Published online May 27, 2025. doi: 10.4254/wjh.v17.i5.104724
Revised: March 13, 2025
Accepted: April 15, 2025
Published online: May 27, 2025
Processing time: 148 Days and 15 Hours
Acute kidney injury (AKI) is present in 30%-40% of hospitalized patients with cirrhosis. Its incidence is higher in patients with severe alcoholic hepatitis, spontaneous bacterial peritonitis, and acute-on-chronic-liver failure (ACLF). Kidney injury is an important landmark event in the natural history of cirrhosis as it is associated with higher mortality. Overwhelming systemic vasodilation, cardiac dysfunction, hypoperfusion, endotoxemia, and direct nephrotoxicity predispose patients with cirrhosis to kidney injury. Infection is present in 25% of patients with decompensated cirrhosis and 35%-40% of patients with ACLF. Advanced cirrhosis with portal hypertension leads to a sluggish portal flow, leading to increased gut congestion, altered gut permeability and bacterial translocations. They drive infection and endotoxemia in such patients. Pathogen-associated molecular patterns activate inflammatory cascades, which leads to further deterioration in hemodynamics and reduced glomerular filtration rate. Infections and pro-inflammatory cytokines like interleukin 6 (IL-6), IL-1, and tumor necrosis factor alpha may directly cause kidney parenchymal injury. The combined effect of dysfunctional albumin and systemic and splanchnic vasodi
Core Tip: The development of acute kidney injury (AKI) changes the trajectory of the natural history of patients with decompensated cirrhosis or acute-on-chronic-liver failure. With the discovery of newer biomarkers and a better under
- Citation: Malakar S, Rungta S, Samanta A, Shamsul Hoda U, Mishra P, Pande G, Roy A, Giri S, Rai P, Mohindra S, Ghoshal UC. Understanding acute kidney injury in cirrhosis: Current perspective. World J Hepatol 2025; 17(5): 104724
- URL: https://www.wjgnet.com/1948-5182/full/v17/i5/104724.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i5.104724
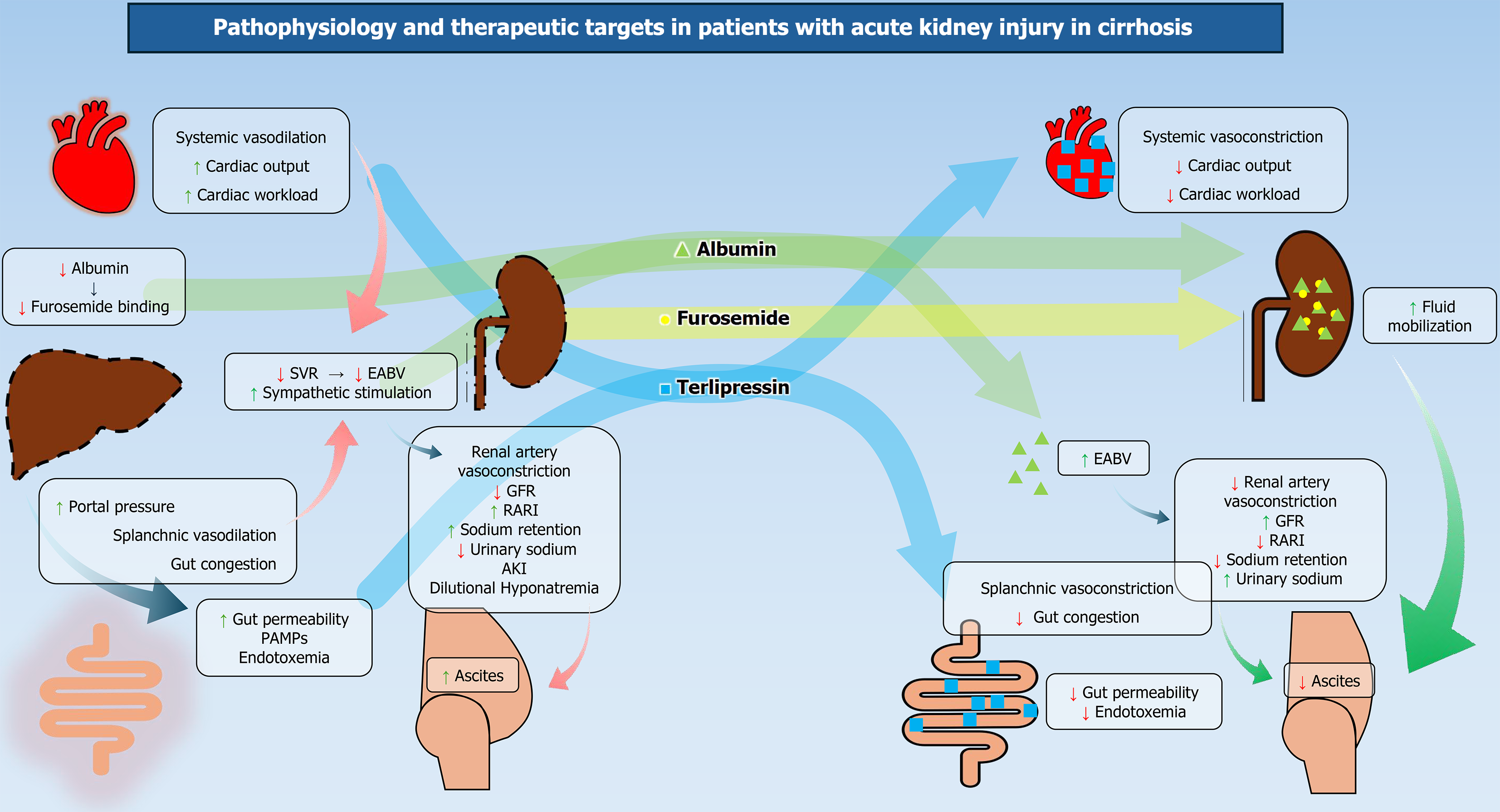
Acute kidney injury (AKI) is associated with higher morbidity and mortality in patients with cirrhosis and imparts a significant healthcare burden[1-3]. The mechanism of AKI in cirrhosis is multifactorial[4,5]. Portal hypertension is associated with cirrhosis-associated immune dysfunction and increased intestinal translocation of bacteria, leading to overwhelming systemic vasodilation[5,6]. The inflammatory cycle perpetuates and enters a vicious cycle, which further deteriorates the hemodynamics, leading to hepatorenal syndrome (HRS)[7-10]. Vasoconstriction and volume expansion are effective in improving arterial blood volume and systemic hemodynamics, thereby improving renal blood flow (Figure 1). By contrast, pre-renal AKI responds only to volume expansion with albumin[11-13]. Hence, the cornerstone of treatment is combining volume expanders, splanchnic and systemic vasoconstrictors[13-15]. Terlipressin also helps to overcome the hemodynamic deficit, improve AKI, and mobilize ascites in patients with cirrhosis[16,17]. Differentiating prerenal or HRS-AKI from acute tubular necrosis (ATN) is important as ATN carries a poor prognosis, and vasoconstrictors can be detrimental in patients with ATN and cirrhosis[13]. Thus, it is important to predict, detect, classify, and treat AKI in its early state, as progressive renal dysfunction is invariably associated with higher mortality in patients with decompensated cirrhosis and acute-on-chronic liver failure (ACLF)[3].
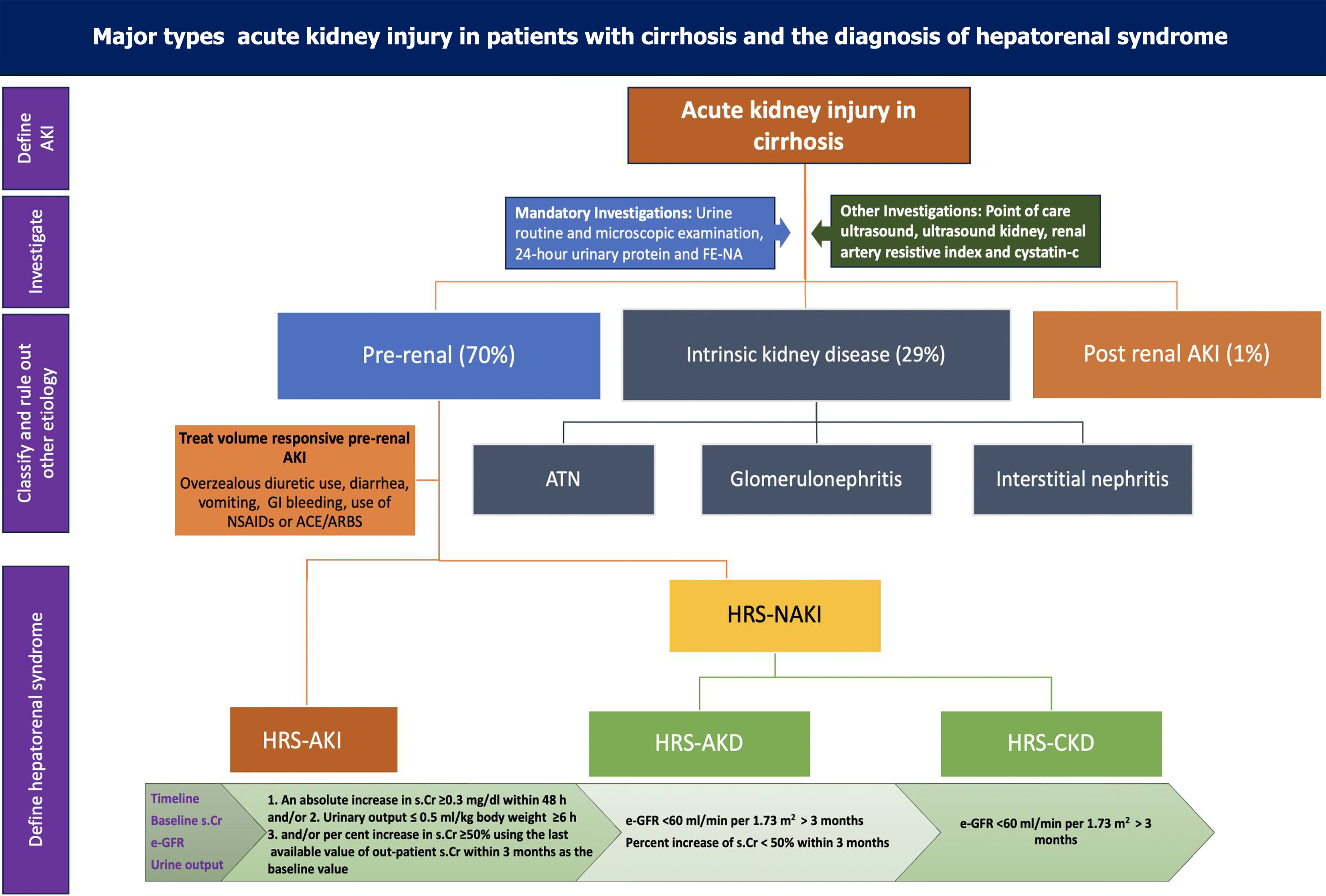
AKI in cirrhosis is diagnosed when there is an absolute increase of serum creatinine by 0.3 mg/dL or an increase of baseline serum creatinine by 50% (Table 1)[14,18]. Whereas Kidney Disease: Improving Global Outcome group defines AKI if any one of these is present in a patient: (1) An increase in serum creatinine by ≥ 0.3 mg/dL within 48 hours; (2) An increase in serum creatinine to > 50%, which is known or presumed to have occurred within the prior 7 days; or (3) Urine output < 0.5 mL/kg/hour which is there for last 6 hours[19]. Lately, the International Club of Ascites (ICA) and Acute Disease Quality Initiative (ADQI) proposed new diagnostic criteria for HRS-AKI (Table 1)[14]. The decline in urine output precedes changes in serum creatinine, and oliguria is considered an independent predictor of worse outcomes. However, creatinine is an unreliable marker to detect kidney dysfunction in patients with cirrhosis due to the higher prevalence of sarcopenia among these patients; thus, newer biomarkers (see new biomarkers section) like cystatin-C have been used in patients with cirrhosis to predict AKI[20,21].
| Key points of diagnosis |
| Cirrhosis with ascites |
| Increase in serum creatinine ≥ 0.3 mg/dL (26.5 mol/L) within 48 hours or ≥ 50% from baseline value known or presumed to have occurred within the prior 7 days and/or urinary output ≤ 0.5 mL/kg for ≥ 6 hours |
| Absence of improvement in serum creatinine and/or urine output within 24 hours following adequate volume resuscitation (when clinically indicated) |
| Absence of strong evidence for alternative explanation as the primary cause of AKI |
After defining AKI, it is important to classify the type of AKI phenotype (Figure 2). Among 30% of hospitalized patients with cirrhosis and AKI, pre-renal AKI is the most common variety[1,2]. A detailed history regarding the use of diuretics, non-steroidal anti-inflammatory drugs (NSAIDs), and angiotensin-converting enzyme (ACE) inhibitors/angiotensin receptor blockers (ARBs) should be documented and discontinued (Figure 2). Prerenal AKI can also be precipitated by overzealous use of diuretics, diarrhea, vomiting, or any other form of blood or fluid loss from the body[22]. Intrinsic kidney disease can be caused by ATN, acute interstitial nephritis, and the use of any contrast during radiological evaluation[23,24]. AKI can evolve into acute kidney disease (AKD) or chronic kidney disease (CKD) if renal injury persists for more than 7 or 90 days, respectively (Figure 2). Concomitant liver and kidney disease can be present in various conditions and can give us a clue about the diagnosis of underlying CKD[24]. Intrinsic kidney disease and hypovolemia should be ruled out before diagnosing HRS-AKI.
Diagnosis of HRS is made when a patient with cirrhosis or acute liver failure meets the criteria of AKI (Table 1), and there is no improvement of renal function after withdrawal of nephrotoxic drugs and 48 hours of volume expansion with albumin[14,25]. However, this time duration is reduced to 24 hours as per the joint ICA and ADQI guidelines (Table 1) mentioned earlier[14]. The fractional excretion of sodium (FENa) is typically low in this group of patients (FENa < 0.2)[26,27]. Neutrophil gelatinase-associated lipocalin (NGAL) has been used to rule out ATN. Still, there is no standard marker to diagnose HRS; it is a diagnosis of exclusion[25]. HRS is a form of functional renal failure when there is kidney dysfunction without any structural damage. Recent data on patients with HRS have challenged the conventional fun
Older subtypes of HRS, HRS-1, and HRS-2, have been reclassified as HRS-AKI and HRS-non-AKI (HRS-NAKI), respectively (Table 1). Patients with cirrhosis and recent worsening of renal function, defined as an increase in creatinine by 0.3 mg/dL in 48 hours or by 50% within a week and/or decreased urine output (< 0.5 mL/kg for 6 hours or more), are classified as HRS-AKI. HRS-NAKI includes HRS-AKD and HRS-CKD based on the previous creatinine and estimated glomerular filtration rate (GFR) (Figure 2). HRS-CKD can be diagnosed if the patient’s baseline creatinine or GFR is available, which was done at least 3 months prior. Persistent GFR < 60 mL/minute/1.73 m2 for more than 3 months should be defined as HRS-CKD if there is no evidence of structural kidney disease (Figure 2). The group of patients who fall between HRS-AKI and HRS-CKD are reclassified as HRS-AKD[14,25] (Table 1).
Routine urine examinations include urine for casts, dysmorphic red blood cells (RBCs), urinary sodium (UNa) and urea (Uurea), and 24-hour urinary protein quantification[26] (Table 2). The FENa is low in patients with cirrhosis without AKI[26]. Very low FENa (< 0.1-0.2) is highly suggestive of HRS[25,26]. FENa is also important to differentiate between HRS-AKI and ATN[26]. The absence of significant proteinuria (24-hour urinary protein < 500 mg/day) and microhematuria (< 50 RBCs/high-power field [HPF]) can indicate that the patient does not have significant structural kidney disease (Table 2). However, recent data suggest that these parameters are not always useful to rule out structural kidney disease[28]. FEUrea is another useful marker to diagnose HRS and to classify the subtypes of AKI in cirrhosis ACLF[29]. FEUrea is highest in ATN, whereas a lower level is seen in pre-renal AKI and HRS. However, FENa performs better than FEUrea in classifying patients with AKI in cirrhosis (Table 2)[27]. FEUrea > 33% suggests ATN, whereas FEUrea < 21% is seen in pre-renal AKI[29]. The use of vasoconstrictors in patients with ATN can be detrimental as it may worsen the glomerular filtration rate[25]. So, it is mandatory to rule out ATN before starting the treatment of HRS. Pre-transplant ATN is associated with higher post-transplant mortality and kidney dysfunction (Table 2)[30].
| Parameters | Pre-renal AKI | Hepatorenal syndrome | Acute tubular necrosis | Comments |
| Clue to diagnose | History of fluid loss or overzealous use of diuretics | Presence of refractory ascites, hyponatremia | Presence of sepsis and hypotension | History cannot be always reliable to diagnose the subtypes of kidney injury |
| Urine sediments[73,74] | The lack of any casts suggests functional renal failure mostly pre-renal AKI[56] | Usually absent | Muddy brown casts and renal tubular epithelial cell casts | Should be interpreted by expert renal pathologists |
| Sensitivity: 73% | RETC and granular cast | |||
| Specificity: 75% | PPV: 100% | |||
| However, bland, hyaline cast may be present | NPV: 40% | |||
| FENa[27,75] | < 1 | < 1 for diagnosing HRS-AKI | Usually > 1 | FENa is unable to distinguish between Pre-renal AKI and HRS-AKI |
| Sensitivity: 90% | Sensitivity: 100% | Sensitivity: 89% | Not validated in patients on diuretics | |
| Specificity: 82% | Specificity: 14% | Specificity: 71% | FENa can be < 1 in patients with cirrhosis without AKI | |
| To differentiate between intrinsic vs pre-renal kidney injury | AASLD | FENa < 0.56 excludes ATN | ||
| < 0.1 suggests HRS[46] | ||||
| FEUrea[29] | < 21 | < 28.7 | > 33 | No standardized cut-off in patients with cirrhosis |
| Sensitivity: 90% | Sensitivity: 75% | < 34 to rule out ATN | ||
| Specificity: 61% | Specificity: 83% | Sensitivity: 70% | ||
| For PRA vs HRS | For non-HRS vs HRS | Specificity: 58% | ||
| NGAL-1[35,36,38,39] | < 110 | < 100 | > 194 mcg/g Cr | |
| Sensitivity: 88% | Sensitivity: 91% | |||
| Specificity: 85% | Specificity: 82% | |||
| Renal artery resistive index[76,77] | Cannot differentiate between prerenal AKI and AKI-HRS | > 0.7 predicts HRS[76] | > 0.8 is suggestive of ATN[77] | RARI is higher in patients with cirrhosis and ascites compared to healthy individuals |
| > 0.77 predicts HRS in cirrhosis | RARI is higher in ATN than in HRS. | |||
| Sensitivity: 100% | ||||
| Specificity: 77% |
The absence of renal parenchymal disease is mandatory to diagnose HRS-AKI[25]. Moreover, in patients with ACLF, sepsis, and acute variceal bleeding, other causes for renal dysfunction may coexist (Table 2). Routine urinary biomarkers
Routine baseline evaluations may not always detect the degree of parenchymal injury[28,30]. Hence, different serum and urinary biomarkers have been used to detect, classify, and prognosticate patients with AKI and cirrhosis[33]. Novel biomarkers can be classified in different ways. Some biomarkers are related to kidney injury (kidney injury molecule-1 [KIM-1]) or cell cycle arrest (tissue inhibitor of metalloproteinase), whereas some may be associated with decreased GFR (cystatin C). Alpha-1 microglobulin, beta-M, and retinol-binding protein (RBP) are increased in AKI because of dimi
| Newer markers | Role in differentiating between ATN and HRS | As a predictor of mortality | Role in diagnosing HRS-AKI | Comments |
| NGAL[35,36,38,39] | 417 mg/g Cr in ATN and 76 ug/g Cr in HRS | uNGAL 110 ng/mL is associated with inpatient mortality[53] | Increased in 1-2 hours after ischaemic renal injury | Can be high in other diseases. Higher synthesis in the presence of sepsis. High in lupus nephritis, IgA nephropathy[41] |
| KIM-1[47,53] | Differentiate HRS from ATN; Cut-off: 15.4 ng/mL (AUC: 0.63); HRS: 3.1 pg/mL | No data | Early rise predicts AKI; Better marker than creatinine[53] | Not widely available |
| Cystatin C[42-45,55] | No data | Serum cystatin-C level of > 1.45 mg/L had the highest 90-day mortality (sensitivity and specificity of 66.7% and 68.4%)[44]. Cystatin-MELD score predicts mortality[45] | Predicts development of AKI in one year[55]; Serum cystatin-C > 1.47 mg/dL is an early marker of AKI in cirrhosis[44,45] | Higher reliability than Cr in patients with sarcopenia |
More than half of patients with ACLF may have underlying kidney injury[48]. Diagnosing and phenotyping AKI in ACLF is challenging as it can be multifactorial[48,49]. Serum cystatin-C and NGAL have been used to detect and prognosticate AKI in patients with ACLF[50]. In another study, soluble cluster of differentiation 163 (CD163) and NGAL were found to predict 28 days of mortality in patients with ACLF[51,52].
Other kidney biomarkers, including L-type fatty acid binding protein, interleukin-18 (IL-18), N-acetyl glycosaminidase, and RBP, are used in clinical trials for research purposes[53-55]. They are not widely available[33].
These newer biomarkers have limitations. Almost all of these biomarkers provide information regarding the damage that already occurred to the renal parenchyma, which might be useful to predict the development of CKD in ACLF patients. With the exception of cystatin-C, other biomarkers are mostly used in clinical trials. There is a great need for the development of cost-effective biomarkers that predict AKI in patients with cirrhosis. Recent developments should focus on discovering newer biomarkers that can monitor the metabolism of renal epithelial cells before their death.
Routine ultrasonography of the kidney is useful for detecting CKD in patients with cirrhosis[56]. Patients with metabolic dysfunction-associated steatotic liver disease (MASLD) and advanced fibrosis are at higher risk of CKD[57]. Bilateral shrunken or enlarged kidneys and loss of corticomedullary differentiation point toward the presence of structural kidney disease[56-58]. Based on ultrasonogram (USG) findings, a diagnosis of CKD can be made in patients with high creatinine who do not have baseline creatinine values.
Renal artery resistive index (RARI) is an important non-invasive parameter to predict pre-renal AKI in patients with cirrhosis. The renin-angiotensin-aldosterone system (RAAS) and the sympathetic nervous system increase renal artery resistance in patients with cirrhosis and refractory ascites. RARI increases proportionately with Child-Turcotte-Pugh (CTP), model for end-stage liver disease (MELD), and MELD-sodium scores in patients with cirrhosis[59]. Lower RARI predicts better renal functions[60]. RARI is higher in patients with cirrhosis compared to healthy individuals. RARI > 0.7 is one of the predictors of AKI in cirrhosis. However, it may be fallacious in patients with systemic hypertension with underlying MASLD-related cirrhosis, intrinsic kidney disease, and generalized atherosclerotic disease[61,62].
RARI is a simple bedside tool to predict AKI in cirrhosis and it can also detect the phenotype of AKI in cirrhosis. A cut-off of > 0.7 can be taken as the predictor of pre-renal AKI in cirrhosis. Very high RARI (> 0.8) is often seen with ATN and may be helpful to differentiate between HRS-AKI and ATN[59,60].
Bedside evaluation of inferior vena cava diameter (IVCD) and IVC collapsibility index (IVCCI) is used in diagnosing systemic hypovolemia in patients with cirrhosis[63]. IVCCI is calculated as (IVCmaximum diameter - IVCminimum diameter)/IVCmaximum diameter and varies between 12% and 42%. IVCCI > 50% and diameter < 0.7 cm suggest hypovolemia[64]. IVCD correlates with right atrial pressure. IVCD > 2 cm and IVCCI < 40% are suggestive of fluid overload status. Albumin infusion in this scenario can be detrimental[65].
In a study by Kaptein et al[64], of the 20 patients with a presumed diagnosis of HRS, 6 had hypovolemia as assessed by USG Doppler. This point-of-care test may help us avoid misclassifying hypovolemic patients as HRS[65]. However, there is no standardized cut-off of IVCD and IVCCI in patients with cirrhosis. Often, it can be fallacious in patients with grade 3 ascites[66].
The presence of cardiac dysfunction and cirrhotic cardiomyopathy (CCM) influences the outcome in patients with ACLF[67]. Baseline cardiac dysfunction is associated with worsening HRS. Cirrhosis is often associated with diastolic failure, electromechanical dissociation, and impaired contractility. Ejection fraction alone is not sufficient to determine the spectrum of cardiac dysfunction[68,69]. Point of care two-dimensional (2D) echocardiography may be useful to estimate systemic vascular resistance index, cardiac index, e'velocity, and the presence of CCM. Very low systemic vascular resistance (SVR) predicts poor outcomes in patients with ACLF. In this group of patients, vasoconstrictors may help to counteract the overwhelming systemic vasodilation[70,71]. A previous study from our group showed that adding a second vasoconstrictor (noradrenaline after terlipressin if SVR < 700 dynes - second/cm5) was beneficial in improving renal function, ascites mobilization, gut permeability, and hemodynamics[7]. Point of care 2D echo may also detect reduced ejection fraction, where we should use albumin more judiciously to avoid volume overload[72].
After proper work-up, the management of renal dysfunction depends on the phenotype of AKI in patients with cirrhosis[73-77]. All offending drugs should be stopped to avoid ongoing nephrotoxicity. It includes beta-blockers, diuretics, ACEIs/ARBs, and NSAIDs[22,23,25,78]. Any potential source of extravascular fluid loss should be identified and corrected (Figure 2). Overzealous use of diuretics precipitates prerenal AKI[22,25]. Any clinical signs of dehydration, such as mean arterial pressure (MAP), pulse, orthostatic changes in blood pressure, capillary refill, skin turgor, decline in urine output, or mentation, should be carefully assessed. Volume replacement usually corrects kidney dysfunction in this scenario. Patients should be thoroughly screened for any infection, especially spontaneous bacterial peritonitis (SBP), pneumonia, and urinary tract infection. Empirical broad-spectrum antibiotics should be started when infection is suspected[78,79]. It has been shown to reduce the incidence of worsening AKI[6,79]. Patients with cirrhosis and septic shock should receive broad-spectrum antibiotics and volume resuscitation. Albumin can be preferred over 0.9% normal saline in patients with cirrhosis and sepsis[80]. A higher MAP goal is desirable in patients with renal dysfunction. Intradialytic hypotension is less in patients with higher MAP compared to patients with lower MAP. However, there is no survival benefit with higher MAP[81].
Pre-renal AKI responds to fluid therapy (Figure 2). After stopping all the nephrotoxic drugs and volume expansion, treatment of HRS can be initiated. Structural kidney disease should be ruled out in such patients.
Terlipressin: Terlipressin administered as a continuous infusion is the drug of choice for patients with HRS[25,82]. It is a pro-drug and is cleaved by endothelial peptidase to its active form. Its active form, lysyl-vasopressin, counteracts overwhelming splanchnic vasodilation (Figure 1) and increases renal blood flow[83]. Up to 20% of patients may have side effects, including diarrhea and abdominal cramps[82,83]. Adverse events are more common in patients with relatively preserved liver function[82]. A recent randomized controlled trial (RCT) (2 mg/24 hours infusion vs 0.5 mg every 4 hours initially then to a maximum dose of 12 mg/day plus albumin 20-40 g/day) has shown that infusion is equally effective in reversing HRS compared to intravenous (IV) bolus injections (76% vs 65%; P non-significant)[84]. However, terlipressin infusion is associated with a lesser rate of adverse events (35% vs 62%; P < 0.025)[84].
In a recently published randomized trial, the e-Terli study by Jindal et al[85] showed that the initiation of terlipressin as early as 12 hours has better outcomes in reversing HRS in ACLF patients. AKI response on day 7 was observed in 68.6% of patients compared to 31.4% of patients in the standard treatment arm.
Noradrenaline: Noradrenaline is an attractive alternative to terlipressin for patients with HRS-AKI. Noradrenaline is given at a dose of 0.5-3 mg/hour in infusion[86]. An RCT has demonstrated that noradrenaline is equally effective in reversing HRS compared to terlipressin. It is also associated with fewer side effects[86]. Patients with HRS-AKI who were managed with noradrenaline had a reversal rate of 43% (39% in the terlipressin group; P = 0.76). In multivariate analysis, a higher CTP score was associated with a lower response to vasopressor. Patients who responded to the treatment had a higher mean arterial pressure, urine volume and urinary sodium and low plasma renin activity. Noradrenaline, with a median dose of 13.1 mg/day, was cheaper than terlipressin (275 vs 975 euros) with similar efficacy. The terlipressin group also reported adverse events such as abdominal pain, peripheral cyanosis, and arrhythmia. However, there was no difference in short-term survival between the two groups. Data comparing noradrenaline and terlipressin for HRS is based on this RCT alone. Further studies and meta-analyses are required to validate its routine use.
In another RCT by Singh et al[87], patients who did not respond to terlipressin after 48 hours were randomized to receive either terlipressin alone or terlipressin combined with noradrenaline. There were no significant differences in the response rate (50% vs 76.7%; P = 0.06) and mortality (36.7% vs 53.3%; P = 0.13) between the two groups. Adverse events were more frequent in the terlipressin group (36.7% vs 13.3%; P < 0.05). SVR-guided titration or adding a second vasoconstrictor can be more useful in this scenario; however, more data are warranted on this issue[7].
Octreotide and midodrine: The combination of octreotide, albumin, and midodrine has been used in patients with HRS-AKI and HRS-NAKI. Previously, it was shown to improve short-term survival and renal function[88]. However, recent data suggest that this combination is not better than terlipressin and albumin regimens in reversing HRS. In an RCT by Cavallin et al[89], 70.4% of patients who received terlipressin (3 mg/day to a maximum of 12 mg/day) and albumin recovered from AKI, compared to 28.6% of patients who received octreotide (100 mcg three times daily to a maximum dose of 200 mcg three times daily and midodrine 7.5 mg three times daily to 12.5 mg three times daily). The results of this RCT suggest that patients with HRS should receive terlipressin if there are no contraindications. Octreotide and midodrine can be used where terlipressin is not available or contraindicated.
The effective blood volume is low in patients with cirrhosis in the background of systemic vasodilation (Figure 1)[90-92]. Reduced albumin and its transcriptionally modified forms bind less to the drugs and cytokines[93,94]. In addition to its role in maintaining oncotic pressure, albumin has many pleiotropic effects in cirrhosis[95]. A large RCT showed the superiority of 5% human albumin over normal saline for initial resuscitation in critically ill patients with cirrhosis and septic shock[6]. In patients with AKI and cirrhosis without any features of shock, volume expansion is done with 1 g/kg/day albumin for 48 hours. A dose of 20-40 g/day is recommended for use with vasoconstrictors in HRS-AKI[25,96]. Patients with CKD and heart failure are at a higher risk of developing volume overload after albumin infusion. Bedside lung ultrasonography, 2D echo, pro-N-terminal-brain natriuretic peptide, and point-of-care ultrasonography (POCUS) of IVCD and IVCCI are useful methods to detect volume overload states in such patients[63,97].
Transjugular intrahepatic portosystemic shunts (TIPS) have been used in refractory ascites and AKI in patients with cirrhosis[25,98]. This group mainly comprises patients with HRS-NAKI (previously called HRS-2). Patients with advanced liver dysfunction managed with TIPS have a higher incidence of hepatic encephalopathy (HE) and mortality[99]. Data on the feasibility and efficacy of TIPS in HRS is limited[99,100], so TIPS is currently not recommended for this indication.
Molecular adsorbent recirculating system and PROMETHEUS are extracorporeal artificial liver support systems[101]. They have been used in patients with liver failure[102]. However, none of these systems demonstrated any mortality benefits[103]. Currently, MARS and PROMETHEUS are not recommended for use in AKI with cirrhosis.
Patients with AKI and cirrhosis who fail to respond to vasoconstrictors and albumin have a very high mortality[82,83]. Liver transplantation or combined liver and kidney transplantation is their only option; however, a significant number of patients die while waiting for LT[104]. So, patients should undergo RRT while on a waiting list for LT because, without transplantation, mortality may reach up to 85% in these patients[104,105].
The presence of AKI, especially pre-transplant ATN, is associated with higher mortality following liver transplant[106]. One-fifth of such patients die within 6 months of transplant[107]. Combined transplants of the liver and kidney are recommended in patients with cirrhosis who are dialysis-dependent or have underlying significant proteinuria and CKD[108]. Current recommendations suggest a combined transplant if a patient with cirrhosis has evidence of CKD (calculated GFR < 60 mL/minute for > 90 days), or sustained AKI (on dialysis at least once a week, or calculated GFR < 25 mL/minute at least once every 7 days) or there are metabolic disorders like hyperoxaluria, atypical hemolytic uremic syndrome, and methylmalonic aciduria[108-110].
Infection is the most common precipitant of ACLF[6,8,111]. Endotoxemia with superimposed bacterial infection worsens hemodynamics, resulting in a reduced GFR[112]. Any decrease in urine output and UNa during the therapy should prompt physicians to look for any bacterial infection[7,113]. A thorough screening for hospital-acquired infection should be done[6,7]. Along with procalcitonin, novel biomarkers like CD64 and presepsin can be used to detect early bacterial infection[114,115]. Low protein in ascites is associated with higher risks of developing SBP[116]. Antibiotic prophylaxis should be started in these patients. Patients with acute variceal bleeding should also receive antibiotic prophylaxis for 7 days[117]. A recent RCT from India showed that norfloxacin prophylaxis is associated with a reduced incidence of infection in patients with ACLF[118]. However, it is currently not recommended for patients with ACLF. IV n-acetyl cysteine (NAC) and granulocyte-colony stimulating factor (G-CSF) have been shown to prevent AKI in cirrhosis[119,120]. They are not currently recommended for this purpose.
Specific indications of antibiotic prophylaxis in patients with cirrhosis are ascites with very low ascitic fluid protein, secondary prophylaxis in SBP, and following acute variceal bleeding in patients with CTP-B and CTP-C cirrhosis.
NAC has been shown to reduce contrast-induced AKI in patients with sepsis and cirrhosis[120]. In an RCT comparing the combination of glucocorticoids and NAC vs glucocorticoids, a significant reduction in the incidence of HRS (25% vs 12%, 0.41, 95% confidence interval [CI]: 0.17-0.98; P = 0.02) and mortality due to HRS (22% vs 9%, 2.79, 95%CI: 1.08-7.42,;P = 0.02) was observed with NAC[121]. However, currently, there are no sufficient data to recommend the use of antioxidants or NAC for the prevention of AKI in patients with ACLF or decompensated cirrhosis.
Albumin has a role in the prevention of AKI in patients undergoing therapeutic paracentesis, especially large volume paracentesis and those having SBP. In an RCT of 80 patients with ACLF, who underwent therapeutic paracentesis of < 5 L, paracentesis-induced circulatory dysfunction was more common in the non-albumin group as compared to the albumin group (70% vs 30%; P = 0.001), including a higher rate of AKI as well (62.5% vs 27.5%; P = 0.003)[9].
Sort et al[122] randomized 126 patients with SBP into two groups: the first group received 20% albumin at a dose of 1.5 g/kg at diagnosis and 1 g/kg on day 3 in infusion + cefotaxime, and the second received cefotaxime alone. The incidence of AKI was higher in the group without albumin (33% vs 10%; P = 0.002), and so were the in-hospital and 90-day mortalities (41% vs 22%; P = 0.03).
Patients with cirrhosis should be counseled about the potential harm of taking NSAIDs, ACEIs/ARBs, other over-the-counter medications, and complementary alternative medicines[123]. About 5% of patients with cirrhosis develop AKI after contrast injection. Lower bicarbonate levels and advanced cirrhosis are risk factors for developing AKI[124]. Albumin, NAC, and IV bicarbonate have been used to prevent AKI in cirrhosis; however, data are limited. Contrast-enhanced imaging should be done judiciously in patients with advanced liver disease.
Currently, few RCTs have evaluated the effect of G-CSF on patients with ACLF, with heterogeneous data from Asian studies contradicting the data from European studies[125,126]. Currently, it is not recommended for the prevention of AKI in ACLF.
Effective arterial blood volume is low in patients with cirrhosis[127]. Any intravascular fluid loss should be corrected promptly. It has been shown to prevent AKI and ATN. IV albumin can be useful in patients with septic shock. Concomitant hepatopulmonary syndrome, portopulmonary hypertension (PPH), and cardiomyopathy reduce cardiac output and activate RAAS. Beta-blockers should be discontinued in patients with PPH[128,129]. Cardiorespiratory comorbidities are often difficult to treat in patients with cirrhosis and may require LT[129].
Concomitant liver and kidney disease can be found in patients with MASLD (diabetic kidney disease), viral hepatitis (glomerulonephritis), primary biliary cholangitis (interstitial nephritis), and alcoholic hepatitis (immunoglobulin A nephropathy)[57,130]. Treating the underlying etiology can reverse the outcome in these patients. Persistent kidney dysfunction and the need for RRT are indications of combined liver and kidney transplantation.
The management of AKI in cirrhosis often requires a multidisciplinary approach. Early detection of AKI may reverse the outcome in patients with cirrhosis. Proper clinical inquiry about nephrotoxic drugs, and proper use of appropriate biomarkers may identify the progression of kidney injury in such patients. POCUS is an evolving diagnostic modality to assess the volume status of patients with cirrhosis and AKI. RARI index can be used in selective groups of patients, however, more data is warranted on POCUS and RARI. Concomitant structural kidney disease may coexist in patients with cirrhosis. Ruling out other etiologies like ATN, structural kidney disease and pre-renal volume responsive AKI is the most important step in diagnosing HRS. The concept that HRS is a functional renal injury has been challenged as some degree of parenchymal injury may coexist with HRS. Routine biomarkers have limited value in diagnosing them. Cystatin-C and NGAL are the most used newer biomarkers to detect structural injury to the liver, however, they are not without limitations. Volume expansion with vasoconstrictors remains the cornerstone of the treatment in patients with HRS. Careful use of terlipressin in AKI is important as it may worsen renal function in patients with ATN. Preventing infection, judicious use of antibiotics and albumin may prevent AKI in patients with cirrhosis. TIPS can be used in a selective group of patients with HRS, however its indication is evolving. Concomitant liver-kidney transplantation can salvage a subset of patients who have persistently low GFR or structural kidney disease requiring hemodialysis. Current research should focus on hepato-renal hemodynamics and early serum or urinary markers to predict AKI in patients with cirrhosis.
The authors express their heartfelt gratitude to all the individuals who contributed their insightful intellectual assistance to this work.
| 1. | Charlton MR, Wall WJ, Ojo AO, Ginès P, Textor S, Shihab FS, Marotta P, Cantarovich M, Eason JD, Wiesner RH, Ramsay MA, Garcia-Valdecasas JC, Neuberger JM, Feng S, Davis CL, Gonwa TA; International Liver Transplantation Society Expert Panel. Report of the first international liver transplantation society expert panel consensus conference on renal insufficiency in liver transplantation. Liver Transpl. 2009;15:S1-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 106] [Article Influence: 6.6] [Reference Citation Analysis (1)] |
| 2. | Low G, Alexander GJ, Lomas DJ. Hepatorenal syndrome: aetiology, diagnosis, and treatment. Gastroenterol Res Pract. 2015;2015:207012. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 3. | Ning Y, Zou X, Xu J, Wang X, Ding M, Lu H. Impact of acute kidney injury on the risk of mortality in patients with cirrhosis: a systematic review and meta-analysis. Ren Fail. 2022;44:1-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 4. | Trebicka J, Amoros A, Pitarch C, Titos E, Alcaraz-Quiles J, Schierwagen R, Deulofeu C, Fernandez-Gomez J, Piano S, Caraceni P, Oettl K, Sola E, Laleman W, McNaughtan J, Mookerjee RP, Coenraad MJ, Welzel T, Steib C, Garcia R, Gustot T, Rodriguez Gandia MA, Bañares R, Albillos A, Zeuzem S, Vargas V, Saliba F, Nevens F, Alessandria C, de Gottardi A, Zoller H, Ginès P, Sauerbruch T, Gerbes A, Stauber RE, Bernardi M, Angeli P, Pavesi M, Moreau R, Clària J, Jalan R, Arroyo V. Addressing Profiles of Systemic Inflammation Across the Different Clinical Phenotypes of Acutely Decompensated Cirrhosis. Front Immunol. 2019;10:476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 136] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 5. | Albillos A, Martin-Mateos R, Van der Merwe S, Wiest R, Jalan R, Álvarez-Mon M. Cirrhosis-associated immune dysfunction. Nat Rev Gastroenterol Hepatol. 2022;19:112-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 213] [Article Influence: 71.0] [Reference Citation Analysis (0)] |
| 6. | Bunchorntavakul C, Chamroonkul N, Chavalitdhamrong D. Bacterial infections in cirrhosis: A critical review and practical guidance. World J Hepatol. 2016;8:307-321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 149] [Article Influence: 16.6] [Reference Citation Analysis (2)] |
| 7. | Pande G, Hatti M, Rai MK, Rai P, Kumar K, Vp K, Nehra A, Kumar S, Ranjan Rout S, Mishra SK, Kumar D, Kumar U, Mishra P, Majeed A, Saraswat VA, Singh K, Singh H, Misra DP, Agarwal V. Response Guided Slow Infusion of Albumin, Vasoconstrictors and Furosemide Improves Ascites Mobilization and Survival in Acute on Chronic Liver Failure: A Proof-of-Concept Study. J Inflamm Res. 2022;15:5027-5039. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 8. | Bajaj JS, Kamath PS, Reddy KR. The Evolving Challenge of Infections in Cirrhosis. N Engl J Med. 2021;384:2317-2330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 125] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 9. | Maiwall R, Chandel SS, Wani Z, Kumar S, Sarin SK. SIRS at Admission Is a Predictor of AKI Development and Mortality in Hospitalized Patients with Severe Alcoholic Hepatitis. Dig Dis Sci. 2016;61:920-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 10. | Erly B, Carey WD, Kapoor B, McKinney JM, Tam M, Wang W. Hepatorenal Syndrome: A Review of Pathophysiology and Current Treatment Options. Semin Intervent Radiol. 2015;32:445-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Patil V, Jain M, Venkataraman J. Paracentesis-induced acute kidney injury in decompensated cirrhosis - prevalence and predictors. Clin Exp Hepatol. 2019;5:55-59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Kulkarni AV, Kumar P, Sharma M, Sowmya TR, Talukdar R, Rao PN, Reddy DN. Pathophysiology and Prevention of Paracentesis-induced Circulatory Dysfunction: A Concise Review. J Clin Transl Hepatol. 2020;8:42-48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 13. | Angeli P, Ginès P, Wong F, Bernardi M, Boyer TD, Gerbes A, Moreau R, Jalan R, Sarin SK, Piano S, Moore K, Lee SS, Durand F, Salerno F, Caraceni P, Kim WR, Arroyo V, Garcia-Tsao G. Diagnosis and management of acute kidney injury in patients with cirrhosis: revised consensus recommendations of the International Club of Ascites. J Hepatol. 2015;62:968-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 527] [Article Influence: 52.7] [Reference Citation Analysis (1)] |
| 14. | Nadim MK, Kellum JA, Forni L, Francoz C, Asrani SK, Ostermann M, Allegretti AS, Neyra JA, Olson JC, Piano S, VanWagner LB, Verna EC, Akcan-Arikan A, Angeli P, Belcher JM, Biggins SW, Deep A, Garcia-Tsao G, Genyk YS, Gines P, Kamath PS, Kane-Gill SL, Kaushik M, Lumlertgul N, Macedo E, Maiwall R, Marciano S, Pichler RH, Ronco C, Tandon P, Velez JQ, Mehta RL, Durand F. Acute kidney injury in patients with cirrhosis: Acute Disease Quality Initiative (ADQI) and International Club of Ascites (ICA) joint multidisciplinary consensus meeting. J Hepatol. 2024;81:163-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 71] [Article Influence: 71.0] [Reference Citation Analysis (0)] |
| 15. | Kulkarni AV, Arab JP, Premkumar M, Benítez C, Tirumalige Ravikumar S, Kumar P, Sharma M, Reddy DN, Simonetto DA, Rao PN. Terlipressin has stood the test of time: Clinical overview in 2020 and future perspectives. Liver Int. 2020;40:2888-2905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 16. | Lefilliatre P, Sogni P, Bertrand V, Del Soldato P, Pateron D, Moreau R, Lebrec D. Aortic hyporeactivity to norepinephrine induced by lipopolysaccharide in cirrhotic rats: beneficial effects of a non-steroidal anti-inflammatory drug coupled with a nitric oxide donor. J Gastroenterol Hepatol. 2001;16:70-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Kulkarni AV, Ravikumar ST, Tevethia H, Premkumar M, Kumar K, Sharma M, Gupta R, Rao PN, Reddy DN. Safety and efficacy of terlipressin in acute-on-chronic liver failure with hepatorenal syndrome-acute kidney injury (HRS-AKI): a prospective cohort study. Sci Rep. 2022;12:5503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 18. | Salerno F, Gerbes A, Ginès P, Wong F, Arroyo V. Diagnosis, prevention and treatment of hepatorenal syndrome in cirrhosis. Postgrad Med J. 2008;84:662-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 356] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 19. | Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120:c179-c184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1436] [Cited by in RCA: 3342] [Article Influence: 257.1] [Reference Citation Analysis (0)] |
| 20. | Fernando S, Polkinghorne KR. Cystatin C: not just a marker of kidney function. J Bras Nefrol. 2020;42:6-7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Chung MY, Jun DW, Sung SA. Diagnostic value of cystatin C for predicting acute kidney injury in patients with liver cirrhosis. Korean J Hepatol. 2010;16:301-307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Russ KB, Stevens TM, Singal AK. Acute Kidney Injury in Patients with Cirrhosis. J Clin Transl Hepatol. 2015;3:195-204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 74] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 23. | Campion D, Ponzo P, Risso A, Caropreso P, Caviglia GP, Sanavia T, Frigo F, Bonetto S, Giovo I, Rizzo M, Martini S, Bugianesi E, Mengozzi G, Marzano A, Manca A, Saracco GM, Alessandria C. A prospective, multicenter, three-cohort study evaluating contrast-induced acute kidney injury (CI-AKI) in patients with cirrhosis. J Hepatol. 2024;80:62-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Gleisner AL, Jung H, Lentine KL, Tuttle-Newhall J. Renal Dysfunction in Liver Transplant Candidates: Evaluation, Classification and Management in Contemporary Practice. J Nephrol Ther. 2012;Suppl 4: 006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 25. | Angeli P, Garcia-Tsao G, Nadim MK, Parikh CR. News in pathophysiology, definition and classification of hepatorenal syndrome: A step beyond the International Club of Ascites (ICA) consensus document. J Hepatol. 2019;71:811-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 282] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 26. | Ariza X, Solà E, Elia C, Barreto R, Moreira R, Morales-Ruiz M, Graupera I, Rodríguez E, Huelin P, Solé C, Fernández J, Jiménez W, Arroyo V, Ginès P. Analysis of a urinary biomarker panel for clinical outcomes assessment in cirrhosis. PLoS One. 2015;10:e0128145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 89] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 27. | Gowda YHS, Jagtap N, Karyampudi A, Rao NP, Deepika G, Sharma M, Gupta R, Tandan M, Ramchandani M, John P, Kulkarni A, Kumar P, Bhaware B, Turpati MV, Reddy DN. Fractional Excretion of Sodium and Urea in Differentiating Acute Kidney Injury Phenotypes in Decompensated Cirrhosis. J Clin Exp Hepatol. 2022;12:899-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 28. | Trawalé JM, Paradis V, Rautou PE, Francoz C, Escolano S, Sallée M, Durand F, Valla D, Lebrec D, Moreau R. The spectrum of renal lesions in patients with cirrhosis: a clinicopathological study. Liver Int. 2010;30:725-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 131] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 29. | Patidar KR, Kang L, Bajaj JS, Carl D, Sanyal AJ. Fractional excretion of urea: A simple tool for the differential diagnosis of acute kidney injury in cirrhosis. Hepatology. 2018;68:224-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 30. | Cullaro G, Verna EC, Lai JC. Association Between Renal Function Pattern and Mortality in Patients With Cirrhosis. Clin Gastroenterol Hepatol. 2019;17:2364-2370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 31. | van Slambrouck CM, Salem F, Meehan SM, Chang A. Bile cast nephropathy is a common pathologic finding for kidney injury associated with severe liver dysfunction. Kidney Int. 2013;84:192-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 158] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 32. | Wadei HM, Geiger XJ, Cortese C, Mai ML, Kramer DJ, Rosser BG, Keaveny AP, Willingham DL, Ahsan N, Gonwa TA. Kidney allocation to liver transplant candidates with renal failure of undetermined etiology: role of percutaneous renal biopsy. Am J Transplant. 2008;8:2618-2626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 33. | Francoz C, Nadim MK, Durand F. Kidney biomarkers in cirrhosis. J Hepatol. 2016;65:809-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 114] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 34. | Lee HA, Seo YS. Current knowledge about biomarkers of acute kidney injury in liver cirrhosis. Clin Mol Hepatol. 2022;28:31-46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 35. | Markwardt D, Holdt L, Steib C, Benesic A, Bendtsen F, Bernardi M, Moreau R, Teupser D, Wendon J, Nevens F, Trebicka J, Garcia E, Pavesi M, Arroyo V, Gerbes AL. Plasma cystatin C is a predictor of renal dysfunction, acute-on-chronic liver failure, and mortality in patients with acutely decompensated liver cirrhosis. Hepatology. 2017;66:1232-1241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 74] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 36. | Barreto R, Elia C, Solà E, Moreira R, Ariza X, Rodríguez E, Graupera I, Alfaro I, Morales-Ruiz M, Poch E, Guevara M, Fernández J, Jiménez W, Arroyo V, Ginès P. Urinary neutrophil gelatinase-associated lipocalin predicts kidney outcome and death in patients with cirrhosis and bacterial infections. J Hepatol. 2014;61:35-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 89] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 37. | Belcher JM, Sanyal AJ, Peixoto AJ, Perazella MA, Lim J, Thiessen-Philbrook H, Ansari N, Coca SG, Garcia-Tsao G, Parikh CR; TRIBE-AKI Consortium. Kidney biomarkers and differential diagnosis of patients with cirrhosis and acute kidney injury. Hepatology. 2014;60:622-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 244] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 38. | Verna EC, Brown RS, Farrand E, Pichardo EM, Forster CS, Sola-Del Valle DA, Adkins SH, Sise ME, Oliver JA, Radhakrishnan J, Barasch JM, Nickolas TL. Urinary neutrophil gelatinase-associated lipocalin predicts mortality and identifies acute kidney injury in cirrhosis. Dig Dis Sci. 2012;57:2362-2370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 125] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 39. | Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, Barasch J, Devarajan P. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol. 2003;14:2534-2543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1227] [Cited by in RCA: 1286] [Article Influence: 61.2] [Reference Citation Analysis (0)] |
| 40. | Kohei J, Ishida H, Tanabe K, Tsuchiya K, Nitta K. Neutrophil gelatinase-associated lipocalin is a sensitive biomarker for the early diagnosis of acute rejection after living-donor kidney transplantation. Int Urol Nephrol. 2013;45:1159-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 41. | Torres-Salido MT, Cortés-Hernández J, Vidal X, Pedrosa A, Vilardell-Tarrés M, Ordi-Ros J. Neutrophil gelatinase-associated lipocalin as a biomarker for lupus nephritis. Nephrol Dial Transplant. 2014;29:1740-1749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 42. | Belcher JM, Sanyal AJ, Garcia-Tsao G, Ansari N, Coca SG, Shlipak MG, Parikh CR. Early trends in cystatin C and outcomes in patients with cirrhosis and acute kidney injury. Int J Nephrol. 2014;2014:708585. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 43. | Jha P, Jha AK, Dayal VM, Jha SK, Kumar A. Baseline serum cystatin C as a marker of acute kidney injury in patients with acute-on-chronic liver failure. Indian J Gastroenterol. 2021;40:563-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 44. | Suksamai A, Chaiprasert A, Chirapongsathorn S. Serum cystatin C as a predictor of 90-day mortality among patients admitted with complications of cirrhosis. JGH Open. 2021;5:607-613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 45. | Maiwall R, Kumar A, Bhardwaj A, Kumar G, Bhadoria AS, Sarin SK. Cystatin C predicts acute kidney injury and mortality in cirrhotics: A prospective cohort study. Liver Int. 2018;38:654-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 46. | Maiwall R, Singh SP, Angeli P, Moreau R, Krag A, Singh V, Singal AK, Tan SS, Puri P, Mahtab M, Lau G, Ning Q, Sharma MK, Rao PN, Kapoor D, Gupta S, Duseja A, Wadhawan M, Jothimani D, Saigal S, Taneja S, Shukla A, Puri P, Govil D, Pandey G, Madan K, Eapen CE, Benjamin J, Chowdhury A, Singh S, Salao V, Yang JM, Hamid S, Shalimar, Jasuja S, Kulkarni AV, Niriella MA, Tevethia HV, Arora V, Mathur RP, Roy A, Jindal A, Saraf N, Verma N, De A, Choudhary NS, Mehtani R, Chand P, Rudra O, Sarin SK. APASL clinical practice guidelines on the management of acute kidney injury in acute-on-chronic liver failure. Hepatol Int. 2024;18:833-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 47. | El-Makarem MAEA, Mahmoud YZ, Moussa MM, El-Saghir SMM, Keryakos HKH. Do old urinary biomarkers have a place in the new definition of hepatorenal syndrome in the Egyptian cirrhotic patients? A single-center experience. Egypt Liver J. 2022;12:23. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 48. | Grønbaek H, Møller HJ, Saliba F, Zeuzem S, Albillos A, Ariza X, Graupera I, Solà E, Amoros A, Pavesi M, Bossen L, Jalan R, Gines P, Arroyo V. Improved prediction of mortality by combinations of inflammatory markers and standard clinical scores in patients with acute-on-chronic liver failure and acute decompensation. J Gastroenterol Hepatol. 2021;36:240-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 49. | Jindal A, Bhadoria AS, Maiwall R, Sarin SK. Evaluation of acute kidney injury and its response to terlipressin in patients with acute-on-chronic liver failure. Liver Int. 2016;36:59-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 50. | Wan ZH, Wang JJ, You SL, Liu HL, Zhu B, Zang H, Li C, Chen J, Xin SJ. Cystatin C is a biomarker for predicting acute kidney injury in patients with acute-on-chronic liver failure. World J Gastroenterol. 2013;19:9432-9438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 35] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 51. | Yewale RV, Ramakrishna BS, Venugopal G, Doraiswami BV, Rajini K. Urine neutrophil gelatinase-associated lipocalin as a biomarker of acute kidney injury and prognosis in decompensated chronic liver disease: A prospective study. Indian J Gastroenterol. 2023;42:106-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 52. | Ariza X, Graupera I, Coll M, Solà E, Barreto R, García E, Moreira R, Elia C, Morales-Ruiz M, Llopis M, Huelin P, Solé C, Fabrellas N, Weiss E, Nevens F, Gerbes A, Trebicka J, Saliba F, Fondevila C, Hernández-Gea V, Fernández J, Bernardi M, Arroyo V, Jiménez W, Deulofeu C, Pavesi M, Angeli P, Jalan R, Moreau R, Sancho-Bru P, Ginès P; CANONIC Investigators, EASL CLIF Consortium. Neutrophil gelatinase-associated lipocalin is a biomarker of acute-on-chronic liver failure and prognosis in cirrhosis. J Hepatol. 2016;65:57-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 102] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 53. | Han WK, Waikar SS, Johnson A, Betensky RA, Dent CL, Devarajan P, Bonventre JV. Urinary biomarkers in the early diagnosis of acute kidney injury. Kidney Int. 2008;73:863-869. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 449] [Cited by in RCA: 400] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 54. | Yoo JJ, Kim SG, Kim YS, Lee B, Lee MH, Jeong SW, Jang JY, Lee SH, Kim HS, Kim YD, Cheon GJ. Estimation of renal function in patients with liver cirrhosis: Impact of muscle mass and sex. J Hepatol. 2019;70:847-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 73] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 55. | Jo SK, Yang J, Hwang SM, Lee MS, Park SH. Role of biomarkers as predictors of acute kidney injury and mortality in decompensated cirrhosis. Sci Rep. 2019;9:14508. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 56. | Ahmed S, Bughio S, Hassan M, Lal S, Ali M. Role of Ultrasound in the Diagnosis of Chronic Kidney Disease and its Correlation with Serum Creatinine Level. Cureus. 2019;11:e4241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 57. | Sun DQ, Jin Y, Wang TY, Zheng KI, Rios RS, Zhang HY, Targher G, Byrne CD, Yuan WJ, Zheng MH. MAFLD and risk of CKD. Metabolism. 2021;115:154433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 222] [Article Influence: 55.5] [Reference Citation Analysis (0)] |
| 58. | Page JE, Morgan SH, Eastwood JB, Smith SA, Webb DJ, Dilly SA, Chow J, Pottier A, Joseph AE. Ultrasound findings in renal parenchymal disease: comparison with histological appearances. Clin Radiol. 1994;49:867-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 59. | Koda M, Murawaki Y, Kawasaki H. Renovascular resistance assessed by color Doppler ultrasonography in patients with chronic liver diseases. J Gastroenterol Hepatol. 2000;15:1424-1429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 60. | Onwuka CC, Ayoola OO, Adekanle O, Famurewa OC, Abidoye IA. Renal arterial resistance index among subjects with liver cirrhosis in a Nigerian population. J Clin Ultrasound. 2021;49:538-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 61. | Darabont R, Mihalcea D, Vinereanu D. Current Insights into the Significance of the Renal Resistive Index in Kidney and Cardiovascular Disease. Diagnostics (Basel). 2023;13:1687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 62. | Radermacher J, Ellis S, Haller H. Renal resistance index and progression of renal disease. Hypertension. 2002;39:699-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 167] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 63. | Premkumar M, Rangegowda D, Kajal K, Khumuckham JS. Noninvasive estimation of intravascular volume status in cirrhosis by dynamic size and collapsibility indices of the inferior vena cava using bedside echocardiography. JGH Open. 2019;3:322-328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 64. | Kaptein EM, Oo Z, Kaptein MJ. Hepatorenal syndrome misdiagnosis may be reduced using inferior vena cava ultrasound to assess intravascular volume and guide management. Ren Fail. 2023;45:2185468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 65. | Velez JCQ, Petkovich B, Karakala N, Huggins JT. Point-of-Care Echocardiography Unveils Misclassification of Acute Kidney Injury as Hepatorenal Syndrome. Am J Nephrol. 2019;50:204-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 66. | Bauman Z, Coba V, Gassner M, Amponsah D, Gallien J, Blyden D, Killu K. Inferior vena cava collapsibility loses correlation with internal jugular vein collapsibility during increased thoracic or intra-abdominal pressure. J Ultrasound. 2015;18:343-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 67. | Kajal K, Premkumar M, Izzy M, Kulkarni AV, Duseja AK, Divyaveer S, Loganathan S, Sihag B, Gupta A, Bahl A, Rathi S, Taneja S, De A, Verma N, Sharma N, Kaur H, Zohmangaihi D, Kumar V, Bhujade H, Chaluvashetty SB, Kalra N. Cirrhotic cardiomyopathy influences clinical outcomes and enhances performance of conventional risk prediction models in acute-on-chronic liver failure with severe sepsis. Aliment Pharmacol Ther. 2023;58:903-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 68. | Møller S, Bernardi M. Interactions of the heart and the liver. Eur Heart J. 2013;34:2804-2811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 322] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 69. | Møller S, Henriksen JH. Cirrhotic cardiomyopathy: a pathophysiological review of circulatory dysfunction in liver disease. Heart. 2002;87:9-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 241] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 70. | Kumar A, Das K, Sharma P, Mehta V, Sharma BC, Sarin SK. Hemodynamic studies in acute-on-chronic liver failure. Dig Dis Sci. 2009;54:869-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 71. | Moller S, Henriksen JH. Cardiovascular complications of cirrhosis. Gut. 2008;57:268-278. [RCA] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 281] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 72. | Koratala A, Ronco C, Kazory A. Albumin Infusion in Patients with Cirrhosis: Time for POCUS-Enhanced Physical Examination. Cardiorenal Med. 2021;11:161-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 73. | Perazella MA, Coca SG, Kanbay M, Brewster UC, Parikh CR. Diagnostic value of urine microscopy for differential diagnosis of acute kidney injury in hospitalized patients. Clin J Am Soc Nephrol. 2008;3:1615-1619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 120] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 74. | Cavanaugh C, Perazella MA. Urine Sediment Examination in the Diagnosis and Management of Kidney Disease: Core Curriculum 2019. Am J Kidney Dis. 2019;73:258-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 91] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 75. | Alsaad AA, Wadei HM. Fractional excretion of sodium in hepatorenal syndrome: Clinical and pathological correlation. World J Hepatol. 2016;8:1497-1501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 76. | Goyal S, Dixit VK, Jain AK, Shukla RC, Ghosh J, Kumar V. Intrarenal resistance index (RI) as a predictor of early renal impairment in patients with liver cirrhosis. Trop Gastroenterol. 2013;34:235-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 77. | Platt JF, Rubin JM, Ellis JH. Acute renal failure: possible role of duplex Doppler US in distinction between acute prerenal failure and acute tubular necrosis. Radiology. 1991;179:419-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 128] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 78. | Zeleke TK, Kemal LK, Mehari EA, Sema FD, Seid AM, Mekonnen GA, Abebe RB. Nephrotoxic drug burden and predictors of exposure among patients with renal impairment in Ethiopia: A multi-center study. Heliyon. 2024;10:e24618. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 79. | Jung ES, Lee JS, Kim MH, Kim NH, Kim KA, Moon YS. [Renal dysfunction after spontaneous bacterial peritonitis in cirrhosis: incidence and risk factors]. Korean J Gastroenterol. 2006;48:401-407. [PubMed] |
| 80. | Philips CA, Maiwall R, Sharma MK, Jindal A, Choudhury AK, Kumar G, Bhardwaj A, Mitra LG, Agarwal PM, Sarin SK. Comparison of 5% human albumin and normal saline for fluid resuscitation in sepsis induced hypotension among patients with cirrhosis (FRISC study): a randomized controlled trial. Hepatol Int. 2021;15:983-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 81. | Maiwall R, Rao Pasupuleti SS, Hidam AK, Kumar A, Tevethia HV, Vijayaraghavan R, Majumdar A, Prasher A, Thomas S, Mathur RP, Kumar G, Sarin SK. A randomised-controlled trial (TARGET-C) of high vs. low target mean arterial pressure in patients with cirrhosis and septic shock. J Hepatol. 2023;79:349-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 25] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 82. | Sanyal AJ, Boyer T, Garcia-Tsao G, Regenstein F, Rossaro L, Appenrodt B, Blei A, Gülberg V, Sigal S, Teuber P; Terlipressin Study Group. A randomized, prospective, double-blind, placebo-controlled trial of terlipressin for type 1 hepatorenal syndrome. Gastroenterology. 2008;134:1360-1368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 446] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 83. | Lebrec D, Moreau R, Cailmail S, Sogni P, Oberti F, Hadengue A. Effects of terlipressin on hemodynamics and oxygen content in conscious portal vein stenosed and cirrhotic rats receiving propranolol. J Hepatol. 1993;17:102-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 84. | Cavallin M, Piano S, Romano A, Fasolato S, Frigo AC, Benetti G, Gola E, Morando F, Stanco M, Rosi S, Sticca A, Cillo U, Angeli P. Terlipressin given by continuous intravenous infusion versus intravenous boluses in the treatment of hepatorenal syndrome: A randomized controlled study. Hepatology. 2016;63:983-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 223] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 85. | Jindal A, Singh H, Kumar G, Arora V, Sharma MK, Maiwall R, Rajan V, Tewathia HV, Vasishtha C, Sarin SK. Early Versus Standard Initiation of Terlipressin for Acute Kidney Injury in ACLF: A Randomized Controlled Trial (eTerli Study). Dig Dis Sci. 2024;69:2204-2214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 86. | Singh V, Ghosh S, Singh B, Kumar P, Sharma N, Bhalla A, Sharma AK, Choudhary NS, Chawla Y, Nain CK. Noradrenaline vs. terlipressin in the treatment of hepatorenal syndrome: a randomized study. J Hepatol. 2012;56:1293-1298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 201] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 87. | Singh V, Jayachandran A, De A, Singh A, Chandel S, Sharma N. Combination of terlipressin and noradrenaline versus terlipressin in hepatorenal syndrome with early non-response to terlipressin infusion: A randomized trial. Indian J Gastroenterol. 2023;42:388-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 88. | Skagen C, Einstein M, Lucey MR, Said A. Combination treatment with octreotide, midodrine, and albumin improves survival in patients with type 1 and type 2 hepatorenal syndrome. J Clin Gastroenterol. 2009;43:680-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 89. | Cavallin M, Kamath PS, Merli M, Fasolato S, Toniutto P, Salerno F, Bernardi M, Romanelli RG, Colletta C, Salinas F, Di Giacomo A, Ridola L, Fornasiere E, Caraceni P, Morando F, Piano S, Gatta A, Angeli P; Italian Association for the Study of the Liver Study Group on Hepatorenal Syndrome. Terlipressin plus albumin versus midodrine and octreotide plus albumin in the treatment of hepatorenal syndrome: A randomized trial. Hepatology. 2015;62:567-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 259] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 90. | Lieberman FL, Denison EK, Reynolds TB. The Relationship Of Plasma Volume, Portal Hypertension, Ascites, And Renal Sodium Retention In Cirrhosis: The Overflow Theory Of Ascites Formation. Ann N Y Acad Sci. 1970;170:202-212. [RCA] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 127] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 91. | Quinlan GJ, Martin GS, Evans TW. Albumin: biochemical properties and therapeutic potential. Hepatology. 2005;41:1211-1219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 600] [Cited by in RCA: 641] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 92. | Figueroa SM, Araos P, Reyes J, Gravez B, Barrera-Chimal J, Amador CA. Oxidized Albumin as a Mediator of Kidney Disease. Antioxidants (Basel). 2021;10:404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 93. | Grüngreiff K, Gottstein T, Reinhold D, Blindauer CA. Albumin Substitution in Decompensated Liver Cirrhosis: Don't Forget Zinc. Nutrients. 2021;13:4011. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 94. | Kouoh F, Gressier B, Luyckx M, Brunet C, Dine T, Cazin M, Cazin JC. Antioxidant properties of albumin: effect on oxidative metabolism of human neutrophil granulocytes. Farmaco. 1999;54:695-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 54] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 95. | Carvalho JR, Verdelho Machado M. New Insights About Albumin and Liver Disease. Ann Hepatol. 2018;17:547-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 132] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 96. | Bai Z, Méndez-Sánchez N, Romeiro FG, Mancuso A, Philips CA, Tacke F, Basaranoglu M, Primignani M, Ibrahim M, Wong YJ, Nery FG, Teschke R, Ferreira CN, Muñoz AE, Pinyopornpanish K, Thevenot T, Singh SP, Mohanty A, Satapathy SK, Ridola L, Maruyama H, Cholongitas E, Levi Sandri GB, Yang L, Shalimar, Yang Y, Villa E, Krag A, Wong F, Jalan R, O'Brien A, Bernardi M, Qi X; Liver Cirrhosis-related Complications (LCC)-International Special Interest Group. Use of albumin infusion for cirrhosis-related complications: An international position statement. JHEP Rep. 2023;5:100785. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 97. | de Mattos ÂZ, Simonetto DA, Terra C, Farias AQ, Bittencourt PL, Pase THS, Toazza MR, de Mattos AA; Alliance of Brazilian Centers for Cirrhosis Care - the ABC Group. Albumin administration in patients with cirrhosis: Current role and novel perspectives. World J Gastroenterol. 2022;28:4773-4786. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (7)] |
| 98. | Testino G, Ferro C, Sumberaz A, Messa P, Morelli N, Guadagni B, Ardizzone G, Valente U. Type-2 hepatorenal syndrome and refractory ascites: role of transjugular intrahepatic portosystemic stent-shunt in eighteen patients with advanced cirrhosis awaiting orthotopic liver transplantation. Hepatogastroenterology. 2003;50:1753-1755. [PubMed] |
| 99. | Rössle M, Gerbes AL. TIPS for the treatment of refractory ascites, hepatorenal syndrome and hepatic hydrothorax: a critical update. Gut. 2010;59:988-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 164] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 100. | Brensing KA, Textor J, Perz J, Schiedermaier P, Raab P, Strunk H, Klehr HU, Kramer HJ, Spengler U, Schild H, Sauerbruch T. Long term outcome after transjugular intrahepatic portosystemic stent-shunt in non-transplant cirrhotics with hepatorenal syndrome: a phase II study. Gut. 2000;47:288-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 286] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 101. | Sponholz C, Matthes K, Rupp D, Backaus W, Klammt S, Karailieva D, Bauschke A, Settmacher U, Kohl M, Clemens MG, Mitzner S, Bauer M, Kortgen A. Molecular adsorbent recirculating system and single-pass albumin dialysis in liver failure--a prospective, randomised crossover study. Crit Care. 2016;20:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 102. | Schaefer B, Schaefer F, Engelmann G, Meyburg J, Heckert KH, Zorn M, Schmitt CP. Comparison of Molecular Adsorbents Recirculating System (MARS) dialysis with combined plasma exchange and haemodialysis in children with acute liver failure. Nephrol Dial Transplant. 2011;26:3633-3639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 103. | Rifai K, Manns MP. Review article: clinical experience with Prometheus. Ther Apher Dial. 2006;10:132-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 104. | Allegretti AS, Parada XV, Eneanya ND, Gilligan H, Xu D, Zhao S, Dienstag JL, Chung RT, Thadhani RI. Prognosis of Patients with Cirrhosis and AKI Who Initiate RRT. Clin J Am Soc Nephrol. 2018;13:16-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 110] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 105. | Wong LP, Blackley MP, Andreoni KA, Chin H, Falk RJ, Klemmer PJ. Survival of liver transplant candidates with acute renal failure receiving renal replacement therapy. Kidney Int. 2005;68:362-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 105] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 106. | Nadim MK, Genyk YS, Tokin C, Fieber J, Ananthapanyasut W, Ye W, Selby R. Impact of the etiology of acute kidney injury on outcomes following liver transplantation: acute tubular necrosis versus hepatorenal syndrome. Liver Transpl. 2012;18:539-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 130] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 107. | Sharma P, Goodrich NP, Zhang M, Guidinger MK, Schaubel DE, Merion RM. Short-term pretransplant renal replacement therapy and renal nonrecovery after liver transplantation alone. Clin J Am Soc Nephrol. 2013;8:1135-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 108. | Hussain SM, Sureshkumar KK. Refining the Role of Simultaneous Liver Kidney Transplantation. J Clin Transl Hepatol. 2018;6:289-295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 109. | Asch WS, Bia MJ. New Organ Allocation System for Combined Liver-Kidney Transplants and the Availability of Kidneys for Transplant to Patients with Stage 4-5 CKD. Clin J Am Soc Nephrol. 2017;12:848-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 110. | Cheng XS, Stedman MR, Chertow GM, Kim WR, Tan JC. Utility in Treating Kidney Failure in End-Stage Liver Disease With Simultaneous Liver-Kidney Transplantation. Transplantation. 2017;101:1111-1119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 111. | Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, Durand F, Gustot T, Saliba F, Domenicali M, Gerbes A, Wendon J, Alessandria C, Laleman W, Zeuzem S, Trebicka J, Bernardi M, Arroyo V; CANONIC Study Investigators of the EASL-CLIF Consortium. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426-1437, 1437.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1720] [Cited by in RCA: 2170] [Article Influence: 180.8] [Reference Citation Analysis (5)] |
| 112. | Helal EM, Sharaf-Eldin M, Abou El Azm AR, Badr Eldin NM, Dawoud MM, Abd-Elsalam S, Ziada DH. Hemodynamic Changes of Hepatic & Renal Vessels in Systemic Bacterial Infection with Fever in HCV Related Cirrhosis. Infect Disord Drug Targets. 2020;20:511-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 113. | Kim MY, Baik SK, Lee SS. Hemodynamic alterations in cirrhosis and portal hypertension. Korean J Hepatol. 2010;16:347-352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 114. | Igna R, Gîrleanu I, Cojocariu C, Muzîca C, Huiban L, Sfarti C, Cuciureanu T, Chiriac S, Sîngeap AM, Petrea OC, Stafie R, Zenovia S, Năstasă R, Stratina E, Rotaru A, Stanciu C, Trifan A, Blaj M. The Role of Presepsin in Diagnosing Infections in Patients with Liver Cirrhosis and Overt Hepatic Encephalopathy. Diagnostics (Basel). 2022;12:2077. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 115. | Pandey G, Singh H, Chaturvedi S, Hatti M, Kumar A, Mishra R, Mishra P, Krishna VP, Bhadauria A, Mohindra S, Misra DP, Saraswat VA, Agarwal V. Utility of neutrophil CD64 in distinguishing bacterial infection from inflammation in severe alcoholic hepatitis fulfilling SIRS criteria. Sci Rep. 2021;11:19726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 116. | de Franchis R, Bosch J, Garcia-Tsao G, Reiberger T, Ripoll C; Baveno VII Faculty. Baveno VII - Renewing consensus in portal hypertension. J Hepatol. 2022;76:959-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1537] [Cited by in RCA: 1494] [Article Influence: 498.0] [Reference Citation Analysis (2)] |
| 117. | Alaniz C, Regal RE. Spontaneous bacterial peritonitis: a review of treatment options. P T. 2009;34:204-210. [PubMed] |
| 118. | Kulkarni AV, Tirumalle S, Premkumar M, Kumar K, Fatima S, Rapole B, Simhadri V, Gora BA, Sasikala M, Gujjarlapudi D, Yelamanchili S, Sharma M, Gupta R, Rao PN, Reddy DN. Primary Norfloxacin Prophylaxis for APASL-Defined Acute-on-Chronic Liver Failure: A Placebo-Controlled Double-Blind Randomized Trial. Am J Gastroenterol. 2022;117:607-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 36] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 119. | Philips CA, Augustine P, Ahamed R, Rajesh S, George T, Valiathan GC, John SK. Role of Granulocyte Colony-stimulating Factor Therapy in Cirrhosis, 'Inside Any Deep Asking Is the Answering'. J Clin Transl Hepatol. 2019;7:371-383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 120. | Ibrahim ES, Sharawy A. Effectiveness of intravenous infusion of N-acetylcysteine in cirrhotic patients undergoing major abdominal surgeries. Saudi J Anaesth. 2015;9:272-278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 121. | Nguyen-Khac E, Thevenot T, Piquet MA, Benferhat S, Goria O, Chatelain D, Tramier B, Dewaele F, Ghrib S, Rudler M, Carbonell N, Tossou H, Bental A, Bernard-Chabert B, Dupas JL; AAH-NAC Study Group. Glucocorticoids plus N-acetylcysteine in severe alcoholic hepatitis. N Engl J Med. 2011;365:1781-1789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 295] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 122. | Sort P, Navasa M, Arroyo V, Aldeguer X, Planas R, Ruiz-del-Arbol L, Castells L, Vargas V, Soriano G, Guevara M, Ginès P, Rodés J. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med. 1999;341:403-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1132] [Cited by in RCA: 1003] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 123. | Yeung E, Yong E, Wong F. Renal dysfunction in cirrhosis: diagnosis, treatment and prevention. MedGenMed. 2004;6:9. [PubMed] |
| 124. | Ul Abideen Z, Mahmud SN, Salih M, Arif A, Ali F, Rasheed A, Zafran M. Contrast-induced Acute Kidney Injury in Patients with Liver Cirrhosis: A Retrospective Analysis. Cureus. 2018;10:e2707. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 125. | Konstantis G, Tsaousi G, Pourzitaki C, Kitsikidou E, Magouliotis DE, Wiener S, Zeller AC, Willuweit K, Schmidt HH, Rashidi-Alavijeh J. Efficacy of Granulocyte Colony-Stimulating Factor in Acute on Chronic Liver Failure: A Systematic Review and Survival Meta-Analysis. J Clin Med. 2023;12:6541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 126. | Engelmann C, Herber A, Franke A, Bruns T, Reuken P, Schiefke I, Zipprich A, Zeuzem S, Goeser T, Canbay A, Berg C, Trebicka J, Uschner FE, Chang J, Mueller T, Aehling N, Schmelzle M, Splith K, Lammert F, Lange CM, Sarrazin C, Trautwein C, Manns M, Häussinger D, Pfeiffenberger J, Galle PR, Schmiedeknecht A, Berg T. Granulocyte-colony stimulating factor (G-CSF) to treat acute-on-chronic liver failure: A multicenter randomized trial (GRAFT study). J Hepatol. 2021;75:1346-1354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 91] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 127. | Henriksen JH, Ring-Larsen H, Christensen NJ. Circulating noradrenaline and central haemodynamics in patients with cirrhosis. Scand J Gastroenterol. 1985;20:1185-1190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 51] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 128. | Rowell LB, Detry JM, Blackmon JR, Wyss C. Importance of the splanchnic vascular bed in human blood pressure regulation. J Appl Physiol. 1972;32:213-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 118] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 129. | Houlihan DD, Holt A, Elliot C, Ferguson JW. Review article: liver transplantation for the pulmonary disorders of portal hypertension. Aliment Pharmacol Ther. 2013;37:183-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 130. | Pouria S, Feehally J. Glomerular IgA deposition in liver disease. Nephrol Dial Transplant. 1999;14:2279-2282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 34] [Article Influence: 1.3] [Reference Citation Analysis (0)] |