Published online Feb 15, 2021. doi: 10.4239/wjd.v12.i2.170
Peer-review started: October 21, 2020
First decision: December 4, 2020
Revised: December 7, 2020
Accepted: December 29, 2020
Article in press: December 29, 2020
Published online: February 15, 2021
Processing time: 93 Days and 18.6 Hours
Telemedicine is defined as the delivery of health services via remote communication and technology. It is a convenient and cost-effective method of intervention, which has shown to be successful in improving glyceamic control for type 2 diabetes patients. The utility of a successful diabetes intervention is vital to reduce disease complications, hospital admissions and associated economic costs.
To evaluate the effects of telemedicine interventions on hemoglobin A1c (HbA1c), systolic blood pressure (SBP), diastolic blood pressure (DBP), body mass index (BMI), post-prandial glucose (PPG), fasting plasma glucose (FPG), weight, cholesterol, mental and physical quality of life (QoL) in patients with type 2 diabetes. The secondary aim of this study is to determine the effect of the following subgroups on HbA1c post-telemedicine intervention; telemedicine characteristics, patient characteristics and self-care outcomes.
PubMed Central, Cochrane Library, Embase and Scopus databases were searched from inception until 18th of June 2020. The quality of the 43 included studies were assessed using the PEDro scale, and the random effects model was used to estimate outcomes and I2 for heterogeneity testing. The mean difference and standard deviation data were extracted for analysis.
We found a significant reduction in HbA1c [-0.486%; 95% confidence interval (CI) -0.561 to -0.410, P < 0.001], DBP (-0.875 mmHg; 95%CI -1.429 to -0.321, P < 0.01), PPG (-1.458 mmol/L; 95%CI -2.648 to -0.268, P < 0.01), FPG (-0.577 mmol/L; 95%CI -0.710 to -0.443, P < 0.001), weight (-0.243 kg; 95%CI -0.442 to -0.045, P < 0.05), BMI (-0.304; 95%CI -0.563 to -0.045, P < 0.05), mental QoL (2.210; 95%CI 0.053 to 4.367, P < 0.05) and physical QoL (-1.312; 95%CI 0.545 to 2.080, P < 0.001) for patients following telemedicine interventions in comparison to control groups. The results of the meta-analysis did not show any significant reductions in SBP and cholesterol in the telemedicine interventions compared to the control groups. The telemedicine characteristic subgroup analysis revealed that clinical treatment models of intervention, as well as those involving telemonitoring, and those provided via modes of videoconference or interactive telephone had the greatest effect on HbA1c reduction. In addition, interventions delivered at a less than weekly frequency, as well as those given for a duration of 6 mo, and those lead by allied health resulted in better HbA1c outcomes. Furthermore, interventions with a focus on biomedical parameters, as well as those with an engagement level > 70% and those with a drop-out rate of 10%-19.9% showed greatest HbA1c reduction. The patient characteristics investigation reported that Hispanic patients with T2DM had a greater HbA1c reduction post telemedicine intervention. For self-care outcomes, telemedicine interventions that resulted in higher post-intervention glucose monitoring and self-efficacy were shown to have better HbA1c reduction.
The findings indicate that telemedicine is effective for improving HbA1c and thus, glycemic control in patients with type 2 diabetes. In addition, telemedicine interventions were also found to significantly improved other health outcomes as well as QoL scores. The results of the subgroup analysis emphasized that interventions in the form of telemonitoring, via a clinical treatment model and with a focus on biomedical parameters, delivered at a less than weekly frequency and 6 mo duration would have the largest effect on HbA1c reduction. This is in addition to being led by allied health, through modes such as video conference and interactive telephone, with an intervention engagement level > 70% and a drop-out rate between 10%-19.9%. Due to the high heterogeneity of included studies and limitations, further studies with a larger sample size is needed to confirm our findings.
Core Tip: The findings indicate that telemedicine is effective for improving hemoglobin A1c (HbA1c) and thus, glycemic control in patients with type 2 diabetes. In addition, telemedicine interventions were also found to significantly improve other health outcomes as well as quality of life scores. The results of the subgroup analysis emphasized that interventions in the form of telemonitoring, via a clinical treatment model and with a focus on biomedical parameters, delivered at a less than weekly frequency and 6 mo duration would have the largest effect on HbA1c reduction. This is in addition to being led by allied health, through modes such as video conference and interactive telephone, with an intervention engagement level > 70% and a drop-out rate between 10%-19.9%.
- Citation: De Groot J, Wu D, Flynn D, Robertson D, Grant G, Sun J. Efficacy of telemedicine on glycaemic control in patients with type 2 diabetes: A meta-analysis. World J Diabetes 2021; 12(2): 170-197
- URL: https://www.wjgnet.com/1948-9358/full/v12/i2/170.htm
- DOI: https://dx.doi.org/10.4239/wjd.v12.i2.170
Type 2 diabetes mellitus (T2DM) is a chronic metabolic disorder in which the pancreas fails to secrete adequate insulin to maintain glucose homeostasis. Blood glucose levels are normally controlled by a series of anabolic and catabolic hormones, primarily insulin and glucagon, respectively[1]. It is evident that T2DM poses an extensive economic burden and negative consequences to health and healthcare systems. In 2014, an estimated 422 million people suffered from diabetes mellitus[2]. This equates to approximately 1 in 11 adults worldwide, 90% of whom suffer from T2DM[3]. The prevalence has nearly quadrupled since 1980 and current projections suggest that 642 million people will suffer from diabetes by 2040[2]. This is mainly attributed to rapid urbanisation, sedentary lifestyles and poor dietary habits[4]. In 2013, the Global Burden of Disease study found that diabetes was the 9th most common cause for reduced life expectancy. Furthermore, 5 million deaths were attributed to diabetes in 2015, equating to 1 death every 6 s[5]. The estimated global economic burden of diabetes mellitus in 2015 was over 1.2 trillion USD[6].
A longitudinal cohort study that followed 117629 female nurses over 20 years found that participants with T2DM at baseline had a 5-times greater risk of myocardial infarction and cerebrovascular disease when compared to those without diabetes[7]. Additionally, diabetes was the leading cause of blindness in individuals aged 20-74 years in 2011 and it was responsible for 44% of end-stage kidney disease and 60% of non-traumatic lower limb amputations[8]. Diabetes can also cause neuropathy through vascular disruption and direct neuronal injury. This may manifest as peripheral neuropathy affecting the extremities or autonomic neuropathy with organ dysfunction[9,10]. Some studies also suggest that T2DM is associated with a greater risk of depression, vascular dementia and Alzheimer’s disease[11,12].
Optimal diabetes management is necessary to slow disease progression, reduce complications and lessen the global healthcare burden[5]. Historically, weight loss and dietary changes have been the primary intervention to decrease visceral adiposity and improve glycaemic control[13,14]. Pharmacological management is also required where lifestyle modifications fail to achieve euglycemia[15]. Glycaemic control is closely monitored using glycosylated hemoglobin A1c (HbA1c) for long-term glycaemic control, vs post-prandial glucose (PPG) and fasting plasma glucose (FPG) for short-term control[16]. Regular testing of the eyes, feet, blood pressure, lipids and urinary albumin excretion are also recommended to screen for possible complications[17].
Despite these interventions, 45% of patients with T2DM fail to achieve the target HbA1c[18]. One major barrier to adequate glycaemic control appears to be patient’s inability to perform adequate self-care, for example, poor adherence to prescribed medications and lifestyle modifications. A recent meta-analysis found that the adherence rate for anti-diabetic medications varied from 38% to 93%[18]. Qualitative studies suggest that poor adherence can be attributed to forgetfulness, medication side effects and insufficient patient education[19]. Self-care has been defined as the formation of knowledge and awareness needed to survive with the nature of a disease in both a health and social context[20,21]. Self-care behaviours relevant to T2DM that assist the disease management include nutrition, physical activity, blood glucose monitoring, medication adherence, disease knowledge, positive behaviour changes and self-efficacy[21]. Research has shown that self-care behaviours are vital to diabetes self-management and have a direct impact on improving glycaemic control, quality of life (QoL) and decreasing incidence of complications[21,22]. Therefore, healthcare workers should aim to promote self-care behaviours in all T2DM interventions[21].
Other potential barriers to optimal glycaemic control in T2DM include inadequate patient outreach, time constraints and overly cautious prescribing habits[23,24]. These barriers have a profound effect on patient outcomes and healthcare costs. Studies have shown that the average patient cost is approximately 2.5-times higher in diabetes patients with a HbA1c greater than 10% compared to those with a HbA1c within the target range[25]. This is primarily due to a higher number of complications and hospitalisations in diabetics with poor glycaemic control[25]. Therefore, it is clear that effective interventions are required to improve patient outcomes and relieve the healthcare burden associated with T2DM.
In recent years, telehealth, also known as telemedicine, has been recognised as an effective way to deliver health services in rural and regional areas because it can be conducted remotely without compromising patient care[26]. Telehealth refers to the delivery of health services with the use of telecommunications and information technology[27]. It aims to maximise access to health services without any additional expense[28]. Telehealth is a broad term that can be classified as synchronous, asynchronous or remote monitoring. Synchronous telehealth refers to the delivery of health services in real time through smart devices, e.g., videoconferencing, mobile phone or computer. Asynchronous telehealth is when data relating to a patient is collected and reviewed at different points in time. Remote monitoring refers to the continuous evaluation of a patient’s clinical status based on specific health readings uploaded by the patient over the phone or online[29].
A review focusing on rural Australians found that telehealth provided better convenience, lower patient costs, improved access to specialist services and reduced hospital admissions[26]. These benefits also apply to patients with reduced mobility and other populations that experience a high degree of isolation[30,31]. Furthermore, telehealth has been shown to improve outcomes in psychiatric patients because it promotes greater self-efficacy and allows patients to maintain existing support networks[32,33]. Service providers also benefit from telehealth due to reduced travel expenses and greater educational opportunities for those working in remote areas[26]. Numerous randomised control trials have been conducted to evaluate the effectiveness of telehealth in patients with T2DM. However, no studies have provided in-depth analysis of the effectiveness of telehealth on health improvement of patients with diabetes[34]. Therefore, this study aimed to utilise a meta-analysis approach to synthesise results from high quality randomised controlled trials, and to comprehensively review literature on the effects of telemedicine interventions on health outcomes for patients with T2DM. The secondary aim is to analyse the effect of telemedicine characteristics, patient characteristics, and self-care outcomes on glycaemic control post-intervention.
The study protocol was registered on the International Prospective Register of Systematic Reviews (PROSPERO) prior to commencing the database search. Published randomised controlled trials (RCTs) that investigated the effect of telemedicine on T2DM were systematically searched on the following databases; Scopus, PubMed Central, Embase and Cochrane until June 18th 2020. Additional records were identified on Proquest Central (Dissertations and Thesis). The search terms were: Type 2 diabetes* AND Telemedicine OR telehealth OR e-health OR eHealth OR m-health OR mHealth AND HbA1c OR glucose OR insulin OR HOMA OR homeostatic model assessment AND Randomised* OR Randomized* OR RCT.
Full-text studies written in English and published between January 1st 1989 and June 18th 2020 were included. The RCTs were required to report at least one primary outcome (HbA1c, insulin or HOMA-IR) and utilise telemedicine intervention in the intervention group(s) to be included. Classic telephone calls were not considered telemedicine. The participants were required to be minimum 18 years of age and have physician diagnosed T2DM or meet the minimum clinical measurements to be diagnosed in the study.
Studies were excluded if the control group contained any component of telemedicine intervention. Studies were excluded if they measured outcomes for less than 24 wk or the data was not separated from individuals with other forms of diabetes, such as Type 1 diabetes. Incomplete post-intervention data and conference abstracts were not included. If the same data set was used in multiple studies, the study with the completed data was included and the other(s) excluded.
Reasonable attempts were made to obtain full-text articles in the cases where it was not available online, including messages to authors on ResearchGate and email. The literature search was performed by De Groot J and Flynn D independently. Any discrepancies in narrowing and excluding studies were resolved in discussion with Robertson D and Sun J.
Results of the searches were exported into EndNote software and duplicates were removed. Titles of the articles were screened to remove clearly irrelevant or non-RCT studies. Abstracts were read from the remaining list and further studies were excluded based on our criteria. For the remainder of studies, full text was reviewed for the purpose of data extraction, with some further exclusion of papers.
PEDRO quality assessment was performed for all studies assessed for full text. Studies were excluded if they were scored ≤ 3 out of 10. A conservative approach was utilised, where if a component could not be confirmed, it was rated 0 for that component. The 10 components were randomisation, concealed allocation, group characteristics similar at baseline, subjects blinded, therapists blinded, assessors blinded, key outcome obtained from 85% of participants, intention to treat analysis, between group statistical analysis, and point measures with measures of variability.
Data extraction was completed by Flynn D and Robertson D, whilst De Groot J and Sun J verified all data entries with the full text of the published papers. The following information was collected from each paper; Table 1 study details, information for the PEDRO quality assessment and the primary and secondary outcomes at both pre- and post-intervention. Primary outcomes were HbA1c, HOMA-IR and insulin. Secondary outcomes were FPG, PPG, body mass index (BMI), weight, waist circumference, systolic blood pressure (SBP), diastolic blood pressure (DBP), total cholesterol (TC), depression, anxiety, QoL. The data was converted to mean and standard deviation where possible and appropriate. Data was converted from median and range if it was considered to have a normal distribution. The data set was excluded if it was not reported as mean ± SD and it was not appropriate to convert, for example a non-normal distribution. Blood glucose was converted from mg/dL to mmol/L and blood lipids were converted from mg/dL to mmol/L. The short form (SF) health survey is used to measure QoL. Although the survey length can vary, each score is standardised from 0-100, 0 being maximum disability and 100 being no disability. The diabetes treatment satisfaction questionnaire was also included with the same standardisation[35]. Mean difference standard deviation was calculated if not provided by the study using the following equation: σ = sqrt (σ12/n1 + σ22/n2). Subgroup information was collected about patient demographics, characteristics about the telemedicine intervention and self-care outcomes.
| Ref. | Number centres, funding | Participants I/C | Design, location (country/territory) | Mean age intervention (SD)/mean age control (SD) (yr) | Sex (M/F) | Intervention type | Control | Measured outcomes | Key results | PEDro | Notes |
| Alanzi et al[36] 2018 | 4, none stated | 10/10 | NB, Saudi Arabia | 8 × (18-40), 2 × (41-50)/9 × (18-40), 1 × (41-50) | 15/5 | Mobile application; Bluetooth transmission of blood glucose, social networking, cognitive behavioral therapy (6 mo) | Usual care | HbA1c | Greater decrease in HbA1c for intervention vs control | 5 | - |
| Arora et al[37] 2014 | 1, Agile Health LLC and McKesson foundation | 64/64 | NB, United States | 50.5 (10.3)/51 (10.2) | 46/82 | Twice daily text messages about self-care and medication adherence (6 mo) | Usual care | HbA1c | No significant differences | 4 | Data converted from median (range)a |
| Bujnowska-Fedak et al[38] 2011 | Multi-centre, none stated | 50/50 | NB, Poland | 53.1 (25.2)/57.5 (27.4) | 51/44 | Transmission of blood glucose to computer network via glucometer, systems sends text to GP (6 mo) | Usual care with glucometer | HbA1c, FPG, PPG, BMI, SBP, DBP, TC | No significant differences | 5 | - |
| Cho et al[39] 2017 | 3, Korean Government | 244/240 | NB, South Korea | 52.9 (9.2)/53.4 (8.7) | 307/177 | Glucometer and blood pressure measurements sent via internet connected device, doctors and nurses send back recommendations (6 mo) | Usual care | HbA1c, FPG, weight, BMI, SBP, DBP, TC, HDL, LDL, TG, WC, PPG, QoL | Greater decrease in HbA1c, PPG and WC for intervention vs control | 6 | - |
| Crowley et al[40] 2016 | 1, Veterans Affairs | 25/25 | NB, United States | 60 (8.4)/60 (9.2) | 48/2 | Glucose testing sent via telephone voice system daily + fortnightly self-management modules via telephone (6 mo) | Usual care | HbA1c, SBP, DBP, Depression | Greater decrease in HbA1c, SBP, DBP for intervention vs control | 6 | - |
| Dafoulas et al[41] 2015 | Multicentre, European Community | 74/80 | SB, Greece | 58.28 (0.93)/64.11 (0.6) | 68/86 | Transmission of blood glucose weekly via mobile app + calls from doctors as required (12 mo) | Usual care | HbA1c, QoL | Greater decrease in HbA1c, QoL physical, QoL mental and physical activity for intervention vs control | 5 | - |
| Dario et al[42] 2017 | 3, European Commission and RENEWING HEALTH project | 208/91 | NB, Italy | 73.05 (5.79)/73.04 (5.28) | 168/131 | Transmission of glucometer via online gateway to doctor | Usual care | HbA1c, QoL, depression, anxiety | No significant differences | 5 | - |
| Fortmann et al[43] 2017 | 4, Mckesson foundation and National center for advancing translational sciences grant | 63/63 | NB, United States | 47.8 (9.0)/49.1 (10.6) | 32/94 | Text messages, up to 3 per day; motivational, educational or call-to-action (6 mo) | Usual care | HbA1c, FPG, TC, HDL, LDL, TG, SBP, DBP, BMI, weight | Greater decrease in HbA1c for intervention vs control | 6 | - |
| Fountoulakis et al[44] 2015 | 2, None stated | 54/26 | NB, Greece | 61.3 (11.4)/63.5 (13.8) | 55/25 | USB-connected modem compatible with glucometer, data transmitted to computers of Department of Endocrinology | Usual care | HbA1c, BMI | Greater decrease in HbA1c for intervention vs control | 5 | Separates T1D and T2D data. Recruitment ‘n’ not provided |
| Holmen et al[45] 2014 | 2, European Union + 6 others | (1) 51/50, (2) 50/50 | NB, Norway | 58.6 (11.8)/55.9 (12.2) | (1) 64/37, (2) 55/45 | (1) mobile phone based self-management system app (automatic blood glucose transmission, input of diet and exercise info, goal managing). (2) Intervention 1 + telephone behaviour-change counselling from nurse for first 4 mo (12 mo) | Usual care | HbA1c, weight | No significant differences | 5 | 3 arm (2 interventions) |
| Jeong et al[46] 2018 | 4, Ministry of Health and Welfare, Republic of Korea and Ministry of Trade, Industry, and Energy South Korea | (1) 113/113, (2)112/113 | NB, South Korea | 53.65 (9.10)/53.16 (9.06) | (1) 64/37, (2) 55/45 | (1) telemonitoring: outpatient clinic + tablet unit with auto blood glucose and weight transmission, diet and exercise monitoring with automated feedback texts. (2) telemedicine: intervention 1 + videoconference outpatient clinic (24 wk) | Usual care | HbA1c, FPG, PPG, BMI, body weight, SBP, DBP, HDL, LDL, TG | Greater decrease in FPG for (1) telemonitoring group and (2) telemedicine group vs control | 6 | 3 arm (2 interventions)a |
| Kempf et al[47] 2017 | Multicentre, Boehringer Ingelheim International and University Dusseldorf | 102/100 | SB, Germany | 59 (9)/60 (8) | 90/77 | Glucometer, weight and pedometer data auto uploaded to online portal + weekly phone calls about lifestyle change and self-management (12 wk, 52 wk follow-up data) | Usual care with self-management guide, scale and step counter | HbA1c, FPG, weight, BMI, SBP, DBP, TC, HDL, LDL, QoL | Greater decease in HbA1c, body weight, BMI, SBP and QoL for intervention vs control | 8 | TG excluded as median (IQR) and skewed |
| Kim et al[48] 2007 | 1, College of Nursing Catholic University of Korea | 30/30 | NB, Korea | 46.8 (8.8)/47.5 (9.1) | 22/29 | Self-monitored glucose and medication use was submitted online and weekly recommendations were sent via text (6 mo) | Usual care | HbA1c, FPG, PPG | Greater decrease in PPG for intervention vs control | 5 | - |
| Kim et al[49] 2008 | 1, Korean Government | 20/20 | NB, Korea | 45.5 (9.1)/48.5 (8) | 16/18 | Medications and self-monitored glucose readings were used to create online medical record + weekly recommendations sent via text message (12 mo) | Usual care | HbA1c, FPG, PPG | Greater decrease in HbA1c for intervention vs control | 5 | - |
| Kim et al[50] 2016 | 1, UB care | 110/110 | NB, China | 52.5 (9.1)/55.6 (10) | 88/94 | Internet-based self-monitoring of blood glucose + recommendations via a website (6 mo) | Usual care | HbA1c, SBP, DBP, TC, TG, HDL, LDL | Greater decrease in HbA1c and FPG for intervention vs control | 4 | - |
| Kim et al[51] 2019 | 3, HealthConnect Co. | 97/94 | NB, South Korea | 60 (8.4)/56.7 (9.1) | 99/73 | Smartphone modules for Bluetooth glucometer, diet, exercise via activity tracker + clinical decision support (24 wk) | Usual care + Manual glucose logbook | HbA1c, FPG, weight, SBP, DBP, TC, TG, LDL, HDL | Greater decrease in HbA1c for intervention vs control | 5 | a |
| Kleinman et al[52] 2017 | 3, Gather Health LLC | 44/46 | NB, India | 48.8 (9)/48 (9.5) | 63/27 | Mobile app provided daily reminders for self-management tasks and data + provider communication and treatment adjustment (6 mo) | Usual care | HbA1c, FPG, BMI | Greater decrease in HbA1c, greater increase self-reported medication adherence and BG testing for intervention vs control | 7 | |
| Lee et al[53] 2020 | 11, Ministery of Science, Technology and Innovation Malaysia | 120/120 | SB, Malaysia | 56.1 (9.2)/56.3 (8.6) | 108/132 | Auto transmission of glucometer data via online portal with automatic feedback + team encouraged self-management monthly + change to medication if required (12 mo) | Usual care | HbA1c, FPG, SBP, DBP, TC, TG, HDL, LDL, QoL | Greater decrease in HbA1c for intervention vs control | 7 | a |
| Lim et al[54] 2016 | 1, Korea healthcare technology R&D project | 50/50 | NB, Korea | 64.3 (5.2)/65.8 (4.7) | 75/25 | Bluetooth glucometer and activity monitor, and dietary and exercise transmission to website + tailored feedback to device or mobile (6 mo) | Usual care + self monitored blood glucose | HbA1c, BMI, SBP, DBP, TC, TG, LDL, HDL, WC, FPG, PPG | Greater decrease in HbA1c for intervention vs control | 5 | - |
| Liou et al[55] 2014 | 6, none stated | 54/41 | NB, Taiwan | 56.6 (7.7)/57 (7.5) | 48/47 | 6 sessions about diet, medication, stress management, goal setting and foot care, including 2 via teleconference (6 mo) | Usual care + 1 in-person education session by nurse | HbA1c, BMI, SBP, DBP, TC, TG, LDL, HDL | Greater decrease in HbA1c for intervention vs control | 5 | TG excluded as log-transformeda |
| Luley et al[56] 2011 | 1, none stated | 35/35 | NB, Germany | 59 (9)/58 (7) | 34/36 | Bluetooth transmission from scales and accelerometer via Homebox to server + weekly feedback and progress via letters + low calorie diet (6 mo) | Usual care + conventional low fat diet | Weight, BMI, HBA1c, TG, HDL, FPG | Greater decrease in HbA1c weight, BMI and FPG for intervention vs control | 5 | a |
| Nicolucci et al[57] 2015 | Multi-centre, MSD Italia grant | 153/149 | NB, Italy | 59.1(10.3)/57.8(8.9) | 94/92 | Bluetooth transmission weight, blood glucose and blood pressure measurements to server via internet + remote support and GP feedback | Usual care | HbA1c, weight, SBP, DBP, TC, HDL, LDL, TG, QoL | Greater decrease of HbA1c and increase of mental summary QoL for intervention vs control | 4 | - |
| Or et al[58] 2020 | 1, Food and Health Bureau | 151/148 | NB, Hong Kong | 63.9 (10.2)/63.7 (9.6) | 192/107 | Bluetooth glucometer and BP monior + website-based technological surrogate nursing care encouraged self-management via tablet with resources (24 wk) | Usual care | HbA1c, SBP, DBP | No significant differences | 7 | - |
| Orsama et al[59] 2013 | 1, Finnish funding agency, Technical Research centre Finland & Bayer HealthCare | 27/29 | NB, Finland | 62.3 (6.5)/61.5 (9.1) | 26/22 | Mobile app transmission of weight, blood glucose, stepcount and blood pressure + automatic feedback with behaviour change focus + website viewing of health record | Usual care | HbA1c, weight, SBP, DBP | Greater decrease in HbA1c and weight for intervention vs control | 5 | Baseline HbA1c up to 2 months were used |
| Pacaud et al[60] 2012 | 1, Lawson Foundation | (1) 18/21, (2) 29/21 | NB, Canada | 52.1 (8.8)/56.3 (8.1) | (1) 10/8, (2) 12/17 | (1) Webstatic: email consults (12 mo). 1A-male, 1B-female. (2) Web Interactive: online chat and email consults. 2A-male, 2B-female. (12 mo) | Usual care + education (face-to-face education, group sessions) | HbA1c, QoL | No significant differences | 5 | 5 arm (2 interventions and separation by sex). Post-test 'n 'not givena |
| Pressman et al[61] 2014 | Multi-centre, Samsung | 118/107 | SB, America | 54.8 (9.8)/56.4 (8.7) | 122/76 | Weekly transmission of blood glucose, blood pressure and weight to case manager via device + tailored telephone feedback (6 mo) | Usual care | Weight, BMI, SBP, LDL, HbA1c | No significant differences | 6 | a |
| Quinn et al[62] 2016 | Multi-centre, University of Maryland and WellDoc | (1) 37/29, (2) 25/27 | NB, United States | 47.3 (6.8)/47.4 (7.5) | (1) 32/34, (2) 27/25 | Mobile phone coaching App, entering blood glucose, diet, medication info with behavioural, motivation or feedback messages + Web portal (12 mo) | Usual care | HbA1c | Greater decease in HbA1c for intervention vs control, no statistical difference for age groups | 4 | 3 arm (separated by age < 55 and > 55) |
| Ramadas et al[63] 2018 | 3, Monash University | 66/62 | NB, Malaysia | 49.6 (10.7)/51.5 (10.3) | 77/51 | Web-based nutrition lesson plan and dietary intervention (12 mo) | Usual care | FBG, HbA1c | No significant differences | 7 | Mean difference provided by study for HbA1ca |
| Rodríguez-Idígoras et al[64] 2009 | Multi-centre, Roche Diagnostics Spain | 161/167 | NB, Spain | 63.32 (11.13)/64.52 (10.32) | 169/159 | Transmission of glucometer data via mobile + mobile contact by patient or healthcare staff when required + teleconsults (12 mo) | Usual care | HbA1c | Greater decrease in HbA1c for intervention vs control | 7 | - |
| Shea et al[65] 2009 | 2, Medicare and Medicaid + 6 more | 844/821 | SB, United States | 70.8 (6.5)/ 70.9 (6.8) | 616/1049 | Home unit with web-enabled computer access to website with education + webcam for videoconferencing + auto uploading glucometer and blood pressure data (5 yr) | Usual care | HbA1c, LDL, SBP, DBP | Greater decrease in HbA1c (years 4-5) and LDL (years 1-4) and SBP (years 1-5) and DBP (years 1-5) for intervention vs control | 5 | Changed from Weinstock 2011, Adjusted mean used |
| Stone et al[66] 2010 | Multi-centre, U.S. Air Force | 64/73 | SB, United States | 3 × (< 45), 38 × (45-65), 23 × (> 65)/4 × (< 45), 43 × (45-65), 26 × (> 65) | 135/2 | Transmission of blood glucose, blood pressure and weight via internet-connected device + monthly phone calls: self-management education, medication changes (6 mo) | Monthly phone call | HbA1c, weight, SBP, DBP, TC, HDL, LDL, TG | Greater decrease in HbA1c for intervention vs control | 7 | - |
| Sun et al[67] 2019 | 1, Science Technology Department Jilin and Jilin University | 44/47 | NB, China | 67.9 (66,71), 68.04 (66, 72) Median (IQR) | 37/54 | Mobile phone application + Bluetooth glucometer + advice every 2 wk via app (6 mo) | Usual care + glucometer + outpatient visits | HbA1c, PPG, FPG, TC, TG, BMI, SBP | Greater decrease in HbA1c and PPG for intervention vs control | 5 | HDL, LDL, BMI, DBP excluded due to median (IQR) and skewed |
| Tang et al[68] 2013 | 1, Agency for Health Research and Quality | 193/189 | SB, United States | 54 (10.7)/53.5 (10.2) | 249/166 | Bluetooth glucometer readings + uploaded nutrition, exercise logs, insulin record online + messages with healthcare team and personalised text and video | Usual care | HbA1c, LDL, weight, SBP, DBP | No significant differences at 12 mo, but greater decrease in HbA1c at 6 months of intervention vs control | 7 | - |
| Vinitha et al[69] 2019 | 5, AstraZeneca Pharma India Ltd | 126/122 | NB, India | 42.4 (8.5)/44.1 (8.9) | 168/80 | Text messages 3 times weekly: education, lifestyle, medication (24 mo) | Usual care | HbA1c, weight, BMI, WC, SBP, DBP, FPG, PPG, TC, HDL, LDL, QoL | Greater decrease in HbA1c, SBP and FPG for intervention vs control | 6 | TG excluded due to median (IQR) and skewed |
| Wang et al[70] 2017 | 1, Science Technology Department Jilin | 106/106 | NB, China | 52.6 (9.1)/54.7 (10.3) | 116/104 | Transfer of glucometer data to health centre via website + receival of information/advice (6 mo) | Usual care | HbA1c, TC, HDL, LDL | Greater decrease in HbA1c, PPG, FPG and TG for intervention vs control | 4 | FPG, TG, BMI, SBP, DBP, PPG excluded due to median (IQR) and skewed |
| Wang et al[71] 2018 | 1, National Center for Clinical and Translational Sciences grant | 11/6 | NB, United States | 58.8 (5.9)/49.2 (10.2) | 7/10 | Smartphone application + Bluetooth scale and glucometer + 12 in-person behaviour-change sessions (6 mo) | Usual care | HbA1c | Greater decrease in HbA1c for intervention vs control | 5 | Paper group intervention not included here |
| Wayne et al[72] 2015 | 2, Public Health Agency Canada | 67/64 | NB, Canada | 53.1 (10.9)/53.3 (11.9) | 28/72 | Mobile phone monitoring and health coaching (6 mo) | Health coaching without mobile | HbA1c, weight, WC, BMI, depression, anxiety, QoL | Greater decrease in BMI and greater increase in QoL (Mental SF-12) for intervention vs control | 5 | - |
| Welch et al[73] 2011 | 1, Baystate Medical Center | 25/21 | NB, United States | 54.4 (10.4)/57.5 (9.5) | 31/15 | Transmission of comprehensive patient data to clinician via internet + 7 in-person visits of 1 h diabetes education (12 mo) | 7 in-person visits of 1 h diabetes education | HbA1c, SBP, DBP, BMI | Greater decrease in HbA1c and SBP for intervention vs control | 4 | Depression excluded due to nil measure of variance |
| Whittemore et al[74] 2020 | 5, none stated | 26/21 | SB, Mexico | 53.9 (9.2)/56.8 (8.3) | 16/31 | 7 weekly self-management group sessions + 6 mo daily text messages about behaviour change + unconnected glucometer (6 mo) | Usual care (waitlist for intervention) | HbA1c, SBP, DBP BMI, depression | No significant differences | 7 | a |
| Wild et al[75] 2016 | 4, Chief Scientist Office Grant | 160/161 | SB, United Kingdom | 60.5 (9.8)/61.4 (9.8) | 214/107 | Bluetooth transmission of glucose, blood pressure and weight to website + called when lifestyle and medication changes required (9 mo) | Usual care | HbA1c, SBP, DBP, weight | Greater decrease in HbA1c, SBP and DBP for intervention vs control | 7 | - |
| Williams et al[76] 2012 | 3, QLD Health, HCF Health and Medical Research Foundation | 60/60 | NB, Australia | 58.4 (8.2)/56.4 (8.3) | 76/44 | Bluetooth Glucometer + Interactive automated telephone system encouraging self-management behaviours (6 mo) | Usual care | HbA1c, QoL | Greater decrease in HbA1c and improved mental HRQL for intervention vs control | 5 | - |
| Xu et al[77] 2020 | 1, National Institutes of Health, Veterans Affairs | 33/32 | NB, United States | 54.6 (1.82)/55.34 (1.94) | 20/44 | Glucose data self reported and collected by automated phone calls/texts, shared with providers with bidirectional communication (12 mo) | Usual care | HbA1c | No significant differences | 5 | Excluded FPG measures as it was patient-reported |
| Yip et al[78] 2002 | 1, none stated | 41/41 | SB, China | 55.29 (8.63)/57.54 (8.52) | 70/52 | 4 educational sessions via videoconference + telephone monitoring (4.5 mo). Outcomes measured at 6 mo) | Usual care | HbA1c | No significant differences | 5 | Additional non-telehealth group not included |
Descriptive words about the intervention, patient demographic and self-care outcomes were collected from each paper. These were then manually coded into subgroups, as defined in the Supplementary Tables 1-4.
| Variables | Studies (n) | Participant (n) | Mean difference | Effect size | Publications Bias | ||||
| MD (95%CI) | Q test | I2 (%) | Effect size (95%CI) | Q test | I2 (%) | Egger’s t (95%CI) | |||
| HbA1c | 47 | 6932 | -0.486 (-0.561, -0.410)c | 2689.381 | 98.290c | -1.523 (-1.896, -1.150)c | 1857.351 | 97.523c | 2.604 (-6.389, -0.815)a |
| SBP | 22 | 4053 | -0.458 (-1.403, 0.486) | 2772.104 | 99.206c | -0.117 (-0.603, 0.370) | 1060.208 | 98.019c | 0.937 (-2.814, 7.432) |
| DBP | 20 | 3764 | -0.875 (-1.429, -0.321)b | 683.275 | 97.219c | -0.376 (-0.743, -0.009)a | 535.388 | 96.451c | 0.896 (-5.695, 2.280) |
| PPG | 6 | 1497 | -1.458 (-2.648, -0.268)b | 437.802 | 98.858c | -1.091 (-1.695, -0.486)c | 256.915 | 98.054 | 0.035 (-9.234, 8.984) |
| FPG | 15 | 2508 | -0.577 (-0.710, -0.443)c | 163.957 | 91.461c | -1.098 (-1.575, -0.622)c | 396.579 | 96.470c | 2.730 (-4.501, -0.524)a |
| Weight | 17 | 3235 | -0.243 (-0.442, -0.045)a | 120.538 | 86.726c | -0.549 (-0.950, -0.149)b | 145.580 | 89.009c | 1.225 (-3.014, 0.814) |
| BMI | 15 | 2357 | -0.304 (-0.563, -0.045)a | 120.110 | 88.344c | -0.346 (-0.514, -0.178)c | 232.373 | 93.975c | 2.963 (-4.376, -0.701)a |
| Cholesterol | 15 | 2951 | -0.070 (-0.141, 0.002) | 492.468 | 97.157c | -0.339 (-0.741, 0.063)a | 379.019 | 96.306c | 1.180 (-7.845, 2.304) |
| Mental QoL | 6 | 634 | 2.210 (0.053, 4.367)a | 842.443 | 99.406c | 0.739 (-0.709, 2.186) | 736.156 | 99.185c | 1.124 (-13.969, 32.978) |
| Physical QoL | 7 | 1467 | 1.312 (0.545, 2.080)c | 210.628 | 97.151c | 1.111 (0.432, 1.790)c | 192.042 | 96.876c | 0.425 (-8.626, 12.046) |
| Subgroups | Studies | Participant | Mean difference | Effect size | ||||
| Q test | Mean difference (95%CI) | I2 (%) | Q test | Effect size (95%CI) | I2 (%) | |||
| Mode of intervention | ||||||||
| Text message | 5 | 469 | 55.331 | -0.591 (-0.892, -0.290)c | 92.771c | 133.957 | -1.377 (-2.503, -0.252)a | 97.014c |
| Mobile app | 12 | 1154 | 321.220 | -0.359 (-0.502, -0.215)c | 96.576c | 281.110 | -1.308 (-2.009, -0.607)c | 96.087c |
| Interactive telephone | 2 | 170 | 9.069 | -0.782 (-1.172, -0.391)c | 88.974b | 2.226 | -2.600 (-3.243, -1.957)c | 55.074 |
| Internet server/computer network | 7 | 1288 | 353.994 | -0.431 (-0.558, -0.304)c | 98.305c | 332.245 | -1.751 (-2.819, -0.684)c | 98.194c |
| Website | 14 | 1875 | 843.158 | -0.539 (-0.706, -0.371)c | 98.458c | 696.131 | -1.747 (-2.587, -0.907)c | 98.133c |
| Video conference | 2 | 164 | 1.687 | -0.845 (-1.144, -0.546)c | 40.719 | 25.261 | -1.450 (-3.323, 0.424) | 96.041c |
| Device or tablet | 5 | 1812 | 332.123 | -0.278 (-0.747, 0.191)c | 98.796c | 162.677 | -0.905 (-1.725, -0.085)a | 97.541c |
| Duration of intervention, months | ||||||||
| ≤ 6 | 6 | 1158 | 222.519 | -0.363 (-0.567, -0.159)c | 97.753c | 239.478 | -1.290 (-2.186, -0.394)b | 97.912c |
| 6 | 20 | 2234 | 1260.455 | -0.626 (-0.766, -0.486)c | 98.493c | 740.442 | -2.054 (-2.712, -1.396)c | 97.434c |
| > 6 | 21 | 2565 | 1173.100 | -0.380 (-0.507, -0.252)c | 98.295c | 674.872 | -1.080 (-1.583, -0.578)c | 97.036c |
| Focus of intervention | ||||||||
| Biomedical parameters | 16 | 2633 | 1830.624 | -0.687 (-0.821, -0.553)c | 99.181c | 844.645 | -2.406 (-3.174, -1.639)c | 98.224c |
| Self-management behaviors | 7 | 334 | 64.189 | -0.360 (-0.805, 0.086) | 90.653c | 139.861 | -0.740 (-1.753, 0.274) | 95.710c |
| Biomedical and self-management | 24 | 3965 | 687.693 | -0.348 (-0.442, -0.253)c | 96.655c | 645.659 | -1.171 (-1.583, -0.759)c | 96.438c |
| Telemedicine method | ||||||||
| Telemonitoring | 19 | 3076 | 1872.902 | -0.553 (-0.662, -0.445)c | 99.039c | 1107.777 | -2.263 (-2.991, -1.534)c | 98.375c |
| Tele-education | 12 | 994 | 125.448 | -0.495 (-0.683, -0.307)c | 91.231c | 289.370 | -1.068 (-1.767, -0.368)b | 96.199c |
| Telemonitoring and tele-education | 16 | 2862 | 501.993 | -0.391 (-0.542, -0.240)c | 97.012c | 287.276 | -1.002 (-1.415, -0.588)c | 94.779c |
| Model of intervention | ||||||||
| Behavior change | 28 | 2773 | 629.401 | -0.452 (-0.575, -0.329)c | 95.710c | 596.338 | -1.041 (-1.426, -0.656)c | 95.472c |
| Treatment | 13 | 2248 | 1518.194 | -0.589 (-0.725, -0.453)c | 99.210c | 710.693 | -2.386 (-3.249, -1.522)c | 98.312c |
| Behavior change and treatment | 6 | 1911 | 448.804 | -0.401 (-0.612, -0.189)c | 98.886c | 332.288 | -1.934 (-3.070, -0.799)c | 98.495c |
| Intervention lead | ||||||||
| Doctor | 11 | 987 | 522.974 | -0.288 (-0.512, -0.064)a | 98.088c | 391.153 | -0.922 (-1.795, -0.048)a | 97.443c |
| Nurse | 15 | 2747 | 868.940 | -0.491 (-0.655, -0.327)c | 98.389c | 474.130 | -1.606 (-2.205, -1.006)c | 97.047c |
| Multi-disciplinary | 17 | 2941 | 1237.843 | -0.534 (-0.643, -0.425)c | 98.707c | 852.270 | -2.038 (-2.702, -1.375)c | 98.123c |
| Allied health | 4 | 257 | 8.094 | -0.939 (-1.651, -0.227)b | 62.935a | 11.197 | -0.600 (-1.137, -0.062)a | 73.208b |
| Dropout rate | ||||||||
| 0%-9.9% | 20 | 2659 | 779.280 | -0.458 (-0.584, -0.333)c | 97.562c | 571.285 | -1.138 (-1.62, -0.657)c | 96.674c |
| 10%-19.9% | 16 | 2795 | 709.022 | -0.645 (-0.786, -0.504)c | 97.884c | 740.889 | -2.329 (-3.107, -1.552)c | 97.975c |
| 20% + | 10 | 994 | 365.413 | -0.297 (-0.472, -0.121)c | 97.537c | 267.256 | -0.902 (-1.632, -0.171)a | 96.632c |
| Frequency of intervention | ||||||||
| Daily | 19 | 2176 | 996.897 | -0.439 (-0.577, -0.302)c | 98.194c | 593.016 | -1.408 (-1.959, -0.857)c | 96.965c |
| < daily to > weekly | 10 | 2005 | 627.889 | -0.307 (-0.462, -0.152)c | 98.567c | 602.249 | -1.452 (-2.300, -0.605)c | 98.506c |
| Weekly | 7 | 1095 | 448.790 | -0.791 (-1.167, -0.416)c | 98.663c | 353.155 | -2.340 (-3.576, -1.105)c | 98.301c |
| < weekly | 5 | 1162 | 30.212 | -0.835 (-1.068, -0.602)c | 86.760c | 131.622 | -2.423 (-3.943, -0.902)b | 96.961c |
| Not mentioned | 6 | 494 | 33.795 | -0.227 (-0.690, 0.237) | 85.205c | 42.744 | -0.360 (-1.010, 0.290) | 88.302c |
| Rate of use/engagement | ||||||||
| ≤ 70% | 5 | 808 | 406.483 | -0.212 (-0.526, 0.102) | 99.016c | 254.620 | -1.060 (-2.422, 0.303) | 98.429c |
| > 70% | 8 | 1287 | 150.709 | -0.576 (-0.696, -0.455)c | 95.355c | 204.974 | -2.492 (-3.514, -1.469)c | 96.585c |
| Not mentioned | 34 | 4837 | 1782.085 | -0.496 (-0.589, -0.403)c | 98.148c | 1231.652 | -1.361 (-1.771, -0.951)c | 97.321c |
| Self-care: Medication adherence | ||||||||
| No significant differences | 4 | 557 | 57.285 | -0.268 (-0.459, -0.077)b | 94.763c | 39.413 | -0.650 (-1.254, -0.047)a | 92.388c |
| Intervention significantly better | 3 | 541 | 22.004 | -0.265 (-0.677, 0.147) | 90.911c | 60.687 | -0.783 (-1.810, 0.245) | 96.704c |
| Not mentioned | 40 | 5834 | 2414.200 | -0.522 (-0.605, -0.44)c | 98.385c | 1538.553 | -1.664 (-2.09, -1.237)c | 97.465c |
| Self -care: Disease knowledge | ||||||||
| No significant differences | 7 | 685 | 33.269 | -0.025 (-0.169, 0.118) | 81.965c | 39.012 | -0.142 (-0.535, 0.252) | 84.620c |
| Intervention significantly better | 2 | 197 | 5.253 | -0.590 (-1.401, 0.222) | 80.963a | 42.653 | -1.220 (-3.543, 1.102) | 97.655c |
| Not mentioned | 38 | 6050 | 2399.254 | -0.530 (-0.611, -0.449)c | 98.458c | 1584.253 | -1.802 (-2.234, -1.37)c | 97.655c |
| Self-care: Behavior change | ||||||||
| No significant differences | 3 | 379 | 0.374 | -0.100 (-0.134, -0.065)c | 0.000 | 10.944 | -0.318 (-0.769, 0.132) | 81.726c |
| Intervention significantly better | 4 | 328 | 94.795 | -0.532 (-1.153, 0.090) | 96.835c | 70.276 | -1.145 (-2.347, 0.057) | 95.731c |
| Not mentioned | 40 | 6225 | 2477.596 | -0.508 (-0.59, -0.427)c | 98.426c | 1678.368 | -1.656 (-2.079, -1.234)c | 97.676c |
| Self-care: Glucose monitoring | ||||||||
| No significant differences | 1 | 299 | 0.000 | -0.100 (-0.136, -0.064)c | 0.000 | 0.000 | -0.634 (-0.866, -0.401)c | 0.000 |
| Intervention significantly better | 3 | 434 | 103.754 | -0.505 (-0.923, -0.087)a | 98.072c | 31.471 | -1.795 (-2.897, -0.693)c | 93.645c |
| Not mentioned | 43 | 6199 | 2441.038 | -0.498 (-0.580, -0.416)c | 98.279c | 1807.229 | -1.527 (-1.942, -1.113)c | 97.676c |
| Self-care: Hospital visits needed | ||||||||
| No significant differences | 2 | 339 | 29.741 | -0.440 (-0.744, -0.137)b | 96.638c | 0.510 | -2.467 (-2.750, -2.184)c | 0.000 |
| Intervention significantly better | 1 | 246 | 0.000 | 0.010 (-0.023, 0.043) | 0.000 | 0.000 | 0.082 (-0.187, 0.351) | 0.000 |
| Not mentioned | 44 | 6347 | 2341.454 | -0.505 (-0.586, -0.424)c | 98.164c | 1692.930 | -1.519 (-1.906, -1.133)c | 97.460c |
| Self-care: self-efficacy | ||||||||
| No significant differences | 6 | 1071 | 90.786 | -0.232 (-0.360, -0.105)c | 94.493c | 71.592 | -0.636 (-1.093, -0.178)b | 93.016c |
| Intervention significantly better | 1 | 47 | 0.000 | -0.810 (-1.013, -0.607)c | 0.000 | 0.000 | -2.290 (-3.029, -1.552)c | 0.000 |
| Not mentioned | 40 | 5814 | 2311.342 | -0.514 (-0.597, -0.430)c | 98.313c | 1633.909 | -1.638 (-2.077, -1.199)c | 97.613c |
| Self-care: nutrition | ||||||||
| No significant differences | 4 | 380 | 42.368 | -0.642 (-0.845, -0.440)c | 92.919c | 7.474 | -2.190 (-2.608, -1.772)c | 59.861 |
| Not mentioned | 43 | 6552 | 2567.224 | -0.466 (-0.546, -0.387)c | 98.364c | 1785.184 | -1.457 (-1.853, -1.061)c | 97.647c |
| Self-care: Exercise | ||||||||
| No significant differences | 2 | 132 | 7.605 | -0.641 (-0.943, -0.338)c | 86.850b | 0.209 | -2.427 (-2.877, -1.976)c | 0.000 |
| Not mentioned | 45 | 6800 | 2636.871 | -0.477 (-0.554, -0.400)c | 98.331c | 1824.510 | -1.485 (-1.866, -1.104)c | 97.588c |
| Racial | ||||||||
| Hispanic | 2 | 148 | 2.118 | -0.867 (-1.052, -0.682)c | 52.782 | 0.502 | -2.791 (-3.245, -2.337)c | 0.000 |
| Not mentioned | 45 | 6784 | 2560.239 | -0.464 (-0.540, -0.388)c | 98.281c | 1804.224 | -1.471 (-1.85, -1.092)c | 97.561c |
| Comorbid disease | ||||||||
| Overweight/obese | 4 | 318 | 50.425 | -1.270 (-1.971, -0.568)c | 94.051c | 50.979 | -2.034 (-3.280, -0.788)c | 94.115c |
| Hypertension | 1 | 299 | 0.000 | -0.100 (-0.136, -0.064)c | 0.000 | 0.000 | -0.634 (-0.866, -0.401)c | 0.000 |
| Not mentioned | 42 | 6315 | 2376.371 | -0.439 (-0.516, -0.361)c | 98.275c | 1787.062 | -1.499 (-1.910, -1.087)c | 97.706c |
Mode of telemedicine intervention subgroups included Text message, Mobile Application, Interactive Telephone System, Online Server, Website, Videoconference, or Tablet/device. Duration of intervention subgroups included < 6 mo, 6 mo, > 6 mo < 12 mo, or > 12 mo. Focus of intervention subgroups included biomedical parameters, self-management behaviours, or combination of biomedical parameters/self-management behaviours. Model of intervention subgroups included behaviour change, clinical treatment, or behaviour change/treatment combination. Type of telemedicine intervention subgroups included telemonitoring, tele-education, or telemonitoring/tele-education combination. Intervention-lead subgroups included doctor, nurse, multi-disciplinary team, or allied health. Drop-out rate subgroups included 0%-9.9%, 10%-19.9%, or > 20%. Frequency of intervention subgroups included daily, < daily > weekly, weekly, or < weekly. Intervention engagement subgroups included < 70% or > 70%.
The self-care components considered for subgroups were related to: medication adherence, disease knowledge, behaviour change, self-glucose monitoring, hospital visits, self-efficacy, nutrition/diet, and exercise. These were categorised into subgroups based on whether there the telemedicine intervention improved significantly more than the control group in each self-care component. Regarding patient characteristic subgroups; racial group subgroups included Hispanic or Chinese, and comorbid disease subgroups included overweight/obese (BMI ≤ 25) and physician-diagnosed hypertension.
The random effects model was used to estimate effect sizes and mean differences in all outcomes and I2 for heterogeneity testing. Publication bias was analysed using Egger regression analysis that P value is more than 0.05 suggesting the publication bias is not significant. Sensitivity analysis was conducted by using removing one study per time and cumulative all studies to identify the results are related to particular study or studies.
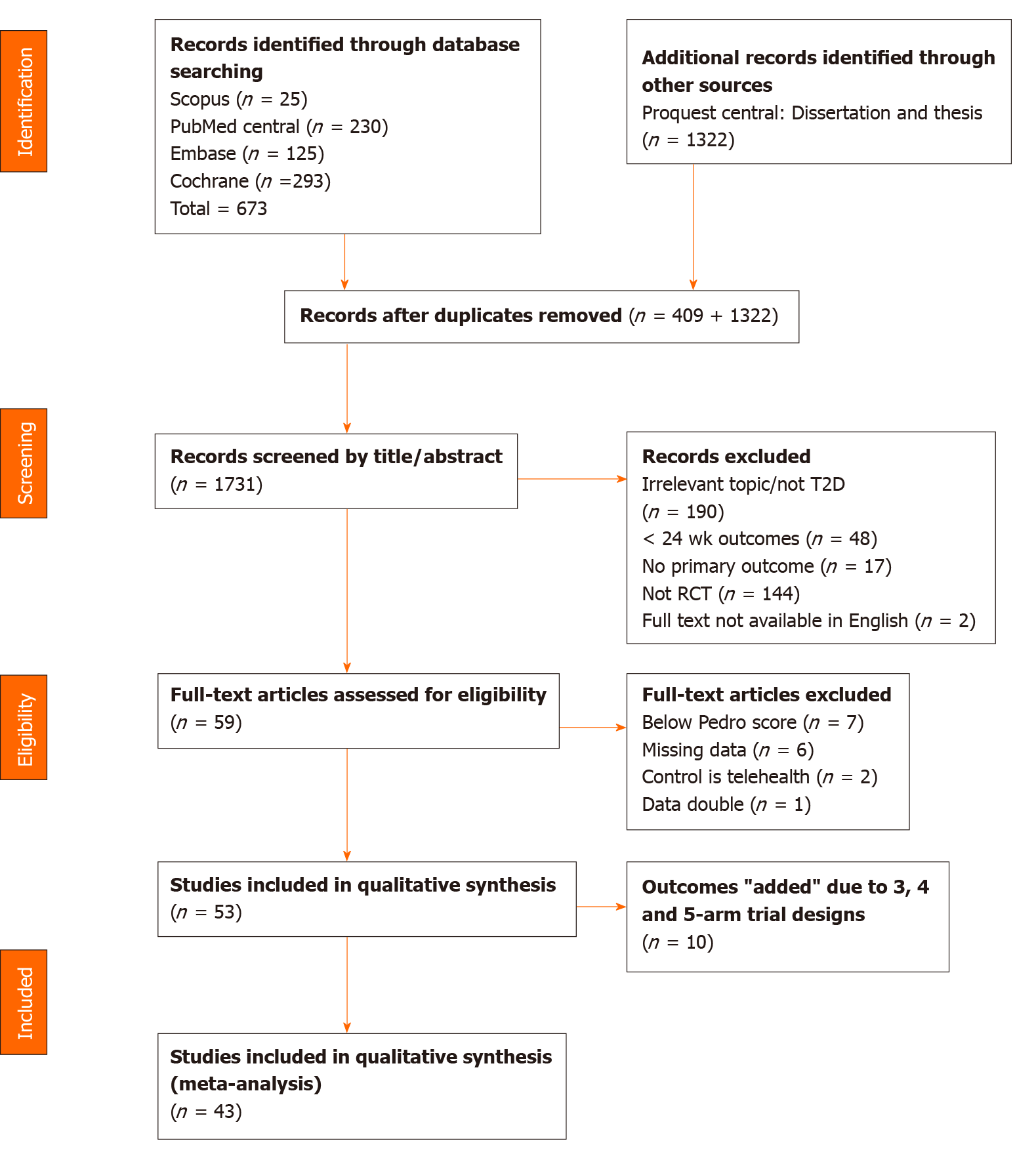
We identified 673 total potential studies from the database search, with 1322 additional records identified through other sources. After removing duplicates, 1731 studies remained to be screened by title and abstract. Full-text screening was conducted on the remaining 59 studies, 16 of which were excluded as a result of the study design or data insufficiency. Due to multiple arm trial designs, the final sample for meta-analysis included 43 eligible studies in quantitative synthesis in total (Figure 1).
The 43 included studies were published between 2002 and 2020, and reported 4365 participants receiving a telemedicine intervention and 4045 participants in the control groups. Participants were recruited from 16 countries including Saudi Arabia, United States, Poland, South Korea, Greece, Italy, Norway, Germany, India, Malaysia, China, Finland, Canada, Spain, Mexico, and Australia. Participants in the intervention group received telemedicine intervention while those in the control group did not. All included studies measured at least one of the required outcome variables, which was consistently HbA1c. The outcomes of SBP, DBP, PPG, FPG, BMI, cholesterol, weight, mental QoL, and physical QoL were also collected from the studies. The demographic characteristics of the participants, intervention and control details, measured outcomes, key results and PEDRO quality assessment of the included studies are summarised and presented in Table 1[36-78].
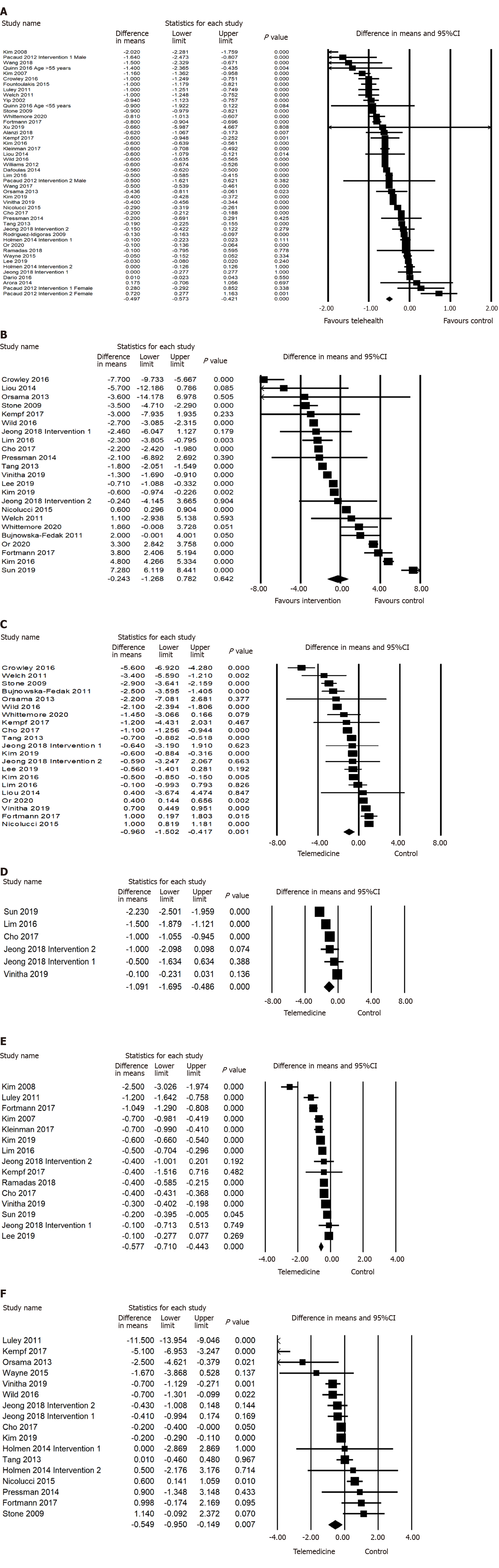
Forty-three studies involving 6932 participants measured HbA1c changes in diabetes patients. The pooled effect on HbA1c was -0.486% [95% confidence interval (CI) -0.561 to -0.410, P < 0.001] in the telemedicine group compared with the usual care group, with a significantly high-level of heterogeneity (I2 = 98.290%, P < 0.001). Our results found that all modes of telemedicine interventions had a significant HbA1c reduction (P < 0.001), six of them with significant heterogeneity (P < 0.001) including text message (-0.591%; 95%CI -0.892 to -0.290, P < 0.001), mobile application (-0.359%; 95%CI -0.502 to -0.215, P < 0.001), interactive telephone (-0.782%; 95%CI -1.172 to -0.391, P < 0.001), internet server/computer network (-0.431%; 95%CI -0.558 to -0.304, P < 0.001), website (-0.539%; 95%CI -0.706 to -0.371, P < 0.001), and device/tablet (-0.278%; 95%CI -0.747 to 0.191, P < 0.001); only videoconference interventions (-0.845%; 95%CI -1.144 to -0.546, P < 0.001) had no significant heterogeneity. A significant HbA1c reduction (P < 0.001) with a significantly high-level of heterogeneity (P < 0.001) was found in all short-term and long-term trials. In addition, interventions of 6 mo duration were observed to have a greater HbA1c reduction (-0.626%; 95%CI -0.766 to -0.486, P < 0.001) compared to trials less than 6 mo, or more than 6 mo. Our results reported that 16 studies had interventions focussed on biomedical parameters only (-0.687%; 95%CI -0.821 to -0.553, P < 0.001) and 24 studies had interventions focussed on both biomedical parameters and self-management behaviours (-0.348%; 95%CI -0.442 to -0.253, P < 0.001) which had a significant HbA1c reduction (P < 0.001) and high level heterogeneity (P < 0.001), compared to 7 studies which had interventions focussed on self-management behaviour only, with no significant HbA1c reduction. Telemonitoring significantly reduced HbA1c (-0.553%; 95%CI -0.662 to -0.445, P < 0.001) and had greater HbA1c reduction compared to those trials using tele-education only (-0.495%; 95%CI -0.683 to -0.307, P < 0.001) and combined telemonitoring and tele-education (-0.391%; 95%CI -0.542 to -0.240, P < 0.001). We also found that those trials with a behaviour change model of intervention (-0.452%; 95%CI -0.575 to -0.329, P < 0.001) and clinical treatment model of intervention (-0.589%; 95%CI -0.725 to -0.453, P < 0.001) reported a significant HbA1c reduction and was associated with heterogeneity (P < 0.001). Six trials conducted a combined behaviour change and treatment model for intervention which also reported a significant HbA1c reduction (-0.401%; 95%CI -0.612 to -0.189, P < 0.001) with heterogeneity (P < 0.001). The telemedicine interventions lead by allied health (-0.939%; 95%CI -1.651 to -0.227, P < 0.01) reported a greater HbA1c reduction compared to those led by doctors (-0.288%; 95%CI -0.512 to -0.064, P < 0.05), nurse lead interventions (-0.491%; 95%CI -0.655 to -0.327, P < 0.001) and led by a multi-disciplinary team (-0.534%; 95%CI -0.643 to -0.425, P < 0.001). Patients who received a weekly intervention (-0.791%; 95%CI -1.167 to -0.416, P < 0.001) and less than weekly (-0.835%; 95%CI -1.068 to -0.602, P < 0.001) reported a greater HbA1c reduction with high heterogeneity (P < 0.001) compared to those patients who received intervention greater than weekly but less than daily (-0.307%; 95%CI -0.462 to -0.152, P < 0.001) and daily interventions (-0.439%; 95%CI -0.577 to -0.302, P < 0.001). The telemedicine interventions with a drop-out rate of 10-19.9% (-0.645%; 95%CI -0.786 to -0.504, P < 0.001) reported a greater HbA1c reduction compared to those with a drop-out rate of 0-9.9% (-0.458%; 95%CI -0.584 to -0.333, P < 0.001), or a drop-out rate 20%+ (-0.297%; 95%CI -0.472 to -0.121, P < 0.001). The telemedicine interventions with an engagement level > 70% (-0.576%; 95%CI -0.696 to –0.455, P < 0.001) a greater HbA1c reduction whilst interventions with engagement levels < 70% did not significantly reduce HbA1c in the analysis.
The self-care outcomes analysis identified that studies with intervention participants with significantly higher self-glucose monitoring (-0.505%; 95%CI -0.923 to -0.087, P < 0.05) with high heterogeneity (P < 0.001), and significantly higher self-efficacy (-0.810%; 95%CI -1.013 to -0.607, P < 0.001) by the end of the intervention showed significant reductions in HbA1c. However, we found that studies where intervention participants had significantly different medication adherence, disease knowledge, hospital visits, nutrition, and behavior change had no effect on HbA1c.
The patient characteristic subgroup analysis revealed that Hispanic participants receiving telemedicine had significantly reduced HbA1c (-0.867%; 95%CI -1.052 to -0.682, P < 0.001), which was greater in comparison to the remainder of the studies (-0.464%; 95%CI -0.540 to -0.388, P < 0.001). Obese participants receiving telemedicine had high heterogeneity (P < 0.001) but significantly decreased HbA1c (-1.270%; 95%CI -1.971 to -0.568, P < 0.001), whilst the hypertensive patients also had decreased HbA1c (-0.100%; 95%CI -0.136 to -0.064, P < 0.001). However, the remainder of studies in comparison had a greater reduction in HbA1c (-0.439%; 95%CI -0.516 to -0.361, P < 0.001), with high heterogeneity (P < 0.001) (Tables 2 and 3).
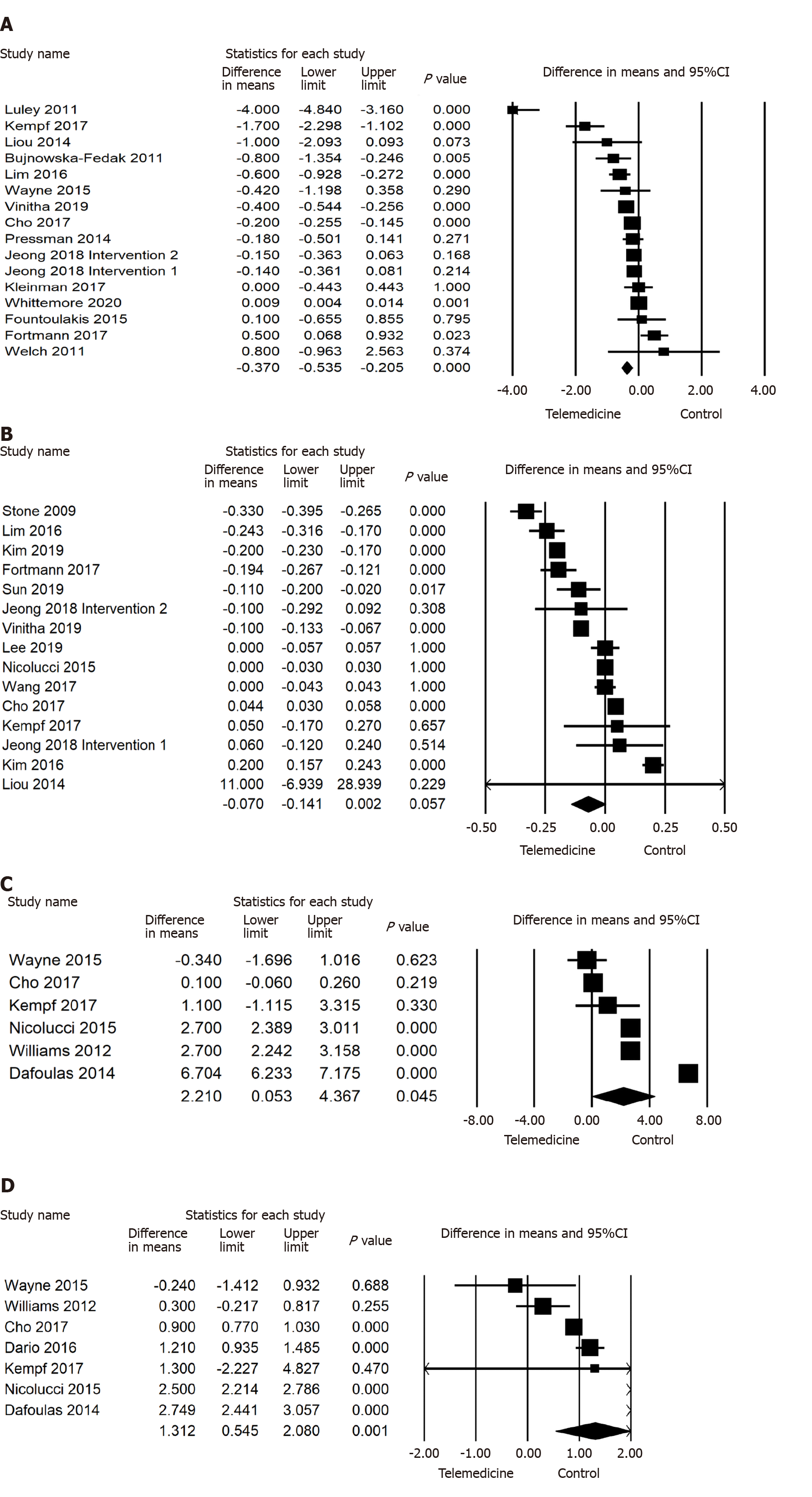
Our results also found significant reductions in DBP (-0.875 mmHg; 95%CI -1.429 to -0.321, P < 0.01), PPG (-1.458 mmol/L; 95%CI -2.648 to -0.268, P < 0.01), FPG (-0.577 mmol/L; 95%CI -0.710 to -0.443, P < 0.001), weight (-0.243 kg; 95%CI -0.442 to -0.045, P < 0.05), BMI (-0.304; 95%CI -0.563 to -0.045, P < 0.05), but increase in Mental QoL (2.210; 95%CI 0.053 to 4.367, P < 0.05) and Physical QoL (1.312; 95%CI 0.545 to 2.080, P < 0.001) for the telemedicine intervention, compared to control. However, twenty-two studies involving 4053 participants were analysed and the pooled effect of SBP was not significant (P > 0.05) in the telemonitoring group compared to the control group. Moreover, 15 studies with 2951 participants reported no significant difference in cholesterol change in the intervention group compared to the control group (Figures 2 and 3).
The study by Shea et al[65] for SBP and DBP was excluded from the analysis due to its significant publication bias. After removing this study, visual inspection of the funnel plots of SBP and DBP (Supplementary Figure 1) showed a symmetrical distribution of the effect size, and no obvious publication bias was found. Egger’s test (Table 2) of SBP (t = 0.937, 95%CI -2.814 to 7.432, P > 0.05), DBP (t = 0.896, 95%CI -5.695 to, 2.280, P > 0.05), PPG (t = 0.035, 95%CI -9.234 to 8.984, P > 0.05), weight (t = 1.225, 95%CI -3.014 to 0.814, P > 0.05), mental QoL (t = 1.124, 95%CI -13.969 to 32.978, P > 0.05) and physical QoL (t = 0.425, 95%CI -8.626 to 12.046, P > 0.05) supported the funnel plot findings indicating that no publication bias was found in the comparison. However, egger’s test (Table 2) of HbA1c (t = 2.604, 95%CI -6.389 to -0.815, P < 0.05) FPG (t = 2.730, 95%CI -4.501 to -0.524, P < 0.05) and BMI (t = 2.963, 95%CI -4.376 to -0.701, P < 0.05) indicated significant publication bias in this comparison. Removing any study did not change the overall significant results, suggesting all included studies are contributing to the overall results.
This meta-analysis systematically reviewed 43 RCTs examining the effects of telemedicine on HbA1c, blood pressure, PPG, FPG, weight, BMI, cholesterol, Mental QoL, and Physical QoL in patients with diabetes. Our research reported that telemedicine interventions have a significant effect on lowering HbA1c; -0.486% (95%CI -0.561 to -0.410, P < 0.001, I2 = 98.290%) in comparison to the controls. The improvement of HbA1c control by telemedicine is consistent with previous research[2,79,80]. In addition, our results also report a significance reduction on DBP (P < 0.01), PPG (P < 0.01), FPG (P < 0.001), weight (P < 0.05), BMI (P < 0.05), Mental QoL (P < 0.05), and Physical QoL (P < 0.001) in the intervention group. However, our results found no significant effect (P < 0.05) on SBP and cholesterol in the intervention group compared to the controls. Although there is significant heterogeneity found in all outcomes, the positive effect of telemedicine is promising. However, the clinical significance of these results must be considered. A 0.875 mmHg reduction in DBP is not very impactful, as normal DBP can range from 80 mmHg or lower[81]. But a PPG reduction of 1.458mmol/L is significant as the upper limit of normal is only 7.8mmol/L[82], which would be a more meaningful change.
In this study, we evaluated the effect of telemedicine intervention characteristics on T2DM in terms of changes to HbA1c. The subgroup analysis showed that interventions provided at a frequency that is less than weekly (-0.835%; 95%CI -1.068 to -0.602, P < 0.001, I2 = 86.760%) provided the greatest reduction in HbA1c. This is an interesting result, with the least frequent intervention revealing the highest glycaemic control. A possible reason for this could be that the participants with less frequent interactions may tend to work towards achieving goals in between each interaction, rather than with a daily or weekly intervention where motivating progress is less experience in each interaction. Patients that received an intervention duration of 6 mo (-0.626%; 95%CI -0.766 to -0.486, P < 0.001) also reported a higher HbA1c reduction. A previous study[83] reported similar results suggesting that 6 mo telemedicine intervention significantly improves HbA1c levels in patients with diabetes patients. In addition, the long-term telemedicine intervention (more than 6 mo) was also found significant for HbA1c improvement, which is consistent with another systematic review[84]. These findings suggested that telemedicine intervention may facilitate HbA1c control for 6 mo or longer.
The impact of telemedicine on HbA1c may be also explained by the different modes of intervention, including text message, mobile app, interactive telephone, internet server, website, video conference and mobile devices[85]. Although all modes of intervention demonstrated significant HbA1c improvement, it is an opportunity to highlight that interactive telephone (-0.782%; 95%CI -1.172 to -0.391, P < 0.001) and videoconference (-0.845%; 95%CI -1.144 to -0.546, P < 0.001) showed a greater HbA1c reduction than other modes of interventions. A study reported similar results to this, theorising that interactive treatment may facilitate remote healthcare on diabetes management[84]. Our results suggest that all modes of intervention are significant for T2DM telemedicine, but perhaps we should pay more attention to the modes of interactive telephone and videoconferencing. However, due to the number of study limitation for videoconference and interactive phone call interventions, more relevant trials are needed for further confirmation.
In the studies we reviewed, telemonitoring interventions, as well as combined telemonitoring and tele-education interventions significantly reduced HbA1c in T2DM patients. In addition, combined behaviour change and clinical treatment models, as well as either behaviour change or clinical treatment models intervention alone, had significantly improved HbA1c levels. Specifically, clinical treatment models of intervention resulted in the greatest decline in HbA1c. Several published studies evidence the positive effect of diabetes treatment by telemonitoring[83,86-88] and tele-education[83,89]. This may be due to healthy behaviour changes[89-92] in addition to medical treatment[93] resulting in disease improvement during the course of telemedicine interventions.
Similarly, telemedicine interventions that focused on biomedical parameters were most effective at reducing HbA1c, followed by biomedical parameters in addition to self-management behaviour focus. The self-management focus alone did not significantly reduce HbA1c, suggesting that the interventions aimed to achieve specific numeric targets (such as HbA1c level, blood glucose level, kilograms of weight loss) are more effective to achieve glycaemic control.
We also found that telemedicine interventions led by allied health, multi-disciplinary or nurse-led interventions showed a better HbA1c control compared to those led by doctors. Due to the characteristics of diabetes management, patients may require not only medications but also lifestyle guidance[79,90,91]. Interventions lead by nurse, allied health and multi-disciplinary teams may provide a better strategy for long-term diabetes treatment. Interestingly, interventions with drop-out rates between 10%-19.9% revealed the greatest reduction in HbA1c. This could be explained by the notion that interventions with lower drop-out rates might retain participants that can be less compliant to the intervention. This is supported by the result that telemedicine interventions with a higher engagement level (> 70%) have greater HbA1c reduction.
Intervention participants that had significantly higher self-glucose monitoring and significantly higher self-efficacy at the end of the intervention showed significantly higher reductions in HbA1c. These results are consistent with a previous meta-analysis[94]. Furthermore, another meta-analysis highlighted that increased self-glucose monitoring results in a reduction of HbA1c and therefore long-term glycemic control[95]. Higher self-efficacy has also been shown to reduce HbA1c[96]. T2DM patients with better self-care knowledge and perform more self-care behaviors will manage their disease more frequently, improving glycemic control and reducing complications[96]. Thus, it would be beneficial to incorporate self-care education and encouragement in the provision of telemedicine interventions for T2DM.
However, most of the trials did not mention the self-care components of nutrition, behaviour change, hospital visits, disease knowledge and medication adherence. Thus, our result on self-care outcomes should be interpreted cautiously. More research of higher methodological quality and larger number of studies are needed to explore whether telemedicine intervention can facilitate self-care development[94].
It is well known that both obesity and T2DM are metabolic diseases that are contributed by an unhealthy lifestyle[97]. In the studies we reviewed, 318 participants were diagnosed with both T2DM and a BMI considered overweight/obese (BMI > 25), which reported a significant HbA1c reduction after telemedicine intervention. There was a similar result for the hypertensive T2DM patients, however there was only 1 study with 299 participants reporting this. There are difficulties determining the number of participants with a BMI > 25 or hypertension in the studies that did not explicitly calculate this. Furthermore, due to the sample size limitation and high heterogeneity, the effectiveness of telemedicine interventions for T2DM and comorbid diseases should be interpreted with caution. Two studies had Hispanic only patients, reporting a total of 318 participants whom had a greater reduction in HbA1c after telemedicine intervention, in comparison to the remainder of studies. Although, the same issue of high heterogeneity and small sample size persists.
This meta-analysis covered various telemedicine interventions for patients with T2DM. We aimed to perform a comprehensive meta-analysis on the effect of telemedicine intervention in the treatment of T2DM. However, our research has some limitations that must be acknowledged. First, we found high heterogeneity for all outcomes and most of the subgroups. The heterogeneity may be caused by the complexity of telemedicine interventions such as duration, strategies, combinations and telemedicine quality. Second, we found that the effectiveness of self-care outcomes is uncertain due to the study number limitations. More trials with large sample sizes and details on self-care outcomes are needed for further investigation. Lastly, although telemedicine interventions showed a significant improvement in mental and physical QoL, the sample size limitation may cause uncertainty and high heterogeneity. Thus, more trials with large sample sizes are needed for further telemedicine evaluation on mental and physical QoL. Considering these limitations, the results of this meta-analysis should be interpreted with caution.
Our findings demonstrated that telemedicine interventions are more effective than the controls for reducing HbA1c in T2DM patients. In addition, the outcomes of DBP, PPG, FPG, weight, BMI, mental QoL and physical QoL improved significantly more in telemedicine interventions when comparing to control. SBP and cholesterol were not significantly different when compared to control. In addition, several components of telehealth characteristics were found to have an effect on glycaemic control through subgroup analysis. The studies that emphasized both behaviour change and treatment models of intervention, as well as interventions delivered via modes such as videoconference and interactive telephone had a larger effect on HbA1c reduction. In addition, less than weekly intervention frequency and intervention durations of 6 mo or longer may obtain a better outcome for glucose control. Interventions focused on biomedical parameters, as well as the method of telemonitoring, and those lead by allied health had a better effect on glucose control. Furthermore, interventions with a drop-out rate between 10%-19.9% and engagement levels of > 70% had the greatest HbA1c reduction.
The patient characteristic investigation reported patients with T2DM that were overweight, as well as Hispanic participants showed a greater HbA1c reduction. The current results also suggest that telehealth interventions that improve glucose monitoring and self-efficacy by the end of the study may be important for self-care development and can result in significant HbA1c reduction. Due to the trial limitation, components including nutrition, exercise, behaviour change, medication adherence, and disease knowledge in telehealth interventions have an uncertain effect on HbA1c. Ultimately, due to the characteristics of telehealth interventions and their positive effect of on diabetes management, we recommend the provision of telehealth interventions with emphasis on patient self-care for better management of T2DM, which may enhance long-term glucose control for patients. In addition, future studies should record more details about telehealth methodology and outcomes, especially self-care, for further evaluation.
Telemedicine is defined as the delivery of health services via remote communication and technology. It is a convenient and cost-effective method of intervention, which has shown to be successful in improving glyceamic control for type 2 diabetes patients. The utility of a successful diabetes intervention is vital to reduce disease complications, hospital admissions and associated economic costs.
There are numerous randomised control trials that evaluate the effectiveness of telemedicine in patients with diabetes. However, no studies have provided an in-depth analysis of the effectiveness of telemedicine for glycaemic control and other health outcomes for type 2 diabetes patients.
This study aimed to utilise a meta-analysis approach to synthesise results from high quality randomised controlled trials, and to comprehensively review literature on the effects of telemedicine interventions on health outcomes for patients with type 2 diabetes. The secondary aim was to analyse the effect of telemedicine characteristics, patient characteristics, and self-care outcomes on glycaemic control.
Fourty-three relevant studies were yielded from PubMed Central, Cochrane Library, Embase and Scopus databases which satisfied quality assessment via the PEDro scale. Mean difference and standard deviation was extracted from pre- and post-intervention data regarding all outcomes of interest, and information for subgroup categories was collected. The random effects model was used to estimate outcomes and I2 was used for heterogeneity testing.
Telemedicine improves hemoglobin A1c (HbA1c), diastolic blood pressure, post-prandial glucose, fasting plasma glucose, weight, body mass index, mental quality of life and physical quality of life score significantly more than control/non-telemedicine interventions.
Subgroup analysis revealed that telemedicine interventions that involved primarily telemonitoring, used a clinical treatment model, delivered via modes such as videoconferencing and interactive telephone, at a rate less frequent than weekly, provided for a duration of 6 mo, led by allied health workers, focussed on biomedical outcomes, had high engagement level and moderate drop out rate were the most effective at reducing HbA1c.
Subgroup analysis about patient characteristics showed that Hispanic patents may benefit more than others in HbA1c reduction. Self-care subgroup analysis demonstrated that telemedicine interventions that significantly improved self-glucose monitoring and self-efficacy more than the control were found to have a higher reduction in HbA1c.
Telemedicine is a useful and effective intervention for type 2 diabetes patients, which improves glycemic control and numerous other health outcomes significantly better than non-telemedicine interventions/controls. Subgroup analysis demonstrated that optimising the characteristics of telemedicine interventions may have a greater effect at improving health outcomes.
In a world where telemedicine is more widely used than ever, it is important to ensure that these services are delivered at a high standard and benefit the participating patients. This study emphasises that telemedicine should be utilised as an effective approach to type 2 diabetes intervention.
Manuscript source: Invited manuscript
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Australia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Nam SM S-Editor: Gao CC L-Editor: A P-Editor: Ma YJ
| 1. | Rines AK, Sharabi K, Tavares CD, Puigserver P. Targeting hepatic glucose metabolism in the treatment of type 2 diabetes. Nat Rev Drug Discov. 2016;15:786-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 265] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 2. | Wu C, Wu Z, Yang L, Zhu W, Zhang M, Zhu Q, Chen X, Pan Y. Evaluation of the clinical outcomes of telehealth for managing diabetes: A PRISMA-compliant meta-analysis. Medicine (Baltimore). 2018;97:e12962. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 3. | Chen L, Magliano DJ, Zimmet PZ. The worldwide epidemiology of type 2 diabetes mellitus--present and future perspectives. Nat Rev Endocrinol. 2011;8:228-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1333] [Cited by in RCA: 1445] [Article Influence: 103.2] [Reference Citation Analysis (0)] |
| 4. | Seuring T, Archangelidi O, Suhrcke M. The Economic Costs of Type 2 Diabetes: A Global Systematic Review. Pharmacoeconomics. 2015;33:811-831. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 557] [Cited by in RCA: 517] [Article Influence: 51.7] [Reference Citation Analysis (0)] |
| 5. | Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14:88-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2249] [Cited by in RCA: 3405] [Article Influence: 486.4] [Reference Citation Analysis (0)] |
| 6. | Bommer C, Heesemann E, Sagalova V, Manne-Goehler J, Atun R, Bärnighausen T, Vollmer S. The global economic burden of diabetes in adults aged 20-79 years: a cost-of-illness study. Lancet Diabetes Endocrinol. 2017;5:423-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 415] [Cited by in RCA: 494] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 7. | Hu FB, Stampfer MJ, Haffner SM, Solomon CG, Willett WC, Manson JE. Elevated risk of cardiovascular disease prior to clinical diagnosis of type 2 diabetes. Diabetes Care. 2002;25:1129-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 356] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 8. | Nolan CJ, Damm P, Prentki M. Type 2 diabetes across generations: from pathophysiology to prevention and management. Lancet. 2011;378:169-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 617] [Cited by in RCA: 626] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 9. | Gonçalves NP, Vægter CB, Andersen H, Østergaard L, Calcutt NA, Jensen TS. Schwann cell interactions with axons and microvessels in diabetic neuropathy. Nat Rev Neurol. 2017;13:135-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 206] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 10. | Vinik AI, Maser RE, Mitchell BD, Freeman R. Diabetic autonomic neuropathy. Diabetes Care. 2003;26:1553-1579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1258] [Cited by in RCA: 1215] [Article Influence: 55.2] [Reference Citation Analysis (0)] |
| 11. | Kodl CT, Seaquist ER. Cognitive dysfunction and diabetes mellitus. Endocr Rev. 2008;29:494-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 526] [Cited by in RCA: 573] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 12. | Stewart R, Liolitsa D. Type 2 diabetes mellitus, cognitive impairment and dementia. Diabet Med. 1999;16:93-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 355] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 13. | Franz MJ, Boucher JL, Rutten-Ramos S, VanWormer JJ. Lifestyle weight-loss intervention outcomes in overweight and obese adults with type 2 diabetes: a systematic review and meta-analysis of randomized clinical trials. J Acad Nutr Diet. 2015;115:1447-1463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 424] [Article Influence: 42.4] [Reference Citation Analysis (0)] |
| 14. | Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348:383-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3042] [Cited by in RCA: 2862] [Article Influence: 130.1] [Reference Citation Analysis (0)] |
| 15. | Nathan DM, Buse JB, Davidson MB, Ferrannini E, Holman RR, Sherwin R, Zinman B; American Diabetes Association; European Association for Study of Diabetes. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32:193-203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2357] [Cited by in RCA: 2284] [Article Influence: 142.8] [Reference Citation Analysis (0)] |
| 16. | Norberg M, Eriksson JW, Lindahl B, Andersson C, Rolandsson O, Stenlund H, Weinehall L. A combination of HbA1c, fasting glucose and BMI is effective in screening for individuals at risk of future type 2 diabetes: OGTT is not needed. J Intern Med. 2006;260:263-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 54] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Graves LE, Donaghue KC. Management of diabetes complications in youth. Ther Adv Endocrinol Metab. 2019;10:2042018819863226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 18. | Polonsky WH, Henry RR. Poor medication adherence in type 2 diabetes: recognizing the scope of the problem and its key contributors. Patient Prefer Adherence. 2016;10:1299-1307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 351] [Cited by in RCA: 471] [Article Influence: 52.3] [Reference Citation Analysis (0)] |
| 19. | Al-Sahouri A, Merrell J, Snelgrove S. Barriers to good glycemic control levels and adherence to diabetes management plan in adults with Type-2 diabetes in Jordan: a literature review. Patient Prefer Adherence. 2019;13:675-693. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 20. | Paterson B, Thorne S. Developmental evolution of expertise in diabetes self-management. Clin Nurs Res. 2000;9:402-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 74] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Shrivastava SR, Shrivastava PS, Ramasamy J. Role of self-care in management of diabetes mellitus. J Diabetes Metab Disord. 2013;12:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 547] [Cited by in RCA: 513] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 22. | Gao J, Wang J, Zheng P, Haardörfer R, Kegler MC, Zhu Y, Fu H. Effects of self-care, self-efficacy, social support on glycemic control in adults with type 2 diabetes. BMC Fam Pract. 2013;14:66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 143] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 23. | Ross SA. Breaking down patient and physician barriers to optimize glycemic control in type 2 diabetes. Am J Med. 2013;126:S38-S48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 113] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 24. | Blonde L. Current challenges in diabetes management. Clin Cornerstone. 2005;7 Suppl 3:S6-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 25. | Menzin J, Korn JR, Cohen J, Lobo F, Zhang B, Friedman M, Neumann PJ. Relationship between glycemic control and diabetes-related hospital costs in patients with type 1 or type 2 diabetes mellitus. J Manag Care Pharm. 2010;16:264-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 117] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 26. | Moffatt JJ, Eley DS. The reported benefits of telehealth for rural Australians. Aust Health Rev. 2010;34:276-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 199] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 27. | Hersh WR, Hickam DH, Severance SM, Dana TL, Pyle Krages K, Helfand M. Diagnosis, access and outcomes: Update of a systematic review of telemedicine services. J Telemed Telecare. 2006;12 Suppl 2:S3-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 80] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 28. | Fritzen K, Basinska K, Rubio-Almanza M, Nicolucci A, Kennon B, Vergès B, Zakrzewska K, Schnell O. Pan-European Economic Analysis to Identify Cost Savings for the Health Care Systems as a Result of Integrating Glucose Monitoring Based Telemedical Approaches Into Diabetes Management. J Diabetes Sci Technol. 2019;13:1112-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 29. | Whitten P, Holtz B, Laplante C. Telemedicine: What have we learned? Appl Clin Inform. 2010;1:132-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 30. | Mair F, Fraser S, Ferguson J, Webster K. Telemedicine via satellite to support offshore oil platforms. J Telemed Telecare. 2008;14:129-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 31. | Wong C, Colven R. Teledermatology in Underserved Populations. Curr Dermatol Rep. 2019;8:91-97. |
| 32. | Averwater NW, Burchfield DC. No place like home: telemonitoring can improve home care. Healthc Financ Manage. 2005;59:46-48, 50, 52. [PubMed] |
| 33. | D'Souza R. Telemedicine for intensive support of psychiatric inpatients admitted to local hospitals. J Telemed Telecare. 2000;6 Suppl 1:S26-S28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 34. | Tchero H, Kangambega P, Briatte C, Brunet-Houdard S, Retali GR, Rusch E. Clinical Effectiveness of Telemedicine in Diabetes Mellitus: A Meta-Analysis of 42 Randomized Controlled Trials. Telemed J E Health. 2019;25:569-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 141] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 35. | Saisho Y. Use of Diabetes Treatment Satisfaction Questionnaire in Diabetes Care: Importance of Patient-Reported Outcomes. Int J Environ Res Public Health. 2018;15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 93] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 36. | Alanzi T, Alanazi NR, Istepanian R, Philip N. Evaluation of the effectiveness of mobile diabetes management system with social networking and cognitive behavioural therapy (CBT) for T2D. Mhealth. 2018;4:35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 37. | Arora S, Peters AL, Burner E, Lam CN, Menchine M. Trial to examine text message-based mHealth in emergency department patients with diabetes (TExT-MED): a randomized controlled trial. Ann Emerg Med. 2014;63:745-54.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 186] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 38. | Bujnowska-Fedak MM, Puchała E, Steciwko A. The impact of telehome care on health status and quality of life among patients with diabetes in a primary care setting in Poland. Telemed J E Health. 2011;17:153-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 39. | Cho JH, Kim HS, Yoo SH, Jung CH, Lee WJ, Park CY, Yang HK, Park JY, Park SW, Yoon KH. An Internet-based health gateway device for interactive communication and automatic data uploading: Clinical efficacy for type 2 diabetes in a multi-centre trial. J Telemed Telecare. 2017;23:595-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 40. | Crowley MJ, Edelman D, McAndrew AT, Kistler S, Danus S, Webb JA, Zanga J, Sanders LL, Coffman CJ, Jackson GL, Bosworth HB. Practical Telemedicine for Veterans with Persistently Poor Diabetes Control: A Randomized Pilot Trial. Telemed J E Health. 2016;22:376-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 41. | Dafoulas GE, Bargiota A, Mavrodi A, Greuel M, Croci A, Dario C. Long-term telemonitoring of patients with DMT2: results of the renewing health cluster 2 multicenter randomized pragmatic trial. Diabetes. 2015;64:A637-A638. |
| 42. | Dario C, Toffanin R, Calcaterra F, Saccavini C, Stafylas P, Mancin S, Vio E. Telemonitoring of Type 2 Diabetes Mellitus in Italy. Telemed J E Health. 2017;23:143-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 43. | Fortmann AL, Gallo LC, Garcia MI, Taleb M, Euyoque JA, Clark T, Skidmore J, Ruiz M, Dharkar-Surber S, Schultz J, Philis-Tsimikas A. Dulce Digital: An mHealth SMS-Based Intervention Improves Glycemic Control in Hispanics With Type 2 Diabetes. Diabetes Care. 2017;40:1349-1355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 109] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 44. | Fountoulakis S, Papanastasiou L, Gryparis A, Markou A, Piaditis G. Impact and duration effect of telemonitoring on ΗbA1c, BMI and cost in insulin-treated Diabetes Mellitus patients with inadequate glycemic control: A randomized controlled study. Hormones (Athens). 2015;14:632-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 45. | Holmen H, Torbjørnsen A, Wahl AK, Jenum AK, Småstuen MC, Arsand E, Ribu L. A Mobile Health Intervention for Self-Management and Lifestyle Change for Persons With Type 2 Diabetes, Part 2: One-Year Results From the Norwegian Randomized Controlled Trial RENEWING HEALTH. JMIR Mhealth Uhealth. 2014;2:e57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 173] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 46. | Jeong JY, Jeon JH, Bae KH, Choi YK, Park KG, Kim JG, Won KC, Cha BS, Ahn CW, Kim DW, Lee CH, Lee IK. Smart Care Based on Telemonitoring and Telemedicine for Type 2 Diabetes Care: Multi-Center Randomized Controlled Trial. Telemed J E Health. 2018;24:604-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 47. | Kempf K, Altpeter B, Berger J, Reuß O, Fuchs M, Schneider M, Gärtner B, Niedermeier K, Martin S. Efficacy of the Telemedical Lifestyle intervention Program TeLiPro in Advanced Stages of Type 2 Diabetes: A Randomized Controlled Trial. Diabetes Care. 2017;40:863-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 89] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 48. | Kim HS, Jeong HS. A nurse short message service by cellular phone in type-2 diabetic patients for six months. J Clin Nurs. 2007;16:1082-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 104] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 49. | Kim SI, Kim HS. Effectiveness of mobile and internet intervention in patients with obese type 2 diabetes. Int J Med Inform. 2008;77:399-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 126] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 50. | Kim HS, Sun C, Yang SJ, Sun L, Li F, Choi IY, Cho JH, Wang G, Yoon KH. Randomized, Open-Label, Parallel Group Study to Evaluate the Effect of Internet-Based Glucose Management System on Subjects with Diabetes in China. Telemed J E Health. 2016;22:666-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 51. | Kim EK, Kwak SH, Jung HS, Koo BK, Moon MK, Lim S, Jang HC, Park KS, Cho YM. The Effect of a Smartphone-Based, Patient-Centered Diabetes Care System in Patients With Type 2 Diabetes: A Randomized, Controlled Trial for 24 Weeks. Diabetes Care. 2019;42:3-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 52. | Kleinman NJ, Shah A, Shah S, Phatak S, Viswanathan V. Improved Medication Adherence and Frequency of Blood Glucose Self-Testing Using an m-Health Platform Versus Usual Care in a Multisite Randomized Clinical Trial Among People with Type 2 Diabetes in India. Telemed J E Health. 2017;23:733-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 77] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 53. | Lee JY, Chan CKY, Chua SS, Ng CJ, Paraidathathu T, Lee KKC, Lee SWH. Telemonitoring and Team-Based Management of Glycemic Control on People with Type 2 Diabetes: a Cluster-Randomized Controlled Trial. J Gen Intern Med. 2020;35:87-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 54. | Lim S, Kang SM, Kim KM, Moon JH, Choi SH, Hwang H, Jung HS, Park KS, Ryu JO, Jang HC. Multifactorial intervention in diabetes care using real-time monitoring and tailored feedback in type 2 diabetes. Acta Diabetol. 2016;53:189-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 55. | Liou JK, Soon MS, Chen CH, Huang TF, Chen YP, Yeh YP, Chang CJ, Kuo SJ, Hsieh MC. Shared care combined with telecare improves glycemic control of diabetic patients in a rural underserved community. Telemed J E Health. 2014;20:175-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 56. | Luley C, Blaik A, Reschke K, Klose S, Westphal S. Weight loss in obese patients with type 2 diabetes: effects of telemonitoring plus a diet combination - the Active Body Control (ABC) Program. Diabetes Res Clin Pract. 2011;91:286-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 57. | Nicolucci A, Cercone S, Chiriatti A, Muscas F, Gensini G. A Randomized Trial on Home Telemonitoring for the Management of Metabolic and Cardiovascular Risk in Patients with Type 2 Diabetes. Diabetes Technol Ther. 2015;17:563-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 58. | Or CK, Liu K, So MKP, Cheung B, Yam LYC, Tiwari A, Lau YFE, Lau T, Hui PSG, Cheng HC, Tan J, Cheung MT. Improving Self-Care in Patients With Coexisting Type 2 Diabetes and Hypertension by Technological Surrogate Nursing: Randomized Controlled Trial. J Med Internet Res. 2020;22:e16769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 59. | Orsama AL, Lähteenmäki J, Harno K, Kulju M, Wintergerst E, Schachner H, Stenger P, Leppänen J, Kaijanranta H, Salaspuro V, Fisher WA. Active assistance technology reduces glycosylated hemoglobin and weight in individuals with type 2 diabetes: results of a theory-based randomized trial. Diabetes Technol Ther. 2013;15:662-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 60. | Pacaud D, Kelley H, Downey AM, Chiasson M. Successful delivery of diabetes self-care education and follow-up through ehealth media. Can J Diabetes. 2012;36:257-262. |
| 61. | Pressman AR, Kinoshita L, Kirk S, Barbosa GM, Chou C, Minkoff J. A novel telemonitoring device for improving diabetes control: protocol and results from a randomized clinical trial. Telemed J E Health. 2014;20:109-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 62. | Quinn CC, Shardell MD, Terrin ML, Barr EA, Park D, Shaikh F, Guralnik JM, Gruber-Baldini AL. Mobile Diabetes Intervention for Glycemic Control in 45- to 64-Year-Old Persons With Type 2 Diabetes. J Appl Gerontol. 2016;35:227-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 63. | Ramadas A, Chan CKY, Oldenburg B, Hussein Z, Quek KF. Randomised-controlled trial of a web-based dietary intervention for patients with type 2 diabetes: changes in health cognitions and glycemic control. BMC Public Health. 2018;18:716. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 64. | Rodríguez-Idígoras MI, Sepúlveda-Muñoz J, Sánchez-Garrido-Escudero R, Martínez-González JL, Escolar-Castelló JL, Paniagua-Gómez IM, Bernal-López R, Fuentes-Simón MV, Garófano-Serrano D. Telemedicine influence on the follow-up of type 2 diabetes patients. Diabetes Technol Ther. 2009;11:431-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 61] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 65. | Shea S, Weinstock RS, Teresi JA, Palmas W, Starren J, Cimino JJ, Lai AM, Field L, Morin PC, Goland R, Izquierdo RE, Ebner S, Silver S, Petkova E, Kong J, Eimicke JP; IDEATel Consortium. A randomized trial comparing telemedicine case management with usual care in older, ethnically diverse, medically underserved patients with diabetes mellitus: 5 year results of the IDEATel study. J Am Med Inform Assoc. 2009;16:446-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 248] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 66. | Stone RA, Rao RH, Sevick MA, Cheng C, Hough LJ, Macpherson DS, Franko CM, Anglin RA, Obrosky DS, Derubertis FR. Active care management supported by home telemonitoring in veterans with type 2 diabetes: the DiaTel randomized controlled trial. Diabetes Care. 2010;33:478-484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 153] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 67. | Sun C, Sun L, Xi S, Zhang H, Wang H, Feng Y, Deng Y, Wang H, Xiao X, Wang G, Gao Y, Wang G. Mobile Phone-Based Telemedicine Practice in Older Chinese Patients with Type 2 Diabetes Mellitus: Randomized Controlled Trial. JMIR Mhealth Uhealth. 2019;7:e10664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 88] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 68. | Tang PC, Overhage JM, Chan AS, Brown NL, Aghighi B, Entwistle MP, Hui SL, Hyde SM, Klieman LH, Mitchell CJ, Perkins AJ, Qureshi LS, Waltimyer TA, Winters LJ, Young CY. Online disease management of diabetes: engaging and motivating patients online with enhanced resources-diabetes (EMPOWER-D), a randomized controlled trial. J Am Med Inform Assoc. 2013;20:526-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 160] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 69. | Vinitha R, Nanditha A, Snehalatha C, Satheesh K, Susairaj P, Raghavan A, Ramachandran A. Effectiveness of mobile phone text messaging in improving glycaemic control among persons with newly detected type 2 diabetes. Diabetes Res Clin Pract. 2019;158:107919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 70. | Wang G, Zhang Z, Feng Y, Sun L, Xiao X, Wang G, Gao Y, Wang H, Zhang H, Deng Y, Sun C. Telemedicine in the Management of Type 2 Diabetes Mellitus. Am J Med Sci. 2017;353:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 71. | Wang J, Cai C, Padhye N, Orlander P, Zare M. A Behavioral Lifestyle Intervention Enhanced With Multiple-Behavior Self-Monitoring Using Mobile and Connected Tools for Underserved Individuals With Type 2 Diabetes and Comorbid Overweight or Obesity: Pilot Comparative Effectiveness Trial. JMIR Mhealth Uhealth. 2018;6:e92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 72. | Wayne N, Perez DF, Kaplan DM, Ritvo P. Health Coaching Reduces HbA1c in Type 2 Diabetic Patients From a Lower-Socioeconomic Status Community: A Randomized Controlled Trial. J Med Internet Res. 2015;17:e224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 146] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 73. | Welch G, Allen NA, Zagarins SE, Stamp KD, Bursell SE, Kedziora RJ. Comprehensive diabetes management program for poorly controlled Hispanic type 2 patients at a community health center. Diabetes Educ. 2011;37:680-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 74. | Whittemore R, Vilar-Compte M, De La Cerda S, Delvy R, Jeon S, Burrola-Méndez S, Pardo-Carrillo M, Lozano-Marrufo A, Pérez-Escamilla R. ¡Sí, Yo Puedo Vivir Sano con Diabetes! A Self-Management Randomized Controlled Pilot Trial for Low-Income Adults with Type 2 Diabetes in Mexico City. Curr Dev Nutr. 2020;4:nzaa074. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 75. | Wild SH, Hanley J, Lewis SC, McKnight JA, McCloughan LB, Padfield PL, Parker RA, Paterson M, Pinnock H, Sheikh A, McKinstry B. Supported Telemonitoring and Glycemic Control in People with Type 2 Diabetes: The Telescot Diabetes Pragmatic Multicenter Randomized Controlled Trial. PLoS Med. 2016;13:e1002098. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 76. | Williams ED, Bird D, Forbes AW, Russell A, Ash S, Friedman R, Scuffham PA, Oldenburg B. Randomised controlled trial of an automated, interactive telephone intervention (TLC Diabetes) to improve type 2 diabetes management: baseline findings and six-month outcomes. BMC Public Health. 2012;12:602. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 77. | Xu R, Xing M, Javaherian K, Peters R, Ross W, Bernal-Mizrachi C. Improving HbA1c with Glucose Self-Monitoring in Diabetic Patients with EpxDiabetes, a Phone Call and Text Message-Based Telemedicine Platform: A Randomized Controlled Trial. Telemed J E Health. 2020;26:784-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 78. | Yip MP. Effectiveness of a nurse coordinated system of telemedicine care for patients with type 2 diabetes mellitus. Ph.D Thesis, Ann Arbor: The Chinese University of Hong Kong (Hong Kong). 2002. |
| 79. | Cradock KA, ÓLaighin G, Finucane FM, Gainforth HL, Quinlan LR, Ginis KA. Behaviour change techniques targeting both diet and physical activity in type 2 diabetes: A systematic review and meta-analysis. Int J Behav Nutr Phys Act. 2017;14:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 218] [Cited by in RCA: 218] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 80. | Lee PA, Greenfield G, Pappas Y. The impact of telehealth remote patient monitoring on glycemic control in type 2 diabetes: a systematic review and meta-analysis of systematic reviews of randomised controlled trials. BMC Health Serv Res. 2018;18:495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 106] [Article Influence: 15.1] [Reference Citation Analysis (1)] |
| 81. | Okamoto R, Kumagai E, Kai H, Shibata R, Ohtsubo T, Kawano H, Fujiwara A, Ito M, Fukumoto Y, Arima H. Effects of lowering diastolic blood pressure to <80 mmHg on cardiovascular mortality and events in patients with coronary artery disease: a systematic review and meta-analysis. Hypertens Res. 2019;42:650-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 82. | Ceriello A, Colagiuri S, Gerich J, Tuomilehto J; Guideline Development Group. Guideline for management of postmeal glucose. Nutr Metab Cardiovasc Dis. 2008;18:S17-S33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 85] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 83. | Greenwood DA, Young HM, Quinn CC. Telehealth Remote Monitoring Systematic Review: Structured Self-monitoring of Blood Glucose and Impact on A1C. J Diabetes Sci Technol. 2014;8:378-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 84. | Marcolino MS, Maia JX, Alkmim MB, Boersma E, Ribeiro AL. Telemedicine application in the care of diabetes patients: systematic review and meta-analysis. PLoS One. 2013;8:e79246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 145] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 85. | Whitehead L, Seaton P. The Effectiveness of Self-Management Mobile Phone and Tablet Apps in Long-term Condition Management: A Systematic Review. J Med Internet Res. 2016;18:e97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 633] [Cited by in RCA: 457] [Article Influence: 50.8] [Reference Citation Analysis (0)] |
| 86. | Kim Y, Park JE, Lee BW, Jung CH, Park DA. Comparative effectiveness of telemonitoring vs usual care for type 2 diabetes: A systematic review and meta-analysis. J Telemed Telecare. 2019;25:587-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 87. | Martínez-Sarriegui I, García-Sáez G, Rigla M, Brugués E, de Leiva A, Gómez EJ, Hernando EM. How continuous monitoring changes the interaction of patients with a mobile telemedicine system. J Diabetes Sci Technol. 2011;5:5-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 88. | Olczuk D, Priefer R. A history of continuous glucose monitors (CGMs) in self-monitoring of diabetes mellitus. Diabetes Metab Syndr. 2018;12:181-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 114] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 89. | Cassimatis M, Kavanagh DJ. Effects of type 2 diabetes behavioural telehealth interventions on glycaemic control and adherence: a systematic review. J Telemed Telecare. 2012;18:447-450. [PubMed] |
| 90. | Pan X, Wang H, Hong X, Zheng C, Wan Y, Buys N, Zhang Y, Sun J. A Group-Based Community Reinforcement Approach of Cognitive Behavioral Therapy Program to Improve Self-Care Behavior of Patients With Type 2 Diabetes. Front Psychiatry. 2020;11:719. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 91. | Yang X, Li Z, Sun J. Effects of Cognitive Behavioral Therapy-Based Intervention on Improving Glycaemic, Psychological, and Physiological Outcomes in Adult Patients With Diabetes Mellitus: A Meta-Analysis of Randomized Controlled Trials. Front Psychiatry. 2020;11:711. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 92. | Hanlon P, Daines L, Campbell C, McKinstry B, Weller D, Pinnock H. Telehealth Interventions to Support Self-Management of Long-Term Conditions: A Systematic Metareview of Diabetes, Heart Failure, Asthma, Chronic Obstructive Pulmonary Disease, and Cancer. J Med Internet Res. 2017;19:e172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 285] [Cited by in RCA: 350] [Article Influence: 43.8] [Reference Citation Analysis (1)] |
| 93. | Al-Haj Mohd MMM, Phung H, Sun J, Morisky DE. Improving adherence to medication in adults with diabetes in the United Arab Emirates. BMC Public Health. 2016;16:857. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 94. | So CF, Chung JW. Telehealth for diabetes self-management in primary healthcare: A systematic review and meta-analysis. J Telemed Telecare. 2018;24:356-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 88] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 95. | Zhu H, Zhu Y, Leung SW. Is self-monitoring of blood glucose effective in improving glycaemic control in type 2 diabetes without insulin treatment: a meta-analysis of randomised controlled trials. BMJ Open. 2016;6:e010524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 96. | Indelicato L, Dauriz M, Santi L, Bonora F, Negri C, Cacciatori V, Targher G, Trento M, Bonora E. Psychological distress, self-efficacy and glycemic control in type 2 diabetes. Nutr Metab Cardiovasc Dis. 2017;27:300-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 97. | Wang Y, Xue H, Huang Y, Huang L, Zhang D. A Systematic Review of Application and Effectiveness of mHealth Interventions for Obesity and Diabetes Treatment and Self-Management. Adv Nutr. 2017;8:449-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 174] [Article Influence: 21.8] [Reference Citation Analysis (0)] |