Published online Jun 15, 2025. doi: 10.4251/wjgo.v17.i6.105570
Revised: March 28, 2025
Accepted: May 8, 2025
Published online: June 15, 2025
Processing time: 115 Days and 5.6 Hours
Finding active lead anti-hepatocellular carcinoma compounds from traditional Chinese medicine has important research value.
To assess the detailed mechanism of oxocrebanine, a compound separated from the traditional Chinese medicinal plant Stephania hainanensis H.S. Lo et Y. Tsoong, and to evaluate its inhibition of the proliferation of human hepatocellular carcinoma cells via apoptosis and autophagy.
MTT, BrdU labeling, and colony formation assays were used to assess the inhibitory effect of oxocrebanine on the growth and proliferation of human hepatocellular carcinoma Hep3B2.1-7 cells. Flow cytometry was used to detect the effect of oxocrebanine on the apoptosis of Hep3B2.1-7 cells. Western blotting was used to assess the expression of apoptosis-related proteins in Hep3B2.1-7 cells. The aforementioned methods were also used to evaluate the effects of oxocrebanine on cell proliferation, autophagy markers, and autophagy-related protein expression levels after adding autophagy inhibitor 3-mA. Furthermore, to verify the anti-hepatocellular carcinoma effect of oxocrebanine in vivo, a nude mouse model was used to investigate the inhibitory effect of oxocrebanine treatment and its mechanism. Apoptosis was detected using a TUNEL assay and the expression of microtubule-associated protein 1 LC3 in tumor specimens was assessed using immunohistochemistry.
Oxocrebanine effectively inhibited the growth of Hep3B2.1-7 cells, whilst upregulating the protein expression of cleaved caspase-3, downregulating poly(ADP-ribose) polymerase 1 protein expression, increasing the levels of Bax and Bcl-2 antagonist/killer 1 protein expression, and decreasing the levels of Bcl-2 and myeloid cell leukemia 1 protein expression, which could promote apoptosis in Hep3B2.1-7 cells. Oxocrebanine promoted the trans
Oxocrebanine can inhibit the proliferation of human hepatocellular carcinoma cells by promoting apoptosis and inducing autophagy in vitro and in vivo.
Core Tip: The present study revealed that oxocrebanine can inhibit the proliferation of hepatocellular carcinoma cells by promoting apoptosis and inducing autophagy both in vitro and in vivo. Oxocrebanine can induce apoptosis in Hep3b2.1-7 cells by upregulating cleaved caspase-3, Bax, and Bak protein expression levels and downregulating PARP1, Bcl-2, and Mcl-1 protein expression levels. The inhibition of Hep3b2.1-7 cell proliferation by oxocrebanine may be related to the induction of protective autophagy. The results of the TUNEL assay and immunohistochemistry also revealed that oxocrebanine induced apoptosis in vivo and increased the expression level of LC3, an autophagy marker.
- Citation: Wang ZW, Pan CY, Wei CL, Liao H, Zhang XP, Zhang CY, Yu L. Oxocrebanine inhibits the proliferation of hepatocellular carcinoma cells by promoting apoptosis and autophagy. World J Gastrointest Oncol 2025; 17(6): 105570
- URL: https://www.wjgnet.com/1948-5204/full/v17/i6/105570.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i6.105570
Hepatocellular carcinoma (HCC) is a primary hepatoma with a high mortality rate, accounting for 75%-85% of primary hepatomas. Globally, China ranks first in hepatoma incidence[1-3]. Studies have reported that the 5-year average survival rate for patients with hepatoma in China is only 12.1%[4,5]. To achieve the goal set in the “Healthy China 2030” plan of increasing the overall 5-year cancer survival rate by 15%[6], it is of significant social importance to find highly effective and low-toxicity anti-HCC drugs. At present, the drugs used to treat HCC in clinical practice mainly include targeted drugs, chemotherapy drugs, and antiviral drugs. Although the above-mentioned drugs have demonstrated some therapeutic effects in clinical practice, challenges such as the low success rate of gene typing for targeted drugs, drug resistance, and toxic side effects remain significant concerns, especially in combination therapy, which can cause severe liver toxicity in patients with HCC[7-9]. Therefore, it is urgent to develop low toxicity and high efficiency anti-HCC drugs, especially by seeking lead compounds or structural optimization from traditional Chinese medicine as a breakthrough point. Exploring anti-HCC active lead compounds from traditional Chinese medicines is one effective path to treat HCC. The Li Chinese medicinal plant, Stephania hainanensis H.S. Lo et Y. Tsoong, commonly used in the Hainan Li ethnic group, is known for its analgesic, anti-swelling, and detoxifying effects, with high efficacy and low side effects. Furthermore, our previous research demonstrated that the Hainan Dichroa febrifuga compound oxocrebanine, which has a planar molecular structure, exerts dual inhibitory effects on topoisomerase and tubulin[10,11]. The present study aimed to assess the growth inhibitory effect of oxocrebanine on HCC cells and evaluate whether the inhibition is related to apoptosis and autophagy. Additionally, the present study aimed to assess the changes in apoptosis and autophagy-related pathway proteins following oxocrebanine treatment to elucidate the mechanism by which oxocrebanine induces apoptosis and autophagy in vitro and in vivo, in order to provide direction and evidence for the development of new drugs for the treatment of HCC.
Hep3B2.1-7 cells (Purchased from Zhejiang Nobo Biological Products Co., Ltd) were cultured with RPMI-1640 (Meilun Bio) for 24 hours. Treatment groups were administered oxocrebanine solutions at concentrations of 5, 10, 20, 40, and 80 μmol/L, while the control group received complete medium alone (Meilun Bio). Drug concentrations were selected based on preliminary experiments to ensure a dose-response range from slight to significant inhibition. Each concentration of oxocrebanine was assessed in six replicates. After 72 hours of cell culture, 10 μL of MTT solution (5 mg/mL) was added to each well. After 4 hours of incubation, the MTT solution was discarded and 150 μL of DMSO was added to each well. The plate was shaken for 10 minutes, and the optical density (OD) value was read using a microplate reader at a wavelength of 490 nm to calculate the cell inhibition rate. The experiment was repeated three times. The cell inhibition rate (%) was calculated as [(mean OD value of control group - mean OD value of treatment group)/mean OD value of control group] × 100.
Using the same experimental groups and drug concentrations as those in the MTT assay, cell proliferation was assessed per the BrdU Proliferation Assay Kit (Frdbio Bio) protocol. The cell proliferation rate (%) was calculated as (mean OD value of BrdU positive group/mean OD value of BrdU negative group) × 100.
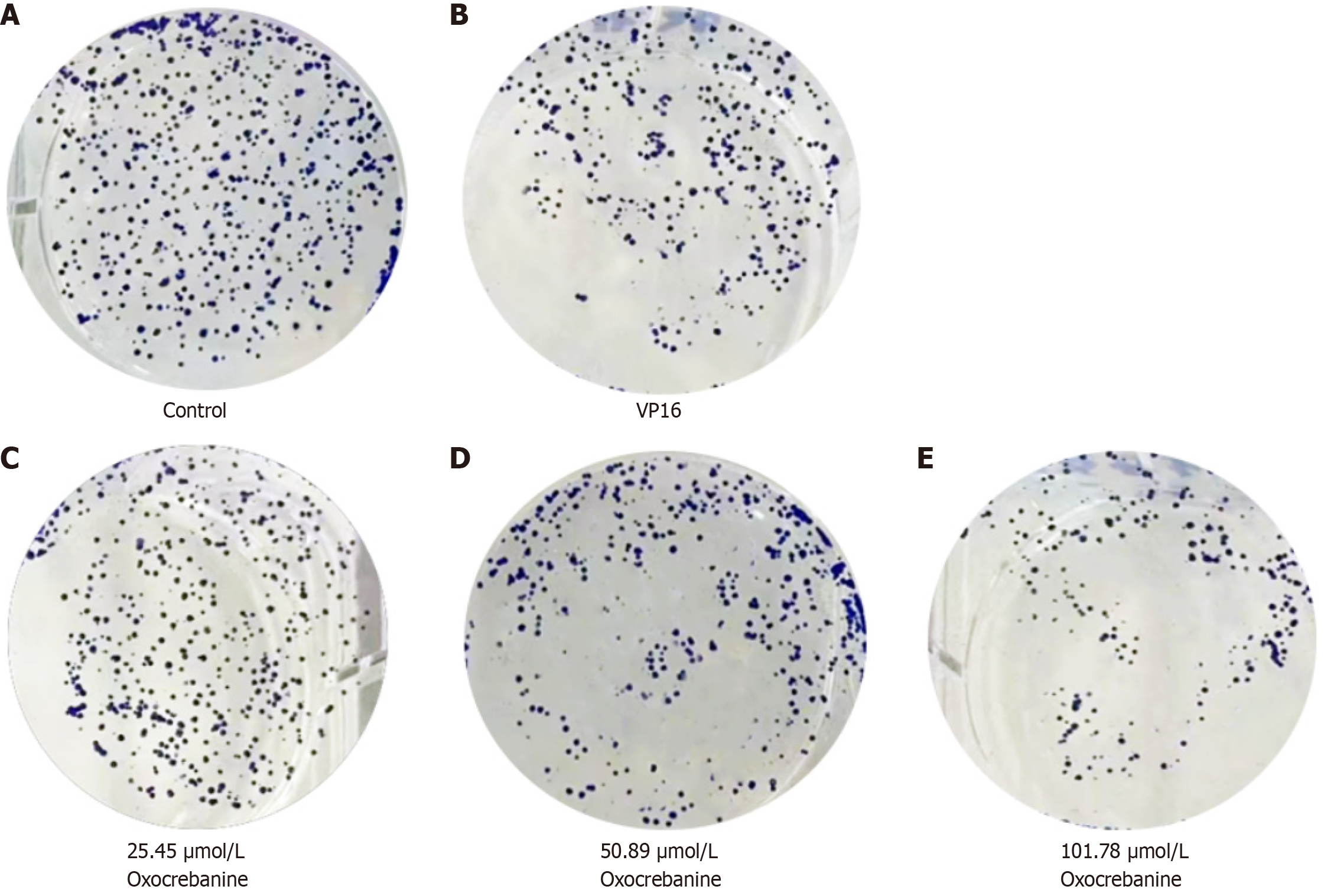
Hep3B2.1-7 cells were treated for 24 hours. Treatment groups were administered oxocrebanine solutions at concentrations of 25.45, 50.89, and 101.79 μmol/L, while the control group was administered 32.54 μmol/L VP16 solution (Shanghai Tongtian Biotechnology Co., Ltd) and cultured in 1 mL complete culture medium (Meilun Bio). After 72 hours of drug treatment, the solutions were discarded, the culture medium was replaced with fresh medium, and the cells were further cultured for 14 days in an incubator. Thereafter, the cells were washed with PBS, fixed with 4% paraformaldehyde for 1 hour, stained with crystal violet for 25 minutes, washed again with PBS, air-dried, and imaged.
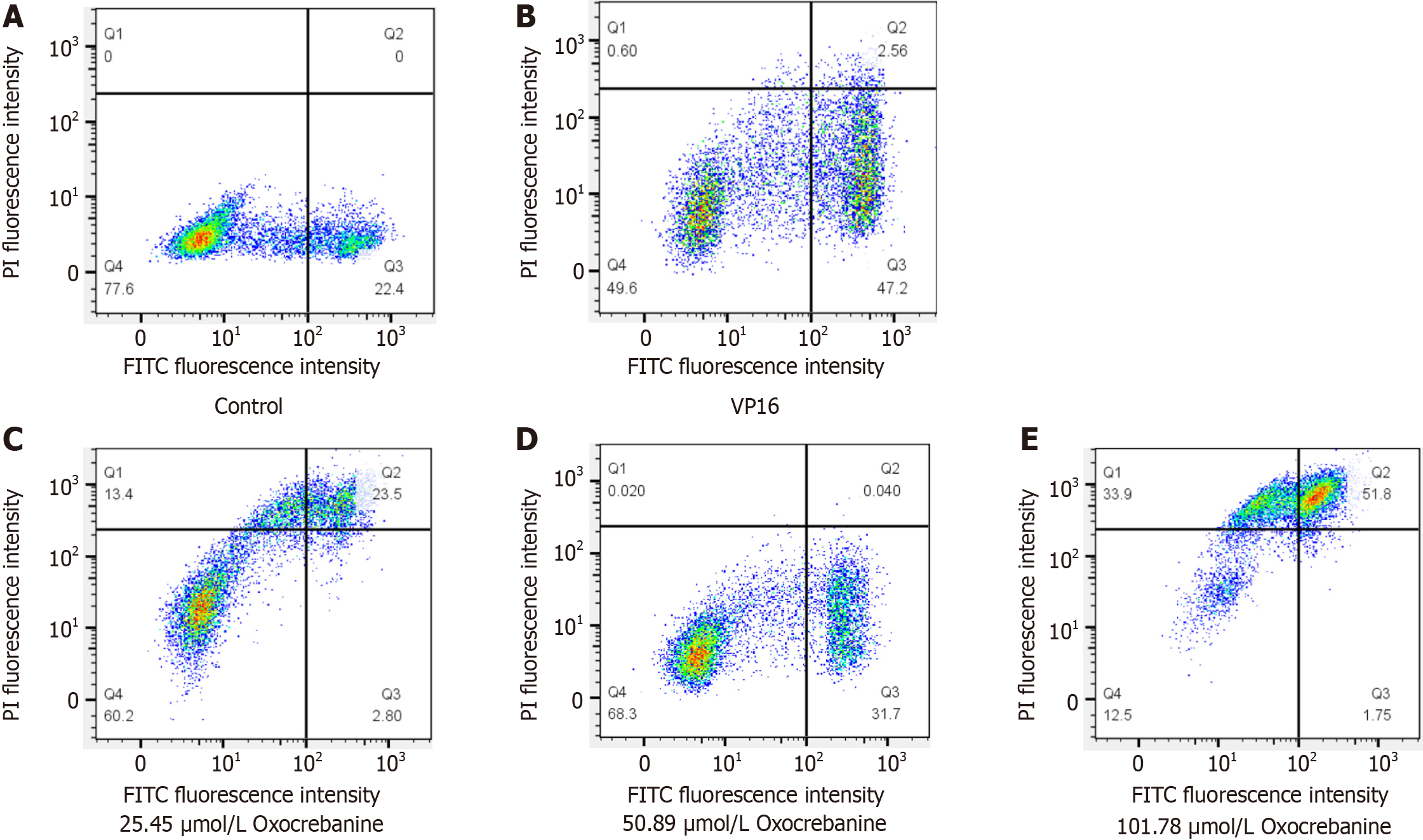
Hep3B2.1-7 cells were treated for 24 hours. Treatment groups were administered oxocrebanine solutions at concentrations of 25.45, 50.89, and 101.79 μmol/L, while the control group was administered 32.54 μmol/L VP16 solution. After 48 hours of incubation, the cells were collected, resuspended in 200 μL of Binding Buffer, and stained with 10 μL of FITC-labeled Annexin V and 5 μL of PI staining solution. The cells were incubated in the dark for 15 minutes and then analyzed using flow cytometry.
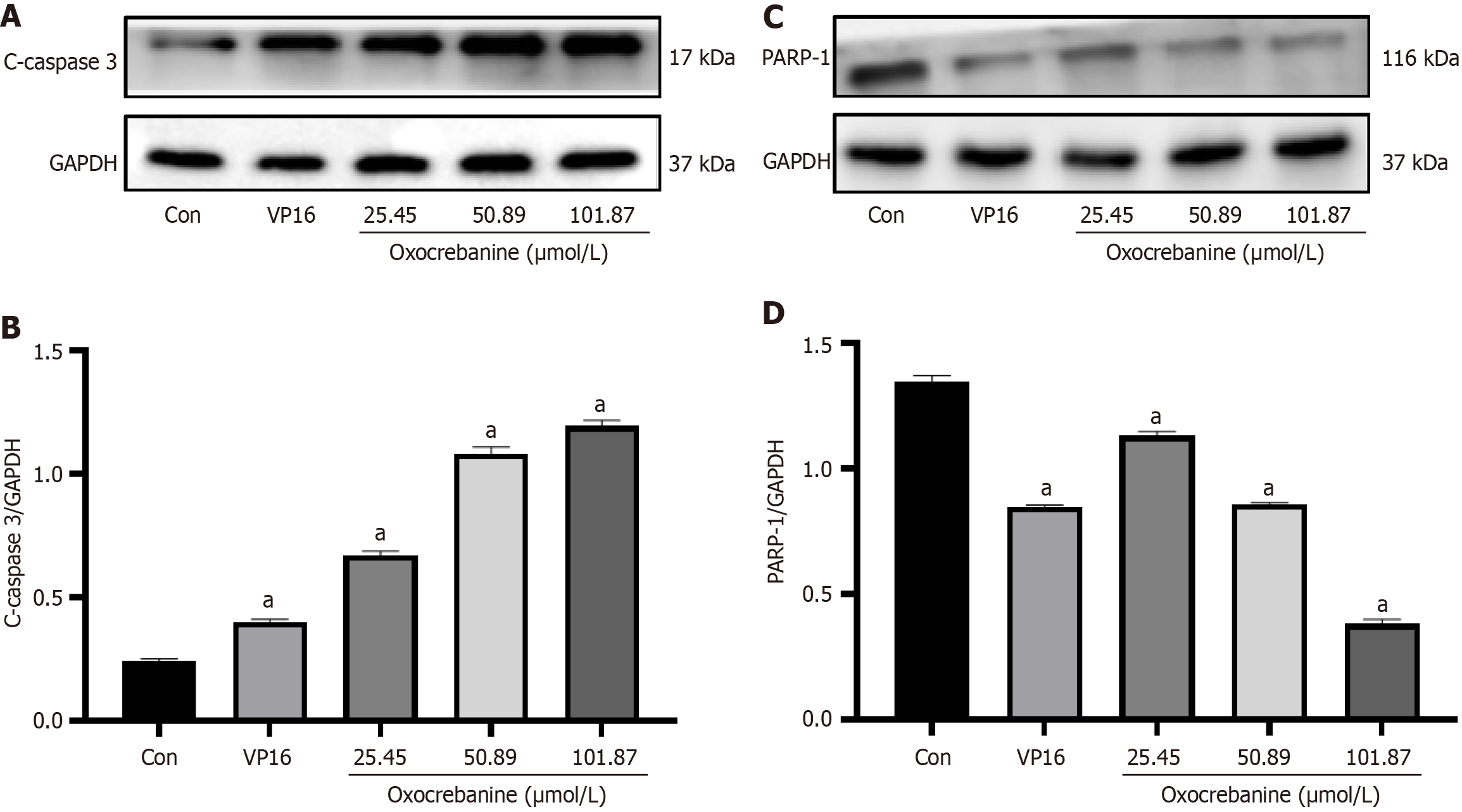
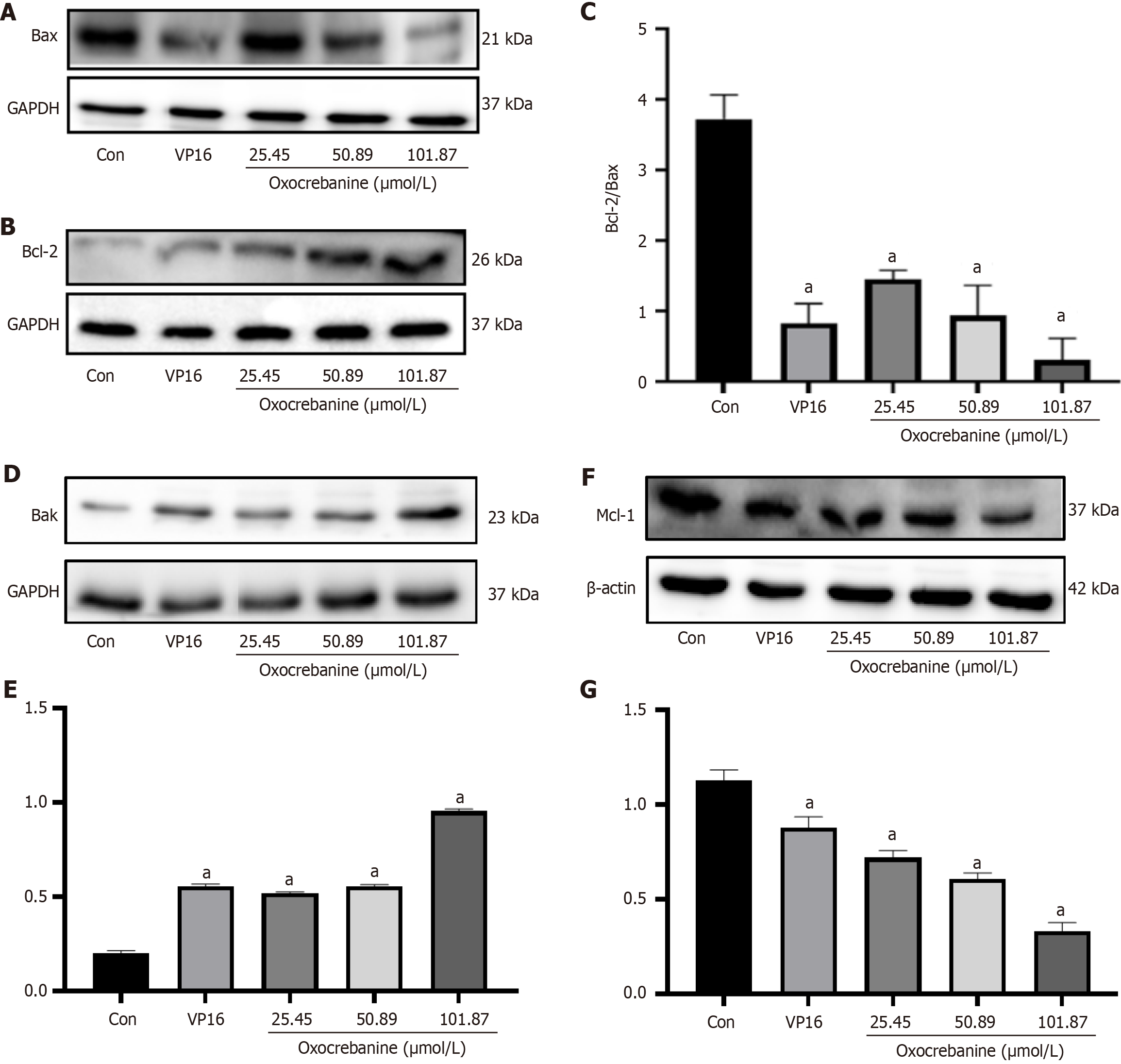
After 24 hours of Hep3B2.1-7 cell culture, the treatment groups and drug concentrations were the same as in PI/Annexin V double staining. After 72 hours, proteins were extracted and quantified. Protein samples were subjected to 10% SDS-PAGE, transferred to PVDF membranes, blocked with 5% skim milk for 2 hours, and incubated overnight at 4 °C with primary antibodies against GAPDH or β-actin, c-caspase3, PARP1, Bcl-2, Bax, Bak and Mcl-1 (Beijing Boaoson Biotechnology Co., Ltd, bs-0755R, bs-2076R, bs-0032R, bs-0081R, bs-20763R, bs-0127R, bs-23315R). The membranes were washed three times with TBST, incubated with HRP secondary antibodies (Beijing Boaoson Biotechnology Co., Ltd, bs-40295G) for 2 hours, washed three times with TBST, and visualized using an ECL luminescence imaging system (General Electric Company of the United States, ImageQuant LAS500). Protein bands were semi-quantitatively analyzed using Image J software (National Institutes of Health).
After 24 hours of Hep3B2.1-7 cell culture, the oxocrebanine + 3-MA group was treated simultaneously with the autophagy inhibitor 3-MA (Selleck) at 4 mmol/L and 50.89 μmol/L oxocrebanine. The control group was cultured in RPMI 1640 medium (Meilun Bio) with 10% fetal bovine serum (Hangzhou Sijiqing Bioengineering Materials Co., Ltd). MTT and BrdU labeling assays were performed as previously described.
The same experimental doses and group assignments used in the aforementioned MTT and BrdU labeling assays in the presence of an autophagy inhibitor were conducted, and Western blot was performed as previously described. The antibodies used included those against GAPDH, microtubule-associated protein 1 LC3-I, and LC3-II (ab192890; Abcam), as well as the eukaryotic translation initiation factor 4EBP1, p-4EBP1, P70S6K, and p-P70S6K (bs-2559R, bs-3018R, bs-5671R, and bs-1426R, respectively; Beijing Boaoson Biotechnology Co., Ltd).
A total of 5 × 106 Hep3B2.1-7 cells in the log growth phase in 200 μL suspension were subcutaneously injected into the dorsal flank of male immunodeficient mice (BALB/cNu/Nu), aged 8-10 weeks. The nude mice (n = 10) were randomly divided into either a control group or a treatment group, with five mice in each group. The mice were fed in specific-pathogen-free animal rooms, and their feed and drinking water were strictly sterilized using high-pressure and high-temperature sterilization methods. During the breeding process, direct contact between human skin and nude mice was avoided, and all items and utensils that came into contact with the nude mice were strictly disinfected. If any animals developed a serious illness or other health problems, such as infectious diarrhea or mousepox, and were unsuitable for the experiments, they were euthanized. Furthermore, the experimental animals were euthanized if the tumor size reached 2 cm. The experiment on the nude mice and related experimental protocols adhered to the 3R principle and were approved by the Medical Ethics Committee of Hainan Medical University (approval No. HYLL-2024-146). When the tumor volume increased to approximately 50 mm3, the nude mice were divided into control and treatment groups (10 mg/kg oxocrebanine). Each group contained five nude mice according to statistical requirements. All drugs were administered intraperitoneally every 3 days for five times. The mice were then observed for 3 days, during which tumor volume was measured every 3 days to plot tumor growth curves for each group. At the end of the observation, each group of nude mice were anesthetized by an intraperitoneal injection of 300 μL of 2% pentobarbital sodium (60 mg/kg), according to pharmacological experimental methodology[12] and literature report[13]. Subsequently, the tumors were imaged using a small animal in vivo live imaging system. The nude mice were then euthanized using 30% vol/minute CO2 (40 cm × 30 cm × 25 cm box), followed by cervical dislocation to confirm death, ensuring that the nude mice quickly lost vital signs and avoiding other animals from seeing the process. Subsequently, the tumor tissues were removed intact, fixed using 4% paraformaldehyde, paraffin embedded, sectioned, and assessed for apoptosis using a TUNEL assay. Expression of the autophagy signature protein LC3 was also evaluated using immunohistochemical staining. In the same way of modeling and administration, another 10 nude mice were divided into control and treatment groups to record the survival time of the tumor-bearing nude mice and generate Kaplan-Meier survival curves. At the end of the experiment, all surviving nude mice were euthanized.
The data are presented as the mean ± SD from three independent experiments. Statistical analyses were performed using SPSS 21.0 for Windows (IBM Corp.). One-sided analysis of variance followed by the Fisher’s least significant difference test was used to analyze the data from different groups. P < 0.05 was considered to indicate a statistically significant difference.
MTT, BrdU labeling, and colony formation assays were used to assess the effect of oxocrebanine on the growth and proliferation of Hep3B2.1-7 cells. The results of the MTT assay are presented in Table 1. After treatment of Hep3B2.1-7 cells with oxocrebanine, the inhibition rate increased significantly in a dose-dependent manner (P < 0.01) compared with the control group, and the IC50 value was calculated to be 50.89 μmol/L. The BrdU labeling assay results indicated that the cell proliferation level decreased significantly (P < 0.01), which also showed a dose-dependent relationship (Table 2). Furthermore, plate colony formation in Hep3B2.1-7 cells decreased significantly after treatment with oxocrebanine (P < 0.01; Table 3 and Figure 1). The results of the aforementioned experiments suggest that oxocrebanine can inhibit the growth and proliferation of Hep3B2.1-7 cells.
The results of PI/Annexin V double staining combined with flow cytometry indicated that, compared with the control group, the apoptosis level of Hep3B2.1-7 cells in the VP16 group was significantly increased (P < 0.01; Table 4 and Figure 2). The apoptosis level in the oxocrebanine group was also significantly increased (P < 0.05 or P < 0.01) in a dose-dependent manner. Moreover, the cells treated with 50.89 μmol/L oxocrebanine exhibited early apoptosis (Figure 2D), whilst late apoptosis was observed at 101.78 μmol/L (Figure 2E).
Western blot analysis was performed to measure the expression levels of cleaved caspase3 and PARP1 proteins in Hep3B2.1-7 cells after treatment with oxocrebanine (Figure 3). Compared with the control group, VP16 treatment significantly upregulated the expression of cleaved caspase3 (P < 0.01) but significantly downregulated the expression of PARP1 (P < 0.01). Similarly, oxocrebanine treatment significantly increased the expression of cleaved caspase3 (P < 0.01) but significantly decreased the expression of PARP1 (P < 0.01).
Western blot analysis was performed to measure the expression levels of the apoptosis-related proteins Bcl-2, Bax, Bak, and Mcl-1 in Hep3B2.1-7 cells after treatment with oxocrebanine. Compared with the control group, VP16 or oxocrebanine significantly reduced the expression levels of Bcl-2 and Bax proteins (P < 0.01; Figure 4A-C). Furthermore, after treatment with VP16 or oxocrebanine, the expression level of Bak protein was significantly increased (P < 0.01), and the expression level of Mcl-1 protein was significantly decreased (P < 0.01; Figure 4D-G).
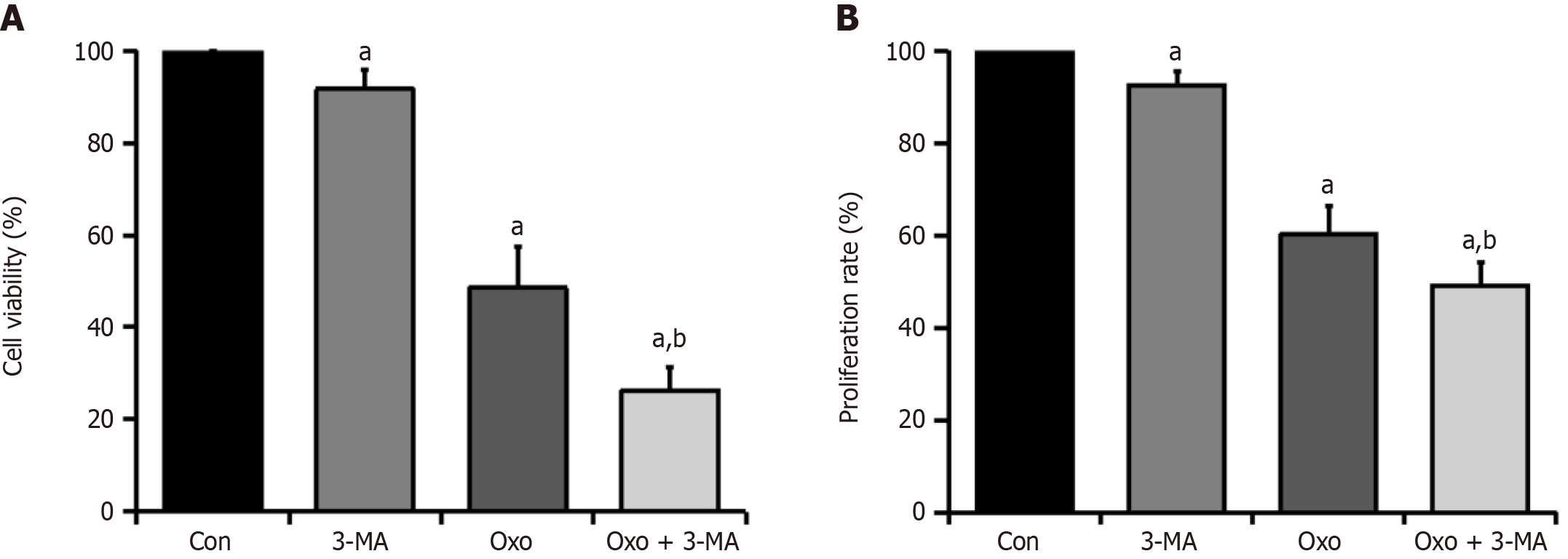
To assess whether oxocrebanine affects the activity of Hep3B2.1-7 cells through the autophagy pathway, MTT and BrdU labeling assays were performed to evaluate the inhibition of HCC cell growth and proliferation after adding the autophagy inhibitor 3-MA. The results of the MTT assay indicated that, compared with the control group, the viability of Hep3B2.1-7 cells significantly decreased after treatment with oxocrebanine alone (P < 0.01; Table 5 and Figure 5A). Compared with the oxocrebanine group, the cell viability in the oxocrebanine + 3-MA group decreased significantly (P < 0.01; Table 5 and Figure 5A). Moreover, the results of the BrdU assay indicated that, compared with the control group, oxocrebanine significantly reduced the proliferation of Hep3B2.1-7 cells (P < 0.01; Table 5 and Figure 5B). Compared with the oxocrebanine group, the proliferation rate in the oxocrebanine + 3-MA group also decreased significantly (P < 0.01; Table 5 and Figure 5B). This indicates that after the addition of the autophagy inhibitor 3-MA, oxocrebanine significantly inhibited the growth and proliferation of Hep3B2.1-7 cells. These results indicate that the inhibition of Hep3B2.1-7 cell proliferation by oxocrebanine may be related to the induction of protective autophagy in HCC cells.
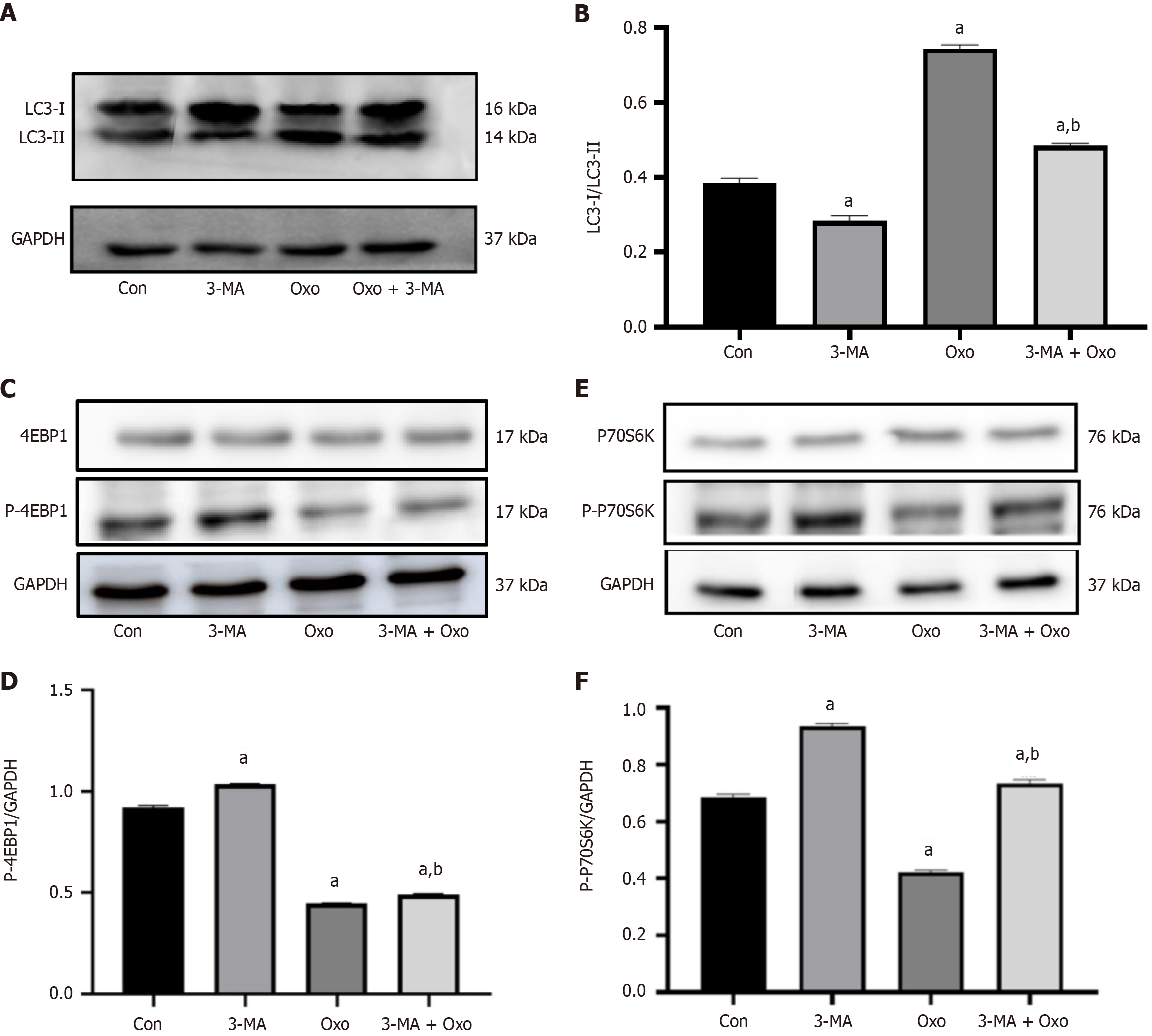
Western blot analysis was performed to measure the LC3- II/LC3-I protein expression ratio in Hep3B2.1-7 cells after administration of the autophagy inhibitor 3-MA and treatment with oxocrebanine. Compared with the control group, the LC3-II/LC3-I ratio in the oxocrebanine group significantly increased (P < 0.01). Compared with oxocrebanine alone, the LC3-II/LC3-I protein expression ratio in the oxocrebanine + 3-MA group significantly decreased (P < 0.01; Figure 6A and B). These results indicate that after treating Hep3B2.1-7 cells with oxocrebanine, the LC3-II/LC3-I ratio increases, and the autophagy inhibitor 3-MA can reverse this process.
Western blot results demonstrated that, compared with the control group, the phosphorylation level of 4EBP1 in Hep3B2.1-7 cells significantly decreased in the oxocrebanine group (P < 0.01). Compared with oxocrebanine alone, the phosphorylation level of 4EBP1 in the oxocrebanine + 3-MA group significantly increased (P < 0.01; Figure 6C and D). The results suggest that oxocrebanine can reduce the phosphorylation level of 4EBP1 in Hep3B2.1-7 cells, and the autophagy inhibitor 3-MA can reverse this process.
Compared with the control group, the phosphorylation level of P70S6K in Hep3B2.1-7 cells significantly decreased in the oxocrebanine group (P < 0.01). Compared with oxocrebanine alone, the phosphorylation level of P70S6K in the oxocrebanine + 3-MA group significantly increased (P < 0.01; Figure 6E and F). These results indicate that oxocrebanine can reduce the phosphorylation level of P70S6K, and the autophagy inhibitor 3-MA can reverse this process.
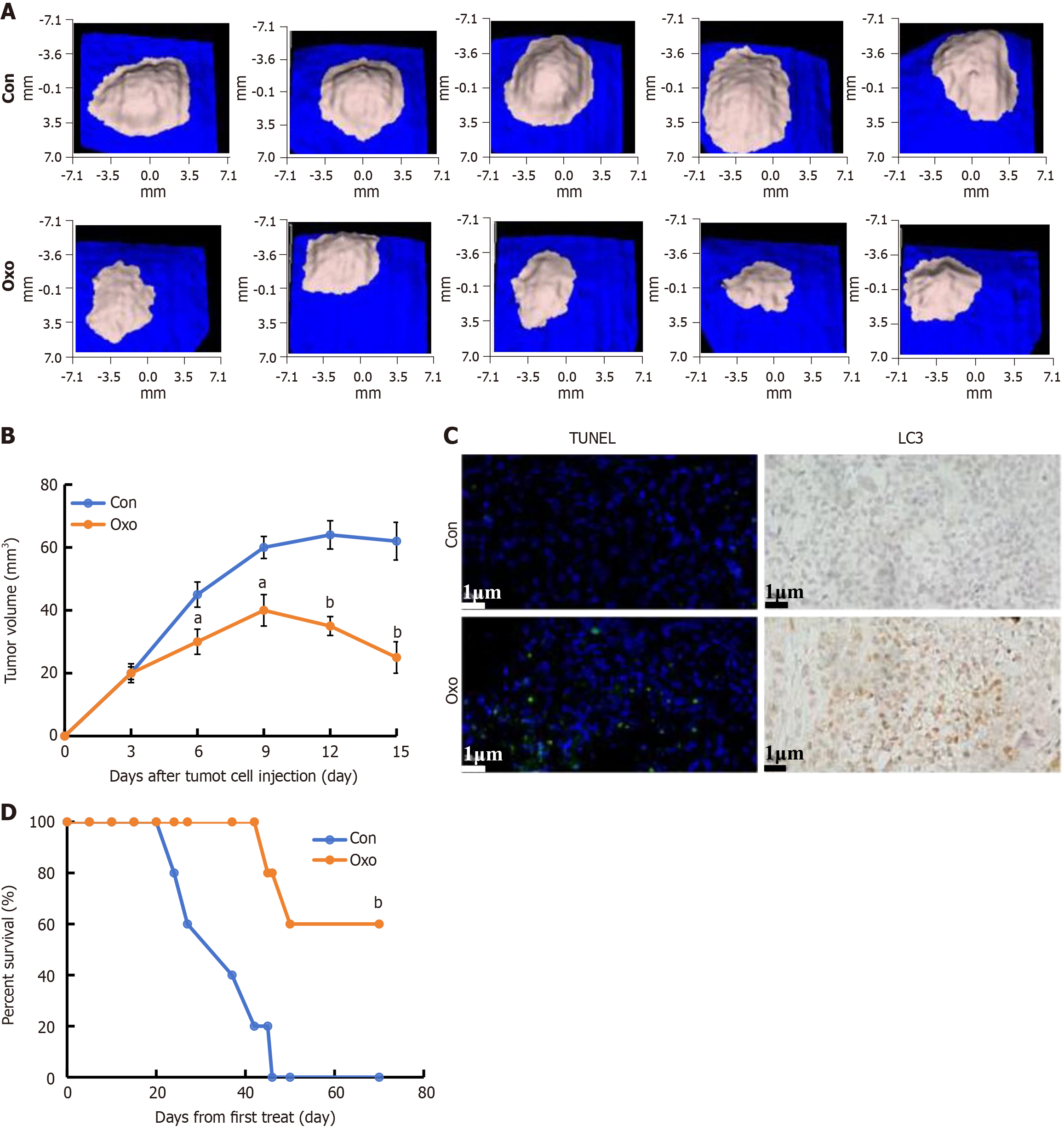
The tumor volume decreased significantly in the oxocrebanine group compared with the control group, and oxocrebanine administration was associated with a significant inhibition of tumor growth in vivo (Figure 7A and B). The TUNEL results revealed significantly increased apoptosis in the oxocrebanine group, and the immunohistochemical staining results demonstrated that the expression level of LC3 in the tumor tissues of mice in the oxocrebanine group was much higher than that of the control group (Figure 7C). This indicates that oxocrebanine can reduce the proliferation of HCC cells in mice through apoptosis and autophagy. Mice in the control group died on days 24, 27, 37, 42, and 46 of treatment, whilst mice in the oxocrebanine group died on days 45 and 50. The remaining three mice survived for > 70 days. On day 42, the survival rate of mice in the oxocrebanine group was 100%, whilst the survival rate in the control group was 20% (Figure 7D). On day 46, the survival rate of mice in the oxocrebanine group was 60%, whilst the survival rate in the control group was 0%. The Kaplan-Meier survival curve demonstrated that oxocrebanine can significantly prolong the survival time of tumor-bearing nude mice (P < 0.01; Figure 7D).
Stephania hainanensis H.S. Lo et Y. Tsoong contains a notable concentration of aporphine alkaloids. Our previous research isolated the aporphine alkaloid oxocrebanine from Stephania hainanensis H.S. Lo et Y. Tsoong, with the molecular formula C20H21O5N and a molecular weight of 355.14, and demonstrated its promising antitumor activity[10,11]. The present study assessed the mechanism by which oxocrebanine inhibits the proliferation of HCC, a prevalent cancer type in tropical regions, such as Hainan Province, Leizhou Peninsula in Guangdong Province, and some areas in Guangxi Province. The results from the MTT, BrdU cell proliferation, and colony formation assays indicate that oxocrebanine effectively inhibits the growth and proliferation of Hep3B2.1-7 cells. The IC50 of oxocrebanine is slightly weaker than that of sorafenib, a targeted drug against HCC. However, sorafenib has cardiovascular toxicity and lead to adverse cardiovascular effects including hypertension, thromboembolism, cardiac ischemia, and left ventricular dysfunction. Unfortunately, the adverse cardiovascular effects caused by sorafenib not only affect solid tumor patients but also limit its application in curing other diseases[13]. Oxocrebanine has similar or slightly better activity compared with Chinese herbal ingredients with significant anti-HCC activity reported recently, such as ginsenosides[14], matrine[15], resveratrol[16], and demethylzeylasteral[17]. The commonly used decoction of root tuber from Stephania hainanensis H.S. Lo et Y. Tsoong has been used to treat liver cancer and precancerous lesions in hospitals of Li Autonomous Prefecture, Hainan Province, China[18]. Previous research results[10] show that oxocrebanine has the characteristics of low toxicity and high efficiency, and has good application prospects. Animal tumor inhibition experiments showed that the body weight of the experimental animals in the oxocrebanine group was slightly reduced, though there was no significant difference compared to the control group, indicating that the toxicity of oxocrebanine is lower. Therefore, as a component of Li ethnomedicine, oxocrebanine has lower toxicity and side effects compared with chemotherapy drugs[19]. Apoptosis and autophagy are two critical cellular pathways that are involved in cell survival or death. The balance between apoptosis and autophagy maintains intracellular homeostasis, but the balance is often dysregulated in many cancers, including HCC[7]. Flow cytometry analysis revealed that oxocrebanine treatment promoted apoptosis in Hep3B2.1-7 cells, suggesting that the inhibition of cell growth and proliferation by oxocrebanine may be related to its induction of apoptosis. Further assessment using Western blotting revealed that oxocrebanine upregulated the expression of the cleaved caspase3 protein whilst downregulating PARP1. Additionally, oxocrebanine upregulated the pro-apoptotic proteins, Bax and Bak, and downregulated the anti-apoptotic proteins, Bcl-2 and Mcl-1, whilst increasing the ratio of Bcl-2 to Bax. These findings suggest that oxocrebanine promotes apoptosis in Hep3B2.1-7 cells. Under several apoptotic stimuli, downstream effectors of p53, such as Bax and Bak, may activate the p53 pathway, increase Bax and Bak expression, and enable their translocation to the cell membrane[20]. There, they can combine with Bcl-2 and Mcl-1, potentially increasing membrane permeability and facilitating the release of apoptosis-related factors, thus directly inducing apoptosis in HCC cells. On the other hand, the activation of the caspase pathway enables caspases to bind with PARP, further activating caspase 3 and promoting apoptosis in Hep3B2.1-7 cells[21]. Autophagy, which serves a major role in cell survival and tumor development, can inhibit or promote tumor proliferation[22]. It could inhibit the proliferation of cancer cells by eliminating tumorigenic protein substrates and damaging organelles[23]. The present study further demonstrated that the inhibitory effect of oxocrebanine on Hep3B2.1-7 cell proliferation may be related to the induction of protective autophagy in HCC cells through the addition of an autophagy inhibitor. The inhibition of autophagy markedly restored cell proliferation in oxocrebanine-treated cells. These results indicated that oxocrebanine induced autophagy in Hep3B2.1-7 cells to inhibit the proliferation of cancer cells. LC3 is a marker of autophagy. During autophagy, cytoplasmic LC3 (LC3-I) undergoes enzymatic cleavage of a small peptide and is converted to membrane-bound LC3-II, indicated by a notable increase in LC3-II in autophagic cells. The LC3-II/LC3-I ratio is commonly used to observe autophagic activity[24,25]. The results of the present study suggest that oxocrebanine increases the LC3-II/LC3-I ratio, indicating an increase in the expression of autophagy marker proteins. However, the autophagy inhibitor 3-MA can reverse this process. The PI3K/Akt/mTOR pathway is a critical signaling pathway in the regulation of autophagy. When inhibited, the phosphorylation of downstream effectors, 4EBP1 and P70S6K, decreases, inducing autophagy[26]. The present research also revealed that oxocrebanine significantly reduced the phosphorylation levels of 4EBP1 and P70S6K. This indicates that oxocrebanine, after acting on Hep3B2.1-7 cells, promotes the conversion of LC3-I to LC3-II, inhibits the phosphorylation of 4EBP1 and P70S6K proteins, and induces autophagy. We speculate that Beclin1, by activating PI3K, regulates the formation of autophagic vesicles, and then induces the lipidation of LC3-I to LC3-II, enabling its translocation to the autophagosome membrane. The autophagosome expands and then induces autophagy in the HCC cells[27,28]. Additionally, oxocrebanine may inhibit the Akt/mTOR signaling pathway, thus reducing the phosphorylation levels of its downstream effectors, 4EBP1 and P70S6K. The autophagy inhibitor 3-MA can reverse this process, further indicating that oxocrebanine can inhibit the proliferation of Hep3B2.1-7 cells through the autophagy pathway. Based on the in vitro experimental results, the present study assessed the anticancer effect and the mechanism of action of oxocrebanine in a subcutaneous transplantation tumor model of human Hep3B2.1-7 cells in nude mice. Oxocrebanine suppressed the growth of Hep3B2.1-7 cells in the tumor-bearing nude mice. The tumor volume of the oxocrebanine group markedly decreased compared with that of the mock-treated and control groups (P < 0.05 and P < 0.01, respectively). Apoptosis is a programmed cell death process, which serves an important role in maintaining the balance between tissues and organs[29]. Most of the tumor tissue cells in the oxocrebanine-treated group underwent apoptosis that was detected using a TUNEL assay, revealing that the decrease in the viability of Hep3B2.1-7 cells was due to the induction of apoptosis. Immunohistochemical staining revealed that LC3 expression in the tumor tissue was reduced, which indicated that oxocrebanine significantly inhibited tumor growth by inducing cell autophagy. These results indicate that oxocrebanine inhibited tumor growth by inducing cell apoptosis and promoting cell autophagy in vivo, which are in line with those obtained in the cell experiments. In summary, the results of the present study demonstrate that oxocrebanine effectively inhibits the growth and proliferation of Hep3B2.1-7 cells. It upregulates the expression of cleaved caspase3, downregulates PARP1, upregulates Bax and Bak, and downregulates Bcl-2 and Mcl-1, leading to apoptosis in Hep3B2.1-7 cells and the inhibition of cell proliferation. The inhibition of Hep3B2.1-7 cell proliferation by oxocrebanine may be related to the induction of protective autophagy in HCC cells. Oxocrebanine also promotes the conversion of LC3-I to LC3-II in Hep3B2.1-7 cells, a process reversed by the autophagy inhibitor 3-MA, which reduces the phosphorylation levels of 4EBP1 and P70S6K, induces autophagy, and thus inhibits cell growth. In addition, there was also an increase in the number of TUNEL positive cells, a marker for apoptosis induction, in the oxocrebanine group. Moreover, immunohistochemistry revealed that the level of LC3 was markedly upregulated after the treatment with oxocrebanine. The present study revealed that oxocrebanine can inhibit the proliferation of HCC cells by promoting apoptosis and inducing autophagy both in vitro and in vivo. These findings raise the possibility of oxocrebanine as a potential new active ingredient of tephania hainanensis H.S. Lo et Y. Tsoong that has anti-HCC properties. However, additional studies are warranted to elucidate other molecular mechanisms which are responsible for the anticancer efficacy of oxocrebanine as a potent anticancer agent against HCC. The research findings may provide a drug option with improved efficacy and reduced toxicity for the clinical treatment of HCC.
In conclusion, oxocrebanine can inhibit the proliferation of human HCC cells by promoting apoptosis and inducing autophagy in vitro and in vivo.
We gratefully acknowledge Professor Xiao-Po Zhang for kindly providing the compounds used in this study.
| 1. | Younossi ZM, Wong G, Anstee QM, Henry L. The Global Burden of Liver Disease. Clin Gastroenterol Hepatol. 2023;21:1978-1991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 200] [Article Influence: 100.0] [Reference Citation Analysis (0)] |
| 2. | Singal AG, Lampertico P, Nahon P. Epidemiology and surveillance for hepatocellular carcinoma: New trends. J Hepatol. 2020;72:250-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 526] [Cited by in RCA: 773] [Article Influence: 154.6] [Reference Citation Analysis (0)] |
| 3. | . Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 143] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 4. | Rumgay H, Arnold M, Ferlay J, Lesi O, Cabasag CJ, Vignat J, Laversanne M, McGlynn KA, Soerjomataram I. Global burden of primary liver cancer in 2020 and predictions to 2040. J Hepatol. 2022;77:1598-1606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 1040] [Article Influence: 346.7] [Reference Citation Analysis (0)] |
| 5. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64295] [Article Influence: 16073.8] [Reference Citation Analysis (174)] |
| 6. | Gao C, Xu J, Liu Y, Yang Y. Nutrition Policy and Healthy China 2030 Building. Eur J Clin Nutr. 2021;75:238-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 7. | Kouroumalis E, Tsomidis I, Voumvouraki A. Pathogenesis of Hepatocellular Carcinoma: The Interplay of Apoptosis and Autophagy. Biomedicines. 2023;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 24] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 8. | de Mattos AZ, Bombassaro IZ, Vogel A, Debes JD. Hepatocellular carcinoma-the role of the underlying liver disease in clinical practice. World J Gastroenterol. 2024;30:2488-2495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Reference Citation Analysis (0)] |
| 9. | Bergquist A, Ekstedt M, Hagström H, Järnerot G, Lindgren S, Nilsson E, Nyhlin N, Rorsman F, Stål P, Werner M; Swehep, Kechagias S. Forty years of successful national research collaboration in liver disease - the Swedish experience. Scand J Gastroenterol. 2024;59:1314-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Yu L, Han S, Lang L, Song H, Zhang C, Dong L, Jia S, Zhang Y, Xiao D, Liu J, Xu Y, Zhang X. Oxocrebanine: A Novel Dual Topoisomerase inhibitor, Suppressed the Proliferation of Breast Cancer Cells MCF-7 by Inducing DNA Damage and Mitotic Arrest. Phytomedicine. 2021;84:153504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Yu L, Jiang CY, Song DX, Yu M, Shang DY, Jiang B, Ji YB and Wang ZW. [Analysis of the Structure Activity Relationship and Inhibition of Tumor Cell Proliferation by Alkaloids in Stephania Hainannensis H.S. Lo et Y. Tsoong]. Liaoning Zhongyi Zazhi. 2020;47:145-154. [DOI] [Full Text] |
| 12. | Guide for the Care and Use of Laboratory Animals. Washington (DC): National Academies Press (US); 2011– . [PubMed] |
| 13. | Li J, Zhang L, Ge T, Liu J, Wang C, Yu Q. Understanding Sorafenib-Induced Cardiovascular Toxicity: Mechanisms and Treatment Implications. Drug Des Devel Ther. 2024;18:829-843. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 14. | Zhou Y, Wang Z, Ren S, Li W. Mechanism of action of protopanaxadiol ginsenosides on hepatocellular carcinoma and network pharmacological analysis. Chin Herb Med. 2024;16:548-557. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 15. | Chang X, Li H, Huang Z, Song C, Zhang Z, Pan W. Matrine suppresses hepatocellular carcinoma tumorigenesis by modulating circ_0055976/miR-1179/lactate dehydrogenase A axis. Environ Toxicol. 2024;39:1481-1493. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 16. | Feng CY, Cai CS, Shi XQ, Zhang ZJ, Su D, Qiu YQ. Resveratrol promotes mitophagy via the MALAT1/miR-143-3p/RRM2 axis and suppresses cancer progression in hepatocellular carcinoma. J Integr Med. 2025;23:79-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 17. | Pan L, Feng F, Wu J, Fan S, Han J, Wang S, Yang L, Liu W, Wang C, Xu K. Demethylzeylasteral targets lactate by inhibiting histone lactylation to suppress the tumorigenicity of liver cancer stem cells. Pharmacol Res. 2022;181:106270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 203] [Article Influence: 67.7] [Reference Citation Analysis (0)] |
| 18. | Wang M, Zhang XM, Fu X, Zhang P, Hu WJ, Yang BY, Kuang HX. Alkaloids in genus stephania (Menispermaceae): A comprehensive review of its ethnopharmacology, phytochemistry, pharmacology and toxicology. J Ethnopharmacol. 2022;293:115248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 19. | Yang XH, Ren LS, Wang GP, Zhao LL, Zhang H, Mi ZG, Bai X. A new method of establishing orthotopic bladder transplantable tumor in mice. Cancer Biol Med. 2012;9:261-265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 20. | Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB, Korsmeyer SJ. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2959] [Cited by in RCA: 3073] [Article Influence: 128.0] [Reference Citation Analysis (0)] |
| 21. | Shimizu S, Narita M, Tsujimoto Y. Bcl-2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC. Nature. 1999;399:483-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1654] [Cited by in RCA: 1660] [Article Influence: 63.8] [Reference Citation Analysis (0)] |
| 22. | Wen X, Klionsky DJ. At a glance: A history of autophagy and cancer. Semin Cancer Biol. 2020;66:3-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 73] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 23. | Singh SS, Vats S, Chia AY, Tan TZ, Deng S, Ong MS, Arfuso F, Yap CT, Goh BC, Sethi G, Huang RY, Shen HM, Manjithaya R, Kumar AP. Dual role of autophagy in hallmarks of cancer. Oncogene. 2018;37:1142-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 418] [Article Influence: 52.3] [Reference Citation Analysis (0)] |
| 24. | Marsden VS, O'Connor L, O'Reilly LA, Silke J, Metcalf D, Ekert PG, Huang DC, Cecconi F, Kuida K, Tomaselli KJ, Roy S, Nicholson DW, Vaux DL, Bouillet P, Adams JM, Strasser A. Apoptosis initiated by Bcl-2-regulated caspase activation independently of the cytochrome c/Apaf-1/caspase-9 apoptosome. Nature. 2002;419:634-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 449] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 25. | Levy JMM, Towers CG, Thorburn A. Targeting autophagy in cancer. Nat Rev Cancer. 2017;17:528-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1382] [Cited by in RCA: 1944] [Article Influence: 243.0] [Reference Citation Analysis (0)] |
| 26. | Mizushima N, Yoshimori T. How to interpret LC3 immunoblotting. Autophagy. 2007;3:542-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1783] [Cited by in RCA: 2146] [Article Influence: 119.2] [Reference Citation Analysis (0)] |
| 27. | Porta C, Paglino C, Mosca A. Targeting PI3K/Akt/mTOR Signaling in Cancer. Front Oncol. 2014;4:64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 759] [Cited by in RCA: 1135] [Article Influence: 103.2] [Reference Citation Analysis (0)] |
| 28. | Kisen GO, Tessitore L, Costelli P, Gordon PB, Schwarze PE, Baccino FM, Seglen PO. Reduced autophagic activity in primary rat hepatocellular carcinoma and ascites hepatoma cells. Carcinogenesis. 1993;14:2501-2505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 96] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 29. | Cheung CS, Chung KK, Lui JC, Lau CP, Hon PM, Chan JY, Fung KP, Au SW. Leachianone A as a potential anti-cancer drug by induction of apoptosis in human hepatoma HepG2 cells. Cancer Lett. 2007;253:224-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |