Published online Jun 27, 2025. doi: 10.4254/wjh.v17.i6.105578
Revised: April 12, 2025
Accepted: May 16, 2025
Published online: June 27, 2025
Processing time: 131 Days and 17.3 Hours
Early transjugular intrahepatic portosystemic shunts (TIPS) is a therapeutic option for acute variceal bleeding (AVB), offering a low risk of rebleeding. However, the long-term outcomes of early TIPS remain unclear.
To evaluate the long-term outcomes for early TIPS compared with standard treatment in patients with cirrhosis and AVB.
We retrospectively analyzed the clinical data of patients with AVB who under
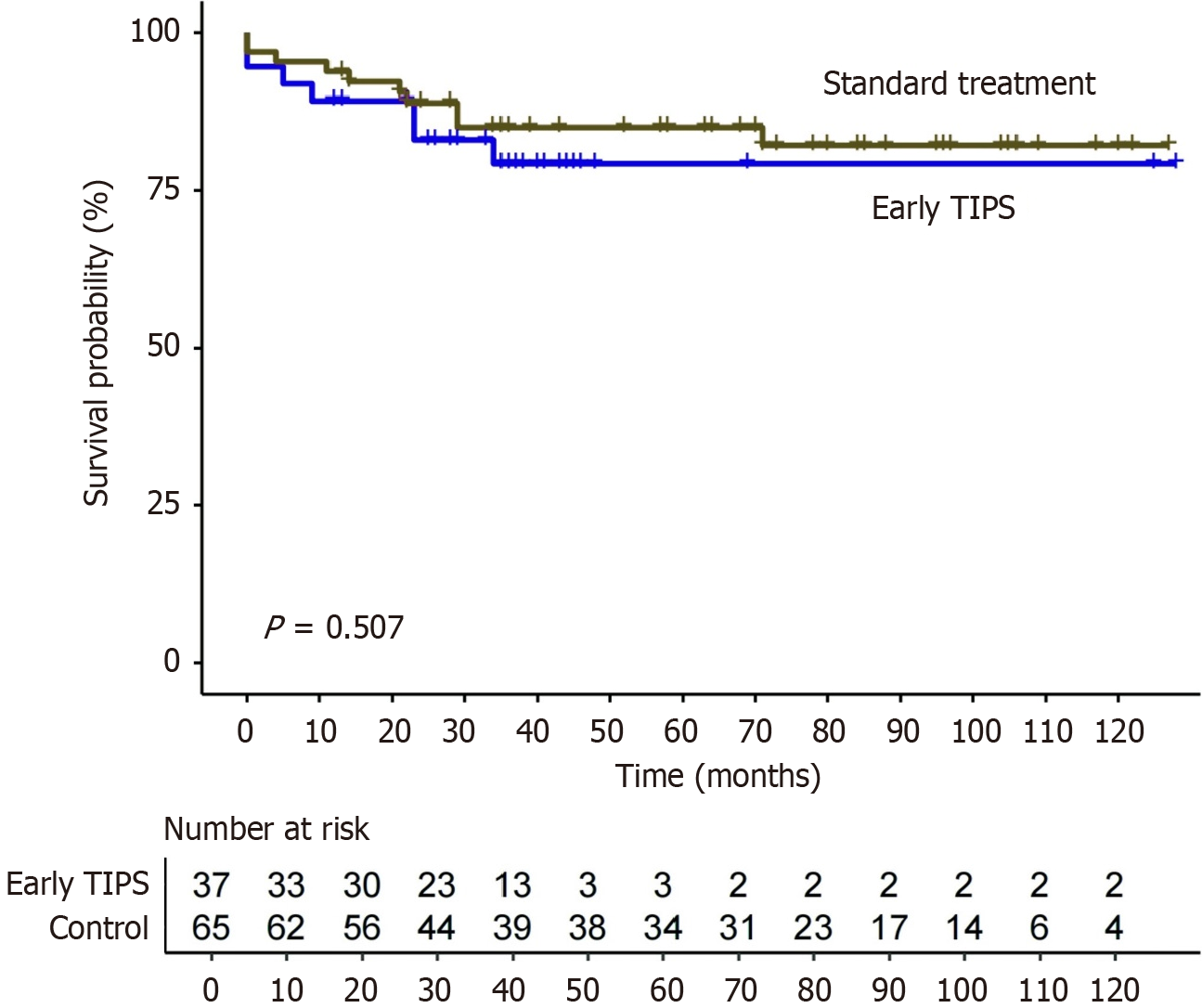
A total of 37 patients with AVB underwent early TIPS, while 65 patients received standard treatment. Compared with the standard treatment group, the rates of uncontrolled bleeding or rebleeding in the early TIPS group were significantly lower (10.8% vs 50.8%, P < 0.001). Over a median follow-up of 46 months, no statistically significant differences were observed in terms of OS (P = 0.507). The presence of comorbidities was identified as an independent predictor of OS (adjusted hazard ratio = 3.81; 95% confidence interval: 1.16-12.46). Notably, new or worsening ascites occurred less frequently in the early TIPS group (13.5% vs 38.5%, P = 0.008). There was no significant difference in the rate of overt hepatic encephalopathy between the two groups (45.9% vs 36.9%, P = 0.372).
While early TIPS is not associated with a long-term survival benefit compared with standard treatment for AVB, it is associated with reduced risks of rebleeding and ascites.
Core Tip: The long-term outcomes of early transjugular intrahepatic portosystemic shunts (TIPS) for acute variceal bleeding (AVB) remain unclear. This retrospective cohort study analyzed the clinical data of patients with AVB who underwent early TIPS or standard treatment. We found that early TIPS was associated with a lower risk of rebleeding and ascites compared with standard treatment for AVB; however, long-term survival did not improve. Moreover, we identified the presence of comorbidities was associated with survival for AVB.
- Citation: Tang X, Liang JB, Wang C, Ma JL, Jia RR, Wang YG, Shi M. Long-term outcomes of early transjugular intrahepatic portosystemic shunts in patients with acute variceal bleeding and cirrhosis. World J Hepatol 2025; 17(6): 105578
- URL: https://www.wjgnet.com/1948-5182/full/v17/i6/105578.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i6.105578
Acute variceal bleeding (AVB) is one of the most severe complications of portal hypertension and is a major cause of mortality in patients with cirrhosis, which presents a significant clinical challenge[1]. Once bleeding occurs, the 6-week mortality exceeds 15%[2]. Advances in understanding of the pathophysiology of AVB have significantly improved its management and prognosis. According to international guidelines, the standard treatment includes careful volume resuscitation, vasoactive drugs, prophylactic antibiotics, and endoscopic band ligation (EBL)[3-5]. However, despite these treatments, hemostasis is not achieved in approximately 10%-15% of patients, thus requiring repeated endoscopic interventions and blood transfusions[6]. Transjugular intrahepatic portosystemic shunts (TIPS) has proven effective in controlling bleeding in these cases[7,8]. However, the 6-week mortality rate remains high, probably due to the deterioration of liver disease and associated organ dysfunction[9].
Early use of TIPS is often considered for high-risk patients, including those with Child-Pugh class C scores < 14 or class B scores > 7 with active bleeding at initial endoscopy. Early TIPS is generally conducted within 72 hours of hospitalization after successful endoscopic treatment. Recent studies have demonstrated its efficacy and safety in the treatment of AVB, with hemostasis success rates of 92%-100% and rebleeding rates of 0% to 12%[10-12]. Lv et al[12] revealed that early TIPS significantly improved short-term transplantation-free survival compared with standard treatment in patients with AVB. However, there is no evidence regarding the long-term outcomes of early TIPS compared to standard treatment for AVB. Therefore, our study aimed to describe and compare the long-term outcomes associated with early TIPS in patients with cirrhosis and AVB.
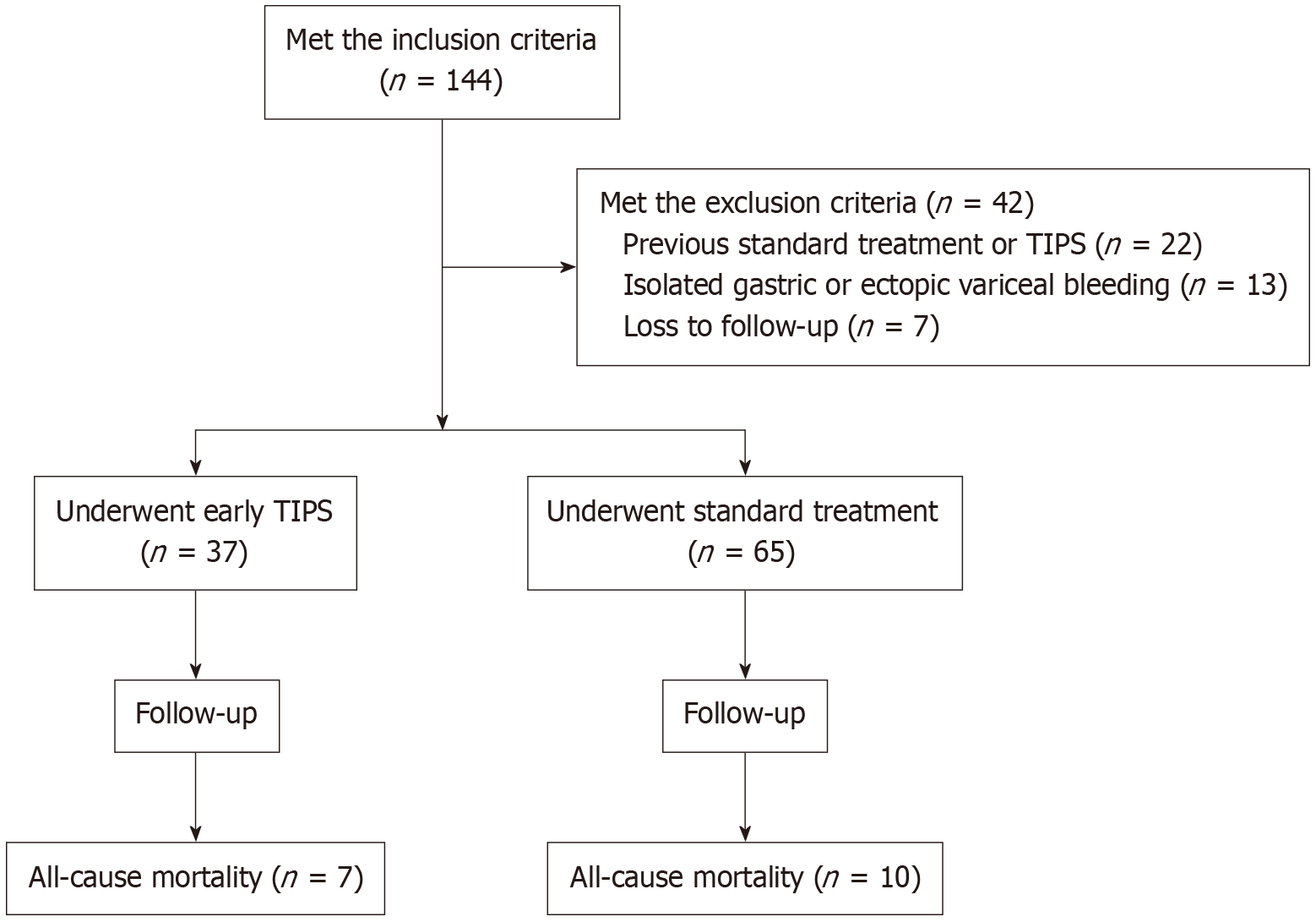
This retrospective cohort study enrolled consecutive patients between January 2014 and December 2023 at Shanghai Tongren Hospital, a tertiary referral center in China. Following were the inclusion criteria: (1) Diagnosis of liver cirrhosis by clinical, laboratory, ultrasound or biopsy findings; (2) Endoscopically proven AVB on the basis of Baveno VII Consensus definitions[3]; and (3) Classified as Child-Pugh class B (> 7 points) or C (< 14 points). Following were the exclusion criteria: (1) Isolated gastric varices or ectopic variceal bleeding; (2) Uncontrolled bleeding at initial endoscopy; (3) Hepatocellular carcinoma exceeding the Milan criteria for liver transplantation; (4) Previous portosystemic shunt, TIPS, or pharmacotherapy combined with endoscopic treatment; (5) Complete portal vein thrombosis; (6) Heart failure; (7) Creatinine level > 3 mg/dL (265 μmol/L); and (8) Unable to complete follow-up or ascertain mortality and the time of death. Patients were categorized into two groups based on whether they underwent early TIPS or received standard treatment. Figure 1 illustrated the patients’ enrollment with flowchart. This study was conducted basing on the Declaration of Helsinki, and the protocol was approved by the Ethics Review Board at Tongren Hospital, Shanghai Jiao Tong University School of Medicine, approval No. K2024-067-01. Prior to any procedure, written informed consent was obtained from all patients.
For eligible patients, early TIPS and standard treatment were offered as treatment options. The potential benefits and risks of each treatment were thoroughly notified. Because the study was retrospective, the treatment option was ultimately decided based on patient care policies and the clinician’s assessment, with consent from the patients. Date on demographic characteristics (age, sex, comorbidities, and etiology of liver disease), laboratory test results (white blood cell count, platelet count, hemoglobin level, international normalized ratio, bilirubin, albumin, and creatinine levels), clinical features (ascites and hepatic encephalopathy at hospitalization) and procedure-related variables (initial endoscopic treatment, initial pharmacological therapy, and duration of hospital stay) were collected from hospital records for retrospective analysis.
All clinical practices were conducted according to the current international consensus[3-5]. Briefly, patients received vasoactive drugs (octreotide, somatostatin, or terlipressin) and prophylactic antibiotics at hospitalization. During the initial endoscopy, the patients underwent EBL within 12 hours after hospitalization. Sclerotherapy was performed in cases where EBL was technically difficult or unfeasible. Vasoactive drugs were used until the TIPS procedure. Early TIPS was performed within 72 hours after the initial endoscopy, and prophylactic antibiotics were administered for 5-7 days. All TIPS were performed under local anesthesia, with of the expanded polytetrafluoroethylene-covered stent initially dilated to 8 mm. If the portal pressure gradient was maintained above 12 mmHg, the stent was further expanded to 10 mm. The patency of TIPS was examined via Doppler ultrasonography. If TIPS stenosis or occlusion was suspected based on the abnormal imaging findings or recurrent portal hypertension, TIPS venography was performed to confirm the diagnosis. In these cases, TIPS revision was performed, which included angioplasty or the placement of an additional expanded polytetrafluoroethylene-covered stent. For patients receiving standard treatment, vasoactive drugs were used for 5 days, and prophylactic antibiotics for 5-7 days. On day 6, treatment with a nonselective beta-blocker was initiated, and EBL was scheduled every 2-4 weeks until varices were eradicated. Additional EBL was recommended for patients with recurrent varices. Uncontrolled bleeding and rebleeding were managed with rescue TIPS.
The patients were hospitalized following early TIPS or standard treatment and monitored for bleeding, chest pain, or signs of infection. Laboratory evaluations were performed to assess for bleeding or hepatic dysfunction before discharge. All patients were followed up at 6 weeks, 3 months and then 6 months, and every 6 months thereafter until death, liver transplantation or December 2024. Physical examination, laboratory evaluation, and liver ultrasound were performed during each surveillance. Clinical data collected during the follow-up period were used for analysis.
The primary outcome measure was overall survival (OS). The secondary outcomes included: (1) Uncontrolled bleeding (≤ 5 days); (2) Early rebleeding (> 5 days to 6 weeks); (3) Late rebleeding (> 6 weeks); (4) The development of overt hepatic encephalopathy (OHE); (5) New or worsening ascites; and (6) Other complications related to portal hypertension. The procedure characteristics were also documented and compared.
The definition of uncontrolled bleeding or rebleeding was based on the criteria from the Baveno VII consensus and European Society of Gastrointestinal Endoscopy guidelines[3,13]. Worsening ascites was defined as persistent ascites requiring large-volume paracentesis or an increase in diuretic dose without complete resolution of ascites[14]. The diagnosis and grading of OHE were based on the West-Haven criteria[15]. Variceal eradication was defined as the absence of varices that could be suctioned into the banding device or their complete disappearance[11].
Categorical variables are expressed as the number of cases and percentages and statistical comparisons were performed using either the χ2 test or Fisher's exact test. The Shapiro-Wilk test was employed to assess the normality of continuous variables. Variables are reported as the means ± SD and were compared using Student’s t-test if followed a normal distribution, or as medians with interquartile range (IQR) and compared using the Mann-Whitney U test if not normally distributed. Estimation and comparison of the survival rates were conducted utilizing the Kaplan-Meier method and log-rank test. Univariate and multivariate Cox regression analyses were employed to identify predictors of survival and to estimate the adjusted hazard ratio (HR) of treatments. In the univariate analysis, variables with P value < 0.20 were included in the multivariate analysis. Statistical significance was set at 0.05 (two-sided). R software (version 4.1.3) and SPSS (version 25.0) were utilized for date analysis.
Out of the 144 patients who fulfilled the inclusion criteria, a total of 102 patients were ultimately enrolled. Among them, 37 patients with advanced cirrhosis underwent early TIPS, and 65 patients underwent standard treatment (Figure 1). There were no cases of technical failure or major complications associated with the TIPS procedure. The clinical and demographic characteristics of the patients are presented in Table 1. The distribution of gender was significantly different between the two groups (P = 0.003). The median model for end-stage liver disease (MELD) score was significantly higher in the standard treatment group than in the early TIPS group (8.0 vs 6.0, P = 0.027). Additionally, the median creatinine levels of standard treatment were significantly higher than those of early TIPS (0.9 vs 0.7 mg/dL, P < 0.001). Other baseline characteristics were comparable between the two treatment groups.
| Characteristics | Early TIPS (n = 37) | Standard treatment (n = 65) | P value |
| Age (year), mean ± SD | 64.5 ± 10.3 | 61.3 ± 10.3 | 0.138 |
| Sex | 0.003 | ||
| Male | 14 (37.8) | 44 (67.7) | |
| Female | 23 (62.2) | 21 (32.3) | |
| Cause of cirrhosis | 0.474 | ||
| HBV | 17 (45.9) | 29 (44.6) | |
| HCV | 3 (8.1) | 6 (9.2) | |
| Alcohol | 1 (2.7) | 8 (12.3) | |
| Autoimmune hepatitis | 4 (10.8) | 8 (12.3) | |
| Other | 12 (32.4) | 14 (21.5) | |
| Comorbidities | |||
| Hypertension | 10 (27.0) | 14 (21.5) | 0.53 |
| Diabetes | 15 (40.5) | 17 (26.2) | 0.132 |
| Cardiovascular disease | 2 (5.4) | 3 (4.6) | 1 |
| Cerebrovascular disease | 3 (8.1) | 3 (4.6) | 0.777 |
| Ascites | 22 (59.5) | 33 (50.8) | 0.397 |
| Child-Pugh score, median (IQR) | 8.0 (8.0-9.0) | 8.0 (8.0-9.0) | 0.275 |
| Child-Pugh class | 0.637 | ||
| Child-Pugh B | 31 (83.8) | 52 (80.0) | |
| Child-Pugh C | 6 (16.2) | 13 (20.0) | |
| MELD score, median (IQR) | 6.0 (1.5-8.5) | 8.0 (3.0-11.0) | 0.027 |
| White blood cell (× 109/L), median (IQR) | 4.0 (2.8-8.0) | 5.9 (3.1-10.9) | 0.127 |
| Hemoglobin (g/L), median (IQR) | 81.0 (70.5-101.5) | 78.0 (66.0-94.0) | 0.364 |
| Platelet count (× 109/L), median (IQR) | 76.0 (49.5-105.5) | 69.0 (45.5-98.5) | 0.335 |
| Albumin (g/L) | 28.7 (6.3) | 28.1 (6.2) | 0.63 |
| Creatinine (mg/dL), median (IQR) | 0.7 (0.6-0.9) | 0.9 (0.7-1.2) | < 0.001 |
| Bilirubin (mg/dL), median (IQR) | 1.2 (0.9-1.9) | 1.5 (1.0-2.1) | 0.106 |
| International normalized ratio, median (IQR) | 1.2 (1.1-1.5) | 1.3 (1.2-1.4) | 0.215 |
| Initial endoscopic treatment | 0.317 | ||
| Endoscopic band ligation | 32 (86.5) | 51 (78.5) | |
| Endoscopic sclerotherapy | 5 (13.5) | 19 (21.5) | |
| Initial vasoactive-drug therapy | 0.613 | ||
| Terlipressin | 11 (29.7) | 14 (21.5) | |
| Somatostatin | 24 (64.9) | 48 (73.8) | |
| Octreotide | 2 (5.4) | 3 (4.6) | |
The outcomes of the procedures are summarized in Table 2. Overall, 4 patients (10.8%) in the early TIPS group and 33 patients (50.8%) in the standard treatment group experienced uncontrolled bleeding or rebleeding (P < 0.001). Specifically, the rates of uncontrolled bleeding (0% vs 13.8%, P = 0.045) and late rebleeding (10.8% vs 32.3%, P = 0.015) were lower in the early TIPS group than in the standard treatment group. However, no significant differences in the rate of early rebleeding were found between the two treatment groups (0% vs 4.6%, P = 0.473). The majority of rebleeding (24 in 37, 64.9%) was due to variceal bleeding.
| Characteristics | Early TIPS (n = 37) | Standard treatment (n = 65) | P value |
| Failure to control bleeding or rebleeding | 4 (10.8) | 33 (50.8) | < 0.001 |
| Uncontrolled bleeding | 0 (0) | 9 (13.8) | 0.045 |
| Early rebleeding | 0 (0) | 3 (4.6) | 0.473 |
| Late rebleeding | 4 (10.8) | 21 (32.3) | 0.015 |
| Cause of bleeding1 | 0.144 | ||
| Varices | 1 (25.0) | 23 (69.7) | |
| Portal hypertensive gastropathy | 1 (25.0) | 4 (12.1) | |
| Gastric ulcer | 0 (0) | 2 (6.1) | |
| Mallory Weiss | 1 (25.0) | 2 (6.1) | |
| Gastrointestinal telangiectasia | 1 (25.0) | 2 (6.1) | |
| Death | 7 (18.9) | 10 (15.4) | 0.645 |
| Cause of death2 | 0.201 | ||
| Liver failure | 3 (42.9) | 3 (30.0) | |
| Multiorgan failure | 2 (28.6) | 1 (10.0) | |
| Hepatocellular carcinoma | 2 (28.6) | 0 (0) | |
| Sepsis/pneumonia | 0 (0) | 3 (30.0) | |
| Gastrointestinal bleeding | 0 (0) | 2 (20.0) | |
| Unrelated to liver disease | 0 (0) | 1 (10.0) | |
| Length of stay | 9.0 (7.0-13.5) | 14.0 (9.0-18.5) | 0.001 |
| Length of stay in ICU, median (IQR) | 1.0 (1.0-3.0) | 1.0 (1.0-3.0) | 0.777 |
| Hospitalization times during follow-up, median (IQR) | 2.0 (1.0-4.0) | 4.0 (2.0-6.0) | 0.003 |
The median length of stay was significantly shorter in the early TIPS group than in the standard treatment group (9.0 vs 14.0 days, P = 0.001). Compared with the standard treatment group, the median hospitalization times during follow-up were significantly fewer in the early TIPS group (2.0 vs 4.0, P = 0.003). However, the median length of stay in the intensive care unit was comparable between the two groups (median, 1.0 vs 1.0 days; P = 0.777).
The overall median follow-up duration was 46 months (IQR: 35-88). The median follow-up duration was 38 months (IQR: 29-44) in the early TIPS group and 71 months (IQR: 35-104) in the standard treatment group. During the follow-up period, 7 patients (18.9%) in the early TIPS group died, as compared with 10 patients (15.4%) in the standard treatment group
The univariate and multivariate analysis results for OS are shown in Table 3. According to the univariate analysis, the crude HR for survival associated with comorbidities was 4.73 [95% confidence interval (CI): 1.54-14.56; P = 0.007]. In the multivariate analysis, the HR for survival associated with comorbidities was 3.81 (95%CI: 1.16-12.46; P = 0.027) after adjusting for patient age, Child-Pugh score, and MELD score.
| Characteristics | Univariate analysis | Multivariate analysis | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Treatment, early TIPS vs standard treatment | 0.72 (0.27-1.92) | 0.511 | - | - |
| Age (years) | 1.05 (1.00-1.11) | 0.049 | 1.04 (0.98-1.10) | 0.22 |
| Sex, male vs female | 0.85 (0.33-2.20) | 0.734 | - | - |
| Comorbidities, yes vs no | 4.73 (1.54-14.56) | 0.007 | 3.81 (1.16-12.46) | 0.027 |
| Bilirubin | 1.30 (0.84-2.01) | 0.237 | - | - |
| Creatinine | 2.24 (0.72-7.01) | 0.165 | - | - |
| Child-Pugh score | 1.56 (1.09-2.21) | 0.014 | 1.40 (0.90-2.15) | 0.132 |
| MELD score | 1.09 (1.01-1.18) | 0.026 | 1.06 (0.98-1.16) | 0.168 |
The complications associated with treatment are shown in Table 4. There were no significant differences between the groups in the terms of rates of hepatocellular carcinoma, spontaneous bacterial peritonitis, hepatopulmonary syndrome, hepatorenal syndrome or other serious adverse events. Compared with the standard treatment group, the rate of new or worsening ascites was significantly lower in the early TIPS group (13.5% vs 38.5%, P = 0.008). The rate of OHE was comparable between the two treatment groups (early TIPS, 45.9% vs standard treatment, 36.9%; P = 0.372). In the early TIPS group, among 17 patients who experienced OHE, the median number of episodes of OHE was 5, compared with a median of 5 episodes among 24 patients in the standard treatment group.
| Characteristics | Early TIPS (n = 37) | Standard treatment (n = 65) | P value |
| Complications of portal hypertension | |||
| OHE | 17 (45.9) | 24 (36.9) | 0.372 |
| New or worsening ascites | 5 (13.5) | 25 (38.5) | 0.008 |
| Hepatocellular carcinoma | 5 (13.5) | 6 (9.2) | 0.735 |
| Spontaneous bacterial peritonitis | 3 (8.1) | 6 (9.2) | 1 |
| Hepatopulmonary syndrome | 0 (0) | 1 (1.5) | 1 |
| Hepatorenal syndrome | 2 (5.4) | 0 (0) | 0.129 |
| Other serious adverse events | |||
| Acute-on-chronic liver failure | 4 (10.8) | 4 (6.2) | 0.647 |
| Portal vein thrombosis | 4 (10.8) | 4 (6.2) | 0.647 |
| Sepsis | 2 (5.4) | 0 (0) | 0.129 |
| Pneumonia | 1 (2.7) | 1 (1.5) | 1 |
The management of AVB poses technical challenges in the context of standard treatment, and may not adequately control bleeding or rebleeding. Recent studies have shown that early TIPS may offer benefits, including elevated hemostasis rates and a decreased risk of rebleeding[10-12]. In the present study, OS was comparable between early TIPS and standard treatment (P = 0.507). However, patients in the early TIPS group experienced significantly shorter hospital stays and fewer times of hospitalization during follow-up. Moreover, the presence of comorbidities (adjusted HR = 3.81; 95%CI: 1.16-12.46) was identified as an independent predictor of survival for AVB. Furthermore, the rate of new or worsening ascites was significantly lower in the early TIPS group than in the standard treatment group (13.5% vs 38.5%, P = 0.008). However, no significant differences were observed in the incidence of OHE (P = 0.372).
One of the major concerns of early TIPS is its uncertain survival benefit when compared with standard treatment. Previous studies reported that only 13% of patients who met the criteria underwent early TIPS, mainly due to a lack of confidence on its impact on survival outcomes[16,17]. Dunne et al[18] reported that early TIPS had no survival effect in high-risk patients with AVB during a 1-year follow-up. However, Lv et al[12] reported that early TIPS improved survival in high-risk patients with AVB during a 2-year follow-up. In contrast, our study demonstrated that OS was comparable between the two treatment groups during a 46-month follow-up.
OHE is a common complication in patients with cirrhosis, particularly following TIPS[19,20]. Similar to the findings of previous studies, almost half of the patients (45.9%) in the early TIPS group developed OHE in our study[11,12]. For patients undergoing early TIPS, OHE poses a significant challenge in terms of morbidity and quality of life However, recent studies have shown that post-TIPS OHE does not negatively impact survival, which may help mitigate concerns regarding the risk of OHE after TIPS[21].
One of the most critical concerns of patients of liver cirrhosis and portal hypertension is intractable variceal bleeding. The annual incidence of AVB ranges from 5% to 15%, with 6-week mortality exceeding 15%[2]. In this study, the early TIPS group had a lower rate of uncontrolled bleeding (0% vs 13.8%, P = 0.045) and late rebleeding (10.8% vs 32.3%, P = 0.015) than standard treatment group. Likewise, other studies demonstrated a similar rate of uncontrolled bleeding or rebleeding (0% to 24.1%) following early TIPS[10-12]. In addition, compared to the standard treatment group, the rates of new or worsening ascites were lower in the early TIPS group (13.5% vs 38.5%, P = 0.008). Thus, although early TIPS may not impact long-term survival in patients with AVB, it may offer distinct benefits in managing bleeding or refractory ascites.
Recent studies have shown improvements in symptoms such as weight loss, anorexia, and fatigue in TIPS-treated patients[22]. These improvements may be attributed to changes in enterohepatic circulation, enhanced absorption, and acceleration of systemic circulation. In this study, significantly fewer hospitalization times during follow-up and shorter hospital stays were observed in the early TIPS group, suggesting that early TIPS may enhance quality of life. Therefore, focusing not only on survival but also on quality of life may more accurately reflect the long-term prognosis of patients treated with TIPS.
The MELD score was lower in the early TIPS group than in the standard treatment group, suggesting a theoretically better prognosis. However, the long-term survival did not improve. This may indicate a two-way effect of TIPS on liver function. In the present study, the median Albumin-bilirubin score significantly improved in 3 months after early TIPS
It is important to acknowledge several limitations in our current study. First, the study was non-randomized and retrospective, which may be affected by patient selection and recall biases. Moreover, some details of the procedure or patient management may have changed. Second, the total number of patients and events during follow-up was small, and the distribution of baseline variables (such as gender and MELD score) were imbalanced; therefore, the statistical power for drawing robust conclusions might be insufficient. Third, although we performed multivariate Cox regression to control for known confounders, there may still be other unmeasured or unknown confounders that could influence the results. Finally, despite adhering to strict inclusion and exclusion criteria, operator selection bias regarding treatment modality may have impacted patient outcomes and prognosis.
In conclusion, early TIPS was not associated with a long-term survival benefit for AVB in patients with cirrhosis. However, early TIPS significantly improved control of variceal bleeding and developing ascites without increasing the occurrence of OHE. For high-risk patients with AVB, early TIPS could be considered a viable treatment option for those who are unable to control bleeding or are at increased risk for refractory ascites.
| 1. | Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet. 2014;383:1749-1761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1139] [Cited by in RCA: 1311] [Article Influence: 119.2] [Reference Citation Analysis (0)] |
| 2. | Carbonell N, Pauwels A, Serfaty L, Fourdan O, Lévy VG, Poupon R. Improved survival after variceal bleeding in patients with cirrhosis over the past two decades. Hepatology. 2004;40:652-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 568] [Cited by in RCA: 527] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 3. | de Franchis R, Bosch J, Garcia-Tsao G, Reiberger T, Ripoll C; Baveno VII Faculty. Baveno VII - Renewing consensus in portal hypertension. J Hepatol. 2022;76:959-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1537] [Cited by in RCA: 1477] [Article Influence: 492.3] [Reference Citation Analysis (2)] |
| 4. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1777] [Cited by in RCA: 1812] [Article Influence: 258.9] [Reference Citation Analysis (2)] |
| 5. | Boike JR, Thornburg BG, Asrani SK, Fallon MB, Fortune BE, Izzy MJ, Verna EC, Abraldes JG, Allegretti AS, Bajaj JS, Biggins SW, Darcy MD, Farr MA, Farsad K, Garcia-Tsao G, Hall SA, Jadlowiec CC, Krowka MJ, Laberge J, Lee EW, Mulligan DC, Nadim MK, Northup PG, Salem R, Shatzel JJ, Shaw CJ, Simonetto DA, Susman J, Kolli KP, VanWagner LB; Advancing Liver Therapeutic Approaches (ALTA) Consortium. North American Practice-Based Recommendations for Transjugular Intrahepatic Portosystemic Shunts in Portal Hypertension. Clin Gastroenterol Hepatol. 2022;20:1636-1662.e36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 135] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 6. | D'Amico G, De Franchis R; Cooperative Study Group. Upper digestive bleeding in cirrhosis. Post-therapeutic outcome and prognostic indicators. Hepatology. 2003;38:599-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 585] [Cited by in RCA: 597] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 7. | Vangeli M, Patch D, Burroughs AK. Salvage tips for uncontrolled variceal bleeding. J Hepatol. 2002;37:703-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 66] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 8. | Azoulay D, Castaing D, Majno P, Saliba F, Ichaï P, Smail A, Delvart V, Danaoui M, Samuel D, Bismuth H. Salvage transjugular intrahepatic portosystemic shunt for uncontrolled variceal bleeding in patients with decompensated cirrhosis. J Hepatol. 2001;35:590-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 134] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 9. | Bosch J. Salvage transjugular intrahepatic portosystemic shunt: is it really life-saving? J Hepatol. 2001;35:658-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Monescillo A, Martínez-Lagares F, Ruiz-del-Arbol L, Sierra A, Guevara C, Jiménez E, Marrero JM, Buceta E, Sánchez J, Castellot A, Peñate M, Cruz A, Peña E. Influence of portal hypertension and its early decompression by TIPS placement on the outcome of variceal bleeding. Hepatology. 2004;40:793-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 328] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 11. | García-Pagán JC, Caca K, Bureau C, Laleman W, Appenrodt B, Luca A, Abraldes JG, Nevens F, Vinel JP, Mössner J, Bosch J; Early TIPS (Transjugular Intrahepatic Portosystemic Shunt) Cooperative Study Group. Early use of TIPS in patients with cirrhosis and variceal bleeding. N Engl J Med. 2010;362:2370-2379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 826] [Cited by in RCA: 842] [Article Influence: 56.1] [Reference Citation Analysis (0)] |
| 12. | Lv Y, Yang Z, Liu L, Li K, He C, Wang Z, Bai W, Guo W, Yu T, Yuan X, Zhang H, Xie H, Yao L, Wang J, Li T, Wang Q, Chen H, Wang E, Xia D, Luo B, Li X, Yuan J, Han N, Zhu Y, Niu J, Cai H, Xia J, Yin Z, Wu K, Fan D, Han G; AVB-TIPS Study Group. Early TIPS with covered stents versus standard treatment for acute variceal bleeding in patients with advanced cirrhosis: a randomised controlled trial. Lancet Gastroenterol Hepatol. 2019;4:587-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 164] [Article Influence: 27.3] [Reference Citation Analysis (1)] |
| 13. | Gralnek IM, Camus Duboc M, Garcia-Pagan JC, Fuccio L, Karstensen JG, Hucl T, Jovanovic I, Awadie H, Hernandez-Gea V, Tantau M, Ebigbo A, Ibrahim M, Vlachogiannakos J, Burgmans MC, Rosasco R, Triantafyllou K. Endoscopic diagnosis and management of esophagogastric variceal hemorrhage: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2022;54:1094-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 124] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 14. | Aithal GP, Palaniyappan N, China L, Härmälä S, Macken L, Ryan JM, Wilkes EA, Moore K, Leithead JA, Hayes PC, O'Brien AJ, Verma S. Guidelines on the management of ascites in cirrhosis. Gut. 2021;70:9-29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 240] [Article Influence: 60.0] [Reference Citation Analysis (0)] |
| 15. | Vilstrup H, Amodio P, Bajaj J, Cordoba J, Ferenci P, Mullen KD, Weissenborn K, Wong P. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60:715-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1583] [Cited by in RCA: 1407] [Article Influence: 127.9] [Reference Citation Analysis (1)] |
| 16. | Thabut D, Pauwels A, Carbonell N, Remy AJ, Nahon P, Causse X, Cervoni JP, Cadranel JF, Archambeaud I, Bramli S, Ehrhard F, Ah-Soune P, Rostain F, Pariente A, Vergniol J, Dupuychaffray JP, Pelletier AL, Skinazi F, Guillygomarc'h A, Vitte RL, Henrion J, Combet S, Rudler M, Bureau C; des Hépato-Gastroentérologues des Hôpitaux Généraux (ANGH); Club Francophone pour l'Etude de l'Hypertension Portale (CFETHTP); CHOC Study Group collaborators:. Cirrhotic patients with portal hypertension-related bleeding and an indication for early-TIPS: a large multicentre audit with real-life results. J Hepatol. 2017;68:73-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 89] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 17. | Hernández-Gea V, Procopet B, Giráldez Á, Amitrano L, Villanueva C, Thabut D, Ibañez-Samaniego L, Silva-Junior G, Martinez J, Genescà J, Bureau C, Trebicka J, Llop E, Laleman W, Palazon JM, Castellote J, Rodrigues S, Gluud LL, Noronha Ferreira C, Barcelo R, Cañete N, Rodríguez M, Ferlitsch A, Mundi JL, Gronbaek H, Hernández-Guerra M, Sassatelli R, Dell'Era A, Senzolo M, Abraldes JG, Romero-Gómez M, Zipprich A, Casas M, Masnou H, Primignani M, Krag A, Nevens F, Calleja JL, Jansen C, Robic MA, Conejo I, Catalina MV, Albillos A, Rudler M, Alvarado E, Guardascione MA, Tantau M, Bosch J, Torres F, Garcia-Pagán JC; International Variceal Bleeding Observational Study Group and Baveno Cooperation. Preemptive-TIPS Improves Outcome in High-Risk Variceal Bleeding: An Observational Study. Hepatology. 2019;69:282-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 96] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 18. | Dunne PDJ, Sinha R, Stanley AJ, Lachlan N, Ireland H, Shams A, Kasthuri R, Forrest EH, Hayes PC. Randomised clinical trial: standard of care versus early-transjugular intrahepatic porto-systemic shunt (TIPSS) in patients with cirrhosis and oesophageal variceal bleeding. Aliment Pharmacol Ther. 2020;52:98-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 19. | Somberg KA, Riegler JL, LaBerge JM, Doherty-Simor MM, Bachetti P, Roberts JP, Lake JR. Hepatic encephalopathy after transjugular intrahepatic portosystemic shunts: incidence and risk factors. Am J Gastroenterol. 1995;90:549-555. [PubMed] |
| 20. | Riggio O, Angeloni S, Salvatori FM, De Santis A, Cerini F, Farcomeni A, Attili AF, Merli M. Incidence, natural history, and risk factors of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt with polytetrafluoroethylene-covered stent grafts. Am J Gastroenterol. 2008;103:2738-2746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 205] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 21. | Nardelli S, Riggio O, Marra F, Gioia S, Saltini D, Bellafante D, Adotti V, Guasconi T, Ridola L, Rosi M, Caporali C, Fanelli F, Roccarina D, Bianchini M, Indulti F, Spagnoli A, Merli M, Vizzutti F, Schepis F. Episodic overt hepatic encephalopathy after transjugular intrahepatic portosystemic shunt does not increase mortality in patients with cirrhosis. J Hepatol. 2024;80:596-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 22. | Dasarathy J, Alkhouri N, Dasarathy S. Changes in body composition after transjugular intrahepatic portosystemic stent in cirrhosis: a critical review of literature. Liver Int. 2011;31:1250-1258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 23. | Sauerbruch T, Mengel M, Dollinger M, Zipprich A, Rössle M, Panther E, Wiest R, Caca K, Hoffmeister A, Lutz H, Schoo R, Lorenzen H, Trebicka J, Appenrodt B, Schepke M, Fimmers R; German Study Group for Prophylaxis of Variceal Rebleeding. Prevention of Rebleeding From Esophageal Varices in Patients With Cirrhosis Receiving Small-Diameter Stents Versus Hemodynamically Controlled Medical Therapy. Gastroenterology. 2015;149:660-8.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 181] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 24. | Holster IL, Tjwa ET, Moelker A, Wils A, Hansen BE, Vermeijden JR, Scholten P, van Hoek B, Nicolai JJ, Kuipers EJ, Pattynama PM, van Buuren HR. Covered transjugular intrahepatic portosystemic shunt versus endoscopic therapy + β-blocker for prevention of variceal rebleeding. Hepatology. 2016;63:581-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 164] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 25. | Korsic S, Stabuc B, Skok P, Popovic P. TIPS vs. endoscopic treatment for prevention of recurrent variceal bleeding: a long-term follow-up of 126 patients. Radiol Oncol. 2021;55:164-171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Zhang F, Zhuge Y, Zou X, Zhang M, Peng C, Li Z, Wang T. Different scoring systems in predicting survival in Chinese patients with liver cirrhosis undergoing transjugular intrahepatic portosystemic shunt. Eur J Gastroenterol Hepatol. 2014;26:853-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |