Published online Jan 15, 2023. doi: 10.4239/wjd.v14.i1.1
Peer-review started: August 24, 2022
First decision: October 21, 2022
Revised: November 4, 2022
Accepted: December 5, 2022
Article in press: December 5, 2022
Published online: January 15, 2023
Processing time: 138 Days and 23.3 Hours
Diabetic foot infections and diabetic foot ulcers (DFU) cause significant suffering and are often recurring. DFU have three important pathogenic factors, namely, microangiopathy causing local tissue anoxia, neuropathy making the foot prone to injuries from trivial trauma, and local tissue hyperglycaemia favouring infection and delaying the wound healing. DFU have been the leading cause for non-traumatic amputations of part or whole of the limb. Western medicines focus mainly on euglycaemia, antimicrobials, debridement and wound cover with grafts, and off-loading techniques. Advances in euglycaemic control, foot care and footwear, systemic antimicrobial therapy, and overall health care access and delivery, have resulted in an overall decrease in amputations. However, the process of wound care after adequate debridement remains a major cost burden globally, especially in developing nations. This process revolves around two basic concerns regarding control/eradication of local infection and promotion of faster healing in a chronic DFU without recurrence. Wound modulation with various dressings and techniques are often a costly affair. Some aspects of the topical therapy with modern/Western medicines are frequently not addressed. Cost of and compliance to these therapies are important as both the wounds and their treatment are “chronic.” Naturally occurring agents/medications from traditional medicine systems have been used frequently in different cultures and nations, though without adequate clinical base/relevance. Traditional Chinese medicine involves restoring yin-yang balance, regulating the ‘chi’, and promoting local blood circulation. Traditional medicines from India have been emphasizing on ‘naturally’ available products to control wound infection and promote all the aspects of wound healing. There is one more group of chemicals which are not pharmaceutical agents but can create acidic milieu in the wound to satisfy the above-mentioned basic concerns. Various natural and plant derived products (e.g., honey, aloe vera, oils, and calendula) and maggots are also used for wound healing purposes. We believe that patients with a chronic wound are so tired physically, emotionally, and financially that they usually accept native traditional medicine which has the same cultural base, belief, and faith. Many of these products have never been tested in accordance to “evidence-based medicine.” There are usually case reports and experience-based reports about these products. Recently, there have been some trials (in vitro and in vivo) to verify the claims of usage of traditional medicines in management of DFU. Such studies show that these natural products enhance the healing process by controlling infection, stimulating granulation tissue, antimicrobial action, promoting fibroblastic activity and collagen deposition, etc. In this review, we attempt to study and analyse the available literature on results of topical traditional medicines, which are usually advocated in the management of DFU. An integrated and ‘holistic’ approach of both modern and traditional medicine may be more acceptable to the patient, cost effective, and easy to administer and monitor. This may also nevertheless lead to further improvement in quality of life and decrease in the rates of amputations for DFU.
Core Tip: The chronicity, cost, and compliance issues complicate the management of diabetic foot ulcers. These patients prefer traditional medicines with the same cultural base and belief. This article focuses on the role of and results of comparative studies about usage of traditional medicines in managing diabetic ulcers. Topical formulations from Chinese and Ayurved systems, honey, plant products, which are commonly used and studied, will be discussed regarding their observed efficacy in wound healing.
- Citation: Rayate AS, Nagoba BS, Mumbre SS, Mavani HB, Gavkare AM, Deshpande AS. Current scenario of traditional medicines in management of diabetic foot ulcers: A review. World J Diabetes 2023; 14(1): 1-16
- URL: https://www.wjgnet.com/1948-9358/full/v14/i1/1.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i1.1
Projections for the increasing prevalence of diabetic patients and prediabetic individuals are alarming. Presently, patients from low- and middle-income countries comprise about 81% of the total prevalence. About 665.5 million people from these nations will be at risk for diabetes by 2045. Nations like China and India will continue to have the maximum number of adult diabetics. Almost a trillion US dollars are spent globally as annual direct expenditures for managing diabetes and its complications[1]. Peripheral vascular and neurological complications in diabetic patients account for more than a third of these expenditures[2]. The world has seen many improvements and advances concerning the diagnosis and management of diabetes. Control for euglycaemia is now an easily achievable goal. The management of diabetes-related complications is still a herculean task. The complex plethora of diabetic foot ulcers (DFU) is one of them. One-third of diabetic patients will eventually develop a foot ulcer sometime in their life[3]. The morbidity and eventual mortality due to DFU are just next to those of cancers[4].
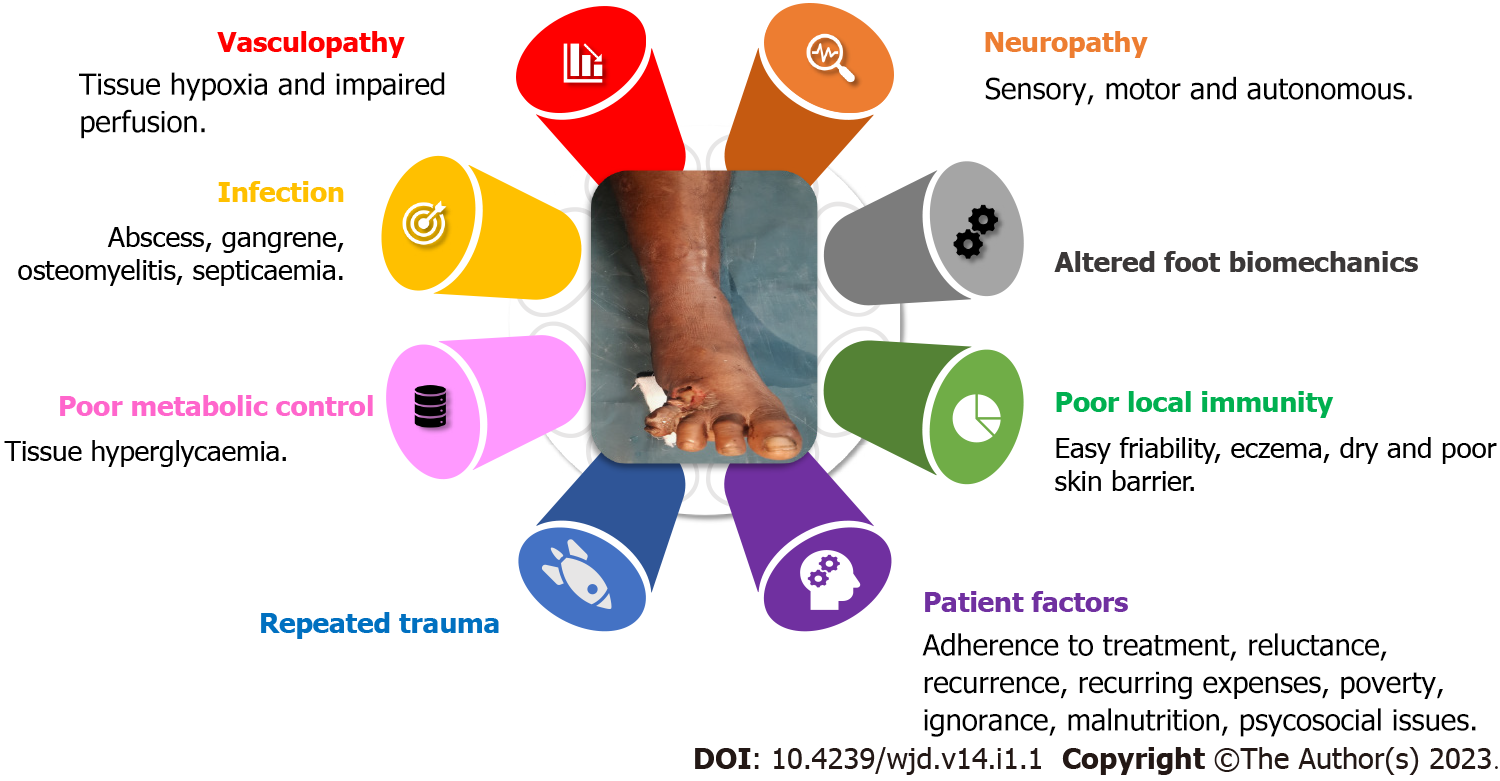
Multiple factors act collaboratively to cause the complex pathology of DFU (Figure 1), especially diabetic microangiopathy and neuropathy[1,5]. Diabetic microangiopathy leads to compromised tissue perfusion and makes the foot prone to poor local immunity, infection, and delayed healing. Diabetic neuropathy, especially sensory loss, makes the foot more prone to repeated trauma and poor foot care. Disturbed glycaemic metabolism makes the tissue prone to infection. Due to peripheral insulin resistance, type 2 diabetics are more likely to suffer from long-term complications of vasculopathy, neuropathy, and diabetic ulcers in the lower limbs[1,6]. Irrespective of age, gender, region, or culture, the pathophysiology of a DFU remains the same. Two or more risk factors, often peripheral neuropathy and frequently vasculopathy, are always present[6] (Figure 1).
DFU are a wound continuum starting from a very superficial ulcer and progressing to a deep-tissue infection and then osteomyelitis. The mere presence of an ulcer or bioburden does not qualify for the definition of DFU. The evidence of manifestations of an inflammatory process in any tissue below the malleoli in a diabetic person is a must. The presence of systemic manifestations of these inflammatory responses itself indicates severe infection[6,7]. Unfortunately, sometimes, despite all possible locoregional treatment, amputating the affected part becomes the sole option. But then this option has a lingering threat of non/delayed healing of the stump.
The wound, on its own, is responsible for producing key molecular regulators like growth factors, chemokines, and cytokines, which affect wound healing[8]. In the wound healing continuum, chronic wounds are stuck in the proliferative phase and are not progressing through the remodelling phase. Chronic wounds have low mitotic activity, highly inflammatory cells, and high protease and cytokine activity[8-10]. These wounds have senescent cells, irresponsive to the signals for clearing inflammation and epithelization[8,9]. The exudate itself is antiproliferative and hampers the extracellular matrix (ECM) production[10]. Undermined edges, friable granulation tissue, foul odour, or exudative floor also denote infection in chronic wounds and contribute to non-responsiveness[8]. Bed preparation in chronic wounds should focus on improving the molecular and cellular environment similar to that in acute healing wounds so that the natural healing can progress[8,11].
Biofilms are complex protective matrix composed of bacterial colonies and various extracellular polymeric substances, sugars, lipids, and glycocalyx, imparting eco-physical and immunological protective and adhesive abilities[12-15]. Biofilm allows the bioburden to flourish, which in turn allows the biofilm to further stabilise, adhere, and progress. Biofilm also contributes to persistence of chronic inflammation (abnormal neutrophilic infiltration and subsequent production of high levels of reactive oxygen species and proteases)[13,14]. It also facilitates horizontal transfer of antimicrobial resistance. Wound exudate also nourishes the biofilm[13]. Apart from the standard definition, biofilm is quandary in terms of detection and evaluation of clearance. Clinical assessment can confirm these issues. Physical debridement of biofilm helps to decrease bioburden and local chronic inflammation, converts the chronicity to a favourable active healing milieu, and improves action of antimicrobials. Available topical drugs like silver, cadexomer, polyhexamethylene biguanide, etc. have variable effects, hence drugs or phytochemicals with hygroscopic or surfactant action are under evaluation[12,13].
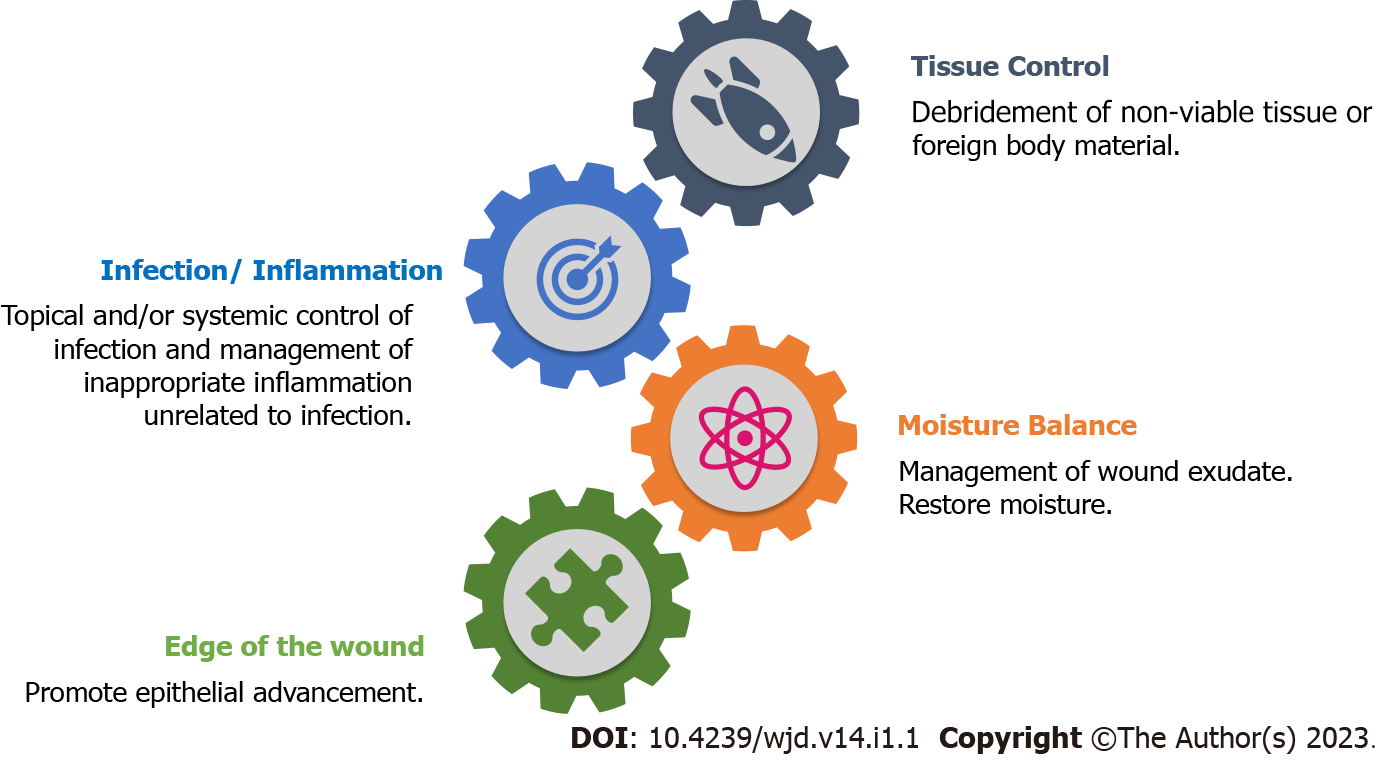
Systemic management is usually the control of hyperglycaemia and coexisting nephropathy and vasculopathy. Wound management in DFU is tailored according to the TIME (Tissue control, Inf-ection/inflammation, Moisture balance, Edge of the wound) concept with the prime aim to restore the balance of key molecular regulators. The TIME concept focuses on tissue control (wound debridement and cleaning), infection/inflammation complex (control of bioburden continuum), moisture-exudate balance, and epithelial advancement (Figure 2)[8,12]. There is a plethora of research on different aspects of DFU (microbiota, risks, care, management, burden, outcome, newer trials, etc.) with an overflowing armamentarium of existing and advanced wound-care products. Irrespective of the choice of dressing material used, its basic function will always be to control the bioburden. The efficient role of one over other dressing materials in wound healing is yet unproven[4,14].
DFU are chronic wounds, and so is their treatment. DFU management cannot be set in rigid protocols due to many issues (resistant strains, polymicrobial infection, appropriate tissue/pus sampling, antibiotic stewardship and misuse, unpredictable recurrence, etc.)[3,16-20]. The adverse effects of topical antimicrobials are concerning[21]. The exact duration of antibiotics for different severities of DFU infections is unknown[8,22]. The cost-factor and compliance issues are significant and interlinked problems when prescribing any DFU treatment[1,3,23-25].
Newer trends show the rising preference for multidisciplinary integration, including biomechanics, biotechnology, and natural material sciences. Sociocultural beliefs sometimes warrant for integrating traditional and Western medicinal options. Evidence defining the valid statistical efficacy of such treatments is lacking. We have observed that patients with DFU are so exhausted physically, emotionally, and financially that they usually accept native traditional medicine with the same cultural base, belief, and faith. Other reasons for medical pluralism are the impulse to try all possible options, fear of side effects of allopathic medicines, cost, recommendations by family members and peers, etc.[25]. We have experienced that people with DFU consider these options patient-friendly because of easy availability, accessibility, and affordability. Many times, care providers also prefer experience-based therapy over evidence-based.
In this narrative review, we attempt to analyse the available literature on topical traditional medicine usually advocated for managing DFU. We have preferred appropriately framed clinical trials and good-quality case series, specific to DFU. We have excluded the literature about personal experiences, multiple therapeutics, individual case reports, and data in non-English languages.
Traditional Chinese medicines (TCM) may be described as multicomponent, multitarget, and multi-effect. TCM perspective involves restoring yin-yang balance, regulating the ‘chi’, and promoting local blood circulation. TCM system suggests that treating DFU should focus on improving and regulating qi, nourishing the yin, resolving the dampness, improving the spleen, and activating stagnant blood[26,27]. TCMs are available and advocated as an oral decoction, foot bath, and topical applicants (ointment and solutions). We have restricted our literature search for topical applications, especially comparative studies.
Various herbs are utilised for producing active compounds or final drugs.
Effects in proliferative phase: A preliminary study on effect of Hongyou ointment and Shengji powder on Wnt signalling pathway proteins, observed that refractory wounds have characteristically abnormal β-catenin expression and over-expressed c-myc and K6. These were downregulated to normal levels after usage of the above formulation[28]. PA-F4 from the ON101 and WH-1 creams, significantly attenuates M1 macrophages by suppressing the NLRP3/interleukin (IL)-1β and IL-6 mediated inflammatory responses[29]. S1 extract of Centella asiatica stimulates fibroblast proliferation, collagen synthesis, and keratinocyte migration[30]. The Kangfuxin solution (KFS), from extracts of Periplaneta americana L., contains many active ingredients (peptides, polyols, and sticky sugar amino acid), which promote granulation tissue, neovascularisation, and neutrophilia, nourishes yin, and has myogenic effects, ultimately promoting rapid wound healing[31].
Antibacterial effects: Although there is no significant data on the actions of individual herbal products for DFU healing, in vitro experiments have shown that extracts from Phellodendri Cortex or Huangbai have an antimicrobial role against Pseudomonas, Staphylococcus aureus (MRSA), Pneumococcus, C. diphtheriae, Streptococcus, etc.[32-34].
Anti-inflammatory effects: Lianqiao has a detoxifying effect and reduces swelling. Jinyinhua soothes the skin itching such as eczema and alleviates swollen ulcers and cellulitis. Wugong also has detoxifying and anti-inflammatory effects[32]. Tangzu Yuyang ointment contains many phytochemical components (berberine, ferulic acid, ginsenoside, phellodendrine, obaculactone, etc.), which exert anti-inflammatory, antibacterial, antioxidant, and analgesic actions on the wound bed[35].
In animal models, Zizhu ointment resulted in rapid wound healing, and downregulated the expression of the Notch4 gene and its target genes and ligands and promoted the proliferation of M2 macrophages and other anti-inflammatory effects[36]. Components of Shenghong liquid (SH) have an anti-inflammatory effect and improve local circulation[37].
Commonly used topical ointment, cream, and solutions[28,32,35-39] for DFU are mentioned in Table 1. The available and accessible literature about human clinical trials[28,32,35,37,39-41] for TCM in DFU is scanty with a very small number of test subjects (Table 2). The therapy is well-tolerated and results are promising but need further high-volume studies.
| Name of formulation | Contents |
| Hongyou ointment[28] | Jiuyi Pellet (Gypsum Fibrosum: hydrargyrum oxydatum crudum), Dong Pellet (main ingredients: sminium), and Vaseline |
| Shengji powder[28] | Gypsum Fibrosuum, Resina Draconis, Resina Olibanum, Myrrh, and Borneolum syntheticum |
| Cortex Phellodendri Compound Fluid[32] | Huangbai (Phellodendron Chinese Schneid), Lianqiao (Forsythia suspensa), Jinyinhua (Lonicera japonica Thunb), Pugongying (Taraxacum mongolicum Hand. -Mazz), and Wugong (Scolopendra) |
| Tangzu Yuyang ointment[35] | Coptis chinensis Franch (Huanglian), Ligusticum chuanxiong Hort. – (Chuanxiong), Atractylodes lancea (Thund.) DC. (Cangzhu), Panax notoginseng (Burk.) F.H. Chen. (Sanqi), Angelica sinensis (Oliv.) Diels. (Danggui), Arnebia euchroma (Royle) Johnst. (Zicao), Phellodendron chinense Schneid. (Huangbo), Rheum officinale Baill. (Dahuang), Borneolum syntheticum (Bingpian), Daemonorops draco Bl. (Xuejie), Gypsum fibrosum praeparatum (Duanshigao), and Sesame oil and Beeswax served as bases |
| Zizhu ointment[36] | Cinnabar (Zhusha), Astragalus mongholicus (Huangqi), Arnebia guttata (Zicao), Donkey hide gelatin (Ejiao), Borneol (Bingpian), and Dragon’s Blood (Xuejie) |
| Shenghong liquid[37] | Radix rehmanniae, Carthamus tinctorius, Coptis chinensis, Rheum officinale, Radix lithospermi, Fructus gardenia, and licorice |
| WH1 and ON101 creams[38,39] | PA-F4 from an extract of Plectranthus amboinicus and S1 from an extract of Centella asiatica. |
| Panchvalka[104,109] | Stem bark of Ficus benghalensis, F. glomerata, F. religiosa, F. virens, and Thespesia populnea |
| Jatyadi tailam[105,109] | Chameli (Jasminum grandiflorum) Neem (Azadirachta indica) Patol (Trichosanthes Dioica), Karanj (Pongamia glabra), Yashtimadhu (Glycyrrhiza glabra), Haridra (Curcuma longa), Daruharidra (Berberis aristate), Kutki (Picrorhiza kurrooa), Manjistha (Rubia cordifolia), Padmakh (Prunus cerasoides), Lodhra (Symplocos racemose), Haritaki (Terminalia chebula), Nilofer (Nymphaea alba), Tutiya (Copper sulfate), Sariva (Hemidesmus indicus), Mom (Wax), Chandan Oil (Santalum album), Kumari oil, and Sesame oil |
| Ref. | Test group | Control group | Results |
| Li et al[28] | Hongyou ointment and Shengji powder (CM group) (n = 27) | WM (mupirocin ointment, growth factor, and vaseline gauze) (n = 26) | Overall effective rate (healed and completely effective) in CM group (22/27, 81.48%) was significantly higher than that in WM group (15/26, 57.69%, P = 0.04). The mean wound healing time was 22.71 ± 5.46 d in CM group vs 26.56 ± 7.56 d in WM group (t = 2.13, P = 0.04) |
| Liu et al[32] | CPCF | KFS | CPCF group: Initial wound area was 7.58 ± 2.13 cm2, improved to 3.83 ± 3.13 cm2 on 14th d, 2.39 ± 2.53 cm2 on 21st d, and 1.18 ± 2.49 cm2 on 28th d. The mean wound area of 7.73 ± 2.11 cm2 in KFS group had improved through 5.66 ± 2.58 cm2 on day 14, 4.42 ± 2.87 cm2 on day 21, and 2.78 ± 3.32 cm2 on 28th d (P < 0.05) |
| Huang et al[39] | ON101 cream (n = 118) | Sodium carboxymethyl cellulose absorbant dressing (n = 112) | At 16 wk, 74 patients (60.7%) of ON101 group and 40 (35.1%) of comparison group had achieved ulcer closure (OR = 2.84; 95%CI 1.66-4.84; P < 0.001). No difference between rates of 50% ulcer reduction at 16 wk (82.8% vs 86.0%) |
| Li et al[35] | TYO (n = 24) | SWT (n = 24) | Improved healing rate of only 4% (37.5% TYO group vs 33.3% SWT group). Significant improvement in TYO group at 12 wk (79.2% vs 41.7%; P = 0.017) and 24 wk (91.7% vs 50%; P = 0.003) |
| Xie et al[37] | SH (n = 30) | Recombinant human basic FGF gel ( n = 30) | Significant reduction in ulcer size by 4, 8, and 12 wk. Ulcer size was reduced from 15.90 ± 3.27 cm2 to 2.75 ± 1.08 cm2 in the SH group and from 15.72 ± 3.11 cm2 to 8.36 ± 2.07 cm2 in controls (P < 0.001) |
| Jiang et al[40] | Jingwanhong ointment | Sulphadiazine zinc ointment (n = 64) | Epithelization was complete by 46.5 ± 15.6 d in Jingwanhong group and 67.9 ± 17.9 d in sulfadiazine zinc group (P < 0.05) |
| Cao et al[41] | Unspecified TCM ointment (n = 20) | Topical ethacridine lactate | Better wound healing at 10, 20, and 30 d in TCM group |
Li et al[28] observed that Hongyou ointment and Shengji powder have a higher overall effective rate, shorter mean healing time, and no adverse reactions or long-term complications when compared to Western medicine.
The Cortex Phellodendri Compound Fluid (CPCF) was superior to the KFS in reducing ulcer area, and increasing growth factor content and total effective rate (P < 0.05). In these groups, serum VEGF (vascular endothelial growth factor), EGF (epidermal growth factor), and bFGF (basic fibroblast growth factor) levels increased significantly after treatment (higher in CPCF group)[32].
Kuo et al[38] studied the effects of WH-1 cream dressings (n = 11) against hydrocolloid dressings (n = 10) in Wagner grade-3 DFU. With no significant difference in the size reduction, improvement of Wagner grade was marginally higher in the WH-1 group but not statistically significant. Huang et al[39] used ON101 cream with same TCM composition as WH-1 cream. The healing rate was statistically significant in the ON101 group, without any significant adverse reactions.
Li et al[35] studied Tangzu Yuyang Ointment (TYO) as an adjuvant to standard wound therapy in Wagner grades 1-3 DFU but could not find any statistical difference in the improvement of ulcer size or Wagner grade nor the incidence of study-related adverse effects or ulcer recurrence. In another study, Wagner grades 1-3 DFU were ultrasonically debrided and the effect of Shenghong liquid (SH) vs recombinant human basic fibroblast growth factor was studied. The SH group had higher and faster rates of ulcer healing[37].
A multicentric study of 229 patients with non-severe ischaemic DFU, observed a statistically significant effective rate (80.54%) in the TCM group compared to the conventional treatment group (68.00%)[42]. Kangfuxin and other extracts from Periplaneta americana are under research for an integrated approach for manufacturing a composite biodegradable hydrogel dressing with promising results in promoting wound healing[43-45].
Apart from dextrose and levulose, honey contains many vitamins, trace minerals, prostaglandins, flavonoids (rutin, kaempferol, catechin, genistein, chrysin, etc.), and polyphenolic compounds (ferulic acid, coumaric acid, gallic acid, abscisic acid, vanillic acid, hydroxycinnamic acid, etc.)[46]. Phenolic acids mainly contribute to the antioxidative and anti-inflammatory actions through various signalling pathways[46].
Honey has antibacterial efficacy against many aerobes and anaerobes[47-51]. This action is attributed to acidic pH, hyperosmolarity, inhibins, antioxidants, H2O2, and various enzymes[46,52-54]. Different studies reported anti-Pseudomonal activity at minimum inhibitory concentrations (MIC) of 11%-40%[50-52,55,56]. Hygroscopic action due to high osmolarity improves lymphatic and blood circulation[57,58]. Hygroscopic action and facilitatory actions for protease activity allow for autolytic debridement[59]. Low concentrations of H2O2 in honey may promote fibroblastic proliferation and neoangiogenesis[60]. Improvements in epithelization, neoangiogenesis, and better collagen synthesis via various growth factors/chemokines (TNF-α, TGF-β, VGEF, IL-6, IL-12, etc.) and signalling pathways, all contribute to the ameliorated healing action of honey[46,61-63].
By using various types of honey in animal studies, many researchers have confirmed faster and higher wound epithelization and contraction, when compared to other topical applicants[64-68].
The various studies comparing honey against povidone[57,69,70] and other dressings[71-74] are summarized in Table 3. Hammouri et al[69] had noted significantly better mean healing time and shorter hospital stay in the honey group as against povidone and H2O2. Fourteen patients had allergic reactions to povidone, but honey dressings were well-tolerated. Eight refractory cases in the povidone group then used honey till healing[69].
| Ref. | Test group | Control group | Results |
| Shukrimi et al[57] | Honey | Povidone iodine | Mean time for "ready for surgical closure" 14.4 d in honey group vs 15.4 d in povidone group (P < 0.005). Less pain and faster improvement in oedema and foul exudation in honey group. No significant changes in bioburden isolation before and after therapy |
| Hammouri et al[69] | Honey and normal saline (n = 100) | Povidone and H2O2 (n = 100) | Mean healing time 21 (7-70) d, hospital stay 13 (7-42) d, and low treatment costs in honey group. In povidone group, the mean healing time was 32 (7-90) d with a mean hospital stay of 23 (7-56) d (P < 0.001) |
| Jan et al[70] | Honey (n = 50) | Povidone iodine (n = 50) | Faster wound healing at various intervals. Healing at end of 8-10 wk: All patients in honey group and 74% in povidone group (P < 0.0001). No difference in amputation rates |
| Imran et al[71] | Honey (n = 179) | Saline dressings (n = 169) | By 120 d, complete healing in 136 (75.97%) wounds in honey group vs 97 (57.39%) wounds in saline group (P < 0.001). Mean wound healing time: 18 (6-120) d in honey group vs 29 (7-120) d in saline group (P < 0.001) |
| Kamaratos et al[72] | Manuka honey (n = 32) | Saline dressings (n = 31) | No statistical difference in the total healed ulcers (97% in honey vs 90% in saline group). Mean healing time: 31 ± 4 d in honey group vs 43 ± 3 d in saline group (P < 0.05) |
| Al Saeed et al[73] | Honey (n = 32) | Tulle grass dressings (n = 27) | Faster wound healing in honey group than simple tulle grass dressings [(61.3% vs 11.5%; P < 0.05) at 6 wk and (87.1% vs 42.3%; P < 0.05) at 6 mo]. Hospital stay and incidence of amputation were also lower in honey group |
| Siavash et al[74] | 5% Royal jelly (bee product) | Placebo | No statistical difference regarding size reduction and complete healing (P > 0.5) |
In a pilot study, Abdelatif used honey in 60 cases of DFU (Wagner grades 1-5). Patients with Wagner grades 1-2 DFU showed a 100% response given the complete healing by 9 wk, whereas those with Wagner grade 3 DFU had a 92% response at the end of 9 wk. The patients with Wagner grades 4-5 DFU underwent surgical debridement followed by topical honey application and showed a 100% response without a need for amputation[75].
Moghazy et al[76] observed that honey dressings significantly reduce the exudation in the wound bed, ulcer dimensions, and surrounding inflammation. In that study, each wound had a different bacterial isolate, but after 2 mo, most wounds (28, 93.3%) had Staphylococcus epidermidis. Pseudomonas aeruginosa was isolated from only two ulcers. The authors noted significant improvement from a severe to favourable grade of the DFU[76]. Kamaratos et al studied the beneficial effects of manuka honey but did not mention the outcome about antibacterial effect against specific bacterium[72].
In an open-label randomized controlled trial (RCT) with three parallel groups (nanosilver, manuka honey, and conventional dressings), cumulative healing, ulcer reduction, and clinical wound infection after 12 wk were measured. Although the nanosilver group had the highest proportion of wound healing, the cumulative healing was not statistically significant (P = 0.26). Rapid ulcer reduction was present in the nanosilver group (97.45% vs 86.24% in the honey group vs 73.91% in the conventional group). Bioburden was significantly reduced in the nanosilver and honey groups. The authors observed that nanosilver and manuka honey were only 1.3 and 1.1 times, respectively, more effective than conventional paraffin tulle dressings[77]. Saeed observed similar results when using nanosilver vs manuka honey dressings[78].
Shrivastava studied the effects of tannin-rich plant extract in one group (n = 69 ulcers) and honey and glycerol in another group (n = 49 ulcers) of a RCT. In that study, 67% of the wounds were DFU. Tannin-rich extracts were better than honey for reducing wound surface area (97.87 vs 33.37%, respectively) and maintaining wound humidity after 6 wk of treatment. The study did not mention the details of progression/outcomes in the DFU patients[79].
Clinical metabolites contributing to antibacterial, anti-inflammatory, and antioxidant actions are lupeol, salicylic acid, urea nitrogen, cinnamomic acid, phenols, sulfur, vitamins, enzymes, mineral, lignin and amino acids, bradykinase, anthraquinones, dihydroxyanthraquinones, saponins, aloin emodin, and many others[80-82].
Nejatzadeh-Barandozi et al[80] and Danish et al[83] studied the antibacterial effect against various Gram-positive and Gram-negative bacteria by disc diffusion techniques. Nejatzadeh-Barandozi et al[80] observed that acetone extracts of aloe vera were better than ethanol or aqueous extracts. The zone of inhibition for acetone and ethanol extracts against Pseudomonas was 19 ± 0.57 mm and 14 ± 0.53 mm, respectively[80]. Aloe vera root and leaf ethanol extracts exhibited good antibacterial activity with a zone of inhibition of > 13 mm at a concentration of 30 mg[83]. Arbab et al[84] also concluded that ethanol extracts are more efficacious than conventional extracts. Antifungal activity was noted against Fusarium and Aspergillus[83]. Goudarzi et al had observed favourable activity against multidrug-resistant P. aeruginosa at a MIC ≤ 400 g/mL[82]. Distillate form of Aloe vera was effective against Staphylococci including methicillin-resistant MRSA and Gram-negative microbes like K. pneumoniae and P. aeruginosa[85]. Aloe vera had a 100% in vitro activity against Pseudomonas compared to vancomycin (72.2%) but less for Staphylococci and Streptococci (75.3% vs 80.5% of vancomycin)[86].
Throughout the study duration, Chithra et al[87] observed that the collagen, protein, and DNA levels were significantly higher in the aloe vera gel group compared to the control group. The period of epithelization was 22.2 ± 92.3 d in the aloe vera gel group and 24.8 ± 92.4 d in the control group (P < 0.05), indicating significantly faster healing[87]. The same authors also noted a positive impact on the production of glycosaminoglycans, especially hyaluronic acid and dermatan sulphate, and proteoglycans in the wound bed[87,88].
Shafaie et al[89] studied the effects of aloe vera on fibroblasts against controls. Fibroblast proliferation and migration were significantly promoted. Aloe-treated fibroblasts were morphologically better and had significantly higher expression of Integrins α1 amd β1 and PECAM-1 gene, which are integral for proliferation, differentiation, migration, and formation of granulation tissue and the ECM. The study concluded that aloe vera is efficient in proliferative, reepithelization, and remodelling phases of wound healing[89].
Takzaree et al[90] observed that the augmented TGF-β gene expression in the aloe vera gel-treated group resulted in rapid formation of granulation tissue, angiogenesis, and epithelialization in rats. Daburkar et al[91] observed significantly higher levels of glycosaminoglycans, rapid wound contraction, and increased breaking (tensile) strength by the ninth day (P < 0.0001) in aloe-treated DFU. In an Indonesian experimental rat study, Sari et al[92] compared the effects of topical aloe vera vs Nigella sativa oil vs untreated group. They noted significantly smaller wounds by the seventh day, better fibroblastic infiltration, and better reepithelization in the aloe vera group[92].
With very few trials, aloe vera had shown promising results in improving healing in DFU, but there are only few human trials[93-95] (Table 4) with small sample size, inadequate data, and low-level evidence. Worasakwutiphong et al[96] conducted a small pilot study by using blended silkworm fibroin and aloe gel extract for the dressing of five hard-to-heal DFU (Wagner grade 1). Spectroscopic analysis and tensile strength analysis showed favourable results. Clinically, three DFU had a significant reduction in wound size and healed within 3 wk. The other two had healed by 4 wk[96].
| Ref. | Test group | Control group | Results |
| Panahi et al[93] | AVO cream | Topical phenytoin | At 4 wk, wound healing scores (overall BJUA score, size, depth, slough, adjacent tissue inflammation) in AVO group were significantly better (P < 0.001) than the pre-treatment score and as compared with the phenytoin group |
| Avijgan et al[94] | Aloe vera ointment with conventional treatment | Only conventional treatment | At 3 mo, 28 (93.3%) patients in aloe vera group vs 14 (46.7%) from control group had complete wound healing (P < 0.05). The overall mean healing time and average cost were significantly lesser in aloe vera group |
| Najafian et al[95] | Aloevera/ Plantavera major gel (n = 20) | Placebo (n = 20) | After 4 wk, significant reduction of ulcer surface in the Plantavera group than in placebo group (P = 0.039). No statistical difference in ulcer depth |
| Tamoli et al[109] | Aerosol sprays (containing Panchvalka Kwatha and Jatyadi Taila) (n = 12) | Standard care (n = 14) | BJUA score: 30.59 ± 7.11 on the first day in herbal group, improved to 23.45 ± 8.79, and 15.32 ± 7.63 on days 30 and 90, respectively. In control group, score: 30.58 ± 8.72 and improved to 21.05 ± 9.78 and 14.92 ± 7.69 on days 30 and 90, respectively. Healing time was better in the aerosol spray group |
| Ajmeer et al[110] | Katupila Kalka (paste of S. leucopyrus leaves) with Tila Taila (sesame oil) (n = 13) | Betadine ointment (n = 10) | Complete healing was noted in 92.3% of cases of group A compared to 20 % of group B. Weekly improvement in exudate and peri-wound skin and size reduction were statistically significant in group A |
Calendula officinalis (Asteraceae family) has been used as a medicinal plant in many traditional systems. Hydroglycolic extracts of calendula flower contain various bioactive terpene alcohols, flavonoids, oligoglycosides, and mono-ester triterpenoids that contribute to the anti-inflammatory, antioxidant, and wound healing actions[97]. Calendula promotes neoangiogenesis via upregulation of VEGF and other angiogenic factors in animal models[98]. Calendula has a broad antimicrobial effect against various Gram-positive and Gram-negative bacteria as well as fungi like Candida and Aspergillus[99].
There are many studies about the efficacy of calendula in non-diabetic wounds but those for DFU are very limited. Carvalho et al[100] conducted a four-arm randomized control trial on diabetes patients with leg ulcers for ulcer size reduction. Four groups, each containing eight patients, were calendula extract oil group, the low-level laser therapy group, a combination of both, and a standard-care control group. At the end of 3 wk, the calendula group did not show a significant reduction from baseline wound size (pre- vs post-treatment). The combination group and laser alone group had statistically better results[100]. Chitosan based hydrogels loaded with calendula have been studied with promising results[101].
Effects of Ageratina pichinchensis extracts (also from Asteraceae family) were compared with those of silver sulfadiazine cream on DFU in a small pilot study. Wounds had healed in a shorter period in the intervention group (65.47 ± 47.08 vs 77.46 ± 50.8 d, P = 0.509)[102].
Principles of Ayurved for managing DFU, focus on restoring tissue perfusion, improving circulation, and controlling the inflammatory processes[103]. The phenolics and tannins in Panchvalka have free-radical scavenging activity comparable to ascorbic acid[104]. Jatyadi oil formulations contain various alkaloids, phenols, tannins, sterols, glycosides, saponins, terpenoids, flavonoids, and anthocyanins which contribute to the anti-inflammatory and antimicrobial activities. Jatyadi oil is effective against Gram-positive microbes, even against MRSA and, to a lesser extent, against Gram-negative bacteria[104,105]. Mandrika et al[105] observed that in vitro, Jatyadi formulations downregulate the proinflammatory cytokines IL-6, IL-1β, and TNF-α, and related chemokines.
Haridra (curcumin) is used in many traditional systems independently as well as in polyherbal formulations. Curcumin has antioxidant and anti-inflammatory (reduction of TNF-α and IL-1 cytokines) properties and promotes granulation formation, fibroblastic migration, collagen synthesis, and epithelization[106].
Polyherbal creams are being studied in animal models and have shown statistically better results than conventional topical creams[107]. Jatyadi Ghrita has a significant role in improving moisture and reepithelization in a rat model study[108]. Only a few comparative studies are available about the role of topical ayurvedic (poly-herbal) preparations (Table 1) in DFU. Data from available trials[109,110] (Table 4) warrants further research. Honey and curcumin are being studied for producing an effective and biodegradable hydrogel sponge for improved wound care[111].
Conventionally available and naturally occurring acids are also used for the topical management of chronic and infected wounds like DFU. The acidic milieu favours wound healing by antimicrobial action as well as promoting inflammatory and proliferative phases[21].
The wound bed is improved into a ground unfavourable for bacterial proliferation, thereby controlling the bioburden. Eliminating biofilms, altering the protease activity, allowing autolytic debridement, decreasing the local toxic effects of bacterial end products, and improving tissue oxygenation, are other notable actions[112-116]. The citric acid (MIC: 500-2500 μg/mL) and hypochlorous acid have antibacterial actions against common culprits in DFU. Despite being very effective against Pseudomonas, the action of acetic acid against other microbes is limited[21]. Boron derivatives (boric acid and sodium pentaborate pentahydrate) have broad antibacterial and antifungal actions[117]. Nagoba et al[118] observed favourable antimicrobial action of citric acid in hard-to-heal DFU with multidrug-resistant MRSA infection.
Citric acid and to some extent, hypochlorous acid both augment fibroblastic growth and promote healthy angiogenesis and epithelization[119,120]. Hyaluronic acid stimulates cell migration, keratinocyte proliferation and maturation, scar remodeling, and free radical scavenging[121]. In vitro and in vivo studies on diabetic rats revealed that low concentrations of boric acid promote the proliferation of dermal cells, stimulate migration, improve expression of ECM proteins, and downregulate the expression of pro-inflammatory nitric oxide synthase and cyclooxygenase-2. Boron derivatives augment the synthesis of various growth factors[117].
Fejfarová et al[122], in a small pilot study of 32 DFU (17 patients received topical 1% acetic acid) found better improvement and easy and cheap usage in the acetic acid group, but the results were not statistically significant. Agrawal et al[123] observed that 1% acetic acid is efficient against many bacteria (MRSA, Pseudomonas spp., Klebsiella, Acinetobacter, E.coli, etc.) and fungi (Candida, Aspergillus, and Cryptococcus spp.). After 14 d of daily acetic acid applications, 64/100 wounds were sterile. Along with the reduction in ulcer size, there were reductions in exudation and surrounding inflammation[123]. Similar effects were also noted with the usage of citric acid in 115 DFU patients with Wagner 1-3 grades[116]. Alginic acid alone is rarely used for DFU, but alginic acid-based preparations are widely used in research and practice.
Despite adequate evidence, the absence of good quality human studies with greater sample size marks the usage of acids in DFU as an unexplored avenue.
Larval or maggot debridement therapy is a biological debridement by the sterile larvae of green bottle fly (Lucilia species)[124-126]. Infection control is achieved by debridement, ingestion of bacteria, and antimicrobial action of the maggot secretions facilitating the action of topical antimicrobials[125-127]. Despite acceptable and relatively painless debriding action, maggots are not socio-culturally accepted as traditional medicine by patients and health-care personnel in many communities. Maggot secretions might induce endothelial proliferation to improve neo-granulation tissue[128]. Maggot therapy related trials in DFU are very few with very small sample size. Overall debridement effect was in 50%-80% patients with an average healing duration of 8-14 wk[125,129]. There is heterogenous data on slough vs total wound area, dose/exposure per cm2, and duration of usage and healing. Definite guidelines and adequate evidence are lacking from the available literature. Leeches have no topical action in diabetic wounds.
DFU have complex pathogenesis and even more complex treatment protocols. Ultimately what matters is salvaging the limb. Refractory wounds with resistant microbes and exhausted resources may benefit from traditional medicine systems, especially if integrated with Western medicine. Experts in such systems should come forward with better trials for the ultimate goal of DFU treatment. The results of available clinical trials on traditional medicines in DFU are not always accessible. Unrestricted availability of observations from such trials will be undoubtedly valuable for researchers around the globe. Traditional medicine systems have a long way to go in terms of large, well-designed, human RCT. Till then, only at the hands of an expert, experience-based DFU treatment may be better than evidence-based treatment.
The authors wish to thank Mr. Vinod Jogdand and Mr. Dipak Badne from Department of Medical Education for their assistance in preparation of the manuscript.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: He Z, China; Pei-Hung L, Taiwan; Wu QN, China S-Editor: Liu GL L-Editor: Wang TQ P-Editor: Liu GL
| 1. | International Diabetes Federation. IDF Diabetes Atlas [Internet]. 10th ed. 2021. Cited 23 August 2022. Available from: www.diabetesatlas.org. |
| 2. | American Diabetes Association. Economic Costs of Diabetes in the U.S. in 2017. Diabetes Care. 2018;41:917-928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1361] [Cited by in RCA: 1729] [Article Influence: 247.0] [Reference Citation Analysis (0)] |
| 3. | Armstrong DG, Boulton AJM, Bus SA. Diabetic Foot Ulcers and Their Recurrence. N Engl J Med. 2017;376:2367-2375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1953] [Cited by in RCA: 2339] [Article Influence: 292.4] [Reference Citation Analysis (2)] |
| 4. | International Diabetes Federation. Clinical Practice Recommendation on the Diabetic Foot: A guide for health care professionals. [Internet]. 2017;1-70. Cited 23 August 2022. Available from: https://www.idf.org/component/attachments/?task=download&id=1152. |
| 5. | Doğruel H, Aydemir M, Balci MK. Management of diabetic foot ulcers and the challenging points: An endocrine view. World J Diabetes. 2022;13:27-36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 51] [Article Influence: 17.0] [Reference Citation Analysis (4)] |
| 6. | International Working Group on the Diabetic Foot. IWGDF Guidelines on the Prevention and Management of Diabetic Foot Disease [Internet]. 2019. Cited 23 August 2022. Available from: https://iwgdfguidelines.org/wp-content/uploads/2019/05/IWGDF-Guidelines-2019.pdf. |
| 7. | Lipsky BA, Senneville É, Abbas ZG, Aragón-Sánchez J, Diggle M, Embil JM, Kono S, Lavery LA, Malone M, van Asten SA, Urbančič-Rovan V, Peters EJG; International Working Group on the Diabetic Foot (IWGDF). Guidelines on the diagnosis and treatment of foot infection in persons with diabetes (IWGDF 2019 update). Diabetes Metab Res Rev. 2020;36 Suppl 1:e3280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 364] [Article Influence: 72.8] [Reference Citation Analysis (0)] |
| 8. | Schultz GS, Sibbald RG, Falanga V, Ayello EA, Dowsett C, Harding K, Romanelli M, Stacey MC, Teot L, Vanscheidt W. Wound bed preparation: a systematic approach to wound management. Wound Repair Regen. 2003;11 Suppl 1:S1-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 830] [Cited by in RCA: 746] [Article Influence: 33.9] [Reference Citation Analysis (1)] |
| 9. | Agren MS, Steenfos HH, Dabelsteen S, Hansen JB, Dabelsteen E. Proliferation and mitogenic response to PDGF-BB of fibroblasts isolated from chronic venous leg ulcers is ulcer-age dependent. J Invest Dermatol. 1999;112:463-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 101] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 10. | Falanga V, Grinnell F, Gilchrest B, Maddox YT, Moshell A. Workshop on the pathogenesis of chronic wounds. J Invest Dermatol. 1994;102:125-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 52] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Falanga V. Classifications for wound bed preparation and stimulation of chronic wounds. Wound Repair Regen. 2000;8:347-352. [PubMed] |
| 12. | Leaper DJ, Schultz G, Carville K, Fletcher J, Swanson T, Drake R. Extending the TIME concept: what have we learned in the past 10 years? Int Wound J. 2012;9 Suppl 2:1-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 224] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 13. | Afonso AC, Oliveira D, Saavedra MJ, Borges A, Simões M. Biofilms in Diabetic Foot Ulcers: Impact, Risk Factors and Control Strategies. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 67] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 14. | Frykberg RG, Banks J. Challenges in the Treatment of Chronic Wounds. Adv Wound Care (New Rochelle). 2015;4:560-582. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1534] [Cited by in RCA: 1440] [Article Influence: 144.0] [Reference Citation Analysis (0)] |
| 15. | Vatan A, Saltoglu N, Yemisen M, Balkan II, Surme S, Demiray T, Mete B, Tabak F; Study Group, Cerrahpasa Diabetic Foot. Association between biofilm and multi/extensive drug resistance in diabetic foot infection. Int J Clin Pract. 2018;72:e13060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 16. | Jaju K, Pichare A, Davane M, Nagoba B. Profile and Antibiotic Susceptibility of Bacterial Pathogens Associated With Diabetic Foot Ulcers From a Rural Area. Wounds. 2019;31:158-162. [PubMed] |
| 17. | Senneville E, Joulie D, Blondiaux N, Robineau O. Surgical techniques for Bone Biopsy in Diabetic Foot Infection, and association between results and treatment duration. J Bone Jt Infect. 2020;5:198-204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Lipsky BA, Dryden M, Gottrup F, Nathwani D, Seaton RA, Stryja J. Antimicrobial stewardship in wound care: a Position Paper from the British Society for Antimicrobial Chemotherapy and European Wound Management Association. J Antimicrob Chemother. 2016;71:3026-3035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 90] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 19. | Lipsky BA, Uçkay İ. Treating Diabetic Foot Osteomyelitis: A Practical State-of-the-Art Update. Medicina (Kaunas). 2021;57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (1)] |
| 20. | Dumville JC, Lipsky BA, Hoey C, Cruciani M, Fiscon M, Xia J. Topical antimicrobial agents for treating foot ulcers in people with diabetes. Cochrane Database Syst Rev. 2017;6:CD011038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 21. | Nagoba B, Gavkare A, Rayate A, Mumbre S, Rao A, Warad B, Nanaware N, Jamadar N. Role of an acidic environment in the treatment of diabetic foot infections: A review. World J Diabetes. 2021;12:1539-1549. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 22. | Uçkay I, Berli M, Sendi P, Lipsky BA. Principles and practice of antibiotic stewardship in the management of diabetic foot infections. Curr Opin Infect Dis. 2019;32:95-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (1)] |
| 23. | Matricali GA, Dereymaeker G, Muls E, Flour M, Mathieu C. Economic aspects of diabetic foot care in a multidisciplinary setting: a review. Diabetes Metab Res Rev. 2007;23:339-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 53] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 24. | Driver VR, Fabbi M, Lavery LA, Gibbons G. The costs of diabetic foot: the economic case for the limb salvage team. J Vasc Surg. 2010;52:17S-22S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 279] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 25. | Nailwal D, B VR, Gupta A. Patterns and predictors of complementary and alternative medicine use in people presenting with the non-communicable disease in an urban health facility, North India. J Public Health Res. 2021;10:2109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Xie XS, Wang YJ, Zuo C, Fan JM, Li XJ. A case report of an effective treatment for diabetic foot ulcers with integration of traditional Chinese medicine and Western medicine. J Diabetes Complications. 2009;23:360-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Li FL, Wang YF, Li X, Li F, Xu R, Chen J, Geng L, Li B. Characteristics and clinical managements of chronic skin ulcers based on traditional chinese medicine. Evid Based Complement Alternat Med. 2012;2012:930192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Li FL, Deng H, Wang HW, Xu R, Chen J, Wang YF, Li X, Fan B, Li B. Effects of external application of Chinese medicine on diabetic ulcers and the expressions of β-catenin, c-myc and K6. Chin J Integr Med. 2011;17:261-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 29. | Leu WJ, Chen JC, Guh JH. Extract From Plectranthus amboinicus Inhibit Maturation and Release of Interleukin 1β Through Inhibition of NF-κB Nuclear Translocation and NLRP3 Inflammasome Activation. Front Pharmacol. 2019;10:573. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 30. | Sawatdee S, Choochuay K, Chanthorn W, Srichana T. Evaluation of the topical spray containing Centella asiatica extract and its efficacy on excision wounds in rats. Acta Pharm. 2016;66:233-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 31. | Wan X, Gen F, Sheng Y, Ou M, Wang F, Peng T, Guo J. Meta-Analysis of the Effect of Kangfuxin Liquid on Diabetic Patients with Skin Ulcers. Evid Based Complement Alternat Med. 2021;2021:1334255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 32. | Liu Y, Li Y, Du Y, Huang T, Zhu C. Multicenter Clinical Trials Analyzing Efficacy and Safety of Topical Cortex Phellodendri Compound Fluid in Treatment of Diabetic Foot Ulcers. Med Sci Monit. 2020;26:e923424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (1)] |
| 33. | Morita Y, Nakashima K, Nishino K, Kotani K, Tomida J, Inoue M, Kawamura Y. Berberine Is a Novel Type Efflux Inhibitor Which Attenuates the MexXY-Mediated Aminoglycoside Resistance in Pseudomonas aeruginosa. Front Microbiol. 2016;7:1223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 34. | Sun Y, Lenon GB, Yang AWH. Phellodendri Cortex: A Phytochemical, Pharmacological, and Pharmacokinetic Review. Evid Based Complement Alternat Med. 2019;2019:7621929. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 35. | Li S, Zhao J, Liu J, Xiang F, Lu D, Liu B, Xu J, Zhang H, Zhang Q, Li X, Yu R, Chen M, Wang X, Wang Y, Chen B. Prospective randomized controlled study of a Chinese herbal medicine compound Tangzu Yuyang Ointment for chronic diabetic foot ulcers: a preliminary report. J Ethnopharmacol. 2011;133:543-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 36. | Huang R, Hu X, Li W, Wang L, Fan W, Han Q, Guo F, Liu G. Zizhu Ointment Accelerates Wound-Healing of Diabetic Ulcers through Promoting M2 Macrophage Polarization via Downregulating the Notch4 Signaling Pathway. Comput Intell Neurosci. 2022;2022:5173836. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 37. | Xie XL, Guan, WJ Tang XY, Chang WL. A combination of ultrasonic debridement and Shenghong wet dressing in patients with chronic ulcers of the lower limbs. J Int Med Res. 2019;47:4656-4663. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 38. | Kuo YS, Chien HF, Lu W. Plectranthus amboinicus and Centella asiatica Cream for the Treatment of Diabetic Foot Ulcers. Evid Based Complement Alternat Med. 2012;2012:418679. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 39. | Huang YY, Lin CW, Cheng NC, Cazzell SM, Chen HH, Huang KF, Tung KY, Huang HL, Lin PY, Perng CK, Shi B, Liu C, Ma Y, Cao Y, Li Y, Xue Y, Yan L, Li Q, Ning G, Chang SC. Effect of a Novel Macrophage-Regulating Drug on Wound Healing in Patients With Diabetic Foot Ulcers: A Randomized Clinical Trial. JAMA Netw Open. 2021;4:e2122607. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 73] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 40. | Jiang YF, Yang C, Jia LJ, Ju S, Wang XM, Gao H, Fu XB, Xu ZR, Lu SL, Han CM. A multicenter randomized study on the clinical effect of Jingwanhong ointment on chronic wounds of feet of diabetic patients as compared with routine treatment. Ganran, Yanzheng, Xiufu. 2015;16:33-36. [DOI] [Full Text] |
| 41. | Cao BL, Miao GZ, Zhu XM, Li CG, Miao J, Wang LQ, Cui ZL, Du QM, Jin J, Wang WC, Guan J, Zhang JM, Tong LF, Jing ZL, Yang JY, Sun GR. Clincal research of traditional Chinese medicine ointment for the treatment of diabetic foot ulcer. Zhongguo Zhongyiyao Xiandai Yuancheng Jiaoyu. 2014;12:11-13. |
| 42. | Li DY, Lv DW, Li X, Yang BH, Zhang ZH, Gan Y, Wang P. A multi-centered, randomize-controlled trial on external treatment of TCM based on syndrome differentiation for non-severe ischemic diabetic foot. Zhonghua Zhongyiyao Zazhi. 2014;29:1266-1269. |
| 43. | Chen Z, Hu Y, Li J, Zhang C, Gao F, Ma X, Zhang J, Fu C, Geng F. A feasible biocompatible hydrogel film embedding Periplaneta americana extract for acute wound healing. Int J Pharm. 2019;571:118707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 44. | He Y, Zhao W, Dong Z, Ji Y, Li M, Hao Y, Zhang D, Yuan C, Deng J, Zhao P, Zhou Q. A biodegradable antibacterial alginate/carboxymethyl chitosan/Kangfuxin sponges for promoting blood coagulation and full-thickness wound healing. Int J Biol Macromol. 2021;167:182-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 125] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 45. | Wang T, Liao Q, Wu Y, Wang X, Fu C, Geng F, Qu Y, Zhang J. A composite hydrogel loading natural polysaccharides derived from Periplaneta americana herbal residue for diabetic wound healing. Int J Biol Macromol. 2020;164:3846-3857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 46. | Talebi M, Talebi M, Farkhondeh T, Samarghandian S. Molecular mechanism-based therapeutic properties of honey. Biomed Pharmacother. 2020;130:110590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 47. | Cooper RA, Molan PC, Harding KG. The sensitivity to honey of Gram-positive cocci of clinical significance isolated from wounds. J Appl Microbiol. 2002;93:857-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 216] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 48. | French VM, Cooper RA, Molan PC. The antibacterial activity of honey against coagulase-negative staphylococci. J Antimicrob Chemother. 2005;56:228-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 99] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 49. | Cooper R, Molan P. The use of honey as an antiseptic in managing Pseudomonas infection. J Wound Care. 1999;8:161-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 83] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 50. | Cooper RA, Halas E, Molan PC. The efficacy of honey in inhibiting strains of Pseudomonas aeruginosa from infected burns. J Burn Care Rehabil. 2002;23:366-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 130] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 51. | Molan PC. The evidence supporting the use of honey as a wound dressing. Int J Low Extrem Wounds. 2006;5:40-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 215] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 52. | Nzeako BC, Hamdi J. Antimicrobial Potential of Honey on some Microbial Isolates. Sultan Qaboos Univ Med J. 2000;2:75-79. |
| 53. | Subrahmanyam M. Topical application of honey in treatment of burns. Br J Surg. 1991;78:497-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 151] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 54. | Wahdan HA. Causes of the antimicrobial activity of honey. Infection. 1998;26:26-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 126] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 55. | Mullai V, Menon T. Bactericidal activity of different types of honey against clinical and environmental isolates of Pseudomonas aeruginosa. J Altern Complement Med. 2007;13:439-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 51] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 56. | Molan PC, Betts JA. Using honey to heal diabetic foot ulcers. Adv Skin Wound Care. 2008;21:313-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 57. | Shukrimi A, Sulaiman AR, Halim AY, Azril A. A comparative study between honey and povidone iodine as dressing solution for Wagner type II diabetic foot ulcers. Med J Malaysia. 2008;63:44-46. [PubMed] |
| 58. | Anastasiou IA, Eleftheriadou I, Tentolouris A, Samakidou G, Papanas N, Tentolouris N. Therapeutic Properties of Honey for the Management of Wounds; Is There a Role in the Armamentarium of Diabetic Foot Ulcer Treatment? Int J Low Extrem Wounds. 2021;20:291-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 59. | Molan PC. Re-introducing honey in the management of wounds and ulcers - theory and practice. Ostomy Wound Manage. 2002;48:28-40. [PubMed] |
| 60. | Zhu G, Wang Q, Lu S, Niu Y. Hydrogen Peroxide: A Potential Wound Therapeutic Target? Med Princ Pract. 2017;26:301-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 100] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 61. | Oryan A, Alemzadeh E, Moshiri A. Biological properties and therapeutic activities of honey in wound healing: A narrative review and meta-analysis. J Tissue Viability. 2016;25:98-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 157] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 62. | Majtan J, Kumar P, Majtan T, Walls AF, Klaudiny J. Effect of honey and its major royal jelly protein 1 on cytokine and MMP-9 mRNA transcripts in human keratinocytes. Exp Dermatol. 2010;19:e73-e79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 63. | Choi DS, Kim S, Lim Y-M, Gwon H-J, Park JS, Nho Y-C, Kwon J. Hydrogel incorporated with chestnut honey accelerates wound healing and promotes early HO-1 protein expression in diabetic (db/db) mice. Tissue Eng Regen Med. 2012;9:36-42. [RCA] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 64. | Gill R, Poojar B, Bairy LK, Praveen KSE. Comparative Evaluation of Wound Healing Potential of Manuka and Acacia Honey in Diabetic and Nondiabetic Rats. J Pharm Bioallied Sci. 2019;11:116-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 65. | Chaudhary A, Bag S, Banerjee P, Chatterjee J. Wound healing efficacy of Jamun honey in diabetic mice model through reepithelialization, collagen deposition and angiogenesis. J Tradit Complement Med. 2020;10:529-543. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 66. | Malkoç M, Yaman SÖ, Imamoğlu Y, İnce İ, Kural BV, Mungan S, Livaoglu M, Yıldız O, Kolaylı S, Orem A. Anti-inflammatory, antioxidant and wound-healing effects of mad honey in streptozotocin-induced diabetic rats. J Apic Res. 2020;59:426-436. [DOI] [Full Text] |
| 67. | Rashidi MK, Mirazi N, Hosseini A. Effect of topical mixture of honey, royal jelly and olive oil-propolis extract on skin wound healing in diabetic rats. Wound Med. 2016;12:6-9. [RCA] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 68. | Demir A, Şimşek T, Engin Ms, Yildiz L. The effect of topical honey dressing on wound healing in Diabetic mice. Gazi Med. 18:110-113. |
| 69. | Hammouri SK. The role of honey in the management of diabetic foot ulcers. J R Med Serv. 2004;11:20-22. |
| 70. | Jan WA, Shah H, Khan M, Fayaz M, Ullah N. Comparison Of Conventional Pyodine Dressing With Honey Dressing For The Treatment Of Diabetic Foot Ulcers. J Postgrad Med Inst. 2012;26:402-407. |
| 71. | Imran M, Hussain MB, Baig M. A Randomized, Controlled Clinical Trial of Honey-Impregnated Dressing for Treating Diabetic Foot Ulcer. J Coll Physicians Surg Pak. 2015;25:721-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 72. | Kamaratos AV, Tzirogiannis KN, Iraklianou SA, Panoutsopoulos GI, Kanellos IE, Melidonis AI. Manuka honey-impregnated dressings in the treatment of neuropathic diabetic foot ulcers. Int Wound J. 2014;11:259-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 73. | Al Saeed M. Therapeutic Efficacy of Conventional Treatment Combined with Manuka Honey in the Treatment of Patients with Diabetic Foot Ulcers: A Randomized Controlled Study. Egypt J Hosp Med. 2013;53:1064-1071. [RCA] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 74. | Siavash M, Shokri S, Haghighi S, Shahtalebi MA, Farajzadehgan Z. The efficacy of topical royal jelly on healing of diabetic foot ulcers: a double-blind placebo-controlled clinical trial. Int Wound J. 2015;12:137-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 75. | Abdelatif M, Yakoot M, Etmaan M. Safety and efficacy of a new honey ointment on diabetic foot ulcers: a prospective pilot study. J Wound Care. 2008;17:108-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 76. | Moghazy AM, Shams ME, Adly OA, Abbas AH, El-Badawy MA, Elsakka DM, Hassan SA, Abdelmohsen WS, Ali OS, Mohamed BA. The clinical and cost effectiveness of bee honey dressing in the treatment of diabetic foot ulcers. Diabetes Res Clin Pract. 2010;89:276-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 77. | Tsang KK, Kwong EW, To TS, Chung JW, Wong TK. A Pilot Randomized, Controlled Study of Nanocrystalline Silver, Manuka Honey, and Conventional Dressing in Healing Diabetic Foot Ulcer. Evid Based Complement Alternat Med. 2017;2017:5294890. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 78. | Al Saeed M. Prospective randomized comparison of controlled release ionic silver hydrophilic dressings and medicated honey-impregnated dressings in treating neuropathic diabetic foot ulcer. Saudi J Heal Sci. 2019;8:25. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 79. | Shrivastava R. Clinical evidence to demonstrate that simultaneous growth of epithelial and fibroblast cells is essential for deep wound healing. Diabetes Res Clin Pract. 2011;92:92-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 80. | Nejatzadeh-Barandozi F. Antibacterial activities and antioxidant capacity of Aloe vera. Org Med Chem Lett. 2013;3:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 120] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 81. | Yazarlu O, Iranshahi M, Kashani HRK, Reshadat S, Habtemariam S, Iranshahy M, Hasanpour M. Perspective on the application of medicinal plants and natural products in wound healing: A mechanistic review. Pharmacol Res. 2021;174:105841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 93] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 82. | Goudarzi M, Fazeli M, Azad M, Seyedjavadi SS, Mousavi R. Aloe vera Gel: Effective Therapeutic Agent against Multidrug-Resistant Pseudomonas aeruginosa Isolates Recovered from Burn Wound Infections. Chemother Res Pract. 2015;2015:639806. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 83. |
Danish P, Ali Q, Hafeez M, Malik A.
ANTIFUNGAL AND ANTIBACTERIAL ACTIVITY OF ALOE VERA PLANT EXTRACT |
| 84. | Arbab S, Ullah H, Weiwei W, Wei X, Ahmad SU, Wu L, Zhang J. Comparative study of antimicrobial action of aloe vera and antibiotics against different bacterial isolates from skin infection. Vet Med Sci. 2021;7:2061-2067. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 85. | Gharibi D, Khosravi M, Hosseini Z, Boroun F, Barzgar SK, Forughi Far A. Antibacterial Effects of Aloe Vera Extracts on some Human and Animal Bacterial Pathogens. J Med Microbiol Infect Dis. 2015;3:6-10. |
| 86. | López Villarreal SM, Elizondo Luévano JH, Pérez Hernández RA, Sánchez García E, Verde Star MJ, Castro Ríos R, Garza Tapia M, Rodríguez Luis OE, Chávez Montes A. Preliminary Study of the Antimicrobial, Anticoagulant, Antioxidant, Cytotoxic, and Anti-Inflammatory Activity of Five Selected Plants with Therapeutic Application in Dentistry. Int J Environ Res Public Health. 2022;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 87. | Chithra P, Sajithlal GB, Chandrakasan G. Influence of aloe vera on the healing of dermal wounds in diabetic rats. J Ethnopharmacol. 1998;59:195-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 148] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 88. | Chithra P, Sajithlal GB, Chandrakasan G. Influence of Aloe vera on the glycosaminoglycans in the matrix of healing dermal wounds in rats. J Ethnopharmacol. 1998;59:179-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 118] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 89. | Shafaie S, Andalib S, Shafaei H, Montaseri A, Tavakolizadeh M. Differential Biological Behavior of Fibroblasts and Endothelial Cells under Aloe vera Gel Culturing. Int J Mol Cell Med. 2020;9:234-246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 90. | Takzaree N, Hadjiakhondi A, Hassanzadeh G, Rouini MR, Manayi A, Zolbin MM. Transforming growth factor-β (TGF-β) activation in cutaneous wounds after topical application of aloe vera gel. Can J Physiol Pharmacol. 2016;94:1285-1290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 91. | Daburkar M, Lohar V, Rathore AS, Bhutada P, Tangadpaliwar S. An in vivo and in vitro investigation of the effect of Aloe vera gel ethanolic extract using animal model with diabetic foot ulcer. J Pharm Bioallied Sci. 2014;6:205-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 92. | Sari Y, Purnawan I, Kurniawan DW, Sutrisna E. A Comparative Study of the Effects of Nigella sativa Oil Gel and Aloe Vera Gel on Wound Healing in Diabetic Rats. J Evid Based Integr Med. 2018;23:2515690X18772804. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 93. | Panahi Y, Izadi M, Sayyadi N, Rezaee R, Jonaidi-Jafari N, Beiraghdar F, Zamani A, Sahebkar A. Comparative trial of Aloe vera/olive oil combination cream versus phenytoin cream in the treatment of chronic wounds. J Wound Care. 2015;24:459-460, 462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 94. | Avijgan M, Kamran A, Abedini A. Effectiveness of Aloe Vera Gel in Chronic Ulcers in Comparison with Conventional Treatments. Iran J Med Sci. 2016;41:S30. [PubMed] |
| 95. | Najafian Y, Khorasani ZM, Najafi MN, Hamedi SS, Mahjour M, Feyzabadi Z. Efficacy of Aloe vera/ Plantago Major Gel in Diabetic Foot Ulcer: A Randomized Double-Blind Clinical Trial. Curr Drug Discov Technol. 2019;16:223-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 96. | Worasakwutiphong S, Termwattanaphakdee T, Kamolhan T, Phimnuan P, Sittichokechaiwut A, Viyoch J. Evaluation of the safety and healing potential of a fibroin-aloe gel film for the treatment of diabetic foot ulcers. J Wound Care. 2021;30:1020-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 97. | Buzzi M, de Freitas F, Winter M. A Prospective, Descriptive Study to Assess the Clinical Benefits of Using Calendula officinalis Hydroglycolic Extract for the Topical Treatment of Diabetic Foot Ulcers. Ostomy Wound Manage. 2016;62:8-24. [PubMed] |
| 98. | Parente LM, Andrade MA, Brito LA, Moura VM, Miguel MP, Lino-Júnior Rde S, Tresvenzol LF, Paula JR, Paulo NM. Angiogenic activity of Calendula officinalis flowers L. in rats. Acta Cir Bras. 2011;26:19-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 99. | Efstratiou E, Hussain AI, Nigam PS, Moore JE, Ayub MA, Rao JR. Antimicrobial activity of Calendula officinalis petal extracts against fungi, as well as Gram-negative and Gram-positive clinical pathogens. Complement Ther Clin Pract. 2012;18:173-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 77] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 100. | Carvalho AF, Feitosa MC, Coelho NP, Rebêlo VC, Castro JG, Sousa PR, Feitosa VC, Arisawa EA. Low-level laser therapy and Calendula officinalis in repairing diabetic foot ulcers. Rev Esc Enferm USP. 2016;50:628-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 101. | Rodríguez-Acosta H, Tapia-Rivera JM, Guerrero-Guzmán A, Hernández-Elizarraráz E, Hernández-Díaz JA, Garza-García JJO, Pérez-Ramírez PE, Velasco-Ramírez SF, Ramírez-Anguiano AC, Velázquez-Juárez G, Velázquez-López JM, Sánchez-Toscano YG, García-Morales S, Flores-Fonseca MM, García-Bustos DE, Sánchez-Chiprés DR, Zamudio-Ojeda A. Chronic wound healing by controlled release of chitosan hydrogels loaded with silver nanoparticles and calendula extract. J Tissue Viability. 2022;31:173-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 102. | Romero-Cerecero O, Zamilpa A, Tortoriello J. Effectiveness and tolerability of a standardized extract from Ageratina pichinchensis in patients with diabetic foot ulcer: a randomized, controlled pilot study. Planta Med. 2015;81:272-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 103. | Indu PP. Diabetic Foot Ulcer - An Ayurvedic Perspective. World J Pharm Med Res. 2018;4:183-187. |
| 104. | Anandjiwala S, Bagul MS, Parabia M, Rajani M. Evaluation of free radical scavenging activity of an ayurvedic formulation, panchvalkala. Indian J Pharm Sci. 2008;70:31-35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 105. | Mandrika I, Kumar S, Zandersone B, Eranezhath SS, Petrovska R, Liduma I, Jezupovs A, Pirags V, Tracevska T. Antibacterial and Anti-Inflammatory Potential of Polyherbal Formulation Used in Chronic Wound Healing. Evid Based Complement Alternat Med. 2021;2021:9991454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 106. | Karri VVSR, Gowthamarajan K, Satish Kumar M, Rajkumar M. Multiple Biological Actions of Curcumin in the Management of Diabetic Foot Ulcer Complications: A Systematic Review. Trop Med Surg. 2015;3:179. [DOI] [Full Text] |
| 107. | Nehete MN, Nipanikar S, Kanjilal AS, Kanjilal S, Tatke PA. Comparative efficacy of two polyherbal creams with framycetin sulfate on diabetic wound model in rats. J Ayurveda Integr Med. 2016;7:83-87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 108. | Jamadagni PS, Jamadagni S, Mukherjee K, Upadhyay S, Gaidhani S, Hazra J. Experimental and histopathological observation scoring methods for evaluation of wound healing properties of Jatyadi Ghrita. Ayu. 2016;37:222-229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 109. | Tamoli S, Ukhalkar V, Acharya GS, Gajre K, Pathak S, Pande S, Solanki Y, Jadhav R, Quadri MJ, Koli N. Wound healing activity of topical herbal aerosol sprays on diabetic and Varicose Ulcers: A randomized, controlled, open labelled, multi-centric clinical trial. J Ayurveda Integr Med. 2022;13:100594. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 110. | Ajmeer AS, Dudhamal TS, Gupta SK. Management of Madhumehajanya Vrana (diabetic wound) with Katupila (Securinega leucopyrus [Willd] Muell.) Kalka. Ayu. 2015;36:351-355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 111. | Momin M, Kurhade S, Khanekar P, Mhatre S. Novel biodegradable hydrogel sponge containing curcumin and honey for wound healing. J Wound Care. 2016;25:364-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 112. | Bjarnsholt T, Alhede M, Jensen PØ, Nielsen AK, Johansen HK, Homøe P, Høiby N, Givskov M, Kirketerp-Møller K. Antibiofilm Properties of Acetic Acid. Adv Wound Care (New Rochelle). 2015;4:363-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 106] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 113. | Prabhu V, Prasadi S, Shivani A, Gore A, Pawar V. Does wound pH modulation with 3% citric acid solution dressing help in wound healing: A pilot study. Saudi Surg J. 2014;2:38-46. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 114. | Tandon S, Singh B, Kapoor S, Mangal S. Comparison of Effect of pH Modulation on Wound Healing with Topical Application of Citric Acid Versus Superoxide Ions. Niger J Surg. 2020;26:122-126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 115. | Nagoba B, Suryawanshi N, Wadher B, Selkar S. Acidic Environment and Wound Healing: A Review. Wounds. 2015;27:5-11. |
| 116. | Nagoba BS, Gandhi RC, Wadher BJ, Rao A, Hartalkar AR, Selkar SP. A simple and effective approach for the treatment of diabetic foot ulcers with different Wagner grades. Int Wound J. 2010;7:153-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 117. | Demirci S, Doğan A, Aydın S, Dülger EÇ, Şahin F. Boron promotes streptozotocin-induced diabetic wound healing: roles in cell proliferation and migration, growth factor expression, and inflammation. Mol Cell Biochem. 2016;417:119-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 118. | Nagoba B, Rawal C, Davane M. Citric acid treatment of a diabetic leg ulcer infected with meticillin-resistant Staphylococcus aureus. J Wound Care. 2022;31:432-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 119. | Nagoba BS, Gandhi RC, Wadher BJ, Potekar RM, Kolhe SM. Microbiological, histopathological and clinical changes in chronic infected wounds after citric acid treatment. J Med Microbiol. 2008;57:681-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 120. | Ragab II, Kamal A. The Effectiveness of Hypochlorous Acid Solution on Healing of Infected Diabetic Foot Ulcers. J Educ Pract. 2017;8:58-71. |
| 121. | Frenkel JS. The role of hyaluronan in wound healing. Int Wound J. 2014;11:159-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 139] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 122. | Fejfarová V, Tibenská H, Niklová J, Bém R, Dubský M, Wosková V, Němcová A, Jirkovská A, Jude E, Lánská V. Benefits of Acidifying Agents in Local Therapy of Diabetic Foot Ulcers Infected by Pseudomonas sp: A Pilot Study. Int J Low Extrem Wounds. 2019;18:262-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 123. | Agrawal KS, Sarda AV, Shrotriya R, Bachhav M, Puri V, Nataraj G. Acetic acid dressings: Finding the Holy Grail for infected wound management. Indian J Plast Surg. 2017;50:273-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 124. | Tian X, Liang XM, Song GM, Zhao Y, Yang XL. Maggot debridement therapy for the treatment of diabetic foot ulcers: a meta-analysis. J Wound Care. 2013;22:462-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 125. | Gunasegaran N, Seah VQH, Ang SY, Aloweni F, Goh WT, Liew AYJ, Tan WX, Tay HT, Chong TT. Maggot debridement therapy in the tropics - Preliminary outcomes from a tertiary hospital. J Tissue Viability. 2022;31:544-551. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 126. | Malekian A, Esmaeeli Djavid G, Akbarzadeh K, Soltandallal M, Rassi Y, Rafinejad J, Rahimi Foroushani A, Farhoud A, Bakhtiary R, Totonchi M. Efficacy of Maggot Therapy on Staphylococcus aureus and Pseudomonas aeruginosa in Diabetic Foot Ulcers: A Randomized Controlled Trial. J Wound Ostomy Continence Nurs. 2019;46:25-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 127. | Valachova I, Bohova J, Kozanek M, Takac P, Majtan J. Lucilia sericata medicinal maggots: a new source of antimicrobial compounds. In book: Microbial pathogens and strategies for combating them: science, technology and education. Formatex Research Center. 2013; 1745-1753. Available from: https://www.researchgate.net/publication/264895259_Lucilia_sericata_medicinal_maggots_a_new_source_of_antimicrobial_compounds. |
| 128. | Sun X, Chen J, Zhang J, Wang W, Sun J, Wang A. Maggot debridement therapy promotes diabetic foot wound healing by up-regulating endothelial cell activity. J Diabetes Complications. 2016;30:318-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 129. | Siavash M, Najjarnezhad A, Mohseni N, Abtahi SM, Karimy A, Sabzevari MH. Efficacy of Maggot Debridement Therapy on Refractory Atypical Diabetic Foot Ulcers: An Open-Label Study. Int J Low Extrem Wounds. 2021;20:315-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |