Published online Feb 15, 2015. doi: 10.4239/wjd.v6.i1.151
Peer-review started: August 29, 2014
First decision: September 30, 2014
Revised: October 9, 2014
Accepted: December 16, 2014
Article in press: December 17, 2014
Published online: February 15, 2015
Processing time: 155 Days and 19.6 Hours
Type 2 diabetes is an emerging health challenge all over the world as a result of urbanization, high prevalence of obesity, sedentary lifestyle and other stress related factors compounded with the genetic prevalence. The health consequences and economic burden of the obesity and related diabetes mellitus epidemic are enormous. Different signaling molecules secreted by adipocytes have been implicated in the development of obesity and associated insulin resistance in type 2 diabetes. Human adiponectin, a 244-amino acid collagen-like protein is solely secreted by adipocytes and acts as a hormone with anti-inflammatory and insulin-sensitizing properties. Adiponectin secretion, in contrast to secretion of other adipokines, is paradoxically decreased in obesity which may be attributable to inhibition of adiponectin gene transcription. There are several mechanisms through which adiponectin may decrease the risk of type 2 diabetes, including suppression of hepatic gluconeogenesis, stimulation of fatty acid oxidation in the liver, stimulation of fatty acid oxidation and glucose uptake in skeletal muscle, and stimulation of insulin secretion. To date, no systematic review has been conducted that evaluate the potential importance of adiponectin metabolism in insulin resistance. In this review attempt has been made to explore the relevance of adiponectin metabolism for the development of diabetes mellitus. This article also identifies this novel target for prospective therapeutic research aiming successful management of diabetes mellitus.
Core tip: Diabetes mellitus and related metabolic disorders like obesity, dyslipidemia are emerging as major global health challenges in recent era. Adiponectin, an adipokine demands profound importance in the field of metabolomics due to its potential role in all these complications. Plasma adiponectin concentration is remarkably lower in subjects with metabolic disorders predicting its significant role as an important biomarker in disease prognosis. We have attempted to enlighten adiponectin function stretching its role as a modulator associating these metabolic obstacles. We believe, this article will surely contribute to the fundamental and clinical research in the field of diabetes and related complications.
- Citation: Ghoshal K, Bhattacharyya M. Adiponectin: Probe of the molecular paradigm associating diabetes and obesity. World J Diabetes 2015; 6(1): 151-166
- URL: https://www.wjgnet.com/1948-9358/full/v6/i1/151.htm
- DOI: https://dx.doi.org/10.4239/wjd.v6.i1.151
Rapid urbanization and change in life style has intensified the prevalence of obesity and dyslipidemia which plays crucial role in developing diabetes mellitus across the globe. Diabetes mellitus is a major public health concern with 382 million individuals being affected worldwide in 2013. Type 2 diabetes mellitus (T2DM) constitutes one of the major forms of diabetes disease burden associated with remarkably accelerated rates of microvascular obstacles and macrovascular disorders. Obesity and its association with developing type 2 diabetes is an interesting area of research for scientists in recent years. Insulin resistance is one of the earliest hallmarks of the pre-diabetic state and results from a complex interplay between obesity-favoring environmental factors, such as unrestricted supply of high-caloric foods and markedly increased sedentary lifestyle combined with a permissive genetic background. The high incidence is attributed to a combination of genetic susceptibility plus adoption of a high-calorie, low-activity lifestyle mainly by urban population.
Adipose tissue was long been identified as an energy storage organ but in recent times extensive studies revealed the role of adipose tissue as an important endocrine organ with a number of metabolic activities; thus its function as a storage organ is now far from reality[1]. Adiponectin, an adipose tissue derived hormone, is lower in obese subjects than their lean counterparts[2]. Epidemiological studies revealed that patients with diabetes and cardiovascular disease (CVD) has lower amount of adiponectin in their serum[3,4], and low serum adiponectin level can be an excellent predictor of developing type 2 diabetes and associated CVD in later stage[5-8]. Thus the role of adiponectin hormone as a potential biomarker for predicting the occurrence of type 2 diabetes is evolving as an interesting area in the study of metabolomics. In this review we aimed to highlight the potential beneficiary function of adiponectin in type 2 diabetes, dyslipidemia and obesity considering both genetic and biochemical approach.
In modern times rapid urbanization and change in lifestyle has increased the prevalence of obesity in manifold, especially the young generation has modified their food habits with high calorie junk foods. Furthermore rapid development of technology has increased the tendency of uptaking sedentary lifestyle with less or no work at all, increasing the chances of getting obese. Obesity which is a major global threat virtually affecting both developed and developing countries. In Central America easy access to high calorie food and adoption of sedentary lifestyle has increased the prevalence of both diabetes and obesity[9] where in developing countries like countries in Latin America[10] and East Asia rise in income has shifted the mass from low calorie whole grain diet to high calorie processed foods which is far energy dense affecting not only the adults but the children and adolescents as well. BMI or body mass index and WC or Waist Circumference is two major parameters to measure obesity[11]. Higher value of BMI (30 kg/m2) and WC increases the risk of type 2 diabetes, high cholesterol, high blood pressure and heart disease.
Type 2 is the most prevalent form of diabetes acco-unting 90%-95% of the cases, especially in developed countries[12]. According to recent estimates of the International Obesity Task Force, up to 1.7 billion people of the world’s population are at a heightened risk of weight-related, non-communicable diseases such as type 2 diabetes which is majorly a lifestyle disorder (International Diabetes Federation, 2004). According to International Diabetes Federation India accounts for the largest number of people (50.8 million) suffering from diabetes in the world, followed by China (43.2 million) and United States (26.8 million). This metabolic syndrome is closely associated with different macro and microvascular disorders[13] (Table 1). The most prevalent diabetic macrovascular complication is Cardiovascular disorder (CVD)[14], which in turn is associated with environmental risk factors as well as genetic predisposition. Antidiabetic drug metformin is particularly useful for overweight and obese diabetic patients. Our earlier report indicates that metformin is particularly useful to restore the antioxidant status of cells hampered in type 2 diabetes stress[15].
| Microvascular complications prevalence | Macrovascular complications prevalence |
| Retinopathy 23.7% | Cardiovascular disease 11.4% |
| Background 20.0% | Peripheral vascular disease 4.0% |
| Proliferative 3.7% | Cerebrovascular accidents 0.9% |
| Nephropathy 5.5% | Hypertension 38.0% |
| Peri-neuropathy 27.5% |
Therefore type 2 diabetes and obesity interplays together to exert more deteriorating effect incorporating other metabolic syndromes such as CVD, dyslipidemia and hypertension[16-25].
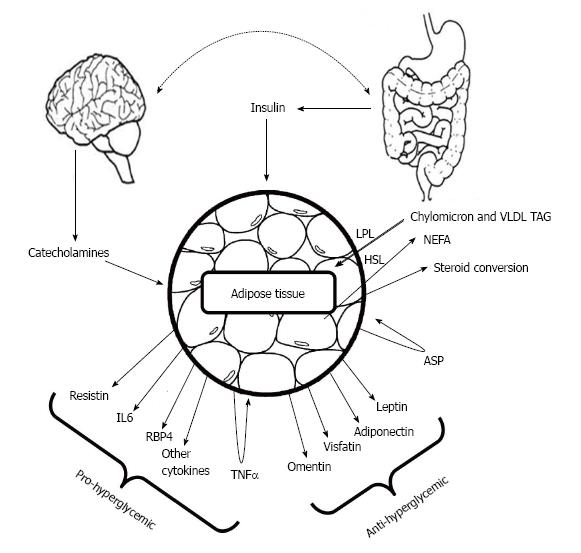
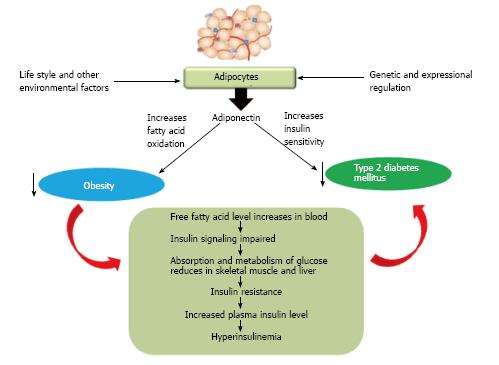
The correlation between rapidly emerging type 2 diabetes and obesity still remained a major question for researcher. It was hypothesized that metabolic dysfunction may cause to acquire obesity which in turn can develop type 2 diabetes. Adipocyte, the major energy storing cell is a storage site of a number of hormones as well whose prime function remains to govern lipid metabolism. Major adipocyte derived hormones are adiponectin, leptin, resistin and visfatin[26]. Leptin and adiponectin exerts positive effect on lowering blood glucose whereas resistin tends to increase blood glucose levels (Figure 1).
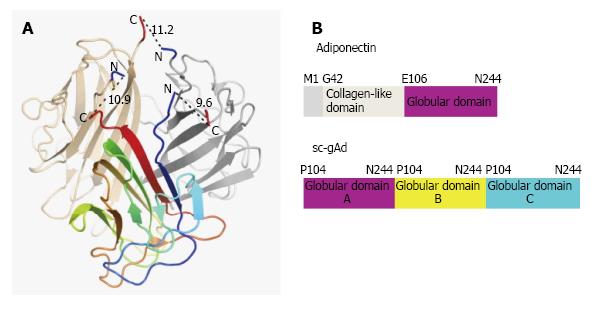
Reported for the first time by Scherer et al[27], 1995, Adiponectin, also known as Acrp30 (adipocyte complement-related protein of 30 kDa) is a protein exclusively secreted by adipocyte having huge structural similarity with C1q[27]. Three monomers (30 kDa) associate together at the globular domain to form the adiponectin trimer, where four to six trimers associate through their collagenous domains to form the high order structure. Monomeric adiponectin has not been observed in the plasma and it is believed to remain within adipocyte[28]. Human adiponectin is encoded by the ADIPOQ gene on the chromosomal locus 3q27 consisting three exons and two introns[29], involved in regulating glucose levels as well as fatty acid breakdown[1]. Mouse adiponectin is a 247 amino acid long protein where human adiponectin is a protein product of 244 amino acids consisting of four domains, an amino-terminal signal sequence, a variable region, a collagenous domain (cAd) consisting of 22 Gly-X-Y repeats, and a carboxy-terminal globular domain (gAd)[27]. It is the most abundant adipokines with its serum concentration ranging from 5 to 30 μg/mL[30].
Structure of single-chain globular domain adiponectin (sc-gAd) is reported (Figure 2), where globular domain is composed of three part A, B and C respectively[30]. The adiponectin protein can undergo proteolytic cleavage and can form the globular form of adiponectin, where the globular head domain has been reported to increase the fatty acid oxidation[33]. Acrp30 is found in two forms in serum; one is low molecular weight (LMW) trimer-dimer where the other one is high molecular weight complex. Oligomer formation of Acrp30 depends on the formation of disulfide bond mediated by Cys-39. Mutation of Cys-39 results in the trimers which can easily undergo proteolytic cleavage in the collagenous domain[34].
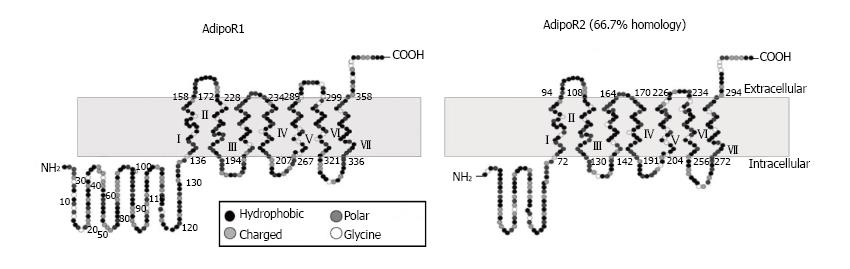
Yamauchi et al[35] reported for the first time about the two adiponectin receptors which can successfully increase AMP kinase and PPAR-alpha ligand activities as well as can accelerate fatty acid oxidation and glucose uptake by adiponectin. These receptors are named as AdipoR1 which is abundantly expressed in skeletal muscle and AdipoR2 which is mainly expressed in the liver (Figure 3)[35,36].
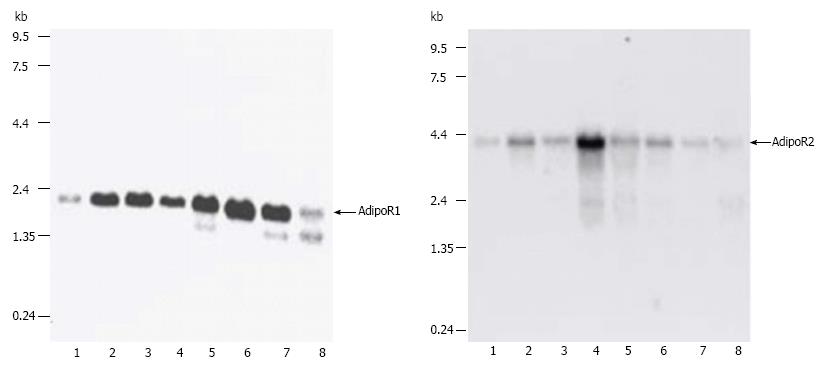
They first successfully performed the cloning of complementary DNAs encoding adiponectin receptors 1 and 2 (AdipoR1 and AdipoR2) by expression cloning[35]. AdipoR1 and AdipoR2 mRNA expression in the liver and skeletal muscle increases after fasting and re-feeding can rapidly restore these to levels equal to the original fed state (Figure 4)[35,36]. Both of these receptors contain seven transmembrane domains but they are structurally and functionally completely distinct from G-protein-coupled receptors. Mild insulin resistance has been observed in both adipoR1 and adipoR2 knocked out mice, but complete abolition of adiponectin activity has been observed in adipoR1/R2 double knockout mice, resulting in increased tissue triglyceride content, inflammation and oxidative stress[37].
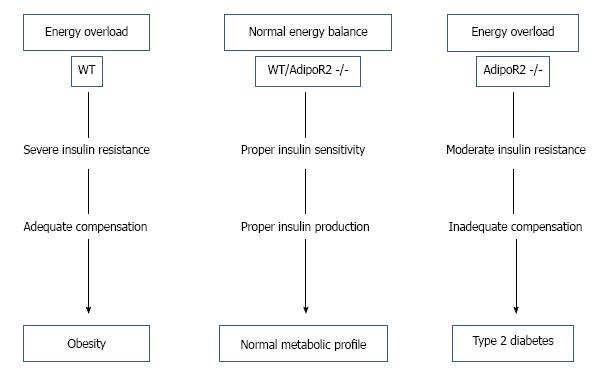
It has been observed by one research group (Figure 5) that abolition of AdipoR2 eradicates β cell replication and neogenesis, thus in presence of high energy diet although it shows moderate insulin sensitivity initially and shows moderate body mass, in later state it tends to develop type 2 diabetes[38]. Insulin resistance consuming high energy diet increases obesity in wild type (WT) mice, where normal energy diet in both WT and AdipoR2 double knocked out mice (AdipoR2 -/-) shows normal metabolism, but AdipoR2 -/- mice with energy overload although shows moderate insulin resistance initially but in later stage develops type 2 diabetes.
In increased oxidation of fatty acids such as in Nonalcoholic steatohepatitis (NASH) the expression levels of AdipoR1/R2 and insulin receptor substrate isoforms 2 (IRS-2) were significantly decreased, whereas IRS-1 was significantly increased[39].
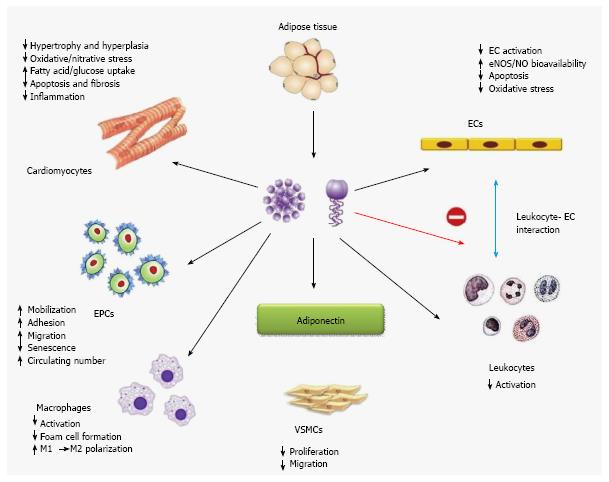
Although it circulates in high concentrations, adiponectin levels are lower in obese subjects than their lean coun-terparts. Apart from negative correlations with measures of adiposity, adiponectin levels are also reduced in association with insulin resistance and type 2 diabetes[40]. Epidemiological studies in different ethnic groups revealed that low level of plasma adiponectin, especially its HMW form can be an important key factor for type 2 diabetes, hypertension, atherosclerosis and myocardial infarction[41]. Other than preventing insulin resistance and adipose tissue inflammation, adiponectin has been associated to exert several cardioprotective roles through direct actions on heart as well as on other vascular cells (Figure 6)[42]. Adiponectin has negative correlation with insulin resistance, along with it maintains negative correlation with plasma triglyceride and low density lipoproteins (LDLs) where it has positive correlation with high density lipoproteins (HDLs)[43]. In this review we will try to elucidate the role of adiponectin in acquiring adiposity in various aspects, i.e., from the metabolomic view to genetic predisposition.
Adipocyte derived adiponectin can modulate the functions of cardiomyocytes, endothelial cells, endothelial progenitor cells, macrophages, leukocytes, and vascular smooth muscles in both endocrine and paracrine manner (Figure 6). Here we will discuss the possible roles of this adipokine in type 2 diabetes mellitus, obesity and dyslipidemia.
Studies in Japan showed that hypertension has a major effect on atherosclerosis and CVD events in persons with high body mass index with T2DM[16]. Adiponectin and its association with lipid metabolism and increased obesity are studied well in many populations.
Adiponectin serves as a central regulatory protein in many metabolic pathways playing crucial role in many metabolic disorders. Its importance as a potential biomarker in type 2 diabetes is increasing rapidly. The major way to estimate plasma adiponectin is by Sandwich ELISA. Lower plasma adiponectin level (< 5 μg/mL) is associated with increasing obesity and acquiring of metabolic disorders.
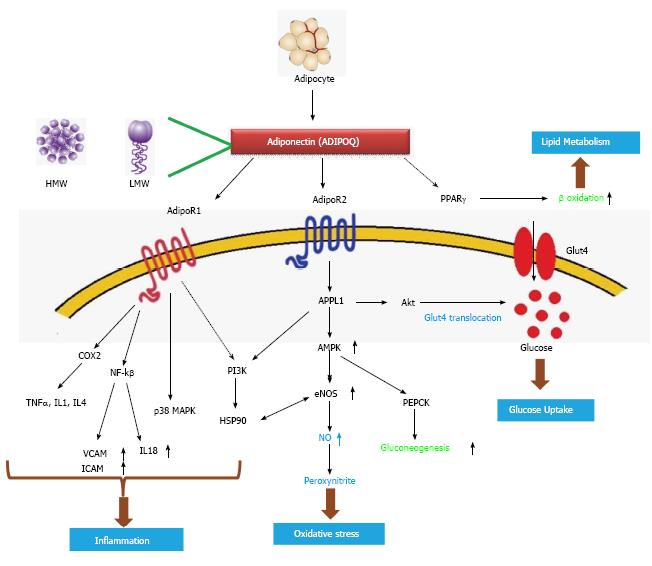
As a key factor of the metabolic pathway: Adiponectin has multifunctional roles in metabolic synchronization (Figure 7). Adiponectin (ADIPOQ) an adipocyte derived hormone activates ADIPOR1 and ADIPOR2, the two adiponectin receptors; it also activates PPARγ ultimately increasing the rate of β oxidation which is a major pathway for lipid metabolism. ADIPOR1 increases the action of number of genes including NF-kβ, TNFα, IL1, IL4. NF-kβ[41] furthermore decreases VCAM1, ICAM1 and IL18 levels; these are important genes involved in inflammation. ADIPOR1 also activates p38MAPK, another gene involved in transcriptional machinery. The action of PI3K is indirectly regulated by ADIPOR1. P13K acts on HSP90 which again increases the action of endothelial nitric oxide synthase (eNOS), which is related to oxidative stress. ADIPOR2 activates APPL1 which up regulates AMP-activated protein kinase 1 (AMPK1) which again up regulates eNOS[40,41] increasing the production of nitric oxide. Elevated AMPK also increases the action of PEPCK ultimately increasing gluconeogenesis. APPL1 works on Akt which increases Glut4 translocation ultimately elevating glucose uptake of the cell[42] (Figure 7). Derived from adipocyte it comes in contact with blood plasma and directly acts on Adipo R1/R2 receptors which further activates/inhibits the downstream genes related to oxidative stress and inflammation. Plasma and hemolysate of patients of type 2 diabetes contains elevated level of protein carbonyl content, which indicates increased oxidative stress[44].
T-cadherin (CDH13) localizes adiponectin to the vascular endothelium. It has been reported that T-cadherin deficiency by siRNA knockdown prevented the ability of adiponectin to promote cellular migration and proliferation[45]. T-cadherin protects from stress-induced pathological cardiac remodeling by binding with adiponectin and activating its cardioprotective functions in mice[46].
Mechanisms of action: Adiponectin exhibits two major mechanisms of action by which it inhibits obesity and type 2 diabetes, one by increasing insulin sensitivity and the other way is to increase fatty acid oxidation.
APPL1, stimulated by adiponectin can interact with both adiponectin receptors and can mediate the down-stream events such as lipid oxidation and membrane translocation of glucose transport 4 (GLUT4), thus increasing glucose uptake (Figure 7), providing a platform for increased insulin sensitization[47]. APPL1 also acts as a mediator of adiponectin signaling pathways by interacting directly with ADIPOR1/ADIPOR2 or signaling proteins, thereby playing critical roles in cell proliferation, apoptosis, cell survival, endosomal trafficking, and chromatin remodelling[48]. APPL1 modulates the insulin signalling pathway by acting with Akt and PI3K[49] (Figure 7).
The major form of storing and transporting fatty acids is triglycerides. Adiponectin has been reported to decrease tissue triglyceride content by increasing the expression of CD36, a fatty acid transporter[50]. Increased tissue TG content activates PI3K and Glut4 increasing glucose uptake, elevating insulin resistance[51]. Thus, lowering of tissue triglyceride content promotes insulin sensitivity. Along with adiponectin has been also reported to increase the expression of PPARα which further lowers the tissue triglyceride content[50]. Some researcher group has demonstrated the role of adiponectin in activating AMPK which can stimulate β oxidation and glucose up taking[52].
It has been established that adiponectin enhances insulin-stimulated IRS-1 tyrosine and Akt phosphorylation. Activation of the LKB1/AMPK/TSC1/2 pathway alleviates the p70S6 kinase-mediated negative regulation of insulin signaling, providing a mechanism by which adiponectin increases insulin sensitivity in cells[53].
Other than playing a crucial role as an insulin sensitizer, adiponectin also defeats obesity and obesity onset type 2 diabetes by increasing fatty acid oxidation. Increased fatty acid oxidation in turn also elevates insulin sensitivity. As stated earlier, adiponectin associated activation of AMPK phosphorylation which in turn implements major role in fatty acid oxidation. In cultured myotubes C2C12, adiponectin treatment has been associated with increased PPARα activity; expression of some downstream genes such as suchasacyl-CoAoxidase and carnitinepalmitoyltransferase1 has been also reported, thus promoting fatty acid oxidation[54]. Adiponectin induces fatty acid oxidation in muscle cells by sequential activation of AMPK, p38 MAPK (mitogen activated protein kinase) and PPARα[54]. It has been studied in humans that LDL activity is correlated positively with plasma adiponectin level, thus LPL may represent a link between low adiponectin levels and dyslipidemia in both nondiabetic individuals and patients with type 2 diabetes[55] where plasma TGs is negatively correlated with LDL activity and positively with diabetic state[56].
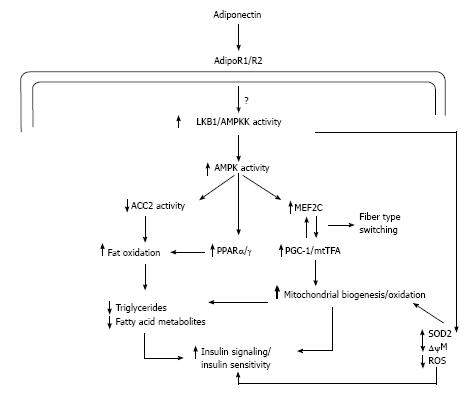
It has been well postulated that subjects with type 2 diabetes has reduced mitochondrial content and decreased electron transport chain activity[57]. Adiponectin has been reported to increase mitochondrial biogenesis and oxidative capacity in mice which in turn is favorable for glucose metabolism as well as fatty acid oxidation as mitochondria is the major cellular site of metabolism[58]. In human model also the adiponectin stimulated mitochondrial biogenesis has been observed[59] (Figure 8).
Adiponectin binds to its receptors which activates AMPK and stimulates the phosphorylation of ACC2 which in turn increases fatty-acid oxidation. As previously discussed adiponectin can activate peroxisome proliferative activated receptor-α (PPARα) stimulating transcription of genes in the fatty-acid oxidation pathway and decreasing triglyceride content in muscle, thus promoting fatty acid oxidation (Figure 8) and improving insulin sensitivity[50,54]. Independent of changes in transcription and mitochondrial mass, the improvements in lipid oxidation occur in less than 6 h in mice[52]. Adiponectin activation of AMPK by upstream kinase AMPK kinase activates transcription of myocyte enhancer factor 2C and phosphorylation of peroxisome proliferative activated receptor γ coactivator 1-α (PGC1α), which in turn increases mitochondrial content, oxidative capacity, and oxidative-fibre type composition. Central to the development of mitochondrial dysfunction is reactive oxygen species (ROS) production, which reacts with DNA, protein, and lipids leading to oxidative damage. ROS production is inversely related to mitochondrial content. Activation of the adiponectin pathway reduces the generation of ROS by two processes: (1) Increasing mitochondrial content, which in turn decreases the workload for each mitochondrion leading to reduced membrane potential (←Δψ) and lower ROS production; and (2) adiponectin increases PGC1α activity which increases the transcription/activity of the antioxidant enzyme SOD2 that decreases super oxide radical (O2•-)[60].
Oxidative stress is a major consequence of type 2 diabetes and obesity related disorders. Previously in our laboratory we had established that hyperglycaemic condition increases the oxygen releasing capacity of haemoglobin which in turn boosts the effect of oxidative stress in diabetes and CVDs[44]. Oxidative stress which is a major indicator of inflammation correlates significantly with adiponectin metabolic pathway. Study by a research group demonstrates that lower adiponectin level is significantly associated with higher inflammatory state[61].
Other than decreasing circulating free fatty acid and lowering triglyceride content adiponectin has been also observed to exert anti-inflammatory and anti-atherogenic effects by reducing TNFα-induced monocyte attachment to endothelial cells and inhibiting platelet derived growth factor-BB to minimize vascular smooth muscle cell proliferation[62]. Most adipokines can exert pro inflammatory effects, among which adiponectin is increasing its importance as a potential inflammatory marker. Obesity is characterized by low grade systemic inflammation[63]. Adiponectin inhibits the action of TNFα which is a key pro inflammatory cytokine in both vascular and cardiac tissue[64]. This novel cytokine has been also reported to decrease the secretion of IL 8 from human aortic endothelial cells (HAEC) stimulated with TNFα, along with it also inhibits IL8 mRNA expression induced by TNFα. Phosphorylation of IkBα is decreased by adiponectin, but phosphorylation of ERK, SAPK/JNK, and p38MAPK remains unaffected[65]. Adiponectin also increases intra-cellular cAMP levels in HAEC and increases PKA activity[65]. The inverse relationship of adiponectin with inflammatory marker CRP has been discussed in the next paragraph of this review.
Thus adiponectin exerts several multitasking roles and combat the prevalence of metabolic disorders like diabetes and obesity. In first step it works as a fascinating insulin sensitizer and in second step it increases fatty acid oxidation. Simultaneously in all above mentioned mode of actions it acts as an important inflammatory marker while playing significant role in minimizing oxidative stress. Thus adiponectin plays affluent role to protect the metabolic harmony of the system through various metabolic pathways and considered as one of the potential biochemical and inflammatory biomarker in metabolic disorders.
Adipocyte is involved with the releasing of another three hormones playing some roles in metabolism; these are leptin, resistin and visfatin. Where low plasma adiponectin has been observed in obesity, leptin levels become significantly higher, having an inverse correlation with adiponectin. Increased subcutaneous fat has been a major determinant of leptin levels. The action of leptin remains to decrease appetite, thermogenesis and increase fatty acid oxidation[66]. The leptin signal is transmitted by the Janus kinase, signal transducer; and activator of transcription pathway decrease glucose, and reduce body weight and fat[66]. One research group showed that adiponectin is more influenced by visceral adipose tissue where leptin is by subcutaneous adipose tissue[62] where fasting glucose, insulin, HOMA-IR and triglyceride has an inverse correlation with adiponectin and leptin maintaining a fairly positive association with these parameters[67]. It is reported that leptin/adiponectin ratio alters in type 2 diabetes as this alteration increases insulin resistance[68]. Another research group reported the plasma leptin/adiponectin ratio as an important atherogenic index[69]. Thus it can be concluded that where adiponectin is a proinflammatory adipokine giving proatherogeneic effect, leptin serves as an antiinflammatory molecule giving a direct antiatherogenic effect.
The plasma level of resistin, a cysteine rich adipokine has been observed to increase in type 2 diabetes but this increase in level is not correlated with insulin resistance and adiposity[70]. Another research group found a decrease in serum resistin value in patients with type 2 diabetes[71]. Where adiponectin level is significantly associated with lipid profile, BMI, resistin levels seem to level independent of these attributes in patients with type 2 diabetes mellitus[72]. Thus the association of resistin with type 2 diabetes, obesity and dyslipidemia is still a new field to explore; and the association of this adipokine with adiponectin is poorly understood.
Visfatin, another adipokine maintains a direct relationship between plasma visfatin levels and type 2 diabetes mellitus. Visfatin binds to the insulin receptor at a site distinct from that of insulin and causes hypogly-caemia by reducing glucose release from liver cells and stimulating glucose utilization in adipocytes and myocytes. Visfatin is upregulated by inflammation and hyperglycaemia and downregulated by insulin[73]. Where the association of visfatin with diabetes mellitus has been well studied its correlation with adiponectin is poorly known. Although it has been postulated in one article that adiponectin maintains a fairly inverse relationship with visfatin[74]. Thus activity of other adipokines with adiponectin is still remained a major field to explore in metabolic syndrome.
Adiponectin which is increasing its importance as a potential biomarker maintains some association with other diabetic biomarkers such as fasting insulin, C- reactive protein (CRP) and homocysteine. Fasting insulin and CRP has been observed to maintain an inverse correlation with adiponectin level[75]. A data observed on Asian Indian obese men revealed that serum adiponectin level is inversely related with fasting insulin and CRP[76]. Both adiponectin and CRP is strongly associated with insulin sensitivity where CRP is more dependent on adiposity[77]. One study group found no significant correlation between plasma homocysteine level and adiponectin in patients with type 2 diabetes[78]. Although an inverse relationship was found between adiponectin and homocysteine in patients with type 1 diabetes but no significant association has been reported in type 2 diabetes[79].
Genetic polymorphisms in ADIPOQ gene and the genes of its receptors has been a major reason for functional defect of this novel adipokine. Genetic polymorphisms of the other genes present in adiponectin metabolic pathway may also alter the functional properties of adiponectin and thus promoting the progression of insulin resistance, dyslipidemia and atherogenesis. These genetic polymorphisms have seen in many ethnic groups. ADIPOQ gene polymorphisms were associated with the risk of T2DM in Chinese Han population[80]. It has been observed that rs2241767AG genotype increases the risk of T2DM in obesity group[80]. A study in south Indian population implies ADIPOQ gene +276 G/T and -3971 A/G polymorphisms are associated with generalized obesity and +349 A/G with central obesity[81].
The polymorphism -1131 T/C in apolipoprotein A5 gene is associated with postprandial hypertriacylgly-cerolemia, elevated small, dense LDL concentrations and oxidative stress in non-obese Korean men[82] and dyslipidemia in Brazilian subjects[83] (Table 2). A significant association of -11391 G/A adiponectin gene polymorphism with waist circumference in diabetic patients has been observed[84]. In white Europeans, +276 G/T was associated with higher serum adiponectin concentrations where -10066 G/A was associated with lower serum adiponectin concentrations[85]. Genetic polymorphisms of ADIPOR1 and ADIPOR2 are also involved in altered function of adiponectin and have been observed by many groups (Table 2). Polymorphisms of other pathway genes like eNOS, NF-kB, PEPCK, IL4 (Table 2) has been also reported to play roles in development of type 2 diabetes, thus they may correlate with adiponectin and regulate its function.
| Ref. | Gene | SL No. | Location | Variation | SNP ID |
| Blech et al[96] | PPARγ | 1 | Intron 1 | Pro12Ala | rs1801282 |
| Blech et al[96]; Ramya et al[81] | ADIPOQ | 1 2 3 4 5 6 7 8 9 | 5’ flanking region Intron 1 Intron 1 Intron 1 Exon 1 coding synonymous Intron 1 Intron 1 5’ flanking region Exon 3 splicing enhancers | −11365 C/G -4522 C/T -3971 A/G +276 G/T +45 T/G +349 A/G +712 G/A -11391 G/A Y111H T/C | rs266729 rs822393 rs822396 rs1501299 rs2241766 rs2241767 rs3774261 rs17300539 rs17366743 |
| Wang et al[97] | ADIPOR1 | 1 2 3 4 | Intron 1 Intron 1 Intron 1 5’ transcription factor binding site | +5646 A/G +5843 A/G -101 T/G -8503C/T | rs1342386 rs1342387 rs2275737 rs6666089 |
| Vaxillaire et al[98] | ADIPOR2 | 1 2 3 4 5 6 | Exon 3 splicing enhancers Intron 1 5’ flanking region Intron 1 Intron 1 Intron 1 | +33371 C/T +26314 A/G -64241 T/G +8645 G/C +14645 A/T -35361 G/A | rs12342 rs767870 rs1029629 rs1468491 rs4766415 rs10773982 |
| Thameem et al[99] | eNOS/NOS3 | 1 2 | Exon 3 splicing enhancers Intron 1 | Glu298Asp -786 T/C | rs1799983 rs2070744 |
| Zhang et al[100] | NF-kB | 1 | 5’ flanking region | -94 insertion/deletion | rs28362491 |
| Rees et al[101] | PEPCK | 1 | 5’ flanking region | -232C/G | rs2071023 |
| Jang et al[82] and Ferreira et al[83] | Apolipoprotein A5 gene (APOA5) | 1 | 5’ flanking region | -1131T/C | rs662799 |
| Ol et al[102] | COX-2 | 1 | 5’ flanking region | -765G/C | rs20417 |
| Ho et al[103] | IL4 | 1 | 5’ flanking region | -590 C/T | rs2243250 |
Adiponectin gene function is not solely dependent on gene polymorphisms rather expression levels of certain genes may modulate its function significantly. Both in type 2 diabetic patients and in animal models of insulin resistance it has been observed that the mRNA expression and secretion of adiponectin is significantly decreased[86,87]. Very low calorie diet has been reported to raise adiponectin mRNA level, whereas re-feeding significantly decreases the mRNA level in morbidly obese women[88]. AdipoR2 mRNA expression in subcutaneous tissue is negatively associated with insulin resistance and metabolic parameters independently of obesity may mediate the improvement of insulin resistance in response to exercise[89]. PPARγ agonist thiazolidinedione has been reported to increase adiponectin level in animal models and human patients[90]. A single nucleotide polymorphism in Pro12Ala in PPARγ is reported to be involved in type 2 diabetes. PPARγ has been found to undergo obesity-induced and protein kinase cdk5-mediated phosphorylation at Ser273 which mediates obesity-induced down-regulation of adiponectin in white adipose tissue[91].
There are certain evidences that adrenomedullin (ADM) may modulate the expression of adiponectin gene. One group of scientists postulated that a genetic variant in ADM gene (rs182052) alters the expression of adiponectin gene and minimizes plasma adiponectin levels[92]. A variation in CDH13 (rs4783244) showed strong associations with total adiponectin and HMW adiponectin in East Asian population where people with this variation have significantly lower adiponectin plasma level, but adiponectin sensitivity tends to increase, eventually maintaining a better metabolic profile[46].
Glucocorticoids are also reported to regulate adi-ponectin gene expression in human adipocytes, where TNFα does not seem to directly inhibit adiponectin synthesis in human adipocytes[93]. SIRT1 and Foxo1, two important genes involved in insulin sensitivity whose low expression leads to impaired Foxo1-C/EBPα complex formation, has been reported to decrease adiponectin expression in obesity and type 2 diabetes[94]. CRP has been reported to suppress adiponectin gene expression partially through the PI3K pathway where decreased production of adiponectin might represent a mechanism by which CRP regulates insulin sensitivity[95].
Genetic polymorphisms which supposed to be a screening tool of adiponectin metabolic disorder may be overpowered by the altered gene expression of adiponectin and related genes. Both of these actions may significantly be associated with low expression of adiponectin which in turn is positively correlated with insulin resistance increasing the prevalence of diabetes and obesity.
Epigenetic association of adiponectin expression is remained a big question to answer. DNA methylation can partly explain the link between the early exposures to a detrimental fetal environment, where the mother is hyperglycemic which may in turn increase the risk to develop obesity and diabetes later in life[104]. One group found significant correlation between the mother blood glucose level and placental DNA methylation at cytosines located at ADIPOQ gene proximal promoter CpG islands[105]. Expression and methylation of ADIPOR1 gene isolated from skeletal muscle cells has been modified after an exercise period of 6 mo in subjects who are first degree relatives of type 2 diabetes patients[106]. But still there are few evidences of the epigenetic modulation of adiponectin and remains a promising field to explore.
Balanced diet with adequate exercise can combat obesity and type 2 diabetes in manifold. Although genetic predisposition is a main key factor of these disorders by still maintaining a well-balanced energy is still a beneficiary supplements in preventing these disorders. Exercise can fairly maintains plasma adiponectin levels and thus promoting insulin sensitivity. One study shows that aerobic exercise increases insulin sensitivity among diabetic patients mediated by adiponectin[107], although drug treatment may be required to normalize plasma adiponectin levels. Adiponectin replenishment therapy is yet not possible as biologically active recombinant adiponectin proteins are inherently unstable and difficult to produce[108]. Certain drug classes such as antidiabetic drugs glitazones and sulfonylureas, and angiotensin receptor blockers, ACE inhibitors and nicotinic acid exert beneficial effects on insulin resistance partly by increasing plasma adiponectin levels. Others such as tetrahydrobiopterin or certain antioxidants are also promising in normalizing plasma adiponectin levels[109]. Omega-3 polyunsaturated fatty acids has been reported to increase plasma adiponectin to leptin ratio in stable coronary artery disease, thus playing a cardioprotective role, might in turn be beneficiary for diabetes and obesity[110]. Thus a healthy life style with some oral supplements may increase adiponectin levels in patients with type 2 diabetes.
Adiponectin, the novel adipocyte has been demonstrated well to play crucial role in obesity and type 2 diabetes mellitus (Figure 9). It is increasing its importance as a potential biomarker in above mentioned diseased state as: (1) It increases insulin sensitivity; (2) It increases fatty acid oxidation; (3) It correlates significantly with oxidative stress; and (4) It acts as an important inflammatory biomarker and up/down regulates many genes in various metabolic pathways. Thus adiponectin could be a novel target for the therapeutic approach to treat diabetes mellitus in near future. Recombinant adiponectin is not effective thus altered expression of adiponectin or related pathway genes could be an effective tool for researchers to mediate its function which in turn may minimize the prevalence of obesity, type 2 diabetes or other metabolic disorders.
We acknowledge Council of Scientific and Industrial Research, Govt. of India for providing research fellowship to one of the author, Ghoshal K.
P- Reviewer: Gómez-Sáez J, Tziomalos K, Uehara Y S- Editor: Tian YL L- Editor: A E- Editor: Lu YJ
| 1. | Maeda K, Okubo K, Shimomura I, Funahashi T, Matsuzawa Y, Matsubara K. cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (AdiPose Most abundant Gene transcript 1). Biochem Biophys Res Commun. 1996;221:286-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1483] [Cited by in RCA: 1443] [Article Influence: 49.8] [Reference Citation Analysis (0)] |
| 2. | Cnop M, Havel PJ, Utzschneider KM, Carr DB, Sinha MK, Boyko EJ, Retzlaff BM, Knopp RH, Brunzell JD, Kahn SE. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia. 2003;46:459-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1014] [Cited by in RCA: 1060] [Article Influence: 48.2] [Reference Citation Analysis (0)] |
| 3. | Ouchi N, Kihara S, Arita Y, Maeda K, Kuriyama H, Okamoto Y, Hotta K, Nishida M, Takahashi M, Nakamura T. Novel modulator for endothelial adhesion molecules: adipocyte-derived plasma protein adiponectin. Circulation. 1999;100:2473-2476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1551] [Cited by in RCA: 1540] [Article Influence: 59.2] [Reference Citation Analysis (0)] |
| 4. | Hotta K, Funahashi T, Arita Y, Takahashi M, Matsuda M, Okamoto Y, Iwahashi H, Kuriyama H, Ouchi N, Maeda K. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 2000;20:1595-1599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2223] [Cited by in RCA: 2228] [Article Influence: 89.1] [Reference Citation Analysis (0)] |
| 5. | Lindsay RS, Funahashi T, Hanson RL, Matsuzawa Y, Tanaka S, Tataranni PA, Knowler WC, Krakoff J. Adiponectin and development of type 2 diabetes in the Pima Indian population. Lancet. 2002;360:57-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 763] [Cited by in RCA: 738] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 6. | Spranger J, Kroke A, Möhlig M, Bergmann MM, Ristow M, Boeing H, Pfeiffer AF. Adiponectin and protection against type 2 diabetes mellitus. Lancet. 2003;361:226-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 775] [Cited by in RCA: 743] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 7. | Pischon T, Girman CJ, Hotamisligil GS, Rifai N, Hu FB, Rimm EB. Plasma adiponectin levels and risk of myocardial infarction in men. JAMA. 2004;291:1730-1737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1309] [Cited by in RCA: 1297] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 8. | Nakamura Y, Shimada K, Fukuda D, Shimada Y, Ehara S, Hirose M, Kataoka T, Kamimori K, Shimodozono S, Kobayashi Y. Implications of plasma concentrations of adiponectin in patients with coronary artery disease. Heart. 2004;90:528-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 190] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 9. | Barcelo A, Gregg EW, Gerzoff RB, Wong R, Perez Flores E, Ramirez-Zea M, Cafiero E, Altamirano L, Ascencio Rivera M, de Cosio G. Prevalence of diabetes and intermediate hyperglycemia among adults from the first multinational study of noncommunicable diseases in six Central American countries: the Central America Diabetes Initiative (CAMDI). Diabetes Care. 2012;35:738-740. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Uauy R, Albala C, Kain J. Obesity trends in Latin America: transiting from under- to overweight. J Nutr. 2001;131:893S-899S. [PubMed] |
| 11. | WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7065] [Cited by in RCA: 8208] [Article Influence: 390.9] [Reference Citation Analysis (0)] |
| 12. | Chakraborty A, Bhattacharyya M. Diabetes, Hypertension and Cardiovascular Disease-An Unsolved Enigma. Phytotherapy in the Management of Diabetes and Hypertension. 2012;85-119. |
| 13. | Ramachandran A. Socio-economic burden of diabetes in India. J Assoc Physicians India. 2007;55 Suppl:9-12. [PubMed] |
| 14. | Cade WT. Diabetes-related microvascular and macrovascular diseases in the physical therapy setting. Phys Ther. 2008;88:1322-1335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 733] [Cited by in RCA: 636] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 15. | Chakraborty A, Chowdhury S, Bhattacharyya M. Effect of metformin on oxidative stress, nitrosative stress and inflammatory biomarkers in type 2 diabetes patients. Diabetes Res Clin Pract. 2011;93:56-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 150] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 16. | Saito I. Epidemiological evidence of type 2 diabetes mellitus, metabolic syndrome, and cardiovascular disease in Japan. Circ J. 2012;76:1066-1073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 17. | Coelho VG, Caetano LF, Liberatore Júnior Rdel R, Cordeiro JA, Souza DR. [Lipid profile and risk factors for cardiovascular diseases in medicine students]. Arq Bras Cardiol. 2005;85:57-62. [PubMed] |
| 18. | Rizk NM, Yousef M. Association of lipid profile and waist circumference as cardiovascular risk factors for overweight and obesity among school children in Qatar. Diabetes Metab Syndr Obes. 2012;5:425-432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Fletcher B, Berra K, Ades P, Braun LT, Burke LE, Durstine JL, Fair JM, Fletcher GF, Goff D, Hayman LL. Managing abnormal blood lipids: a collaborative approach. Circulation. 2005;112:3184-3209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 133] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 20. | Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837-1847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6201] [Cited by in RCA: 6263] [Article Influence: 232.0] [Reference Citation Analysis (0)] |
| 21. | Brezinka V, Padmos I. Coronary heart disease risk factors in women. Eur Heart J. 1994;15:1571-1584. [PubMed] |
| 22. | Grundy SM, Benjamin IJ, Burke GL, Chait A, Eckel RH, Howard BV, Mitch W, Smith SC, Sowers JR. Diabetes and cardiovascular disease: a statement for healthcare professionals from the American Heart Association. Circulation. 1999;100:1134-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1310] [Cited by in RCA: 1296] [Article Influence: 49.8] [Reference Citation Analysis (0)] |
| 23. | Stone PH, Muller JE, Hartwell T, York BJ, Rutherford JD, Parker CB, Turi ZG, Strauss HW, Willerson JT, Robertson T. The effect of diabetes mellitus on prognosis and serial left ventricular function after acute myocardial infarction: contribution of both coronary disease and diastolic left ventricular dysfunction to the adverse prognosis. The MILIS Study Group. J Am Coll Cardiol. 1989;14:49-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 339] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 24. | Singer DE, Moulton AW, Nathan DM. Diabetic myocardial infarction. Interaction of diabetes with other preinfarction risk factors. Diabetes. 1989;38:350-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Smith JW, Marcus FI, Serokman R. Prognosis of patients with diabetes mellitus after acute myocardial infarction. Am J Cardiol. 1984;54:718-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 130] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 26. | Rondinone CM. Adipocyte-derived hormones, cytokines, and mediators. Endocrine. 2006;29:81-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 27. | Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270:26746-26749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2259] [Cited by in RCA: 2299] [Article Influence: 76.6] [Reference Citation Analysis (0)] |
| 28. | Chandran M, Phillips SA, Ciaraldi T, Henry RR. Adiponectin: more than just another fat cell hormone? Diabetes Care. 2003;26:2442-2450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 670] [Cited by in RCA: 649] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 29. | Siitonen N, Pulkkinen L, Lindström J, Kolehmainen M, Eriksson JG, Venojärvi M, Ilanne-Parikka P, Keinänen-Kiukaanniemi S, Tuomilehto J, Uusitupa M. Association of ADIPOQ gene variants with body weight, type 2 diabetes and serum adiponectin concentrations: the Finnish Diabetes Prevention Study. BMC Med Genet. 2011;12:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 112] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 30. | Min X, Lemon B, Tang J, Liu Q, Zhang R, Walker N, Li Y, Wang Z. Crystal structure of a single-chain trimer of human adiponectin globular domain. FEBS Lett. 2012;586:912-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 31. | Mohamed-Ali V, Pinkney JH, Coppack SW. Adipose tissue as an endocrine and paracrine organ. Int J Obes Relat Metab Disord. 1998;22:1145-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 616] [Cited by in RCA: 599] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 32. | Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature. 2006;444:847-853. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1730] [Cited by in RCA: 1587] [Article Influence: 83.5] [Reference Citation Analysis (0)] |
| 33. | Fruebis J, Tsao TS, Javorschi S, Ebbets-Reed D, Erickson MR, Yen FT, Bihain BE, Lodish HF. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc Natl Acad Sci USA. 2001;98:2005-2010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 670] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 34. | Pajvani UB, Du X, Combs TP, Berg AH, Rajala MW, Schulthess T, Engel J, Brownlee M, Scherer PE. Structure-function studies of the adipocyte-secreted hormone Acrp30/adiponectin. Implications fpr metabolic regulation and bioactivity. J Biol Chem. 2003;278:9073-9085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 798] [Cited by in RCA: 820] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 35. | Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, Sugiyama T, Miyagishi M, Hara K, Tsunoda M. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2258] [Cited by in RCA: 2310] [Article Influence: 105.0] [Reference Citation Analysis (0)] |
| 36. | Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev. 2005;26:439-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1824] [Cited by in RCA: 1808] [Article Influence: 90.4] [Reference Citation Analysis (0)] |
| 37. | Yamauchi T, Nio Y, Maki T, Kobayashi M, Takazawa T, Iwabu M, Okada-Iwabu M, Kawamoto S, Kubota N, Kubota T. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med. 2007;13:332-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 968] [Cited by in RCA: 1055] [Article Influence: 58.6] [Reference Citation Analysis (0)] |
| 38. | Liu Y, Michael MD, Kash S, Bensch WR, Monia BP, Murray SF, Otto KA, Syed SK, Bhanot S, Sloop KW. Deficiency of adiponectin receptor 2 reduces diet-induced insulin resistance but promotes type 2 diabetes. Endocrinology. 2007;148:683-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 87] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 39. | Matsunami T, Sato Y, Ariga S, Sato T, Shimomura T, Kashimura H, Hasegawa Y, Yukawa M. Regulation of synthesis and oxidation of fatty acids by adiponectin receptors (AdipoR1/R2) and insulin receptor substrate isoforms (IRS-1/-2) of the liver in a nonalcoholic steatohepatitis animal model. Metabolism. 2011;60:805-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 40. | Lihn AS, Pedersen SB, Richelsen B. Adiponectin: action, regulation and association to insulin sensitivity. Obes Rev. 2005;6:13-21. [PubMed] |
| 41. | Zhu W, Cheng KK, Vanhoutte PM, Lam KS, Xu A. Vascular effects of adiponectin: molecular mechanisms and potential therapeutic intervention. Clin Sci (Lond). 2008;114:361-374. [PubMed] |
| 42. | Xu A, Vanhoutte PM. Adiponectin and adipocyte fatty acid binding protein in the pathogenesis of cardiovascular disease. Am J Physiol Heart Circ Physiol. 2012;302:H1231-H1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 92] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 43. | Yamamoto Y, Hirose H, Saito I, Tomita M, Taniyama M, Matsubara K, Okazaki Y, Ishii T, Nishikai K, Saruta T. Correlation of the adipocyte-derived protein adiponectin with insulin resistance index and serum high-density lipoprotein-cholesterol, independent of body mass index, in the Japanese population. Clin Sci (Lond). 2002;103:137-142. [PubMed] |
| 44. | Saha A, Adak S, Chowdhury S, Bhattacharyya M. Enhanced oxygen releasing capacity and oxidative stress in diabetes mellitus and diabetes mellitus-associated cardiovascular disease: a comparative study. Clin Chim Acta. 2005;361:141-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 45. | Parker-Duffen JL, Nakamura K, Silver M, Kikuchi R, Tigges U, Yoshida S, Denzel MS, Ranscht B, Walsh K. T-cadherin is essential for adiponectin-mediated revascularization. J Biol Chem. 2013;288:24886-24897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 127] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 46. | Denzel MS, Scimia MC, Zumstein PM, Walsh K, Ruiz-Lozano P, Ranscht B. T-cadherin is critical for adiponectin-mediated cardioprotection in mice. J Clin Invest. 2010;120:4342-4352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 259] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 47. | Mao X, Kikani CK, Riojas RA, Langlais P, Wang L, Ramos FJ, Fang Q, Christ-Roberts CY, Hong JY, Kim RY. APPL1 binds to adiponectin receptors and mediates adiponectin signalling and function. Nat Cell Biol. 2006;8:516-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 523] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 48. | Deepa SS, Dong LQ. APPL1: role in adiponectin signaling and beyond. Am J Physiol Endocrinol Metab. 2009;296:E22-E36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 218] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 49. | Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261-1274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4880] [Cited by in RCA: 4799] [Article Influence: 266.6] [Reference Citation Analysis (0)] |
| 50. | Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3510] [Cited by in RCA: 3499] [Article Influence: 145.8] [Reference Citation Analysis (0)] |
| 51. | Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest. 2000;106:171-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1893] [Cited by in RCA: 1865] [Article Influence: 74.6] [Reference Citation Analysis (0)] |
| 52. | Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288-1295. [PubMed] |
| 53. | Wang C, Mao X, Wang L, Liu M, Wetzel MD, Guan KL, Dong LQ, Liu F. Adiponectin sensitizes insulin signaling by reducing p70 S6 kinase-mediated serine phosphorylation of IRS-1. J Biol Chem. 2007;282:7991-7996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 151] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 54. | Yoon MJ, Lee GY, Chung JJ, Ahn YH, Hong SH, Kim JB. Adiponectin increases fatty acid oxidation in skeletal muscle cells by sequential activation of AMP-activated protein kinase, p38 mitogen-activated protein kinase, and peroxisome proliferator-activated receptor alpha. Diabetes. 2006;55:2562-2570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 424] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 55. | von Eynatten M, Schneider JG, Humpert PM, Rudofsky G, Schmidt N, Barosch P, Hamann A, Morcos M, Kreuzer J, Bierhaus A. Decreased plasma lipoprotein lipase in hypoadiponectinemia: an association independent of systemic inflammation and insulin resistance. Diabetes Care. 2004;27:2925-2929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 102] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 56. | De Vries R, Wolffenbuttel BH, Sluiter WJ, van Tol A, Dullaart RP. Post-heparin plasma lipoprotein lipase, but not hepatic lipase activity, is related to plasma adiponectin in type 2 diabetic patients and healthy subjects. Clin Lab. 2005;51:403-409. [PubMed] |
| 57. | Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes. 2002;51:2944-2950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1699] [Cited by in RCA: 1735] [Article Influence: 75.4] [Reference Citation Analysis (0)] |
| 58. | Iwabu M, Yamauchi T, Okada-Iwabu M, Sato K, Nakagawa T, Funata M, Yamaguchi M, Namiki S, Nakayama R, Tabata M. Adiponectin and AdipoR1 regulate PGC-1alpha and mitochondria by Ca(2+) and AMPK/SIRT1. Nature. 2010;464:1313-1319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 684] [Cited by in RCA: 787] [Article Influence: 52.5] [Reference Citation Analysis (0)] |
| 59. | Civitarese AE, Ukropcova B, Carling S, Hulver M, DeFronzo RA, Mandarino L, Ravussin E, Smith SR. Role of adiponectin in human skeletal muscle bioenergetics. Cell Metab. 2006;4:75-87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 184] [Cited by in RCA: 177] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 60. | Maassen JA, Janssen GM, Lemkes HH. Mitochondrial diabetes mellitus. J Endocrinol Invest. 2002;25:477-484. [PubMed] |
| 61. | Chen SJ, Yen CH, Huang YC, Lee BJ, Hsia S, Lin PT. Relationships between inflammation, adiponectin, and oxidative stress in metabolic syndrome. PLoS One. 2012;7:e45693. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 88] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 62. | Park KG, Park KS, Kim MJ, Kim HS, Suh YS, Ahn JD, Park KK, Chang YC, Lee IK. Relationship between serum adiponectin and leptin concentrations and body fat distribution. Diabetes Res Clin Pract. 2004;63:135-142. [PubMed] |
| 63. | Ouchi N, Walsh K. Adiponectin as an anti-inflammatory factor. Clin Chim Acta. 2007;380:24-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 664] [Cited by in RCA: 632] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 64. | Ouchi N, Walsh K. A novel role for adiponectin in the regulation of inflammation. Arterioscler Thromb Vasc Biol. 2008;28:1219-1221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 65. | Kobashi C, Urakaze M, Kishida M, Kibayashi E, Kobayashi H, Kihara S, Funahashi T, Takata M, Temaru R, Sato A. Adiponectin inhibits endothelial synthesis of interleukin-8. Circ Res. 2005;97:1245-1252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 188] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 66. | Yadav A, Kataria MA, Saini V, Yadav A. Role of leptin and adiponectin in insulin resistance. Clin Chim Acta. 2013;417:80-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 444] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 67. | Matsubara M, Maruoka S, Katayose S. Inverse relationship between plasma adiponectin and leptin concentrations in normal-weight and obese women. Eur J Endocrinol. 2002;147:173-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 345] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 68. | Oda N, Imamura S, Fujita T, Uchida Y, Inagaki K, Kakizawa H, Hayakawa N, Suzuki A, Takeda J, Horikawa Y. The ratio of leptin to adiponectin can be used as an index of insulin resistance. Metabolism. 2008;57:268-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 147] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 69. | Satoh N, Naruse M, Usui T, Tagami T, Suganami T, Yamada K, Kuzuya H, Shimatsu A, Ogawa Y. Leptin-to-adiponectin ratio as a potential atherogenic index in obese type 2 diabetic patients. Diabetes Care. 2004;27:2488-2490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 163] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 70. | Hasegawa G, Ohta M, Ichida Y, Obayashi H, Shigeta M, Yamasaki M, Fukui M, Yoshikawa T, Nakamura N. Increased serum resistin levels in patients with type 2 diabetes are not linked with markers of insulin resistance and adiposity. Acta Diabetol. 2005;42:104-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 71. | Yang J, Li M, Wu CY, Wang H, Xu QS, Deng JY. [Reduced resistin levels in patients with type 2 diabetes mellitus]. Zhonghua Yixue Zazhi. 2003;83:1471-1474. [PubMed] |
| 72. | Shetty GK, Economides PA, Horton ES, Mantzoros CS, Veves A. Circulating adiponectin and resistin levels in relation to metabolic factors, inflammatory markers, and vascular reactivity in diabetic patients and subjects at risk for diabetes. Diabetes Care. 2004;27:2450-2457. [PubMed] |
| 73. | Adeghate E. Visfatin: structure, function and relation to diabetes mellitus and other dysfunctions. Curr Med Chem. 2008;15:1851-1862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 127] [Article Influence: 7.5] [Reference Citation Analysis (1)] |
| 74. | El-Hini SH, Mohamed FI, Hassan AA, Ali F, Mahmoud A, Ibraheem HM. Visfatin and adiponectin as novel markers for evaluation of metabolic disturbance in recently diagnosed rheumatoid arthritis patients. Rheumatol Int. 2013;33:2283-2289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 75. | Adam FM, Nara MG, Adam JM. Fasting insulin, adiponectin, hs-CRP levels, and the components of metabolic syndrome. Acta Med Indones. 2006;38:179-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 76. | Vikram NK, Misra A, Pandey RM, Dwivedi M, Luthra K. Adiponectin, insulin resistance, and C-reactive protein in postpubertal Asian Indian adolescents. Metabolism. 2004;53:1336-1341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 77. | Putz DM, Goldner WS, Bar RS, Haynes WG, Sivitz WI. Adiponectin and C-reactive protein in obesity, type 2 diabetes, and monodrug therapy. Metabolism. 2004;53:1454-1461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 78. | Sakuta H, Suzuki T, Yasuda H, Ito T. Adiponectin levels and cardiovascular risk factors in Japanese men with type 2 diabetes. Endocr J. 2005;52:241-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 79. | Heilman K, Zilmer M, Zilmer K, Kool P, Tillmann V. Elevated plasma adiponectin and decreased plasma homocysteine and asymmetric dimethylarginine in children with type 1 diabetes. Scand J Clin Lab Invest. 2009;69:85-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 80. | Du W, Li Q, Lu Y, Yu X, Ye X, Gao Y, Ma J, Cheng J, Cao Y, Du J. Genetic variants in ADIPOQ gene and the risk of type 2 diabetes: a case-control study of Chinese Han population. Endocrine. 2011;40:413-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 81. | Ramya K, Ayyappa KA, Ghosh S, Mohan V, Radha V. Genetic association of ADIPOQ gene variants with type 2 diabetes, obesity and serum adiponectin levels in south Indian population. Gene. 2013;532:253-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 92] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 82. | Jang Y, Kim JY, Kim OY, Lee JE, Cho H, Ordovas JM, Lee JH. The -1131T--& gt; C polymorphism in the apolipoprotein A5 gene is associated with postprandial hypertriacylglycerolemia; elevated small, dense LDL concentrations; and oxidative stress in nonobese Korean men. Am J Clin Nutr. 2004;80:832-840. [PubMed] |
| 83. | Ferreira CN, Carvalho MG, Fernandes AP, Santos IR, Rodrigues KF, Lana AM, Almeida CR, Loures-Vale AA, Gomes KB, Sousa MO. The polymorphism -1131T& gt; C in apolipoprotein A5 gene is associated with dyslipidemia in Brazilian subjects. Gene. 2013;516:171-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 84. | Hasani-Ranjbar S, Amoli MM, Tabatabaei-Malazy O, Rumi Y, Tavakkoly-Bazzaz J, Samimi H, Abbasifarid E. Effect of adiponectin gene polymorphisms on waist circumference in patients with diabetes. J Diabetes Metab Disord. 2012;11:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 85. | AlSaleh A, O’Dell SD, Frost GS, Griffin BA, Lovegrove JA, Jebb SA, Sanders TA. Single nucleotide polymorphisms at the ADIPOQ gene locus interact with age and dietary intake of fat to determine serum adiponectin in subjects at risk of the metabolic syndrome. Am J Clin Nutr. 2011;94:262-269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 86. | Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, Tataranni PA. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86:1930-1935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 843] [Cited by in RCA: 664] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 87. | Yang WS, Lee WJ, Funahashi T, Tanaka S, Matsuzawa Y, Chao CL, Chen CL, Tai TY, Chuang LM. Weight reduction increases plasma levels of an adipose-derived anti-inflammatory protein, adiponectin. J Clin Endocrinol Metab. 2001;86:3815-3819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 253] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 88. | Liu YM, Lacorte JM, Viguerie N, Poitou C, Pelloux V, Guy-Grand B, Coussieu C, Langin D, Basdevant A, Clément K. Adiponectin gene expression in subcutaneous adipose tissue of obese women in response to short-term very low calorie diet and refeeding. J Clin Endocrinol Metab. 2003;88:5881-5886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 89. | Blüher M, Williams CJ, Klöting N, Hsi A, Ruschke K, Oberbach A, Fasshauer M, Berndt J, Schön MR, Wolk A. Gene expression of adiponectin receptors in human visceral and subcutaneous adipose tissue is related to insulin resistance and metabolic parameters and is altered in response to physical training. Diabetes Care. 2007;30:3110-3115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 70] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 90. | Maeda N, Takahashi M, Funahashi T, Kihara S, Nishizawa H, Kishida K, Nagaretani H, Matsuda M, Komuro R, Ouchi N. PPARgamma ligands increase expression and plasma concentrations of adiponectin, an adipose-derived protein. Diabetes. 2001;50:2094-2099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1280] [Cited by in RCA: 1252] [Article Influence: 52.2] [Reference Citation Analysis (0)] |
| 91. | Choi JH, Banks AS, Estall JL, Kajimura S, Boström P, Laznik D, Ruas JL, Chalmers MJ, Kamenecka TM, Blüher M. Anti-diabetic drugs inhibit obesity-linked phosphorylation of PPARgamma by Cdk5. Nature. 2010;466:451-456. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 780] [Cited by in RCA: 721] [Article Influence: 48.1] [Reference Citation Analysis (0)] |
| 92. | Wong HK, Ong KL, Leung RY, Cheung TT, Xu A, Lam TH, Lam KS, Cheung BM. Plasma level of adrenomedullin is influenced by a single nucleotide polymorphism in the adiponectin gene. PLoS One. 2013;8:e70335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 93. | Degawa-Yamauchi M, Moss KA, Bovenkerk JE, Shankar SS, Morrison CL, Lelliott CJ, Vidal-Puig A, Jones R, Considine RV. Regulation of adiponectin expression in human adipocytes: effects of adiposity, glucocorticoids, and tumor necrosis factor alpha. Obes Res. 2005;13:662-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 148] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 94. | Qiao L, Shao J. SIRT1 regulates adiponectin gene expression through Foxo1-C/enhancer-binding protein alpha transcriptional complex. J Biol Chem. 2006;281:39915-39924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 278] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 95. | Yuan G, Chen X, Ma Q, Qiao J, Li R, Li X, Li S, Tang J, Zhou L, Song H. C-reactive protein inhibits adiponectin gene expression and secretion in 3T3-L1 adipocytes. J Endocrinol. 2007;194:275-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 96. | Blech I, Katzenellenbogen M, Katzenellenbogen A, Wainstein J, Rubinstein A, Harman-Boehm I, Cohen J, Pollin TI, Glaser B. Predicting diabetic nephropathy using a multifactorial genetic model. PLoS One. 2011;6:e18743. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 97. | Wang H, Zhang H, Jia Y, Zhang Z, Craig R, Wang X, Elbein SC. Adiponectin receptor 1 gene (ADIPOR1) as a candidate for type 2 diabetes and insulin resistance. Diabetes. 2004;53:2132-2136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 76] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 98. | Vaxillaire M, Dechaume A, Vasseur-Delannoy V, Lahmidi S, Vatin V, Leprêtre F, Boutin P, Hercberg S, Charpentier G, Dina C. Genetic analysis of ADIPOR1 and ADIPOR2 candidate polymorphisms for type 2 diabetes in the Caucasian population. Diabetes. 2006;55:856-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 65] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 99. | Thameem F, Puppala S, Arar NH, Stern MP, Blangero J, Duggirala R, Abboud HE. Endothelial nitric oxide synthase (eNOS) gene polymorphisms and their association with type 2 diabetes-related traits in Mexican Americans. Diab Vasc Dis Res. 2008;5:109-113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 100. | Zhang D, Li L, Zhu Y, Zhao L, Wan L, Lv J, Li X, Huang P, Wei L, Ma M. The NFKB1 -94 ATTG insertion/deletion polymorphism (rs28362491) contributes to the susceptibility of congenital heart disease in a Chinese population. Gene. 2013;516:307-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 101. | Rees SD, Britten AC, Bellary S, O’Hare JP, Kumar S, Barnett AH, Kelly MA. The promoter polymorphism -232C/G of the PCK1 gene is associated with type 2 diabetes in a UK-resident South Asian population. BMC Med Genet. 2009;10:83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 102. | Ol KK, Agachan B, Gormus U, Toptas B, Isbir T. Cox-2 gene polymorphism and IL-6 levels in coronary artery disease. Genet Mol Res. 2011;10:810-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 103. | Ho KT, Shiau MY, Chang YH, Chen CM, Yang SC, Huang CN. Association of interleukin-4 promoter polymorphisms in Taiwanese patients with type 2 diabetes mellitus. Metabolism. 2010;59:1717-1722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 104. | Houde AA, Hivert MF, Bouchard L. Fetal epigenetic programming of adipokines. Adipocyte. 2013;2:41-46. [PubMed] |
| 105. | Bouchard L, Hivert MF, Guay SP, St-Pierre J, Perron P, Brisson D. Placental adiponectin gene DNA methylation levels are associated with mothers’ blood glucose concentration. Diabetes. 2012;61:1272-1280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 145] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 106. | Nitert MD, Dayeh T, Volkov P, Elgzyri T, Hall E, Nilsson E, Yang BT, Lang S, Parikh H, Wessman Y. Impact of an exercise intervention on DNA methylation in skeletal muscle from first-degree relatives of patients with type 2 diabetes. Diabetes. 2012;61:3322-3332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 283] [Cited by in RCA: 284] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 107. | Yokoyama H, Emoto M, Araki T, Fujiwara S, Motoyama K, Morioka T, Koyama H, Shoji T, Okuno Y, Nishizawa Y. Effect of aerobic exercise on plasma adiponectin levels and insulin resistance in type 2 diabetes. Diabetes Care. 2004;27:1756-1758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 69] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 108. | Gu W, Li Y. The therapeutic potential of the adiponectin pathway. BioDrugs. 2012;26:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 109. | Antonopoulos AS, Lee R, Margaritis M, Antoniades C. Adiponectin as a regulator of vascular redox state: therapeutic implications. Recent Pat Cardiovasc Drug Discov. 2011;6:78-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 110. | Mostowik M, Gajos G, Zalewski J, Nessler J, Undas A. Omega-3 polyunsaturated fatty acids increase plasma adiponectin to leptin ratio in stable coronary artery disease. Cardiovasc Drugs Ther. 2013;27:289-295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (1)] |