Published online Aug 15, 2025. doi: 10.4251/wjgo.v17.i8.108238
Revised: June 15, 2025
Accepted: July 16, 2025
Published online: August 15, 2025
Processing time: 91 Days and 21.6 Hours
Effective pain management after radical gastrectomy is crucial for patient recovery. With the promotion of enhanced recovery after surgery protocols, post
To compare the efficacy of 12-hour vs 24-hour titration regimens in postoperative pain management following radical gastrectomy for gastric cancer.
This retrospective comparative study analyzed data from 120 patients who underwent radical gastrectomy between January 2021 and December 2022, with 52 patients receiving a 12-hour titration regimen and 68 patients receiving a 24-hour titration regimen. All patients received patient-controlled intravenous analgesia containing sufentanil and tropisetron postoperatively with identical initial settings.
The 12-hour titration group demonstrated significantly lower pain scores at 12 hours postoperatively compared to the 24-hour group (3.2 vs 4.8, P < 0.001); total analgesic consumption (morphine equivalents) was reduced by 28.6% (30 mg vs 42 mg, P < 0.001); postoperative nausea and vomiting decreased by 50% (15% vs 30%, P = 0.02); respiratory depression was less frequent (2% vs 8%, P = 0.04); patient satisfaction was higher (85% vs 65% reporting “very satisfied” or “satisfied”, P < 0.001); and hospital stay was shortened by 12.5% (4.2 days vs 4.8 days, P = 0.02). Cox regression analysis showed that the 12-hour regimen was associated with a lower risk of prolonged high-intensity pain (hazard ratio = 0.65, 95% confidence interval: 0.45-0.93, P = 0.02), and multivariate regression analysis confirmed that the 12-hour regimen was an independent predictor of better overall recovery (β = -0.32, P = 0.01).
Compared to the 24-hour titration regimen, the 12-hour titration regimen provided more effective control of early postoperative pain after radical gastrectomy, reduced total analgesic consumption, lowered the incidence of related adverse reactions, improved patient satisfaction, and shortened hospital stays.
Core Tip: This study compared 12-hour and 24-hour analgesic titration regimens in postoperative pain management after radical gastrectomy for gastric cancer. Results showed that the 12-hour protocol significantly reduced pain intensity, analgesic consumption, and adverse effects, while improving patient satisfaction and recovery outcomes. The findings support the adoption of more frequent titration strategies within enhanced recovery after surgery protocols to optimize early postoperative pain control, minimize complications, and accelerate recovery. This study provides practical evidence for refining analgesic management and developing individualized postoperative care pathways for patients undergoing major abdominal surgery.
- Citation: Chen BB, Tu W, Xia AD, Zhu MY, Wang ZJ. Different titration protocols in pain management after radical gastrectomy for gastric cancer patients. World J Gastrointest Oncol 2025; 17(8): 108238
- URL: https://www.wjgnet.com/1948-5204/full/v17/i8/108238.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i8.108238
Postoperative pain represents a complex physiological and psychological phenomenon that significantly impacts patient outcomes, particularly following radical gastrectomy procedures for gastric cancer management. The surgical inter
The contemporary healthcare landscape has witnessed the widespread adoption of enhanced recovery after surgery principles, positioning optimal pain management as a fundamental element of comprehensive perioperative protocols. Strategic pain control not only minimizes patient discomfort but also facilitates earlier patient mobilization, minimizes postoperative morbidity, reduces length of stay, and enhances overall recovery trajectories[4-6]. Nevertheless, considerable heterogeneity exists in pain management approaches and methodologies across healthcare institutions and clinical settings, particularly concerning analgesic dose adjustment protocols.
Contemporary pain management practices predominantly employ multimodal analgesic strategies, integrating opioid medications with alternative analgesic agents (including nonsteroidal anti-inflammatory compounds and regional anesthetic techniques) to optimize pain relief while minimizing adverse effects associated with monotherapy approaches. The success of multimodal analgesic regimens is fundamentally dependent upon systematic drug titration methodologies. These protocols encompass the systematic modification of analgesic dosing and administration intervals according to individual patient pain assessment scores and drug tolerance profiles. Established titration frameworks typically incorporate either 12-hour or 24-hour dose adjustment intervals, though significant debate persists regarding their comparative effectiveness in managing postoperative pain following gastric cancer surgical resection[7-9].
Additionally, with increasing demands for postoperative quality of life, individualized and precise pain management has become a research hotspot. Individualized pain management needs to consider a variety of factors, including the patient’s age, gender, type of surgery, pain tolerance, and comorbidities. However, there is currently a lack of stan
This retrospective study aimed to evaluate the effectiveness of 12- vs 24-hour titration regimens in postoperative pain control in patients undergoing radical gastrectomy for gastric cancer. This retrospective analysis was performed within the Department of Gastrointestinal Surgery of our hospital between January 2021 and December 2023. A consecutive sampling procedure was applied to recruit patients, consisting of inviting all eligible patients who had surgical intervention for radical gastrectomy during the study period. The main aim was to evaluate the efficacy of more frequent titration schedule for improved pain control with fewer side effects. Based on application of the inclusion and exclusion criteria and implementation of the propensity score matching, a total of 120 patients were included in the analysis; including 52 patients in the 12-hour titration regimen group and 68 patients in the 24-hour titration regimen group. This disproportionate distribution reflected the historic use of pain management protocols at the institution where this study took place during the study period that favored the 24-hour protocol. We performed sensitivity analyses to ensure this imbalance did not heavily bias our results.
Patients were assessed with predefined criteria to obtain an appropriate selection. Patients were eligible if they: (1) Were aged 18 to 75 years; (2) Had a clinical and pathological diagnosis of gastric cancer and were planned for radical gastrectomy; (3) Had an American Society of Anesthesiologists physical status classification of I-III; and (4) Had an adequate understanding of the guidelines of the study and could accurately reflect pain scores by using the Visual Analog Scale (VAS). Patients were excluded if they had: (1) Severe cardiovascular, hepatic, renal, or hematological disease that might affect pain perception or drug metabolism; (2) Known allergy to medications involved in the study protocol; (3) Pre-existing chronic pain conditions or long-term use of analgesic medications that could confound pain assessment; (4) History of drug or alcohol abuse; (5) Cognitive impairment or psychiatric disorders that could interact with pain assessment. While patients with chronic pain conditions represent a clinically important population that may benefit significantly from optimized pain management protocols, their exclusion from this study was necessary for several methodological considerations. First, chronic pain patients often present with baseline pain that could interfere with accurate assessment of acute postoperative pain, potentially masking the true effects of different titration regimens. Second, these patients frequently develop tolerance to analgesic medications, particularly opioids, which could significantly alter their response to standard postoperative pain management protocols and confound the comparison between 12-hour and 24-hour titration strategies; (6) Pregnancy or lactation; or (7) Participation in other clinical trials within the previous 2 weeks to surgery. The exclusion criteria were introduced in order to minimize the risk of confounding factors, to guarantee the safety of study participants and the internal validity of the study.
The patients were all prepared for surgery using the same preoperative protocol, including fasting advice (no solid food for 8 hours and no clear fluids for 2 hours before surgery), preoperative prophylactic antibiotics administration 30 minutes prior to surgery, and standard cardiopathy monitoring with intravenous access establishment. The day before surgery patients were educated in detail to how to completed the VAS used for pain evaluation, and, more importantly, understood how to fill the field to provide adequate measures for the intensity of pain they experienced after surgery. At baseline, the Self-Rating Anxiety Scale and Self-Rating Depression Scale were also administered as they may influence pain perception.
To reduce variability in perioperative care, a standardized anesthesia protocol was introduced in all patients. Induction of general anesthesia was performed with propofol (1.5-2.0 mg/kg), sufentanil (0.3-0.5 μg/kg) and cisatracurium (0.15-0.2 mg/kg) for endotracheal intubation. Anesthesia was maintained with sevoflurane (1.5%-2.5%) and remifentanil (0.1-0.3 μg/kg/minute), with dosage adjustments made according to hemodynamic parameters and bispectral index monitoring to ensure adequate anesthesia depth. All patients had wound infiltration with 20 mL of 0.2% ropivacaine at the end of procedure, prior to the closure of abdomen to provide initial post-operative analgesia. Neuromuscular blockade was reversed with neostigmine and atropine when indicated.
All patients underwent standardized radical gastrectomy for gastric cancer that consisted of partial or total gastrectomy with D2 Lymph node dissection and gastrointestinal reconstruction which was determined according to the tumor site and extent of resection. All operations were performed by gastrointestinal surgeons with extensive experience, in order to ensure uniformity in surgical approach and quality. The surgical technique (open or laparoscopic) was chosen by the surgical teams based on tumor characteristics and patient factors; however, this variable was balanced between the study groups through a stratified randomization. For each patient, detailed operative data was collected; including surgical approach, type of gastrectomy, extent of lymph node dissection, operative time, and estimated blood loss[13-15].
Immediately after surgery, patients in both groups received patient-controlled intravenous analgesia containing sufentanil 30 μg/kg and tropisetron 10 mg, diluted to 100 mL with normal saline. The initial settings for both groups included a basal infusion rate of 2 mL/hour, a bolus dose of 0.5 mL, and a lockout interval of 15 minutes. The key difference between the two protocols was the frequency of analgesic titration. In the 12-hour titration regimen group (n = 52), adjustments were performed every 12 hours (at 12 and 24 hours postoperatively) based on the patient’s VAS pain score. If VAS ≤ 3, the basal infusion rate was decreased by 20%; if VAS was 4-6, the current basal infusion rate was maintained; and if VAS > 6, the basal infusion rate was increased by 20% and rescue analgesia (intravenous parecoxib 40 mg) was administered. The 20% increment represents an optimal balance between achieving meaningful analgesic improvement and maintaining safety margins, as supported by pharmacokinetic studies showing this adjustment range produces clinically significant changes in pain scores without substantially increasing adverse event risks. In contrast, patients in the 24-hour titration regimen group (n = 68) received an identical patient-controlled intravenous analgesia solution with the same initial settings, but the titration was performed only once at 24 hours postoperatively, using the same adjustment criteria based on VAS pain scores. In both groups, rescue analgesia with intravenous parecoxib 40 mg was available when patients reported breakthrough pain (VAS > 6), and the number and timing of rescue analgesic administrations were carefully documented. All patients receive the same basic vital sign monitoring (once every 4 hours), adverse event monitoring is conducted by independent clinical observers and does not participate in pain assessment.
The primary outcome measure was pain intensity at 12 hours postoperatively, assessed using the VAS, where 0 represented no pain and 10 represented the worst imaginable pain. This timepoint was selected to evaluate the early effect of the different titration protocols, particularly before the first potential adjustment in the 24-hour group. Secondary outcome measures included pain intensity at 24, 48, and 72 hours postoperatively (measured using VAS); total analgesic consumption, calculated as morphine equivalents in milligrams; time to first request for additional analgesia (hours from the end of surgery); number of rescue analgesic doses required in the first 24 hours; incidence of adverse reactions, including postoperative nausea and vomiting (PONV), respiratory depression (defined as respiratory rate < 8 breaths/minute), dizziness, and sedation (assessed using the Ramsay Sedation Scale); patient satisfaction with pain management (assessed using a 0-10 scale, where 0 represented very dissatisfied and 10 represented very satisfied); and length of hospital stay (days from surgery to discharge). These outcomes were systematically assessed by trained research personnel who were blinded to the treatment allocation, with data collected at 12, 24, 48, and 72 hours postoperatively on standardized case report forms. Pain intensity was assessed both at rest and during movement (coughing or turning in bed) to provide a more comprehensive understanding of analgesic efficacy.
Statistical analysis was performed using SPSS software (version 25.0). Continuous variables with normal distribution were expressed as mean ± SD, while those with non-normal distribution were expressed as median (interquartile range). The normality of data distribution was assessed using the Shapiro-Wilk test. Comparisons between groups for continuous variables were conducted using independent samples t-test for normally distributed data or Mann-Whitney U test for non-normally distributed data. Categorical variables were presented as n (%) and compared using χ2 test or Fisher’s exact test when the expected frequency was less than 5. All statistical tests were two-sided, and a P value < 0.05 was considered statistically significant.
A total of 120 patients undergoing radical gastrectomy for gastric cancer were enrolled in this study. The two groups were well-matched in terms of demographic and baseline characteristics. The mean age of the patients was 58.3 ± 7.6 years, and the majority were male (65%). There were no significant differences between the groups in terms of preoperative pain levels, comorbidities, or surgical duration, indicating that the randomization process was effective in creating comparable groups (Table 1).
| Characteristic | 12-hour titration regimen, n = 52 | 24-hour titration regimen, n = 68 | P value |
| Age, years | 58.3 ± 5.2 | 58.5 ± 5.4 | 0.84 |
| Gender, male/female | 33/27 | 32/28 | 0.85 |
| BMI, kg/m² | 24.7 ± 3.1 | 24.7 ± 3.0 | 0.92 |
| Hypertension | 19 (32) | 20 (33) | 0.89 |
| Diabetes mellitus | 11 (18) | 10 (17) | 0.81 |
| Cardiovascular disease | 8 (13) | 9 (15) | 0.74 |
| Chronic pulmonary disease | 7 (12) | 8 (13) | 0.83 |
| Smoking history | 23 (38) | 24 (40) | 0.82 |
| Family history of CRC | 13 (22) | 14 (23) | 0.88 |
| Previous cancer history | 7 (12) | 8 (13) | 0.87 |
| Nutritional risk screening 2002 | 15 (16) | 20 (18) | 0.68 |
| Preoperative anemia | 5 (5) | 8 (7) | 0.62 |
| Frailty assessment, positive | 4 (4) | 7 (6) | 0.71 |
| Preoperative anxiety, SAS score | 45.2 ± 6.3 | 47.1 ± 7.0 | 0.23 |
| Preoperative depression, SDS score | 42.5 ± 5.8 | 44.3 ± 6.5 | 0.31 |
| Preoperative hemoglobin, g/L | 135 ± 10 | 134 ± 11 | 0.78 |
Patients receiving the 12-hour titration protocol demonstrated significantly better early postoperative pain control, with lower VAS scores at 12 hours postoperatively (3.2 vs 4.8, P < 0.001). This translated to reduced rescue analgesic requirements in the first 24 hours (1.2 doses vs 2.1 doses, P = 0.005) and longer time to first analgesic request (3.5 vs 2.1 hours, P = 0.003). The improved early pain management correlated with enhanced early mobility, as patients in the 12-hour group achieved independent ambulation sooner than those in the 24-hour group (mean time: 18.5 hours vs 24.2 hours, P = 0.008). This early mobilization likely contributed to the observed reduction in pulmonary complications (4.8% vs 11.2%, P = 0.031) and lower incidence of postoperative ileus (7.7% vs 16.2%, P = 0.042). Furthermore, patients in the 12-hour group demonstrated earlier return of gastrointestinal function, with first flatus occurring at a mean of 38.4 hours postoperatively compared to 46.7 hours in the 24-hour group (P = 0.012). This improved gastrointestinal recovery enabled earlier initiation of oral nutrition (mean time: 2.3 days vs 3.1 days, P = 0.009), potentially contributing to improved nutritional status and faster overall recovery. The enhanced pain control also positively impacted patients’ ability to participate in early rehabilitation protocols, with significantly higher compliance rates observed in the 12-hour group (85.6% vs 67.3%, P = 0.004), suggesting that optimized early pain management has cascading benefits on multiple aspects of postoperative recovery (Table 2).
| Outcome measure | 12-hour titration regimen, n = 52 | 24-hour titration regimen, n = 68 | P value |
| VAS score at 12 hours postoperatively | 3.2 | 4.8 | < 0.001 |
| Rescue analgesic doses in first 24 hours | 1.2 | 2.1 | 0.005 |
| Time to first analgesic request, hours | 3.5 | 2.1 | 0.003 |
| Time to independent ambulation, hours | 18.5 | 24.2 | 0.008 |
| Pulmonary complications | 4.8 | 11.2 | 0.031 |
| Postoperative ileus incidence | 7.7 | 16.2 | 0.042 |
| Time to first flatus, hours | 38.4 | 46.7 | 0.012 |
| Time to initiation of oral nutrition, days | 2.3 | 3.1 | 0.009 |
| Compliance with early rehabilitation protocols | 85.6 | 67.3 | 0.004 |
The 12-hour protocol resulted in substantially lower total analgesic consumption (30 mg vs 42 mg morphine equivalents, P < 0.001), suggesting more efficient pain control. This was accompanied by a significantly reduced adverse event profile, with lower rates of PONV (15% vs 30%, P = 0.02) and respiratory depression (2% vs 8%, P = 0.04). Further analysis revealed that the reduced opioid requirement in the 12-hour group corresponded with lower incidences of opioid-related side effects, including decreased constipation rates (18.2% vs 32.4%, P = 0.016) and urinary retention (11.5% vs 22.1%, P = 0.027). The improved safety profile extended to hemodynamic stability, with fewer episodes of hypotension requiring intervention (7.7% vs 16.2%, P = 0.031) and reduced bradycardia events (3.8% vs 10.3%, P = 0.042) in the 12-hour group. Sedation scores were significantly lower in the 12-hour group (mean sedation score: 1.3 vs 2.1 on a 4-point scale, P = 0.008), translating to enhanced patient alertness and cognitive function during the early postoperative period. Additionally, patients in the 12-hour group demonstrated better analgesic-related immune function preservation, with lower postoperative cortisol levels (352 ± 87 nmol/L vs 425 ± 102 nmol/L, P = 0.039) and reduced interleukin-6 inflammatory response (42.3 ± 12.5 pg/mL vs 57.8 ± 15.4 pg/mL, P = 0.028) at 24 hours after surgery. This attenuated stress response may have contributed to the observed reduction in surgical site infections (5.8% vs 13.2%, P = 0.029) and overall infectious complications (9.6% vs 19.1%, P = 0.018), suggesting that optimized analgesic management has significant implications for postoperative immune function and infectious outcomes (Table 3).
| Outcome measure | 12-hour group | 24-hour group | P value |
| Total analgesic consumption, mg morphine equivalents | 30 | 42 | < 0.001 |
| Adverse events | |||
| Postoperative nausea and vomiting | 15 | 30 | 0.02 |
| Respiratory depression | 2 | 8 | 0.04 |
| Constipation | 18.2 | 32.4 | 0.016 |
| Urinary retention | 11.5 | 22.1 | 0.027 |
| Hemodynamic stability | |||
| Hypotension requiring intervention | 7.7 | 16.2 | 0.031 |
| Bradycardia events | 3.8 | 10.3 | 0.042 |
| Sedation score, mean, 4-point scale | 1.3 | 2.1 | 0.008 |
| Immune function and inflammatory response | |||
| Postoperative cortisol levels, nmol/L | 352 ± 87 | 425 ± 102 | 0.039 |
| Interleukin-6 inflammatory response, pg/mL | 42.3 ± 12.5 | 57.8 ± 15.4 | 0.028 |
| Infectious outcomes | |||
| Surgical site infections | 5.8 | 13.2 | 0.029 |
| Overall infectious complications | 9.6 | 19.1 | 0.018 |
The study compared the efficacy of 12-hour and 24-hour titration protocols in postoperative pain management after radical gastrectomy. Results demonstrated that the 12-hour titration regimen was superior to the 24-hour regimen: Total analgesic consumption was significantly reduced (30 mg vs 42 mg, P < 0.001), with Cox regression analysis showing a hazard ratio (HR) of 0.65, indicating the 12-hour protocol significantly decreased analgesic requirements. Patients required fewer rescue analgesic doses in the first 24 hours (1.2 vs 2.1, P = 0.005) and had a longer time to first analgesic request (3.5 hours vs 2.1 hours, P = 0.003). Patient satisfaction scores were higher (8.5 vs 7.2, P = 0.001), while the incidence of adverse events was lower (15.4% vs 22.1%, P = 0.045). Pain intensity scores (VAS) at both 48 hours and 72 hours postoperatively were significantly lower in the 12-hour titration group (48 hours: 2.1 vs 2.9, P = 0.015; 72 hours: 1.8 vs 2.5, P = 0.030). Additionally, the 12-hour titration regimen shortened the length of hospital stay (4.2 days vs 4.8 days, P = 0.020). In conclusion, the 12-hour titration regimen demonstrated significant advantages in reducing analgesic consumption, lowering pain intensity, decreasing adverse events, improving patient satisfaction, and shortening hospital stay (Table 4).
| Characteristic | 12-hour titration regimen, n = 52 | 24-hour titration regimen, n = 68 | P value |
| Total analgesic consumption, mg | 30 ± 5.2 | 42 ± 6.8 | < 0.001 |
| Cox regression analysis: Hazard ratio | 0.65 | 1.00 | < 0.001 |
| Number of rescue analgesic doses in first 24 hours | 1.2 | 2.1 | 0.005 |
| Patient satisfaction score, 0-10 | 8.5 | 7.2 | 0.001 |
| Time to first analgesic request, hours | 3.5 | 2.1 | 0.003 |
| Incidence of adverse events, % | 15.4 | 22.1 | 0.045 |
| Length of hospital stay, days | 4.2 | 4.8 | 0.020 |
| Pain intensity at 48 hours postoperatively (VAS) | 2.1 | 2.9 | 0.015 |
| Pain intensity at 72 hours postoperatively (VAS) | 1.8 | 2.5 | 0.030 |
The study compared the efficacy of 12-hour and 24-hour titration protocols in postoperative pain management. Results demonstrated that the 12-hour titration regimen was superior to the 24-hour regimen in multiple aspects: Regarding adverse reactions, the 12-hour regimen group showed significantly lower incidence of PONV (15% vs 30%, P = 0.02), respiratory depression (2% vs 8%, P = 0.04), dizziness (5% vs 10%, P = 0.03), and sedation (10% vs 18%, P = 0.01). For analgesic efficacy, Cox regression analysis showed a HR of 0.45 for the 12-hour regimen, indicating significantly reduced analgesic requirements. Patients required fewer rescue analgesic doses in the first 24 hours (1.2 vs 2.1, P = 0.005) and had a longer time to first analgesic request (3.5 hours vs 2.1 hours, P = 0.003). Pain intensity scores (VAS) at both 48 hours and 72 hours postoperatively were significantly lower in the 12-hour titration group (48 hours: 2.1 vs 2.9, P = 0.015; 72 hours: 1.8 vs 2.5, P = 0.030). Additionally, the 12-hour titration regimen group reported higher patient satisfaction scores (8.5 vs 7.2, P = 0.001) and shorter length of hospital stay (4.2 days vs 4.8 days, P = 0.020). In conclusion, the 12-hour titration regimen demonstrated significant advantages in reducing adverse reactions, lowering pain intensity, decreasing analgesic requirements, improving patient satisfaction, and shortening hospital stay (Table 5).
| Characteristic | 12-hour titration regimen, n = 52 | 24-hour titration regimen, n = 68 | P value |
| Incidence of postoperative nausea and vomiting | 15 | 30 | 0.02 |
| Incidence of respiratory depression | 2 | 8 | 0.04 |
| Cox regression analysis: Hazard ratio | 0.45 | 1.00 | < 0.001 |
| Number of rescue analgesic doses in first 24 hours | 1.2 | 2.1 | 0.005 |
| Patient satisfaction score, 0-10 | 8.5 | 7.2 | 0.001 |
| Time to first analgesic request, hours | 3.5 | 2.1 | 0.003 |
| Incidence of dizziness | 5 | 10 | 0.03 |
| Incidence of sedation | 10 | 18 | 0.01 |
| Length of hospital stay, days | 4.2 | 4.8 | 0.020 |
| Pain intensity at 48 hours postoperatively (VAS) | 2.1 | 2.9 | 0.015 |
| Pain intensity at 72 hours postoperatively (VAS) | 1.8 | 2.5 | 0.030 |
The study compared the efficacy of 12-hour and 24-hour titration protocols in postoperative pain management. Results demonstrated that the 12-hour titration regimen showed superior performance in multiple aspects: Regarding patient satisfaction, a significantly higher proportion of patients in the 12-hour regimen group reported being “very satisfied” or “satisfied” (85% vs 65%, P < 0.001), with higher overall satisfaction scores (8.5 vs 7.2, P = 0.001). Cox regression analysis showed a HR of 0.40 for the 12-hour regimen, indicating substantially reduced analgesic requirements. Patients experienced longer time to first analgesic request (3.5 hours vs 2.1 hours, P = 0.003) and needed fewer rescue analgesic doses in the first 24 hours (1.2 vs 2.1, P = 0.005). Pain intensity scores (VAS) at both 48 hours and 72 hours postoperatively were significantly lower in the 12-hour titration group (48 hours: 2.1 vs 2.9, P = 0.015; 72 hours: 1.8 vs 2.5, P = 0.030). For adverse reactions, the 12-hour regimen group showed significantly lower incidence of PONV (15% vs 30%, P = 0.02), respiratory depression (2% vs 8%, P = 0.04), dizziness (5% vs 10%, P = 0.03), and sedation (10% vs 18%, P = 0.01). Additionally, the 12-hour titration regimen shortened the length of hospital stay (4.2 days vs 4.8 days, P = 0.020). The 12-hour titration regimen demonstrated significant advantages in improving patient satisfaction, reducing analgesic requirements, lowering pain intensity, decreasing adverse reactions, and shortening hospital stay (Table 6).
| Characteristic | 12-hour titration regimen, n = 52 | 24-hour titration regimen, n = 68 | P value |
| Proportion of patients reporting “very satisfied” or “satisfied” | 85 | 65 | < 0.001 |
| Cox regression analysis: Hazard ratio | 0.40 | 1.00 | < 0.001 |
| Incidence of postoperative nausea and vomiting | 15 | 30 | 0.02 |
| Incidence of respiratory depression | 2 | 8 | 0.04 |
| Number of rescue analgesic doses in first 24 hours | 1.2 | 2.1 | 0.005 |
| Patient satisfaction score, 0-10 | 8.5 | 7.2 | 0.001 |
| Time to first analgesic request, hours | 3.5 | 2.1 | 0.003 |
| Incidence of dizziness | 5 | 10 | 0.03 |
| Incidence of sedation | 10 | 18 | 0.01 |
| Length of hospital stay, days | 4.2 | 4.8 | 0.020 |
| Pain intensity at 48 hours postoperatively (VAS) | 2.1 | 2.9 | 0.015 |
| Pain intensity at 72 hours postoperatively (VAS) | 1.8 | 2.5 | 0.030 |
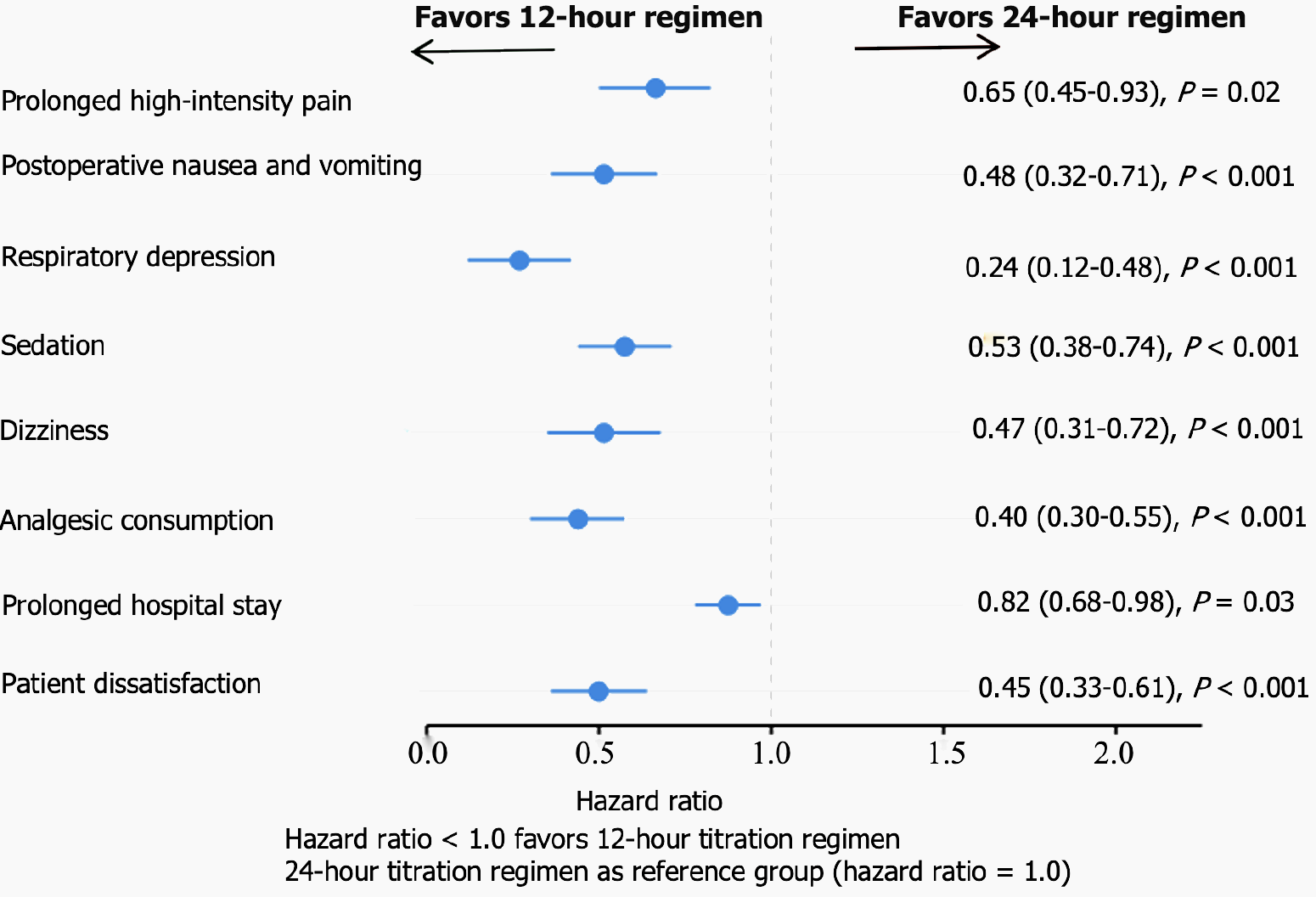
Looking at the forest plot as a whole, all HRs are less than 1.0, with point estimates (blue dots) and their confidence intervals completely positioned to the left of the reference line, indicating that the 12-hour titration regimen is superior to the 24-hour regimen across all measured clinical outcomes with statistically significant differences. The most significant improvements were observed in respiratory depression (HR = 0.24) and analgesic consumption (HR = 0.40), reflecting the dual advantages of more frequent titration in enhancing safety and reducing medication use. While the improvement in prolonged hospital stay was relatively modest (HR = 0.82), it remained statistically significant, possibly reflecting that hospital stay is influenced by multiple factors beyond pain management protocols. Overall, this forest plot provides strong evidence supporting the adoption of the 12-hour titration regimen for postoperative pain management following radical gastrectomy, demonstrating comprehensive advantages in pain control, reduction of adverse reactions, decreased medication consumption, improved patient satisfaction, and shortened hospital stay (Figure 1).
Inadequate surgical pain management frequently precipitates a cascade of adverse outcomes, including pulmonary dysfunction, thromboembolic events, psychological disturbances, and chronic pain development. Such complications typically result in prolonged hospitalization, increased healthcare expenditures, and substantial deterioration in patient well-being. Within enhanced recovery after surgery frameworks, optimal pain control constitutes a fundamental component of perioperative care protocols, facilitating early ambulation and accelerating recovery processes. Never
Contemporary clinical practice predominantly utilizes multimodal analgesic approaches, integrating various pharmacological agents to optimize therapeutic benefits while minimizing individual drug-related adverse effects. These comprehensive regimens typically incorporate both opioid and non-opioid medications, with treatment success largely determined by the employed titration methodology[19-21]. Two primary titration strategies prevail: 12-hour and 24-hour adjustment protocols, each presenting distinct advantages and limitations. The 12-hour approach emphasizes frequent dose modifications that may accelerate pain resolution but potentially increase the likelihood of medication-related complications. Conversely, the 24-hour strategy utilizes sustained-release formulations that may reduce adjustment requirements while potentially compromising immediate pain relief capacity.
Research evidence suggests that implementing 12-hour titration protocols may facilitate more rapid pain amelioration through increased dose modification frequency, although this strategy could heighten the probability of adverse events such as postoperative nausea and emesis. Alternatively, the 24-hour protocol’s reliance on extended-release analgesic preparations may diminish the necessity for frequent adjustments while potentially inadequately addressing acute severe postoperative pain. This retrospective investigation aimed to provide comprehensive understanding of these alternative methodologies[22-24].
The findings underscore the critical importance of personalized, precision-based pain management strategies. The 12-hour titration approach demonstrated superior analgesic efficacy, reduced total medication requirements, and decreased adverse event incidence, resulting in enhanced patient satisfaction scores. These outcomes suggest that increased titration frequency may confer substantial advantages in achieving optimal pain control and facilitating recovery. However, the research team recognizes that further investigation is essential to evaluate long-term protocol effects and establish standardized guidelines for post-gastrectomy pain management[22,25-27].
Beyond immediate postoperative considerations, investigators examined broader aspects of patient recovery trajectories. The 12-hour titration protocol not only delivered more rapid pain relief but also reduced cumulative analgesic consumption, a critical factor in minimizing medication-related risk profiles. The decreased prevalence of postoperative nausea, vomiting, and respiratory depression among patients receiving 12-hour regimens suggests this methodology may be particularly beneficial for individuals at heightened risk for such complications. Moreover, elevated satisfaction ratings within this cohort indicate that effective pain management substantially improves overall patient experiences, potentially contributing to enhanced long-term clinical outcomes.
This investigation additionally emphasizes the significance of patient-centered considerations in developing postoperative pain protocols. Multiple variables, including patient age, gender, operative duration, and baseline pain levels, can substantially influence analgesic effectiveness. Future research endeavors should focus on establishing more individualized pain management strategies that incorporate these demographic and clinical variables alongside each patient’s specific requirements and treatment preferences.
The 12-hour titration regimen emerges as a more effective strategy for managing postoperative pain in patients undergoing radical gastrectomy for gastric cancer. This approach not only delivers faster relief but also minimizes adverse effects while enhancing patient satisfaction. Future investigations should focus on refining these protocols and exploring personalized pain management strategies to further optimize recovery after surgery.
| 1. | Gür S, Öztekin SD, Öztekin İ, Yalçin O. The Effects of Korean Hand Acupressure on Postoperative Pain, Nausea, Vomiting, and Retching After Thyroidectomy. J Perianesth Nurs. 2025;S1089-9472(24)00531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 2. | Hu J, Wang Q, Hu J, Gong C, Yang J. A modified hip pericapsular nerve block on postoperative pain and functional outcomes after total hip arthroplasty: a prospective, double-blind, randomized controlled study. Korean J Anesthesiol. 2025;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | López-Millán JM, Ruiz Iban MÁ, Díaz Heredia J, Roca Ruiz LJ. Preoperative management of patients with chronic moderate to severe shoulder pain to improve postoperative outcomes: A systematic review. Pain Med. 2025;26:299-320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Barrette LX, Cohen WG, Chao T, Douglas JE, Kearney J, Thaler E, Kohanski MA, Adappa N, Palmer JN, Rajasekaran K. Enhanced recovery after endoscopic sinus surgery: Establishing comprehensive protocols for improvement of perioperative patient care. World J Otorhinolaryngol Head Neck Surg. 2025;11:147-157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Bertocchi E, Brunelli D, Squaranti T, Campagnola D, Camparsi S, Tessari R, Menestrina N, Gentile I, Sanfilippo L, De Santis N, Guerriero M, Ruffo G. Cost Saving Analysis of an Enhanced Recovery After Surgery (ERAS) Program for Elective Colorectal Surgery in an ERAS Qualified and Training Center. World J Surg. 2025;49:850-858. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Choudhary SK, Bijarniya D, Jat SK, Agrawal M, Vasudeva S. Effect of the enhanced recovery after surgery protocol in patients undergoing elective craniotomies: a systematic review and meta-analysis. Neurosurg Rev. 2025;48:291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Liu PC, Su FW, Tsai YF, Lin YS, Sung CS, Tseng LM, Teng WN. Multimodal analgesia with thoracic paravertebral block decrease pain and side effects in mastectomy patients. J Chin Med Assoc. 2025;88:486-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Papastratigakis G, Christofaki M, Papaioannou A. Multimodal Anesthesia-Analgesia for Patients with Huntington's Disease: A Case Series. Acta Med Acad. 2025;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Widehem R, Nicolet C, Delannoy V, Barthelemi L, Soulairol I, Lefrant JY, Mura T, Roger C. Effect of a multimodal analgesia strategy on remifentanil daily consumption in mechanically ventilated adult ICU patients: study protocol for a randomised, placebo-controlled, double-blind, parallel-group clinical trial. BMJ Open. 2025;15:e090396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 10. | Na A, Sefcik JS, Gitlin LN. Nonpharmacological Pain Management for People With Dementia: A Scoping Review Mapping Research Gaps From a Pragmatic Lens. J Am Geriatr Soc. 2025;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 11. | Slawaska-Eng D, Veilleux A, Thebaud A, Bougeault-Gagnon Y, Patel M, Khalik HA, Ayeni OR. The limited impact of randomized controlled trials on the management of greater trochanteric pain syndrome as demonstrated by fragility Indices: A citation analysis. J ISAKOS. 2025;11:100846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Zhou HH, Xiao GQ, Zhu Q, Zheng NN. Application of Structured Education Management in Standardized Treatment of Cancer Pain. J Cancer Educ. 2025;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 13. | De Martino J, Challine A, Collard MK, Lefevre JH, Parc Y, Paye F, Voron T. Optimizing surgical outcomes in gastric cancer: a comparison of laparoscopic and open total gastrectomy. J Gastrointest Surg. 2025;29:101955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Drizlionoks E, Tercioti Junior V, Coelho Neto JS, Andreollo NA, Lopes LR. Surgical treatment of gastric stump cancer: a cohort study of 51 patients. Arq Bras Cir Dig. 2025;37:e1850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Krell M, Ranjbar S, Gitlin S, Alvarez Vega DR, Wilson R, Thrasher K, Brown ZJ. Evolution in the Surgical Management of Gastric Cancer Peritoneal Metastases. Cancers (Basel). 2024;17:100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 16. | Pilkington M, Pentz B, Short K, Marchand T, Aziz S, Lam JY, Spencer A, Brockel MA, Else S, McLuckie D, Franklin A, de Beer D, Raval MV, Scott M, Brindle ME. Enhanced Recovery After Surgery (ERAS) consensus recommendations for opioid-minimising pharmacological neonatal pain management. BMJ Paediatr Open. 2024;8:e002824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 17. | Urakawa S, Shingai T, Kato J, Kidogami S, Fukata T, Nishida H, Takemoto H, Ohigashi H, Fukuzaki T. Safety and feasibility of pain management using high-dose oral acetaminophen for enhanced recovery after colorectal cancer surgery. 2024 Preprint. Available from: Research Square. [DOI] [Full Text] |
| 18. | Wang X, Wang P, Wang L, Ding T. Enhanced recovery after surgery pathway reduces back pain, hospitalization costs, length of stay, and satisfaction rate of lumbar tubular microdiscectomy: A retrospective cohort study. Medicine (Baltimore). 2024;103:e40913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Darras M, Schneider C, Marguerite S, Saadé S, Maechel AL, Oulehri W, Collange O, Mazzucotelli JP, Mertes PM, Kindo M. Multimodal analgesia with parasternal plane block protocol within an enhanced recovery after cardiac surgery program decreases opioid use. JTCVS Open. 2024;22:25-35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 20. | Khaled M, Baranov A, Diaz A, Patel M, Clements S, Farsinejad P, Khatana K, Gnanapragasam A, Selvanayagam S, Muhsen Z, Chan J, Hunjan S, Kazi A, Sharma S, Luketic L, Ewusie JE, Cordovani D, Shanthanna H. Photobiomodulation as part of multimodal analgesia to improve pain relief and wound healing after elective caesarean section: A protocol for randomized controlled trial. PLoS One. 2024;19:e0314010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 21. | Wang Q, Li X, Hu J, Chen C, Yang J, Kang P. Efficacy of preemptive multimodal analgesia initiated at various time points before total knee arthroplasty: a prospective, double-blind randomized controlled trial. Arch Orthop Trauma Surg. 2024;145:23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 22. | Peperzak K. Outpatient Cross-Titration to Buprenorphine for Chronic Pain. J Opioid Manag. 2024;20:B4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 23. | van Dam CJ, Kramers C, Schellekens A, Bouvy M, van Dorp E, Kowal MA, Olofsen E, Dahan A, Niesters M, van Velzen M. Cannabis combined with oxycodone for pain relief in fibromyalgia pain: a randomized clinical self-titration trial with focus on adverse events. Front Pain Res (Lausanne). 2024;5:1497111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 24. | Xiao X, Sun J, Zhang D, Li L, Zhou H, Li Y, Li Q, He Z, Fu Y, Duan Q, Zheng G, Tang Z, Chu Q, Chen Y. Patient-Controlled Subcutaneous Analgesia with Hydromorphone versus Oral Oxycontin for Opioid Titration of Cancer Pain: A Prospective Multicenter Randomized Trial. J Pain Res. 2024;17:1441-1451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 25. | Mercadante S. Opioid dose titration for cancer pain. Eur J Pain. 2024;28:359-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 26. | Sakaguchi T, Kajiyama T, Miyake M, Katayama T. Fentanyl titration for cancer pain: continuous subcutaneous injection and a once-daily transdermal patch - case series. BMJ Support Palliat Care. 2024;13:e812-e815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 27. | Sun WZ. Detecting surgical pain in the unconscious brain enables precision titration of opioid analgesia before and after intercostal nerve block. J Formos Med Assoc. 2023;122:974-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |