Copyright
©The Author(s) 2023.
World J Diabetes. May 15, 2023; 14(5): 494-511
Published online May 15, 2023. doi: 10.4239/wjd.v14.i5.494
Published online May 15, 2023. doi: 10.4239/wjd.v14.i5.494
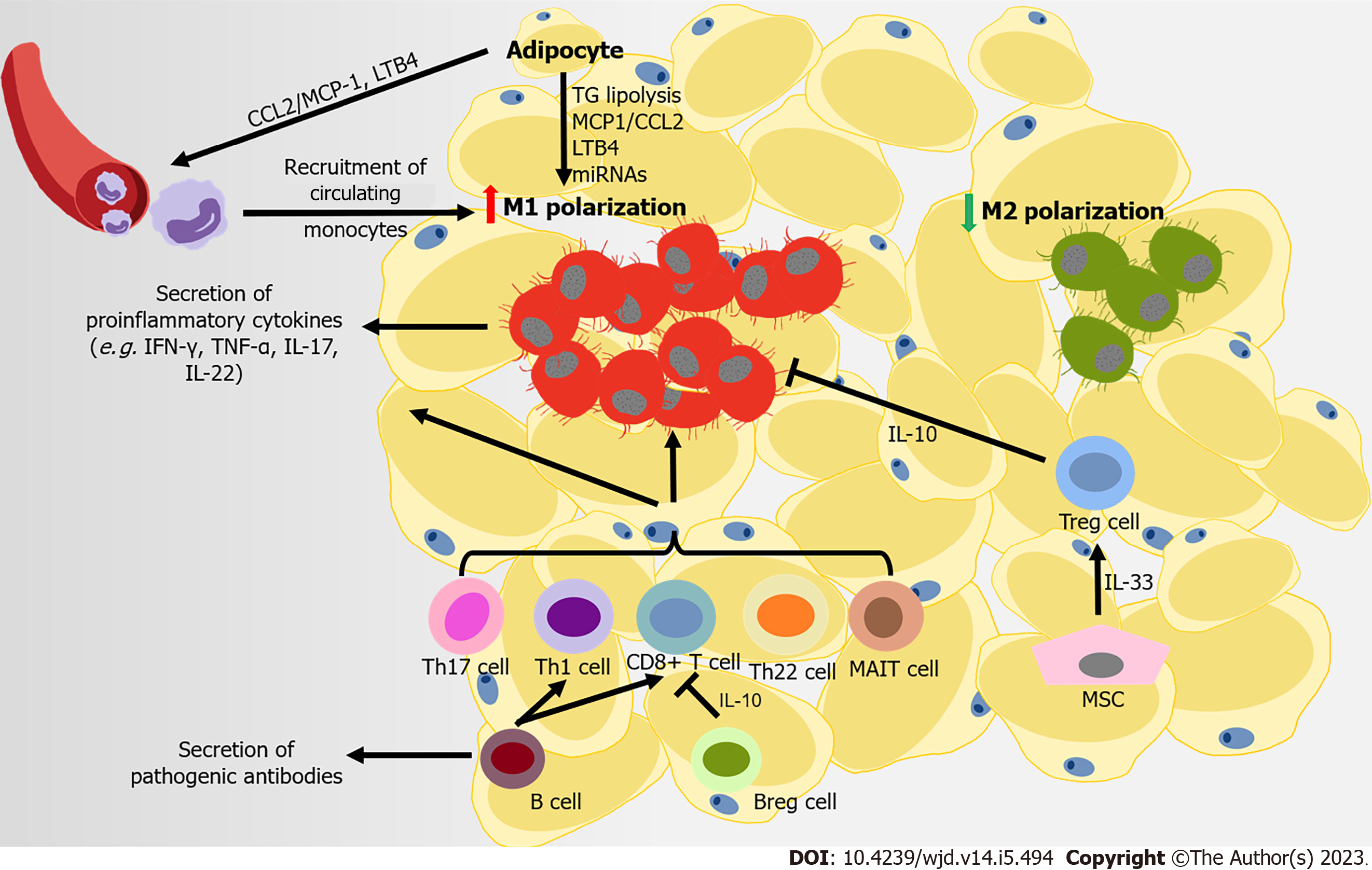
Figure 2 Immune attack and inflammation in the white adipose tissue during obesity-related type 2 diabetes.
At the later stage of obesity, recruited monocytes mainly contribute to the accumulation of macrophages in adipose tissue, following the secretion of monocyte chemoattractant protein-1 (MCP1) and leukotriene B4 (LTB4) by adipocytes to the microenvironment. Free fatty acids from the diet and in triglyceride (TG) lipolysis in adipocytes promote M1-like polarization. Several adipocyte-derived microRNAs also regulate the immune balance between M1- and M2-macrophage polarization. CD8+ T cells, pro-inflammatory CD4+ T cells (T helper type 1 [Th1], Th17, and Th22) and mucosal-associated invariant T cells are also recruited into adipose tissue, promoting M1-like polarization. Regulatory B cells (Bregs) and regulatory T cells (Tregs) can negatively control the local inflammation by secreting interleukin-10 (IL-10), but B cells contribute to systemic inflammation by activating CD8+ and Th1 cells, and releasing pathogenic antibodies. Some mesenchymal stromal cells in visceral adipose tissue can improves insulin resistance and inflammation in adipose tissues through expanding and sustaining the resident Treg population via IL-33 secretion.
- Citation: Wang HW, Tang J, Sun L, Li Z, Deng M, Dai Z. Mechanism of immune attack in the progression of obesity-related type 2 diabetes. World J Diabetes 2023; 14(5): 494-511
- URL: https://www.wjgnet.com/1948-9358/full/v14/i5/494.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i5.494