Published online Jul 15, 2025. doi: 10.4239/wjd.v16.i7.108121
Revised: May 19, 2025
Accepted: June 23, 2025
Published online: July 15, 2025
Processing time: 90 Days and 23.5 Hours
Type 2 diabetes mellitus (T2DM), a chronic metabolic disease with a high global incidence, has become a serious public health challenge. China has the largest number of T2DM patients worldwide, imposing a significant economic burden on the healthcare system. T2DM is closely associated with insulin resistance, impaired pancreatic B cell function, and disordered glucose and lipid metabolism, which can lead to various complications, reducing patients' quality of life and increasing the risk of disability and death. Thus, finding effective preventive and intervention measures is crucial. Exercise therapy, a key part of diabetes management, has gained attention in recent years, with many studies indicating its benefits for blood glucose control and other aspects in diabetic patients.
To assess the effectiveness of combined resistance and aerobic exercise interventions on blood glucose control and metabolic indicators in patients with T2DM and to explore their application in diabetes management.
Systematic searches were conducted using PubMed, EMBASE, Cochrane Library, and Chinese databases for relevant randomized controlled trials (RCTs). The inclusion criteria were participants aged ≥ 18 years with T2DM and the intervention involved combined resistance and aerobic exercise for ≥ 8 weeks. The primary outcome indicators were fasting blood glucose, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), triglycerides (TG), glycated hemoglobin A1c (HbA1c), and total cholesterol (TC) levels. Data analysis was performed using RevMan software, and the interventional effects were assessed using weighted mean differences or standardized mean differences (SMD).
Six RCTs meeting the inclusion criteria were included, with a total sample size of 366 participants. The meta-analysis results showed that combined resistance and aerobic exercise significantly improved several metabolic indicators in patients with T2DM. Specific results were as follows: (1) For fasting blood glucose, combined exercise was more effective than aerobic exercise alone [SMD = 1.22; 95% confidence interval (95%CI): 0.70, 1.74; P < 0.00001]; (2) LDL-C levels were significantly reduced by the combined intervention (SMD = 1.45; 95%CI: 1.18-1.72; P < 0.00001); (3) The combined intervention significantly increased HDL-C levels (SMD = 1.42; 95%CI: 0.98-1.87; P < 0.00001); (4) The combined intervention significantly reduced TG levels (SMD = 1.12; 95%CI: 0.85-1.39; P < 0.00001; (5) No statistically significant difference was observed in HbA1c between the combined and the aerobic exercise group (SMD = −0.03; 95%CI: -1.09 to 1.04; P < 0.00001); and (6) The combined exercise intervention group significantly reduced TC levels (SMD = 2.66; 95%CI: 1.93-3.38; P < 0.00001). The subgroup analysis results suggest that the effect of exercise interventions may be influenced by various factors, including the patient's age, baseline blood glucose levels, and exercise intensity.
Combined resistance and aerobic exercise intervention significantly improved fasting blood glucose, LDL-C, HDL-C, TG, and TC levels in patients with T2DM, especially in terms of blood glucose control and cardiovascular risk, demonstrating better outcomes than aerobic exercise alone.
Core Tip: Combined resistance and aerobic exercise significantly improves glycemic control and metabolic indicators in patients with type 2 diabetes, including fasting blood glucose, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides, and total cholesterol levels. Although no significant improvement was observed in glycated hemoglobin A1c levels, combined exercise remains a promising therapeutic strategy for diabetes management.
- Citation: Ma JC, Shu S, Chen TX, Bai HJ, Yang Y, Ding XW. Intervention effect of combined resistance and aerobic exercise on type 2 diabetes: A meta-analysis. World J Diabetes 2025; 16(7): 108121
- URL: https://www.wjgnet.com/1948-9358/full/v16/i7/108121.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i7.108121
Type 2 diabetes mellitus (T2DM) is a chronic metabolic disease with a high incidence worldwide that has become a serious public health issue. According to the latest report from the International Diabetes Federation (IDF), the global number of patients with diabetes exceeds 537 million, over 90% of whom suffer from T2DM, and is projected to reach 783 million by 2045[1]. China has the largest number of patients with T2DM worldwide, which imposes a significant economic burden on its healthcare system[2]. T2DM is closely associated with insulin resistance, impaired pancreatic B cell function, and disordered glucose and lipid metabolism, which can lead to various complications, such as cardiovascular diseases, diabetic nephropathy, and diabetic retinopathy, significantly reducing patients' quality of life and increasing the risk of disability and death[3]. While some clinical trials suggest the superior efficacy of combined exercise in glycemic control[4,5], others indicate that excessive high-intensity interval training may impair mitochondrial respiratory function in skeletal muscle, thereby disrupting normal metabolic processes and exacerbating insulin resistance and glycemic variability[6,7]. These discrepancies may stem from variations in study design, such as differences in exercise protocols (e.g., intensity, duration) or participant characteristics [e.g., age, baseline glycated hemoglobin A1c (HbA1c) levels]. Therefore, the identification of effective prevention and intervention measures is a current research topic. Exercise therapy, an important modality of diabetes management, has been widely recognized in recent years[8]. Many studies have shown that physical exercise improves glycemic control in patients with diabetes, effectively alleviates insulin resistance, promotes pancreatic function, and reduces the risk of cardiovascular diseases[9]. The American Diabetes Association guidelines recommend at least 150 minutes of moderate-intensity aerobic exercise or at least two sessions of resistance training per week to significantly improve glycemic levels and cardiovascular risk factors in patients with T2DM[10]. Traditional views hold that aerobic exercise can effectively improve cardiopulmonary function and increase muscle glucose uptake and utilization, thereby lowering blood glucose levels. Resistance training enhances muscle strength and mass, significantly improving insulin sensitivity and promoting glucose metabolism[11]. Recently, several studies have focused on the interventional effects of combined resistance and aerobic exercises on T2DM, suggesting that combined exercise may improve insulin sensitivity, glycemic control, and metabolic indicators. However, the results of existing studies show certain differences and controversies, with a few indicating that combined exercises are superior to single forms of exercise, whereas others suggest no significant advantages. This meta-analysis aimed to systematically review existing studies on combined resistance and aerobic exercise intervention in T2DM, and to clarify the efficacy of combined exercise intervention in improving glycemic control and metabolism-related indicators in patients with T2DM.
The search strategy is shown in Table 1. We systematically searched multiple Chinese and English databases, including PubMed, EMBASE, Cochrane Library, China National Knowledge Infrastructure, Wanfang Database, and Chinese Medical Journal Database. The search strategy combined subject terms (MeSH terms) and free terms to ensure coverage of all relevant studies. Subject terms for Chinese database searches include: "resistance training”, "aerobic exercise”, "combined exercise”, "exercise intervention”, "T2DM”, "diabetes”, "efficacy”, "effectiveness”, "safety”, and "randomized controlled trials". The specific search strategy for English databases is as follows: "Resistance training" OR "Strength training" OR "Resistance exercise" OR "Strength exercise" OR "Aerobic exercise" OR "Aerobic training" OR "Combined training" OR "Combined exercise" OR "Resistance-aerobic exercise" OR "Aerobic-resistance exercise" OR "Exercise intervention" OR "Physical activity" AND ("Type 2 Diabetes Mellitus" OR "Diabetes Mellitus Type 2" OR "T2DM" OR "Non-insulin-dependent diabetes mellitus" OR "NIDDM" OR "Type II Diabetes") AND ("Effectiveness" OR "Efficacy" OR "Clinical effect" OR "Therapeutic effect" OR "Glycemic control" OR "Blood glucose control" OR "HbA1c" OR "Glucose metabolism") AND ("Safety" OR "Adverse events" OR "Adverse effects" OR "Side effects" OR "Safety profile") AND ("RCT" OR "Randomized controlled trial" OR "Clinical trial" OR "Randomized trial").
| Type of database | Search term combination | Screening conditions |
| English databases | "Resistance training" OR "Strength training" OR "Resistance exercise" OR "Strength exercise" OR "Aerobic exercise" OR "Aerobic training" OR "Combined training" OR "Combined exercise" OR "Resistance-aerobic exercise" OR "Aerobic-resistance exercise" OR "Exercise intervention" OR "Physical activity" AND ("Type 2 Diabetes Mellitus" OR "Diabetes Mellitus Type 2" OR "T2DM" OR "Non-insulin-dependent diabetes mellitus" OR "NIDDM" OR "Type II Diabetes") AND ("Effectiveness" OR "Efficacy" OR "Clinical effect" OR "Therapeutic effect" OR "Glycemic control" OR "Blood glucose control" OR "HbA1c" OR "Glucose metabolism") AND ("Safety" OR "Adverse events" OR "Adverse effects" OR "Side effects" OR "Safety profile") AND ("RCT" OR "Randomized controlled trial" OR "Clinical trial" OR "Randomized trial") | (1) Randomized controlled trials; (2) Patients with T2DM aged 18 or older; (3) Compare resistance exercise with aerobic exercise lasting at least 8 weeks, with preset frequency, intensity, and duration; and (4) Primary outcome indicators include at least one of the following and provide usable data: Fasting blood glucose level; low-density lipoprotein cholesterol level; high-density lipoprotein cholesterol level; triglycerides level; HbA1c level; total cholesterol level |
| Chinese databases | "Resistance training”, "aerobic exercise”, "combined exercise”, "exercise intervention”, "T2DM”, "diabetes”, "efficacy”, "effectiveness”, "safety”, and "randomized controlled trials" |
(1) Studies are randomized controlled trials (RCTs); (2) Participants are patients with T2DM aged 18 or older; (3) The trial compares resistance exercise with aerobic exercise lasting at least 8 weeks, with preset frequency, intensity, and duration; and (4) The primary outcome indicators include at least one of the following and provide usable data: (a) Fasting blood glucose; (b) Low-density lipoprotein cholesterol (LDL-C) level; (c) High-density lipoprotein cholesterol (HDL-C) level; (d) Triglycerides (TG) level; (e) HbA1c level; and (f) Total cholesterol (TC) level.
Non-RCTs, trials without a control group, case reports, experiential reports, literature with data errors or ambiguities, literature reviews, clinical pharmacological studies, and animal experiments.
Two evaluators independently searched, extracted the data, and assessed the methodological quality. Discrepancies were resolved by cross-verification and discussion or by arbitration of a third reviewer. Quality assessment of the literature followed the Cochrane RCT quality evaluation criteria, which included random allocation methods, allocation con
All statistical analyses were conducted using the RevMan 5.3 software (Oxford, United Kingdom). For continuous variables, the weighted mean difference or standardized mean difference (SMD) was used as an effect size indicator to compare differences between the intervention and control groups. The Cochrane Collaboration's RevMan 5.3 software will be used for meta-analysis. Odds ratios were used as outcome indicators for dichotomous variables, whereas SMD was used for continuous variables, both expressed with 95% confidence intervals (95%CI). If the studies were homogeneous (P > 0.05, I2 < 50%), a fixed-effects model was used; otherwise, a random-effects model was used. If heterogeneity was significant, a random-effects model was used, and publication bias was assessed using funnel plots.
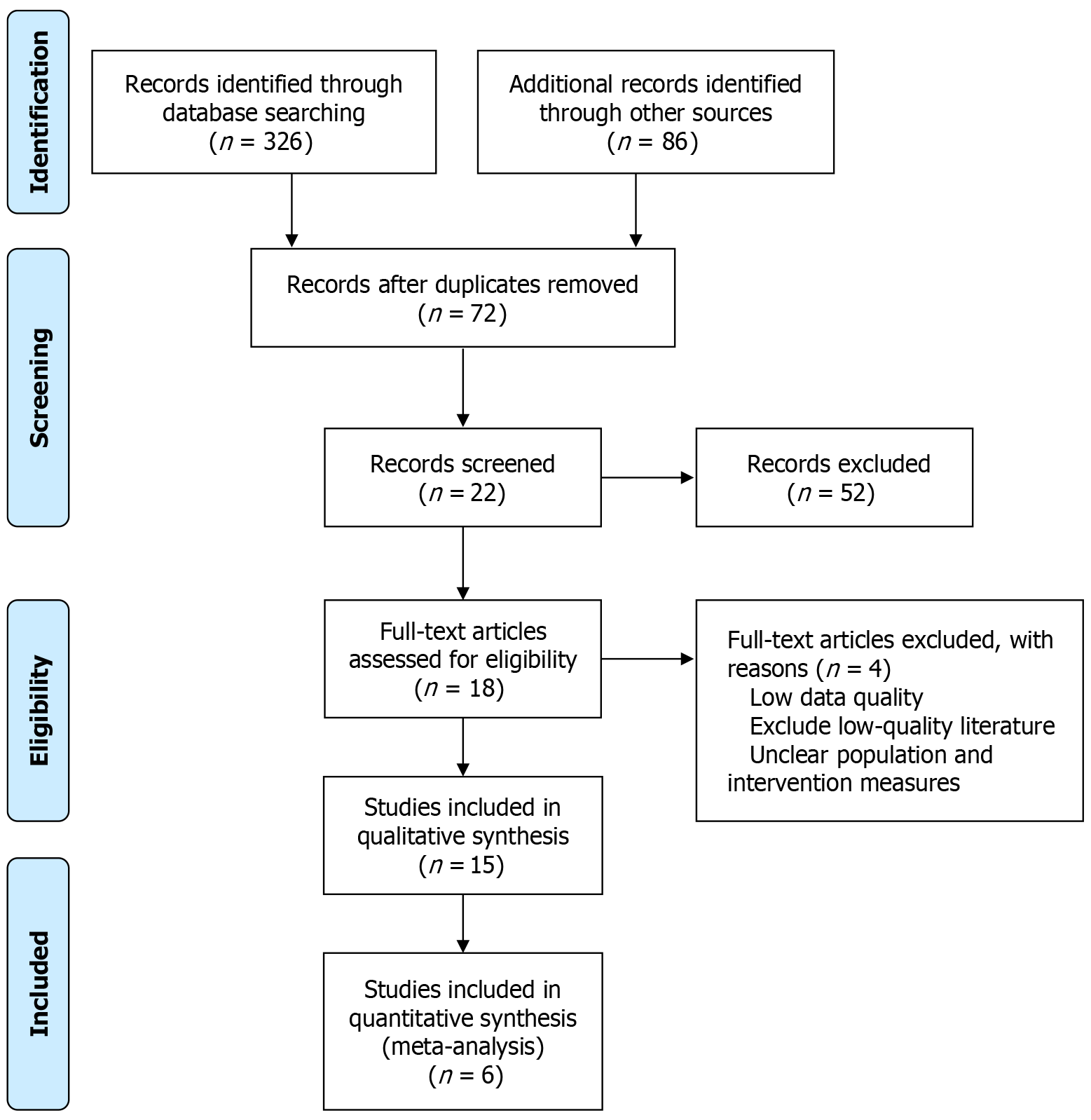
A systematic search of PubMed, EMBASE, Web of Science, and Cochrane Library identified 412 potential studies. After initial screening by title and abstract and removal of duplicates, 72 studies were retained. Further screening excluded 52 studies because of low quality or inconsistency with the research objectives. Of the remaining 18 studies, 4 were excluded because of incomplete data or inconsistencies with the research objectives. Six studies met the inclusion criteria and were included in this meta-analysis. The literature selection process is illustrated in Figure 1.
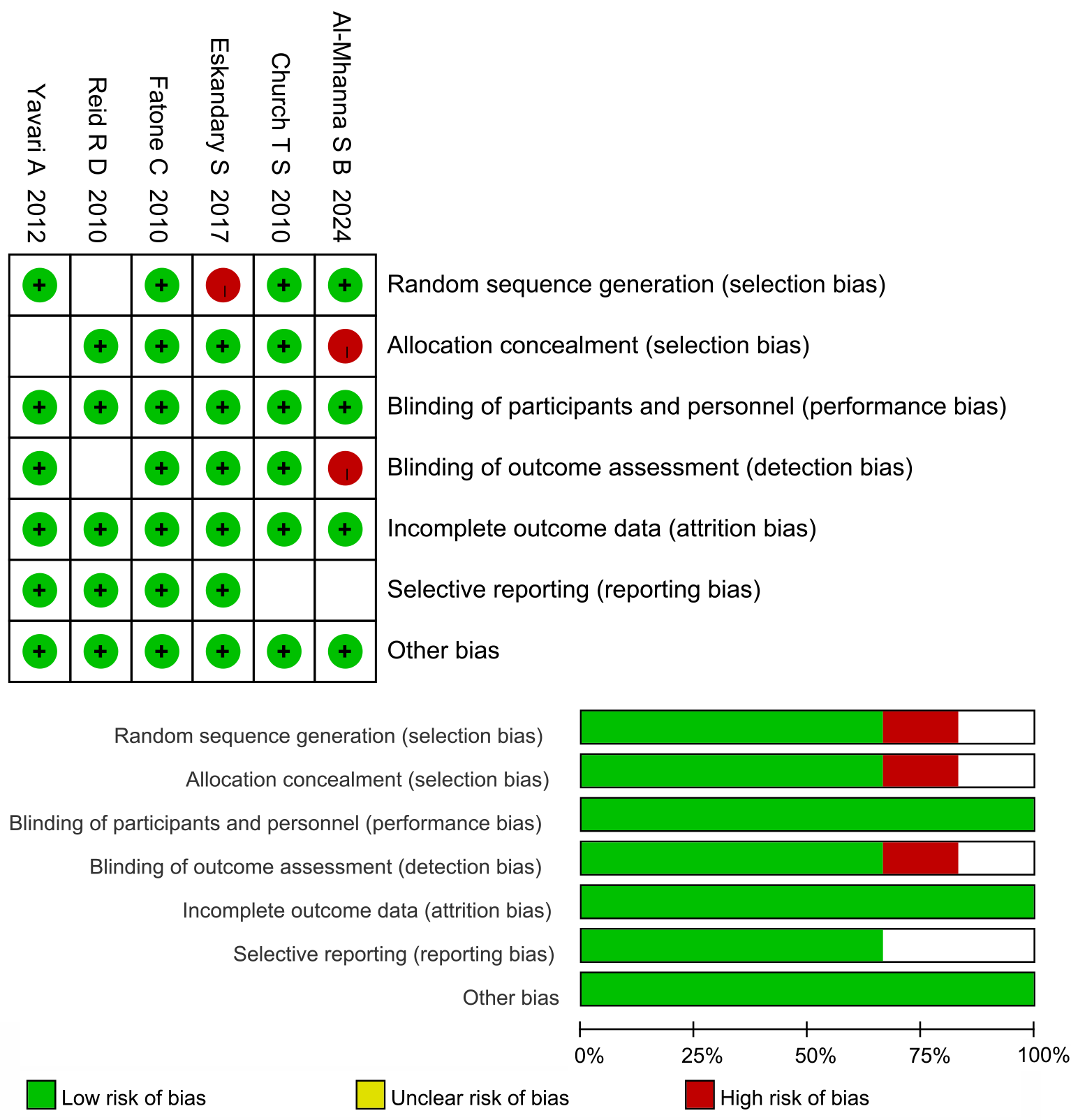
Of the six included studies[12-17], four were of high methodological quality (grade A) and two were of moderate quality (grade B). Four studies provided detailed descriptions of the methods used; one reported allocation concealment, and another had comparable outcome indicators. The results of the quality evaluation are shown in Figure 2.
This analysis included six clinical studies[12-17] with a total sample size of 366 patients. All studies were high-quality RCTs (most scored A). The age range of the study population was 30-75 years, with a long disease duration in both the treatment and control groups. The disease duration in the treatment group ranged from 1 to 25 years, whereas that in the control group ranged from 1 to 6 years. The treatment primarily combined resistance training and aerobic exercise for 12 months. The main outcome indicators included biochemical indicators such as fasting blood glucose, LDL-C, HDL-C, TG, HbA1c, and TC levels. All studies compared the effects of combined resistance and aerobic exercise with those of aerobic exercise alone and analyzed different age groups and health statuses (Table 2).
| Ref. | Sample (male/female) | Age | Outcome | Exercise method | Duration exercise program (month) | Resistance exercise-aerobic exercise combination (year) | Value of reference | ||
| Treatment group (resistance exercise-aerobic exercise combination) | Control group | ||||||||
| Yavari et al[12], 2012 | 52/46 | 56-75 | (1), (2), (4), (5) | Resistance band exercises, including 10 different movements, with 3 sets of 15-20 repetitions per movement | Gait training at an intensity of 3.6-5.2 metabolic equivalents | Simple exercise: Slow walking | 12 | 2-19/1-6 | B |
| Church et al[13], 2010 | 69/68 | ≥ 34 | (1), (2), (5), (6) | 5 resistance training machines, with 3 sets of 8 repetitions per machine, resistance ranging from 60% of baseline to approximately 2.5 kg | Cycling ergometer at an intensity of 50%-85% of VO2 peak | Jogging for 30 minutes | 12 | 2-3/1-6 | A |
| Reid et al[14], 2010 | 60/62 | 30-55 | (1), (2), (3) | 9 exercises using machines and free weights, with 3 sets of 10 repetitions per exercise | Treadmill, upright stationary bike, recumbent stationary bike, and elliptical trainer at an intensity of 65%-70% of predicted heart rate | Treadmill for 20 minutes | 12 | 2-25/1-6 | A |
| Eskandary et al[15], 2017 | 37/37 | ≥ 30 | (1), (2), (4), (5), (6) | 7 exercises using weight machines, with 2-3 sets of 7-9 repetitions at maximum weight | Cycling or treadmill at an intensity of 60%-75% of maximum heart rate | Simple exercise: Slow walking | 12 | 1-6 | A |
| Al-Mhanna et al[16], 2024 | 63/63 | < 43 | (1), (2), (3), (5) | 8 exercises using weight machines, with 2-3 sets of 6-8 repetitions per exercise | Cycling ergometer at an intensity of 65%-85% of heart rate reserve | Simple aerobic exercise for 20 minutes + 10 minutes of slow walking | 12 | 2-19 | A |
| Fatone et al[17], 2010 | 42/37 | ≥ 32 | (1), (2), (4), (5), (6) | No machine-based exercises, with 3 sets of 8-10 repetitions per exercise | Treadmill, elliptical machine, or cycle ergometer at an intensity of 60%-75% of maximum heart rate | Simple exercise: Slow walking | 12 | 1-4/1-5 | B |
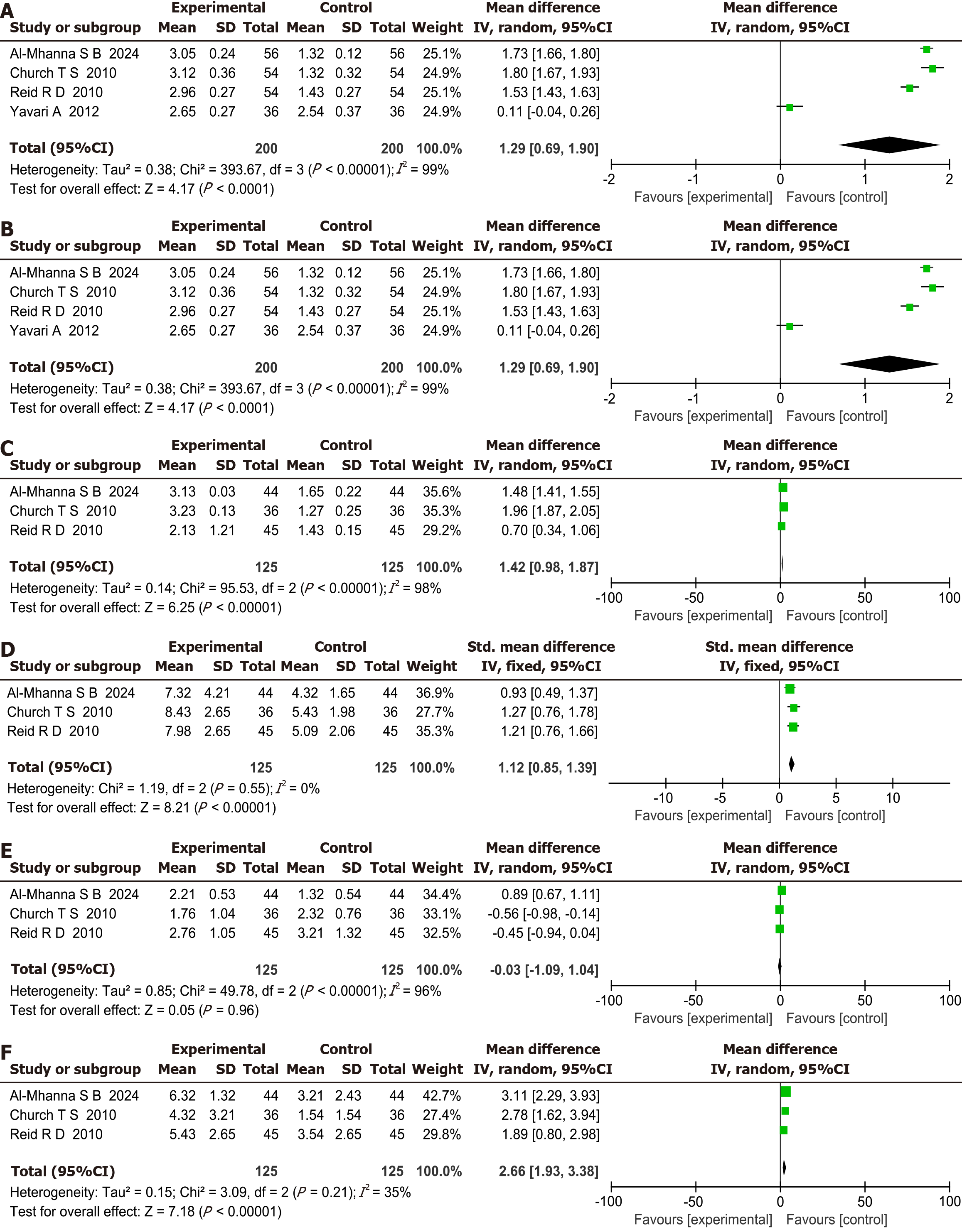
Fasting blood glucose comparison: This meta-analysis included six studies that compared fasting blood glucose levels in patients with T2DM between the two groups. A P value of 0.001 and I2 of 75% indicated heterogeneity among the studies. The pooled effect size using a fixed-effects model showed SMD of 1.22, 95%CI: 0.70-1.74, indicating that combined aerobic and resistance exercise was more effective than aerobic exercise alone, with a statistically significant difference (P < 0.00001), as shown in Figure 3A.
LDL-C comparison: This meta-analysis included three homogeneous studies on improvements in LDL-C levels in patients with T2DM. The pooled effect size was SMD of 1.45, 95%CI: 1.18-1.72, indicating that combined aerobic and resistance exercise was significantly more effective than aerobic exercise alone (P < 0.00001), as shown in Figure 3B.
HDL-C comparison: This meta-analysis included five studies that compared HDL-C levels in patients with T2DM between the two groups. An I2 value of 98% indicated significant heterogeneity. The pooled effect size, using a random-effects model, had an SMD of 1.42, 95%CI: 0.98-1.87, indicating that combined aerobic and resistance exercise was more effective than aerobic exercise alone, with a statistically significant difference (P < 0.00001), as shown in Figure 3C.
TG comparison: This meta-analysis included three studies that compared TG levels in patients with T2DM between the two groups. An I2 value of 0% indicated no heterogeneity. The pooled effect size, using a fixed-effects model, had an SMD of 1.12, 95%CI: 0.85-1.39, indicating that combined aerobic and resistance exercise was more effective than aerobic exercise alone, with a statistically significant difference (P < 0.00001), as shown in Figure 3D.
HbA1c comparison: This meta-analysis included three studies that compared HbA1c levels in patients with T2DM between the two groups. A P value < 0.00001 and I2 of 96% indicated heterogeneity among studies. The pooled effect size, using a random-effects model, showed an SMD of -0.03, 95%CI: -1.09 to 1.04, indicating that combined aerobic and resistance exercise was more effective than aerobic exercise alone; however, the difference was not statistically significant (P < 0.00001), as shown in Figure 3E.
TC comparison: This meta-analysis included three studies that compared TC levels in patients with T2DM between the two groups. A P value of 0.21 and I2 of 35% indicated no homogeneity among studies. The pooled effect size, using a fixed-effects model, showed an SMD of 2.66, 95%CI: 1.93 to 3.38, indicating that combined aerobic and resistance exercise was more effective than aerobic exercise alone, with a statistically significant difference (P < 0.00001), as shown in Figure 3F.
Subgroup analysis: To further explore the impact of different factors on the effect of the combined resistance-aerobic exercise intervention in type 2 diabetes, a subgroup analysis was performed. The participants were grouped according to age, baseline glycemic control (fasting blood glucose), and exercise intensity. Younger patients (30-50 years old) often had better intervention results than older ones, especially in terms of fasting blood glucose, LDL-C, HDL-C, and TC levels. Although the effect was weaker in the older patients, the intervention remained effective. For baseline glycemic control, patients with poor glycemic control (fasting blood glucose > 8 mmol/L) showed more significant metabolic improvements after combined resistance and aerobic exercise, particularly in LDL-C, HDL-C, and TC levels. In terms of exercise intensity, the high-intensity group generally outperformed the low-to-moderate-intensity group in terms of metabolic indicators, particularly in improving HDL-C and TC levels. These subgroup findings suggest that the effectiveness of the combined resistance-aerobic exercise intervention in type 2 diabetes might be influenced by patient age, baseline blood glucose level, and exercise intensity. Therefore, in diabetes management, customized exercise plans are needed to maximize the treatment benefits, as shown in Table 3.
| Factor | Fasting blood glucose | LDL-C | HDL-C | TG | HbA1c | TC |
| Age (years) | ||||||
| 30-50 | 1.35 (0.75, 1.95) | 1.60 (1.25, 1.95) | 1.50 (1.05, 1.95) | 1.25 (0.80, 1.70) | -0.10 (-1.10, 0.90) | 2.80 (2.05, 3.55) |
| > 50 | 1.00 (0.50, 1.50) | 1.30 (1.05, 1.55) | 1.30 (0.90, 1.70) | 1.00 (0.70, 1.30) | 0.05 (-0.95, 1.05) | 2.40 (1.75, 3.05) |
| Fasting blood glucose | ||||||
| > 8 mmol/L | 1.50 (1.00, 2.00) | 1.75 (1.40, 2.10) | 1.40 (1.00, 1.80) | 1.15 (0.85, 1.45) | -0.05 (-1.10, 1.00) | 2.90 (2.30, 3.50) |
| < 8 mmol/L | 0.95 (0.40, 1.50) | 1.10 (0.80, 1.40) | 1.25 (0.80, 1.70) | 1.05 (0.70, 1.40) | 0.05 (-0.95, 1.05) | 2.50 (1.85, 3.15) |
| Exercise intensity | ||||||
| High | 1.25 (0.80, 1.70) | 1.50 (1.25, 1.75) | 1.55 (1.10, 2.00) | 1.10 (0.80, 1.40) | -0.05 (-1.05, 0.95) | 2.75 (2.10, 3.40) |
| Low-to moderate | 1.10 (0.50, 1.70) | 1.25 (1.00, 1.50) | 1.10 (0.70, 1.50) | 1.05 (0.70, 1.40) | 0.00 (-1.00, 1.00) | 2.50 (1.85, 3.15) |
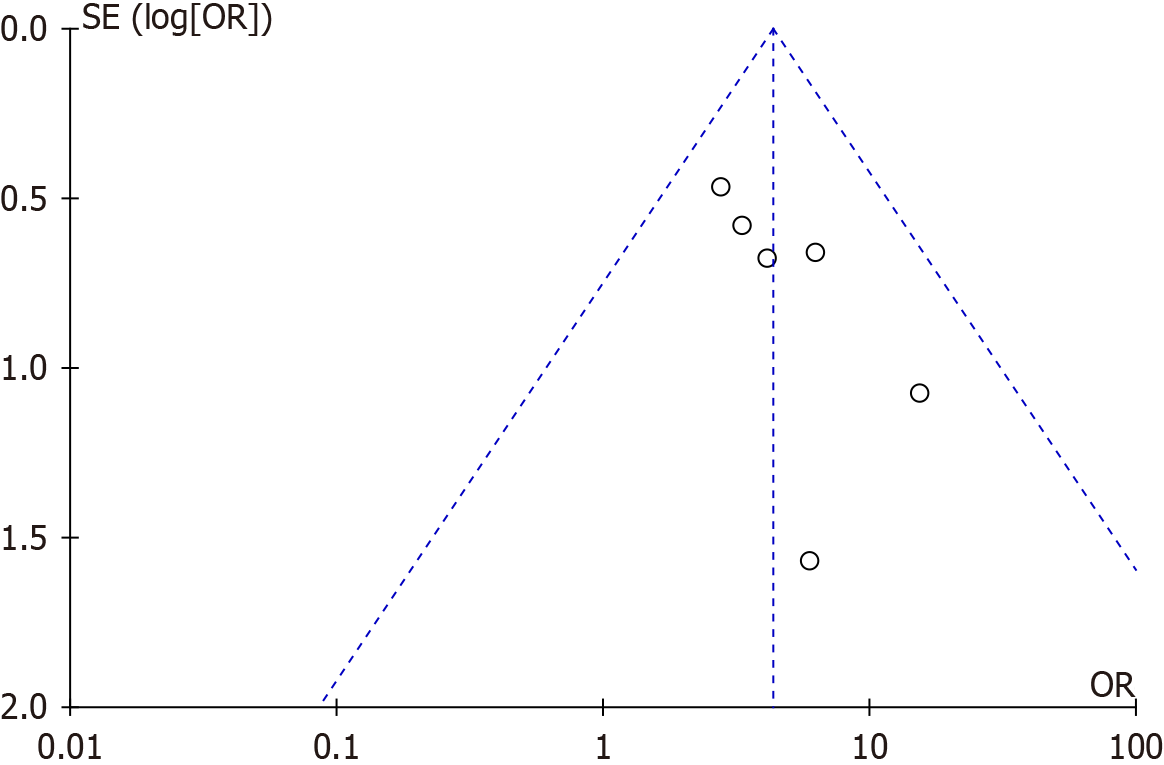
Publication bias of the combined resistance and aerobic exercise intervention for T2DM was assessed and the results are shown in Figure 4. The included studies showed a symmetrical distribution in the funnel plot, indicating minimal publication bias among the studies. Most of the scatter points were concentrated in the upper part of the funnel plot, suggesting good representation and accuracy of the studies included. In summary, no obvious publication bias was observed.
T2DM is a major global chronic metabolic disease and a growing public health concern. According to the IDF, over 537 million people worldwide have diabetes, approximately 90% of whom have T2DM, and this number is expected to rise even further in the coming decades[18]. This meta-analysis, encompassing six RCTs, evaluated the effects of combined resistance and aerobic exercise on blood sugar levels and metabolic indicators in patients with T2DM. The findings revealed that combined resistance-aerobic exercise was more effective than aerobic exercise alone in reducing the levels of several metabolic indicators, including fasting blood glucose, LDL-C, HDL-C, TG, and TC. However, the reduction in HbA1c level was not statistically significant, indicating that combined exercise may not offer a substantial advantage in this specific outcome.
This study shows that combined resistance-aerobic exercise significantly improves fasting blood glucose and lipid levels, potentially due to the comprehensive regulatory effects of exercise on metabolic pathways. Moderate-intensity continuous training exerts time-specific effects on lipid metabolism in adolescents, with findings indicating that exercise performed at different time periods differentially modulates blood lipid parameters such as TC, LDL, and HDL[19]. This study showed that combined resistance and aerobic exercise has a significant advantage in controlling fasting blood glucose, a key indicator of blood sugar control that reflects pancreatic function and insulin sensitivity. Several factors influence fasting blood glucose levels, including insulin secretion, insulin resistance, and hepatic glucose production[20,21]. High-intensity aerobic exercise improves muscle glucose uptake and utilization, and lowers fasting blood glucose levels. Research shows that regular aerobic exercise improves fasting blood glucose in patients with T2DM, with exercise intensity directly proportional to the effect[22]. Resistance training enhances insulin sensitivity and lowers fasting blood glucose levels by increasing muscle mass and strength[23]. Our results are consistent with those of previous studies demonstrating the benefits of combined resistance and aerobic exercise in improving fasting blood glucose levels. However, some studies have indicated that aerobic-only exercise can also significantly improve fasting blood glucose levels; therefore, the advantage of combined exercise may depend on specific exercise programs, exercise intensity, and individual patient differences.
LDL-C and HDL-C play important roles in cardiovascular health. Exercise, especially a combination of resistance and aerobic, can significantly improve lipid levels[24,25]. Resistance training promotes fat oxidation and lowers LDL-C by increasing muscle mass and metabolism[26]. Meanwhile, aerobic exercise enhances cardiopulmonary function and fat metabolism, thereby increasing HDL-C levels. High TG levels are closely related to insulin resistance and an increased risk of cardiovascular disease[27]. Exercise, particularly aerobic, can effectively lower TG levels[28,29]. Resistance training also contributes to increased muscle mass and metabolism, which in turn accelerates fat breakdown and utilization, thereby reducing TG levels. In this study, combined resistance-aerobic exercise significantly lowered TG levels in patients with T2DM, supporting previous findings. However, the extent of TG reduction varies across studies owing to differences in intervention and exercise intensity. HbA1c reflects the average blood sugar level over the past 2-3 months[30]. Our meta-analysis showed that combined resistance and aerobic exercises did not significantly lower HbA1c levels, which aligns with the findings of some studies. Our finding of non-significant HbA1c reduction contrasts with prior studies reporting HbA1c improvements[31]. This discrepancy may arise from shorter intervention durations in included trials (e.g., 12 months vs. longer-term studies) or heterogeneity in baseline HbA1c levels across populations. Possible reasons include that HbA1c changes require long-term intervention and more precise, individualized treatment. HbA1c response may also be influenced by baseline diabetes control, intervention intensity, and participant compliance. TC levels, an important lipid indicator linked to cardiovascular disease, did not show significant improvement in this study. Blood glucose variability is an independent predictor of cardiovascular events. Future research should use continuous glucose monitoring to assess the long-term effects of exercise on blood glucose stability[32]. This study shows that combined exercise is highly effective for diabetes management, in line with the integrated health strategy advocated by Chen et al[33]. Future diabetes management must integrate exercise with other lifestyle changes to fully improve metabolic indicators and long-term outcomes.
The limitations of this study are the small number of trials included and the high heterogeneity across studies, which might have affected the reliability of the conclusions. Factors such as exercise intensity, intervention duration, patient demographics, and differences in the study design could have led to varying interventional effects. Future research should include larger sample sizes, more standardized interventions, and multicenter RCTs to further verify the effects of combined resistance-aerobic exercise on patients with T2DM and assess the impact of different intervention types, durations, and population characteristics. The high heterogeneity (I² = 96%) observed in HbA1c analysis underscores the need for standardized exercise protocols and larger cohorts to minimize confounding factors such as adherence variability or co-interventions (e.g., dietary adjustments).
This meta-analysis addresses a critical gap in the literature by consolidating evidence from six RCTs with rigorous inclusion criteria, resolving inconsistencies in prior studies through subgroup analyses (e.g., age, exercise intensity), and demonstrating the differential efficacy of combined exercise across metabolic outcomes. Combined resistance and aerobic exercises have significant therapeutic effects on glycemic control and metabolic indicators in patients with T2DM, particularly in improving fasting blood glucose, LDL-C, HDL-C, and TG levels. Despite the lack of significant difference in HbA1c levels, combined exercise remains an effective means of diabetes management.
| 1. | Ali H, Abu-Farha M, Hammad MM, Devarajan S, Bahbahani Y, Al-Khairi I, Cherian P, Alsairafi Z, Vijayan V, Al-Mulla F, Attar AA, Abubaker J. Potential Role of N-Cadherin in Diagnosis and Prognosis of Diabetic Nephropathy. Front Endocrinol (Lausanne). 2022;13:882700. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 2. | Awoke T, Kiflu DA, Tadesse DA. The Effect of Aerobic and Combined, Aerobic-anaerobicexercise on Obese Diabetic Patients. 2024 Preprint. [DOI] [Full Text] |
| 3. | Kaikhosro Doulatyari P, Ghahramani M, Mozaffari K. Investigating the Effect of Aerobic and Resistance Training on Insulin Resistance and Some Cardiovascular Disease Risk Factors in Type 2 Diabetes Mellitus Patients: A Systematic Review. J Clin Res Paramed Sci. 2023;12:e134510. [DOI] [Full Text] |
| 4. | Amare F, Alemu Y, Enichalew M, Demilie Y, Adamu S. Effects of aerobic, resistance, and combined exercise training on body fat and glucolipid metabolism in inactive middle-aged adults with overweight or obesity: a randomized trial. BMC Sports Sci Med Rehabil. 2024;16:189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Aldahr MHS, Abd El-Kader SM. Impact of exercise on renal function, oxidative stress, and systemic inflammation among patients with type 2 diabetic nephropathy. Afr Health Sci. 2022;22:286-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Shambrook P, Kingsley MI, Taylor NF, Wundersitz DW, Wundersitz CE, Paton CD, Gordon BA. A comparison of acute glycaemic responses to accumulated or single bout walking exercise in apparently healthy, insufficiently active adults. J Sci Med Sport. 2020;23:902-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Flockhart M, Nilsson LC, Tais S, Ekblom B, Apró W, Larsen FJ. Excessive exercise training causes mitochondrial functional impairment and decreases glucose tolerance in healthy volunteers. Cell Metab. 2021;33:957-970.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 134] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 8. | Park W, Jung WS, Hong K, Kim YY, Kim SW, Park HY. Effects of Moderate Combined Resistance- and Aerobic-Exercise for 12 Weeks on Body Composition, Cardiometabolic Risk Factors, Blood Pressure, Arterial Stiffness, and Physical Functions, among Obese Older Men: A Pilot Study. Int J Environ Res Public Health. 2020;17:7233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 9. | Annibalini G, Lucertini F, Agostini D, Vallorani L, Gioacchini A, Barbieri E, Guescini M, Casadei L, Passalia A, Del Sal M, Piccoli G, Andreani M, Federici A, Stocchi V. Concurrent Aerobic and Resistance Training Has Anti-Inflammatory Effects and Increases Both Plasma and Leukocyte Levels of IGF-1 in Late Middle-Aged Type 2 Diabetic Patients. Oxid Med Cell Longev. 2017;2017:3937842. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 10. | Abdollahpour Alni M, Nikookheslat SD. The effect of 12 weeks aerobic, resistance and combined trainings on peripheral vascular disease in type 2 diabetes with peripheral neuropathy in men. Obes Med. 2022;34:100439. [DOI] [Full Text] |
| 11. | Kwon HR, Min KW, Ahn HJ, Seok HG, Lee JH, Park GS, Han KA. Effects of Aerobic Exercise vs. Resistance Training on Endothelial Function in Women with Type 2 Diabetes Mellitus. Diabetes Metab J. 2011;35:364-373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 12. | Yavari A, Najafipoor F, Aliasgharzadeh A, Niafar M, Mobasseri M. Effect of aerobic exercise, resistance training or combined training on glycaemic control and cardiovascular risk factors in patients with type 2 diabetes. Biol Sport. 2012;29:135-143. [RCA] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 13. | Church TS, Blair SN, Cocreham S, Johannsen N, Johnson W, Kramer K, Mikus CR, Myers V, Nauta M, Rodarte RQ, Sparks L, Thompson A, Earnest CP. Effects of aerobic and resistance training on hemoglobin A1c levels in patients with type 2 diabetes: a randomized controlled trial. JAMA. 2010;304:2253-2262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 715] [Cited by in RCA: 647] [Article Influence: 43.1] [Reference Citation Analysis (0)] |
| 14. | Reid RD, Tulloch HE, Sigal RJ, Kenny GP, Fortier M, McDonnell L, Wells GA, Boulé NG, Phillips P, Coyle D. Effects of aerobic exercise, resistance exercise or both, on patient-reported health status and well-being in type 2 diabetes mellitus: a randomised trial. Diabetologia. 2010;53:632-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 15. | Eskandary S, Rahimi E. Effects of eight weeks aerobic training, resistance training and concurrent training on the metabolic syndrome and HbA1c in men with type 2 diabetes. Phys Activity Horm. 2017;1:51-64. |
| 16. | Al-Mhanna SB, Batrakoulis A, Wan Ghazali WS, Mohamed M, Aldayel A, Alhussain MH, Afolabi HA, Wada Y, Gülü M, Elkholi S, Abubakar BD, Rojas-Valverde D. Effects of combined aerobic and resistance training on glycemic control, blood pressure, inflammation, cardiorespiratory fitness and quality of life in patients with type 2 diabetes and overweight/obesity: a systematic review and meta-analysis. PeerJ. 2024;12:e17525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 17. | Fatone C, Guescini M, Balducci S, Battistoni S, Settequattrini A, Pippi R, Stocchi L, Mantuano M, Stocchi V, De Feo P. Two weekly sessions of combined aerobic and resistance exercise are sufficient to provide beneficial effects in subjects with Type 2 diabetes mellitus and metabolic syndrome. J Endocrinol Invest. 2010;33:489-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Earnest CP, Johannsen NM, Swift DL, Gillison FB, Mikus CR, Lucia A, Kramer K, Lavie CJ, Church TS. Aerobic and strength training in concomitant metabolic syndrome and type 2 diabetes. Med Sci Sports Exerc. 2014;46:1293-1301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 19. | Zhang H, Liu J, Cui M, Chai H, Chen L, Zhang T, Mi J, Guan H, Zhao L. Moderate-intensity continuous training has time-specific effects on the lipid metabolism of adolescents. J Transl Int Med. 2023;11:57-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 20. | Al-Mhanna SB, Rocha-Rodriguesc S, Mohamed M, Batrakoulis A, Aldhahi MI, Afolabi HA, Yagin FH, Alhussain MH, Gülü M, Abubakar BD, Ghazali WSW, Alghannam AF, Badicu G. Effects of combined aerobic exercise and diet on cardiometabolic health in patients with obesity and type 2 diabetes: a systematic review and meta-analysis. BMC Sports Sci Med Rehabil. 2023;15:165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 35] [Reference Citation Analysis (0)] |
| 21. | Okamoto T, Masuhara M, Ikuta K. Combined aerobic and resistance training and vascular function: effect of aerobic exercise before and after resistance training. J Appl Physiol (1985). 2007;103:1655-1661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 61] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 22. | Nery C, Moraes SRA, Novaes KA, Bezerra MA, Silveira PVC, Lemos A. Effectiveness of resistance exercise compared to aerobic exercise without insulin therapy in patients with type 2 diabetes mellitus: a meta-analysis. Braz J Phys Ther. 2017;21:400-415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 23. | Chen S, Zhou K, Shang H, Du M, Wu L, Chen Y. Effects of concurrent aerobic and resistance training on vascular health in type 2 diabetes: a systematic review and meta-analysis. Front Endocrinol (Lausanne). 2023;14:1216962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 24. | Larose J, Sigal RJ, Khandwala F, Kenny GP. Comparison of strength development with resistance training and combined exercise training in type 2 diabetes. Scand J Med Sci Sports. 2012;22:e45-e54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 25. | Kang SJ, Ko KJ, Baek UH. Effects of 12 weeks combined aerobic and resistance exercise on heart rate variability in type 2 diabetes mellitus patients. J Phys Ther Sci. 2016;28:2088-2093. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 26. | Montero D, Vinet A, Roberts CK. Effect of combined aerobic and resistance training versus aerobic training on arterial stiffness. Int J Cardiol. 2015;178:69-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 27. | Bosomworth NJ. Indications for omega-3 fatty acid supplementation in prevention of cardiovascular disease: From fish to pharmaceuticals. Can Fam Physician. 2023;69:459-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 28. | Bellavere F, Cacciatori V, Bacchi E, Gemma ML, Raimondo D, Negri C, Thomaseth K, Muggeo M, Bonora E, Moghetti P. Effects of aerobic or resistance exercise training on cardiovascular autonomic function of subjects with type 2 diabetes: A pilot study. Nutr Metab Cardiovasc Dis. 2018;28:226-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 29. | Balducci S, Zanuso S, Cardelli P, Salvi L, Bazuro A, Pugliese L, Maccora C, Iacobini C, Conti FG, Nicolucci A, Pugliese G; Italian Diabetes Exercise Study (IDES) Investigators. Effect of high- versus low-intensity supervised aerobic and resistance training on modifiable cardiovascular risk factors in type 2 diabetes; the Italian Diabetes and Exercise Study (IDES). PLoS One. 2012;7:e49297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 30. | Soumitra Mondal, Muluken Gebeyehu. Effects of Aerobic and Resistance Exercises on Selected Physiological Biochemical and Anthropometric Variables among Type 2 Diabetic Patients in Dilla, Ethiopia. Indian J Public Health Res Dev. 2021;12:229-237. [DOI] [Full Text] |
| 31. | Vidanage D, Prathapan S, Hettiarachchi P, Wasalathanthri S. Impact of aerobic exercises on taste perception for sucrose in patients with type 2 diabetes mellitus; A randomized controlled trial. BMC Endocr Disord. 2022;22:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 32. | Yang X, Su G, Zhang T, Yang H, Tao H, Du X, Dong J. Comparison of admission glycemic variability and glycosylated hemoglobin in predicting major adverse cardiac events among type 2 diabetes patients with heart failure following acute ST-segment elevation myocardial infarction. J Transl Int Med. 2024;12:188-196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 33. | Chen W, Tang Y, Si Y, Tu B, Xiao F, Bian X, Xu Y, Qin Y. Association of life's essential 8 with prevalence and all-cause mortality of chronic kidney disease among US adults: Results from the National Health and Nutrition Examination Survey (2015-2018). J Transl Int Med. 2024;12:581-591. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |