Published online May 15, 2025. doi: 10.4251/wjgo.v17.i5.104802
Revised: February 22, 2025
Accepted: March 14, 2025
Published online: May 15, 2025
Processing time: 126 Days and 7.3 Hours
Monomorphic epitheliotropic intestinal T-cell lymphoma (MEITL) is an uncom
A 78-year-old female with recurrent vomiting and abdominal distension was admitted to our hospital. Magnetic resonance and 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) examinations revealed multiple segmental malignant tumors in the small intestine with me
Magnetic resonance imaging serves as a valuable tool in detecting malignant tumor lesions of MEITL, whereas 18F-FDG PET/CT offers additional assistance in tumor staging and assessing treatment efficacy.
Core Tip: Monomorphic epitheliotropic intestinal T-cell lymphoma is an uncommon and highly aggressive form of lymphoma, making it challenging to distinguish this disease from other intestinal-related disease in clinical practice. In this case, we present the magnetic resonance and 18F-fluorodeoxyglucose positron emission tomography/computed tomography details of a 78-year-old female patient who presented with recurrent vomiting and abdominal distension. After completing three cycles of reduced-dose cyclophosphamide, vinorelbine, and prednisone regimen chemotherapy, the follow-up 18F-fluorodeoxyglucose positron emission tomography/computed tomography revealed partial response in treatment efficacy. We further summarized the imaging findings of monomorphic epitheliotropic intestinal T-cell lymphoma reported previously in the literature.
- Citation: Tang WJ, Luo SH, Wu Z, Kang Y, Lan B, Zhang ZQ, Zhong JY, Zhong JP, Wen CJ. Imaging findings of primary monomorphic epitheliotropic intestinal T-cell lymphoma: A case report. World J Gastrointest Oncol 2025; 17(5): 104802
- URL: https://www.wjgnet.com/1948-5204/full/v17/i5/104802.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i5.104802
Monomorphic epitheliotropic intestinal T-cell lymphoma (MEITL) is an uncommon and highly aggressive form of lymphoma originating in the intraepithelial lymphocytes of the gastrointestinal tract and accounts for less than 1% of all non-Hodgkin’s lymphomas[1]. In accordance with the 2022 5th edition of the World Health Organization classification of hematologic malignancies, intestinal T-cell and natural killer cell lymphoid proliferations and lymphomas are classified into five subtypes: Enteropathy-associated T-cell lymphoma (EATL), MEITL, indolent clonal T-cell lymphoproliferative disorder of the gastrointestinal tract, indolent natural killer cell lymphoproliferative disorder of the gastrointestinal tract, and intestinal T-cell lymphoma not otherwise specified[2]. MEITL, previously referred to as type II EATL, is more prevalent among Asian populations and is not associated with celiac disease[3]. However, type I EATL is more common in Western regions and is linked to celiac disease[3]. The early clinical symptoms of MEITL are insidious, with most patients being diagnosed at an advanced stage and having a median survival time of only 7 months[4]. Treatment options for non-Hodgkin’s lymphomas include: Chemotherapy, radiation therapy, immune checkpoint inhibitors, bispecific T-cell engagers, chimeric antigen receptor T cell therapy and allogeneic hematopoietic stem cell transplantation[5]. Owing to the rarity of MEITL, only a handful of medical imaging reports exist in the literature, which poses significant challenges for clinical diagnosis. 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) is considered the standard imaging modality for the diagnosis, staging and response assessment of lymphoma. Under these circumstances, we present a patient with MEITL who underwent 18F-FDG PET/CT, magnetic resonance (MR) and gastroduodenoscopy examinations. We further discuss the differential diagnosis and therapeutic approaches of MEITL, aiming to foster a deeper understanding of the disease.
A 78-year-old female patient was admitted with recurrent vomiting and abdominal distension for more than 20 days.
The patient developed discomfort with vomiting 20 days prior, usually 1-2 hours after eating. The vomit consisted of gastric contents accompanied by abdominal distension without fever, chills, diarrhea, abdominal pain or other discom
There was no special abnormality in the patient’s past medical history.
The patient reported no family history of malignant tumors.
Her vital signs were as follows: Temperature 36.8 °C, blood pressure 117/65 mmHg, heart rate 76 bpm, and respiratory rate 20 rpm. Physical examination revealed no abnormal signs.
Laboratory tests revealed increased white blood cells (11.64 × 109/L), β2-microglobulin (3.26 μg/mL) and platelet count (466 × 109/L); decreased red blood cells (3.77 × 1012/L) and hemoglobin (101 g/L); and a positive fecal occult blood test. β2-microglobulin is abundant in lymphocytes and therefore considered a major marker of lymphocyte proliferative diseases. In clinical practice, elevated levels of β2-microglobulin associated with poor prognosis in lymphoma patients[6].
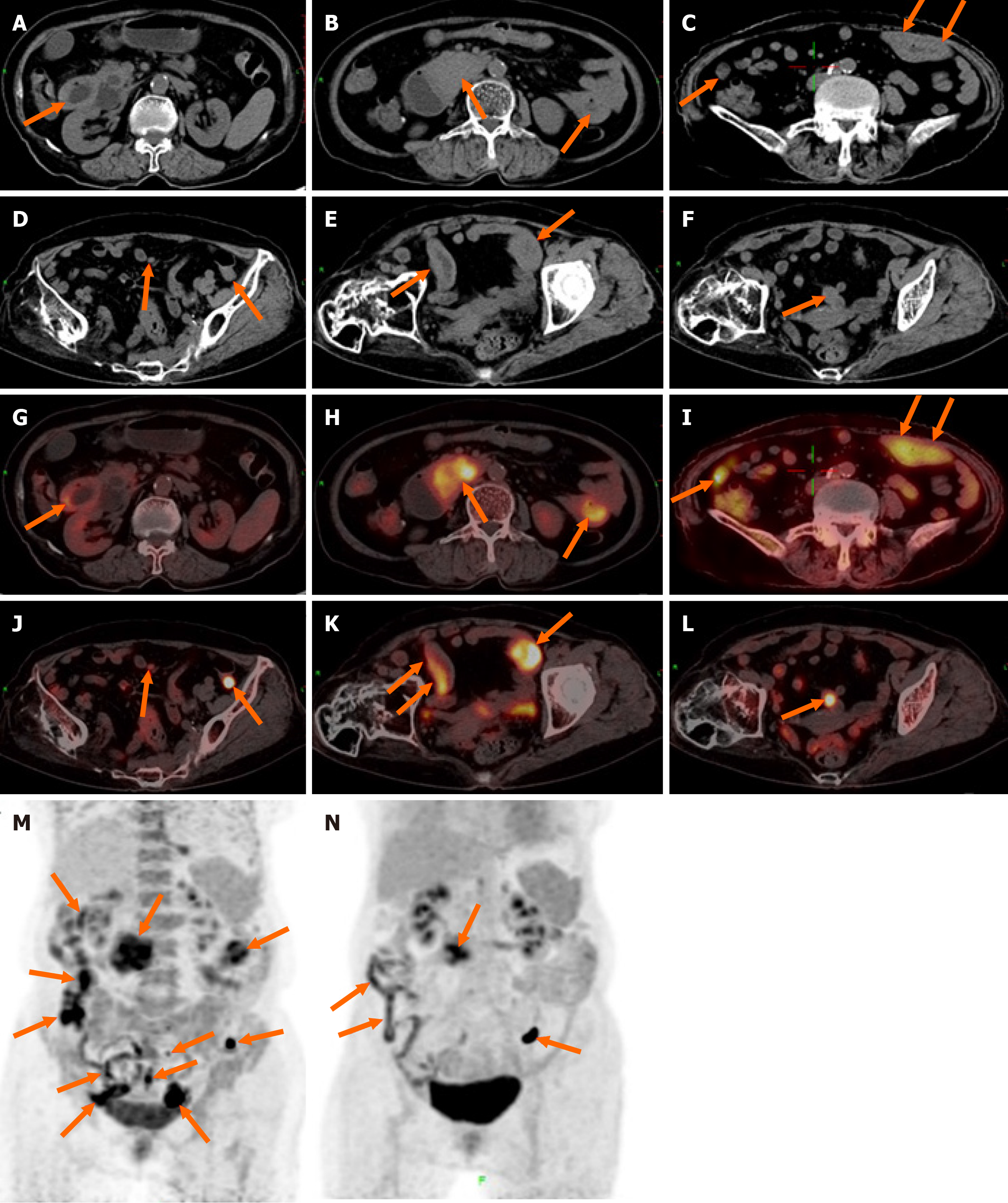
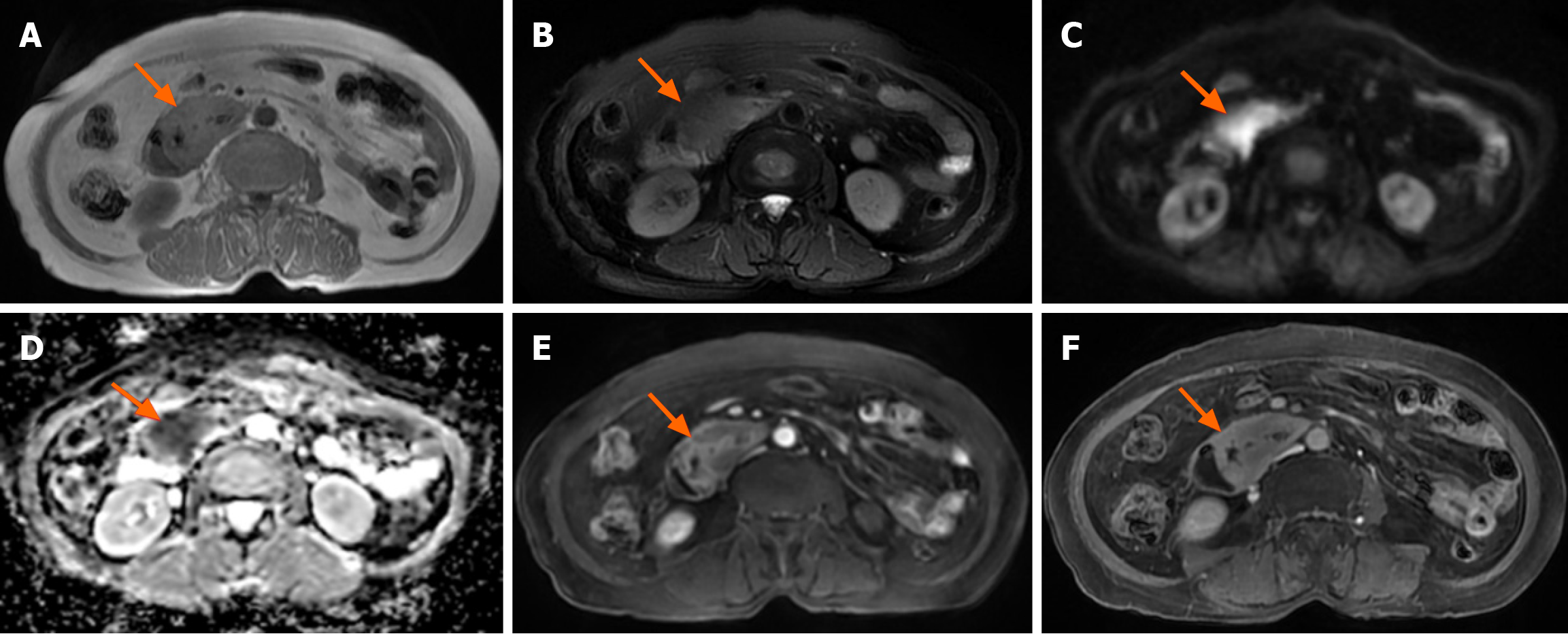
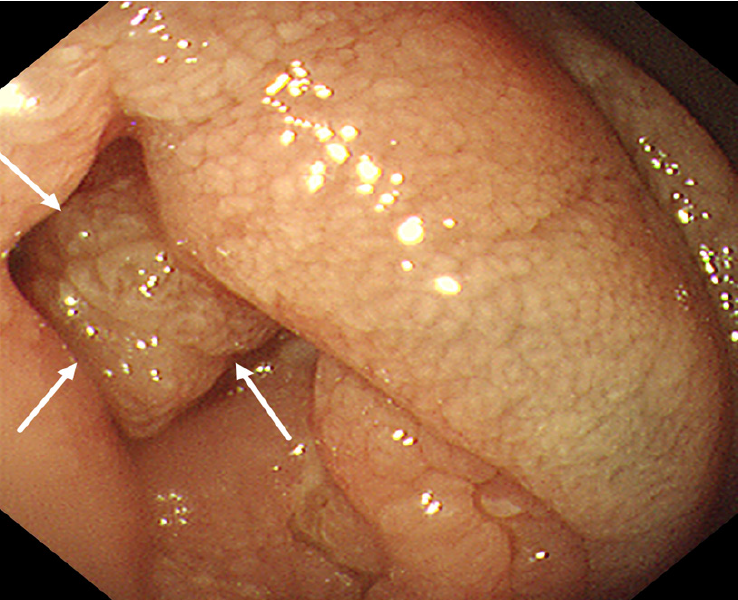
18F-FDG PET/CT revealed multiple segmental thickening in the descending and horizontal segments of the duodenum, jejunum, and ileum. The horizontal segment of the duodenum had the largest lesion, approximately 5 cm × 4 cm in size. The intestinal tract showed aneurysmal dilatation with stenosis and obstruction of the intestinal lumen, and the maximal standardized uptake value (SUVmax) was 9.2 (Figure 1). The SUVmax reflects the uptake of 18F-FDG and the metabolic activity of the lesion. Multiple enlarged lymph nodes were found in the abdominal and pelvic mesentery. The largest lesion was 0.9 cm × 0.7 cm in size, with an SUVmax of 3.1 (Figure 1). A plain MR scan of the upper abdomen revealed that the larger lesion in the horizontal segment of the duodenum presented an iso-signal on T1 weighted imaging (T1WI) and an iso- or hypo-signal on T2WI. It was hyperintense on diffusion-weighted imaging, and the apparent diffusion coefficient was 0.8 × 10-3 mm2/second. Post-contrast MR imaging (MRI) demonstrated delayed and homogeneous enhancement (Figure 2). Gastroduodenoscopy revealed cauliflower-like masses in the duodenal lumen, marked narrowing and obstruction of the intestinal lumen (Figure 3).
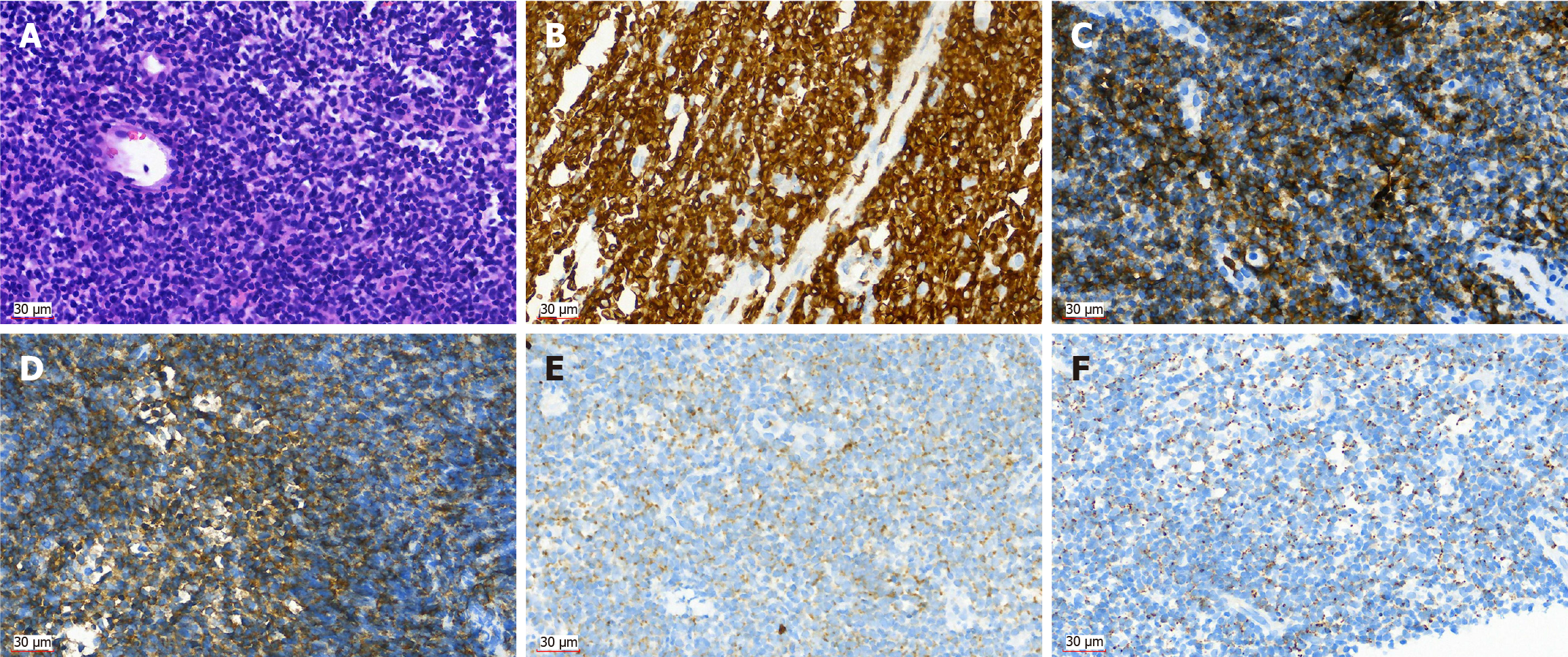
An endoscopic biopsy of the lesion located in the descending segment of the duodenum suggested a T-lymphoproliferative lesion. Microscopically, the atypical lymphoid cells were diffusely proliferated, with round or irregular nuclei, thick chromatin, and scattered plasma cells. The immunohistochemical marker results were as follows: CD2 (+), CD3 (+), CD5 (+), CD7 (+), CD8 (+), CD56 (+), CD4 (-), CD20 (-), cytokeratin (-), T-cell intracellular antigen 1 (+), granzyme B (+), Epstein-Barr virus-encoded small RNA (-), and Ki-67 (60%+) (Figure 4). Gene rearrangement suggested that T cell receptor β and T cell receptor γ were positive. Bone marrow aspiration biopsy did not reveal evidence of neoplastic involvement.
The final pathology diagnosis revealed MEITL (Figure 4). 18F-FDG PET/CT revealed retroperitoneal and mesenteric lymph node metastasis. According to the Lugano staging criteria, version 2014, the stage was II.
Given the patient's advanced age, we used a reduced-dose cyclophosphamide, vinorelbine, and prednisone (CVP) chemotherapy regimen: Cyclophosphamide 500 mg/m2 (day 1), vinorelbine 20 mg/m2 (day 1), and prednisone 60 mg/m2 (day 1-day 5). The cycle of treatment lasted for 21 days.
After the first course of chemotherapy, the patient’s clinical symptoms were significantly relieved. After completing three cycles of chemotherapy, the patient underwent a follow-up 18F-FDG PET/CT scan, revealing a decrease in multiple lesions in the small intestine as well as a reduction in the enlarged mesenteric lymph nodes (Figure 1). The efficacy was evaluated as a partial response. The patient was still alive after 6 months of follow-up.
According to the 2022 World Health Organization classification of hematologic malignancies, MEITL is a rare subtype of intestinal T-cell proliferative lymphoma formerly known as type II ETAL[2]. To our knowledge, approximately 300 cases have been reported worldwide to date. MEITL is caused by the malignant proliferation of intraepithelial lymphocytes, with an average age of approximately 60 years at first diagnosis and a male to female ratio of approximately 2:1[1,7]. The most common site of MEITL is the small intestine[7]. The early clinical symptoms of MEITL are insidious and can manifest as nonspecific intestinal symptoms, such as abdominal pain and bowel dilatation[1]. Therefore, most MEITLs are found at an advanced stage with an extremely poor prognosis: the median survival time of MEITLs is only 7 months, and the 1-year overall survival rate is only 36%[4].
Currently, there are few imaging reports on MEITL. The previously reported cases are shown in Table 1. According to previous reports, MEITL can involve the whole intestine, with increased FDG uptake in the lesion and an SUV ranged 3.3 to 10.2[8]. Our patient also presented with multiple lesions in the small intestine, with a SUVmax of 9.2, which is consistent with previous reports in the literature. Intestinal lymphoma typically does not involve organ metastasis. However, in rare instances, MEITL may metastasize to the lung, liver, central nervous system and bone marrow[8-11]. MR examination shows iso- or hypo-signals on T2WI and limited diffusion on diffusion-weighted imaging, which is attributable to the abundance of tumor cells and the diminished concentration of extracellular fluid. On an enhanced scan, MEITL exhibits delayed and homogeneous enhancement, which is similar to the performance in a previous report[8]. These findings suggest that MEITL is generally characterized by the absence of necrosis. A search of the PubMed database found five case reports of MEITL that presented with MRI and/or 18F-FDG PET/CT findings (Table 1)[8-10,12,13].
| Ref. | Age/sex | Symptom | Location | Imaging findings | Treatment | Outcome, month |
| [8] | 48/F | Abdominal pain, diarrhea, distension, dysuria, frequency, and urgency | Entire small bowel, part of the colon, and rectum. Infiltration of the spleen, skeleton, liver and some mesenteric lymph nodes | Iso or hypo-intensity on T2WI, reduced diffusion on DWI, low signal intensity on ADC map, and markedly homogeneous enhancement. Increased FDG-uptake (SUVmax 4.0-10.2). The spleen, skeleton, liver and some swollen mesenteric lymph nodes were also FDG-avid | Only received anti-infection, nutrition support, and hormone therapy | 3 (dead) |
| [9] | 74/M | Acute onset of left hemiparesis and diarrhea | The small intestinal of the pelvic cavity. Involving the lung and brain | PET-MRI revealed high 18F-FDG accumulation in a mass in the pelvic cavity (SUVmax of 12.9), the right cerebral hemisphere with SUVmax of 8.3, bilateral lungs (SUVmax of 11), and mediastinal lymph nodes (SUVmax of 8.6) | Transferred to outpatient clinic for palliative care | Absent |
| [10] | 57/M | Bloody diarrhea, abdominal pain, and urological symptoms, including dysuria, frequency and urgency | The ileocecum, entire colon, and rectum | Increased FDG activity (SUVmax of 14.5). Multiple pulmonary nodules and ground-glass opacities in both lungs with intense FDG uptake (SUVmax of 14.8) and abnormal hypermetabolism in the prostate (SUVmax of 5.6) | Chemotherapy | 1.5 (dead) |
| [12] | 56 (ranged 39-70)/8M, 4F | Abdominal pain, abdominal distension, ascites, intestinal obstruction, diarrhea, intestinal perforation, weight loss and gastrointestinal bleeding | The stomach, small bowel and large bowel. Lymph nodes and other organs were involved in 58% of cases. Thoracic and brain were frequently involved | The SUVmax of the stomach, small bowel and large bowel lesions varied from 3.6 to 8.7. The SUVmax of regional lymph nodes varying from 0.9 to 5.3 | Chemotherapy | The median survival was 13 (1-136) months |
| [13] | 61/M; 35/F | Upper abdominal pain and intermittent black stool; abdominal distention | Small intestine and upper sigmoid colon | Increased metabolism in 18F-FDG PET/CT. Regional lymph nodes were also metabolically active | CHOP combined with chidamide; surgery combined with ifosfamide, etoposide, and vincristine and chidamide | 15 (dead); 17 (alive) |
MEITL should be differentiated from Crohn’s disease (CD), intestinal B-cell lymphoma and intestinal carcinoma in imaging diagnosis. The present patient presented with multiple segmental thickening of the small intestine, similar to the imaging findings of “skip distribution” in patients with CD. Although CD also manifests as intestinal wall thickening and FDG uptake, there is no obvious intestinal dilatation. Epelboym et al[14] reported that the average SUV ratio (SUVmax/liver SUVmean) in eight patients with CD before treatment was 3.16 (range 1.88-4.6). The SUV ratio in our patient was 5.4, which was greater than that in patients with CD. On contrast-enhanced CT, CD may present enlargement and thickening of the mesenteric vessels. Moreover, the onset of CD is gradual, and the course of the disease is relatively long, CD can be complicated by abscesses and fistula formation and can involve extraintestinal organs, such as the mouth, eyes and joints[15]. The characteristic finding of CD under endoscopy is cobblestone-like ulceration[15]. Most intestinal B-cell lymphomas are localized lesions, and the SUVmax (mean ± SD) of B-cell lymphoma (14.1 ± 6.4) is significantly greater than that of T-cell lymphoma (7.6 ± 3.9)[16], which is helpful for their differentiation. The focus of intestinal carcinoma is relatively limited, causing focal intestinal concentric stenosis or asymmetric intestinal stenosis. Intestinal carcinoma rarely involves multiple segments; however, it is prone to invade the surrounding fat space and adjacent organs and hematogenously metastasize to the liver and lungs. MEITL is more likely to manifest as aneurysmal dilatation at multiple sites and generally does not result in fat space infiltration around the intestine.
The imaging differential diagnosis of MEITL from ETAL is difficult because of their rarity and limited reference data. On PET/CT, EATL typically presents as hypermetabolic lesions (SUVmax of 6.4-8.0), combined with a background of diffuse low FDG uptake (SUVmax of 2.2-4.6) related to celiac disease[17]. However, MEITL lesions present as discrete hypermetabolic lesions without a background of low FDG uptake, as they are not associated with celiac disease[12]. The final diagnosis depends on pathological examination. There is an absence of standard treatment guidelines for MEITL because of its rarity. In previous reports, cyclophosphamide, doxorubicin, vincristine, and prednisone was the most commonly used chemotherapy[18]. For elderly EMITL patients, our reduced-dose CVP chemotherapy regimen also appeared to be an appropriate treatment, and the patient achieved a partial response after treatment. However, further follow-up studies with larger sample sizes are needed.
This article comprehensively describes the imaging findings of MEITL. MRI serves as a valuable tool in detecting malignant MEITL lesions, whereas 18F-FDG PET/CT offers additional assistance in tumor staging and assessing treatment efficacy. For elderly EMITL patients, a reduced dose of the CVP regimen is an alternative therapeutic approach. Given the rarity of MEITL, no standard treatment method for MEITL exists, highlighting the need to establish standardized treatment protocols in future studies.
| 1. | Chen C, Gong Y, Yang Y, Xia Q, Rao Q, Shao Y, Zhu L, Zhang J, Li X, Ji P, Zhai B, Zhang X, Zhang Z. Clinicopathological and molecular genomic features of monomorphic epitheliotropic intestinal T-cell lymphoma in the Chinese population: a study of 20 cases. Diagn Pathol. 2021;16:114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 2. | Ferry JA, Hill B, Hsi ED. Mature B, T and NK-cell, plasma cell and histiocytic/dendritic cell neoplasms: classification according to the World Health Organization and International Consensus Classification. J Hematol Oncol. 2024;17:51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 3. | Lucioni M, Fraticelli S, Santacroce G, Bonometti A, Aronico N, Sciarra R, Lenti MV, Bianchi PI, Neri G, Feltri M, Neri B, Ferrario G, Riboni R, Corazza GR, Vanoli A, Arcaini L, Paulli M, Di Sabatino A. Clinical and Histopathological Features of an Italian Monocentric Series of Primary Small Bowel T-Cell Lymphomas. Cancers (Basel). 2023;15:2743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 4. | van Vliet C, Spagnolo DV. T- and NK-cell lymphoproliferative disorders of the gastrointestinal tract: review and update. Pathology. 2020;52:128-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 5. | Sonkin D, Thomas A, Teicher BA. Cancer treatments: Past, present, and future. Cancer Genet. 2024;286-287:18-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 197] [Reference Citation Analysis (0)] |
| 6. | Zhang MC, Zhou M, Song Q, Wang S, Shi Q, Wang L, Yan FH, Qu JM, Zhao WL. Clinical features and outcomes of pulmonary lymphoma: A single center experience of 180 cases. Lung Cancer. 2019;132:39-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Mutzbauer G, Maurus K, Buszello C, Pischimarov J, Roth S, Rosenwald A, Chott A, Geissinger E. SYK expression in monomorphic epitheliotropic intestinal T-cell lymphoma. Mod Pathol. 2018;31:505-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 8. | Zhang X, Liu S, Liu Y, Lv Z, Yang J. 18F-Fluorodeoxyglucose PET/CT and MRI Imaging Characteristics of Monomorphic Epitheliotropic Intestinal T-cell Lymphoma: A case report. Curr Med Imaging. 2023. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 9. | Suzuki Y, Minemura H, Tomita H, Saito M, Watanabe N, Umeda T, Kawamata T, Rikimaru M, Morimoto J, Koizumi T, Togawa R, Sato Y, Hirai K, Uematsu M, Nikaido T, Fukuhara N, Fukuhara A, Sato S, Saito J, Kanazawa K, Tanino Y, Shibata Y. Monomorphic Epitheliotropic Intestinal T-cell Lymphoma Involving the Lung and Brain: A Rare Case Study. Intern Med. 2020;59:2559-2563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Li C, He Y. (18)F-FDG PET/CT findings in a rare monomorphic epitheliotropic intestinal T cell lymphoma. Eur J Nucl Med Mol Imaging. 2021;48:649-650. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 11. | Ikeda C, Maruyama D, Oka H, Fujino T, Maeshima AM, Matsushita H. Bone marrow involvement by monomorphic epitheliotropic intestinal T-cell lymphoma. Br J Haematol. 2019;187:10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Chan TSY, Lee E, Khong PL, Tse EWC, Kwong YL. Positron emission tomography computed tomography features of monomorphic epitheliotropic intestinal T-cell lymphoma. Hematology. 2018;23:10-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Liu TZ, Zheng YJ, Zhang ZW, Li SS, Chen JT, Peng AH, Huang RW. Chidamide based combination regimen for treatment of monomorphic epitheliotropic intestinal T cell lymphoma following radical operation: Two case reports. World J Clin Cases. 2020;8:1278-1286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (2)] |
| 14. | Epelboym Y, Shyn PB, Chick JFB, Hamilton MJ, OʼConnor SD, Silverman SG, Kim CK. Crohn Disease: FDG PET/CT Before and After Initial Dose of Anti-Tumor Necrosis Factor Therapy to Predict Long-term Response. Clin Nucl Med. 2017;42:837-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Hisabe T, Hirai F, Matsui T, Watanabe M. Evaluation of diagnostic criteria for Crohn's disease in Japan. J Gastroenterol. 2014;49:93-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Chan WK, Au WY, Wong CY, Liang R, Leung AY, Kwong YL, Khong PL. Metabolic activity measured by F-18 FDG PET in natural killer-cell lymphoma compared to aggressive B- and T-cell lymphomas. Clin Nucl Med. 2010;35:571-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 17. | Hoffmann M, Vogelsang H, Kletter K, Zettinig G, Chott A, Raderer M. 18F-fluoro-deoxy-glucose positron emission tomography (18F-FDG-PET) for assessment of enteropathy-type T cell lymphoma. Gut. 2003;52:347-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 66] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Bissessur AS, Zhou JC, Xu L, Li ZQ, Ju SW, Jia YL, Wang LB. Surgical management of monomorphic epitheliotropic intestinal T-cell lymphoma followed by chemotherapy and stem-cell transplant: A case report and review of the literature. World J Gastrointest Oncol. 2022;14:2273-2287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |