Published online Oct 15, 2021. doi: 10.4251/wjgo.v13.i10.1492
Peer-review started: January 28, 2021
First decision: June 4, 2021
Revised: June 16, 2021
Accepted: July 14, 2021
Article in press: July 14, 2021
Published online: October 15, 2021
Processing time: 258 Days and 9.7 Hours
Gastric cancer (GC) is one of the leading causes of cancer-related death world
To investigate the effects of Rab5a overexpression on the tumorigenesis of GC.
First, the expression levels of Rab5a and Rab4a in primary tumorous tissues of GC patients diagnosed between 2015 and 2018 were analyzed. Then we constructed HGC-27 cell lines overexpressing green fluorescent protein-Rab5a or red fluorescent protein-Rab4a and investigated the interaction between Rab5a or Rab4a using Western blotting, co-immunoprecipitation, confocal microscopy, and colocalization analysis. Finally, epidermal growth factor-stimulated proliferation of these cell lines was analyzed using cell counting kit-8 cell viability assay.
Compared with normal gastric tissues, the expression levels of Rab5a and Rab4a increased progressively both in paracancerous tissues and in advanced cancerous tissues. Epidermal growth factor could promote the proliferation of HGC-27 cells, especially Rab5a-overexpressing HGC-27 cells. Notably, Rab5a and Rab4a co-overexpression promoted the proliferation of HGC-27 cells to the greatest extent. Further analysis identified a direct interaction between Rab5a and Rab4a in HGC-27 cells.
Co-overexpression of Rab5a and Rab4a in GC may promote the endosomal recycling of epidermal growth factor receptor, which in turn contributes to poor prognosis and tumor progression in GC patients. Inhibition of Rab5a or Rab4a expression might be a promising therapy for refractory GC.
Core Tip: Firstly, our data proved that Rab5a can directly interact with Rab4a. Se
- Citation: Cao GJ, Wang D, Zeng ZP, Wang GX, Hu CJ, Xing ZF. Direct interaction between Rab5a and Rab4a enhanced epidermal growth factor-stimulated proliferation of gastric cancer cells. World J Gastrointest Oncol 2021; 13(10): 1492-1505
- URL: https://www.wjgnet.com/1948-5204/full/v13/i10/1492.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v13.i10.1492
The incidence of gastric cancer (GC) in China ranks second only after lung cancer[1]. It was reported that there are about 400000 new GC cases in China every year, accounting for 42% of the world’s total GC new cases[1]. Although specific inhibitors against epidermal growth factor receptor (EGFR), vascular endothelial growth factor, and phosphatidylinositol 3-kinase signaling pathways have already been utilized in the treatment of GC, 25%-40% of patients with stage II-IV GC still suffer from post-treatment recurrence[2-4]. Therefore, there is an urgent need to elucidate the mecha
Rab and Rab-related factors have been established as the main regulators of the activity of membrane trafficking and signal transduction pathways, which in turn regulate cell growth, survival, and programmed cell death or apoptosis[5-7]. Among Rab and Rab-related factors, Rab5 was first found to be a member involved in sorting pathways and endocytic trafficking. There are three subtypes of Rab5 in eukaryotes, namely Rab5a, Rab5b, and Rab5c[7]. These three subtypes can stimulate early endosome fusion and regulate the endocytosis of transferrin[8]. Rab5a is highly expressed in human breast cancer[9], ovarian cancer[10], lung cancer[11], oral cancer[12], hepatocellular carcinoma[13], and gliomas tissues[14]. Its overexpression is correlated with tumor malignancy and metastasis and may affect cell proliferation by regulating the activity of epidermal growth factor (EGF) signaling. Upon EGF stimulation, Rab5a can bind with its downstream effector PTB domain and leucine zipper motif 1 (APPL1), which leads to the transportation of APPL1 from the cy
Previous studies have found that Rab5a is significantly overexpressed in GC tissues, and that overexpression of Rab5a may be related to the advancement of GC[19-21]. However, the underlying mechanisms remain unclear. To investigate the implication of Rab5a overexpression on GC development, in this study, we compared the expression levels of Rab5a and Rab4a in GC tissues with those in intestinal metaplasia tissue and surrounding normal gastric mucosa and found that the cancerous tissues exhibited significantly higher levels of Rab5a and Rab4a (P < 0.01). Additionally, we found that Rab5a and Rab4a could directly interact with each other in HGC-27 cells. These results suggest that overexpression of Rab5a and Rab4a in GC may promote the endosomal recycling of EGFR, thereby enhancing the signal transduction and effects of EGF stimulation. This study may provide new ideas for the study of the occurrence and treatment of GC.
Primary tumor specimens were obtained from 42 GC patients who underwent resection between 2015 and 2018 in Huashan Hospital. Patients who had received chemotherapy or radiotherapy prior to surgery were excluded. The histological diagnosis was evaluated according to the World Health Organization guidelines[22]. Clinical data were obtained from medical records. Patients aged from 32 to 81 years (60 ± 12 years, mean ± SD) were included in this study. The tumor stage was classified according to the eighth edition of tumor node metastasis (TNM) classification of the Union for International Cancer Control[23]. Twenty patients (47.62%) were classified as early stage (stages I and II), while 22 patients (52.38%) were classified as advanced stage (stages III and IV) according to the clinical TNM. As for the histological grade, 27 patients (64.28%) were graded as grade I or II, while 15 (35.71%) were grade III or IV. With regard to metastasis, lymph node metastasis was observed in 22 patients (52.38%), while 20 patients (47.62%) were without metastasis.
All tissue sections were deparaffinized, rehydrated, and blocked as described by Crosby et al[24]. The sections were then incubated with antibodies (specified below) overnight at 4 °C. After washing with phosphate buffered saline (PBS), the sections were incubated with horseradish peroxidase-labeled goat anti-rabbit antibody (1: 200; Santa Cruz Biotechnology, Dallas, TX, United States) for 1 h at room temperature. After repeated washing with PBS, the antigen-antibody complex was visualized with substrate chromogen (DAB; Dako Cytomation, Carpinteria, CA, United States) and counterstained with hematoxylin. Antibodies used in this study included rabbit polyclonal antibody against Rab5a, rabbit polyclonal antibody against Rab4a, rabbit polyclonal antibody against APPL1, and rabbit polyclonal antibody against histone deacetylase 1. All these antibodies were purchased from Santa Cruz Biotechnology. For grading purpose, the percentage of stained samples was determined based on immunohistochemical staining results. Samples in which no cell was stained were defined as “negative”. “Weak positive” was defined as < 10% of the tumor cells positively stained, moderate positive was defined as 10%-50% of the tumor cells positively stained, and strong positive was defined as > 50% of the tumor cells positively stained.
To construct a lentiviral plasmid overexpressing red fluorescent protein-Rab4a, upstream primer 5’-CCG GAATTC GCCACC ATGTCGCAGACGGCCATGTC-3’ and downstream primer 5’-CCG GGATCC CTAACAACCACACTCCTGAGCGTTC-3’ were used to isolate Rab4a complementary DNA (cDNA). Then the polymerase chain reaction products were digested and inserted into the pGMLV-mCherry-puro vector (Genomeditech Inc., Shanghai, China). For the construction of lentiviral plasmid overexpressing Rab5a, two primers were synthesized, including 5’-CCG GAATTC GCCACCATGGCTAGTCGAGGCGCAA-3’ and 5’-CCG GGATCC TTAGTTACTACAACACTGATTCCTGGTTGGTT-3’, for amplification of Rab5a cDNA. The po
Human GC cell line HGC-27 was purchased from the cell bank at the Institute of Biochemistry and Cell Biology (Shanghai Institute for Biological Sciences, Chinese Academy of Sciences, Shanghai, China). The cells were cultured in Roswell Park Memorial Institute 1640 medium supplemented with 10% fetal bovine serum (Gibco, Grand Island, NY, United States), streptomycin (100 μg/mL), and penicillin (100 U/mL) in a humidified atmosphere of 5% CO2 at 37°C. For overexpression of green fluorescent protein-Rab5a or/and red fluorescent protein-Rab4a, HGC-27 cells were treated with the aforementioned supernatant containing lentiviruses.
Immunoblotting analysis was carried out using standard methods. Lysates prepared from control and transfected cells were separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis, and transferred onto polyvinylidene fluoride membranes (Millipore Corporation, Billerica MA, United States). The membranes were incubated with rabbit anti-human Rab5a (1: 300) or Rab4a antibody (1:1000) overnight at 4℃. After washing polyvinylidene fluoride membranes with PBS, they were incubated with horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G (1:2000) for 2 h. After further washing, the immunoblots were developed using the enhanced chemiluminescence reagents (Amersham Life Science, Piscataway, NJ, United States). Each experiment was repeated three times.
Cell lysates were sonicated for 30 min before centrifugation at 12000 rpm for 10 min at 4°C. For co-immunoprecipitation, lysates were incubated with 20-40 μL of washed protein G agarose beads (Roche, Basel, Switzerland) for 3 h at 4°C to reduce non
The transfected HGC-27 cell lines were cultured for live-cell confocal imaging on four- or eight-well chambered glass slides. Representative confocal images were recorded using Leica SP8 confocal microscope equipped with an HCX PL APO 63X/1.4 oil objective (Leica Microsystems, Wetzlar, Germany). Image acquisition was conducted at 1240 × 1240 pixels using sequential scanning for each channel. After background correction, RGB profile intensity and Manders’ correlation coefficient were used to measure the degree of colocalization between Rab5a and Rab4a with the EzColocalization tool in the National Institutes of Health ImageJ software (version: 2.1.0/1.53c)[25].
Viability of HGC-27 cells was measured using cell counting kit (CCK)-8 (Dojindo, Rockville, MD, United States). Briefly, cells were seeded into six 96-well plates at the density of 1000 cells per well. For each cell line, two parallel samples were prepared, one was directly stimulated with 10 ng/mL EGF, the other remained untreated. The plates were cultured in a 5% CO2 incubator at 37 °C. The proliferation of cells was evaluated at 0 h, 24 h, 30 h, 48 h, 54 h, and 72 h respectively by adding 5 μL CCK-8 solution (Fluka, Buchs, Switzerland) into each well. After that, the optical density of each well was measured at 450 nm and 630 nm using a microplate reader. All experiments were performed in triplicates.
All images were acquired using identical acquisition parameters, and all data are presented as means ± standard error. Significance was determined using student’s t-test or Kruskal-Wallis Test. Values of aP < 0.05, bP < 0.01, and cP < 0.001 were considered indicative of significance. All statistical analyses were performed with R software packages, version 3.6.3 (The R Foundation).
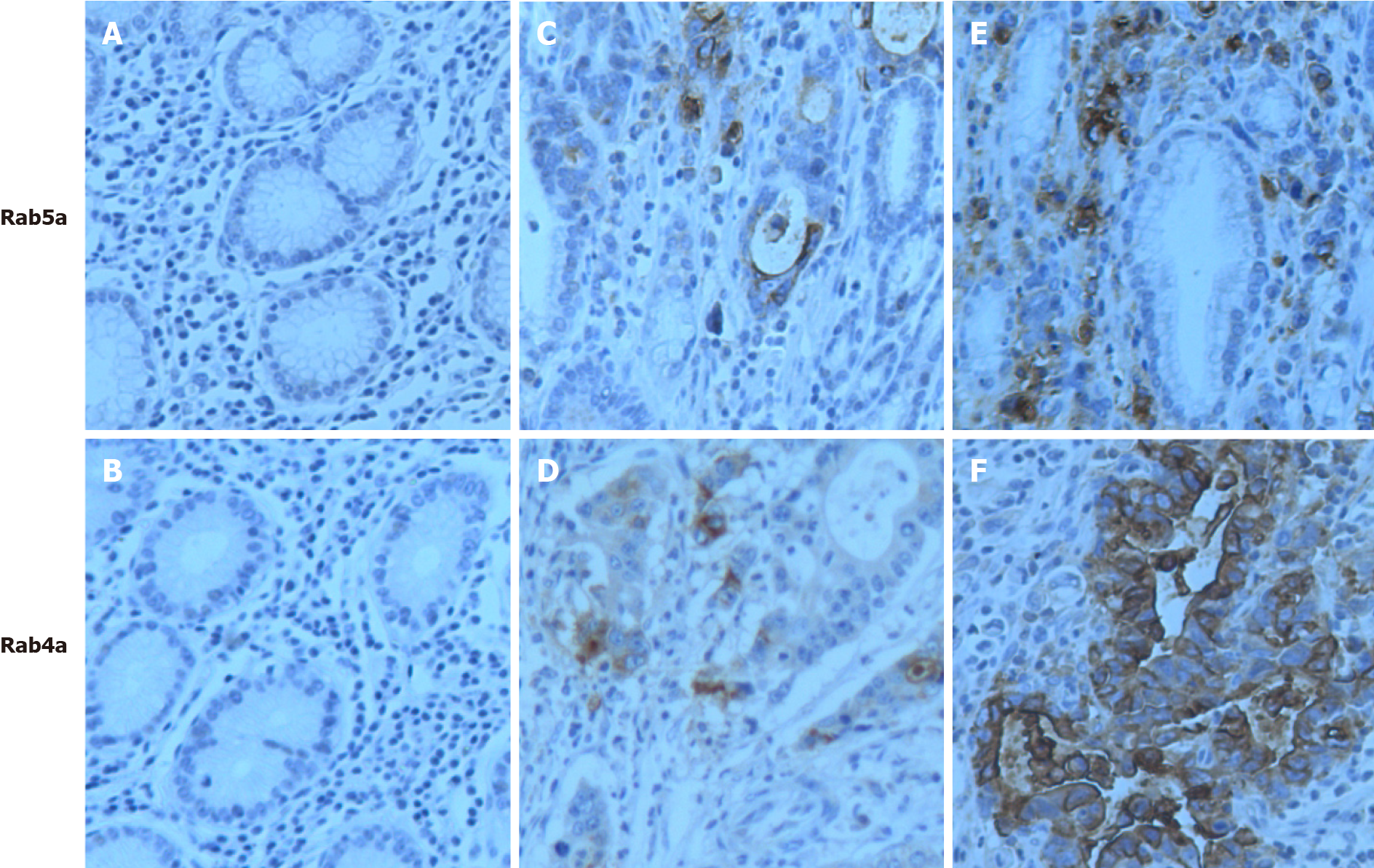
Our immunohistochemical staining analysis revealed “negative” (-) (Figure 1A and B) and “weak positive” (+) levels of Rab5a and Rab4a in normal and paracancerous gastric tissues, respectively (Figure 1C and D). In the epithelial cells of GC tissues, Rab5a and Rab4a were both overexpressed (+++) in the cytoplasm or on cell membrane (Figure 1E and F).
The 42 GC specimens were classified into different clinical stages, and their immunohistochemical phenotypes were examined (Table 1): Among the 42 GC specimens, the overall Rab5a positive rate was 76.2%, while the overall Rab4a positive rate was 85.7%; among the 20 early-stage GC specimens, the Rab5a positive rate was 60.0%, and the Rab4a positive rate was 70.0%; the expression levels of Rab5a and Rab4a were further enhanced in advanced-stage GC specimens, with positive rates of 90.9% and 100% being observed for Rab5a and Rab4a, respectively. The difference between the two groups was statistically significant (P < 0.05). Notably, the expression level of Rab5a increased from negative (-), to positive (+), to strong positive (++), and to very strong positive (+++) with the up stage of GC (P < 0.01). For Rab4a, a similar trend was observed. Taken together, these data suggest that Rab5a and Rab4a may be involved in GC development. To verify these results, we further analyzed the expression patterns of Rab5a and Rab4a in normal gastric mucosa, gastric tissues adjacent to the tumors, early-stage GC tumor tissues, and advanced-stage GC tumor tissues. As shown in Table 2, the Rab5a and Rab4a positive rates were 20% and 10% in normal gastric mucosa, 35.3% and 40% in adjacent tissues, 60% and 70% in early-stage GC (I, II) tissues, and 90.9% and 100% in advanced-stage GC tissues, respectively. Moreover, the expression levels of Rab5a and Rab4a were significantly different among groups (P < 0.01) (Table 2). Collectively, these results confirmed that the expression levels of Rab5a and Rab4a were progressively enhanced with the advan
| Classification | Clinical stages | Histological classification | Metastasis | ||||
| Advanced | Early | I + II | III + IV | Yes | No | ||
| Number of cases | 22 | 20 | 27 | 15 | 22 | 20 | |
| Gender | Male | 18 | 12 | 18 | 12 | 16 | 14 |
| Female | 4 | 8 | 9 | 3 | 6 | 6 | |
| Age | 60 ± 11 | 62 ± 17 | 59 ± 12 | 62 ± 11 | 63 ± 10 | 57 ± 13 | |
| Rab5 | - | 2 | 8 | 9 | 0 | 0 | 6 |
| + | 6 | 10 | 11 | 4 | 12 | 10 | |
| ++ | 10 | 2 | 7 | 8 | 10 | 4 | |
| +++ | 4 | 0 | 0 | 3 | 2 | 0 | |
| Statistics (χ2) | 13.87 | 12.963 | 10.476 | ||||
| P value | < 0.01 | < 0.01 | 0.015 | ||||
| Rab4 | - | 0 | 6 | 7 | 0 | 0 | 8 |
| + | 6 | 12 | 12 | 6 | 4 | 8 | |
| ++ | 12 | 2 | 8 | 6 | 12 | 4 | |
| +++ | 4 | 0 | 0 | 3 | 6 | 0 | |
| Statistics (χ2) | 19.091 | 9.644 | 19.282 | ||||
| P value | < 0.01 | 0.022 | < 0.01 | ||||
| HDAC1 | APPL1 | Rab4a | Rab5a | |||||||||||||
| - | + | ++ | +++ | - | + | ++ | +++ | - | + | ++ | +++ | - | + | ++ | +++ | |
| Normal gastric tissue | 20 | 0 | 0 | 0 | 12 | 8 | 0 | 0 | 18 | 2 | 0 | 0 | 16 | 4 | 0 | 0 |
| Paracancerous tissue | 24 | 10 | 0 | 0 | 24 | 10 | 0 | 0 | 20 | 14 | 0 | 0 | 22 | 12 | 0 | 0 |
| Early-stage gastric cancer | 4 | 8 | 8 | 0 | 5 | 10 | 5 | 0 | 6 | 12 | 2 | 0 | 8 | 10 | 2 | 0 |
| Advanced-stage gastric cancer | 0 | 8 | 10 | 4 | 2 | 6 | 10 | 4 | 0 | 6 | 12 | 4 | 2 | 6 | 10 | 4 |
| Statistics (χ2) | 59.162 | 43.456 | 58.338 | 45.719 | ||||||||||||
| P value | < 0.001 | < 0.001 | < 0.001 | < 0.001 | ||||||||||||
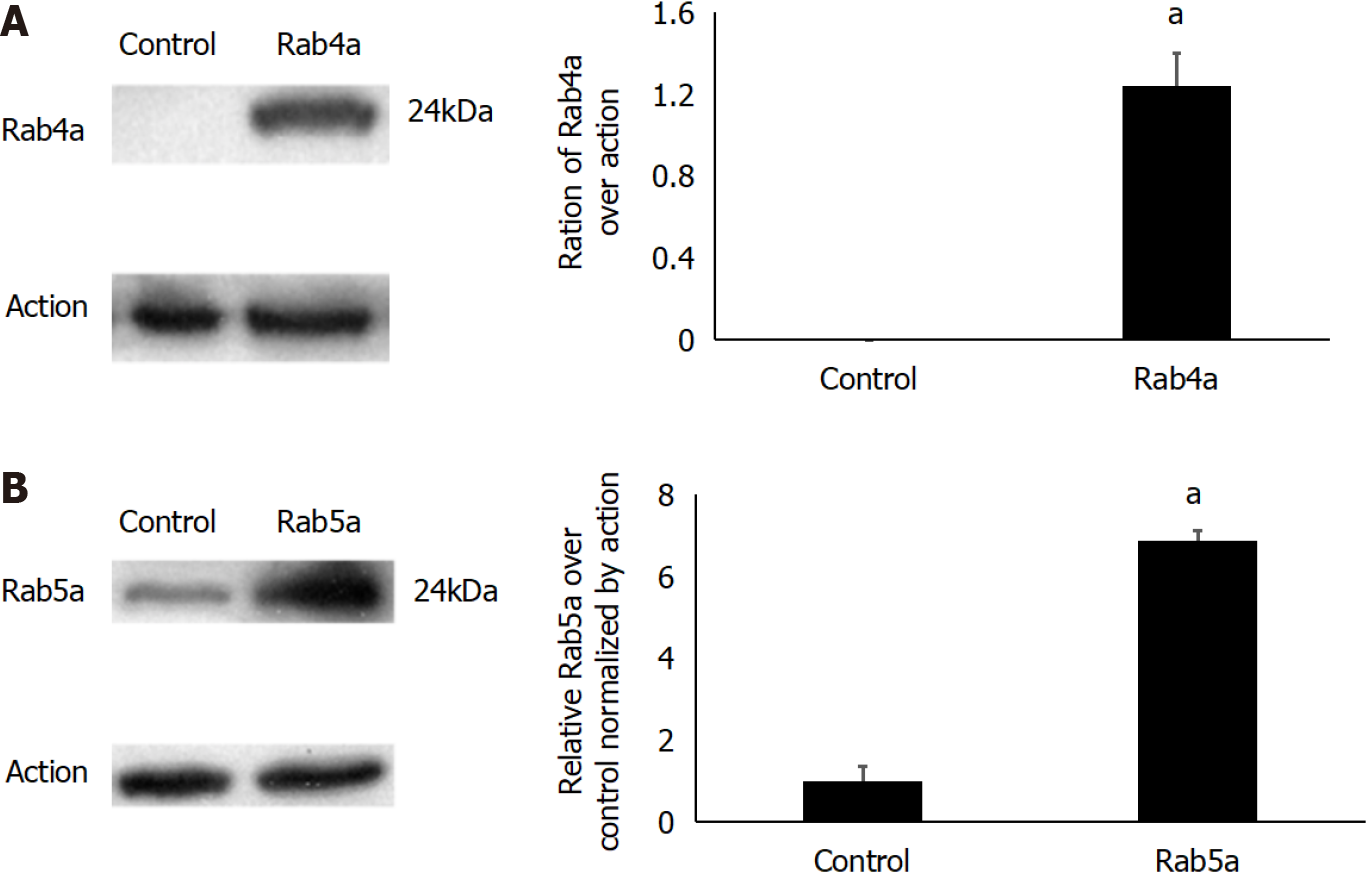
The lentiviral plasmids were first constructed to transfect transiently 293T cells; the expression levels of Rab5a and Rab4a were determined by western blot (Figure 2). After transfection, the level of Rab4a was significantly improved (Figure 2A), while that of Rab5a was moderately enhanced (about 5-8-fold as high as the endogenous expression level) (Figure 2B). The results showed that the endogenous Rab4a cannot even be detected by western blot in the 293T cell line (Figure 2A), while Rab5a expression is minimal (Figure 2B), and that the transfection was successful.
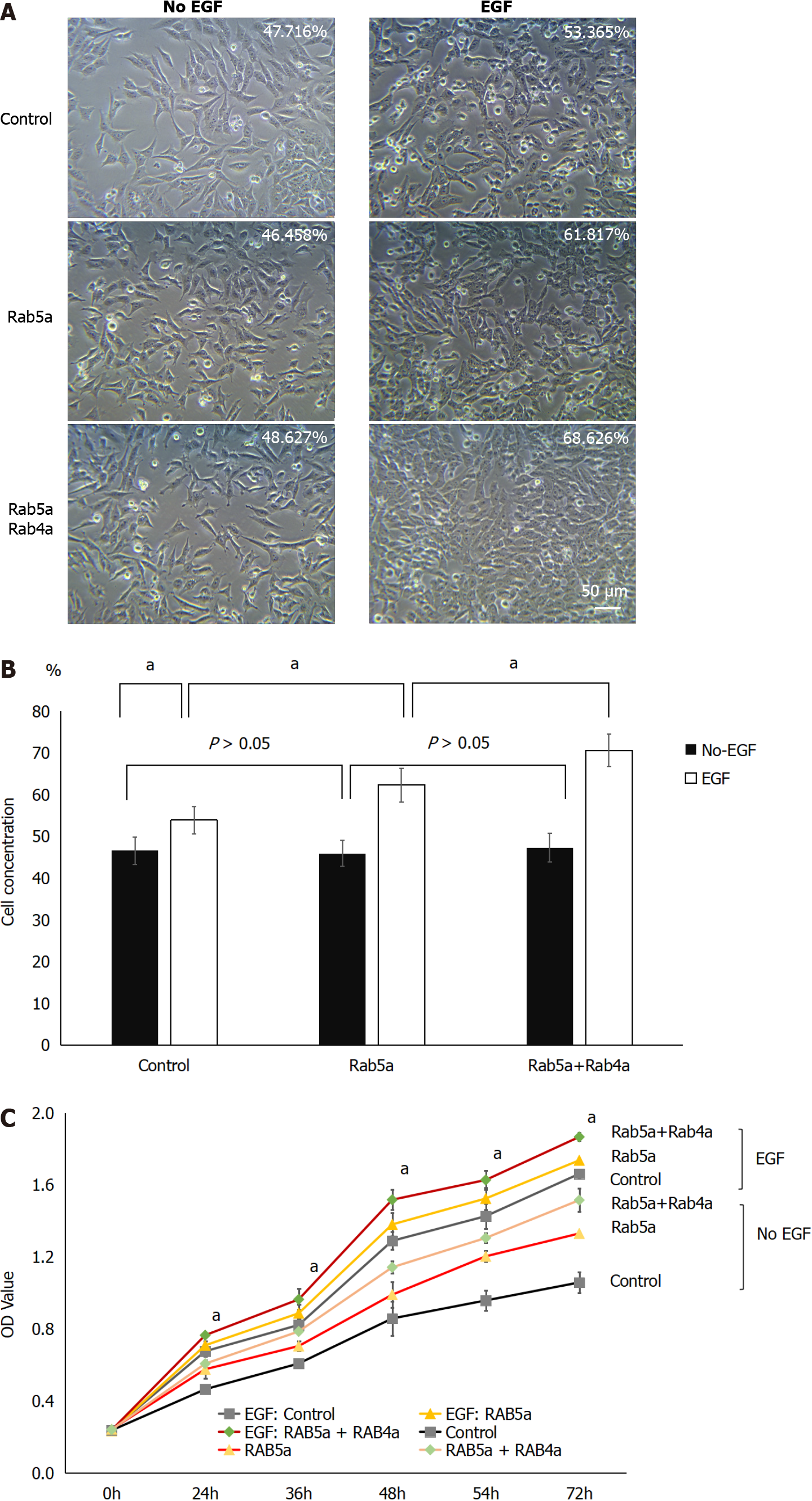
Before EGF administration, similar HGC-27 cell densities were observed in the Control group, Rab5a overexpression group, and Rab5a + Rab4a overexpression group. However, after 24 h of EGF stimulation, cell proliferation was found to increase significantly in all the groups. Among them, the Rab5a + Rab4a overexpression group had the highest cell density, followed by the Rab5a overexpression group, and finally the Control group (Figure 3A and B). There were statistically significant differences in cell density before and after (Figure 3A) EGF stimulation in all the groups (P < 0.05). In addition, 24 h after EGF stimulation, there were statistical differences between the Rab5a overexpression group and the Control group (P < 0.05) and between the Rab5a + Rab4a overexpression group and the Rab5a overexpression group (Figure 3A) (P < 0.05).
To characterize further EGF-stimulated HGC-27 cell proliferation, the cell viability of each group was then evaluated by CCK-8 assay as previously described[26]. Upon EGF stimulation, cell proliferation in each group was increased significantly (Figure 3C, P < 0.05). Among all the groups, the Rab5a + Rab4a overexpression group showed the largest increase in cell viability. These results indicated that Rab5a overexpression can significantly promote EGF-mediated cell proliferation, and this effect was further enhanced by Rab4a overexpression.
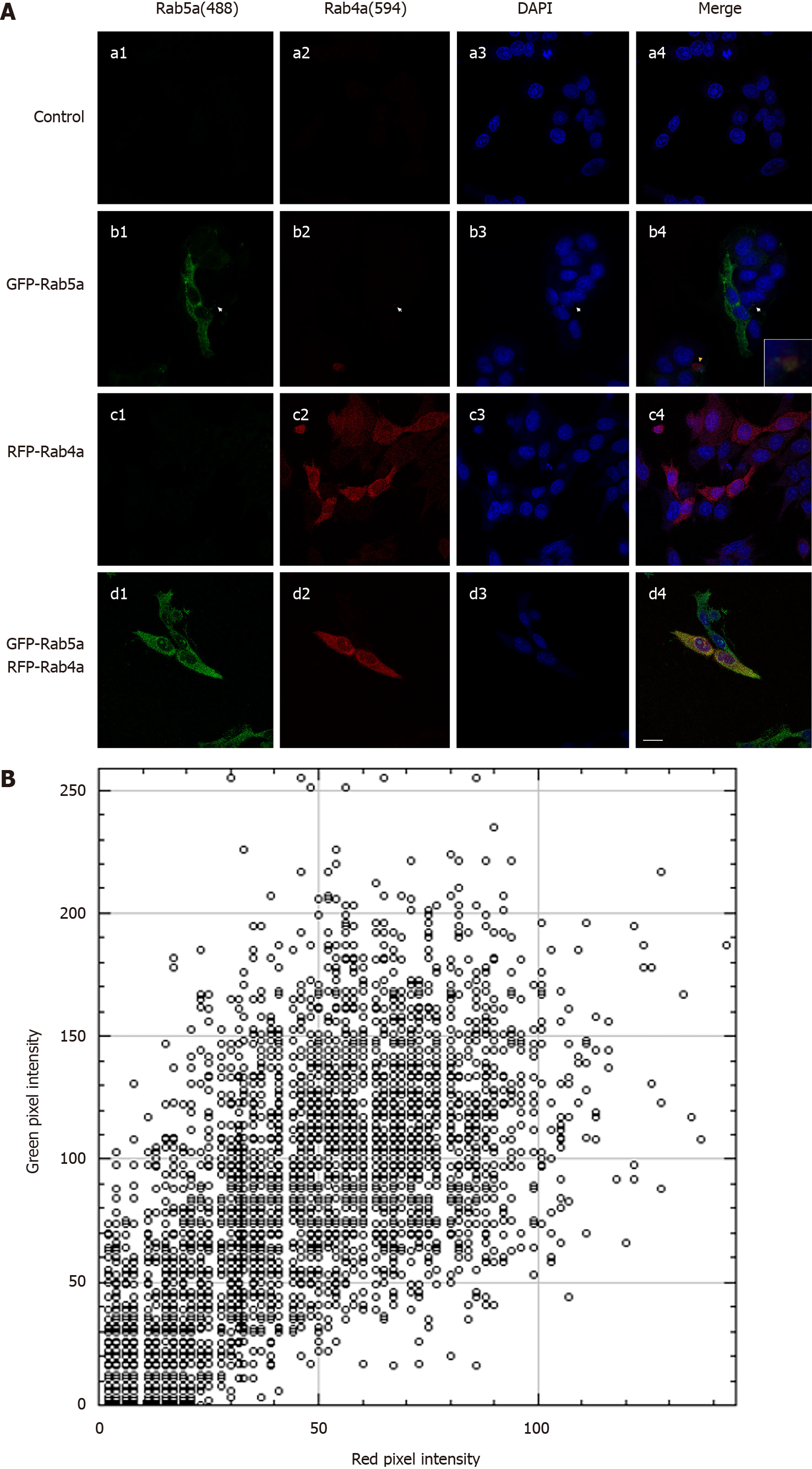
Under normal circumstances, HGC-27 cells express a low level of Rab5a and even a lower level of Rab4a (Figure 4A: a1-a4). We found that Rab5a overexpression resulted in a significantly increased level of endogenous Rab4a and that the two proteins co-localized in endosomes (Figure 4A: b1-b4). On the contrary, Rab4a overexpression did not significantly affect the protein level of Rab5a (Figure 4A: c1-c4). In HGC-27 cells of the Rab5a + Rab4a overexpression group, the two proteins co-localized in a large number of endosomes (Figure 4A: d1-d4). Quantitative analysis of the co-localization between Rab5a and Rab4a with ImageJ showed (Figure 4B) that despite background noises in the red and green fluorescence channels, a clear overlap between the two colors was observed. The above results suggest that Rab5a could directly interact with Rab4a to increase its protein level in HGC-27 cells.
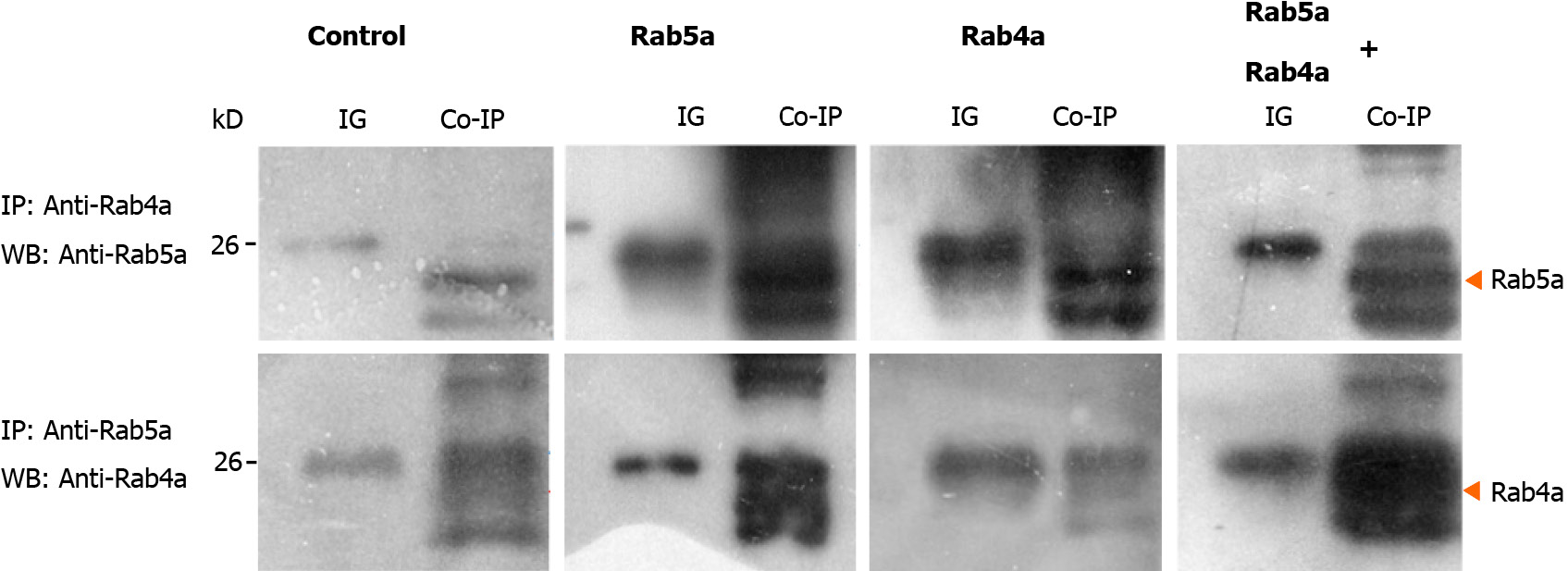
In order to verify the aforementioned observations, we performed immunoprecipitation experiments in HGC-27 cells. As shown in Figure 5A and E, although Rab5a and Rab4a exhibited low endogenous expression levels in HGC-27 cells, an interaction between the two proteins was observed. This result confirmed a direct interaction between endogenous Rab5a and Rab4a proteins. In the Rab5a + Rab4a overexpression cells, a stronger interaction between the two guanosine triphosphatases was observed as expected (Figure 5D and H). Interestingly, in HGC-27 cells overexpressing Rab5a alone, although there is no artificial expression of Rab4a protein, the co-precipitation of Rab5a and Rab4a was still clearly observed (Figure 5B and F). On the contrary, in HGC-27 cells overexpressing Rab4a alone, the co-precipitation of Rab5a and Rab4a was weak (Figure 5C and G). Collectively, these results indicated that overexpression of Rab5a promoted the expression of endogenous Rab4a in HGC-27 cells and that the two proteins could directly interact with each other.
Rab proteins are small guanosine triphosphate-binding proteins. They belong to the largest Ras subfamily and are ubiquitously expressed in all eukaryotes[27]. Rab5 protein was first cloned from a pheochromocytoma cDNA library by Zahraoui et al[28] in 1989. The first subtype was named Rab5a. Rab5a can stimulate early endosome fusion and regulate transferrin endocytosis[29,30]. More studies have shown that overexpression of Rab5 is implicated human breast cancer[9], ovarian cancer[10], lung cancer[11], oral cancer[12], hepatocellular carcinoma[13], and gliomas[14]. Rab4a is another subtype of Rab proteins. It is mainly located in the reticular coated vesicles, early endosomes, and circulatory endosomes and is an important regulator of endocytosis and circulation[31-34]. It was found that the protein levels of Rab5a and Rab4a in malignant tumor cells were significantly higher than those in paracancerous tissues, which is consistent with the results of this study. Barbieri et al[29] found that EGF stimulation induced a specific, rapid, and transient activation of Rab5a in NR6 cells and that the activation of endocytosis by EGF was enhanced by expression of Rab5a, but not Rab5b or Rab5c. Similar to this finding, an inhibition on EGFR endocytosis had been observed by fluorescent assay in porcine aortic endothelial cells expressing a dominant negative Rab5a mutant and EGFR[35]. Fukui et al[36] found that Rab5a enhanced EGF-EGFR signal transmission in hepatocellular carcinoma and promoted the metastasis of hepatocellular carcinoma. Zhao et al[10] found that EGF combined with EGFR could accelerate ovarian cancer cell proliferation through shuttling Rab5a and APPL1 from the plasmalemma into the nucleus. Yang et al[37] found that the increased expression of Rab5a protein in 123 breast cancer patients was associated with axillary LN metastasis. Tubbesing et al[38] found that Rab4 could increase EGFR activation in breast cancer cells.
In this study, we found that EGF can stimulate HGC-27 GC cell proliferation and that overexpression of Rab5a or Rab5a + Rab4a further enhanced the effect of EGF. Interestingly, the proliferation rate was the highest in the Rab5a + Rab4a group. The results of confocal microscopy suggested that Rab5a co-localized with Rab4a in endosomes. This observation was subsequently verified by our co-immunoprecipitation experiments, in which we found that Rab5a could directly interact with Rab4a in HGC-27 cells. There are very few reports about interaction between Rab5a and Rab4a[34,39]. De Renzis et al[40] discovered in 2002 that activated Rab5a could interact with Rab4a via recruiting Rabenosyn-5 and a series of Rab effector proteins.
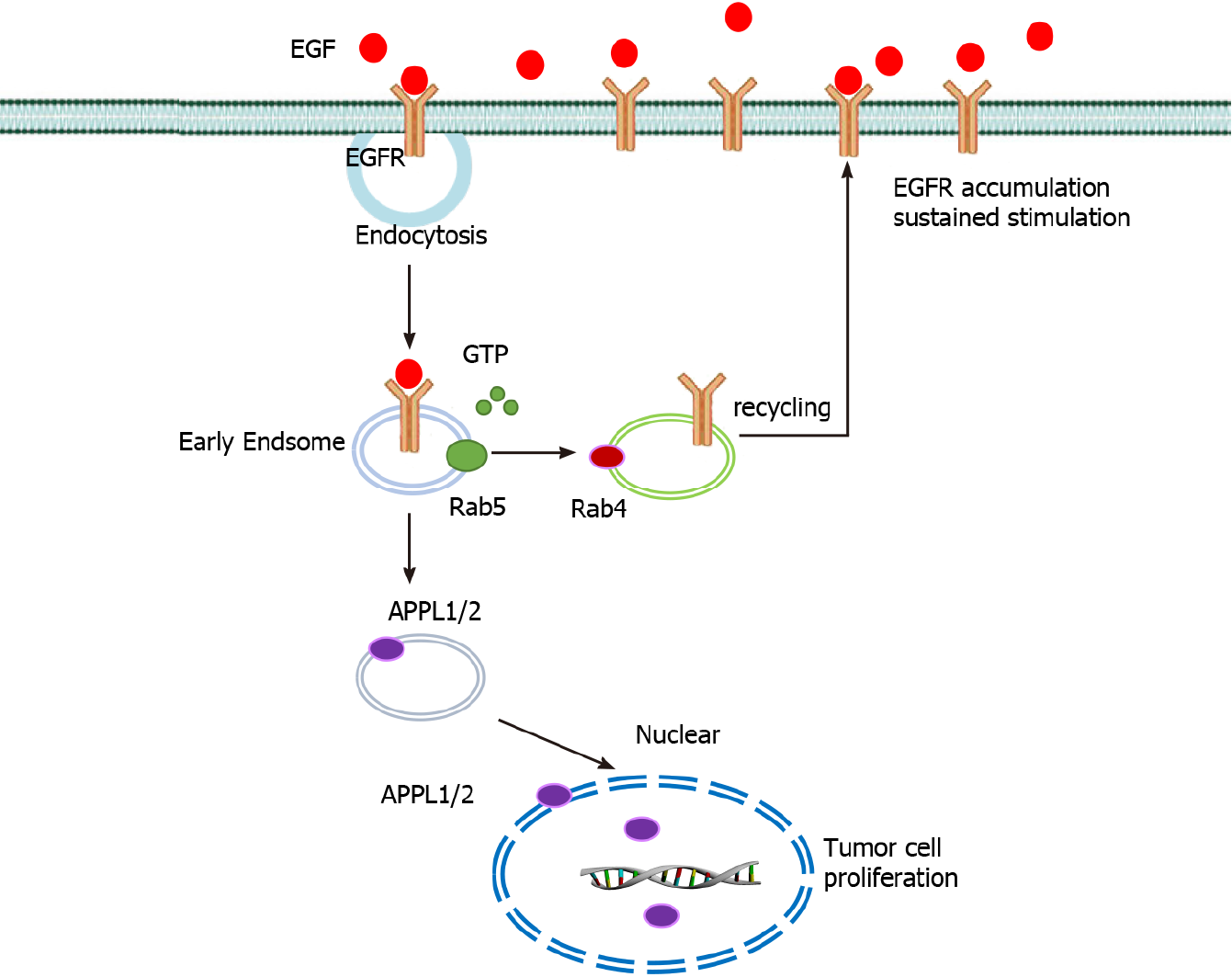
In conclusion, these results suggests that the interaction between EGFR and Rab5a is enhanced after EGF stimulation and that the direct interaction between Rab5a and Rab4a enhances the endosomal circulation of EGFR and thereby maintains its expression level on the cell membrane. This can in turn enhance the effects of EGF, increase the malignancy of GC cells, and enhance their proliferation ability (Figure 6). Therefore, inhibiting the expression of Rab5a and Rab4a might be a new anti-tumor approach.
Co-overexpression of Rab5a and Rab4a in GC may promote the endosomal recycling of EGFR, which in turn contributes to poor prognosis and tumor progression in GC patients. Inhibition of Rab5a or Rab4a expression might be a promising therapy for refractory GC.
Gastric cancer (GC) is a common malignant tumor of digestive system with a poor overall prognosis.
The purpose of our study is to explore the pathogenesis of GC and improve the prognosis of GC.
We constructed HGC-27 cell lines overexpressing green fluorescent protein-Rab5a or red fluorescent protein-Rab4a and tissue samples of 42 patients with GC.
Western blot, co-immunoprecipitation, immunohistochemistry, immunoprecipitation assay, cell culture, confocal microscopy, etc. were utilized.
Rab5a and Rab4a were overexpressed in GC tissues. The direct interaction between Rab5a and Rab4a enhanced epidermal growth factor-stimulated proliferation of GC cells.
Co-overexpression of Rab5a and Rab4a in GC may promote the endosomal recycling of epidermal growth factor receptor, which in turn contributes to poor prognosis and tumor progression in GC patients. Inhibition of Rab5a or Rab4a expression might be a promising therapy for refractory GC.
To explore the pathogenesis of GC and improve the prognosis of GC.
We thank Doctor Zhen Zhao (Minhang Hospital, Fudan University) for optimization of experimental scheme.
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: Chinese Anti-Cancer Association, No. Qtcs1245.
Specialty type: Cell biology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Losurdo G S-Editor: Zhang H L-Editor: Filipodia P-Editor: Yuan YY
| 1. | Cai Q, Zhu C, Yuan Y, Feng Q, Feng Y, Hao Y, Li J, Zhang K, Ye G, Ye L, Lv N, Zhang S, Liu C, Li M, Liu Q, Li R, Pan J, Yang X, Zhu X, Li Y, Lao B, Ling A, Chen H, Li X, Xu P, Zhou J, Liu B, Du Z, Du Y, Li Z; Gastrointestinal Early Cancer Prevention & Treatment Alliance of China (GECA). Development and validation of a prediction rule for estimating gastric cancer risk in the Chinese high-risk population: a nationwide multicentre study. Gut. 2019;68:1576-1587. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 149] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 2. | Lee J, Lim DH, Kim S, Park SH, Park JO, Park YS, Lim HY, Choi MG, Sohn TS, Noh JH, Bae JM, Ahn YC, Sohn I, Jung SH, Park CK, Kim KM, Kang WK. Phase III trial comparing capecitabine plus cisplatin versus capecitabine plus cisplatin with concurrent capecitabine radiotherapy in completely resected gastric cancer with D2 lymph node dissection: the ARTIST trial. J Clin Oncol. 2012;30:268-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 486] [Cited by in RCA: 580] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 3. | Cristescu R, Lee J, Nebozhyn M, Kim KM, Ting JC, Wong SS, Liu J, Yue YG, Wang J, Yu K, Ye XS, Do IG, Liu S, Gong L, Fu J, Jin JG, Choi MG, Sohn TS, Lee JH, Bae JM, Kim ST, Park SH, Sohn I, Jung SH, Tan P, Chen R, Hardwick J, Kang WK, Ayers M, Hongyue D, Reinhard C, Loboda A, Kim S, Aggarwal A. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat Med. 2015;21:449-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1071] [Cited by in RCA: 1578] [Article Influence: 157.8] [Reference Citation Analysis (0)] |
| 4. | Bang YJ, Kim YW, Yang HK, Chung HC, Park YK, Lee KH, Lee KW, Kim YH, Noh SI, Cho JY, Mok YJ, Ji J, Yeh TS, Button P, Sirzén F, Noh SH; CLASSIC trial investigators. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. 2012;379:315-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1267] [Cited by in RCA: 1291] [Article Influence: 99.3] [Reference Citation Analysis (0)] |
| 5. | Seabra MC, Mules EH, Hume AN. Rab GTPases, intracellular traffic and disease. Trends Mol Med. 2002;8:23-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 363] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 6. | Cong XX, Gao XK, Rao XS, Wen J, Liu XC, Shi YP, He MY, Shen WL, Shen Y, Ouyang H, Hu P, Low BC, Meng ZX, Ke YH, Zheng MZ, Lu LR, Liang YH, Zheng LL, Zhou YT. Rab5a activates IRS1 to coordinate IGF-AKT-mTOR signaling and myoblast differentiation during muscle regeneration. Cell Death Differ. 2020;27:2344-2362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 7. | Nagano M, Toshima JY, Siekhaus DE, Toshima J. Rab5-mediated endosome formation is regulated at the trans-Golgi network. Commun Biol. 2019;2:419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 8. | Bucci C, Lütcke A, Steele-Mortimer O, Olkkonen VM, Dupree P, Chiariello M, Bruni CB, Simons K, Zerial M. Co-operative regulation of endocytosis by three Rab5 isoforms. FEBS Lett. 1995;366:65-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 131] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 9. | Arriagada C, Silva P, Millet M, Solano L, Moraga C, Torres VA. Focal adhesion kinase-dependent activation of the early endocytic protein Rab5 is associated with cell migration. J Biol Chem. 2019;294:12836-12845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Zhao Z, Liu XF, Wu HC, Zou SB, Wang JY, Ni PH, Chen XH, Fan QS. Rab5a overexpression promoting ovarian cancer cell proliferation may be associated with APPL1-related epidermal growth factor signaling pathway. Cancer Sci. 2010;101:1454-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 11. | Bidkhori G, Narimani Z, Hosseini Ashtiani S, Moeini A, Nowzari-Dalini A, Masoudi-Nejad A. Reconstruction of an integrated genome-scale co-expression network reveals key modules involved in lung adenocarcinoma. PLoS One. 2013;8:e67552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 89] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 12. | Zhang D, Lu C, Ai H. Rab5a is overexpressed in oral cancer and promotes invasion through ERK/MMP signaling. Mol Med Rep. 2017;16:4569-4576. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Geng D, Zhao W, Feng Y, Liu J. Overexpression of Rab5a promotes hepatocellular carcinoma cell proliferation and invasion via FAK signaling pathway. Tumour Biol. 2016;37:3341-3347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Zhou X, Xie S, Wu S, Qi Y, Wang Z, Zhang H, Lu D, Wang X, Dong Y, Liu G, Yang D, Shi Q, Bian W, Yu R. Golgi phosphoprotein 3 promotes glioma progression via inhibiting Rab5-mediated endocytosis and degradation of epidermal growth factor receptor. Neuro Oncol. 2017;19:1628-1639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 15. | Goto-Silva L, McShane MP, Salinas S, Kalaidzidis Y, Schiavo G, Zerial M. Retrograde transport of Akt by a neuronal Rab5-APPL1 endosome. Sci Rep. 2019;9:2433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 16. | Diggins NL, Webb DJ. APPL1 is a multifunctional endosomal signaling adaptor protein. Biochem Soc Trans. 2017;45:771-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 17. | Galvez T, Gilleron J, Zerial M, O'Sullivan GA. SnapShot: Mammalian Rab proteins in endocytic trafficking. Cell. 2012;151:234-234.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 18. | Frittoli E, Palamidessi A, Marighetti P, Confalonieri S, Bianchi F, Malinverno C, Mazzarol G, Viale G, Martin-Padura I, Garré M, Parazzoli D, Mattei V, Cortellino S, Bertalot G, Di Fiore PP, Scita G. A RAB5/RAB4 recycling circuitry induces a proteolytic invasive program and promotes tumor dissemination. J Cell Biol. 2014;206:307-328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 109] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 19. | Erratum for the Research Article: "Circulating tumor DNA methylation profiles enable early diagnosis, prognosis prediction, and screening for colorectal cancer" by H. Luo, Q. Zhao, W. Wei, L. Zheng, S. Yi, G. Li, W. Wang, H. Sheng, H. Pu, H. Mo, Z. Zuo, Z. Liu, C. Li, C. Xie, Z. Zeng, W. Li, X. Hao, Y. Liu, S. Cao, W. Liu, S. Gibson, K. Zhang, G. Xu, R.-h. Xu. Sci Transl Med. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Li Y, Sun X, Ji D, Kong X, Liu D, Zhao Z, Yan J, Chen S. Expression of Rab5a correlates with tumor progression in pancreatic carcinoma. Virchows Arch. 2017;470:527-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 21. | Yuan W, Liu B, Wang X, Li T, Xue H, Mo X, Yang S, Ding S, Han W. CMTM3 decreases EGFR expression and EGF-mediated tumorigenicity by promoting Rab5 activity in gastric cancer. Cancer Lett. 2017;386:77-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 22. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2554] [Cited by in RCA: 2430] [Article Influence: 486.0] [Reference Citation Analysis (3)] |
| 23. | Paner GP, Stadler WM, Hansel DE, Montironi R, Lin DW, Amin MB. Updates in the Eighth Edition of the Tumor-Node-Metastasis Staging Classification for Urologic Cancers. Eur Urol. 2018;73:560-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 434] [Article Influence: 62.0] [Reference Citation Analysis (0)] |
| 24. | Adell T, Barberán S, Sureda-Gómez M, Almuedo-Castillo M, de Sousa N, Cebrià F. Immunohistochemistry on Paraffin-Embedded Planarian Tissue Sections. Methods Mol Biol. 2018;1774:367-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Stauffer W, Sheng H, Lim HN. EzColocalization: An ImageJ plugin for visualizing and measuring colocalization in cells and organisms. Sci Rep. 2018;8:15764. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 174] [Cited by in RCA: 204] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 26. | Tominaga H, Ishiyama M, Ohseto F, Sasamoto K, Hamamoto T, Suzuki K, Watanabe M. A water-soluble tetrazolium salt useful for colorimetric cell viability assay. Anal Commun. 1999;36:47-50. [RCA] [DOI] [Full Text] [Cited by in Crossref: 470] [Cited by in RCA: 472] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 27. | Stenmark H, Olkkonen VM. The Rab GTPase family. Genome Biol. 2001;2:REVIEWS3007. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 461] [Cited by in RCA: 515] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 28. | Zahraoui A, Touchot N, Chardin P, Tavitian A. The human Rab genes encode a family of GTP-binding proteins related to yeast YPT1 and SEC4 products involved in secretion. J Biol Chem. 1989;264:12394-12401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 246] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 29. | Barbieri MA, Roberts RL, Gumusboga A, Highfield H, Alvarez-Dominguez C, Wells A, Stahl PD. Epidermal growth factor and membrane trafficking. EGF receptor activation of endocytosis requires Rab5a. J Cell Biol. 2000;151:539-550. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 196] [Cited by in RCA: 241] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 30. | de Hoop MJ, Huber LA, Stenmark H, Williamson E, Zerial M, Parton RG, Dotti CG. The involvement of the small GTP-binding protein Rab5a in neuronal endocytosis. Neuron. 1994;13:11-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 128] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 31. | Bielli A, Thörnqvist PO, Hendrick AG, Finn R, Fitzgerald K, McCaffrey MW. The small GTPase Rab4A interacts with the central region of cytoplasmic dynein light intermediate chain-1. Biochem Biophys Res Commun. 2001;281:1141-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 79] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 32. | Nagelkerken B, Van Anken E, Van Raak M, Gerez L, Mohrmann K, Van Uden N, Holthuizen J, Pelkmans L, Van Der Sluijs P. Rabaptin4, a novel effector of the small GTPase rab4a, is recruited to perinuclear recycling vesicles. Biochem J. 2000;346 Pt 3:593-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 33. | Barbarin A, Frade R. Procathepsin L secretion, which triggers tumour progression, is regulated by Rab4a in human melanoma cells. Biochem J. 2011;437:97-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 34. | Nag S, Rani S, Mahanty S, Bissig C, Arora P, Azevedo C, Saiardi A, van der Sluijs P, Delevoye C, van Niel G, Raposo G, Setty SRG. Rab4A organizes endosomal domains for sorting cargo to lysosome-related organelles. J Cell Sci. 2018;131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 35. | Galperin E, Sorkin A. Visualization of Rab5 activity in living cells by FRET microscopy and influence of plasma-membrane-targeted Rab5 on clathrin-dependent endocytosis. J Cell Sci. 2003;116:4799-4810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 67] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 36. | Fukui K, Tamura S, Wada A, Kamada Y, Igura T, Kiso S, Hayashi N. Expression of Rab5a in hepatocellular carcinoma: Possible involvement in epidermal growth factor signaling. Hepatol Res. 2007;37:957-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 37. | Yang PS, Yin PH, Tseng LM, Yang CH, Hsu CY, Lee MY, Horng CF, Chi CW. Rab5A is associated with axillary lymph node metastasis in breast cancer patients. Cancer Sci. 2011;102:2172-2178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 38. | Tubbesing K, Ward J, Abini-Agbomson R, Malhotra A, Rudkouskaya A, Warren J, Lamar J, Martino N, Adam AP, Barroso M. Complex Rab4-Mediated Regulation of Endosomal Size and EGFR Activation. Mol Cancer Res. 2020;18:757-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 39. | Rai A, Goody RS, Müller MP. Multivalency in Rab effector interactions. Small GTPases. 2019;10:40-46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 40. | de Renzis S, Sönnichsen B, Zerial M. Divalent Rab effectors regulate the sub-compartmental organization and sorting of early endosomes. Nat Cell Biol. 2002;4:124-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 301] [Article Influence: 13.1] [Reference Citation Analysis (0)] |