Published online Apr 15, 2017. doi: 10.4239/wjd.v8.i4.143
Peer-review started: September 23, 2016
First decision: October 20, 2016
Revised: January 6, 2017
Accepted: January 16, 2017
Article in press: January 18, 20117
Published online: April 15, 2017
Processing time: 202 Days and 20.2 Hours
To assess in rodent and human adipocytes the antilipolytic capacity of hexaquis(benzylammonium) decavanadate (B6V10), previously shown to exert antidiabetic effects in rodent models, such as lowering free fatty acids (FFA) and glucose circulating levels.
Adipose tissue (AT) samples were obtained after informed consent from overweight women undergoing plastic surgery. Comparison of the effects of B6V10 and reference antilipolytic agents (insulin, benzylamine, vanadate) on the lipolytic activity was performed on adipocytes freshly isolated from rat, mouse and human AT. Glycerol release was measured using colorimetric assay as an index of lipolytic activity. The influence of B6V10 and reference agents on glucose transport into human fat cells was determined using the radiolabelled 2-deoxyglucose uptake assay.
In all the species studied, B6V10 exhibited a dose-dependent inhibition of adipocyte lipolysis when triglyceride breakdown was moderately enhanced by β-adrenergic receptor stimulation. B6V10 exerted on human adipocyte a maximal lipolysis inhibition of glycerol release that was stronger than that elicited by insulin. However, B6V10 did not inhibit basal and maximally stimulated lipolysis. When incubated at dose ≥ 10 μmol/L, B6V10 stimulated by twofold the glucose uptake in human fat cells, but - similarly to benzylamine - without reaching the maximal effect of insulin, while it reproduced one-half of the insulin-stimulation of lipogenesis in mouse fat cells.
B6V10 exerts insulin-like actions in adipocytes, including lipolysis inhibition and glucose transport activation. B6V10 may be useful in limiting lipotoxicity related to obesity and insulin resistance.
Core tip: This study investigates in murine and human adipocytes the antilipolytic properties of a conjugate of benzylamine and decavanadate (B6V10), already reported to lower hyperglycaemia in diabetic rodents. Data indicated that the conjugate dose-dependently inhibited submaximal activation of lipolysis in all the species studied. Such antilipolytic action deals with the in vivo FFA-lowering properties already described for B6V10 in diabetic rats. B6V10 also activated lipogenesis and glucose transport in fat cells. B6V10 should therefore be useful in preventing the lipotoxicity constituted by the unrestrained lipolytic activity of insulin-resistant adipocytes in obese individuals presenting type 2 diabetes, a state named diabesity.
- Citation: Carpéné C, Garcia-Vicente S, Serrano M, Marti L, Belles C, Royo M, Galitzky J, Zorzano A, Testar X. Insulin-mimetic compound hexaquis (benzylammonium) decavanadate is antilipolytic in human fat cells. World J Diabetes 2017; 8(4): 143-153
- URL: https://www.wjgnet.com/1948-9358/full/v8/i4/143.htm
- DOI: https://dx.doi.org/10.4239/wjd.v8.i4.143
In obesity, the excessive enlargement of the adipose tissue (AT) is often associated with type 2 diabetes and morbid complications, especially when the hypertrophied fat depots are located in the intra-abdominal cavity (known as visceral fat). More than a decade ago, the links between fatness and altered glucose and lipid handling led to propose the term diabesity to define a complex disease distinct from “healthy” obesity[1]. The function of AT is not restricted to lipid storage: Indeed, it is also an endocrine organ, secreting numerous adipokines. Therefore, the excess of AT can be associated with insulin resistance[2,3], endocrine, metabolic and inflammatory disturbances, increasing the risk of co-morbidities, such as hypertension and dyslipidaemia. However, all these disorders, known as metabolic syndrome[4], not only co-exist with hypertrophied lipid storage but also with excessive lipid mobilization since the entire lipid turnover is dysregulated in diabesity. In fact, the circulating levels of the products of adipocyte lipolysis, namely free fatty acids (FFA) and glycerol, are dramatically elevated in obese individuals[5]. Such increase is likely resulting from a defective responsiveness of the adipocytes to the antilipolytic action of insulin. It is important to mention that insulin not only stimulates triglyceride synthesis, but also inhibits triglyceride breakdown, lowering basal lipolysis in fat cells and reducing FFA and glycerol blood levels. Therefore, in obese subjects with insulin resistance, the hypertrophied adipocytes release excessive amounts of FFA, which are not a good fuel supply to the other organs, and even hamper carbohydrate utilization. This contributes to maintaining insulin resistance and its deleterious outcomes. Especially when occurring in visceral AT, such excessive lipolysis results in a high flux of FFA toward the liver, causing hepatosteatosis, inflammation, and worsening dyslipidemia. It is admitted that subjects with visceral fat have higher postprandial FFA and are at a higher risk of fatty liver disease and hepatic insulin resistance[6-8]. Indeed, clinical studies have demonstrated that the insulin resistance occurring in excessive AT affects metabolic parameters and increases liver damage[9]. Excessive FFA also have toxic effects in other organs (e.g., alteration of insulin secretion in pancreas[10]), that contribute to exacerbate hyperinsulinemia and insulin resistance. At the cellular level, excessive FFA supply impairs mitochondrial function and leads to abnormal lipid oxidation, further disturbing lipid turnover and cell survival. All these effects of excessive FFA belong to a network of mechanisms currently defined as lipotoxicity[11].
Since unrestrained AT lipolysis results in increased fatty acid release, leading to lipotoxicity, the search for antilipolytic drugs has been re-considered recently as a promising approach to delay and/or reverse the onset of insulin resistance in diabesity. Consequently, many pharmacological agents are under investigation with the objective of reproducing and surpassing the beneficial effects of the classical antilipolytic agent Acipimox, reported to transiently alleviate insulin resistance in obese subjects[12]. Agonists of Gi-protein coupled receptors endowed with such antilipolytic properties have been reviewed elsewhere[5]. In this context, we aimed to verify in adipocytes the antilipolytic properties of a potential antidiabetic agent previously characterized as an insulin-mimicker on its basis to activate glucose transport in adipocytes from rodent models[13].
Our interest was therefore focused in searching how an arylalkylamine vanadium salt, endowed with insulin-like actions regarding glucose disposal[14], was able to directly reduce the lipolytic activity of freshly isolated adipocytes. Our previous studies showed that hexaquis(benzylammonium) decavanadate, the formula of which is (C7H10N)6V10O28.2H2O, is a salt conjugate of benzylamine and decavanate (B6V10) acting as a substrate for semicarbazide sensitive amine oxidase/vascular adhesion protein-1 (SSAO/VAP-1)[15]. This enzyme is abundant at the surface of adipocytes and generates hydrogen peroxide when oxidizing its amine substrates. In the presence of B6V10, SSAO/VAP-1 also generated substantial amount of peroxovanadium, which, via phosphatase inhibition, was able to trigger insulin signalling downstream of the insulin receptor and to activate glucose transport in rodent adipocytes in the complete absence of insulin[16]. Chronic administration of B6V10 to rat or mouse models of diabetes substantially lowered blood glucose levels[13]. In addition, B6V10 normalized the plasma concentration of non-esterified fatty acids in severely diabetic rats[13]. Our present study consisted in a comparative approach testing under various conditions the putative antilipolytic actions of B6V10 in murine and human adipocytes.
We first tested increasing doses of B6V10 (0.1 to 100 μmol/L) on the triglyceride breakdown (lipolysis releasing FFA and glycerol) in rat adipocytes. Then a broader range of B6V10 doses (1 nmol/L to 100 μmol/L) was tested on the lipolytic and lipogenic responses of mouse adipocytes. Finally, our observations showed for the first time in human adipocytes a substantial antilipolytic action of supramicromolar doses of B6V10, which also activated glucose uptake.
Adipocytes were isolated from samples of subcutaneous adipose tissue obtained from women undergoing abdominal lipectomy under the control of plastic surgery staff of Rangueil Hospital (Toulouse, France). A total of 13 overweight women (age range: 30-48 year, BMI = 25.9 ± 1.1 kg/m2) were incorporated in the study following agreement of the INSERM guidelines and local ethic committee. The surgically removed pieces of human adipose tissue were placed in sterile plastic box, and transferred in less than one hour to the laboratory. The samples were immediately subjected to collagenase digestion at 37 °C to obtain freshly isolated adipose cells. To do so, pieces of adipose tissue were minced with scissors in Krebs-Ringer salt solution pH 7.5 containing 15 mmol/L sodium bicarbonate, 10 mmol/L HEPES and 3.5% of fat-depleted bovine serum albumin (KRBHA), and 5.5 mmol/L glucose. For the cell preparations used for glucose uptake assays, glucose was replaced by 2 mmol/L pyruvate. After digestion with 1 mg/mL collagenase type II for approximately 45 min under agitation, buoyant adipocytes were separated by filtration through a 300 μmol/L mesh-screen and carefully washed in fresh medium to obtain adipocyte suspensions as previously described[17]. Final adipocyte suspensions averaged 14.5 ± 1.4 mg cell lipids/400 μL unless otherwise stated.
The same procedure as above was applied for rat and mouse adipocyte preparations. A total of 10 male Wistar rats were purchased at Charles River (L’Arbresle, France) and were sacrificed according to INSERM guidelines for adipocyte preparation as previously reported[18]. Rat adipocytes were used at 15.3 ± 1.0 mg lipids/400 μL for the preliminary tests. Adipocytes were isolated from intra-abdominal adipose tissues obtained from male and female C57BL/6 mice. A total of 12 mice were used as already described[19] for the preparation of adipocyte suspensions that averaged 13.3 ± 0.8 mg cell lipids/400 μL.
Filtration of digested adipose tissue, fat cell separation and incubation were performed in disposable plastic wares at 37 °C, as described[17]. All the tested agents were added to 400 μL of fat cell suspension in KRBHA under the form of 4 μL of a dilution extemporaneously done to reach the final indicated concentration. The agents were incubated with the fat cells at 37 °C under constant, gentle, shaking during 90 min. Incubations were stopped by placing the incubation tubes on ice. As already documented, lipolytic activity was determined by using glycerol release as an index[20], since FFA release follows parallel variations in our experimental conditions[21]. Once the buoyant adipocytes were frosted, 150 μL of medium were removed for glycerol spectrophotometric measurement at 340 nm, after addition of 1.5 mL of chromogenic mixture (0.6 mmol/L NAD, 1.4 mmol/L ATP, 0.2 mol/L glycine, 1 mol/L hydrazine, with 15 unit/mL glycerol phosphate dehydrogenase, and 0.6 unit/mL glycerokinase, pH 9.8), as previously described[22].
An isotopic dilution of [3H]-2-deoxyglucose (2-DG) was added at a final concentration of 0.1 mmol/L (approximately 1300000 dpm/vial) to 400 μL of cell suspension after 45 min preincubation with the tested agents. Human fat cells were incubated for additional 10 min and then stopped with 100 μL of 100 μmol/L cytochalasin B. Aliquotes (200 μL) of shaken cell suspension were immediately centrifuged in microtubes containing dinonyl phthalate of density 0.98 g/mL, which allowed to separate the adipocytes as previously described[23]. The radiolabelled hexose internalized in viable fat cells (upper part of the tubes) was then counted in scintillation vials. The extracellular 2-DG present was determined using adipocytes whose transport activity was previously blocked by cytochalasin B at time 0. It did not exceed 1% of the maximum 2-DG uptake in the presence of insulin and was subtracted from the assays.
De novo lipogenic activity was determined by quantifying the D-[3-3H]-glucose incorporation into lipids in mouse fat cells, according to[21]. They were incubated at 37 °C for 120 min in the same incubation medium as above, only containing only 0.6 mmol/L of isotopic glucose dilution as source of carbohydrates. The same vials were used for incubation, lipid extraction in an organic mixture for liquid scintillation (InstaFluorPlus) and counting of the labelled neo-synthesized lipids, following a procedure adapted from Moody et al[24].
Hexaquis(benzylammonium) decavanadate (B6V10) was synthesized and purified by Fernando Albericio and coworkers as previously detailed[16] and kindly given by Genmedica (Barcelona, Spain). Benzylamine, sodium orthovanadate, (-)-isoprenaline hydrochloride (isoproterenol), atrial natriuretic peptide (ANP), collagenase type II and other reagents were from Sigma-Aldrich (Saint Quentin Fallavier, France). 2-DG and D-3-[3H]-glucose were from Perkin Elmer (Boston, MA, United States).
Results are presented as means ± standard error of the means (SEM) of (n) observations. Statistical analysis for comparisons between B6V10 and respective control used Student’s t test.
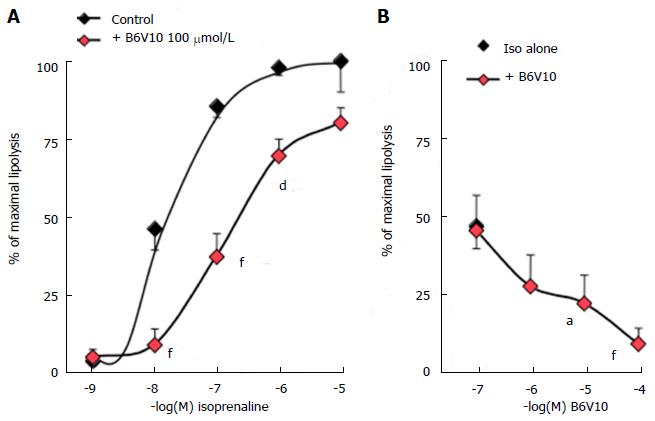
It is necessary to moderately activate lipolysis to detect whether a putative antilipolytic agent is able to limit triglyceride breakdown. This approach was first performed in rat adipocytes. The β-adrenergic agonist isoprenaline increased lipolytic activity in a typical concentration-dependent manner, and reached maximal activation at 1-10 μmmol/L. Addition of 100 μmmol/L of hexaquis(benzylammonium) decavanadate (B6V10) to increasing doses of isoprenaline impaired the β-adrenergic stimulation, clearly shifting the dose-response curve (Figure 1A). Noteworthy, the conjugate B6V10 did not alter the maximal effect of the highest isoprenaline dose. Similarly, the lowest dose of isoprenaline did not activate lipolysis sufficiently to allow any detection of B6V10 effect. Then, increasing concentrations of B6V10 were tested against an intermediate dose of isoprenaline (10 nmmol/L). In this condition, B6V10 dose-dependently inhibited the lipolytic activation induced by the β-agonist (Figure 1B). The conjugate therefore exhibited a clear and rapid antilipolytic effect in rat adipocytes, a cell model in which B6V10 has been already reported to mimic another insulin action: Glucose transport activation[13].
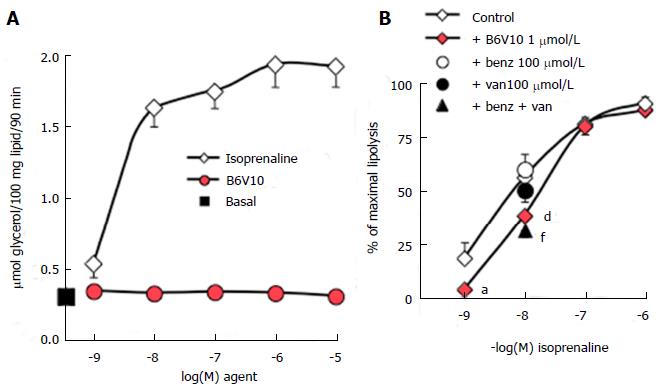
Further studies performed on mouse adipocytes confirmed that increasing doses of B6V10 did not affect basal lipolysis, which was readily activated by isoprenaline (Figure 2A). Such lack of effect indicated that the conjugate was not lipolytic. However, other recognized antilipolytic agents, including insulin, were also unable to lower basal glycerol release (not shown). Consistent with our data obtained using rat adipocytes, activation of lipolytic activity was required to unmask putative antilipolytic effects. Consequently, B6V10 was tested at 1 μmmol/L in the presence of increasing doses of isoprenaline (Figure 2B). B6V10 did not impair the maximal lipolysis promoted by 0.1 and 1 μmmol/L of the β-adrenergic agonist, but it impaired the submaximal stimulation by 1 nmol/L and 10 nmol/L isoprenaline. When tested separately, the components of B6V10, benzylamine and sodium orthovanadate, did not alter the lipolytic effect of 10 nmol/L isoprenaline, while their combination at 100 μmol/L each was as antilipolytic as B6V10 (Figure 2B).
Thus, the antilipolytic action of B6V10 was detectable only when lipolysis was mildly activated by the β-adrenergic agonist isoprenaline. This could suggest that B6V10 was acting by antagonizing activation of β-adrenergic receptors. To ascertain that an antagonism at β-adrenergic receptors was not mandatory to observe a response to the conjugate, we verified its direct effect on glucose utilization in mouse fat cells. When tested alone at 10 μmol/L, B6V10 reproduced 49.0% ± 7.8% of the de novo lipogenic action of 100 nmol/L insulin, which was equivalent to a threefold increase over the basal values of the incorporation of radiolabelled glucose into the lipids of mouse adipocytes (n = 5, not shown). At 100 μmol/L, B6V10 reached 85.0% ± 3.5% of the maximal lipogenic effect of insulin. These data supported that the conjugate was active per se on adipocytes through a mechanism distinct from antagonism at β-adrenoceptors, since these G-coupled receptors were not activated during the test of lipogenic activity. Moreover, this verification confirmed our previous characterization of B6V10 as an insulin-mimicking agent, acting through a hydrogen peroxide-dependent mechanism on the stimulation of glucose transport into fat cells[13]. In this context, the antilipolytic effect and the lipogenic effects of B6V10 could be considered as additional facets to the multiple B6V10 insulin-mimicking properties.
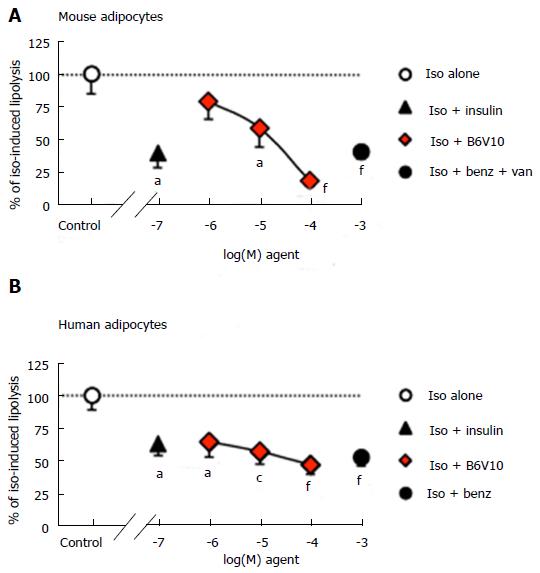
Antilipolytic effects of insulin and B6V10 were then compared in mouse fat cells and in human adipocytes. In mouse, lipolysis was moderately activated by 10 nmol/L isoprenaline, reaching 1.28 ± 0.11 μmoles glycerol released/100 mg cell lipids/90 min. This sub-maximal stimulation of lipolysis represented an optimal condition to detect any putative induction or blockade by the tested agents. Figure 3 shows that the antilipolytic effect of a relatively high dose of insulin (100 nmol/L) was significant although incomplete. A similar partial antilipolytic effect was observed with 1 mmol/L benzylamine only in the presence of 0.1 mmol/L vanadate. The dose-dependent antilipolytic effect of B6V10 led to a stronger lipolysis inhibition than with insulin or benzylamine, at least when the conjugate was tested at 100 μmol/L (Figure 3A).
In freshly prepared human adipocyte suspensions, basal lipolysis was maximally activated by 10 μmol/L of isoprenaline (532% ± 107% of basal) but was unaltered by insulin alone (89% ± 16% of basal, n = 10, not shown). The stimulation of glycerol release by the dose of isoprenaline used in mice (10 nmol/L) was also submaximal. This dose triggered the production of 0.67 ± 0.08 μmol of glycerol/100 mg of cell lipids/90 min in human adipocyte preparations, while basal release was 0.20 ± 0.07 μmol glycerol/100 mg lipids/90 min. This lipolytic activation was considered as a 100% reference for testing the influence of insulin (100 nmol/L), benzylamine (1 mmol/L), or increasing doses of B6V10 (1-100 μmol/L) (Figure 3B). All these agents partially but significantly limited the β-adrenergic-induced lipolysis. When tested at 1 mmol/L, antilipolytic activity of benzylamine was as efficient as 100 μmol/L B6V10. The addition of vanadium did not enhance its effect (not shown).
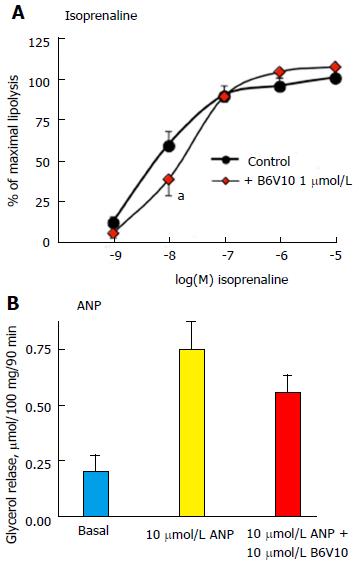
Further analyses of the B6V10 antilipolytic effect were performed on human adipocytes and showed that 1 μmol/L of the conjugate could not impair the maximal lipolysis stimulation by 0.1, 1 or 10 μmol/L isoprenaline, while it impaired the submaximal β-adrenergic activation of glycerol release (Figure 4A). Similarly, no significant inhibition by B6V10 was detected on 1 nmol/L isoprenaline, when glycerol release values were close to basal levels. Moreover, B6V10 tended to limit the maximal effect of another strong lipolytic stimulator: The ANP, only active in human adipocytes[25,26] (Figure 4B).
In agreement with our data obtained in rodent adipocytes, micromolar doses of B6V10 were only limiting moderate lipolysis activation in human adipocytes. Thus, B6V10 appears to essentially hamper modest lipolytic activations, as those corresponding to the physiological modulation of triglyceride breakdown during interprandial cycles of energy supply and energy demand.
Lastly, we explored whether the conjugate B6V10 could activate glucose transport in human adipocytes alongside its repression of triglyceride breakdown. In fact, previous studies that have demonstrated an insulin-like action of B6V10 on hexose uptake were restricted to murine adipocytes[13].
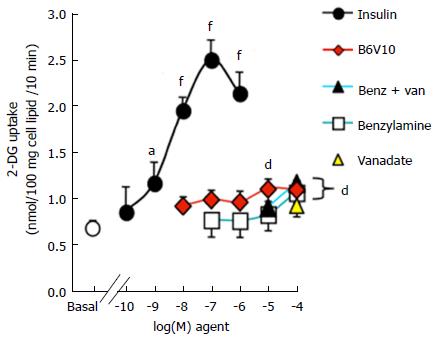
Here we show that freshly isolated human adipocyte preparations were highly sensitive to insulin, since 100 nmol/L of the hormone induced a four-fold increase in basal 2-deoxyglucose uptake (Figure 5). There was no significant effect of B6V10 on glucose uptake when added at inframicromolar doses. However, at 10 and 100 μmol/L, B6V10 reproduced approximately one third of the insulin stimulation of glucose transport, resulting in a highly significant activation. Sodium orthovanadate did not stimulate glucose uptake at 100 μmol/L and had no synergic effect with 10 or 100 μmol/L benzylamine. Indeed, when present at 0.1 mmol/L, benzylamine was as effective as 10 μmol/L B6V10 at stimulating hexose transport (Figure 5).
The property of B6V10, a conjugate of benzylamine and decavanadate, to lower blood glucose has been reported together with in vitro demonstration of its ability to activate glucose transport and insulin signalling in adipocytes[13,14], while its ability to lower circulating FFAs in diabetic rats remains unexplained. Here we report for the first time that B6V10 is directly antilipolytic in murine and human adipocytes, a major finding regarding the growing interest for antilipolytic agents that may limit the harmful outcomes of lipotoxicity in diabesity[11,27,28]. Our observation using 1 μmol/L of the conjugate in human adipocytes, adds therefore a new insight to the development of vanadium-containing antidiabetic compounds, although it remains to avoid an overlap between their therapeutic and toxic doses. Accordingly, we are discussing below about the interest of inhibiting adipocyte lipolysis to reduce lipotoxicity, and about the possibility to develop vanadium-containing antidiabetic and anti-obesity agents that could become “drugable”, two issues independently covered in very recent reviews[5,29].
In diabesity, when AT has developed an insulin resistance, the fatty acid storage in hypertrophied adipocytes under the form of triglycerides becomes limited due to a decrease in lipogenic and antilipolytic action of insulin. Such reduced insulin responsiveness derepresses lipolysis in adipocytes and leads to ectopic FFA deposition (in liver, vessels, muscles, endocrine glands), which in turn hampers glucose utilization and lipid oxidation in all these organs. To combat this lipotoxicity, there is a need to control excessive FFA mobilization from AT that requires the characterization of potent antilipolytic factors and constitutes a novel therapeutic approach for the treatment of obesity complications. In fact, there is mounting evidence that antilipolytic agents limiting the release of non-esterified fatty acid and glycerol into the blood stream, should be considered as antidiabetic or anti-obesity agents[5]. In this regard, the reversal of lipotoxicity is proposed to contribute to the beneficial effects of old drugs, such as pioglitazone. These “novel” properties are added to the anti-inflammatory properties of pioglitazone that improve metabolic and secretory functions in adipocytes and β-cells[27], and lead to re-examine this old antihyperglycemic agent as a treatment for non-alcoholic fatty liver disease (NAFLD) that often complicates type 2 diabetes[28]. It is important to note that lipotoxicity can lead to severe NAFLD even when insulin resistance in AT is not concomitant with obesity. Indeed, the transgenic mice carrying fat-specific knockout of the insulin receptor are characterized by severe atrophy of fat depots, pronounced diabetes, and marked fatty liver disease[30]. Thus, it seems safer to limit ectopic lipid deposition by restricting excessive FFA release and blunting insulin resistance in adipocytes, even at the expense of maintaining adipose mass, than to overstimulate fat store mobilization. One has to keep in mind that one of the most powerful lipolytic agents, TNF-α, does not help in mitigating the deleterious outcomes of insulin resistance: On the opposite, it strongly desensitizes to insulin action and promotes inflammation.
Since we previously reported that chronic treatment with B6V10 lowered plasma FFA in diabetic rats[13], we asked whether this agent could be effective in lowering adipocyte lipolysis. In this comparative work, we brought compelling evidence that the conjugate inhibits lipolysis in rodent adipocytes with an efficiency greater than the ones obtained with its components used separately (benzylamine and decavanadate). Indeed, we have previously characterized B6V10 as an agent that exerts in adipocytes potent insulin-mimetic effects downstream the insulin receptor, in a manner that is sensitive to SSAO/VAP-1 inhibition and which reproduces the synergism between benzylamine and vanadate[13,14]. The effective doses of B6V10 in inhibiting lipolysis in rodent adipocytes are superimposable to those necessary for glucose transport stimulation. Moreover, our de novo lipogenesis experiments add to the list of B6V10 insulin-like effects[14] its capacity to activate glucose incorporation into the neosynthesized lipids in mouse adipocytes.
The fact that B6V10 was unable to impair maximal activation of lipolysis in all the models studied is not a concern since the amplitude of increased lipolytic activity of adipocytes is much lower in pathological states of insulin resistance than the activation that physiologically emerges during prolonged fasting or cold exposure[31]. Noteworthy, human adipocytes exhibited both lipolysis inhibition and glucose uptake activation in response to B6V10. Our data clearly show that the in vitro antilipolytic effect of a relatively high dose of insulin was not complete in subcutaneous adipocytes of sedentary women. Since insulin inhibited only by one-third of the response to isoprenaline, and since this effect was fully reproduced by 1 μmol/L of B6V10 (i.e., at a dose only tenfold higher than that necessary for insulin), the conjugate can be definitely considered as a good insulin mimicker. Increasing the dose of B6V10 up to 100 μmol/L resulted in a higher but partial inhibition of lipolysis. Therefore, B6V10 could surpass insulin-like antilipolytic action in adipocytes from overweight subjects, who exhibited weak insulin sensitivity, although being non-obese and non-diabetic. Yet, the insulin antilipolytic response appeared to be more altered in these individuals than the insulin activation of glucose transport. The latter reached a four-fold stimulation of basal uptake in our conditions, which does not denote a fully developed insulin-resistant state for human fat cells[32]. Describing the exact onset of these defects was not in the scope of our studies, but deserves to be performed in future clinical studies, taking into account the influence of gender and fat depot anatomical location. Actually, it can be noticed that the maximal antilipolytic response to B6V10 was lower in human adipocytes than in rat and mouse models.
B6V10 is one of the promising antihyperglycaemic agents belonging to the wide family of vanadium derivatives. It can be summarized that, once ingested, vanadium is found in the organism under a cationic (vanadyl) or anionic (vanadate) form, the latter resembling to a phosphate group. In fact, orthovanadate (H2VO4-) interacts with a pleiad of cellular components interacting with H2PO4-, e.g., enzymes influenced by (de)phosphorylation state. Yet, the ability of vanadium to mimic insulin actions in rat adipocytes has been reported in the 80s and univocally confirmed in all the insulin-sensitive tissues expressing GLUT4. Furthermore, we observed that vanadate and vanadyl were equally efficient in totally inhibiting rat adipocyte lipolysis at 1 mmol/L[33]. The current issue of vanadium pharmacology is to take advantage of these insulin-like properties without the concerns raised by the high degree of vanadium toxicity (due to accumulation in tissues like the kidneys and bones); in other terms: Lowering the risk/benefit ratio[29]. Among the various improvements raised by studies of chemico-biological interactions of vanadium derivatives[34], the vanadium peroxides, or pervanadates, formed by mixing vanadium and H2O2[16], have shown an increase in the potency for insulinomimetic actions in adipocytes[29]. With pervanadates, the effective doses were lowered from millimolar to micromolar range, as they are irreversible inhibitors of various phosphatases and act on target cells at much lower doses than vanadate. Recently, we synthesized and characterized salts composed by arylalkylamines combined with decavanadate that permitted to lower the effective antidiabetic dose of vanadium to non-toxic levels. The more active compound of this series, namely B6V10, mixes decavanadate, a complex form that increases adipocyte glucose uptake more potently than other vanadium forms[35], with benzylamine, also behaving as an insulin mimicker in human fat cells[36]. These two halves were already described to act synergistically, especially in fat cells where benzylamine is oxidized by the highly expressed SSAO/VAP-1, thereby generating hydrogen peroxide[37], which in turn reacts with vanadate to generate peroxovanadate. This compound then inhibits protein tyrosine phosphatases and triggers glucose carrier translocation and hexose transport activation[38]. This cascade of events results in a substantial antihyperglycaemic action in diabetic rodents that is more potent than the separate effects of benzylamine and vanadate[39]. All these insulin-like actions disappear when SSAO/VAP-1 is pharmacologically inhibited or genetically invalidated[40]. Thus, when B6V10 undergoes oxidation by SSAO/VAP-1, it generates peroxovanadate and acts in vitro[16] as well as in vivo[14] to trigger antidiabetic actions. By releasing the real active vanadium-based ligands that interact with phosphatases near the target cells, the B6V10 is therefore a mean to improve decavanate “speciation” (see review from Scior and coworkers for further details[29]) and to circumvent the concerns raised by decavanadate toxicology[41].
Our in vitro analysis reveals a potent antilipolytic action of B6V10, which might be helpful in combating the lipotoxicity that participates to diabesity complications. Several concerns to this therapeutic potential could be raised since our experiments were performed only in adipocytes isolated from subcutaneous abdominal depots of overweight women.
The first concern could be the relevance of our observations for visceral adipocytes from massively obese subjects, considered as more harmful. Indeed, clinical studies have demonstrated that impaired triglyceride storage also occurs in the subcutaneous AT of insulin-resistant individuals when compared to their BMI-matched controls classified as insulin-sensitive[42]. Using deuterated water prolonged administration and functional exploration of subcutaneous AT, these studies elegantly indicated that, during the onset of type 2 diabetes in humans, there was a clear defect in insulin suppression of lipolysis and activation of de novo lipogenesis in the subcutaneous adipocytes themselves.
A second concern could be raised regarding the fact that we have only determined glycerol release as an index of lipolysis, while lipotoxicity is mainly supported by excessive FFA release. Previous studies on AT lipolysis and insulin sensitivity have evidenced a tight relationship between spontaneous glycerol production by human AT explants and insulin resistance in a large cohort of subjects presenting a wide range of BMI[43]. According to Girousse et al[43], both lipolysis end-products, glycerol and FFAs, were equivalent to show that partial inhibition of AT lipolysis improves insulin sensitivity[43].
Another limitation regarding the maximal antilipolytic capacity of B6V10 is that it is not complete and can be surpassed by various stronger antilipolytic agents (such as nicotinic acid, purinergic or α2-adrenergic agonists, see[32]). However, these agents are unable to activate glucose uptake in human adipocytes (C. Carpéné unpublished observations) and do not offer the dual interest of B6V10 to lower both circulating glucose and lipids.
Lastly, insulin also plays lipogenic and antilipolytic actions when infused into the hypothalamus of rats[44]. Whether B6V10 also mimics insulin actions in the brain, in a manner that could influence its antidiabetic and lipid-lowering action during chronic treatment remains unknown and deserves further in vivo studies in insulin-resistant models.
Though being clearly antilipolytic in human adipocytes, 1 μmol/L B6V10 was not more effective than 1 mmol/L benzylamine, and the combination of vanadate with benzylamine did not lead to the synergism found in rat adipocytes. Indeed, in human AT, the maximal antilipolytic effect of B6V10 was comparable to that of benzylamine, already described to hamper about one-half of stimulated lipolysis[36]. Regarding the glucose uptake in human adipocytes, B6V10 is clearly stimulating, but there is no synergism between its components, benzylamine and vanadate, each one reproducing at 100 μmol/L the effect of 10 μmol/L conjugate. This is in apparent agreement with the proposed lack of glucose transport activation by decavanadate in human adipocytes[35], and confirms the absence of potentiation between SSAO/VAP-1 substrates and vanadium regarding glucose transport in human adipocytes[33]. Therefore, while noticeable synergism between SSAO/VAP-1 substrates and decavanadate occurs when using B6V10 in murine adipocytes, this apparently does not work as well in human fat cells, for a reason that remains to be elucidated.
Consequently, our comparative approach indicates that B6V10 cannot be immediately considered for clinical application as an efficient mean to increase the benefit/risk ratio of vanadium regarding its therapeutic antidiabetic indication. Nevertheless, it must be noted that, although B6V10 is not the most potent and powerful antilipolytic agents described so far in human adipocytes, it combines two insulin-like actions: Limiting lipolysis and increasing glucose uptake. In this regard, it should be considered as a valuable candidate to further develop an approach based on the mitigation of lipotoxicity in diabesity. This adds an alternative to classical antilipolytic agents proposed to limit lipotoxicity, such as nicotinic acid (Acipimox)[12], or lipase inhibitors[5]. Another consequence of our depicted interspecies differences is that the exploration of the antilipolytic properties in human adipose cells deserves to be applied to other vanadium conjugates recently tested with success on diabetic rodents, such as those combining metformin and decavanadate[45].
In conclusion, the conjugate of benzylamine and decavanadate B6V10 exerts insulin-like actions in human adipocytes, including lipolysis inhibition and glucose transport activation.
We thank the staff of plastic surgery of Rangueil Hospital (Toulouse, F) for providing us with surgical samples from abdominal lipectomies and Anne Bouloumié for helpful discussions. The authors also thank Anais Briot for careful perusal of the manuscript and editorial improvements.
Insulin resistance of adipocytes in hypertrophied fat depots leads to an increased lipolytic activity releasing in the circulation excessive amounts of free fatty acids (FFA) that accumulates under the form of triglyceride-rich ectopic lipid droplets in liver and muscles. The conjugate salt hexaquis(benzylammonium) decavanadate has been reported to lower circulating glucose and FFA in diabetic rodents, but its direct action of adipocyte lipolytic activity has never been assessed.
This in vitro approach definitely brings evidence that B6V10 reproduces the rapid antilipolytic action of insulin in murine and human fat cells. At 100 μmol/L, B6V10 even surpasses the maximal inhibition of lipolysis induced by the pancreatic hormone. Since the molecule also stimulates glucose uptake in human adipocytes and has been demonstrated to exert antihyperglycemic actions in murine models of diabetes, it can be qualified as insulin mimicker.
In vitro, B6V10 exerts various insulin-like actions in human adipocytes including lipolysis inhibition and glucose uptake activation. This conjugate salt of benzylamine and decavanadate has the potential to alleviate the deleterious complications linked to the insulin resistance of adipocyte antilipolytic/lipogenic activities emerging in morbid obese and diabetic patients, and could be considered as a potential antidiabetic agent.
B6V10 could be useful as an auxiliary therapy in limiting the lipotoxicity related to obesity and insulin resistance. Its chronic administration might delay ectopic fat deposition and should reduce hepatic steatosis whether active in obese patients at doses acting in fat stores without exerting adverse effects elsewhere in the organism.
ANP: Atrial natriuretic peptide; AT: Adipose tissue; BMI: Body mass index; B6V10: Hexaquis(benzylammonium) decavanadate; FFA: Free fatty acids; GLUT4: Insulin-sensitive glucose transporter; H2O2: Hydrogen peroxide; SEM: Standard error of the mean; SSAO/VAP-1: Semicarbazide-sensitive amine oxidase, identical to VAP-1 (vascular adhesion protein-1).
Manuscript presents solid data and is of good quality.
Manuscript source: Invited manuscript
Specialty type: Endocrinology and metabolism
Country of origin: France
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Efanov AM, Raghow RS S- Editor: Kong JX L- Editor: A E- Editor: Wu HL
| 1. | Astrup A, Finer N. Redefining type 2 diabetes: 'diabesity' or 'obesity dependent diabetes mellitus'? Obes Rev. 2000;1:57-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 253] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 2. | Schenk S, Saberi M, Olefsky JM. Insulin sensitivity: modulation by nutrients and inflammation. J Clin Invest. 2008;118:2992-3002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 843] [Cited by in RCA: 861] [Article Influence: 50.6] [Reference Citation Analysis (0)] |
| 3. | Chakraborti CK. Role of adiponectin and some other factors linking type 2 diabetes mellitus and obesity. World J Diabetes. 2015;6:1296-1308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 58] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 4. | Lee MJ, Wu Y, Fried SK. Adipose tissue heterogeneity: implication of depot differences in adipose tissue for obesity complications. Mol Aspects Med. 2013;34:1-11. [PubMed] |
| 5. | Morigny P, Houssier M, Mouisel E, Langin D. Adipocyte lipolysis and insulin resistance. Biochimie. 2016;125:259-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 320] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 6. | Wajchenberg BL, Giannella-Neto D, da Silva ME, Santos RF. Depot-specific hormonal characteristics of subcutaneous and visceral adipose tissue and their relation to the metabolic syndrome. Horm Metab Res. 2002;34:616-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 268] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 7. | Gastaldelli A, Cusi K, Pettiti M, Hardies J, Miyazaki Y, Berria R, Buzzigoli E, Sironi AM, Cersosimo E, Ferrannini E. Relationship between hepatic/visceral fat and hepatic insulin resistance in nondiabetic and type 2 diabetic subjects. Gastroenterology. 2007;133:496-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 413] [Cited by in RCA: 451] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 8. | Liu A, McLaughlin T, Liu T, Sherman A, Yee G, Abbasi F, Lamendola C, Morton J, Cushman SW, Reaven GM. Differential intra-abdominal adipose tissue profiling in obese, insulin-resistant women. Obes Surg. 2009;19:1564-1573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 9. | Lomonaco R, Ortiz-Lopez C, Orsak B, Webb A, Hardies J, Darland C, Finch J, Gastaldelli A, Harrison S, Tio F. Effect of adipose tissue insulin resistance on metabolic parameters and liver histology in obese patients with nonalcoholic fatty liver disease. Hepatology. 2012;55:1389-1397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 339] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 10. | Lee Y, Hirose H, Ohneda M, Johnson JH, McGarry JD, Unger RH. Beta-cell lipotoxicity in the pathogenesis of non-insulin-dependent diabetes mellitus of obese rats: impairment in adipocyte-beta-cell relationships. Proc Natl Acad Sci USA. 1994;91:10878-10882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 590] [Cited by in RCA: 622] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 11. | Saponaro C, Gaggini M, Carli F, Gastaldelli A. The Subtle Balance between Lipolysis and Lipogenesis: A Critical Point in Metabolic Homeostasis. Nutrients. 2015;7:9453-9474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 265] [Cited by in RCA: 367] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 12. | Santomauro AT, Boden G, Silva ME, Rocha DM, Santos RF, Ursich MJ, Strassmann PG, Wajchenberg BL. Overnight lowering of free fatty acids with Acipimox improves insulin resistance and glucose tolerance in obese diabetic and nondiabetic subjects. Diabetes. 1999;48:1836-1841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 308] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 13. | García-Vicente S, Yraola F, Marti L, González-Muñoz E, García-Barrado MJ, Cantó C, Abella A, Bour S, Artuch R, Sierra C. Oral insulin-mimetic compounds that act independently of insulin. Diabetes. 2007;56:486-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 14. | Zorzano A, Palacín M, Marti L, García-Vicente S. Arylalkylamine vanadium salts as new anti-diabetic compounds. J Inorg Biochem. 2009;103:559-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Yraola F, Zorzano A, Albericio F, Royo M. Structure-activity relationships of SSAO/VAP-1 arylalkylamine-based substrates. ChemMedChem. 2009;4:495-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Yraola F, García-Vicente S, Marti L, Albericio F, Zorzano A, Royo M. Understanding the mechanism of action of the novel SSAO substrate (C7NH10)6(V10O28).2H2O, a prodrug of peroxovanadate insulin mimetics. Chem Biol Drug Des. 2007;69:423-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Mercader J, Wanecq E, Chen J, Carpéné C. Isopropylnorsynephrine is a stronger lipolytic agent in human adipocytes than synephrine and other amines present in Citrus aurantium. J Physiol Biochem. 2011;67:443-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 18. | Iffiú-Soltész Z, Prévot D, Carpéné C. Influence of prolonged fasting on monoamine oxidase and semicarbazide-sensitive amine oxidase activities in rat white adipose tissue. J Physiol Biochem. 2009;65:11-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Carpéné C, Gomez-Zorita S, Gupta R, Grès S, Rancoule C, Cadoudal T, Mercader J, Gomez A, Bertrand C, Iffiu-Soltész Z. Combination of low dose of the anti-adipogenic agents resveratrol and phenelzine in drinking water is not sufficient to prevent obesity in very-high-fat diet-fed mice. Eur J Nutr. 2014;53:1625-1635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Visentin V, Morin N, Fontana E, Prévot D, Boucher J, Castan I, Valet P, Grujic D, Carpéné C. Dual action of octopamine on glucose transport into adipocytes: inhibition via beta3-adrenoceptor activation and stimulation via oxidation by amine oxidases. J Pharmacol Exp Ther. 2001;299:96-104. [PubMed] |
| 21. | Les F, Deleruyelle S, Cassagnes LE, Boutin JA, Balogh B, Arbones-Mainar JM, Biron S, Marceau P, Richard D, Nepveu F. Piceatannol and resveratrol share inhibitory effects on hydrogen peroxide release, monoamine oxidase and lipogenic activities in adipose tissue, but differ in their antilipolytic properties. Chem Biol Interact. 2016;258:115-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 22. | Visentin V, Prévot D, Marti L, Carpéné C. Inhibition of rat fat cell lipolysis by monoamine oxidase and semicarbazide-sensitive amine oxidase substrates. Eur J Pharmacol. 2003;466:235-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Iglesias-Osma MC, Bour S, Garcia-Barrado MJ, Visentin V, Pastor MF, Testar X, Marti L, Enrique-Tarancon G, Valet P, Moratinos J. Methylamine but not mafenide mimics insulin-like activity of the semicarbazide-sensitive amine oxidase-substrate benzylamine on glucose tolerance and on human adipocyte metabolism. Pharmacol Res. 2005;52:475-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Moody AJ, Stan MA, Stan M, Gliemann J. A simple free fat cell bioassay for insulin. Horm Metab Res. 1974;6:12-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 279] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 25. | Sengenès C, Berlan M, De Glisezinski I, Lafontan M, Galitzky J. Natriuretic peptides: a new lipolytic pathway in human adipocytes. FASEB J. 2000;14:1345-1351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 175] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 26. | Moro C, Crampes F, Sengenes C, De Glisezinski I, Galitzky J, Thalamas C, Lafontan M, Berlan M. Atrial natriuretic peptide contributes to physiological control of lipid mobilization in humans. FASEB J. 2004;18:908-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 121] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 27. | Agrawal NK, Kant S. Targeting inflammation in diabetes: Newer therapeutic options. World J Diabetes. 2014;5:697-710. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 125] [Cited by in RCA: 139] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 28. | Cusi K. Treatment of patients with type 2 diabetes and non-alcoholic fatty liver disease: current approaches and future directions. Diabetologia. 2016;59:1112-1120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 107] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 29. | Scior T, Guevara-Garcia JA, Do QT, Bernard P, Laufer S. Why antidiabetic vanadium complexes are not in the pipeline of “Big Pharma” drug research? A critical review. Curr Med Chem. 2016;23:2874-2891. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 30. | Softic S, Boucher J, Solheim MH, Fujisaka S, Haering MF, Homan EP, Winnay J, Perez-Atayde AR, Kahn CR. Lipodystrophy Due to Adipose Tissue-Specific Insulin Receptor Knockout Results in Progressive NAFLD. Diabetes. 2016;65:2187-2200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 94] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 31. | Galitzky J, Nibbelink M, Lafontan M, Ambid L, Carpéné C. Cold-exposure reduces adiposity and increases thermogenic capacity in guinea pigs despite their lack of adipocyte β3-adrenergic responsiveness. Fundam Clin Pharmacol. 1995;9:74. |
| 32. | Carpéné C, Galitzky J, Saulnier-Blache JS. Short-term and rapid effects of lysophosphatidic acid on human adipose cell lipolytic and glucose uptake activities. AIMS Molec Sci. 2016;3:222-237. [RCA] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 33. | Missaoui S, Abello V, Prévot D, Testar X, Carpéné C. Comparison of the insulin-like effects of vanadate, vanadyl, and tungstate in rodent and human fat cells. in: Metal Ions in Biology and Medecine Eds: P Collery, John Libbey Eurotext, Paris. 2008;10:776-781. |
| 34. | Pereira MJ, Carvalho E, Eriksson JW, Crans DC, Aureliano M. Effects of decavanadate and insulin enhancing vanadium compounds on glucose uptake in isolated rat adipocytes. J Inorg Biochem. 2009;103:1687-1692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 35. | Aureliano M. Recent perspectives into biochemistry of decavanadate. World J Biol Chem. 2011;2:215-225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 46] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 36. | Morin N, Lizcano JM, Fontana E, Marti L, Smih F, Rouet P, Prévot D, Zorzano A, Unzeta M, Carpéné C. Semicarbazide-sensitive amine oxidase substrates stimulate glucose transport and inhibit lipolysis in human adipocytes. J Pharmacol Exp Ther. 2001;297:563-572. [PubMed] |
| 37. | Marti L, Abella A, De La Cruz X, García-Vicente S, Unzeta M, Carpéné C, Palacín M, Testar X, Orozco M, Zorzano A. Exploring the binding mode of semicarbazide-sensitive amine oxidase/VAP-1: identification of novel substrates with insulin-like activity. J Med Chem. 2004;47:4865-4874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 38. | Enrique-Tarancón G, Castan I, Morin N, Marti L, Abella A, Camps M, Casamitjana R, Palacín M, Testar X, Degerman E. Substrates of semicarbazide-sensitive amine oxidase co-operate with vanadate to stimulate tyrosine phosphorylation of insulin-receptor-substrate proteins, phosphoinositide 3-kinase activity and GLUT4 translocation in adipose cells. Biochem J. 2000;350 Pt 1:171-180. [PubMed] |
| 39. | Marti L, Abella A, Carpéné C, Palacín M, Testar X, Zorzano A. Combined treatment with benzylamine and low dosages of vanadate enhances glucose tolerance and reduces hyperglycemia in streptozotocin-induced diabetic rats. Diabetes. 2001;50:2061-2068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 51] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 40. | Bour S, Prévot D, Guigné C, Stolen C, Jalkanen S, Valet P, Carpéné C. Semicarbazide-sensitive amine oxidase substrates fail to induce insulin-like effects in fat cells from AOC3 knockout mice. J Neural Transm (Vienna). 2007;114:829-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 41. | Aureliano M. Decavanadate Toxicology and Pharmacological Activities: V10 or V1, Both or None? Oxid Med Cell Longev. 2016;2016:6103457. [PubMed] |
| 42. | Allister CA, Liu LF, Lamendola CA, Craig CM, Cushman SW, Hellerstein MK, McLaughlin TL. In vivo 2H2O administration reveals impaired triglyceride storage in adipose tissue of insulin-resistant humans. J Lipid Res. 2015;56:435-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 43. | Girousse A, Tavernier G, Valle C, Moro C, Mejhert N, Dinel AL, Houssier M, Roussel B, Besse-Patin A, Combes M. Partial inhibition of adipose tissue lipolysis improves glucose metabolism and insulin sensitivity without alteration of fat mass. PLoS Biol. 2013;11:e1001485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 170] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 44. | Scherer T, O'Hare J, Diggs-Andrews K, Schweiger M, Cheng B, Lindtner C, Zielinski E, Vempati P, Su K, Dighe S. Brain insulin controls adipose tissue lipolysis and lipogenesis. Cell Metab. 2011;13:183-194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 211] [Cited by in RCA: 188] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 45. | Treviño S, Sánchez-Lara E, Sarmiento-Ortega VE, Sánchez-Lombardo I, Flores-Hernández JÁ, Pérez-Benítez A, Brambila-Colombres E, González-Vergara E. Hypoglycemic, lipid-lowering and metabolic regulation activities of metforminium decavanadate (H2Metf)3 [V10O28]·8H2O using hypercaloric-induced carbohydrate and lipid deregulation in Wistar rats as biological model. J Inorg Biochem. 2015;147:85-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |