Copyright
©The Author(s) 2023.
World J Diabetes. May 15, 2023; 14(5): 494-511
Published online May 15, 2023. doi: 10.4239/wjd.v14.i5.494
Published online May 15, 2023. doi: 10.4239/wjd.v14.i5.494
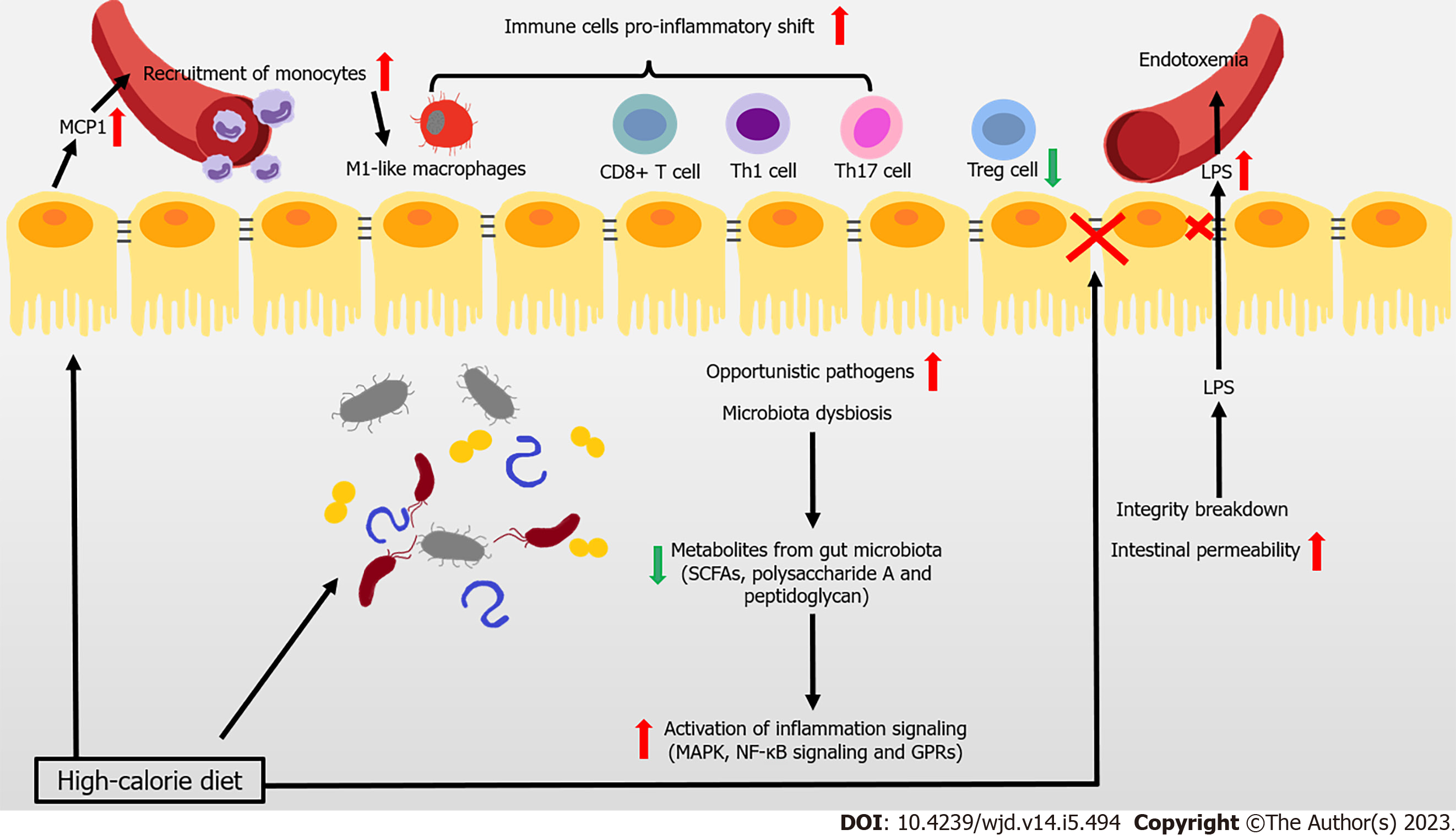
Figure 1 Immune attack and inflammation in the gut during obesity-related type 2 diabetes.
In the context of obesity and type 2 diabetes mellitus, overnutrition leads to the reduced gut microbiota, and even the increase of opportunistic pathogens. At the same time, the occurrence of decreased metabolites levels with anti-inflammatory effects, is accompanied by the activation of inflammation signaling. During obesity, imbalance of pro- and anti-inflammatory immune cells occurs in the gut. The intestinal epithelial cell-produced monocyte chemoattractant protein-1 (MCP1) recruits the circulating monocytes to the gut and they shift to the pro-inflammatory phenotype. High fat diet also induces a pro-inflammatory shift in T cells, accompanied with decreased regulatory T cells. Immunoglobulin A (IgA)-secreting immune cells and IgA secretion are both decreased. High-calorie diet and several recruited immune cells also impair intestinal barrier and increase intestinal epithelial and gut vascular permeability, leading to the leakage of microbiota-derived molecules (such as lipopolysaccharide [LPS]) into blood. High levels of LPS and other bacterial products cause endotoxemia and inflammation in multiple organs that further aggravate the metabolic diseases. GPR: G protein-coupled receptor; SCFAs: Short-chain fatty acids.
- Citation: Wang HW, Tang J, Sun L, Li Z, Deng M, Dai Z. Mechanism of immune attack in the progression of obesity-related type 2 diabetes. World J Diabetes 2023; 14(5): 494-511
- URL: https://www.wjgnet.com/1948-9358/full/v14/i5/494.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i5.494