Published online Feb 27, 2015. doi: 10.4254/wjh.v7.i2.276
Peer-review started: August 23, 2014
First decision: November 14, 2014
Revised: December 16, 2014
Accepted: January 9, 2015
Article in press: Janurary 12, 2015
Published online: February 27, 2015
Processing time: 175 Days and 14.2 Hours
AIM: To evaluate the relationship between hepatocellular carcinoma (HCC) vascularity and grade; to describe patterns and vascular/histopathological variations of post-transplantation recurrence.
METHODS: This retrospective study included 165 patients (143 men, 22 women; median age 56.8 years, range 28-70.4 years) transplanted for HCC who had a follow-up period longer than 2 mo. Pre-transplantation dynamic computed tomography or magnetic resonance examinations were retrospectively reviewed, classifying HCC imaging enhancement pattern into hypervascular and hypovascular based on presence of wash-in during arterial phase. All pathologic reports of the explanted livers were reviewed, collecting data about HCC differentiation degree. The association between imaging vascular pattern and pathological grade was estimated using the Fisher exact test. All follow-up clinical and imaging data were reviewed for evidence of recurrence. Recurrence rate was calculated and imaging features of recurrent tumor were collected, classifying early and late recurrences based on timing (< or ≥ 2 years after transplantation) and intrahepatic, extrahepatic and both intrahepatic and extrahepatic recurrences based on location. All intrahepatic recurrences were classified as hypervascular or hypovascular and the differentiation degree was collected where available. The presence of variations in imaging enhancement pattern and pathological grade between the primary tumor and the intrahepatic recurrence was evaluated and the association between imaging and histopatholgical variations was estimated by using the χ2 test.
RESULTS: Of the 163 patients with imaging evidence of viable tumor, 156 (95.7%) had hypervascular and 7 (4.3%) hypovascular HCC. Among the 125 patients with evidence of viable tumor in the explanted liver, 19 (15.2%) had grade 1, 56 (44.8%) grade 2, 40 (32%) grade 3 and 4 (3.2%) grade 4 HCC, while the differentiation degree was not assessable for 6 patients (4.8%). A significant association was found between imaging vascularity and pathological grade (P = 0.035). Post-transplantation recurrence rate was 14.55% (24/165). All recurrences occurred in patients who had a hypervascular primary tumor. Three patients (12.5%) experienced late recurrence; the location of the first recurrence was extrahepatic in 14 patients (58.3%), intrahepatic in 7 patients (29.2%) and both intrahepatic and extrahepatic in 3 patients (12.5%). Two patients had a variation in imaging characteristics between the primary HCC (hypervascular) and the intrahepatic recurrent HCC (hypovascular), while 1 patient had a variation of histopathological characteristics (from moderate to poor differentiation), however no association was found between imaging and histopathological variations.
CONCLUSION: A correlation was found between HCC grade and vascularity; some degree of variability may exist between the primary and the recurrence imaging/histopathological characteristics, apparently not correlated.
Core tip: During hepatocarcinogenesis, besides the differentiation loss, blood supply changes occur. Recently, a correlation between higher histopathological grades and hypervascular dynamic-imaging enhancement pattern has been demonstrated. Hepatocellular carcinoma recurrence after transplantation is relatively common, however the issue of possible variations in imaging and histopathology between the primary and the recurrent tumor, and particularly the relationship between enhancement and grade changes, has never been investigated. We demonstrated a correlation between vascularity and pathological grade in a large population of transplanted patients, and some degree of variability between the primary and the recurrent tumor vascularity was found, though not associated with histopathological changes.
- Citation: Pecchi A, Besutti G, Santis MD, Giovane CD, Nosseir S, Tarantino G, Benedetto FD, Torricelli P. Post-transplantation hepatocellular carcinoma recurrence: Patterns and relation between vascularity and differentiation degree. World J Hepatol 2015; 7(2): 276-284
- URL: https://www.wjgnet.com/1948-5182/full/v7/i2/276.htm
- DOI: https://dx.doi.org/10.4254/wjh.v7.i2.276
Hepatocellular carcinoma (HCC) is the most frequent primary hepatic malignancy[1], and it represents an important health issue due to its increasing incidence and poor survival[2]. HCC usually arises in the background of chronic liver diseases, in particular cirrhosis is the substrate of HCC in 80%-90% of cases[3]. Carcinogenesis in HCC is typically a multistep process, that comprehends low-grade and high grade dysplastic nodules possibly with neoplastic foci, early HCC, and overt HCC. Pathologically, HCC is graded based on differentiation degree into four degrees of cellular dysplasia and architectural tissue disarrangement (well-differentiated, moderately differentiated, poorly differentiated and undifferentiated, grade 1 to 4)[4]. During the hepatocarcinogenesis multistage process, besides the size growth and the loss of differentiation of the nodule, blood supply changes progressively occur, so that the hepatocellular nodule becomes more and more dependent on newly formed arteries and in parallel less dependent on the portal contribution[3].
Contrast-enhanced dynamic imaging, performed both with multidetector CT (MDCT) and magnetic resonance imaging (MRI), provides information about HCC vascularity. In particular, two different hemodynamic patterns have been featured: the typical, much more common, hypervascular pattern, and the less frequent hypovascular variant of HCC, which shows no arterial phase hypervascularity[5]. Recently, some studies have compared contrast enhanced dynamic imaging findings with histopathological differentiation degree, showing a correlation between higher pathological grades and hypervascular enhancement pattern[6-10], even though some investigators have reported a subsequent decrease in arterial blood supply in the late stage of HCC development (grade ≥ 3)[7-9].
Liver transplantation (LT) is the preferred treatment for selected patients with HCC. However, even after the introduction of selection criteria such as Milan Criteria[11], HCC recurrence rate after LT has been estimated to be 8%-17%[12-15]. Many investigators have reported on the spectrum of imaging findings of HCC recurrence after LT, distinguishing different patterns on the basis of recurrence location[12,13,16] or timing[14]. In particular, the majority of tumor burden in recurrent HCC is typically in extrahepatic locations[12,15,16] and the average time to recurrence ranges between 1 and 2 years after LT[14,16]. The hemodynamic imaging characteristics of recurred HCC have been scarcely reported, however, similarly to the primary HCC, the majority of recurred HCC appear as hypervascular lesions.
To our knowledge, the issue of possible variations in imaging and histopathological characteristics between the primary and the recurrent HCC, particularly referring to the relationship between enhancement pattern changes and differentiation degree changes, has never been investigated. The preliminary objective of this study was to evaluate the relationship between HCC contrast-enhanced dynamic imaging pattern and pathological differentiation degree in a population of patients transplanted for HCC. Additional aims were to describe the patterns and imaging features of HCC recurrence after LT and to evaluate the variations in imaging and histopathological characteristics between the primary HCC and the intrahepatic recurrence, particularly elucidating whether differentiation degree variations may justify contrast-enhanced imaging pattern changes.
Between October 2004 and November 2011, a total of 172 consecutive patients with known HCC underwent LT at our hospital. During this period, another patient, transplanted without known HCC, had pathologic diagnosis of incidental HCC in the liver explant. Of these 173 patients, 8 patients were excluded because of a short follow up period (≤ 2 mo), as a result of perioperative mortality. The remaining 165 patients (143 men, 22 women; median age 56.8 years, range 28-70.4 years), who had a follow-up period longer than 2 mo, were included. Fifteen of them were HIV-infected patients. Clinical data about the etiology of the underlying liver disease, were collected. Table 1 summarizes demographic and clinical characteristics of the included patients.
| Demographic and clinical characteristics (n = 165) | ||||
| Sex | Male (%) | 143 (86.7) | ||
| Female (%) | 22 (13.3) | |||
| Median age (range) | 56.8 (28; 70.4) | |||
| HIV + (%) | 15 (9.1) | |||
| Etiology of the underlying hepatic disease | Viral (%) | 136 (82.4) | HBV-related | 28 (17.0) |
| HCV-related | 92 (55.8) | |||
| Mixed | 16 (9.7) | |||
| Not viral (%) | 29 (17.6) | Alcoholic | 16 (9.7) | |
| Cryptogenetic | 6 (3.6) | |||
| Other | 7 (4.2) | |||
All available MDCT and MRI dynamic hepatic examinations were reviewed, selecting those with evidence of viable tumor, and thus where vascularity was assessable. MDCT examinations were performed using a 64-slice CT scanner (Lightspeed VCT, GE Medical Systems, Milwaukee, Wisconsin, United States) with contrast enhancement and bolus-tracking technique to obtain a multiphase (arterial, portal and hepatic venous phases) examination after an unenhanced scan. Image reconstruction was obtained with a 2.5 mm slice thickness and a 2.5 mm interval. Dynamic MRI studies were conducted on a 1.5-T high field magnet (Philips Achieva, Philips Medical System, Best, The Netherlands) with a Phased Array coil. The protocol included axial T1- and T2-weighted sequences with and without fat suppression and axial dynamic three-dimensional T1-weighted fat-suppressed GRE sequences obtained before and after a bolus injection of gadopentetate dimeglumine (Gd-DOTA) in arterial, portal and hepatic venous phases.
Pre-transplantation imaging examinations were retrospectively reviewed by two experienced radiologists by consensus reading, both blinded to the results of the pathologic reports. From the last pre-LT examination with evidence of viable tumor, data about the HCC enhancement pattern were collected. In particular, based on the presence or absence of wash-in, which was defined as present when the lesion was hyperattenuating compared to the surrounding hepatic parenchyma during the arterial phase, lesions were classified into hypervascular and hypovascular.
A pathologist experienced with liver pathologies reviewed all pathologic reports of the explanted livers, collecting data about the differentiation degree, scored according to the World Health Organization criteria[4] into well-differentiated (Grade 1), moderately differentiated (Grade 2), poorly differentiated (Grade 3), and undifferentiated (Grade 4) types. When different degrees were reported in the same explanted liver, the prevailing grade (the one demonstrated by the larger number of nodules) was considered.
All available postoperative dynamic imaging examinations (MDCT or MRI) were retrospectively reviewed for evidence of recurrent HCC. Proof of recurrence was made on the basis of biopsy or growth of new lesions with appropriate radiologic features, combined with rising AFP levels or with negative work-up for another primary malignancy. Imaging features of recurrent HCC were collected. Based on recurrence timing, recurrences were divided into early (< 2 years after LT) and late (≥ 2 years after LT). With respect to tumor location at the moment of the first recurrence, three different patterns were distinguished: intrahepatic recurrence (allograft itself), extrahepatic recurrence and both intrahepatic and extrahepatic recurrence.
All follow-up dynamic imaging examinations were also reviewed to describe the enhancement pattern of all intrahepatic recurrences (those that occurred at the moment of the first recurrence and subsequent ones), in particular classifying lesions into hypervascular and hypovascular based on the presence of wash-in.
Intrahepatic recurrence histopathological differentiation degree was obtained by a review of the available pathological reports (in case of biopsy or resection of the recurred HCC).
The association between pretransplantation HCC enhancement pattern (hypervascular or hypovascular) and explanted liver HCC differentiation degree was evaluated by using the Fisher exact test. Recurrence rate was calculated. The presence of variations in imaging features (enhancement pattern) and histopathological characteristics (differentiation degree) between the primary and the intrahepatic recurred HCC was evaluated and the association between imaging and histopatholgical variations was estimated by using the χ2 test. For all statistical analyses, a P < 0.05 was considered to indicate a statistically significant difference.
The statistical methods of this study were reviewed by Marta Di Nicola from Department of Experimental and Clinical Sciences, Laboratory of Biostatistics, University of Chieti, Italy.
Of the 165 patients who were included, 2 had no evidence of viable tumor in all dynamic imaging examinations performed within 6 mo to LT. Of the remaining 163 patients in whom evaluation of enhancement pattern was possible, 156 (95.7%) had evidence of hypervascular HCC (Table 2). Of these 163 patients, 125 patients had evidence of viable tumor in the explanted liver, while the remaining 38 patients had completely necrotic nodules as a result of pre-transplantation loco-regional therapies performed after imaging examinations. The distribution of the pathological differentiation degree of the primary HCC over the 125 patients who had viable tumor is summarized in Table 2. The differentiation degree was not assessable for 6 patients (4.8%). Different degrees were shown in the same explanted liver in 5 cases, and in such cases the prevailing grade was considered.
| Pre-LT imaging and transplant pathology | ||
| Pre-LT imaging | ||
| Enhancement pattern (n = 163) | Hypervascular (%) | 156 (95.7) |
| Hypovascular (%) | 7 (4.3) | |
| Histopathology | ||
| Differentiation degree (n = 125) | Grade 1 (%) | 19 (15.2) |
| Grade 2 (%) | 56 (44.8) | |
| Grade 3 (%) | 40 (32) | |
| Grade 4 (%) | 4 (3.2) | |
| Not assessable (%) | 6 (4.8) | |
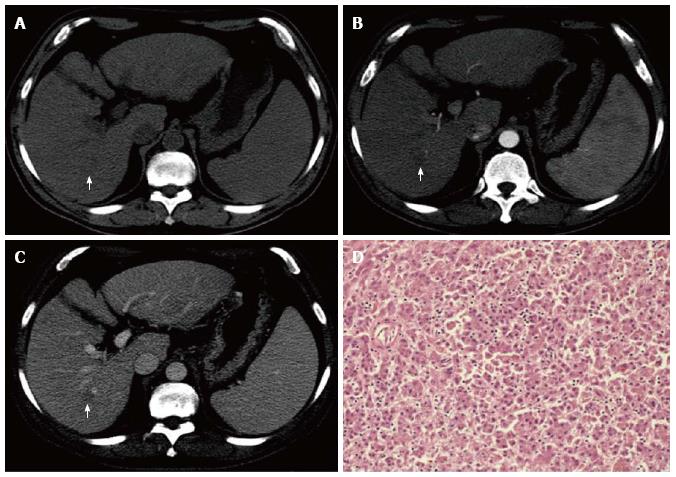
Both enhancement pattern and differentiation degree were available for 113 patients. Among them, a significant association was found between imaging enhancement pattern and histopathological differentiation degree (P = 0.035). As shown in Table 3, 50% (3/6) of the patients whit a hypovascular HCC had a well-differentiated tumor, vs 14% (15/107) of those with a hypervascular HCC. An explicative case of a well-differentiated HCC which was characterized by an atypical enhancement pattern in the pre-LT dynamic MDCT examination is depicted in Figure 1.
| Dynamic imaging enhancement pattern and histopathological differentiation degree (n = 113) | |||||
| Enhancement pattern | Grade 1 | Grade 2 | Grade 3 | Grade 4 | P value |
| Hypovascular | 3 | 1 | 1 | 1 | 0.035 |
| Hypervascular | 15 | 52 | 37 | 3 | |
Of the 165 patients included, 24 (14.55%) had evidence of HCC recurrence after the LT. The 1-, 3-, 5-years cumulative disease-free survival rates according to the Kaplan-Meier method were 92.96%, 83.9% and 82.84%, respectively. The mean duration of recurrence-free survival was 40.15 mo.
Time to development of the recurrence ranged from 1.55 to 41.85 mo after LT, with a median value of 12.36 mo. Three patients (12.5%) experienced late recurrence (≥ 2 years after LT), with a rate of 1.7% (3/175). All recurrences occurred in patients who had a hypervascular primary HCC, while none of the patients with hypovascular primary HCC had a recurrence after LT.
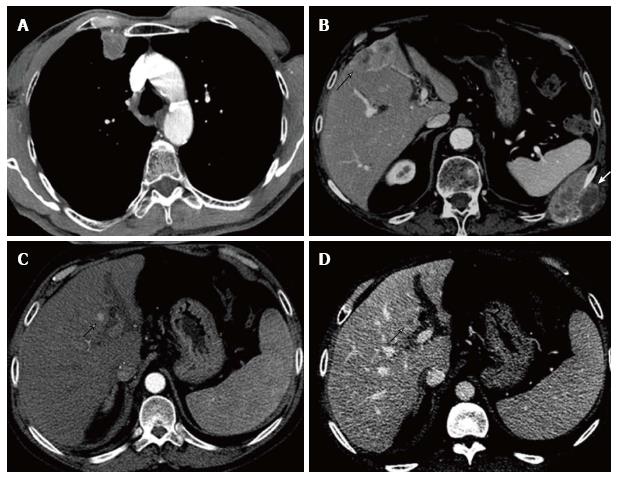
With respect to recurrence location at the moment of the first recurrence, 14 patients (58.3%) showed extrahepatic recurrence, 7 patients (29.2%) had intrahepatic recurrence and 3 patients (12.5%) showed both intrahepatic and extrahepatic recurrence. Eight patients had more than one recurrence site at the moment of the first recurrence. Only one of the 3 patients with late recurrence had intrahepatic recurrence. Recurrence patterns and most common imaging features of recurrence are further described in Table 4 and some recurrence cases illustrated in Figure 2. When extrahepatic recurrences had a solid component which was large enough to allow vascularity evaluation, they showed a contrast enhancement similar to the primary hypervascular HCC, hence usually already evident in the arterial phase of the examination. Frequently they also presented with some necrotic intralesional component.
| Recurrence patterns (n = 24) | ||
| Intrahepatic | 7 (29.2%) | |
| Solitary nodule | 3 (12.5%) | |
| Multifocal lesions | 4 (16.7%) | |
| Extrahepatic | 14 (58.3%) | |
| Lung | 9 (37.5%) | |
| Solitary nodule | 4 (16.7%) | |
| Multiple nodules | 5 (20.8%) | |
| Consolidation | / | |
| Bone | 5 (20.8%) | |
| Osteolytic | 5 (20.8%) | |
| Osteoblastic | / | |
| Lymph nodes | 2 (8.3%) | |
| Brain | 1 (4.2%) | |
| Spleen | 1 (4.2%) | |
| Adrenal | 1 (4.2%) | |
| Intrahepatic and extrahepatic | 3 (12.5%) | |
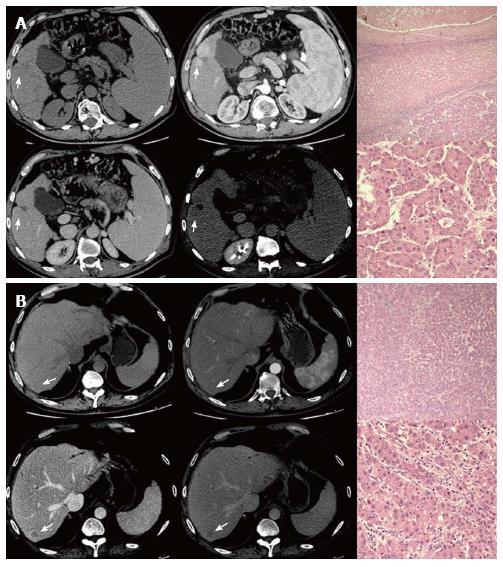
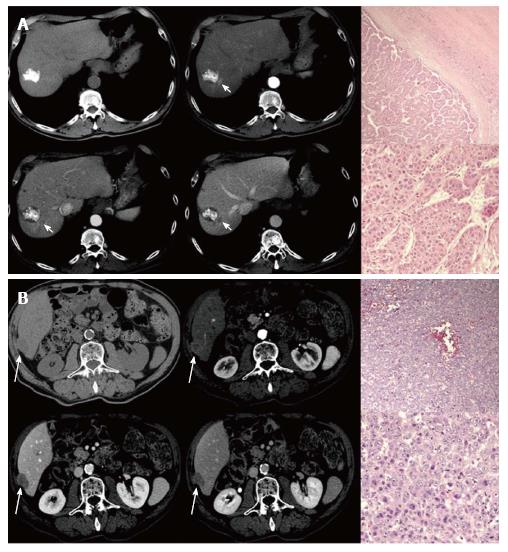
Among the 14 patients who didn’t show intrahepatic recurrence at the moment of the first recurrence, 4 had evidence of hepatic recurrence in further follow-up imaging examinations. On a total of 14 patients who firstly or subsequently developed an intrahepatic recurrent HCC, 12 had hypervascular and 2 had hypovascular recurrent HCCs, classified based on the presence of wash-in. Evidence of wash out was shown by all intrahepatic recurrences. Nine of them underwent liver biopsy with histological diagnosis of HCC, 4 scored as moderately and 5 scored as poorly-differentiated. Two patients (14.3%) showed a variation in imaging characteristics between the primary HCC (hypervascular) and the intrahepatic recurrent HCC (hypovascular). However, they didn’t show any variation in histopathological characteristics (Figure 3). Only 1 patient, who had hypervascular enhancement pattern on pre-LT imaging as well as on recurrence imaging, had a variation of histopathological characteristics (from moderate to poor differentiation) (Figure 4). Therefore, no association was found between imaging and histopathological variations.
It is well known that during HCC development progressive changes in the vascular supply occur[3]: as dysplastic nodules undergo malignant transformation, abnormal neoplastic arterial supply increases while portal supply decreases. Classic HCC is exclusively supplied by the hepatic artery and lacks a portal venous supply, leading, on dynamic imaging examinations, to the typical hypervascular pattern characterized by both wash-in in the arterial phase and wash-out in portal or delayed phases. Less frequently HCCs present as hypovascular lesions, enhancing less than the surrounding liver both on arterial and venous phase imaging, probably as a result of a dual blood supply, both arterial and portal[17,18].
Some investigators have recently reported a correlation between histopathologic grade and HCC blood supply in radiological and pathologic analyses. A tendency towards higher grades in tumors with hypervascular pattern was demonstrated[6-10], however there is some evidence that in the late stage of HCC development, the arterial blood supply decreases again[7-9].
In our population, comparatively with previous studies[9], the majority of HCCs were moderately or poorly differentiated, almost all of them being characterized by a typical hypervascular pattern. On the contrary, the few atypical hypovascular HCCs were predominantly distributed in the well-differentiated group, resulting in 50% of patients with hypovascular HCC vs only 15% of those with typical HCC having a grade 1 tumor. Therefore, despite the fact that in our population a very small number of patients had evidence of atypical HCC (4.3%), a correlation was found between differentiation degree and enhancement pattern (P = 0.035).
Recently, some authors have hypothesized the utility of imaging pattern as a prognostic factor for tumor outcome after locoregional treatment[19] or surgery[20,21]. In our study, the pre-LT imaging examinations of all the 24 patients who experienced HCC recurrence after LT showed hypervascular tumors, and all of them were graded as moderately or poorly differentiated on histopathology (n = 14 grade 2, n = 9 grade 3, n = 1 not assessable). No patient with hypovascular HCC at pre-LT imaging developed post-LT recurrence, not even the few of them with a high histopathological degree. These findings suggest, albeit the number of cases is limited, a lower tendency towards recurrence and a longer recurrence-free survival in patients with hypovascular rather than hypervascular HCCs, underlying the potential prognostic role of vascular supply along with the pathological grade.
In our population the tumor recurrence rate after transplantation was 14.55%, which is similar to the rates observed in other studies with comparable follow-up period and in which Milan selection criteria were adopted[12,14,15]. Extrahepatic recurrence was the most common recurrence pattern with respect to tumor location, comparatively with other studies in which the focus was on the appearance of early recurrence[12,15,16]. Consistently with previous studies[14], the median time to recurrence was 12.36 mo and late recurrence was less common, representing only 12.5% of cases, with a rate of 1.7%.
Focusing on the variations in imaging and histopathological characteristics between the primary and the intrahepatic recurred HCC, among the 14 patients with intrahepatic recurrence, enhancement pattern changes (from hypervascular to hypovascular) were experienced by two patients and histopathological changes (from moderate to poor differentiation degree) were recorded in one patient, underlying that some degree of variability may exist between the primary and the recurred HCC. Even though the number of patients who had changes in imaging or pathological characteristics is small, it is to be noted that no correlation was found between enhancement and differentiation degree changes. In particular, the two patients who changed enhancement pattern did not show any variation in histopathology between the primary and the recurred HCC. On the other hand, in one patient a shift from moderately to poorly differentiated HCC was observed, while no change in the enhancement pattern was registered. This result agrees with the higher prevalence of hypervascular pattern among both grade 2 and 3 HCCs.
Some limitations of this study should be noted. First, it was designed retrospectively. Second, in the correlation analysis between imaging pattern and differentiation degree of the primary tumor, wash-out was not considered, while recently some studies have focused on hypervascular HCCs signal intensity during portal venous phase as an additional predictive factor for differentiation degree[22]. Finally, this study lacked a genomic and immunophenotypical analysis of both the primary and the recurrent HCC.
In conclusion, this study confirms the recently explored correlation between HCC differentiation degree and dynamic-imaging enhancement pattern. Moreover, our preliminary results show that some degree of variability may exist between the primary and the recurred HCC imaging characteristics. If tumoral histopathological characteristics do not seem to justify enhancement pattern variations, a possible explanation is perhaps to be found in changes which occur in liver parenchymal structure and vascularity following transplantation[23]. However, more studies with larger patient groups are needed to better explore the presence of and the reasons for enhancement pattern changes between the primary and the recurred HCC.
A correlation between histopathological grade and vascularity of hepatocellular carcinoma (HCC) has been recently demonstrated. More frequently HCC is hypervascular, an enhancement pattern that usually corresponds to moderate or poor differentiation degree. Liver transplantation is the optimal treatment for HCC, however recurrence rate still ranges from 8% to 17%. HCC recurrence is usually similar to the primary tumor, but some variability in enhancement pattern and histopathological characteristics may be expected.
The relation between HCC pathological grade and vascularity introduces the possible role of imaging enhancement pattern as a prognostic factor for tumor outcome after transplantation. Moreover, in the field of HCC recurrence after transplantation, the reasons for variability between the primary tumor and the recurrence characteristics are still to be understood.
The recently introduced correlation between HCC vascularity and differentiation degree has been discussed in other studies, however the authors aimed to better explore this relationship by applying it to the issue of HCC recurrence after transplantation.
The most relevant future application of the study is the possible use of imaging enhancement pattern as a prognostic factor for tumor recurrence after transplantation; moreover the study of recurrence variability may allow to better understand the factors involved in post-transplantation recurrence.
HCC is the most common type of liver tumor, arising from hepatocytes and typically originating in patients with chronic liver diseases; liver transplantation is the optimal, potentially curative treatment for HCC; recurrence of hepatocellular carcinoma after transplantation reflects hematogenous spread, lymphatic spread and peritoneal seeding of neoplastic cells.
A very interesting topic, with a somewhat novel approach that justifies thee small number of patients included.
P- Reviewer: Abbasoglu O, Boucek C, Dirchwolf M S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Ayuso C, Rimola J, García-Criado A. Imaging of HCC. Abdom Imaging. 2012;37:215-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 2. | Shanbhogue AK, Prasad SR, Takahashi N, Vikram R, Sahani DV. Recent advances in cytogenetics and molecular biology of adult hepatocellular tumors: implications for imaging and management. Radiology. 2011;258:673-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 3. | Trevisani F, Cantarini MC, Wands JR, Bernardi M. Recent advances in the natural history of hepatocellular carcinoma. Carcinogenesis. 2008;29:1299-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 121] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 4. | Ishak KG, Anthony PP, Sobin LH. Histological typing of tumors of the liver. World Health Organization, Geneva. Available from: http: //whqlibdoc.who.int/publications/1994/3540581545_eng.pdf. |
| 5. | Bolondi L, Gaiani S, Celli N, Golfieri R, Grigioni WF, Leoni S, Venturi AM, Piscaglia F. Characterization of small nodules in cirrhosis by assessment of vascularity: the problem of hypovascular hepatocellular carcinoma. Hepatology. 2005;42:27-34. [PubMed] |
| 6. | Tarhan NC, Hatipoğlu T, Ercan E, Bener M, Keleş G, Başaran C, Bilezikçi B. Correlation of dynamic multidetector CT findings with pathological grades of hepatocellular carcinoma. Diagn Interv Radiol. 2011;17:328-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Yoon SH, Lee JM, So YH, Hong SH, Kim SJ, Han JK, Choi BI. Multiphasic MDCT enhancement pattern of hepatocellular carcinoma smaller than 3 cm in diameter: tumor size and cellular differentiation. AJR Am J Roentgenol. 2009;193:W482-W489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 101] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 8. | Asayama Y, Yoshimitsu K, Nishihara Y, Irie H, Aishima S, Taketomi A, Honda H. Arterial blood supply of hepatocellular carcinoma and histologic grading: radiologic-pathologic correlation. AJR Am J Roentgenol. 2008;190:W28-W34. [PubMed] |
| 9. | Jang HJ, Kim TK, Burns PN, Wilson SR. Enhancement patterns of hepatocellular carcinoma at contrast-enhanced US: comparison with histologic differentiation. Radiology. 2007;244:898-906. [PubMed] |
| 10. | Lee JH, Lee JM, Kim SJ, Baek JH, Yun SH, Kim KW, Han JK, Choi BI. Enhancement patterns of hepatocellular carcinomas on multiphasicmultidetector row CT: comparison with pathological differentiation. Br J Radiol. 2012;85:e573-e583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 11. | Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-699. [PubMed] |
| 12. | Kim YS, Lim HK, Rhim H, Lee WJ, Joh JW, Park CK. Recurrence of hepatocellular carcinoma after liver transplantation: patterns and prognostic factors based on clinical and radiologic features. AJR Am J Roentgenol. 2007;189:352-358. [PubMed] |
| 13. | Lee CH, Brubaker LM, Gerber DA, Ku YM, Kim YH, Shin SS, Semelka RC. MRI findings of recurrent hepatocellular carcinoma after liver transplantation: preliminary results. J Magn Reson Imaging. 2011;33:1399-1405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Chok KS, Chan SC, Cheung TT, Chan AC, Fan ST, Lo CM. Late recurrence of hepatocellular carcinoma after liver transplantation. World J Surg. 2011;35:2058-2062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 15. | Chan KM, Chou HS, Wu TJ, Lee CF, Yu MC, Lee WC. Characterization of hepatocellular carcinoma recurrence after liver transplantation: perioperative prognostic factors, patterns, and outcome. Asian J Surg. 2011;34:128-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Roayaie S, Schwartz JD, Sung MW, Emre SH, Miller CM, Gondolesi GE, Krieger NR, Schwartz ME. Recurrence of hepatocellular carcinoma after liver transplant: patterns and prognosis. Liver Transpl. 2004;10:534-540. [PubMed] |
| 17. | Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208-1236. [PubMed] |
| 18. | Nakashima Y, Nakashima O, Hsia CC, Kojiro M, Tabor E. Vascularization of small hepatocellular carcinomas: correlation with differentiation. Liver. 1999;19:12-18. [PubMed] |
| 19. | Kawamura Y, Ikeda K, Seko Y, Hosaka T, Kobayashi M, Saitoh S, Kumada H. Heterogeneous type 4 enhancement of hepatocellular carcinoma on dynamic CT is associated with tumor recurrence after radiofrequency ablation. AJR Am J Roentgenol. 2011;197:W665-W673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 20. | Choi JW, Lee JM, Kim SJ, Yoon JH, Baek JH, Han JK, Choi BI. Hepatocellular carcinoma: imaging patterns on gadoxetic acid-enhanced MR Images and their value as an imaging biomarker. Radiology. 2013;267:776-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 135] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 21. | Chung GE, Lee JH, Yoon JH, Myung SJ, Lee K, Jang JJ, Lee JM, Kim SH, Suh KS, Kim YJ. Prognostic implications of tumor vascularity and its relationship to cytokeratin 19 expression in patients with hepatocellular carcinoma. Abdom Imaging. 2012;37:439-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Okamoto D, Yoshimitsu K, Nishie A, Tajima T, Asayama Y, Ishigami K, Hirakawa M, Ushijima Y, Kakihara D, Nakayama T. Enhancement pattern analysis of hypervascular hepatocellular carcinoma on dynamic MR imaging with histopathological correlation: validity of portal phase imaging for predicting tumor grade. Eur J Radiol. 2012;81:1116-1121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 23. | Navasa M, Feu F, García-Pagán JC, Jiménez W, Llach J, Rimola A, Bosch J, Rodés J. Hemodynamic and humoral changes after liver transplantation in patients with cirrhosis. Hepatology. 1993;17:355-360. [PubMed] |