Published online Jun 27, 2025. doi: 10.4254/wjh.v17.i6.106849
Revised: March 25, 2025
Accepted: May 21, 2025
Published online: June 27, 2025
Processing time: 109 Days and 17.4 Hours
Non-alcoholic fatty liver disease (NAFLD), also referred to as metabolic-asso
Core Tip: Non-alcoholic fatty liver disease (NAFLD) is among the most prevalent chronic liver conditions that has been recognized as an emerging global health problem. Evidence suggests that the gut microbiota plays a critical role in the pathophysiology of NAFLD by influencing liver inflammation through its metabolites. Identifying gut microbial and metabolic signatures opens new avenues for early diagnosis and therapeutic interventions. Microbiota-targeted therapies, including diet, probiotics, prebiotics, synbiotics, and fecal microbiota transplantation, hold promise for alleviating NAFLD and improving patient outcomes.
- Citation: Pandey H, Goel P, Srinivasan VM, Tang DWT, Wong SH, Lal D. Gut microbiota in non-alcoholic fatty liver disease: Pathophysiology, diagnosis, and therapeutics. World J Hepatol 2025; 17(6): 106849
- URL: https://www.wjgnet.com/1948-5182/full/v17/i6/106849.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i6.106849
Non-alcoholic fatty liver disease (NAFLD), also referred to as metabolic-associated fatty liver disease (MAFLD), is defined as an excessive accumulation of intracellular hepatic fat without consuming significant amounts of alcohol[1]. The novel and non-stigmatizing nomenclature for NAFLD is metabolic dysfunction-associated steatotic liver disease (MASLD)[2], although its use in current literature is limited. Therefore, the term NAFLD is being adhered to in the present manuscript. NAFLD has been recognized as an emerging global health problem and a leading cause of chronic liver diseases[3]. NAFLD affects 25% to 30% of the global population[4], with a prevalence of 25% and 34% in Western countries and Asia, respectively[5,6]. NAFLD results in liver inflammation, which progresses to non-alcoholic steatohepatitis (NASH) and ultimately to liver cirrhosis and hepatocellular carcinoma (HCC)[7]. NAFLD is the third leading cause of HCC[8]. Although only 10% of individuals with NAFLD develop liver cirrhosis and HCC, the disease imposes a significant economic burden[9]. NAFLD is considered a multisystem disease due to its association with a higher risk of metabolic disorders such as type 2 diabetes, obesity, and cardiovascular and chronic kidney diseases[7,10]. Although NAFLD is strongly linked to metabolic syndrome[11,12], it may develop even in the absence of metabolic syndrome[13]. NAFLD is also associated with an increased risk for sarcopenia, which is the loss of skeletal muscle mass, strength, and function[14]. NAFLD often progresses asymptomatically, with clinical symptoms appearing in patients only in the last-stage of liver disease[15]. The primary pathological feature of NASH is inflammation, which significantly contributes to the onset of NASH and its advancement to cirrhosis and HCC.
The pathophysiology of NAFLD is complex, involving both genetic and environmental factors[16]. Factors contributing to NAFLD include obesity, lack of physical activity, high-fat diet (HFD), de novo lipogenesis, lipotoxicity, insulin resistance, epigenetic impairment, gut barrier dysfunction, and gut microbiota dysbiosis[4,17-21]. Insulin resistance and obesity are crucial pathophysiological factors in the onset and progression of NAFLD, which is often considered the hepatic manifestation of metabolic syndrome.
The current methods for NAFLD diagnosis include biopsy, imaging techniques such as fibroscan, ultrasound, and magnetic resonance imaging, and serum biomarkers. Biopsy is invasive and carries potential risks, while imaging techniques and serum biomarkers have limited diagnostic accuracy and poor predictive value[22-25]. Therefore, there is an urgent need for robust and accurate noninvasive tools for the early NAFLD diagnosis. This review explores the relationship between the gut microbiota and the onset and progression of NAFLD. We also discuss the potential of microbiota in the diagnosis, treatment, and prevention of NAFLD.
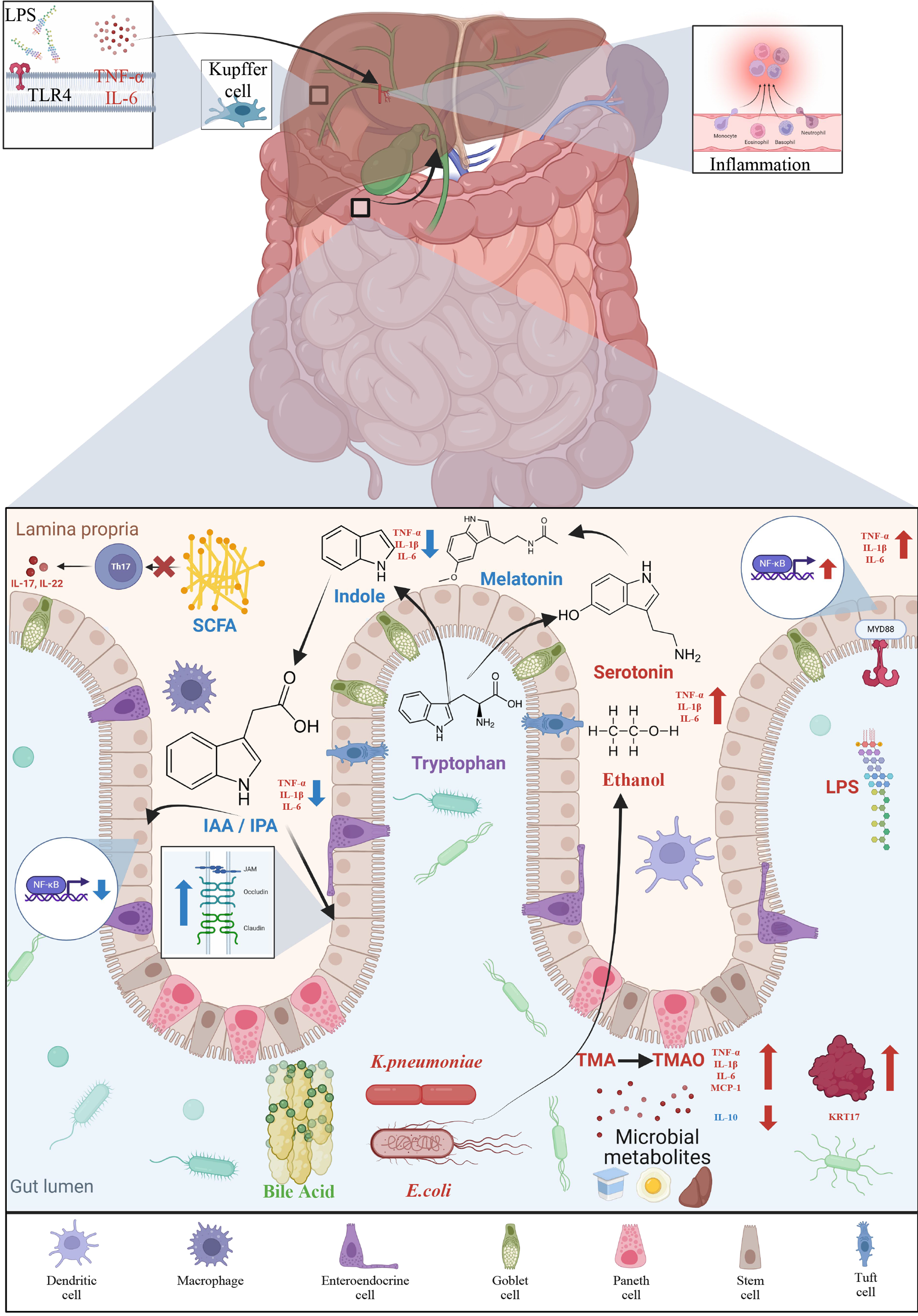
The gut-liver axis is the bidirectional relationship between the gut and the liver, which are connected through the hepatic portal vein, bile tract, and systemic circulation and involve various signals and interactions that influence both gastrointestinal (GI) and liver health[26]. This complex communication network plays a crucial role in metabolic processes, immune responses, and the overall maintenance of metabolic homeostasis. The gut-liver axis also forms a key link between the gut microbiota and the liver. The liver receives most of its blood supply from the hepatic portal vein and the hepatic artery. Nearly 70%-75% of the blood supplied to the liver is delivered through the hepatic portal vein, which transports nutrient-rich blood from the GI tract and spleen to the liver. In addition to nutrients, the hepatic portal vein also transports bacteria and toxins to the liver. The metabolites and products of the gut microbiota reach the liver via the hepatic portal vein, while bioactive molecules such as bile acids (BAs), produced by the liver, reach the intestine to regulate gut microbiota[27]. The gut-liver axis is critical in NAFLD pathogenesis[28] and its disruption is increasingly recognized as a significant contributor to the disease. Therefore, understanding the gut-liver axis and its role in NAFLD pathogenesis has important clinical implications. The multiple-hit hypothesis also supports the role of the gut-liver axis in NAFLD development, proposing that the onset and progression of the disease are due to a combination of various contributing factors. Moreover, the interaction between the “mucus layer-soil” and “gut bacteria-seed” is an important factor that determines the pathogenesis of NAFLD[29].
The human gut microbiota consists of approximately 40 trillion microorganisms that inhabit the human GI tract[30,31], weighing approximately 1-2 kg[32]. Gut microbiota includes bacteria, archaea, fungi, protozoa, and viruses[33]. While 40% of the core microbiota is shared among humans, the remaining 60% usually varies[30]. Firmicutes, Bacteroidetes, Actinobacteria, Proteobacteria, and Fusobacteria are the dominant bacterial phyla in humans[34]. The dominant phyla in the colon are Bacteroidetes and Actinobacteria, while the dominant phylum in the small intestine is Firmicutes[35,36].
The gut microbiota is essential for normal gut physiology and homeostasis and is closely linked to health and disease[37-41]. The gut microbiota is also associated with energy metabolism and storage in the liver. Published data agree on the role of gut microbiota in the development of metabolic disorders such as obesity[42,43], diabetes[44], cardiovascular[45], and chronic liver diseases[46]. Obesity is the most prevalent metabolic disorder. The gut microbiota plays a crucial role in the onset of obesity and associated metabolic disorders[47]. Gut microbiota is known to affect liver lipid me
A growing body of evidence highlights the crucial role of the gut microbiota and their metabolites in the onset and progression of NAFLD[28,52]. Under normal conditions, the intestinal barrier prevents the movement of microbes and their products to the liver. An alteration in gut microbiota, known as dysbiosis, is caused due to an imbalance between symbiotic and opportunistic microbes. Many factors contribute to dysbiosis, including high-fat and high-sugar diets, lack of fiber, indiscriminate use of antibiotics, infections, and inflammation. Dysbiosis results in impairing intestinal tight junctions. This results in increased intestinal permeability, allowing harmful bacteria and their metabolites to enter the liver, thereby stimulating hepatic immune cells and triggering inflammatory pathways and NAFLD[53-55]. Bacterial components, particularly lipopolysaccharide (LPS), a cell component of Gram-negative bacteria, cause metabolic endotoxemia[56] by triggering hepatic expression of toll-like receptor 4 (TLR4) and secretion of interleukin-8 (IL-8), causing inflammatory reactions[57]. These inflammatory reactions further alter the intestinal microecosystem, leading to a vicious cycle of NAFLD progression[58]. Identifying gut microbiota signature and metabolic profiles in NAFLD patients may provide novel biomarkers for accurate diagnosis and staging of the disease.
Various methods are available to study microbial diversity[59]. Next-generation sequencing methods are the methods of choice to explore microbial diversity from the human gut. Gut microbial diversity is a crucial determinant of human health. Several studies have indicated that NAFLD is associated with reduced gut microbial diversity[60,61]. Astbury et al[61] reported reduced α- and β-diversity in NASH patients, with or without cirrhosis, compared to healthy controls. They also observed an abundance of the genus Collinsella and a decrease in Ruminococcaceae in NASH subjects. A cross-sectional study with 87 children with biopsy-proven NAFLD found a reduced α-diversity of fecal microbiomes in children with NAFLD and NASH with a higher abundance of Prevotella copri in those with severe fibrosis[62]. Another cross-sectional study involving 73 participants found decreased bacterial α-diversity in subjects with NAFLD, with a higher Firmicutes-to-Bacteroidetes ratio and reduced abundance of Bacteroidetes, Prevotella, Gemmiger, and Oscillospira[63]. Wang et al[64] found that NAFLD was associated with reduced diversity and a phylum-level changes in the fecal microbiome. There was a 24% reduction in Firmicutes, particularly SCFA-producing genera, and a 20% increase in Bacteroidetes. A recent meta-analysis encompassing 54 studies with 8894 participants reported a moderate decrease in α-diversity indices such as Shannon and Chao1 among NAFLD patients[65].
Several studies have reported phylum-level changes in NAFLD[66,67] and have identified gut microbiome signatures associated with the disease[60,68-70] (Table 1). An important feature associated with NAFLD and other metabolic disorders is a decline in the abundance of commensal bacteria indicative of a healthy gut, such as Akkermansia muciniphila and Faecalibacterium prausnitzii[71-75] and an increased abundance of endotoxin-producing bacteria, such as Enterobacter, Escherichia coli, and Klebsiella pneumoniae[76-82]. Escherichia coli O157:H7 has been shown to have a damaging effect on tight junctions, thereby increasing intestinal permeability[82]. Jiang et al[83] found an increased abundance of Escherichia, Lactobacillus, Streptococcus, and Anaerobacter in NAFLD patients compared to healthy subjects. The abundance of Bacteroides also increases significantly in patients with NASH and fibrosis[84]. A significant association exists between Ruminococcus and hepatic steatosis[85], and the abundance of Ruminococcus is notably increased in patients with fibrosis[84]. Contrary to this, Da Silva et al[86] found a lower abundance of Ruminococcus in simple steatosis and NASH patients. Ruminococcus gnavus produces an inflammatory polysaccharide that stimulates the production of inflammatory cytokines by dendritic cells[87]. Ruminococcus gnavus is therefore implicated in inflammatory diseases such as Crohn’s disease and may also play a key role in NAFLD development.
| Number of participants | Microbiota alterations | Ref. |
| NASH (n = 65), HC (n = 76) | Increased Collinsella, decreased Ruminococcaceae in NASH | [61] |
| NAFLD (n = 87), obesity without NAFLD (n = 37) | High abundance of Prevotella copri in severe fibrosis | [62] |
| Obese youth with NAFLD (n = 44), without NAFLD (n = 29) | Higher Firmicutes-to-Bacteroidetes ratio and lower abundance of Bacteroidetes, Prevotella, Gemmiger, and Oscillospira in NAFLD | [63] |
| NAFLD (n = 43), HC (n = 83) | Reduction in Firmicutes and Clostridia, increase in Bacteroidetes and Bacteroidia, decrease in Lachnospiraceae, Ruminococcaceae, Lactobacillaceae, and Peptostreptococcaceae in NAFLD | [64] |
| HS (n = 56), HC (n = 49) | Fewer Lachnospiraceae and Ruminococcaceae, enrichment of Acidaminococcus, Escherichia spp., Bacteroides spp. | [66] |
| Stage 0-2 NAFLD-related fibrosis | Eubacterium rectale and Bacteroides vulgatus abundant in mild/moderate NAFLD, Bacteroides vulgatus and Escherichia coli most abundant in advanced fibrosis. Ruminococcus obeum CAG:39, Ruminococcus obeum, and Eubacterium rectale significantly lower in advanced fibrosis | [67] |
| NAFLD (n =25), HC (n = 22) | Escherichia_Shigella, Lachnospiraceae_Incertae_Sedis, and Blautia more abundant, Prevotella decreased in NAFLD. Higher abundance of genus Blautia and the corresponding Lachnospiraceae family in NASH. Higher abundance of Escherichia_Shigella and the corresponding Enterobacteriaceae family in fibrosis | [60] |
| NASH (n = 46), HC (n = 38) | Significant reduction in Akkermansia muciniphila and increase in Enterobacteriaceae in NASH | [74] |
| Total (n = 1148), NAFLD (n = 205) | Significant reduction in family Ruminococcaceae and the genus Faecalibacterium in NAFLD | [75] |
| NAFLD-cirrhosis (n = 27), non-NAFLD control (n = 54) | Enrichment in Negativicutes, reduction in Clostridia | [68] |
| SS (n = 11), NASH (n = 22), HC (n = 17) | Lower percentage of Bacteroidetes (Bacteroidetes to total bacteria counts) in NASH than SS and HC | [69] |
| Severe steatosis (n = 36), fibrosis | Significantly reduction in relative abundance of fecal Clostridium sensu stricto in liver fibrosis | [70] |
| NAFLD (n = 60), chronic viral hepatitis (n = 32), HC (n = 50) | Escherichia coli predominant bacterium in NAFLD | [79] |
| NAFLD (n = 29), HC (n = 25) | Expansion of Escherichia_Shigella in NAFLD | [81] |
| NAFLD (n = 57) | Bacteroides abundance significantly increased, Prevotella abundance decreased in NASH and F ≥ 2. Ruminococcus abundance significantly higher in F ≥ 2 patients | [84] |
| Total (n = 1355), steatosis (n = 472) | Coprococcus, Ruminococcus gnavus increased in steatosis | [85] |
| SS (n = 15), NASH (n = 24), HC (n = 28) | Less abundance of Bacteroidetes and Firmicutes, more abundance of Lactobacillus and Lactobacillaceae in NAFLD compared to HC. Lower abundance of Ruminococcus, Faecalibacterium prausnitzii and Coprococcus in both NASH and SS patients compared to HC | [86] |
| NAFLD (n = 13), obese no NAFLD | More abundance of Gammaproteobacteria and Prevotella in NAFLD | [88] |
| NASH (n = 16), HC (n = 8) | More abundance of Bacteroidetes (Bacteroides and Prevotella genus) in NASH | [89] |
| MAFLD (n = 32), HC (n = 30) | Increased abundance of Prevotella, Bacteroides, and Escherichia-Shigella in MAFLD | [90] |
| Obese with NAFLD (n = 36), obese without NAFLD (n = 17), HC (n = 20) | Decreased abundance of Blautia, Alkaliphilus, Flavobacterium, and Akkermansia in obese subjects, with or without NAFLD, and increased abundance of Streptococcus in NAFLD | [92] |
| NAFLD (n = 90), HC (n = 90) | Increase Slackia, Dorea formicigenerans Methanobrevibacter and Phascolarctobacterium in NAFLD | [93] |
| MAFLD (n = 81), HC (n = 25) | Enrichment of Dorea, Lactobacillus and Megasphaera in MAFLD | [94] |
| Mild NAFLD (n = 33), moderate NAFLD (n = 20), severe NAFLD | Enrichment of Faecalibacterium, Subdoligranulum, Haemophilus, and Roseburia in NAFLD | [97] |
| NAFL (n = 14), NASH (n = 18), HC | Higher abundance of Fusobacteria and Fusobacteriaceae in NASH compared to NAFL and HCs | [98] |
| NAFLD-related cirrhosis and HCC | Higher abundance of Enterobacteriaceae and Streptococcus and a reduction in Akkermansia in cirrhosis. Higher abundance of Bacteroides and Ruminococcaceae, reduction in Bifidobacterium in HCC | [8] |
| NAFLD (n = 98), first-degree relatives (n = 105) | Enrichment of Streptococcus and Megasphaera in NAFLD-cirrhosis | [99] |
| NAFLD-HCC (n = 32), NAFLD-cirrhosis (n = 28), non-NAFLD control (n = 30) | Significant enrichment of Bacteroides xylanisolvens, Ruminococcus gnavus, and Clostridium bolteae in NAFLD-HCC and NAFLD-cirrhosis | [100] |
An increased abundance of Prevotella has been observed in patients with NAFLD and NASH[88,89]. Contrary to this, several studies have found a lower abundance of Prevotella in NAFLD, NASH, and fibrosis patients[60,84] and a higher abundance in healthy individuals[83]. Further research is warranted to establish Prevotella abundance in fatty liver diseases. A study involving 32 MAFLD patients found that there was an increase in the relative abundance of Fusobacteriota, Bacteroidota, Pseudomonadota, and a decrease in Bacillota. At the genus level, there was an increase in the relative abundances of Escherichia-Shigella, Prevotella, and Bacteroides[90]. In a meta-analysis, Li et al[91] concluded that an increase in Streptococcus, Escherichia, and Prevotella, and a decrease in Ruminococcus, Coprococcus, and Faecalibacterium could represent the microbial signature of NAFLD.
Nistal et al[92] compared the gut microbiota of obese patients, with or without NAFLD, to healthy controls. They found a decreased abundance of Blautia, Alkaliphilus, Flavobacterium, and Akkermansia in obese subjects, with or without NAFLD, and an increased abundance of Streptococcus in patients with NAFLD. A community-based cohort study identified microbial signatures associated with NAFLD risk such as Slackia, Dorea formicigenerans, Methanobrevibacter, and Phascolarctobacterium[93]. A study involving 81 MAFLD patients found that Dorea, Lactobacillus, and Megasphaera were enriched in the MAFLD group, while Ruminococcus obeum and Alistipes were more abundant in healthy individuals[94]. A two-sample Mendelian randomization analysis revealed that Christensenellaceae, Lactobacillaceae, and Intestinibacter were negatively correlated, while Actinomycetales, Coriobacteriia, Ruminococcaceae_UCG005, and Oxalobacteraceae were positively correlated with NAFLD[95]. A recent study established a causal relationship between the abundance of eight gut microbiota species and progression of NAFLD[96]. These include the order Actinomycetales, NB1n, the family Ruminococcaceae, Actinomycetaceae, and Oxalobacteraceae, which were positively correlated; the Christensenellaceae R7 group, Lactobacillaceae, and Intestinibacter were negatively correlated with the onset of NAFLD. Recently, Yang et al[97] reported that four bacterial genera, Haemophilus, Faecalibacterium, Roseburia, and Subdoligranulum, along with acetic acid and butyric acid, effectively distinguished NAFLD patients from healthy individuals.
In addition to microbiota signatures associated with NAFLD, several studies have also explored microbiota signatures associated with NASH, fibrosis, and cirrhosis. Rau et al[98] found that patients with NASH exhibited a greater abundance of Fusobacteria and Fusobacteriaceae compared to NAFLD and healthy controls. Ponziani et al[8] found that the fecal microbiota of patients with NAFLD-related cirrhosis showed an increased abundance of Enterobacteriaceae and Streptococcus and a decreased abundance of Akkermansia. Caussy et al[99] reported an enrichment of Streptococcus and Me
Numerous studies across different NAFLD patient populations have reported discrepant microbiota signatures, making it difficult to establish a consensus on the microbiota associated with NAFLD. These discrepancies found in microbiota signatures across studies are mainly attributed to sequencing and diagnostic tools, geographical locations, ethnicity, population characteristics, and disease spectrum[11]. Nevertheless, comparing these studies may provide useful insights into the microbiota signatures that can effectively help diagnose NAFLD onset and progression.
Gut microbiota-derived products, called microorganism-associated molecular patterns (MAMPs), play a crucial role in NAFLD progression[101]. MAMPs include LPS, peptidoglycan, flagellin, lipoproteins, lipoteichoic acid, and DNA. These MAMPs trigger pattern-recognition receptors, such as toll-like receptors (TLRs). TLR signaling is triggered in NAFLD, and TLRs are involved in the development of steatosis and fibrosis[102]. TLRs associated with NASH include TLR2, TLR4, TLR5, and TLR9[103-105]. TLR4- or TLR9-deficient mice fed on HFD or choline-deficient diet were effectively protected from hepatic steatosis[103,106], suggesting a strong association between TLR activation and NAFLD.
TLR2 is the receptor for peptidoglycan and lipoteichoic acid, which are components of the cell walls of Gram-positive bacteria. TLR2-deficient mice on a choline-deficient amino acid-defined diet showed lower expression of proinflammatory cytokine and were resistant to diet-induced steatohepatitis[107]. TLR4, the receptor for LPS, activates the MyD88/nuclear factor kappa B (NF-κB) cascade, triggering the secretion of pro-inflammatory cytokines like IL-6 and tumor necrosis factor-α (TNF-α), leading to inflammation and liver fibrosis[58]. TLR4 can also activate NF-κB through the toll/IL-1 receptor domain-containing adapter protein[108]. Several studies have reported an increase in LPS and TLR4 in the liver of NASH subjects[109-111]. Wild-type mice on a standard diet receiving continuous low doses of LPS developed hepatic steatosis and insulin resistance[56]. Similarly, NAFLD mice receiving LPS injections showed an increase in proinflammatory cytokines and liver injury[112,113]. Humans with NAFLD also show elevated LPS levels[114]. De
TLR signaling induces the secretion of pro-inflammatory cytokines, TNF-α and IL-1β, key mediators in the onset and progression of NAFLD. This is evident from the fact that mice deficient in TLR2, 4, and 9 expressed low proinflammatory cytokines[106,107]. Hepatic macrophages (Kupffer cells) are the primary source of these proinflammatory cytokines[119]. TNF-α not only regulates lipid metabolism but also impairs insulin signaling, resulting in insulin resistance and elevated insulin levels[120]. It also promotes cholesterol accumulation in hepatocytes[121]. Elevated insulin promotes hepatic lipogenesis and the influx of free fatty acid into hepatocytes, while insulin resistance promotes the release of fatty acids from adipocytes and prevents their uptake by adipocytes[122]. Fat-laden hepatocytes have elevated expression of apoptosis signal-regulating kinase-1, c-Jun N-terminal kinase, and pro-apoptotic genes such as Bax, mediating TNF-α-induced apoptosis[123]. In line with these findings, TNF-α signaling-deficient mice are found to be resistant to diet-induced NAFLD[124]. IL-1β regulates lipid metabolism and promotes the accumulation of fats in hepatocytes[125], resulting in NAFLD. Consistent with these findings, IL-1β signaling-deficient mice were found to be effectively protected against steatosis and liver fibrosis[126].
The microbiota-derived metabolites can either activate or suppress signaling pathways, thereby influencing the pro
SCFAs, including propionate, acetate, and butyrate, are produced through microbial fermentation of complex car
Butyrate exerts an anti-inflammatory effect by inhibiting T helper type 17 cells through the activation of regulatory T cells in the intestine, thereby reducing pro-inflammatory signaling pathways[141]. Gut dysbiosis lead to a reduction in butyrate levels, which in turn contributes to low-grade inflammation. Butyrate also plays crucial role in preserving intestinal integrity. In NAFLD, decreased butyrate levels increase gut permeability, facilitating bacterial and LPS translocation[28]. Studies done in animal models have also showed that oral administration of butyrate enhances the intestinal barrier integrity and reduces pathological liver alterations[142-146]. In mice with HFD-induced NAFLD, butyrate mitigates inflammation and fat accumulation by promoting the growth of beneficial bacteria and enhancing the GI barrier[146]. Furthermore, butyrate intervention in HFD-fed mice increase SCFA-producing bacteria, reduces pathogenic bacteria and serum endotoxin levels, and inhibits the expression of proinflammatory cytokines[147]. Administration of sodium butyrate can prevent the progression from simple steatosis to steatohepatitis[145].
Administration of sodium acetate has been shown to have a hepatoprotective effect against nicotine-induced hepatic steatosis[148]. Acetate also reduces hepatic and intramuscular triglyceride content and inhibits lipid accumulation by suppressing fatty acid synthesis and enhancing fatty acid oxidation[149]. Administration of sodium propionate, sodium acetate, or sodium butyrate to methionine- and choline-deficient (MCD) diet-fed NASH mice alleviates NASH by lowering serum levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), liver cholesterol, and triglycerides[143].
Primary BAs are produced by the liver and are secreted into the duodenum with bile. BAs and the gut microbiota engage in a bidirectional interaction[150]. The gut microbiota converts primary BAs into secondary BAs. The intestinal bacteria, particularly Bifidobacterium, Lactobacillus, Clostridium, Bacteroides, and Listeria, produce bile salt hydrolases that deconjugate BAs[151]. The deconjugated BAs are then converted into secondary BAs by 7α-dehydroxylase, primarily produced by Clostridium and Eubacterium.
BAs help preserve intestinal barrier function, thereby preventing bacterial translocation[152]. Acting as signaling molecules, BAs bind to various nuclear receptors[153,154], which play an important role in energy regulation. Dysbiosis in the gut microbiota decreases the synthesis of secondary BAs, which dysregulates nuclear receptors, contributing to the development of NAFLD[152]. BAs regulate hepatic steatosis by activating farnesoid X receptor (FXR) and Takeda G protein-coupled receptor 5 (TGR5)[155]. Intestinal FXR stimulates the release of fibroblast growth factor 15/19 (FGF15/19), which acts on the liver, muscle, and adipose tissue[156]. Overexpression of FGF15 reduces fat mass in mice[157], whereas its knockout promotes hepatic steatosis in HFD-fed mice[158]. Disruption of intestine-specific FXR has been shown to reduce hepatic triglyceride accumulation in a murine model of HFD-induced NAFLD[159]. FXR activation decreases fatty acid uptake and synthesis while increasing fatty acid oxidation[160]. It also triggers peroxisome proliferator-activated receptor α (PPARα) expression, promoting β-oxidation and decreasing lipid accumulation[161]. Mice lacking FXR exhibit elevated serum BAs, cholesterol, triglycerides, and hepatic cholesterol and triglycerides[162]. TGR5 activation promotes the release of glucagon-like peptide-1 from intestinal enteroendocrine cells, enhancing insulin secretion and improving glucose tolerance[163]. TGR5 also suppresses the NF-κB pathway, and TGR5 knockout mice exhibit more severe hepatic necrosis and inflammation[164]. Furthermore, TGR5 activation by BAs inhibits nod-like receptor protein 3 inflammasome, thereby elevating inflammation[165].
Serum concentrations of both primary and secondary BAs are elevated in patients with NAFLD[166]. NASH is characterized by decreased secondary BAs, increased total primary BAs, and elevated conjugated to unconjugated chenodeoxycholate, cholate, and total primary BAs[167]. A metabolomic study revealed alterations in the plasma BAs in NAFLD patients, with plasma 7-keto deoxycholic acid levels associated with advanced-stage hepatic fibrosis and plasma 7-keto lithocholic acid linked to NASH[168]. Increased taurocholate has also been associated with higher grades of steatosis. Elevated levels of BAs and propionate have been reported in non-obese NAFLD patients with fibrosis[169]. A cohort study found that the ratio of circulating conjugated chenodeoxycholic acids to muricholic acids was elevated in NASH patients[170]. Recently, Wang et al[171] showed that tauroursodeoxycholic acid, a synthetic bile salt, reduced obesity and hepatic lipid accumulation, while also enhancing intestinal barrier function and gut microbial diversity.
Tryptophan is an essential amino acid that must be supplemented through diet. Gut microbes directly influence the three major Trp metabolism pathways, which produce serotonin, kynurenine, and indole derivatives[172]. Tryptophan metabolites can play either a positive or negative role in NAFLD. Alterations in tryptophan and its metabolites have been observed in NAFLD-cirrhosis[68]. Gut microbiota metabolizes unabsorbed tryptophan into indole and its derivatives, such as IPA and IAA, which improve gut barrier function and modulate the immune response via aryl hydrocarbon receptor[173]. Indole and its derivatives are also known for their anti-inflammatory effects[174]. Numerous studies have highlighted the anti-inflammatory potential of indole. Knudsen et al[175] showed that indole reduced markers of hepatic damage and hepatic expression of genes involved in inflammation and macrophage activation in ob/ob mice. Beaumont et al[176] demonstrated that the oral administration of indole reduced LPS-induced increase in proinflammatory mediators and prevented LPS-induced changes in cholesterol metabolism. Zhao et al[177] reported that IPA inhibited NF-κB signaling and reduced the levels of proinflammatory cytokines. Additionally, studies have also shown that indole enhances intestinal integrity, and there is a negative correlation between indole and hepatic fat content[178,179]. NAFLD has been linked to reduced production of indole and its derivatives[179,180]. IPA levels were found to be lower in liver fibrosis patients compared to controls[181]. Administration of IPA can increase the expression of tight junction proteins, maintain intestinal epithelium homeostasis, reduce hepatic inflammation and liver injury, and modulate gut microbiota in HFD-fed rats[177]. It has also been shown to lower fasting blood glucose levels, plasma insulin levels, and insulin resistance in mice[182]. A recent study demonstrated lower levels of IPA and IAA in the feces of patients with hepatic steatosis compared to healthy controls[183]. Moreover, the administration of IPA and IAA improved hepatic steatosis and inflammation in an animal model by inhibiting the NF-κB signaling pathway.
Ritze et al[184] found that oral tryptophan supplementation attenuated experimental NAFLD in mice, probably by stabilizing the intestinal barrier and improving the dysregulated intestinal serotonergic system. IAA administration can reduce HFD-induced oxidative stress, plasma total cholesterol, low-density lipoprotein cholesterol (LDL-C), and inflammatory response of the liver in a mouse model[185]. A study involving 58 obese patients who underwent sleeve gastrostomy found that the levels of IAA increased post-surgery, and plasma IAA levels were negatively correlated with liver fat attenuation[186]. Another indole derivative, NecroX-7, was shown to reduce serum AST and ALT, hepatic steatosis and fibrosis, and lipid peroxidation in ob/ob mice[187].
Serotonin [5-hydroxytryptamine (5-HT)] is a tryptophan metabolite produced by the action of tryptophan hydroxylase. Studies have identified 5-HT as a risk factor in the pathological progression of NASH. Wang et al[188] showed that 5-HT increased lipid accumulation and expression of lipogenesis-related genes, while the absence of 5-HT prevented hepatic lipid accumulation and the expression of inflammatory factors. Choi et al[189] demonstrated that inhibiting serotonin synthesis alleviates hepatic steatosis by reducing liver 5-HT (serotonin) receptor 2A signaling. Moreover, tryptophan hydroxylase and 5-HT (serotonin) receptor 2A knockdown mice exhibit resistance to HFD-induced hepatic steatosis. In contrast to 5-HT’s pro-inflammatory effects, its metabolite melatonin has been shown to have anti-inflammatory properties. Melatonin reduces serum and liver triglyceride levels and inhibits LPS-induced hepatic fat buildup in mice[190]. It also improves NAFLD phenotypes, and reduces the expression of pro-inflammatory cytokines, lowers oxidative stress levels, ALT, fasting plasma glucose, body and liver weight, and LDL-C[191,192]. In a randomized controlled trial involving seventy-four patients with NAFLD, melatonin significantly decreased levels of pro-inflammatory cytokines, hepatic inflammation, and improved fat metabolism parameters[193]. Another randomized, double-blind, placebo-controlled clinical trial involving forty-five patients found that melatonin significantly improved fatty liver grade, ALT, AST, weight, waist, abdominal circumference, and both systolic and diastolic blood pressure[194].
The gut microbiota ferments dietary nutrients like choline and carnitine to produce TMA, which is subsequently converted into TMA N-oxide (TMAO) in the liver[195]. TMAO has been associated with several chronic diseases, such as diabetes, insulin resistance, liver steatosis, hypertension, atherosclerosis, heart failure, and cancer. The association between TMAO and NAFLD was first established by Chen et al[196], who found that TMAO levels in NAFLD patients were 4.17 times higher than in healthy controls. Elevated TMAO levels are linked to obesity and the severity of MAFLD[197]. TMAO promotes inflammation in adipose tissue[198,199], increases fasting insulin resistance and pro-inflammatory cytokine monocyte chemoattractant protein-1, and decreases anti-inflammatory cytokine IL-10 in adipose tissue[199]. It has also been shown to increase triglyceride levels and the expression of liver fibrosis-related genes, particularly KRT17, in fatty liver cells in vitro[200]. A cohort study of 5292 participants found that the plasma TMAO levels were linked to all-cause mortality in patients with NAFLD[201]. TMAO exacerbates hepatic steatosis by modulating BA metabolism. A case-control study involving biopsy-proven NAFLD patients identified a positive correlation between TMAO levels and serum levels of total BAs[202]. This study also found that TMAO administration deteriorated liver function and elevated hepatic triglyceride and lipogenesis in HFD-fed mice. TMAO binds to the endoplasmic reticulum stress kinase protein kinase RNA-like endoplasmic reticulum kinase and triggers the unfolded protein response (UPR)[203]. UPR is also triggered by the buildup of misfolded proteins in the endoplasmic reticulum and is known to be activated in various liver diseases, including hepatic steatosis[204]. These findings suggest that TMAO plays a key role in the progression and development of NAFLD through UPR activation.
TMAO is an early biomarker of NAFLD, with a specific cut-off level identifying individuals at high risk of the disease[205]. A cohort study of 357 Mexican obese patients with varying stages of NAFLD found a significant association between TMAO and choline levels and the risk of NASH[206]. In contrast, Zhao et al[207] reported that oral TMAO reduced ALT, AST, and hepatic and serum cholesterol levels, restored gut flora diversity, and attenuated steatohepatitis in HFD and high-cholesterol diet-fed rats. A newly identified metabolite, N,N,N-trimethyl-5-aminovaleric acid, has been found to be elevated in patients with hepatic steatosis[208].
Ethanol is produced in the small intestine by gut microbiota acting on dietary carbohydrates and is subsequently processed by the liver through the action of alcohol dehydrogenases. A potential link exists between blood ethanol levels and alterations in the gut microbiota[209]. Zhu et al[78] reported significantly elevated blood ethanol levels and an increased abundance of alcohol-producing bacteria, such as Escherichia, in NASH patients compared to healthy individuals and obese non-NASH patients. Michail et al[88] found notably higher ethanol levels in children with NAFLD compared to controls. Engstler et al[210] also observed significantly elevated fasting ethanol levels in children with NAFLD compared to controls. Further studies in mice suggested that elevated ethanol levels may be due to insulin-dependent impairments in alcohol dehydrogenase activity rather than increased endogenous ethanol synthesis. In a prospective clinical study, Meijnikman et al[211] measured ethanol levels in 146 individuals and used selective ADH inhibition to show that elevated portal vein ethanol concentrations, driven by gut microbiota (e.g., Lactobacillaceae), increase with NAFLD progression and play a significant role in its pathogenesis. Additionally, the high-alcohol-pro
Diet not only affects the progression of various metabolic disorders but also the gut microbiota composition and intestinal barrier function, suggesting that an unhealthy diet may contribute to NAFLD progression[213]. Several studies have shown that a Western diet increases, while a Mediterranean diet (MD) decreases, intrahepatic fat accumulation[214,215]. Western diet induces NASH in male mice, exhibiting features matching human steatohepatitis, such as hepatic inflammation, steatosis, and fibrosis[216]. Additionally, Western diet increases the risk of NAFLD by 56%, while the Prudent and MDs reduce the risk by 22% and 23%, respectively[217].
MD is rich in nutrients, particularly monounsaturated fatty acids, polyunsaturated fatty acids, and fiber, all of which have beneficial effects on lipid metabolism and fatty liver disease[218]. Randomized controlled trials have confirmed the efficacy of MD in reducing total cholesterol, serum triglycerides, hepatic steatosis, and liver cirrhosis in NAFLD patients[219-221] (Table 2). Additionally, MD has also been shown to improve insulin resistance[222,223]. MD is considered a safe and effective means of treating NAFLD patients. A meta-analysis further confirmed that MD significantly reduced the fatty liver index and insulin resistance in patients with NAFLD[224]. In a randomized clinical trial involving children, MD and a low-fat diet reduced hepatic steatosis, hepatic enzymes, and insulin resistance[225]. Another randomized clinical trial with adolescents similarly demonstrated a significant reduction in hepatic steatosis, serum transaminase levels, and insulin resistance with MD and a low-fat diet[226].
| Number of participants | Dietary intervention | Outcome | Ref. |
| n = 74 | Melatonin | Significant decrease in levels of pro-inflammatory cytokines, inflammation in liver and improvement in parameters of fat metabolism | [193] |
| n = 45 | Melatonin | Significant improvement in the grade of fatty liver, ALT, AST, weight, waist and abdominal circumference and systolic and diastolic blood pressure | [194] |
| n = 18 | MD | Significant reduction in mean body weight, waist circumference, ALT and AST | [220] |
| n = 49 | MD or LFD | Reduction in hepatic steatosis and liver enzymes with MD and LFD. Improvements in total cholesterol, serum triglyceride, and glycated hemoglobin with MD | [221] |
| n = 56 | MD or ML | Weight reduction with MD and ML. Significant improvement in ALT levels and liver stiffness with ML and liver stiffness with MD | [222] |
| n = 44 | MD or LFD | Significant decrease in hepatic steatosis, serum transaminase levels, and insulin resistance with MD and LFD | [227] |
| n = 27 | KD or LFD | Significantly reduction in liver fat with both KD and LFD | [233] |
| n = 37 | High animal or plant protein diet | Reduction in liver fat within 6 weeks, no change in body weight | [251] |
| n = 40 | Low free sugar diet | Reduction in hepatic steatosis and ALT level | [249] |
| n = 29 | Low dietary sugar diet | Reduction in hepatic de novo lipogenesis, hepatic fat and fasting insulin | [250] |
The ketogenic diet (KD) involves a significant reduction in carbohydrate intake. Ketosis is achieved when carbohydrate intake is below 50 g per day, regardless of fat and calorie intake[227]. KD has been shown to reduce intrahepatic triglycerides by 31% and hepatic insulin resistance by 58%[228]. However, various risk factors are associated with KD. For example, Muyyarikkandy et al[229] showed that long-term KD intervention can aggravate hepatic mitochondrial dy
A high dietary fiber intake improves metabolic parameters associated with NAFLD[233] and promotes the growth of beneficial bacteria, such as Bifidobacterium, Lactobacillus, and Bacteroidetes, and SCFA-producing bacteria[234,235]. In contrast, the MCD diet has been shown to significantly reduce Bifidobacterium and Lactobacillus in feces[236]. Choline affects the composition of the gut microbiota[237]. Choline plays a critical role in lipid metabolism and prevents fat buildup in the liver. Additionally, choline is also metabolized by gut microbes to dimethylamine and TMA, which are subsequently converted into TMAO in the liver, leading to hepatic inflammation and damage[238]. Furthermore, choline-deficient diet has been associated with the development of NASH and dysbiosis of the gut microbiota[239-241].
HFD is associated with an increased abundance of Proteobacteria, such as Desulfovibrionaceae and Enterobacteriaceae[56,242]. Proteobacteria can increase LPS levels or disrupt the intestinal barrier[243,244], contributing to the development of NAFLD. A high intake of dietary cholesterol accelerates the development of spontaneous NAFLD and triggers liver inflammation in mice[245]. A higher fat fraction has been linked to lower α-diversity of the gut microbiota[246]. In contrast, a low intake of dietary saturated fatty acids in humans is associated with increased microbial diversity, ir
A diet high in animal or plant protein has been shown to reduce liver fat by 36% to 48% without affecting body weight[250]. Such a diet can also lower hepatic enzymes, reduce markers of inflammation, decrease serum keratin 18 levels and improve insulin sensitivity. Recently, Zhou et al[251] demonstrated that isoleucine-restricted diets could reverse HFD-induced weight gain, hepatic inflammation, increased fasting glucose levels, and improved hepatic insulin resistance, lipid metabolism, and gluconeogenesis disorder.
Berberine, a quaternary ammonium compound found in some plants, exhibits potent antibacterial activity. Studies suggest that berberine can modulate gut microbiota and offers therapeutic potential for treating metabolic diseases[252,253]. Similarly, resveratrol, a natural polyphenol found in many plants such as grapes may help prevent metabolic diseases by lowering blood glucose and lipid levels[254]. This effect is partly attributed to its ability to modify gut microbiota.
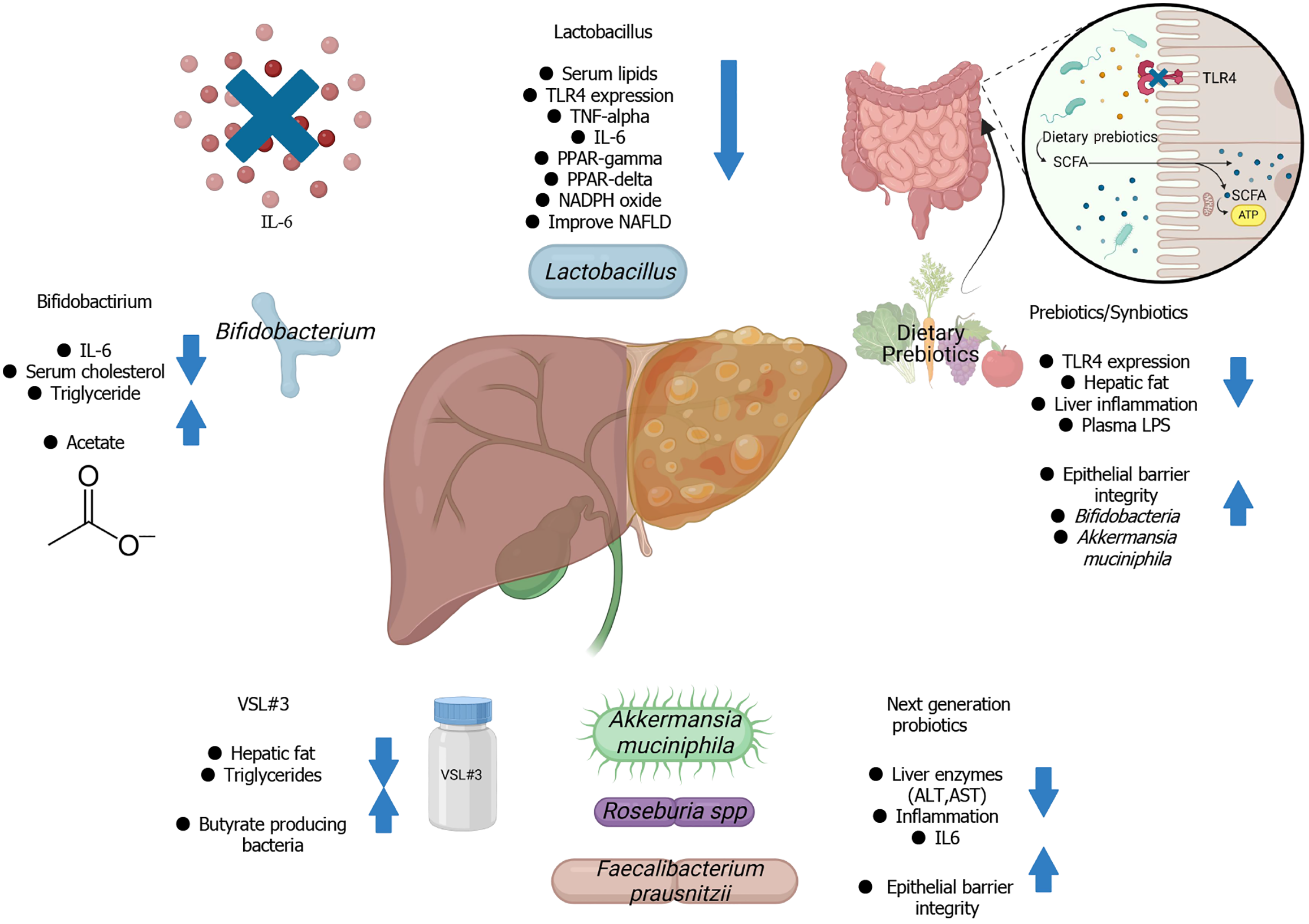
Currently, there are no Food and Drug Administration-approved drug therapies for NAFLD. Lifestyle modifications, including dietary interventions and exercise, and pharmacotherapy, including the use of metformin, thiazolidinediones, statins, and ursodeoxycholic acid, are the only known treatments of NAFLD[255]. Restoring gut homeostasis in NAFLD patients through microbiota-targeted therapies has emerged as a promising alternative approach for managing and treating the disease (Figure 2). Prebiotics, probiotics, and synbiotics have been shown to improve hepatic alterations associated with hypercholesterolemia by modulating the expression of genes related to β-oxidation and lipogenesis[256]. Strategies aimed at modulating the gut microbiota through prebiotics, probiotics, synbiotics, and FMT may offer potential therapeutic options for managing NAFLD (Table 3).
| Number of participants | Intervention | Outcome | Ref. |
| n = 20 | L. rhamnosus GG | Significant decrease in ALT and antipeptidoglycan-polysaccharide antibodies, no effect on BMI Z score and visceral fat | [280] |
| n = 44 | VSL#3 | Improvement in NAFLD and reduction in triglycerides and insulin resistance | [311] |
| n = 28 | L. bulgaricus and S. thermophilus | Reduction in the levels of ALT, AST, and GGT | [315] |
| n = 20 | Lepicol probiotic (L. plantarum, L. delbrueckii, L. acidophilus, L. rhamnosus and B. bifidum) | Reduction in intrahepatic triglyceride and serum AST, no change in waist circumference, BMI, and glucose and lipid levels | [316] |
| n = 72 | Probiotic yogurt containing L. acidophilus La5 and B. lactis Bb12 | Reduction in the levels of ALT, AST, total cholesterol, and LDL cholesterol | [317] |
| n = 42 | Lactocare (L. casei, L. acidophilus, L. rhamnosus, L. bulgaricus, B. breve, B. longum, S. thermophilus) | Reduction in insulin, insulin resistance and inflammation markers such as TNF-α and IL-6 | [318] |
| n = 64 | Probiotic mixture (L. acidophilus ATCC B3208, B. lactis DSMZ 32269, B. bifidum ATCC SD6576, and L. rhamnosus DSMZ 21690) | Reduction in waist circumference, cholesterol, triglycerides, LDL-C, ALT and AST. No change in weight, BMI, and BMI Z score | [319] |
| n = 65 | Probiotic mixture (L. acidophilus, L. rhamnosus, L. paracasei, Pediococcus pentosaceus, B. lactis, and B. breve) | Reduction in body weight, total body fat, and intrahepatic fat | [320] |
| n = 58 | “Symbiter” containing 14 probiotic belonging to Bifidobacterium, Lactobacillus, Lactococcus, and Propionibacterium | Reduction in fatty liver index, liver stiffness, serum AST, GGT, TNF-α and IL-6 | [321] |
| n = 39 | Multi-strain probiotics containing six different Lactobacillus and Bifidobacterium spp. | Stabilization of the mucosal immune function. No effect on hepatic steatosis and fibrosis levels | [322] |
| n = 40 | Akkermansia muciniphila | Improvement in insulin sensitivity, reduction in insulinemia, plasma total cholesterol, levels of the markers for liver dysfunction and inflammation | [334] |
| n = 22 | Inulin-type fructans | Increased fecal Bifidobacterium. No effect on liver fat contents, liver function tests, and metabolic and inflammatory mediators | [375] |
| n = 197 | Resistant starch | Reduction in intrahepatic triglyceride content and liver enzymes | [382] |
| n = 66 | Synbiotic (B. longum with FOS) | Significant reduction in inflammatory markers, LDL cholesterol, steatosis, and NASH activity index | [395] |
| n = 52 | Protexin (L. casei, L. rhamnosus, L. acidophilus, L. bulgaricus, B. breve, B. longum, S. thermophilus with prebiotic FOS) | Significant attenuation in inflammatory markers, such as ALT, AST, GGT, high-sensitivity CRP, TNF-α, and NF-κB p65 | [397] |
| n = 50 | Protexin (L. casei, L. rhamnosus, L. acidophilus, L. bulgaricus, B. breve, B. longum, S. thermophilus with prebiotic FOS) | Reduction in inflammatory mediators, hepatic steatosis and fibrosis | [398] |
| n = 104 | Synbiotic (FOS and B. animalis subspecies lactis BB-12) | Alteration in fecal microbiota. No effect on liver fat content or markers of liver fibrosis | [406] |
| n = 21 | Fecal microbiota transplantation | Reduction in small intestinal permeability. No improvement in insulin resistance and hepatic proton density fat fraction | [418] |
Probiotics are living microorganisms that provide multiple health benefits to the host if administered adequately[257]. These health benefits include inhibition of pathogenic bacteria, regulation of the immune system, reduction in blood cholesterol, and enhancement of the intestinal mucosal barrier function[258,259]. Probiotics work by modifying the gut microbiota and producing the metabolites that inhibit pathogenic microbes, thus help prevent intestinal infections[260-262]. The effects of probiotics depend on the factors such as the duration of treatment, cocktail combination, and probiotic dosage. Lactobacillus and Bifidobacterium are among the widely used probiotics and have demonstrated potential in treating NAFLD. Both are known to improve glucose-insulin homeostasis and reduce hepatic steatosis by modulating gut microbiota[263].
Lactobacillus: Studies have shown that Lactobacillus exhibits a hypocholesterolemic effect[264-266] suggesting its po
L. plantarum MB452 has been shown to enhance intestinal barrier function by upregulating genes involved in tight junction signaling[270]. L. plantarum NCU116 administration has demonstrated the ability to restore liver function and reduce fat accumulation, and decrease proinflammatory cytokines in the liver[271]. Oral gavage of L. plantarum ZJUIDS14 to HFD-induced NAFLD mice alleviates liver damage, insulin resistance, and hepatic steatosis. It also strengthens intestinal barrier integrity, regulates gut microbiota, and improves mitochondrial function[272]. Additionally, L. plan
L. rhamnosus GG inhibits NF-κB signaling, reducing the effects of pro-inflammatory cytokines on epithelial barrier integrity[275]. Administration of L. rhamnosus GG has been shown to decrease hepatic fat content, serum triglyceride and cholesterol levels, and expression of pro-inflammatory genes and suppress FXR and FGF15 signaling[276]. It also reduces portal LPS and duodenal IkappaB protein levels while restoring the abundance of duodenal tight junction protein in mice with high-fructose diet-induced NAFLD[277]. L. rhamnosus GG treatment improves hepatic steatosis, liver injury, adipose inflammation, glucose intolerance, and insulin resistance[278]. In a randomized controlled trial involving twenty obese children, administration of L. rhamnosus GG significantly decreased ALT levels and antipeptidoglycan-polysaccharide antibodies without affecting body mass index (BMI) Z score and visceral fat[279].
L. gasseri BNR17 is known to reduce body weight, white adipose tissue weight, serum leptin, and insulin, while increasing the expression of fatty acid oxidation-related genes[280]. Naudin et al[281] found that oral gavage of L. lactis subsp. cremoris to mice on a Western diet resulted in reduced hepatic steatosis, inflammation, and weight gain; lower serum cholesterol and BMI; and increased glucose tolerance. L. coryniformis subsp. torquens T3 has been shown to inhibit obesity and liver inflammation, while alleviating hepatic fat buildup in mice with NAFLD[282]. Additionally, L. coryniformis can reduce the abundance of harmful bacteria, enhance the intestinal barrier, and increase SCFA content. L. johnsonii BS15 protects HFD-induced NAFLD mice from hepatic steatosis and hepatocyte apoptosis[283]. BS15 also lowers serum LPS levels, inhibits insulin resistance, and decreases intestinal permeability. Oral administration of L. reuteri GMNL-263 decreases levels of serum glucose, insulin, lipids, and hepatic injury markers, along with improving liver lipid profile in high fructose-induced hepatic steatosis in mice[284]. GMNL-263 also reduces TNF-α and IL-6 levels in adipose tissue and increases the number of Bifidobacterium and Lactobacillus. The therapeutic effects of Lactobacillus are not limited to live bacteria; heat-killed L. brevis SBL88 has been shown to significantly reduce hepatic fat droplets without altering the gut microbiota but by modulating lipid and insulin metabolism[285]. Despite the potential of Lactobacillus in NAFLD treatment, some studies have shown contrasting results. In a double-blind placebo-controlled study, it was found that L. acidophilus did not have any effect on serum lipid levels[286]. Furthermore, many studies have observed an increased abundance of Lactobacillus in patients with NAFLD[94].
Bifidobacterium: Bifidobacterium is another widely used probiotic. Studies have found a decrease in the abundance of Bifidobacterium in NAFLD patients[78,242]. Therefore, restoring the abundance of Bifidobacterium through probiotic supplementation could be a potential alternative treatment for NAFLD. Bifidobacterium has been shown to improve visceral fat, cholesterol levels, and insulin resistance[287,288]. It can alleviate NAFLD through the activation of the sirtuin inhibitor (sirtuin 1)-mediating signaling pathway[289].
B. animalis ssp. lactis GCL2505 has demonstrated an anti-metabolic syndrome effects in HFD-fed mice. Oral administration of GCL2505 has been shown to reduce visceral fat accumulation, improve glucose tolerance, and increase acetate levels[290]. Administration of B. adolescentis and B. pseudocatenulatum CECT 7765 has been shown to reduce visceral fat accumulation, liver steatosis, serum cholesterol, triglyceride, glucose, and IL-6 and decrease insulin sensitivity in HFD-fed rats[287,291]. B. longum has proven to be more efficient than L. acidophilus in attenuating liver fat accumulation[292]. An et al[293] found antiobesity and lipid-lowering effects of three Bifidobacterium spp. (B. longum SPM 1207, B. longum SPM 1205, and B. pseudocatenulatum SPM 1204) in HFD-induced obese rats. A recent study found that B. lactis Probio-M8 could improve liver damage and hepatic steatosis in HFD-fed rats[294]. Furthermore, M8 modulated the gut microbiota by increasing the abundance of Bifidobacterium and decreasing the abundance of Bilophila, Lachnoclostridium, GCA-900066225, and Phascolarctobacterium.
Saccharomyces boulardii: Saccharomyces boulardii is a fungal probiotic that provides a conducive environment for the growth of Lactobacillus and Bifidobacterium[295]. S. boulardii modulates gut microbiota and the immune system, exhibits antitumor activity, inhibits pathogen growth and inflammation, and maintains the SCFA levels and intestinal barrier integrity[296-298]. Several studies have highlighted the potential of S. boulardii in NAFLD treatment. Everard et al[299] found that oral administration of S. boulardii in obese mice for 4 weeks led to reduced body weight, fat mass, and hepatic steatosis and modulated gut microbial composition. Yang et al[300] reported that S. boulardii gavage to MCD diet-fed mice resulted in improved liver function, less hepatic steatosis, decreased inflammation, increased microbiota diversity, and maintained gut integrity.
Probiotic cocktails: Many studies have explored the potential of a combination of two or more probiotic strains in NAFLD treatment. A cocktail of B. infantis, L. acidophilus, and Bacillus cereus has been shown to delay the progression of NAFLD by suppressing the LPS/TLR4 signaling[301]. Oral administration of L. lactis and Pediococcus pentosaceus can mitigate NAFLD progression by modulating the gut environment[130]. Consumption of kefir, a probiotic drink with more than 50 species of lactic acid bacteria and yeast, prevents obesity and NAFLD by modulating gut microbiota and promoting fatty acid oxidation[302]. Oral administration of Symbiter, a concentrated biomass of 14 probiotic bacteria combined with omega-3, has been shown to decrease hepatic triglyceride and hepatic steatosis[303].
VSL#3, a widely used mixture of eight live freeze-dried probiotic strains, has been confirmed as an effective the
In addition to live probiotic cocktails, cell-free and fermentation extracts of various probiotics have also been explored for treatment of NAFLD. Administration of cell-free supernatants of four probiotic strains, L. salivarius Ls-33, L. acidophilus NCFM, B. lactis HN019, and B. lactis 420 has been shown to increase tight junction integrity[82]. A probiotic mixture consisting of L. acidophilus, L. plantarum, Bifidobacterium bifidum, Bacillus subtilis fermentation extract, Aspergillus oryzae fermentation extract, and maltodextrin has been shown to improve lipid profiles, and reduce TNF-α and IL-6 levels in rats fed a HFD and sucrose diet[313].
Clinical data and randomized controlled trials have supported the efficacy of probiotics in the treatment of NAFLD[314-321] (Table 3). Systematic reviews and meta-analyses have further highlighted the potential of probiotics in treating metabolic diseases, including NAFLD[322-325]. However, a systematic review by Tarantino and Finelli[326] concluded that drawing a definitive conclusions regarding the therapeutic efficacy of probiotics and prebiotics in NAFLD and obesity remains challenging due to the lack of high-quality clinical studies.
Next-generation probiotics: Next-generation probiotics (NGPs) are unconventional probiotics that have demonstrated enhanced therapeutic effects in NAFLD. Akkermansia muciniphila is a mucin degrader and an important component of healthy gut microbiota, which plays a crucial role in preserving the gut barrier[72] and produces SCFAs[327]. A. muciniphila is a promising NGP that is inversely associated with metabolic disorders such as obesity, diabetes, and cardiometabolic diseases[328]. It has been shown to prevent fatty liver disease and decrease serum triglycerides in mice, reinforce the epithelial barrier, and reduce fat deposition and obesity in humans and mice[329,330]. It has also been found to efficiently reverse hepatic steatosis, inflammation, and liver injury in mice[331]. The beneficial effects of A. muciniphila are attributed to increased mitochondrial oxidation, BA metabolism, and L-aspartate levels in the liver, as well as modulation of the gut microbiota. Kim et al[332] found that oral administration of A. muciniphila led to a significant reduction in serum triglyceride, ALT, and IL-6 in obese mice. In a randomized controlled trial, it was found that oral administration of A. muciniphila improved insulin sensitivity and decreased insulinemia, lowered plasma total cholesterol, and reduced the markers of liver dysfunction and inflammation[333]. Daily oral supplementation of 1010A. muciniphila has been found to be safe and well tolerated. A recent study also showed that A. muciniphila outperformed VSL#3 in reducing hepatic fat content in NAFLD mice[334].
Faecalibacterium prausnitzii is a constituent of the healthy gut microbiota in humans, known for its ability to ferment dietary fibers and produce SCFAs. F. prausnitzii synthesizes a metabolite called microbial anti-inflammatory molecule, which helps maintain intestinal barrier function and elevates the expression of tight junction proteins[335]. When combined with Roseburia intestinalis and Bacteroides faecis, F. prausnitzii can further enhance the epithelial barrier integrity[336]. Oral administration of F. prausnitzii in HFD-fed mice has been shown to decrease hepatic fat content, lower hepatic enzymes, reduce adipose tissue inflammation, and increase fatty acid oxidation and adiponectin signaling[337].
Clostridium butyricum MIYAIRI 588 is a butyrate-producing bacterium traditionally used in humans for the treatment of diarrhea and constipation. MIYAIRI 588 has been shown to prevent NAFLD progression by reducing hepatic fat de
Parabacteroides distasonis has emerged as a promising agent for NAFLD, primarily through its modulation of metabolic pathways and the gut-liver axis. Wei et al[341] demonstrated that P. distasonis alleviates hepatic steatosis and inflammation in mice by inhibiting pro-inflammatory signaling and restoring gut barrier function through its metabolite pentadecanoic acid. Additionally, pentadecanoic acid has been shown to reduce liver fat content and weight in the TANGO randomized controlled trial[342]. It also boosts fatty acid oxidation via γ-linolenic acid production, activating PPARα signaling[343]. Beyond NAFLD, P. distasonis has demonstrated therapeutic potential in obesity and type 2 diabetes by improving glucose homeostasis, insulin sensitivity, and systemic inflammation through the production of SCFAs and succinate[344]. Recent studies have highlighted the therapeutic potential of other bacteria that can be used as NGPs, such as Limosilactobacillus mucosae (L. mucosae)[345], Bacteroides thetaiotaomicron[346], and Lactiplantibacillus plantarum ZDY2013[347]. L. mucosae is effective in reducing body weight, liver weight, adipose tissue weight, total cholesterol, triacylglycerol, and LDL-C in NAFLD mice[345]. Additionally, L. mucosae can decrease the relative abundance of Ruminococcaceae and increase the abundance of Akkermansia. Bacteroides thetaiotaomicron has been shown to reduce fat accumulation, hyperlipidemia, insulin resistance, and body weight, and prevent hepatic steatohepatitis and liver injury in HFD-fed mice[346]. It also modulates the gut microbial composition and decreases the Firmicutes to Bacteroidetes ratio. L. plantarum ZDY2013 administration improves insulin resistance, reduces fat accumulation in the liver, restores liver function, and regulates oxidative stress[347].
Prebiotics are dietary components, particularly indigestible carbohydrates that maintain a healthy gut microbiota by providing an optimal environment for the growth of beneficial microbes[348,349]. Studies have shown that prebiotic administration increases the plethora of beneficial microbes such as Akkermansia, Faecalibacterium, Bifidobacterium, Lactobacillus, and Roseburia, while decreasing pathogenic microbes[350-354]. Complex carbohydrates, such as fructo-oligosaccharides (FOS), galacto-oligosaccharides (GOS), inulin, polydextrose, and fructans, are fermented by specific gut microbiota to produce SCFAs.
FOS is known to improve liver pathology, inhibit adipocyte enlargement, and increase fecal concentrations of butyrate and acetate and serum concentrations of propionate[355]. Additionally, FOS administration decreases the expression of genes related to inflammation and lipid metabolism. Studies have shown that dietary FOS can decrease steatohepatitis by restoring intestinal epithelial barrier function and normal GI microflora[356]. Pachikian et al[357] reported that FOS reduced hepatic triglyceride buildup in mice and altered gut microflora by increasing caecal Bifidobacterium spp. and decreasing Roseburia spp. Matsumoto et al[358] reported that FOS could decrease hepatic steatosis, liver inflammation, and expression of TLR4 in an MCD mouse model of NASH. This was accompanied by an increase in fecal SCFAs and im
GOS increases the abundance of Bifidobacterium[363]. A blend of GOS and FOS (9:1) has been shown to increase both the abundance and proportion of bifidobacteria in healthy infants[364]. A double-blind, randomized study involving 45 overweight adults having ≥ 3 risk factors linked to metabolic syndrome found that GOS administration increased the number of fecal bifidobacteria and fecal secretory immunoglobulin A, while decreasing total cholesterol, insulin, plasma C-reactive protein, and fecal calprotectin[365].
Inulin is a fructan found naturally in a variety of plants. It can decrease plasma triacylglycerol concentrations and hepatic lipogenesis[366] and increase the abundance of Bifidobacterium[367]. Inulin administration results in the pro
Lactulose, a synthetic sugar commonly used for the treatment of constipation, promotes the growth of beneficial bacteria[375]. A study on humans has shown that lactulose increases fecal bifidobacterial counts without significantly affecting Lactobacillus, fecal BAs, and neutral sterols[376]. A study by Fan et al[377] found that while lactulose could not completely prevent steatohepatitis development, it could improve hepatic inflammation in rats with steatohepatitis induced by HFD. Chitin-glucan has been shown to decrease hepatic triglyceride accumulation, weight gain induced by HFD, and hypercholesterolemia[378]. Additionally, a combination of prebiotic isomalto-oligosaccharides and antioxidant lycopene has been found to work synergistically in preventing nonalcoholic fatty liver-like symptoms[379]. Dietary isomalt has been shown to attenuate hepatic steatosis, restore intestinal epithelial integrity, improve the abundance of Akkermansia, suppress Acinetobacter and Corynebacterium_1, and reduce fecal acetate concentrations, while elevating propionate and butyrate in rats[380]. A randomized, placebo-controlled clinical trial involving 197 participants found that resistant starch intervention reduced intrahepatic triglyceride content and liver enzymes[381].
Many recent studies have explored the potential of plant polysaccharides and bioactives in NAFLD treatment. Zhang et al[382] demonstrated that polysaccharides from Smilax china reduced serum and hepatic lipid levels, and reversed HFD-induced increases in body, liver, and adipose tissue weights. The polysaccharide intervention upregulated the genes related to lipolysis, downregulated the genes related to lipogenesis, and restored gut microbiota. Huang et al[383] found that large-leaf yellow tea polysaccharides decreased bile salt hydrolase-producing genera and increased taurine metabolism genera and the levels of conjugated BAs. Hao et al[384] reported that Crataegus pinnatifida polysaccharide effectively reduced liver steatosis, decreased serum levels of triglyceride, total cholesterol, AST, ALT, and LDL-C, in
Synbiotics, a combination of pro- and prebiotics[349], have shown potential in modulating gut microbiota composition and improving markers of liver health. L. rhamnosus GG and L. plantarum WCFS1, combined with anthraquinone from Cassia obtusifolia L., have been shown to reduce blood lipid levels, improve insulin resistance, and enhance intestinal mucosal barrier function in NAFLD rats[387]. L. paracasei N1115 and FOS have been shown to alleviate HFD-induced hepatic steatosis, slow the progression of cirrhosis, reduce serum triglyceride and cholesterol, fasting blood glucose and insulin, and improve intestinal barrier function by increasing the expression of tight junction components[388]. FOS and a probiotic cocktail, including L. rhamnosus, L. casei, L. bulgaricus, B. longum, and Streptococcus thermophilus, can reduce liver stiffness, ALT, and serum cholesterol[389]. Administration of Lactobacillus reuteri with guar gum and inulin for three months in NASH patients resulted in reductions in steatosis, weight, waist circumference, and BMI, although it did not improve intestinal permeability or LPS levels[390]. Daily consumption of synbiotic yogurt containing B. animalis and inulin has been shown to reduce NAFLD grade and levels of ALT, AST, alkaline phosphatase, and gamma-glutamyl transferase[391]. A synbiotic consisting of L. paracasei, arabinogalactan, and FOS has been shown to improve insulin resistance, slow NAFLD progression, reduce cytokines and TLR expression, preserve gut barrier integrity, and increase the abundance of Enterobacteriales and Escherichia coli in colonic mucosa[392]. A. muciniphila and quercetin administration in the NAFLD mouse model has been shown to attenuate steatosis by modulating gut microbiota and enhancing hepatic expression of BA synthesis[393]. A randomized controlled trial using B. longum with FOS reported significant reductions in inflammatory markers, LDL-C, steatosis, and NASH activity index[394]. Protexin, a synbiotic containing seven probiotic bacteria belonging to lactobacilli, bifidobacteria, and S. thermophilus with prebiotic FOS, has shown promising results in NAFLD treatment[395-398].
Many recent studies have highlighted the therapeutic potential of different synbiotic blends in treating NAFLD. Zhang et al[399] demonstrated that a synbiotic combination of Bifidobacterium and S. thermophilus with inulin improved total triglyceride, cholesterol (LDL and high-density lipoprotein cholesterol), insulin resistance, and restored the intestinal barrier function and inflammation in an early NAFLD mouse model. Ralli et al[400] found that a combination of silymarin, piperine, and fulvic acid, along with a probiotic blend of Bifidobacterium adolescentis, Bifidobacterium bifidum, Lactobacillus casei, and L. rhamnosus, reduced hepatic fat accumulation and improved gut microbiota composition in an NAFLD mouse model. Gao et al[401] reported that aqueous extract from Chrysanthemum morifolium combined with a probiotic blend containing L. rhamnosus GG, L. rhamnosus CNCMl-4036, L. rhamnosus BPL1-15, and B. lactis BPL-1 effectively reduced body weight and hepatic steatosis, improved glucose intolerance and insulin resistance, and mo
Systematic reviews and meta-analyses have also confirmed the utility of synbiotics in NAFLD treatment[402-404]. A recent systematic review and meta-analysis reported that synbiotic treatment in NAFLD patients led to a reduction in liver enzymes, lowered cholesterol and LDL levels, improved glucose profiles, and decreased pro-inflammatory cy
FMT is a biotherapeutic procedure that restores and repairs gut microbial diversity[406]. In FMT, microbial populations are transplanted from a healthy donor to an unhealthy recipient[407]. The potential of FMT can be highlighted by studies showing that germ-free mice develop hepatic steatosis on receiving FMT from NASH-affected mice, suggesting that donor characteristics can be transferred to the recipient through FMT[408]. Additionally, germ-free mice that received FMT from HFD- fed mice exhibited intestinal epithelial barrier disruption[409].
The efficacy of FMT has been demonstrated in human and animal studies. Zhou et al[410] found that FMT from healthy mice protected HFD-fed mice from steatohepatitis. The procedure also led to an increased abundance of Christensenellaceae and Lactobacillus, a rise in caecal butyrate concentration, and enhanced expression of intestinal tight junction protein zonula occludens-1. Furthermore, FMT reduced hepatic triglyceride and cholesterol and lowered pro-inflammatory cytokines. FMT from lean donors to recipients with metabolic syndrome has been shown to improve insulin sensitivity, increase abundance of butyrate-producing intestinal microbiota, and result in changes in plasma metabolites[411,412].
Clinical trials have found that FMT is generally safe, with no significant adverse effects, and leads to sustained shifts in the recipient’s microbiota toward that of the donor[413]. Allogenic FMT using feces from metabolic syndrome donors has been shown to decrease insulin sensitivity in recipients with metabolic syndrome[414]. Even a short one week FMT intervention has proven effective in improving liver disease severity indices[415]. In a double-blind randomized con
Although FMT is generally considered a safe therapeutic procedure, it is not entirely free from potential adverse effects and complications[419,420]. Most side effects are usually mild, including abdominal pain, diarrhea, constipation, and low-grade fevers, which typically resolve within a few days[421]. However, more severe and life-threatening events, such as sepsis, bacteremia, ileus, or perforation, have also been reported. One notable adverse event involved two recipients developing extended-spectrum beta-lactamase-producing E. coli bacteremia, following FMT, with one of these patients subsequently dying[422]. FMT has also been associated with an increased risk of transmitting microbiome-associated chronic diseases, including autoimmune, GI, and cardiometabolic disorders[423-426]. It therefore becomes an absolute necessity to carry out donor screening before FMT to avoid the transfer of potentially life-threatening infections from the donor’s microbiota.
A growing body of evidence suggests a strong link between alterations in gut microbiota and the pathophysiology of NAFLD. Identifying specific microbiota and metabolic profiles could serve as valuable marker for the development of gut microbiota-based diagnostic tests, providing reliable and accurate diagnosis of NAFLD. Studies have identified microbiota and metabolic signatures associated with different stages of NAFLD, including NASH and cirrhosis. However, the variability in microbial signatures across different studies underscores the need for further research. A particularly important area of focus is understanding the role of certain bacteria in progression of NAFLD and exploring their potential diagnostic implications in distinguishing between the early and advanced stages of the disease.
Further research into the role of the gut microbiota in maintaining gut barrier function and regulating the immune system is essential for fully understanding its involvement in NAFLD. Targeted microbiota manipulation through dietary interventions, probiotics, prebiotics, synbiotics, and FMT represents a promising strategy for NAFLD treatment. However, despite the promising results, further research involving larger cohorts are needed to confirm the efficacy and safety of these microbiota-targeted therapies and to develop personalized treatment approaches for NAFLD. Furthermore, integrating machine learning and artificial intelligence with microbial and metabolic signatures could become a powerful tool for early detection and diagnosis of NAFLD.
| 1. | Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism. 2016;65:1038-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1490] [Cited by in RCA: 2119] [Article Influence: 235.4] [Reference Citation Analysis (1)] |
| 2. | Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal AJ, Kanwal F, Romero D, Abdelmalek MF, Anstee QM, Arab JP, Arrese M, Bataller R, Beuers U, Boursier J, Bugianesi E, Byrne CD, Castro Narro GE, Chowdhury A, Cortez-Pinto H, Cryer DR, Cusi K, El-Kassas M, Klein S, Eskridge W, Fan J, Gawrieh S, Guy CD, Harrison SA, Kim SU, Koot BG, Korenjak M, Kowdley KV, Lacaille F, Loomba R, Mitchell-Thain R, Morgan TR, Powell EE, Roden M, Romero-Gómez M, Silva M, Singh SP, Sookoian SC, Spearman CW, Tiniakos D, Valenti L, Vos MB, Wong VW, Xanthakos S, Yilmaz Y, Younossi Z, Hobbs A, Villota-Rivas M, Newsome PN; NAFLD Nomenclature consensus group. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology. 2023;78:1966-1986. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1567] [Cited by in RCA: 1320] [Article Influence: 660.0] [Reference Citation Analysis (0)] |
| 3. | Powell EE, Wong VW, Rinella M. Non-alcoholic fatty liver disease. Lancet. 2021;397:2212-2224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 461] [Cited by in RCA: 1684] [Article Influence: 421.0] [Reference Citation Analysis (33)] |
| 4. | Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, George J, Bugianesi E. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4054] [Cited by in RCA: 3797] [Article Influence: 542.4] [Reference Citation Analysis (2)] |
| 5. | Kasper P, Martin A, Lang S, Kütting F, Goeser T, Demir M, Steffen HM. NAFLD and cardiovascular diseases: a clinical review. Clin Res Cardiol. 2021;110:921-937. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 384] [Article Influence: 76.8] [Reference Citation Analysis (1)] |
| 6. | Yip TC, Lee HW, Chan WK, Wong GL, Wong VW. Asian perspective on NAFLD-associated HCC. J Hepatol. 2022;76:726-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 88] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 7. | European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2290] [Cited by in RCA: 3181] [Article Influence: 353.4] [Reference Citation Analysis (4)] |
| 8. | Ponziani FR, Bhoori S, Castelli C, Putignani L, Rivoltini L, Del Chierico F, Sanguinetti M, Morelli D, Paroni Sterbini F, Petito V, Reddel S, Calvani R, Camisaschi C, Picca A, Tuccitto A, Gasbarrini A, Pompili M, Mazzaferro V. Hepatocellular Carcinoma Is Associated With Gut Microbiota Profile and Inflammation in Nonalcoholic Fatty Liver Disease. Hepatology. 2019;69:107-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 474] [Article Influence: 79.0] [Reference Citation Analysis (1)] |
| 9. | Maestri M, Santopaolo F, Pompili M, Gasbarrini A, Ponziani FR. Gut microbiota modulation in patients with non-alcoholic fatty liver disease: Effects of current treatments and future strategies. Front Nutr. 2023;10:1110536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 39] [Reference Citation Analysis (0)] |
| 10. | Byrne CD, Targher G. NAFLD: a multisystem disease. J Hepatol. 2015;62:S47-S64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1516] [Cited by in RCA: 2140] [Article Influence: 214.0] [Reference Citation Analysis (0)] |
| 11. | Aron-Wisnewsky J, Vigliotti C, Witjes J, Le P, Holleboom AG, Verheij J, Nieuwdorp M, Clément K. Gut microbiota and human NAFLD: disentangling microbial signatures from metabolic disorders. Nat Rev Gastroenterol Hepatol. 2020;17:279-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 691] [Article Influence: 138.2] [Reference Citation Analysis (0)] |
| 12. | Hrncir T, Hrncirova L, Kverka M, Hromadka R, Machova V, Trckova E, Kostovcikova K, Kralickova P, Krejsek J, Tlaskalova-Hogenova H. Gut Microbiota and NAFLD: Pathogenetic Mechanisms, Microbiota Signatures, and Therapeutic Interventions. Microorganisms. 2021;9:957. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 114] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 13. | Singh SP, Anirvan P, Reddy KR, Conjeevaram HS, Marchesini G, Rinella ME, Madan K, Petroni ML, Al-Mahtab M, Caldwell SH, Aithal GP, Hamid SS, Farrell GC, Satapathy SK, Duseja A, Acharya SK, Dassanayake AS, Goh KL. Non-alcoholic fatty liver disease: Not time for an obituary just yet! J Hepatol. 2021;74:972-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 14. | Li AA, Kim D, Ahmed A. Association of Sarcopenia and NAFLD: An Overview. Clin Liver Dis (Hoboken). 2020;16:73-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 15. | Long Q, Luo F, Li B, Li Z, Guo Z, Chen Z, Wu W, Hu M. Gut microbiota and metabolic biomarkers in metabolic dysfunction-associated steatotic liver disease. Hepatol Commun. 2024;8:e0310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 21] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 16. | Seko Y, Yamaguchi K, Itoh Y. The genetic backgrounds in nonalcoholic fatty liver disease. Clin J Gastroenterol. 2018;11:97-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 17. | Neuschwander-Tetri BA. Hepatic lipotoxicity and the pathogenesis of nonalcoholic steatohepatitis: the central role of nontriglyceride fatty acid metabolites. Hepatology. 2010;52:774-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 726] [Cited by in RCA: 843] [Article Influence: 56.2] [Reference Citation Analysis (0)] |
| 18. | Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005-2023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2413] [Cited by in RCA: 2613] [Article Influence: 201.0] [Reference Citation Analysis (1)] |
| 19. | Pelusi S, Valenti L. Hepatic fat as clinical outcome and therapeutic target for nonalcoholic fatty liver disease. Liver Int. 2019;39:250-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 20. | Tilg H, Adolph TE, Moschen AR. Multiple Parallel Hits Hypothesis in Nonalcoholic Fatty Liver Disease: Revisited After a Decade. Hepatology. 2021;73:833-842. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 249] [Article Influence: 62.3] [Reference Citation Analysis (0)] |
| 21. | Cerreto M, Santopaolo F, Gasbarrini A, Pompili M, Ponziani FR. Bariatric Surgery and Liver Disease: General Considerations and Role of the Gut-Liver Axis. Nutrients. 2021;13:2649. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 22. | Zhou JH, Cai JJ, She ZG, Li HL. Noninvasive evaluation of nonalcoholic fatty liver disease: Current evidence and practice. World J Gastroenterol. 2019;25:1307-1326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 173] [Cited by in RCA: 161] [Article Influence: 26.8] [Reference Citation Analysis (3)] |
| 23. | Tamaki N, Ajmera V, Loomba R. Non-invasive methods for imaging hepatic steatosis and their clinical importance in NAFLD. Nat Rev Endocrinol. 2022;18:55-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 151] [Article Influence: 50.3] [Reference Citation Analysis (0)] |
| 24. | Kramer H, Pickhardt PJ, Kliewer MA, Hernando D, Chen GH, Zagzebski JA, Reeder SB. Accuracy of Liver Fat Quantification With Advanced CT, MRI, and Ultrasound Techniques: Prospective Comparison With MR Spectroscopy. AJR Am J Roentgenol. 2017;208:92-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 179] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 25. | Castera L, Friedrich-Rust M, Loomba R. Noninvasive Assessment of Liver Disease in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2019;156:1264-1281.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 619] [Cited by in RCA: 1037] [Article Influence: 172.8] [Reference Citation Analysis (0)] |
| 26. | Han H, Jiang Y, Wang M, Melaku M, Liu L, Zhao Y, Everaert N, Yi B, Zhang H. Intestinal dysbiosis in nonalcoholic fatty liver disease (NAFLD): focusing on the gut-liver axis. Crit Rev Food Sci Nutr. 2023;63:1689-1706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 80] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 27. | Kurdi P, Kawanishi K, Mizutani K, Yokota A. Mechanism of growth inhibition by free bile acids in lactobacilli and bifidobacteria. J Bacteriol. 2006;188:1979-1986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 257] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 28. | Chen J, Vitetta L. Gut Microbiota Metabolites in NAFLD Pathogenesis and Therapeutic Implications. Int J Mol Sci. 2020;21:5214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 208] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 29. | Zhang B, Li J, Fu J, Shao L, Yang L, Shi J. Interaction between mucus layer and gut microbiota in non-alcoholic fatty liver disease: Soil and seeds. Chin Med J (Engl). 2023;136:1390-1400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 30. | Pandey H, Tang DWT, Wong SH, Lal D. Gut Microbiota in Colorectal Cancer: Biological Role and Therapeutic Opportunities. Cancers (Basel). 2023;15:866. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 64] [Reference Citation Analysis (0)] |
| 31. | Pandey H, Jain D, Tang DWT, Wong SH, Lal D. Gut microbiota in pathophysiology, diagnosis, and therapeutics of inflammatory bowel disease. Intest Res. 2024;22:15-43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 28] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 32. | Cui X, Ye L, Li J, Jin L, Wang W, Li S, Bao M, Wu S, Li L, Geng B, Zhou X, Zhang J, Cai J. Metagenomic and metabolomic analyses unveil dysbiosis of gut microbiota in chronic heart failure patients. Sci Rep. 2018;8:635. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 145] [Cited by in RCA: 242] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 33. | Rowan-Nash AD, Korry BJ, Mylonakis E, Belenky P. Cross-Domain and Viral Interactions in the Microbiome. Microbiol Mol Biol Rev. 2019;83:e00044-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 84] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 34. | Tap J, Mondot S, Levenez F, Pelletier E, Caron C, Furet JP, Ugarte E, Muñoz-Tamayo R, Paslier DL, Nalin R, Dore J, Leclerc M. Towards the human intestinal microbiota phylogenetic core. Environ Microbiol. 2009;11:2574-2584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 627] [Cited by in RCA: 635] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 35. | Peterson DA, Frank DN, Pace NR, Gordon JI. Metagenomic approaches for defining the pathogenesis of inflammatory bowel diseases. Cell Host Microbe. 2008;3:417-427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 404] [Cited by in RCA: 385] [Article Influence: 22.6] [Reference Citation Analysis (1)] |
| 36. | Faith JJ, Guruge JL, Charbonneau M, Subramanian S, Seedorf H, Goodman AL, Clemente JC, Knight R, Heath AC, Leibel RL, Rosenbaum M, Gordon JI. The long-term stability of the human gut microbiota. Science. 2013;341:1237439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1329] [Cited by in RCA: 1454] [Article Influence: 121.2] [Reference Citation Analysis (0)] |
| 37. | Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A. 2004;101:15718-15723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4530] [Cited by in RCA: 4417] [Article Influence: 210.3] [Reference Citation Analysis (4)] |
| 38. | Bäckhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915-1920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3394] [Cited by in RCA: 3545] [Article Influence: 177.3] [Reference Citation Analysis (5)] |
| 39. | Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9:313-323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3922] [Cited by in RCA: 3465] [Article Influence: 216.6] [Reference Citation Analysis (0)] |
| 40. | Boleij A, Tjalsma H. Gut bacteria in health and disease: a survey on the interface between intestinal microbiology and colorectal cancer. Biol Rev Camb Philos Soc. 2012;87:701-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 109] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 41. | Sommer F, Anderson JM, Bharti R, Raes J, Rosenstiel P. The resilience of the intestinal microbiota influences health and disease. Nat Rev Microbiol. 2017;15:630-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 683] [Article Influence: 85.4] [Reference Citation Analysis (0)] |
| 42. | Mehal WZ. The Gordian Knot of dysbiosis, obesity and NAFLD. Nat Rev Gastroenterol Hepatol. 2013;10:637-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 118] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 43. | Escobedo G, López-Ortiz E, Torres-Castro I. Gut microbiota as a key player in triggering obesity, systemic inflammation and insulin resistance. Rev Invest Clin. 2014;66:450-459. [PubMed] |
| 44. | Delzenne NM, Cani PD, Everard A, Neyrinck AM, Bindels LB. Gut microorganisms as promising targets for the management of type 2 diabetes. Diabetologia. 2015;58:2206-2217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 198] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 45. | Kitai T, Tang WHW. The Role and Impact of Gut Microbiota in Cardiovascular Disease. Rev Esp Cardiol (Engl Ed). 2017;70:799-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 46. | Wieland A, Frank DN, Harnke B, Bambha K. Systematic review: microbial dysbiosis and nonalcoholic fatty liver disease. Aliment Pharmacol Ther. 2015;42:1051-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 164] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 47. | Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7222] [Cited by in RCA: 6410] [Article Influence: 337.4] [Reference Citation Analysis (0)] |
| 48. | Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9796] [Cited by in RCA: 8761] [Article Influence: 461.1] [Reference Citation Analysis (1)] |
| 49. | Zambell KL, Fitch MD, Fleming SE. Acetate and butyrate are the major substrates for de novo lipogenesis in rat colonic epithelial cells. J Nutr. 2003;133:3509-3515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 116] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 50. | Samuel BS, Shaito A, Motoike T, Rey FE, Backhed F, Manchester JK, Hammer RE, Williams SC, Crowley J, Yanagisawa M, Gordon JI. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc Natl Acad Sci U S A. 2008;105:16767-16772. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1270] [Cited by in RCA: 1150] [Article Influence: 67.6] [Reference Citation Analysis (0)] |
| 51. | Cani PD, Delzenne NM. The role of the gut microbiota in energy metabolism and metabolic disease. Curr Pharm Des. 2009;15:1546-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 625] [Cited by in RCA: 651] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 52. | Le Roy T, Llopis M, Lepage P, Bruneau A, Rabot S, Bevilacqua C, Martin P, Philippe C, Walker F, Bado A, Perlemuter G, Cassard-Doulcier AM, Gérard P. Intestinal microbiota determines development of non-alcoholic fatty liver disease in mice. Gut. 2013;62:1787-1794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 601] [Cited by in RCA: 715] [Article Influence: 59.6] [Reference Citation Analysis (0)] |
| 53. | Albillos A, de Gottardi A, Rescigno M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J Hepatol. 2020;72:558-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 542] [Cited by in RCA: 1265] [Article Influence: 253.0] [Reference Citation Analysis (1)] |
| 54. | Ji Y, Yin Y, Sun L, Zhang W. The Molecular and Mechanistic Insights Based on Gut-Liver Axis: Nutritional Target for Non-Alcoholic Fatty Liver Disease (NAFLD) Improvement. Int J Mol Sci. 2020;21:3066. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 91] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 55. | Chen L, Tao X, Zeng M, Mi Y, Xu L. Clinical and histological features under different nomenclatures of fatty liver disease: NAFLD, MAFLD, MASLD and MetALD. J Hepatol. 2024;80:e64-e66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 43] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 56. | Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget A, Delmée E, Cousin B, Sulpice T, Chamontin B, Ferrières J, Tanti JF, Gibson GR, Casteilla L, Delzenne NM, Alessi MC, Burcelin R. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761-1772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4095] [Cited by in RCA: 4564] [Article Influence: 253.6] [Reference Citation Analysis (1)] |
| 57. | Ma J, Zhou Q, Li H. Gut Microbiota and Nonalcoholic Fatty Liver Disease: Insights on Mechanisms and Therapy. Nutrients. 2017;9:1124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 140] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 58. | Arab JP, Martin-Mateos RM, Shah VH. Gut-liver axis, cirrhosis and portal hypertension: the chicken and the egg. Hepatol Int. 2018;12:24-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 155] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 59. | Pandey H, Lal D. Exploring Bacterial Diversity: How Far Have We Reached? Advancements of Microbiology. 2023;62:117-131. [DOI] [Full Text] |
| 60. | Shen F, Zheng RD, Sun XQ, Ding WJ, Wang XY, Fan JG. Gut microbiota dysbiosis in patients with non-alcoholic fatty liver disease. Hepatobiliary Pancreat Dis Int. 2017;16:375-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 435] [Cited by in RCA: 411] [Article Influence: 51.4] [Reference Citation Analysis (1)] |
| 61. | Astbury S, Atallah E, Vijay A, Aithal GP, Grove JI, Valdes AM. Lower gut microbiome diversity and higher abundance of proinflammatory genus Collinsella are associated with biopsy-proven nonalcoholic steatohepatitis. Gut Microbes. 2020;11:569-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 144] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 62. | Schwimmer JB, Johnson JS, Angeles JE, Behling C, Belt PH, Borecki I, Bross C, Durelle J, Goyal NP, Hamilton G, Holtz ML, Lavine JE, Mitreva M, Newton KP, Pan A, Simpson PM, Sirlin CB, Sodergren E, Tyagi R, Yates KP, Weinstock GM, Salzman NH. Microbiome Signatures Associated With Steatohepatitis and Moderate to Severe Fibrosis in Children With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2019;157:1109-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 199] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 63. | Monga Kravetz A, Testerman T, Galuppo B, Graf J, Pierpont B, Siebel S, Feinn R, Santoro N. Effect of Gut Microbiota and PNPLA3 rs738409 Variant on Nonalcoholic Fatty Liver Disease (NAFLD) in Obese Youth. J Clin Endocrinol Metab. 2020;105:e3575-e3585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 64. | Wang B, Jiang X, Cao M, Ge J, Bao Q, Tang L, Chen Y, Li L. Altered Fecal Microbiota Correlates with Liver Biochemistry in Nonobese Patients with Non-alcoholic Fatty Liver Disease. Sci Rep. 2016;6:32002. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 274] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 65. | Su X, Chen S, Liu J, Feng Y, Han E, Hao X, Liao M, Cai J, Zhang S, Niu J, He S, Huang S, Lo K, Zeng F. Composition of gut microbiota and non-alcoholic fatty liver disease: A systematic review and meta-analysis. Obes Rev. 2024;25:e13646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 22] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 66. | Hoyles L, Fernández-Real JM, Federici M, Serino M, Abbott J, Charpentier J, Heymes C, Luque JL, Anthony E, Barton RH, Chilloux J, Myridakis A, Martinez-Gili L, Moreno-Navarrete JM, Benhamed F, Azalbert V, Blasco-Baque V, Puig J, Xifra G, Ricart W, Tomlinson C, Woodbridge M, Cardellini M, Davato F, Cardolini I, Porzio O, Gentileschi P, Lopez F, Foufelle F, Butcher SA, Holmes E, Nicholson JK, Postic C, Burcelin R, Dumas ME. Molecular phenomics and metagenomics of hepatic steatosis in non-diabetic obese women. Nat Med. 2018;24:1070-1080. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 303] [Cited by in RCA: 491] [Article Influence: 70.1] [Reference Citation Analysis (0)] |
| 67. | Loomba R, Seguritan V, Li W, Long T, Klitgord N, Bhatt A, Dulai PS, Caussy C, Bettencourt R, Highlander SK, Jones MB, Sirlin CB, Schnabl B, Brinkac L, Schork N, Chen CH, Brenner DA, Biggs W, Yooseph S, Venter JC, Nelson KE. Gut Microbiome-Based Metagenomic Signature for Non-invasive Detection of Advanced Fibrosis in Human Nonalcoholic Fatty Liver Disease. Cell Metab. 2017;25:1054-1062.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 551] [Cited by in RCA: 753] [Article Influence: 94.1] [Reference Citation Analysis (0)] |
| 68. | Oh TG, Kim SM, Caussy C, Fu T, Guo J, Bassirian S, Singh S, Madamba EV, Bettencourt R, Richards L, Yu RT, Atkins AR, Huan T, Brenner DA, Sirlin CB, Downes M, Evans RM, Loomba R. A Universal Gut-Microbiome-Derived Signature Predicts Cirrhosis. Cell Metab. 2020;32:878-888.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 198] [Article Influence: 39.6] [Reference Citation Analysis (1)] |
| 69. | Mouzaki M, Comelli EM, Arendt BM, Bonengel J, Fung SK, Fischer SE, McGilvray ID, Allard JP. Intestinal microbiota in patients with nonalcoholic fatty liver disease. Hepatology. 2013;58:120-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 496] [Cited by in RCA: 556] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 70. | Lanthier N, Rodriguez J, Nachit M, Hiel S, Trefois P, Neyrinck AM, Cani PD, Bindels LB, Thissen JP, Delzenne NM. Microbiota analysis and transient elastography reveal new extra-hepatic components of liver steatosis and fibrosis in obese patients. Sci Rep. 2021;11:659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 71. | Shi Z, Lei H, Chen G, Yuan P, Cao Z, Ser HL, Zhu X, Wu F, Liu C, Dong M, Song Y, Guo Y, Chen C, Hu K, Zhu Y, Zeng XA, Zhou J, Lu Y, Patterson AD, Zhang L. Impaired Intestinal Akkermansia muciniphila and Aryl Hydrocarbon Receptor Ligands Contribute to Nonalcoholic Fatty Liver Disease in Mice. mSystems. 2021;6:e00985-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 72. | Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, Guiot Y, Derrien M, Muccioli GG, Delzenne NM, de Vos WM, Cani PD. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A. 2013;110:9066-9071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2639] [Cited by in RCA: 3316] [Article Influence: 276.3] [Reference Citation Analysis (0)] |
| 73. | Santacruz A, Collado MC, García-Valdés L, Segura MT, Martín-Lagos JA, Anjos T, Martí-Romero M, Lopez RM, Florido J, Campoy C, Sanz Y. Gut microbiota composition is associated with body weight, weight gain and biochemical parameters in pregnant women. Br J Nutr. 2010;104:83-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 564] [Cited by in RCA: 631] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 74. | Özkul C, Yalınay M, Karakan T, Yılmaz G. Determination of certain bacterial groups in gut microbiota and endotoxin levels in patients with nonalcoholic steatohepatitis. Turk J Gastroenterol. 2017;28:361-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 75. | Iino C, Endo T, Mikami K, Hasegawa T, Kimura M, Sawada N, Nakaji S, Fukuda S. Significant decrease in Faecalibacterium among gut microbiota in nonalcoholic fatty liver disease: a large BMI- and sex-matched population study. Hepatol Int. 2019;13:748-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 76. | Fei N, Bruneau A, Zhang X, Wang R, Wang J, Rabot S, Gérard P, Zhao L. Endotoxin Producers Overgrowing in Human Gut Microbiota as the Causative Agents for Nonalcoholic Fatty Liver Disease. mBio. 2020;11:e03263-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 127] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 77. | Fei N, Zhao L. An opportunistic pathogen isolated from the gut of an obese human causes obesity in germfree mice. ISME J. 2013;7:880-884. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 422] [Cited by in RCA: 542] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 78. | Zhu L, Baker SS, Gill C, Liu W, Alkhouri R, Baker RD, Gill SR. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: a connection between endogenous alcohol and NASH. Hepatology. 2013;57:601-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1015] [Cited by in RCA: 1282] [Article Influence: 106.8] [Reference Citation Analysis (1)] |
| 79. | Kapil S, Duseja A, Sharma BK, Singla B, Chakraborti A, Das A, Ray P, Dhiman RK, Chawla Y. Small intestinal bacterial overgrowth and toll-like receptor signaling in patients with non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2016;31:213-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 135] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 80. | Zhang Y, Jiang W, Xu J, Wu N, Wang Y, Lin T, Liu Y, Liu Y. E. coli NF73-1 Isolated From NASH Patients Aggravates NAFLD in Mice by Translocating Into the Liver and Stimulating M1 Polarization. Front Cell Infect Microbiol. 2020;10:535940. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 81. | Xin FZ, Zhao ZH, Liu XL, Pan Q, Wang ZX, Zeng L, Zhang QR, Ye L, Wang MY, Zhang RN, Gong ZZ, Huang LJ, Sun C, Shen F, Jiang L, Fan JG. Escherichia fergusonii Promotes Nonobese Nonalcoholic Fatty Liver Disease by Interfering With Host Hepatic Lipid Metabolism Through Its Own msRNA 23487. Cell Mol Gastroenterol Hepatol. 2022;13:827-841. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 82. | Putaala H, Salusjärvi T, Nordström M, Saarinen M, Ouwehand AC, Bech Hansen E, Rautonen N. Effect of four probiotic strains and Escherichia coli O157:H7 on tight junction integrity and cyclo-oxygenase expression. Res Microbiol. 2008;159:692-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 84] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 83. | Jiang W, Wu N, Wang X, Chi Y, Zhang Y, Qiu X, Hu Y, Li J, Liu Y. Dysbiosis gut microbiota associated with inflammation and impaired mucosal immune function in intestine of humans with non-alcoholic fatty liver disease. Sci Rep. 2015;5:8096. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 473] [Cited by in RCA: 453] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 84. | Boursier J, Mueller O, Barret M, Machado M, Fizanne L, Araujo-Perez F, Guy CD, Seed PC, Rawls JF, David LA, Hunault G, Oberti F, Calès P, Diehl AM. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology. 2016;63:764-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 763] [Cited by in RCA: 1051] [Article Influence: 116.8] [Reference Citation Analysis (0)] |
| 85. | Alferink LJM, Radjabzadeh D, Erler NS, Vojinovic D, Medina-Gomez C, Uitterlinden AG, de Knegt RJ, Amin N, Ikram MA, Janssen HLA, Kiefte-de Jong JC, Metselaar HJ, van Duijn CM, Kraaij R, Darwish Murad S. Microbiomics, Metabolomics, Predicted Metagenomics, and Hepatic Steatosis in a Population-Based Study of 1,355 Adults. Hepatology. 2021;73:968-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 86. | Da Silva HE, Teterina A, Comelli EM, Taibi A, Arendt BM, Fischer SE, Lou W, Allard JP. Nonalcoholic fatty liver disease is associated with dysbiosis independent of body mass index and insulin resistance. Sci Rep. 2018;8:1466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 202] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 87. | Henke MT, Kenny DJ, Cassilly CD, Vlamakis H, Xavier RJ, Clardy J. Ruminococcus gnavus, a member of the human gut microbiome associated with Crohn's disease, produces an inflammatory polysaccharide. Proc Natl Acad Sci U S A. 2019;116:12672-12677. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 236] [Cited by in RCA: 539] [Article Influence: 89.8] [Reference Citation Analysis (0)] |
| 88. | Michail S, Lin M, Frey MR, Fanter R, Paliy O, Hilbush B, Reo NV. Altered gut microbial energy and metabolism in children with non-alcoholic fatty liver disease. FEMS Microbiol Ecol. 2015;91:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 236] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 89. | Sobhonslidsuk A, Chanprasertyothin S, Pongrujikorn T, Kaewduang P, Promson K, Petraksa S, Ongphiphadhanakul B. The Association of Gut Microbiota with Nonalcoholic Steatohepatitis in Thais. Biomed Res Int. 2018;2018:9340316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 90. | Yang L, Dai Y, He H, Liu Z, Liao S, Zhang Y, Liao G, An Z. Integrative analysis of gut microbiota and fecal metabolites in metabolic associated fatty liver disease patients. Front Microbiol. 2022;13:969757. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 91. | Li F, Ye J, Shao C, Zhong B. Compositional alterations of gut microbiota in nonalcoholic fatty liver disease patients: a systematic review and Meta-analysis. Lipids Health Dis. 2021;20:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 70] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 92. | Nistal E, Sáenz de Miera LE, Ballesteros Pomar M, Sánchez-Campos S, García-Mediavilla MV, Álvarez-Cuenllas B, Linares P, Olcoz JL, Arias-Loste MT, García-Lobo JM, Crespo J, González-Gallego J, Jorquera Plaza F. An altered fecal microbiota profile in patients with non-alcoholic fatty liver disease (NAFLD) associated with obesity. Rev Esp Enferm Dig. 2019;111:275-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 93. | Leung H, Long X, Ni Y, Qian L, Nychas E, Siliceo SL, Pohl D, Hanhineva K, Liu Y, Xu A, Nielsen HB, Belda E, Clément K, Loomba R, Li H, Jia W, Panagiotou G. Risk assessment with gut microbiome and metabolite markers in NAFLD development. Sci Transl Med. 2022;14:eabk0855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 73] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 94. | Yang C, Xu J, Xu X, Xu W, Tong B, Wang S, Ji R, Tan Y, Zhu Y. Characteristics of gut microbiota in patients with metabolic associated fatty liver disease. Sci Rep. 2023;13:9988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 95. | Li Y, Liang X, Lyu Y, Wang K, Han L, Wang Y, Sun J, Chi C. Association between the gut microbiota and nonalcoholic fatty liver disease: A two-sample Mendelian randomization study. Dig Liver Dis. 2023;55:1464-1471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 96. | Pan T, Su L, Zhang Y, Yi F, Chen Y. Impact of gut microbiota on nonalcoholic fatty liver disease: insights from a leave-one-out cross-validation study. Front Microbiol. 2023;14:1320279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 97. | Yang C, Wu J, Yang L, Hu Q, Li L, Yang Y, Hu J, Pan D, Zhao Q. Altered gut microbial profile accompanied by abnormal short chain fatty acid metabolism exacerbates nonalcoholic fatty liver disease progression. Sci Rep. 2024;14:22385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 98. | Rau M, Rehman A, Dittrich M, Groen AK, Hermanns HM, Seyfried F, Beyersdorf N, Dandekar T, Rosenstiel P, Geier A. Fecal SCFAs and SCFA-producing bacteria in gut microbiome of human NAFLD as a putative link to systemic T-cell activation and advanced disease. United European Gastroenterol J. 2018;6:1496-1507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 245] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 99. | Caussy C, Tripathi A, Humphrey G, Bassirian S, Singh S, Faulkner C, Bettencourt R, Rizo E, Richards L, Xu ZZ, Downes MR, Evans RM, Brenner DA, Sirlin CB, Knight R, Loomba R. A gut microbiome signature for cirrhosis due to nonalcoholic fatty liver disease. Nat Commun. 2019;10:1406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 239] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 100. | Behary J, Amorim N, Jiang XT, Raposo A, Gong L, McGovern E, Ibrahim R, Chu F, Stephens C, Jebeili H, Fragomeli V, Koay YC, Jackson M, O'Sullivan J, Weltman M, McCaughan G, El-Omar E, Zekry A. Gut microbiota impact on the peripheral immune response in non-alcoholic fatty liver disease related hepatocellular carcinoma. Nat Commun. 2021;12:187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 231] [Cited by in RCA: 292] [Article Influence: 73.0] [Reference Citation Analysis (0)] |
| 101. | Ganz M, Szabo G. Immune and inflammatory pathways in NASH. Hepatol Int. 2013;7 Suppl 2:771-781. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 137] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 102. | Miyake Y, Yamamoto K. Role of gut microbiota in liver diseases. Hepatol Res. 2013;43:139-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 103. | Miura K, Kodama Y, Inokuchi S, Schnabl B, Aoyama T, Ohnishi H, Olefsky JM, Brenner DA, Seki E. Toll-like receptor 9 promotes steatohepatitis by induction of interleukin-1beta in mice. Gastroenterology. 2010;139:323-34.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 527] [Cited by in RCA: 623] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 104. | Szabo G, Velayudham A, Romics L Jr, Mandrekar P. Modulation of non-alcoholic steatohepatitis by pattern recognition receptors in mice: the role of toll-like receptors 2 and 4. Alcohol Clin Exp Res. 2005;29:140S-145S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 112] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 105. | Vijay-Kumar M, Aitken JD, Carvalho FA, Cullender TC, Mwangi S, Srinivasan S, Sitaraman SV, Knight R, Ley RE, Gewirtz AT. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science. 2010;328:228-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1496] [Cited by in RCA: 1589] [Article Influence: 105.9] [Reference Citation Analysis (0)] |
| 106. | Rivera CA, Adegboyega P, van Rooijen N, Tagalicud A, Allman M, Wallace M. Toll-like receptor-4 signaling and Kupffer cells play pivotal roles in the pathogenesis of non-alcoholic steatohepatitis. J Hepatol. 2007;47:571-579. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 566] [Cited by in RCA: 560] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 107. | Miura K, Yang L, van Rooijen N, Brenner DA, Ohnishi H, Seki E. Toll-like receptor 2 and palmitic acid cooperatively contribute to the development of nonalcoholic steatohepatitis through inflammasome activation in mice. Hepatology. 2013;57:577-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 230] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 108. | Soares JB, Pimentel-Nunes P, Roncon-Albuquerque R, Leite-Moreira A. The role of lipopolysaccharide/toll-like receptor 4 signaling in chronic liver diseases. Hepatol Int. 2010;4:659-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 265] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 109. | Nguyen-Lefebvre AT, Horuzsko A. Kupffer Cell Metabolism and Function. J Enzymol Metab. 2015;1:101. [PubMed] |
| 110. | Carpino G, Del Ben M, Pastori D, Carnevale R, Baratta F, Overi D, Francis H, Cardinale V, Onori P, Safarikia S, Cammisotto V, Alvaro D, Svegliati-Baroni G, Angelico F, Gaudio E, Violi F. Increased Liver Localization of Lipopolysaccharides in Human and Experimental NAFLD. Hepatology. 2020;72:470-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 265] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 111. | Sharifnia T, Antoun J, Verriere TG, Suarez G, Wattacheril J, Wilson KT, Peek RM Jr, Abumrad NN, Flynn CR. Hepatic TLR4 signaling in obese NAFLD. Am J Physiol Gastrointest Liver Physiol. 2015;309:G270-G278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 194] [Article Influence: 19.4] [Reference Citation Analysis (1)] |
| 112. | Imajo K, Fujita K, Yoneda M, Nozaki Y, Ogawa Y, Shinohara Y, Kato S, Mawatari H, Shibata W, Kitani H, Ikejima K, Kirikoshi H, Nakajima N, Saito S, Maeyama S, Watanabe S, Wada K, Nakajima A. Hyperresponsivity to low-dose endotoxin during progression to nonalcoholic steatohepatitis is regulated by leptin-mediated signaling. Cell Metab. 2012;16:44-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 275] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 113. | Kudo H, Takahara T, Yata Y, Kawai K, Zhang W, Sugiyama T. Lipopolysaccharide triggered TNF-alpha-induced hepatocyte apoptosis in a murine non-alcoholic steatohepatitis model. J Hepatol. 2009;51:168-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 136] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 114. | Harte AL, da Silva NF, Creely SJ, McGee KC, Billyard T, Youssef-Elabd EM, Tripathi G, Ashour E, Abdalla MS, Sharada HM, Amin AI, Burt AD, Kumar S, Day CP, McTernan PG. Elevated endotoxin levels in non-alcoholic fatty liver disease. J Inflamm (Lond). 2010;7:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 239] [Cited by in RCA: 294] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 115. | Pappo I, Bercovier H, Berry EM, Haviv Y, Gallily R, Freund HR. Polymyxin B reduces total parenteral nutrition-associated hepatic steatosis by its antibacterial activity and by blocking deleterious effects of lipopolysaccharide. JPEN J Parenter Enteral Nutr. 1992;16:529-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 116. | Vijay-Kumar M, Sanders CJ, Taylor RT, Kumar A, Aitken JD, Sitaraman SV, Neish AS, Uematsu S, Akira S, Williams IR, Gewirtz AT. Deletion of TLR5 results in spontaneous colitis in mice. J Clin Invest. 2007;117:3909-3921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 233] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 117. | Shen B, Gu T, Shen Z, Zhou C, Guo Y, Wang J, Li B, Xu X, Li F, Zhang Q, Cai X, Dong H, Lu L. Escherichia coli Promotes Endothelial to Mesenchymal Transformation of Liver Sinusoidal Endothelial Cells and Exacerbates Nonalcoholic Fatty Liver Disease Via Its Flagellin. Cell Mol Gastroenterol Hepatol. 2023;16:857-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 22] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 118. | Jiang W, Sun R, Zhou R, Wei H, Tian Z. TLR-9 activation aggravates concanavalin A-induced hepatitis via promoting accumulation and activation of liver CD4+ NKT cells. J Immunol. 2009;182:3768-3774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 119. | Miura K, Yang L, van Rooijen N, Ohnishi H, Seki E. Hepatic recruitment of macrophages promotes nonalcoholic steatohepatitis through CCR2. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1310-G1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 405] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 120. | Hotamisligil GS, Murray DL, Choy LN, Spiegelman BM. Tumor necrosis factor alpha inhibits signaling from the insulin receptor. Proc Natl Acad Sci U S A. 1994;91:4854-4858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 829] [Cited by in RCA: 850] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 121. | Ma KL, Ruan XZ, Powis SH, Chen Y, Moorhead JF, Varghese Z. Inflammatory stress exacerbates lipid accumulation in hepatic cells and fatty livers of apolipoprotein E knockout mice. Hepatology. 2008;48:770-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 194] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 122. | Cawthorn WP, Sethi JK. TNF-alpha and adipocyte biology. FEBS Lett. 2008;582:117-131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 627] [Cited by in RCA: 578] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 123. | Zhang W, Kudo H, Kawai K, Fujisaka S, Usui I, Sugiyama T, Tsukada K, Chen N, Takahara T. Tumor necrosis factor-alpha accelerates apoptosis of steatotic hepatocytes from a murine model of non-alcoholic fatty liver disease. Biochem Biophys Res Commun. 2010;391:1731-1736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 124. | Salles J, Tardif N, Landrier JF, Mothe-Satney I, Guillet C, Boue-Vaysse C, Combaret L, Giraudet C, Patrac V, Bertrand-Michel J, Denis P, Chardigny JM, Boirie Y, Walrand S. TNFα gene knockout differentially affects lipid deposition in liver and skeletal muscle of high-fat-diet mice. J Nutr Biochem. 2012;23:1685-1693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 125. | Stienstra R, Saudale F, Duval C, Keshtkar S, Groener JE, van Rooijen N, Staels B, Kersten S, Müller M. Kupffer cells promote hepatic steatosis via interleukin-1beta-dependent suppression of peroxisome proliferator-activated receptor alpha activity. Hepatology. 2010;51:511-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 370] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 126. | Kamari Y, Shaish A, Vax E, Shemesh S, Kandel-Kfir M, Arbel Y, Olteanu S, Barshack I, Dotan S, Voronov E, Dinarello CA, Apte RN, Harats D. Lack of interleukin-1α or interleukin-1β inhibits transformation of steatosis to steatohepatitis and liver fibrosis in hypercholesterolemic mice. J Hepatol. 2011;55:1086-1094. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 241] [Cited by in RCA: 239] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 127. | Aragonès G, Colom-Pellicer M, Aguilar C, Guiu-Jurado E, Martínez S, Sabench F, Antonio Porras J, Riesco D, Del Castillo D, Richart C, Auguet T. Circulating microbiota-derived metabolites: a "liquid biopsy? Int J Obes (Lond). 2020;44:875-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 128. | Hu C, Wang T, Zhuang X, Sun Q, Wang X, Lin H, Feng M, Zhang J, Cao Q, Jiang Y. Metabolic analysis of early nonalcoholic fatty liver disease in humans using liquid chromatography-mass spectrometry. J Transl Med. 2021;19:152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 129. | Liu L, Zhao J, Zhang R, Wang X, Wang Y, Chen Y, Feng R. Serum untargeted metabolomics delineates the metabolic status in different subtypes of non-alcoholic fatty liver disease. J Pharm Biomed Anal. 2021;200:114058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 130. | Yu JS, Youn GS, Choi J, Kim CH, Kim BY, Yang SJ, Lee JH, Park TS, Kim BK, Kim YB, Roh SW, Min BH, Park HJ, Yoon SJ, Lee NY, Choi YR, Kim HS, Gupta H, Sung H, Han SH, Suk KT, Lee DY. Lactobacillus lactis and Pediococcus pentosaceus-driven reprogramming of gut microbiome and metabolome ameliorates the progression of non-alcoholic fatty liver disease. Clin Transl Med. 2021;11:e634. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 94] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 131. | Morrison DJ, Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes. 2016;7:189-200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1566] [Cited by in RCA: 2373] [Article Influence: 263.7] [Reference Citation Analysis (0)] |
| 132. | Machiels K, Joossens M, Sabino J, De Preter V, Arijs I, Eeckhaut V, Ballet V, Claes K, Van Immerseel F, Verbeke K, Ferrante M, Verhaegen J, Rutgeerts P, Vermeire S. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut. 2014;63:1275-1283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1036] [Cited by in RCA: 1335] [Article Influence: 121.4] [Reference Citation Analysis (3)] |
| 133. | Venkatraman A, Ramakrishna BS, Pulimood AB. Butyrate hastens restoration of barrier function after thermal and detergent injury to rat distal colon in vitro. Scand J Gastroenterol. 1999;34:1087-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 134. | Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly-Y M, Glickman JN, Garrett WS. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341:569-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2937] [Cited by in RCA: 3959] [Article Influence: 329.9] [Reference Citation Analysis (1)] |
| 135. | Chang PV, Hao L, Offermanns S, Medzhitov R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc Natl Acad Sci U S A. 2014;111:2247-2252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1079] [Cited by in RCA: 1514] [Article Influence: 137.6] [Reference Citation Analysis (0)] |
| 136. | Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, Schilter HC, Rolph MS, Mackay F, Artis D, Xavier RJ, Teixeira MM, Mackay CR. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461:1282-1286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2007] [Cited by in RCA: 2399] [Article Influence: 149.9] [Reference Citation Analysis (0)] |
| 137. | Singh N, Gurav A, Sivaprakasam S, Brady E, Padia R, Shi H, Thangaraju M, Prasad PD, Manicassamy S, Munn DH, Lee JR, Offermanns S, Ganapathy V. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity. 2014;40:128-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1198] [Cited by in RCA: 1694] [Article Influence: 154.0] [Reference Citation Analysis (0)] |
| 138. | Kondo T, Kishi M, Fushimi T, Kaga T. Acetic acid upregulates the expression of genes for fatty acid oxidation enzymes in liver to suppress body fat accumulation. J Agric Food Chem. 2009;57:5982-5986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 205] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 139. | Lin HV, Frassetto A, Kowalik EJ Jr, Nawrocki AR, Lu MM, Kosinski JR, Hubert JA, Szeto D, Yao X, Forrest G, Marsh DJ. Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PLoS One. 2012;7:e35240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 760] [Cited by in RCA: 926] [Article Influence: 71.2] [Reference Citation Analysis (0)] |
| 140. | Liu W, Luo X, Tang J, Mo Q, Zhong H, Zhang H, Feng F. A bridge for short-chain fatty acids to affect inflammatory bowel disease, type 1 diabetes, and non-alcoholic fatty liver disease positively: by changing gut barrier. Eur J Nutr. 2021;60:2317-2330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 68] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 141. | Chen J, Vitetta L. Inflammation-Modulating Effect of Butyrate in the Prevention of Colon Cancer by Dietary Fiber. Clin Colorectal Cancer. 2018;17:e541-e544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 94] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 142. | Ye J, Lv L, Wu W, Li Y, Shi D, Fang D, Guo F, Jiang H, Yan R, Ye W, Li L. Butyrate Protects Mice Against Methionine-Choline-Deficient Diet-Induced Non-alcoholic Steatohepatitis by Improving Gut Barrier Function, Attenuating Inflammation and Reducing Endotoxin Levels. Front Microbiol. 2018;9:1967. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 149] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 143. | Deng M, Qu F, Chen L, Liu C, Zhang M, Ren F, Guo H, Zhang H, Ge S, Wu C, Zhao L. SCFAs alleviated steatosis and inflammation in mice with NASH induced by MCD. J Endocrinol. 2020;245:425-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 141] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 144. | Jin CJ, Sellmann C, Engstler AJ, Ziegenhardt D, Bergheim I. Supplementation of sodium butyrate protects mice from the development of non-alcoholic steatohepatitis (NASH). Br J Nutr. 2015;114:1745-1755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 114] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 145. | Baumann A, Jin CJ, Brandt A, Sellmann C, Nier A, Burkard M, Venturelli S, Bergheim I. Oral Supplementation of Sodium Butyrate Attenuates the Progression of Non-Alcoholic Steatohepatitis. Nutrients. 2020;12:951. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 146. | Zhou D, Pan Q, Xin FZ, Zhang RN, He CX, Chen GY, Liu C, Chen YW, Fan JG. Sodium butyrate attenuates high-fat diet-induced steatohepatitis in mice by improving gut microbiota and gastrointestinal barrier. World J Gastroenterol. 2017;23:60-75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 342] [Cited by in RCA: 329] [Article Influence: 41.1] [Reference Citation Analysis (6)] |
| 147. | Zhai S, Qin S, Li L, Zhu L, Zou Z, Wang L. Dietary butyrate suppresses inflammation through modulating gut microbiota in high-fat diet-fed mice. FEMS Microbiol Lett. 2019;366:fnz153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 71] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 148. | Dangana EO, Omolekulo TE, Areola ED, Olaniyi KS, Soladoye AO, Olatunji LA. Sodium acetate protects against nicotine-induced excess hepatic lipid in male rats by suppressing xanthine oxidase activity. Chem Biol Interact. 2020;316:108929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 149. | Liu L, Fu C, Li F. Acetate Affects the Process of Lipid Metabolism in Rabbit Liver, Skeletal Muscle and Adipose Tissue. Animals (Basel). 2019;9:799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 150. | Chen J, Thomsen M, Vitetta L. Interaction of gut microbiota with dysregulation of bile acids in the pathogenesis of nonalcoholic fatty liver disease and potential therapeutic implications of probiotics. J Cell Biochem. 2019;120:2713-2720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 103] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 151. | Song Z, Cai Y, Lao X, Wang X, Lin X, Cui Y, Kalavagunta PK, Liao J, Jin L, Shang J, Li J. Taxonomic profiling and populational patterns of bacterial bile salt hydrolase (BSH) genes based on worldwide human gut microbiome. Microbiome. 2019;7:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 185] [Cited by in RCA: 319] [Article Influence: 53.2] [Reference Citation Analysis (0)] |
| 152. | Lorenzo-Zúñiga V, Bartolí R, Planas R, Hofmann AF, Viñado B, Hagey LR, Hernández JM, Mañé J, Alvarez MA, Ausina V, Gassull MA. Oral bile acids reduce bacterial overgrowth, bacterial translocation, and endotoxemia in cirrhotic rats. Hepatology. 2003;37:551-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 249] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 153. | Houten SM, Watanabe M, Auwerx J. Endocrine functions of bile acids. EMBO J. 2006;25:1419-1425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 448] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 154. | Hylemon PB, Zhou H, Pandak WM, Ren S, Gil G, Dent P. Bile acids as regulatory molecules. J Lipid Res. 2009;50:1509-1520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 527] [Cited by in RCA: 530] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 155. | Chiang JYL, Ferrell JM. Bile acid receptors FXR and TGR5 signaling in fatty liver diseases and therapy. Am J Physiol Gastrointest Liver Physiol. 2020;318:G554-G573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 262] [Article Influence: 52.4] [Reference Citation Analysis (0)] |
| 156. | Schumacher JD, Guo GL. Pharmacologic Modulation of Bile Acid-FXR-FGF15/FGF19 Pathway for the Treatment of Nonalcoholic Steatohepatitis. Handb Exp Pharmacol. 2019;256:325-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 157. | Tomlinson E, Fu L, John L, Hultgren B, Huang X, Renz M, Stephan JP, Tsai SP, Powell-Braxton L, French D, Stewart TA. Transgenic mice expressing human fibroblast growth factor-19 display increased metabolic rate and decreased adiposity. Endocrinology. 2002;143:1741-1747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 379] [Cited by in RCA: 429] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 158. | Alvarez-Sola G, Uriarte I, Latasa MU, Fernandez-Barrena MG, Urtasun R, Elizalde M, Barcena-Varela M, Jiménez M, Chang HC, Barbero R, Catalán V, Rodríguez A, Frühbeck G, Gallego-Escuredo JM, Gavaldà-Navarro A, Villarroya F, Rodriguez-Ortigosa CM, Corrales FJ, Prieto J, Berraondo P, Berasain C, Avila MA. Fibroblast growth factor 15/19 (FGF15/19) protects from diet-induced hepatic steatosis: development of an FGF19-based chimeric molecule to promote fatty liver regeneration. Gut. 2017;66:1818-1828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 131] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 159. | Jiang C, Xie C, Li F, Zhang L, Nichols RG, Krausz KW, Cai J, Qi Y, Fang ZZ, Takahashi S, Tanaka N, Desai D, Amin SG, Albert I, Patterson AD, Gonzalez FJ. Intestinal farnesoid X receptor signaling promotes nonalcoholic fatty liver disease. J Clin Invest. 2015;125:386-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 555] [Cited by in RCA: 540] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 160. | Xi Y, Li H. Role of farnesoid X receptor in hepatic steatosis in nonalcoholic fatty liver disease. Biomed Pharmacother. 2020;121:109609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 73] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 161. | Pineda Torra I, Claudel T, Duval C, Kosykh V, Fruchart JC, Staels B. Bile acids induce the expression of the human peroxisome proliferator-activated receptor alpha gene via activation of the farnesoid X receptor. Mol Endocrinol. 2003;17:259-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 361] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 162. | Sinal CJ, Tohkin M, Miyata M, Ward JM, Lambert G, Gonzalez FJ. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell. 2000;102:731-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1341] [Cited by in RCA: 1399] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 163. | Thomas C, Gioiello A, Noriega L, Strehle A, Oury J, Rizzo G, Macchiarulo A, Yamamoto H, Mataki C, Pruzanski M, Pellicciari R, Auwerx J, Schoonjans K. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009;10:167-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1223] [Cited by in RCA: 1393] [Article Influence: 87.1] [Reference Citation Analysis (0)] |
| 164. | Wang YD, Chen WD, Yu D, Forman BM, Huang W. The G-protein-coupled bile acid receptor, Gpbar1 (TGR5), negatively regulates hepatic inflammatory response through antagonizing nuclear factor κ light-chain enhancer of activated B cells (NF-κB) in mice. Hepatology. 2011;54:1421-1432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 391] [Cited by in RCA: 373] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 165. | Guo C, Xie S, Chi Z, Zhang J, Liu Y, Zhang L, Zheng M, Zhang X, Xia D, Ke Y, Lu L, Wang D. Bile Acids Control Inflammation and Metabolic Disorder through Inhibition of NLRP3 Inflammasome. Immunity. 2016;45:802-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 525] [Article Influence: 58.3] [Reference Citation Analysis (0)] |
| 166. | Jiao N, Baker SS, Chapa-Rodriguez A, Liu W, Nugent CA, Tsompana M, Mastrandrea L, Buck MJ, Baker RD, Genco RJ, Zhu R, Zhu L. Suppressed hepatic bile acid signalling despite elevated production of primary and secondary bile acids in NAFLD. Gut. 2018;67:1881-1891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 516] [Article Influence: 73.7] [Reference Citation Analysis (1)] |
| 167. | Puri P, Daita K, Joyce A, Mirshahi F, Santhekadur PK, Cazanave S, Luketic VA, Siddiqui MS, Boyett S, Min HK, Kumar DP, Kohli R, Zhou H, Hylemon PB, Contos MJ, Idowu M, Sanyal AJ. The presence and severity of nonalcoholic steatohepatitis is associated with specific changes in circulating bile acids. Hepatology. 2018;67:534-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 308] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 168. | Nimer N, Choucair I, Wang Z, Nemet I, Li L, Gukasyan J, Weeks TL, Alkhouri N, Zein N, Tang WHW, Fischbach MA, Brown JM, Allayee H, Dasarathy S, Gogonea V, Hazen SL. Bile acids profile, histopathological indices and genetic variants for non-alcoholic fatty liver disease progression. Metabolism. 2021;116:154457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 96] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 169. | Lee G, You HJ, Bajaj JS, Joo SK, Yu J, Park S, Kang H, Park JH, Kim JH, Lee DH, Lee S, Kim W, Ko G. Distinct signatures of gut microbiome and metabolites associated with significant fibrosis in non-obese NAFLD. Nat Commun. 2020;11:4982. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 247] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 170. | Chen J, Zheng M, Liu J, Luo Y, Yang W, Yang J, Liu J, Zhou J, Xu C, Zhao F, Su M, Zang S, Shi J; Chinese NAFLD Clinical Research Network (CNAFLD CRN). Ratio of Conjugated Chenodeoxycholic to Muricholic Acids is Associated with Severity of Nonalcoholic Steatohepatitis. Obesity (Silver Spring). 2019;27:2055-2066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 171. | Wang H, Guo Y, Han W, Liang M, Xiao X, Jiang X, Yu W. Tauroursodeoxycholic Acid Improves Nonalcoholic Fatty Liver Disease by Regulating Gut Microbiota and Bile Acid Metabolism. J Agric Food Chem. 2024;72:20194-20210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 172. | Agus A, Planchais J, Sokol H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe. 2018;23:716-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 834] [Cited by in RCA: 1811] [Article Influence: 301.8] [Reference Citation Analysis (1)] |
| 173. | Modoux M, Rolhion N, Mani S, Sokol H. Tryptophan Metabolism as a Pharmacological Target. Trends Pharmacol Sci. 2021;42:60-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 179] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 174. | Rothhammer V, Mascanfroni ID, Bunse L, Takenaka MC, Kenison JE, Mayo L, Chao CC, Patel B, Yan R, Blain M, Alvarez JI, Kébir H, Anandasabapathy N, Izquierdo G, Jung S, Obholzer N, Pochet N, Clish CB, Prinz M, Prat A, Antel J, Quintana FJ. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat Med. 2016;22:586-597. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1000] [Cited by in RCA: 1098] [Article Influence: 122.0] [Reference Citation Analysis (0)] |
| 175. | Knudsen C, Neyrinck AM, Leyrolle Q, Baldin P, Leclercq S, Rodriguez J, Beaumont M, Cani PD, Bindels LB, Lanthier N, Delzenne NM. Hepatoprotective Effects of Indole, a Gut Microbial Metabolite, in Leptin-Deficient Obese Mice. J Nutr. 2021;151:1507-1516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 176. | Beaumont M, Neyrinck AM, Olivares M, Rodriguez J, de Rocca Serra A, Roumain M, Bindels LB, Cani PD, Evenepoel P, Muccioli GG, Demoulin JB, Delzenne NM. The gut microbiota metabolite indole alleviates liver inflammation in mice. FASEB J. 2018;32:fj201800544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 150] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 177. | Zhao ZH, Xin FZ, Xue Y, Hu Z, Han Y, Ma F, Zhou D, Liu XL, Cui A, Liu Z, Liu Y, Gao J, Pan Q, Li Y, Fan JG. Indole-3-propionic acid inhibits gut dysbiosis and endotoxin leakage to attenuate steatohepatitis in rats. Exp Mol Med. 2019;51:1-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 166] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 178. | Shimada Y, Kinoshita M, Harada K, Mizutani M, Masahata K, Kayama H, Takeda K. Commensal bacteria-dependent indole production enhances epithelial barrier function in the colon. PLoS One. 2013;8:e80604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 204] [Cited by in RCA: 284] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 179. | Ma L, Li H, Hu J, Zheng J, Zhou J, Botchlett R, Matthews D, Zeng T, Chen L, Xiao X, Athrey G, Threadgill DW, Li Q, Glaser S, Francis H, Meng F, Li Q, Alpini G, Wu C. Indole Alleviates Diet-Induced Hepatic Steatosis and Inflammation in a Manner Involving Myeloid Cell 6-Phosphofructo-2-Kinase/Fructose-2,6-Biphosphatase 3. Hepatology. 2020;72:1191-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 92] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 180. | Krishnan S, Ding Y, Saedi N, Choi M, Sridharan GV, Sherr DH, Yarmush ML, Alaniz RC, Jayaraman A, Lee K. Gut Microbiota-Derived Tryptophan Metabolites Modulate Inflammatory Response in Hepatocytes and Macrophages. Cell Rep. 2018;23:1099-1111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 259] [Cited by in RCA: 434] [Article Influence: 72.3] [Reference Citation Analysis (1)] |
| 181. | Sehgal R, Ilha M, Vaittinen M, Kaminska D, Männistö V, Kärjä V, Tuomainen M, Hanhineva K, Romeo S, Pajukanta P, Pihlajamäki J, de Mello VD. Indole-3-Propionic Acid, a Gut-Derived Tryptophan Metabolite, Associates with Hepatic Fibrosis. Nutrients. 2021;13:3509. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 182. | Abildgaard A, Elfving B, Hokland M, Wegener G, Lund S. The microbial metabolite indole-3-propionic acid improves glucose metabolism in rats, but does not affect behaviour. Arch Physiol Biochem. 2018;124:306-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 84] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 183. | Min BH, Devi S, Kwon GH, Gupta H, Jeong JJ, Sharma SP, Won SM, Oh KK, Yoon SJ, Park HJ, Eom JA, Jeong MK, Hyun JY, Stalin N, Park TS, Choi J, Lee DY, Han SH, Kim DJ, Suk KT. Gut microbiota-derived indole compounds attenuate metabolic dysfunction-associated steatotic liver disease by improving fat metabolism and inflammation. Gut Microbes. 2024;16:2307568. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 47] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 184. | Ritze Y, Bárdos G, Hubert A, Böhle M, Bischoff SC. Effect of tryptophan supplementation on diet-induced non-alcoholic fatty liver disease in mice. Br J Nutr. 2014;112:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 185. | Ji Y, Gao Y, Chen H, Yin Y, Zhang W. Indole-3-Acetic Acid Alleviates Nonalcoholic Fatty Liver Disease in Mice via Attenuation of Hepatic Lipogenesis, and Oxidative and Inflammatory Stress. Nutrients. 2019;11:2062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 180] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 186. | Wang Y, Wang G, Bai J, Zhao N, Wang Q, Zhou R, Li G, Hu C, Li X, Tao K, Xia Z, Wang G. Role of Indole-3-Acetic Acid in NAFLD Amelioration After Sleeve Gastrectomy. Obes Surg. 2021;31:3040-3052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 187. | Chung HK, Kim YK, Park JH, Ryu MJ, Chang JY, Hwang JH, Lee CH, Kim SH, Kim HJ, Kweon GR, Kim KS, Shong M. The indole derivative NecroX-7 improves nonalcoholic steatohepatitis in ob/ob mice through suppression of mitochondrial ROS/RNS and inflammation. Liver Int. 2015;35:1341-1353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 188. | Wang L, Fan X, Han J, Cai M, Wang X, Wang Y, Shang J. Gut-Derived Serotonin Contributes to the Progression of Non-Alcoholic Steatohepatitis via the Liver HTR2A/PPARγ2 Pathway. Front Pharmacol. 2020;11:553. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 189. | Choi W, Namkung J, Hwang I, Kim H, Lim A, Park HJ, Lee HW, Han KH, Park S, Jeong JS, Bang G, Kim YH, Yadav VK, Karsenty G, Ju YS, Choi C, Suh JM, Park JY, Park S, Kim H. Serotonin signals through a gut-liver axis to regulate hepatic steatosis. Nat Commun. 2018;9:4824. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 117] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 190. | Chen X, Zhang C, Zhao M, Shi CE, Zhu RM, Wang H, Zhao H, Wei W, Li JB, Xu DX. Melatonin alleviates lipopolysaccharide-induced hepatic SREBP-1c activation and lipid accumulation in mice. J Pineal Res. 2011;51:416-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 191. | Sun H, Wang X, Chen J, Song K, Gusdon AM, Li L, Bu L, Qu S. Melatonin improves non-alcoholic fatty liver disease via MAPK-JNK/P38 signaling in high-fat-diet-induced obese mice. Lipids Health Dis. 2016;15:202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 192. | Yu Y, Chen D, Zhao Y, Zhu J, Dong X. Melatonin ameliorates hepatic steatosis by inhibiting NLRP3 inflammasome in db/db mice. Int J Immunopathol Pharmacol. 2021;35:20587384211036819. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 193. | Celinski K, Konturek PC, Slomka M, Cichoz-Lach H, Brzozowski T, Konturek SJ, Korolczuk A. Effects of treatment with melatonin and tryptophan on liver enzymes, parameters of fat metabolism and plasma levels of cytokines in patients with non-alcoholic fatty liver disease--14 months follow up. J Physiol Pharmacol. 2014;65:75-82. [PubMed] |
| 194. | Bahrami M, Cheraghpour M, Jafarirad S, Alavinejad P, Asadi F, Hekmatdoost A, Mohammadi M, Yari Z. The effect of melatonin on treatment of patients with non-alcoholic fatty liver disease: a randomized double blind clinical trial. Complement Ther Med. 2020;52:102452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 195. | Coutinho-Wolino KS, de F Cardozo LFM, de Oliveira Leal V, Mafra D, Stockler-Pinto MB. Can diet modulate trimethylamine N-oxide (TMAO) production? Eur J Nutr. 2021;60:3567-3584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 65] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 196. | Chen YM, Liu Y, Zhou RF, Chen XL, Wang C, Tan XY, Wang LJ, Zheng RD, Zhang HW, Ling WH, Zhu HL. Associations of gut-flora-dependent metabolite trimethylamine-N-oxide, betaine and choline with non-alcoholic fatty liver disease in adults. Sci Rep. 2016;6:19076. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 161] [Cited by in RCA: 254] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 197. | Barrea L, Muscogiuri G, Annunziata G, Laudisio D, de Alteriis G, Tenore GC, Colao A, Savastano S. A New Light on Vitamin D in Obesity: A Novel Association with Trimethylamine-N-Oxide (TMAO). Nutrients. 2019;11:1310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 198. | Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, Smith JD, DiDonato JA, Chen J, Li H, Wu GD, Lewis JD, Warrier M, Brown JM, Krauss RM, Tang WH, Bushman FD, Lusis AJ, Hazen SL. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19:576-585. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2783] [Cited by in RCA: 3164] [Article Influence: 263.7] [Reference Citation Analysis (0)] |
| 199. | Gao X, Liu X, Xu J, Xue C, Xue Y, Wang Y. Dietary trimethylamine N-oxide exacerbates impaired glucose tolerance in mice fed a high fat diet. J Biosci Bioeng. 2014;118:476-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 271] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 200. | Nian F, Zhu C, Jin N, Xia Q, Wu L, Lu X. Gut microbiota metabolite TMAO promoted lipid deposition and fibrosis process via KRT17 in fatty liver cells in vitro. Biochem Biophys Res Commun. 2023;669:134-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 22] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 201. | Flores-Guerrero JL, Post A, van Dijk PR, Connelly MA, Garcia E, Navis G, Bakker SJL, Dullaart RPF. Circulating trimethylamine-N-oxide is associated with all-cause mortality in subjects with nonalcoholic fatty liver disease. Liver Int. 2021;41:2371-2382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 202. | Tan X, Liu Y, Long J, Chen S, Liao G, Wu S, Li C, Wang L, Ling W, Zhu H. Trimethylamine N-Oxide Aggravates Liver Steatosis through Modulation of Bile Acid Metabolism and Inhibition of Farnesoid X Receptor Signaling in Nonalcoholic Fatty Liver Disease. Mol Nutr Food Res. 2019;63:e1900257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 154] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 203. | Chen S, Henderson A, Petriello MC, Romano KA, Gearing M, Miao J, Schell M, Sandoval-Espinola WJ, Tao J, Sha B, Graham M, Crooke R, Kleinridders A, Balskus EP, Rey FE, Morris AJ, Biddinger SB. Trimethylamine N-Oxide Binds and Activates PERK to Promote Metabolic Dysfunction. Cell Metab. 2019;30:1141-1151.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 264] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 204. | Malhi H, Kaufman RJ. Endoplasmic reticulum stress in liver disease. J Hepatol. 2011;54:795-809. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 980] [Cited by in RCA: 953] [Article Influence: 68.1] [Reference Citation Analysis (0)] |
| 205. | Barrea L, Annunziata G, Muscogiuri G, Di Somma C, Laudisio D, Maisto M, de Alteriis G, Tenore GC, Colao A, Savastano S. Trimethylamine-N-oxide (TMAO) as Novel Potential Biomarker of Early Predictors of Metabolic Syndrome. Nutrients. 2018;10:1971. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 198] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 206. | León-Mimila P, Villamil-Ramírez H, Li XS, Shih DM, Hui ST, Ocampo-Medina E, López-Contreras B, Morán-Ramos S, Olivares-Arevalo M, Grandini-Rosales P, Macías-Kauffer L, González-González I, Hernández-Pando R, Gómez-Pérez F, Campos-Pérez F, Aguilar-Salinas C, Larrieta-Carrasco E, Villarreal-Molina T, Wang Z, Lusis AJ, Hazen SL, Huertas-Vazquez A, Canizales-Quinteros S. Trimethylamine N-oxide levels are associated with NASH in obese subjects with type 2 diabetes. Diabetes Metab. 2021;47:101183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 77] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 207. | Zhao ZH, Xin FZ, Zhou D, Xue YQ, Liu XL, Yang RX, Pan Q, Fan JG. Trimethylamine N-oxide attenuates high-fat high-cholesterol diet-induced steatohepatitis by reducing hepatic cholesterol overload in rats. World J Gastroenterol. 2019;25:2450-2462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 64] [Cited by in RCA: 64] [Article Influence: 10.7] [Reference Citation Analysis (1)] |
| 208. | Zhao M, Zhao L, Xiong X, He Y, Huang W, Liu Z, Ji L, Pan B, Guo X, Wang L, Cheng S, Xu M, Yang H, Yin Y, Garcia-Barrio MT, Chen YE, Meng X, Zheng L. TMAVA, a Metabolite of Intestinal Microbes, Is Increased in Plasma From Patients With Liver Steatosis, Inhibits γ-Butyrobetaine Hydroxylase, and Exacerbates Fatty Liver in Mice. Gastroenterology. 2020;158:2266-2281.e27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 105] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 209. | Cope K, Risby T, Diehl AM. Increased gastrointestinal ethanol production in obese mice: implications for fatty liver disease pathogenesis. Gastroenterology. 2000;119:1340-1347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 265] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 210. | Engstler AJ, Aumiller T, Degen C, Dürr M, Weiss E, Maier IB, Schattenberg JM, Jin CJ, Sellmann C, Bergheim I. Insulin resistance alters hepatic ethanol metabolism: studies in mice and children with non-alcoholic fatty liver disease. Gut. 2016;65:1564-1571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 94] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 211. | Meijnikman AS, Davids M, Herrema H, Aydin O, Tremaroli V, Rios-Morales M, Levels H, Bruin S, de Brauw M, Verheij J, Kemper M, Holleboom AG, Tushuizen ME, Schwartz TW, Nielsen J, Brandjes D, Dirinck E, Weyler J, Verrijken A, De Block CEM, Vonghia L, Francque S, Beuers U, Gerdes VEA, Bäckhed F, Groen AK, Nieuwdorp M. Microbiome-derived ethanol in nonalcoholic fatty liver disease. Nat Med. 2022;28:2100-2106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 123] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 212. | Yuan J, Chen C, Cui J, Lu J, Yan C, Wei X, Zhao X, Li N, Li S, Xue G, Cheng W, Li B, Li H, Lin W, Tian C, Zhao J, Han J, An D, Zhang Q, Wei H, Zheng M, Ma X, Li W, Chen X, Zhang Z, Zeng H, Ying S, Wu J, Yang R, Liu D. Fatty Liver Disease Caused by High-Alcohol-Producing Klebsiella pneumoniae. Cell Metab. 2019;30:675-688.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 322] [Article Influence: 53.7] [Reference Citation Analysis (0)] |
| 213. | Cotillard A, Kennedy SP, Kong LC, Prifti E, Pons N, Le Chatelier E, Almeida M, Quinquis B, Levenez F, Galleron N, Gougis S, Rizkalla S, Batto JM, Renault P; ANR MicroObes consortium, Doré J, Zucker JD, Clément K, Ehrlich SD. Dietary intervention impact on gut microbial gene richness. Nature. 2013;500:585-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1189] [Cited by in RCA: 1297] [Article Influence: 108.1] [Reference Citation Analysis (0)] |
| 214. | Merra G, Noce A, Marrone G, Cintoni M, Tarsitano MG, Capacci A, De Lorenzo A. Influence of Mediterranean Diet on Human Gut Microbiota. Nutrients. 2020;13:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 245] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 215. | Montemayor S, Mascaró CM, Ugarriza L, Casares M, Llompart I, Abete I, Zulet MÁ, Martínez JA, Tur JA, Bouzas C. Adherence to Mediterranean Diet and NAFLD in Patients with Metabolic Syndrome: The FLIPAN Study. Nutrients. 2022;14:3186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 49] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 216. | Yang M, Qi X, Li N, Kaifi JT, Chen S, Wheeler AA, Kimchi ET, Ericsson AC, Rector RS, Staveley-O'Carroll KF, Li G. Western diet contributes to the pathogenesis of non-alcoholic steatohepatitis in male mice via remodeling gut microbiota and increasing production of 2-oleoylglycerol. Nat Commun. 2023;14:228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 46] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 217. | Hassani Zadeh S, Mansoori A, Hosseinzadeh M. Relationship between dietary patterns and non-alcoholic fatty liver disease: A systematic review and meta-analysis. J Gastroenterol Hepatol. 2021;36:1470-1478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 107] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 218. | Kastorini CM, Milionis HJ, Esposito K, Giugliano D, Goudevenos JA, Panagiotakos DB. The effect of Mediterranean diet on metabolic syndrome and its components: a meta-analysis of 50 studies and 534,906 individuals. J Am Coll Cardiol. 2011;57:1299-1313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 722] [Cited by in RCA: 736] [Article Influence: 52.6] [Reference Citation Analysis (0)] |
| 219. | Biolato M, Manca F, Marrone G, Cefalo C, Racco S, Miggiano GA, Valenza V, Gasbarrini A, Miele L, Grieco A. Intestinal permeability after Mediterranean diet and low-fat diet in non-alcoholic fatty liver disease. World J Gastroenterol. 2019;25:509-520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 59] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 220. | Properzi C, O'Sullivan TA, Sherriff JL, Ching HL, Jeffrey GP, Buckley RF, Tibballs J, MacQuillan GC, Garas G, Adams LA. Ad Libitum Mediterranean and Low-Fat Diets Both Significantly Reduce Hepatic Steatosis: A Randomized Controlled Trial. Hepatology. 2018;68:1741-1754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 149] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 221. | Katsagoni CN, Papatheodoridis GV, Ioannidou P, Deutsch M, Alexopoulou A, Papadopoulos N, Papageorgiou MV, Fragopoulou E, Kontogianni MD. Improvements in clinical characteristics of patients with non-alcoholic fatty liver disease, after an intervention based on the Mediterranean lifestyle: a randomised controlled clinical trial. Br J Nutr. 2018;120:164-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 100] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 222. | Plaz Torres MC, Aghemo A, Lleo A, Bodini G, Furnari M, Marabotto E, Miele L, Giannini EG. Mediterranean Diet and NAFLD: What We Know and Questions That Still Need to Be Answered. Nutrients. 2019;11:2971. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 71] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 223. | Romero-Gómez M, Zelber-Sagi S, Trenell M. Treatment of NAFLD with diet, physical activity and exercise. J Hepatol. 2017;67:829-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 844] [Cited by in RCA: 926] [Article Influence: 115.8] [Reference Citation Analysis (0)] |
| 224. | Kawaguchi T, Charlton M, Kawaguchi A, Yamamura S, Nakano D, Tsutsumi T, Zafer M, Torimura T. Effects of Mediterranean Diet in Patients with Nonalcoholic Fatty Liver Disease: A Systematic Review, Meta-Analysis, and Meta-Regression Analysis of Randomized Controlled Trials. Semin Liver Dis. 2021;41:225-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 56] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 225. | Akbulut UE, Isik IA, Atalay A, Eraslan A, Durmus E, Turkmen S, Yurttas AS. The effect of a Mediterranean diet vs. a low-fat diet on non-alcoholic fatty liver disease in children: a randomized trial. Int J Food Sci Nutr. 2022;73:357-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 226. | Yurtdaş G, Akbulut G, Baran M, Yılmaz C. The effects of Mediterranean diet on hepatic steatosis, oxidative stress, and inflammation in adolescents with non-alcoholic fatty liver disease: A randomized controlled trial. Pediatr Obes. 2022;17:e12872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 35] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 227. | Phinney SD, Bistrian BR, Wolfe RR, Blackburn GL. The human metabolic response to chronic ketosis without caloric restriction: physical and biochemical adaptation. Metabolism. 1983;32:757-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 117] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 228. | Luukkonen PK, Dufour S, Lyu K, Zhang XM, Hakkarainen A, Lehtimäki TE, Cline GW, Petersen KF, Shulman GI, Yki-Järvinen H. Effect of a ketogenic diet on hepatic steatosis and hepatic mitochondrial metabolism in nonalcoholic fatty liver disease. Proc Natl Acad Sci U S A. 2020;117:7347-7354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 163] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 229. | Muyyarikkandy MS, McLeod M, Maguire M, Mahar R, Kattapuram N, Zhang C, Surugihalli C, Muralidaran V, Vavilikolanu K, Mathews CE, Merritt ME, Sunny NE. Branched chain amino acids and carbohydrate restriction exacerbate ketogenesis and hepatic mitochondrial oxidative dysfunction during NAFLD. FASEB J. 2020;34:14832-14849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 230. | Anekwe CV, Chandrasekaran P, Stanford FC. Ketogenic Diet-induced Elevated Cholesterol, Elevated Liver Enzymes and Potential Non-alcoholic Fatty Liver Disease. Cureus. 2020;12:e6605. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 231. | Risi R, Tozzi R, Watanabe M. Beyond weight loss in nonalcoholic fatty liver disease: the role of carbohydrate restriction. Curr Opin Clin Nutr Metab Care. 2021;24:349-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 232. | Crabtree CD, Kackley ML, Buga A, Fell B, LaFountain RA, Hyde PN, Sapper TN, Kraemer WJ, Scandling D, Simonetti OP, Volek JS. Comparison of Ketogenic Diets with and without Ketone Salts versus a Low-Fat Diet: Liver Fat Responses in Overweight Adults. Nutrients. 2021;13:966. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 233. | Krawczyk M, Maciejewska D, Ryterska K, Czerwińka-Rogowska M, Jamioł-Milc D, Skonieczna-Żydecka K, Milkiewicz P, Raszeja-Wyszomirska J, Stachowska E. Gut Permeability Might be Improved by Dietary Fiber in Individuals with Nonalcoholic Fatty Liver Disease (NAFLD) Undergoing Weight Reduction. Nutrients. 2018;10:1793. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 234. | Pérez-Montes de Oca A, Julián MT, Ramos A, Puig-Domingo M, Alonso N. Microbiota, Fiber, and NAFLD: Is There Any Connection? Nutrients. 2020;12:3100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 235. | Zhu W, Zhou Y, Tsao R, Dong H, Zhang H. Amelioratory Effect of Resistant Starch on Non-alcoholic Fatty Liver Disease via the Gut-Liver Axis. Front Nutr. 2022;9:861854. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 236. | Okubo H, Sakoda H, Kushiyama A, Fujishiro M, Nakatsu Y, Fukushima T, Matsunaga Y, Kamata H, Asahara T, Yoshida Y, Chonan O, Iwashita M, Nishimura F, Asano T. Lactobacillus casei strain Shirota protects against nonalcoholic steatohepatitis development in a rodent model. Am J Physiol Gastrointest Liver Physiol. 2013;305:G911-G918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 85] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 237. | Spencer MD, Hamp TJ, Reid RW, Fischer LM, Zeisel SH, Fodor AA. Association between composition of the human gastrointestinal microbiome and development of fatty liver with choline deficiency. Gastroenterology. 2011;140:976-986. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 498] [Cited by in RCA: 545] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 238. | Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, Wu Y, Schauer P, Smith JD, Allayee H, Tang WH, DiDonato JA, Lusis AJ, Hazen SL. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57-63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4469] [Cited by in RCA: 4057] [Article Influence: 289.8] [Reference Citation Analysis (0)] |
| 239. | Corbin KD, Zeisel SH. Choline metabolism provides novel insights into nonalcoholic fatty liver disease and its progression. Curr Opin Gastroenterol. 2012;28:159-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 349] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 240. | Ye JZ, Li YT, Wu WR, Shi D, Fang DQ, Yang LY, Bian XY, Wu JJ, Wang Q, Jiang XW, Peng CG, Ye WC, Xia PC, Li LJ. Dynamic alterations in the gut microbiota and metabolome during the development of methionine-choline-deficient diet-induced nonalcoholic steatohepatitis. World J Gastroenterol. 2018;24:2468-2481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 49] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 241. | Schneider KM, Mohs A, Kilic K, Candels LS, Elfers C, Bennek E, Schneider LB, Heymann F, Gassler N, Penders J, Trautwein C. Intestinal Microbiota Protects against MCD Diet-Induced Steatohepatitis. Int J Mol Sci. 2019;20:308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 242. | Zhang C, Zhang M, Wang S, Han R, Cao Y, Hua W, Mao Y, Zhang X, Pang X, Wei C, Zhao G, Chen Y, Zhao L. Interactions between gut microbiota, host genetics and diet relevant to development of metabolic syndromes in mice. ISME J. 2010;4:232-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 651] [Cited by in RCA: 831] [Article Influence: 51.9] [Reference Citation Analysis (1)] |
| 243. | Weglarz L, Dzierzewicz Z, Skop B, Orchel A, Parfiniewicz B, Wiśniowska B, Swiatkowska L, Wilczok T. Desulfovibrio desulfuricans lipopolysaccharides induce endothelial cell IL-6 and IL-8 secretion and E-selectin and VCAM-1 expression. Cell Mol Biol Lett. 2003;8:991-1003. [PubMed] |
| 244. | Loubinoux J, Mory F, Pereira IA, Le Faou AE. Bacteremia caused by a strain of Desulfovibrio related to the provisionally named Desulfovibrio fairfieldensis. J Clin Microbiol. 2000;38:931-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 83] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 245. | Gao X, Lin X, Xin Y, Zhu X, Li X, Chen M, Huang Z, Guo H. Dietary cholesterol drives the development of nonalcoholic steatohepatitis by altering gut microbiota mediated bile acid metabolism in high-fat diet fed mice. J Nutr Biochem. 2023;117:109347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 246. | Stanislawski MA, Lozupone CA, Wagner BD, Eggesbø M, Sontag MK, Nusbacher NM, Martinez M, Dabelea D. Gut microbiota in adolescents and the association with fatty liver: the EPOCH study. Pediatr Res. 2018;84:219-227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 247. | Schoeler M, Ellero-Simatos S, Birkner T, Mayneris-Perxachs J, Olsson L, Brolin H, Loeber U, Kraft JD, Polizzi A, Martí-Navas M, Puig J, Moschetta A, Montagner A, Gourdy P, Heymes C, Guillou H, Tremaroli V, Fernández-Real JM, Forslund SK, Burcelin R, Caesar R. The interplay between dietary fatty acids and gut microbiota influences host metabolism and hepatic steatosis. Nat Commun. 2023;14:5329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 51] [Reference Citation Analysis (0)] |
| 248. | Schwimmer JB, Ugalde-Nicalo P, Welsh JA, Angeles JE, Cordero M, Harlow KE, Alazraki A, Durelle J, Knight-Scott J, Newton KP, Cleeton R, Knott C, Konomi J, Middleton MS, Travers C, Sirlin CB, Hernandez A, Sekkarie A, McCracken C, Vos MB. Effect of a Low Free Sugar Diet vs Usual Diet on Nonalcoholic Fatty Liver Disease in Adolescent Boys: A Randomized Clinical Trial. JAMA. 2019;321:256-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 190] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 249. | Cohen CC, Li KW, Alazraki AL, Beysen C, Carrier CA, Cleeton RL, Dandan M, Figueroa J, Knight-Scott J, Knott CJ, Newton KP, Nyangau EM, Sirlin CB, Ugalde-Nicalo PA, Welsh JA, Hellerstein MK, Schwimmer JB, Vos MB. Dietary sugar restriction reduces hepatic de novo lipogenesis in adolescent boys with fatty liver disease. J Clin Invest. 2021;131:e150996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 250. | Markova M, Pivovarova O, Hornemann S, Sucher S, Frahnow T, Wegner K, Machann J, Petzke KJ, Hierholzer J, Lichtinghagen R, Herder C, Carstensen-Kirberg M, Roden M, Rudovich N, Klaus S, Thomann R, Schneeweiss R, Rohn S, Pfeiffer AF. Isocaloric Diets High in Animal or Plant Protein Reduce Liver Fat and Inflammation in Individuals With Type 2 Diabetes. Gastroenterology. 2017;152:571-585.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 190] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 251. | Zhou X, Hao R, Zhu X, Tan X, Li D. Isoleucine‐restricted diets improve high‐fat diet‐induced nonalcoholic fatty liver disease via regulating insulin resistance and gut microbiota. Food Frontiers. 2024;5:893-906. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 252. | Zhang X, Zhao Y, Zhang M, Pang X, Xu J, Kang C, Li M, Zhang C, Zhang Z, Zhang Y, Li X, Ning G, Zhao L. Structural changes of gut microbiota during berberine-mediated prevention of obesity and insulin resistance in high-fat diet-fed rats. PLoS One. 2012;7:e42529. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 342] [Cited by in RCA: 400] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 253. | Xu JH, Liu XZ, Pan W, Zou DJ. Berberine protects against diet-induced obesity through regulating metabolic endotoxemia and gut hormone levels. Mol Med Rep. 2017;15:2765-2787. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 254. | Qiao Y, Sun J, Xia S, Tang X, Shi Y, Le G. Effects of resveratrol on gut microbiota and fat storage in a mouse model with high-fat-induced obesity. Food Funct. 2014;5:1241-1249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 270] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 255. | Duvnjak M, Tomasic V, Gomercic M, Smircic Duvnjak L, Barsic N, Lerotic I. Therapy of nonalcoholic fatty liver disease: current status. J Physiol Pharmacol. 2009;60 Suppl 7:57-66. [PubMed] |
| 256. | Alves CC, Waitzberg DL, de Andrade LS, Dos Santos Aguiar L, Reis MB, Guanabara CC, Júnior OA, Ribeiro DA, Sala P. Prebiotic and Synbiotic Modifications of Beta Oxidation and Lipogenic Gene Expression after Experimental Hypercholesterolemia in Rat Liver. Front Microbiol. 2017;8:2010. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 257. | Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, Calder PC, Sanders ME. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11:506-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4055] [Cited by in RCA: 5557] [Article Influence: 505.2] [Reference Citation Analysis (2)] |
| 258. | Tojo R, Suárez A, Clemente MG, de los Reyes-Gavilán CG, Margolles A, Gueimonde M, Ruas-Madiedo P. Intestinal microbiota in health and disease: role of bifidobacteria in gut homeostasis. World J Gastroenterol. 2014;20:15163-15176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 309] [Cited by in RCA: 339] [Article Influence: 30.8] [Reference Citation Analysis (5)] |
| 259. | Sánchez B, Delgado S, Blanco-Míguez A, Lourenço A, Gueimonde M, Margolles A. Probiotics, gut microbiota, and their influence on host health and disease. Mol Nutr Food Res. 2017;61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 463] [Cited by in RCA: 650] [Article Influence: 72.2] [Reference Citation Analysis (0)] |
| 260. | Gillor O, Etzion A, Riley MA. The dual role of bacteriocins as anti- and probiotics. Appl Microbiol Biotechnol. 2008;81:591-606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 300] [Cited by in RCA: 239] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 261. | Mackowiak PA. Recycling metchnikoff: probiotics, the intestinal microbiome and the quest for long life. Front Public Health. 2013;1:52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 163] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 262. | Mills JP, Rao K, Young VB. Probiotics for prevention of Clostridium difficile infection. Curr Opin Gastroenterol. 2018;34:3-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 107] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 263. | Wang J, Tang H, Zhang C, Zhao Y, Derrien M, Rocher E, van-Hylckama Vlieg JE, Strissel K, Zhao L, Obin M, Shen J. Modulation of gut microbiota during probiotic-mediated attenuation of metabolic syndrome in high fat diet-fed mice. ISME J. 2015;9:1-15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 450] [Cited by in RCA: 534] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 264. | Nguyen TD, Kang JH, Lee MS. Characterization of Lactobacillus plantarum PH04, a potential probiotic bacterium with cholesterol-lowering effects. Int J Food Microbiol. 2007;113:358-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 236] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 265. | Wang Y, Xu N, Xi A, Ahmed Z, Zhang B, Bai X. Effects of Lactobacillus plantarum MA2 isolated from Tibet kefir on lipid metabolism and intestinal microflora of rats fed on high-cholesterol diet. Appl Microbiol Biotechnol. 2009;84:341-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 140] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 266. | Wang J, Zhang H, Chen X, Chen Y, Menghebilige, Bao Q. Selection of potential probiotic lactobacilli for cholesterol-lowering properties and their effect on cholesterol metabolism in rats fed a high-lipid diet. J Dairy Sci. 2012;95:1645-1654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 111] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 267. | Wagnerberger S, Spruss A, Kanuri G, Stahl C, Schröder M, Vetter W, Bischoff SC, Bergheim I. Lactobacillus casei Shirota protects from fructose-induced liver steatosis: a mouse model. J Nutr Biochem. 2013;24:531-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 97] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 268. | Naito E, Yoshida Y, Makino K, Kounoshi Y, Kunihiro S, Takahashi R, Matsuzaki T, Miyazaki K, Ishikawa F. Beneficial effect of oral administration of Lactobacillus casei strain Shirota on insulin resistance in diet-induced obesity mice. J Appl Microbiol. 2011;110:650-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 166] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 269. | Sohn W, Jun DW, Lee KN, Lee HL, Lee OY, Choi HS, Yoon BC. Lactobacillus paracasei Induces M2-Dominant Kupffer Cell Polarization in a Mouse Model of Nonalcoholic Steatohepatitis. Dig Dis Sci. 2015;60:3340-3350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 270. | Anderson RC, Cookson AL, McNabb WC, Park Z, McCann MJ, Kelly WJ, Roy NC. Lactobacillus plantarum MB452 enhances the function of the intestinal barrier by increasing the expression levels of genes involved in tight junction formation. BMC Microbiol. 2010;10:316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 251] [Cited by in RCA: 333] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 271. | Li C, Nie SP, Zhu KX, Ding Q, Li C, Xiong T, Xie MY. Lactobacillus plantarum NCU116 improves liver function, oxidative stress and lipid metabolism in rats with high fat diet induced non-alcoholic fatty liver disease. Food Funct. 2014;5:3216-3223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 97] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 272. | Cao F, Ding Q, Zhuge H, Lai S, Chang K, Le C, Yang G, Valencak TG, Li S, Ren D. Lactobacillus plantarum ZJUIDS14 alleviates non-alcoholic fatty liver disease in mice in association with modulation in the gut microbiota. Front Nutr. 2022;9:1071284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 273. | Zhao Z, Chen L, Zhao Y, Wang C, Duan C, Yang G, Niu C, Li S. Lactobacillus plantarum NA136 ameliorates nonalcoholic fatty liver disease by modulating gut microbiota, improving intestinal barrier integrity, and attenuating inflammation. Appl Microbiol Biotechnol. 2020;104:5273-5282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 92] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 274. | Park EJ, Lee YS, Kim SM, Park GS, Lee YH, Jeong DY, Kang J, Lee HJ. Beneficial Effects of Lactobacillus plantarum Strains on Non-Alcoholic Fatty Liver Disease in High Fat/High Fructose Diet-Fed Rats. Nutrients. 2020;12:542. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 275. | Donato KA, Gareau MG, Wang YJJ, Sherman PM. Lactobacillus rhamnosus GG attenuates interferon-{gamma} and tumour necrosis factor-alpha-induced barrier dysfunction and pro-inflammatory signalling. Microbiology (Reading). 2010;156:3288-3297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 156] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 276. | Kim B, Park KY, Ji Y, Park S, Holzapfel W, Hyun CK. Protective effects of Lactobacillus rhamnosus GG against dyslipidemia in high-fat diet-induced obese mice. Biochem Biophys Res Commun. 2016;473:530-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 99] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 277. | Ritze Y, Bárdos G, Claus A, Ehrmann V, Bergheim I, Schwiertz A, Bischoff SC. Lactobacillus rhamnosus GG protects against non-alcoholic fatty liver disease in mice. PLoS One. 2014;9:e80169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 223] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 278. | Liu Q, Liu Y, Li F, Gu Z, Liu M, Shao T, Zhang L, Zhou G, Pan C, He L, Cai J, Zhang X, Barve S, McClain CJ, Chen Y, Feng W. Probiotic culture supernatant improves metabolic function through FGF21-adiponectin pathway in mice. J Nutr Biochem. 2020;75:108256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 279. | Vajro P, Mandato C, Licenziati MR, Franzese A, Vitale DF, Lenta S, Caropreso M, Vallone G, Meli R. Effects of Lactobacillus rhamnosus strain GG in pediatric obesity-related liver disease. J Pediatr Gastroenterol Nutr. 2011;52:740-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 248] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 280. | Kang JH, Yun SI, Park MH, Park JH, Jeong SY, Park HO. Anti-obesity effect of Lactobacillus gasseri BNR17 in high-sucrose diet-induced obese mice. PLoS One. 2013;8:e54617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 150] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 281. | Naudin CR, Maner-Smith K, Owens JA, Wynn GM, Robinson BS, Matthews JD, Reedy AR, Luo L, Wolfarth AA, Darby TM, Ortlund EA, Jones RM. Lactococcus lactis Subspecies cremoris Elicits Protection Against Metabolic Changes Induced by a Western-Style Diet. Gastroenterology. 2020;159:639-651.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 73] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 282. | Song W, Wang T, Cui X, Li L, Chen B, Li Y, Yue T. Lactobacillus coryniformis subsp. torquens T3 alleviates non-alcoholic fatty liver disease via reconstruction of the gut microbiota and redox system. J Sci Food Agric. 2023;103:6814-6825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 283. | Xin J, Zeng D, Wang H, Ni X, Yi D, Pan K, Jing B. Preventing non-alcoholic fatty liver disease through Lactobacillus johnsonii BS15 by attenuating inflammation and mitochondrial injury and improving gut environment in obese mice. Appl Microbiol Biotechnol. 2014;98:6817-6829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 111] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 284. | Hsieh FC, Lee CL, Chai CY, Chen WT, Lu YC, Wu CS. Oral administration of Lactobacillus reuteri GMNL-263 improves insulin resistance and ameliorates hepatic steatosis in high fructose-fed rats. Nutr Metab (Lond). 2013;10:35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 187] [Cited by in RCA: 182] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 285. | Hayashi H, Sawada K, Tanaka H, Muro K, Hasebe T, Nakajima S, Okumura T, Fujiya M. The effect of heat-killed Lactobacillus brevis SBL88 on improving selective hepatic insulin resistance in non-alcoholic fatty liver disease mice without altering the gut microbiota. J Gastroenterol Hepatol. 2023;38:1847-1854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 286. | Lewis SJ, Burmeister S. A double-blind placebo-controlled study of the effects of Lactobacillus acidophilus on plasma lipids. Eur J Clin Nutr. 2005;59:776-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 68] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 287. | Chen J, Wang R, Li XF, Wang RL. Bifidobacterium adolescentis supplementation ameliorates visceral fat accumulation and insulin sensitivity in an experimental model of the metabolic syndrome. Br J Nutr. 2012;107:1429-1434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 205] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 288. | Shin HS, Park SY, Lee DK, Kim SA, An HM, Kim JR, Kim MJ, Cha MG, Lee SW, Kim KJ, Lee KO, Ha NJ. Hypocholesterolemic effect of sonication-killed Bifidobacterium longum isolated from healthy adult Koreans in high cholesterol fed rats. Arch Pharm Res. 2010;33:1425-1431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 289. | Ren T, Huang C, Cheng M. Dietary blueberry and bifidobacteria attenuate nonalcoholic fatty liver disease in rats by affecting SIRT1-mediated signaling pathway. Oxid Med Cell Longev. 2014;2014:469059. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 290. | Aoki R, Kamikado K, Suda W, Takii H, Mikami Y, Suganuma N, Hattori M, Koga Y. A proliferative probiotic Bifidobacterium strain in the gut ameliorates progression of metabolic disorders via microbiota modulation and acetate elevation. Sci Rep. 2017;7:43522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 177] [Cited by in RCA: 160] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 291. | Cano PG, Santacruz A, Trejo FM, Sanz Y. Bifidobacterium CECT 7765 improves metabolic and immunological alterations associated with obesity in high-fat diet-fed mice. Obesity (Silver Spring). 2013;21:2310-2321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 163] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 292. | Xu RY, Wan YP, Fang QY, Lu W, Cai W. Supplementation with probiotics modifies gut flora and attenuates liver fat accumulation in rat nonalcoholic fatty liver disease model. J Clin Biochem Nutr. 2012;50:72-77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 96] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 293. | An HM, Park SY, Lee DK, Kim JR, Cha MK, Lee SW, Lim HT, Kim KJ, Ha NJ. Antiobesity and lipid-lowering effects of Bifidobacterium spp. in high fat diet-induced obese rats. Lipids Health Dis. 2011;10:116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 223] [Cited by in RCA: 303] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 294. | Fan W, Tang K, Deng Y, Zheng C, Pan M, Pi D, Liang Z, Zhen J, Yang Q, Zhang Y. Bifidobacterium lactis Probio‐M8 prevents nonalcoholic fatty liver disease in high‐fat diet‐fed rats: The potential role in modulating gut microbiota. Food Bioeng. 2024;3:29-40. [RCA] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 295. | Kelesidis T, Pothoulakis C. Efficacy and safety of the probiotic Saccharomyces boulardii for the prevention and therapy of gastrointestinal disorders. Therap Adv Gastroenterol. 2012;5:111-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 226] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 296. | Mcfarland LV. Common Organisms and Probiotics: Saccharomyces boulardii. In: Floch MH, Ringel Y, Walker WA. The Microbiota in Gastrointestinal Pathophysiology. United States: Academic Press, 2017: 145-164. [DOI] [Full Text] |
| 297. | Fakruddin M, Hossain MN, Ahmed MM. Antimicrobial and antioxidant activities of Saccharomyces cerevisiae IFST062013, a potential probiotic. BMC Complement Altern Med. 2017;17:64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 100] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 298. | Schneider SM, Girard-Pipau F, Filippi J, Hebuterne X, Moyse D, Hinojosa GC, Pompei A, Rampal P. Effects of Saccharomyces boulardii on fecal short-chain fatty acids and microflora in patients on long-term total enteral nutrition. World J Gastroenterol. 2005;11:6165-6169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 74] [Cited by in RCA: 81] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 299. | Everard A, Matamoros S, Geurts L, Delzenne NM, Cani PD. Saccharomyces boulardii administration changes gut microbiota and reduces hepatic steatosis, low-grade inflammation, and fat mass in obese and type 2 diabetic db/db mice. mBio. 2014;5:e01011-e01014. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 160] [Cited by in RCA: 204] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 300. | Yang AM, Lin CY, Liu SH, Syu GD, Sun HJ, Lee KC, Lin HC, Hou MC. Saccharomyces Boulardii Ameliorates Non-alcoholic Steatohepatitis in Mice Induced by a Methionine-Choline-Deficient Diet Through Gut-Liver Axis. Front Microbiol. 2022;13:887728. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 301. | Xue L, He J, Gao N, Lu X, Li M, Wu X, Liu Z, Jin Y, Liu J, Xu J, Geng Y. Probiotics may delay the progression of nonalcoholic fatty liver disease by restoring the gut microbiota structure and improving intestinal endotoxemia. Sci Rep. 2017;7:45176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 200] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 302. | Kim DH, Kim H, Jeong D, Kang IB, Chon JW, Kim HS, Song KY, Seo KH. Kefir alleviates obesity and hepatic steatosis in high-fat diet-fed mice by modulation of gut microbiota and mycobiota: targeted and untargeted community analysis with correlation of biomarkers. J Nutr Biochem. 2017;44:35-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 121] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 303. | Kobyliak N, Falalyeyeva T, Bodnar P, Beregova T. Probiotics Supplemented with Omega-3 Fatty Acids are More Effective for Hepatic Steatosis Reduction in an Animal Model of Obesity. Probiotics Antimicrob Proteins. 2017;9:123-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 304. | Li Z, Yang S, Lin H, Huang J, Watkins PA, Moser AB, Desimone C, Song XY, Diehl AM. Probiotics and antibodies to TNF inhibit inflammatory activity and improve nonalcoholic fatty liver disease. Hepatology. 2003;37:343-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 658] [Cited by in RCA: 702] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 305. | Esposito E, Iacono A, Bianco G, Autore G, Cuzzocrea S, Vajro P, Canani RB, Calignano A, Raso GM, Meli R. Probiotics reduce the inflammatory response induced by a high-fat diet in the liver of young rats. J Nutr. 2009;139:905-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 186] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 306. | Ma X, Hua J, Li Z. Probiotics improve high fat diet-induced hepatic steatosis and insulin resistance by increasing hepatic NKT cells. J Hepatol. 2008;49:821-830. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 317] [Cited by in RCA: 306] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 307. | Mencarelli A, Cipriani S, Renga B, Bruno A, D'Amore C, Distrutti E, Fiorucci S. VSL#3 resets insulin signaling and protects against NASH and atherosclerosis in a model of genetic dyslipidemia and intestinal inflammation. PLoS One. 2012;7:e45425. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 90] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 308. | Jena PK, Sheng L, Li Y, Wan YY. Probiotics VSL#3 are effective in reversing non-alcoholic steatohepatitis in a mouse model. Hepatobiliary Surg Nutr. 2020;9:170-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 309. | Loguercio C, Federico A, Tuccillo C, Terracciano F, D'Auria MV, De Simone C, Del Vecchio Blanco C. Beneficial effects of a probiotic VSL#3 on parameters of liver dysfunction in chronic liver diseases. J Clin Gastroenterol. 2005;39:540-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 340] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 310. | Alisi A, Bedogni G, Baviera G, Giorgio V, Porro E, Paris C, Giammaria P, Reali L, Anania F, Nobili V. Randomised clinical trial: The beneficial effects of VSL#3 in obese children with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2014;39:1276-1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 343] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 311. | Solga SF, Buckley G, Clark JM, Horska A, Diehl AM. The effect of a probiotic on hepatic steatosis. J Clin Gastroenterol. 2008;42:1117-1119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 312. | Velayudham A, Dolganiuc A, Ellis M, Petrasek J, Kodys K, Mandrekar P, Szabo G. VSL#3 probiotic treatment attenuates fibrosis without changes in steatohepatitis in a diet-induced nonalcoholic steatohepatitis model in mice. Hepatology. 2009;49:989-997. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 210] [Cited by in RCA: 204] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 313. | Al-Muzafar HM, Amin KA. Probiotic mixture improves fatty liver disease by virtue of its action on lipid profiles, leptin, and inflammatory biomarkers. BMC Complement Altern Med. 2017;17:43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 78] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 314. | Aller R, De Luis DA, Izaola O, Conde R, Gonzalez Sagrado M, Primo D, De La Fuente B, Gonzalez J. Effect of a probiotic on liver aminotransferases in nonalcoholic fatty liver disease patients: a double blind randomized clinical trial. Eur Rev Med Pharmacol Sci. 2011;15:1090-1095. [PubMed] |
| 315. | Wong VW, Won GL, Chim AM, Chu WC, Yeung DK, Li KC, Chan HL. Treatment of nonalcoholic steatohepatitis with probiotics. A proof-of-concept study. Ann Hepatol. 2013;12:256-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 212] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 316. | Nabavi S, Rafraf M, Somi MH, Homayouni-Rad A, Asghari-Jafarabadi M. Effects of probiotic yogurt consumption on metabolic factors in individuals with nonalcoholic fatty liver disease. J Dairy Sci. 2014;97:7386-7393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 163] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 317. | Sepideh A, Karim P, Hossein A, Leila R, Hamdollah M, Mohammad E G, Mojtaba S, Mohammad S, Ghader G, Seyed Moayed A. Effects of Multistrain Probiotic Supplementation on Glycemic and Inflammatory Indices in Patients with Nonalcoholic Fatty Liver Disease: A Double-Blind Randomized Clinical Trial. J Am Coll Nutr. 2016;35:500-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 87] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 318. | Famouri F, Shariat Z, Hashemipour M, Keikha M, Kelishadi R. Effects of Probiotics on Nonalcoholic Fatty Liver Disease in Obese Children and Adolescents. J Pediatr Gastroenterol Nutr. 2017;64:413-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 189] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 319. | Ahn SB, Jun DW, Kang BK, Lim JH, Lim S, Chung MJ. Randomized, Double-blind, Placebo-controlled Study of a Multispecies Probiotic Mixture in Nonalcoholic Fatty Liver Disease. Sci Rep. 2019;9:5688. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 141] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 320. | Kobyliak N, Abenavoli L, Mykhalchyshyn G, Kononenko L, Boccuto L, Kyriienko D, Dynnyk O. A Multi-strain Probiotic Reduces the Fatty Liver Index, Cytokines and Aminotransferase levels in NAFLD Patients: Evidence from a Randomized Clinical Trial. J Gastrointestin Liver Dis. 2018;27:41-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 177] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 321. | Mohamad Nor MH, Ayob N, Mokhtar NM, Raja Ali RA, Tan GC, Wong Z, Shafiee NH, Wong YP, Mustangin M, Nawawi KNM. The Effect of Probiotics (MCP(®) BCMC(®) Strains) on Hepatic Steatosis, Small Intestinal Mucosal Immune Function, and Intestinal Barrier in Patients with Non-Alcoholic Fatty Liver Disease. Nutrients. 2021;13:3192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 109] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 322. | Ma YY, Li L, Yu CH, Shen Z, Chen LH, Li YM. Effects of probiotics on nonalcoholic fatty liver disease: a meta-analysis. World J Gastroenterol. 2013;19:6911-6918. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 280] [Cited by in RCA: 254] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 323. | Koutnikova H, Genser B, Monteiro-Sepulveda M, Faurie JM, Rizkalla S, Schrezenmeir J, Clément K. Impact of bacterial probiotics on obesity, diabetes and non-alcoholic fatty liver disease related variables: a systematic review and meta-analysis of randomised controlled trials. BMJ Open. 2019;9:e017995. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 176] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 324. | Sharpton SR, Maraj B, Harding-Theobald E, Vittinghoff E, Terrault NA. Gut microbiome-targeted therapies in nonalcoholic fatty liver disease: a systematic review, meta-analysis, and meta-regression. Am J Clin Nutr. 2019;110:139-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 144] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 325. | Yang R, Shang J, Zhou Y, Liu W, Tian Y, Shang H. Effects of probiotics on nonalcoholic fatty liver disease: a systematic review and meta-analysis. Expert Rev Gastroenterol Hepatol. 2021;15:1401-1409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 326. | Tarantino G, Finelli C. Systematic review on intervention with prebiotics/probiotics in patients with obesity-related nonalcoholic fatty liver disease. Future Microbiol. 2015;10:889-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 103] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 327. | Zhai Q, Feng S, Arjan N, Chen W. A next generation probiotic, Akkermansia muciniphila. Crit Rev Food Sci Nutr. 2019;59:3227-3236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 283] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 328. | Cani PD, de Vos WM. Next-Generation Beneficial Microbes: The Case of Akkermansia muciniphila. Front Microbiol. 2017;8:1765. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 507] [Cited by in RCA: 698] [Article Influence: 87.3] [Reference Citation Analysis (0)] |
| 329. | Dao MC, Everard A, Aron-Wisnewsky J, Sokolovska N, Prifti E, Verger EO, Kayser BD, Levenez F, Chilloux J, Hoyles L; MICRO-Obes Consortium, Dumas ME, Rizkalla SW, Doré J, Cani PD, Clément K. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut. 2016;65:426-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1101] [Cited by in RCA: 1316] [Article Influence: 146.2] [Reference Citation Analysis (0)] |
| 330. | Plovier H, Everard A, Druart C, Depommier C, Van Hul M, Geurts L, Chilloux J, Ottman N, Duparc T, Lichtenstein L, Myridakis A, Delzenne NM, Klievink J, Bhattacharjee A, van der Ark KC, Aalvink S, Martinez LO, Dumas ME, Maiter D, Loumaye A, Hermans MP, Thissen JP, Belzer C, de Vos WM, Cani PD. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat Med. 2017;23:107-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 967] [Cited by in RCA: 1461] [Article Influence: 162.3] [Reference Citation Analysis (0)] |
| 331. | Rao Y, Kuang Z, Li C, Guo S, Xu Y, Zhao D, Hu Y, Song B, Jiang Z, Ge Z, Liu X, Li C, Chen S, Ye J, Huang Z, Lu Y. Gut Akkermansia muciniphila ameliorates metabolic dysfunction-associated fatty liver disease by regulating the metabolism of L-aspartate via gut-liver axis. Gut Microbes. 2021;13:1-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 191] [Article Influence: 47.8] [Reference Citation Analysis (0)] |
| 332. | Kim S, Lee Y, Kim Y, Seo Y, Lee H, Ha J, Lee J, Choi Y, Oh H, Yoon Y. Akkermansia muciniphila Prevents Fatty Liver Disease, Decreases Serum Triglycerides, and Maintains Gut Homeostasis. Appl Environ Microbiol. 2020;86:e03004-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 135] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 333. | Depommier C, Everard A, Druart C, Plovier H, Van Hul M, Vieira-Silva S, Falony G, Raes J, Maiter D, Delzenne NM, de Barsy M, Loumaye A, Hermans MP, Thissen JP, de Vos WM, Cani PD. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: a proof-of-concept exploratory study. Nat Med. 2019;25:1096-1103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 774] [Cited by in RCA: 1429] [Article Influence: 238.2] [Reference Citation Analysis (0)] |
| 334. | Wu T, Zeng Z, Yu Y. Role of Probiotics in Gut Microbiome and Metabolome in Non-Alcoholic Fatty Liver Disease Mouse Model: A Comparative Study. Microorganisms. 2024;12:1020. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 335. | Xu J, Liang R, Zhang W, Tian K, Li J, Chen X, Yu T, Chen Q. Faecalibacterium prausnitzii-derived microbial anti-inflammatory molecule regulates intestinal integrity in diabetes mellitus mice via modulating tight junction protein expression. J Diabetes. 2020;12:224-236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 125] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 336. | Mohebali N, Ekat K, Kreikemeyer B, Breitrück A. Barrier Protection and Recovery Effects of Gut Commensal Bacteria on Differentiated Intestinal Epithelial Cells In Vitro. Nutrients. 2020;12:2251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 337. | Munukka E, Rintala A, Toivonen R, Nylund M, Yang B, Takanen A, Hänninen A, Vuopio J, Huovinen P, Jalkanen S, Pekkala S. Faecalibacterium prausnitzii treatment improves hepatic health and reduces adipose tissue inflammation in high-fat fed mice. ISME J. 2017;11:1667-1679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 181] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 338. | Endo H, Niioka M, Kobayashi N, Tanaka M, Watanabe T. Butyrate-producing probiotics reduce nonalcoholic fatty liver disease progression in rats: new insight into the probiotics for the gut-liver axis. PLoS One. 2013;8:e63388. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 222] [Cited by in RCA: 261] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 339. | Seo M, Inoue I, Tanaka M, Matsuda N, Nakano T, Awata T, Katayama S, Alpers DH, Komoda T. Clostridium butyricum MIYAIRI 588 improves high-fat diet-induced non-alcoholic fatty liver disease in rats. Dig Dis Sci. 2013;58:3534-3544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 340. | Ukena SN, Singh A, Dringenberg U, Engelhardt R, Seidler U, Hansen W, Bleich A, Bruder D, Franzke A, Rogler G, Suerbaum S, Buer J, Gunzer F, Westendorf AM. Probiotic Escherichia coli Nissle 1917 inhibits leaky gut by enhancing mucosal integrity. PLoS One. 2007;2:e1308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 310] [Cited by in RCA: 349] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 341. | Wei W, Wong CC, Jia Z, Liu W, Liu C, Ji F, Pan Y, Wang F, Wang G, Zhao L, Chu ESH, Zhang X, Sung JJY, Yu J. Parabacteroides distasonis uses dietary inulin to suppress NASH via its metabolite pentadecanoic acid. Nat Microbiol. 2023;8:1534-1548. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 99] [Article Influence: 49.5] [Reference Citation Analysis (0)] |
| 342. | Chooi YC, Zhang QA, Magkos F, Ng M, Michael N, Wu X, Volchanskaya VSB, Lai X, Wanjaya ER, Elejalde U, Goh CC, Yap CPL, Wong LH, Lim KJ, Velan SS, Yaligar J, Muthiah MD, Chong YS, Loo EXL, Eriksson JG; TANGO Study Group. Effect of an Asian-adapted Mediterranean diet and pentadecanoic acid on fatty liver disease: the TANGO randomized controlled trial. Am J Clin Nutr. 2024;119:788-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 343. | Guo M, Jiang X, Ouyang H, Zhang X, Zhang S, Wang P, Bi G, Wu T, Zhou W, Liang F, Yang X, Fan S, Fang J, Chen P, Bi H. Parabacteroides distasonis promotes liver regeneration by increasing β-hydroxybutyric acid (BHB) production and BHB-driven STAT3 signals. Acta Pharm Sin B. 2025;15:1430-1446. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 344. | Duan J, Li Q, Cheng Y, Zhu W, Liu H, Li F. Therapeutic potential of Parabacteroides distasonis in gastrointestinal and hepatic disease. MedComm (2020). 2024;5:e70017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 345. | Dang D, Li B, Ding M, Ross RP, Stanton C, Zhao J, Yang B, Chen W. Limosilactobacillus mucosae FZJTZ26M3 prevents NAFLD in mice through modulation of lipid metabolism and gut microbiota dysbiosis. Food Sci Hum Well. 2024;13:1589-1601. [DOI] [Full Text] |
| 346. | Li H, Wang XK, Tang M, Lei L, Li JR, Sun H, Jiang J, Dong B, Li HY, Jiang JD, Peng ZG. Bacteroides thetaiotaomicron ameliorates mouse hepatic steatosis through regulating gut microbial composition, gut-liver folate and unsaturated fatty acids metabolism. Gut Microbes. 2024;16:2304159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 34] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 347. | Teng Q, Lv H, Peng L, Ren Z, Chen J, Ma L, Wei H, Wan C. Lactiplantibacillus plantarum ZDY2013 Inhibits the Development of Non-Alcoholic Fatty Liver Disease by Regulating the Intestinal Microbiota and Modulating the PI3K/Akt Pathway. Nutrients. 2024;16:958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 348. | Lim CC, Ferguson LR, Tannock GW. Dietary fibres as "prebiotics": implications for colorectal cancer. Mol Nutr Food Res. 2005;49:609-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 105] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 349. | Pandey KR, Naik SR, Vakil BV. Probiotics, prebiotics and synbiotics- a review. J Food Sci Technol. 2015;52:7577-7587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 752] [Cited by in RCA: 645] [Article Influence: 64.5] [Reference Citation Analysis (0)] |
| 350. | Azcarate-Peril MA, Ritter AJ, Savaiano D, Monteagudo-Mera A, Anderson C, Magness ST, Klaenhammer TR. Impact of short-chain galactooligosaccharides on the gut microbiome of lactose-intolerant individuals. Proc Natl Acad Sci U S A. 2017;114:E367-E375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 144] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 351. | Maier TV, Lucio M, Lee LH, VerBerkmoes NC, Brislawn CJ, Bernhardt J, Lamendella R, McDermott JE, Bergeron N, Heinzmann SS, Morton JT, González A, Ackermann G, Knight R, Riedel K, Krauss RM, Schmitt-Kopplin P, Jansson JK. Impact of Dietary Resistant Starch on the Human Gut Microbiome, Metaproteome, and Metabolome. mBio. 2017;8:e01343-17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 164] [Cited by in RCA: 214] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 352. | Costabile A, Fava F, Röytiö H, Forssten SD, Olli K, Klievink J, Rowland IR, Ouwehand AC, Rastall RA, Gibson GR, Walton GE. Impact of polydextrose on the faecal microbiota: a double-blind, crossover, placebo-controlled feeding study in healthy human subjects. Br J Nutr. 2012;108:471-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 353. | So D, Whelan K, Rossi M, Morrison M, Holtmann G, Kelly JT, Shanahan ER, Staudacher HM, Campbell KL. Dietary fiber intervention on gut microbiota composition in healthy adults: a systematic review and meta-analysis. Am J Clin Nutr. 2018;107:965-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 442] [Article Influence: 63.1] [Reference Citation Analysis (0)] |
| 354. | Xie X, He Y, Li H, Yu D, Na L, Sun T, Zhang D, Shi X, Xia Y, Jiang T, Rong S, Yang S, Ma X, Xu G. Effects of prebiotics on immunologic indicators and intestinal microbiota structure in perioperative colorectal cancer patients. Nutrition. 2019;61:132-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 91] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 355. | Takai A, Kikuchi K, Ichimura M, Tsuneyama K, Moritoki Y, Matsumoto K, Tsunashima H, Onda T, Kuniyoshi N, Nariyama T, Ohyatsu S, Kubota J, Nagumo K, Sato S, Hara M, Miyakawa H. Fructo-oligosaccharides ameliorate steatohepatitis, visceral adiposity, and associated chronic inflammation via increased production of short-chain fatty acids in a mouse model of non-alcoholic steatohepatitis. BMC Gastroenterol. 2020;20:46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 356. | Huang X, Chen Q, Fan Y, Yang R, Gong G, Yan C, Song Y, Zhang B, Xi S, Huang Y, Xu H. Fructooligosaccharides attenuate non-alcoholic fatty liver disease by remodeling gut microbiota and association with lipid metabolism. Biomed Pharmacother. 2023;159:114300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 357. | Pachikian BD, Essaghir A, Demoulin JB, Catry E, Neyrinck AM, Dewulf EM, Sohet FM, Portois L, Clerbaux LA, Carpentier YA, Possemiers S, Bommer GT, Cani PD, Delzenne NM. Prebiotic approach alleviates hepatic steatosis: implication of fatty acid oxidative and cholesterol synthesis pathways. Mol Nutr Food Res. 2013;57:347-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 358. | Matsumoto K, Ichimura M, Tsuneyama K, Moritoki Y, Tsunashima H, Omagari K, Hara M, Yasuda I, Miyakawa H, Kikuchi K. Fructo-oligosaccharides and intestinal barrier function in a methionine-choline-deficient mouse model of nonalcoholic steatohepatitis. PLoS One. 2017;12:e0175406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 359. | Borges Haubert NJ, Marchini JS, Carvalho Cunha SF, Suen VM, Padovan GJ, Jordao AA Junior, Marchini Alves CM, Marchini JF, Vannucchi H. Choline and Fructooligosaccharide: Non-alcoholic Fatty Liver Disease, Cardiac Fat Deposition, and Oxidative Stress Markers. Nutr Metab Insights. 2015;8:1-6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 360. | Cani PD, Possemiers S, Van de Wiele T, Guiot Y, Everard A, Rottier O, Geurts L, Naslain D, Neyrinck A, Lambert DM, Muccioli GG, Delzenne NM. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut. 2009;58:1091-1103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1735] [Cited by in RCA: 1888] [Article Influence: 118.0] [Reference Citation Analysis (1)] |
| 361. | Bomhof MR, Parnell JA, Ramay HR, Crotty P, Rioux KP, Probert CS, Jayakumar S, Raman M, Reimer RA. Histological improvement of non-alcoholic steatohepatitis with a prebiotic: a pilot clinical trial. Eur J Nutr. 2019;58:1735-1745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 114] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 362. | Daubioul CA, Horsmans Y, Lambert P, Danse E, Delzenne NM. Effects of oligofructose on glucose and lipid metabolism in patients with nonalcoholic steatohepatitis: results of a pilot study. Eur J Clin Nutr. 2005;59:723-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 135] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 363. | Canfora EE, van der Beek CM, Hermes GDA, Goossens GH, Jocken JWE, Holst JJ, van Eijk HM, Venema K, Smidt H, Zoetendal EG, Dejong CHC, Lenaerts K, Blaak EE. Supplementation of Diet With Galacto-oligosaccharides Increases Bifidobacteria, but Not Insulin Sensitivity, in Obese Prediabetic Individuals. Gastroenterology. 2017;153:87-97.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 156] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 364. | Holscher HD, Faust KL, Czerkies LA, Litov R, Ziegler EE, Lessin H, Hatch T, Sun S, Tappenden KA. Effects of prebiotic-containing infant formula on gastrointestinal tolerance and fecal microbiota in a randomized controlled trial. JPEN J Parenter Enteral Nutr. 2012;36:95S-105S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 365. | Vulevic J, Juric A, Tzortzis G, Gibson GR. A mixture of trans-galactooligosaccharides reduces markers of metabolic syndrome and modulates the fecal microbiota and immune function of overweight adults. J Nutr. 2013;143:324-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 240] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 366. | Letexier D, Diraison F, Beylot M. Addition of inulin to a moderately high-carbohydrate diet reduces hepatic lipogenesis and plasma triacylglycerol concentrations in humans. Am J Clin Nutr. 2003;77:559-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 140] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 367. | Fehlbaum S, Prudence K, Kieboom J, Heerikhuisen M, van den Broek T, Schuren FHJ, Steinert RE, Raederstorff D. In Vitro Fermentation of Selected Prebiotics and Their Effects on the Composition and Activity of the Adult Gut Microbiota. Int J Mol Sci. 2018;19:3097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 125] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 368. | Weitkunat K, Schumann S, Petzke KJ, Blaut M, Loh G, Klaus S. Effects of dietary inulin on bacterial growth, short-chain fatty acid production and hepatic lipid metabolism in gnotobiotic mice. J Nutr Biochem. 2015;26:929-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 143] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 369. | Aoki R, Onuki M, Hattori K, Ito M, Yamada T, Kamikado K, Kim YG, Nakamoto N, Kimura I, Clarke JM, Kanai T, Hase K. Commensal microbe-derived acetate suppresses NAFLD/NASH development via hepatic FFAR2 signalling in mice. Microbiome. 2021;9:188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 99] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 370. | Catry E, Bindels LB, Tailleux A, Lestavel S, Neyrinck AM, Goossens JF, Lobysheva I, Plovier H, Essaghir A, Demoulin JB, Bouzin C, Pachikian BD, Cani PD, Staels B, Dessy C, Delzenne NM. Targeting the gut microbiota with inulin-type fructans: preclinical demonstration of a novel approach in the management of endothelial dysfunction. Gut. 2018;67:271-283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 141] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 371. | Chambers ES, Viardot A, Psichas A, Morrison DJ, Murphy KG, Zac-Varghese SE, MacDougall K, Preston T, Tedford C, Finlayson GS, Blundell JE, Bell JD, Thomas EL, Mt-Isa S, Ashby D, Gibson GR, Kolida S, Dhillo WS, Bloom SR, Morley W, Clegg S, Frost G. Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut. 2015;64:1744-1754. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 940] [Cited by in RCA: 956] [Article Influence: 95.6] [Reference Citation Analysis (0)] |
| 372. | Chong CYL, Orr D, Plank LD, Vatanen T, O'Sullivan JM, Murphy R. Randomised Double-Blind Placebo-Controlled Trial of Inulin with Metronidazole in Non-Alcoholic Fatty Liver Disease (NAFLD). Nutrients. 2020;12:937. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 373. | Dewulf EM, Cani PD, Claus SP, Fuentes S, Puylaert PG, Neyrinck AM, Bindels LB, de Vos WM, Gibson GR, Thissen JP, Delzenne NM. Insight into the prebiotic concept: lessons from an exploratory, double blind intervention study with inulin-type fructans in obese women. Gut. 2013;62:1112-1121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 531] [Cited by in RCA: 585] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 374. | Reshef N, Gophna U, Reshef L, Konikoff F, Gabay G, Zornitzki T, Knobler H, Maor Y. Prebiotic Treatment in Patients with Nonalcoholic Fatty Liver Disease (NAFLD)-A Randomized Pilot Trial. Nutrients. 2024;16:1571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 375. | Salminen S, Salminen E. Lactulose, lactic acid bacteria, intestinal microecology and mucosal protection. Scand J Gastroenterol Suppl. 1997;222:45-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 97] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 376. | Bouhnik Y, Attar A, Joly FA, Riottot M, Dyard F, Flourié B. Lactulose ingestion increases faecal bifidobacterial counts: a randomised double-blind study in healthy humans. Eur J Clin Nutr. 2004;58:462-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 104] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 377. | Fan JG, Xu ZJ, Wang GL. Effect of lactulose on establishment of a rat non-alcoholic steatohepatitis model. World J Gastroenterol. 2005;11:5053-5056. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 39] [Cited by in RCA: 40] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 378. | Neyrinck AM, Possemiers S, Verstraete W, De Backer F, Cani PD, Delzenne NM. Dietary modulation of clostridial cluster XIVa gut bacteria (Roseburia spp.) by chitin-glucan fiber improves host metabolic alterations induced by high-fat diet in mice. J Nutr Biochem. 2012;23:51-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 203] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 379. | Singh DP, Khare P, Zhu J, Kondepudi KK, Singh J, Baboota RK, Boparai RK, Khardori R, Chopra K, Bishnoi M. A novel cobiotic-based preventive approach against high-fat diet-induced adiposity, nonalcoholic fatty liver and gut derangement in mice. Int J Obes (Lond). 2016;40:487-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 380. | Yang Z, Su H, Chen X, Ni Z, Tao H, Jiang Y, Peng L. Isomalt attenuates hepatic steatosis in rats via modulating gut microbiota and its metabolic function. J Funct Foods. 2024;112:105963. [DOI] [Full Text] |
| 381. | Ni Y, Qian L, Siliceo SL, Long X, Nychas E, Liu Y, Ismaiah MJ, Leung H, Zhang L, Gao Q, Wu Q, Zhang Y, Jia X, Liu S, Yuan R, Zhou L, Wang X, Li Q, Zhao Y, El-Nezami H, Xu A, Xu G, Li H, Panagiotou G, Jia W. Resistant starch decreases intrahepatic triglycerides in patients with NAFLD via gut microbiome alterations. Cell Metab. 2023;35:1530-1547.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 72] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 382. | Zhang W, Yu L, Yang Q, Zhang J, Wang W, Hu X, Li J, Zheng G. Smilax China L. polysaccharide prevents HFD induced-NAFLD by regulating hepatic fat metabolism and gut microbiota. Phytomedicine. 2024;127:155478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 383. | Huang Y, Chen H, Chen J, Wu Q, Zhang W, Li D, Lu Y, Chen Y. Yellow tea polysaccharides protect against non-alcoholic fatty liver disease via regulation of gut microbiota and bile acid metabolism in mice. Phytomedicine. 2024;133:155919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 384. | Hao P, Yang X, Yin W, Wang X, Ling Y, Zhu M, Yu Y, Chen S, Yuan Y, Quan X, Xu Z, Zhang J, Zhao W, Zhang Y, Song C, Xu Q, Qin S, Wu Y, Shu X, Wei K. A study on the treatment effects of Crataegus pinnatifida polysaccharide on non-alcoholic fatty liver in mice by modulating gut microbiota. Front Vet Sci. 2024;11:1383801. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 385. | Wu Y, Yin W, Hao P, Chen Y, Yu L, Yu X, Wu Y, Li X, Wang W, Zhou H, Yuan Y, Quan X, Yu Y, Hu B, Chen S, Zhou Z, Sun W. Polysaccharide from Panax japonicus C.A. Mey prevents non-alcoholic fatty liver disease development based on regulating liver metabolism and gut microbiota in mice. Int J Biol Macromol. 2024;260:129430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 386. | Zuo G, Chen M, Zuo Y, Liu F, Yang Y, Li J, Zhou X, Li M, Huang JA, Liu Z, Lin Y. Tea Polyphenol Epigallocatechin Gallate Protects Against Nonalcoholic Fatty Liver Disease and Associated Endotoxemia in Rats via Modulating Gut Microbiota Dysbiosis and Alleviating Intestinal Barrier Dysfunction and Related Inflammation. J Agric Food Chem. 2024;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 387. | Mei L, Tang Y, Li M, Yang P, Liu Z, Yuan J, Zheng P. Co-Administration of Cholesterol-Lowering Probiotics and Anthraquinone from Cassia obtusifolia L. Ameliorate Non-Alcoholic Fatty Liver. PLoS One. 2015;10:e0138078. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 388. | Yao F, Jia R, Huang H, Yu Y, Mei L, Bai L, Ding Y, Zheng P. Effect of Lactobacillus paracasei N1115 and fructooligosaccharides in nonalcoholic fatty liver disease. Arch Med Sci. 2019;15:1336-1344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 389. | Manzhalii E, Virchenko O, Falalyeyeva T, Beregova T, Stremmel W. Treatment efficacy of a probiotic preparation for non-alcoholic steatohepatitis: A pilot trial. J Dig Dis. 2017;18:698-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 83] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 390. | Ferolla SM, Couto CA, Costa-Silva L, Armiliato GN, Pereira CA, Martins FS, Ferrari Mde L, Vilela EG, Torres HO, Cunha AS, Ferrari TC. Beneficial Effect of Synbiotic Supplementation on Hepatic Steatosis and Anthropometric Parameters, But Not on Gut Permeability in a Population with Nonalcoholic Steatohepatitis. Nutrients. 2016;8:397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 85] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 391. | Bakhshimoghaddam F, Shateri K, Sina M, Hashemian M, Alizadeh M. Daily Consumption of Synbiotic Yogurt Decreases Liver Steatosis in Patients with Nonalcoholic Fatty Liver Disease: A Randomized Controlled Clinical Trial. J Nutr. 2018;148:1276-1284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 103] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 392. | Raso GM, Simeoli R, Iacono A, Santoro A, Amero P, Paciello O, Russo R, D'Agostino G, Di Costanzo M, Canani RB, Calignano A, Meli R. Effects of a Lactobacillus paracasei B21060 based synbiotic on steatosis, insulin signaling and toll-like receptor expression in rats fed a high-fat diet. J Nutr Biochem. 2014;25:81-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 393. | Juárez-Fernández M, Porras D, Petrov P, Román-Sagüillo S, García-Mediavilla MV, Soluyanova P, Martínez-Flórez S, González-Gallego J, Nistal E, Jover R, Sánchez-Campos S. The Synbiotic Combination of Akkermansia muciniphila and Quercetin Ameliorates Early Obesity and NAFLD through Gut Microbiota Reshaping and Bile Acid Metabolism Modulation. Antioxidants (Basel). 2021;10:2001. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 71] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 394. | Malaguarnera M, Vacante M, Antic T, Giordano M, Chisari G, Acquaviva R, Mastrojeni S, Malaguarnera G, Mistretta A, Li Volti G, Galvano F. Bifidobacterium longum with fructo-oligosaccharides in patients with non alcoholic steatohepatitis. Dig Dis Sci. 2012;57:545-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 333] [Article Influence: 25.6] [Reference Citation Analysis (1)] |
| 395. | Shavakhi A, Minakari M, Firouzian H, Assali R, Hekmatdoost A, Ferns G. Effect of a Probiotic and Metformin on Liver Aminotransferases in Non-alcoholic Steatohepatitis: A Double Blind Randomized Clinical Trial. Int J Prev Med. 2013;4:531-537. [PubMed] |
| 396. | Eslamparast T, Poustchi H, Zamani F, Sharafkhah M, Malekzadeh R, Hekmatdoost A. Synbiotic supplementation in nonalcoholic fatty liver disease: a randomized, double-blind, placebo-controlled pilot study. Am J Clin Nutr. 2014;99:535-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 287] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 397. | Mofidi F, Poustchi H, Yari Z, Nourinayyer B, Merat S, Sharafkhah M, Malekzadeh R, Hekmatdoost A. Synbiotic supplementation in lean patients with non-alcoholic fatty liver disease: a pilot, randomised, double-blind, placebo-controlled, clinical trial. Br J Nutr. 2017;117:662-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 170] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 398. | Asgharian A, Askari G, Esmailzade A, Feizi A, Mohammadi V. The Effect of Symbiotic Supplementation on Liver Enzymes, C-reactive Protein and Ultrasound Findings in Patients with Non-alcoholic Fatty Liver Disease: A Clinical Trial. Int J Prev Med. 2016;7:59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 70] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 399. | Zhang C, Fang T, Shi L, Wang Y, Deng X, Wang J, Zhou Y. The synbiotic combination of probiotics and inulin improves NAFLD though modulating gut microbiota. J Nutr Biochem. 2024;125:109546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 20] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 400. | Ralli T, Ahmad S, Saifi Z, Alhalmi A, Aeri V, Aqil M, Kohli K. Exploring the therapeutic potential of silymarin-based herbal remedy (prebiotic) and probiotic blend in a mouse model of NAFLD: Insights into gut microbiota modulation and liver health. Heliyon. 2024;10:e33505. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 401. | Gao X, Zhu Z, Bao Y, Li Y, Zhu W, He X, Ge X, Huang W, Wang H, Wei W, Du J, Chen L, Li H, Sheng L. Chrysanthemum morifolium Ramat extract and probiotics combination ameliorates metabolic disorders through regulating gut microbiota and PPARα subcellular localization. Chin Med. 2024;19:76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Reference Citation Analysis (0)] |
| 402. | Liu L, Li P, Liu Y, Zhang Y. Efficacy of Probiotics and Synbiotics in Patients with Nonalcoholic Fatty Liver Disease: A Meta-Analysis. Dig Dis Sci. 2019;64:3402-3412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 79] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 403. | Cai J, Dong J, Chen D, Ye H. The effect of synbiotics in patients with NAFLD: a systematic review and meta-analysis. Therap Adv Gastroenterol. 2023;16:17562848231174299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 404. | Fadhilah J, Zariyah H, Pramono A, Purnomo HD, Syauqy A, Afifah DN, Fadhillah FS, Purwanti RA. Effect of synbiotic supplementation on liver function, metabolic profile and gut microbiota in non-alcoholic fatty liver disease (NAFLD): A meta-analysis of randomized controlled trials. Clin Nutr Open Sci. 2024;56:128-151. [DOI] [Full Text] |
| 405. | Scorletti E, Afolabi PR, Miles EA, Smith DE, Almehmadi A, Alshathry A, Childs CE, Del Fabbro S, Bilson J, Moyses HE, Clough GF, Sethi JK, Patel J, Wright M, Breen DJ, Peebles C, Darekar A, Aspinall R, Fowell AJ, Dowman JK, Nobili V, Targher G, Delzenne NM, Bindels LB, Calder PC, Byrne CD. Synbiotics Alter Fecal Microbiomes, But Not Liver Fat or Fibrosis, in a Randomized Trial of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2020;158:1597-1610.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 156] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 406. | Smits LP, Bouter KE, de Vos WM, Borody TJ, Nieuwdorp M. Therapeutic potential of fecal microbiota transplantation. Gastroenterology. 2013;145:946-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 473] [Article Influence: 39.4] [Reference Citation Analysis (1)] |
| 407. | van Nood E, Vrieze A, Nieuwdorp M, Fuentes S, Zoetendal EG, de Vos WM, Visser CE, Kuijper EJ, Bartelsman JF, Tijssen JG, Speelman P, Dijkgraaf MG, Keller JJ. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368:407-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2582] [Cited by in RCA: 2678] [Article Influence: 223.2] [Reference Citation Analysis (0)] |
| 408. | Chiu CC, Ching YH, Li YP, Liu JY, Huang YT, Huang YW, Yang SS, Huang WC, Chuang HL. Nonalcoholic Fatty Liver Disease Is Exacerbated in High-Fat Diet-Fed Gnotobiotic Mice by Colonization with the Gut Microbiota from Patients with Nonalcoholic Steatohepatitis. Nutrients. 2017;9:1220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 123] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 409. | Mouries J, Brescia P, Silvestri A, Spadoni I, Sorribas M, Wiest R, Mileti E, Galbiati M, Invernizzi P, Adorini L, Penna G, Rescigno M. Microbiota-driven gut vascular barrier disruption is a prerequisite for non-alcoholic steatohepatitis development. J Hepatol. 2019;71:1216-1228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 230] [Cited by in RCA: 459] [Article Influence: 76.5] [Reference Citation Analysis (0)] |
| 410. | Zhou D, Pan Q, Shen F, Cao HX, Ding WJ, Chen YW, Fan JG. Total fecal microbiota transplantation alleviates high-fat diet-induced steatohepatitis in mice via beneficial regulation of gut microbiota. Sci Rep. 2017;7:1529. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 305] [Cited by in RCA: 295] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 411. | Vrieze A, Van Nood E, Holleman F, Salojärvi J, Kootte RS, Bartelsman JF, Dallinga-Thie GM, Ackermans MT, Serlie MJ, Oozeer R, Derrien M, Druesne A, Van Hylckama Vlieg JE, Bloks VW, Groen AK, Heilig HG, Zoetendal EG, Stroes ES, de Vos WM, Hoekstra JB, Nieuwdorp M. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143:913-6.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1881] [Cited by in RCA: 2016] [Article Influence: 155.1] [Reference Citation Analysis (0)] |
| 412. | Kootte RS, Levin E, Salojärvi J, Smits LP, Hartstra AV, Udayappan SD, Hermes G, Bouter KE, Koopen AM, Holst JJ, Knop FK, Blaak EE, Zhao J, Smidt H, Harms AC, Hankemeijer T, Bergman JJGHM, Romijn HA, Schaap FG, Olde Damink SWM, Ackermans MT, Dallinga-Thie GM, Zoetendal E, de Vos WM, Serlie MJ, Stroes ESG, Groen AK, Nieuwdorp M. Improvement of Insulin Sensitivity after Lean Donor Feces in Metabolic Syndrome Is Driven by Baseline Intestinal Microbiota Composition. Cell Metab. 2017;26:611-619.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 531] [Cited by in RCA: 681] [Article Influence: 85.1] [Reference Citation Analysis (0)] |
| 413. | Allegretti JR, Kassam Z, Mullish BH, Chiang A, Carrellas M, Hurtado J, Marchesi JR, McDonald JAK, Pechlivanis A, Barker GF, Miguéns Blanco J, Garcia-Perez I, Wong WF, Gerardin Y, Silverstein M, Kennedy K, Thompson C. Effects of Fecal Microbiota Transplantation With Oral Capsules in Obese Patients. Clin Gastroenterol Hepatol. 2020;18:855-863.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 206] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 414. | de Groot P, Scheithauer T, Bakker GJ, Prodan A, Levin E, Khan MT, Herrema H, Ackermans M, Serlie MJM, de Brauw M, Levels JHM, Sales A, Gerdes VE, Ståhlman M, Schimmel AWM, Dallinga-Thie G, Bergman JJ, Holleman F, Hoekstra JBL, Groen A, Bäckhed F, Nieuwdorp M. Donor metabolic characteristics drive effects of faecal microbiota transplantation on recipient insulin sensitivity, energy expenditure and intestinal transit time. Gut. 2020;69:502-512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 222] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 415. | Philips CA, Pande A, Shasthry SM, Jamwal KD, Khillan V, Chandel SS, Kumar G, Sharma MK, Maiwall R, Jindal A, Choudhary A, Hussain MS, Sharma S, Sarin SK. Healthy Donor Fecal Microbiota Transplantation in Steroid-Ineligible Severe Alcoholic Hepatitis: A Pilot Study. Clin Gastroenterol Hepatol. 2017;15:600-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 262] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 416. | Smits LP, Kootte RS, Levin E, Prodan A, Fuentes S, Zoetendal EG, Wang Z, Levison BS, Cleophas MCP, Kemper EM, Dallinga-Thie GM, Groen AK, Joosten LAB, Netea MG, Stroes ESG, de Vos WM, Hazen SL, Nieuwdorp M. Effect of Vegan Fecal Microbiota Transplantation on Carnitine- and Choline-Derived Trimethylamine-N-Oxide Production and Vascular Inflammation in Patients With Metabolic Syndrome. J Am Heart Assoc. 2018;7:e008342. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 173] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 417. | Craven L, Rahman A, Nair Parvathy S, Beaton M, Silverman J, Qumosani K, Hramiak I, Hegele R, Joy T, Meddings J, Urquhart B, Harvie R, McKenzie C, Summers K, Reid G, Burton JP, Silverman M. Allogenic Fecal Microbiota Transplantation in Patients With Nonalcoholic Fatty Liver Disease Improves Abnormal Small Intestinal Permeability: A Randomized Control Trial. Am J Gastroenterol. 2020;115:1055-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 228] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 418. | Stols-Gonçalves D, Mak AL, Madsen MS, van der Vossen EWJ, Bruinstroop E, Henneman P, Mol F, Scheithauer TPM, Smits L, Witjes J, Meijnikman AS, Verheij J, Nieuwdorp M, Holleboom AG, Levin E. Faecal Microbiota transplantation affects liver DNA methylation in Non-alcoholic fatty liver disease: a multi-omics approach. Gut Microbes. 2023;15:2223330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 38] [Reference Citation Analysis (0)] |
| 419. | Dailey FE, Turse EP, Daglilar E, Tahan V. The dirty aspects of fecal microbiota transplantation: a review of its adverse effects and complications. Curr Opin Pharmacol. 2019;49:29-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 77] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 420. | Baxter M, Colville A. Adverse events in faecal microbiota transplant: a review of the literature. J Hosp Infect. 2016;92:117-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 131] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 421. | Wang ZK, Yang YS, Chen Y, Yuan J, Sun G, Peng LH. Intestinal microbiota pathogenesis and fecal microbiota transplantation for inflammatory bowel disease. World J Gastroenterol. 2014;20:14805-14820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 76] [Cited by in RCA: 91] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 422. | DeFilipp Z, Bloom PP, Torres Soto M, Mansour MK, Sater MRA, Huntley MH, Turbett S, Chung RT, Chen YB, Hohmann EL. Drug-Resistant E. coli Bacteremia Transmitted by Fecal Microbiota Transplant. N Engl J Med. 2019;381:2043-2050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 575] [Cited by in RCA: 833] [Article Influence: 138.8] [Reference Citation Analysis (0)] |
| 423. | Sekirov I, Russell SL, Antunes LC, Finlay BB. Gut microbiota in health and disease. Physiol Rev. 2010;90:859-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2501] [Cited by in RCA: 2749] [Article Influence: 183.3] [Reference Citation Analysis (1)] |
| 424. | Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau AL, Griffin NW, Lombard V, Henrissat B, Bain JR, Muehlbauer MJ, Ilkayeva O, Semenkovich CF, Funai K, Hayashi DK, Lyle BJ, Martini MC, Ursell LK, Clemente JC, Van Treuren W, Walters WA, Knight R, Newgard CB, Heath AC, Gordon JI. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science. 2013;341:1241214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2415] [Cited by in RCA: 2725] [Article Influence: 227.1] [Reference Citation Analysis (0)] |
| 425. | Alang N, Kelly CR. Weight gain after fecal microbiota transplantation. Open Forum Infect Dis. 2015;2:ofv004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 270] [Cited by in RCA: 302] [Article Influence: 30.2] [Reference Citation Analysis (1)] |
| 426. | Gregory JC, Buffa JA, Org E, Wang Z, Levison BS, Zhu W, Wagner MA, Bennett BJ, Li L, DiDonato JA, Lusis AJ, Hazen SL. Transmission of atherosclerosis susceptibility with gut microbial transplantation. J Biol Chem. 2015;290:5647-5660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 378] [Article Influence: 34.4] [Reference Citation Analysis (0)] |