Published online May 15, 2023. doi: 10.4239/wjd.v14.i5.494
Peer-review started: December 28, 2022
First decision: February 20, 2023
Revised: March 6, 2023
Accepted: March 30, 2023
Article in press: March 30, 2023
Published online: May 15, 2023
Processing time: 138 Days and 8.3 Hours
Obesity and overweight are widespread issues in adults, children, and adolescents globally, and have caused a noticeable rise in obesity-related complications such as type 2 diabetes mellitus (T2DM). Chronic low-grade inflammation is an important promotor of the pathogenesis of obesity-related T2DM. This proinflammatory activation occurs in multiple organs and tissues. Immune cell-mediated systemic attack is considered to contribute strongly to impaired insulin secretion, insulin resistance, and other metabolic disorders. This review focused on highlighting recent advances and underlying mechanisms of immune cell infiltration and inflammatory responses in the gut, islet, and insulin-targeting organs (adipose tissue, liver, skeletal muscle) in obesity-related T2DM. There is current evidence that both the innate and adaptive immune systems contribute to the development of obesity and T2DM.
Core Tip: Obesity is closely associated with the occurrence and development of insulin resistance and type 2 diabetes mellitus (T2DM). Previous studies have demonstrated the important role of immune cell infiltration and inflammatory response in obesity-related T2DM. This review presents immune responses in the gut with respect to metabolic challenges. We also highlight the effects of immune attacks and proinflammatory shifts on insulin-secreting and targeting organs.
- Citation: Wang HW, Tang J, Sun L, Li Z, Deng M, Dai Z. Mechanism of immune attack in the progression of obesity-related type 2 diabetes. World J Diabetes 2023; 14(5): 494-511
- URL: https://www.wjgnet.com/1948-9358/full/v14/i5/494.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i5.494
Globally, obesity and associated complications are widespread. Over the past 40 years, the impact of this non-contagious disease has spread from high-income countries to low- and middle-income countries, with its prevalence nearly tripling globally. Statistics from the World Health Organization in 2016 showed that 13% of the global adult population is obese, and more than 1.9 billion adults are overweight. The prevalence and degree of overweight and obese children and adolescents have also noticeably risen, generating concern for future years. Up to 2025, it is estimated that about 20% of the global population will be obese[1,2]. Widespread obesity among adults and adolescents will lead to a striking increase in obesity-driven health complications such as type 2 diabetes mellitus (T2DM), as most T2DM patients tend to be overweight or obese[3,4].
The close correlation of obesity with T2DM has generated broad research interests of researchers. Although the pathophysiological mechanisms linking obesity to T2DM remain unclear, many studies have suggested that immune attack induced by overnutrition in multiple organs strongly contributes to insulin resistance (IR), lipotoxicity, and glucotoxicity. In this review, we examine recent advances and underlying mechanisms of local and systemic immune attack and chronic low-grade inflammation in T2DM induced by obesity.
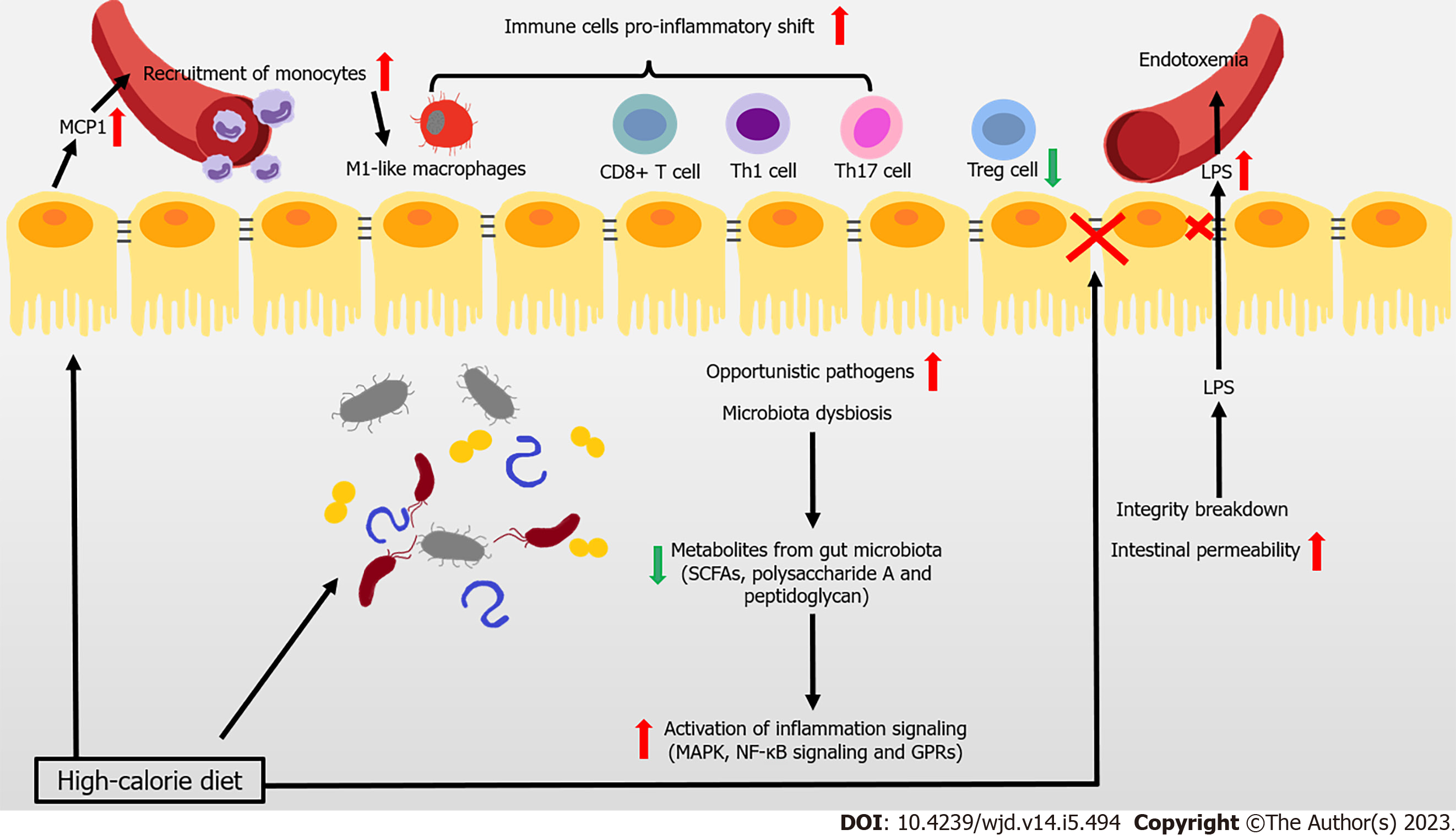
Most patients with T2DM are obese or overweight. These two states represent the disrupted condition of energy homeostasis in the body, due to chronic excessive calorie intake over expenditure. The gut is the first important “station” through which high-calorie food enters the body. There is recent widespread evidence that disturbance to the gut (particularly the dysbiosis of gut microbiota, imbalance of immune cells, and impaired gut barrier function) hinders the immune response and contributes to the development of obesity related IR and T2DM (Figure 1).
The composition of gut microbiota is complex, with high variability across individuals. This composition can be altered by changes to diet, and is closely associated with the development of disease. Reduced gene richness of gut microbiota is a common phenomenon caused by modern dietary structure, and might be associated with dyslipidemia, severe IR, and low-grade local or systemic inflammation[5,6]. Existing studies have shown that after introducing microbiota from obese donors to germ-free mice, lipid accumulation and IR arose. This result demonstrated the close association between the gut microbiota and metabolic disorders in obesity-related T2DM[7,8]. Changes to metabolites caused by an altered gut microbiome help mediate the link between the host and gut microbiome. Short-chain fatty acids (SCFAs) are the products of undigested dietary fibers degraded by gut bacteria, and include acetate, propionate, and butyrate. These SCFAs have anti-inflammatory properties, in particular, butyrate[9,10]. Metagenome-wide studies have shown that the dysbiosis of gut bacteria occurs in patients with T2DM, in which the abundance of butyrate-producing bacteria declines, while that of opportunistic pathogens increases[11,12]. For instance, the administration of commercial Bifidobacterium strains reduces body weight gain and downregulates inflammation, by reshaping intestinal gene signatures in mice[13]. Many studies have shown that the anti-inflammatory effects of butyrate are mainly achieved by inhibiting mitogen-activated protein kinase pathways and nuclear factor kappa B (NF-κB) in intestinal epithelial cells, which reduce the secretion of proinflammatory mediators and molecules involved in the homing of inflammatory cells[14]. The metabolite-sensitive G protein-coupled receptor (GPR) and its ligands strongly affect anti-inflammatory responses, with SCFA functioning being partially mediated by their receptors GPR41 and GPR43[15-17]. In addition to SCFAs, bacteria from the phylum Bacteroidetes produce glycan from fiber modulating immune function to protect against inflammation, such as polysaccharide A and peptidoglycan[18]. Thus, the anti-inflammatory responses involving SCFAs and other microbial-related metabolites in the intestine are likely weakened in the gut, and are likely closely associated with the development of obesity and T2DM.
The infiltration and proinflammatory shift of immune cells contribute to the inflammation of the intestine under metabolic challenge. In mice and obese humans, high-fat diet (HFD) induces chemokine (C-C motif) ligand 2/monocyte chemoattractant protein-1 (CCL2/MCP-1) production to rise in epithelial cells, which recruit monocytes to the gut, shifting to the proinflammatory phenotype[19,20]. Macrophage-specific deletion of C-C chemokine receptor type 2 (CCR2) ameliorates insulin sensitivity and glucose tolerance, confirming the association between the infiltration of proinflammatory macrophages and obesity-induced metabolic disorders[19]. Moreover, HFD also induces a proinflammatory shift in T cells, with elevated interferon gamma (IFN-γ)-producing CD4+, CD8+ T cells, and interleukin 17 (IL-17)-producing γδ T cells, along with decreased regulatory T cells (Tregs)[21]. Tregs are one lineage of CD4+ T cells. These cells are involved in maintaining immune homeostasis and restricting excessive immune responses. T helper 17 (Th17) cells might secrete IL-17A, IL-17F, IL-21, and IL-22. Several strains of Clostridia help with the expansion and differentiation of Tregs, by providing bacterial antigens and an environment rich in transforming growth factor beta, contributing to the immunological homeostasis of the gut[22,23]. Lactobacillus reuteri, Bacteroides fragilis, B. hetaiotaomicron, Clostridium, and Faecalibacterium prausnitzii promote the differentiation of Tregs. Segmented filamentous bacteria are required for Th17 cells to develop in the gut. Furthermore, SCFAs improve the Treg/Th17 balance, and induce IL-22 production in CD4+ T cells and innate lymphoid cells (ILCs), maintaining intestinal homeostasis[17,24,25].
Many studies have shown that serum lipopolysaccharide (LPS) levels rise in T2DM patients, with a triggering factor to IR and diabetes being identified that is closely associated with intestinal integrity and permeability[26,27]. One recent study of 128 obese human subjects showed that the abundance of Escherichia coli, an important producer of LPS, was higher in obese patients with T2DM compared with the lean patients[28]. LPS is recognized by Toll-like receptors (TLRs) of the innate immune system, leading to the aggregation of macrophages and activation of the NF-κB inflammatory signaling pathway. This process triggers systemic immune and inflammatory responses that aggravate IR[14,29]. In general, a healthy intestinal barrier protects the organism from the passage of microbes. However, the intestinal barrier of people with T2DM is disturbed, leading to the uncontrolled passage of LPS and microbiota-derived molecules, and subsequent endotoxemia and chronic inflammation[30]. In particular, obese mice have fewer immunoglobulin A (IgA)-secreting immune cells and lower IgA secretion and glucose metabolism disorders arise in obese IgA-deficient mice. Administering metformin and bariatric surgery augment cellular and stool IgA levels[31]. Obese patients with T2DM exhibit a lower expression of intestinal tight junction genes and interference with the WNT/β-catenin signaling pathway, both of which are linked increased intestinal epithelial and gut vascular permeability[31-33]. Several immune cells (such as mucosal-associated invariant T cells [MAIT]) also impair gut integrity by inducing the dysbiosis of microbiota[34]. IL-1β can increase barrier permeability in intestinal epithelial cells, whereas IL-22 is considered a protector of maintaining intestinal barrier integrity[35-37]. Reduced integrity and higher intestinal permeability of the intestine promote the translocation of microbiota-derived molecules from the intestinal lumen to the bloodstream. This process triggers the activation of lamina propria macrophages in the intestine, causing LPS levels to rise in the blood.
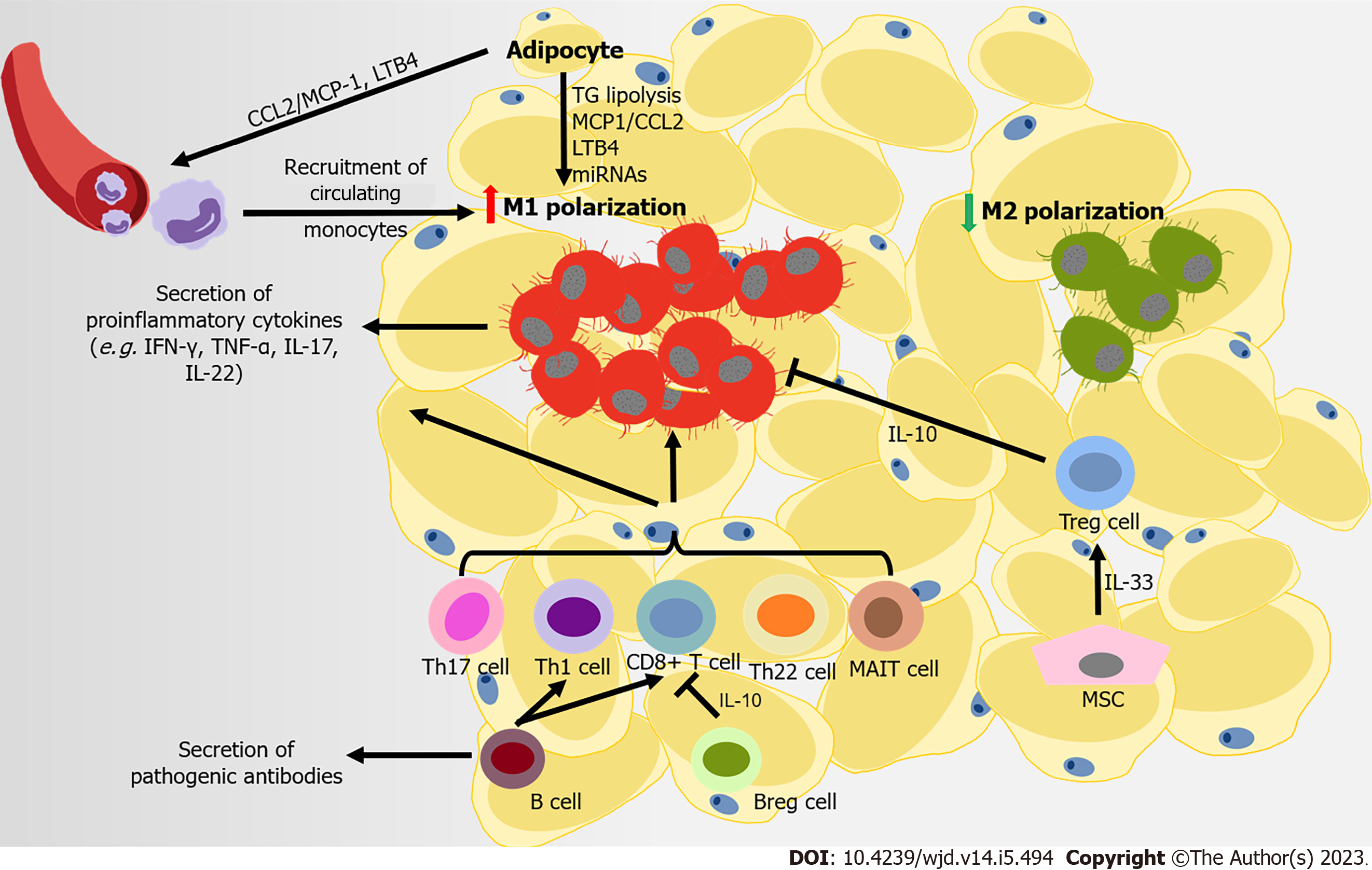
Eating more calorie-dense foods combined with less exercise promotes the development of obesity. In both mice and humans, excess energy is stored in white adipose tissues (ATs) (WAT), which serves as the immune and endocrine organ containing mature adipocytes, adipocyte precursor cells (also called adipose stromal cells), and immune cells. Obesity causes a persistent low-grade inflamed condition in these expanding adipose depots, and the simultaneous infiltration of immune cells in the stromal vascular fraction and systematic metabolic disorders. The inflammatory storm driven by dysfunctional WAT disrupts its normal function and that of other insulin-sensitive organs. Consequently, this process contributes to the pathophysiological mechanisms of IR and T2DM (Figure 2). However, in obese subjects with T2DM, this immune attack appears to be stronger. Obese patients with T2DM have a higher degree of inflammation at both the systemic and AT level compared to patients with normal glucose tolerance. This phenomenon is characterized by aggravated macrophage infiltration in WAT, with elevated IL-6 levels and CD4+ T cell numbers in serum[38].
Macrophages are representative immune cells of the innate immune system, and were first studied in relation to the process of immune infiltration in WAT. The infiltration and activation of macrophages is beginning to be recognized as a pivotal instigator of meta-inflammation. Normally, M2 anti-inflammatory macrophages are the main type in WAT[39,40]. However, as obesity develops, instead of the M2-phenotype, M1 proinflammatory macrophages in AT gradually increase (up to 40% of cells in AT), leading to a proinflammatory state in WAT[40-42]. The greater increase in M1-like polarized mac-rophages results in their being responsible for almost all secretions of tumor necrosis factor alpha (TNF-α) and IL-6 in WAT. In turn, this process impairs the insulin signaling pathway, leading to IR, both locally and systemically[43]. Initially, the proliferation of resident macrophages dominates the accumulation of macrophages in WAT. Then at the later stage of obesity, recruited monocytes con
Aside from macrophages, adaptive immune cells are involved in the pathogenesis of obesity-related T2DM. In HFD-induced obese mice, CD8+ T cells are recruited into AT, promoting M1-like polarization[40,54,55]. However, different categories of CD4+ T cells have various functions in AT[56]. Proinflammatory CD4+ T cells (Th1, Th17, and Th22) are important promoters of the development of obesity-associated metabolic disorders. These cells produce proinflammatory cytokines (IFN-γ, TNF-α, IL-17, and IL-22), and are involved in the recruitment and activation of M1 macrophages[57-60]. MAIT are innate-like T cells that express a semi-invariant T cell receptor, which promote inflammation in AT by inducing M1 macrophage polarization. This process leads to IR and impaired glucose and lipid metabolism[34]. Conversely, Tregs provide an essential accessory function that prevents systemic metabolic disorders, through suppressing the expression of MCP-1 in adipocytes to limit M1 macrophage infiltration via IL-10 and other insulin-sensitizing factors. However, the development of Tregs in WAT seems to depend on insulin signaling. Insulin signaling drives the transition of CD73loST2 (IL-33 receptor) hiadipose Treg subsets, which might also suppress inflammation in WAT via the hypoxia inducible factor 1 alpha–mediator complex subunit 23–peroxisome proliferator-activated receptor gamma axis[61]. Furthermore, AT B cells also negatively control local inflammation by secreting IL-10 (secreted by Bregs) and other soluble factors. B cells also contribute to systemic inflammation by activating CD8+ and Th1 cells, and releasing pathogenic antibodies[62-65]. B cells from obese mice consistently produce a proinflammatory cytokine profile compared to those from lean controls[66]. B cells transferred from obese mice induce the development of IR in B cell-deficient lean mice. By contrast, B cell depletion in mice restores aberrant immune cell composition and improves metabolic capacity in WAT[67]. T-bet B cells are B cells lacking cluster of differentiation 21 (CD21) and CD23. These cells accumulate in humans that have an elevated body mass index, and in mice with higher body weight. Mice without T-bet B cells have lower weight gain and M1 macrophage infiltration in WAT[68,69]. Thus, regulation of the adaptive immune system is related to the inflammation of AT in obesity. Adaptive immune cells are involved in AT IR in obesity-related T2DM; however, some of these effects may be achieved through promoting the polarization of M1-like macrophages.
Recent studies have shown that other types of cells in AT also participate in regulating immune balance. Mesenchymal cells contribute towards shaping immune responses and maintaining immune homeostasis in WAT. Mesenchymal cells express IL-7, IL-33, and CCL19, which recruit both innate and adaptive lymphocytes. IL-33 is produced by particular mesenchymal stromal cells in visceral AT (VAT), IL-33 improves IR and inflammation in AT, possibly through expanding and sustaining the resident Treg population[70-73]. Administering IL-33 helps combat obesity, by markedly increasing the fraction of group 2 ILCs and eosinophil, and improving WAT browning[74].
However, the distribution of AT appears to be closely related to the occurrence and progression of metabolic diseases. It has been universally accepted that central body fat deposition and injured function of AT are closer associated with obesity-related metabolic diseases than fat mass in the whole body. Generally, AT is divided into abdominal subcutaneous AT, femoral subcutaneous AT (FSAT, main type of lower-body AT), VAT, according to their different location. SAT is the largest AT depot. The expansion of FSAT and adipocyte hyperplasia from precursor cells are considered to be a healthier alterative of AT in meeting elevated storage energy demands. However, any damage to these approaches leads to the accumulation of fat in upper body AT and organs, which causes “lipotoxicity” in other insulin-sensitive organs, as well as systemic IR and a higher risk of T2DM. Several studies have found that SAT may have a more beneficial metabolic phenotypes, notably its accumulation in lower-body[75,76]. Upper body AT (especially VAT) is usually characterized by more rapid storage of energy and a higher lipolysis rate than lower-body, which contributes to systemic FFA levels[77]. Interestingly, a recent study revealed that expanded adipocytes, lower SAT oxygenation, inflammation infiltration in SAT, and elevated FFA release, these changes in SAT that were considered harmful, seemed to be unrelated to the occurrence of obesity-induced IR[76,78]. Collectively, expansion and inflammation in VAT, rather than SAT, are the culprit involved in obesity-related metabolic diseases. Therefore, the effects of abdominal WAT accumulation are of more concern.
Metabolically healthy obesity (MHO) is a subgroup of obesity, which does not have an universally accepted definition. In most studies, MHO presented without the following features: dyslipidemia, IR, impaired glucose metabolism, and overt T2DM. Compared with metabolically unhealthy obesity (MUO), MHO usually has more expandability of SAT, less ectopic fat accumulation, normal concentration of inflammatory markers, and preserved better β-cell function, and insulin sensitivity[79-81]. Systematically, decreased concentrations of C-reactive protein, TNF-α, IL-6, and plasminogen activator inhibitor-1 were found in the MHO subjects than MUO individuals[82]. Changes to the distribution and function of AT might also strongly contribute to the conversion of these two states. Excess caloric storage demand leads to the overload of SAT and ectopic fat accumulation and this ectopic fat deposition will eventually cause the transition from MHO to MUO[79]. Besides, many studies have revealed that less immune cells infiltration (such as proinflammatory macrophages and T lymphocytes) and cytokines production in MHO than in MUO, usually along with the increased VAT mass[83-86]. Improved antioxidant capacity and diminished oxidative stress could be also observed in MHO subjects than in MUO people[87,88].
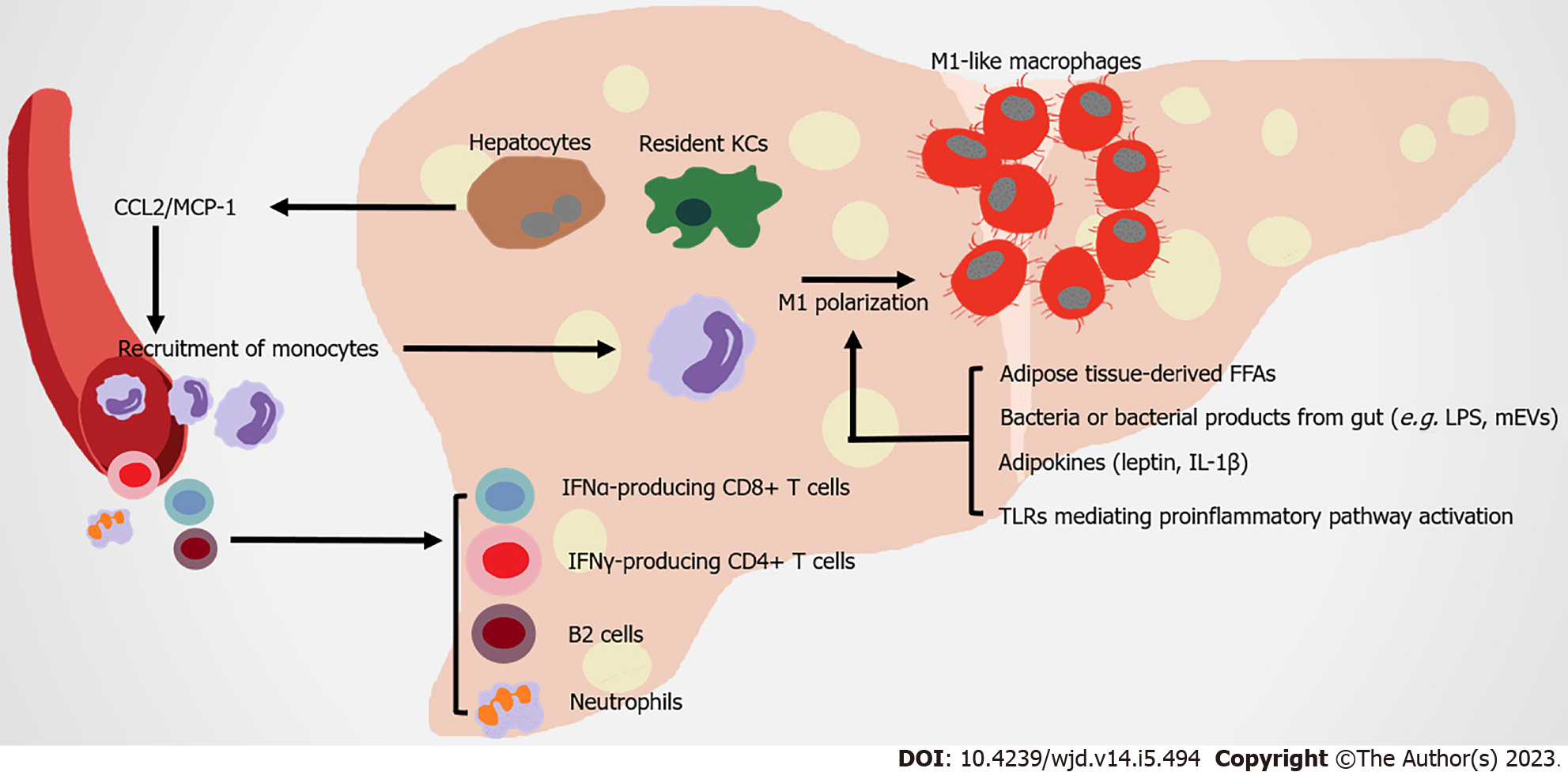
The liver is the metabolic center of nutrients and drugs in the body. It receives material supplied from the gut via the portal vein, proinflammatory immune cells and cytokines from circulation, which strongly impact its physiological function (Figure 3).
Liver macrophages contribute to obesity-related hepatic IR by producing both inflammatory and non-inflammatory factors. Hepatic macrophages include resident macrophages (Kupffer cells [KCs], high expression of F4/80 and C-type lectin domain family 4 member F) and recruited hepatic macrophages (RHMs), high expression of CD11b and CCR2. RHMs are derived from circulating Lyc6+ monocytes, which are recruited by steatosis hepatocytes and KCs secreting CCL2/MCP-1[89-92]. Although the ratio of KC to RHM is different in the liver of healthy mice and humans, as obesity develops, hepatic RHMs noticeably increase. These RHMs serve as a main promoter of inflammation injury in the liver, by producing chemokines and cytokines (in both humans and mice), which are related to obesity induced IR[93-95]. Multiple mechanisms are involved in the proinflammatory activation of hepatic macrophages. In obese individuals, FFAs overflow from obese AT contributes to the activation of resident hepatic macrophages[96]. Leptin and adiponectin from expanded AT have contrasting actions on KCs. The former stimulates proinflammatory and profibrogenic cytokines in KCs, whereas the latter modifies KCs towards anti-inflammatory phenotypes[97,98]. AT-derived proinflammatory cytokines (such as IL-1β) contribute to the chronic activation of hepatic NF-κB, promoting the development of nonalcoholic steatohepatitis (NASH)[99]. KCs highly express scavenger, complement, and pattern recognition receptors, including TLRs. Intestinal permeability rises during obesity, leading to the translocation of bacteria or their products to the portal circulation. These substances are recognized by TLRs in macrophages, which activate NF-κB, IFN regulatory factors and other downstream transcriptional factors to induce inflammatory responses[100]. Microbe-related products, including extracellular vesicles (mEVs) containing gut microbial DNA, that leak from gut reach the liver, and exacerbate obesity-associated hepatic inflammation and IR. Vsig4+ macrophages and CRIg+ macrophages efficiently clear mEVs through a complementary component C3-dependent mechanism; however, HFD impairs these benefits[101,102]. CD68 serves as a marker for macrophages residing in the liver; however, this indicator is not sufficient for distinguishing them from monocyte-derived cells. The utilization of single-cell sequencing allows their origin, function, and associated inflammatory phenotype to be clearly distinguished. Two distinct populations of intrahepatic CD68 macrophages exist in human livers. CD68MARCO+++− cells are characterized by the enriched expression of LYZ, CSTA, and CD74, which represent their proinflammatory function. The CD68MARCO macrophage subset is similar to resident KCs, inducing immune tolerance[103]. Counter to expectation, KCs in diet-induced steatohepatitis probably participate in reparation pathways, not proinflammatory function[104]. However, KCs and RHMs both shift towards a proinflammatory phenotype[105]. Overall, the types and functions of liver macrophages are still under investigation.
Nonalcoholic fatty liver disease (NAFLD), obesity, and T2DM are closely related in terms of pathogenesis. The prevalence of NAFLD is higher in subjects with obesity compared to lean subjects[106,107]. T2DM is also closely associated with NAFLD and its severe form NASH. Most T2DM patients suffer from NAFLD[108-110]. NAFLD, particularly NASH, usually leads to more severe hepatic IR that negatively affects T2DM development[111]. In NASH mice, KC is gradually replaced by RHM. Although RHM could respond to local environmental clues and develops a KC-like transcriptomic profile, this profile is not identical to original healthy KCs[90]. In healthy subjects, KCs inhibit monocyte and macrophage recruitment by secreting IL-10 and promoting immune tolerance through inducing Tregs and programmed death-ligand 1 expression. However, when NASH happens, injured hepato
The histopathology hallmarks of human NASH include the infiltration of neutrophils with MPO-positive immunoreactivity[99]. Neutrophil extracellular traps (NETs) are extracellular web-like structures of decondensed chromatin with cytosolic and granule proteins. These structures are important in hepatic chronic inflammatory conditions. NET blockade significantly decreases the infiltration of RHMs and neutrophils[121].
Moreover, recent studies have focused on elucidating the role of adaptive immunity cells in liver inflammation under metabolic challenge. The accumulation of B cells (especially B2 cells) and T cells in liver arises in more than half of NASH patients[122-124]. B cell-activating factor levels in the circulation are elevated in NASH patients compared to those with simple steatosis. This phenomenon is associated with more advanced IR, more severe steatohepatitis and fibrosis[123,125,126]. The contribution of B cells to the progression of NASH could be attributed to the production of proinflammatory mediators and their antigen-presenting capabilities[122]. Interfering with B2 cells reduces the Th1 cell activation of liver CD4+ T cells and IFN-γ production[123]. In both humans and mice, IFN-γ-producing CD4+ T cells and IFN-α-producing CD8+ T cells increase in the liver, promoting IR under diet-induced metabolic stress[127,128]. Thus, the infiltration of adaptive immunity cells in liver strongly affect inflammatory mechanisms during the development of NASH.
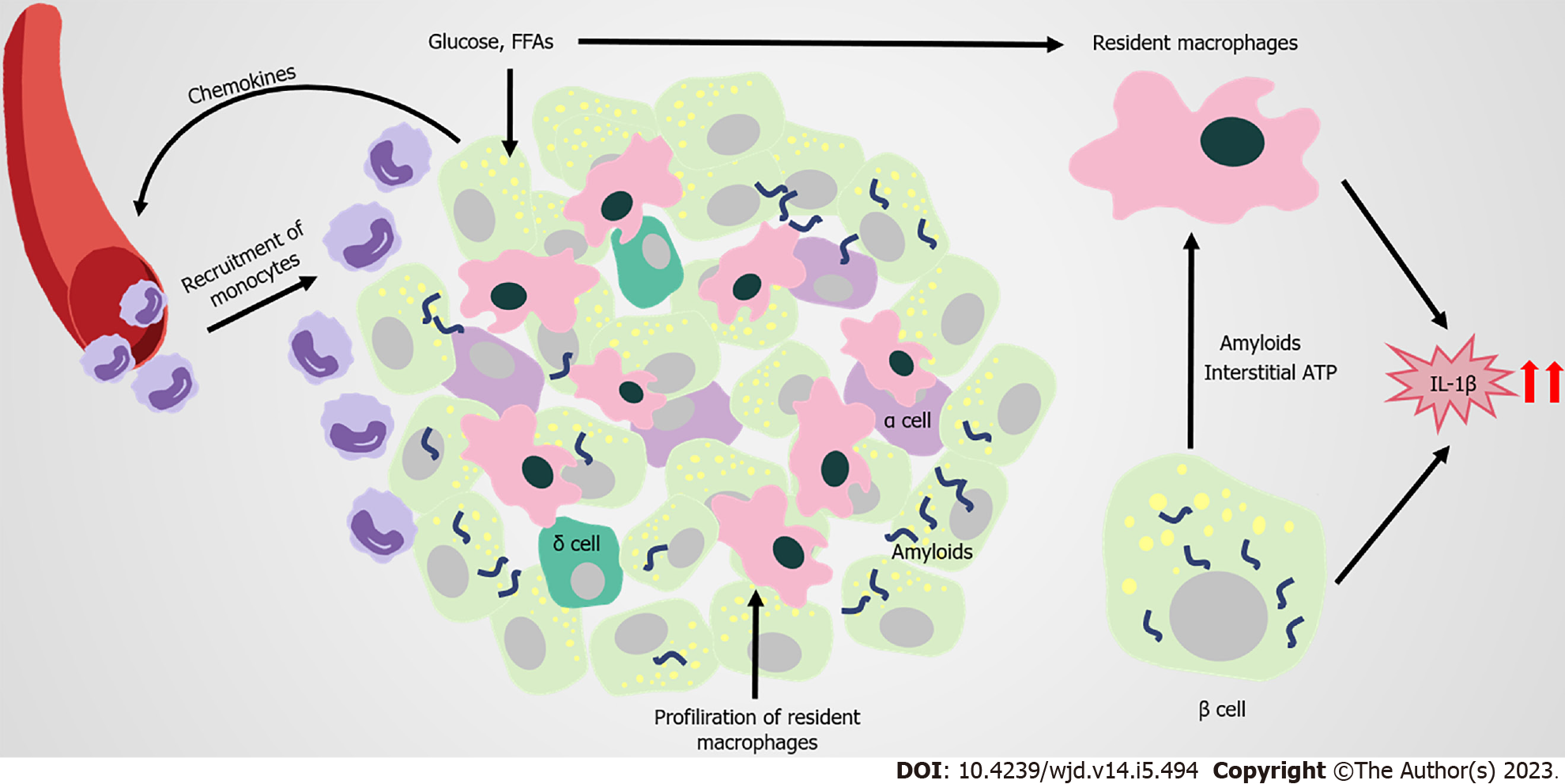
In the pathophysiologic process of islets of obesity and T2DM, innate immune cells are important, especially macrophages. Increased infiltration of resident macrophages and transformation towards a proinflammatory phenotype contributes to obesity and T2DM islets, the extent of which is generally correlated with β-cell dysfunction[129-131] (Figure 4). Islet macrophages express F4/80, CD11c, major histocompatibility complex class II, CD64, CD11b, CX3C motif chemokine receptor 1, CD68, and lysozyme[132]. At the early stage of obesity, resident macrophages enhance the compensatory proliferation of β cells, mediated by platelet-derived growth factor (PDGF)-PDGF receptor signaling[129]. As the disease progresses, CD68-positive macrophages are elevated in T2DM islets[130,133,134]. The proliferation of resident macrophages causes them to accumulate in islets with elevated inflammatory cytokines and chemokines (such as IL-1β, TNF-α), impairing the hyperplasia and dysfunction of β cells[131]. Overall, changes to the number and function of islet macrophages affect the pathogenesis of obesity and T2DM.
However, the factors that trigger the infiltration and proinflammation polarization of macrophages in islets remain unclear. Β cells are potentially one of the early responders in the altered islet microenvironment. In obesity, β cells recruit Ly6C+ monocytes to the islets by producing chemokines, despite these recruited monocytes remaining at the boundary of the exocrine and endocrine pancreas[129]. Amyloid deposition in islets is a typical pathological feature of T2DM, and is also a strong stimulus for macrophage-mediated NLRP3 inflammasome activation and IL-1β production[135-137]. In amyloid-positive T2DM islets, the number of macrophages greatly increases, with CD68 and inducible nitric oxide synthase-positive[134]. Macrophages that are resident to islets act as heightened sensors of interstitial ATP levels. Consequently, glucose-activated insulin and ATP co-secretion of β cells might trigger cytokine production from macrophages[138]. Macrophages resident to islets are in contact with blood vessels, probably protecting against inflammatory moieties from blood by extending their filopodias; however, high concentrations of glucose in T2DM limit this method of capture[139,140]. In addition, GRP92 activation in islet macrophages promotes conversion to the anti-inflammatory phenotype, and improves β-cell function[141]. The accumulation of intestinal mEVs causes CD11c+ macrophages to increase, with elevated IL-1β in islets impairing insulin secretion. Vsig4+ macrophages in islets block intestine-derived mEV via a C3-mediated mechanism. By contrast, obesity causes a marked decrease in Vsig4+ macrophages[142].
IL-1β is a key proinflammatory cytokine that clearly increases in T2DM islets. Although macrophages are considered to be the major producers of IL-1β in obesity islets, for which the potential mechanism has been identified, β cells also produce IL-1β[129,137]. Glucose-induced IL-1β auto-stimulation in β cells might contribute to glucotoxicity in T2DM islets[143,144]. However, IL-1β on β cells seem to have varied effects. For instance, low concentrations of IL-1β help to increase β-cell proliferation and improve insulin secretion following glucose stimulation. By contrast, high concentrations of IL-1β promote inflammation in islets, and might be closely related to the development of pre-diabetes and T2DM[145-147]. The IL-1R antagonist (IL-1Ra) also declines in T2DM β cells, pushing the IL-1/IL-1Ra balance towards a proinflammatory state[148-151]. The vaccine and responsive miRNA targeting IL1β are promising approaches for treating T2DM, by restoring β-cell mass, inhibiting β-cell apoptosis, and increasing insulin secretion[152-154]. Thus, antagonizing IL-1β is a potential target for T2DM treatment.
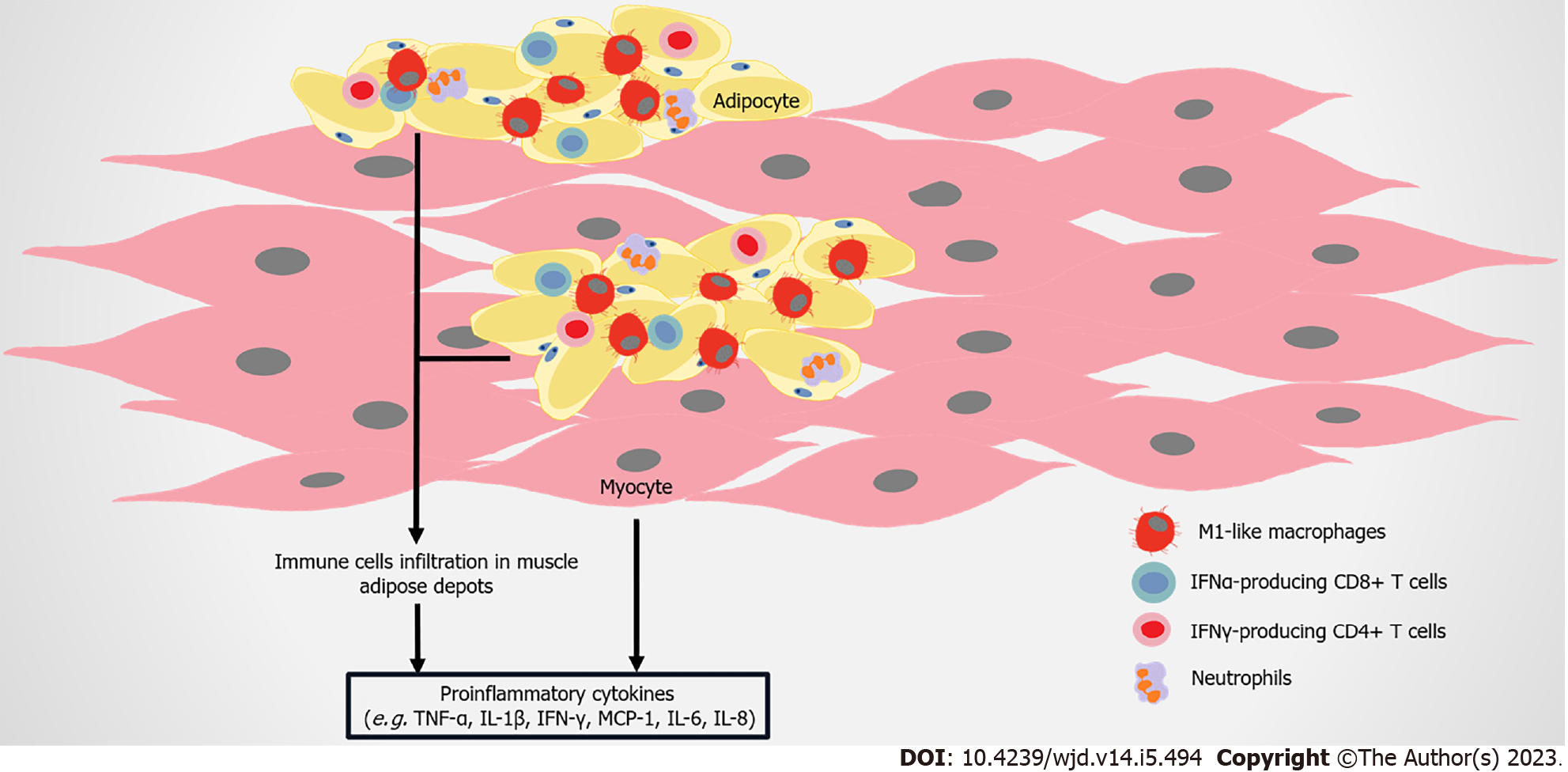
As skeletal muscle is the principle organ for glucose disposal, IR in this tissue becomes a crucial determinant of obesity and T2DM-related metabolic disorders[155,156]. Immune attack and inflammatory responses in skeletal muscle also regulate IR formation (Figure 5). CD11c-expressing proinflammatory macrophages, monocytes, and neutrophils are higher in the skeletal muscle of HFD-induced mice compared to the control[157,158]. More macrophages markers are found in the skeletal muscle of healthy subjects after HFD administration, with the development of IR[159,160]. In obese T2DM patients, the number of CD68+ macrophages is elevated in skeletal muscle[158,161]. Total T cells and αβ T cells, containing CD8+ T cells and IFN-γ–producing CD4+ cells are higher in the skeletal muscle of obese mice compared to control mice[162]. FFAs induce or synergize with macrophages to aggravate the inflammatory response in muscle cells, resulting in IR[163-165]. These immune cells infiltrate skeletal muscle, and accumulate in muscle AT between myocytes and the surrounding muscle, leading to higher levels of local proinflammatory cytokines, such as TNF-α, IL-1β, and IFN-γ[158,160,166].
Similar to adipocytes, skeletal muscle cells produce MCP-1, IL-6, IL-8, TNF-α, and other molecules, and part of these molecules lead to the infiltration of macrophages, inducing IR[157,167]. Muscle biopsies show that the gene expression of inflammatory cytokines (such as TNF-α) is upregulated in IR subjects[168]. Compared with non-diabetic subjects, more IL-6, IL-8, IL-15, TNF-α, growth related oncogene α, MCP-1, and follistatin are released by skeletal muscle cells from T2DM patients[169]. Aerobic exercise reduces the infiltration of macrophage in skeletal muscles, and improves insulin sensitivity and elevates the production of anti-inflammatory cytokine IL-10[170]. IL-10 attenuates macrophage infiltration and cytokine response in skeletal muscle, mitigating diet-induced IR[160]. Interestingly, while IL-6 usually promotes inflammation, acute IL-6 treatment in skeletal muscle strengthens insulin-stimulated glucose disposal in humans, possibly mediated by AMP-activated protein kinase signaling[171,172]. Therefore, the exact role of myokines in the metabolism of skeletal muscle needs to be further clarified.
TLRs are also present in skeletal muscle. The expression and signaling of TLR4 is elevated in the muscle of IR patients[173]. LPS-induced IR in skeletal muscle entirely depends on TLR4[174]. The inhibition or deletion of TLR4 prevents acute hyperlipidemia-induced skeletal muscle IR[175,176]. Palmitate induces myeloid differentiation primary response 88 and TLR2 receptor to combine in mouse myotube cells, providing the foundation for inflammation and IR[177]. Therefore, TLRs are also involved in activating proinflammatory factors on skeletal muscle cells.
Overall, many studies support the association of obesity and related-T2DM with increased inflammation of skeletal muscle in rodents and humans. The greater infiltration of macrophages and T cells, and their polarization towards proinflammatory phenotypes, means they act as primary promoters in increasing the inflammation of skeletal muscle. Skeletal muscle cell-secreting myokines also exhibit proinflammatory effects during the development of obesity and T2DM.
Chronic low-grade inflammation involving the immune system is a typical feature of obesity-associated T2DM. It generates an inflammatory storm affecting multiple organs and tissues throughout the body. Adaptive activation of the immune system usually stems from an energy imbalance in the body induced by excess calorie intake. However, as the imbalance continues to grow, parenchymal cells and immune cells (in particular, macrophages/monocytes), and their cross-talk, promote the inflammatory response and the development of T2DM by exacerbating IR. Targeting immune cells and relative inflammatory responses is an effective treatment of obesity and associated T2DM.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cai L, United States; Hejazi J, Iran; Park JH, South Korea S-Editor: Zhang H L-Editor: Filipodia P-Editor: Yu HG
| 1. | Loos RJF, Yeo GSH. The genetics of obesity: from discovery to biology. Nat Rev Genet. 2022;23:120-133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 613] [Article Influence: 204.3] [Reference Citation Analysis (1)] |
| 2. | Jebeile H, Kelly AS, O'Malley G, Baur LA. Obesity in children and adolescents: epidemiology, causes, assessment, and management. Lancet Diabetes Endocrinol. 2022;10:351-365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 465] [Article Influence: 155.0] [Reference Citation Analysis (0)] |
| 3. | Daousi C, Casson IF, Gill GV, MacFarlane IA, Wilding JP, Pinkney JH. Prevalence of obesity in type 2 diabetes in secondary care: association with cardiovascular risk factors. Postgrad Med J. 2006;82:280-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 218] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 4. | Hu FB, Manson JE, Stampfer MJ, Colditz G, Liu S, Solomon CG, Willett WC. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med. 2001;345:790-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1801] [Cited by in RCA: 1742] [Article Influence: 72.6] [Reference Citation Analysis (0)] |
| 5. | Cotillard A, Kennedy SP, Kong LC, Prifti E, Pons N, Le Chatelier E, Almeida M, Quinquis B, Levenez F, Galleron N, Gougis S, Rizkalla S, Batto JM, Renault P; ANR MicroObes consortium, Doré J, Zucker JD, Clément K, Ehrlich SD. Dietary intervention impact on gut microbial gene richness. Nature. 2013;500:585-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1189] [Cited by in RCA: 1294] [Article Influence: 107.8] [Reference Citation Analysis (0)] |
| 6. | Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G, Almeida M, Arumugam M, Batto JM, Kennedy S, Leonard P, Li J, Burgdorf K, Grarup N, Jørgensen T, Brandslund I, Nielsen HB, Juncker AS, Bertalan M, Levenez F, Pons N, Rasmussen S, Sunagawa S, Tap J, Tims S, Zoetendal EG, Brunak S, Clément K, Doré J, Kleerebezem M, Kristiansen K, Renault P, Sicheritz-Ponten T, de Vos WM, Zucker JD, Raes J, Hansen T; MetaHIT consortium, Bork P, Wang J, Ehrlich SD, Pedersen O. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500:541-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2727] [Cited by in RCA: 3202] [Article Influence: 266.8] [Reference Citation Analysis (2)] |
| 7. | Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau AL, Griffin NW, Lombard V, Henrissat B, Bain JR, Muehlbauer MJ, Ilkayeva O, Semenkovich CF, Funai K, Hayashi DK, Lyle BJ, Martini MC, Ursell LK, Clemente JC, Van Treuren W, Walters WA, Knight R, Newgard CB, Heath AC, Gordon JI. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science. 2013;341:1241214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2415] [Cited by in RCA: 2719] [Article Influence: 226.6] [Reference Citation Analysis (0)] |
| 8. | Fei N, Zhao L. An opportunistic pathogen isolated from the gut of an obese human causes obesity in germfree mice. ISME J. 2013;7:880-884. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 422] [Cited by in RCA: 541] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 9. | Xu YH, Gao CL, Guo HL, Zhang WQ, Huang W, Tang SS, Gan WJ, Xu Y, Zhou H, Zhu Q. Sodium butyrate supplementation ameliorates diabetic inflammation in db/db mice. J Endocrinol. 2018;238:231-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 111] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 10. | Chang PV, Hao L, Offermanns S, Medzhitov R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc Natl Acad Sci U S A. 2014;111:2247-2252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1079] [Cited by in RCA: 1507] [Article Influence: 137.0] [Reference Citation Analysis (0)] |
| 11. | Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, Liang S, Zhang W, Guan Y, Shen D, Peng Y, Zhang D, Jie Z, Wu W, Qin Y, Xue W, Li J, Han L, Lu D, Wu P, Dai Y, Sun X, Li Z, Tang A, Zhong S, Li X, Chen W, Xu R, Wang M, Feng Q, Gong M, Yu J, Zhang Y, Zhang M, Hansen T, Sanchez G, Raes J, Falony G, Okuda S, Almeida M, LeChatelier E, Renault P, Pons N, Batto JM, Zhang Z, Chen H, Yang R, Zheng W, Yang H, Wang J, Ehrlich SD, Nielsen R, Pedersen O, Kristiansen K. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490:55-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3971] [Cited by in RCA: 4821] [Article Influence: 370.8] [Reference Citation Analysis (1)] |
| 12. | Karlsson FH, Tremaroli V, Nookaew I, Bergström G, Behre CJ, Fagerberg B, Nielsen J, Bäckhed F. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature. 2013;498:99-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1766] [Cited by in RCA: 2141] [Article Influence: 178.4] [Reference Citation Analysis (0)] |
| 13. | Kim G, Yoon Y, Park JH, Park JW, Noh MG, Kim H, Park C, Kwon H, Kim Y, Sohn J, Park S, Im SK, Chung HY, Nam MH, Kwon JY, Kim IY, Kim YJ, Baek JH, Kim HS, Weinstock GM, Cho B, Lee C, Fang S, Park H, Seong JK. Bifidobacterial carbohydrate/nucleoside metabolism enhances oxidative phosphorylation in white adipose tissue to protect against diet-induced obesity. Microbiome. 2022;10:188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 14. | Blaak EE, Canfora EE, Theis S, Frost G, Groen AK, Mithieux G, Nauta A, Scott K, Stahl B, van Harsselaar J, van Tol R, Vaughan EE, Verbeke K. Short chain fatty acids in human gut and metabolic health. Benef Microbes. 2020;11:411-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 193] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 15. | Kim MH, Kang SG, Park JH, Yanagisawa M, Kim CH. Short-chain fatty acids activate GPR41 and GPR43 on intestinal epithelial cells to promote inflammatory responses in mice. Gastroenterology. 2013;145:396-406.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 576] [Cited by in RCA: 763] [Article Influence: 63.6] [Reference Citation Analysis (0)] |
| 16. | Lu Y, Fan C, Li P, Lu Y, Chang X, Qi K. Short Chain Fatty Acids Prevent High-fat-diet-induced Obesity in Mice by Regulating G Protein-coupled Receptors and Gut Microbiota. Sci Rep. 2016;6:37589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 284] [Cited by in RCA: 451] [Article Influence: 50.1] [Reference Citation Analysis (0)] |
| 17. | Yang W, Yu T, Huang X, Bilotta AJ, Xu L, Lu Y, Sun J, Pan F, Zhou J, Zhang W, Yao S, Maynard CL, Singh N, Dann SM, Liu Z, Cong Y. Intestinal microbiota-derived short-chain fatty acids regulation of immune cell IL-22 production and gut immunity. Nat Commun. 2020;11:4457. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 415] [Cited by in RCA: 645] [Article Influence: 129.0] [Reference Citation Analysis (0)] |
| 18. | Potrykus M, Czaja-Stolc S, Stankiewicz M, Kaska Ł, Małgorzewicz S. Intestinal Microbiota as a Contributor to Chronic Inflammation and Its Potential Modifications. Nutrients. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 60] [Article Influence: 15.0] [Reference Citation Analysis (1)] |
| 19. | Kawano Y, Nakae J, Watanabe N, Kikuchi T, Tateya S, Tamori Y, Kaneko M, Abe T, Onodera M, Itoh H. Colonic Pro-inflammatory Macrophages Cause Insulin Resistance in an Intestinal Ccl2/Ccr2-Dependent Manner. Cell Metab. 2016;24:295-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 146] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 20. | Rohm TV, Fuchs R, Müller RL, Keller L, Baumann Z, Bosch AJT, Schneider R, Labes D, Langer I, Pilz JB, Niess JH, Delko T, Hruz P, Cavelti-Weder C. Obesity in Humans Is Characterized by Gut Inflammation as Shown by Pro-Inflammatory Intestinal Macrophage Accumulation. Front Immunol. 2021;12:668654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 21. | Luck H, Tsai S, Chung J, Clemente-Casares X, Ghazarian M, Revelo XS, Lei H, Luk CT, Shi SY, Surendra A, Copeland JK, Ahn J, Prescott D, Rasmussen BA, Chng MH, Engleman EG, Girardin SE, Lam TK, Croitoru K, Dunn S, Philpott DJ, Guttman DS, Woo M, Winer S, Winer DA. Regulation of obesity-related insulin resistance with gut anti-inflammatory agents. Cell Metab. 2015;21:527-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 284] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 22. | Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, Fukuda S, Saito T, Narushima S, Hase K, Kim S, Fritz JV, Wilmes P, Ueha S, Matsushima K, Ohno H, Olle B, Sakaguchi S, Taniguchi T, Morita H, Hattori M, Honda K. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500:232-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1875] [Cited by in RCA: 2134] [Article Influence: 177.8] [Reference Citation Analysis (2)] |
| 23. | Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, Takahashi M, Fukuda NN, Murakami S, Miyauchi E, Hino S, Atarashi K, Onawa S, Fujimura Y, Lockett T, Clarke JM, Topping DL, Tomita M, Hori S, Ohara O, Morita T, Koseki H, Kikuchi J, Honda K, Hase K, Ohno H. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2951] [Cited by in RCA: 3775] [Article Influence: 314.6] [Reference Citation Analysis (0)] |
| 24. | Wang L, Beier UH, Akimova T, Dahiya S, Han R, Samanta A, Levine MH, Hancock WW. Histone/protein deacetylase inhibitor therapy for enhancement of Foxp3+ T-regulatory cell function posttransplantation. Am J Transplant. 2018;18:1596-1603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 25. | Liu YJ, Tang B, Wang FC, Tang L, Lei YY, Luo Y, Huang SJ, Yang M, Wu LY, Wang W, Liu S, Yang SM, Zhao XY. Parthenolide ameliorates colon inflammation through regulating Treg/Th17 balance in a gut microbiota-dependent manner. Theranostics. 2020;10:5225-5241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 214] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 26. | Fuke N, Nagata N, Suganuma H, Ota T. Regulation of Gut Microbiota and Metabolic Endotoxemia with Dietary Factors. Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 176] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 27. | Gomes JMG, Costa JA, Alfenas RCG. Metabolic endotoxemia and diabetes mellitus: A systematic review. Metabolism. 2017;68:133-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 224] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 28. | Yang K, Niu J, Zuo T, Sun Y, Xu Z, Tang W, Liu Q, Zhang J, Ng EKW, Wong SKH, Yeoh YK, Chan PKS, Chan FKL, Miao Y, Ng SC. Alterations in the Gut Virome in Obesity and Type 2 Diabetes Mellitus. Gastroenterology. 2021;161:1257-1269.e13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 108] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 29. | Mohammad S, Thiemermann C. Role of Metabolic Endotoxemia in Systemic Inflammation and Potential Interventions. Front Immunol. 2020;11:594150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 179] [Cited by in RCA: 257] [Article Influence: 64.3] [Reference Citation Analysis (0)] |
| 30. | Horton F, Wright J, Smith L, Hinton PJ, Robertson MD. Increased intestinal permeability to oral chromium (51 Cr) -EDTA in human Type 2 diabetes. Diabet Med. 2014;31:559-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 88] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 31. | Luck H, Khan S, Kim JH, Copeland JK, Revelo XS, Tsai S, Chakraborty M, Cheng K, Tao Chan Y, Nøhr MK, Clemente-Casares X, Perry MC, Ghazarian M, Lei H, Lin YH, Coburn B, Okrainec A, Jackson T, Poutanen S, Gaisano H, Allard JP, Guttman DS, Conner ME, Winer S, Winer DA. Gut-associated IgA(+) immune cells regulate obesity-related insulin resistance. Nat Commun. 2019;10:3650. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 142] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 32. | Rohr MW, Narasimhulu CA, Rudeski-Rohr TA, Parthasarathy S. Negative Effects of a High-Fat Diet on Intestinal Permeability: A Review. Adv Nutr. 2020;11:77-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 391] [Article Influence: 78.2] [Reference Citation Analysis (0)] |
| 33. | Mouries J, Brescia P, Silvestri A, Spadoni I, Sorribas M, Wiest R, Mileti E, Galbiati M, Invernizzi P, Adorini L, Penna G, Rescigno M. Microbiota-driven gut vascular barrier disruption is a prerequisite for non-alcoholic steatohepatitis development. J Hepatol. 2019;71:1216-1228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 230] [Cited by in RCA: 456] [Article Influence: 76.0] [Reference Citation Analysis (0)] |
| 34. | Toubal A, Kiaf B, Beaudoin L, Cagninacci L, Rhimi M, Fruchet B, da Silva J, Corbett AJ, Simoni Y, Lantz O, Rossjohn J, McCluskey J, Lesnik P, Maguin E, Lehuen A. Mucosal-associated invariant T cells promote inflammation and intestinal dysbiosis leading to metabolic dysfunction during obesity. Nat Commun. 2020;11:3755. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 112] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 35. | Wang X, Ota N, Manzanillo P, Kates L, Zavala-Solorio J, Eidenschenk C, Zhang J, Lesch J, Lee WP, Ross J, Diehl L, van Bruggen N, Kolumam G, Ouyang W. Interleukin-22 alleviates metabolic disorders and restores mucosal immunity in diabetes. Nature. 2014;514:237-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 374] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 36. | Rawat M, Nighot M, Al-Sadi R, Gupta Y, Viszwapriya D, Yochum G, Koltun W, Ma TY. IL1B Increases Intestinal Tight Junction Permeability by Up-regulation of MIR200C-3p, Which Degrades Occludin mRNA. Gastroenterology. 2020;159:1375-1389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 152] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 37. | Al-Sadi RM, Ma TY. IL-1beta causes an increase in intestinal epithelial tight junction permeability. J Immunol. 2007;178:4641-4649. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 473] [Cited by in RCA: 463] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 38. | van Beek L, Lips MA, Visser A, Pijl H, Ioan-Facsinay A, Toes R, Berends FJ, Willems van Dijk K, Koning F, van Harmelen V. Increased systemic and adipose tissue inflammation differentiates obese women with T2DM from obese women with normal glucose tolerance. Metabolism. 2014;63:492-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 39. | Li C, Menoret A, Farragher C, Ouyang Z, Bonin C, Holvoet P, Vella AT, Zhou B. Single cell transcriptomics based-MacSpectrum reveals novel macrophage activation signatures in diseases. JCI Insight. 2019;5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 86] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 40. | Vijay J, Gauthier MF, Biswell RL, Louiselle DA, Johnston JJ, Cheung WA, Belden B, Pramatarova A, Biertho L, Gibson M, Simon MM, Djambazian H, Staffa A, Bourque G, Laitinen A, Nystedt J, Vohl MC, Fraser JD, Pastinen T, Tchernof A, Grundberg E. Single-cell analysis of human adipose tissue identifies depot and disease specific cell types. Nat Metab. 2020;2:97-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 287] [Article Influence: 57.4] [Reference Citation Analysis (0)] |
| 41. | Lumeng CN, Deyoung SM, Bodzin JL, Saltiel AR. Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes. 2007;56:16-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 765] [Cited by in RCA: 775] [Article Influence: 43.1] [Reference Citation Analysis (0)] |
| 42. | Moussa K, Gurung P, Adams-Huet B, Devaraj S, Jialal I. Increased eosinophils in adipose tissue of metabolic syndrome. J Diabetes Complications. 2019;33:535-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 43. | Feng Z, Zhu L, Wu J. RAGE signalling in obesity and diabetes: focus on the adipose tissue macrophage. Adipocyte. 2020;9:563-566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 44. | Zheng C, Yang Q, Cao J, Xie N, Liu K, Shou P, Qian F, Wang Y, Shi Y. Local proliferation initiates macrophage accumulation in adipose tissue during obesity. Cell Death Dis. 2016;7:e2167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 126] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 45. | Li P, Oh DY, Bandyopadhyay G, Lagakos WS, Talukdar S, Osborn O, Johnson A, Chung H, Maris M, Ofrecio JM, Taguchi S, Lu M, Olefsky JM. LTB4 promotes insulin resistance in obese mice by acting on macrophages, hepatocytes and myocytes. Nat Med. 2015;21:239-247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 248] [Cited by in RCA: 254] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 46. | Amano SU, Cohen JL, Vangala P, Tencerova M, Nicoloro SM, Yawe JC, Shen Y, Czech MP, Aouadi M. Local proliferation of macrophages contributes to obesity-associated adipose tissue inflammation. Cell Metab. 2014;19:162-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 425] [Cited by in RCA: 484] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 47. | Li B, Leung JCK, Chan LYY, Yiu WH, Tang SCW. A global perspective on the crosstalk between saturated fatty acids and Toll-like receptor 4 in the etiology of inflammation and insulin resistance. Prog Lipid Res. 2020;77:101020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 89] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 48. | Shan B, Shao M, Zhang Q, Hepler C, Paschoal VA, Barnes SD, Vishvanath L, An YA, Jia L, Malladi VS, Strand DW, Gupta OT, Elmquist JK, Oh D, Gupta RK. Perivascular mesenchymal cells control adipose-tissue macrophage accrual in obesity. Nat Metab. 2020;2:1332-1349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 49. | Cho YK, Son Y, Kim SN, Song HD, Kim M, Park JH, Jung YS, Ahn SY, Saha A, Granneman JG, Lee YH. MicroRNA-10a-5p regulates macrophage polarization and promotes therapeutic adipose tissue remodeling. Mol Metab. 2019;29:86-98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 50. | Miranda K, Yang X, Bam M, Murphy EA, Nagarkatti PS, Nagarkatti M. MicroRNA-30 modulates metabolic inflammation by regulating Notch signaling in adipose tissue macrophages. Int J Obes (Lond). 2018;42:1140-1150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 81] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 51. | Zhu D, Johnson TK, Wang Y, Thomas M, Huynh K, Yang Q, Bond VC, Chen YE, Liu D. Macrophage M2 polarization induced by exosomes from adipose-derived stem cells contributes to the exosomal proangiogenic effect on mouse ischemic hindlimb. Stem Cell Res Ther. 2020;11:162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 92] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 52. | Pan Y, Hui X, Hoo RLC, Ye D, Chan CYC, Feng T, Wang Y, Lam KSL, Xu A. Adipocyte-secreted exosomal microRNA-34a inhibits M2 macrophage polarization to promote obesity-induced adipose inflammation. J Clin Invest. 2019;129:834-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 340] [Article Influence: 56.7] [Reference Citation Analysis (0)] |
| 53. | Tam TH, Chan KL, Boroumand P, Liu Z, Brozinick JT, Bui HH, Roth K, Wakefield CB, Penuela S, Bilan PJ, Klip A. Nucleotides released from palmitate-activated murine macrophages attract neutrophils. J Biol Chem. 2020;295:4902-4911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 54. | Jiang E, Perrard XD, Yang D, Khan IM, Perrard JL, Smith CW, Ballantyne CM, Wu H. Essential role of CD11a in CD8+ T-cell accumulation and activation in adipose tissue. Arterioscler Thromb Vasc Biol. 2014;34:34-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 55. | Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, Otsu M, Hara K, Ueki K, Sugiura S, Yoshimura K, Kadowaki T, Nagai R. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15:914-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1571] [Cited by in RCA: 1717] [Article Influence: 107.3] [Reference Citation Analysis (0)] |
| 56. | Zhao Y, Lin L, Li J, Xiao Z, Chen B, Wan L, Li M, Wu X, Hin Cho C, Shen J. CD4(+) T cells in obesity and obesity-associated diseases. Cell Immunol. 2018;332:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 57. | Zeng C, Shi X, Zhang B, Liu H, Zhang L, Ding W, Zhao Y. The imbalance of Th17/Th1/Tregs in patients with type 2 diabetes: relationship with metabolic factors and complications. J Mol Med (Berl). 2012;90:175-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 195] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 58. | Duffaut C, Zakaroff-Girard A, Bourlier V, Decaunes P, Maumus M, Chiotasso P, Sengenès C, Lafontan M, Galitzky J, Bouloumié A. Interplay between human adipocytes and T lymphocytes in obesity: CCL20 as an adipochemokine and T lymphocytes as lipogenic modulators. Arterioscler Thromb Vasc Biol. 2009;29:1608-1614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 180] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 59. | Touch S, Clément K, André S. T Cell Populations and Functions Are Altered in Human Obesity and Type 2 Diabetes. Curr Diab Rep. 2017;17:81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 60. | Guo H, Xu BC, Yang XG, Peng D, Wang Y, Liu XB, Cui CR, Jiang YF. A High Frequency of Peripheral Blood IL-22(+) CD4(+) T Cells in Patients With New Onset Type 2 Diabetes Mellitus. J Clin Lab Anal. 2016;30:95-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 61. | Li Y, Lu Y, Lin SH, Li N, Han Y, Huang Q, Zhao Y, Xie F, Guo Y, Deng B, Tsun A, Du J, Li D, Sun J, Shi G, Zheng F, Su X, Duan S, Zheng SG, Wang G, Tong X, Li B. Insulin signaling establishes a developmental trajectory of adipose regulatory T cells. Nat Immunol. 2021;22:1175-1185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 61] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 62. | Ivanov S, Merlin J, Lee MKS, Murphy AJ, Guinamard RR. Biology and function of adipose tissue macrophages, dendritic cells and B cells. Atherosclerosis. 2018;271:102-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 63. | Nishimura S, Manabe I, Takaki S, Nagasaki M, Otsu M, Yamashita H, Sugita J, Yoshimura K, Eto K, Komuro I, Kadowaki T, Nagai R. Adipose Natural Regulatory B Cells Negatively Control Adipose Tissue Inflammation. Cell Metab. 2013;18:759-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 178] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 64. | García-Hernández MH, Rodríguez-Varela E, García-Jacobo RE, Hernández-De la Torre M, Uresti-Rivera EE, González-Amaro R, Portales-Pérez DP. Frequency of regulatory B cells in adipose tissue and peripheral blood from individuals with overweight, obesity and normal-weight. Obes Res Clin Pract. 2018;12:513-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 65. | Winer DA, Winer S, Shen L, Wadia PP, Yantha J, Paltser G, Tsui H, Wu P, Davidson MG, Alonso MN, Leong HX, Glassford A, Caimol M, Kenkel JA, Tedder TF, McLaughlin T, Miklos DB, Dosch HM, Engleman EG. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat Med. 2011;17:610-617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 848] [Cited by in RCA: 795] [Article Influence: 56.8] [Reference Citation Analysis (0)] |
| 66. | DeFuria J, Belkina AC, Jagannathan-Bogdan M, Snyder-Cappione J, Carr JD, Nersesova YR, Markham D, Strissel KJ, Watkins AA, Zhu M, Allen J, Bouchard J, Toraldo G, Jasuja R, Obin MS, McDonnell ME, Apovian C, Denis GV, Nikolajczyk BS. B cells promote inflammation in obesity and type 2 diabetes through regulation of T-cell function and an inflammatory cytokine profile. Proc Natl Acad Sci U S A. 2013;110:5133-5138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 385] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 67. | Camell CD, Günther P, Lee A, Goldberg EL, Spadaro O, Youm YH, Bartke A, Hubbard GB, Ikeno Y, Ruddle NH, Schultze J, Dixit VD. Aging Induces an Nlrp3 Inflammasome-Dependent Expansion of Adipose B Cells That Impairs Metabolic Homeostasis. Cell Metab. 2019;30:1024-1039.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 155] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 68. | Hägglöf T, Vanz C, Kumagai A, Dudley E, Ortega V, Siller M, Parthasarathy R, Keegan J, Koenigs A, Shute T, Leadbetter EA. T-bet(+) B cells accumulate in adipose tissue and exacerbate metabolic disorder during obesity. Cell Metab. 2022;34:1121-1136.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 48] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 69. | Frasca D, Diaz A, Romero M, Blomberg BB. Phenotypic and Functional Characterization of Double Negative B Cells in the Blood of Individuals With Obesity. Front Immunol. 2021;12:616650. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 70. | Spallanzani RG, Zemmour D, Xiao T, Jayewickreme T, Li C, Bryce PJ, Benoist C, Mathis D. Distinct immunocyte-promoting and adipocyte-generating stromal components coordinate adipose tissue immune and metabolic tenors. Sci Immunol. 2019;4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 173] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 71. | Li C, Spallanzani RG, Mathis D. Visceral adipose tissue Tregs and the cells that nurture them. Immunol Rev. 2020;295:114-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 72. | Han JM, Wu D, Denroche HC, Yao Y, Verchere CB, Levings MK. IL-33 Reverses an Obesity-Induced Deficit in Visceral Adipose Tissue ST2+ T Regulatory Cells and Ameliorates Adipose Tissue Inflammation and Insulin Resistance. J Immunol. 2015;194:4777-4783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 145] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 73. | Jimenez MT, Michieletto MF, Henao-Mejia J. A new perspective on mesenchymal-immune interactions in adipose tissue. Trends Immunol. 2021;42:375-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 74. | Ding X, Luo Y, Zhang X, Zheng H, Yang X, Liu M. IL-33-driven ILC2/eosinophil axis in fat is induced by sympathetic tone and suppressed by obesity. J Endocrinol. 2016;231:35-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 75. | Pinnick KE, Nicholson G, Manolopoulos KN, McQuaid SE, Valet P, Frayn KN, Denton N, Min JL, Zondervan KT, Fleckner J; MolPAGE Consortium, McCarthy MI, Holmes CC, Karpe F. Distinct developmental profile of lower-body adipose tissue defines resistance against obesity-associated metabolic complications. Diabetes. 2014;63:3785-3797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 141] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 76. | Koh HE, van Vliet S, Pietka TA, Meyer GA, Razani B, Laforest R, Gropler RJ, Mittendorfer B. Subcutaneous Adipose Tissue Metabolic Function and Insulin Sensitivity in People With Obesity. Diabetes. 2021;70:2225-2236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 77. | Karpe F, Pinnick KE. Biology of upper-body and lower-body adipose tissue--link to whole-body phenotypes. Nat Rev Endocrinol. 2015;11:90-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 361] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 78. | Klöting N, Fasshauer M, Dietrich A, Kovacs P, Schön MR, Kern M, Stumvoll M, Blüher M. Insulin-sensitive obesity. Am J Physiol Endocrinol Metab. 2010;299:E506-E515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 569] [Cited by in RCA: 637] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 79. | Blüher M. Metabolically Healthy Obesity. Endocr Rev. 2020;41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 570] [Article Influence: 114.0] [Reference Citation Analysis (0)] |
| 80. | Iacobini C, Pugliese G, Blasetti Fantauzzi C, Federici M, Menini S. Metabolically healthy versus metabolically unhealthy obesity. Metabolism. 2019;92:51-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 281] [Article Influence: 46.8] [Reference Citation Analysis (0)] |
| 81. | Stefan N, Häring HU, Hu FB, Schulze MB. Metabolically healthy obesity: epidemiology, mechanisms, and clinical implications. Lancet Diabetes Endocrinol. 2013;1:152-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 485] [Cited by in RCA: 611] [Article Influence: 50.9] [Reference Citation Analysis (0)] |
| 82. | Phillips CM, Perry IJ. Does inflammation determine metabolic health status in obese and nonobese adults? J Clin Endocrinol Metab. 2013;98:E1610-E1619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 212] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 83. | Esser N, L'homme L, De Roover A, Kohnen L, Scheen AJ, Moutschen M, Piette J, Legrand-Poels S, Paquot N. Obesity phenotype is related to NLRP3 inflammasome activity and immunological profile of visceral adipose tissue. Diabetologia. 2013;56:2487-2497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 185] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 84. | Bigornia SJ, Farb MG, Mott MM, Hess DT, Carmine B, Fiscale A, Joseph L, Apovian CM, Gokce N. Relation of depot-specific adipose inflammation to insulin resistance in human obesity. Nutr Diabetes. 2012;2:e30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 85. | Pandolfi JB, Ferraro AA, Sananez I, Gancedo MC, Baz P, Billordo LA, Fainboim L, Arruvito L. ATP-Induced Inflammation Drives Tissue-Resident Th17 Cells in Metabolically Unhealthy Obesity. J Immunol. 2016;196:3287-3296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 93] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 86. | McLaughlin T, Liu LF, Lamendola C, Shen L, Morton J, Rivas H, Winer D, Tolentino L, Choi O, Zhang H, Hui Yen Chng M, Engleman E. T-cell profile in adipose tissue is associated with insulin resistance and systemic inflammation in humans. Arterioscler Thromb Vasc Biol. 2014;34:2637-2643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 268] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 87. | Lwow F, Dunajska K, Milewicz A, Jedrzejuk D, Kik K, Szmigiero L. Effect of moderate-intensity exercise on oxidative stress indices in metabolically healthy obese and metabolically unhealthy obese phenotypes in postmenopausal women: a pilot study. Menopause. 2011;18:646-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 88. | Cӑtoi AF, Pârvu AE, Andreicuț AD, Mironiuc A, Crӑciun A, Cӑtoi C, Pop ID. Metabolically Healthy versus Unhealthy Morbidly Obese: Chronic Inflammation, Nitro-Oxidative Stress, and Insulin Resistance. Nutrients. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 89. | Baeck C, Wehr A, Karlmark KR, Heymann F, Vucur M, Gassler N, Huss S, Klussmann S, Eulberg D, Luedde T, Trautwein C, Tacke F. Pharmacological inhibition of the chemokine CCL2 (MCP-1) diminishes liver macrophage infiltration and steatohepatitis in chronic hepatic injury. Gut. 2012;61:416-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 466] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 90. | Seidman JS, Troutman TD, Sakai M, Gola A, Spann NJ, Bennett H, Bruni CM, Ouyang Z, Li RZ, Sun X, Vu BT, Pasillas MP, Ego KM, Gosselin D, Link VM, Chong LW, Evans RM, Thompson BM, McDonald JG, Hosseini M, Witztum JL, Germain RN, Glass CK. Niche-Specific Reprogramming of Epigenetic Landscapes Drives Myeloid Cell Diversity in Nonalcoholic Steatohepatitis. Immunity. 2020;52:1057-1074.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 303] [Article Influence: 60.6] [Reference Citation Analysis (0)] |
| 91. | Scott CL, Guilliams M. The role of Kupffer cells in hepatic iron and lipid metabolism. J Hepatol. 2018;69:1197-1199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 66] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 92. | Lee KJ, Kim MY, Han YH. Roles of heterogenous hepatic macrophages in the progression of liver diseases. BMB Rep. 2022;55:166-174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 93. | Barreby E, Chen P, Aouadi M. Macrophage functional diversity in NAFLD - more than inflammation. Nat Rev Endocrinol. 2022;18:461-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 146] [Article Influence: 48.7] [Reference Citation Analysis (0)] |
| 94. | Morinaga H, Mayoral R, Heinrichsdorff J, Osborn O, Franck N, Hah N, Walenta E, Bandyopadhyay G, Pessentheiner AR, Chi TJ, Chung H, Bogner-Strauss JG, Evans RM, Olefsky JM, Oh DY. Characterization of distinct subpopulations of hepatic macrophages in HFD/obese mice. Diabetes. 2015;64:1120-1130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 136] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 95. | Gadd VL, Skoien R, Powell EE, Fagan KJ, Winterford C, Horsfall L, Irvine K, Clouston AD. The portal inflammatory infiltrate and ductular reaction in human nonalcoholic fatty liver disease. Hepatology. 2014;59:1393-1405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 343] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 96. | Rosso C, Kazankov K, Younes R, Esmaili S, Marietti M, Sacco M, Carli F, Gaggini M, Salomone F, Møller HJ, Abate ML, Vilstrup H, Gastaldelli A, George J, Grønbæk H, Bugianesi E. Crosstalk between adipose tissue insulin resistance and liver macrophages in non-alcoholic fatty liver disease. J Hepatol. 2019;71:1012-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 143] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 97. | Li Z, Lin H, Yang S, Diehl AM. Murine leptin deficiency alters Kupffer cell production of cytokines that regulate the innate immune system. Gastroenterology. 2002;123:1304-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 79] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 98. | Fukushima J, Kamada Y, Matsumoto H, Yoshida Y, Ezaki H, Takemura T, Saji Y, Igura T, Tsutsui S, Kihara S, Funahashi T, Shimomura I, Tamura S, Kiso S, Hayashi N. Adiponectin prevents progression of steatohepatitis in mice by regulating oxidative stress and Kupffer cell phenotype polarization. Hepatol Res. 2009;39:724-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 79] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 99. | Liang W, Lindeman JH, Menke AL, Koonen DP, Morrison M, Havekes LM, van den Hoek AM, Kleemann R. Metabolically induced liver inflammation leads to NASH and differs from LPS- or IL-1β-induced chronic inflammation. Lab Invest. 2014;94:491-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 100. | Marra F, Svegliati-Baroni G. Lipotoxicity and the gut-liver axis in NASH pathogenesis. J Hepatol. 2018;68:280-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 411] [Cited by in RCA: 607] [Article Influence: 86.7] [Reference Citation Analysis (0)] |
| 101. | Luo Z, Ji Y, Gao H, Gomes Dos Reis FC, Bandyopadhyay G, Jin Z, Ly C, Chang YJ, Zhang D, Kumar D, Ying W. CRIg(+) Macrophages Prevent Gut Microbial DNA-Containing Extracellular Vesicle-Induced Tissue Inflammation and Insulin Resistance. Gastroenterology. 2021;160:863-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 73] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 102. | Luo Z, Ji Y, Zhang D, Gao H, Jin Z, Yang M, Ying W. Microbial DNA enrichment promotes liver steatosis and fibrosis in the course of non-alcoholic steatohepatitis. Acta Physiol (Oxf). 2022;235:e13827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 103. | MacParland SA, Liu JC, Ma XZ, Innes BT, Bartczak AM, Gage BK, Manuel J, Khuu N, Echeverri J, Linares I, Gupta R, Cheng ML, Liu LY, Camat D, Chung SW, Seliga RK, Shao Z, Lee E, Ogawa S, Ogawa M, Wilson MD, Fish JE, Selzner M, Ghanekar A, Grant D, Greig P, Sapisochin G, Selzner N, Winegarden N, Adeyi O, Keller G, Bader GD, McGilvray ID. Single cell RNA sequencing of human liver reveals distinct intrahepatic macrophage populations. Nat Commun. 2018;9:4383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 592] [Cited by in RCA: 1012] [Article Influence: 144.6] [Reference Citation Analysis (0)] |
| 104. | McGettigan B, McMahan R, Orlicky D, Burchill M, Danhorn T, Francis P, Cheng LL, Golden-Mason L, Jakubzick CV, Rosen HR. Dietary Lipids Differentially Shape Nonalcoholic Steatohepatitis Progression and the Transcriptome of Kupffer Cells and Infiltrating Macrophages. Hepatology. 2019;70:67-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 89] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 105. | Xiong X, Kuang H, Ansari S, Liu T, Gong J, Wang S, Zhao XY, Ji Y, Li C, Guo L, Zhou L, Chen Z, Leon-Mimila P, Chung MT, Kurabayashi K, Opp J, Campos-Pérez F, Villamil-Ramírez H, Canizales-Quinteros S, Lyons R, Lumeng CN, Zhou B, Qi L, Huertas-Vazquez A, Lusis AJ, Xu XZS, Li S, Yu Y, Li JZ, Lin JD. Landscape of Intercellular Crosstalk in Healthy and NASH Liver Revealed by Single-Cell Secretome Gene Analysis. Mol Cell. 2019;75:644-660.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 589] [Cited by in RCA: 545] [Article Influence: 90.8] [Reference Citation Analysis (0)] |
| 106. | Guagnano MT, D'Ardes D, Ilaria R, Santilli F, Schiavone C, Bucci M, Cipollone F. Non-Alcoholic Fatty Liver Disease and Metabolic Syndrome in Women: Effects of Lifestyle Modifications. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 107. | Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5322] [Cited by in RCA: 7517] [Article Influence: 835.2] [Reference Citation Analysis (0)] |
| 108. | Portillo-Sanchez P, Bril F, Maximos M, Lomonaco R, Biernacki D, Orsak B, Subbarayan S, Webb A, Hecht J, Cusi K. High Prevalence of Nonalcoholic Fatty Liver Disease in Patients With Type 2 Diabetes Mellitus and Normal Plasma Aminotransferase Levels. J Clin Endocrinol Metab. 2015;100:2231-2238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 388] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 109. | Williamson RM, Price JF, Glancy S, Perry E, Nee LD, Hayes PC, Frier BM, Van Look LA, Johnston GI, Reynolds RM, Strachan MW; Edinburgh Type 2 Diabetes Study Investigators. Prevalence of and risk factors for hepatic steatosis and nonalcoholic Fatty liver disease in people with type 2 diabetes: the Edinburgh Type 2 Diabetes Study. Diabetes Care. 2011;34:1139-1144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 312] [Cited by in RCA: 294] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 110. | Strey CBM, de Carli LA, Fantinelli M, Gobbato SS, Bassols GF, Losekann A, Coral GP. Impact of Diabetes Mellitus and Insulin on Nonalcoholic Fatty Liver Disease in the Morbidly Obese. Ann Hepatol. 2018;17:585-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 111. | Lomonaco R, Bril F, Portillo-Sanchez P, Ortiz-Lopez C, Orsak B, Biernacki D, Lo M, Suman A, Weber MH, Cusi K. Metabolic Impact of Nonalcoholic Steatohepatitis in Obese Patients With Type 2 Diabetes. Diabetes Care. 2016;39:632-638. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 114] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 112. | Thibaut R, Gage MC, Pineda-Torra I, Chabrier G, Venteclef N, Alzaid F. Liver macrophages and inflammation in physiology and physiopathology of non-alcoholic fatty liver disease. FEBS J. 2022;289:3024-3057. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 55] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 113. | Kisseleva T, Brenner D. Molecular and cellular mechanisms of liver fibrosis and its regression. Nat Rev Gastroenterol Hepatol. 2021;18:151-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 1150] [Article Influence: 287.5] [Reference Citation Analysis (0)] |
| 114. | Kim SY, Jeong JM, Kim SJ, Seo W, Kim MH, Choi WM, Yoo W, Lee JH, Shim YR, Yi HS, Lee YS, Eun HS, Lee BS, Chun K, Kang SJ, Kim SC, Gao B, Kunos G, Kim HM, Jeong WI. Pro-inflammatory hepatic macrophages generate ROS through NADPH oxidase 2 via endocytosis of monomeric TLR4-MD2 complex. Nat Commun. 2017;8:2247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 188] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 115. | Miura K, Yang L, van Rooijen N, Brenner DA, Ohnishi H, Seki E. Toll-like receptor 2 and palmitic acid cooperatively contribute to the development of nonalcoholic steatohepatitis through inflammasome activation in mice. Hepatology. 2013;57:577-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 230] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 116. | Hu J, Wang H, Li X, Liu Y, Mi Y, Kong H, Xi D, Yan W, Luo X, Ning Q, Wang X. Fibrinogen-like protein 2 aggravates nonalcoholic steatohepatitis via interaction with TLR4, eliciting inflammation in macrophages and inducing hepatic lipid metabolism disorder. Theranostics. 2020;10:9702-9720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 73] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 117. | Mridha AR, Wree A, Robertson AAB, Yeh MM, Johnson CD, Van Rooyen DM, Haczeyni F, Teoh NC, Savard C, Ioannou GN, Masters SL, Schroder K, Cooper MA, Feldstein AE, Farrell GC. NLRP3 inflammasome blockade reduces liver inflammation and fibrosis in experimental NASH in mice. J Hepatol. 2017;66:1037-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 546] [Cited by in RCA: 823] [Article Influence: 102.9] [Reference Citation Analysis (0)] |
| 118. | Garcia-Martinez I, Santoro N, Chen Y, Hoque R, Ouyang X, Caprio S, Shlomchik MJ, Coffman RL, Candia A, Mehal WZ. Hepatocyte mitochondrial DNA drives nonalcoholic steatohepatitis by activation of TLR9. J Clin Invest. 2016;126:859-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 375] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 119. | Mridha AR, Haczeyni F, Yeh MM, Haigh WG, Ioannou GN, Barn V, Ajamieh H, Adams L, Hamdorf JM, Teoh NC, Farrell GC. TLR9 is up-regulated in human and murine NASH: pivotal role in inflammatory recruitment and cell survival. Clin Sci (Lond). 2017;131:2145-2159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 120. | Wang F, Stappenbeck F, Tang LY, Zhang YE, Hui ST, Lusis AJ, Parhami F. Oxy210, a Semi-Synthetic Oxysterol, Exerts Anti-Inflammatory Effects in Macrophages via Inhibition of Toll-like Receptor (TLR) 4 and TLR2 Signaling and Modulation of Macrophage Polarization. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 121. | van der Windt DJ, Sud V, Zhang H, Varley PR, Goswami J, Yazdani HO, Tohme S, Loughran P, O'Doherty RM, Minervini MI, Huang H, Simmons RL, Tsung A. Neutrophil extracellular traps promote inflammation and development of hepatocellular carcinoma in nonalcoholic steatohepatitis. Hepatology. 2018;68:1347-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 357] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 122. | Barrow F, Khan S, Fredrickson G, Wang H, Dietsche K, Parthiban P, Robert S, Kaiser T, Winer S, Herman A, Adeyi O, Mouzaki M, Khoruts A, Hogquist KA, Staley C, Winer DA, Revelo XS. Microbiota-Driven Activation of Intrahepatic B Cells Aggravates NASH Through Innate and Adaptive Signaling. Hepatology. 2021;74:704-722. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 133] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 123. | Bruzzì S, Sutti S, Giudici G, Burlone ME, Ramavath NN, Toscani A, Bozzola C, Schneider P, Morello E, Parola M, Pirisi M, Albano E. B2-Lymphocyte responses to oxidative stress-derived antigens contribute to the evolution of nonalcoholic fatty liver disease (NAFLD). Free Radic Biol Med. 2018;124:249-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 92] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 124. | Sutti S, Albano E. Adaptive immunity: an emerging player in the progression of NAFLD. Nat Rev Gastroenterol Hepatol. 2020;17:81-92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 282] [Cited by in RCA: 280] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 125. | Himoto T, Fujita K, Nomura T, Tani J, Morishita A, Yoneyama H, Haba R, Masaki T. Verification of B-lymphocyte activating factor's involvement in the exacerbation of insulin resistance as well as an autoimmune response in patients with nonalcoholic steatohepatitis and patients with HCV-related chronic liver disease. Diabetol Metab Syndr. 2017;9:45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 126. | Miyake T, Abe M, Tokumoto Y, Hirooka M, Furukawa S, Kumagi T, Hamada M, Kawasaki K, Tada F, Ueda T, Hiasa Y, Matsuura B, Onji M. B cell-activating factor is associated with the histological severity of nonalcoholic fatty liver disease. Hepatol Int. 2013;7:539-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 127. | Inzaugarat ME, Ferreyra Solari NE, Billordo LA, Abecasis R, Gadano AC, Cherñavsky AC. Altered phenotype and functionality of circulating immune cells characterize adult patients with nonalcoholic steatohepatitis. J Clin Immunol. 2011;31:1120-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 98] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 128. | Ghazarian M, Revelo XS, Nøhr MK, Luck H, Zeng K, Lei H, Tsai S, Schroer SA, Park YJ, Chng MHY, Shen L, D'Angelo JA, Horton P, Chapman WC, Brockmeier D, Woo M, Engleman EG, Adeyi O, Hirano N, Jin T, Gehring AJ, Winer S, Winer DA. Type I Interferon Responses Drive Intrahepatic T cells to Promote Metabolic Syndrome. Sci Immunol. 2017;2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 152] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 129. | Ying W, Lee YS, Dong Y, Seidman JS, Yang M, Isaac R, Seo JB, Yang BH, Wollam J, Riopel M, McNelis J, Glass CK, Olefsky JM, Fu W. Expansion of Islet-Resident Macrophages Leads to Inflammation Affecting β Cell Proliferation and Function in Obesity. Cell Metab. 2019;29:457-474.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 185] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 130. | Ehses JA, Perren A, Eppler E, Ribaux P, Pospisilik JA, Maor-Cahn R, Gueripel X, Ellingsgaard H, Schneider MK, Biollaz G, Fontana A, Reinecke M, Homo-Delarche F, Donath MY. Increased number of islet-associated macrophages in type 2 diabetes. Diabetes. 2007;56:2356-2370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 552] [Cited by in RCA: 585] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 131. | Cucak H, Grunnet LG, Rosendahl A. Accumulation of M1-like macrophages in type 2 diabetic islets is followed by a systemic shift in macrophage polarization. J Leukoc Biol. 2014;95:149-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 114] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 132. | Calderon B, Carrero JA, Ferris ST, Sojka DK, Moore L, Epelman S, Murphy KM, Yokoyama WM, Randolph GJ, Unanue ER. The pancreas anatomy conditions the origin and properties of resident macrophages. J Exp Med. 2015;212:1497-1512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 186] [Cited by in RCA: 229] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 133. | Yu S, Cheng Y, Zhang L, Yin Y, Xue J, Li B, Gong Z, Gao J, Mu Y. Treatment with adipose tissue-derived mesenchymal stem cells exerts anti-diabetic effects, improves long-term complications, and attenuates inflammation in type 2 diabetic rats. Stem Cell Res Ther. 2019;10:333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 100] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 134. | Kamata K, Mizukami H, Inaba W, Tsuboi K, Tateishi Y, Yoshida T, Yagihashi S. Islet amyloid with macrophage migration correlates with augmented β-cell deficits in type 2 diabetic patients. Amyloid. 2014;21:191-201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 135. | Westwell-Roper CY, Ehses JA, Verchere CB. Resident macrophages mediate islet amyloid polypeptide-induced islet IL-1β production and β-cell dysfunction. Diabetes. 2014;63:1698-1711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 182] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 136. | Masters SL, Dunne A, Subramanian SL, Hull RL, Tannahill GM, Sharp FA, Becker C, Franchi L, Yoshihara E, Chen Z, Mullooly N, Mielke LA, Harris J, Coll RC, Mills KH, Mok KH, Newsholme P, Nuñez G, Yodoi J, Kahn SE, Lavelle EC, O'Neill LA. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1β in type 2 diabetes. Nat Immunol. 2010;11:897-904. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1102] [Cited by in RCA: 1032] [Article Influence: 68.8] [Reference Citation Analysis (0)] |
| 137. | Westwell-Roper C, Denroche HC, Ehses JA, Verchere CB. Differential Activation of Innate Immune Pathways by Distinct Islet Amyloid Polypeptide (IAPP) Aggregates. J Biol Chem. 2016;291:8908-8917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 138. | Weitz JR, Makhmutova M, Almaça J, Stertmann J, Aamodt K, Brissova M, Speier S, Rodriguez-Diaz R, Caicedo A. Mouse pancreatic islet macrophages use locally released ATP to monitor beta cell activity. Diabetologia. 2018;61:182-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 72] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 139. | Zinselmeyer BH, Vomund AN, Saunders BT, Johnson MW, Carrero JA, Unanue ER. The resident macrophages in murine pancreatic islets are constantly probing their local environment, capturing beta cell granules and blood particles. Diabetologia. 2018;61:1374-1383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 140. | Ferris ST, Zakharov PN, Wan X, Calderon B, Artyomov MN, Unanue ER, Carrero JA. The islet-resident macrophage is in an inflammatory state and senses microbial products in blood. J Exp Med. 2017;214:2369-2385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 86] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 141. | de Souza CO, Paschoal VA, Sun X, Vishvanath L, Zhang Q, Shao M, Onodera T, Chen S, Joffin N, Bueno LM, Gupta RK, Oh DY. GPR92 activation in islet macrophages controls β cell function in a diet-induced obesity model. J Clin Invest. 2022;132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 142. | Gao H, Luo Z, Ji Y, Tang K, Jin Z, Ly C, Sears DD, Mahata S, Ying W. Accumulation of microbial DNAs promotes to islet inflammation and β cell abnormalities in obesity in mice. Nat Commun. 2022;13:565. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 57] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 143. | Böni-Schnetzler M, Thorne J, Parnaud G, Marselli L, Ehses JA, Kerr-Conte J, Pattou F, Halban PA, Weir GC, Donath MY. Increased interleukin (IL)-1beta messenger ribonucleic acid expression in beta -cells of individuals with type 2 diabetes and regulation of IL-1beta in human islets by glucose and autostimulation. J Clin Endocrinol Metab. 2008;93:4065-4074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 255] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 144. | Maedler K, Sergeev P, Ris F, Oberholzer J, Joller-Jemelka HI, Spinas GA, Kaiser N, Halban PA, Donath MY. Glucose-induced beta cell production of IL-1beta contributes to glucotoxicity in human pancreatic islets. J Clin Invest. 2002;110:851-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 444] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 145. | Maedler K, Schumann DM, Sauter N, Ellingsgaard H, Bosco D, Baertschiger R, Iwakura Y, Oberholzer J, Wollheim CB, Gauthier BR, Donath MY. Low concentration of interleukin-1beta induces FLICE-inhibitory protein-mediated beta-cell proliferation in human pancreatic islets. Diabetes. 2006;55:2713-2722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 129] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 146. | Golden TN, Simmons RA. Immune dysfunction in developmental programming of type 2 diabetes mellitus. Nat Rev Endocrinol. 2021;17:235-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 147. | Zhao G, Dharmadhikari G, Maedler K, Meyer-Hermann M. Possible role of interleukin-1β in type 2 diabetes onset and implications for anti-inflammatory therapy strategies. PLoS Comput Biol. 2014;10:e1003798. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 148. | Ehses JA, Lacraz G, Giroix MH, Schmidlin F, Coulaud J, Kassis N, Irminger JC, Kergoat M, Portha B, Homo-Delarche F, Donath MY. IL-1 antagonism reduces hyperglycemia and tissue inflammation in the type 2 diabetic GK rat. Proc Natl Acad Sci U S A. 2009;106:13998-14003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 274] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 149. | Maedler K, Sergeev P, Ehses JA, Mathe Z, Bosco D, Berney T, Dayer JM, Reinecke M, Halban PA, Donath MY. Leptin modulates beta cell expression of IL-1 receptor antagonist and release of IL-1beta in human islets. Proc Natl Acad Sci U S A. 2004;101:8138-8143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 206] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 150. | Glas R, Sauter NS, Schulthess FT, Shu L, Oberholzer J, Maedler K. Purinergic P2X7 receptors regulate secretion of interleukin-1 receptor antagonist and beta cell function and survival. Diabetologia. 2009;52:1579-1588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 75] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 151. | Böni-Schnetzler M, Häuselmann SP, Dalmas E, Meier DT, Thienel C, Traub S, Schulze F, Steiger L, Dror E, Martin P, Herrera PL, Gabay C, Donath MY. β Cell-Specific Deletion of the IL-1 Receptor Antagonist Impairs β Cell Proliferation and Insulin Secretion. Cell Rep. 2018;22:1774-1786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 152. | Zha J, Chi XW, Yu XL, Liu XM, Liu DQ, Zhu J, Ji H, Liu RT. Interleukin-1β-Targeted Vaccine Improves Glucose Control and β-Cell Function in a Diabetic KK-Ay Mouse Model. PLoS One. 2016;11:e0154298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 153. | Zhang Y, Yu XL, Zha J, Mao LZ, Chai JQ, Liu RT. Therapeutic vaccine against IL-1β improved glucose control in a mouse model of type 2 diabetes. Life Sci. 2018;192:68-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 154. | Sun Y, Zhou S, Shi Y, Zhou Y, Zhang Y, Liu K, Zhu Y, Han X. Inhibition of miR-153, an IL-1β-responsive miRNA, prevents beta cell failure and inflammation-associated diabetes. Metabolism. 2020;111:154335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 155. | DeFronzo RA, Tripathy D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care. 2009;32 Suppl 2:S157-S163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1183] [Cited by in RCA: 1439] [Article Influence: 89.9] [Reference Citation Analysis (0)] |
| 156. | Sylow L, Tokarz VL, Richter EA, Klip A. The many actions of insulin in skeletal muscle, the paramount tissue determining glycemia. Cell Metab. 2021;33:758-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 180] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 157. | Wei Y, Chen K, Whaley-Connell AT, Stump CS, Ibdah JA, Sowers JR. Skeletal muscle insulin resistance: role of inflammatory cytokines and reactive oxygen species. Am J Physiol Regul Integr Comp Physiol. 2008;294:R673-R680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 171] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 158. | Fink LN, Costford SR, Lee YS, Jensen TE, Bilan PJ, Oberbach A, Blüher M, Olefsky JM, Sams A, Klip A. Pro-inflammatory macrophages increase in skeletal muscle of high fat-fed mice and correlate with metabolic risk markers in humans. Obesity (Silver Spring). 2014;22:747-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 145] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 159. | Boon MR, Bakker LE, Haks MC, Quinten E, Schaart G, Van Beek L, Wang Y, Van Schinkel L, Van Harmelen V, Meinders AE, Ottenhoff TH, Van Dijk KW, Guigas B, Jazet IM, Rensen PC. Short-term high-fat diet increases macrophage markers in skeletal muscle accompanied by impaired insulin signalling in healthy male subjects. Clin Sci (Lond). 2015;128:143-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 160. | Hong EG, Ko HJ, Cho YR, Kim HJ, Ma Z, Yu TY, Friedline RH, Kurt-Jones E, Finberg R, Fischer MA, Granger EL, Norbury CC, Hauschka SD, Philbrick WM, Lee CG, Elias JA, Kim JK. Interleukin-10 prevents diet-induced insulin resistance by attenuating macrophage and cytokine response in skeletal muscle. Diabetes. 2009;58:2525-2535. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 283] [Cited by in RCA: 300] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 161. | Khan IM, Perrard XY, Brunner G, Lui H, Sparks LM, Smith SR, Wang X, Shi ZZ, Lewis DE, Wu H, Ballantyne CM. Intermuscular and perimuscular fat expansion in obesity correlates with skeletal muscle T cell and macrophage infiltration and insulin resistance. Int J Obes (Lond). 2015;39:1607-1618. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 135] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 162. | Khan IM, Dai Perrard XY, Perrard JL, Mansoori A, Wayne Smith C, Wu H, Ballantyne CM. Attenuated adipose tissue and skeletal muscle inflammation in obese mice with combined CD4+ and CD8+ T cell deficiency. Atherosclerosis. 2014;233:419-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 75] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 163. | Varma V, Yao-Borengasser A, Rasouli N, Nolen GT, Phanavanh B, Starks T, Gurley C, Simpson P, McGehee RE Jr, Kern PA, Peterson CA. Muscle inflammatory response and insulin resistance: synergistic interaction between macrophages and fatty acids leads to impaired insulin action. Am J Physiol Endocrinol Metab. 2009;296:E1300-E1310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 174] [Cited by in RCA: 170] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 164. | Samokhvalov V, Bilan PJ, Schertzer JD, Antonescu CN, Klip A. Palmitate- and lipopolysaccharide-activated macrophages evoke contrasting insulin responses in muscle cells. Am J Physiol Endocrinol Metab. 2009;296:E37-E46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 165. | Pillon NJ, Arane K, Bilan PJ, Chiu TT, Klip A. Muscle cells challenged with saturated fatty acids mount an autonomous inflammatory response that activates macrophages. Cell Commun Signal. 2012;10:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 166. | Patsouris D, Cao JJ, Vial G, Bravard A, Lefai E, Durand A, Durand C, Chauvin MA, Laugerette F, Debard C, Michalski MC, Laville M, Vidal H, Rieusset J. Insulin resistance is associated with MCP1-mediated macrophage accumulation in skeletal muscle in mice and humans. PLoS One. 2014;9:e110653. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 167. | Sell H, Dietze-Schroeder D, Kaiser U, Eckel J. Monocyte chemotactic protein-1 is a potential player in the negative cross-talk between adipose tissue and skeletal muscle. Endocrinology. 2006;147:2458-2467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 166] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 168. | Saghizadeh M, Ong JM, Garvey WT, Henry RR, Kern PA. The expression of TNF alpha by human muscle. Relationship to insulin resistance. J Clin Invest. 1996;97:1111-1116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 495] [Cited by in RCA: 489] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 169. | Ciaraldi TP, Ryan AJ, Mudaliar SR, Henry RR. Altered Myokine Secretion Is an Intrinsic Property of Skeletal Muscle in Type 2 Diabetes. PLoS One. 2016;11:e0158209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 170. | Li N, Shi H, Guo Q, Gan Y, Zhang Y, Jia J, Zhang L, Zhou Y. Aerobic Exercise Prevents Chronic Inflammation and Insulin Resistance in Skeletal Muscle of High-Fat Diet Mice. Nutrients. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 171. | Al-Khalili L, Bouzakri K, Glund S, Lönnqvist F, Koistinen HA, Krook A. Signaling specificity of interleukin-6 action on glucose and lipid metabolism in skeletal muscle. Mol Endocrinol. 2006;20:3364-3375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 183] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 172. | Carey AL, Steinberg GR, Macaulay SL, Thomas WG, Holmes AG, Ramm G, Prelovsek O, Hohnen-Behrens C, Watt MJ, James DE, Kemp BE, Pedersen BK, Febbraio MA. Interleukin-6 increases insulin-stimulated glucose disposal in humans and glucose uptake and fatty acid oxidation in vitro via AMP-activated protein kinase. Diabetes. 2006;55:2688-2697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 576] [Cited by in RCA: 642] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 173. | Reyna SM, Ghosh S, Tantiwong P, Meka CS, Eagan P, Jenkinson CP, Cersosimo E, Defronzo RA, Coletta DK, Sriwijitkamol A, Musi N. Elevated toll-like receptor 4 expression and signaling in muscle from insulin-resistant subjects. Diabetes. 2008;57:2595-2602. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 279] [Cited by in RCA: 295] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 174. | Hussey SE, Liang H, Costford SR, Klip A, DeFronzo RA, Sanchez-Avila A, Ely B, Musi N. TAK-242, a small-molecule inhibitor of Toll-like receptor 4 signalling, unveils similarities and differences in lipopolysaccharide- and lipid-induced inflammation and insulin resistance in muscle cells. Biosci Rep. 2012;33:37-47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 175. | Radin MS, Sinha S, Bhatt BA, Dedousis N, O'Doherty RM. Inhibition or deletion of the lipopolysaccharide receptor Toll-like receptor-4 confers partial protection against lipid-induced insulin resistance in rodent skeletal muscle. Diabetologia. 2008;51:336-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 91] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 176. | Lang CH, Silvis C, Deshpande N, Nystrom G, Frost RA. Endotoxin stimulates in vivo expression of inflammatory cytokines tumor necrosis factor alpha, interleukin-1beta, -6, and high-mobility-group protein-1 in skeletal muscle. Shock. 2003;19:538-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 169] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 177. | Senn JJ. Toll-like receptor-2 is essential for the development of palmitate-induced insulin resistance in myotubes. J Biol Chem. 2006;281:26865-26875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 201] [Article Influence: 10.6] [Reference Citation Analysis (0)] |