Published online May 15, 2025. doi: 10.4251/wjgo.v17.i5.103594
Revised: January 22, 2025
Accepted: March 14, 2025
Published online: May 15, 2025
Processing time: 172 Days and 7.7 Hours
ANAPC1, a key regulator of the ubiquitination in tumour development, has not been thoroughly studied in hepatocellular carcinoma (HCC).
To elucidate the expression of ANAPC1 in HCC and its potential regulatory mechanism related to ubiquitination.
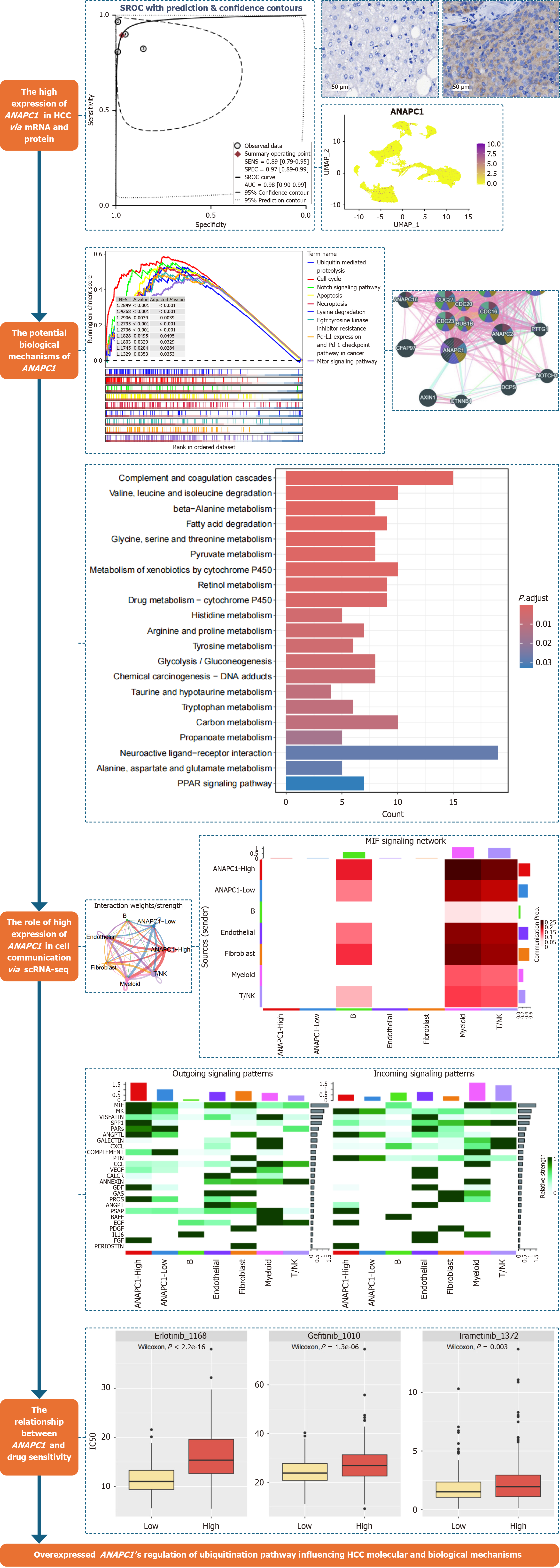
Bulk RNA (RNA sequencing and microarrays), immunohistochemistry (IHC) tissues, and single-cell RNA sequencing (scRNA-seq) data were integrated to comprehensively investigate ANAPC1 expression in HCC. Clustered regularly interspaced short palindromic repeats analysis was performed to assess growth in HCC cell lines following ANAPC1 knockout. Enrichment analyses were conducted to explore the functions of ANAPC1. ScRNA-seq data was used to examine the cell cycle and metabolic levels. CellChat analysis was applied to investigate the interactions between ANAPC1 and different cell types. The relationship between ANAPC1 expre
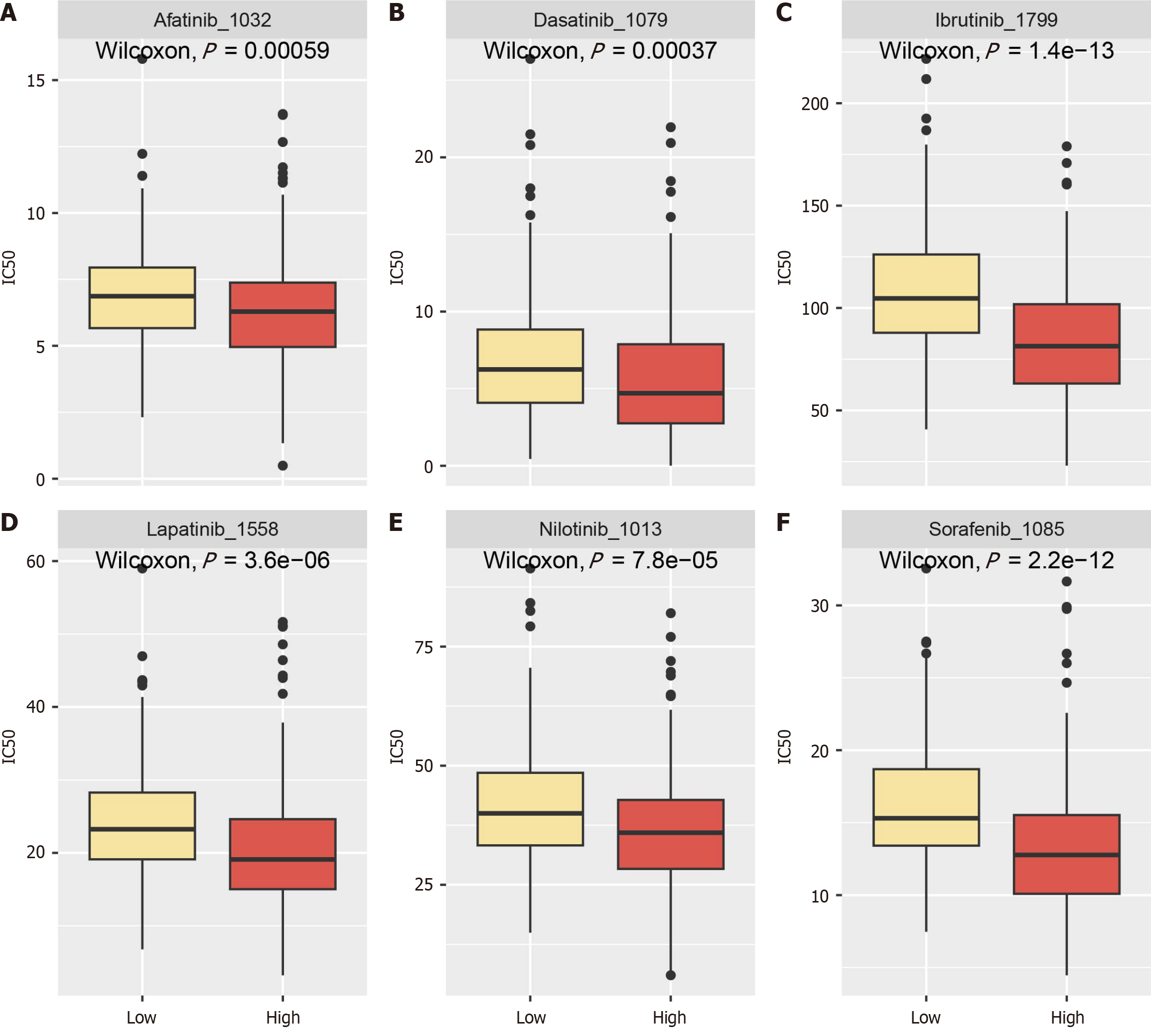
ANAPC1 messenger RNA was found to be upregulated in bulk RNA, IHC tissues samples and malignant hepatocytes. The proliferation of JHH2 cell lines was most significantly inhibited after ANAPC1 knockdown. In biological pathways, the development of HCC was found to be linked to the regulation of ubiquitin-mediated proteolysis. Additionally, scRNA-seq results indicated that highly expressed ANAPC1 was in the G2/M phase, with increased glycolysis/gluconeogenesis activity. A CellChat analysis showed that ANAPC1 was associated with the regulation of the migration inhibitory factor-(cluster of differentiation 74 + C-X-C chemokine receptor type 4) pathway. Higher ANAPC1 expression correlated with stronger effects of sorafenib, dasatinib, ibrutinib, lapatinib, nilotinib and afatinib.
The high expression level of ANAPC1 may regulate the cell cycle and metabolic levels of HCC through the ubiqui
Core Tip: ANAPC1, a key ubiquitination regulator in tumours, remains unstudied in hepatocellular carcinoma (HCC). This first multicenter study on ANAPC1 in HCC shows its high expression in bulk RNA (n = 3913, including RNA sequencing and microarray), immunohistochemistry (n = 632) and single-cell RNA sequencing (15999 malignant hepatocytes). Clustered regularly interspaced short palindromic repeats knockout of ANAPC1 inhibited HCC cell proliferation. Gene set enrichment analysis, gene ontology and Kyoto encyclopedia of genes and genomes analyses revealed the role of the high expression of ANAPC1 in ubiquitin-mediated protein degradation, which may affect the G2/M phase and glycolysis, regulate migration inhibitory factor signalling and enhance HCC sensitivity to sorafenib, dasatinib, ibrutinib, lapatinib, nilotinib and afatinib.
- Citation: Tang YX, Wu WZ, Zhou SS, Zeng DT, Zheng GC, He RQ, Qin DY, Huang WY, Chen JT, Dang YW, Tang YL, Chi BT, Zhan YT, Chen G. Exploring the potential function of high expression of ANAPC1 in regulating ubiquitination in hepatocellular carcinoma. World J Gastrointest Oncol 2025; 17(5): 103594
- URL: https://www.wjgnet.com/1948-5204/full/v17/i5/103594.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i5.103594
Liver cancer, a malignant tumour that significantly threatens human health globally, has garnered considerable attention in medical research due to its increasing incidence and mortality rates. Influenced by various factors, the number of liver cancer cases in China reached 367700 in 2022, making it the fourth most common cancer in the country[1,2]. Hepatocellular carcinoma (HCC), the most common subtype of liver cancer, accounts for 75% to 85% of cases and poses a severe challenge to public health[3]. The prognosis for HCC remains unfavorable, primarily due to the fact that most patients are diagnosed at advanced stages, and the available treatment options are limited[4-6]. Therefore, an in-depth study of biomarkers and molecular mechanisms associated with the occurrence and progression of HCC is crucial to achieve early and accurate diagnosis, optimize treatment plans and improve treatment outcomes.
The APC1 protein, encoded by ANAPC1, is the largest subunit of the E3 ubiquitin ligase anaphase-promoting complex/cyclosome (APC/C) and plays a significant role in cell cycle regulation and metabolic pathway control[7-11]. Studies have shown that ANAPC1 is widely expressed in various cancer cells[12]. High expression levels of ANAPC1 have been found to be closely related to poor prognosis in T-cell lymphoma and T-cell acute lymphoblastic leukemia[9]. A high copy number of ANAPC1 was found to be related to poor prognosis in the pan-kidney cohort[13]. In addition, in a difference analysis between the cisplatin-sensitive bladder cancer (BC) cell line T24 and the cisplatin-resistant BC cell line T24R2, the high expression level of ANAPC1 was identified as a gene that affects the therapeutic effect[14]. However, the specific role of ANAPC1 in HCC remains unexplored, providing ample research opportunities to investigate its potential value in HCC.
This research integrates bulk RNA [including RNA sequencing (RNA-seq) and microarray], immunohistochemistry (IHC) and single-cell RNA sequencing (scRNA-seq) to analyze the expression levels of ANAPC1 at the messenger RNA (mRNA) and protein levels. Using clustered regularly interspaced short palindromic repeats (CRISPR), the research explores the relationship between ANAPC1 expression and cell proliferation and investigates potential signalling pathways through which ANAPC1 regulates HCC. This includes ANAPC1’s function in cell cycle stage regulation, metabolic pathway modulation and cell communication analysis in high ANAPC1 expression scenarios. Finally, the study analyses the relationship between ANAPC1 expression and drug sensitivity.
The HCC bulk RNA datasets (including RNA-seq and microarray datasets) used in this study were sourced from multiple public databases, including The Cancer Genome Atlas (TCGA), the Gene Expression Omnibus, the Genotype-Tissue Expression, the Array Express, and the Sequence Read Archive platforms. For inclusion in the study, each dataset was required to contain human samples, ANAPC1 expression data, at least three samples in both HCC and non-cancer control groups, and samples not subjected to any drug treatment or similar interventions. Datasets that contained duplicate samples or non-transcriptomic data or that lacked expression matrix data were excluded from the study. Due to batch effects across different platforms, we employed the sva and limma packages in R. Specifically, we utilized the “combat” function to merge data within the same platform and applied the “normalizebetweenarrays” function for data normalization. Finally, we visualized the results using boxplots to assess the effectiveness of the batch effect correction. In this way, we effectively eliminated the batch effects and consolidated expression data matrices from the same platform[15,16]. Detailed screening information is provided in Supplementary Figure 1.
We collected 953 internal tissue samples (473 HCC samples and 480 non-cancer control samples) from the First Affiliated Hospital of Guangxi Medical University, Yulin Red Cross Hospital, and Lingshan Hospital. A standardized protocol was used to perform IHC staining. First, the samples were fixed with formalin, embedded in paraffin, and dewaxed with ethylenediaminetetraacetic acid buffer. Endogenous peroxidase activity was then blocked. An invitrogen rabbit anti-ANAPC1 polyclonal antibody (Thermo Fisher Scientific, Catalog No. PA5-83639; 1:500) was applied (stored at 4 °C). Throughout the experiment, the slides were washed with phosphate-buffered saline to ensure cleanliness and accuracy. Following staining, the tissue microarrays were incubated, stained, dehydrated, sealed, and stored at room temperature. The samples were scored according to the colour intensity (no staining: 0, light yellow: 1, tan yellow: 2, and brown-black: 3) and the percentage of positive cells (≤ 5%: 0, 6%-25%: 1, 26%-50%: 2, 51%-75%: 3, and ≥ 76%: 4)[17]. The overall IHC score (the product of the color intensity and percentage of positive cells scores) was independently determined by two experienced pathologists. The ethics committees of the First Affiliated Hospital of Guangxi Medical University (No. 2022-KT-GuiWei-074), Lingshan County People’s Hospital (No. Z2090523), and the Red Cross Hospital of Yulin City (No. 2024-8) approved the study. All participants provided informed consent. Reference was made to proteomic data provided by the Proteomic Data Commons to enrich the study’s content.
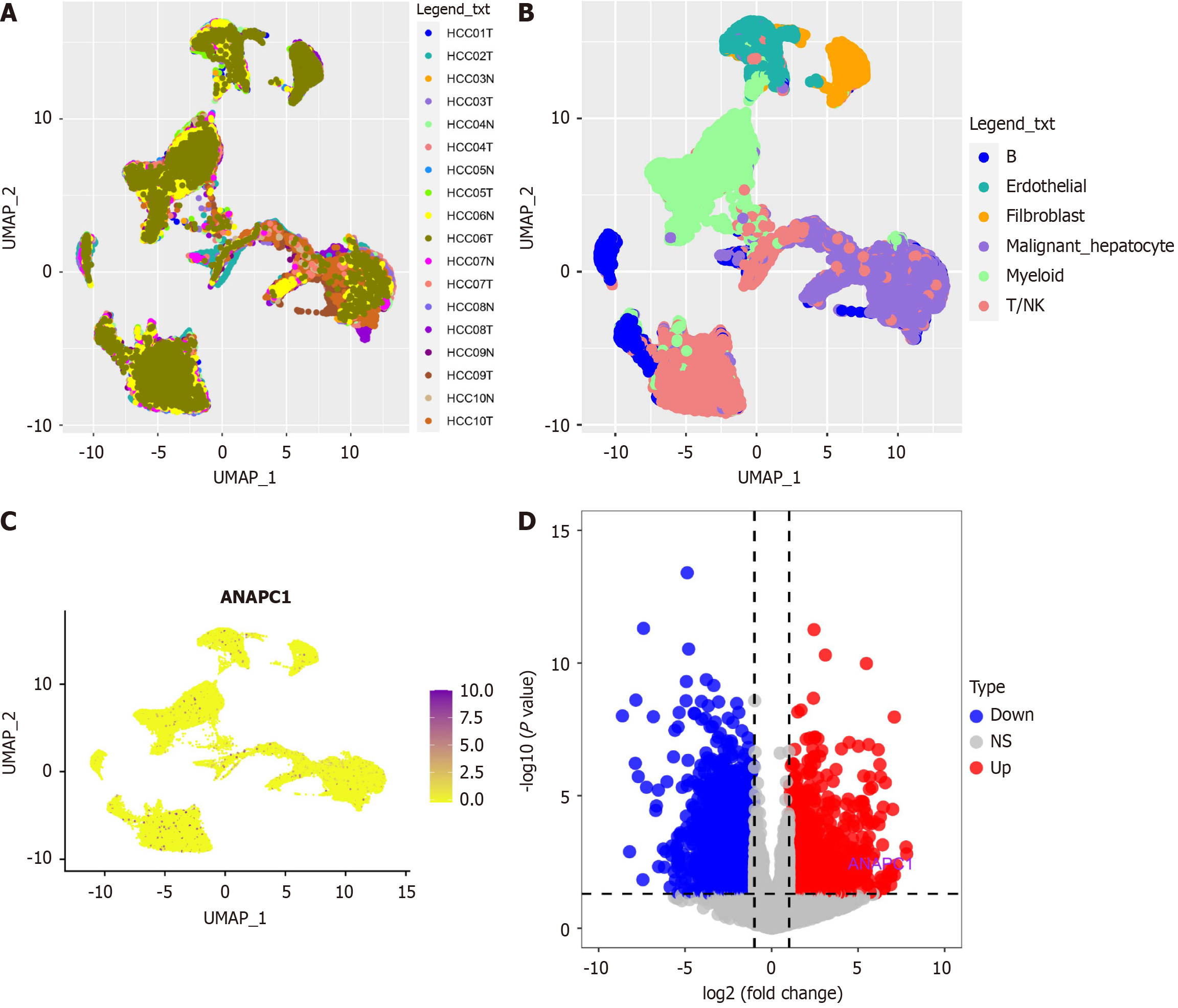
To investigate the role that ANAPC1 plays in malignant liver cells, cells that met the criteria of 200 < nFeature_RNA < 8000 and percent mt < 10 were selected from the GSE149614 dataset. Dimensionality reduction and clustering at 0.4 resolution were performed using the uniform manifold approximation and projection (UMAP) algorithm. Malignant liver cells were identified with reference to endothelial cells and fibroblasts using cell type annotation[18] and the infercnv package[19]. Endothelial cells, fibroblasts, B cells, T/natural killer (NK) cells, myeloid cells, and malignant liver cells were ultimately identified, and the expression of ANAPC1 in these cell types was determined. Finally, a pseudobulk analysis was performed to identify genes differentially expressed between the normal group and the HCC groups of malignant liver cells.
To explore the effect of ANAPC1 on cell growth, the functional effect scores associated with knocking out ANAPC1 in multiple HCC cell lines were collected using the Cancer Data Analysis Platform at the University of Alabama at Birmingham website. Additionally, the dependency scores associated with ANAPC1 in these HCC cell lines were obtained from the Cancer Cell Line Encyclopedia website[20].
To study the potential regulatory mechanisms of ANAPC1 in HCC, the protein-protein interaction network of ANAPC1 was explored using GeneMANIA (https://genemania.org/). We identified biological pathways and molecular mechanisms linked to ANAPC1 in HCC by performing a Kyoto Encyclopedia of Genes and Genomes (KEGG) gene enrichment analysis using TCGA mRNA data. We identified significantly differentially expressed genes by combining the collected mRNA expression profiles, and calculating the standardized mean difference (SMD). Additionally, we performed a Pearson correlation analysis to identify the genes that correlated with ANAPC1. The criteria were as follows: Correlation coefficient (r) ≥ 0.5 and P < 0.05 (satisfied on at least 11 platforms) and r ≤ -0.4 and P < 0.05 (satisfied on at least six platforms)[21].
Two sets of intersecting genes were analyzed: Genes that positively correlated genes with ANAPC1 and upregulated differentially expressed genes (SMD > 0, P < 0.05), and genes that negatively correlated genes with ANAPC1 and downregulated differentially expressed genes (SMD < 0, P < 0.05). The intersecting genes were subjected to enrichment analyses (i.e., gene ontology, KEGG, and reactome analyses) to elucidate the potential regulatory mechanisms of ANAPC1[22].
To investigate ANAPC1 expression during the different stages of the cell cycle, we performed a cell cycle analysis of malignant hepatocytes from the scRNA-seq GSE149614 dataset.
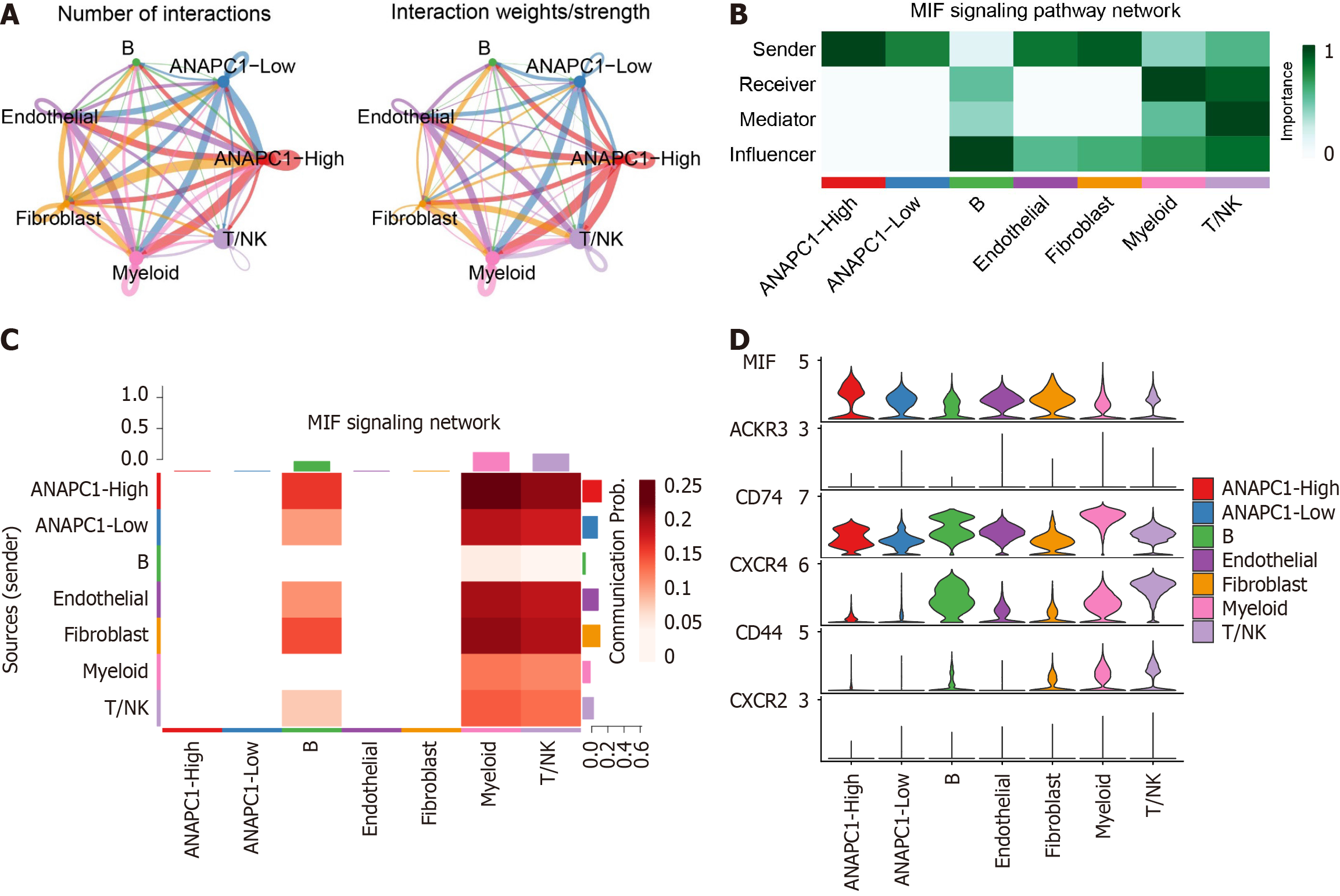
To further investigate the metabolic role that ANAPC1 plays in the regulation of metabolic activity in HCC, malignant liver cells from the GSE149614 dataset were first subclustered into two groups based on ANAPC1 expression levels (high vs low expression). The scMetabolism package was used to analyze the metabolic activity of two groups. Additionally, we analyzed the effect that ANAPC1 has on cell communication. For this, we used CellChat package to examine ligand-receptor interactions in groups of cells with high and low ANAPC1 expression across different cell types[23].
To further investigate the correlation between ANAPC1 expression and the response of HCC to tyrosine kinase inhibitors (TKIs), we analyzed ANAPC1 expression in the TCGA HCC dataset. Data from the GDSC 2.0 platform were analyzed using the oncoPredict package to predict the impact of ANAPC1 expression on the efficacy of TKIs with the aim of to elucidating its potential role in TKI therapy[24,25].
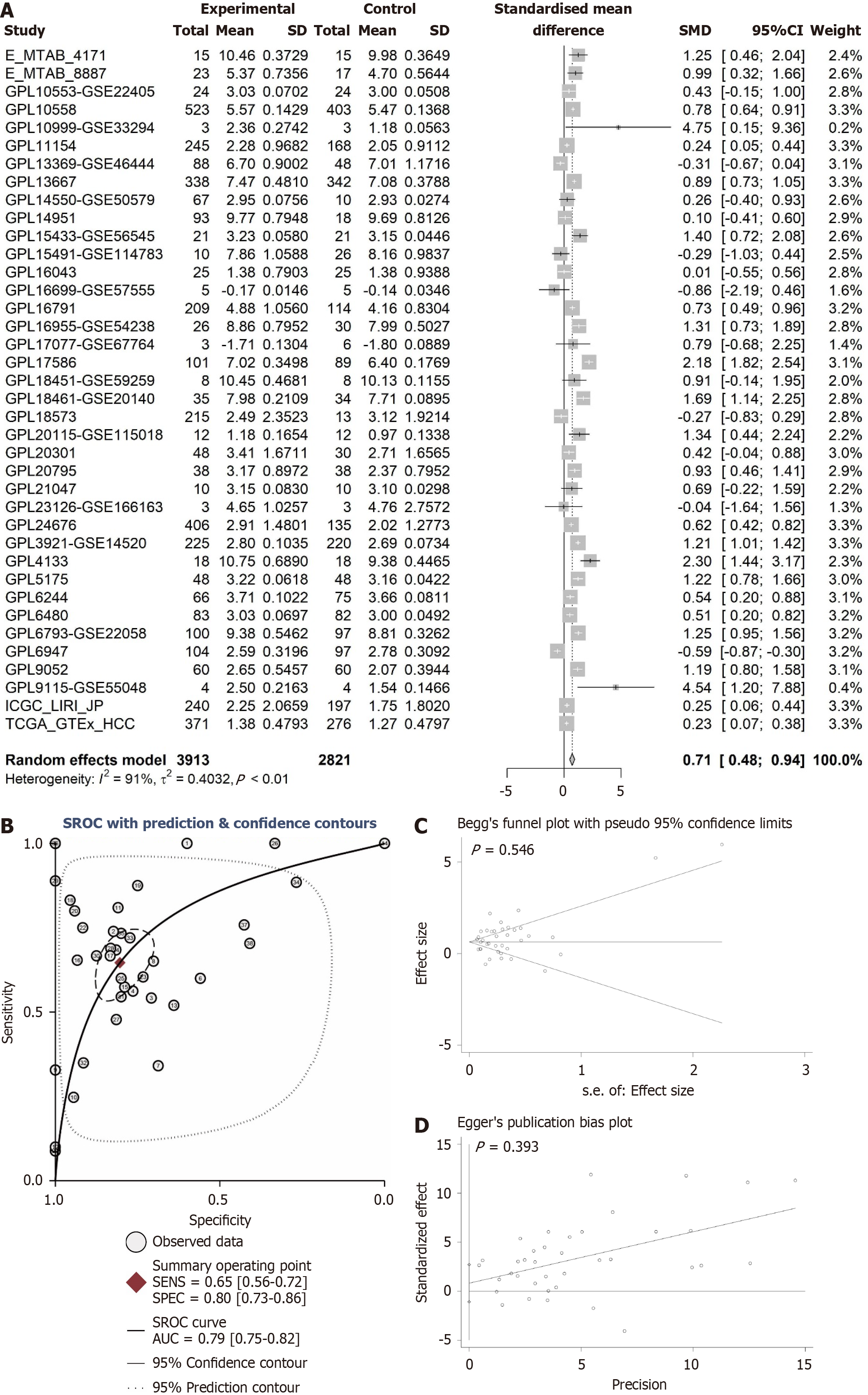
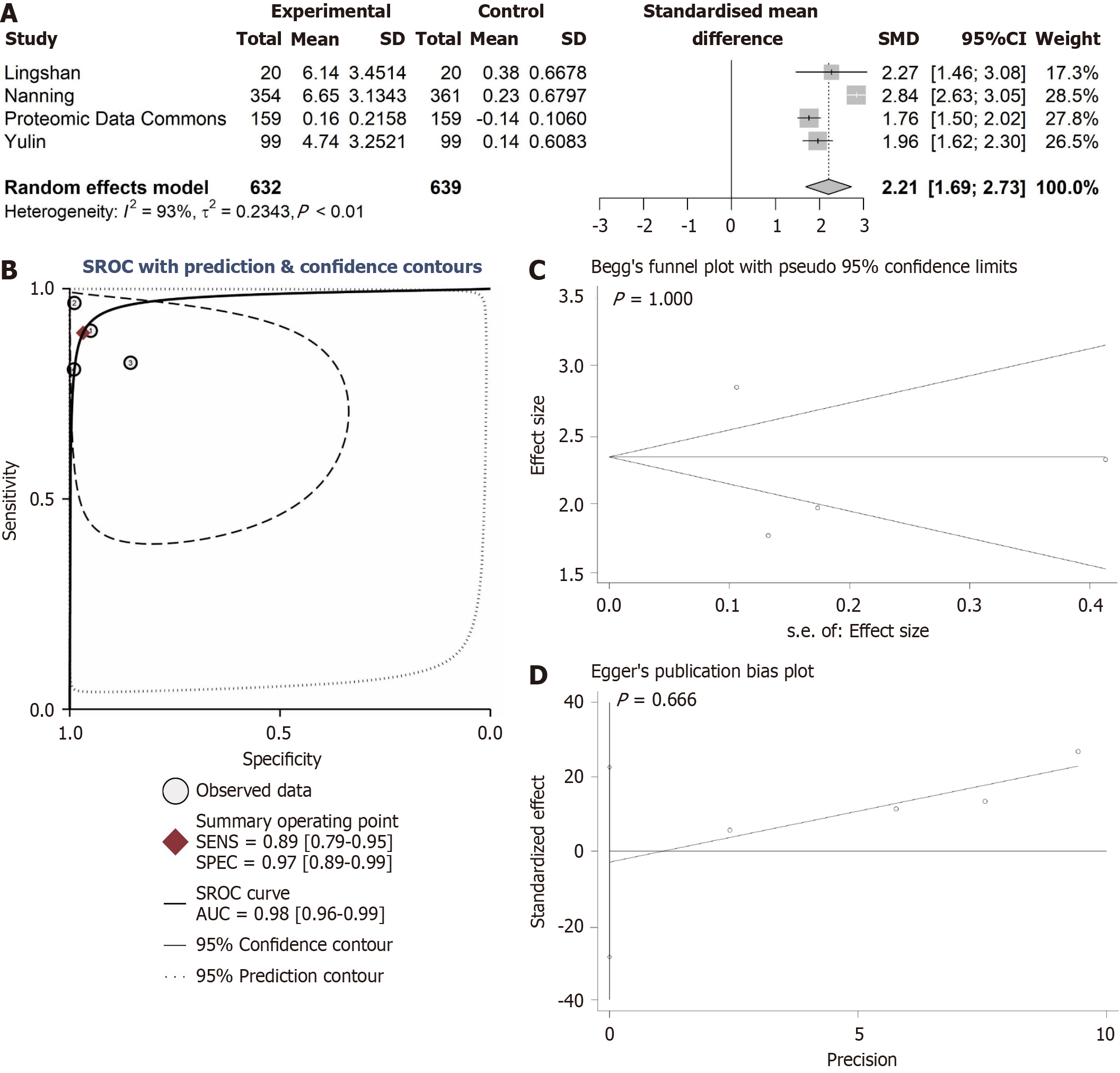
In this study, the Wilcoxon rank-sum test was employed to assess the differences in ANAPC1 mRNA and protein expression in HCC. A fixed-effects model was selected based on heterogeneity (I² < 50%); otherwise, a random-effects model was used to calculate the SMD. Receiver operating characteristic (ROC) curves were generated using the pROC package, and a summary ROC (sROC) curve was constructed using STATA 16.0 to evaluate ANAPC1 expression levels based on the area under the curve (AUC). A larger AUC reflects a more pronounced expression difference[26]. A significance level of P < 0.05, indicated a statistically significant differences, except for Begg’s and Egger’s tests. The flowchart of this study is shown in Figure 1.
The violin plots, calculated using the Wilcoxon test, illustrate the differences in ANAPC1 expression levels between the non-HCC and HCC groups, while ROC analysis further validates these expression differences. The integration of the violin plot and ROC analysis suggests a significant difference in ANAPC1 mRNA expression across 18 platforms (P < 0.05, AUC > 0.7, Supplementary Figures 2-7). The integrated analysis of 3913 HCC samples revealed high ANAPC1 mRNA expression [SMD = 0.71, 95% confidence interval (CI): 0.48-0.94, Figure 2A]. The sROC also demonstrated high ANAPC1 mRNA expression (AUC = 0.79, Figure 2B). Neither the Begg’s test nor the Egger’s test revealed significant publication bias (P > 0.05, Figure 2C and D).
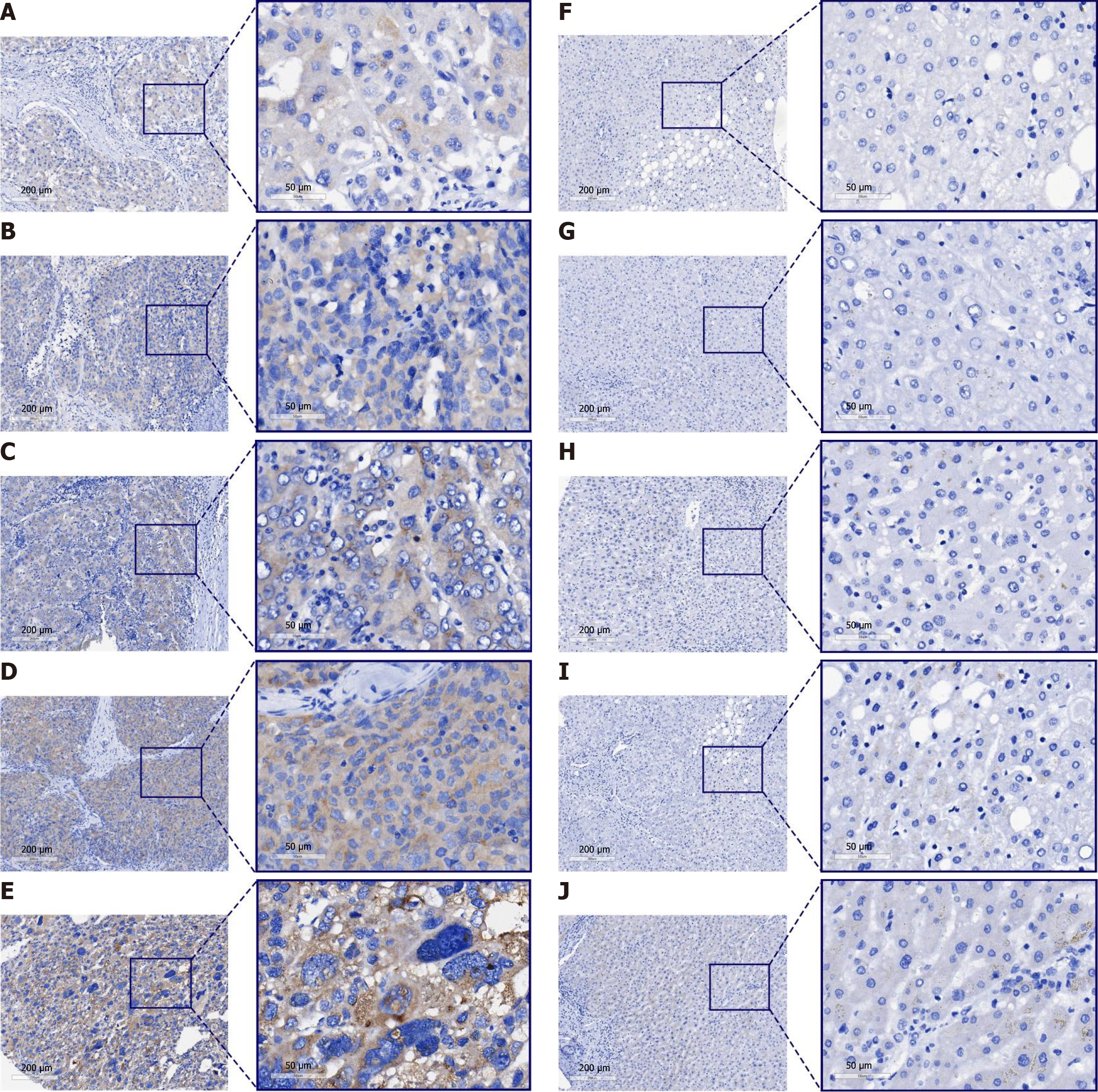
The expression of ANAPC1 protein in HCC was explored by integrating internal tissue microarrays and external proteomics. Under the microscope, ANAPC1 staining showed strong positivity in HCC samples, whereas in the non-HCC group, ANAPC1 staining showed weak positivity (Figure 3). The protein expression data across all four groups revealed a statistically significant difference in ANAPC1 protein expression between the HCC and non-HCC groups, as determined by the Wilcoxon test (P < 0.05, AUC > 0.7, Supplementary Figures 8 and 9). Compared to non-HCC tissues, the integrated expression of ANAPC1 protein in 632 HCC tissues was significantly higher (SMD = 2.21, 95%CI: 1.69-2.73, Figure 4A). The sROC demonstrated high ANAPC1 protein expression (AUC = 0.98, Figure 4B). The integrated analysis results also indicated no significant publication bias (P > 0.05, Figure 4C and D).
ANAPC1 expression was the highest in the SNU398 cell line, while the most pronounced growth inhibition upon ANAPC1 knockdown was observed in the JHH2 cell line (Supplementary Figure 10).
Based on the GSE14694 dataset, data dimensionality reduction using the UMAP algorithm revealed the distribution of 10 HCC samples and 10 non-HCC samples (Figure 5A). The infercnv analysis ultimately identified 15990 malignant hepatocytes and displayed the cell type distribution, highlighting elevated expression of ANAPC1 in malignant hepatocytes (Figure 5B and C). The volcano plot generated from the pseudobulk analysis revealed that ANAPC1 is a significantly upregulated differentially expressed gene (Figure 5D).
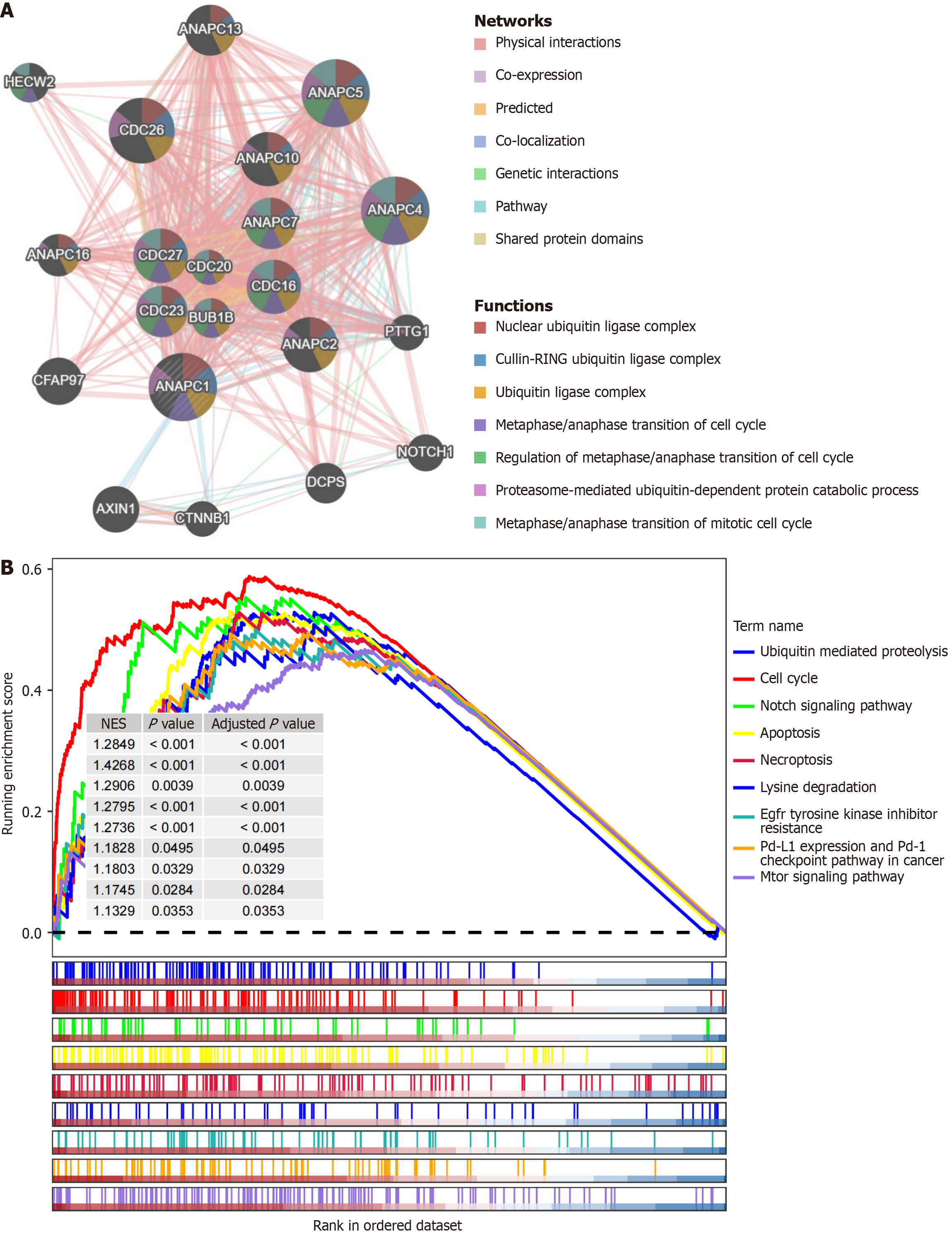
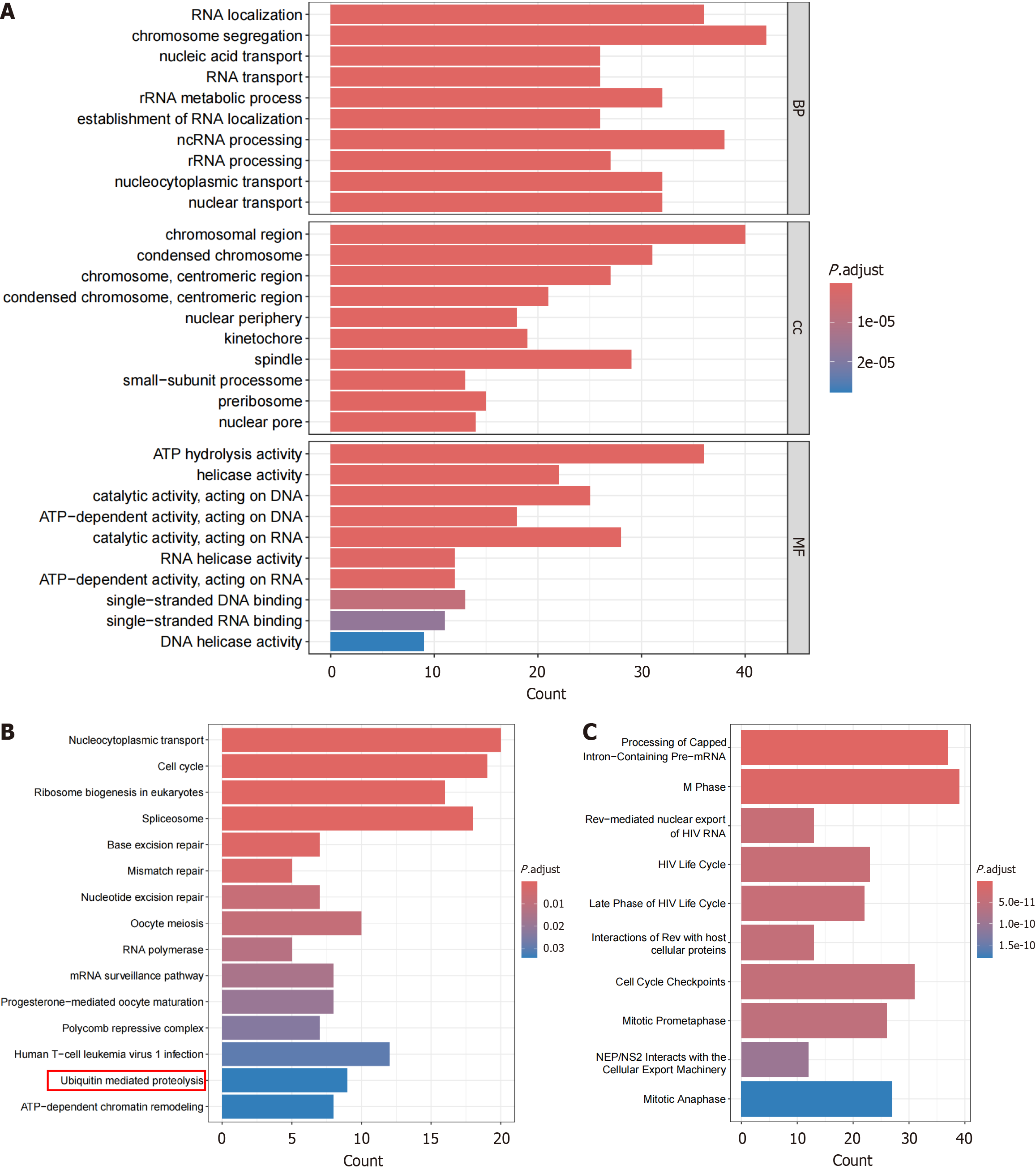
The protein-protein interaction network indicated that ANAPC1-related genes were enriched in pathways such as the cullin-RING ubiquitin ligase complex, the ubiquitin ligase complex, and the regulation of metaphase/anaphase transition of the cell cycle (Figure 6A). The gene set enrichment analysis revealed that ANAPC1 was associated with pathways such as ubiquitin-mediated proteolysis, the cell cycle, and epidermal growth factor receptor (EGFR) TKI resistance (Figure 6B).
The intersection of highly expressed differentially expressed genes calculated by SMD and ANAPC1-positively corre
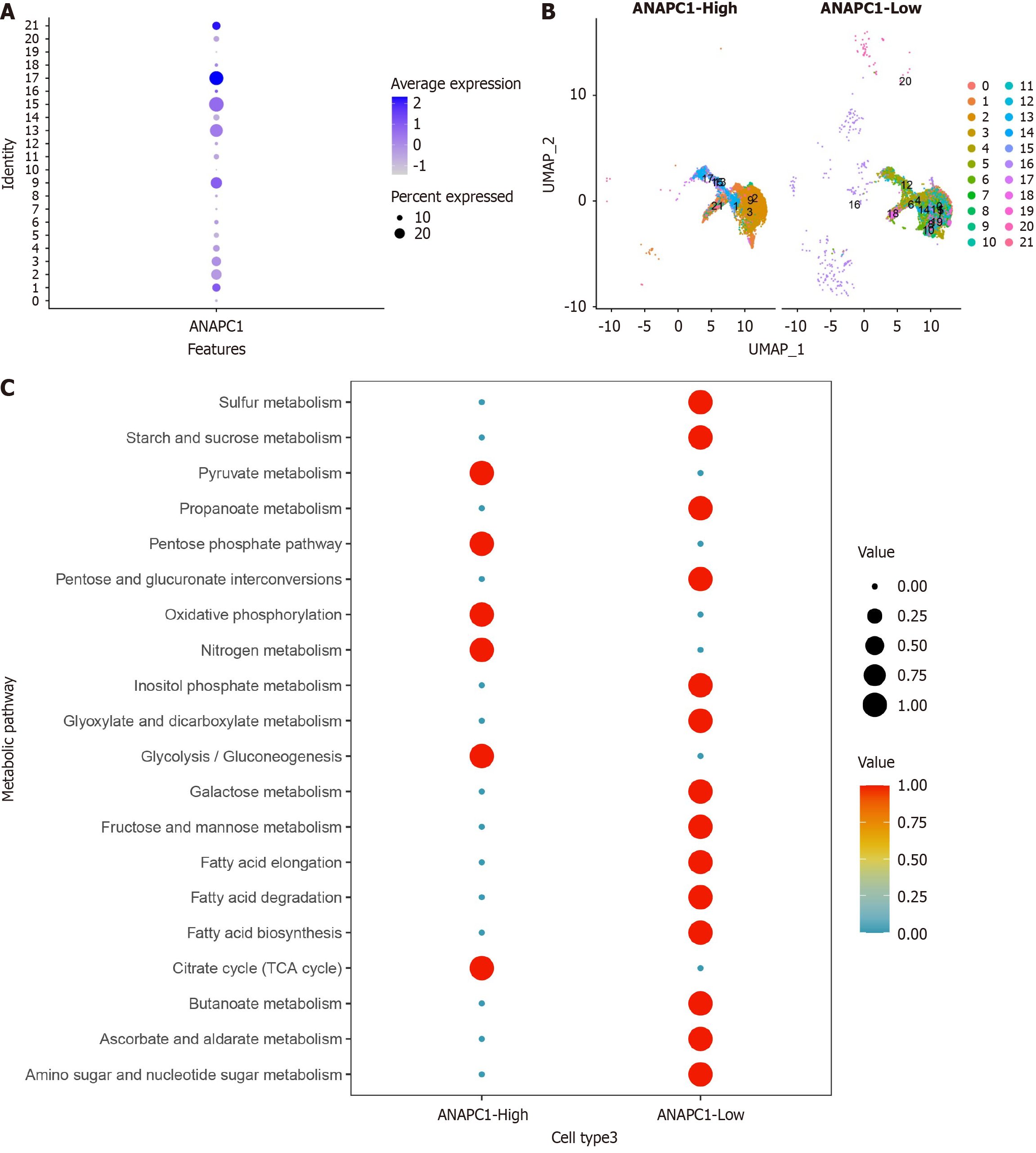
The intersection of low-expressed differential genes and ANAPC1-negative correlation genes resulted in 412 low-expressed negative correlation genes. The enrichment analysis showed that these genes were gathering in pathways such as pyruvate metabolism, glycolysis/gluconeogenesis, and tyrosine metabolism (Supplementary Figure 11). After pairwise comparison using Wilcoxon rank sum test, in malignant hepatocytes under scRNA-seq, ANAPC1 was the most highly expressed during the G2M phase of the cell cycle (Supplementary Figure 12).
After secondary clustering of malignant hepatocytes, the clusters were categorized into two groups based on ANAPC1 expression levels: High ANAPC1 expression and low ANAPC1 expression (Figure 8A and B). Metabolic analysis revealed that high ANAPC1 expression was more concentrated in metabolic pathways such as pyruvate metabolism and glyco
The strength/quantity of cell type interactions in cell communication were analyzed based on ANAPC1 expression grouping (Figure 9A). Mechanism analysis showed that the activity of the macrophage migration inhibitory factor (MIF) pathway was enhanced in the ANAPC1 high expression group, and MIF-[cluster of differentiation 74 (CD74) + C-X-C chemokine receptor type 4 (CXCR4)] may be a key ligand receptor (Supplementary Figure 13). Subsequently, we focused on studying the MIF pathway and found that the ANAPC1 high expression group played the most important interaction in the pathway (Figure 9B and C). Within the MIF pathway-associated genes, MIF exhibited elevated expression in the ANAPC1 high-expression group (Figure 9D).
Drug sensitivity analysis showed that higher ANAPC1 expression correlated with increased sensitivity of samples to sorafenib, dasatinib, ibrutinib, lapatinib, nilotinib and afatinib (Figure 10).
There are no reports in the literature on the regulatory relationship between ANAPC1 and HCC. This research was the first time data from multiple centers and levels have been combined to examine ANAPC1 mRNA and the expression of protein in HCC from the perspectives of bulk RNA, IHC and scRNA-seq. It comprehensively investigated the high ANAPC1 expression in HCC, explored its role in regulating the G2/M phase of the cell cycle and analyzed the central regulation of glycolysis-related pathways by ANAPC1. This is relevant to ANAPC1’s latent regulatory role in the ubiquitination pathway as a ubiquitin protein. Cell communication analysis revealed that the MIF signal may also have been a potential pathway through which ANAPC1 regulated ubiquitination. Furthermore, higher ANAPC1 expression correlated with stronger effects of sorafenib, dasatinib, ibrutinib, lapatinib, nilotinib and afatinib, suggesting its potential value in HCC therapy. This study confirmed for the first time the high ANAPC1 expression in HCC and further elucidated its potential regulatory role in ubiquitination in HCC, providing valuable insights into the exploration of the oncogenic role of ANAPC1 in HCC.
Abnormal expression of ANAPC1 may be associated with poor disease prognosis. ANAPC1 has been shown to be significantly under expressed in bone and muscle tissues of patients with osteoporosis, affecting both disease progression and osteogenic differentiation[8]. Additionally, ANAPC1 has been shown to be widely expressed in many types of cancer cells[12]. In T-cell lymphoma and T-cell acute lymphoblastic leukemia, higher ANAPC1 expression has been associated with worse patient prognosis[9]. In a mature azoxymethane/dextran sulfate sodium-induced colitis-associated cancer preclinical mouse model, ANAPC1, as part of DNA damage response genes, showed reduced expression, which might indicate cancer progression in colonic tissues[27]. Moreover, in the pan-kidney cohort, ANAPC1 acted as an oncogenic driver gene, with higher copy numbers suggesting poor prognosis[13]. From a therapeutic perspective, high ANAPC1 expression has been shown to be a potential cisplatin resistance gene in breast cancer[14]. Unfortunately, there have been no studies on the relationship between HCC and ANAPC1. This study is the first to comprehensively analyze the high expression levels of ANAPC1 in HCC, integrating 3913 HCC samples from 38 platforms, 15990 malignant hepatocytes, and 4 protein datasets containing 632 HCC samples. This multi-perspective and multi-center approach combines both mRNA and protein data to provide a thorough investigation of ANAPC1 in HCC. The ANAPC1 high-expression group also showed sensitivity to partial TKIs treatment, suggesting that high ANAPC1 expression may be a key factor in the progression of HCC and a potential therapeutic target. Given the association between high ANAPC1 expression and TKI sensitivity, ANAPC1 could serve as a predictive biomarker for patient stratification in clinical trials, helping identify individuals who are more likely to respond to targeted therapies. Incorporating ANAPC1 expression levels into clinical trial designs could enhance personalized treatment approaches, improving both efficacy and patient outcomes. Moreover, ANAPC1 may act as a feature gene, enabling clinicians to better identify high-risk HCC patients and tailor therapeutic strategies accordingly. This study lays the groundwork for future clinical investigations and the potential integration of ANAPC1 in personalized medicine for HCC.
ANAPC1 may have regulated the progression of HCC through the ubiquitination pathway. As the largest subunit of the ubiquitin E3 ligase APC/C, it evidently played a key role in the regulation of ubiquitination[7-11]. Ubiquitination-regulated protein turnover through the ubiquitin-proteasome system has been shown to influence cell cycle progression, cell growth, apoptosis and metabolism[11,28,29]. It has been shown that ANAPC1, as the largest subunit of the cyclosome, plays a crucial part in the assembly and functional regulation of the cyclosome in response to cell cycle arrest and stress and that it has a certain role in the regulation of ubiquitination[30]. Additionally, APC/C-Cdh1 has been shown to regulate the glycolytic pathway in astrocytes, targeting the glycolytic enzyme 6-phosphofructo-2,6-bisphosphate-3 for degradation[11].
However, no studies have analyzed the role of ANAPC1 in HCC in depth. Based on cell cycle and metabolic analysis, we found that high expression of ANAPC1 was concentrated in the G2/M phase and was associated with the occurrence of glycolysis, suggesting that the ubiquitination regulation by ANAPC1 may be key to disease progression. ANAPC1 expression, predominantly observed during the G2/M phase, suggested its potential role in promoting cell proliferation and accelerating the progression of HCC. With increased expression of ANAPC1, it might have influenced changes in ubiquitination, thereby regulating the cell cycle and glycolysis levels and promoting the progression of HCC. Further
This study has several limitations that warrant consideration. First, while we have proposed theoretical insights into the ubiquitination-related regulatory pathways of ANAPC1, these inferences require further validation through more rigorous experimental approaches, such as gene knockdown or overexpression models using CRISPR technology, as well as in vitro and in vivo drug response assays. Additionally, although we have hypothesized a regulatory relationship between ANAPC1 and CXCR4, further exploration using relevant data is necessary to substantiate this hypothesis. Furthermore, the reliability of scRNA-seq analysis is heavily influenced by the quality and depth of the data set utilized. For example, the GSE149614 dataset may not adequately represent all cell subsets associated with HCC. Therefore, it is essential to integrate additional single-cell datasets for further analysis.
This study revealed for the first time that ANAPC1 might affect the cell cycle and regulate glycolysis in HCC through the ubiquitination pathway, thereby promoting the occurrence and development of HCC.
The authors thank the Guangxi Zhuang Autonomous Region Clinical Medicine Research Center for Molecular Pathology and Intelligent Pathology Precision Diagnosis.
| 1. | Zheng RS, Chen R, Han BF, Wang SM, Li L, Sun KX, Zeng HM, Wei WW, He J. [Cancer incidence and mortality in China, 2022]. Zhonghua Zhong Liu Za Zhi. 2024;46:221-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 155] [Reference Citation Analysis (0)] |
| 2. | Xia M, Chen J, Hu Y, Qu B, Bu Q, Shen H. miR-10b-5p promotes tumor growth by regulating cell metabolism in liver cancer via targeting SLC38A2. Cancer Biol Ther. 2024;25:2315651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 3. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64684] [Article Influence: 16171.0] [Reference Citation Analysis (177)] |
| 4. | Lehrich BM, Delgado ER. Lipid Nanovesicle Platforms for Hepatocellular Carcinoma Precision Medicine Therapeutics: Progress and Perspectives. Organogenesis. 2024;20:2313696. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 5. | Rui T, Wang K, Xiang A, Guo J, Tang N, Jin X, Lin Y, Liu J, Zhang X. Serum Exosome-Derived piRNAs Could Be Promising Biomarkers for HCC Diagnosis. Int J Nanomedicine. 2023;18:1989-2001. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 6. | Wang Y, Deng B. Hepatocellular carcinoma: molecular mechanism, targeted therapy, and biomarkers. Cancer Metastasis Rev. 2023;42:629-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 169] [Article Influence: 84.5] [Reference Citation Analysis (0)] |
| 7. | Ajeawung NF, Nguyen TTM, Lu L, Kucharski TJ, Rousseau J, Molidperee S, Atienza J, Gamache I, Jin W, Plon SE, Lee BH, Teodoro JG, Wang LL, Campeau PM. Mutations in ANAPC1, Encoding a Scaffold Subunit of the Anaphase-Promoting Complex, Cause Rothmund-Thomson Syndrome Type 1. Am J Hum Genet. 2019;105:625-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 8. | Malavašič P, Polajžer S, Lovšin N. Anaphase-Promoting Complex Subunit 1 Associates with Bone Mineral Density in Human Osteoporotic Bone. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 9. | Chen C, Nie D, Huang Y, Yu X, Chen Z, Zhong M, Liu X, Wang X, Sui S, Liu Z, Tan J, Yu Z, Li Y, Zeng C. Anticancer effects of disulfiram in T-cell malignancies through NPL4-mediated ubiquitin-proteasome pathway. J Leukoc Biol. 2022;112:919-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 10. | Yan D, He Q, Pei L, Yang M, Huang L, Kong J, He W, Liu H, Xu S, Qin H, Lin T, Huang J. The APC/C E3 ligase subunit ANAPC11 mediates FOXO3 protein degradation to promote cell proliferation and lymph node metastasis in urothelial bladder cancer. Cell Death Dis. 2023;14:516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Reference Citation Analysis (0)] |
| 11. | Almeida A, Jimenez-Blasco D, Bolaños JP. Cross-talk between energy and redox metabolism in astrocyte-neuron functional cooperation. Essays Biochem. 2023;67:17-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 39] [Reference Citation Analysis (0)] |
| 12. | Jörgensen PM, Gräslund S, Betz R, Ståhl S, Larsson C, Höög C. Characterisation of the human APC1, the largest subunit of the anaphase-promoting complex. Gene. 2001;262:51-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Melloy PG. The anaphase-promoting complex: A key mitotic regulator associated with somatic mutations occurring in cancer. Genes Chromosomes Cancer. 2020;59:189-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Kim SH, Ho JN, Jin H, Lee SC, Lee SE, Hong SK, Lee JW, Lee ES, Byun SS. Upregulated expression of BCL2, MCM7, and CCNE1 indicate cisplatin-resistance in the set of two human bladder cancer cell lines: T24 cisplatin sensitive and T24R2 cisplatin resistant bladder cancer cell lines. Investig Clin Urol. 2016;57:63-72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 15. | Jiang H, Zhang X, Wu Y, Zhang B, Wei J, Li J, Huang Y, Chen L, He X. Bioinformatics identification and validation of biomarkers and infiltrating immune cells in endometriosis. Front Immunol. 2022;13:944683. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 33] [Reference Citation Analysis (0)] |
| 16. | Gao L, Xiong DD, Yang X, Li JD, He RQ, Huang ZG, Lai ZF, Liu LM, Luo JY, Du XF, Zeng JH, Li MF, Li SH, Dang YW, Chen G. The expression characteristics and clinical significance of ACP6, a potential target of nitidine chloride, in hepatocellular carcinoma. BMC Cancer. 2022;22:1244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 17. | Zhang W, Liang ZQ, He RQ, Huang ZG, Wang XM, Wei MY, Su HL, Liu ZS, Zheng YS, Huang WY, Zhang HJ, Dang YW, Li SH, Cheng JW, Chen G, He J. The upregulation and transcriptional regulatory mechanisms of Extra spindle pole bodies like 1 in bladder cancer: An immunohistochemistry and high-throughput screening Evaluation. Heliyon. 2024;10:e31192. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 18. | Lu Y, Yang A, Quan C, Pan Y, Zhang H, Li Y, Gao C, Lu H, Wang X, Cao P, Chen H, Lu S, Zhou G. A single-cell atlas of the multicellular ecosystem of primary and metastatic hepatocellular carcinoma. Nat Commun. 2022;13:4594. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 241] [Reference Citation Analysis (0)] |
| 19. | Chen K, Wang Y, Hou Y, Wang Q, Long D, Liu X, Tian X, Yang Y. Single cell RNA-seq reveals the CCL5/SDC1 receptor-ligand interaction between T cells and tumor cells in pancreatic cancer. Cancer Lett. 2022;545:215834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 62] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 20. | Chandrashekar DS, Karthikeyan SK, Korla PK, Patel H, Shovon AR, Athar M, Netto GJ, Qin ZS, Kumar S, Manne U, Creighton CJ, Varambally S. UALCAN: An update to the integrated cancer data analysis platform. Neoplasia. 2022;25:18-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 1325] [Article Influence: 441.7] [Reference Citation Analysis (0)] |
| 21. | Schober P, Boer C, Schwarte LA. Correlation Coefficients: Appropriate Use and Interpretation. Anesth Analg. 2018;126:1763-1768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1999] [Cited by in RCA: 3909] [Article Influence: 558.4] [Reference Citation Analysis (0)] |
| 22. | Dai W, Guo C, Wang Y, Li Y, Xie R, Wu J, Yao B, Xie D, He L, Li Y, Huang H, Wang Y, Liu S. Identification of hub genes and pathways in lung metastatic colorectal cancer. BMC Cancer. 2023;23:323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 23. | Zhang W, Chen XS, Wei Y, Wang XM, Chen XJ, Chi BT, Huang LQ, He RQ, Huang ZG, Li Q, Chen G, He J, Wu M. Overexpressed KCNK1 regulates potassium channels affecting molecular mechanisms and biological pathways in bladder cancer. Eur J Med Res. 2024;29:257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 24. | Gao C, Chang L, Xu T, Li X, Chen Z. AKR1C1 overexpression leads to lenvatinib resistance in hepatocellular carcinoma. J Gastrointest Oncol. 2023;14:1412-1433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 25. | Wang H, Sun P, Yao R, Zhang W, Zhou X, Yao J, He K. Comprehensive pan-cancer analysis of PTGES3 and its prognostic role in hepatocellular carcinoma. Front Oncol. 2023;13:1158490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 26. | Rong MH, Li JD, Zhong LY, Huang YZ, Chen J, Xie LY, Qin RX, He XL, Zhu ZH, Huang SN, Zhou XG. CCNB1 promotes the development of hepatocellular carcinoma by mediating DNA replication in the cell cycle. Exp Biol Med (Maywood). 2022;247:395-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 27. | Sharp SP, Malizia RA, Walrath T, D'Souza SS, Booth CJ, Kartchner BJ, Lee EC, Stain SC, O'Connor W Jr. DNA damage response genes mark the early transition from colitis to neoplasia in colitis-associated colon cancer. Gene. 2018;677:299-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 28. | De Silva ARI, Page RC. Ubiquitination detection techniques. Exp Biol Med (Maywood). 2023;248:1333-1346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 29. | Cruz Walma DA, Chen Z, Bullock AN, Yamada KM. Ubiquitin ligases: guardians of mammalian development. Nat Rev Mol Cell Biol. 2022;23:350-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 110] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 30. | Yanagida M, Yamashita YM, Tatebe H, Ishii K, Kumada K, Nakaseko Y. Control of metaphase-anaphase progression by proteolysis: cyclosome function regulated by the protein kinase A pathway, ubiquitination and localization. Philos Trans R Soc Lond B Biol Sci. 1999;354:1559-69; discussion 1569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 31. | Kim MS, Kim SH, Yang SH, Kim MS. miR-4487 Enhances Gefitinib-Mediated Ubiquitination and Autophagic Degradation of EGFR in Non-Small Cell Lung Cancer Cells by Targeting USP37. Cancer Res Treat. 2022;54:445-457. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 32. | Li K, Peng ZY, Wang R, Li X, Du N, Liu DP, Zhang J, Zhang YF, Ma L, Sun Y, Tang SC, Ren H, Yang YP, Sun X. Enhancement of TKI sensitivity in lung adenocarcinoma through m6A-dependent translational repression of Wnt signaling by circ-FBXW7. Mol Cancer. 2023;22:103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 49] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 33. | Kotb RM, Ibrahim SS, Mostafa OM, Shahin NN. Potential role of CXCR4 in trastuzumab resistance in breast cancer patients. Biochim Biophys Acta Mol Basis Dis. 2022;1868:166520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 34. | Fumagalli A, Zarca A, Neves M, Caspar B, Hill SJ, Mayor F Jr, Smit MJ, Marin P. CXCR4/ACKR3 Phosphorylation and Recruitment of Interacting Proteins: Key Mechanisms Regulating Their Functional Status. Mol Pharmacol. 2019;96:794-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 35. | Wang Z, Zhang L, Qiao A, Watson K, Zhang J, Fan GH. Activation of CXCR4 triggers ubiquitination and down-regulation of major histocompatibility complex class I (MHC-I) on epithelioid carcinoma HeLa cells. J Biol Chem. 2008;283:3951-3959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 36. | Tsunoda T, Riku M, Yamada N, Tsuchiya H, Tomita T, Suzuki M, Kizuki M, Inoko A, Ito H, Murotani K, Murakami H, Saeki Y, Kasai K. ENTREP/FAM189A2 encodes a new ITCH ubiquitin ligase activator that is downregulated in breast cancer. EMBO Rep. 2022;23:e51182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |