Published online May 15, 2023. doi: 10.4239/wjd.v14.i5.594
Peer-review started: February 28, 2023
First decision: March 14, 2023
Revised: March 20, 2023
Accepted: April 12, 2023
Article in press: April 12, 2023
Published online: May 15, 2023
Processing time: 76 Days and 7.9 Hours
Intracranial and extracranial artery stenosis is associated with cerebral infarction. Vascular calcification and atherosclerosis are the main causes of stenosis and major risk factors for cardiovascular and cerebrovascular events in patients with type 2 diabetes mellitus (T2DM). Bone turnover biomarkers (BTMs) are associated with vascular calcification, atherosclerosis, glucose, and lipid metabolism.
To investigate the association of circulating BTM levels with severe intracranial and extracranial artery stenosis in patients with T2DM.
For this cross-sectional study including 257 T2DM patients, levels of the BTMs serum osteocalcin (OC), C-terminal cross-linked telopeptide of type I collagen (CTX), and procollagen type I N-peptide were measured by electrical chemiluminescent immunoassay, and artery stenosis was assessed by color Doppler and transcranial Doppler. Patients were grouped according to the existence and location (intracranial vs. extracranial) of artery stenosis. Correlations between BTM levels, previous stroke, stenosis location, and glucose and lipid metabolism were analyzed.
T2DM patients with severe artery stenosis had a higher frequency of previous stroke and levels of all three tested BTMs (all P < 0.05) than patients without. Some differences in OC and CTX levels were observed according to the location of artery stenosis. Significant associations were also observed between BTM levels and some glucose and lipid homeostasis parameters. On multivariate logistic regression analysis, all BTMs were significant predictors of artery stenosis in T2DM patients with and without adjustment for confounding factors (all P < 0.001), and receiver operating characteristic curve analysis demonstrated the ability of BTM levels to predict artery stenosis in T2DM patients.
BTM levels were found to be independent risk factors for severe intracranial and extracranial artery stenosis and were differentially associated with glucose and lipid metabolism in patients with T2DM. Therefore, BTMs may be promising biomarkers and potential therapeutic targets for artery stenosis.
Core Tip: The occurrence of cerebral infarction is associated with severe intracranial and extracranial artery stenosis; thus, this study aims to identify risk factors for severe intracranial and extracranial artery stenosis in patients with type 2 diabetes mellitus (T2DM) to prevent the occurrence of cerebral infarction. Our study found that Bone turnover biomarkers (BTMs) are associated with the risk of arterial stenosis, probably due to the relationship between BTMs and glucose and lipid metabolism disorders. Detection of BTMs in patients with T2DM may help reduce the occurrence of cardiovascular disease.
- Citation: Si SC, Yang W, Luo HY, Ma YX, Zhao H, Liu J. Association of bone turnover biomarkers with severe intracranial and extracranial artery stenosis in type 2 diabetes mellitus patients. World J Diabetes 2023; 14(5): 594-605
- URL: https://www.wjgnet.com/1948-9358/full/v14/i5/594.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i5.594
Intracranial and extracranial artery stenosis is an established predictor of cerebral infarction[1]. Therefore, a clear understanding of risk factors for intracranial and extracranial artery stenosis is important for the prevention of cerebrovascular events. Atherosclerosis is a critical stage of arterial stenosis, and abnormal bone metabolism is associated with the development of atherosclerosis[2]. Vascular calcification is one of the pathologic mechanisms of atherogenesis, and it is a programmed form of osteogenesis that is induced by inflammatory cytokines in blood vessels. The pathological process involves the trans-differentiation of vascular smooth muscle cells into osteoblasts, which are then associated with the activation of vascular osteogenesis, enhanced bone turnover, and abnormal mineral metabolism.
Atherosclerotic calcification is an independent predictor of the mortality and morbidity of cardiovascular and cerebrovascular diseases[3]. The extent and severity of vascular calcification are a reflection of the atherosclerotic plaque burden, and as an outcome of atherosclerosis, vascular calcification causes vascular sclerosis and dysfunction and is accepted as the basic pathological process in many cardiovascular diseases[4]. Vascular calcification is an active and regulated process with similarities to bone formation and is mediated by many of the same processes that promote bone formation[5]. Calcification of atherosclerotic plaques is considered a complex physiological process mediated by both inhibitor and promoter interactions, including between osteoclast- and osteoblast-like arterial cells. Plaque calcification is an important factor in plaque instability, which is conducive to plaque rupture and thrombosis. Accordingly, it is a predictor of future cardiovascular events.
Research shows that patients with type 2 diabetes mellitus (T2DM) who experience a transient ischemic attack or an anterior circulation ischemic stroke are more likely to have a lipid-rich necrotic core and vascular plaques with calcification, and the biological processes of bone formation and calcified atherosclerotic plaque share many common features in patients with T2DM[6]. Additionally, vascular calcification is considered a major risk factor for T2DM and is always associated with cardiovascular complications.
Bone turnover biomarkers (BTMs) are indicators of bone metabolism and may be related to the progression of vascular calcification. Bone metabolism is associated with dyslipidemia and diabetes, which are both important risk factors for atherosclerosis. BTM levels are also related to glucose and lipid metabolism[7]. Thus, BTM levels may be useful biomarkers for assessing the risk of cardiovascular events in patients with T2DM.
Osteocalcin (OC) is a biochemical marker of bone formation that specifically mediates bone mineralization and is expressed mainly by osteoblasts. Osteoblasts are also known to secrete endocrine factors that regulate insulin production and adipose tissue metabolism. OC can extend the metabolic endocrine function of bone with obvious extraosseous effects and is vital for not only bone metabolism but also lipid and glucose metabolism. OC can directly regulate energy metabolism, including glucose and lipids, and is involved in the process of vascular calcification affecting the progression of atherosclerosis[8]. OC is also associated with atherosclerotic disease specifically in patients with T2DM[9]. Another BTM, the C-terminal cross-linked telopeptide of type I collagen (CTX), is a marker of bone resorption. Recent clinical research identified CTX as an independent predictor of increased common carotid artery wall intima - media thickness in the elderly population[10]. The BTM procollagen type I N-peptide (PINP) was shown to predict the risk of myocardial infarction in older male patients[11].
Clinical studies to date have mainly studied the associations between different BTMs and atherosclerosis, whereas few studies have explored the correlations between BTM levels and severe intracranial and extracranial artery stenosis in patients with T2DM. Thus, we aimed to determine whether BTMs are associated with intracranial and extracranial atherosclerosis and investigate the value of BTMs as potential indicators for risk assessment and intervention targets for severe intracranial and extracranial artery stenosis in patients with T2DM.
This cross-sectional study included 257 consecutive patients with T2DM who were hospitalized in our facility between January 2018 and December 2019 and underwent evaluation of intracranial and extracranial arteries. Data were recorded by trained physicians who used a standardized questionnaire to collect medical history information, including age, sex, and history of stroke, coronary artery disease (CAD), hypertension, dyslipidemia, smoking, and alcohol consumption. Diseases were recorded based on codes from the International Classification, Ninth Revision. Physical parameters, including height, weight, and body mass index (BMI), were measured. T2DM was diagnosed according to criteria recommended by the American Diabetes Association in 1997[12]. T2DM diagnosis was based on fasting plasma glucose ≥ 7.0 mmol/L or plasma glucose ≥ 11.1 mmol/L or hemoglobin (HbA1c) ≥ 6.5%. Patients were excluded if they were taking any medication that might influence bone metabolism, such as steroids, thiazides, vitamin D or calcium supplements, antiresorptive or hormone therapy, or were known to have any of the following conditions: acute diabetic complications, malignant tumor, infection, hepatic failure, chronic kidney disease, heart failure, thyroid disease, parathyroid disease, or gastrointestinal disease. The ethics committee of Xuanwu Hospital of Capital Medical University approved this study. All participants provided informed consent.
After overnight fasting for 12 h, peripheral venous blood samples were collected early the next morning for all patients. Fasting plasma glucose (FPG), triglyceride (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), serum creatinine (Scr), blood urea nitrogen (Bun), and uric acid (UA) levels were measured by an automatic analyzer. The glycated HbA1c level was measured by high-pressure liquid chromatography. Insulin (INS), C-peptide (CP), OC, CTX, and PINP levels were determined using an electrical chemiluminescent immunoassay.
Sonographers performed color Doppler ultrasound to obtain images of the common carotid arteries, external carotid arteries, internal carotid arteries, and subclavian arteries. Severe extracranial stenosis was defined as the narrowing of vessel diameter by ≥ 70% in any of these arteries. Sonographers also performed transcranial Doppler ultrasound to obtain images of the anterior cerebral arteries, middle cerebral arteries, posterior cerebral arteries, basilar arteries, and vertebral arteries. Severe intracranial artery stenosis was defined as the narrowing of vessel diameter by ≥ 70% in any of these arteries. According to the results of these examinations, patients were assigned to four groups: No severe intracranial and extracranial artery stenosis (NOCS) group, only severe intracranial artery stenosis (ICAS) group, only severe extracranial artery stenosis (ECAS) group, and combined severe intracranial and extracranial artery stenosis (COAS) group. For analysis, the AS group included all patients with severe intracranial and/or extracranial artery stenosis (ICAS + ECAS + COAS groups).
Normally distributed data are presented as the mean ± SD, and data with a skewed distribution are presented as the median (interquartile range). Categorical variables are shown as frequencies (percentages). We used the Mann–Whitney U test to compare mean values between the NOCS and AS groups. The Kruskal–Wallis test was used to compare mean values among the NOCS, ICAS, ECAS, and COAS groups. When no subgroup had an expected count below five, we used the Pearson χ2 test. If any subgroup had an expected count below five, we used Fisher’s exact test. We performed Spearman correlation analysis to calculate correlation coefficients between BTM levels and glucose and lipid parameters. We used multivariate logistic regression models to estimate the association of artery stenosis with BTM levels. Receiver operating characteristic (ROC) curves and the corresponding area under the curve (AUC) values were used to examine the ability of BTM levels to predict the incidence of artery stenosis. All statistical analyses were performed with the SPSS Statistical Package (version 26.0). The results were considered statistically significant if the corresponding P value was less than 0.05.
Among the 257 T2DM patients included in this study, 136 were female and 121 were male. The mean age of all participants was 66 ± 11 years (range, 36–93 years). The average duration of T2DM among all patients was 12.0 ± 13.0 years. Overall, 87.2% (n = 224) had no stenosis (NOCS group), and 12.8% (n = 33) had some form of stenosis (AS group). The clinical and laboratory parameters for all patients and the groups with and without artery stenosis are presented in Table 1. Compared with the NOCS group, the AS group had a higher percentage of patients with a history of stroke (9.8% vs 27.3%, P = 0.004) and higher levels of HbA1c, OC, CTX, and PINP (all P < 0.05).
| Characteristics | All patients (n = 257) | NOCS group (n = 224) | AS group (n = 33) | P value |
| Age (yr) | 66.0 (11.0) | 66.0 (11.0) | 68.0 (12.0) | 0.236 |
| Male, n (%) | 121 (47.1) | 103 (46.0) | 18 (54.5) | 0.358 |
| Duration of T2DM (yr) | 12.0 (13.0) | 12.0 (14.0) | 13.0 (11.0) | 0.438 |
| Risk factors, n (%) | ||||
| 189 (73.5) | 165 (73.7) | 24 (72.7) | 0.910 | |
| 124 (48.2) | 112 (50.0) | 12 (36.4) | 0.143 | |
| 74 (28.8) | 63 (28.3) | 11 (33.3) | 0.548 | |
| 31 (12.1) | 22 (9.8) | 9 (27.3) | 0.004 | |
| 74 (28.8) | 67 (29.9) | 7 (21.2) | 0.303 | |
| 64 (24.9) | 59 (26.3) | 5 (15.2) | 0.165 | |
| BMI (kg/m2) | 25.76 (3.57) | 25.78 (3.59) | 25.76 (3.45) | 0.597 |
| Glucose (mmol/L) | 7.48 (3.58) | 7.49 (3.53) | 6.72 (4.25) | 0.563 |
| HbA1c (%) | 7.60 (2.50) | 7.60 (2.60) | 8.40 (2.90) | 0.037 |
| INS (µIU/mL) | 12.21 (12.71) | 12.00 (10.71) | 16.82 (18.93) | 0.159 |
| CP (ng/mL) | 2.33 (1.52) | 2.33 (1.53) | 2.40 (1.89) | 0.754 |
| TC (mmol/L) | 4.26 (1.50) | 4.27 (1.41) | 4.23 (2.25) | 0.721 |
| TG (mmol/L) | 1.48 (1.38) | 1.50 (1.39) | 1.41 (1.36) | 0.433 |
| LDL-C (mmol/L) | 2.38 (1.27) | 2.39 (1.20) | 2.27 (1.79) | 0.781 |
| HDL-C (mmol/L) | 1.12 (0.47) | 1.13 (0.47) | 1.04 (0.38) | 0.320 |
| Scr (µmol/L) | 62.0 (24.0) | 62.0 (22.8) | 60.0 (43.0) | 0.144 |
| Bun (mmol/L) | 5.98 (2.30) | 5.92 (2.12) | 6.82 (3.60) | 0.137 |
| UA (µmol/L) | 341.0 (126.5) | 342.0 (127.3) | 339.0 (1520) | 0.823 |
| OC (ng/mL) | 10.60 (5.87) | 10.27 (5.87) | 12.63 (7.07) | 0.001 |
| CTX (pg/mL) | 0.31 (0.21) | 0.29 (0.19) | 0.39 (0.30) | 0.007 |
| PINP (pg/mL) | 34.40 (20.96) | 33.39 (20.37) | 43.92 (25.50) | 0.010 |
The clinical and laboratory parameters of patients categorized further based on the location of artery stenosis are presented in Table 2. Among the 33 patients with T2DM with artery stenosis, 7.4% (n = 19) had only severe intracranial artery stenosis (ICAS group), 3.1% (n = 8) had only severe extracranial artery stenosis (ECAS group), and 2.3% (n = 6) had both severe intracranial and extracranial artery stenosis (COAS group). Compared with that in the NOCS group, the frequency of previous strokes was higher in all three groups (21.1% in ICAS group vs 25.0% in ECAS group vs 50.0% in COAS group vs 9.8% in NOCS, P = 0.009). Compared with the NOCS group, the ICAS group had a lower frequency of hyperlipidemia, and the ECAS group had a higher frequency of hyperlipidemia (21.1% in the ICAS group vs 75.0% in the ECAS group vs 50% in the NOCS group, P = 0.029). The OC levels differed significantly among the four groups (P = 0.012). Further statistical analyses determined that the OC levels in the COAS and ICAS groups were higher than those in the NOCS group (P = 0.034 and 0.039, respectively). While the ECAS group showed a trend toward higher OC levels compared with the NOCS group, the difference was not significant (P = 0.077). Similarly, the CTX levels differed among the four groups (P = 0.027), and further statistical analysis showed that the CTX level in the COAS group was higher than that in the NOCS group (P = 0.017). The ICAS group also showed a trend toward higher CTX levels than the NOCS group, but this difference did not reach statistical significance (P = 0.062). The PINP concentrations were comparable among the four groups, although a weak trend toward a reduced PINP was seen in the NOCS group (P = 0.059).
| Characteristics | NOCS group (n = 224) | ICAS group (n = 19) | ECAS group (n = 8) | COAS group (n = 6) | P value |
| Age (yr) | 66.0 (11.0) | 65.0 (16.0) | 67.5 (13.0) | 71.0 (10.0) | 0.373 |
| Male, n (%) | 103 (46.0) | 10 (52.6) | 5 (62.5) | 3 (50) | 0.780 |
| Duration of T2DM (yr) | 12.0 (14.0) | 15.0 (11.0) | 18.0 (20.8) | 11.5 (7.0) | 0.635 |
| Risk factors, n (%) | |||||
| Hypertension | 165 (73.7) | 13 (68.4) | 5 (62.5) | 6 (100) | 0.527 |
| Hyperlipidemia | 112 (50.0) | 4 (21.1) | 7 (75.0) | 2 (33.3) | 0.029 |
| CAD | 63 (28.3) | 7 (36.8) | 2 (25.0) | 2 (33.3) | 0.826 |
| Previous stroke | 22 (9.8) | 4 (21.1) | 2 (25.0) | 3 (50.0) | 0.009 |
| Current smoker | 67 (29.9) | 4 (21.1) | 1 (12.5) | 2 (33.3) | 0.682 |
| Current drinker | 59 (26.3) | 4 (21.1) | 1 (12.5) | 0 (0) | 0.554 |
| BMI (kg/m2) | 25.78 (3.59) | 26.06 (3.90) | 25.32 (2.33) | 23.96 (12.48) | 0.821 |
| FPG (mmol/L) | 7.49 (3.53) | 7.83 (4.13) | 7.13 (6.89) | 5.76 (2.48) | 0.446 |
| HbA1c (%) | 7.60 (2.60) | 7.90 (3.10) | 9.15 (1.70) | 7.90 (1.80) | 0.067 |
| INS (µIU/mL) | 12.00 (10.71) | 16.99 (19.11) | 12.78 (19.72) | 15.90 (25.32) | 0.334 |
| CP (ng/mL) | 2.33 (1.53) | 2.52 (1.45) | 1.56 (2.95) | 2.03 (2.31) | 0.599 |
| TC (mmol/L) | 4.27 (1.41) | 3.85 (1.98) | 4.92 (2.35) | 4.35 (2.25) | 0.535 |
| TG (mmol/L) | 1.50 (1.39) | 1.59 (1.31) | 1.29 (1.31) | 1.16 (1.85) | 0.585 |
| LDL-C (mmol/L) | 2.39 (1.20) | 2.27 (1.76) | 2.62 (1.88) | 2.01 (2.27) | 0.943 |
| HDL-C (mmol/L) | 1.13 (0.47) | 0.98 (0.29) | 1.25 (1.21) | 1.04 (0.43) | 0.170 |
| Scr (µmol/L) | 62.0 (22.8) | 59.0 (48.0) | 78.5 (44.8) | 60.0 (40.3) | 0.499 |
| Bun (mmol/L) | 5.92 (2.12) | 6.36 (2.74) | 7.92 (6.76) | 6.57 (5.00) | 0.419 |
| UA (µmol/L) | 342.0 (127.3) | 358.0 (174.0) | 322.5 (153.3) | 303.0 (136.3) | 0.746 |
| OC (ng/mL) | 10.27 (5.87) | 12.76 (8.14) | 11.89 (4.02) | 15.15 (11.43) | 0.012 |
| CTX (pg/mL) | 0.29 (0.19) | 0.41 (0.29) | 0.35 (0.23) | 0.65 (0.51) | 0.027 |
| PINP (pg/mL) | 33.39 (20.37) | 41.25 (24.71) | 47.22 (39.85) | 51.28 (37.96) | 0.059 |
We applied four multivariate logistic regression models to identify independent predictors of artery stenosis (Table 3). With the unadjusted Model 1, the OC level was found to be significantly associated with an increased risk of artery stenosis [odds ratio (OR) = 1.123; 95%CI: 1.049–1.203; P = 0.001]. A similar association was observed with the model further adjusted for age and sex (Model 2, OR = 1.117; 95%CI: 1.041–1.199; P = 0.002). With further adjustment of the model for hypertension, hyperlipidemia, CAD, duration of T2DM, smoking status, and drinking status, the OC level remained a predictor of high risk for artery stenosis (Model 3, OR = 1.117; 95%CI: 1.039–1.201; P = 0.003). A significant association between the OC level and artery stenosis persisted after further adjustment of the model for BMI, Scr, Bun, UA, and HbA1c (Model 4, OR = 1.109; 95%CI: 1.029–1.196; P = 0.007). CTX and PINP levels were also significantly associated with artery stenosis in all models with adjustment for different potential confounding factors (all P < 0.05).
| Variable | Model | OR (95%CI) | P value |
| OC | Model 1 | 1.123 (1.049-1.203) | 0.001 |
| Model 2 | 1.117 (1.041-1.199) | 0.002 | |
| Model 3 | 1.117 (1.039-1.201) | 0.003 | |
| Model 4 | 1.109 (1.029-1.196) | 0.007 | |
| CTX | Model 1 | 9.750 (1.759-54.059) | 0.009 |
| Model 2 | 8.674 (1.529-49.211) | 0.015 | |
| Model 3 | 10.833 (1.725-68.037) | 0.011 | |
| Model 4 | 8.526 (1.189-61.119) | 0.033 | |
| PINP | Model 1 | 1.024 (1.006-1.042) | 0.010 |
| Model 2 | 1.022 (1.003-1.041) | 0.025 | |
| Model 3 | 1.022 (1.003-1.042) | 0.021 | |
| Model 4 | 1.020 (1.001-1.040) | 0.044 |
The results of the Spearman correlation analysis of associations between BTM levels and parameters of glucose and lipid metabolism are presented in Table 4. This analysis showed that the OC level was associated with the levels of TC (r = 0.185; P = 0.003) and LDL-C (r = 0.213; P = 0.001). Additionally, the CTX level was associated with the levels of FPG (r = –0.134; P = 0.031), TC (r = 0.147; P = 0.018), and LDL-C (r = 0.197; P = 0.003). The PINP level was associated with the levels of FPG (r = –0.141; P = 0.024) and LDL-C (r = 0.129; P = 0.039).
| Variable | OC | CTX | PINP | |||
| r | P value | r | P value | r | P value | |
| FPG | -0.102 | 0.103 | -0.134 | 0.031 | -0.141 | 0.024 |
| HbA1c | -0.012 | 0.846 | -0.022 | 0.724 | -0.012 | 0.845 |
| INS | -0.050 | 0.425 | -0.086 | 0.172 | -0.031 | 0.628 |
| CP | 0.021 | 0.741 | 0.045 | 0.473 | 0.067 | 0.286 |
| TC | 0.185 | 0.003 | 0.147 | 0.018 | 0.112 | 0.074 |
| TG | 0.020 | 0.753 | -0.058 | 0.351 | -0.041 | 0.514 |
| LDL-C | 0.213 | 0.001 | 0.187 | 0.003 | 0.129 | 0.039 |
| HDL-C | 0.020 | 0.745 | 0.062 | 0.319 | 0.015 | 0.811 |
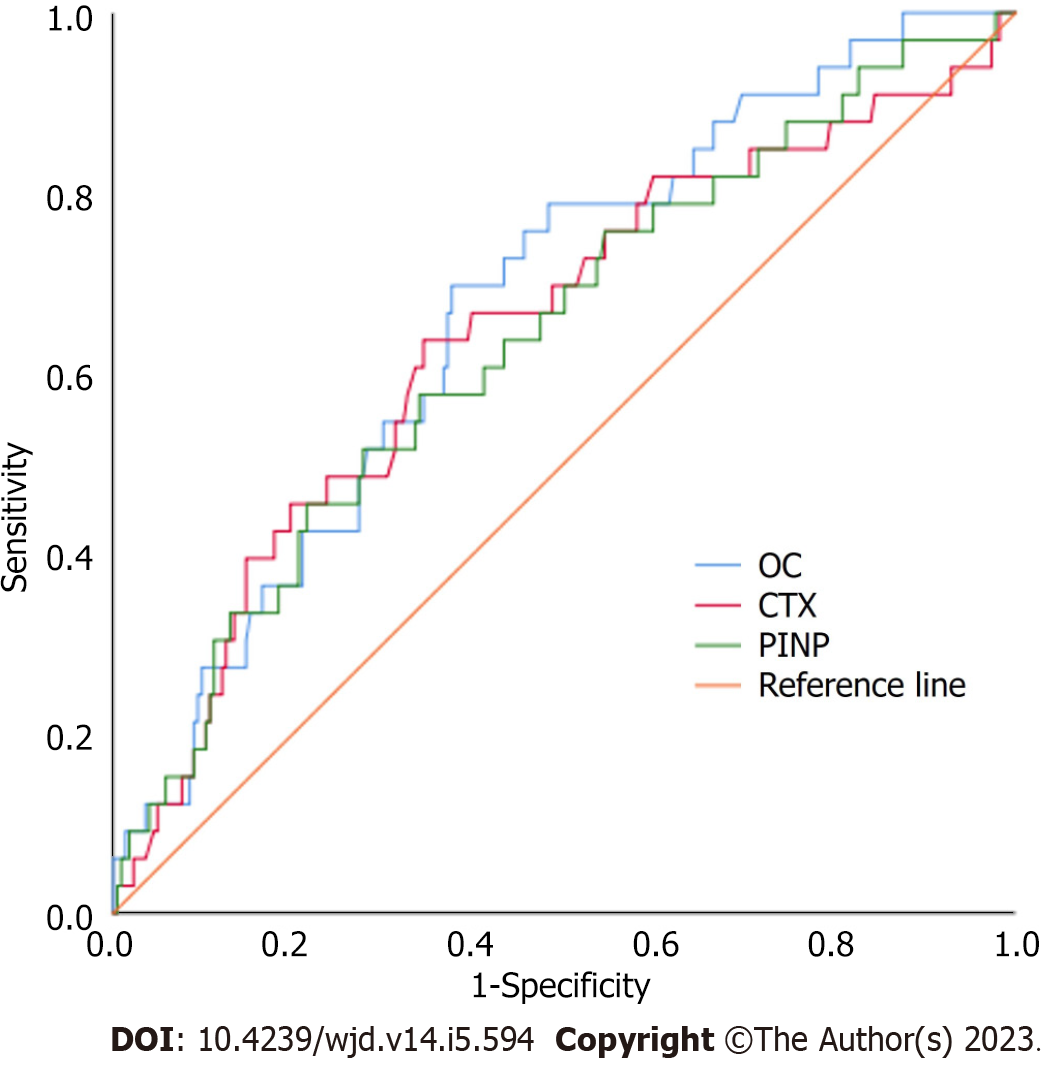
The ROC curves for the abilities of BTM levels to predict the incidence of artery stenosis in patients with T2DM are shown in Figure 1. The AUC (95%CI) values were 0.673 (0.580, 0.766) for OC, 0.644 (0.538, 0.751) for CTX, and 0.639 (0.536, 0.742) for PINP. All three BTMs were found to be significant predictors of artery stenosis risk among the patients with T2DM included in this study (P < 0.001).
In this study, we explored the associations among BTM levels, previous stroke, and the burden and location of intracranial and extracranial artery stenosis in patients with T2DM. We also investigated the correlations among BTM levels and parameters of glucose and lipid metabolism in patients with T2DM. From the cross-sectional data analyzed in this study, we observed a considerable incidence of artery stenosis among patients with T2DM. Moreover, T2DM patients with artery stenosis were more likely to have a history of stroke than those without artery stenosis, regardless of the location of artery stenosis, and the highest incidence of previous stroke was observed among patients with both severe intracranial and extracranial artery stenosis (COAS group). We also found that BTM levels showed significant correlations with the risk of severe intracranial and extracranial artery stenosis. In this study, circulating OC, CTX, and PINP concentrations were significantly higher in T2DM patients with artery stenosis (AS group) than in those without artery stenosis (NOCS group, Table 1). Some differences were observed among the trends in BTMs according to the location of artery stenosis. OC levels were higher in all subgroups with artery stenosis, whereas CTX levels were only higher in the COAS and ICAS groups than in the NOCS group. Our analyses indicated that all three BTMs were independent risk factors for severe intracranial and extracranial artery stenosis in patients with T2DM, in support of our hypothesis, and these associations were independent of possible confounders. Based on their correlation with indicators of glucose and lipid metabolism, the elevated BTM levels identified a particularly unfavorable metabolic profile, mostly related to dyslipidemia. Higher BTM levels were associated with elevated LDL-C, and the OC level was positively correlated with the TC level. However, the CTX level was negatively correlated with FPG and positively correlated with TC. Furthermore, the PINP level was negatively correlated with FPG. These findings suggest that BTM levels can reflect altered glucose and lipid metabolism as well as atherosclerosis. The role of BTMs in bone metabolism along with their influence on glucose and lipid homeostasis and stroke risk confirms the tight interaction of the cardiovascular–bone metabolism axis. Overall, the present study suggests that BTMs may represent novel biomarkers of accelerated atherosclerosis in patients with T2DM, offering promising tools for cardiovascular risk stratification among patients with T2DM.
T2DM is a common metabolic disease, and its incidence continues to increase worldwide. The incidence of atherosclerosis in patients with T2DM is high, and large vessel disease due to atherosclerosis seriously threatens the life and health of patients with T2DM. Thus, effective strategies for the prevention and treatment of large vessel disease in the context of T2DM are needed to improve public health globally. Even with reductions in traditional risk factors for cardiovascular disease (CVD), achieved via better blood lipid control and smoking cessation, the incidence of CVD remains high. Moreover, blood glucose control does not completely reduce the mortality attributed to atherosclerotic and vascular calcification-related CVD among patients with T2DM. Therefore, other pathological mechanisms of atherosclerosis need to be characterized to support the discovery of therapeutic targets that can be used to identify, diagnose, evaluate, and treat patients with T2DM at high risk of atherosclerosis as early as possible to improve the prognosis of patients with T2DM.
With research advances related to bone health and vascular lesions, the significance and key role of the bone–vascular axis in vascular lesions are increasingly recognized. The instability of atherosclerotic plaques is associated with a higher level of calcification, and severe vascular calcification can be regarded as a nonspecific marker of atherosclerosis. The calcified atherosclerosis burden is considered the main predictor of risk for CVD events and death[13]. T2DM is associated with greater intraplaque calcification volume and a greater proportion of calcification within a plaque. Vascular calcification was also shown to be a powerful risk factor for cardiovascular death in patients with T2DM and results in the development of severe atherosclerosis[14]. Likely, several important mediators of bone mineral homeostasis are also involved in the development of arterial calcification.
Several mineralization markers have been identified in atheromatic plaques[15]. Currently, the effects of BTM levels on atherosclerosis or plaque calcification remain unclear, with conflicting data reported in the literature. Previous studies have mainly examined the relationship between BTM levels and the prevalence of atherosclerotic diseases and found that BTM levels may be predictors of cardiovascular risk in T2DM patients[16]. In patients with T2DM, OC was identified as an independent risk factor for carotid atherosclerosis[17] and shown to play a role in CVD[18]. OC was also found to be deposited at sites of vascular calcification[19]. Another study found that progressive calcification of atherosclerotic plaques was accompanied by a significant increase in OC[20]. Type I collagen is a major collagen component of the intima, media, and adventitia of blood vessel walls and is significantly increased in atherosclerosis. PINP is a precursor of type I collagen, and CTX is the carboxyl-terminal degradation product of type I collagen. Thus, the expression levels of both BTMs are increased in atherosclerosis accordingly. Abnormal matrix collagen turnover in vessel walls eventually causes arterial stiffness, which is associated with CVD risk, and increased arterial stiffness is associated with both collagen degradation (CTX) and synthesis[21]. Increased osteoclast activity has been observed in T2DM[22], and CTX expression was found in areas of intimal hyperplasia and late-calcified plaques[23]. Gafane et al[24] found a positive association between large artery stiffness and the CTX level. Another study found that OC and CTX levels are related to an increased risk of cardiac and carotid calcified plaque development[25]. However, research on the correlation between BTM levels and severe intracranial and extracranial stenosis in patients with T2DM is lacking. The present study found that BTM levels were significantly associated with the risk of artery stenosis in unadjusted and fully adjusted models accounting for potential confounders. These results suggest for the first time that BTMs are independent risk factors for severe intracranial and extracranial stenosis in patients with T2DM. Notably, these associations were independent of other known cardiovascular risk factors in T2DM. Additionally, our results indicate that BTMs may participate in and contribute to the pathogenesis and progression of atherosclerosis.
The mechanisms by which BTMs influence atherosclerosis remain unclear. One possibility is that OC plays an important role in glucose metabolism[26,27]. Consistently, in this study, OC was significantly associated with glucose regulation. We also observed that PINP and CTX levels were negatively correlated with FPG. A previous study found that PINP is negatively correlated with FPG and HbA1c[28]. We also found that all tested BTMs were associated with indicators of dyslipidemia. Barchetta et al[29] also reported an association between OC and TC. We also observed differences in glucose and lipid metabolism about artery stenosis in patients with T2DM. Patients with artery stenosis had higher levels of HbA1c than those without artery stenosis. However, indicators of dyslipidemia differed between patients with intracranial vs extracranial artery stenosis in our study. Jin et al[30] found that hyperlipidemia was an independent predictor of extracranial artery stenosis but not intracranial artery stenosis, and in the present study, a higher frequency of hyperlipidemia was observed in the ECAS group.
Metabolic variables may represent the underlying mechanism for the association between BTMs and atherosclerosis, as increased BTM levels correlated with several metabolic risk factors and increased atherosclerosis. BTMs may influence glucose and lipid homeostasis, while cardiovascular risk factors can regulate the expression of mineralization markers and promote the calcification process.
The strengths of the present study include overall profiling of BTM levels in patients with T2DM, which has not been reported previously, and extensive correlation analyses between BTM levels and indicators of glucose metabolism, lipid metabolism, and vascular disease, which ultimately allowed us to identify independent associations between BTM levels and atherosclerosis in patients with T2DM. However, this study also has several limitations. Due to its cross-sectional design, we could not establish causal relationships among the correlating factors. Further prospective studies with relevant clinical endpoints are needed to clarify whether BTMs play a causal role in the development of atherosclerosis and to assess the effect of BTM-lowering therapies on the development of atherosclerosis. We plan to carry out large-scale longitudinal studies in the future. Finally, in vivo animal studies are required to elucidate the role of BTMs in the pathogenic mechanisms of atherosclerosis and plaque calcification.
Based on our findings that BTM levels are strongly correlated with artery stenosis risk and dis-turbance of glucose and lipid metabolism, BTMs should be investigated as indicators for predicting the risk of CVD and as potential therapeutic targets for artery stenosis in patients with T2DM. Multimodal imaging technologies can identify intracranial and extracranial atherosclerosis, which indicates an increased risk of ischemic events or artery stenosis. The combination of such imaging analyses and measurement of serum BTM levels offers a promising strategy for evaluating the diagnosis, pathogenesis, and progression of intracranial and extracranial stenosis. Therefore, we should test BTMs in patients with T2DM, complete vascular examinations in patients with elevated BTMs, and conduct active monitoring, follow-up, and treatment in this part of the population.
Elevated BTM levels correlated with an increased risk of artery stenosis in patients with T2DM as well as with several indicators of metabolic syndrome. Accordingly, BTM levels may serve as circulating endocrine markers that reflect the regulation of glucose and lipid metabolism, thereby reflecting the risk of vascular disease in patients with T2DM. BTMs may also represent potential therapeutic targets for atherosclerosis, and additional research is warranted to explore the underlying mechanism linking BTMs and atherosclerosis. Patients with high BTM levels should be considered a high-risk group in efforts to prevent cardiovascular events.
Intracranial and extracranial artery stenosis is associated with cerebral infarction. Vascular calcification and atherosclerosis are the main causes of stenosis and major risk factors for cardiovascular and cerebrovascular events in patients with type 2 diabetes mellitus (T2DM).
Our study found that bone turnover biomarkers (BTMs) are associated with the risk of arterial stenosis, probably due to the relationship between BTMs and glucose and lipid metabolism disorders. Detection of BTMs in patients with T2DM may help reduce the occurrence of cardiovascular disease.
This study aimed to investigate the association of circulating BTM levels with severe intracranial and extracranial artery stenosis in patients with T2DM.
After overnight fasting for 12 h, peripheral venous blood samples were collected early the next morning for all patients. Fasting plasma glucose, triglyceride, total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, serum creatinine, blood urea nitrogen, and uric acid levels were measured by an automatic analyzer. The glycated hemoglobin (HbA1c) level was measured by high-pressure liquid chromatography. Insulin, C-peptide, osteocalcin (OC), C-terminal cross-linked telopeptide of type I collagen (CTX), and procollagen type I N-peptide (PINP) levels were determined using an electrical chemiluminescent immunoassay.
Among the 257 T2DM patients included in this study, 136 were female and 121 were male. The mean age of all participants was 66 ± 11 years. The average duration of T2DM among all patients was 12.0 ± 13.0 years. Overall, 87.2% had no stenosis, and 12.8% had some form of stenosis. Compared with the no severe intracranial and extracranial artery stenosis group, the artery stenosis group had a higher percentage of patients with a history of stroke and higher levels of HbA1c, OC, CTX, and PINP.
Elevated BTM levels correlated with an increased risk of artery stenosis in patients with T2DM as well as with several indicators of metabolic syndrome. Accordingly, BTM levels may serve as circulating endocrine markers that reflect the regulation of glucose and lipid metabolism, thereby reflecting the risk of vascular disease in patients with T2DM.
BTMs may also represent potential therapeutic targets for atherosclerosis, and additional research is warranted to explore the underlying mechanism linking BTMs and atherosclerosis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chen JT, Taiwan; Corcillo A, United Kingdom; Yoon S, South Korea S-Editor: Wang JL L-Editor: A P-Editor: Chen YX
| 1. | Jiang C, Zhang J, Zhu J, Wang X, Wen Z, Zhao X, Yuan C; CARE-II Investigators. Association between coexisting intracranial artery and extracranial carotid artery atherosclerotic diseases and ipsilateral cerebral infarction: a Chinese Atherosclerosis Risk Evaluation (CARE-II) study. Stroke Vasc Neurol. 2021;6:595-602. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Thompson B, Towler DA. Arterial calcification and bone physiology: role of the bone-vascular axis. Nat Rev Endocrinol. 2012;8:529-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 247] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 3. | Bos D, Leening MJ, Kavousi M, Hofman A, Franco OH, van der Lugt A, Vernooij MW, Ikram MA. Comparison of Atherosclerotic Calcification in Major Vessel Beds on the Risk of All-Cause and Cause-Specific Mortality: The Rotterdam Study. Circ Cardiovasc Imaging. 2015;8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 92] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 4. | Demer LL, Tintut Y. Vascular calcification: pathobiology of a multifaceted disease. Circulation. 2008;117:2938-2948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 676] [Cited by in RCA: 800] [Article Influence: 47.1] [Reference Citation Analysis (0)] |
| 5. | Towler DA. Commonalities Between Vasculature and Bone: An Osseocentric View of Arteriosclerosis. Circulation. 2017;135:320-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | Wagenknecht LE, Divers J, Register TC, Russell GB, Bowden DW, Xu J, Langefeld CD, Lenchik L, Hruska KA, Carr JJ, Freedman BI. Bone Mineral Density and Progression of Subclinical Atherosclerosis in African-Americans With Type 2 Diabetes. J Clin Endocrinol Metab. 2016;101:4135-4141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Bellissimo MP, Roberts JL, Jones DP, Liu KH, Taibl KR, Uppal K, Weitzmann MN, Pacifici R, Drissi H, Ziegler TR, Alvarez JA. Metabolomic Associations with Serum Bone Turnover Markers. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 8. | Yang SW, Hennessy RR, Khosla S, Lennon R, Loeffler D, Sun T, Liu Z, Park KH, Wang FL, Lerman LO, Lerman A. Circulating osteogenic endothelial progenitor cell counts: new biomarker for the severity of coronary artery disease. Int J Cardiol. 2017;227:833-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Reyes-Garcia R, Rozas-Moreno P, Jimenez-Moleon JJ, Villoslada MJ, Garcia-Salcedo JA, Santana-Morales S, Muñoz-Torres M. Relationship between serum levels of osteocalcin and atherosclerotic disease in type 2 diabetes. Diabetes Metab. 2012;38:76-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Leli C, Pasqualini L, Vaudo G, Gaggioli S, Scarponi AM, Mannarino E. Carotid intima-media thickness and bone turnover: the role of C-terminal telopeptide of type I collagen. Intern Emerg Med. 2010;5:127-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Yeap BB, Alfonso H, Chubb SA, Byrnes E, Beilby JP, Ebeling PR, Allan CA, Schultz C, Hankey GJ, Golledge J, Flicker L, Norman PE. Proportion of Undercarboxylated Osteocalcin and Serum P1NP Predict Incidence of Myocardial Infarction in Older Men. J Clin Endocrinol Metab. 2015;100:3934-3942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 12. | Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 115] [Reference Citation Analysis (0)] |
| 13. | Allison MA, Hsi S, Wassel CL, Morgan C, Ix JH, Wright CM, Criqui MH. Calcified atherosclerosis in different vascular beds and the risk of mortality. Arterioscler Thromb Vasc Biol. 2012;32:140-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 166] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 14. | Lei MH, Wu YL, Chung SL, Chen CC, Chen WC, Hsu YC. Coronary Artery Calcium Score Predicts Long-Term Cardiovascular Outcomes in Asymptomatic Patients with Type 2 Diabetes. J Atheroscler Thromb. 2021;28:1052-1062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 15. | Scimeca M, Anemona L, Granaglia A, Bonfiglio R, Urbano N, Toschi N, Santeusanio G, Schiaroli S, Mauriello S, Tancredi V, Schillaci O, Bonanno E, Mauriello A. Plaque calcification is driven by different mechanisms of mineralization associated with specific cardiovascular risk factors. Nutr Metab Cardiovasc Dis. 2019;29:1330-1336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 16. | Zwakenberg SR, van der Schouw YT, Schalkwijk CG, Spijkerman AMW, Beulens JWJ. Bone markers and cardiovascular risk in type 2 diabetes patients. Cardiovasc Diabetol. 2018;17:45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Sheng L, Cao W, Cha B, Chen Z, Wang F, Liu J. Serum osteocalcin level and its association with carotid atherosclerosis in patients with type 2 diabetes. Cardiovasc Diabetol. 2013;12:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Levinger I, Brennan-Speranza TC, Zulli A, Parker L, Lin X, Lewis JR, Yeap BB. Multifaceted interaction of bone, muscle, lifestyle interventions and metabolic and cardiovascular disease: role of osteocalcin. Osteoporos Int. 2017;28:2265-2273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 19. | Tyson KL, Reynolds JL, McNair R, Zhang Q, Weissberg PL, Shanahan CM. Osteo/chondrocytic transcription factors and their target genes exhibit distinct patterns of expression in human arterial calcification. Arterioscler Thromb Vasc Biol. 2003;23:489-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 407] [Cited by in RCA: 398] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 20. | Polonskaya YV, Kashtanova EV, Murashov IS, Volkov AM, Kurguzov AV, Chernyavsky AM, Ragino YI. Associations of Osteocalcin, Osteoprotegerin, and Calcitonin with Inflammation Biomarkers in Atherosclerotic Plaques of Coronary Arteries. Bull Exp Biol Med. 2017;162:726-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Cotie LM, Currie KD, McGill GM, Cameron AJ, McFadden AS, Phillips SM, MacDonald MJ. Associations between measures of vascular structure and function and systemic circulating blood markers in humans. Physiol Rep. 2016;4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Gilbert MP, Pratley RE. The impact of diabetes and diabetes medications on bone health. Endocr Rev. 2015;36:194-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 137] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 23. | Barascuk N, Skjøt-Arkil H, Register TC, Larsen L, Byrjalsen I, Christiansen C, Karsdal MA. Human macrophage foam cells degrade atherosclerotic plaques through cathepsin K mediated processes. BMC Cardiovasc Disord. 2010;10:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 24. | Gafane LF, Schutte R, Kruger IM, Schutte AE. Large artery stiffness and carotid intima-media thickness in relation to markers of calcium and bone mineral metabolism in African women older than 46 years. J Hum Hypertens. 2015;29:152-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Liu D, Chen L, Dong S, Peng Z, Yang H, Chen Y, Li L, Zhou H, Zhou R. Bone mass density and bone metabolism marker are associated with progression of carotid and cardiac calcified plaque in Chinese elderly population. Osteoporos Int. 2019;30:1807-1815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Cipriani C, Colangelo L, Santori R, Renella M, Mastrantonio M, Minisola S, Pepe J. The Interplay Between Bone and Glucose Metabolism. Front Endocrinol (Lausanne). 2020;11:122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 123] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 27. | Ma H, Lin H, Hu Y, Li X, He W, Jin X, Gao J, Zhao N, Gao X. Serum levels of osteocalcin in relation to glucose metabolism and carotid atherosclerosis in Chinese middle-aged and elderly male adults: the Shanghai Changfeng Study. Eur J Intern Med. 2014;25:259-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | Li W, Liu X, Liu L, Zhang L, Li M, Liu R, Li T, Chen E, Liu S. Relationships of Serum Bone Turnover Markers With Metabolic Syndrome Components and Carotid Atherosclerosis in Patients With Type 2 Diabetes Mellitus. Front Cardiovasc Med. 2022;9:824561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 29. | Barchetta I, Ceccarelli V, Cimini FA, Bertoccini L, Fraioli A, Alessandri C, Lenzi A, Baroni MG, Cavallo MG. Impaired bone matrix glycoprotein pattern is associated with increased cardio-metabolic risk profile in patients with type 2 diabetes mellitus. J Endocrinol Invest. 2019;42:513-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Jin H, Peng Q, Nan D, Lv P, Liu R, Sun W, Teng Y, Liu Y, Fan C, Xing H, Xu K, Huang Y. Prevalence and risk factors of intracranial and extracranial artery stenosis in asymptomatic rural residents of 13 villages in China. BMC Neurol. 2017;17:136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |