Published online Aug 15, 2025. doi: 10.4239/wjd.v16.i8.108166
Revised: May 20, 2025
Accepted: July 2, 2025
Published online: August 15, 2025
Processing time: 129 Days and 19.5 Hours
Diabetic foot ulcers (DFUs) represent a common and serious complication of diabetes, characterized by impaired wound healing and an increased risk of infection. These infections severely impact patient health, necessitating extensive medical intervention, and increasing the risk of amputation. Vitamin D (VD) plays a critical role in immune regulation and tissue repair.
To investigate the effects of VD supplementation on infection rates, wound hea
A randomized controlled trial was conducted involving 120 patients with DFUs. Participants were randomly assigned to either a control group (n = 60), which received standard care without VD supplementation, or an intervention group (n = 60), which received 2000 IU of oral VD3 (cholecalciferol) daily for 12 weeks. The primary outcomes included the incidence and severity of infections, whereas the secondary outcomes included wound healing rate, serum 25-hydroxyvitamin D level, levels of immune markers (cathelicidin, interleukin-6 and tumor necrosis factor-α), and adverse events, such as hypercalcemia.
The incidence of infection was significantly lower in the VD supplementation group (25%) compared with the control group (45%) (P = 0.01). Severe infections requiring systemic antibiotics or hospitalization were also less frequent in the VD supplementation group. Wound healing was notably enhanced in the VD supplementation group, with a 60% reduction in ulcer size compared with a 35% reduction in the control group (P < 0.01). Serum 25(OH)D level significantly increased in the VD supplementation group (from 16.5 ng/mL to 35.2 ng/mL), confirming the efficacy of VD supplementation. Immune function improved, as demonstrated by a 30% rise in cathelicidin level and a 20% decline in levels of pro-inflammatory cytokines. No adverse effects, including hypercalcemia, were reported.
The VD supplementation effectively reduced infection rate, promoted wound healing, and strengthened immune responses in patients with DFUs. These findings support the incorporation of VD as a safe and effective adjunctive therapy in the clinical management of DFUs.
Core Tip: Diabetic foot ulcers (DFUs) are a highly prevalent and serious complication of diabetes, characterized by slow wound healing and an increased risk of infection. These infections severely impact patient health, often requiring extensive medical intervention and increasing the risk of amputation. Vitamin D plays a critical role in immune regulation and wound healing. This study found that vitamin D supplementation effectively reduced infection rates, enhanced wound healing, and strengthened immune responses in DFU cases. These findings suggest that vitamin D is a beneficial and safe adjunct to standard DFU care, potentially mitigating infection-related complications and improving clinical outcomes.
- Citation: Gao YQ, Gao YH, Xing JH. Vitamin D supplementation reduces infection rate and promotes wound healing in patients with diabetic foot ulcers. World J Diabetes 2025; 16(8): 108166
- URL: https://www.wjgnet.com/1948-9358/full/v16/i8/108166.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i8.108166
Diabetic foot ulcers (DFUs) are one of the most severe and prevalent complications in adult patients with diabetes mellitus, affecting approximately 15%-25% of diabetic cases during their lifetime[1]. These ulcers typically develop due to peripheral neuropathy, impaired blood circulation, and deficient wound healing mechanisms, leading to chronic open sores primarily located on the feet. DFUs are a leading cause of hospitalization and play a critical role in the increasing incidence of lower limb amputations[2]. DFU infections pose a distinct clinical challenge, as they can exacerbate ulcer severity, prolong wound healing, and aggravate the risk of serious systemic complications, including life-threatening sepsis[3].
The heightened susceptibility to infection in patients with DFUs stems primarily from impaired immune responses and vascular complications caused by persistent hyperglycemia[4]. Elevated blood glucose levels in diabetics negatively impact multiple immune components of the body, particularly neutrophils and macrophages, proving essential for infection control. Hyperglycemia disrupts neutrophil functions, including chemotaxis and oxidative burst, prolonging the inflammatory phase and impairing wound healing[5]. Macrophage viability is similarly compromised, resulting in deficient interleukin (IL) expression. This weakened immune response is further aggravated by the presence of polymicrobial microflora, including gram-negative bacteria, increasing the complexity and severity of the wound infection in patients with DFU[6].
Peripheral vascular disease and neuropathy, which are common in diabetics, also play fundamental roles in the development and progression of DFUs. These conditions reduce lower limb blood flow, limiting oxygen, nutrient, and immune cell delivery to ulcer sites, as well as diminishing the body’s ability to combat infections[7]. Chronic hy
Recent studies have shown that vitamin D (VD) plays a crucial role in modulating the immune system and enhancing wound healing, extending beyond its traditional functions in calcium regulation and bone health. It is instrumental in the production of antimicrobial peptides, such as cathelicidin, which are vital for innate immunity, as they disrupt bacterial cell walls and prevent the spread of infection[10-12]. VD's immunomodulatory effects are evident in both the innate and adaptive immune systems, where it influences the production of defensin β2 and cathelicidin by various immune cells, including macrophages and keratinocytes[12]. This modulation helps reduce excessive inflammation, a critical factor in chronic wounds (e.g., DFUs), where high levels of inflammation can hinder the healing process and increase the risk of infection[11].
VD supplementation can effectively promote wound healing, particularly in cases of VD deficiency. Furthermore, the role of VD in regulating inflammatory cytokines and inhibiting pro-inflammatory cell proliferation highlights its potential in managing inflammatory diseases and enhancing immune responses to infection[11]. Despite these promising findings, large-scale clinical studies are necessary to assess VD as a standard therapy.
Research has demonstrated a significant association between VD deficiency and adverse clinical outcomes in diabetics, particularly those with chronic wounds. Cases with low serum VD levels exhibit increased susceptibility to recurrent infections, delayed wound healing, and severe complications, including osteomyelitis and limb amputation[13]. This association is clinically significant, as diabetics frequently present with VD deficiency due to multiple contributing factors, such as limited sunlight exposure, inadequate dietary intake, and impaired renal function[14]. Therefore, investigating the therapeutic potential of VD supplementation on infection rates and wound healing in DFUs is an important area of clinical research.
DFUs present a substantial clinical challenge because of their pronounced susceptibility to infections, impaired healing rate, and elevated risk of severe complications, including amputation[15]. Although advancements in wound care and infection control have been achieved, persistent infections in DFUs remain a significant barrier to successful treatment. Considering the critical role of VD in enhancing immune responses and tissue repair, investigating its therapeutic potential for improving DFU outcomes warrants significant attention[8]. While substantial evidence exists regarding VD's antimicrobial and immunomodulatory properties, particularly its capacity to stimulate antimicrobial peptide production and to regulate inflammatory responses, its specific effects on infection control in DFUs remain insufficiently investigated[16]. Previous research has examined the general benefits of VD on immune function and skeletal health, while comparatively few studies have addressed its precise role in wound healing and infection prevention particularly in diabetics[17]. This research gap is particularly important given the high prevalence of VD deficiency among diabetics and its potential impact on wound healing[18].
This study aimed to eliminate this critical gap by investigating whether VD supplementation can reduce infection rate and improve healing outcomes in patients with DFUs. By concentrating on a well-defined diabetic population, the study provided an opportunity to directly evaluate the potential benefits of VD supplementation in enhancing innate immune responses to bacterial infections and promoting tissue repair in chronic wounds. Notably, all participants enrolled in this trial exhibited deficient or insufficient serum 25(OH)D levels at baseline, highlighting the clinical significance of assessing VD supplementation in this high-risk group.
A total of 120 adult cases diagnosed with DFUs were recruited from the First Affiliated Hospital of Zhengzhou University (Zhengzhou, China). All participants initially presented with moderate-to-severe DFU infections and received a standardized 4-week course of systemic antibiotics according to institutional protocols before randomization. Participants were then randomly assigned to either the VD group (n = 60) or the control group (n = 60) using a random number table to evaluate outcomes following initial infection control. Both investigators and participants were not blinded to the interventions. The inclusion criteria were as follows: (1) Confirmed diagnosis of diabetes and the presence of at least one persistent DFU lasting more than four weeks despite conventional wound treatment; (2) Age > 18 years; and (3) Voluntary participation. The exclusion criteria were as follows: (1) Chronic renal insufficiency; (2) Hypercalcemia; (3) Hyperphosphatemia; (4) Known allergy to VD; and (5) An ankle-brachial index < 0.4. The study protocol was approved by the Ethics Committee of the First Affiliated Hospital of Zhengzhou University (Approval No. 2025-KY-0093-002). All the participants provided written informed consent prior to enrollment. The trial was not registered at public platforms.
Baseline information was collected from each participant before the intervention, covering the following domains.
Demographic data: Age, gender, and duration of diabetes diagnosis.
Ulcer characteristics: Ulcer size (in cm²) and severity were documented and categorized according to the Infectious Diseases Society of America (IDSA) Diabetic Foot Infection Classification system.
VD level: Baseline serum 25-hydroxyvitamin D (25(OH)D) level was measured to identify VD deficiency or insufficiency. The average baseline VD level was 16.5 ± 4.8 ng/mL in the VD group and 17.1 ± 5.0 ng/mL in the control group, demonstrating comparable VD status across groups.
Glycemic control: Glycated hemoglobulin A1c (HbA1c) level was documented for each participant, with mean values of 8.5% ± 1.2% in the VD supplementation group and 8.6% ± 1.3% in the control group.
Additional health measures: Supplementary health measures, such as blood pressure and body mass index (BMI), were documented, and no statistically significant differences were identified between the two groups.
Participants in the VD group received a daily oral dosage of 2000 IU of VD3 (cholecalciferol) in capsule form for 12 weeks, with the objective of elevating serum VD level to an appropriate range (> 30 ng/mL). The control group received placebo capsules identical in appearance to those given to the treatment group. Both groups continued to receive standard wound care, including routine wound cleaning, debridement, dressing changes, and offloading techniques, in accordance with the clinical guidelines for DFU management. To ensure adherence to the supplementation protocol, participants attended biweekly follow-up visits, during which pill counts were conducted, and any adverse events were monitored and documented throughout the study. Serum 25[OH]D level was measured at both the beginning and end of the intervention to assess the efficacy of VD supplementation in the intervention group.
The primary outcome was the incidence of new or recurrent infections during the 12-week period following antibiotic treatment. All patients had received a standardized 4-week course of systemic antibiotics prior to randomization, ensuring resolution of initial infections. The study specifically assessed whether adjunctive VD supplementation could reduce reinfection or exacerbation rates compared with standard care alone. Although all participants initially presented with moderate or severe DFU infections, each received standardized antibiotic therapy for 4 weeks prior to randomization. The primary endpoint, defined as ‘infection incidence’, referred to any new or recurrent infections occurring during the intervention phase. Infection recurrence was defined based on the presence of clinical signs and confirmed by microbial culture. Infection rates were reported as the number of infections per 100 patient-days to account for the study duration and variability in follow-up. Clinical assessments were performed at each follow-up visit and included evaluation of signs, such as erythema, swelling, purulent discharge, and increased local temperature at the ulcer site. Wound swabs were collected for microbiological analysis to confirm bacterial presence. Cultures were used to identify bacterial species and to classify infections as either monomicrobial or polymicrobial. Antimicrobial susceptibility testing was performed using standard disc diffusion methods, as per CLSI guidelines. Infection severity was graded using standardized scoring systems, including the Diabetic Foot Infection Classification developed by the IDSA.
The secondary outcomes of this study included the wound healing rate, serum 25[OH]D concentration, immunological markers, and VD supplementation-associated adverse events. Each outcome was assessed at baseline, at the 6-week midpoint, and at the end of the 12-week intervention period.
Wound healing was assessed by measuring ulcer size at baseline, 6 weeks, and 12 weeks. Healing progress was quantified as the percentage reduction in ulcer area from baseline, providing a standardized metric for evaluating wound improvement over time. At each assessment point, digital planimetry was used to determine the ulcer area in square centimeters (cm²). Complete ulcer closure was also documented as an indicator of wound healing.
To assess the efficacy of VD supplementation, serum 25(OH)D level was measured at baseline and throughout the intervention. Blood samples were collected and analyzed using ELISA to quantify the serum 25(OH)D level. The target for the VD group was to achieve serum VD level greater than 30 ng/mL at study completion. Baseline serum 25(OH)D concentrations were comparable between the two groups.
The influence of VD supplementation on immune function was evaluated by analyzing specific immunological markers, including cathelicidin and proinflammatory cytokines IL-6 and tumor necrosis factor-α (TNF-α). Blood samples collected at baseline and after the intervention were examined to determine potential improvements in immune response. Cathelicidin concentration was measured using an ELISA kit.
To ensure the safety of VD supplementation at 2000 IU/day, participants were closely monitored for adverse events, with special emphasis on detecting signs of hypercalcemia. Serum calcium level was measured at each follow-up visit, and any side effects, such as gastrointestinal discomfort or allergic reactions were documented. No cases of hypercalcemia or other significant adverse events were reported in either group, indicating that VD supplementation was well tolerated and appeared safe in the study population.
Statistical analysis was conducted using SPSS 27.0 software (IBM Corp., Armonk, NY, United States), with the significance level set at P < 0.05. To enhance accuracy, 95% confidence intervals (CIs) were calculated. The primary objective was to compare the incidence and rate of infection between the VD and control groups using χ2 test, and infection rates were standardized per 100 patient-days to account for differences in follow-up duration. The relative reduction risk (RRR) and corresponding 95%CI were calculated to assess the impact of VD supplementation on the infection risk. The secondary outcomes were evaluated as follows: Wound healing, measured by the reduction in ulcer size over time, was analyzed within groups at baseline, 6 weeks, and 12 weeks using paired t-test, while between-group comparisons at each time point were conducted using independent t-test. Complete ulcer closure rates were compared between the two groups using the χ2 test. Serum 25[OH]D level was assessed within groups by paired t-test for pre- and post-intervention comparisons, and between-group differences at study completion were evaluated using independent t-test. Changes in levels of immune markers, including cathelicidin and pro-inflammatory cytokines (IL-6 and TNF-α), were analyzed in groups using paired t-test, and post-intervention between-group comparisons were performed via independent t-test. Adverse events, such as hypercalcemia, were described qualitatively due to their low incidence, precluding inferential statistical analysis. Continuous data were presented as mean ± SD, while categorical variables were expressed as percentage. Effect sizes were calculated where appropriate to emphasize the clinical relevance of the findings.
A total of 120 patients with DFUs were recruited and randomly assigned to either the VD supplementation group (n = 60) or control group (n = 60). Their baseline characteristics were evenly distributed throughout the groups, assuring comparability and reducing potential bias in outcome assessment. Patients’ mean age was 65.4 ± 8.3 years in the VD supplementation group and 64.7 ± 7.9 years in the control group. The sex distribution was comparable, with 53.3% and 46.7% of patients in the VD supplementation group being male and female, respectively, and 55.0% and 45.0% of patients in the control group being male and female, respectively. The mean duration of diabetes was 12.1 ± 5.6 years in the VD supplementation group and 11.8 ± 5.2 years in the control group.
Participants were presented with persistent DFUs for over four weeks despite conventional treatment. The average ulcer size at baseline was 5.6 ± 2.1 cm² in the VD supplementation group and 5.4 ± 2.3 cm² in the control group, showing no statistically significant difference between the two groups (P = 0.62). The severity of ulcers, assessed using the IDSA Diabetic Foot Infection Classification, was similar between the two groups, and the majority of cases exhibited moderate-to-severe infections. Baseline serum VD (25-hydroxyvitamin D) concentrations was comparable in both groups (16.5 ± 4.8 and 17.1 ± 5.0 ng/mL in the VD supplementation and control groups, respectively, P = 0.47). Baseline blood VD levels indicated that all participants were either deficient (< 20 ng/mL) or inadequate (20-30 ng/mL), highlighting the necessity for intervention. The mean HbA1c level also was comparable (VD group: 8.5% ± 1.2%; Control: 8.6% ± 1.3%; P = 0.74). Other essential baseline variables, including BMI and blood pressure, were similar between the two groups (Table 1).
| Baseline characteristics | VD group (n = 60) | Control group (n = 60) | P value |
| Mean age/(years) | 65.4 ± 8.3 | 64.7 ± 7.9 | - |
| Sex distribution | 53.3% male, 46.7% female | 55.0% male, 45.0% female | - |
| Duration of diabetes (years) | 12.1 ± 5.6 | 11.8 ± 5.2 | - |
| Ulcer size (cm²) | 5.6 ± 2.1 | 5.4 ± 2.3 | 0.62 |
| Ulcer severity | Moderate to severe | Moderate to severe | - |
| Baseline serum 25-(OH)D levels | 16.5 ± 4.8 ng/mL | 17.1 ± 5.0 ng/mL | 0.47 |
| HbA1c (%) | 8.5 ± 1.2 | 8.6 ± 1.3 | 0.74 |
| Other factors (BP, BMI) | Comparable across both groups | Comparable across both groups | - |
The baseline parameters of the study population were well balanced, providing a solid foundation for assessing the impact of VD supplementation on infection rates and wound healing in patients with DFUs.
The principal outcome of this study was the incidence of infections in DFUs between the VD supplementation group and the control group. During the 12-week intervention period, the VD supplementation group had a significantly reduced incidence of infections compared with the control group (Table 2).
| Infection outcome | VD group (n = 60) | Control group (n = 60) | P value | Absolute risk reduction |
| Infection incidence (%) | 25% (15/60) | 45% (27/60) | 0.01 | 20% |
| Infection rate per 100 patient-days | 3.2 | 5.8 | 0.01 | — |
| Relative risk reduction | 44% | — | — | — |
| 95% confidence interval (infection incidence) | 18.0% to 32.0% | 36.0% to 54.0% | — | — |
| Severe infections requiring antibiotics (%) | 10% (6/60) | 25% (15/60) | 0.03 | 15% |
| Hospitalization due to infection (%) | 5% (3/60) | 13% (8/60) | — | 8% |
The VD supplementation group exhibited an infection incidence of 25% (95%CI: 18.0%-32.0%; 15 out of 60 participants), whereas the control group demonstrated a 45% incidence (95%CI: 36.0%-54.0%; 27 out of 60 participants). Infection rates were 3.2 infections per 100 patient-days in the VD supplementation group vs 5.8 infections per 100 patient-days in the control group, and this difference reached statistical significance (P = 0.01), corresponding to a 44% RRR in the VD cohort. Moreover, participants receiving VD supplementation experienced fewer severe infections requiring systemic antibiotic treatment or hospitalization. Only 10% (6 of 60) of participants in the VD supplementation group required systemic antibiotics vs 25% (15 of 60) in the control group (P = 0.03). Hospitalization due to infection-related complications occurred in 5% of participants in the VD supplementation group vs 13% in the control group. These findings indicated that VD supplementation could not only reduce overall infection rate, but could also mitigate infection severity, thereby decreasing the need for intensive medical interventions (Table 2).
These results indicate a possible protective function of VD in infection control among diabetic cases with chronic wounds, as VD treatment has been linked to a significant decrease in the incidence and severity of DFUs.
Wound healing: One of the secondary outcomes of the present study was the rate of wound healing, which was assessed by measuring the reduction in ulcer size at baseline, 6, and 12 weeks. Participants in the VD supplementation group experienced significantly faster wound healing compared with those in the control group (Table 3).
| Secondary outcome | VD group (n = 60) | Control group (n = 60) | P value |
| Wound healing | |||
| Baseline ulcer size (cm²) | 5.6 | 5.4 | - |
| Ulcer size at 6 weeks (cm²) | 3.6 (35% reduction from baseline) | 4.3 (20% reduction from baseline) | - |
| Ulcer size at 12 weeks (cm²) | 2.2 (60% reduction from baseline) | 3.5 (35% reduction from baseline) | < 0.01 |
| Complete ulcer closure (%) | 20% | 10% | - |
| Serum 25(OH)D levels (ng/mL) | |||
| Baseline | 16.5 ± 4.8 | 17.1 ± 5.0 | 0.47 |
| Post-intervention (12 weeks) | 35.2 ± 7.5 | 18.3 ± 5.2 | < 0.001 |
| Immune markers | |||
| Cathelicidin (change from baseline) | +30% | No significant change | < 0.01 |
| Pro-inflammatory cytokines (IL-6, TNF-α) | -20% | No significant change | 0.02 |
| Adverse events | |||
| Hypercalcemia | 0 cases | 0 cases | - |
| Other side effects | None reported | None reported | - |
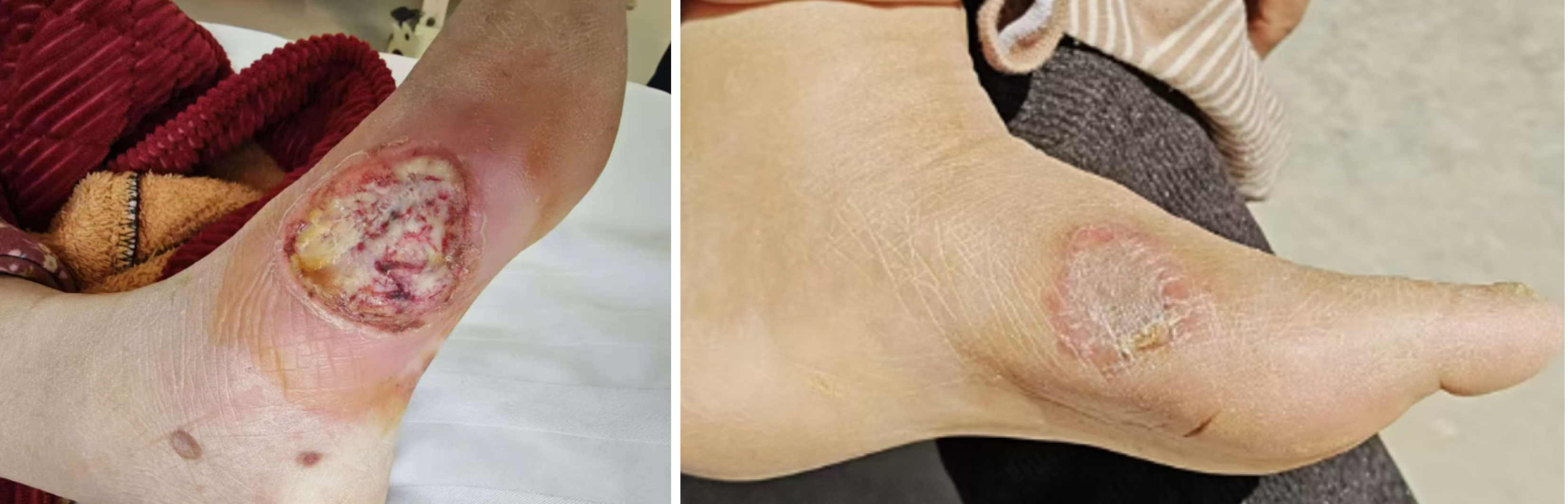
Initially, the average ulcer size was 5.6 cm² in the VD supplementation group and 5.4 cm² in the control group. After 6 weeks, the average ulcer size in the VD supplementation group was reduced by 35%, resulting in a mean size of 3.6 cm². In contrast, the control group showed only a 20% reduction, with a mean size of 4.3 cm² at the 6-week follow-up. By the study’s conclusion at 12 weeks, ulcers in the VD supplementation group was reduced by 60%, with a mean size of 2.2 cm², vs a 35% reduction in the control group, which had a mean ulcer size of 3.5 cm² (P < 0.01). Complete ulcer closure was achieved in 20% of participants in the VD supplementation group (Figure 1), while only 10% of participants in the control group reached full healing by the end of the study. These findings indicate that VD supplementation is associated with significantly accelerated wound healing in patients with DFUs (Table 3).
Serum 25(OH)D level: The efficacy of VD supplementation was evaluated by measuring serum 25(OH)D level at baseline and throughout the intervention. At baseline, the average serum 25(OH)D level was 16.5 ± 4.8 ng/mL in the VD supplementation group and 17.1 ± 5.0 ng/mL in the control group, with no statistically significant difference (P = 0.47). After 12 weeks, the VD supplementation group exhibited a notable elevation in serum 25(OH)D level, attaining a mean of 35.2 ± 7.5 ng/mL, thereby demonstrating sufficiency (> 30 ng/mL). The control group exhibited no significant change, with a post-intervention mean of 18.3 ± 5.2 ng/mL (P < 0.001). The results validated that VD supplementation significantly elevated serum 25(OH)D level to an adequate range in the intervention group (Table 3).
The influence of VD supplementation on immune function was evaluated by monitoring alterations in immune markers, such as cathelicidin, defensins, and pro-inflammatory cytokines (IL-6 and TNF-α). After 12 weeks, participants who received VD demonstrated a significant 30% increase in cathelicidin level from baseline (P < 0.01), indicating the enhanced antimicrobial defense. Additionally, the levels of pro-inflammatory cytokines (IL-6 and TNF-α) were reduced by 20% in the VD supplementation group, demonstrating a reduction in wound healing-associated chronic inflammation (P = 0.02). Conversely, the levels of these markers in the control group did not significantly vary. These results indicated that VD supplementation improved immune function in patients with DFUs, potentially promoting infection control and wound healing (Table 3).
Participants were monitored for VD supplementation-associated adverse events throughout the study, with particular attention to the signs of hypercalcemia. No notable adverse events were documented in either group. In the VD supplementation group, blood calcium concentration was consistently maintained within the normal range (8.5-10.5 mg/dL) throughout the study, and no cases with hypercalcemia were identified. Additionally, none of the participants in the VD supplementation group experienced side effects, such as gastrointestinal discomfort or allergic reactions. These findings demonstrated that VD supplementation at the prescribed dose (2000 IU/day) was well-tolerated and appeared safe for this patient population (Table 3).
This randomized controlled trial (RCT) demonstrated good evidence that VD supplementation could significantly reduce infection rates and accelerate wound healing in patients with DFUs. The baseline comparability between the intervention and control groups strengthened the validity of the findings and supported a causal interpretation of the observed effects. The study contributes to the expanding evidence base demonstrating the immunomodulatory and wound-healing benefits of correcting VD deficiency in vulnerable diabetic populations.
The mean baseline 25(OH)D levels in both groups confirmed a significant deficiency, aligning with existing research that highlights the high prevalence of VD insufficiency in diabetics, particularly those with chronic wounds. A previous study reported that a lower VD level is associated with an increased infection rate and delayed wound healing in patients with DFU[19]. This highlights the clinical importance of identifying and correcting VD deficiency in this vulnerable population.
Baseline HbA1c level was elevated in both groups, indicating poor glycemic control, a well-established contributor to delayed healing, and an increased infection risk in patients with DFUs. Chen et al[20] emphasized that poor glycemic control impairs immune function and prolongs ulcer duration. The similarity in glycemic status in both groups in this study strengthens the ability to isolate the effects of VD supplementation from variations in glucose control.
This study provided novel evidence that VD supplementation could significantly reduce the incidence and severity of infection in patients with DFUs. A 44% RRR was found in the VD supplementation group compared with that in the control group. The findings of the present study align with Badralany et al’s results[19], who similarly reported a reduction in infection rates in patients receiving VD supplementation. Notably, this study presented additional insights by incorporating both clinical infection assessments and microbial cultures based on standardized diagnostic criteria. Furthermore, fewer cases in the intervention group required systemic antibiotics or hospitalization, highlighting the clinical relevance of these findings.
The strength of this study lies in its comprehensive evaluation of the impact of VD on infection outcomes, immune modulation, and wound healing in a randomized controlled framework, specifically concentrated on DFUs. While prior studies have separately explored the role of VD supplementation in enhancing immunity or accelerating tissue repair[21], this trial is among the first to simultaneously assess infection incidence, wound healing, and levels of immune biomarkers (cathelicidin, IL-6, and TNF-α). This integrated approach uniquely contributes to the understanding of the multifaceted therapeutic benefits of VD supplementation.
Wound healing was significantly improved in the VD supplementation group, with a 60% reduction in ulcer size compared with 35% in the control group. Complete ulcer closure was more frequent in the VD supplementation group. This result supports Kurian et al’s viewpoint[21], who demonstrated accelerated wound healing in diabetics following VD therapy. The improvement in wound healing noted in this study is likely linked to the immunomodulatory effects, reduced inflammation, and improved antimicrobial activity.
Immune marker analysis confirmed proposed mechanism of action. Participants in the intervention group showed a 30% increase in cathelicidin, a key antimicrobial peptide, and a 20% reduction in pro-inflammatory cytokines (IL-6 and TNF-α). These immunological shifts align with Chrisdianto et al’s findings[22], who demonstrated the role of VD in enhancing innate immunity and attenuating chronic inflammation. The reduction in inflammatory burden may promote tissue regeneration and reduce the risk of infection progression.
Recent research has underscored the critical role of VD supplementation in managing DFUs and associated immune dysfunction in patients with type 2 diabetes. Gul et al[23] and Kanwal et al[24] both found significant associations between a low serum VD level and the prevalence of DFUs, with the latter reporting a mean VD level of 31.62 nmol/L in patients with DFUs compared to 38.38 nmol/L in those without. Furthermore, VD is closely linked to DFUs and has been identified as an independent protective factor against their development[25]. Liu et al[26] also highlighted that severe VD deficiency increases susceptibility to DFUs, suggesting that VD supplementation can improve ulcer outcomes. These findings align with the broader implications of VD deficiency on adverse diabetic outcomes, as pointed out by Todorova et al[27], emphasizing the need for effective management of VD level in diabetics.
The safety and tolerability of daily 2000 IU VD 3 supplementation have been confirmed in multiple studies, which reported no adverse events, including hypercalcemia. For instance, a comparative study involving cancer patients found that both 1000 and 2000 IU doses significantly increased serum 25-hydroxyvitamin D level without causing hyper
Despite the strengths of this RCT, it has several limitations. Firstly, the microbiological profile of the ulcers was not systematically analyzed, which limited our ability to correlate specific pathogens with infection outcomes. Secondly, although all participants received standard wound care, slight variations in local practices might influence the healing trajectories. Thirdly, the relatively short 12-week follow-up precluded the assessment of long-term ulcer recurrence or sustained immunological benefits. Finally, although the safety of VD supplementation was rigorously monitored, the study may have been underpowered to detect rare adverse events. Future research should address these limitations by including longer follow-up periods, conducting pathogen-specific analyses, and performing multicenter validation to enhance the generalizability of the findings.
In conclusion, the findings suggested that VD supplementation appeared as a promising adjunctive strategy for improving DFU outcomes. By integrating clinical, microbiological, and immunological endpoints, this study provided a more comprehensive understanding of how correcting VD deficiency may reduce the risk of infection, enhance innate immunity, and promote wound repair. Given its safety, affordability, and accessibility, routine screening and targeted supplementation of VD should be strongly considered in the multidisciplinary management of DFUs, especially in VD deficient individuals. Future research with longer follow-up and detailed pathogen profiling will further clarify its therapeutic potential.
This research indicated that VD administration at 2000 IU/day markedly decreased infection rates, accelerated wound healing, and improved immune responses in patients with DFUs. Participants in the VD group not only experienced a lower incidence of infection, but also exhibited fewer severe infections requiring medical intervention. The significant elevation in serum 25(OH)D level, along with improvement in immune markers, highlights the efficacy of VD supplementation in modulating immune responses and promoting wound healing. The absence of adverse events, including hypercalcemia, further confirms its safety profile. These findings suggest that VD may serve as a safe and effective adjunct to standard DFU care, providing a noninvasive strategy to enhance clinical outcomes and reduce the morbidity associated with chronic diabetic wounds.
| 1. | Kim A. Daily Life Management Guidelines for Diabetic Foot Patients. J Korean Diabetes. 2023;24:214-220. [DOI] [Full Text] |
| 2. | Del Core MA, Ahn J, Lewis RB, Raspovic KM, Lalli TAJ, Wukich DK. The Evaluation and Treatment of Diabetic Foot Ulcers and Diabetic Foot Infections. Foot Ankle Orthop. 2018;3. [RCA] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 3. | Albert S. Cost-effective management of recalcitrant diabetic foot ulcers. Clin Podiatr Med Surg. 2002;19:483-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Matheson EM, Bragg SW, Blackwelder RS. Diabetes-Related Foot Infections: Diagnosis and Treatment. Am Fam Physician. 2021;104:386-394. [PubMed] |
| 5. | R AS, Nambi N, Radhakrishnan L, Prasad MK, Ramkumar KM. Neutrophil Migration is a Crucial Factor in Wound Healing and the Pathogenesis of Diabetic Foot Ulcers: Insights into Pharmacological Interventions. Curr Vasc Pharmacol. 2024;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 6. | Banerjee T, Rai S, Singh A, Goswami AG, Ansari MA, Pratap A, Basu S, Shukla VK. Poor Glycemic Control Is Associated With Lower Interleukin-2 (IL-2) Levels and Low Macrophage Viability in Chronic Diabetic Foot Ulcers (DFUs). Int J Low Extrem Wounds. 2023;15347346231182793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 7. | Yousif D, Yousif Z, Joseph P. Diabetic Foot Ulcer Neuropathy, impaired vasculature, and immune responses. Diabetic Foot Ulcers - Pathogenesis, Innovative Treatments and AI Applications. United Kingdom: Intech Open, 2024. [DOI] [Full Text] |
| 8. | Mohsin F, Javaid S, Tariq M, Mustafa M. Molecular immunological mechanisms of impaired wound healing in diabetic foot ulcers (DFU), current therapeutic strategies and future directions. Int Immunopharmacol. 2024;139:112713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 9. | Vu BG, Stach CS, Salgado-Pabón W, Diekema DJ, Gardner SE, Schlievert PM. Superantigens of Staphylococcus aureus from patients with diabetic foot ulcers. J Infect Dis. 2014;210:1920-1927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Gunville CF, Mourani PM, Ginde AA. The role of vitamin D in prevention and treatment of infection. Inflamm Allergy Drug Targets. 2013;12:239-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 112] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 11. | Kamen DL, Tangpricha V. Vitamin D and molecular actions on the immune system: modulation of innate and autoimmunity. J Mol Med (Berl). 2010;88:441-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 381] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 12. | Rizkia CP. Vitamin D and Its Role in Modulating Immune System: A Narrative Literature Review. Open Access Indones J Med Rev. 2023;3:356-361. [DOI] [Full Text] |
| 13. | Papaioannou I, Pantazidou G, Kokkalis Z, Georgopoulos N, Jelastopulu E. Vitamin D Deficiency in Elderly With Diabetes Mellitus Type 2: A Review. Cureus. 2021;13:e12506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Tang W, Chen L, Ma W, Chen D, Wang C, Gao Y, Ran X. Association between vitamin D status and diabetic foot in patients with type 2 diabetes mellitus. J Diabetes Investig. 2022;13:1213-1221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 15. | Akkus G, Sert M. Diabetic foot ulcers: A devastating complication of diabetes mellitus continues non-stop in spite of new medical treatment modalities. World J Diabetes. 2022;13:1106-1121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 75] [Cited by in RCA: 53] [Article Influence: 17.7] [Reference Citation Analysis (7)] |
| 16. | Golpour A, Bereswill S, Heimesaat MM. Antimicrobial and Immune-Modulatory Effects of Vitamin D Provide Promising Antibiotics-Independent Approaches to Tackle Bacterial Infections - Lessons Learnt from a Literature Survey. Eur J Microbiol Immunol (Bp). 2019;9:80-87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 17. | Rebelos E, Tentolouris N, Jude E. The Role of Vitamin D in Health and Disease: A Narrative Review on the Mechanisms Linking Vitamin D with Disease and the Effects of Supplementation. Drugs. 2023;83:665-685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 57] [Reference Citation Analysis (0)] |
| 18. | Zhang JJ, Yu HC, Geng TT, Zhang JJ, Zhou XT, Wang YX, Zhang BF, Yang K, Franco OH, Liao YF, Liu G, Pan A. Serum 25-hydroxyvitamin D concentrations, vitamin D receptor polymorphisms, and risk of infections among individuals with type 2 diabetes: a prospective cohort study. Am J Clin Nutr. 2024;120:398-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 19. | Badralany F, Nurul Ratna Mutu Manikam. Effects of vitamin d administration for wound healing in patients with diabetic foot ulcers: Evidence based case report. Ind J Clin Nutr Physic. 2024;6:1-11. [DOI] [Full Text] |
| 20. | Chen W, Liu L, Hu F. Efficacy of vitamin D supplementation on glycaemic control in type 2 diabetes: An updated systematic review and meta-analysis of randomized controlled trials. Diabetes Obes Metab. 2024;26:5713-5726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 21. | Kurian SJ, Miraj SS, Benson R, Munisamy M, Saravu K, Rodrigues GS, Rao M. Vitamin D Supplementation in Diabetic Foot Ulcers: A Current Perspective. Curr Diabetes Rev. 2021;17:512-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 22. | Chrisdianto A, Airlangga PS, Wirabuana B, Iskandar RPD. Vitamin D and Wound Recovery: Illuminating the Path to Enhanced Healing in Diabetic Patients. Pharmacogn J. 2024;16:485-491. [DOI] [Full Text] |
| 23. | Gul MM, Khan SU, Malik T, Aijaz W, Imran M, Bendari A. Association between vitamin d levels and diabetic foot complications in individuals diagnosed with type 2 diabetes mellitus. JPTCP. 2024. [DOI] [Full Text] |
| 24. | Kanwal M, Soomro A, Hussain W, Sonam, Yousuf T, Shaikh Z. Association between Vitamin D Status and Diabetic Foot in Patients of Type 2 Diabetes Mellitus. PJHS. 2022. [DOI] [Full Text] |
| 25. | Wang F, Zhou L, Zhu D, Yang C. A Retrospective Analysis of the Relationship Between 25-OH-Vitamin D and Diabetic Foot Ulcer. Diabetes Metab Syndr Obes. 2022;15:1347-1355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 26. | Liu L, Zhang F, Jamali M, Guimarães NS, Radkhah N, Jamilian P, Wang Q. The role of vitamin D in diabetic foot ulcer; an umbrella review of meta-analyses. Front Nutr. 2024;11:1454779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 27. | Todorova AS, Jude EB, Dimova RB, Chakarova NY, Serdarova MS, Grozeva GG, Tsarkova PV, Tankova TI. Vitamin D Status in a Bulgarian Population With Type 2 Diabetes and Diabetic Foot Ulcers. Int J Low Extrem Wounds. 2022;21:506-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 28. | Dědečková E, Viták R, Jirásko M, Králová M, Topolčan O, Pecen L, Fürst T, Brož P, Kučera R. Vitamin D(3) Supplementation: Comparison of 1000 IU and 2000 IU Dose in Healthy Individuals. Life (Basel). 2023;13:808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 29. | Kuznia S, Czock D, Kopp-Schneider A, Caspari R, Fischer H, Laetsch DC, Slavic M, Brenner H, Schöttker B. Efficacy and Safety of a Personalized Vitamin D(3) Loading Dose Followed by Daily 2000 IU in Colorectal Cancer Patients with Vitamin D Insufficiency: Interim Analysis of a Randomized Controlled Trial. Nutrients. 2022;14:4546. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 30. | Sha S, Degen M, Vlaski T, Fan Z, Brenner H, Schöttker B. The Safety Profile of Vitamin D Supplements Using Real-World Data from 445,493 Participants of the UK Biobank: Slightly Higher Hypercalcemia Prevalence but Neither Increased Risks of Kidney Stones nor Atherosclerosis. Nutrients. 2024;16:2251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |