCM. Fengmi was procured from Beijing Tongrentang Co., Ltd China. The above herbs were authenticated by Dr. Hong-Cheng Lin and Dr. Hao Xu. For the in vitro experiments, HQD freeze-dried powder was prepared as described below. Dissolve the above herbs with pure water and boil twice, each for 30 minutes. Anhydrous ethanol was then gradually added to the filtrate until a final concentration of 75% was reached (1 volume of the liquid to 3 volumes of anhydrous ethanol). Seal the container and subject it to ultrasonic agitation, then incubate at 4 °C for 24-48 hours. Subject the sample to centrifugation at 12000 rpm for 10 minutes, followed by supernatant collection. Transfer it into 1.5 mL EP tubes and utilize a vacuum centrifuge to evaporate the ethanol at 38 °C, resulting in a freeze-dried powder form of the filtrate. For the in vivo experiments, HQD water extract were extracted as described below. Dissolve the above herbs with pure water and boil twice, each for 30 minutes. The two extracts were then combined and concentrated on specific concentration. The mice were given HQD orally.
The HQD aqueous extract obtained above was analyzed by ultrahigh performance liquid chromatography (UHPLC) Q-Exactive HFX at Applied protein Technology Co., Ltd. (Shanghai, China). The brief steps are as follows: HQD extracts were analyzed utilizing a Vanquish UHPLC system (Thermo Scientific, Waltham, MA, United States) equipped with an HSS-T3 column (100 mm × 2.1 mm, 1.8 μm particles size; Waters), maintained at a column compartment temperature of 35 °C. Mobile phase A is H2O + 0.1% formic acid and mobile phase B is acetonitrile + 0.1% formic acid (liquid chromatography-mass spectrometry grade solvents, Fisher chemical). The separation of samples was carried out at a flow rate of 0.3 mL/minute according to the following gradient: 1 minute isocratic at 5% B, up to 98 B in 16 minutes, back to 5% B in 0.5 minute and then 2.5 minutes isocratic at 5% B. The Q-Exactive HFX mass spectrometer (Thermo Fisher Scientific, Bremen, Germany) was coupled to the UHPLC system. Mass spectra were collected in both positive and negative electrospray ionization modes using data-dependent acquisition mode with a mass range of m/z 90-1300. The tandem mass spectrometry spectra were collected from the top 10 most intense MS1 ions. The step normalized high-energy dissociation collision energies of 20, 40, and 60 units were applied. The capillary temperature is set at 320 °C whereas the temperature of the probe heater is maintained at 350 °C. The raw data was converted to mzXML format using ProteoWizard and then analyzed using XCMS software.
For the animal experiments, 6-to-8-week-old Kunming mice were sourced from Zhuhai Bestest Biotechnology Co., Ltd, China. Animals were kept in a controlled setting at 23 °C and reared for 1 week on a 12 hours dark/light cycle. After a one-week acclimatization in standard laboratory conditions, the experimental procedures were initiated. The 50 mice were randomly categorized into 5 experimental groups: (1) Control group (CON, n = 10); (2) STC model (administered loperamide hydrochloride (LOP, cat: KM11838, KKL, United States), n = 10); (3) Low dosage HQD treatment group (administered LOP and low dosage HQD, 0.6 g/100 g, n = 10); (4) Medium dosage HQD treatment group (administered LOP and medium dosage HQD, 1.2 g/100 g at a dose based on equivalent doses in mice and humans calculated by experimental pharmacological methods, n = 10); and (5) High dosage HQD treatment group (administered LOP and high dosage HQD, 2.4 g/100 g, n = 10). Ten days following the modeling, intestinal charcoal transit assessments were performed on the various groups (three mice per group), and colon tissues were harvested for single-cell isolation (three mice from the CON, STC, and medium dosage HQD groups). Additionally, fresh colon tissues (one sample from CON, STC, and medium dosage HQD groups) were collected, rinsed with phosphate buffered saline (PBS), embedded in optimal cutting temperature compound, and rapidly frozen using dry ice. After the optimal cutting temperature compound is entirely frozen, the tissue may be maintained at -80 °C for further ST-seq. The colon tissues from CON, STC, and medium dosage HQD groups were collected fixed in formalin for further hematoxylin and eosin (HE) staining, Alcian blue-periodic acid-Schiff (AB-PAS) staining, and immunohistochemistry.
Except for the CON group, the mice in other four groups received 1 mg/100 g of LOP via oral gavage twice daily for five consecutive days to induce a model of STC. The CON group was administered an equivalent volume of sterile water as a placebo intervention. After successful modeling, the STC group received LOP along with 10 g/L sodium carboxymethyl cellulose via oral gavage for a further five consecutive days. For the HQD group, in addition to continuing to receive LOP, they also received low (0.6 g/100 g), medium (1.2 g/100 g, equivalent human dose), and high (2.4 g/100 g) dosage HQD water extract via oral gavage once daily for a further five consecutive days.
On the 10th day of the experiment, the mice underwent to a 12-hour fasting period without water deprivation. Subsequently, activated charcoal (0.2 g/mL) was incorporated into the drug solution and delivered through oral gavage. Thirty minutes later, carbon dioxide anesthesia was employed to euthanize the mice. The mesentery was carefully dissected from the intestinal cavity, and intestinal specimens were collected from the pyloric region to the ileocecal junction. The total length of the small intestine as well as the distance from the pylorus to the leading edge of the charcoal, was measured. The intestinal charcoal transit ratio was calculated as the ratio of the charcoal meal transit distance to the total length of small intestinal length and as follows: Intestinal transit ratio = (charcoal meal transit distance/total small intestinal length) × 100%.
The fresh colon tissue was washed with cold PBS (cat: 10-040-CVRC, Corning, NY, United States), minced on ice, and then subjected to enzymatic digestion. For every 0.2 g of tissue, the enzyme mixture consisted of 0.1 mL of collagenase D (cat: 11088858001, Roche, Switzerland) at a concentration of 20 mg/mL and 0.3 mL of Dispase II (cat: 04942078001, Roche, Switzerland) at 10 mg/mL. The mixture was incubated at 37 °C while shaking at 150 rpm for 60 minutes. After dissociation, the cell suspension was filtered with a 70 μm cell strainer and centrifuged at 300 g for 7 minutes under 4 °C conditions. Discard the supernatant and lyse the red blood cells as necessary. The cell pellet was resuspended in cold 0.04% bovine serum albumin (cat: 9048-46-8, BBI, Shanghai, China)/PBS for scRNA-seq. The cells were stained with acridine orange/propidium iodide and evaluated for viability.
The formalin-fixed, paraffin-embedded tissue samples from STC patients were collected through resection of colon at the sixth affiliated hospital of Sun Yat-sen University from January 2019 to December 2023. The control group included patients who underwent colectomy were collected because of colorectal cancer. The Ethics Committee of the Sixth Affiliated Hospital of Sun Yat-sen University provided ethical approval for the use of patient materials in this study (No. 2024ZSLYEC-100). Since this was not an interventional trial, patients were not directly involved in the study’s design or the determination of outcome measures.
The goblet-like cell line LS174T was purchased from Wuhan Zishan Biotechnology Co., Ltd (cat: STCC10816). The culture medium was RPMI 1640 medium (cat: 10-040-CVRC, CORNING, NY, United States) supplemented with 10% fetal bovine serum (Pricella, Wuhan, China) and 1% penicillin-streptomycin solution (ThermoFisher, MA, United States). The cells were placed in a humidifier chamber at 37 °C and 5% CO2. The cell experiment was divided into five groups: Control group, lipopolysaccharide (LPS, cat: L4391, Merck, Darmstadt, Germany) group (1 μg/mL), HQD low dose (2 mg/mL), HQD medium dose (4 mg/mL), and HQD high dose group (8 mg/mL). For LPS group, the LPS powder was solubilized in PBS and diluted to the required concentration to treat cells for 24 hours. HQD freeze-dried powder was reconstituted in PBS and adjusted to the appropriate concentration. For HQD low dose, HQD medium dose, and HQD high dose group, LPS solution and HQD solution were added to stimulate cells for 24 hours.
Cells were resuspended with a radio immunoprecipitation assay lysis buffer containing a protease inhibitor and phosphorylase inhibitor, then lysed on ice for 30 minutes. Protein quantification was conducted using a bicinchoninic acid kit, and protein denaturation was achieved by boiling in a metal bath at 100 °C for 10 minutes, after which the samples were stored at -20 °C. Proteins were separated on a 10% sodium-dodecyl sulfate gel electrophoresis gel, transferred to a nitrocellulose filter membrane, enclosed in blocking buffer for 1 hour at room temperature, and the primary antibody [IKB alpha, cat: ET1603-6, HuaBio, Hangzhou, China; phospho-nuclear factor κB (phospho-NF-κB) p65, S529, cat: ET1604-27, HuaBio, Hangzhou, China; NF-κB p65, cat: ET1603, HuaBio, Hangzhou, China; and phospho-IKB alpha, S32/S36, cat: HA722770, HuaBio, Hangzhou, China] was incubated overnight at 4 °C. Each membrane underwent three 5-minute washes with tris-buffered saline with Tween, then incubated with the secondary antibody for 1-2 hours at room temperature. Subsequently, the membrane was washed and scanned. Glyceraldehyde-3-phosphate dehydrogenase [recombinant anti-glyceraldehyde-3-phosphate dehydrogenase antibody, horseradish peroxidase (HRP) conjugated, cat: ZB15004-HRP, Servicebio, Wuhan, China] was employed as internal reference to assess the gray value of protein bands.
To detect the inhibitory effect of HQD on the NF-κB signaling pathway in LS174T cells, we measured the translocation of p65 into the nucleus. LS174T cells were evenly seeded in confocal dishes. The cells were then incubated with 1 μg/mL of LPS for 24 hours in the continued presence or absence of HQD. The cell density was maintained at a low level to avoid cell contact interfering with morphological observations. After 24 hours, the medium was removed, and the cells were fixed with Triton X-100 (cat: 9002-93-1, Solarbio, Beijing, China) solution and sealed with 4% bovine serum albumin for 1 hour. The primary antibody was then added into the confocal dishes, which were then incubated in a 37 °C incubator for 2 to 3 hours. The second fluorescent antibody [Alexa Fluor® 488-conjugated goat anti-rabbit immunoglobulin G (H+L), cat: GB25303, Servicebio, Wuhan, China] was added and incubated in a 37 °C incubator for 1 hour. Next, 40 μL 2-(4-amidinophenyl)-6-indolecarbamidine dihydrochloride (cat: G1407, Servicebio, Wuhan, China) was added to each confocal dish to label the nuclei. Finally, the experimental results were obtained through immunofluorescence.
The mouse colon tissues were fixed and then dehydrated, embedded in paraffin wax, sectioned 5 μm thick section. Following dewaxing and rehydration, the tissue sections were immersed in hematoxylin solution for 5 minutes, followed by sequential soaking in ethanol of varying concentrations five times and a final rinse with distilled water. The eosin solution was stained for 3 minutes followed by dehydration with xylene and graded alcohol. Following this, the tablet was sealed using neutral resin and analyzed under microscopic observation. The stained slides were examined for histopathological changes using a light microscope.
The procedure for preparing paraffin sections includes dewaxing to water. Staining is done according to the AB-PAS dye set (Servicebio, cat: G1049) provided by the reagent manufacturer. The staining procedure involves applying AB-PAS C for 15 minutes followed by rinsing until colorless, then staining with AB-PAS B for 15 minutes with additional rinses, and finally using AB-PAS A for 30 minutes in the dark before rinsing. After dehydration through ethanol and xylene, the sections are sealed with neutral gum and examined under a microscope for analysis.
The colon tissue sections were immersed in xylene solution to remove paraffin, rehydrated, and then subjected to antigen retrieval using 10 mmol/L EDTA antigen repair solution (pH 9.0, cat: ZLI-9067, ZSGB-Bio, Beijing, China). The tissue sections were subsequently incubated with peroxidase-blocking solution for 15 minutes and protein blocking solution for 20 minutes. All sections were incubated overnight at 4 °C with primary antibodies against p65 (cat: ET1603, HuaBio, Hangzhou, China), AQP3 (cat: AF5222, Affinity Biosciences, Changzhou, China), claudin-1 (cat: 13050-1-AP, Proteintech, Wuhan, China), and mucin 2 (MUC2, cat: ET1704-06, HuaBio, Hangzhou, China), respectively. Following this, tissue sections were incubated with an HRP-conjugated secondary antibody [HRP conjugated goat anti-rabbit immunoglobulin G (H+L), cat: GB23303, Servicebio, Wuhan, China]. Immunoreactivity was visualized with a chromogenic substrate (diaminobenzidine, cat: ZLI-9019, ZSGB-Bio, Beijing, China). Finally, tissue specimens were stained with hematoxylin solution to clearly distinguish nuclei from the cytoplasm. Six fields of view for mucosal epithelium were randomly selected under the microscope. All images were analyzed the average optical density using ImageJ Fiji software (NIH, Maryland, United States).
The BD Rhapsody system was utilized to capture transcriptomic data from single cells of the mice colon tissue. Single-cell capture was accomplished by employing a limited dilution method to randomly distribute a single-cell suspension into over 200000 microwells. The process for preparing whole transcriptome libraries adhered to the BD Rhapsody single-cell whole-transcriptome amplification workflow. The libraries were quantified using a high sensitivity DNA chip (Agilent) on a Bioanalyzer 2200 and the Qubit high sensitivity DNA assay (Thermo Fisher Scientific). All libraries were sequenced by T7 MGI on a 150 bp paired-end run.
Spatial transcriptomics assays were conducted on quality-controlled tissue sections in accordance with the protocol of the Visium CytAssist Fresh Frozen Section Spatial Gene Expression (10x Genomics). In brief, tissue sections were mounted on Sigma-Aldrich Poly Prep Slides and allowed to dry overnight, which facilitated optimal attachment. Subsequently, the sections were stained using HE and high-resolution images were captured at a 20 × magnification using Leica Aperio Versa 8 whole slide scanner for detailed visualization. For sections treated with HE staining, crosslinks were immediately reversed and a probe panel covering the entire mouse transcriptome was added. After hybridization of the probe pairs to their target transcripts, the slides were then positioned on the Visium CytAssist instrument for permeabilization and RNase treatment. Subsequently, the ligated probes underwent hybridization with spatially barcoded oligonucleotides, which allowed for the collection of transcriptomic data in a spatial context. Finally, the prepared spatial transcriptomics libraries were sequenced using the Illumina NovaSeq 6000 system.
Following the acquisition of raw sequencing data, we employed the fastp tool (with default parameters) to filter adapter sequences and filter out low-quality reads. After that, unique molecular identifier (UMI) tool was employed for scRNA-seq analysis to ascertain the cell barcode whitelist. The clean UMI-based data was aligned to the mouse genome reference using the STAR alignment tool, with tailored parameters from the UMI-tools standard pipeline employed to calculate UMI counts for each sample. Subsequently, we performed quality control and further analysis using the Seurat package[20] in R software. For further analysis, cells with fewer than 200 or exceeding 6000 expressed genes were excluded from the analysis, along with those exhibiting a mitochondrial UMI rate exceeding 30%. The remaining data were normalized and scaled using the top 2000 high-variable genes. Principal component analysis was performed based on the scaled data, and the top 20 principal components were used to construct uniform manifold approximation and projection (UMAP) and visualization[21]. Canonical marker genes were utilized to annotate the major cell types. To further identify specific epithelial cell subtypes, we selected clusters corresponding to epithelial cell and performed UMAP analysis and marker gene analysis again.
To identify differentially expressed genes (DEGs) for each cluster, we employed the FindAllMarkers function with its default settings. To identify DEGs between two groups, the FindMarkers function was employed. The DEGs were subjected to a filtering process based on specific criteria: Wilcoxon Rank Sum test, min. pct = 0.1, P value < 0.05, |logfc.threshold| > 0.25. To explore the biological functions related to the DEGs, we utilized the clusterProfiler package[22] to conduct gene ontology (GO) enrichment analysis. We separately conducted GO enrichment analysis for the top 200 up-regulated and down-regulated DEGs. Enrichment analysis results for biological processes were chosen according to a predefined statistical criterion (qvalueCutoff = 0.05). The results were visualized as bar plots using clusterProfiler and ggplot2 R packages.
We employed a fast per-ranked gene set enrichment analysis (GSEA) using fgsea R package to conduct functional enrichment analysis for group-specific DEGs. We fed each DEGs list, area under the curve value of each gene, and the gene sets to the ‘fgsea’ function (nperm = 1000). A positive normalized enrichment score indicates that a specific functional gene set is enriched at the beginning of the ranked list, suggesting upregulation in the specific cell population compared with others. Conversely, a negative normalized enrichment score means certain functional gene set is enriched towards the end of the ranked list, indicating down regulation in the specific cell population relative to other cells.
To explore potential expression programs of epithelial cells, we applied non-negative factorization through the GeneNMF package across all samples. Initially, expression counts for each sample were normalized using the Seurat NormalizeData function, adhering to its default parameter configurations. Highly variable genes were detected through the Seurat FindVariableFeature function, and a subset of 2000 highly variable genes was chosen for subsequent analysis. We analyzed the most commonly co-expressed gene modules in each sample using the multiNMF function. The gene programs identified through this process were then aggregated into metaprograms using the getMetaPrograms function, ultimately resulting in the identification of eight metaprograms.
The activities of transcription factors (TFs) for each group were assessed using DoRothEA package[23]. We initiated our assessment by employing run_Viper function to process the regulons and determine TF activity. TFs were ranked according to the variance of their associated Viper scores, enabling the identification of those with the most significant variability in activity. Heatmap was utilized to depict the variations in TFs between the compared groups.
Monocle 2[24] was employed to investigate the transcriptional dynamics among cell subtypes. we created a CellDataSet object with its standard settings. The differentialGeneTest function was employed to detect variable genes, which were subsequently filtered using a q-value cutoff of less than 0.01. The DDRTree algorithm, along with the orderCells function, was utilized to perform dimensional reduction and cell ordering. To perform pseudotemporal inference, Seurat-analyzed cells were subsequently imported into Monocle 3. Pre-processing in Monocle 3 package follows the methodology detailed by Cao et al[25]. Subsequently, to shed more light on the biological processes underpinning epithelial proliferation and differentiation, we leveraged Monocle 3 to identify modules of co-expressed genes, which helped in elucidating the complex gene expression patterns within these processes.
Further analysis and visualization for ST data were conducted in R with the Seurat package[20]. The anchor-based integration workflow from the Seurat package was employed to merge ST-seq data with scRNA-seq data, assigning a prediction score to each spot based on subtypes identified in the single-cell analysis. The TransferData function in the Seurat package was utilized to visualize the predicted cell type composition and subtypes derived from scRNA-seq data at the spot level for each tissue section. Additionally, we employed the gene set variance analysis function to assess the activity of specific pathways for each spot within our ST-seq data.
Data were presented as mean ± SE and experimental data were analyzed using Graph pad Prism Version 8 software. Unpaired t-tests were employed for comparisons between two groups, while one-way analysis of variance followed by Dunnett’s test was used for multiple group comparisons. P values of P < 0.05 were considered statistically significant.
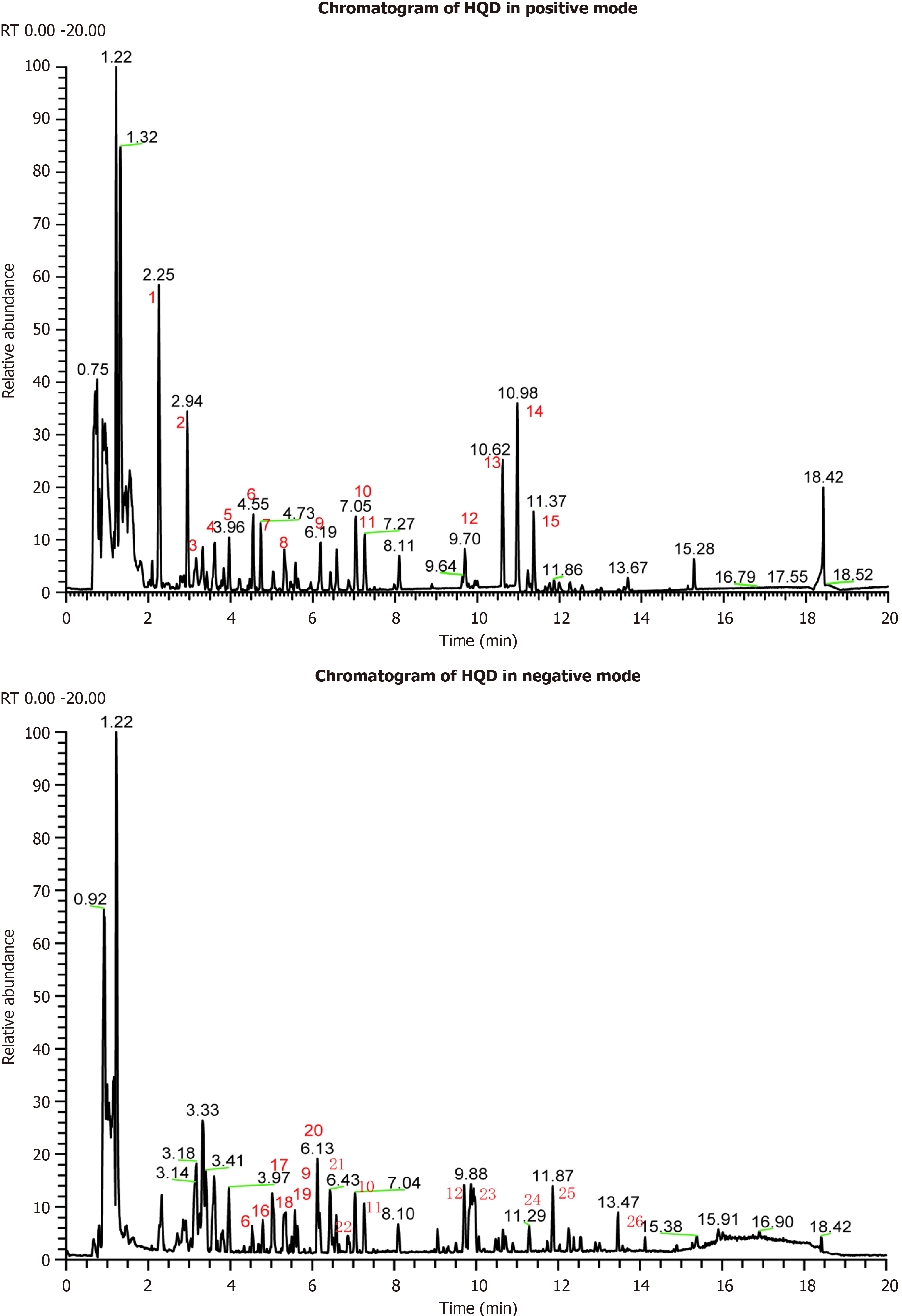
The chemical profiles of the HQD aqueous extract were determined utilizing both positive and negative ionization modes, with the corresponding chromatograms being shown in Figure 1. The identification of 1828 components was achieved through the integration of accurate molecular weight data, secondary ion cleavage profiles, and corroboration with database and published literature, including calycosin-7-o-beta-d-glucoside, 6’’-O-malonylglycitin, astragaloside IV, astragaloside I, calycosin, nobiletin, tangeritin, hesperidin, N-trans-coumaroyloctopamine, astragaloside II, linoleate, luteolin, isomucronulatol 7-o-glucoside, astragaloside III, etc. The identified components are detailed in Supplementary Table 1.
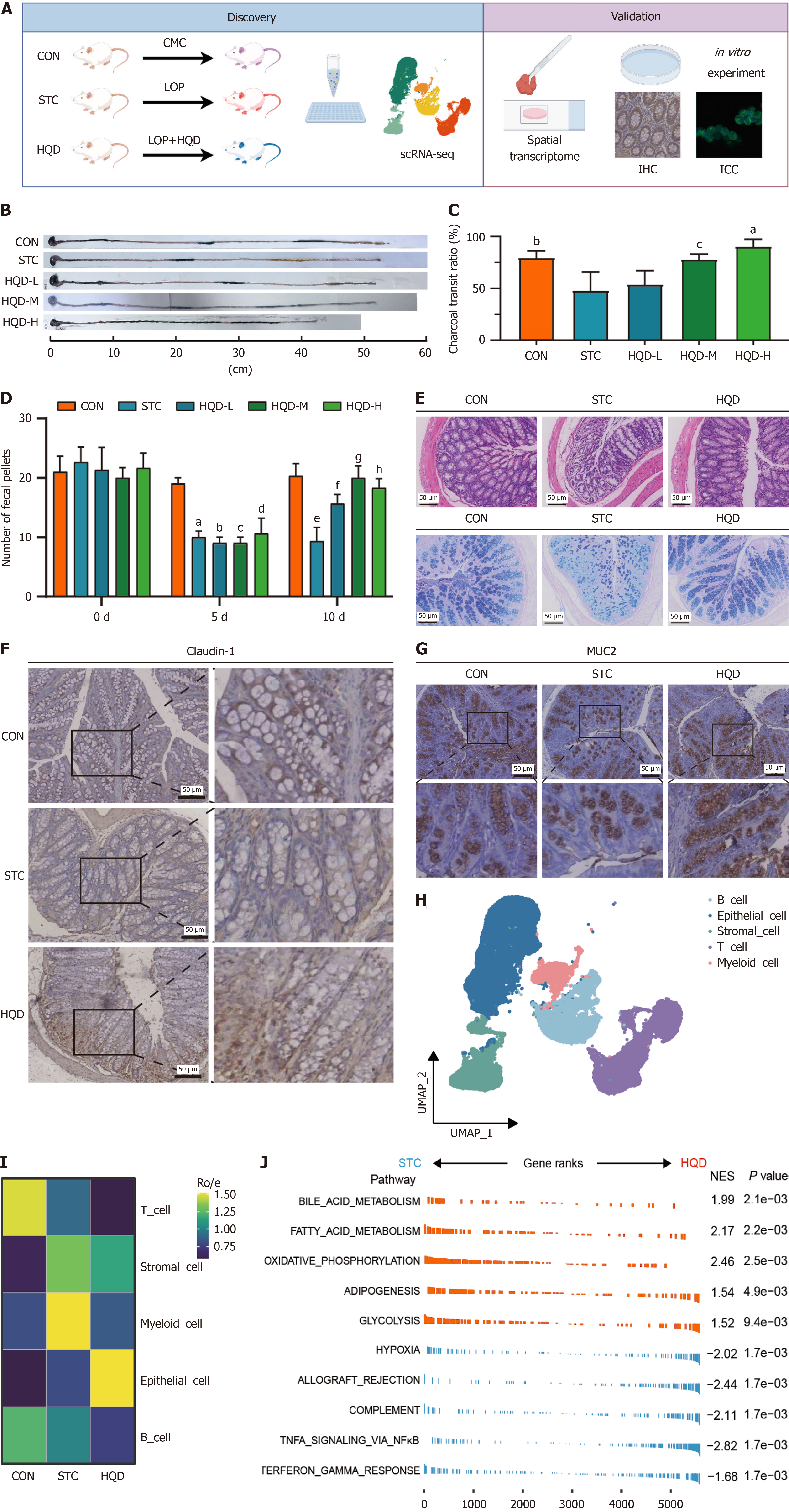
The mice were divided into the CON, STC, and HQD (low, medium, and high dosage) groups. An STC mice model was induced by LOP, and the HQD group received treatment with HQD (Figure 2A). Compared to the CON group, LOP reduced the intestinal charcoal transit ratio and the number of fecal pellets in the STC group, which indicated the efficacious establishment of the STC mice model. Treatment with HQD in LOP-induced STC mice led to improved defecation (Figure 2B-D). LOP-induced constipation led to damaged colonic mucosa, decreased secretion of goblet cells and mucus, disorganized villi morphology, and decreased claudin-1 (intestinal tight junction proteins) expression in mice (Figure 2E and F, Supplementary Figure 1A). Decreased claudin-1 expressions were also observed in the colonic tissues of STC patients (Supplementary Figure 1B and C). We determined by immunohistochemical staining that MUC2 secreted by epithelial cells in STC mice was reduced (Figure 2G, Supplementary Figure 1D), and MUC2 expression was also found to be reduced in the colonic tissues of STC patients (Supplementary Figure 1E and F). However, HQD treatment could improve defecation and protect intestinal mucosal barrier in STC mice (Figure 2B-G). This suggests that HQD may alleviate constipation by improving intestinal mucosal barrier.
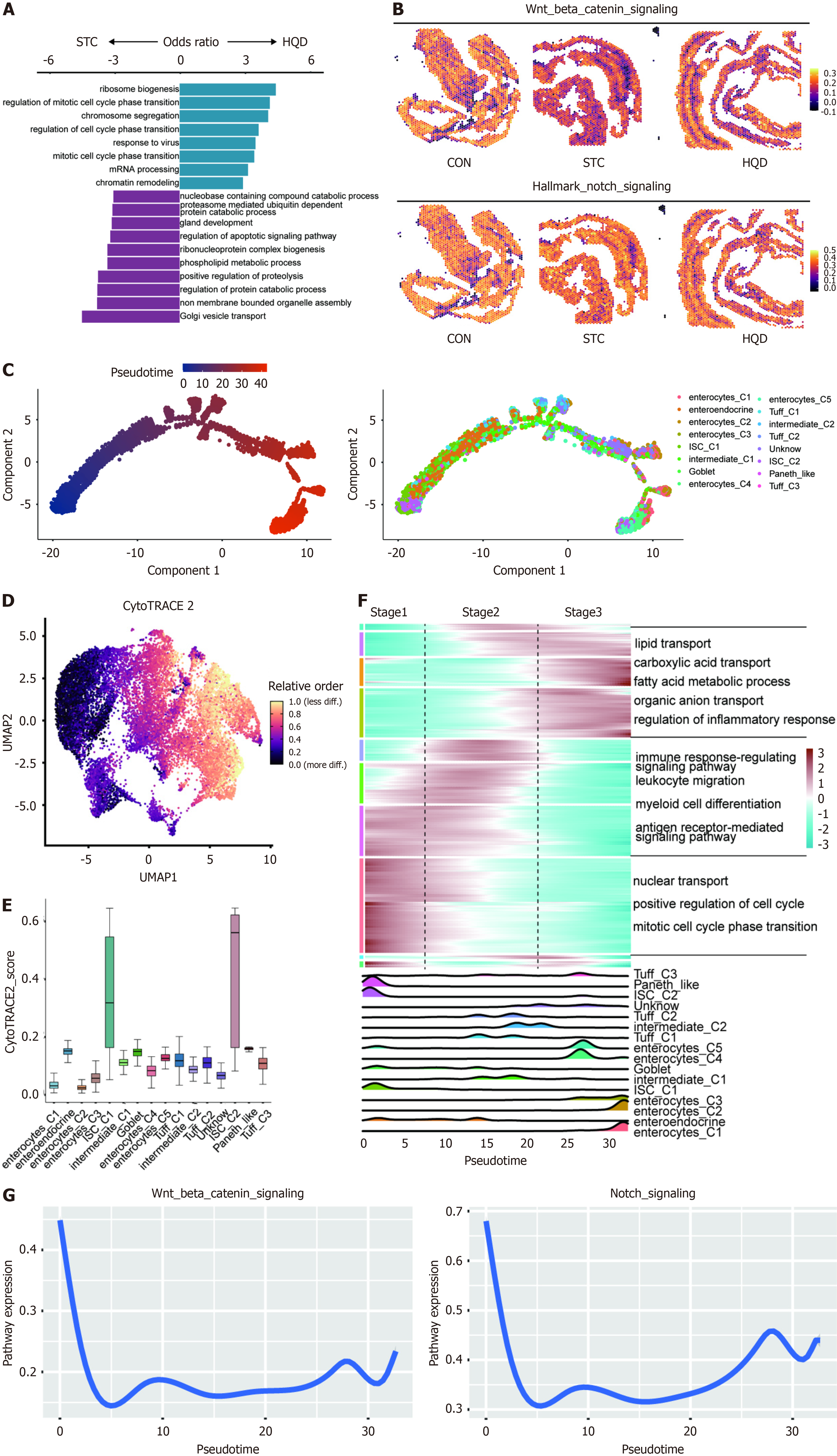
To explore the mechanism of composition and functional changes of intestinal mucosal barrier in STC and the therapeutic mechanism of HQD, we performed scRNA-seq and ST-seq on colon tissues of STC mice. First, we performed scRNA-seq on nine colon tissues from CON, STC, and HQD groups (3 mice in each group). After quality control, 21250 cells from the CON group, 13436 cells from the STC group, and 21947 cells from the HQD group were retained for further analysis (Figure 2H, Supplementary Figure 1G). We identified five major cell types (Figure 2H). Interestingly, myeloid cells were more abundant in the STC group (Figure 2I, Supplementary Figure 1H). Following HQD treatment, we observed a downregulation of inflammation-associated pathways in STC group, including the NF-κB signaling pathway, interferon-gamma response, and complement pathways, while metabolic pathway activities were significantly upregulated in HQD group (Figure 2J). Furthermore, most cell types including myeloid cells, T cells, stromal cells, and B cells in the STC group enriched the NF-κB signal pathway, while epithelial cells, stromal cells, and B cells in the HQD group enriched the pathways related to cell proliferation signaling (Supplementary Figure 1I and J). These findings reveal that STC induced by LOP caused functional changes in colon tissue cells, and HQD exerts its therapeutic effect by regulating the function of these cells.
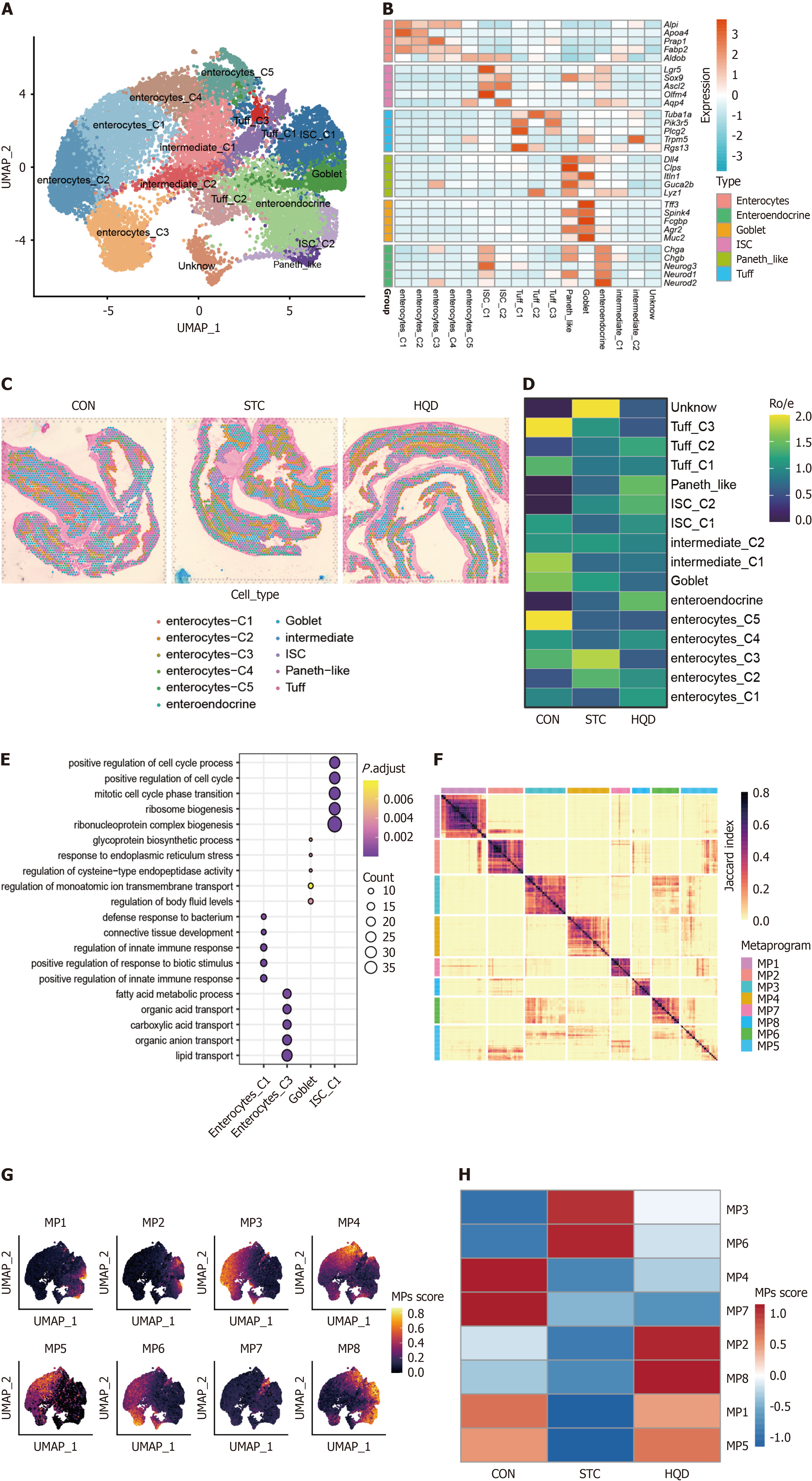
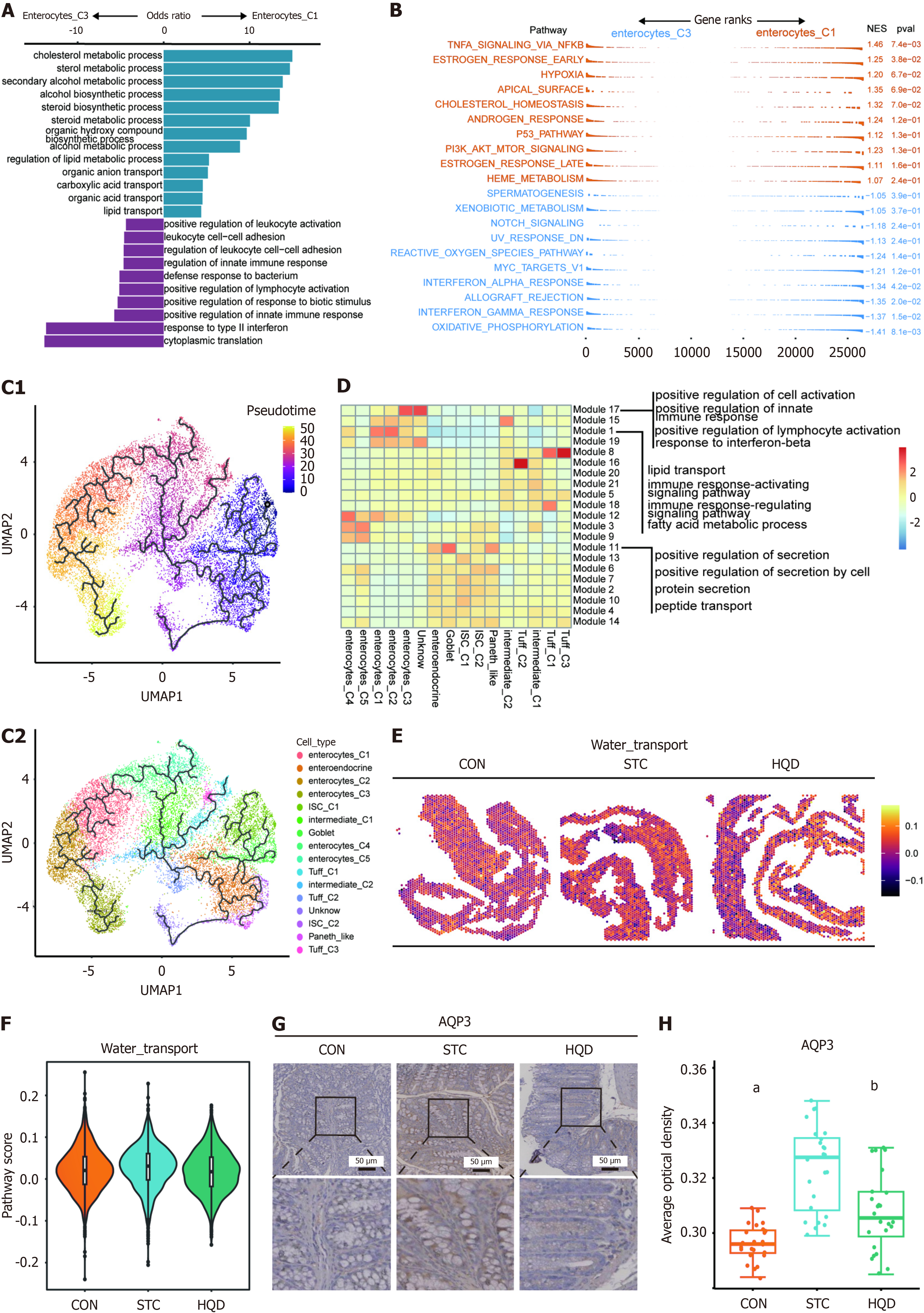
To further clarify the diversity of colon epithelial cells, we performed additional clustering on the epithelial cells from the aforementioned three groups. Specifically, we re-clustered the epithelial cells into 16 clusters, assigned identities based on classical markers, and identified six subtypes, namely enterocytes, intestinal stem cells (ISCs), Tuff cells, Paneth-like cells, goblet cells, enteroendocrine cells (Figure 3A and B). Each subtype exhibits a unique expression pattern, highlighting the complexity of the epithelial cell landscape. Utilizing single-cell sequencing data, we identified identical epithelial cell types in three spatial transcriptomics data (Figure 3C, Supplementary Figure 2A). Each cell subtype was distributed across different groups (Figure 3D). In comparison to the CON group, the proportion of Enterocyte_C1 within the STC group exhibited a reduction, which was partially restored after treatment with HQD (Figure 3D). Compared to the CON group, the STC group exhibited an elevated proportion of Enterocyte_C3, a change that was partially reversed after HQD treatment (Figure 3D). Compared with the STC group, the proportion of ISCs increased after HQD treatment, especially ISC_C1 cells were more enriched in the CON and HQD groups (Figure 3D). To comprehensively investigate the functional diversity of epithelial cells, we performed GO enrichment analysis. The results demonstrated that enterocyte_C1 exhibited significant enrichment in substance transport pathways, enterocyte_C3 displayed enrichment in immune response pathways, and ISC_C1 was notably enriched in pathways related to cell proliferation (Figure 3E).
To elucidate the potential molecular characteristics driving epithelial heterogeneity, we employed non-negative matrix factorization to uncover potential transcriptional programs of epithelial cells. We identified 8 transcriptional program clusters/meta-programs (MP) of epithelial cells (Figure 3F). MP1 was associated with secretory pathways, indicating its secretory-related epithelial status; MP2 was linked to cell cycle pathways; MP6 was characterized by immune response and ion transport pathway; while MP3 were indicative of defense and immune response pathways (Supplementary Table 2). Notably, specific cell subtypes demonstrated increased expression of particular MP genes, with MP1 mainly expressed in goblet cells, MP2 mainly expressed in ISCs, MP6 mainly expressed in enterocyte_C3, and MP3 mainly expressed in enterocyte_C1 and enterocyte_C2 (Supplementary Figure 2B). Upon projection into UMAP space, these MP-related epithelial subtypes demonstrated a strong alignment with their corresponding MP activities, further highlighting the observed heterogeneity within the colon epithelium (Figure 3G). Notably, MP1 and MP2 were suppressed in the STC group but were restored in the HQD group. Conversely, MP3 and MP6 were more highly expressed in the STC group but were downregulated following HQD treatment (Figure 3H). This suggests that goblet cell-related functions were inhibited in the STC group, and HQD treatment could restore these functions. The presence of immune-inflammatory related pathways in MP3 and MP6 indicates immune and inflammatory activation in STC. Overall, our research elucidates the epithelial heterogeneity induced by LOP and demonstrates the protective effects of HQD treatment, which modulates functions associated with epithelial cell secretion and immune-inflammatory responses.
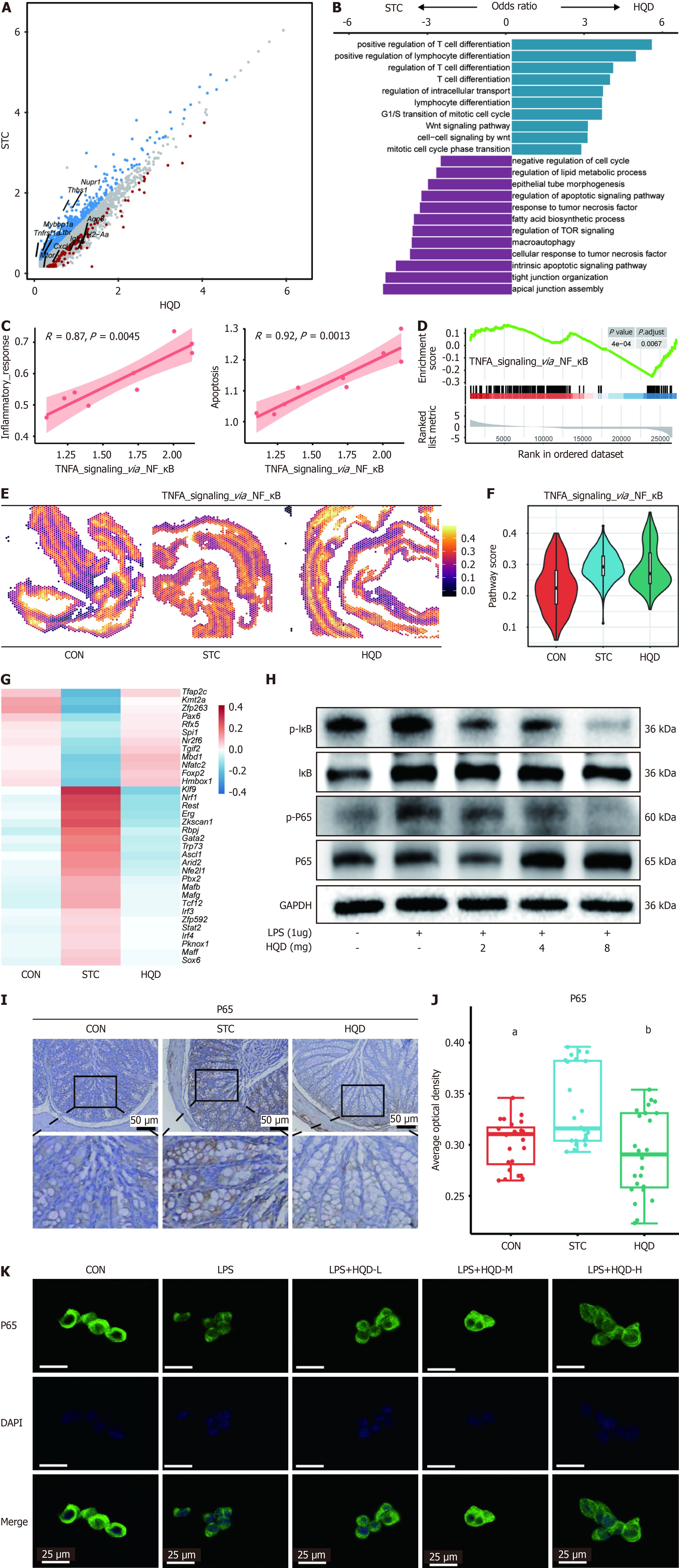
A significant alteration in the expression of the goblet cell-specific MP1 module underscores the critical role of goblet cells in the mechanism by which HQD alleviates STC (Figure 3H, Supplementary Figure 2B). To explore the potential role of goblet cells, we identified DEGs between goblet cells in the HQD and STC groups (Figure 4A). Our analysis identified that genes enriched in STC comprise nuclear protein transcription regulator 1, thrombospondin 1, MYB binding protein 1a, tumor necrosis factor receptor superfamily, member 1a, and lymphotoxin B receptor, whereas genes with elevated expression in the HQD group include Aqp8, histocompatibility 2 class II antigen A alpha, interferon gamma induced GTP hydrolases, mechanistic target of rapamycin kinase, and C-X-C motif chemokine ligand 1. Further GO enrichment analysis of goblet cell-related DEGs revealed that these genes were enriched in pathways associated with the regulation of apoptotic signaling, response to tumor necrosis factor, and the negative regulation of the cell cycle in the STC group. In contrast, in the HQD group, they were enriched in pathways related to lymphocyte differentiation and the regulation of intracellular transport (Figure 4B). These data indicate that goblet cells in the colons of STC mice exhibit a stronger tendency for apoptosis, which significantly affects the mucin secretion function of the colonic mucosal epithelium.
Notably, our findings revealed a significant positive correlation between the inflammatory response/the apoptosis pathway and the NF-κB pathway in goblet cells (Figure 4C). Further GSEA analysis revealed that goblet cells in the STC group exhibited significant enrichment in NF-κB pathways compared to the HQD group, indicating a stronger inflammatory response in the STC group (Figure 4D). Additionally, we observed significant enrichment of the NF-κB pathway in STC samples during the ST-seq analysis, with its activity being notably suppressed following HQD treatment (Figure 4E and F). In the ST-seq analysis, we also observed that goblet cell proliferation pathway score in the STC group was significantly lower than that in the HQD group (Supplementary Figure 3A and B). Because the functional status of goblet cells changes with HQD treatment, we performed DoRothEA (TF) analysis on goblet cell clusters and found that kruppel-like factor 9 was the most active in STC goblet cells (Figure 4G). Previous studies have shown that kruppel-like factor 9 functions as a pro-inflammatory TF that enhances toll like receptor 2 expression, thereby facilitating the activation of inflammation-related signaling pathways[26]. In addition, Erg and interferon regulatory factor 4 were the most active in the STC group but were significantly downregulated after HQD treatment (Figure 4G). Previous research has also revealed the key role of Erg and interferon regulatory factor 4 in regulating the activity of the NF-κB pathway[27,28]. Western blot analysis further confirmed that the activity of the NF-κB pathway was inhibited after HQD treatment of LS174T cells (Figure 4H). An immunohistochemistry analysis revealed that NF-κB P65 expression in the colons of STC mice was significantly increased, subsequently decreasing following HQD treatment (Figure 4I and J) and P65 expression was also found to be increased in the colonic tissues of STC patients (Supplementary Figure 3C and D). P65 was similarly elevated in the colons of STC patients. Moreover, the aforementioned result was confirmed through immunocytochemistry analysis of nuclear translocation of NF-κB P65 in LPS induced LS174T cells. We observed a similar finding that HQD treatment inhibited the translocation of NF-κB P65 into the nucleus (Figure 4K). In summary, our results indicate that HQD treatment suppresses the activity of the inflammation-related NF-κB pathway and apoptosis pathway in goblet cells.
ISCs exhibit significant self-renewal capacity and are capable of differentiating into various specialized cell types found in the intestinal epithelium. Cell composition analysis (Ro/e) revealed a decreasing trend of ISC_C1 in STC, which was restored after HQD treatment (Figure 3D). To further elucidate the underlying mechanisms of HQD treatment, we performed DEG and GO enrichment analysis on ISC_C1, showing that ISC_C1 in HQD enriched cell cycle-related pathway, while STC exhibited higher expression of apoptosis pathway (Figure 5A, Supplementary Figure 4A). Notably, we also found in the ST-seq data that ISCs after HQD treatment had higher Wnt/β-catenin pathway, Notch pathway score, and E2F_targets pathway compared to ISCs in the STC group (Figure 5B, Supplementary Figure 4B).
To further determine the impact of HQD treatment on the fate of epithelial cells, we applied the monocle2 algorithm to establish a pseudo-time trajectory, revealing the differentiation path of epithelial cells. Trajectory analysis results showed that epithelial cells differentiated from ISC cells into goblet cells and enterocytes (Figure 5C). We then used CytoTRACE to predict the differentiation state of each epithelial subtype and confirmed that ISCs had a significantly higher differentiation potential than other epithelial subtypes (Figure 5D and E, Supplementary Figure 4C). These results suggest that ISCs may act as the progenitor source for intestinal epithelial cell lineages. We next investigated transcriptional alterations related to epithelial differentiation and observed that epithelial cell clusters could be divided into three stages, with the first stage enriching for cell cycle signaling pathways. The second stage enriched in immune regulation pathways, indicating their potential role in regulating intestinal immunity. Interestingly, genes and pathways involved in substance transport-related were upregulated in the third stage, suggesting a higher degree of functional differentiation in these cells (Figure 5F). Notably, we found that the Wnt/β-catenin signaling pathway and Notch pathway were most active in the cells of the first stage and gradually decreased as the cells differentiated, indicating their potential important role in regulating the differentiation of ISC cells into mature intestinal epithelial cells (Figure 5G). We further compared the activity of the Wnt/β-catenin pathway and Notch pathway in epithelial cells among the three groups and found that the epithelium of the STC group had the lowest activity of the Wnt/β-catenin pathway and Notch pathway, which was restored after HQD treatment (Supplementary Figure 4D). Overall, our findings indicate that LOP-induced STC inhibited the proliferation and differentiation of ISCs, while HQD treatment restored the functionality of ISCs.
It is noteworthy that we identified two groups of enterocytes with opposing distribution trends; Enterocyte_C1 was enriched in the HQD group, while Enterocyte_C3 was enriched in the STC group (Figure 3D). This suggests that they play different roles during the HQD treatment process. We initially conducted a DEG analysis on these two cell types, revealing that Enterocyte_C1 highly expresses genes involved in substance transport such as solute carrier family 27 member 2, solute carrier family 22 member 4, NPC intracellular cholesterol transporter 1, and Aqp8, whereas Enterocyte_C3 highly expresses chemokines like C-X-C motif chemokine ligand 16 and C-C motif chemokine ligand 28 (Supplementary Figure 5A). Further enrichment analysis of DEGs for Enterocyte_C1 and Enterocyte_C3 showed that these genes in Enterocyte_C1 are enriched in pathways related to organic anion transport and lipid transport, while the Enterocyte_C3 group is enriched in pathways related to positive regulation of leukocyte activation and regulation of leukocyte cell-cell adhesion (Figure 6A). GSEA analysis indicated that compared to Enterocyte_C1, Enterocyte_C3 is enriched in interferon gamma response and interferon alpha response (Figure 6B). To further investigate the changes in gene expression patterns of these two cell clusters, trajectory analysis was performed using the monocle 3 R package. Based on CytoTRACE results, ISC_C1 was considered as the starting point for epithelial cell differentiation, with Enterocyte_C3 at the end point of differentiation, and Enterocyte_C1 showing a trend towards differentiating into Enterocyte_C3 (Figure 6C). We continued to examine gene modules in our dataset’s pseudotime projection. Of particular interest were module 1 and module 17, representing a set of genes that are strongly enriched in Enterocyte_C1 and Enterocyte_C3, respectively. Functional enrichment analysis of genes in module 1 identified pathway involving lipid transport and fatty acid metabolic processes, suggesting that these pathways may contribute to alternative Enterocyte_C1 fate trajectories (Figure 6D). Gens in module 17 revealed the enrichment of immune-related pathways such as response to interferon-beta and regulation of innate immune response, indicating that these pathways may be key modules in the differentiation process of Enterocyte_C3 (Figure 6D). It is noteworthy that we found higher activity of water transport pathway in STC group in ST-seq data (Figure 6E and F). Immunohistochemistry revealed increased levels of AQP3 in the STC group mice (Figure 6G and H). This alteration was also observed in the colonic tissues of patients with STC (Supplementary Figure 5B and C). HQD reduced the expression of AQP3 in the SCT mice (Figure 6G and H). In summary, our findings highlight two groups of intestinal cells with different functions. STC resulted in an increase in immune-related Enterocyte_C3 cells, and after HQD treatment, there was an increase in Enterocyte_C1 linked to substance transport.
The management of chronic constipation often involves the use of laxatives and other medications, which are associated with notable side effects[29]. This emphasizes the need for alternative treatment options. TCM has emerged as a promising approach for alleviating constipation, with numerous human and animal studies demonstrating positive outcomes[30-32]. Nevertheless, the exact underlying mechanisms remain largely unknown. This study shows that HQD significantly improves intestinal motility, alleviates the histology and function of intestinal mucosal barrier, and inhibits the activation of the NF-κB pathway in epithelial cells. These findings suggest that HQD may exert therapeutic effects on STC by modulating the intestinal mucosal barrier.
Previous researches have observed that the constipated animals exhibit damage to the intestinal mucosal barrier[6-9]. These studies showed that the mucosal layer of the constipated animals was significantly thinner, and the crypts were shortened, the protein expression level of MUC2 and claudin-1 were reduced. Our results were consistent with previous studies. The a defective function of intestinal mucosal barrier is closely associated with inflammation[33]. Inflammation causes disruption of the intestinal mucosal barrier, leading to increased permeability[34]. Research has indicated that inflammatory responses are involved in the pathophysiology of constipation[35]. However, there has been a lack of investigations utilizing methodologies such as single-cell sequencing and spatial transcriptomics to examine the cellular dynamics of the intestinal mucosal barrier at a single-cell resolution. Our study not only elucidates the remodeling of mucosa epithelium in constipation model at a single-cell resolution but also reveals the specific mechanisms by which HQD exerts its therapeutic effects through the modulation of mucosa epithelium. This represents a preliminary application of integrating single-cell transcriptomics with spatial mapping in STC research, providing improved resolution for characterizing cellular dynamics and the spatial organization of epithelial subtypes during disease progression and treatment. Compared to conventional bulk tissue analysis, our multimodal approach enables more precise identification of specific cell populations and their functional changes. The colonic mucosal epithelial cell population comprises columnar enterocytes, goblet cells, ISCs, and enteroendocrine cell. Utilizing scRNA-seq and spatial transcriptomics, we identified these cell subtypes and found that they experience distinct functional perturbations in the context of STC. Moreover, we observed that HQD broadly impacts these epithelial cell subtypes, alleviating inflammation within the intestinal mucosa.
Goblet cells are responsible for secreting mucus, which plays an important role in lubricating the intestines, protecting the mucosa, and forming the mucosal barrier. In the colon tissues of constipation mice model and patients with STC, we found the impairment of goblet cell. This may be one of the reasons for constipation. Further analysis of goblet cells showed a decrease in their numbers, impaired function, and reduced secretion of MUC2 in constipation mice. This is consistent with previous studies[36,37]. We further discovered that in constipation, the activation of the NF-κB pathway, inflammatory response pathway, and apoptosis pathway in goblet cells is correlated. This may be the reason for the decrease in the number and dysfunction of goblet cells. Previous studies on intestinal inflammation have shown that the activation of the NF-κB signaling pathway in inflamed tissues results in goblet cell dysfunction, which impacts mucus formation and ultimately compromises the integrity of the intestinal mucosal barrier[38]. The components of HQD, such as Astragalus and grossamide (a representative lignanamide in hemp seed), can inhibit the NF-κB signaling pathway, thereby alleviating inflammatory responses[39,40]. In our study, we demonstrated through cellular experiments that HQD effectively inhibits the NF-κB signaling pathway. This inhibition may provide insight into the therapeutic mechanisms by which HQD exerts its beneficial effects.
ISCs are located at the base of colonic crypts and play a crucial role in maintaining the integrity and functionality of the colonic mucosa through proliferation and differentiation. Under steady-state conditions, ISCs maintain tissue regeneration by balancing self-renewal and differentiation, enabling them to give rise to various mature colonic epithelial cell types, including absorptive enterocytes, goblet cells, and enteroendocrine cells. Our study revealed that the apoptosis pathway in ISCs is activated in constipation mice, suggesting a disruption in the normal physiological functions of these ISCs, which subsequently leads to damage of the intestinal mucosal barrier. Treatment with HQD resulted in the restoration of the proliferative capacity of ISCs. We further analysis the key pathways involved in the proliferation and differentiation of ISCs, particularly the Wnt/β-catenin and Notch pathways, which were affected by both constipation and HQD treatment during the differentiation process into mature epithelial cells, including goblet cells. Notably, HQD reinstated the Wnt/β-catenin and Notch pathways that had been suppressed in constipation mice, providing a potential explanation for the observed increase in goblet cell numbers. In summary, these findings suggest that the functional mechanism of HQD in regulating ISCs may be associated with the Wnt/β-catenin and Notch signaling pathways.
Enterocytes are a key type of intestinal epithelial cell that play a vital role in nutrient absorption. These cells facilitate the transfer of nutrients from the intestinal lumen into the bloodstream through specialized transport and channel proteins located in their membranes. Enterocytes also are essential for maintaining the balance of water and electrolytes within the intestine. Additionally, enterocytes are capable of secreting antimicrobial peptides and other immune-regulatory molecules, which assist in defending against pathogens while also participating in the modulation of intestinal immune responses. In this study, we identified two functionally specific types of enterocytes (C1 and C3). Enterocyte C1 primarily focuses on nutrient transport and metabolism. In contrast, enterocyte C3 is more closely associated with immune and inflammatory functions. Notably, in constipation mice, enterocyte C3 cells constituted a larger proportion, which correlates with the significant inflammation observed in the colons of these mice. This further underscores the evident inflammation in the colons of constipated mice, which alters the functional state of enterocytes. Moreover, intestinal inflammation can enhance the permeability of the intestinal mucosal epithelium[34]. We observed alterations in the expression of AQPs in mice with constipation, a change that was also evident in the colonic tissues of patients diagnosed with STC. However, the relationship between AQPs and constipation remains controversial and even contradictory. Some studies have indicated an increase in AQP3 expression in the colonic tissues of patients and animal model with constipation[41,42], while others reported opposite results[43,44]. Our research, utilizing various experimental methods including scRNA-seq and immunohistochemistry, demonstrated that AQP3 levels are elevated in both mice and patients with constipation. The contradictory AQP3 expression patterns may arise from: (1) Intestinal region-dependent functional diversity of AQP3; (2) Heterogeneity in pathological states of constipation across studies; and (3) Distinct regulatory mechanisms (hormonal, inflammatory, oxidative) governing its expression. As above mentioned, HQD exhibits anti-inflammatory effects. In our study of enterocytes, treatment with HQD similarly alleviated inflammation in these cells.
The combination of scRNA-seq and ST-seq technologies in this study establishes a new paradigm for investigating complex herbal formulations. The application of these technologies has allowed us to uncover novel insights into the pathophysiology of STC and the mechanisms of HQD treatment at a single-cell resolution. This approach overcomes the historical limitations of bulk tissue analysis in TCM research, enabling precise identification of cellular targets and pathway modulations underlying formula efficacy. This study has several limitations. First, while the mouse model replicates key pathological features of STC, differences in physiological environments between mice and humans may affect the clinical translatability of the results. Second, although the cell lines used are representative, the in vitro environment lacks intricate cellular interactions and signaling pathways present in the in vivo microenvironment. Future studies will prioritize the use of human-relevant models, such as organoids, and conduct multicenter clinical trials to further validate the clinical application of HQD.
In summary, this study indicates that HQD can improve intestinal motility dysfunction in the STC mouse model, promote intestinal mucosal barrier repair, and alleviate inflammation in intestinal mucosa epithelium. The protective effects of HQD may be mediated through the inhibition of the NF-κB signaling pathway.
| 1. | Mugie SM, Benninga MA, Di Lorenzo C. Epidemiology of constipation in children and adults: a systematic review. Best Pract Res Clin Gastroenterol. 2011;25:3-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 665] [Cited by in RCA: 567] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 2. | Rao SS, Rattanakovit K, Patcharatrakul T. Diagnosis and management of chronic constipation in adults. Nat Rev Gastroenterol Hepatol. 2016;13:295-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 222] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 3. | Yang L, Wang Y, Zhang Y, Li W, Jiang S, Qian D, Duan J. Gut microbiota: a new avenue to reveal pathological mechanisms of constipation. Appl Microbiol Biotechnol. 2022;106:6899-6913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 4. | Wang JK, Yao SK. [Research Progress of Intestinal Mucosal Barrier Function in Chronic Constipation]. Yixue Zongshu. 2021;27:4070-4075. [DOI] [Full Text] |
| 5. | Jensen SK, Pærregaard SI, Brandum EP, Jørgensen AS, Hjortø GM, Jensen BAH. Rewiring host-microbe interactions and barrier function during gastrointestinal inflammation. Gastroenterol Rep (Oxf). 2022;10:goac008. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 6. | Li T, Hu M, Jiang C, Zhang D, Gao M, Xia J, Miao M, Shi G, Li H, Zhang J, Yin Z. Laxative effect and mechanism of Tiantian Capsule on loperamide-induced constipation in rats. J Ethnopharmacol. 2021;266:113411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Li C, Nie SP, Zhu KX, Xiong T, Li C, Gong J, Xie MY. Effect of Lactobacillus plantarum NCU116 on loperamide-induced constipation in mice. Int J Food Sci Nutr. 2015;66:533-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 8. | Zhai X, Lin D, Zhao Y, Yang X. Bacterial Cellulose Relieves Diphenoxylate-Induced Constipation in Rats. J Agric Food Chem. 2018;66:4106-4117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 9. | Wen Y, Zhan Y, Tang SY, Liu F, Wang QX, Kong PF, Tang XG. Zhizhu Decoction Alleviates Intestinal Barrier Damage via Regulating SIRT1/FoxO1 Signaling Pathway in Slow Transit Constipation Model Mice. Chin J Integr Med. 2023;29:809-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 10. | Wang X, Guo R, Yu Z, Zikela L, Li J, Li S, Han Q. Torreya grandis Kernel Oil Alleviates Loperamide-Induced Slow Transit Constipation via Up-Regulating the Colonic Expressions of Occludin/Claudin-1/ZO-1 and 5-HT3R/5-HT4R in BALB/c Mice. Mol Nutr Food Res. 2024;68:e2300615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 11. | Kim JE, Song HJ, Choi YJ, Jin YJ, Roh YJ, Seol A, Park SH, Park JM, Kang HG, Hwang DY. Improvement of the intestinal epithelial barrier during laxative effects of phlorotannin in loperamide-induced constipation of SD rats. Lab Anim Res. 2023;39:1. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 12. | Fan YB, Wu ZJ. [Clinical Research Progress on Traditional Chinese Medicine Therapy for Slow Transit Constipation]. Liaoning Zhongyiyao Daxue Xuebao. 2013;15:266-269. [DOI] [Full Text] |
| 13. | Wang Y, Li C, Song SH, Zhang YM. [Research on Key Information of Classic Famous Prescription Huangqitang and Its Modern Clinical Application]. Zhongguo Shiyan Fangjixue Zazhi. 2024;30:187-196. [DOI] [Full Text] |
| 14. | He FH, Liu YZ, Wu Y, Liang LJ, Xu XM, Xie ZH. [Clinical study on modified Huangqi decoction in treating senile functional constipation of qi deficiency type]. Zhongyaocai. 2015;38:410-412. [DOI] [Full Text] |
| 15. | Lu Q, Huang ZW, Ye MM. [Treatment of functional constipation in elderly patients with the TCM comprehensive therapy based on modified Huangqi decoction]. Jilin Zhongyiyao. 2023;43:1316-1320. [DOI] [Full Text] |
| 16. | Liao YY, Chen HY, Li WL, Wei ZJ, Zhang CB, Zhang Y, Huang ZF, Zhang JR, Li JL. [Therapeutic effect of food therapy combined with Jiajian Huangqi Decoction on senile functional constipation]. Tianjin Zhongyiyao. 2019;36:153-155. [DOI] [Full Text] |
| 17. | He Q, Han C, Huang L, Yang H, Hu J, Chen H, Dou R, Ren D, Lin H. Astragaloside IV alleviates mouse slow transit constipation by modulating gut microbiota profile and promoting butyric acid generation. J Cell Mol Med. 2020;24:9349-9361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 18. | Chen H, Wan X, He Q, Xiao G, Zheng Y, Luo M, Yang C, Ren D, Lu L, Peng H, Lin H. Single-cell RNA sequencing reveals cellular dynamics and therapeutic effects of astragaloside IV in slow transit constipation. Biomol Biomed. 2024;24:871-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 19. | Yu KQ, Wu J, Gong HL, He HB, Yang CM. Systematic evaluation on Chinese patent medicine or prescription based on Astragalus membranaceus for functional constipation with qi deficiency type. Tianjin Zhongyiyao. 2019;14:1360-1367. [DOI] [Full Text] |
| 20. | Stuart T, Butler A, Hoffman P, Hafemeister C, Papalexi E, Mauck WM 3rd, Hao Y, Stoeckius M, Smibert P, Satija R. Comprehensive Integration of Single-Cell Data. Cell. 2019;177:1888-1902.e21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10603] [Cited by in RCA: 9430] [Article Influence: 1571.7] [Reference Citation Analysis (0)] |
| 21. | Becht E, McInnes L, Healy J, Dutertre CA, Kwok IWH, Ng LG, Ginhoux F, Newell EW. Dimensionality reduction for visualizing single-cell data using UMAP. Nat Biotechnol. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2977] [Cited by in RCA: 2861] [Article Influence: 408.7] [Reference Citation Analysis (0)] |
| 22. | Wu T, Hu E, Xu S, Chen M, Guo P, Dai Z, Feng T, Zhou L, Tang W, Zhan L, Fu X, Liu S, Bo X, Yu G. clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation (Camb). 2021;2:100141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 522] [Cited by in RCA: 4723] [Article Influence: 1180.8] [Reference Citation Analysis (0)] |
| 23. | Garcia-Alonso L, Holland CH, Ibrahim MM, Turei D, Saez-Rodriguez J. Benchmark and integration of resources for the estimation of human transcription factor activities. Genome Res. 2019;29:1363-1375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 432] [Cited by in RCA: 557] [Article Influence: 92.8] [Reference Citation Analysis (0)] |
| 24. | Qiu X, Mao Q, Tang Y, Wang L, Chawla R, Pliner HA, Trapnell C. Reversed graph embedding resolves complex single-cell trajectories. Nat Methods. 2017;14:979-982. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2722] [Cited by in RCA: 2806] [Article Influence: 350.8] [Reference Citation Analysis (0)] |
| 25. | Cao J, Spielmann M, Qiu X, Huang X, Ibrahim DM, Hill AJ, Zhang F, Mundlos S, Christiansen L, Steemers FJ, Trapnell C, Shendure J. The single-cell transcriptional landscape of mammalian organogenesis. Nature. 2019;566:496-502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2656] [Cited by in RCA: 2456] [Article Influence: 409.3] [Reference Citation Analysis (0)] |
| 26. | Chang Z, Li H. KLF9 deficiency protects the heart from inflammatory injury triggered by myocardial infarction. Korean J Physiol Pharmacol. 2023;27:177-185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 27. | Hoesel B, Schmid JA. The complexity of NF-κB signaling in inflammation and cancer. Mol Cancer. 2013;12:86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2313] [Cited by in RCA: 2526] [Article Influence: 210.5] [Reference Citation Analysis (0)] |
| 28. | Wong RWJ, Tan TK, Amanda S, Ngoc PCT, Leong WZ, Tan SH, Asamitsu K, Hibi Y, Ueda R, Okamoto T, Ishida T, Iida S, Sanda T. Feed-forward regulatory loop driven by IRF4 and NF-κB in adult T-cell leukemia/lymphoma. Blood. 2020;135:934-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 29. | Ihara E, Manabe N, Ohkubo H, Ogasawara N, Ogino H, Kakimoto K, Kanazawa M, Kawahara H, Kusano C, Kuribayashi S, Sawada A, Takagi T, Takano S, Tomita T, Noake T, Hojo M, Hokari R, Masaoka T, Machida T, Misawa N, Mishima Y, Yajima H, Yamamoto S, Yamawaki H, Abe T, Araki Y, Kasugai K, Kamiya T, Torii A, Nakajima A, Nakada K, Fukudo S, Fujiwara Y, Miwa H, Kataoka H, Nagahara A, Higuchi K. Evidence-Based Clinical Guidelines for Chronic Constipation 2023. Digestion. 2025;106:62-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Reference Citation Analysis (0)] |
| 30. | Wang L, Wu F, Hong Y, Shen L, Zhao L, Lin X. Research progress in the treatment of slow transit constipation by traditional Chinese medicine. J Ethnopharmacol. 2022;290:115075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 32] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 31. | Lin LW, Fu YT, Dunning T, Zhang AL, Ho TH, Duke M, Lo SK. Efficacy of traditional Chinese medicine for the management of constipation: a systematic review. J Altern Complement Med. 2009;15:1335-1346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 32. | Tuohongerbieke A, Wang H, Wu J, Wang Z, Dong T, Huang Y, Zhu D, Sun D, Tsim KWK. Xiao Cheng Qi Decoction, an Ancient Chinese Herbal Mixture, Relieves Loperamide-Induced Slow-Transit Constipation in Mice: An Action Mediated by Gut Microbiota. Pharmaceuticals (Basel). 2024;17:153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 33. | Sánchez de Medina F, Romero-Calvo I, Mascaraque C, Martínez-Augustin O. Intestinal inflammation and mucosal barrier function. Inflamm Bowel Dis. 2014;20:2394-2404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 313] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 34. | Luissint AC, Parkos CA, Nusrat A. Inflammation and the Intestinal Barrier: Leukocyte-Epithelial Cell Interactions, Cell Junction Remodeling, and Mucosal Repair. Gastroenterology. 2016;151:616-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 395] [Article Influence: 43.9] [Reference Citation Analysis (0)] |
| 35. | Mokhtare M, Alimoradzadeh R, Agah S, Mirmiranpour H, Khodabandehloo N. The Association between Modulating Inflammatory Cytokines and Constipation of Geriatrics in Iran. Middle East J Dig Dis. 2017;9:228-234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 36. | Zhan Y, Wen Y, Du LJ, Wang XX, Tang SY, Kong PF, Huang WG, Tang XG. Effects of Maren Pills on the Intestinal Microflora and Short-Chain Fatty Acid Profile in Drug-Induced Slow Transit Constipation Model Rats. Front Pharmacol. 2022;13:804723. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 37. | Kim JE, Lee MR, Park JJ, Choi JY, Song BR, Son HJ, Choi YW, Kim KM, Hong JT, Hwang DY. Quercetin promotes gastrointestinal motility and mucin secretion in loperamide-induced constipation of SD rats through regulation of the mAChRs downstream signal. Pharm Biol. 2018;56:309-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 38. | Chen S, Liu H, Li Z, Tang J, Huang B, Zhi F, Zhao X. Epithelial PBLD attenuates intestinal inflammatory response and improves intestinal barrier function by inhibiting NF-κB signaling. Cell Death Dis. 2021;12:563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 39. | Ryu M, Kim EH, Chun M, Kang S, Shim B, Yu YB, Jeong G, Lee JS. Astragali Radix elicits anti-inflammation via activation of MKP-1, concomitant with attenuation of p38 and Erk. J Ethnopharmacol. 2008;115:184-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 117] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 40. | Luo Q, Yan X, Bobrovskaya L, Ji M, Yuan H, Lou H, Fan P. Anti-neuroinflammatory effects of grossamide from hemp seed via suppression of TLR-4-mediated NF-κB signaling pathways in lipopolysaccharide-stimulated BV2 microglia cells. Mol Cell Biochem. 2017;428:129-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 41. | Sun LL, Jiang HB, Liu BY, Li WD, Du AL, Luo XQ, Li XQ. Effects of rhein on intestinal transmission, colonic electromyography and expression of aquaporin-3 by colonic epithelium cells in constipated mice. Int J Clin Exp Pathol. 2018;11:614-623. [PubMed] |
| 42. | Yuan WT, Yang HF, Zhang ZY, Liu JB. [Expression and significance of aquaporin 3 and aquaporin 9 in colonic mucosa of patients with functional constipation]. Zhonghua Wei Chang Wai Ke Za Zhi. 2008;11:57-60. [PubMed] [DOI] [Full Text] |
| 43. | Zhi H, Yuan WT. [Expression of aquaporin 3, 4, and 8 in colonic mucosa of rat models with slow transit constipation]. Zhonghua Wei Chang Wai Ke Za Zhi. 2011;14:459-461. [PubMed] [DOI] [Full Text] |
| 44. | Zhan Y, Tang X, Xu H, Tang S. Maren Pills Improve Constipation via Regulating AQP3 and NF-κB Signaling Pathway in Slow Transit Constipation In Vitro and In Vivo. Evid Based Complement Alternat Med. 2020;2020:9837384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |