Published online May 27, 2014. doi: 10.4254/wjh.v6.i5.326
Revised: February 8, 2014
Accepted: April 11, 2014
Published online: May 27, 2014
Processing time: 207 Days and 8.5 Hours
A new treatment paradigm for hepatitis C is that the treatment must include an existing direct-acting antiviral agent, namely, a protease inhibitor (PI) combined with PEGylated interferon-α and ribavirin. The currently marketed PIs and PIs in clinical trials have different mechanisms of action. The development of new PIs aims for an improved safety profile and higher effectiveness. This article reviews NS3/4A protease inhibitors, focusing on major criteria such as their effectiveness and safety. Specific attention is paid to dosing regimens and adverse event profiles of PIs administered in clinical settings.
Core tip: This article reviews NS3/4A protease inhibitors, focusing on major criteria such as effectiveness and safety. Specific attention is paid to dosing regimens and adverse event profiles of protease inhibitor administered in clinical settings.
- Citation: Bakulin I, Pasechnikov V, Varlamicheva A, Sannikova I. NS3 protease inhibitors for treatment of chronic hepatitis C: Efficacy and safety. World J Hepatol 2014; 6(5): 326-339
- URL: https://www.wjgnet.com/1948-5182/full/v6/i5/326.htm
- DOI: https://dx.doi.org/10.4254/wjh.v6.i5.326
Since 2011, increased attention has been given to “direct” antiviral agents for chronic hepatitis C (HCV). Combined treatment with PEGylated interferon-α (PEG-IFNα) and ribavirin cannot be considered a standard treatment for type 1 HCV anymore. A new treatment paradigm is that the treatment must include an existing direct-acting antiviral agent (DAA), namely, protease inhibitor (PI) combined with PEG-IFNα and ribavirin.
The currently marketed PIs and PIs in clinical trials (CTs) have different mechanisms of action. The development of new PIs aims for an improved safety profile and higher effectiveness[1]. An ideal combination is an interferon-free therapy with oral once-daily agents that are highly effective and well tolerated, do not interact with the majority of well-known therapeutics, and can be used to treat concomitant disorders. The recent evolution of DAA has included a considerable improvement in their effectiveness since 2011, and in most cases, antiviral treatment (AVT) duration has decreased.
This article reviews NS3/4A protease inhibitors, focusing on major criteria such as effectiveness and safety. Specific attention is paid to the dosing regimens and adverse event (AE) profiles with PIs administered in clinical settings.
Since 2011, we have seen an increase in the value placed on “direct” antiviral agents for HCV. Most of these agents are at various CT stages, with some already being integrated in routine clinical practice as a treatment standard for type 1 HCV patients (Table 1). In 2011, FDA and ЕМА approved the first DAA-telaprevir and boceprevir - for HCV treatment in patients infected with type 1 HCV. Randomized CTs have shown that triple therapy is not only significantly more effective for type 1 HCV patients but that it is also the only alternative for patients with previous AVT failure. One should note that in Russia, telaprevir was approved in December 2012 and boceprevir in May 2013.
| NS3/4A protease inhibitors | Polymerase inhibitors | Inhibitors | ||
| Nucleotides/nucleosides | Non-nucleoside | NS5A | Cyclophilin | |
| Generation I: Boceprevir (Merck)1 Telaprevir (Vertex)1 | PSI-7977 (Pharmasset)1 PSI-938 (Pharmasset)1 Mericitabine (Roche/ Genentech)1 IDX-184 (Idenix) | Filibuvir (Pfizer)1 VX-222 (Vertex)1 Tegobuvir (Gilead)1 ANA-598 (Anadys)1 ABT-072 (Abbott)1 ABT-333 (Abbott)1 | Daclatasvir (Bristol-Myers Squibb) GS-5885 (Gilead)1 | Alisporivir (Novartis)1 SCY-635 (Scynexis)1 |
| Generation II: Simeprevir (Tibotec) BI 201335 (Boehringer Ingelheim) Danoprevir (Roche/Genentech), studied with Ritonavir; Vaniprevir (Merck)2 BMS-650032 (Bristol-Myers Squibb)2 GS-9451 (Gilead)1 GS-9256 (Gilead)2 ACH-1625 (Achillion)1 ABT-450 (Abbott) MK-5172 (Merck)1 | ||||
NS3/4А serine protease inhibitors are divided into two classes. The first generation includes the well-studied telaprevir and boceprevir. By the time their phase III CT was completed, these agents were already acknowledged as new AVT standards for type 1 HCV patients.
NS3/4A protease has a crucial role in the replication cycle of hepatitis C virus. It cleaves polyprotein in four sequential active sites, forming the N-terminal proteins NS4A, NS4B, NS5A and NS5B. Regarding its chemical properties, this enzyme is related to the serine protease group. For instance, it can cleave and inactivate the host proteins Trif and Cardif. Both of these proteins are important in the responses to interferon (IFN) mediated by the receptors TLR3 and RIG-I, respectively[2,3]. Additionally, NS3 is not only a protease but also a component of the replication complex for viral RNA, acting as an RNA-helicase and nucleotide triphosphatase (NTPase). Due to its impressive set of functions, NS3 protease is an attractive target for HCV therapy. The HCV RNA replication cycle and targets for direct-acting antivirals have been thoroughly described in publications by Moradpour and Pawlotsky[4,5]. Clinical trials have studied several promising molecules that inhibit HCV protease.
Table 2 reviews published CT results for ultra-novel NS3/4a PIs, including their efficacy and safety parameters[6].
| PI | Genotype | PI treatment duration (wk) | Treatment duration (wk) | Treatment regimen | PEG-IFNα | Publication date |
| Telaprevir | 1а/1b/1с/unknown | 12/8 | 20/24/44/48 | 750 mg TID | (+) | 2011 |
| Boceprevir | 1а/1b/unknown | 24/32/44 | 28/36/48 | 800 mg TID | (+) | 2011 |
| Daclatasvir | 1а/1b | 24 | 24 | 60 mg/d | (+/-) | 2012 |
| Asunaprevir | 1а/1b | 24 | 24 | 600 mg BID | (+/-) | 2012 |
| АВТ-450 | 1а/1b | 12 | 12 | 250/150 mg/d | (-) | 2013 |
Telaprevir efficacy was studied in phase II and III CTs (Table 3)[6-8].
| RCT | Dose frequency | Duration | SVR | Possible AE |
| Prove 1 | Each 8 h, 6 t/d | 24 wk: 12 wk of triple therapy, 12 wk of conventional treatment | 61% | Rash, anemia, nausea, diarrhea |
| Advance | Every 8 h, 6 t/d | 24-48 wk: 8-12 wk of viral response-based treatment followed by conventional treatment | 69%-75% | Rash, anemia, nausea, diarrhea |
| Illuminate | Every 8 h, 6 t/d | 24-48 wk: 12 wks of viral response-based treatment: 12 wk of triple therapy followed by conventional treatment | 64%-92% | Rash, anemia, nausea, diarrhea |
| Optimize | Every 12 h, 6 t/d | 24-48 wk: 12 wk of viral response-based treatment: 12 wk of triple therapy followed by conventional treatment for 12 to 36 wk | 58%-81% (depending on fibrosis stage) | Rash, anemia, nausea |
For telaprevir-based AVT, the following regimens are used: Treatment-naive patients and relapsers: Telaprevir is started from treatment day 1 and is always combined with conventional treatment of PEG-IFN/RBV for 12 wk. If no viremia is present (HCV RNA-negative) at 4 and 12 wk, treatment duration is 24 wk. If viral load is detected (HCV RNA-positive) at 4 or 12 wk, treatment duration is 48 wk. Null responders or partial responders as well as liver cirrhosis patients: The only option for triple therapy is telaprevir for 12 wk, with a total AVT duration of 48 wk. Telaprevir-based regimens have clear algorithms for AVT early discontinuation[9]. Triple therapy must be totally canceled in the following cases: HCV RNA above 1000 IU/mL at 4 and 12 wk on triple therapy; HCV RNA-positive at treatment week 24; Viral breakthrough and/or viral load increase.
The above rules are unambiguous and must be strictly followed because they are evidence-based results that were developed after multicenter randomized CTs. If HCV RNA is present in the titers above, it indicates that the AVT is ineffective when continued treatment has no clinical or cost-effective rationale. Moreover, ongoing treatment might result in resistant strain development, as indicated by phase II and III CTs showing relapses and no viral response.
The AE control algorithm is important for telaprevir-based treatment. The safety profile of triple therapy has a higher AE number vs conventional treatment, which in the future, may be a limiting factor for first-generation PI use (Table 4).
| Agent RCT | Telaprevir | ||||
| Advance | Realize | Illuminate | |||
| PR | T8/12PR | PR48 | (lead-in) T12PR48 | T12PR24/48 | |
| Serious AE | 7% | 9% | 5% | 12% | 9% |
| Discontinued AVT due to AE | 7% | 10% | 3% | 15%-11% | 18% |
| Anorectal symptoms | 4% | 8%-13% | 7% | 15%-12% | - |
| Taste disturbances | - | - | 6% | 12% | - |
| Anemia | 19% | 39%-37% | 15% | 30%-36% | 39% |
| Severe neutropenia | 19% | 17%-14% | 11% | 14%-13% | - |
| Rash | 24% | 35%-37% | 19% | 37%-36% | 37% |
| Fatigue | 57% | 58%-57% | 40% | 55%-50% | 68% |
| Pruritus | 36% | 45%-50% | 27% | 52%-50% | 51% |
| Nausea | 31% | 40%-43% | 23% | 35%-33% | 47% |
| Diarrhea | 22% | 32%-28% | 14% | 25%-26% | 30% |
Triple-therapy AEs have been reviewed in CT results and other recent publications[10]. Therefore, it seems necessary to dwell on some of them because developing AEs might require changes in patient management (PI or AVT discontinuation) or may be difficult to control in clinical settings.
Telaprevir has the following common AEs: rash, anemia and anorectal signs (as shown by the ADVANCED and REALIZE trials).
Telaprevir-based triple therapy increases the anemia rate by 15% to 21% vs control. The severe anemia rate is comparable among study arms and results in discontinued treatment in 2%-4% of cases (Table 5).
| RCT | Dose frequency | Duration | SVR | Possible AE |
| SPRINT 1 | 12 pills for 3 intakes | 28-wk triple therapy vs 4-wk lead-in phase | 54%-56% | Metal taste, anemia |
| 48-wk triple therapy vs 4-wk lead-in phase | 67%-75% | |||
| SPRINT 2 | 12 pills for 3 intakes | 28-48 wk: ''viral response-based treatment''; ''lead-in period''; if HCV RNA (-) by week 8 and 24, to stop at week 28; if HCV RNA (+), 20 wk of double therapy | 67% And 44% were given abridged AVT | Taste disturbances, anemia, neutropenia |
Anemia development in the compared arms is not a negative prognostic criterion for SVR. Currently, the main method for anemia control is ribavirin dose adjustment. Some experts consider that an Hb below 7.5 g/dL implies complete triple therapy discontinuation. However, the CUPIC study showed that AVT can be continued if erythropoietin and blood transfusions are used[11,12].
Rash is considered a specific AE for telaprevir-based therapy and results in 5%-7% of treatment discontinuation cases. In 50% of cases, rash appears within the first 4 wk of treatment, but rash can develop during the whole course of treatment. In some rare cases, skin signs can be classified as serious AEs.
The rash treatment algorithm depends on its severity (evaluated on the body surface involved). Mild to moderate rash is an indication for antihistamine agents, local steroid ointments and avoiding sunlight. It does not require stopping triple therapy. For severe rash, it is recommended to stop telaprevir, and conventional treatment can be continued with the provision of effective treatment with steroids (locally) and antihistamines. In case of progression and severe skin signs, treatment must be canceled.
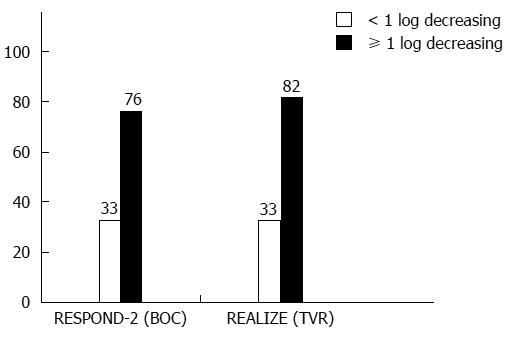
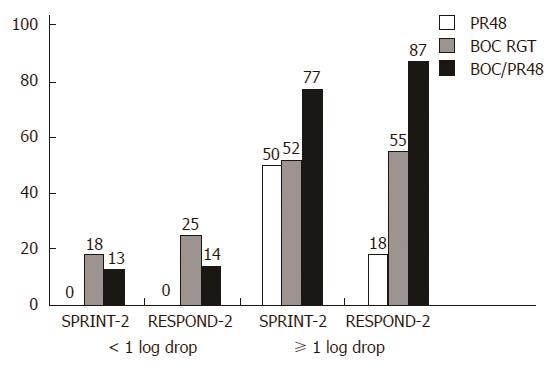
Boceprevir’s efficacy was studied in phase II/III CTs (Table 5)[1,6]. Considering a new term, i.e., the lead-in period or lead-in phase, several issues should be discussed. Triple therapy development has provided data on new sensitive response predictors. Assessing the viral load decrease after a 4-wk lead-in can allow for an accurate assessment of a patient’s chances to reach SVR (Figure 1): A lead-in period enables researchers to assess conventional treatment tolerance and prognosis if PEG-IFN/RBV is safe to use. RCT results (RESPOND-2 and PROVE-2) show that a lead-in period lowers the viral load before the onset of triple therapy and delineates a patient group that should receive a shorter AVT duration. These diminish the probability of mutant, PI-resistant HCV strain development. If the viral load drops by more than 2 log10, this indicates high patient sensitivity to IFNα and ribavirin, which is a rationale to continue AVT as a standard treatment. However, it seems that the main objective of the lead-in period is to discern the patient groups in which conventional AVT appears to be less effective in a prognostic sense, and triple therapy makes it possible to avoid unjustified treatment costs and a non-mandatory pharmaceutical load when double therapy is continued (Figure 2).
An important issue is strict compliance with discontinuation rules for triple therapy. For instance, ineffective boceprevir-based triple AVT should stopped in time to prevent the development of boceprevir-resistant HCV strains. The entire triple therapy should be canceled in the following cases (algorithm for early treatment discontinuation): (1) if the HCV RNA is above IU/mL at week 12 of AVT (triple therapy week 8); (2) if there is no aviremia at week 24 of AVT (triple therapy week 20); and (3) in case of viral breakthrough and/or viral load increase by 1 log10.
The SPRINT-2 and RESPOND-2 data show that boceprevir use increases the rate of taste disturbances, anemia and neutropenia[6,13] (Table 6).
| AgentRCT | Boceprevir | |||
| SPRINT-2 | RESPOND-2 | |||
| PR48 | PR4/ PRB24/44 | PR48 | PR4/ PRB32/44 | |
| Serious AE | 9% | 11%-12% | 5% | 10%-14% |
| Discontinued AVT due to AE | 16% | 12%-16% | 2% | 8%-12% |
| Anorectal symptoms | - | - | - | - |
| Taste disturbances | 18% | 37%-43% | 11% | 43%-45% |
| Anemia | 29% | 49% | 20% | 43%-46% |
| Severe neutropenia | 14% | 24%-25% | 9% | 19%-20% |
| Rash | 23% | 25%-24% | 5% | 17%-14% |
| Fatigue | 60% | 53%-57% | 50% | 53.7%-57.1% |
| Pruritus | 27% | 24%-26% | 17.50% | 18.5%-19.3% |
| Nausea | 42% | 48%-43% | 37.50% | 43.8%-39.1% |
| Diarrhea | 22% | 22%-27% | 15% | 22.8%-23% |
The development of second-generation NS3/4A protease inhibitors resulted in some hopes of improving treatment outcomes in type 1 HCV patients. This group of agents has some advantages compared to the 1st-generation NS3/4A protease inhibitors (telaprevir and boceprevir): first, the dosing mode (once a day) and second, a better tolerance profile (fewer adverse events). However, the fact that both groups of agents have both a common viral genotype as their target and similar resistance profiles restrain us from considering 2nd-generation NS3/4A protease inhibitors to be a new class of HCV protease inhibitors. Nevertheless, modern publications still use this term for a range of new therapeutic agents with improved pharmacokinetics. It is possible that after some CTs are completed, 2nd-generation NS3/4A protease inhibitors will replace the 1st-generation agents when combined with PEG-IFN/RBV, thereby becoming the 1st generation of DAA in regimens of so-called interferon-free HCV treatment.
Simeprevir (TMC435; Tibotec, Beerse, Belgium; Medivir Pharmaceuticals, Stockholm, Sweden; Janssen, Beerse, Belgium) is one of the 2nd-generation NS3/4A protease inhibitors. Simeprevir has passed phase I to III trials in patients with 1a and 1b HCV genotypes.
Phase I and II trials demonstrated potential antiviral activity for TMC435, as well as its efficacy and tolerability. TMC435’s pharmacokinetic properties enable its use in once-a-day dosing[14].
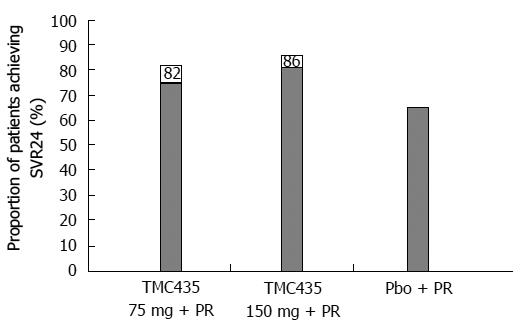
To study TMC435’s efficacy and safety, a phase IIb trial, PILLAR [Protease Inhibitor TMC435 trial assessing the optimaL dose and duration as once daiLy Anti-viral Regimen] (TMC435-C205; NCT00882908), was organized. Conducted in 13 countries in Europe, North America and Australia, it enrolled 368 naive patients with genotype 1 treated with simeprevir combined with PEG-IFN/RBV for 24 or 48 wk. Two doses of simeprevir (75 mg vs 150 mg) and treatment durations (12 wk vs 24 wk) were compared. The final analysis of the PILLAR study showed that TMC435 given in combination with PEG-IFN/RBV to naive patients with genotype 1 HCV resulted in a SVR rate that significantly exceeded that observed in patients treated with the placebo + PEG-IFN/RBV combination (Figure 3).
In 2 patient arms treated with TMC435 at 75 mg/d, the patient percentage reaching SVR in varied from 75% (12 wk) to 82% (24 wk); with TMC435 at 150 mg/d, this percentage varied from 81% (12 wk) to 86% (24 wk). In the comparison arm, the percentage of placebo-treated patients reaching SVR in 24 wk or less amounted to 65%[15].
A new publication[16] analyzing the PILLAR study results indicated that SVR determined in 24 wk after planned treatment completion (SVR24) varied in the range of 74.7%-86.1% for all simeprevir arms vs 64.9% in the control. All arms treated with simeprevir for 12 or 24 wk showed a significant difference in SVR24 parameters, excluding the arm given 75 mg/d for 24 wk. Rapid virologic response (HCV RNA < 25 IU/mL, undetermined level at treatment week 4) was reached in the SMV-treated arms in 68.0%-75.6% of cases, compared to 5.2% in the placebo-treated controls. The criteria for a shortened treatment course (RGT criteria) were met in 79.2%-86.1% of the SMV-treated patients completing treatment in 24 wk. SVR24 was reached in 85.2%-95.6% of patients in these groups[16].
An international, randomized, double-blinded, controlled phase IIb study, ASPIRE (TMC435-C206; NCT00980330), aimed to evaluate the efficacy, tolerability, safety and pharmacokinetics of TMC435 given in combination with PEG-IFN/RBV[17]. ASPIRE enrolled 462 treatment-experienced genotype 1 HCV patients. This arm included partial responders, prior relapsers, and patients with significant fibrosis or cirrhosis (Metavir, F4 stage). Patients were randomized to receive 100 or 150 mg of simeprevir OD or placebo for 12, 24 or 48 wk. The study arms with simeprevir treatment for 12 or 24 wk later continued treatment with PEG-IFN/RBV (control) only up to 48 wk. The SVR rate was significantly higher in all simeprevir-treated arms compared to those treated with PEG-IFN/RBV only. The best results were reached in patients treated with simeprevir 150 mg/d. For instance, SVR in prior relapsers reached 85% vs 37% in the control, 75% and 19% in the two subgroups of partial responders, and 51% and 19% in the two subgroups of non-responders[18]. It is important to note that a high SVR24 (31%) was found with simeprevir-based therapy in subgroups of liver cirrhosis patients and those with a previous null response, i.e., those who traditionally are considered difficult to treat.
Viral breakthrough (in 42 of 43 patients) or infection relapse (in 34 of 36 patients) was associated with viral resistance development. Viral breakthrough has been noted in most studies when using protease inhibitors in combination with conventional treatment[18]. Genotype 1a patients more often have mutation R155 as the only mutation or with other mutations, while genotype 1b patients have mutation D168V[18].
At the moment, 3 Phase III clinical studies to study simeprevir’s efficacy are known: (1) in treatment-naive HCV patients: QUEST-1 and QUEST-2; (2) in relapsers after previous PEG-IFN/RBV treatment: PROMISE; and (3) in null-responders: ATTAIN.
QUEST-1 enrolled 394 genotype 1 HCV patients with F0-F4 fibrosis (METAVIR scale) stratified by HCV subtype and genotype IL28B[19]. Patients were randomized to a simeprevir dose of 150 mg/d or placebo combined with PEG-IFN/RBV for 12 wk followed by PEG-IFN/RBV monotherapy. A treatment duration of 24 or 48 wk in the simeprevir arm and the placebo arm depended on treatment response at wk 4 and 12. If virus was undetected in the blood (HCV RNA < 25 IU/mL) at weeks 4 and 12, the patient met the short treatment criteria (RGT-criteria), and the treatment was finished at week 24. Treatment for 48 wk was recommended for patients who were treated with placebo instead of simeprevir. The majority of simeprevir-treated patients were compliant with the RGT criteria (85%) and completed treatment at week 24. The rapid response rate (RVR) reached 80% in the patients treated with simeprevir in combination with PEG-IFN/RBV and 12% in the patients treated with placebo and PEG-IFN/RBV. Simeprevir combined with PEG-IFN/RBV resulted in HCV elimination in more patients compared to the combination of placebo and PEG-IFN/RBV (80% vs 50%, P < 0.001). The relapse rate in the simeprevir/PEG-IFN/RBV arm was less than that in the placebo/PEG-IFN/RBV arm (9% vs 21%), as was the percentage of treatment failures (9% vs 34%). The QUEST-1 study showed that simeprevir 150 mg/d OD given along with PEG-IFN/RBV provided a high SVR12 rate, making it possible to decrease the treatment duration to 24 wk in a majority of patients (85%).
QUEST-2, a randomized, double-blinded, placebo-controlled study (NCT01290679), enrolled approximately 400 treatment-naive patients with genotype 1 HCV[20]. Patients were stratified according to genotype 1 subtype and host genotype (IL28B). They were given simeprevir (150 mg OD) combined with PEG-IFN/RBV (both of PEG-IFN type) or placebo combined with PEG-IFN for 12 wk followed by a PEG-IFN/RBV regimen. A treatment duration of 24 or 48 wk in both patient arms depended on the treatment response at weeks 4 and 12. If virus was undetected in the blood (HCV RNA < 25 IU/mL) at weeks 4 and 12, the patient met the short treatment criteria (RGT-criteria) and the treatment was finished at week 24. Treatment for 48 wk was recommended for patients who were treated with placebo instead of simeprevir. A majority of simeprevir-treated patients complied with the RGT criteria (91%) and completed treatment at 24 wk. The rapid virologic rate (RVR) was 79% in simeprevir/PEG-IFN/RBV patients and 13% in patients treated with placebo/PEG-IFN/RBV. Simeprevir combined with PEG-IFN/RBV provided HCV elimination in more patients than did the combination of placebo with PEG-IFN/RBV (SVR12 rate: 81% vs 50%, P < 0.001). The relapse rate in the simeprevir/PEG-IFN/RBV arm was lower than that in the placebo/PEG-IFN/RBV arm (13% vs 24%), as was the rate of treatment failure (7% vs 32%). The QUEST-2 study showed that simeprevir 150 mg/d OD given along with PEG-IFN/RBV provides a high SVR12 rate, making it possible to decrease the treatment duration to 24 wk in a majority of patients (91%).
The objective of the phase III trial PROMISE (TMC435-HPC3007) was to study the efficacy, safety and tolerability of simeprevir combined with PEG-IFN/RBV in patients infected with genotype 1 HCV and treatment failure. The study enrolled 393 prior relapsers after treatment. Approximately 40% of patients had the 1a subtype of the HCV genotype, approximately 75% of them had the unfavorable genotype IL28B, 15% had considerable liver fibrosis (stage F3), and 15% had diagnosed liver cirrhosis (stage F4). Patients were given 150 mg of simeprevir combined with PEG-IFN/RBV for 12 wk, followed by PEG-IFN/RBV only for another 12 wk. At this point, the patients either stopped treatment based on the RGT criteria (no virus in blood at treatment week 4 and 12) or continued PEG-IFN/RBV treatment up to week 48. In the control arm, placebo combined with PEG-IFN/RBV was given for 12 wk; up to week 48, they were given basic treatment, i.e., PEG-IFN/RBV. A total of 77% of the simeprevir-treated patients and 3% of the control group developed rapid treatment response in 4 wk (RVR). At the end of treatment, the responses were very high: 97% in the simeprevir group and 72% in the control. The majority of patients (93%) complied with the RGT criteria (treatment termination at week 24); in this group, 83% of patients reached SVR during the following 12 wk of basic treatment (SVR12). Among the remaining 7% of simeprevir-treated patients who did not comply with the RGT criteria and continued treatment to week 48, SVR12 was reached in only 32%. Among patients with HCV subtype 1a, SVR12 was reached in 70% of the simeprevir-treated group and in 28% of the placebo group; for subtype 1b, these values were 86% and 43%, respectively. The IL28B CC genotype was associated with a better response to simeprevir-based triple therapy vs control (SVR12 was 89% vs 53%). SVR12 for the СТ genotype was 79% vs 34%. SVR12 for the TT genotype was 65% vs 19%. Regardless of fibrosis severity, the SVR rate in the simeprevir-treated arms was higher than in control. For instance, with fibrosis stage F0-F2 (absent to moderate), the SVR rate was 82%; with significant fibrosis, it was 73%; and with liver cirrhosis, it was 74%. In the control group, SVR reached 41%, 20% and 26%, respectively. Ineffective treatment was noted in 3% of simeprevir-treated patients and in 27% of control patients. Relapse after treatment completion was found in 19% and 48%, respectively. Thus, in case of relapse after conventional therapy with PEG-IFN/RBV in genotype 1 HCV patients, treatment with simeprevir and PEG-IFN/RBV provided a high cure rate: 79% of patients reached SVR12.
The phase III trial ATTAIN (NCT01485991) is studying the efficacy of simeprevir plus PEG-IFN/RBV and telaprevir plus PEG-IFN/RBV in patients with a failed attempt at HCV eradication after conventional therapy (PEG-IFN/RBV). ATTAIN is projected to be finished in 2014.
The profile of adverse events recorded in the PILLAR study[15,16] was similar between the group treated with simeprevir and the group with conventional treatment. For instance, comparing patients treated with simeprevir/PEG-IFN/RBV vs placebo/PEG-IFN/RBV, an adverse event rate > 10% was recorded for fatigue (42.4% and 48.1%, respectively), flu-like syndrome (31.7% and 37.7%, respectively), itching (31.1% and 45.5%, respectively), headache (46.0% and 51.9%, respectively), nausea (27.8% and 27.3%, respectively), rash (21.0% and 23.4%, respectively), anemia (20.4% and 20.8%, respectively), neutropenia (24.3% and 20.8%, respectively).
The majority of adverse events recorded in patients treated with simeprevir/PEG-IFN/RBV in the ASPIRE study were also observed in patients treated with disease-modifying therapy PEG-IFN/RBV (fatigue, flu-like syndrome, itching, headache, nausea) and were similar to the patient control group[17,18]. Adverse events requiring the discontinuation of at least one of the therapeutics in the study were reported in 4%-10.4% of the patients treated with simeprevir/PEG-IFN/RBV combination compared to 13% of the control group. Serious adverse events (SAEs) were detected at similar rates in patients treated with the combination of simeprevir and disease-modifying therapy (3.8%-11.5%) and in the patients treated with placebo combined with disease-modifying therapy (13%). Anemia developed in 19.0%-22.1% of patients treated with simeprevir + PEG-IFN/RBV and in 20.8% of patients treated with placebo combined with PEG-IFN/RBV. In both arms, anemia did not result in discontinued treatment. Skin rash of any type was reported in 23.4%-30.8% of patients treated with simeprevir + PEG-IFN/RBV and in 20.8% of in patients treated with placebo + PEG-IFN/RBV. Rash resulting in discontinued treatment was noted in only 3 cases (2 patients of the simeprevir + PEG-IFN/RBV arm and 1 patient of the placebo + PEG-IFN/RBV arm). Insignificant, isolated and reversible increase of both bilirubin types (direct and indirect) in blood serum was found in patients treated with simeprevir + PEG-IFN/RBV. Because elevated plasma activity of alanine aminotransferase (ALT) and alkaline phosphatase (ALP) was not associated with the simultaneous elevation of bilirubin, the elevated serum ALT in the majority of patients was interpreted as a developed biochemical response during the treatment.
In the PILLAR and ASPIRE studies, fatigue as a treatment-related adverse event was reported in 63%-65% of treatment-naive and 97% of treated patients[21,22]. In both trials, fatigue severity according to the Fatigue Severity Scale increased with treatment duration. However, fatigue disappeared more quickly in treatment-naive simeprevir-treated patients than it did in patients treated with PEG-IFN/RBV only. Considering the follow-up period of 72 wk after treatment completion, these differences were statistically significant (P < 0.001).
These trials also showed lower the quality of life in patients according to the health-related quality of life (HRQoL) scale. Treatment-naive simeprevir-treated patients showed a faster quality of life improvement compared to the group treated with PEG-IFN/RBV only[21,22].
Simeprevir was well tolerated by patients enrolled in the QUEST-1, QUEST-2, and PROMISE studies[19-23]. The total AE incidence was similar in the arms treated with simeprevir + PEG-IFN/RBV and placebo + PEG-IFN/RBV.
Discontinued treatment due to adverse events in both arms was found in 3% of patients[19]. The grade 3-4 adverse event rate was 23% in the simeprevir + PEG-IFN/RBV patient arm and 29% in the PEG-IFN/RBV arm. The most common adverse events in the simeprevir and placebo arms were fatigue (40% and 38%, respectively), headache (31% and 37%, respectively), and itching (21% and 11%, respectively). Simeprevir intake was associated with transient moderately elevated bilirubin that was not associated with elevated aminotransferases or alkaline phosphatase. Rash and photosensitivity were slightly more common in patients who were treated with simeprevir compared to the patients receiving placebo (27% vs 20% and 4% vs 1%, respectively). During the PROMISE study, a shorter treatment duration resulted in lower fatigue intensity and a faster return to normal activity among patients treated with simeprevir and PEG-IFN/RBV.
Simeprevir seems to be preferable when choosing HCV treatment compared to telaprevir or boceprevir because it is advantageous with regard to dosing regimen (once a day), tolerance and safety (no rash or anemia). All three phase III trials, QUEST-1, QUEST-2, and PROMISE, showed a high infection cure rate (79%-81% SVR12). Importantly, the addition of simeprevir to PEG-IFN/RBV was associated with a higher SVR rate without a significant increase in fatigue severity or decrease in quality of life. Moreover, the shorter-duration antiviral treatment with simeprevir was associated with a higher SVR rate and a shorter period of worsened quality of life.
Faldaprevir (BI 201335, Boehringer Ingelheim Pharmaceuticals, Ingelheim, Germany) is a 2nd-generation NS3/4A protease inhibitor with once-a-day dosing.
Faldaprevir’s efficacy, tolerance and safety were studied in genotype 1 HCV patients in multiple phase II and III clinical trials (SILEN-C1, SILEN-C2, SILEN-C3, STARTVerso™1).
SILEN-C1 and SILEN-C2 were phase II randomized clinical studies with the objective of examining BI 201335’s efficacy and safety in combination with PEG-IFN/RBV in treatment-naive patients[22] and in treatment failures (partial or non-responders)[24] infected with genotype 1 HCV. Both trials studied the effectiveness of a 3-d lead-in phase with PEG-IFN/RBV. The lead-in period was used when studying boceprevir’s efficacy in combination with PEG-IFN/RBV in genotype 1 HCV patients[13,25]. This treatment phase was expected to lower the probability of developing HCV resistance during the treatment.
SILEN-C1 enrolled 429 treatment-naive patients infected with genotype 1 HCV[22]. Four study arms were made: for 24 wk, patients were administered a combination of PEG-IFN/RBV with placebo (control group), faldaprevir 120 mg OD with a 3-d PEG-IFN/RBV lead-in (LI) phase, faldaprevir 240 mg OD with LI, or faldaprevir 240 mg OD without LI followed by PEG-IFN/RBV therapy up to the total 24 wk. If a patient taking faldaprevir 240 mg complied with the RGT criteria (HCV RNA < 25 IU/mL at week 4, undetectable viral load at weeks 8-20), the treatment was discontinued at week 24. The rest of the patients continued PEG-IFN/RBV therapy up to week 48. The SVR rate was 56%, 72%, 72% and 84%, respectively, for the four arms. In total, 92% of patients with the RGT in the faldaprevir 240 mg OD arms reached SVR, irrespective of the PEG-IFN/RBV duration, and 82% of patients with genotype 1a treated with faldaprevir 240 mg OD reached SVR, compared to 47% in the placebo group.
SILEN-C2 enrolled 288 patients without liver cirrhosis who were partial or null-responders to previous HCV treatment[24]. All three arms were treated with faldaprevir combined with PEG-IFN/RBV for 48 wk: 240 mg OD with a 3-d PEG-IFN/RBV lead-in, 240 mg OD without LI, or 240 mg BID with LI. Patients treated with faldaprevir 240 mg OD/LI and reaching HCV RNA < 25 IU/mL by week 4 and undetectable HCV at weeks 8-20 were randomized again. Some of them stopped treatment at week 24, and the others continued PEG-IFN/RBV therapy up to week 48. The SVR rate in prior partial responders was 32%, 50% and 42% in the arms given faldaprevir 240 mg OD with LI, faldaprevir 240 mg OD without LI, and 240 mg BID with LI, respectively. The SVR rate in prior null responders was 21%, 35% and 29% in the respective arms. In patients given faldaprevir 240 mg OD with lead-in (LI) and an AVT duration of 24 wk, the percentage of patients with RGT who reached SVR24 was 43%, while in those continuing treatment up to week 48 it was 72% (Р = 0.035).
Summarizing the results of these two trials, we should note that due to unclear reasons, the patient arms treated with a 3-d lead-in phase (lead-in arms) experienced a treatment effectiveness that was significantly lower, which was a basis for refusing such management to limit the chances of developing faldaprevir resistance.
SILEN-C3 enrolled 159 treatment-naive patients with genotype 1 HCV. Patients were randomized into two arms: 12 and 24 wk of treatment with 120 mg of BI 201335 OD combined with PEG-IFN/RBV. Liver cirrhosis was found in approximately 12% of patients at treatment onset; 48% of the first arm patients and 37% of the second arm patients had subtype 1a HCV, and 46% and 53%, respectively, had subtype 1b HCV. Both patient arms had a lead-in period of 3 d of PEG-IFN/RBV prior to starting BI 201335 therapy. Patients with an early rapid virologic response (eRVR), meaning unquantifiable HCV RNA at week 4 and undetectable load at weeks 8-18, stopped therapy. The rest of the patients continued treatment with PEG-IFN/RBV only up to week 48. SVR rates were similar for both AVT types (65% vs 73%) and in patients with eRVR (82% vs 81%).
The STARTVerso™ (placebo-controlled, double blinded, phase III) trial studied the efficacy and safety of faldaprevir combined with PEG-IFN/RBV in 652 patients previously not treated with AVT and with HCV subtypes 1a and 1b, including patients with compensated liver cirrhosis[26]. The patients were divided into three arms: placebo combined with PEG-IFN/RBV for 24 wk, faldaprevir 120 mg OD combined with PEG-IFN/RBV for 12 or 24 wk (RGT arm), and faldaprevir 240 mg OD combined with PEG-IFN/RBV for 12 wk. Patients complying with the RGT criteria (HCV RNA < 25 IU/mL at week 4 and undetectable load at week 8) and treated with faldaprevir combined with PEG-IFN/RBV stopped treatment at week 24. Patients who did not comply with the RGT criteria who were treated with placebo/PEG-IFN/RBV were given PEG-IFN/RBV treatment only up to week 48. The primary endpoint was reaching SVR within 12 wk of the planned treatment completion (SVR12). Patients given faldaprevir OD in combination with PEG-IFN/RBV (120 and 240 mg) reached SVR12 in 79% and 80% of cases, respectively. Compared with these 2 arms, the placebo/PEG-IFN/RBV arm had a SVR12 rate of 52% (P < 0.0001). In the RGT arm, early rapid response was seen in 87% and 89% of faldaprevir-treated patients (120 and 240 mg, respectively). Those patients were fully compliant with the criteria for treatment shortening. Treated for 12 wk with faldaprevir and 24 wk with PEG-IFN/RBV alone, 86% and 89% of this patient arm (120 and 240 mg, respectively) reached SVR12. Thus, the STARTVerso™ trial showed that it is possible for a majority of patients (88%) to shorten the treatment to 24 wk with considerable HCV elimination compared to patients treated with PEG-IFN/RBV only for 48 wk.
All phase II studies of the SILEN-C series reported that the differences in AE patterns and rates were not significant, including rash, photosensitivity, nausea, vomiting and diarrhea. As in the trials of other PIs, faldaprevir for HCV treatment was associated with transitory elevation of non-conjugated bilirubin. With a faldaprevir OD regimen, significant AEs developed less frequently compared to a BID regimen.
In the phase III trial STARTVerso™[26], all drugs were discontinued in 4% of patients in the placebo arm, 4% in the faldaprevir 120 mg arm, and 5% in the faldaprevir 240 mg arm. Faldaprevir only was discontinued in 1% of patients in the 120 mg arm and 3% in the 240 mg arm. Serious adverse events developed in in 6%, 7% and 7% of patients in the respective study arms. Grade 3 rash (severe) was reported in < 1% in each of the study arms. The rate of Hb drop within first 24 wk (Hb ≤ 8.5 g/dL) was similar in all arms (2%, 3% and 3%, respectively).
Faldaprevir OD combined with PEG-IFN/RBV provides a high SVR rate in HCV patients along with good tolerance and safety.
Danoprevir (RG7277; Roche, Basle, Switzerland; InterMune Pharmaceuticals, Brisbane, CA, United States) is a 2nd-generation NS3/4A protease inhibitor of macrocyclic origin with the same activity toward HCV genotypes 1, 4 and 6 (in vitro)[27,28]. Phase I clinical studies showed the high antiviral activity of danoprevir for genotype 1 HCV. Danoprevir in that category of patients was administered as monotherapy, combined with PEG-IFN/RBV, or combined with a HCV polymerase inhibitor, i.e., mericitabine, in an interferon-free regimen[29-32].
The phase II trial DAUPHINE[33] studied the efficacy of three danoprevir doses (50, 100 and 200 mg) boosted with ritonavir 100 mg taken BID in combination with PEG-IFN/RBV (RGT). Ritonavir addition is known to increase the PI blood concentration, thereby suppressing CYP3A activity. Twelve weeks after treatment completion, the SVR12 rate was 93% in genotype 1 HCV patients treated with danoprevir 200 mg BID combined with PEG-IFN/RBV. Danoprevir 100 mg/d provided a SVR12 rate of 83%, and 50 mg/d 67%. The effectiveness of danoprevir 200 mg BID combined with PEG-IFN/RBV was not affected by HCV genotype subtype (1a vs 1b) or IL28B genotype (CC vs non-CC).
The objective of the randomized, placebo-controlled, parallel-group phase II trial ATLAS (NCT00963885) was to study the efficacy and safety of RGT danoprevir combined with PEG-IFN/RBV for 12 wk compared to PEG-IFN/RBV in naive genotype 1 HCV patients[34]. It was an international study, with sites in North America (31 sites), Europe (8 sites) and Australia (3 sites). Patients who had not previously been treated for HCV (treatment-naïve patients) were randomized into 4 groups. For 12 wk, patients were given danoprevir (300 mg every 8 h, 600 mg every 12 h or 900 mg every 12 h) or placebo in combination with PEG-IFN/RBV. Follow-up treatment included PEG-IFN/RBV therapy only. Patients with an extended rapid virologic response (eRVR) (RNA < 15 IU/mL for 4-20 wk) stopped treatment at week 24. Patients without eRVR continued PEG-IFN/RBV therapy for 48 wk. The main criterion for assessing efficacy was SVR within 24 wk after treatment completion. The SVR rate was 68% in patients treated with danoprevir 300 mg, 85% in danoprevir 600 mg and 76% in danoprevir 900 mg, compared with 42% in placebo-treated patients. RGT was found in 71 patients given danoprevir 600 mg combined with PEG-IFN/RBV, and SVR was found in 96%.
In the ATLAS study, serious adverse events were reported for 7%-8% of danoprevir-treated patients and for 19% of placebo-treated patients. Four danoprevir-treated patients had transient ALT elevation. The highest danoprevir dose (900 mg) resulted in grade 4 ALT elevation that, in turn, required therapy discontinuation in the relevant patient arm.
Danoprevir combined with PEG-IFN/RBV resulted in a high SVR rate in genotype 1 HCV patients. However, high danoprevir doses can result in prominent ALT elevation requiring AVT discontinuation.
One of the most considerable achievements in AVT development with PI combinations (if not the most important) is the phase IIa trials with asunaprevir (ASV, BMS-650032, 600 mg; BID) and daclatasvir (DCV, NS5A inhibitor, 60 mg; OD) combined with conventional therapy and a comparison group (with no conventional treatment).
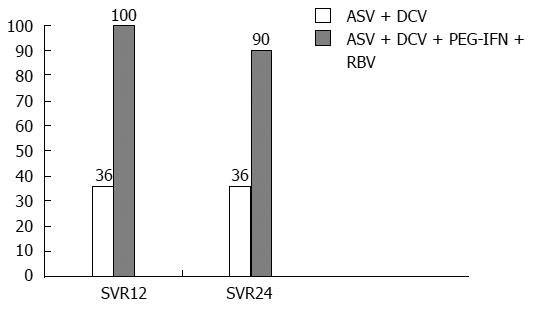
A phase II CT with the above combination, the AI447-011 study, showed its efficacy for one of the most complex HCV-infected patient groups: non-responders with zero prior virologic response (HCV RNA decreased less than 2 log10 by week 12 of conventional therapy). This group appears to be the most complex from the point of view of antiviral regimen selection because null responders should be considered insensitive to IFN-based agents. That study’s results show that a combination of direct-acting antivirals is the only therapeutic option for this patient category. The design of the AI447-011 study involved an efficacy comparison in 2 patient arms: Arm 1 was given the combination of ASV + DCV, and arm 2 was given ASV+ DCV + conventional therapy. The treatment duration was 24 wk; one of the important exclusion criteria was liver cirrhosis[35] (Figure 4). The study reported the seemingly unreachable SVR of 90% among null responders under complex therapy (ASV+ DCV + conventional therapy).
Even more impressive results were shown by Japanese researchers, Suzuki et al[36] (2012), who also used an ASV + DCV regimen in null-responders (n = 21); their comparison group included patients (n = 22) with contraindicated IFN-based therapy (Table 7). Their data showed an SVR of 90.5% in group 1. Additionally, this CT showed the prognostic value of EVR: all EVR patients reached SVR.
| Null response(n = 21) | Contraindications to IFN-basedtherapy (n = 22) | |
| RVR4 | 20 (95.2) | 15 (68.2) |
| RVR12 | 19 (90.5) | 14 (63.6) |
| SVR24 | 19 (90.5) | 14 (63.6) |
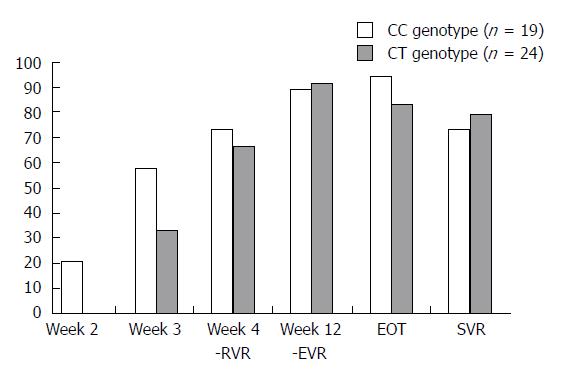
The study of the ASV + DCV combination drew the following conclusions: IL28B polymorphism appears to lose its SVR predictive value; the majority of patients with aviremia after 2 wk treatment had the CC-genotype, without significant SVR differences in patients with different IL28B genotypes (Figure 5).
The study of the ASV+DCV combination reported relatively more often AEs related to moderate headache, nasopharyngitis exacerbation elevated aminotransferases, and diarrhea. Laboratory AEs were moderate and severe [grade 3-4 (G3-4)] impairments related to elevated transaminase activity. Serious adverse events were found in 6 patients: mild and moderate pyrexia (G2-3), moderate gastroenteritis (in 2 patients); hyperbilirubinemia (G4). In all patients pyrexia disappeared in 4 to 10 d after AVT cancellation. Hyperbilirubinemia and cytolytic syndrome resolved within 4 wk after treatment discontinuation (Table 8). We should note that in the studies of ASV + DCV + conventional therapy combinations, the AE structure showed a prevalence of disorders caused by conventional treatment.
| Lok et al[35] AI447-011 | Suzuki et al[36] | |||
| ASV + DCV + conventional therapy(n = 10) | ASV + DCV(n = 11) | Null response (n = 21) | Contraindications to IFN-based therapy (n = 22) | |
| Diarrhea | 70.0% | 72.7% | 43% | 9% |
| Fatigue | 70.0% | 54.5% | -1 | -1 |
| Headache | 50.0% | 45.5% | 38% | 27% |
| Nausea | 50.0% | 18.2% | -1 | -1 |
| Coughing | 20.0% | 27.3% | -1 | -1 |
| Subfebrile temperature | 27.3% | 10.0% | 14% | 23% |
Asunaprevir-based AVT regimens are highly effective (above 90%) in the most challenging patient category (null responders); the safety profile of the given AVT regimen was mainly not different from PEG-IFN/RBV.
Agent АВТ-450 (AbbVie) is used only in combination with the non-nucleoside inhibitor NS5B (АВТ-333), ribavirin and ritonavir. Therefore, АВТ-450 efficacy and safety should be considered only a multicomponent “achievement”.
The clinical efficacy of an АВТ-450-based AVT treatment was published as the results of the AVIATOR study, a phase IIa CT, by Poordad et al[37] (2013). The genotype 1 patient population mainly included treatment-naive patients (66%, n = 33), while partial responders and null responders comprised 34% of the population (n = 17). АВТ-450 was not used as monotherapy. The dosing of the inhibitor combination depended on the studied population. The given study used a combination of NS5B (АВТ-333), ribavirin and ritonavir coupled with various АВТ-450 doses for 12 wk. The results showed that АВТ-450 was effective at 150 mg OD: the SVR rate amounted to 93%, compared to 95% in the comparison group with АВТ-450 250 mg OD (Table 9). The phase IIb study of the combination (2013) showed comparable efficacy in similar study arms: treatment-naive 89% and 96%, null responders 89 and 95%, respectively. It should be noted that the SVR rate for АВТ-450-based AVT did not depend on IL28B polymorphism (Table 10).
| Study arm | n | Genotype | Status | Combination | Duration | Treatment regimen | SVR |
| Total: group 1 + 2 | 33 | 1а/1b (28/5) | Naive | АВТ-450 + ritonavir + АВТ-333 + ribavirin | 12 wk | АВТ-450, 250 mg/d or 150 mg/d + ritonavir, 100 mg/d; АВТ-333, 400 mg BID; ribavirin, body weight-based | 93%-95% |
| 3 | 17 | 1а/1b (16/1) | partial virologic response, null response | АВТ-450 + ritonavir + АВТ-333 + ribavirin | 12 wk | АВТ-450, 150 mg/d; ritonavir, 100 mg/d; АВТ-333, 400 mg BID; ribavirin, body weight-based | 47% |
| Study arm | Status | CC-genotype | CT-genotype | TT-genotype | SVR |
| 1 | Naive | 10/9 | 7/7 | 2/2 | 95% |
| 2 | Naive | 5/4 | 7/7 | 2/2 | 93% |
| 3 | Partial virologic response, null response | 0/0 | 12/6 | 5/2 | 47% |
There were no noted specific AEs from АВТ-450-based AVT. The most common were headache, fatigue, nausea (Table 11). However, good tolerance in its totality is related not only to АВТ-450 but also to other combination constituents[38]. It is worth mentioning that despite the presence of ribavirin in the combination, anemia was not a frequent AE deserving special attention.
| AEs with incidence above 20% | AE incidence |
| Headache | 14%-26% |
| Fatigue | 35%-47% |
| Insomnia | 0-26% |
| Nausea | 21%-24% |
| Rash | 6%-21% |
АВТ-450 + ritonavir + АВТ-333 + ribavirin in phase IIa and IIb studies was highly effective in HCV patients with the following criteria: genotype 1, both treatment-naive patients and null responders, no liver cirrhosis. At the moment, studies for optimal treatment duration are ongoing. Regarding safety, this combination was well tolerated, and possible AEs were mostly related to asthenia syndrome. Specific AEs were not detected in the studies.
GS-9256 was used only in combination with the non-nucleoside inhibitor tegobuvir (GS-9190). Therefore, GS-9256’s efficacy and safety should be considered only in a multicomponent treatment.
Another representative of the PI class, GS-9256 (Gilead) was studied in combination with the non-nucleoside inhibitor tegobuvir (GS-9190) and ribavirin. The combination of 4 agents (GS-9256 + tegobuvir + ribavirin + PEG-IFNα) was used as a comparison group. According to the phase II study by Zeuzem et al[39] (2010), the SVR rate was comparable in the absence vs. the presence of PEG-IFNα: 100% vs 94%. The SVR rate of the two-component regimen (only with direct-acting antivirals) amounted to 67% (Table 12).
| GS-9256 + tegobuvir(n = 15) | GS-9256 + tegobuvir + ribavirin by weight (n = 13) | GS-9256 + tegobuvir + ribavirin by weight + PEG-IFNα(n = 14) | |
| Week 4, RVR | 1/15 (7) | 6/13 (46) | 10/14 (71) |
| Week 12, EVR | 3/15 (20) | 8/13 (62) | 14/14 (100) |
| Week 24, SVR | 10/15 (67) | 13/13 (100) | 13/14 (94) |
Patients taking GS-9256 + tegobuvir 40 mg showed good tolerance, and the majority of AEs were of medium severity. No specific AEs were found. Conversely, the comparison arm (GS-9256 + tegobuvir + ribavirin by weight + PEG-IFNа) developed common AEs typical for PEG-IFN/RBV regimens (Table 13). During the CT period, 2 cases of serious AEs were reported: bursitis (infected) and vasovagal attack, which the investigators interpreted as not related to the studied agent. No laboratory impairments of serious AE (G4) type were found.
| GS-9256 + tegobuvir | GS-9256 + tegobuvir + ribavirin by weight | GS-9256 + tegobuvir + ribavirin by weight + PEG-IFN1 | |
| AE incidence | 50% | 93% | 81%-100% |
| Anemia | 0 | 0 | 0-13% |
| Eye pain | 0 | 0 | 0-13% |
| Diarrhea | 19% | 20% | 6%-40% |
| Nausea | 13% | 20% | 6%-40% |
| Flu-like syndrome | 0 | 0 | 44%-80% |
| Fatigue | 6% | 33% | 13%-33% |
| Headache | 31% | 47% | 13%-40% |
| Insomnia | 0 | 20% | 6%-13% |
| Dry skin | 0 | 13% | 0% |
| Pruritus | 6% | 20% | 0%-7% |
In general, GS-9256 + tegobuvir + ribavirin is highly effective in treatment-naive genotype 1 HCV patients. An analysis of the phase II study results indicated a good safety profile for combinations including GS-9256.
The appearance in clinical settings of the first direct-acting antivirals to treat HCV provided improved effectiveness and decreased AVT duration in a majority of genotype 1 patients.
The addition of protease inhibitors has been beneficial for HCV patients. The SVR rate of 40%-50% obtained using conventional treatment was significantly improved (up to 80%) through the addition of telaprevir or boceprevir in triple therapy regimens with the provision that AVT is administered following all main approaches and principles (e.g., accounting for contraindications, potential drug-drug interactions, adherence to AVT regimens depending on initial patient parameters)[10].
Another solid argument for the development of new combined regimens that include direct-acting antivirals is the high rate of hematological AEs, especially ribavirin-induced anemia. When new AVTs for genotype 1 HCV are introduced, most attention should be paid to the regimens, such as by excluding PEG-IFNα to expand the treatment groups and including patients with contraindications to IFN and IFN intolerance.
Our review shows that combinations of direct-acting antivirals can become a novel therapeutic standard not only for patients with contraindicated IFN therapy. PI combinations with other direct-acting antivirals can improve the SVR rates in non-responders with prior partial virologic response and, more importantly, in those with prior null virologic response (Table 14).
| Ref. | n | Combination | Duration | SVR |
| Zeuzem et al[39] | 37 | Telaprevir-based triple therapy | 48 wk | 33% |
| Bacon et al[25] | 58 | Boceprevir-based triple therapy | 48 wk | 52% |
| Lok et al[35] | 11 | DCV + ASV | 24 wk | 36% |
| Lok et al[35] | 10 | DCV + ASV + conventional therapy | 24 wk | 90% |
| Suzuki et al[36] | 21 | |||
| Poordad et al[37] | 7 | ABT-450 + ritonavir + АВТ-333 + ribavirin | 12 wk | 43% |
When triple therapy appeared, it was the only combination available for null responders. However, the evident barriers were interferon intolerance and issues with concomitant treatment selection to avoid drug interactions. Currently, asunaprevir-based combinations are the treatment of choice for null responders. They have a SVR rate of 90%, and in case of interferon intolerance, patients can be offered antiviral АВТ-450-based regimens. In any case, the era of direct-acting antivirals assumes interferon-free therapy. Once, supposedly ideal regimens for HCV treatment implied interferon-free combinations. Now, the emergence of direct-acting antivirals makes it possible to develop optimally dosed treatments and completely exclude clinically significant AEs related to interferon use.
P- Reviewers: Liu J, Takaguchi K, Tziomalos K S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
| 1. | Vachon ML, Dieterich DT. The era of direct-acting antivirals has begun: the beginning of the end for HCV? Semin Liver Dis. 2011;31:399-409. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 2. | Zignego AL, Craxì A. Extrahepatic manifestations of hepatitis C virus infection. Clin Liver Dis. 2008;12:611-636, ix. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 3. | Chevaliez S, Pawlotsky JM. Diagnosis and management of chronic viral hepatitis: antigens, antibodies and viral genomes. Best Pract Res Clin Gastroenterol. 2008;22:1031-1048. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 4. | Moradpour D, Penin F, Rice CM. Replication of hepatitis C virus. Nat Rev Microbiol. 2007;5:453-463. [PubMed] [Cited in This Article: ] |
| 5. | Pawlotsky JM, Chevaliez S, McHutchison JG. The hepatitis C virus life cycle as a target for new antiviral therapies. Gastroenterology. 2007;132:1979-1998. [PubMed] [Cited in This Article: ] |
| 6. | Swan T. Hepatitis C Drug Development Catapults Onward. 2013 Pipeline Report. Available from: http://www.pipelinereport.org/2013/hcv. [Cited in This Article: ] |
| 7. | Jacobson IM, McHutchison JG, Dusheiko G, Di Bisceglie AM, Reddy KR, Bzowej NH, Marcellin P, Muir AJ, Ferenci P, Flisiak R. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364:2405-2416. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1866] [Cited by in F6Publishing: 1835] [Article Influence: 141.2] [Reference Citation Analysis (0)] |
| 8. | Sherman KE, Flamm SL, Afdhal NH, Nelson DR, Sulkowski MS, Everson GT, Fried MW, Adler M, Reesink HW, Martin M. Response-guided telaprevir combination treatment for hepatitis C virus infection. N Engl J Med. 2011;365:1014-1024. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 592] [Cited by in F6Publishing: 628] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 9. | Wilby KJ, Greanya ED, Ford JA, Yoshida EM, Partovi N. A review of drug interactions with boceprevir and telaprevir: implications for HIV and transplant patients. Ann Hepatol. 2012;11:179-185. [PubMed] [Cited in This Article: ] |
| 10. | Bakulin IG, Fedulenkova LV, Sidorova IO. Safety of Boceprevir and Telaprevir in patients with chronic hepatitis C during triple therapy. Hepatol Forum. 2012;2:16-20. [Cited in This Article: ] |
| 11. | Roberts SK, Andreone P, Pol S, Younossi Z, Diago M, Lawitz E, Focaccia R, Foster G, Horban A, Lonjon-DomanecoI . Impact of anemia and ribavirin dose reduction on SVR to a telaprevir-based regimen in patients with HCV genotype 1 and prior peginterferon/ribavirin treatment failure in the phase III REALIZE study. Hepatology. 2011;54:1007A-1008. [Cited in This Article: ] |
| 12. | Harrington PR, Zeng W, Naeger LK. Clinical relevance of detectable but not quantifiable hepatitis C virus RNA during boceprevir or telaprevir treatment. Hepatology. 2012;55:1048-1057. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 13. | Poordad F, McCone J, Bacon BR, Bruno S, Manns MP, Sulkowski MS, Jacobson IM, Reddy KR, Goodman ZD, Boparai N. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1195-1206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1948] [Cited by in F6Publishing: 1951] [Article Influence: 150.1] [Reference Citation Analysis (0)] |
| 14. | Manns M, Reesink H, Berg T, Dusheiko G, Flisiak R, Marcellin P, Moreno C, Lenz O, Meyvisch P, Peeters M. Rapid viral response of once-daily TMC435 plus pegylated interferon/ribavirin in hepatitis C genotype-1 patients: a randomized trial. Antivir Ther. 2011;16:1021-1033. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 15. | Fried M, Buti M, Dore GJ, Flisiak R, Ferenci P, Jacobson IM, Marcellin P, Manns MP, Nikitin I, Poordad FF. TMC435 in combination with peginterferon and ribavirin in treatment naiıve HCV genotype 1 patients: final analysis of the PILLAR phase IIb study. Hepatology. 2011;54:1429A. [Cited in This Article: ] |
| 16. | Fried MW, Buti M, Dore GJ, Flisiak R, Ferenci P, Jacobson I, Marcellin P, Manns M, Nikitin I, Poordad F. Once-daily simeprevir (TMC435) with pegylated interferon and ribavirin in treatment-naïve genotype 1 hepatitis C: the randomized PILLAR study. Hepatology. 2013;58:1918-1929. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 216] [Cited by in F6Publishing: 239] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 17. | Foster GR, Fried MW, Hezode C, Hirschfield GM, Nikitin I, Poordad F, Lenz O, Peeters M, Sekar V, De SmedtG. The Aspire Trial: TMC435 in Treatment-experienced Patients with Genotype-1 HCV infection Who Have Failed Previous Pegifn/rbv Treatment. J Hepatol. 2011;54:S546. [DOI] [Cited in This Article: ] |
| 18. | Lenz O, Fevery B, Vijgen L, Verbeeck J, Peeters M, Beumont-Mauviel M, Zeuzem S, Picchio G. TMC 435 in patients infected with HCV genotype 1 who have failed previous pegylated interferon/ribavirin treatment: virologic analysis of the ASPIRE trial. 47th Annual Meeting of the European Association for the Study of the Liver; 2012 Apr 18-22. Spain: European Association for the Study of the Liver; . [Cited in This Article: ] |
| 19. | Jacobson IM, Dore GJ, Foster GR, Fried MW, Radu M, Rafalskiy VV, Moroz L, Craxì A, Peeters M, Lenz O. Simeprevir (TMC435) with Peginterferon/Ribavirin for Chronic HCV Genotype-1 Infection in Treatment-Naive Patients: Results From QUEST-1, a Phase III Trial. 2013 May 18-21. Orlando: Digestive Disease Week; . [Cited in This Article: ] |
| 20. | Poordad F, Manns MP, Marcellin P, Affonso de Araujo ES, Buti M, Horsmans Y, Janczewska E, Villamil F, Peeters M, Lenz O. Simeprevir (TMC435) with Peginterferon/Ribavirin for Treatment of Chronic HCV Genotype-1 Infection in Treatment-Naive Patients: Results From QUEST-2, a Phase III Trial. 48th Annual Meeting of the European Association for the Study of the Liver; 2013 May 18-21. Orlando: Digestive Disease Week; . [Cited in This Article: ] |
| 21. | Scott J, Rosa K, Fu M, Cerri K, Peeters M, Beumont- Mauviel M, Gilles L. Improved SVR with Simeprevir (TMC435) Associated with reduced time with patient-reported fatigue in treatment-naive, HCV-infected patients in the PILLAR phase IIb trial. J Hepatol. 2013;58:S371-372. [Cited in This Article: ] |
| 22. | Sulkowski MS, Asselah T, Lalezari J, Ferenci P, Fainboim H, Leggett B, Bessone F, Mauss S, Heo J, Datsenko Y. Faldaprevir combined with pegylated interferon alfa-2a and ribavirin in treatment-naïve patients with chronic genotype 1 HCV: SILEN-C1 trial. Hepatology. 2013;57:2143-2154. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 88] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 23. | Lawitz E, Forns X, Zeuzem S, Gane E, Bronowicki J-P, Andreone P, Horban A, Brown A, Peeters M, Lenz O. Simeprevir (TMC435) With Peginterferon/Ribavirin for Treatment of Chronic HCV Genotype 1 Infection in Patients Who Relapsed After Previous Interferon-Based Therapy: Results From PROMISE, a Phase III Trial. 2013 May 18-21. Orlando: Digestive Disease Week; . [DOI] [Cited in This Article: ] [Cited by in Crossref: 209] [Cited by in F6Publishing: 212] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 24. | Sulkowski MS, Bourlière M, Bronowicki JP, Asselah T, Pawlotsky JM, Shafran SD, Pol S, Mauss S, Larrey D, Datsenko Y. Faldaprevir combined with peginterferon alfa-2a and ribavirin in chronic hepatitis C virus genotype-1 patients with prior nonresponse: SILEN-C2 trial. Hepatology. 2013;57:2155-2163. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 25. | Bacon BR, Gordon SC, Lawitz E, Marcellin P, Vierling JM, Zeuzem S, Poordad F, Goodman ZD, Sings HL, Boparai N. Boceprevir for previously treated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1207-1217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1287] [Cited by in F6Publishing: 1288] [Article Influence: 99.1] [Reference Citation Analysis (0)] |
| 26. | Ferenci P, Asselah T, Foster GR, Zeuzem S, Sarrazin C, Moreno C, Ouzan D, Maevskaya M, Calinas F, Morano LE. Faldaprevir plus pegylated interferon alfa-2a and Ribavirin in chronic hcv genotype-1 treatment-naïve Patients: final results from STARTVerso1, a randomised, Double-blind, placebo-controlled phase III trial. J Hepatol. 2013;58:S569. [Cited in This Article: ] |
| 27. | Gottwein JM, Scheel TK, Jensen TB, Ghanem L, Bukh J. Differential efficacy of protease inhibitors against HCV genotypes 2a, 3a, 5a, and 6a NS3/4A protease recombinant viruses. Gastroenterology. 2011;141:1067-1079. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 115] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 28. | Imhof I, Simmonds P. Genotype differences in susceptibility and resistance development of hepatitis C virus to protease inhibitors telaprevir (VX-950) and danoprevir (ITMN-191). Hepatology. 2011;53:1090-1099. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 29. | Gane EJ, Roberts SK, Stedman CA, Angus PW, Ritchie B, Elston R, Ipe D, Morcos PN, Baher L, Najera I. Oral combination therapy with a nucleoside polymerase inhibitor (RG7128) and danoprevir for chronic hepatitis C genotype 1 infection (INFORM-1): a randomised, double-blind, placebo-controlled, dose-escalation trial. Lancet. 2010;376:1467-1475. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 269] [Cited by in F6Publishing: 220] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 30. | Forestier N, Larrey D, Guyader D, Marcellin P, Rouzier R, Patat A, Smith P, Bradford W, Porter S, Blatt L. Treatment of chronic hepatitis C patients with the NS3/4A protease inhibitor danoprevir (ITMN-191/RG7227) leads to robust reductions in viral RNA: a phase 1b multiple ascending dose study. J Hepatol. 2011;54:1130-1136. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 31. | Forestier N, Larrey D, Marcellin P, Guyader D, Patat A, Rouzier R, Smith PF, Qin X, Lim S, Bradford W. Antiviral activity of danoprevir (ITMN-191/RG7227) in combination with pegylated interferon α-2a and ribavirin in patients with hepatitis C. J Infect Dis. 2011;204:601-608. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 32. | Larrey D, Carenco C, Guyader D, Boyer N, Benhamou Y, Pageaux GP, Rouzier R, Marcellin P. Sustained virological response after 14-day treatment with danoprevir and 48-week treatment with pegylated interferon-α2a (40 KD) plus ribavirin. Antivir Ther. 2012;17:927-932. [PubMed] [Cited in This Article: ] |
| 33. | Everson G, Cooper C, Hézode C, Shiffman ML, Yoshida E, Beltran-Jaramillo T, Ferenci P, Zeuzem S, Brunda M, Shulman N. Rapid and sustained achievement of undetectable HCV RNA during treatment with ritonavir-boosted danoprevir/PEG-IFNa-2A/RBV in HCV genotype 1 or 4 patients: Dauphine week 36 interim analysis. 47th Annual Meeting of the European Association for the Study of Liver Disease; 2012 Apr 18-22. Barcelona, Spain: Digestive Disease Week; . [Cited in This Article: ] |
| 34. | Marcellin P, Cooper C, Balart L, Larrey D, Box T, Yoshida E, Lawitz E, Buggisch P, Ferenci P, Weltman M. Randomized controlled trial of danoprevir plus peginterferon alfa-2a and ribavirin in treatment-naïve patients with hepatitis C virus genotype 1 infection. Gastroenterology. 2013;145:790-800.e3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 35. | Lok AS, Gardiner DF, Lawitz E, Martorell C, Everson GT, Ghalib R, Reindollar R, Rustgi V, McPhee F, Wind-Rotolo M. Preliminary study of two antiviral agents for hepatitis C genotype 1. N Engl J Med. 2012;366:216-224. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 478] [Cited by in F6Publishing: 471] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 36. | Suzuki Y, Ikeda K, Suzuki F, Toyota J, Karino Y, Chayama K, Kawakami Y, Ishikawa H, Watanabe H, Hu W. Dual oral therapy with daclatasvir and asunaprevir for patients with HCV genotype 1b infection and limited treatment options. J Hepatol. 2013;58:655-662. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 191] [Cited by in F6Publishing: 210] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 37. | Poordad F, Lawitz E, Kowdley KV, Cohen DE, Podsadecki T, Siggelkow S, Heckaman M, Larsen L, Menon R, Koev G. Exploratory study of oral combination antiviral therapy for hepatitis C. N Engl J Med. 2013;368:45-53. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 238] [Cited by in F6Publishing: 250] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 38. | Zeng QL, Zhang JY, Zhang Z, Wang LF, Wang FS. Sofosbuvir and ABT-450: terminator of hepatitis C virus? World J Gastroenterol. 2013;19:3199-3206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 17] [Cited by in F6Publishing: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 39. | Zeuzem S, Buggisch P, Agarwal K, Marcellin P, Sereni D, Klinker H, Moreno C, Zarski JP, Horsmans Y, Mo H. The protease inhibitor, GS-9256, and non-nucleoside polymerase inhibitor tegobuvir alone, with ribavirin, or pegylated interferon plus ribavirin in hepatitis C. Hepatology. 2012;55:749-758. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 105] [Article Influence: 8.8] [Reference Citation Analysis (0)] |