Published online Oct 15, 2022. doi: 10.4239/wjd.v13.i10.888
Peer-review started: June 18, 2022
First decision: July 14, 2022
Revised: July 23, 2022
Accepted: September 12, 2022
Article in press: September 12, 2022
Published online: October 15, 2022
Processing time: 117 Days and 18.8 Hours
Gestational diabetes mellitus (GDM) is a metabolic disease with an increasing annual incidence rate. Our previous observational study found that pregnant women with GDM had mild cognitive decline.
To analyze the changes in metabonomics in pregnant women with GDM and explore the mechanism of cognitive function decline.
Thirty GDM patients and 30 healthy pregnant women were analyzed. Solid-phase microextraction gas chromatography/mass spectrometry was used to detect organic matter in plasma and urine samples. Statistical analyses were conducted using principal component analysis and partial least squares discriminant analysis.
Differential volatile metabolites in the serum of pregnant women with GDM included hexanal, 2-octen-1-ol, and 2-propanol. Differential volatile metabolites in the urine of these women included benzene, cyclohexanone, 1-hexanol, and phenol. Among the differential metabolites, the conversion of 2-propanol to acetone may further produce methylglyoxal. Therefore, 2-propanol may be a potential marker for serum methylglyoxal.
2-propanol may be a potential volatile marker to evaluate cognitive impairment in pregnant women with GDM.
Core Tip: Gas chromatography-mass spectrometry was used in a metabonomics analysis to determine the changes in volatile metabolites in pregnant women with gestational diabetes mellitus (GDM) and to explore the mechanism of cognitive function decline in these women. 2-propanol was identified as a potential volatile marker to evaluate cognitive impairment in pregnant women with GDM.
- Citation: Sana SRGL, Chen GM, Lv Y, Guo L, Li EY. Metabonomics fingerprint of volatile organic compounds in serum and urine of pregnant women with gestational diabetes mellitus. World J Diabetes 2022; 13(10): 888-899
- URL: https://www.wjgnet.com/1948-9358/full/v13/i10/888.htm
- DOI: https://dx.doi.org/10.4239/wjd.v13.i10.888
Gestational diabetes mellitus (GDM) is a metabolic disorder in which hyperglycemia develops during pregnancy in women who did not previously have diabetes[1]. The incidence of GDM varies in different countries and regions, ranging from 1 to 30% of pregnancies, and is highest in Africa, Asia, and India[2]. Epidemiological evidence indicates a continuous increase in the incidence of GDM worldwide. The presence of hyperglycemia during gestation is often associated with various abnormalities, such as obesity, cardiovascular disease, preeclampsia, and even stillbirth. GDM diagnosed at 24 to 28 wk of gestation reportedly affects fetal development[3]. The substantial effect that GDM can have on maternal and fetal health necessitates the development of a screening method for predictive and diagnostic biomarkers of GDM in early stages of pregnancy[4].
Refinements of metabonomics research methods have led to the widespread use of the mature technology in various fields, including studies of disease mechanisms and diagnosis, treatment, treatment effects, and prevention. It is helpful to analyze the changes in metabolic substances caused by pathophysiological changes in diseases[5]. Metabonomics clinical research mainly obtains relevant differential substances by analyzing patients' serum, urine, and feces. There are many metabonomics technologies, and each has shortcomings. However, the use of the technology in combination with another technology, such as liquid/gas chromatography-mass spectrometry (LC/GC-MS), can improve their respective advantages and compensate for the shortcomings of each technology. This integration also improves the metabonomic method and can obtain more reliable clinical sample data. Sample analyses involved principal component analysis (PCA), partial least squares-discriminant analysis (PLS-DA), and orthogonal projections to latent structures-DA. These analyses have identified differential metabolites. Bioinformatics database analysis of the relevant metabolic pathway can explain the possible metabolic mechanisms and pathophysiological changes and verify the biomarkers related to the disease mechanism.
We previously reported that patients with GDM have mild cognitive decline, but the underlying mechanism remains unclear[6]. A causative association of cognitive decline with metabolic abnorm
Patients aged 18 to 35 years with American Society of Anesthesiologists physical status I-II were enrolled. Thirty women with GDM who were diagnosed, followed, and treated at the First Affiliated Hospital of Harbin Medical University were included in the study. Thirty age-matched pregnant women without diabetes constituted the normal pregnancy (NP) group. All patients and volunteers read and signed informed consent forms before enrollment in the study. The study protocol was approved by the Ethics Committee of the First Affiliated Hospital of Harbin Medical University and registered with the Chinese Clinical Trial Registry (registration number: ChiCTR2000038703).
GDM was diagnosed with at least one abnormal result during the oral glucose tolerance test: Plasma glucose during fasting ≥ 92 mg/dL (5.1 mmol/L) or ≥ 180 mg/dL (10.0 mmol/L) at 1 h or ≥ 153 mg/dL (8.5 mmol/L) at 2 h. Patients with pre-gestational type 1 (T1) or type 2 (T2) DM were not included in the study. Patients with unnatural pregnancy or a gestational period of < 37 wk or > 41 wk were excluded. Subjects on medications affecting cognitive function, including corticosteroids, antidepressants, or antiepileptics, were also not included. Subjects with chronic metabolic, endocrine, inflammatory diseases, cancer, drug or alcohol dependency, history of major brain abnormalities (e.g., tumors and hydrocephaly), epilepsy, or Parkinson’s disease were excluded. The psychological status of pregnant women was assessed using the Hamilton Depression Scale; a score > 7 indicated the potential for depression and was the final exclusion criterion.
On the survey data, all the enrolled patients underwent routine medical history inquiries, physical examinations, and laboratory measurements. The clinical research coordinators used a standard questionnaire to collect information on demographic characteristics and medical history (Figure 1). All pregnant women were instructed to maintain their usual physical activity and diet for at least 3 d before the survey. After overnight fasting for ≥ 10 h, venous blood and urine samples were collected and stored at -80 °C. All parameters were measured within 6 mo of sample collection.
A 75 μM extraction head was used. The coating material was carbon molecular sieve of polydimethylsiloxane. An automatic sample injector was used for heating and extraction. A puncture made in the liquid sample bottle allowed injection. In the headspace extraction method, the extraction temperature was 40 °C, and the extraction time was 20 min. After the extraction and concentration of the samples were completed, the automatic sampling device inserted the extraction head into the GC-MS injection port for analysis.
All analyses were performed on a model QP2010 GC/MS (Shimadzu) equipped with a DB-5MS PLOT column (length: 30 m; inner diameter: 0.250 μm; film thickness, 0.25 mm; Agilent Technologies). The injections were performed in splitless mode, with a splitless time of 1 min. The injector temperature was set at 200 °C, and helium was used as the carrier gas at a flow rate of 2 mL/min. The temperature in the column was maintained at 40 °C for 2 min to condense hydrocarbons. The temperature was then increased to 200 °C at a rate of 70 °C /min and held for 1 min. Subsequently, the temperature was increased to 230 °C at 20 °C/min and maintained for 3 min. MS analyses were performed in full-scan mode with an associated m/z range of 35–200 amu. An ionization energy of 70 eV was used for each measurement, and the ion source was maintained at 200 °C.
Statistical analyses were performed using the SIMCA-p + 11 software. Differences in volatile organic carbons (VOCs) between groups were tested using PLS-DA and PCA. SIMCA-p software was used to prevent overfitting by applying default seven-round cross-validation. Additionally, permutation tests using 200 iterations were performed to further validate the supervised model. Potential metabolic biomarkers were selected based on variable importance in the projection values calculated from the PLS-DA model. For all data analyses, P < 0.05 indicated statistical significance. The area under the curve (AUC) of the combined biomarkers and sensitivity and specificity calculations were performed using R language software 3.2 (R Development Core Team 2011).
Sixty pregnant women participated in this study, including 30 pregnant women with GDM in the GDM group and 30 healthy pregnant women in the NP group. Body weight, blood glucose level, and hemoglobin A1c level in the GDM group were significantly higher than of those in the NP group (P < 0.05) (Table 1).
| GDM | NP | t | P value | |
| Sample | 30 | 30 | ||
| Age (yr) | 28.38 ± 2.52 | 29.14 ± 3.61 | 0.95 | 0.35 |
| Height (cm) | 162.34 ± 4.69 | 164.61 ± 5.36 | 1.75 | 0.09 |
| Weight (kg) | 76.33 ± 9.16 | 74.05 ± 8.97 | 0.98 | 0.33 |
| Glucose (mmol/L) | 4.825 ± 1.03 | 3.39 ± 0.56 | 6.70 | < 0.001 |
| Hba1c (%) | 5.93 ± 0.73 | 4.86 ± 0.93 | 4.50 | < 0.001 |
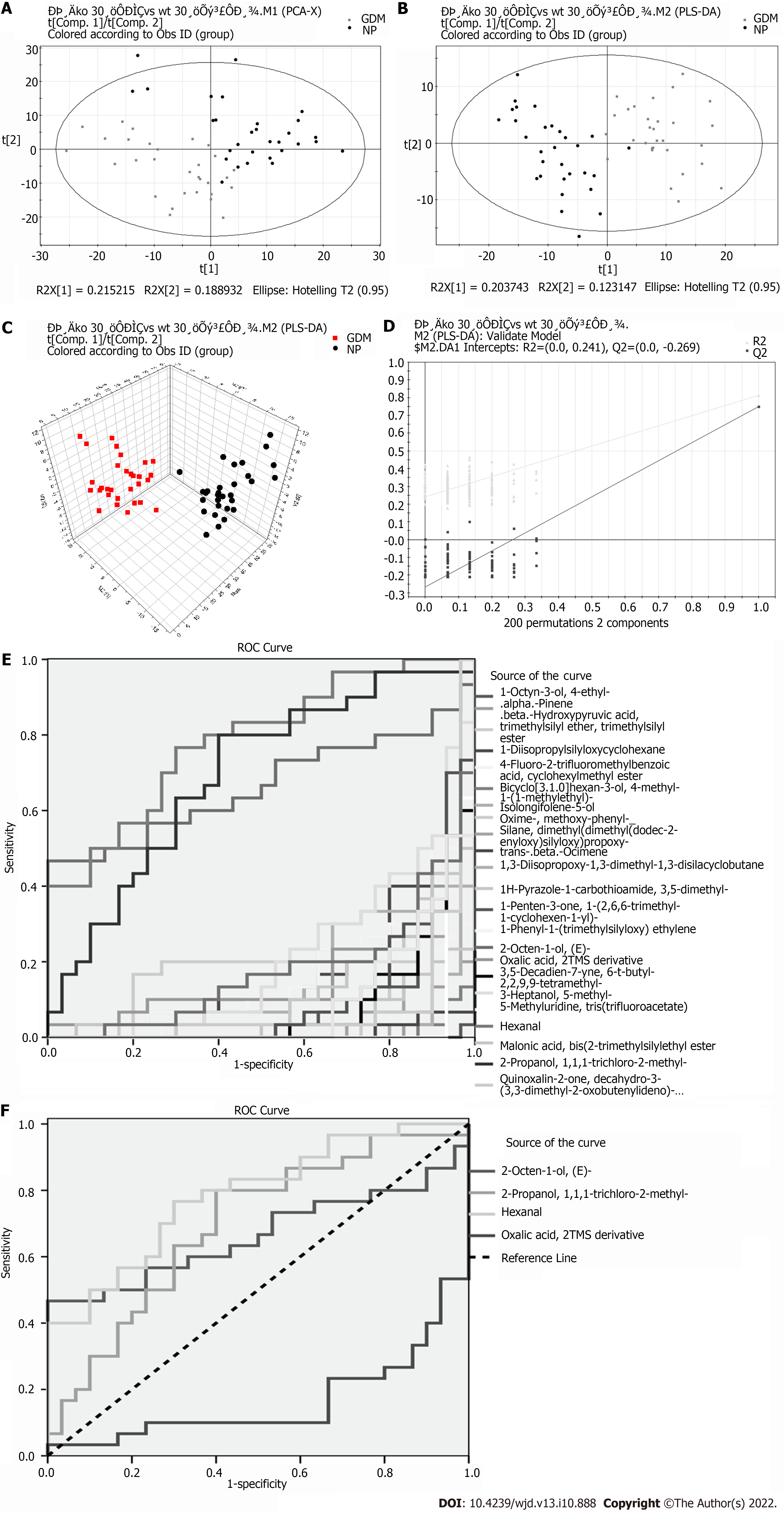
Eighteen significant differential metabolites were evident between the GDM and NP groups (Table 2). Comparing the GDM and NP groups revealed a good separation trend in the two groups in the two-dimensional PCA score diagram (Figure 2A). When a single prediction component and three orthogonal components were used, the PLS-DA score map (R2X[1] = 0.203743, R2X[2] = 0.123147, T2 = 0.95) revealed a good separation effect of the data of the GDM and NP groups (Figure 2B and C). Additionally, 200 iterations were conducted to test the supervision model. The R2 and Q2 values calculated from the converted data were lower than their original verification values [R2 = (0.0, 0.241), Q2 = (0.0, -0.269)], which proved the effectiveness of the supervision model (Figure 2D). The receiver operating characteristic (ROC) curves showed that the AUC of the three VOCs was greater than 0.5; the closer it was to 1, the better was the diagnostic effect (Figure 2E and F).
| Differential metabolite | VIP | P value | Time | FC (GDM/NP) |
| 1-Octyn-3-ol, 4-ethyl- | 2.2718 | 0.0000 | 9.1501 | -0.3956 |
| 4-Fluoro-2-trifluoromethylbenzoic acid, cyclohexylmethyl ester | 1.6588 | 0.0000 | 4.3833 | -0.3885 |
| Bicyclo[3.1.0]hexan-3-ol, 4-methyl-1-(1-methylethyl)- | 2.11317 | 0.0000 | 8.6667 | -0.4162 |
| Isolongifolene-5-ol | 1.85815 | 0.0000 | 18.1917 | -0.6099 |
| Oxime-, methoxy-phenyl- | 1.06288 | 0.0001 | 6.3083 | -0.2675 |
| Trans-beta-Ocimene | 2.01398 | 0.0000 | 6.9250 | -0.3406 |
| 1H-Pyrazole-1-carbothioamide, 3,5-dimethyl- | 1.66239 | 0.0000 | 4.7250 | -0.5068 |
| 1-Penten-3-one, 1-(2,6,6-trimethyl-1-cyclohexen-1-yl)- | 1.38245 | 0.0000 | 18.8000 | -0.9345 |
| 2-Octen-1-ol, (E)- | 1.11297 | 0.0287 | 8.0417 | 0.5487 |
| 2-Propanol, 1,1,1-trichloro-2-methyl- | 1.32854 | 0.0000 | 7.8167 | -0.2366 |
| 3,5-Decadien-7-yne, 6-t-butyl-2,2,9,9-tetramethyl- | 1.51135 | 0.0000 | 18.7750 | -0.5299 |
| 3-Heptanol, 5-methyl- | 1.2384 | 0.0001 | 5.3235 | -0.2684 |
| 5-Methyluridine, tris(trifluoroacetate) | 2.04962 | 0.0000 | 10.6833 | -0.2948 |
| Hexanal | 1.09786 | 0.0001 | 4.0858 | 0.3192 |
| Malonic acid, bis (2-trimethylsilylethyl ester | 1.59984 | 0.0000 | 19.5083 | -0.3655 |
| Oxalic acid, 2TMS derivative | 1.13131 | 0.0097 | 16.6477 | 0.1542 |
| Quinoxalin-2-one, decahydro-3-(3,3-dimethyl-2-oxobutenylideno)- | 1.73099 | 0.0000 | 7.3270 | -0.0682 |
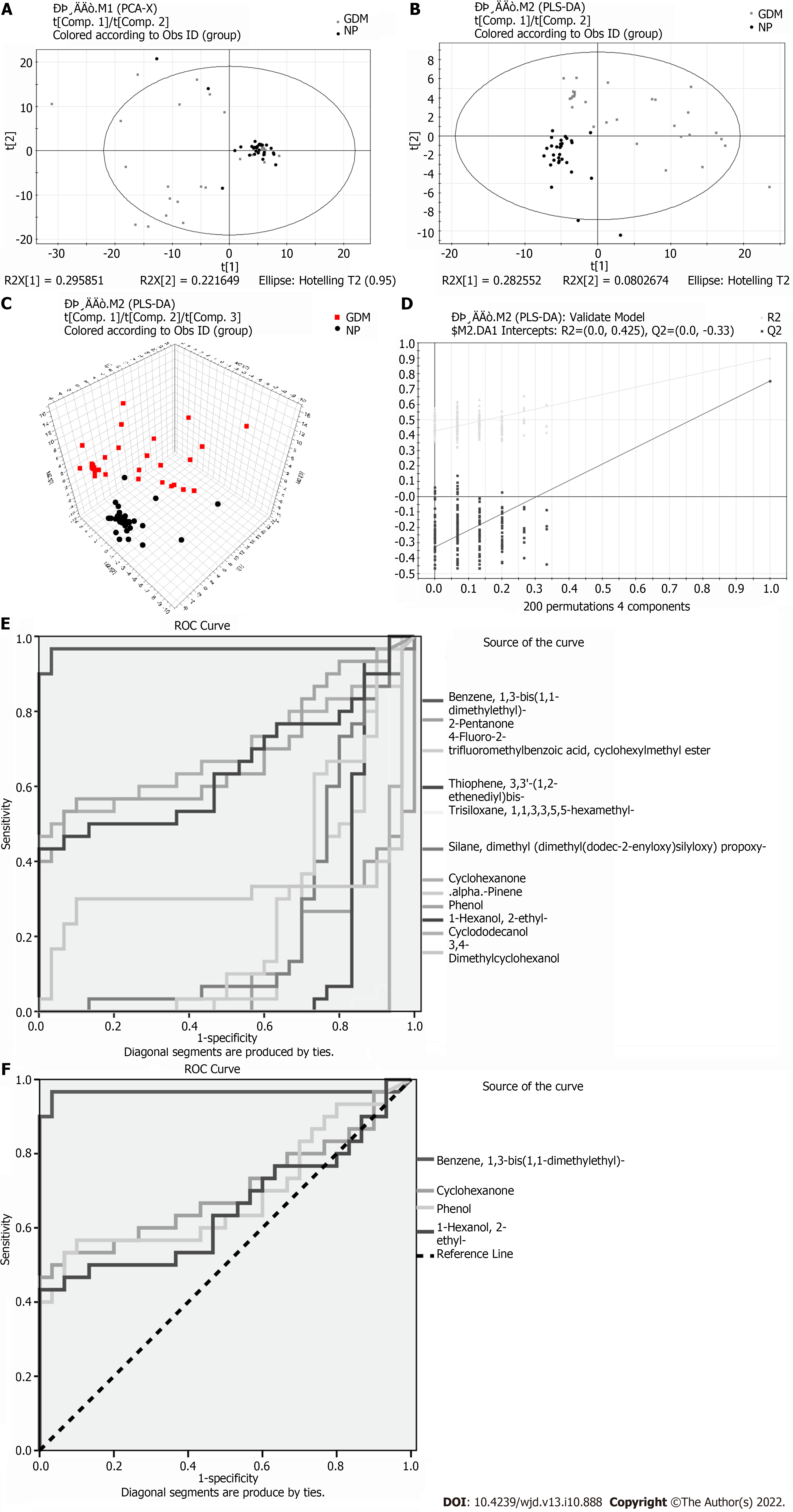
Eleven meaningfully differential metabolites were found between the GDM and NP groups (Table 3). Comparison between the GDM and NP groups revealed a good separation trend in the two-dimensional PCA score diagram (Figure 3A). When using a single prediction component and three orthogonal components, the PLS-DA score map (R2X[1] = 0.295851, R2X[2] = 0.221649, T2 = 0.95) showed that the data from the GDM and NP groups also had a good separation effect (Figure 3B and C). Additionally, 200 iterative permutations were conducted to test the supervision model. The R2 value and Q2 value calculated from the converted data were lower than their original verification values [R2 = (0.0, 0.425), Q2 = (0.0, -0.33)], which proved the effectiveness of the supervision model (Figure 3D). The ROC curves (Figure 3E and F) were the same as those described previously.
| Differential metabolite | Similarity | VIP | P value | Time | FC (GDM/Ctrl) |
| Benzene, 1,3-bis(1,1-dimethylethyl)- | 88 | 2.87589 | 0.0000 | 13.927 | 0.560880597 |
| 2-Pentanone | 87 | 2.2746 | 0.0000 | 3.017 | -0.077266923 |
| 4-Fluoro-2-trifluoromethylbenzoic acid, cyclohexylmethyl ester | 59 | 1.93204 | 0.0001 | 4.368 | -0.339022989 |
| Thiophene, 3,3'-(1,2-ethenediyl)bis- | 58 | 1.62894 | 0.0000 | 4.371 | -0.184457329 |
| Cyclohexanone | 95 | 1.13467 | 0.0081 | 6.018 | 0.935083138 |
| .alpha.-Pinene | 94 | 1.51705 | 0.0015 | 6.916 | -0.221670672 |
| Phenol | 98 | 1.02127 | 0.0135 | 7.970 | 1.492584113 |
| 1-Hexanol, 2-ethyl- | 96 | 1.16684 | 0.0444 | 9.118 | 0.693139386 |
| Cyclododecanol | 83 | 2.53908 | 0.0000 | 12.365 | -0.41722675 |
| 3,4-Dimethylcyclohexanol | 78 | 2.33881 | 0.0207 | 13.488 | -0.094482061 |
Investigations of metabolic substances in body fluids are an important supplement to the path
The following five compounds were highly expressed in the GDM group: 2-octen-1-ol; 2-propanol, 1,1,1-trichloro-2-methyl; hexanal; oxalic acid, 2TMS derivative; and oxime-, methoxy-phenyl. Most of these compounds are alcohols or aldehydes.
Aldehydes are active carbonyl organic molecules that are widely present in the body. They have variable structures. The structures of over 20 types of active aldehydes have been determined and studied. These include hexanal found in this study[7]. ROC curves for hexanal correlated well with the specificity and sensitivity of GDM (AUC > 0.5). The findings implicate hexanal as a potential marker of GDM.
Active aldehydes are mainly produced during lipid and glucose metabolism (including enzymatic and non-enzymatic pathways). The enzyme pathway usually involves an aldehyde intermediate or by-product produced during glucose and lipid metabolism in vivo[8]. This is also consistent with the disorder of active aldehyde metabolism observed in pregnant women with GDM. Under pathological conditions, aldehyde metabolism is disordered, resulting in abundant accumulation of aldehyde and formation of an aldehyde microenvironment[9]. Aldehyde metabolism disorders are involved in the occurrence and development of various diseases. Active aldehydes are closely related to the pathogenesis of endocrine diseases. Our previous study found that serum methylglyoxal (MGO) levels in pregnant women with GDM were significantly higher than those in healthy pregnant women[6]. MGO-induced insulin dysfunction (reduced secretion and increased resistance) may directly cause vascular dysfunction, which is a common complication of diabetes. In patients with neurodegenerative diseases, the levels of active aldehydes are also increased significantly[10]. The levels of 4-hydroxynonenal and acrolein in the brain of patients with mild cognitive impairment and early Alzheimer's disease are increased, and the neurotoxicity of acrolein is time- and concentration-dependent[11,12]. In addition, MGO can be detected in the arterial wall of a rat model of middle cerebral artery ischemia-reperfusion[13].
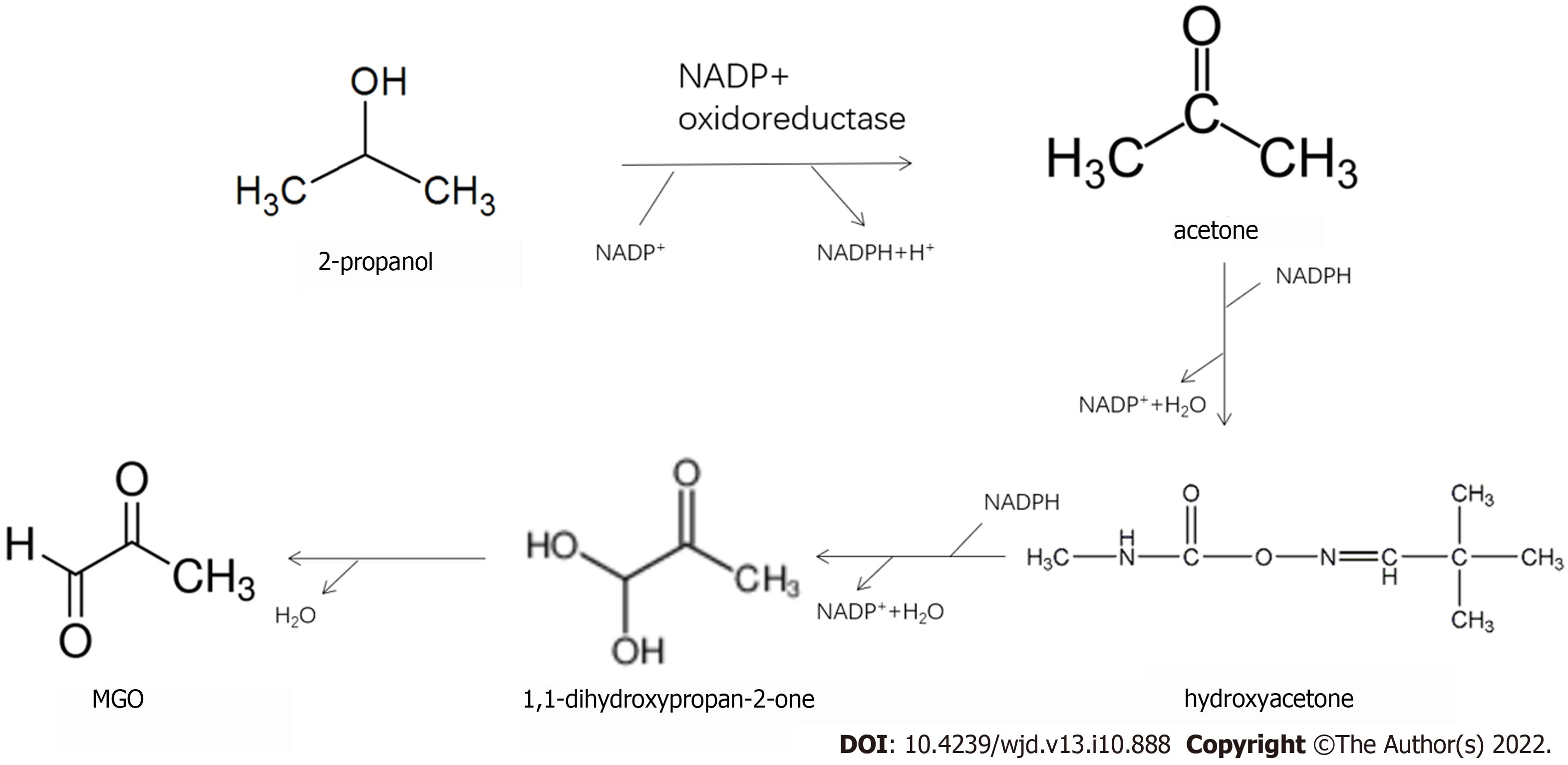
In a metabonomic study, GC-ion mobility spectrometry was used to analyze changes in exhaled VOCs in mild cognitive impairment, Alzheimer's disease, and normal control groups. Six compounds (tentatively acetone, 2-propanol, 2-butanone, hexanal, heptanaldehyde, and 1-butanol) play key roles in the diagnosis of mild cognitive impairment and Alzheimer's disease[14]. In addition to the detection of cognitive-related volatile substances, such as 2-propanol and hexanal, our analyses also revealed the metabolic pathways of 2-propanol and MGO (Figure 4). Therefore, the increase in serum 2-propanol levels in pregnant women with GDM may be a potential marker of MGO and cognitive decline.
In the urine analysis, 11 different metabolites were detected. Five of these were highly expressed in the GDM group: Benzene; 1,3-bis(1,1-dimethylethyl)-cyclohexanone; phenol; 1-hexanol, 2-ethyl; and 3,4-dimethylcyclohexanol. The ROC curves of these volatile substances correlated well, with AUCs > 0.5. The AUC for benzene was close to 0.9, and the AUCs for cyclohexanone, 1-hexanol, and phenol exceeded 0.5. Under the action of cytochrome P450 monooxygenase, human benzene is oxidized into toxic epoxy benzene, which combines with glutathione to form phenylmercaptouric acid. The latter is metabolized into phenol, catechol, hydroquinone, and other compounds and finally discharged from the body in the form of a sulfate conjugate or glucosidic acid. Cyclohexanone may be formed by oxidation of cyclohexane. Different amounts of cyclohexanone have been found in the exhaled breath of healthy individuals and patients with chronic obstructive pulmonary disease[15]. The levels of ethylhexyl alcohol in the blood and exhalate are reportedly reduced in patients with thyroid papillary cancer and colorectal cancer, respectively. This may be due to the consumption of ethylhexanol during tumor cell proliferation. Various lung cancer cells release 2-ethyl-1-hexanol[16]. These substances may also be related to the metabolic changes caused by oxidative stress and inflammatory changes in pregnant women with GDM.
An increasing number of metabonomic analyses of urine reflect the potential value of urine as an excreted product that can be collected non-invasively for analysis. Metabonomic methods for urinalysis can yield relatively complete metabonomic profiles and provide a new analytical approach for the diagnosis and mechanistic analysis of diseases. Metabolism of oral hypertension drugs, such as losartan, in patients with T2DM studied by GC has shown that plasma metabolites do not change, whereas urine metabolites (sorbitol and inositol) change significantly[17]. Diaz et al[18] analyzed the changes in urine metabolism in pregnant women in early, middle, and late pregnancy and found 21 different metabolites, including choline, creatinine, and lactate[18]. Other metabonomics analyses of the urinary metabolites of GDM pregnant women correlated p-inositol phosphate polysaccharide (P-IPG) with maternal blood glucose and pointed out that P-IPG may be a potential marker of insulin resistance in pregnant women with GDM[19]. Changes in urinary metabolites were observed in GDM patients from 8 to 16 wk postpartum[20]. The authors found that the longer the pregnancy cycle, the higher the lactose content in the urine samples of GDM pregnant women. However, lactose decreased rapidly after termination of pregnancy; in contrast, the blood concentrations of glucose and citric acid increased[20].
In this study, differential volatile metabolites in the serum and urine of pregnant women with GDM were detected by SPME. Volatile substances, such as hexanal, 2-octen-1-ol, and 2-propanol, were found in the serum of pregnant women with GDM. ROC curves indicated that they had a good correlation with GDM, which may be potential markers. Additional analyses demonstrated the metabolic conversion of 2-propanol in GDM serum to MGO, which could cause systemic damage. Thus, 2-propanol may be a potential marker of MGO. This should be investigated further. Volatile substances, such as benzene, cyclohexanone, 1-hexanol, and phenol, were found in the urine of pregnant women with GDM. However, their metabolic sources require further study.
Differential volatile metabolites in the serum of pregnant women with GDM mainly include hexanal, 2-octen-1-ol, and 2-propanol. The differential volatile metabolites in the urine of pregnant women with GDM include benzene, cyclohexanone, 1-hexanol, and phenol.
Gestational diabetes mellitus (GDM) is a metabolic disorder in which hyperglycemia develops during pregnancy in non-diabetic women.
Gas chromatography-mass spectrometry (GC-MS) was used to analyze changes in metabonomics in pregnant women with GDM and to explore the mechanism of cognitive function decline in pregnant women with GDM.
To study the cognitive function of pregnant women with GDM and to identify potential volatile markers to evaluate the cognitive impairment of pregnant women with GDM.
Solid-phase microextraction GC-MS analysis was used to detect organic matter in plasma and urine samples. The statistical methods used were principal component analysis and partial least squares-discriminant analysis.
Differential volatile metabolites in the serum of pregnant women with GDM mainly included hexanal, 2-octen-1-ol, and 2-propanol. The differential volatile metabolites in the urine of pregnant women with GDM included benzene, cyclohexanone, 1-hexanol, and phenol.
Of 2-propanol may be a potential volatile marker to evaluate the cognitive impairment of pregnant women with GDM.
The study of perinatal cognitive decline is worthwhile, especially in women with GDM. The key is the prevention and treatment of the disease. Whether 2-propanol can be used as a therapeutic target requires further investigation.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Dąbrowski M, Poland; Jovandaric M, Serbia; Suravajhala PN, India S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Cai YX
| 1. | Radzicka S, Pietryga M, Iciek R, Brązert J. The role of visfatin in pathogenesis of gestational diabetes (GDM). Ginekol Pol. 2018;89:518-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (1)] |
| 2. | McIntyre HD, Catalano P, Zhang C, Desoye G, Mathiesen ER, Damm P. Gestational diabetes mellitus. Nat Rev Dis Primers. 2019;5:47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 487] [Cited by in RCA: 983] [Article Influence: 163.8] [Reference Citation Analysis (1)] |
| 3. | Kim W, Park SK, Kim YL. Gestational diabetes mellitus diagnosed at 24 to 28 wk of gestation in older and obese Women: Is it too late? PLoS One. 2019;14:e0225955. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 4. | Artzi NS, Shilo S, Hadar E, Rossman H, Barbash-Hazan S, Ben-Haroush A, Balicer RD, Feldman B, Wiznitzer A, Segal E. Prediction of gestational diabetes based on nationwide electronic health records. Nat Med. 2020;26:71-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 151] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 5. | Pietzner M, Stewart ID, Raffler J, Khaw KT, Michelotti GA, Kastenmüller G, Wareham NJ, Langenberg C. Plasma metabolites to profile pathways in noncommunicable disease multimorbidity. Nat Med. 2021;27:471-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 132] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 6. | Sana S, Deng X, Guo L, Wang X, Li E. Cognitive Dysfunction of Pregnant Women with Gestational Diabetes Mellitus in Perinatal Period. J Healthc Eng. 2021;2021:2302379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Nelson MM, Baba SP, Anderson EJ. Biogenic Aldehydes as Therapeutic Targets for Cardiovascular Disease. Curr Opin Pharmacol. 2017;33:56-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Phelps RL, Metzger BE, Freinkel N. Carbohydrate metabolism in pregnancy. XVII. Diurnal profiles of plasma glucose, insulin, free fatty acids, triglycerides, cholesterol, and individual amino acids in late normal pregnancy. Am J Obstet Gynecol. 1981;140:730-736. [PubMed] |
| 9. | Goodacre R. Metabolomics shows the way to new discoveries. Genome Biol. 2005;6:354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Shamsaldeen YA, Mackenzie LS, Lione LA, Benham CD. Methylglyoxal, A Metabolite Increased in Diabetes is Associated with Insulin Resistance, Vascular Dysfunction and Neuropathies. Curr Drug Metab. 2016;17:359-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 11. | Park MW, Cha HW, Kim J, Kim JH, Yang H, Yoon S, Boonpraman N, Yi SS, Yoo ID, Moon JS. NOX4 promotes ferroptosis of astrocytes by oxidative stress-induced lipid peroxidation via the impairment of mitochondrial metabolism in Alzheimer's diseases. Redox Biol. 2021;41:101947. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 417] [Article Influence: 104.3] [Reference Citation Analysis (0)] |
| 12. | Shuster SO, Fica-Contreras SM, Hedges JS, Henning NJ, Choi S. Comparison of the reaction of methylglyoxal (MGO) with murine and human amyloid beta (Aβ): Insights into a mechanism of Alzheimer's disease (AD). Biochem Biophys Res Commun. 2020;533:1298-1302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Fang L, Li X, Zhong Y, Yu J, Yu L, Dai H, Yan M. Autophagy protects human brain microvascular endothelial cells against methylglyoxal-induced injuries, reproducible in a cerebral ischemic model in diabetic rats. J Neurochem. 2015;135:431-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 14. | Tiele A, Wicaksono A, Daulton E, Ifeachor E, Eyre V, Clarke S, Timings L, Pearson S, Covington JA, Li X. Breath-based non-invasive diagnosis of Alzheimer's disease: a pilot study. J Breath Res. 2020;14:026003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 15. | Mochalski P, Sponring A, King J, Unterkofler K, Troppmair J, Amann A. Release and uptake of volatile organic compounds by human hepatocellular carcinoma cells (HepG2) in vitro. Cancer Cell Int. 2013;13:72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 16. | Sponring A, Filipiak W, Mikoviny T, Ager C, Schubert J, Miekisch W, Amann A, Troppmair J. Release of volatile organic compounds from the lung cancer cell line NCI-H2087 in vitro. Anticancer Res. 2009;29:419-426. [PubMed] |
| 17. | Yuan K, Kong H, Guan Y, Yang J, Xu G. A GC-based metabonomics investigation of type 2 diabetes by organic acids metabolic profile. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;850:236-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 51] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Diaz SO, Barros AS, Goodfellow BJ, Duarte IF, Carreira IM, Galhano E, Pita C, Almeida Mdo C, Gil AM. Following healthy pregnancy by nuclear magnetic resonance (NMR) metabolic profiling of human urine. J Proteome Res. 2013;12:969-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 19. | Scioscia M, Kunjara S, Gumaa K, McLean P, Rodeck CH, Rademacher TW. Urinary excretion of inositol phosphoglycan P-type in gestational diabetes mellitus. Diabet Med. 2007;24:1300-1304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Sachse D, Sletner L, Mørkrid K, Jenum AK, Birkeland KI, Rise F, Piehler AP, Berg JP. Metabolic changes in urine during and after pregnancy in a large, multiethnic population-based cohort study of gestational diabetes. PLoS One. 2012;7:e52399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |