Published online May 15, 2017. doi: 10.4239/wjd.v8.i5.202
Peer-review started: October 28, 2016
First decision: December 1, 2016
Revised: December 15, 2016
Accepted: March 12, 2017
Article in press: March 13, 2017
Published online: May 15, 2017
Processing time: 202 Days and 0.4 Hours
To determine lipid species that change in response to a change in dairy consumption. In addition, to investigate whether dairy associated lipid species are correlated with changes in measures of vascular structure and function.
A 12-mo randomised controlled trial was conducted to determine the effect of increased consumption of fruit, vegetables and dairy, compared to usual diet, on measures of vascular structure and function in adults with type 1 and type 2 diabetes (n = 108). This paper comprises post-hoc analyses investigating the relationship between dairy intake, serum lipid species and vascular health. Central and peripheral blood pressure, carotid femoral pulse wave velocity, augmentation index, serum lipid species and dietary intake were measured at baseline and 3-mo. Common carotid artery intima media thickness was measured at baseline and 12-mo.
Serum lipid species [lysophosphatidylcholine (LPC) 14:0, LPC 15:0, LPC 16:1, phosphatidylcholine (PC) 29:0 PC 30:0, PC 31:0 and cholesterol ester (CE) 14:0] were associated with the change in full fat dairy consumption (rho 0.19-0.25; P < 0.05). The 3-mo change in some lipids was positively associated with the 3-mo change in central systolic [LPC 14:0 (rho 0.30; P = 0.007), PC 30:0 (rho 0.28; P = 0.010)] and diastolic blood pressure [LPC 14:0 (rho 0.32; P = 0.004), LPC 15:0 (rho 0.23; P = 0.04), LPC 16:1 (rho 0.23; P = 0.035), PC 29:0 (rho 0.28; P = 0.01), PC 30:0 (rho 0.36; P = 0.001), PC 31:0 (rho 0.30; P = 0.007)] and 12-mo change in common carotid artery intimal medial thickness [CE 14:0 (rho 0.22; P = 0.02)]. Pulse wave velocity and augmentation index were unrelated to dairy and lipid species.
An increase in dairy associated lipids appears to be associated with an increase in blood pressure and common carotid intimal medial thickness.
Core tip: We have examined the relationship between changes in dairy intake, lipid species and vascular function. Although it was expected that an increase in dairy intake would lower blood pressure and be associated with improvements in vascular structure we found that increases in lipid species associated with dairy (LPC 14:0, LPC 15:0, LPC 16:1, CE 14:0) were associated with adverse changes in these parameters. Dairy does not appear to be beneficial in people with diabetes.
- Citation: Petersen KS, Keogh JB, Lister N, Weir JM, Meikle PJ, Clifton PM. Association between dairy intake, lipids and vascular structure and function in diabetes. World J Diabetes 2017; 8(5): 202-212
- URL: https://www.wjgnet.com/1948-9358/full/v8/i5/202.htm
- DOI: https://dx.doi.org/10.4239/wjd.v8.i5.202
By 2030 it is projected that 7.7% of the world’s population will have diabetes, which is an increase of 54% since 2010[1]. Individuals with type 1 and type 2 diabetes are two-to-three times more likely to develop cardiovascular disease (CVD) compared with the general population[2-4]. In Australia, in 2010 approximately 30% of all deaths in people with type 1 and type 2 diabetes were due to CVD[5]. Poor diet is the leading contributor to the global burden of disease[6] and better dietary quality is associated with lower rates of CVD[7,8]. The most recent evidence from prospective cohort studies suggests that dairy consumption is protective against CVD[9]. Although uncertainty remains about the vascular effects of dairy fatty acids[10].
Arterial stiffness measured by augmentation index and carotid femoral pulse wave velocity (cfPWV) are independent predictors of CVD[11,12]. Similarly, carotid intima media thickness (IMT) is an early measure of atherosclerosis that predicts CVD[13,14]. Epidemiological studies have indicated that higher consumption of dairy products is associated with lower carotid IMT[15,16] and less arterial stiffening[15,17-19].
Self-reported dietary intake is limited by inaccurate reporting, which is well-documented in the general population and people with diabetes[20]. Biomarkers of dietary intake, including dairy consumption, remove the reliance on self-reported dietary data. Previously it has been shown that fatty acids of ruminant origin are correlated with self-reported dietary intake of dairy products[19,21].
The aim is to determine serum lipid species that change in response to a change in dairy consumption. Based on our previous analysis[19] we hypothesise that the following lipid species will reflect a change in dairy consumption: Cholesterol ester (CE) 14:0, CE 15:0, lysophosphatidylcholine (LPC) 14:0, LPC 15:0, LPC 17:1, phosphatidylcholine (PC) 29:0, PC 30:0, PC 31:0, PC 31:1, PC 33:0, PC 33:1, PC 33:3, PC 35:0, sphingomyelin (SM) 31:1, SM 32:0, triacylglycerol (TG) 16:0/16:0/16:0. The secondary aims are to determine the correlation between the change in dairy associated lipid species and 3-mo change in peripheral and central blood pressure, augmentation index and cfPWV. The 12-mo change in common carotid artery intima media thickness (CCA-IMT) will also be correlated with the change in lipid species.
As previously reported, a 12-mo randomised controlled trial was conducted to determine the effect of improving dietary quality on CCA-IMT, compared to a control group continuing on their habitual diet, in a cohort of people with type 1 and type 2 diabetes[22]. Briefly, participants were randomised to increase consumption of fruit (+1 serve/day), vegetables (+2 serves/day) and dairy (+1 serve/day), regardless of usual intake or to continue on their usual diet. One serve of dairy was either 250 mL of milk or 200 g of yoghurt and no advice was given regarding the fat content. Dietary counselling was provided by a dietitian. Ethics approval was obtained from the University of South Australian Human Research Ethics Committee and the participants provided written informed consent. The trial was registered with the Australian New Zealand Clinical Trials Registry (ACTRN12613000251729) on 04/09/2014.
A post hoc analysis was conducted that comprised a subsample of the cohort (n = 108) that had serum lipidomic analysis performed at baseline and 3-mo. A food frequency questionnaire (FFQ) was used to measure dietary intake at baseline and 3-mo and a fasting serum sample was collected for measurement of lipid species. Participants had peripheral and central blood pressure, augmentation index and cfPWV measured at baseline and 3-mo. In addition, CCA-IMT was measured at baseline and 12-mo. The intervention to increase dairy by one serve per day was only partially successful with 34 people in the intervention group increasing and 17 decreasing their dairy consumption, while in the control group 23 increased and 34 reduced their dairy intake. Thus overall 57 people increased and 51 decreased dairy intake so the analyses were performed without regard for allocation to intervention or control.
Subjects above 18 years of age with diagnosed type 1 or type 2 diabetes for any duration managed with diet, oral hypoglycaemic agents (OHA) and/or insulin were recruited from August 2012 until December 2013 from a database of volunteers, public advertisements and a recruitment company (Intuito Market Research, Adelaide, South Australia). Exclusion criteria were: Unstable CVD requiring active intervention, heart failure, significant renal impairment (eGFR < 30 mL/min), liver disease, cancer or allergic/intolerant/dislike of fruit, vegetables or dairy.
Lipid analysis was performed on fasting serum by liquid chromatography, electrospray ionization-tandem mass spectrometry as previously published[23]. In total, 342 individual lipid species were measured from 23 classes.
Habitual dietary intake was measured using the electronic version of the Dietary Questionnaire for Epidemiological Studies Version 2 FFQ. The FFQ includes questions on the types of milk (fat content), cheese (hard, firm, soft, ricotta, cottage cheese, cream cheese or low fat cheese) and spreads (butter) used. Questions about the quantity of milk consumed per day (none, < 250 mL, 250-500 mL, 500-750 mL, > 750 mL) and the frequency of cheese, yoghurt and ice-cream consumption are also included. This FFQ has been found to have relatively good agreement with a 3-d weighed food record in the general population[24] and in people with type 1 and type 2 diabetes[25].
Anthropometric measurements: Height was measured using a stadiometer (SECA, Hamburg, Germany) to the nearest 0.1 cm while barefoot/flat footwear. Weight was measured to the nearest 0.05 kg using calibrated electronic scales (SECA, Hamburg, Germany) while the participants were barefoot/light footwear and wore light clothing.
Peripheral blood pressure: Clinic blood pressure was measured using an automated sphygmomanometer (SureSigns VS3; Philips, North Ryde, Sydney, Australia) once the participant had been seated for 5 min. A normal sleeve (16 cm × 52 cm) was used for an arm circumference of 24-32 cm and a large sleeve (16 cm × 70 cm) for an arm circumference of 32-42 cm. A minimum of four consecutive readings were taken at 1 min intervals. The first reading was discarded and the following three consistent measurements, i.e., systolic blood pressure within a range of 1.3 kPa, were used.
Common carotid artery intima media thickness: The measurements of the carotid artery were taken using B mode ultra-sound by one operator, with an intra-observer coefficient of variation (CV) of 4.4% (n = 34). The participants were supine with their head positioned at 45 degrees away from the side of the neck being measured. A high resolution ultrasound machine with a 12 MHz transducer was used (Samsung Medison MySono U6, South Korea). A 1 cm region of the IMT on the far wall of the distal common carotid artery on both sides was measured using automatic edge detection software (Samsung Medison MySono U6 Auto IMT, South Korea) as recommended in the Mannheim Carotid Intima- Media Thickness Consensus Paper (2004-2006-2011)[26]. Areas of plaque, defined as a 50% greater IMT than the surrounding IMT or IMT > 1.5 mm, were not imaged. Three seconds clips were captured and the mean of 10 measurements taken from each of these clips was averaged for a mean and mean maximum CCA-IMT value at baseline and 12 mo.
Central blood pressure and augmentation index: A SphygmoCor® XCEL (AtCor Medical, West Ryde, Sydney, Australia) was used to measure central blood pressure and augmentation index. A cuff was placed over the brachial artery on the right arm and measurements were completed after the participants had been quietly resting for 5 min. Three consecutive measurements were taken and the average calculated. All of the measurements were taken by one operator with a CV of 4.2% (n = 28).
Pulse wave velocity: A SphygmoCor® XCEL (AtCor Medical, West Ryde, Sydney, Australia) was used to measure cfPWV. The tonometer was placed on the right carotid artery and the cuff on the right femoral artery. A 10-s recording of the carotid-femoral waveform was taken. Three measurements were performed at each time-point and an average taken. The measurements were taken by two operators; the intra-observer CVs were 4.2% (n = 28) and 7.3% (n = 11), respectively and the inter-observer CV was 5.0% (n = 18).
Data are presented as mean ± SD. The change in dairy intake between baseline and 3-mo was not different between the groups so the cohort was analysed as a whole. Paired samples t-tests were used to determine the change in dairy intake, anthropometric measures, blood pressure, biochemistry and vascular measurements over time.
Change in lipid species containing fatty acids 15:0, 16:1 and 17:0 known to be of ruminant origin[27-29] (CE 15:0, CE 16:1, CE 17:0, diacylglycerol (DG) 16:1/18:1, LPC 15:0, LPC 16:1, LPC 17:0, TG 15:0/18:1/16:0, TG 15:0/18:1/18:1, TG 16:1/16:1/16:1, TG 16:1/16:1/18:0, TG 16:1/16:1/18:1, TG 16:1/18:1/18:1, TG 16:1/18:1/18:2, TG 17:0/16:0/16:1, TG 17:0/16:0/18:0, TG 17:0/18:1/14:0, TG 17:0/18:1/16:0, TG 17:0/18:1/16:1, TG 17:0/18:1/18:1, TG 17:0/18:2/16:0) and those we have previously shown to be correlated with dairy consumption[19] (CE 14:0, CE 15:0, LPC 14:0, LPC 15:0, LPC 17:1, PC 29:0, PC 30:0, PC 31:0, PC 31:1, PC 33:0, PC 33:1, PC 33:3, PC 35:0, SM 31:1, SM 32:0, TG 16:0/16:0/16:0) were correlated with the 3-mo change in full fat dairy consumption. Spearman’s correlation was used to correlate the change in dairy consumption with the change in lipid species because the data did not have a normal distribution. Lipid species that were correlated with the change in full fat dairy consumption were correlated with the change in vascular measurements. Both absolute and percent change (change/baseline × 100) are presented. No adjustment for multiple testing were made with this analysis. Secondary analyses involving all of the lipid species (> 300) were conducted. Spearman’s correlation was used to determine the association between the change in lipid species and the change in vascular measures. In addition, baseline concentrations of the lipid species were correlated with the change in vascular measures. These secondary analyses were corrected for multiple comparisons using the Benjamini Hochberg approach[30]. Analyses were performed using SPSS (version 19, 2010, SPSS Inc, Chicago, IL, United States). Statistical significance was set at P < 0.05. The statistical methods of this study were reviewed by Ms Kylie Lange from the University of Adelaide, Australia.
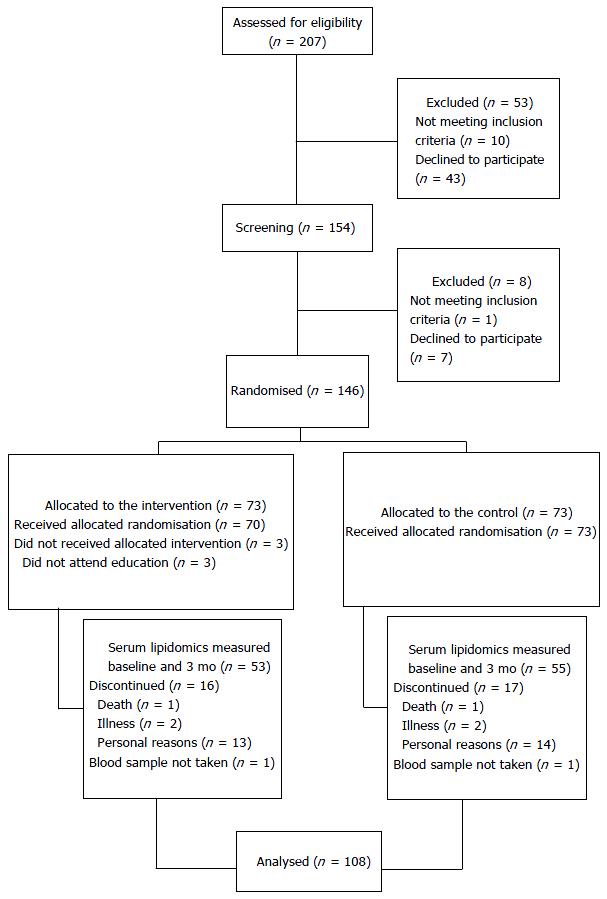
A total of 108 participants were involved in these analyses (Figure 1). Baseline characteristics of the participants included in this subsample were not different from non-participants with regards to age, weight, BMI, peripheral blood pressure, central systolic blood pressure, mean or mean maximum CCA-IMT. Central diastolic blood pressure, augmentation index, cfPWV and HbA1c were higher (P < 0.05) in the non-participants compared with the participants. Baseline characteristics of the participants are presented in Table 1.
| Characteristic | Cohort (n = 108) |
| Age (yr) | 58 ± 14 |
| Weight (kg) | 96.2 ± 20.6 |
| BMI (kg/m2) | 32.6 ± 6.4 |
| Sex | |
| Female | 40 (37) |
| Male | 68 (63) |
| Diabetes type | |
| Type 1 | 14 (13) |
| Type 2 | 94 (87) |
| Time since diabetes diagnosis (yr) | |
| Type 1 | 21 ± 13 |
| Type 2 | 8 ± 7 |
| Smoking status | |
| Never smoked | 56 (52) |
| Past smoker | 47 (43) |
| Current smoker | 5 (5) |
| Smoking pack years (years)a | 10.5 ± 16.3 |
| Prescribed anti-hypertensive medication | 66 (61) |
| Prescribed lipid lowering medication | 61 (57) |
| Diabetes treatment | |
| None | 22 (20) |
| OHA | 49 (46) |
| Insulin | 16 (15) |
| OHA + insulin | 21 (19) |
| Presence of Microalbuminuria1 | 14 (13) |
| HbA1c | 7.1 ± 1.2 |
Table 2 shows consumption of dairy products measured using the FFQ. There was no significant change in total or full fat dairy consumption over time. Yoghurt consumption increased while cheese and butter consumption decreased. There was no effect of the treatment on dairy consumption or lipid levels, so we were able to examine the association between the change in dairy intake and the change in lipid species in the entire cohort to identify dairy associated lipid species. And we further analysed the correlation between the change in dairy associated lipid species and vascular measurements.
Table 3 shows the results of correlating the change in lipid species containing 15:0, 16:1 or 17:0 or those identified as correlating with dairy intake in the previous cross-sectional study with the change in full fat dairy consumption (milk, yoghurt, cheese, butter and ice-cream). No adjustments were made.
| Change in lipid species | rho value | P value |
| LPC 14:0 | 0.25 | 0.01 |
| LPC 15:0 | 0.21 | 0.03 |
| LPC 16:1 | 0.22 | 0.02 |
| PC 29:0 | 0.22 | 0.02 |
| PC 30:0 | 0.23 | 0.02 |
| PC 31:0 | 0.19 | 0.049 |
| CE 14:0 | 0.24 | 0.01 |
Table 4 shows the anthropometric measurements, blood pressure, biochemistry and vascular measurements at baseline and follow-up in the whole group. Measurements of central blood pressure and augmentation index were performed on 82 participants due to equipment availability. cfPWV was performed on 70 participants due to technical difficulties because of obesity. The lipid species that were shown to be correlated with total full fat dairy consumption (LPC 14:0, LPC 15:0, LPC 16:1, PC 29:0, PC 30:0, PC 31:0, CE 14:0) were then correlated with the vascular measurements that changed over time.
| Baseline | 3 mo | P value for change | |
| Weight (kg) | 96.2 ± 20.6 | 95.9 ± 20.6 | 0.40 |
| Peripheral systolic blood pressure (kPa) | 16.9 ± 2.0 | 16.9 ± 1.9 | 0.75 |
| Peripheral diastolic blood pressure (kPa) | 9.4 ± 1.3 | 9.4 ± 1.3 | 0.69 |
| Peripheral mean arterial pressure (kPa) | 12 ± 1.3 | 12 ± 1.3 | 0.69 |
| Peripheral pulse pressure (kPa) | 7.4 ± 1.3 | 7.4 ± 1.7 | 0.93 |
| Central systolic blood pressure (kPa) | 16.8 ± 1.3 | 16.1 ± 1.9 | 0.004 |
| Central diastolic blood pressure (kPa) | 10.9 ± 1.3 | 10.5 ± 1.2 | 0.002 |
| Central mean arterial pressure (kPa) | 13.2 ± 1.5 | 12.8 ± 1.3 | 0.002 |
| Central pulse pressure (kPa) | 5.9 ± 1.7 | 5.8 ± 1.7 | 0.09 |
| Central augmented pressure (kPa) | 1.2 ± 0.6 | 1.1 ± 0.8 | 0.07 |
| Augmentation index (%) | 19 ± 7 | 21 ± 12 | 0.005 |
| cfPWV (m/s) | 9.4 ± 1.7 | 9.4 ± 1.8 | 0.63 |
| Pulse transit time (m/s) | 60 ± 10 | 60 ± 9 | 0.95 |
| CCA-IMT (mm) | 0.72 ± 0.12 | 0.71 ± 0.121 | 0.002 |
| Total cholesterol (mmol/L) | 3.7 ± 1.0 | 3.7 ± 1.0 | 0.59 |
| HDL cholesterol (mmol/L) | 1.2 ± 0.3 | 1.2 ± 0.3 | 0.54 |
| LDL cholesterol (mmol/L) | 2.0 ± 0.8 | 2.0 ± 0.7 | 0.40 |
| Triglycerides (mmol/L) | 1.1 ± 0.9 | 1.1 ± 0.9 | 0.53 |
| Glucose (mmol/L) | 7.3 ± 2.9 | 7.3 ± 2.7 | 0.95 |
| High sensitivity C reactive protein (mg/L) | 2.5 ± 2.4 | 2.4 ± 2.4 | 0.80 |
Table 5 presents the results of these analyses including both absolute and percent change in each parameter. Percent and absolute change in LPC 14:0, PC 30:0 and PC 31:0 were correlated with percent and absolute change in central systolic and diastolic blood pressure and central mean arterial pressure. Change in central diastolic blood pressure was correlated with the change in LPC 15:0, LPC 16:1, PC 29:0 and PC 31:0. Change in central mean arterial pressure was also correlated with the change in PC 31:0. The absolute and percent change in CCA-IMT was correlated with the absolute and percent change in CE 14:0 only. There were no lipid species that were associated with the change in augmentation index or cfPWV. Change in full fat dairy consumption itself was not associated with the change in any of the vascular measurements. When the change in all of the lipid species (n > 300) was examined after correction for multiple comparisons, there were no significant correlations between any of the lipid species and the change in central systolic and diastolic blood pressure, central mean arterial pressure, augmentation index or CCA-IMT.
| Absolute change | Percent change | |||
| rho value | P value | rho value | P value | |
| Central systolic blood pressure | ||||
| LPC 14:0 | 0.30 | 0.007 | 0.28 | 0.01 |
| PC 30:0 | 0.28 | 0.010 | 0.31 | 0.004 |
| PC 31:0 | 0.20 | 0.07 | 0.22 | 0.046 |
| Central diastolic blood pressure | ||||
| LPC 14:0 | 0.32 | 0.004 | 0.32 | 0.00 |
| LPC 15:0 | 0.23 | 0.04 | 0.22 | 0.046 |
| LPC 16:1 | 0.23 | 0.035 | 0.21 | 0.06 |
| PC 29:0 | 0.28 | 0.01 | 0.34 | 0.002 |
| PC 30:0 | 0.36 | 0.001 | 0.40 | 0.001 |
| PC 31:0 | 0.30 | 0.007 | 0.31 | 0.004 |
| Central mean arterial pressure | ||||
| LPC 14:0 | 0.30 | 0.007 | 0.29 | 0.009 |
| PC 29:0 | 0.21 | 0.06 | 0.25 | 0.025 |
| PC 30:0 | 0.31 | 0.005 | 0.33 | 0.002 |
| PC 31:0 | 0.24 | 0.033 | 0.24 | 0.028 |
| Augmentation index | ||||
| Nil significant | ||||
| cfPWV | ||||
| Nil significant | ||||
| CCA-IMT | ||||
| CE 14:0 | 0.22 | 0.02 | 0.23 | 0.02 |
Baseline concentration of PC 34:4 (rho = -0.32; P < 0.0001), PC 32:2 (rho = -0.33; P < 0.0001), GM3 ganglioside (GM3) 16:0 (rho = -0.34; P < 0.0001), GM3 18:0 (rho = -0.38; P = 0.0001) and total GM3 (rho = -0.36; P = 0.0003) were inversely associated with the change in CCA-IMT, after adjustment for multiple comparisons. The change in central systolic and diastolic blood pressure, central mean arterial pressure, augmentation index or cfPWV were not associated with the concentration of any lipid species at baseline after correction for multiple comparisons.
These analyses show that a change in the concentration of a number of serum lipid species (LPC 14:0, LPC 15:0, LPC 16:1, PC 29:0 PC 30:0, PC 31:0, CE 14:0), previously shown to be correlated with dairy consumption, were associated with a change in full fat dairy consumption in the present study. One or more of these lipid species were positively associated with the 3-mo change in central blood pressure and 12-mo change in CCA-IMT in this cohort with type 1 and type 2 diabetes.
Fatty acids C 15:0 and C 17:0 are not produced endogenously and are constituents of dairy fat. The fatty acid C 14:0 is produced endogenously but is also a component of dairy fat. Therefore these fatty acids in plasma or for C 14:0 in adipose tissue are considered biomarkers of dairy intake[28]. In the present study change in LPC 15:0, LPC 14:0 and CE 14:0 were weakly positively correlated with the change in consumption of full fat dairy. LPC concentration in milk fat is < 1% of the total PC concentration and therefore dairy would be an unlikely direct source of LPC species[21]. LPC lipid species are predominately produced endogenously through a number of different processes including from PC by phospholipase A2 enzyme and the oxidation of LDL particles[31,32].
In a randomised cross-over study, consumption of a full fat dairy diet containing non-fermented products (butter, cream or ice cream) was associated with higher plasma concentrations of sphingomyelin compared with low-fat dairy intake[33]. Plasma concentrations of odd chain phosphatidylcholine (15:0 and 17:0) were increased with consumption of both full fat fermented (including yoghurt and cheese) and non-fermented dairy products. In this study vascular measurements were not performed. Previously we showed a cross-sectional association between serum lipid species and consumption of full fat dairy in people with type 1 and type 2 diabetes but serum lipid species were not associated with arterial stiffness despite dairy consumption being inversely associated with cfPWV[19]. In addition, baseline CCA-IMT was not associated with any serum lipid species. In the present study, changes in serum lipid species were associated with the change in central blood pressure (LPC 14:0, LPC 15:0, LPC 16:1, PC 29:0, PC 30:0, PC 31:0) and the change in CCA-IMT (CE 14:0). In addition, baseline concentration of PC 34:4, PC 32:2, GM3 16:0, GM3 18:0 and total GM3 were associated with the change in CCA-IMT such that higher baseline levels were associated with greater CCA-IMT regression. GM3 accumulation has been observed in intimal atherosclerotic lesions in the carotid artery and aorta[34] and serum GM3 has been identified as a potential marker of early atherosclerosis[35]. Therefore this finding suggests that those with greater atherosclerotic burden at baseline had greater CCA-IMT regression which is consistent with our previous finding[22].
This study shows that when dairy associated serum lipids species were reduced at 3-mo there was a reduction in central blood pressure and CCA-IMT. Conversely when there was an increase in these serum lipid species central blood pressure increased. This finding is not consistent with previous research showing an inverse association between dairy consumption and blood pressure[17,36]. Machin et al[36] showed that consuming a high dairy diet (non-fat) for 4 wk reduced central blood pressure and cfPWV compared with a high fruit diet. The relationship we observed whereby an increase in LPC 14:0, LPC 15:0 and LPC 16:1 was associated with an increase in central blood pressure may be explained by the proinflammatory and atherogenic properties of non-omega 3 polyunsaturated fatty acid enriched LPC[37]. LPCs act on endothelial cells, smooth muscle cells, monocytes, macrophages and T-cells in a number of ways to inhibit endothelial relaxation and up-regulate production of inflammatory and adhesion molecules[32,38]. Inflammation, measured by CRP, has been positively associated with blood pressure and development of hypertension[39,40], which may explain our findings in the current study. As such, our results may not be due to the fatty acid composition per se but fatty acid metabolism and resulting lipid species. Previously total phospholipid C 15:0 has been inversely associated with blood pressure and coronary heart disease[41]. Although it remains unclear what the mechanism behind this finding is and this should be explored in the future.
Ruminant fatty acids C 15:0 and C 17:0 do not have the same physiological effects as other saturated fatty acids (although they have not been tested in isolation) and are not associated with increased risk of cardiovascular outcomes and may actually be protective[42,43]. Dairy products also contain many other components such calcium, magnesium, phosphorus, potassium and bioactive peptides that may contribute to the cardio-protective effect of dairy consumption[44-46]. In a four-way cross-over study it was shown that the calcium content of dairy products counteracts the fat content to attenuate the increase in total and LDL cholesterol, without reducing HDL cholesterol[44]. The synergist effect of the different components in dairy products may explain why dairy consumption was not associated with any of the vascular measurements in the current study despite the observed association between the lipid species and these measurements.
Nestel et al[21] showed that the phospholipid classes LPC and lysoalkylphosphatidylcholine were associated with measures of insulin sensitivity and insulin resistance. In addition, full fat dairy consumption was associated with phospholipid fatty acids C 15:0, C 16:1 and C 18:1 n-7 but there was no relationship detected between dairy consumption and insulin sensitivity or resistance measures. This is similar to the findings of the present study whereby the change in dairy consumption was not associated with the change in central blood pressure or CCA-IMT, but the change in a number of lipid species was associated with both the change in dairy intake and the change in central blood pressure and CCA-IMT. This may be because lipid analysis provides a more objective measure of intake and is not limited by the measurement error associated with self-reported dietary intake, although it may be due to a lack of statistical power.
Limitations of this study are that it is observational and therefore causation cannot be established. In addition, just over half of the cohort were prescribed lipid lowering medication which may confound the results. Finally, this study comprises a small sample size and findings should be replicated in a larger cohort. We also did not have lipidomic data at 12-mo but we assumed changes early in the observation period would be required in order see changes in IMT at 12-mo.
In conclusion, in this cohort with type 1 and type 2 diabetes a number of serum lipid species that were associated with a change in full fat dairy consumption were also correlated with the 3-mo change in central blood pressure and 12-mo change in CCA-IMT.
The authors of this study would like to acknowledge the contributions of study participants and the staff of the University of South Australia. We would like to thank Dr. Eva Pedersen for her help with data collection and providing nutritional counselling.
People with diabetes are at higher risk of cardiovascular disease than the general population. And poor diet is a leading contributor to the development of cardiovascular disease. At present the effect of dairy consumption on vascular health is unclear. In this study the authors aimed to determine lipid species that change in response to a change in dairy consumption. In addition, to investigate whether dairy associated lipid species are correlated with changes in measures of vascular structure and function.
Measurement of dietary intake is challenging due to inaccurate reporting, which is well documented in people with diabetes. Previously it has been shown that serum lipid species of ruminant origin are correlated with dairy consumption. In this study, the authors investigated the association between dairy intake measured by dietary questionnaire and serum lipid species and vascular health.
These analyses show that a change in the concentration of a number of serum lipid species (LPC 14:0, LPC 15:0, LPC 16:1, PC 29:0 PC 30:0, PC 31:0, CE 14:0), previously shown to be correlated with dairy consumption, were associated with a change in full fat dairy consumption. One or more of these lipid species were positively associated with the 3-mo change in central blood pressure and 12-mo change in common carotid artery intima media thickness (CCA-IMT) in this cohort with type 1 and type 2 diabetes.
Due to the observational nature of this research these findings are hypothesis generating and should be confirmed in the future.
CCA-IMT is visualized using B mode ultrasound. The intima-media complex is the area of tissue starting at the luminal edge of the artery and ending at the boundary between the media and the adventitia. CCA-IMT is a measure of early atherosclerosis. Augmentation index and carotid femoral pulse wave velocity are non-invasive measures of arterial stiffness.
In the manuscript, the authors conducted a subanalysis of a previous randomized trial addressing the role of diary intake on vascular function and blood pressure parameters. They documented a significant relationship between blood pressure and augmentation index and certain lipid parameters. The study is well conducted with a huge number of parameters analysed and the results are intriguing.
Manuscript source: Invited manuscript
Specialty type: Endocrinology and metabolism
Country of origin: Australia
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Neves MF, Qi L, van Bilsen M, Verdoia M S- Editor: Gong ZM L- Editor: A E- Editor: Li D
| 1. | Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4438] [Cited by in RCA: 4368] [Article Influence: 291.2] [Reference Citation Analysis (4)] |
| 2. | Carson AP, Tanner RM, Yun H, Glasser SP, Woolley JM, Thacker EL, Levitan EB, Farkouh ME, Rosenson RS, Brown TM. Declines in coronary heart disease incidence and mortality among middle-aged adults with and without diabetes. Ann Epidemiol. 2014;24:581-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Juutilainen A, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Similarity of the impact of type 1 and type 2 diabetes on cardiovascular mortality in middle-aged subjects. Diabetes Care. 2008;31:714-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 133] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 4. | Lind M, Svensson A-M, Kosiborod M, Gudbjörnsdottir S, Pivodic A, Wedel H, Dahlqvist S, Clements M, Rosengren A. Glycemic Control and Excess Mortality in Type 1 Diabetes. N Engl J Med. 2014;371:1972-1982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 575] [Cited by in RCA: 641] [Article Influence: 58.3] [Reference Citation Analysis (0)] |
| 5. | Harding JL, Shaw JE, Peeters A, Guiver T, Davidson S, Magliano DJ. Mortality Trends Among People With Type 1 and Type 2 Diabetes in Australia: 1997-2010. Diabetes Care. 2014;37:2579-2586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 121] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 6. | Forouzanfar MH, Alexander L, Anderson HR, Bachman VF, Biryukov S, Brauer M, Burnett R, Casey D, Coates MM, Cohen A. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. The. Lancet. 2015;386:2287-2323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2079] [Cited by in RCA: 1825] [Article Influence: 182.5] [Reference Citation Analysis (0)] |
| 7. | Iqbal R, Anand S, Ounpuu S, Islam S, Zhang X, Rangarajan S, Chifamba J, Al-Hinai A, Keltai M, Yusuf S. Dietary Patterns and the Risk of Acute Myocardial Infarction in 52 Countries: Results of the INTERHEART Study. Circulation. 2008;118:1929-1937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 292] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 8. | Brunner EJ, Mosdøl A, Witte DR, Martikainen P, Stafford M, Shipley MJ, Marmot MG. Dietary patterns and 15-y risks of major coronary events, diabetes, and mortality. Am J Clin Nutr. 2008;87:1414-1421. [PubMed] |
| 9. | Qin L-Q, Xu J-Y, Han S-F, Zhang Z-L, Zhao Y-Y, Szeto IM. Dairy consumption and risk of cardiovascular disease: an updated meta-analysis of prospective cohort studies. Asia Pac J Clin Nutr. 2015;24:90-100. [PubMed] |
| 10. | Mozaffarian D. Dietary and Policy Priorities for Cardiovascular Disease, Diabetes, and Obesity A Comprehensive Review. Circulation. 2016;133:187-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1337] [Cited by in RCA: 1464] [Article Influence: 162.7] [Reference Citation Analysis (0)] |
| 11. | Vlachopoulos C, Aznaouridis K, O’Rourke MF, Safar ME, Baou K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with central haemodynamics: a systematic review and meta-analysis. Eur Heart J. 2010;31:1865-1871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1133] [Cited by in RCA: 1039] [Article Influence: 69.3] [Reference Citation Analysis (0)] |
| 12. | Ben-Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, Boutouyrie P, Cameron J, Chen CH, Cruickshank JK. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol. 2014;63:636-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1125] [Cited by in RCA: 1355] [Article Influence: 112.9] [Reference Citation Analysis (0)] |
| 13. | Schwartz SM, deBlois D, O’Brien ERM. The Intima: Soil for Atherosclerosis and Restenosis. Circ Res. 1995;77:445-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 596] [Cited by in RCA: 591] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 14. | Lorenz MW, Polak JF, Kavousi M, Mathiesen EB, Völzke H, Tuomainen T-P, Sander D, Plichart M, Catapano AL, Robertson CM. Carotid intima-media thickness progression to predict cardiovascular events in the general population (the PROG-IMT collaborative project): a meta-analysis of individual participant data. The Lancet. 2012;379:2053-2062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 462] [Cited by in RCA: 451] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 15. | Recio-Rodriguez J, Gomez-Marcos M, Patino-Alonso M-C, Sanchez A, Agudo-Conde C, Maderuelo-Fernandez J, Garcia-Ortiz L, on behalf of the EVIDENT Group. Association between fat amount of dairy products with pulse wave velocity and carotid intima-media thickness in adults. Nutr J. 2014;13:37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Ivey KL, Lewis JR, Hodgson JM, Zhu K, Dhaliwal SS, Thompson PL, Prince RL. Association between yogurt, milk, and cheese consumption and common carotid artery intima-media thickness and cardiovascular disease risk factors in elderly women. Am J Clin Nutr. 2011;94:234-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 17. | Crichton GE, Elias MF, Dore GA, Abhayaratna WP, Robbins MA. Relations Between Dairy Food Intake and Arterial Stiffness. Hypertension. 2012;59:1044-1051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 18. | Livingstone KM, Lovegrove JA, Cockcroft JR, Elwood PC, Pickering JE, Givens DI. Does Dairy Food Intake Predict Arterial Stiffness and Blood Pressure in Men?: Evidence from the Caerphilly Prospective Study. Hypertension. 2013;61:42-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 19. | Petersen KS, Keogh JB, Meikle PJ, Garg ML, Clifton PM. Dietary predictors of arterial stiffness in a cohort with type 1 and type 2 diabetes. Atherosclerosis. 2015;238:175-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Sallé A, Ryan M, Ritz P. Underreporting of Food Intake in Obese Diabetic and Nondiabetic Patients. Diabetes Care. 2006;29:2726-2727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 50] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | Nestel PJ, Straznicky N, Mellett NA, Wong G, De Souza DP, Tull DL, Barlow CK, Grima MT, Meikle PJ. Specific plasma lipid classes and phospholipid fatty acids indicative of dairy food consumption associate with insulin sensitivity. Am J Clin Nutr. 2014;99:46-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 22. | Petersen KS, Clifton PM, Blanch N, Keogh JB. Effect of improving dietary quality on carotid intima media thickness in subjects with type 1 and type 2 diabetes: a 12-mo randomized controlled trial. Am J Clin Nutr. 2015;102:771-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Weir JM, Wong G, Barlow CK, Greeve MA, Kowalczyk A, Almasy L, Comuzzie AG, Mahaney MC, Jowett JBM, Shaw J. Plasma lipid profiling in a large population-based cohort. J Lipid Res. 2013;54:2898-2908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 296] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 24. | Hodge A, Patterson AJ, Brown WJ, Ireland P, Giles G. The Anti Cancer Council of Victoria FFQ: relative validity of nutrient intakes compared with weighed food records in young to middle-aged women in a study of iron supplementation. Aust N Z J Public Health. 2000;24:576-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 487] [Cited by in RCA: 519] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 25. | Petersen KS, Smith JM, Clifton PM, Keogh JB. Dietary intake in adults with type 1 and type 2 diabetes: validation of the Dietary Questionnaire for Epidemiological Studies version 2 FFQ against a 3-d weighed food record and 24-h urinalysis. Br J Nutr. 2015;114:2056-2063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Touboul P, Hennerici M, Meairs S, Adams H, Amarenco P, Bornstein N, Csiba L, Desvarieux M, Ebrahim S, Hernandez Hernandez R. Mannheim carotid intima-media thickness and plaque consensus (2004-2006-2011). An update on behalf of the advisory board of the 3rd, 4th and 5th watching the risk symposia, at the 13th, 15th and 20th European Stroke Conferences, Mannheim, Germany, 2004, Brussels, Belgium, 2006, and Hamburg, Germany, 2011. Cerebrovasc Dis. 2012;34:290-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 903] [Cited by in RCA: 1272] [Article Influence: 97.8] [Reference Citation Analysis (0)] |
| 27. | Wolk A, Vessby B, Ljung H, Barrefors P. Evaluation of a biological marker of dairy fat intake. Am J Clin Nutr. 1998;68:291-295. [PubMed] |
| 28. | Wolk A, Furuheim M, Vessby B. Fatty Acid Composition of Adipose Tissue and Serum Lipids Are Valid Biological Markers Of Dairy Fat Intake in Men. J Nutr. 2001;131:828-833. [PubMed] |
| 29. | Micha R, King IB, Lemaitre RN, Rimm EB, Sacks F, Song X, Siscovick DS, Mozaffarian D. Food sources of individual plasma phospholipid trans fatty acid isomers: the Cardiovascular Health Study. Am J Clin Nutr. 2010;91:883-893. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 30. | Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J Roy Statist Soc Ser B. 1995;57:289-300. [DOI] [Full Text] |
| 31. | Sato H, Kato R, Isogai Y, Saka G-i, Ohtsuki M, Taketomi Y, Yamamoto K, Tsutsumi K, Yamada J, Masuda S, Ishikawa Y, Ishii T, Kobayashi T, Ikeda K, Taguchi R, Hatakeyama S, Hara S, Kudo I, Itabe H, Murakami M. Analyses of Group III Secreted Phospholipase A2 Transgenic Mice Reveal Potential Participation of This Enzyme in Plasma Lipoprotein Modification, Macrophage Foam Cell Formation, and Atherosclerosis. J Biol Chem. 2008;283:33483-33497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 99] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 32. | Takayuki M, Tsuneo K, Katsuo K. Role of Lysophosphatidylcholine (LPC) in Atherosclerosis. Curr Med Chem. 2007;14:3209-3220. [RCA] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 266] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 33. | Nestel PJ, Mellett N, Pally S, Wong G, Barlow CK, Croft K, Mori TA, Meikle PJ. Effects of low-fat or full-fat fermented and non-fermented dairy foods on selected cardiovascular biomarkers in overweight adults. Br J Nutr. 2013;110:2242-2249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 34. | Bobryshev YV, Lord RSA, Golovanova NK, Gracheva EV, Zvezdina ND, Sadovskaya VL, Prokazova NV. Incorporation and localisation of ganglioside GM3 in human intimal atherosclerotic lesions. Biochim Biophys Acta. 1997;1361:287-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 35. | Inokuchi JI. GM3 and diabetes. Glycoconj J. 2014;31:193-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 36. | Machin DR, Park W, Alkatan M, Mouton M, Tanaka H. Effects of non-fat dairy products added to the routine diet on vascular function: A randomized controlled crossover trial. Nutr Metab Cardiovasc Dis. 2015;25:364-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 37. | Akerele OA, Cheema SK. Fatty acyl composition of lysophosphatidylcholine is important in atherosclerosis. Medical Hypotheses. 2015;85:754-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 38. | Schmitz G, Ruebsaamen K. Metabolism and atherogenic disease association of lysophosphatidylcholine. Atherosclerosis. 2010;208:10-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 277] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 39. | Emerging Risk Factors Collaboration. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. The Lancet. 2010;375:132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1615] [Cited by in RCA: 1853] [Article Influence: 123.5] [Reference Citation Analysis (0)] |
| 40. | Sesso HD, Wang L, Buring JE, Ridker PM, Gaziano JM. Comparison of Interleukin-6 and C-Reactive Protein for the Risk of Developing Hypertension in Women. Hypertension. 2007;49:304-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 131] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 41. | de Oliveira Otto MC, Nettleton JA, Lemaitre RN, M . Steffen L, Kromhout D, Rich SS, Y. Tsai M, Jacobs DR, Mozaffarian D. Biomarkers of Dairy Fatty Acids and Risk of Cardiovascular Disease in the Multi-Ethnic Study of Atherosclerosis. J Am Heart Assoc. 2013;2:e000092. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 88] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 42. | Dawczynski C, Kleber ME, März W, Jahreis G, Lorkowski S. Saturated fatty acids are not off the hook. Nutr Metab Cardiovasc Dis. 2015;25:1071-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 43. | Khaw K-T, Friesen MD, Riboli E, Luben R, Wareham N. Plasma Phospholipid Fatty Acid Concentration and Incident Coronary Heart Disease in Men and Women: The EPIC-Norfolk Prospective Study. PLoS Med. 2012;9:e1001255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 191] [Cited by in RCA: 210] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 44. | Lorenzen JK, Jensen SK, Astrup A. Milk minerals modify the effect of fat intake on serum lipid profile: results from an animal and a human short-term study. Br J Nutr. 2014;111:1412-1420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 45. | FitzGerald RJ, Murray BA, Walsh DJ. Hypotensive Peptides from Milk Proteins. J Nutr. 2004;134:980S-988S. |
| 46. | Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, Bray GA, Vogt TM, Cutler JA, Windhauser MM. A Clinical trial of the effects of dietary patterns on blood pressure. N Engl J Med. 1997;336:1117-1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3896] [Cited by in RCA: 3719] [Article Influence: 132.8] [Reference Citation Analysis (0)] |