Published online Dec 27, 2022. doi: 10.4240/wjgs.v14.i12.1350
Peer-review started: September 16, 2022
First decision: October 20, 2022
Revised: October 29, 2022
Accepted: December 6, 2022
Article in press: December 6, 2022
Published online: December 27, 2022
Processing time: 102 Days and 4.9 Hours
The only potential curative treatment for patients with pancreatic cancer is sur
To elucidate the associations of body composition measures derived from pre
One hundred fifteen patients undergoing pancreatic resection with curative intent for pancreatic cancer were retrospectively enrolled. A preoperative CT scan at the third lumbar vertebral level was performed to measure the skeletal muscle index (SMI), mean skeletal muscle radiodensity, subcutaneous adipose tissue index, and visceral to subcutaneous adipose tissue area ratio. The clinical and pathological data were collected. The effects of these factors on long-term survival were eva
Among the five body composition measures, only low SMI independently pre
Low preoperative SMI was more prevalent in elderly patients and was associated with a poor prognosis among pancreatic cancer patients, especially elderly patients.
Core Tip: Measures of body composition have been regarded as a reliable prognostic predictor for cancer patients after surgery, but further research is needed. In this study, we showed that low preoperative skeletal muscle index (SMI) potentially predicts the prognosis of pancreatic cancer patients. We also revealed that low SMI was more prevalent in elderly patients and was associated with a poor prognosis among elderly patients after pancreatic cancer resection.
- Citation: Cai ZW, Li JL, Liu M, Wang HW, Jiang CY. Low preoperative skeletal muscle index increases the risk of mortality among resectable pancreatic cancer patients: A retrospective study. World J Gastrointest Surg 2022; 14(12): 1350-1362
- URL: https://www.wjgnet.com/1948-9366/full/v14/i12/1350.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v14.i12.1350
Pancreatic cancer is among the most aggressive malignancies, representing one of the leading causes of cancer-related deaths worldwide[1,2]. The only potential curative treatment modality for patients with pancreatic cancer currently available is surgery. However, approximately 80% of patients are estimated to present with either unresectable or metastatic disease at the time of the first admission[3]. In addition, the prognosis remains poor even for the small subset of patients with a localized, resectable tumor, with only 20% surviving for 5 years following surgery[4]. In this context, multiple factors, such as the tumor status, administration of adjuvant chemotherapy, and surgical radicality, have been recognized as tumor-related prognostic factors. Therefore, the characterization of these prognostic factors may help stratify patients for better individualized treatment and improve long-term survival outcomes.
According to the literature, body mass index (BMI) calculated from height and weight is a valuable indicator of body size. Recently, the relationship between obesity and pancreatic cancer has been extensively studied[5]. Data derived from clinical trials and meta-analyses have consistently shown that obesity (BMI > 25 kg/m2) is associated with poor survival outcomes, but some studies have reported inconsistent associations with overweight or lower levels of obesity[6-8]. This discrepancy may be explained by the fact that BMI measures the relation of weight to height without assessing individual components of the body, such as muscle and adipose tissue, or components of weight with differing associations with survival.
Currently, a paucity of studies have explored measures of body composition mainly in patients with resectable pancreatic cancer[9-11]. Sarcopenia, an age-dependent decrease in skeletal muscle volume, was initially described in 1989[12]. Based on the recent consensus from the European Working Group on Sarcopenia in Older People and the Asian Working Group for Sarcopenia, computed tomography (CT) imaging at the level of the third lumbar vertebra is an effective imaging modality for the clinical detection of sarcopenia[13,14]. Several previous studies have documented that sarcopenia, which is mainly established by the presence of low muscle quantity and quality, is significantly associated with a poor prognosis for advanced pancreatic cancer patients[15-20].
Furthermore, numerous epidemiological and fundamental studies have provided evidence to support a possible link between obesity and pancreatic cancer[8,21,22]. Measures of body composition reflecting obesity, including visceral obesity [namely, a high visceral adipose tissue index (VATI)] and low skeletal muscle radiodensity (SMD) (a measure of muscle quality indicative of adipose tissue deposition in muscle fibers and reduced function), have been established as useful prognostic indicators[9-11,23]. Sarcopenia was recently identified as an independent prognostic factor, especially for elderly patients with esophageal cancer[24]. However, at present, no similar studies have been performed in patients with resectable pancreatic cancer. Therefore, we examined associations between cancer prognosis and the measures of body composition, including muscle mass, muscle radiodensity, and adiposity, in a retrospective cohort. Specifically, we analyzed the effects of measures of body composition on the prognosis of patients in different age groups.
A retrospective review was performed on the records from all patients who underwent pancreatectomy for resectable pancreatic ductal adenocarcinoma (PDAC) at a single institution from January 2015 to December 2019. Patients with abdominal CT scans captured within 1 wk before surgery that were available for analysis were included. Patients who had a history of abdominal surgery were excluded from the study. A total of 115 patients were finally enrolled in this study. Preoperative demographics, body weight, height, and laboratory data, including leukocytes, neutrophils, lymphocytes, platelets, albumin, and carbohydrate antigen 19-9 levels, were obtained from the electronic media database. Systemic inflammatory indicators were defined as follows: neutrophil-lymphocyte ratio (absolute neutrophil count divided by absolute lymphocyte count), platelet-to-lymphocyte ratio (absolute platelet count divided by absolute lymphocyte count), and systemic immune-inflammation index (SIII, platelet count × neutrophil-lymphocyte ratio). Tumor size, tumor location, pathological TNM staging (according to AJCC 8th edition), tumor differentiation grade, presence of perineural invasion, status of the resection margin, lymph node metastasis, and adjuvant chemotherapy were also recorded for analysis. This study was approved by the Institutional Review Board of Huadong Hospital Affiliated to Fudan University.
Cross-sectional CT images of the third lumbar vertebra were analyzed using Slice-O-Matic software (v.5. Tomovision, Montreal, Quebec, Canada)[17,25]. In detail, various body composition parameters were measured, including subcutaneous adipose tissue area, visceral adipose tissue area, and skeletal muscle area. The following tissue Hounsfield unit (HU) thresholds were employed: -190 to -30 for subcutaneous adipose tissue, -150 to -50 for visceral adipose tissue, and -29 to 150 for skeletal muscle. As described previously, the body composition evaluation included the subcutaneous adipose tissue index (SATI), VATI, and skeletal muscle index (SMI), which were named and calculated from subcutaneous adipose tissue area, visceral adipose tissue area, and skeletal muscle area divided by height in meters squared (cm2/m2), respectively[9]. The visceral to subcutaneous adipose tissue area ratio (VSR) was calculated to assess the abdominal adipose tissue distribution, and skeletal muscle radiodensity was measured from the mean CT value (HU) of the whole skeletal muscle area to assess muscle quality. This procedure was performed by two experienced investigators who were blinded to the clinical characteristics of the participants.
Based on the considerable differences in body composition between sexes, sex-specific cutoff values were established using receiver operating characteristic curves for each body composition parameter[10,26]. The cutoff values were selected based on the best accuracy of 1-year mortality; thus, the cutoff value for SMI (cm2/m2) in males was 45.16 [area under the curve (AUC) = 0.650] and 34.65 (AUC = 0.698) in females. The cutoff values for mean muscle radiodensity (HU) in males and females were 35.8 (AUC = 0.538) and 30.47 (AUC = 0.793), respectively. The SMI and mean muscle radiodensity cutoff points possessing the maximum absolute value of the log-rank statistic were used to establish the incidence of low SMI and low SMD. In addition, the cutoff values were 0.86 (AUC = 0.574) and 1.06 (AUC = 0.639) for the VSR, 28.70 (AUC = 0.639) and 49.72 (AUC = 0.691) for the VATI (cm2/m2), and 43.99 (AUC = 0.599) and 48.76 (AUC = 0.635) for the SATI (cm2/m2) in males and females, respectively. The classifications of underweight, normal weight, and obesity were based on definitions applied to Asian populations[9]. The analyses were performed using the following BMI categories: < 20.0 kg/m2, underweight; 20.0-24.9 kg/m2, normal weight; and ≥ 25.0 kg/m2, obese. According to the World Health Organization, 65 years was the established cutoff to define people as elderly[27].
Patient characteristics were summarized as counts and percentages for categorical variables and means and standard deviations for continuous variables. Differences in variables between groups were analyzed using Pearson’s χ2test for categorical variables or the independent t test for continuous variables. Overall survival (OS) was defined as the interval from the first diagnosis until death, and recurrence-free survival (RFS) was calculated from the date of surgery to the date of recurrence or metastasis. Cumulative OS and RFS were estimated using the Kaplan-Meier method, and differences between groups were calculated using the log-rank test. The optimal high vs low values of inflammatory indicators were defined by examining a grid of cutoff values and choosing the cutoff value with the lowest -2 log-likelihood[28]. The effects of body composition parameters and clinicopathological factors on OS or RFS were evaluated using a Cox proportional hazards model. All variables with P < 0.05 in the univariate analysis were included in the multivariate analysis. The results were presented as hazard ratios (HRs) with 95% confidence intervals (CIs). All statistical analyses were conducted using SPSS (SPSS 25.0, IBM Inc., Chicago, IL, United States) and GraphPad Prism (GraphPad Software 8.0.1, San Diego, CA, United States). P values < 0.05 were considered statistically significant.
The clinicopathological characteristics of the 115 enrolled patients were summarized (Table 1). According to the sex-specific cutoff values, 38 (33.3%) patients were characterized by low SMI, whereas 26 (22.6%) patients were characterized by low SMD. Patients with low SMI were older, more likely to be male, had poorly differentiated carcinoma, and had a tumor located in the pancreatic head compared to those with high SMI. Additionally, patients with low SMI more frequently had lower albumin levels. In addition, an older age and perineural invasion were highly predominant among patients with low SMD. Notably, we observed that neither SMI nor SMD was significantly associated with the TNM stage or indicators of systemic inflammation, such as the neutrophil-lymphocyte ratio, platelet-to-lymphocyte ratio, and SIII.
| Characteristics | Total (n = 115) | Low SMI | High SMI | P value | Low SMD | High SMD | P value |
| Age | 65.1 (9.0) | 68.8 (9.3) | 63.3 (8.4) | 0.002b | 70.8 (7.0) | 63.5 (8.9) | < 0.001c |
| Male sex | 71 (61.7) | 29 (76.3) | 42 (54.5) | 0.024a | 13 (50.0) | 58 (65.2) | 0.162 |
| BMI in kg/m2 | 22.7 (3.3) | 20.9 (3.1) | 23.6 (3.0) | < 0.001c | 24.0 (4.0) | 22.3 (2.9) | 0.016a |
| < 20.0 | 21(18.3) | 14 (36.8) | 7 (9.1) | 5 (19.2) | 16 (18.0) | ||
| 20.0-24.9 | 69 (60.0) | 20 (52.6) | 49 (63.6) | 10 (38.5) | 59 (66.3) | ||
| ≥ 25.0 | 25 (21.7) | 4 (10.5) | 21 (27.3) | 0.001b | 11 (42.3) | 14 (15.7) | 0.01a |
| Albumin in g/dL | 42.5 (5.6) | 40.6 (4.1) | 43.5 (6.1) | 0.01a | 41.7 (7.7) | 42.8 (4.9) | 0.393 |
| Tumor location | |||||||
| Head | 67 (58.3) | 29 (76.3) | 38 (49.4) | 18 (69.2) | 49 (55.1) | ||
| Body + tail | 48 (41.7) | 9 (23.7) | 39 (50.6) | 0.006b | 8 (30.8) | 40 (44.9) | 0.197 |
| Tumor size in cm | 3.6 (1.4) | 3.7 (1.7) | 3.3 (1.2) | 0.942 | 3.3 (1.3) | 3.6 (1.4) | 0.602 |
| Differentiation | |||||||
| Well | 5 (4.3) | 1 (2.7) | 4 (5.3) | 3 (12.0) | 4 (4.6) | ||
| Moderate | 100 (87.0) | 31 (83.8) | 69 (92.0) | 19 (76.0) | 81 (93.1) | ||
| Poor | 7 (6.1) | 5 (13.5) | 2 (2.7) | 0.04a | 3 (12.0) | 2 (2.3) | 0.774 |
| Nodal metastases | 64 (55.7) | 20 (52.6) | 44 (57.1) | 0.647 | 12 (46.2) | 52 (58.4) | 0.268 |
| Perineural invasion1 | 81 (86.2) | 27 (87.1) | 54 (85.7) | 0.855 | 25 (100) | 56 (81.2) | 0.046a |
| R1 resection | 13 (11.3) | 3 (7.9) | 10 (13.2) | 0.602 | 3 (11.5) | 10 (11.4) | 1.000 |
| Adjuvant therapy | 84 (73.0) | 24 (63.2) | 60 (77.9) | 0.093 | 18 (69.2) | 66 (74.2) | 0.618 |
| TNM stage | |||||||
| I | 43 (37.4) | 14 (36.8) | 29 (37.7) | 14 (53.8) | 29 (32.6) | ||
| II | 57 (49.6) | 19 (50.0) | 38 (49.4) | 10 (38.5) | 47 (52.8) | ||
| III | 15 (13.0) | 5 (13.1) | 10 (13.0) | 0.996 | 2 (7.6) | 13 (14.6) | 0.135 |
| CA19-9 > 200 KU/L | 71 (61.7) | 24 (66.7) | 47 (63.5) | 0.746 | 21 (84.0) | 50 (58.8) | 0.021a |
| SMA in cm2 | 123.0 (30.6) | 108.4 (19.9) | 130.2 (32.4) | < 0.001c | 113.2 (28.4) | 125.9 (30.7) | 0.056 |
| SMD in HU | 39.4 (8.6) | 38.5 (10.0) | 39.8 (7.8) | 0.462 | 28.5 (5.1) | 42.6 (6.5) | < 0.001c |
| SMI in cm2/m2 | 44.6 (9.8) | 38.1 (5.3) | 47.7 (10.0) | < 0.001c | 41.1 (9.0) | 45.6 (9.9) | 0.033a |
| VAT in cm2 | 113.4 (66.5) | 89 (50.2) | 125.4 (70.5) | < 0.001c | 151.0 (60.2) | 102.4 (64.5) | 0.001b |
| SAT in cm2 | 121.4 (64.6) | 100.5 (45.4) | 131.7 (70.2) | 0.014a | 160.0 (89.3) | 110.1 (50.7) | < 0.001c |
| NLR > 2.6 | 44 (38.3) | 16 (42.1) | 28 (36.4) | 0.551 | 13 (50.0) | 31 (34.8) | 0.162 |
| PLR > 108 | 77 (67.0) | 27 (71.1) | 50 (64.9) | 0.512 | 21 (80.8) | 56 (62.9) | 0.089 |
| SIII > 400 | 68 (59.1) | 24 (63.2) | 44 (57.1) | 0.537 | 17 (65.4) | 51 (57.3) | 0.461 |
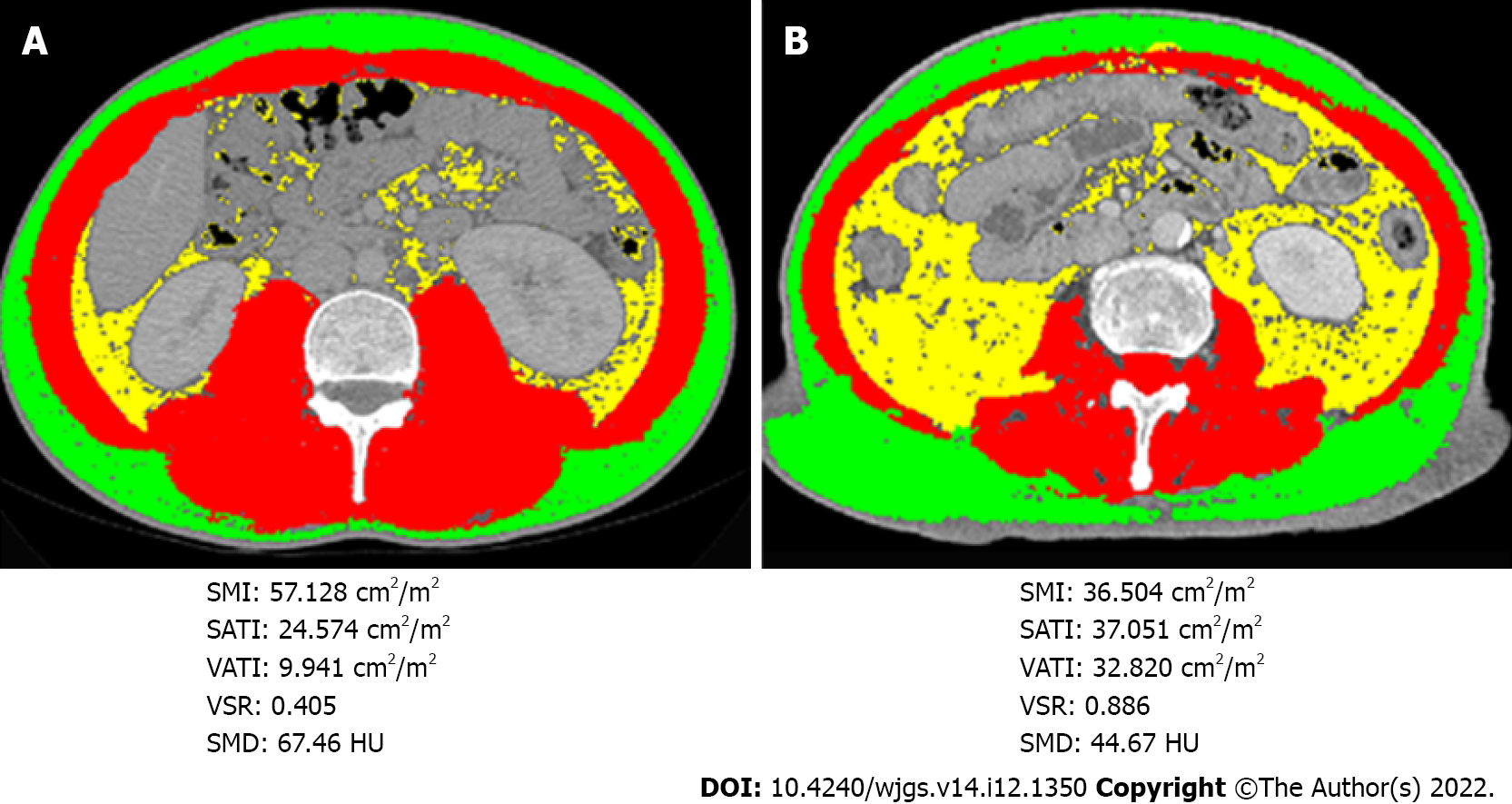
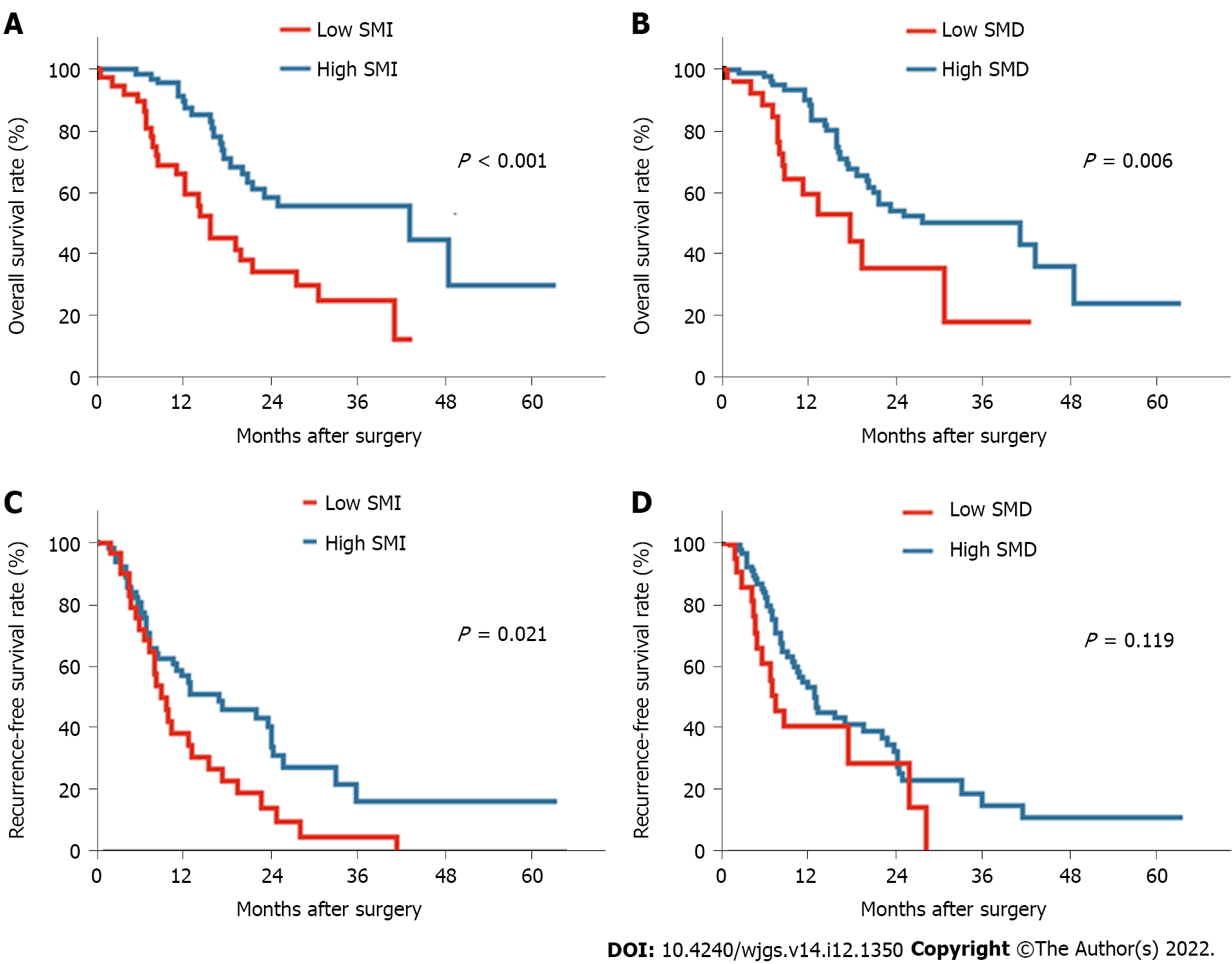
First, the prognostic value of the widely used measurement BMI was assessed. Both obesity and underweight were related to a worse prognosis for pancreatic cancer patients than normal weight; however, a significant difference in survival was not observed between the obese and underweight groups (Supplementary Figure 1). Notably, two normal weight patients with similar BMIs showed rather different body composition parameters from CT-based measurements, including SMI, SMD, VATI, SATI, and VSR (Figure 1). These findings indicated that BMI may not be an appropriate indicator to assess the correlation between body composition and the prognosis. Thus, the correlation between body composition parameters based on CT scans and the survival prognosis was further evaluated. Patients with low preoperative SMI and low SMD experienced a shorter OS than those with high SMI (P < 0.001; Figure 2A) and high SMD (P = 0.006; Figure 2B). Shorter RFS was also observed among patients with low SMI (P = 0.021; Figure 2C) but not among those with low SMD (P = 0.119; Figure 2D). Meanwhile, high VATI, high SATI, and high VSR were not related to either poor OS or RFS outcomes (Supplementary Figure 2).
In the univariate risk analysis of factors associated with mortality (Table 2), low SMI, low SMD, high carbohydrate antigen 19-9 levels, high platelet-to-lymphocyte ratio, high SIII, lymph node metastasis, and absence of adjuvant chemotherapy were identified as risk factors for mortality after tumor resection. Remarkably, low SMI remained an independent risk factor for mortality in the multivariate analysis (HR: 2.307; 95%CI: 1.210-4.402; P = 0.011), indicating that a low muscle quantity was a significant risk factor for mortality. We also observed higher mortality among patients with low SMD, although this finding was not significant (HR: 2.093; 95%CI: 1.000-4.379; P = 0.050).
| Factor | Univariate analysis | P value | Multivariate analysis | P value |
| HR (95%CI) | HR (95%CI) | |||
| Age ≥ 65 yr | 1.661 (0.905-3.051) | 0.102 | ||
| Male sex | 0.686 (0.371-1.267) | 0.228 | ||
| BMI < 20.0 underweight | 0.534 (0.262-1.085) | 0.083 | ||
| BMI ≥ 25.0 obesity | 0.631 (0.322-1.233) | 0.178 | ||
| Albumin < 3.8 g/dL | 1.146 (0.550-2.388) | 0.716 | ||
| Low SMI | 2.805 (1.559-5.045) | 0.001b | 2.307 (1.210-4.402) | 0.011a |
| Low SMD | 2.395 (1.261-4.550) | 0.008b | 2.093 (1.000-4.379) | 0.05 |
| Tumor location, head | 1.620 (0.870-3.019) | 0.128 | ||
| Tumor size > 2 cm | 1.107 (0.494-2.480) | 0.805 | ||
| Differentiation poor | 3.102 (0.947-10.158) | 0.061 | ||
| Nodal metastases | 1.853 (1.022-3.358) | 0.042a | 2.308 (1.200-4.442) | 0.012a |
| Perineural invasion | 2.303 (0.701-7.564) | 0.169 | ||
| R1 Resection | 1.165 (0.415-3.267) | 0.772 | ||
| TNM stage III | 1.515 (0.673-3.414) | 0.316 | ||
| Adjuvant therapy | 0.528 (0.284-0.980) | 0.043a | 0.480 (0.242-0.952) | 0.036a |
| CA19-9 > 200 KU/L | 1.891 (1.021-3.504) | 0.043a | 0.851 (0.428-1.689) | 0.644 |
| NLR > 2.6 | 1.630 (0.911-2.916) | 0.099 | ||
| PLR > 108 | 2.208 (1.150-4.238) | 0.017a | 1.296 (0.517-3.250) | 0.58 |
| SIII > 400 | 1.911 (1.044-3.500) | 0.036a | 1.338 (0.586-3.057) | 0.489 |
Furthermore, low SMI, lymph node metastasis, and high SIII, but not low SMD, were identified as risk factors based on the univariate analysis of risk factors associated with RFS (Table 3). The multivariate analysis showed that low SMI continued to be independently associated with tumor recurrence (HR: 1.907; 95%CI: 1.147-3.171; P = 0.013).
| Factor | Univariate analysis | P value | Multivariate analysis | P value |
| HR (95%CI) | HR (95%CI) | |||
| Age > 65 yr | 1.661 (0.905-3.051) | 0.102 | ||
| Male sex | 0.631 (0.382-1.041) | 0.071 | ||
| BMI < 20.0 underweight | 1.216 (0.660-2.240) | 0.530 | ||
| BMI ≥ 25.0 obesity | 1.823 (0.969-3.430) | 0.062 | ||
| Albumin < 3.8 g/dL | 0.871 (0455-1.668) | 0.677 | ||
| Low SMI | 1.784 (1.083-2.939) | 0.023a | 1.907 (1.147-3.171) | 0.013a |
| Low SMD | 1.567 (0.885-2.773) | 0.123 | ||
| Tumor location, head | 1.327 (0.795-2.215) | 0.278 | ||
| Tumor size > 2 cm | 1.611 (0.767-3.382) | 0.208 | ||
| Differentiation poor | 0.978 (0.238-4.022) | 0.976 | ||
| Nodal metastases | 1.897 (1.134-3.174) | 0.015a | 1.922 (1.129-3.272) | 0.016a |
| Perineural invasion | 1.886 (0.841-4.231) | 0.124 | ||
| R1 Resection | 1.593 (0.754-3.365) | 0.222 | ||
| TNM stage III | 1.703 (0.833-3.483) | 0.144 | ||
| Adjuvant therapy | 0.866 (0.479-1.567) | 0.635 | ||
| CA19-9 in Ku/L > 200 | 1.439 (0.855-2.421) | 0.170 | ||
| NLR > 2.6 | 1.071 (0.648-1.769) | 0.789 | ||
| PLR > 108 | 1.612 (0.955-2.722) | 0.074 | ||
| SIII > 400 | 1.827 (1.097-3.043) | 0.021a | 1.655 (0.984-2.785) | 0.058 |
| Platelets > 310 × 109 | 1.245 (0.533-2.910) | 0.613 |
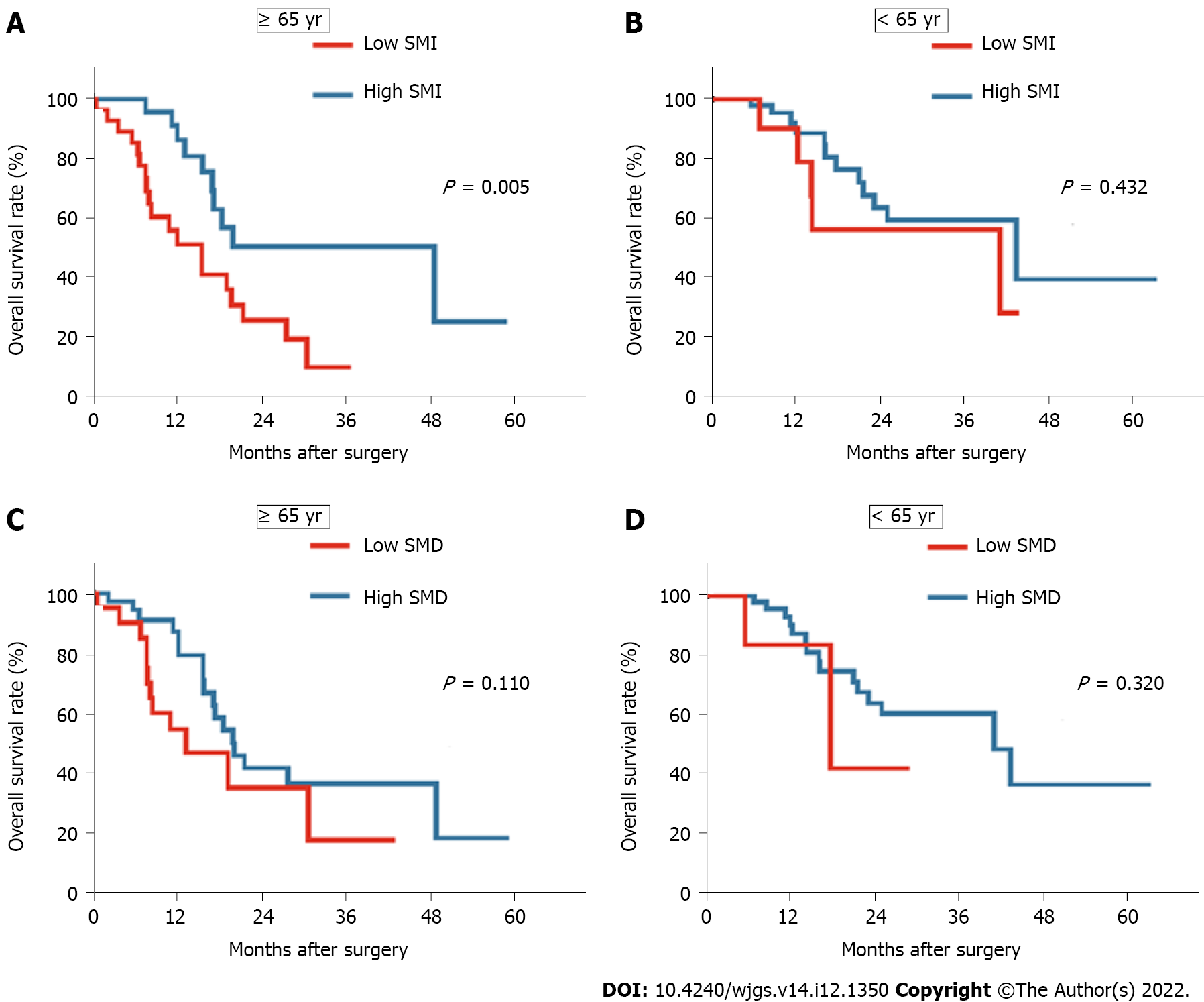
A significant difference in age was observed between the groups with and without low SMI or SMD. We performed subgroup analyses based on age and body composition to further elucidate the potential effect of body composition on the prognosis of elderly patients with PDAC. First, we identified that 48.2% (n = 27) of elderly patients and 18.6% (n = 11) of young patients were characterized by low SMI. In addition, 35.7% (n = 20) of elderly patients and 10.2% (n = 6) of young patients were identified as having low SMD. Compared to young patients, a larger proportion of patients aged 65 years and older had a low SMI or SMD. Moreover, among elderly patients, the presence of low SMI was associated with a significantly decreased OS compared with those with high SMI (P = 0.005; Figure 3A). Furthermore, an almost comparable OS was observed between groups with a high and low SMI among patients younger than 65 years (P = 0.432; Figure 3B). Meanwhile, among the different age groups, the OS and RFS rates in the low SMD group did not differ significantly from those in the high SMD group (P = 0.110 and P = 0.320, respectively; Figure 3C and D). These findings indicated that a low preoperative SMI was more prevalent in elderly patients and was associated with a poor prognosis among pancreatic cancer patients, especially elderly patients.
In this retrospective study, we assessed preoperative body composition measures from clinically acquired CT scans and comprehensively analyzed the effects of these measures on mortality and cancer recurrence in our single cohort of patients undergoing surgical resection for PDAC. Our findings revealed that low SMI was highly prevalent and associated with a significantly increased risk of death and cancer recurrence among pancreatic cancer patients. We also identified that elderly patients exhibited a higher risk of low SMI than younger patients. Based on the results of the subgroup analysis of mortality and recurrence stratified by age, we noted that low SMI was a valuable predictor of mortality, specifically in the elderly patient subgroup. Thus, prognostic measures may be easily integrated into routine clinical care using new software to generate a highly accurate measure of SMI from clinically collected CT scans.
Results from our cohort and some other cohorts of patients with resectable pancreatic cancer indicated that elderly patients are more vulnerable to sarcopenia (usually defined by a low SMI) than young patients[15,23,29]. Most experts believe that age-related sarcopenia is an inevitable part of aging[30]. The aging-related denervation process exerts a strong effect on quantitative changes in muscle, such as loss of muscle fibers and atrophy, and qualitative changes in muscle affecting protein function, the repair process, and coordinated contractility and resilience to stress, leading to a loss of muscle volume and function in older age[31]. However, our results were inconsistent with some findings from previous studies that no significant difference in age was observed between the subgroups with and without sarcopenia[10,32]. This difference may be attributed to the cutoff values used for low SMI. In addition, the cutoff value for sarcopenia in previous studies that showed between-cohort age-related differences were considerably lower than the corresponding value in studies that did not show any significant difference in age between sarcopenia and non-sarcopenia cohorts. Currently, no established consensus value is available for CT-based sarcopenia (namely, low SMI) in Asian populations. Therefore, more large-scale studies are needed in the future to establish a consensus cutoff value for sarcopenia in Asian populations and confirm these observations.
We further identified that low preoperative SMI was an independent prognostic factor for mortality and cancer recurrence for PDAC patients after pancreatectomy. These findings are consistent with previous reports describing the effect of low SMI on PDAC patients[10,15,32,33]. More importantly, we found that low SMI exerted a greater adverse effect on the prognosis among elderly patients. In contrast, no remarkable association between low SMI and mortality was identified among nonelderly patients. Likewise, Nakashima et al[24] revealed that low SMI was significantly associated with patients with esophageal cancer aged 65 years and older but not with those younger than 65 years[24].
Furthermore, comparable OS and RFS rates were observed among both elderly and young patients with low SMI. Additionally, low SMI contributes to a long-term prognosis that is similar to that of lymph node metastasis in elderly patients. Several studies have reported that sarcopenia is associated with insulin resistance, vitamin D deficiency, increased inflammatory cytokine levels, such as tumor necrosis factor-α and interleukin-6, and decreased concentrations of myokines, such as interleukin-15[34-37]. Notably, all of the aforementioned inflammatory cytokines are related to the progression of pancreatic cancer[38-42]. Thus, the quantity of skeletal muscle may be linked to the prognosis of patients with PDAC through various mechanisms.
Recently, with the gradual increase in the incidence of PDAC among elderly patients and the aging population, the number of pancreatic cancer patients with sarcopenia is estimated to increase steadily[43]. Therefore, an early preoperative diagnosis of low SMI/sarcopenia coupled with early intervention is essential for elderly patients to extend their OS and simultaneously promote a good quality of life, which would also conserve large amounts of medical resources.
In this study, we did not observe a relationship between survival and SMD (a surrogate of muscle quality) in patients with pancreatic cancer. To our knowledge, only one previous study of patients with unresectable pancreatic cancer and distal cholangiocarcinoma reported a significant relationship between SMD and the tumor prognosis[11]. In that study, low SMD was defined operationally as a mean skeletal muscle radiodensity of < 33 HU in patients with a BMI ≥ 25 kg/m2 and < 41 HU in patients with a BMI < 25 kg/m2 across the orthogonal view. Moreover, the prevalence of low SMD (55.3%) was much higher among patients in the previous study than among patients in the present study (22.6%). These results imply that fatty infiltration into muscle may be a hallmark of more advanced cancer but is not as predominant in cancer at an earlier stage.
However, despite these promising results, we acknowledge several limitations of the present study. First, this study was performed retrospectively at a single center with a relatively small sample size; hence, the potential for selection bias exists. Therefore, larger prospective cohort studies are needed to confirm these findings. Second, we must consider whether our cutoff values are adequate to define low SMI or sarcopenia. Although the cutoff value must be determined from a normal population of people of different ages, a unified standard is unavailable for the general Asian populations. Moreover, no specific cutoff values for pancreatic cancer were available to identify patients with sarcopenia. Here, we determined the cutoff values in this population using receiver operating characteristic curves, which is considered a more accurate method than the use of standard deviations to set cutoff values. However, more studies are still needed to explore more specific indicators that may reflect muscle function and are not limited by race or region.
In summary, low preoperative SMI, which is simply diagnosed through routine staging CT scans, was associated with a poor prognosis, especially among elderly patients with pancreatic cancer. Thus, the early identification of aging-specific factors, such as SMI, enables early interventions to ameliorate clinical outcomes in elderly patients with pancreatic cancer.
The only potential curative treatment for patients with pancreatic cancer is surgery; however, the prognosis remains poor.
Measures of body composition based on computed tomography (CT) scans have been established as reliable predictors of the prognosis of cancer patients after surgery, but further research focusing on pancreatic cancer is needed.
To elucidate the associations of body composition measures derived from preoperative CT scans with the prognosis of patients with pancreatic cancer.
One hundred fifteen patients undergoing pancreatic resection with curative intent for pancreatic cancer were retrospectively enrolled. The preoperative CT scan at the third lumbar vertebral level was measured for skeletal muscle index (SMI), mean skeletal muscle radiodensity, subcutaneous adipose tissue index, visceral adipose tissue index, and subcutaneous adipose tissue area ratio. The clinical and pathological data were collected. The effects of these factors on long-term survival were evaluated.
Among the five body composition measures, only low SMI independently predicted overall survival (OS) [hazard ratio (HR): 2.307; 95% confidence interval (CI): 1.210-4.402] and recurrence-free survival (HR: 1.907; 95%CI: 1.147-3.171). Furthermore, patients with low SMI (vs high SMI) were older (68.8 ± 9.3 years vs 63.3 ± 8.4 years); low SMI was present in 27 of 56 patients (48.2%) aged 65 years and older and in 11 of 59 younger patients (18.6%). In addition, subgroup analyses revealed that the correlation between low SMI and OS was observed only in patients aged 65 years and older.
Low preoperative SMI was more prevalent in elderly patients and was associated with a poor prognosis among pancreatic cancer patients, especially elderly patients.
The early identification of aging-specific factors, such as low SMI, allows for the possibility to facilitate early interventions to ameliorate clinical outcomes in elderly patients with pancreatic cancer.
We sincerely thank Xin-Xin Xu and Dr. He Li for their guidance on this manuscript.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Pandey NM, India; Sato H, Japan S-Editor: Fan JR L-Editor: Filipodia P-Editor: Fan JR
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55828] [Article Influence: 7975.4] [Reference Citation Analysis (132)] |
| 2. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12667] [Cited by in RCA: 15314] [Article Influence: 3062.8] [Reference Citation Analysis (4)] |
| 3. | Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605-1617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2078] [Cited by in RCA: 2206] [Article Influence: 147.1] [Reference Citation Analysis (2)] |
| 4. | Mizrahi JD, Surana R, Valle JW, Shroff RT. Pancreatic cancer. Lancet. 2020;395:2008-2020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 869] [Cited by in RCA: 1673] [Article Influence: 334.6] [Reference Citation Analysis (1)] |
| 5. | Brown JC, Meyerhardt JA. Obesity and Energy Balance in GI Cancer. J Clin Oncol. 2016;34:4217-4224. [PubMed] |
| 6. | Greenlee H, Unger JM, LeBlanc M, Ramsey S, Hershman DL. Association between Body Mass Index and Cancer Survival in a Pooled Analysis of 22 Clinical Trials. Cancer Epidemiol Biomarkers Prev. 2017;26:21-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 139] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 7. | Mintziras I, Miligkos M, Wächter S, Manoharan J, Maurer E, Bartsch DK. Sarcopenia and sarcopenic obesity are significantly associated with poorer overall survival in patients with pancreatic cancer: Systematic review and meta-analysis. Int J Surg. 2018;59:19-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 147] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 8. | Majumder K, Gupta A, Arora N, Singh PP, Singh S. Premorbid Obesity and Mortality in Patients With Pancreatic Cancer: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2016;14:355-368.e; quiz e32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 9. | Fujiwara N, Nakagawa H, Kudo Y, Tateishi R, Taguri M, Watadani T, Nakagomi R, Kondo M, Nakatsuka T, Minami T, Sato M, Uchino K, Enooku K, Kondo Y, Asaoka Y, Tanaka Y, Ohtomo K, Shiina S, Koike K. Sarcopenia, intramuscular fat deposition, and visceral adiposity independently predict the outcomes of hepatocellular carcinoma. J Hepatol. 2015;63:131-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 571] [Cited by in RCA: 565] [Article Influence: 56.5] [Reference Citation Analysis (0)] |
| 10. | Okumura S, Kaido T, Hamaguchi Y, Kobayashi A, Shirai H, Yao S, Yagi S, Kamo N, Hatano E, Okajima H, Takaori K, Uemoto S. Visceral Adiposity and Sarcopenic Visceral Obesity are Associated with Poor Prognosis After Resection of Pancreatic Cancer. Ann Surg Oncol. 2017;24:3732-3740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 129] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 11. | Rollins KE, Tewari N, Ackner A, Awwad A, Madhusudan S, Macdonald IA, Fearon KC, Lobo DN. The impact of sarcopenia and myosteatosis on outcomes of unresectable pancreatic cancer or distal cholangiocarcinoma. Clin Nutr. 2016;35:1103-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 155] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 12. | Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr. 1997;127:990S-991S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1263] [Cited by in RCA: 1450] [Article Influence: 51.8] [Reference Citation Analysis (0)] |
| 13. | Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, Cooper C, Landi F, Rolland Y, Sayer AA, Schneider SM, Sieber CC, Topinkova E, Vandewoude M, Visser M, Zamboni M; Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16-31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6646] [Cited by in RCA: 7800] [Article Influence: 1300.0] [Reference Citation Analysis (1)] |
| 14. | Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS, Chou MY, Chen LY, Hsu PS, Krairit O, Lee JS, Lee WJ, Lee Y, Liang CK, Limpawattana P, Lin CS, Peng LN, Satake S, Suzuki T, Won CW, Wu CH, Wu SN, Zhang T, Zeng P, Akishita M, Arai H. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc. 2014;15:95-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2330] [Cited by in RCA: 2967] [Article Influence: 269.7] [Reference Citation Analysis (0)] |
| 15. | Ninomiya G, Fujii T, Yamada S, Yabusaki N, Suzuki K, Iwata N, Kanda M, Hayashi M, Tanaka C, Nakayama G, Sugimoto H, Koike M, Fujiwara M, Kodera Y. Clinical impact of sarcopenia on prognosis in pancreatic ductal adenocarcinoma: A retrospective cohort study. Int J Surg. 2017;39:45-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 79] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 16. | van Dijk DP, Bakens MJ, Coolsen MM, Rensen SS, van Dam RM, Bours MJ, Weijenberg MP, Dejong CH, Olde Damink SW. Low skeletal muscle radiation attenuation and visceral adiposity are associated with overall survival and surgical site infections in patients with pancreatic cancer. J Cachexia Sarcopenia Muscle. 2017;8:317-326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 184] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 17. | Sato H, Goto T, Hayashi A, Kawabata H, Okada T, Takauji S, Sasajima J, Enomoto K, Fujiya M, Oyama K, Ono Y, Sugitani A, Mizukami Y, Okumura T. Prognostic significance of skeletal muscle decrease in unresectable pancreatic cancer: Survival analysis using the Weibull exponential distribution model. Pancreatology. 2021;21:892-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Emori T, Itonaga M, Ashida R, Tamura T, Kawaji Y, Hatamaru K, Yamashita Y, Shimokawa T, Koike M, Sonomura T, Kawai M, Kitano M. Impact of sarcopenia on prediction of progression-free survival and overall survival of patients with pancreatic ductal adenocarcinoma receiving first-line gemcitabine and nab-paclitaxel chemotherapy. Pancreatology. 2022;22:277-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 19. | Uemura S, Iwashita T, Ichikawa H, Iwasa Y, Mita N, Shiraki M, Shimizu M. The impact of sarcopenia and decrease in skeletal muscle mass in patients with advanced pancreatic cancer during FOLFIRINOX therapy. Br J Nutr. 2021;125:1140-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 20. | Asama H, Ueno M, Kobayashi S, Fukushima T, Kawano K, Sano Y, Tanaka S, Nagashima S, Morimoto M, Ohira H, Maeda S. Sarcopenia: Prognostic Value for Unresectable Pancreatic Ductal Adenocarcinoma Patients Treated With Gemcitabine Plus Nab-Paclitaxel. Pancreas. 2022;51:148-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 21. | Incio J, Liu H, Suboj P, Chin SM, Chen IX, Pinter M, Ng MR, Nia HT, Grahovac J, Kao S, Babykutty S, Huang Y, Jung K, Rahbari NN, Han X, Chauhan VP, Martin JD, Kahn J, Huang P, Desphande V, Michaelson J, Michelakos TP, Ferrone CR, Soares R, Boucher Y, Fukumura D, Jain RK. Obesity-Induced Inflammation and Desmoplasia Promote Pancreatic Cancer Progression and Resistance to Chemotherapy. Cancer Discov. 2016;6:852-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 331] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 22. | Cai Z, Liang Y, Xing C, Wang H, Hu P, Li J, Huang H, Wang W, Jiang C. Cancerassociated adipocytes exhibit distinct phenotypes and facilitate tumor progression in pancreatic cancer. Oncol Rep. 2019;42:2537-2549. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 23. | Okumura S, Kaido T, Hamaguchi Y, Fujimoto Y, Masui T, Mizumoto M, Hammad A, Mori A, Takaori K, Uemoto S. Impact of preoperative quality as well as quantity of skeletal muscle on survival after resection of pancreatic cancer. Surgery. 2015;157:1088-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 212] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 24. | Nakashima Y, Saeki H, Nakanishi R, Sugiyama M, Kurashige J, Oki E, Maehara Y. Assessment of Sarcopenia as a Predictor of Poor Outcomes After Esophagectomy in Elderly Patients With Esophageal Cancer. Ann Surg. 2018;267:1100-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 144] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 25. | Kays JK, Shahda S, Stanley M, Bell TM, O'Neill BH, Kohli MD, Couch ME, Koniaris LG, Zimmers TA. Three cachexia phenotypes and the impact of fat-only loss on survival in FOLFIRINOX therapy for pancreatic cancer. J Cachexia Sarcopenia Muscle. 2018;9:673-684. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 113] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 26. | Zore T, Palafox M, Reue K. Sex differences in obesity, lipid metabolism, and inflammation-A role for the sex chromosomes? Mol Metab. 2018;15:35-44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 152] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 27. | Macchini M, Chiaravalli M, Zanon S, Peretti U, Mazza E, Gianni L, Reni M. Chemotherapy in elderly patients with pancreatic cancer: Efficacy, feasibility and future perspectives. Cancer Treat Rev. 2019;72:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 28. | Aziz MH, Sideras K, Aziz NA, Mauff K, Haen R, Roos D, Saida L, Suker M, van der Harst E, Mieog JS, Bonsing BA, Klaver Y, Koerkamp BG, van Eijck CH. The Systemic-immune-inflammation Index Independently Predicts Survival and Recurrence in Resectable Pancreatic Cancer and its Prognostic Value Depends on Bilirubin Levels: A Retrospective Multicenter Cohort Study. Ann Surg. 2019;270:139-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 197] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 29. | Peng YC, Wu CH, Tien YW, Lu TP, Wang YH, Chen BB. Preoperative sarcopenia is associated with poor overall survival in pancreatic cancer patients following pancreaticoduodenectomy. Eur Radiol. 2021;31:2472-2481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 71] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 30. | Dhillon RJ, Hasni S. Pathogenesis and Management of Sarcopenia. Clin Geriatr Med. 2017;33:17-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 342] [Article Influence: 48.9] [Reference Citation Analysis (0)] |
| 31. | Larsson L, Degens H, Li M, Salviati L, Lee YI, Thompson W, Kirkland JL, Sandri M. Sarcopenia: Aging-Related Loss of Muscle Mass and Function. Physiol Rev. 2019;99:427-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 935] [Cited by in RCA: 1010] [Article Influence: 168.3] [Reference Citation Analysis (0)] |
| 32. | Gruber ES, Jomrich G, Tamandl D, Gnant M, Schindl M, Sahora K. Sarcopenia and sarcopenic obesity are independent adverse prognostic factors in resectable pancreatic ductal adenocarcinoma. PLoS One. 2019;14:e0215915. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 33. | Choi MH, Yoon SB, Lee K, Song M, Lee IS, Lee MA, Hong TH, Choi MG. Preoperative sarcopenia and post-operative accelerated muscle loss negatively impact survival after resection of pancreatic cancer. J Cachexia Sarcopenia Muscle. 2018;9:326-334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 113] [Article Influence: 16.1] [Reference Citation Analysis (1)] |
| 34. | Lutz CT, Quinn LS. Sarcopenia, obesity, and natural killer cell immune senescence in aging: altered cytokine levels as a common mechanism. Aging (Albany NY). 2012;4:535-546. [PubMed] |
| 35. | Kelley DE, Goodpaster BH, Storlien L. Muscle triglyceride and insulin resistance. Annu Rev Nutr. 2002;22:325-346. [PubMed] |
| 36. | Gilsanz V, Kremer A, Mo AO, Wren TA, Kremer R. Vitamin D status and its relation to muscle mass and muscle fat in young women. J Clin Endocrinol Metab. 2010;95:1595-1601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 120] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 37. | Zoico E, Rossi A, Di Francesco V, Sepe A, Olioso D, Pizzini F, Fantin F, Bosello O, Cominacini L, Harris TB, Zamboni M. Adipose tissue infiltration in skeletal muscle of healthy elderly men: relationships with body composition, insulin resistance, and inflammation at the systemic and tissue level. J Gerontol A Biol Sci Med Sci. 2010;65:295-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 164] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 38. | Robinson DW Jr, Eisenberg DF, Cella D, Zhao N, de Boer C, DeWitte M. The prognostic significance of patient-reported outcomes in pancreatic cancer cachexia. J Support Oncol. 2008;6:283-290. [PubMed] |
| 39. | Friess H, Guo XZ, Nan BC, Kleeff J, Büchler MW. Growth factors and cytokines in pancreatic carcinogenesis. Ann N Y Acad Sci. 1999;880:110-121. [PubMed] |
| 40. | Hamada S, Masamune A, Yoshida N, Takikawa T, Shimosegawa T. IL-6/STAT3 Plays a Regulatory Role in the Interaction Between Pancreatic Stellate Cells and Cancer Cells. Dig Dis Sci. 2016;61:1561-1571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 41. | Barreto SG, Neale RE. Vitamin D and pancreatic cancer. Cancer Lett. 2015;368:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 42. | Mutgan AC, Besikcioglu HE, Wang S, Friess H, Ceyhan GO, Demir IE. Insulin/IGF-driven cancer cell-stroma crosstalk as a novel therapeutic target in pancreatic cancer. Mol Cancer. 2018;17:66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 86] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 43. | DeSantis CE, Miller KD, Dale W, Mohile SG, Cohen HJ, Leach CR, Goding Sauer A, Jemal A, Siegel RL. Cancer statistics for adults aged 85 years and older, 2019. CA Cancer J Clin. 2019;69:452-467. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 276] [Cited by in RCA: 241] [Article Influence: 40.2] [Reference Citation Analysis (0)] |