Published online Aug 15, 2025. doi: 10.4251/wjgo.v17.i8.105321
Revised: March 25, 2025
Accepted: June 16, 2025
Published online: August 15, 2025
Processing time: 208 Days and 16.6 Hours
Gastric cancer (GC) is a widespread malignancy and associated with high rates of morbidity and mortality worldwide.
To examine the functional role of long non-coding RNAs small nucleolar RNA host gene 5 (SNHG5) and its regulation of miR-92a-3p and B-cell translocation gene 2 (BTG2) in GC progression.
Quantitative reverse transcription PCR and western blot analysis determined the expression of SNHG5, miR-92a-3p, and BTG2 in GC and adjacent non-neoplastic mucosa. Dual-luciferase assays demonstrated interactions of SNHG5 with miR-92a-3p and BTG2. AGS cells were transfected with SNHG5 overexpression and miR-92a-3p knockdown models. Various assays, including CCK-8, colony formation, scratch wound healing, and Transwell assays, were used to determine cell proliferation and migration. An experimental model of a xenograft mouse was used to determine in vivo tumor growth. At the same time histological changes were evaluated by hematoxylin and eosin staining, with western blot analysis used to evaluate signaling pathway protein expression.
BTG2 and SNHG5 were downregulated in GC tissues, and miR-92a-3p was upregulated. Overexpression of SNHG5 or knockdown of miR-92a-3p reduced GC cell proliferation and migration, and increased BTG2 expression while decreasing PI3K/AKT signaling activity. The dual-luciferase assays demonstrated direct binding of miR-92a-3p to SNHG5 and BTG2. Tumor volume and weight were significantly reduced in mice transplanted with AGS cells treated with miR-92a-3p inhibitor or SNHG5 overexpression compared with control AGS cells. Hematoxylin and eosin staining revealed that treated tumors exhibited degenerative characteristics, including irregular morphology and nucleolysis.
LncRNA SNHG5 inhibited GC cell growth and migration by modulating the PI3K/AKT pathway via the miR-92a-3p/BTG2 axis.
Core Tip: This study emphasized the role of long non-coding RNAs small nucleolar RNA host gene 5 (SNHG5) as a tumor suppression factor in gastric cancer (GC). SNHG5 acted as a competing endogenous RNA by reducing levels of miR-92a-3p, leading to increased levels of B-cell translocation gene 2, a tumor suppressor gene. This ultimately leads to the inhibition of GC cell proliferation and migration by inhibiting the PI3K/AKT signaling pathway. This is the first time that the SNHG5/miR-92a-3p/B-cell translocation gene 2 axis has been discovered to contribute to the progression of GC. This molecular understanding aids in providing an attractive target for therapy and will contribute to a framework of RNA-based approaches to diagnosing and treating GC.
- Citation: Mao QQ, Zhang ML, Zhong L, Xu XD, Wang XH, Pan DY, Zhou FS, Huang JX, Zhao XG, Chen JJ, Jiang XY, Sun X, Ding WQ. LncRNA SNHG5 modulates cell proliferation and migration through the miR-92a-3p/BTG2 axis in gastric cancer by the PI3K/AKT pathway. World J Gastrointest Oncol 2025; 17(8): 105321
- URL: https://www.wjgnet.com/1948-5204/full/v17/i8/105321.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i8.105321
Gastric cancer (GC) is one of the most prevalent and deadliest cancers globally. The cancer burden, morbidity burden, and mortality burden of GC is high and significant and is even more relevant for populations in East Asia[1]. There have not been many recent advances in diagnostic and therapeutic measures. However, advanced GC has one of the worst prognoses compared with other advanced cancers.
Recent advances in cancer studies have focused on understanding the critical regulatory roles of non-coding RNAs (ncRNAs), which may have key roles in regulating the initiation and promotion of GC[2]. The ncRNAs include microRNAs (miRNAs) and long ncRNAs (lncRNAs). miR-92a-3p is the most upregulated miRNA in GC tissues, and it is associated with increased proliferation of the tumor, invasiveness of the cancer, and worse clinical prognoses[3]. Similarly, lncRNAs are currently being investigated regarding cancer pathways, including the small nucleolar RNA host gene 5 (SNHG5). SNHG5 has been investigated throughout other cancers; however, its functional role in GC is currently understudied[4].
There is evidence that lncRNA SNHG5 is downregulated in GC tissues and serum compared with normal adjacent tissues, suggesting it likely has an anti-tumor role. However, the downstream molecular interactions of SNHG5 and clinical implications are not defined. B-cell translocation gene 2 (BTG2) is a tumor suppressor gene downregulated in various types of cancers, including thymic, prostate, and hepatocellular carcinoma[5]. Furthermore, BTG2 inhibits proliferation and induces apoptosis in GC[6,7].
Bioinformatics analyses from databases like StarBase show that SNHG5 potentially sponges miR-92a-3p, which targets BTG2, suggesting there may be an SNHG5/miR-92a-3p/BTG2 regulatory network that regulates malignancy in GC. However, there is much to learn with the completion of functional studies to confirm this regulatory network.
This study investigated the relationship between lncRNA SNHG5, miR-92a-3p, and BTG2 in GC. It will contribute to a better understanding of GC pathogenesis and provide new possible molecular targets for further applications in precision medicine and RNA decoy-based therapeutics.
Between February 2022 and October 2023, we acquired nearby non-cancerous tissues and GC tissues from a cohort of 8 patients with a diagnosis of GC. All tissue collections were conducted with protocols approved by the hospital’s Institutional Review Board after a thorough ethics review. All patients provided written informed consent prior to any sample collection in accordance with the Declaration of Helsinki and institutional ethical policies. Quantitative PCR (qPCR) was performed on both GC and corresponding normal tissues to investigate the expression patterns of miR-92a-3p, lncRNA SNHG5, and BTG2.
The AGS cells were procured from the American Type Culture Collection and cultured in Dulbecco’s Modified Eagle Medium, a high-glucose formulation enriched with 100 μg/mL of streptomycin, 10% fetal bovine serum, and 100 units/mL of penicillin.
To establish various experimental groups in the GC cell line AGS, we performed transfections with plasmids including the negative control (NC), overexpression of lncRNA SNHG5 (OE-lncRNA SNHG5), and knockdown of miR-92a-3p (sh-miR-92a-3p). On the day prior, the cells were plated and allowed to grow until they achieved about 40% confluence. On the day of transfection, the culture medium was switched to a serum-deprived version, and 2 μL of Lipofectamine 3000 was introduced into each well. This mixture was brought up to a final volume of 125 μL with Dulbecco’s Modified Eagle Medium. Subsequently, 8 μg of the respective plasmid was introduced into each well, and a 20-min incubation at room temperature was allowed for the mixture to promote complex aggregation. Subsequently, 250 μL of the transfection reagent solution was aliquoted into each well, ensuring thorough mixing. The cells were then transferred to a 37 °C incubator for a period of 5 h. After this interval the transfection mixture was aspirated, and the cells were replenished with complete growth medium for continued incubation for an additional 48 h.
Following transfection of the AGS cells, the culture medium was meticulously suctioned off, followed by two rounds of cell washing with PBS to remove any residual medium. Afterward, 100 μL of lysis buffer was introduced to each well, and the cells were lysed with gentle rocking to collect the lysate. Subsequently, 20 μL of the cellular lysis buffer and 100 μL of the LAR II reagent were introduced to the lysate on a luminometer-compatible plate, mixed thoroughly to ensure homogeneity, and the luciferase activity was measured. The reaction was terminated by introducing 100 μL of the stopping solution per well as the final step, and the luciferase activity was quantified by calculating the ratio of luminescence before and after the addition of the stop solution.
Four male BALB/c nude mice aged 4-6 weeks were selected for the in vivo experiment. The animals were randomly assigned to receive subcutaneous injections of AGS cells that were either untreated or transfected with either the NC plasmid, OE-lncRNA SNHG5 plasmid, or sh-miR-92a-3p plasmid. This resulted in establishing four groups: The blank control; NC; lncRNA SNHG5 overexpression; and miR-92a-3p knockdown groups.
Prior to injection, the AGS cells were resuspended in sterile normal saline to achieve a suitable concentration. A syringe delivered the cell mixture subcutaneously to the ventral flank regions of the mice. The growth of the tumors was supervised by consistently measuring their dimensions at 4-day intervals. Following a 28-day monitoring period, the animals were euthanized humanely. The tumor tissues were then harvested for further analysis, including measurement, photography, and weighing.
The tumor tissues were fixed in a 10% formalin buffer solution and processed for histological examination through routine methods, including paraffin embedding and sectioning. The resulting paraffin sections underwent deparaffinization and rehydration, followed by hematoxylin and eosin staining to reveal cellular architecture and tissue morphology. Post-staining, the sections were subjected to an ethanol gradient for dehydration, cleared with xylene, and mounted with a neutral balsam medium for enhanced transparency and preservation. The gastric tissue pathological changes in the mice from each experimental group were subsequently observed using a light microscope with a × 200 magnification.
The GC cells underwent a 24-h incubation period for attachment and initial growth before transfection. After transfection, at 12-h intervals up to 72 h, the wells were filled with serum-free medium. Subsequently, 10 μL CCK-8 was introduced into each well to assess cellular viability. Two hours later, a plate reader was used to measure the absorbance at 450 nm.
Following a 24-h transfection period, the cells were harvested using enzymatic dissociation with trypsin and neutralized with pancreatic enzymes to create a single-cell suspension. Subsequently, an aliquot of 500 cells was sampled and plated in triplicate in petri dishes for each experimental group. The cultures were nurtured at a uniform temperature of 37 °C in a CO2-humidified chamber and preserved at a 95% humidity level for a span of 7 days. Once the incubation was complete, the cells underwent fixation with a 4% paraformaldehyde solution. Following fixation, they were stained with a 1% crystal violet solution to visualize the colonies. Clonal colonies, defined as groups of more than 10 cells, were enumerated under a microscope. The cloning efficiency was calculated as a percentage by taking the ratio of the number of resulting colonies to the initial cell seeding density and then multiplying by 100 to convert it into a percentage.
Cells were evenly distributed into 6-well plates for cultivation in fetal bovine serum-supplemented medium until reaching approximately 90% confluence. A sterile pipette tip was quickly drawn across the monolayer to create a uniform scratch. Subsequently, we replaced the medium in the plates with serum-free medium and incubated them for a period of 24 h. Cell migration images were captured using an Olympus CKX41 inverted microscope (Tokyo, Japan) and analyzed with ImageJ software provided by the National Institutes of Health (Bethesda, MD, United States). To determine the migration rate (MR), we employed the following formula: Migration rate = (A - B)/A × 100%, where A denotes the initial gap width at the 0-h mark and B denotes the gap width after a 24-h culture period.
In our study total RNA was isolated from AGS cells of each experimental group using Trizol reagent. Reverse transcription of RNA to cDNA was carried out using a commercial kit. Subsequently, qPCR analysis was conducted with the resulting cDNA and gene-specific primers, following established qPCR protocols. GAPDH and U6 snRNA served as endogenous controls for data normalization. The qPCR conditions began with an initial denaturation step at 95 °C for 30 s, followed by 40 cycles of 94 °C for 30 s, annealing at 65 °C for 30 s, and a final denaturation at 94 °C for 30 s. The 2-ΔΔCT method was applied to determine the relative gene expression levels, adjusting for variations in initial template amounts. The primers for BTG2 were 5’-CCTACCCCTACCCACCAGAA-3’ (forward) and 5’-TTTACCGCTTTGCCTCGTCT-3’ (reverse). The primers for GAPDH were 5’-ACCACAGTCCATGCCATCAC-3’ (forward) and 5’-TCCACCACCCTGTTGCTGTA-3’ (reverse).
Lysis was performed in myocardial tissue or cells using RIPA buffer to extract total protein (Thermo Scientific, United States). SDS-PAGE was first employed for protein separation, followed by membrane transfer onto PVDF and blocking with non-fat dry milk. Incubation with the primary antibodies was performed overnight at a dilution ratio of 1:2000. The primary antibodies included a rabbit polyclonal to AKT (phospho T308) (Cat No. ab38449, Abcam, 1:10000), rabbit monoclonal mAb to AKT (Cat No. ab314110, Abcam, 1:1000), p-PI3K (Cat No. ab ab302958, Abcam, 1:100000), rabbit mAb to PI3K (product number 17366, Abcam, 1:1000), and rabbit pAb to GAPDH as a loading control (Cat No. 10494-1-AP, Proteintech, 1:40000). An HRP-linked secondary antibody was then applied for 60 min, after which the membranes were developed using an ECL substrate to visualize the protein bands.
Statistical evaluations were conducted utilizing SPSS version 17.0 and GraphPad software version 5.0. Data from the experiments were depicted as the mean ± SD, and the independent samples t-test was utilized to evaluate mean value differences between the two groups.
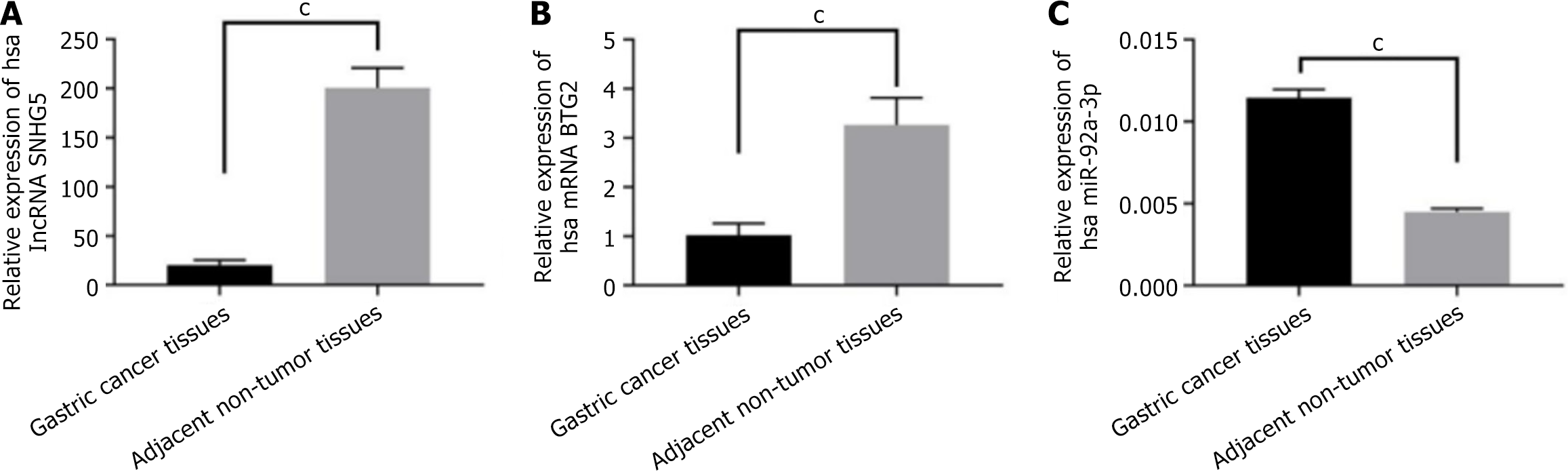
To identify the levels of BTG2, miR-92a-3p, and lncRNA SNHG5 in GC, tumor tissues and adjacent non-tumor tissues from patients with GC were subjected to qPCR, and the results showed that the expression of lncRNA SNHG5 was significantly decreased in GC tumor tissues (Figure 1A). Notably, BTG2 expression was significantly lower in GC tumor tissues than in the adjacent non-tumor tissues (Figure 1B), while there was an observed increase of miR-92a-3p (Figure 1C).
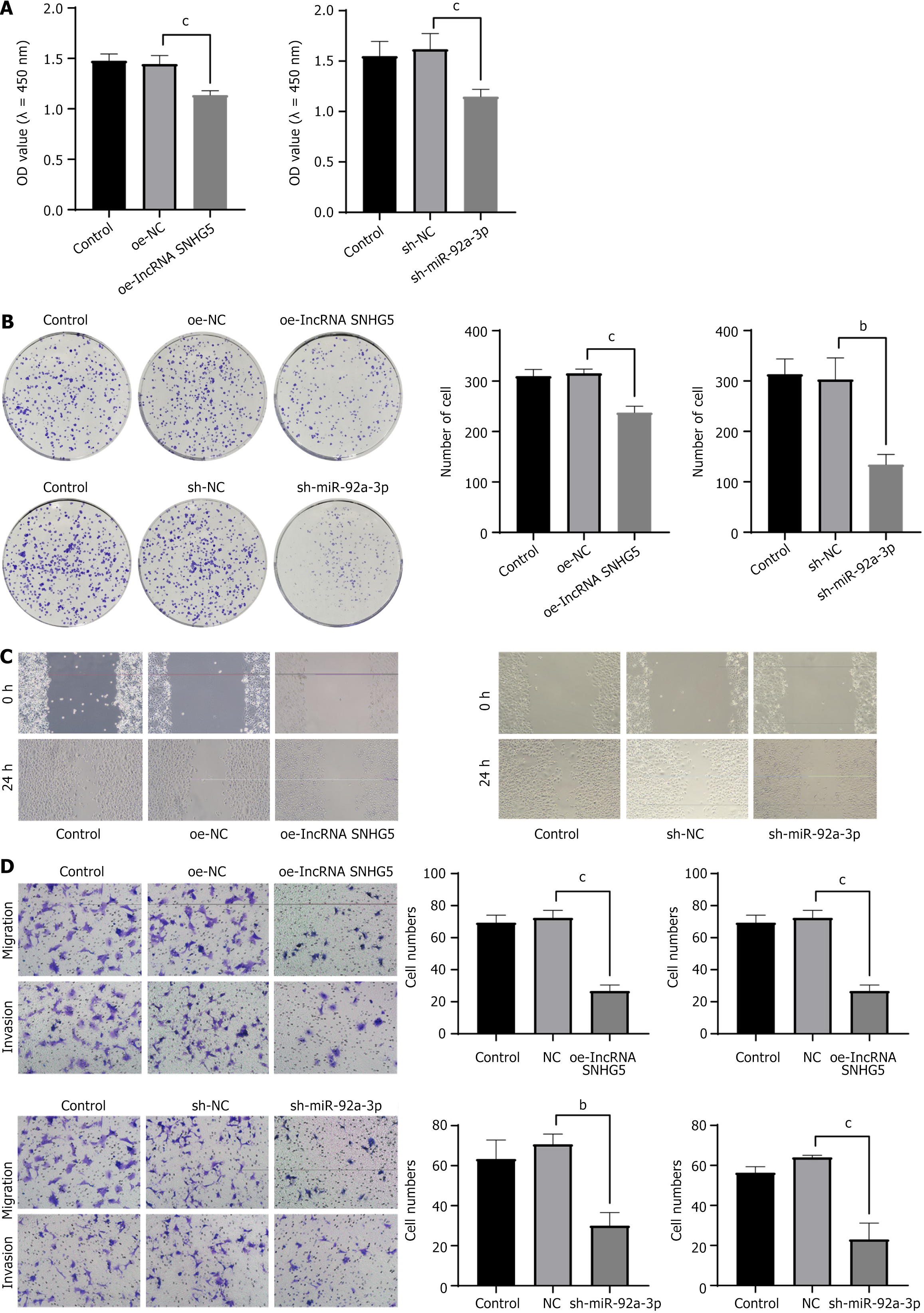
The data presented in the figure demonstrates that overexpression of lncRNA SNHG5 coupled with the suppression of miR-92a-3p resulted in a significant reduction in the viability (Figure 2A and B) by CCK-8 assay and colony formation and decreased migratory capacity by scratch and Transwell assay (Figure 2C and D) of GC cells as opposed to the control group.
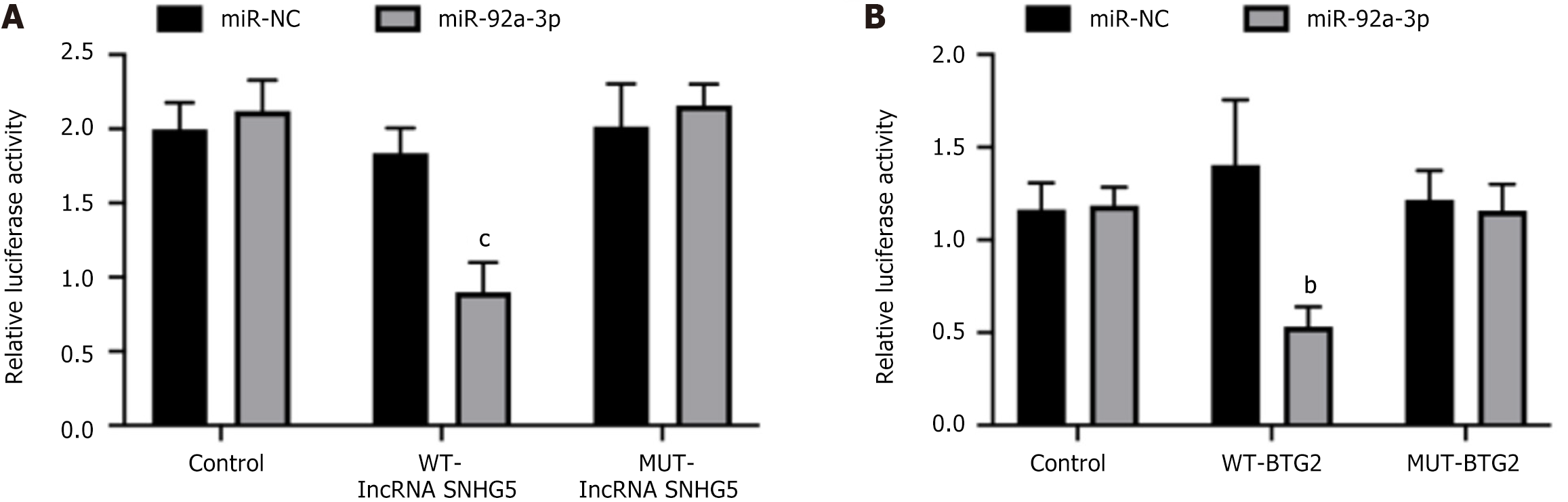
To further explore the molecular basis of how lncRNA SNHG5 suppressed the growth of GC, we employed the miRDB and TargetScan databases to pinpoint possible downstream targets of lncRNA SNHG5. Our findings revealed that lncRNA SNHG5 and miR-92a-3p share complementary sequences. We performed a luciferase reporter gene assay using GC cells to validate the direct binding between miR-92a-3p and lncRNA SNHG5. The results indicated that the introduction of miR-92a-3p mimics along with the wild-type (WT) lncRNA SNHG5 vector significantly decreased luciferase activity when compared with the mutant (MUT) lncRNA SNHG5 vector (Figure 3A).
In addition, we performed a database search to identify the direct targets of miR-92a-3p and found that NID1 could be a candidate target gene and was subsequently confirmed using a luciferase assay. It is noteworthy that the luciferase activity in AGS cells that were cotransfected with the WT-BTG2 vector along with miR-92a-3p mimics exhibited a marked decrease compared with those transfected with the MUT-BTG2 vector. In summary, miR-92a-3p appeared to serve as a downstream effector of lncRNA SNHG5, and BTG2 was directly targeted by miR-92a-3p, suggesting an indirect regulatory pathway from lncRNA SNHG5 to BTG2 (Figure 3B).
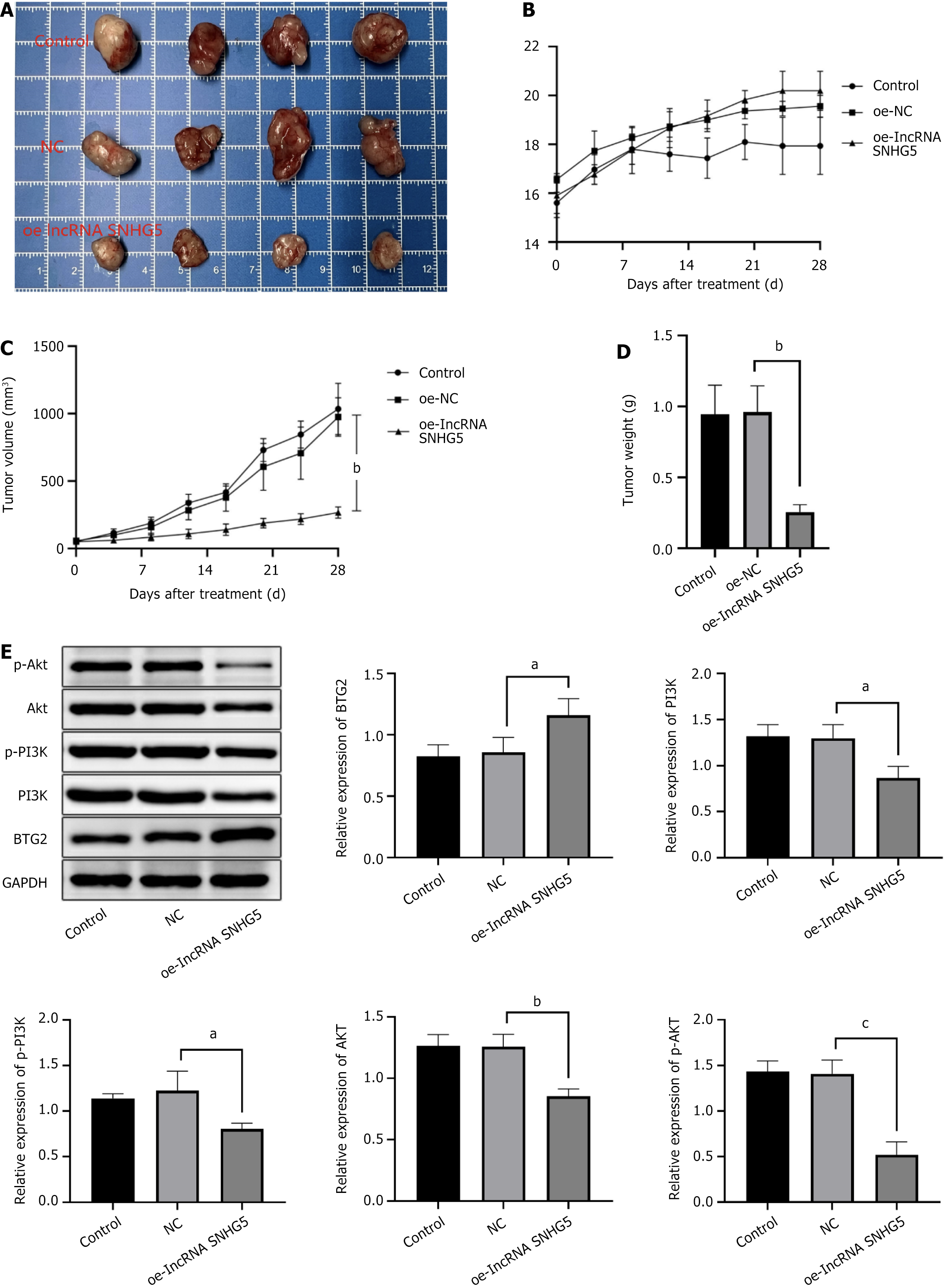
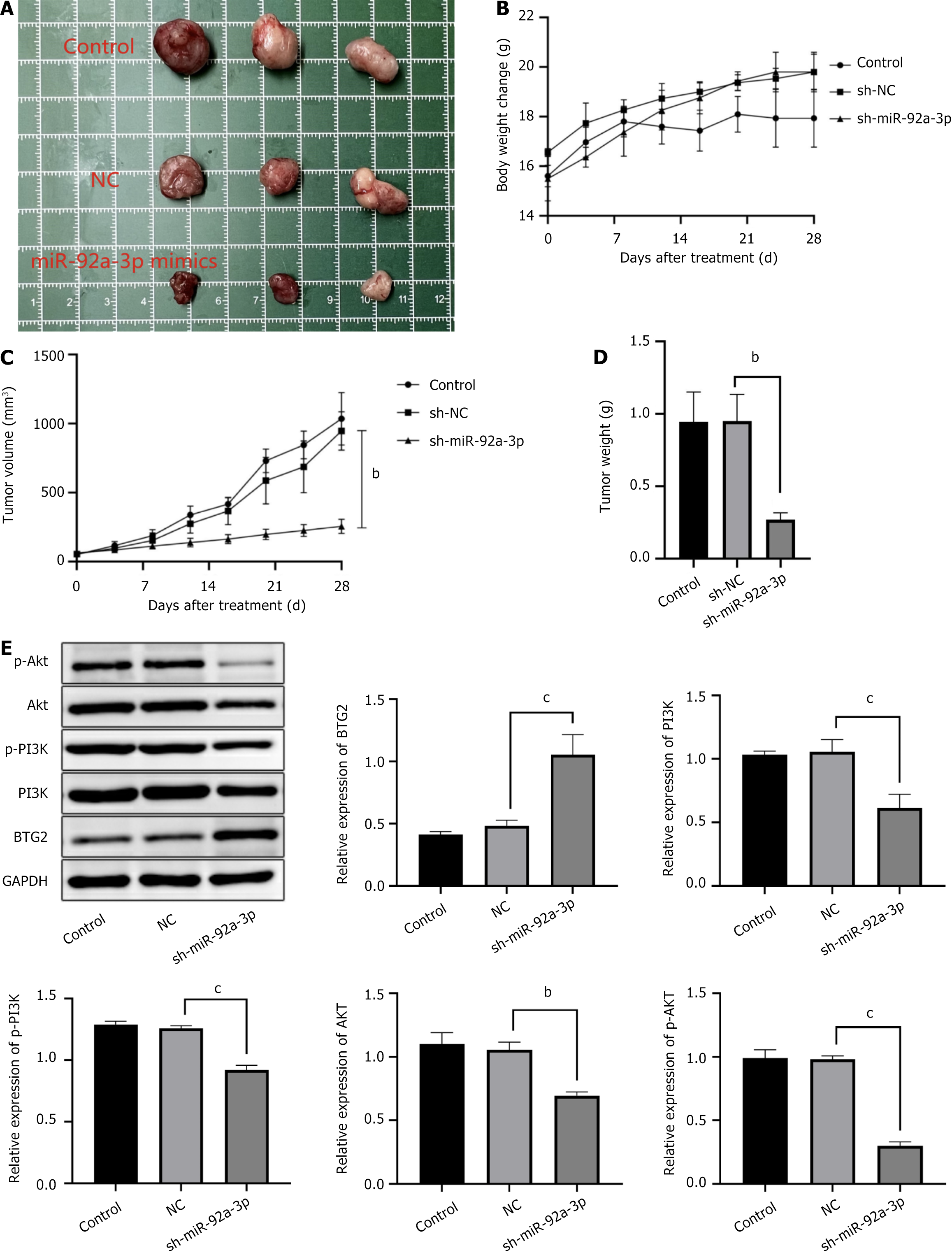
We established a xenograft mouse model to explore the impact of lncRNA SNHG5 on GC within a living system. The figure illustrates that the OE-lncRNA SNHG5 (Figure 4) and the sh-miR-92a-3p (Figure 5) resulted in a substantial reduction in both the weight and dimensions of the tumors. The tumor cells in the tissue displayed irregular morphologies and evidence of nucleolar disintegration. Additionally, upon OE-lncRNA SNHG5 and the sh-miR-92a-3p transfection, the expression of BTG2 within the tumor tissues of the mice was observed. In contrast, the protein levels of AKT, PI3K, phosphorylated PI3K, and phosphorylated AKT were reduced.
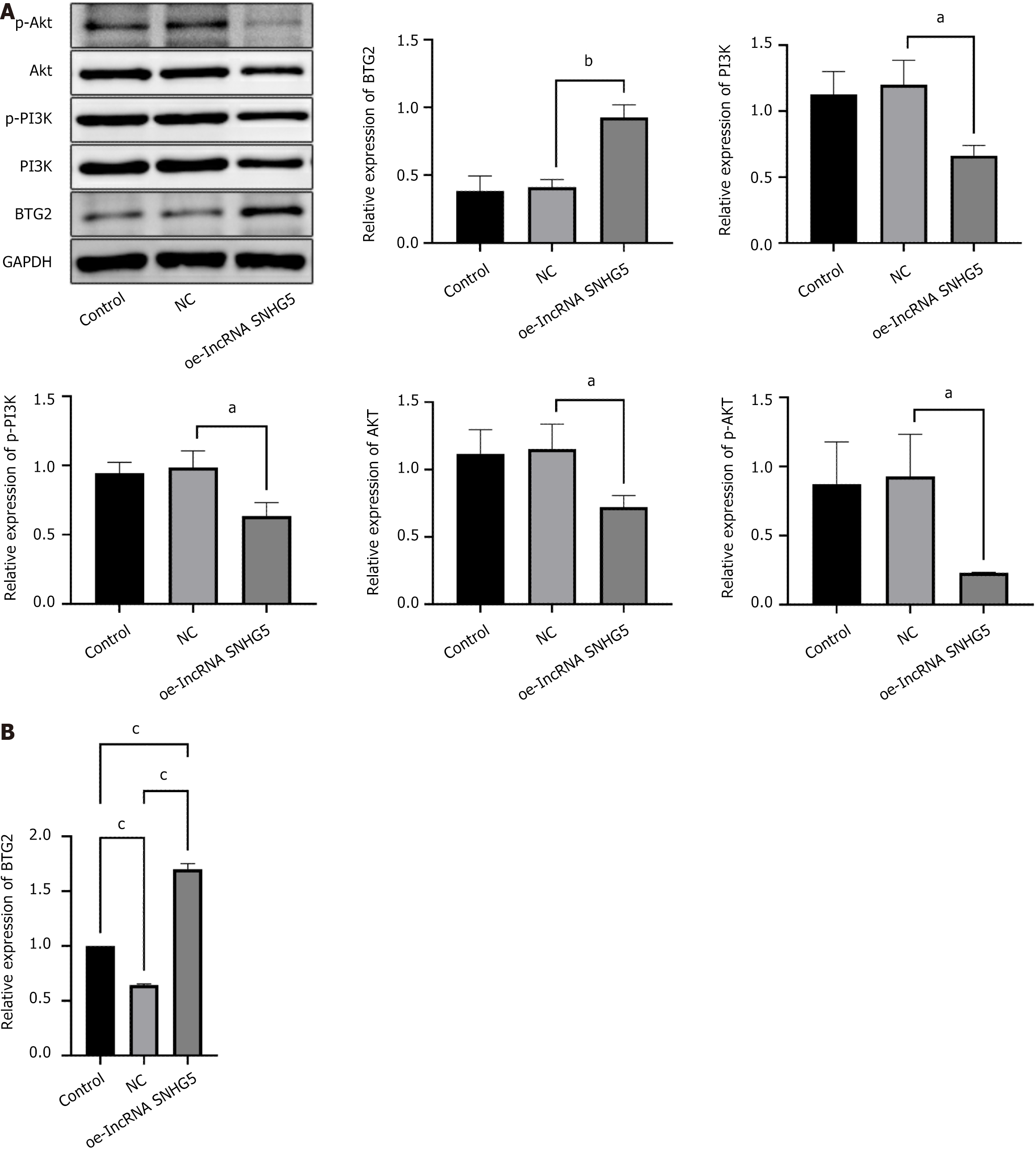
Upon OE-lncRNA SNHG5 transfection we evaluated the levels of proteins associated with miR-92a-3p, BTG2, and the PI3K/AKT signaling pathway. The comparative analysis revealed that in the lncRNA SNHG5 overexpression group a notable decrease in the levels of miR-92a-3p were observed when juxtaposed with the NC group. In parallel there was an observed upregulation of both BTG2 mRNA and protein levels. Conversely, the protein levels of PI3K, AKT, p-AKT, and p-PI3K were notably diminished (Figure 6).
The overexpression of SNHG5 potentially exerts its inhibitory influence on miR-92a-3p expression, subsequently inhibiting the activation of the PI3K/AKT signaling pathway and upregulating BTG2. This orchestrated molecular modulation is hypothesized to underlie the suppressive effects on GC cell proliferation and migration.
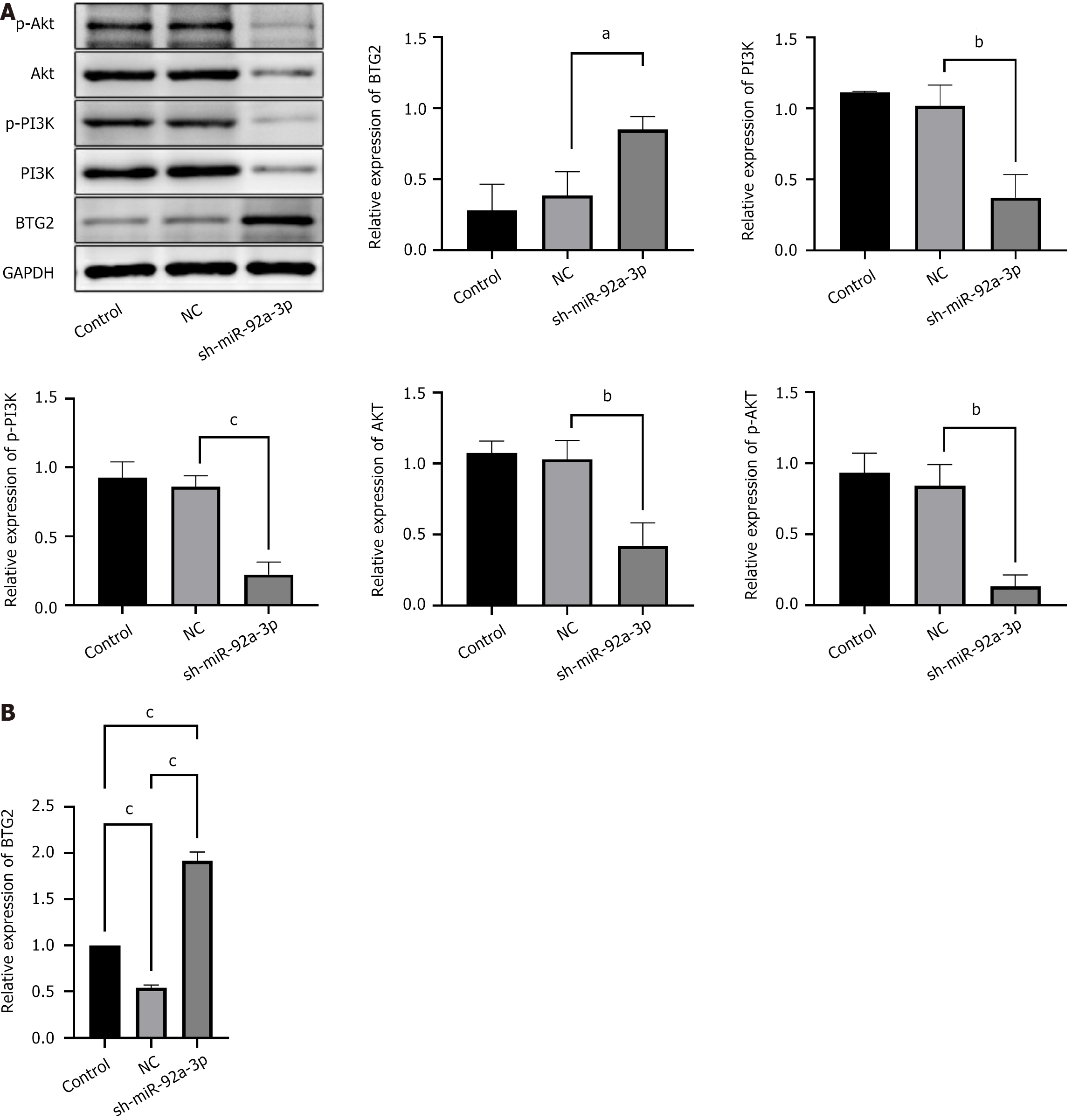
The miR-92a-3p knockout group showed increased BTG2 levels, whereas the expression of phosphorylated PI3K, phosphorylated AKT, PI3K, and AKT proteins was found to be diminished (Figure 7). Due to its knockdown, the reduction in miR-92a-3p expression is hypothesized to lessen its inhibitory effect on BTG2 and the PI3K/AKT signaling cascade. As a result this manipulation is associated with an elevation in BTG2 levels and a suppression of the PI3K/AKT signaling activity.
The competing endogenous RNA (ceRNA) mechanism is a widely recognized regulatory network within cells, encompassing various RNA species, such as mRNA, lncRNA, and circRNA. These RNA types modulate the expression levels of the others by sharing microRNA response elements (MREs). The ceRNA hypothesis has garnered considerable attention in oncology, with substantial evidence highlighting its vital role in tumor initiation, growth, and metastatic spread[8]. miRNAs mediate post-transcriptional control by interacting with MREs on the target mRNA transcripts. This interaction can lead to the degradation of the target mRNA or the inhibition of its translation[9].
Within the context of the ceRNA mechanism, ncRNAs, including lncRNAs and circRNAs act as molecular ‘sponges’ by sharing MREs with specific miRNAs, thereby adsorbing and antagonizing the effects of miRNAs. This interaction allows mRNAs originally targeted for suppression by miRNAs to evade this regulation, leading to an upregulation of the matching target genes[10]. In the field of oncology, the ceRNA hypothesis is crucial for modulating the expression of genes that govern cell cycle advancement and cell multiplication, encompassing genes for cyclins and proteins that participate in signaling pathways linked to proliferation[11,12], thereby either promoting or inhibiting tumor cell growth and division.
The lncRNA SNHG5 has emerged as a critical player in the pathogenesis of various malignancies, including GC[13,14]. A study by Li et al[5] assessed the lncRNA SNHG5 expression in the non-neoplastic tissue adjacent to GC lesions from patients. They also assessed the association between serum lncRNA SNHG5 levels and various clinicopathological characteristics, which indicated a decreased expression of lncRNA SNHG5 in pre-surgical patients with GCs, particularly among those with unfavorable prognoses. Additionally, it has been observed that overexpression of METase can promote apoptosis and autophagy by upregulating lncRNA SNHG5 levels in both tumor tissues and cell lines, thus exerting an inhibitory effect on tumor progression[15].
Li et al[5] reported that the expression of lncRNA SNHG5 in the serum of patients with GC was downregulated and lower than that in the benign gastrosia group and healthy control group. lncRNA SNHG5 was correlated with drinking history and TNM stage. Synthesizing these findings, lncRNA SNHG5 emerges as a candidate therapeutic target for GC. However, additional studies are required to thoroughly understand the regulatory mechanisms and functional roles of lncRNA SNHG5.
In our study we found decreased expression of lncRNA SNHG5 in GC tissues. Cell growth was assessed using the CCK-8 assay and a clonogenic assay, whereas cell motility was determined through the Transwell migration test and scratch wound closure assay. The findings underscored enhanced cell growth and mobility in the group with elevated lncRNA SNHG5 expression. The results of the in vivo experiments showed that a significantly reduced tumor growth rate, size, and weight along with cellular irregularities and nucleolysis in the tumor tissue cells with lncRNA SNHG5 overexpression.
Previous studies have shown that lncRNAs function as ceRNAs by sponging miRNA to negatively regulate the expression of miRNAs and their downstream target genes. Based on computational analyses and an extensive literature review, miR-92a-3p was identified as a potential downstream mediator of lncRNA SNHG5. This miRNA was implicated in the proliferation and invasiveness of GC cells[16]. Notably, Lu et al[17] has proposed that the serum concentration of miR-92a-3p could serve as a potential biomarker for the early detection of GC. Moreover, it has been shown that the increased levels of miR-92a-3p can lead to the downregulation of E-cadherin, thereby enhancing the migratory ability of GC cells[18].
Zhu et al[19] declared that the miR-92a-3p/HIP1R axis could regulate the PI3K/AKT pathway, inhibit proliferation, migration, and invasion, and induce apoptosis in pancreatic cancer cells. Our results showed that miR-92a-3p knockdown could alleviate GC progression. We successfully developed a dual-luciferase reporter system that included both the WT and MUT versions of the lncRNA SNHG5 gene. Utilizing this system, we conducted a dual-luciferase reporter assay to evaluate the interaction between lncRNA SNHG5 and miR-92a-3p. Our findings revealed a significant reduction in luciferase activity in the WT lncRNA SNHG5 reporter when cotransfected with miR-92a-3p mimics. This result strongly suggests that lncRNA SNHG5 directly targets and downregulates miR-92a-3p.
BTG2 is acknowledged as a tumor suppressor that plays a pivotal role in inhibiting the growth of cancer cells and inducing cell death in various types of cancer[20-22]. The BTG2 protein exerts various functions, encompassing the control of cell cycle progression, cell multiplication, apoptosis, DNA repair processes, and cellular differentiation[23,24]. BTG2 exerts its anti-tumor effects by modulating the activity of transcription factors and proteins associated with EMT, thereby significantly impeding the invasive and metastatic potential of tumor cells[25,26].
In our research, BTG2 was downregulated in GC tissues. lncRNA SNHG5 overexpression and miR-92a-3p knockdown decreased GC progression in vivo by regulating BTG2 expression. To discover the possible mediation of impact of miR-92a-3p on GC cell growth via BTG2, we performed transfections of AGS cells with constructs containing miR-92a-3p mimics, WT-BTG2, and MUT-BTG2. Subsequently, dual-luciferase reporter assays were conducted. The outcomes offered conclusive proof that miR-92a-3p has a direct targeting effect on BTG2. These results suggest that lncRNA SNHG5 could be a crucial modulator in regulating GC onset and progression, potentially through the indirect control of BTG2.
The PI3K/AKT signaling pathway is an essential regulatory axis that governs cellular survival, proliferation, and motility[27,28]. Upon activation at the plasma membrane, PI3K catalyzes the transformation of phosphatidylinositol 4,5-bisphosphate into phosphatidylinositol 3,4,5-trisphosphate. This transformation is a pivotal event that leads to the assembly and activation of AKT kinase. Once triggered, AKT acts as a central regulator, influencing numerous biological processes that can enhance cell growth, survival, and metabolic activities and prevent cell death by phosphorylating numerous downstream effectors[29]. In neoplastic cells the PI3K/AKT signaling cascade hyperactivation is frequently observed. It is characterized by enhanced cell proliferation and an increased resistance to apoptosis that collectively contribute to the promotion of tumorigenesis and tumor progression[30-32].
Our study showed that lncRNA SNHG5 overexpression and miR-92a-3p knockdown inhibited the PI3K/AKT signaling pathway. These results suggest that modulating the PI3K/AKT pathway may be a key mechanism through which the lncRNA SNHG5/miR-92a-3p/BTG2 network influences the growth potential of GC cells. Based on the aforementioned findings, this study demonstrated that lncRNA SNHG5 modulated the targeted binding of miR-92a-3p to BTG2. Additionally, it modulated the functionality of the PI3K/AKT signaling cascade. These results reveal the regulatory mechanism underlying the lncRNA SNHG5/miR-92a-3p/BTG2 network in GC, its impact on GC cell proliferation and invasion, and the PI3K/AKT signaling pathway. This novel insight enhances our understanding of GC pathogenesis and provides valuable information for future research on GC treatment strategies.
Future investigations should explore interactions between the lncRNA SNHG5/miR-92a-3p/BTG2 network and other crucial regulatory factors and signaling pathways, aiming to develop more comprehensive and effective combination therapy approaches for improved therapeutic outcomes. While this study provides initial evidence for the role of the lncRNA SNHG5/miR-92a-3p/BTG2 axis in GC, limitations include the reliance on a single cell line and a relatively small sample size. Future studies with larger, diverse cohorts and multiple cell lines are needed to validate these findings. Additionally, the clinical relevance of these molecular interactions requires further exploration in patient populations.
| 1. | Ooi WF, Xing M, Xu C, Yao X, Ramlee MK, Lim MC, Cao F, Lim K, Babu D, Poon LF, Lin Suling J, Qamra A, Irwanto A, Qu Zhengzhong J, Nandi T, Lee-Lim AP, Chan YS, Tay ST, Lee MH, Davies JO, Wong WK, Soo KC, Chan WH, Ong HS, Chow P, Wong CY, Rha SY, Liu J, Hillmer AM, Hughes JR, Rozen S, Teh BT, Fullwood MJ, Li S, Tan P. Epigenomic profiling of primary gastric adenocarcinoma reveals super-enhancer heterogeneity. Nat Commun. 2016;7:12983. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 107] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 2. | Yu H, Rong L. Emerging role of long non-coding RNA in the development of gastric cancer. World J Gastrointest Oncol. 2018;10:260-270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 3. | Tao XC, Zhang XY, Sun SB, Wu DQ. miR‑92a contributes to cell proliferation, apoptosis and doxorubicin chemosensitivity in gastric carcinoma cells. Oncol Rep. 2019;42:313-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Li YH, Hu YQ, Wang SC, Li Y, Chen DM. LncRNA SNHG5: A new budding star in human cancers. Gene. 2020;749:144724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 5. | Li X, Du Y, Wang Y. The value of LncRNA SNHG5 as a marker for the diagnosis and prognosis of gastric cancer. Am J Transl Res. 2021;13:5420-5427. [PubMed] |
| 6. | Xu Z, Wang Y, Xiong J, Cui F, Wang L, Peng H. NUSAP1 knockdown inhibits cell growth and metastasis of non-small-cell lung cancer through regulating BTG2/PI3K/Akt signaling. J Cell Physiol. 2020;235:3886-3893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 7. | Li DY, Lin FF, Li GP, Zeng FC. Exosomal microRNA-15a from ACHN cells aggravates clear cell renal cell carcinoma via the BTG2/PI3K/AKT axis. Kaohsiung J Med Sci. 2021;37:973-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 8. | Mao M, Zhang J, Xiang Y, Gong M, Deng Y, Ye D. Role of exosomal competitive endogenous RNA (ceRNA) in diagnosis and treatment of malignant tumors. Bioengineered. 2022;13:12156-12168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 32] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 9. | O'Brien J, Hayder H, Zayed Y, Peng C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front Endocrinol (Lausanne). 2018;9:402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2111] [Cited by in RCA: 3361] [Article Influence: 480.1] [Reference Citation Analysis (0)] |
| 10. | Han TS, Hur K, Cho HS, Ban HS. Epigenetic Associations between lncRNA/circRNA and miRNA in Hepatocellular Carcinoma. Cancers (Basel). 2020;12:2622. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 122] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 11. | Xu Q, Jia X, Wu Q, Shi L, Ma Z, Ba N, Zhao H, Xia X, Zhang Z. Esomeprazole affects the proliferation, metastasis, apoptosis and chemosensitivity of gastric cancer cells by regulating lncRNA/circRNA-miRNA-mRNA ceRNA networks. Oncol Lett. 2020;20:329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 12. | Liu YM, Cao Y, Zhao PS, Wu LY, Lu YM, Wang YL, Zhao JF, Liu XG. CircCCNB1 silencing acting as a miR-106b-5p sponge inhibited GPM6A expression to promote HCC progression by enhancing DYNC1I1 expression and activating the AKT/ERK signaling pathway. Int J Biol Sci. 2022;18:637-651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 13. | Li Y, Hu J, Guo D, Ma W, Zhang X, Zhang Z, Lu G, He S. LncRNA SNHG5 promotes the proliferation and cancer stem cell-like properties of HCC by regulating UPF1 and Wnt-signaling pathway. Cancer Gene Ther. 2022;29:1373-1383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 42] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 14. | Xing X, Xu T, Liu B, Guo Q. LncRNA SNHG5 can Regulate the Proliferation and Migration of Diffuse Large B Cell Lymphoma Progression via Targeting miR-181-5p/XIAP. J Cancer. 2022;13:784-792. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 15. | Xin L, Zhou LQ, Liu L, Yuan YW, Zhang HT, Zeng F. METase promotes cell autophagy via promoting SNHG5 and suppressing miR-20a in gastric cancer. Int J Biol Macromol. 2019;122:1046-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 16. | Mao QQ, Chen JJ, Xu WJ, Zhao XZ, Sun X, Zhong L. miR-92a-3p promotes the proliferation and invasion of gastric cancer cells by targeting KLF2. J Biol Regul Homeost Agents. 2020;34:1333-1341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 17. | Lu X, Lu J, Wang S, Zhang Y, Ding Y, Shen X, Jing R, Ju S, Chen H, Cong H. Circulating serum exosomal miR-92a-3p as a novel biomarker for early diagnosis of gastric cancer. Future Oncol. 2021;17:907-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 18. | 18 Zhang G, Li S, Lu J, Ge Y, Wang Q, Ma G, Zhao Q, Wu D, Gong W, Du M, Chu H, Wang M, Zhang A, Zhang Z. LncRNA MT1JP functions as a ceRNA in regulating FBXW7 through competitively binding to miR-92a-3p in gastric cancer. Mol Cancer. 2018;17:87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 155] [Cited by in RCA: 221] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 19. | Zhu S, Xu H, Chen R, Shen Q, Yang D, Peng H, Tong J, Fu Q. DNA methylation and miR-92a-3p-mediated repression of HIP1R promotes pancreatic cancer progression by activating the PI3K/AKT pathway. J Cell Mol Med. 2023;27:788-802. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Gao SS, Yang XH, Wang M. Inhibitory effects of Bcell translocation gene 2 on skin cancer cells via the Wnt/βcatenin signaling pathway. Mol Med Rep. 2016;14:3464-3468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Song J, Xu F, An L, Yin Y, Liu J, Chai J, Yang Y, Li M, Jia Q, Wang Z. BTG2 suppresses the growth and metastasis of cervical squamous cell carcinoma. Pathol Res Pract. 2023;247:154577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 22. | Zhang XZ, Chen MJ, Fan PM, Jiang W, Liang SX. BTG2 Serves as a Potential Prognostic Marker and Correlates with Immune Infiltration in Lung Adenocarcinoma. Int J Gen Med. 2022;15:2727-2745. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 23. | Yang DL, Gan ML, Tan Y, Ge GH, Li Q, Jiang YZ, Tang GQ, Li MZ, Wang JY, Li XW, Zhang SH, Zhu L. MiR-222-3p Regulates the Proliferation and Differentiation of C2C12 Myoblasts by Targeting BTG2. Mol Biol. 2019;53:38-44. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Velayutham N, Calderon MU, Alfieri CM, Padula SL, van Leeuwen FN, Scheijen B, Yutzey KE. Btg1 and Btg2 regulate neonatal cardiomyocyte cell cycle arrest. J Mol Cell Cardiol. 2023;179:30-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 25. | Hwang SS, Lim J, Yu Z, Kong P, Sefik E, Xu H, Harman CCD, Kim LK, Lee GR, Li HB, Flavell RA. mRNA destabilization by BTG1 and BTG2 maintains T cell quiescence. Science. 2020;367:1255-1260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 72] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 26. | Kim SH, Jung IR, Hwang SS. Emerging role of anti-proliferative protein BTG1 and BTG2. BMB Rep. 2022;55:380-388. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 27. | Feng Y, Hua X, Niu R, Du Y, Shi C, Zhou R, Chen FH. ROS play an important role in ATPR inducing differentiation and inhibiting proliferation of leukemia cells by regulating the PTEN/PI3K/AKT signaling pathway. Biol Res. 2019;52:26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 28. | Zeng J, Chen M, Yang Y, Wu B. A novel hypoxic lncRNA, HRL-SC, promotes the proliferation and migration of human dental pulp stem cells through the PI3K/AKT signaling pathway. Stem Cell Res Ther. 2022;13:286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 29. | Karami Fath M, Ebrahimi M, Nourbakhsh E, Zia Hazara A, Mirzaei A, Shafieyari S, Salehi A, Hoseinzadeh M, Payandeh Z, Barati G. PI3K/Akt/mTOR signaling pathway in cancer stem cells. Pathol Res Pract. 2022;237:154010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 76] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 30. | Fang Y, Ji W, Yan C. Research Progress of PI3K/PTEN/AKT Signaling Pathway Associated with Renal Cell Carcinoma. Dis Markers. 2022;2022:1195875. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 31. | Aguayo F, Perez-Dominguez F, Osorio JC, Oliva C, Calaf GM. PI3K/AKT/mTOR Signaling Pathway in HPV-Driven Head and Neck Carcinogenesis: Therapeutic Implications. Biology (Basel). 2023;12:672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 32. | Noorolyai S, Shajari N, Baghbani E, Sadreddini S, Baradaran B. The relation between PI3K/AKT signalling pathway and cancer. Gene. 2019;698:120-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 408] [Article Influence: 68.0] [Reference Citation Analysis (0)] |