Published online Jun 15, 2025. doi: 10.4251/wjgo.v17.i6.107021
Revised: April 15, 2025
Accepted: May 19, 2025
Published online: June 15, 2025
Processing time: 92 Days and 5.7 Hours
Although previous findings indicated that pathological assessment of tumor budding (TB), desmoplastic reaction (DR), and tumor-infiltrating lymphocytes (TILs) may play a role in determining tumor behavior in many malignancies, the relationship between TB, DR, and TILs in patients with pancreatic ductal adenocarcinoma (PDAC) is still unknown.
To evaluate relationships of TB, DR, and TILs with histopathological parameters and determine their prognostic value in patients with PDAC.
The study cohort comprised 100 patients diagnosed with PDAC. Peritumoral budding (PTB) and intratumoral budding (ITB) were assessed according to the International Tumor Budding Consensus Conference guidelines. DR was classified based on stromal maturation. TILs were evaluated semiquantitatively with a 5% cutoff. Additionally, cases were categorized into two groups according to lymphocyte density: No/Low lymphocytes and medium/high lymphocytes.
A significant correlation was observed between ITB and PTB (r = 0.890). Higher PTB was associated with fewer TILs and immature stroma (P < 0.001). PTB and TILs were significantly related to tumor dimension, lymphovascular invasion, lymph node metastasis (LNM), and stage (P < 0.005). ITB was also associated with the presence of lymph node involvement. The results of the univariate analysis revealed a significant correlation between poor survival rates and the presence of lymphovascular invasion, LNM, PTB, ITB, and TILs according to scoring (P < 0.001). The multivariate analysis revealed LNM, PTB, ITB, and TILs according to scoring as independent prognostic factors.
TB assessment stratified patients with PDAC. PTB-ITB correlation showed diagnostic relevance of ITB in biopsy specimens. The prognostic significance of DR and interplay with TIL subsets warrant further investigation.
Core Tip: Pancreatic ductal adenocarcinoma is a highly aggressive malignancy, necessitating novel histopathological markers for better prognostic stratification. This study evaluated tumor budding, desmoplastic reaction, and tumor-infiltrating lymphocytes in patients with pancreatic ductal adenocarcinoma. Peritumoral budding (PTB) correlated with tumor size, while intratumoral budding (ITB) was linked to lymph node involvement. Both PTB and ITB were independent prognostic factors for poor survival. Additionally, high PTB and ITB levels were associated with immature stroma, suggesting a role in epithelial-mesenchymal transition. These findings highlighted the prognostic value of tumor budding and its potential integration into pathology reporting protocols.
- Citation: Alpsoy A, Yavuz A, Simsek K, Altunay B, Karaca M, Unal B, Bassorgun CI, Tatli AM, Elpek GO. Evaluation of tumor budding, desmoplastic reaction, and lymphocytic infiltration in predicting survival for pancreatic ductal adenocarcinoma. World J Gastrointest Oncol 2025; 17(6): 107021
- URL: https://www.wjgnet.com/1948-5204/full/v17/i6/107021.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i6.107021
Pancreatic adenocarcinoma (PDAC) is one of the most lethal malignancies worldwide and by 2030 is expected to be the second leading cause of cancer-related mortality[1,2]. The primary treatment is surgical excision in combination with multiagent chemotherapy[3]. Despite significant improvements in treatment modalities, tumor recurrence after radical resection remains considerable, and long-term survival remains less than 10%[4,5]. Moreover, the various outcomes of patients with similar stages reflect the heterogeneous nature of these tumors and necessitate new parameters to better predict the clinical course and treatment[6].
In recent years, significant evidence has indicated that the epithelial-mesenchymal transition (EMT) process is associated with aggressive behavior in many cancers[7]. Tumor budding (TB) is a marker of EMT. It is an important parameter that is associated with adverse clinicopathological features and decreased survival in many types of solid tumors including gastrointestinal cancers[8,9]. Currently, TB is used in routine reporting protocols as an independent prognostic parameter in patients with colorectal cancer (CRC)[10]. Although several groups have reported that TB is negatively correlated with overall survival in patients with PDAC[7,11], this metric is not included in any classification or protocol and requires further study.
Recent studies have shown that the tumor microenvironment (TME) has a significant effect on tumor progression. Excessive fibrous or connective tissue formation around a tumor, known as a desmoplastic reaction (DR), is a host-related factor[12] and has been identified as a factor that influences tumor behavior in solid cancers. In CRC, tumor classification according to the desmoplastic stroma type [hematoxylin and eosin (H&E) staining] has important prognostic implications[13]. Tumors with a DR consisting of myxoid stroma or thick, eosinophilic collagen fibers are associated with a worse prognosis. In contrast, those with a DR featuring mature, thin collagen fibers have a better prognosis.
In patients with PDAC who have a poor prognosis the tumor characteristically stimulates excessive production of fibroblasts, collagen, and other stromal components, resulting in the formation of a rigid desmoplastic stroma that prevents drugs from entering the tumor, thus increasing drug resistance[14]. Therefore, studies have been conducted to understand the mechanism of desmoplasia and fibrosis to identify new treatment targets in PDAC[15-17]. However, the effect of histopathological grading of the DR on tumor behavior is unknown.
Similar to other solid malignancies, the interplay between stromal and tumor cells in the TME influences immune cells in PDAC[18]. There is a relationship between subsets of tumor-infiltrating lymphocytes (TILs) and prognosis[6,19,20]. However, the proportions of TILs in H&E-stained sections and their associations with TB, DR, and survival have not been thoroughly investigated.
There were three main objectives for this pathological evaluation-based study. The first objective was to elucidate the relationships between TB, DR, and TILs and traditional clinicopathological factors and patient prognosis. The second objective was to determine the relationships among these variables. The third objective was to determine whether these parameters can be used as independent markers to predict survival.
With the approval of the Institutional Review Board, the data of 276 consecutive patients who underwent radical pancreatectomy for PDAC at our center between 2005 and 2020 were reviewed. Patients were removed if they had received preoperative chemotherapy and/or radiotherapy, in order to exclude treatment-related tissue modifications (n = 176), or if they experienced in-hospital mortality. The study group included 100 patients with PDAC.
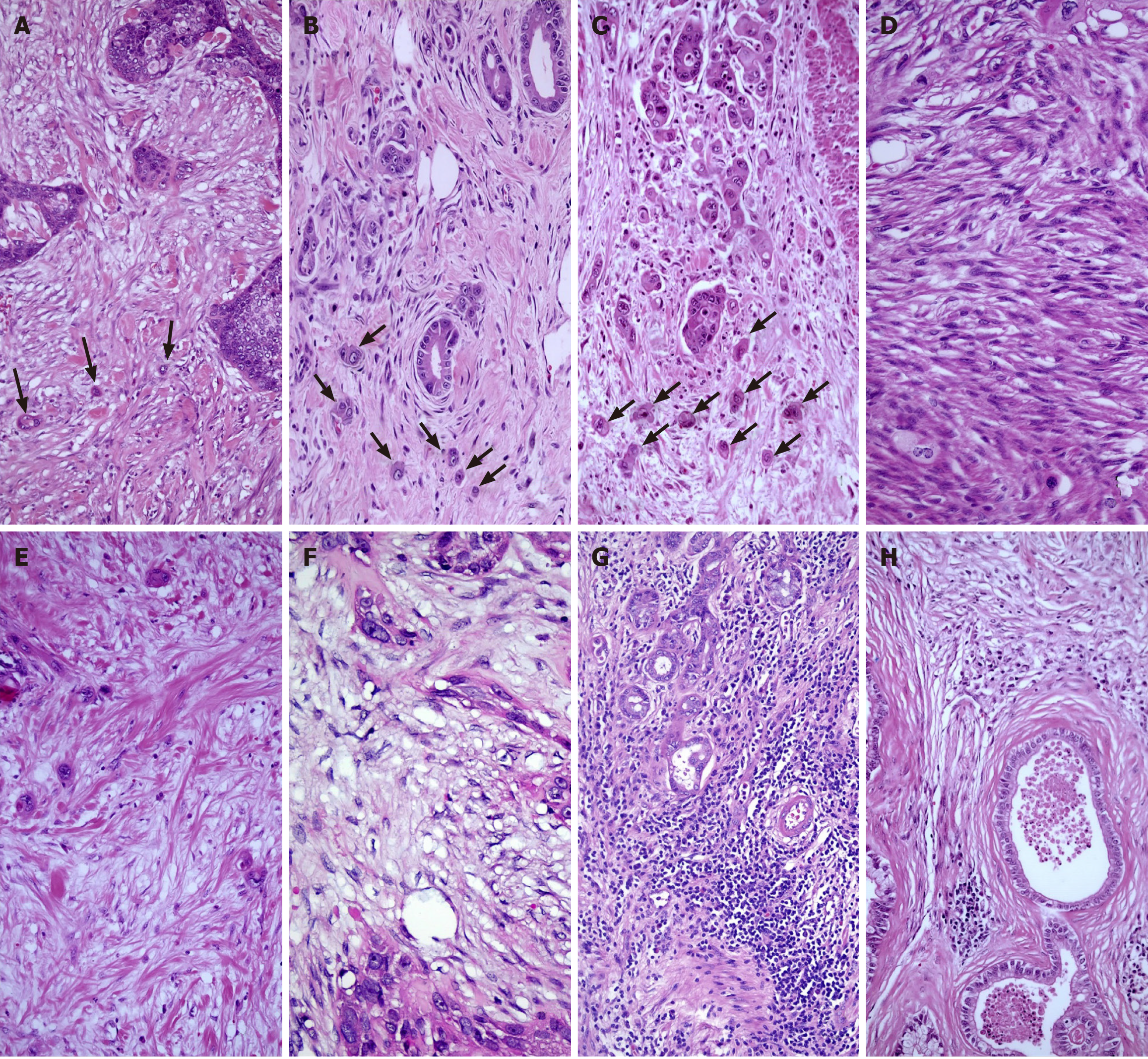
The assessment of both peritumoral budding (PTB) and intratumoral budding (ITB) was performed according to the International Tumor Budding Consensus Conference recommendations[21]. The count was determined in a standardized field area of 0.785 mm2 at × 200 magnification. PTB and ITB were categorized into three grades: Grade 1 (0-4, TB1); grade 2 (5-9, TB2); and grade 3 (≥ 10, TB3) (Figure 1A-C).
DR can be categorized into three groups depending on the maturation of the tumor stroma[22]. Mature-type DR comprises fine collagen fibers in multiple layers (DR1). Intermediate-type DR contains keloid-like collagen (DR2). Immature-type DR consists of myxoid stroma (DR3), which completely occupies a × 40 objective lens field on slides (Figure 1D-F).
TILs were scored by quantifying the percentage of TILs within the stromal region and the invasive borders of tumors, excluding carcinoma cells[23]. The counting process focused on lymphocytes and did not consider polymorphonuclear leukocytes (Figure 1G and H). The average percentage of TILs was recorded (pTILs). Furthermore, the level of TILs was assessed as absent, low, moderate, or high (scored as 0, 1, 2, and 3, respectively) (sTILs) by counting as previously suggested in PDAC[22,24].
The assessment of statistical data was performed using SPSS version 27 (IBM Corp., Armonk, NY, United States). Spearman’s correlation test was used to determine the relationships between variables. The χ2 test was used to examine categorical data. Univariate analysis, including survival analysis, was estimated using the Kaplan-Meier method. The log-rank test was used to compare survival rates. A Cox proportional hazards regression model was applied for multivariate analysis. A P value < 0.05 was considered to be statistically significant.
Table 1 summarizes the demographic data of the study group. The patients were divided into two groups according to mean patient age and mean tumor diameter.
| Factor | n | PTB1 | PTB2 | PTB3 | ITB1 | ITB2 | ITB3 | DR1 | DR2 | DR3 | ↓pTILs | ↑pTILs | ↓sTILs | ↑sTILs |
| Age, years | ||||||||||||||
| < 61.75 ± 12.40 | 40 | 10 (55.6) | 10 (33.3) | 20 (38.5) | 5 (31.3) | 11 (61.1) | 24 (36.4) | 8 (30.8) | 14 (51.9) | 18 (38.3) | 24 (35.3) | 16 (50.0) | 27 (37.5) | 13 (46.4) |
| ≥ 61.75 ± 12.40 | 60 | 8 (44.4) | 20 (66.7) | 32 (61.5) | 11 (68.8) | 7 (39.9) | 42 (63.6) | 18 (69.2) | 13 (48.1) | 29 (61.7) | 44 (64.7) | 16 (50.0) | 45 (62.5) | 15 (53.6) |
| Sex | ||||||||||||||
| Female | 46 | 8 (44.4) | 10 (33.3) | 28 (53.8) | 6 (37.5) | 7 (38.9) | 33 (50.0) | 10 (38.5) | 14 (51.9) | 22 (46.8) | 32 (47.1) | 14 (43.8) | 33 (45.8) | 13 (46.4) |
| Male | 54 | 10 (55.6) | 20 (66.7) | 24 (46.2) | 10 (62.5) | 11 (61.1) | 33 (50.0) | 16 (61.5) | 13 (48.1) | 25 (53.2) | 36 (52.9) | 18 (56.3) | 39 (54.2) | 15 (53.6) |
| Location | ||||||||||||||
| Head | 55 | 10 (55.6) | 14 (46.7) | 31 (59.6) | 10 (62.5) | 7 (38.9) | 38 (57.6) | 14 (53.8) | 11 (40.7) | 30 (63.8) | 35 (51.5) | 20 (62.5) | 42 (58.3) | 13 (46.4) |
| Corpus | 27 | 5 (27.8) | 12 (40.0) | 10 (19.2) | 5 (31.3) | 7 (38.9) | 15 (22.7) | 8 (30.8) | 7 (25.9) | 12 (25.5) | 18 (26.5) | 9 (28.1) | 16 (22.2) | 11 (39.3) |
| Tail | 18 | 3 (16.6) | 4 (13.3) | 11 (21.2) | 1 (6.2) | 4 (22.2) | 13 (19.7) | 4 (15.4) | 9 (33.4) | 5 (10.6) | 15 (22.0) | 3 (9.4) | 14 (19.4) | 4 (14.3) |
| Diameter, cm | ||||||||||||||
| < 1.43 ± 0.60 | 56 | 13 (72.2) | 12 (40.0) | 31 (59.6) | 9 (56.2) | 9 (50.0) | 38 (57.6) | 12 (46.2) | 16 (59.3) | 28 (59.6) | 34 (50.0) | 22 (68.8) | 40 (55.6) | 16 (57.1) |
| ≥ 1.43 ± 0.60 | 44 | 5 (27.8) | 18 (60.0) | 21 (40.4) | 7 (43.8) | 9 (50.0) | 28 (42.4) | 14 (53.8) | 11 (40.7) | 19 (40.4) | 34 (50.0) | 10 (31.2) | 32 (44.4) | 12 (42.9) |
| RM | ||||||||||||||
| R0 | 81 | 16 (88.9) | 22 (73.3) | 43 (82.7) | 22 (84.6) | 20 (74.1) | 39 (83.0) | 22 (84.6) | 20 (74.1) | 39 (83.0) | 54 (79.4) | 27 (84.4) | 59 (81.9) | 22 (78.6) |
| R1 | 19 | 2 (11.1) | 8 (26.7) | 9 (17.3) | 4 (15.4) | 7 (25.9) | 8 (17.0) | 4 (15.4) | 7 (25.9) | 8 (17.0) | 14 (20.6) | 5 (15.6) | 13 (18.1) | 6 (21.4) |
| Invasion | ||||||||||||||
| T1 | 28 | 11 (61.1) | 4 (13.3) | 13 (25.0)a | 5 (31.3) | 4 (22.2) | 19 (28.8) | 7 (26.9) | 5 (18.6) | 16 (34.0) | 14 (20.6) | 14 (43.8)b | 20 (27.8) | 8 (28.6) |
| T2 | 42 | 6 (33.3) | 15 (50.0) | 21 (40.4) | 8 (50.0) | 10 (55.6) | 24 (36.4) | 11 (42.3) | 11 (40.7) | 20 (42.6) | 29 (42.6) | 13 (40.6) | 27 (37.5) | 15 (53.6) |
| T3 | 26 | 1 (5.6) | 11 (36.7) | 14 (26.9) | 3 (18.8) | 3 (16.7) | 20 (30.3) | 7 (26.9) | 10 (37.0) | 9 (19.1) | 21 (30.9) | 5 (15.6) | 22 (30.5) | 4 (14.2) |
| T4 | 4 | 0 | 0 | 4 (7.7) | 0 | 1 (5.5) | 3 (4.5) | 1 (3.9) | 1 (3.7) | 2 (4.3) | 4 (5.9) | 0 | 3 (4.2) | 1 (3.6) |
| Node status | ||||||||||||||
| N0 | 56 | 14 (77.8) | 20 (66.7) | 22 (42.3)b | 13 (81.2) | 13 (72.2) | 30 (45.4) | 17 (65.4) | 14 (51.9) | 25 (53.2) | 32 (47.0) | 24 (75.0)b | 35 (48.6) | 21 (75.0)b |
| N1 | 22 | 2 (11.1) | 7 (23.3) | 13 (25.0) | 1 (6.3) | 2 (11.1) | 19 (28.8) | 3 (11.5) | 7 (25.9) | 12 (25.5) | 18 (26.5) | 4 (12.5) | 16 (22.2) | 6 (21.4) |
| N2 | 22 | 2 (11.1) | 3 (10.0) | 17 (32.7) | 2 (12.5) | 3 (16.7) | 17 (25.8) | 6 (23.1) | 6 (22.2) | 10 (21.3) | 18 (26.5) | 4 (12.5) | 21 (29.2) | 1 (3.6) |
| LNM | ||||||||||||||
| Absent | 56 | 14 (77.8) | 20 (66.7) | 22 (42.3)a | 13 (81.3) | 13 (72.2) | 30 (45.5)b | 17 (65.4) | 14 (51.9) | 25 (53.2) | 32 (47.1) | 24 (75.0)a | 35 (48.6) | 21 (75.0)b |
| Present | 44 | 4 (22.2) | 10 (33.3) | 30 (57.7) | 3 (18.8) | 5 (27.8) | 36 (54.5) | 9 (34.6) | 13 (48.1) | 22 (48.6) | 36 (52.9) | 8 (25.0) | 37 (51.4) | 7 (25.0) |
| Metastasis | ||||||||||||||
| Absent | 88 | 17 (94.4) | 27 (90.0) | 44 (84.6) | 15 (93.8) | 16 (88.9) | 57 (86.4) | 21 (80.8) | 23 (85.2) | 44 (93.6) | 58 (85.3) | 30 (93.8) | 62 (86.1) | 26 (92.9) |
| Present | 12 | 1 (5.6) | 3 (10.0) | 8 (15.4) | 1 (6.2) | 2 (11.1) | 9 (13.6) | 5 (19.2) | 4 (14.8) | 3 (6.4) | 10 (14.7) | 2 (6.3) | 10 (13.9) | 2 (7.1) |
| Stage | ||||||||||||||
| I | 29 | 9 (50.0) | 7 (23.3) | 13 (25.0)b | 9 (56.3) | 5 (27.8) | 15 (22.7) | 7 (26.9) | 6 (22.2) | 16 (34.0) | 14 (20.6) | 15 (46.9)a | 20 (27.8) | 9 (32.1)b |
| II | 25 | 5 (27.8) | 12 (40.0) | 8 (15.4) | 3 (18.8) | 7 (38.9) | 15 (22.7) | 8 (30.8) | 9 (33.2) | 8 (17.0) | 15 (22.1) | 10 (31.3) | 13 (18.1) | 12 (42.9) |
| III | 34 | 3 (16.6) | 8 (26.7) | 23 (44.2) | 3 (18.8) | 4 (22.2) | 27 (40.9) | 6 (23.1) | 8 (29.6) | 20 (42.6) | 29 (42.6) | 5 (15.6) | 29 (40.2) | 5 (17.9) |
| IV | 12 | 1 (5.6) | 3 (10.0) | 8 (15.4) | 1 (6.3) | 2 (11.1) | 9 (13.6) | 5 (19.2) | 4 (14.8) | 3 (6.4) | 10 (14.7) | 2 (6.3) | 10 (13.9) | 2 (7.1) |
| Grade | ||||||||||||||
| G1 | 35 | 6 (33.3) | 12 (40.0) | 17 (32.7) | 9 (56.3) | 5 (27.8) | 21 (31.8) | 11 (42.3) | 9 (33.3) | 15 (31.9) | 24 (35.3) | 11 (34.4) | 23 (31.9) | 12 (42.9) |
| G2 | 54 | 10 (55.6) | 14 (46.7) | 30 (57.7) | 6 (37.5) | 10 (55.6) | 38 (57.6) | 12 (46.2) | 16 (59.3) | 26 (55.3) | 39 (57.4) | 15 (46.9) | 39 (54.2) | 15 (53.5) |
| G3 | 11 | 2 (11.1) | 4 (13.3) | 5 (9.6) | 1 (6.2) | 3 (16.7) | 7 (10.6) | 3 (11.5) | 2 (7.4) | 6 (12.8) | 5 (7.4) | 6 (18.8) | 10 (13.9) | 1 (3.6) |
| LVI | ||||||||||||||
| Absent | 55 | 15 (83.3) | 18 (60.0) | 22 (42.3)a | 11 (68.8) | 13 (72.2) | 31 (40.0) | 16 (61.5) | 13 (48.1) | 26 (55.3) | 32 (47.1) | 23 (71.9)b | 34 (47.2) | 21 (75.0)b |
| Present | 45 | 3 (16.7) | 12 (40.0) | 30 (57.7) | 5 (31.2) | 5 (27.8) | 35 (53.0) | 10 (38.5) | 14 (51.9) | 21 (44.7) | 36 (52.9) | 9 (28.1) | 38 (52.8) | 7 (25.0) |
| PNI | ||||||||||||||
| Absent | 25 | 7 (38.9) | 10 (33.3) | 8 (15.4) | 6 (37.5) | 6 (33.3) | 13 (19.7) | 7 (26.9) | 7 (25.9) | 11 (23.4) | 15 (22.1) | 10 (31.3) | 17 (23.6) | 8 (28.6) |
| Present | 75 | 11 (61.1) | 20 (66.7) | 44 (84.6) | 10 (62.5) | 12 (66.7) | 53 (80.3) | 19 (73.1) | 20 (74.1) | 36 (76.6) | 53 (77.9) | 22 (68.8) | 55 (76.4) | 20 (71.4) |
| Survival | ||||||||||||||
| Deceased | 68 | 6 (33.3) | 18 (60.0) | 44 (84.6)a | 0 | 13 (72.2) | 55 (83.3)a | 15 (57.7) | 20 (74.1) | 33 (70.2) | 50 (73.5) | 18 (56.3) | 57 (79.2) | 11 (39.3)a |
| Alive | 32 | 12 (66.7) | 12 (40.0) | 8 (15.4) | 16 (100) | 5 (27.8) | 11 (16.7) | 11 (42.3) | 7 (25.9) | 14 (29.8) | 18 (26.5) | 14 (43.8) | 15 (20.8) | 17 (60.7) |
| PTB | ||||||||||||||
| PTB1 | 18 | - | - | - | 10 (62.5) | 3 (16.7) | 5 (7.6) | 7 (26.9) | 2 (7.4) | 9 (19.1)b | 7 (10.3) | 11 (34.4)b | 11 (15.3) | 7 (25.0)b |
| PTB2 | 30 | - | - | - | 5 (31.2) | 14 (77.8) | 11 (16.5) | 12 (46.2) | 8 (29.6) | 10 (21.3) | 20 (29.4) | 10 (31.3) | 18 (25.0) | 12 (42.9) |
| PTB3 | 52 | - | - | - | 1 (6.3) | 1 (5.5) | 50 (75.9) | 7 (26.9) | 17 (63.0) | 28 (59.6) | 41 (60.3) | 11 (34.4) | 43 (59.7) | 9 (32.1) |
| ITB | ||||||||||||||
| ITB1 | 16 | - | - | - | - | - | - | 10 (38.5) | 1 (3.7) | 5 (10.6)a | 7 (10.3) | 9 (28.1)b | 6 (8.3) | 10 (37.5)a |
| ITB2 | 18 | - | - | - | - | - | - | 5 (19.2) | 6 (22.2) | 7 (14.9) | 11 (16.2) | 7 (21.9) | 10 (13.9) | 8 (28.6) |
| ITB3 | 66 | - | - | - | - | - | - | 11 (42.3) | 20 (74.1) | 35 (74.5) | 50 (73.5) | 16 (50.0) | 56 (77.8) | 10 (35.7) |
| DR | ||||||||||||||
| DR1 | 26 | - | - | - | - | - | - | - | - | - | 15 (22.1) | 11 (34.4) | 19 (26.4) | 7 (25.0) |
| DR2 | 27 | - | - | - | - | - | - | - | - | - | 19 (27.9) | 8 (25.0) | 17 (23.6) | 10 (34.7) |
| DR3 | 47 | - | - | - | - | - | - | - | - | - | 34 (50.0) | 13 (40.6) | 36 (50.0) | 11 (39.3) |
| Total | 100 | 18 | 30 | 52 | 16 | 18 | 66 | 26 | 27 | 47 | 68 | 32 | 72 | 28 |
The mean of the pTILs was 9.46 (median: 5, standard deviation: 4.81, minimum: 0, maximum: 82). The patients were separated into two groups, according to the median cutoff, as low-pTILs and high-pTILs. No stromal lymphocytes were observed in 40 cases. Low, moderate, and high levels of lymphocyte infiltration were detected in 38, 18, and 14 cases, respectively. Cases were classified into two groups, low-sTILs (no or low stromal lymphocytes) and high-sTILs (moderate or high stromal lymphocytes), for further analyses.
A strong relationship was observed between the PTB and ITB scores (r = 0.890). Moreover, DR3 cases were more common (59.6%) in the PTB3 and ITB3 groups (P < 0.05). In addition, 60.3% and 73.5% of patients in the low-pTIL group had high PTB and ITB scores, respectively, indicating an inverse relationship between TB and pTILs (P < 0.001). Similar correlations were also observed with sTILs (P < 0.05). The DR was not correlated with pTILs and sTILs.
Tumors with larger diameters more frequently exhibited higher levels of PTBs and lower pTILs and sTILs. Similarly, a high PTB grade was positively correlated with lymph node metastasis (LNM) and lymphovascular invasion, whereas an inverse relationship was found with high TILs (pTILs and sTILs). In addition, advanced-stage tumors exhibited a higher PTB grade and lower pTILs and sTILs more frequently than early-stage tumors. ITB was also positively correlated with LNM (Table 1).
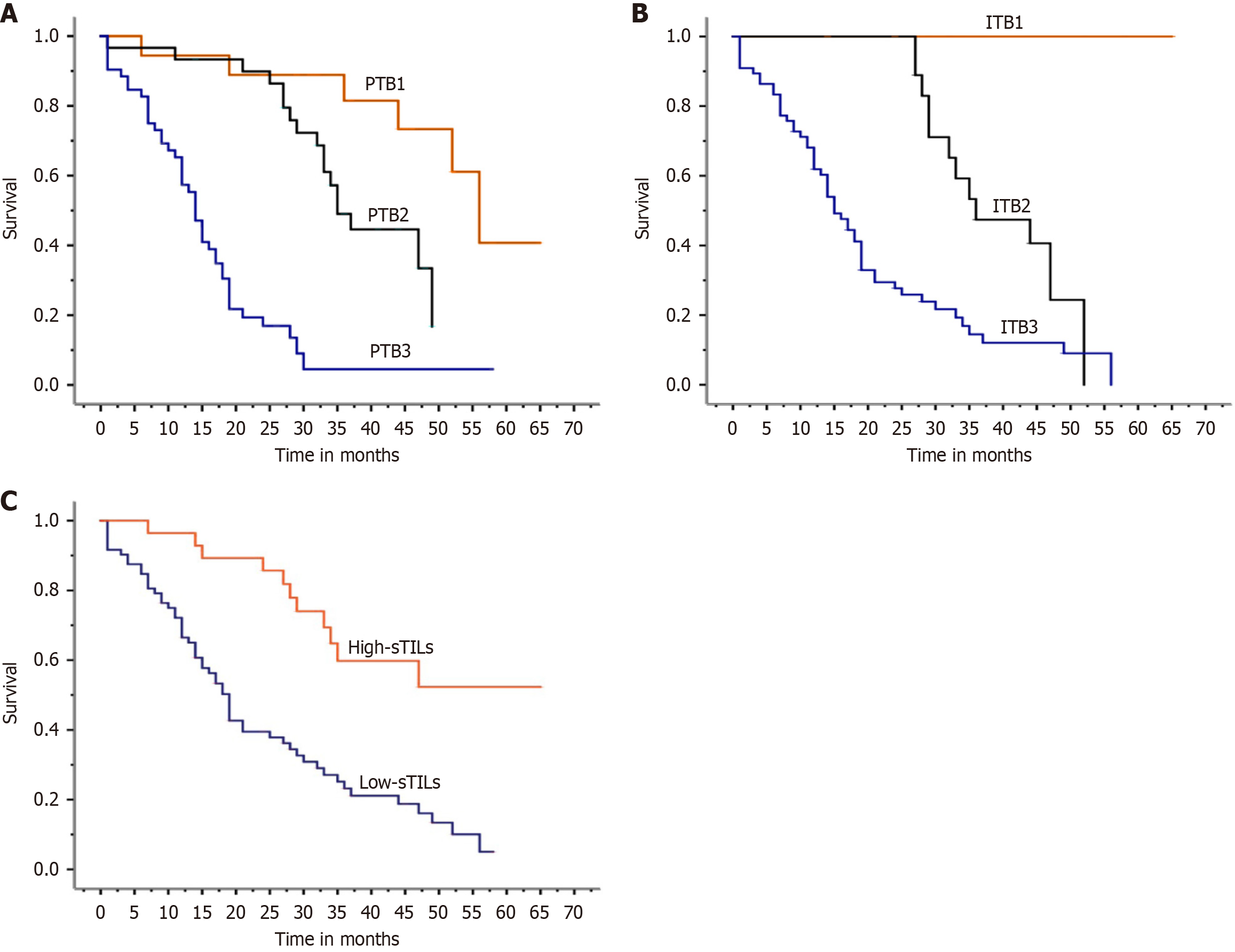
Follow-up data were available for all patients. The mean follow-up time was 24.93 ± 16.9 months (median: 22.5 months, range: 1-65 months). According to the univariate analysis, ITB, PTB, LNM, DR, stage, and sTILs were identified as risk factors for poor prognosis (P < 0.05; Table 2, Figure 2). The relationships between age, sex, tumor diameter, and outcomes were not significantly different (P > 0.05). After multivariate analysis, the PTB and ITB scores and sTILs were determined to be independent prognostic factors (Table 2).
| Factors | n | Univariate survival analysis | Univariate Cox regression | Multivariate Cox regression | ||||||
| Mean survival | Median survival | P value | HR | P value | 95%CI | P value | ||||
| Estimate ± SE | 95%CI | Estimate ± SE | 95%CI | |||||||
| Age, years | ||||||||||
| < 61.75 ± 12.40 | 40 | 39.5 ± 2.8 | 33.900-45.000 | 44.0 ± 6.4 | 31.400-56.500 | 0.220 | 1.232 | 0.483 | 0.688-2.207 | 0.184 |
| ≥ 61.75 ± 12.40 | 60 | 45.8 ± 2.6 | 40.700-51.000 | 47.0 ± 1.8 | 43.400-50.500 | |||||
| Sex | ||||||||||
| Male | 46 | 43.0 ± 3.6 | 35.900-50.200 | 47.0 ± 7.2 | 32.800-61.100 | 0.810 | 0.863 | 0.616 | 0.485-1.535 | 0.592 |
| Female | 54 | 44.4 ± 2.5 | 39.500-49.300 | 46.0 ± 1.5 | 42.900-49.000 | |||||
| Diameter, cm | ||||||||||
| < 1.43 ± 0.60 | 56 | 44.0 ± 38.6 | 38.600-49.400 | 47.0 ± 1.8 | 43.400-50.500 | 0.730 | 0.850 | 0.606 | 0.246-1.528 | 0.541 |
| ≥ 1.43 ± 0.60 | 44 | 42.2 ± 3.0 | 36.300-48.100 | 44.0 ± 3.0 | 38.000-49.900 | |||||
| Location | ||||||||||
| Head | 55 | 29.1 ± 3.0 | 23.000-35.100 | 21.0 ± 2.9 | 15.100-26.800 | 0.590 | 1.523 | 0.582 | 1.081-2.404 | 0.053 |
| Corpus | 27 | 36.6 ± 28.8 | 28.800-44.400 | 37.0 ± 6.5 | 24.100-49.000 | |||||
| Tail | 18 | 21.7 ± 3.5 | 14.800-28.600 | 17.0 ± 6.3 | 4.500-29.400 | |||||
| RM | ||||||||||
| R0 | 81 | 15.1 ± 3.6 | 8.000-22.100 | 6.0 ± 1.3 | 3.200-8.700 | 0.640 | 0.396 | 0.253 | 0.179-1.877 | 0.071 |
| R1 | 19 | 8.6 ± 1.4 | 5.700-11.440 | 8.0 ± 2.9 | 2.300-13.700 | |||||
| Invasion | ||||||||||
| T1 | 28 | 33.0 ± 3.5 | 26.000-40.000 | 32.0 ± 3.1 | 25.700-38.200 | 0.320 | 0.739 | 0.116 | 0.507-1.078 | 0.197 |
| T2 | 42 | 33.5 ± 2.9 | 27.700-39.200 | 36.0 ± 3.1 | 29.800-42.200 | |||||
| T3 | 26 | 42.0 ± 4.0 | 34.100-49.900 | 49.0 ± 9.3 | 30.600-67.300 | |||||
| T4 | 4 | 29.0 | 29.000-29.000 | 29.0 | - | |||||
| Node status | ||||||||||
| N0 | 56 | 19.0 ± 5.7 | 7.800-30.200 | 5.0 ± 1.0 | 3.000-6.900 | 0.550 | 1.585 | < 0.020 | 0.523-2.096 | 0.961 |
| N1 | 22 | 9.6 ± 1.1 | 7.300-11.900 | 9.0 ± 1.5 | 5.900-12.000 | |||||
| N2 | 22 | 7.6 ± 1.1 | 5.300-9.900 | 8.0 ± 1.8 | 4.400-11.600 | |||||
| LNM | ||||||||||
| Absent | 56 | 45.8 ± 2.3 | 41.300-50.400 | 48.0 ± 1.6 | 44.800-51.100 | < 0.001 | 1.388 | < 0.049 | 0.874-12.741 | 0.821 |
| Present | 44 | 38.1 ± 4.3 | 29.600-46.600 | 36.0 ± 2.3 | 31.400-40.500 | |||||
| Metastasis | ||||||||||
| Absent | 88 | 43.2 ± 2.0 | 39.100-47.300 | 46.0 ± 1.4 | 43.100-48.900 | 0.680 | 1.968 | 0.137 | 0.244-13.121 | 0.802 |
| Present | 12 | 41.1 ± 6.1 | 29.000-53.200 | - | - | |||||
| Stage | ||||||||||
| I | 29 | 42.3 ± 2.9 | 36.500-48.000 | 45.0 ± 1.5 | 41.900-48.000 | 0.350 | 1.102 | 0.041 | 0.321-1.602 | 0.212 |
| II | 25 | 48.5 ± 3.7 | 41.100-55.900 | 48.0 ± 2.7 | 42.600-53.300 | |||||
| III | 34 | 38.2 ± 4.9 | 28.400-47.900 | 36.0 ± 3.9 | 28.300-43.600 | |||||
| IV | 12 | 41.1 ± 6.1 | 29.000-53.200 | - | - | |||||
| Grade | ||||||||||
| Low | 35 | 47.3 ± 3.1 | 41.100-53.600 | 47.0 ± 1.5 | 43.900-50.000 | 0.140 | 0.748 | 0.210 | 0.475-1.177 | 0.265 |
| Moderate | 54 | 42.5 ± 2.8 | 37.000-48.000 | 48.0 ± 2.9 | 42.300-53.700 | |||||
| High | 11 | 35.3 ± 5.2 | 24.900-45.600 | 36.0 ± 5.0 | 26.100-45.900 | |||||
| LVI | ||||||||||
| Absent | 55 | 45.9 ± 2.4 | 41.100-50.600 | 47.0 ± 2.0 | 42.800-51.100 | 0.001 | 2.011 | 0.030 | 0.438-89.230 | 0.176 |
| Present | 45 | 38.6 ± 3.8 | 31.200-46.100 | 36.0 ± 4.8 | 26.400-45.500 | |||||
| PNI | ||||||||||
| Absent | 25 | 42.9 ± 2.6 | 37.800-48.100 | 47.0 ± 1.0 | 45.000-48.900 | 0.950 | 0.095 | 0.950 | 0.891-4.182 | 0.143 |
| Present | 75 | 43.5 ± 2.7 | 38.200-48.800 | 45.0 ± 3.2 | 38.600-51.300 | |||||
| PTB | ||||||||||
| PTB1 | 18 | 51.8 ± 4.3 | 43.400-60.300 | 56.0 ± 3.8 | 48.500-63.400 | < 0.001 | 3.065 | < 0.001 | 1.561-5.334 | < 0.001 |
| PTB2 | 30 | 37.0 ± 2.4 | 32.300-41.700 | 35.0 ± 2.3 | 30.400-39.500 | |||||
| PTB3 | 52 | 15.9 ± 1.9 | 12.100-19.6 | 14.0 ± 0.8 | 12.000-15.900 | |||||
| ITB | ||||||||||
| ITB1 | 16 | 44.4 ± 3.6 | 37.300-51.500 | 46.0 ± 1.8 | 42.400-49.500 | < 0.001 | 5.558 | < 0.001 | 2.905-10.634 | < 0.001 |
| ITB2 | 18 | 40.2 ± 2.3 | 35.500-44.900 | 44.0 ± 6.4 | 31.400-56.500 | |||||
| ITB3 | 66 | 29.1 ± 2.6 | 23.900-34.200 | 28.0 ± 3.2 | 21.600-34.300 | |||||
| DR | ||||||||||
| DR1 | 26 | 43.7 ± 3.3 | 37.100-50.300 | 45.0 ± 6.5 | 32.100-57.800 | 0.680 | 1.445 | 0.075 | 0.964-2.168 | 0.340 |
| DR2 | 27 | 40.3 ± 3.1 | 34.200-46.500 | 48.0 | - | |||||
| DR3 | 47 | 44.7 ± 3.3 | 38.200-51.300 | 48.0 ± 4.6 | 38.800-57.100 | |||||
| pTILs | ||||||||||
| ↓pTILs | 68 | 27.5 ± 2.6 | 22.300-32.700 | 21.0 ± 4.8 | 11.600-30.400 | 0.630 | 1.681 | 0.349 | 0.567-4.987 | 0.403 |
| ↑pTILs | 32 | 35.3 ± 3.7 | 28.100-45.500 | 37.0 ± 9.8 | 17.700-56.300 | |||||
| sTILs | ||||||||||
| ↓sTILs | 72 | 23.6 ± 2.2 | 19.400-27.900 | 19.0 ± 1.4 | 16.400-21.500 | < 0.001 | 0.476 | 0.022 | 0.205-1.105 | 0.044 |
| ↑sTILs | 28 | 47.7 ± 4.1 | 39.200-55.600 | 27.0 ± 4.5 | 18.200-35.800 | |||||
In this study, the PTB grade was associated with tumor diameter, nodal status, and stage. Moreover, the PTB and ITB grades strongly correlated with LNM. However, we did not observe a relationship between TB and distant metastasis. The results of previous studies examining these parameters are variable. While one study achieved similar results as ours[25], TB was associated with different parameters in other studies[26,27]. These differences can be explained by the number and selection of cases and the methods used to detect TB.
There are consistent results that TB is unrelated to distant metastasis. It is an interesting observation, especially considering the relationship between the EMT and metastatic potential of tumors[26,28]. In a recent elegant study, Lohneis et al[27] explained this difference with the term “partial EMT”. They emphasized that although the buds appear separate from the primary tumor mass in two-dimensional sections, they are connected to the main tumor mass in three-dimensional reconstructions. They also observed that the partial E-cadherin and beta-catenin expression in these cells is not completely eliminated, indicating that there is no transition to the complete mesenchymal phenotype. These data indicated that routine evaluation of the use of TB in the prediction of distant metastasis in patients with PDAC is limited. In addition, the presence of complete EMT in the TB area should be supported by immunohistochemical methods. Nevertheless, despite the differences among studies, our results highlighted that TB might reflect aggressive tumor behavior in patients with PDAC.
We observed that a lower TB grade was correlated with a better overall survival. Moreover, TB was an independent prognostic parameter that predicts survival in patients with PDAC. This finding confirmed the previous findings of the prognostic relevance of TB in these tumors, suggesting that this parameter has the potential to be included in the standard pathology reporting protocol for PDAC[7,11,28]. However, the most critical obstacle for including TB is the absence of a standard determination method. In this study, TB was determined using the method recommended by the International Tumor Budding Consensus Conference[21]. There is a lack of complete agreement between the recent data and therefore no consensus for evaluating TB in patients with PDAC. For example, one evaluation of TB in H&E-stained sections found reliability[11], while another noted that pancytokeratin immunohistochemistry at 10 high-powered fields was superior to H&E in terms of reproducibility and interobserver agreement[29]. In addition, the use of different cutoff values and groupings in the analysis of TB limits the ability to draw conclusions. Therefore, more studies should be performed to improve the reproducibility of TB evaluation, including the implementation of artificial intelligence-based applications[30].
In previous studies, a topographic distinction was not performed in the evaluation of TB, indicating that the distinction of PTB in PDAC resection samples was not always clear and that the boundaries were often blurred[29]. However, we evaluated PTB and ITB separately and observed that both were independent markers for determining tumor behavior. Moreover, we noted that ITB grade was a more potent prognostic factor than PTB grade. Conversely, the strong association between PTB and ITB warrants further research to determine the value of evaluating ITB grade in small biopsies for the determination of tumor behavior and treatment strategies in patients with unresectable PDAC.
Our results indicated that high levels of both PTB and ITB are associated with immature DR in PDAC, supporting their association with the EMT. However, this parameter was not associated with clinicopathological factors and failed to predict survival. New evidence has enhanced our understanding of the impact of the TME on PDAC and other types of cancer[7,14,31,32]. Multiple biological interactions within the TME promote the rapid proliferation and dissemination of cancer cells[33]. The increasing importance of stromal cells in facilitating a conducive environment for tumor growth has opened a viable avenue for therapeutic intervention[12,13,18].
There is increasing interest in targeting the TME to overcome the limited efficacy of conventional chemotherapy as a potential therapeutic option for treating PDAC, which has a significant amount of stroma[14,15]. Recent studies have demonstrated that multiple cellular processes play a role in the formation of DR and fibrosis in PDAC[14]. These discoveries may lead to the development of sophisticated diagnostic instruments and novel therapeutic approaches.
Although previous studies have addressed the influence of stromal density on the clinical outcome of patients with PDAC[31], the prognostic value of utilizing DR for determining stromal maturity and its relationship with TB have not been thoroughly investigated. The prognostic impact of DR has been investigated in CRC by Ueno et al[22], who discovered that patients with intermediate and immature stroma experience worse clinical outcomes than those with mature stroma.
Most studies on DR in gastrointestinal system tumors, including ours, have focused on the least mature form of stromal tissue. In addition, we investigated the maturity level of the stromal tissue at both the invasive front and the main tumor mass. Recently, Hacking et al[34] presented a revised three-stage approach to assess DR in CRC and categorized patients into risk groups that are linked to disease-free survival. This method categorizes DR by the total content of the immature desmoplastic stromal reaction. Further studies are needed to conduct direct comparative assessments between the methodologies and prognosis in patients with PDAC.
PDAC is a multifaceted combination of immunological, stromal, and tumor cells. It is distinguished by a compact stroma that promotes communication between the tumor and the immune system in the TME. The interaction between stromal and tumor cells affects the presence of immune cells in the TME. The desmoplastic region is comprised of immune cells, including tumor-associated macrophages, and a significant population of fibroblasts/myofibroblasts, primarily pancreatic stellate cells, which play a role in fibrosis[14,18].
The protumorigenic microenvironment is composed mainly of highly fibrotic stroma and is characterized by a significant presence of immunosuppressive cells[14]. Considerable data on the relationships among TIL subsets, TB, and tumor behavior in patients with PDAC have been obtained recently[19,20]. In an elegant study, Wartenberg et al[6] demonstrated that the combination of low TB, low stromal FOXP3 counts, the presence of tertiary lymphoid tissue, and the absence of CDKN2A mutations confers a significant survival advantage in patients with PDAC. These data indicated the importance of immune host responses that correlate with tumor characteristics, including TB, and may help in the recognition of PDAC subtypes with prognostic/predictive significance.
In our study, we observed a similar inverse correlation between TB and TILs. Additionally, our findings indicated that sTILs served as an independent prognostic factor, confirming recent research that highlighted the significant role of TILs in the TME of PDAC. However, we did not find a similar relationship between pTILs and prognosis, suggesting that variations in the methods used to quantify TILs in H&E-stained sections of PDAC may lead to different results.
Counting TILs in H&E-stained tissue sections is a quick and simple method to provide valuable insights into tumor behavior in various solid tumors, particularly in breast cancer. However, the role of TIL counts in PDAC prognosis has not been extensively investigated, and a standard method has not been established. Recent evidence suggests that intratumoral tertiary lymphoid structures are associated with prolonged overall survival and disease-free survival in PDAC, highlighting the important impact of lymphocytes on the progression of this disease[35]. The findings of our study, which further reinforce the important association between TILs and LNM, lymphovascular invasion, and TB, support their important role in the TME, their influence on disease progression, and their potential in treatment strategies.
The results on TIL counts and their prognostic significance using various methods have been inconsistent. For example, another study utilized a different method than we did and found no correlation between TILs and disease progression[36]. In contrast, another study concluded that TIL assessment when conducted according to specific scoring systems was an independent indicator for prognostic evaluation[24]. Our study also revealed a more detailed relationship between TIL levels and patient outcomes with this alternative scoring method. Our data suggest that higher TIL levels may be associated with more favorable prognostic outcomes in specific subgroups. These findings highlighted the importance of improving assessment techniques to increase the predictive power of TILs in clinical settings.
Although a significant association between TB grade and TILs was found in this study, we did not observe an association between TILs and DR grade. These findings are in contrast with a recent study that indicated the role of the tumor stroma in the interaction of tumor cells with the immune system in the TME[33]. This difference indicates that the applicability of the methods we used to determine TILs in PDAC is limited and should not exclude the significance of recent studies in which lymphocyte subtypes were more extensively studied. Considering the intricate interplay between immune factors and tumor phenotypes in PDAC, additional research is necessary to elucidate the associations between DR and TIL subsets. These investigations will enhance our understanding of the underlying mechanisms and identify potential variables that could modulate these interactions.
A limitation of this study was that it was conducted within a single center, leading to a relatively small sample size. This may limit its ability to detect nuanced relationships or differences. Another limitation was the retrospective nature of the study. Retrospective studies cannot exclude potential selection biases, which limits generalizability to other populations and settings. Therefore, it is imperative to conduct multicenter prospective studies and external validation to substantiate these findings.
The absence of a standardized evaluation method to evaluate TB, DR, and TILs in patients with PDAC may have also led to variability in our results. While we followed the procedures recommended in the current literature and guidelines, the lack of consensus for these histopathological findings may impact the capacity to reproduce and compare our findings with other investigations. In addition, the diverse behavior of PDAC necessitates a comprehensive investigation encompassing a broad spectrum of potential prognostic markers. We examined a limited number of prognostic markers that while significant can only encompass some aspects of patient outcomes.
Our study group comprised only patients diagnosed with PDAC (not otherwise specified) because there are a limited number of patients with other tumor subtypes available. Therefore, additional research is needed to explore how TB, DR, and TILs interact across different types of PDAC. Understanding these relationships is essential for gaining insights into tumor behavior and patient survival, particularly follow-up and treatment options. Despite these limitations the prognostic significance of TB and DR in patients with PDAC will benefit future research and may lead to the inclusion of these parameters in pathological reporting protocols.
Both the ITB and PTB grade are powerful prognostic factors, and the significant relationship between them suggests that evaluating the ITB in biopsies can be used as a valuable parameter for determining tumor behavior and treatment strategies, especially in patients who are not suitable for surgery. However, these data should be evaluated in further studies with more extensive patient series. A high PTB and ITB grade were associated with immature DR and supports their association with EMT. However, this parameter was not associated with clinicopathological factors and failed to predict survival, necessitating further investigations. Further studies with larger sample sizes are needed to confirm the findings from this study and increase the applicability of the results. In addition, multicenter retrospective studies across multiple countries and hospitals to determine the consistency of the association between TB, DR, and TILs in PDAC are required. This approach could provide a comprehensive understanding of the interactions between these variables and their influence on patient outcomes. By pooling data from diverse populations, researchers can ultimately improve treatment strategies for those affected by PDAC.
| 1. | Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71:7-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8287] [Cited by in RCA: 11914] [Article Influence: 2978.5] [Reference Citation Analysis (4)] |
| 2. | Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913-2921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5379] [Cited by in RCA: 5124] [Article Influence: 465.8] [Reference Citation Analysis (0)] |
| 3. | Bahra M, Pratschke J, Klein F, Neuhaus P, Boas-Knoop S, Puhl G, Denecke T, Pullankavumkal JR, Sinn M, Riess H, Pelzer U. Cytoreductive Surgery for Pancreatic Cancer Improves Overall Outcome of Gemcitabine-Based Chemotherapy. Pancreas. 2015;44:930-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Raut CP, Tseng JF, Sun CC, Wang H, Wolff RA, Crane CH, Hwang R, Vauthey JN, Abdalla EK, Lee JE, Pisters PW, Evans DB. Impact of resection status on pattern of failure and survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Ann Surg. 2007;246:52-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 446] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 5. | Strobel O, Neoptolemos J, Jäger D, Büchler MW. Optimizing the outcomes of pancreatic cancer surgery. Nat Rev Clin Oncol. 2019;16:11-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 597] [Article Influence: 99.5] [Reference Citation Analysis (0)] |
| 6. | Wartenberg M, Zlobec I, Perren A, Koelzer VH, Gloor B, Lugli A, Karamitopoulou E. Accumulation of FOXP3+T-cells in the tumor microenvironment is associated with an epithelial-mesenchymal-transition-type tumor budding phenotype and is an independent prognostic factor in surgically resected pancreatic ductal adenocarcinoma. Oncotarget. 2015;6:4190-4201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 7. | Kohler I, Bronsert P, Timme S, Werner M, Brabletz T, Hopt UT, Schilling O, Bausch D, Keck T, Wellner UF. Detailed analysis of epithelial-mesenchymal transition and tumor budding identifies predictors of long-term survival in pancreatic ductal adenocarcinoma. J Gastroenterol Hepatol. 2015;30 Suppl 1:78-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 8. | Ulase D, Heckl S, Behrens HM, Krüger S, Röcken C. Prognostic significance of tumour budding assessed in gastric carcinoma according to the criteria of the International Tumour Budding Consensus Conference. Histopathology. 2020;76:433-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 9. | Zlobec I, Lugli A. Tumour budding in colorectal cancer: molecular rationale for clinical translation. Nat Rev Cancer. 2018;18:203-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 10. | Lugli A, Zlobec I, Berger MD, Kirsch R, Nagtegaal ID. Tumour budding in solid cancers. Nat Rev Clin Oncol. 2021;18:101-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 220] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 11. | Karamitopoulou E, Wartenberg M, Zlobec I, Cibin S, Worni M, Gloor B, Lugli A. Tumour budding in pancreatic cancer revisited: validation of the ITBCC scoring system. Histopathology. 2018;73:137-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 12. | Chan TS, Shaked Y, Tsai KK. Targeting the Interplay Between Cancer Fibroblasts, Mesenchymal Stem Cells, and Cancer Stem Cells in Desmoplastic Cancers. Front Oncol. 2019;9:688. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 87] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 13. | Yoshida Y, Nakanishi Y, Mitsuhashi T, Yamamoto H, Hayashi MO, Oba M, Nitta T, Ueno T, Yamada T, Ono M, Kuwabara S, Hatanaka Y, Hirano S. Postoperative Prognosis According to Pathologic Categorization of Desmoplastic Reaction in Patients with Extrahepatic Cholangiocarcinoma. Ann Surg Oncol. 2023;30:7348-7357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 14. | Sato H, Hara T, Meng S, Tsuji Y, Arao Y, Saito Y, Sasaki K, Kobayashi S, Doki Y, Eguchi H, Ishii H. Multifaced roles of desmoplastic reaction and fibrosis in pancreatic cancer progression: Current understanding and future directions. Cancer Sci. 2023;114:3487-3495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 15. | Hsu SK, Jadhao M, Liao WT, Chang WT, Hung CT, Chiu CC. Culprits of PDAC resistance to gemcitabine and immune checkpoint inhibitor: Tumour microenvironment components. Front Mol Biosci. 2022;9:1020888. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 16. | Gu A, Li J, Qiu S, Hao S, Yue ZY, Zhai S, Li MY, Liu Y. Pancreatic cancer environment: from patient-derived models to single-cell omics. Mol Omics. 2024;20:220-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Gu A, Li J, Li MY, Liu Y. Patient-derived xenograft model in cancer: establishment and applications. MedComm (2020). 2025;6:e70059. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 18. | Muller M, Haghnejad V, Schaefer M, Gauchotte G, Caron B, Peyrin-Biroulet L, Bronowicki JP, Neuzillet C, Lopez A. The Immune Landscape of Human Pancreatic Ductal Carcinoma: Key Players, Clinical Implications, and Challenges. Cancers (Basel). 2022;14:995. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 44] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 19. | Orhan A, Vogelsang RP, Andersen MB, Madsen MT, Hölmich ER, Raskov H, Gögenur I. The prognostic value of tumour-infiltrating lymphocytes in pancreatic cancer: a systematic review and meta-analysis. Eur J Cancer. 2020;132:71-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 118] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 20. | Panahi M, Rezagholizadeh F, Mollazadehghomi S, Farhangnia P, Niya MHK, Ajdarkosh H, Tameshkel FS, Heshmati SM. The association between CD3(+) and CD8(+)tumor-infiltrating lymphocytes (TILs) and prognosis in patients with pancreatic adenocarcinoma. Cancer Treat Res Commun. 2023;35:100699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 21. | Lugli A, Kirsch R, Ajioka Y, Bosman F, Cathomas G, Dawson H, El Zimaity H, Fléjou JF, Hansen TP, Hartmann A, Kakar S, Langner C, Nagtegaal I, Puppa G, Riddell R, Ristimäki A, Sheahan K, Smyrk T, Sugihara K, Terris B, Ueno H, Vieth M, Zlobec I, Quirke P. Recommendations for reporting tumor budding in colorectal cancer based on the International Tumor Budding Consensus Conference (ITBCC) 2016. Mod Pathol. 2017;30:1299-1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 575] [Cited by in RCA: 725] [Article Influence: 90.6] [Reference Citation Analysis (0)] |
| 22. | Ueno H, Kanemitsu Y, Sekine S, Ishiguro M, Ito E, Hashiguchi Y, Kondo F, Shimazaki H, Mochizuki S, Kajiwara Y, Shinto E, Yamamoto J. Desmoplastic Pattern at the Tumor Front Defines Poor-prognosis Subtypes of Colorectal Cancer. Am J Surg Pathol. 2017;41:1506-1512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 90] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 23. | Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, Wienert S, Van den Eynden G, Baehner FL, Penault-Llorca F, Perez EA, Thompson EA, Symmans WF, Richardson AL, Brock J, Criscitiello C, Bailey H, Ignatiadis M, Floris G, Sparano J, Kos Z, Nielsen T, Rimm DL, Allison KH, Reis-Filho JS, Loibl S, Sotiriou C, Viale G, Badve S, Adams S, Willard-Gallo K, Loi S; International TILs Working Group 2014. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol. 2015;26:259-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1473] [Cited by in RCA: 2214] [Article Influence: 201.3] [Reference Citation Analysis (0)] |
| 24. | Lianyuan T, Dianrong X, Chunhui Y, Zhaolai M, Bin J. The predictive value and role of stromal tumor-infiltrating lymphocytes in pancreatic ductal adenocarcinoma (PDAC). Cancer Biol Ther. 2018;19:296-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 25. | Petrova E, Zielinski V, Bolm L, Schreiber C, Knief J, Thorns C, Bronsert P, Timme-Bronsert S, Bausch D, Perner S, Keck T, Wellner U. Tumor budding as a prognostic factor in pancreatic ductal adenocarcinoma. Virchows Arch. 2020;476:561-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 26. | El Amrani M, Corfiotti F, Corvaisier M, Vasseur R, Fulbert M, Skrzypczyk C, Deshorgues AC, Gnemmi V, Tulasne D, Lahdaoui F, Vincent A, Pruvot FR, Van Seuningen I, Huet G, Truant S. Gemcitabine-induced epithelial-mesenchymal transition-like changes sustain chemoresistance of pancreatic cancer cells of mesenchymal-like phenotype. Mol Carcinog. 2019;58:1985-1997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 27. | Lohneis P, Sinn M, Klein F, Bischoff S, Striefler JK, Wislocka L, Sinn BV, Pelzer U, Oettle H, Riess H, Denkert C, Bläker H, Jühling A. Tumour buds determine prognosis in resected pancreatic ductal adenocarcinoma. Br J Cancer. 2018;118:1485-1491. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 28. | Liu DN, Lv A, Tian ZH, Tian XY, Guan XY, Dong B, Zhao M, Hao CY. Superior mesenteric artery margin in pancreaticoduodenectomy for pancreatic adenocarcinoma. Oncotarget. 2017;8:7766-7776. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 29. | Karamitopoulou E, Esposito I, Zlobec I, Insilla AC, Wartenberg M, Schaeffer DF, Kalloger S, La Rosa S, Sempoux C, Ramos Centeno I, Lohneis P. Reproducibility of tumor budding assessment in pancreatic cancer based on a multicenter interobserver study. Virchows Arch. 2021;478:719-726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Zhou T, Man Q, Li X, Xie Y, Hou X, Wang H, Yan J, Wei X, Bai W, Liu Z, Liu J, Hao J. Artificial intelligence-based comprehensive analysis of immune-stemness-tumor budding profile to predict survival of patients with pancreatic adenocarcinoma. Cancer Biol Med. 2023;20:196-217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 31. | Karamitopoulou E, Haemmig S, Baumgartner U, Schlup C, Wartenberg M, Vassella E. MicroRNA dysregulation in the tumor microenvironment influences the phenotype of pancreatic cancer. Mod Pathol. 2017;30:1116-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 32. | Wang J, Liao Z. Research progress of microrobots in tumor drug delivery. Food Med Homol. 2024;1:9420025. [DOI] [Full Text] |
| 33. | Pernot S, Evrard S, Khatib AM. The Give-and-Take Interaction Between the Tumor Microenvironment and Immune Cells Regulating Tumor Progression and Repression. Front Immunol. 2022;13:850856. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 34. | Hacking S, Ebare K, Angert M, Lee L, Vitkovski T, Thomas R, Chavarria H, Jin C, Nasim M. Immature Stroma and Prognostic Profiling in Colorectal Carcinoma: Development and Validation of Novel Classification Systems. Pathol Res Pract. 2020;216:152970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 35. | Hiraoka N, Ino Y, Yamazaki-Itoh R, Kanai Y, Kosuge T, Shimada K. Intratumoral tertiary lymphoid organ is a favourable prognosticator in patients with pancreatic cancer. Br J Cancer. 2015;112:1782-1790. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 155] [Cited by in RCA: 274] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 36. | Hart PA, Smyrk TC, Bamlet WR, Chari ST. Impact of Intratumoral Inflammation on Survival After Pancreatic Cancer Resection. Pancreas. 2016;45:123-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |