Published online Aug 26, 2025. doi: 10.4252/wjsc.v17.i8.107124
Revised: May 11, 2025
Accepted: July 4, 2025
Published online: August 26, 2025
Processing time: 149 Days and 23.8 Hours
Tendon tissue engineering requires biomimetic scaffolds and mechanical cues to direct mesenchymal stem cell differentiation toward tenogenic lineages. Bone marrow-derived mesenchymal stem cells (BMSCs), aligned nanofiber scaffolds, and cyclic uniaxial stretching can be used to create a functional engineered ligament tissue.
To investigate the effects of aligned nanofiber scaffolds and cyclic stretch on BMSC tenogenesis for ligament engineering.
BMSCs were cultured on aligned and random poly-lactic acid nanofiber scaffolds under static and cyclic tensile conditions (0.5 Hz, 2% strain, 2 hours/day) for 7 days using a mechanical loading system (CFILLOAD-300). The Ras homolog gene family (Rho)-associated coiled coil-containing kinase (ROCK) inhibitor Y27632 was applied to explore its role in tenogenic differentiation. Scaffold morphology was assessed by scanning electron microscopy, while cell morp
Scanning electron microscopy revealed that aligned scaffolds provided consistent directional structure, whereas random scaffolds displayed a disordered fiber arrangement. Confocal microscopy showed that under static conditions, BMSCs on aligned scaffolds grew parallel to fiber alignment, while those on random scaffolds grew randomly. Under cyclic tensile strain, BMSCs on both scaffold types exhibited elongation along the direction of strain, adopting a spindle-shaped morphology. Cyclic uniaxial strain enhanced cell viability and metabolic activity based on CCK-8 assay results and upregulated ligament-specific gene and protein expression on aligned scaffolds compared to static conditions. BMSCs on aligned scaffolds under tensile strain showed the highest expression of tenogenic markers, suggesting a synergistic effect of scaffold alignment and mechanical loading. ROCK inhibition with Y27632 upregulated alternative signaling pathways (focal adhesion kinase and runt-related transcription factor 2), further promoting tenogenic differentiation.
Aligned nanofiber scaffolds combined with cyclic tensile strain provide an optimal environment for guiding BMSC differentiation toward ligamentous lineages, as assessed by increased expression of ligament-specific markers. Mechanical stimulation (uniaxial stretching) significantly influences BMSC tenogenic differentiation, and the combined use of aligned nanofibers and tensile strain further enhances this effect. The ROCK pathway plays a regulatory role in this process, though its precise mechanisms require further investigation.
Core Tip: This study investigated the role of aligned nanofiber scaffolds and cyclic stretch in promoting Bone marrow-derived mesenchymal stem cell (BMSC) differentiation towards ligamentous tissue. We demonstrate that cyclic tensile strain, when applied to BMSCs on aligned nanofiber scaffolds, enhances tenogenic differentiation, as demonstrated by increased expression of collagen and tenogenic markers. These findings suggest that mechanical cues and scaffold alignment are critical in guiding BMSC differentiation for ligament tissue engineering, providing valuable insights for developing strategies to improve ligament regeneration.
- Citation: Yang CW, Zhang YQ, Chang H, Gao R, Chen D, Yao H. Aligned nanofiber scaffolds combined with cyclic stretch facilitate mesenchymal stem cell differentiation for ligament engineering. World J Stem Cells 2025; 17(8): 107124
- URL: https://www.wjgnet.com/1948-0210/full/v17/i8/107124.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v17.i8.107124
The regenerative potential of mesenchymal stem cells (MSCs) in tendon tissue engineering has gained significant attention due to their ability to differentiate into tenocyte-like cells under specific microenvironmental cues[1]. One crucial factor in guiding MSCs towards tenogenic differentiation is scaffold design, which provides both structural and biochemical cues that mimic native tendon tissue[2-5]. Aligned nanofiber scaffolds are effective in directing MSC alignment and enhancing differentiation, as their anisotropic structure mimics the native tendon extracellular matrix[6-8]. The combination of scaffold alignment and mechanical stimulation, such as cyclic uniaxial stretch, further promotes tenogenic differentiation by simulating physiological tendon loading conditions[4,6,9,10].
Recent studies have explored various mechanical and biochemical stimuli to enhance the tenogenic potential of MSCs. Cyclic stretching has been shown to upregulate tenogenic markers in MSCs, such as collagen I, collagen III, and tenascin C[11,12]. Mechanical loading activates key pathways involved in tenogenesis, including Ras homolog gene family (Rho)-associated coiled coil-containing kinase (ROCK), focal adhesion kinase (FAK), and runt-related transcription factor 2 (RUNX2), which regulate cytoskeletal organization and cellular alignment[13]. While ROCK inhibition hinders MSC differentiation towards tendon lineages, its role may vary depending on other factors, such as scaffold structure and mechanical conditions[9].
This study combines aligned nanofiber scaffolds with cyclic uniaxial stretch to create a biomimetic environment for MSC differentiation, to elucidate the effects of scaffold alignment, tensile strain, and ROCK inhibition on tenogenic differentiation. Using both gene and protein expression analyses, this research provided insights into the potential synergistic effects of scaffold alignment and mechanical strain in promoting ligament-like MSC differentiation, challenging previous findings that ROCK inhibition suppresses this process[14]. The results offer valuable implications for designing effective tendon tissue engineering strategies by leveraging both structural and mechanical cues.
Aligned and random poly-lactic acid (PLA) nanofiber scaffolds were fabricated by dissolving PLA at 20% (w/w) in a dichloromethane and N,N-dimethylformamide mixture (2:1 w/w). Electrospinning was conducted at a rate of 2 mL/hour with a needle-to-collector distance of 15 cm and 18 kV voltage. Aligned fibers were collected on a rotating mandrel, while random fibers were collected on a stationary plate. Both scaffold types were dried and stored. Scanning electron microscopy (SEM) was used to observe fiber morphology. Mechanical properties, including Young’s modulus, fracture strength, and elongation, were assessed using a tensile tester at 10 mm/minute, with a maximum load of 10 N. Wett
Bone marrow-derived MSCs (BMSCs) were isolated from 2-week-old New Zealand white rabbits. Following euthanasia via ear vein injection of ketamine (1-2 mL), femurs and tibias were harvested under sterile conditions. The marrow cavity was flushed with Dulbecco’s modified Eagle’s medium (Gibco, A3161002, NY, United States) supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin (100 mg/mL) to collect bone marrow. The suspension was cultured at 37 °C in 5% CO2. Non-adherent cells were removed after 48 hours, and adherent cells were cultured to 80%-90% con
Aligned and random nanofiber scaffolds were cut into 9 cm × 1.5 cm strips and sterilized under ultraviolet light for 2 hours. Each strip was embedded in silicone culture chambers within a mechanical stretching device (CFILLOAD-300), pretreated with ethanol, and washed with phosphate buffered saline (PBS). BMSCs were seeded at 20000 cells/cm2 and cultured in a humidified incubator at 37 °C, 5% CO2. After 2 days, scaffolds were subjected to uniaxial cyclic tensile strain (2% strain at 0.5 Hz for 2 hours daily) for 7 days. Static controls were maintained without strain.
To assess BMSC viability, scaffolds were cut into 1.4 cm × 1.4 cm squares and sterilized, and seeded on aligned, random, and control surfaces (12-well plates). Cell viability was evaluated at 4 hours, 1 day, 4 days, and 7 days using the CCK-8 assay, which measures cellular metabolic activity. At each time point, the medium was aspirated, and the scaffolds mounted on Cellcrown fixtures, transferred to new 12-well plates, washed with PBS, and incubated with fresh culture medium containing 10% CCK-8 for 2 hours at 37 °C. After incubation, 200 μL CCK-8 solution was transferred to a 96-well plate, and optical density was measured at 450 nm.
After 1 and 7 days of culture, scaffolds were processed for SEM to evaluate BMSC morphology. Samples were gently washed with PBS, fixed with 2.5% glutaraldehyde for 30 minutes, dehydrated through graded ethanol, and dried. Samples were mounted on stubs, sputter-coated with gold, and imaged.
For confocal microscopy, BMSCs were cultured for 1 and 7 days, then fixed, permeabilized, and stained with rhodamine-phalloidin for F-actin and 4’,6-diamidino-2-phenylindole (DAPI) for nuclei. The scaffolds were mounted on slides and imaged to analyze cytoskeletal arrangement and cell alignment relative to scaffold orientation and tensile strain.
Total RNA was extracted from BMSCs on scaffolds after 7 days of culture. cDNA synthesis was performed, and quantitative polymerase chain reaction assessed the expression of tenogenic markers, including collagen type I alpha 2 (COL1A2), collagen type III alpha 1 (COL3A1), tenascin C, and tenomodulin, with GAPDH as an internal control. Fold changes in gene expression relative to static controls were calculated by the ΔΔCt method[9].
After 7 days of culture, scaffolds mounted on Cellcrown fixtures and scaffolds subjected to mechanical stretching (using the mechanical stretching machine) were carefully removed. The scaffolds on the stretching machine were in silicone wells, while those in the Cellcrown were in 12-well plates. Both sets of scaffolds were washed twice with PBS to remove any residual medium. The scaffolds were transferred to sterile culture dishes, gently cut into smaller pieces with sterile scissors, and placed into 2 mL EP tubes. The samples were homogenized, and protein was extracted using RIPA lysis buffer containing 10% phenylmethanesulfonyl fluoride. Protein concentration was measured using a BCA kit, and the protein was stored at -80 °C for later analysis. Western blotting was performed, and proteins were detected using specific primary antibodies against collagen I (BIOSS, BS-10423R, Beijing, China), collagen III (BIOSS, BS-0549R, Beijing, China), tenascin C (BIOSS, BS-1039R, Beijing, China), and tenomodulin (BIOSS, BS-7525R, Beijing, China), with β-actin (BIOSS, BS-20735R, Beijing, China) as a loading control. Protein bands were visualized and quantified.
All experimental data were expressed as mean ± SD. Statistical significance was determined using one-way analysis of variance with post-hoc tests (P < 0.01 considered significant) using SPSS version24.0 software.
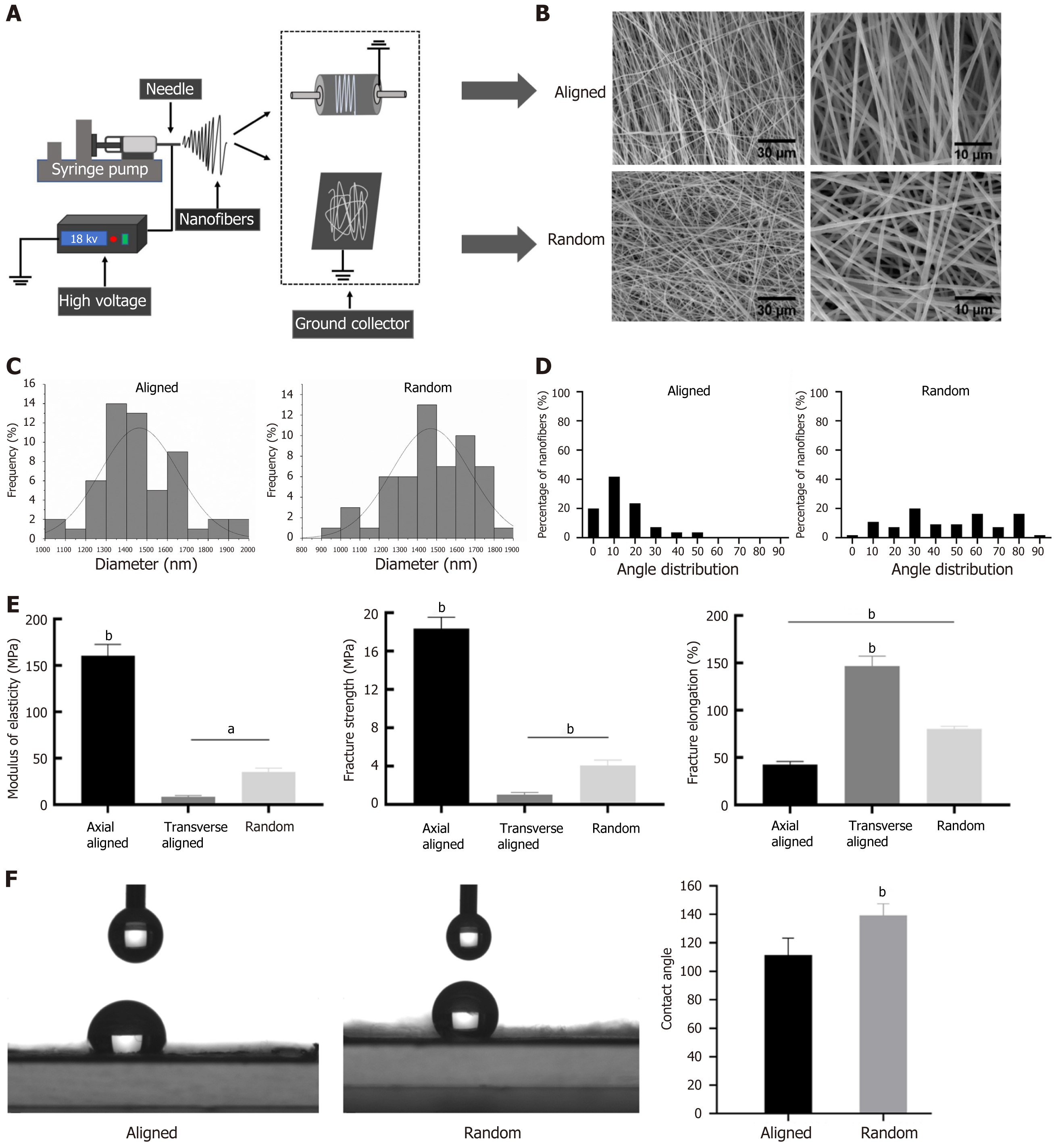
We prepared random and aligned nanofiber scaffolds using an electrospinning setup with either a rotating or stationary collector, resulting in distinct fiber orientations (Figure 1A). Both scaffold types presented a uniform, smooth texture, with an approximate thickness of 100 μm, and appeared as consistent, milky-white films (Supplementary Figure 1). SEM images illustrate structural differences between the scaffolds, with aligned fibers displaying a parallel arrangement and random fibers exhibiting a disordered configuration (Figure 1B). Image analysis at 3000 × and 1000 × magnification showed that both types of nanofibers maintained similar diameter ranges, averaging 1460.71 ± 188.76 nm for aligned scaffolds and 1469.92 ± 205.35 nm for random scaffolds, indicating no significant difference in fiber thickness (Figure 1C, Supplementary Table 1).
Angle distribution analysis highlighted clear differences in orientation: Aligned scaffolds primarily displayed a narrow angle range (0°-30°), confirming their uniform alignment, while fibers in random scaffolds show a broader, nearly uniform angle distribution from 0° to 90° (Figure 1D). Mechanical testing further revealed that aligned scaffolds exhibited higher modulus of elasticity, fracture strength, and fracture elongation compared to transversely aligned and random fibers, suggesting that alignment enhances mechanical performance along the fiber direction (Figure 1E). Contact angle measurements indicated that random fibers had a higher contact angle than aligned fibers, reflecting greater hydrophobicity in the random orientation (Figure 1F), which aligned with the hyd
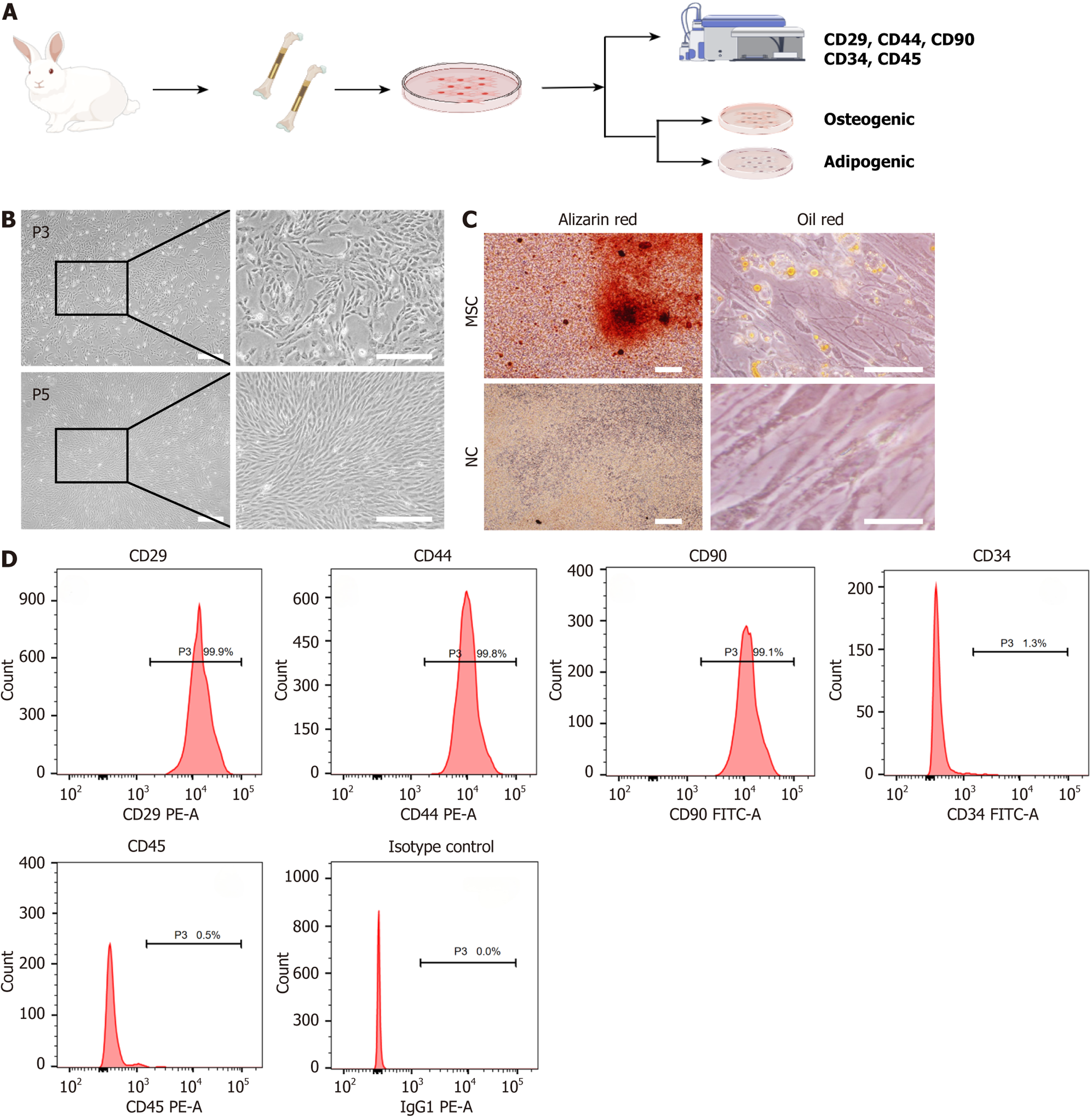
We systematically isolated, cultured, and characterized rabbit BMSCs to verify their multipotency and phenotype (Figure 2A). We obtained primary BMSCs using the whole bone marrow adherence method, with cells initially displaying a polygonal or cobblestone shape after 2 days of culture. By 8-10 days, cell confluence reached > 90%. Following subculturing at a 1:3 ratio, BMSCs achieved 90%-95% confluence within 4-5 days, and by passage 3, most cells had adopted a spindle or polygonal morphology, with some retaining a cobblestone shape. At passage 5, cells grew in a radial or vortex pattern with a predominantly spindle-shaped morphology, indicating optimal cell state (Figure 2A and B).
We performed differentiation assays to verify the multipotent potential of BMSCs. When cultured in osteogenic induction medium for 21 days, BMSCs displayed significant calcium nodule deposition, as indicated by positive Alizarin Red staining, while the control group showed no such staining (Figure 2C). For adipogenic differentiation, BMSCs cultured in adipogenic induction medium for 14 days developed variously sized lipid droplets in the cytoplasm, visua
Flow cytometry analysis of surface markers confirmed the MSC phenotype, with high expression of CD29 (99.9%), CD90 (99.1%), and CD44 (99.8%), and low expression of CD34 (1.3%) and CD45 (0.5%), consistent with MSC characteristics (Figure 2D). These results confirm the successful isolation, typical morphology, and multipotent differentiation capability of rabbit-derived BMSCs.
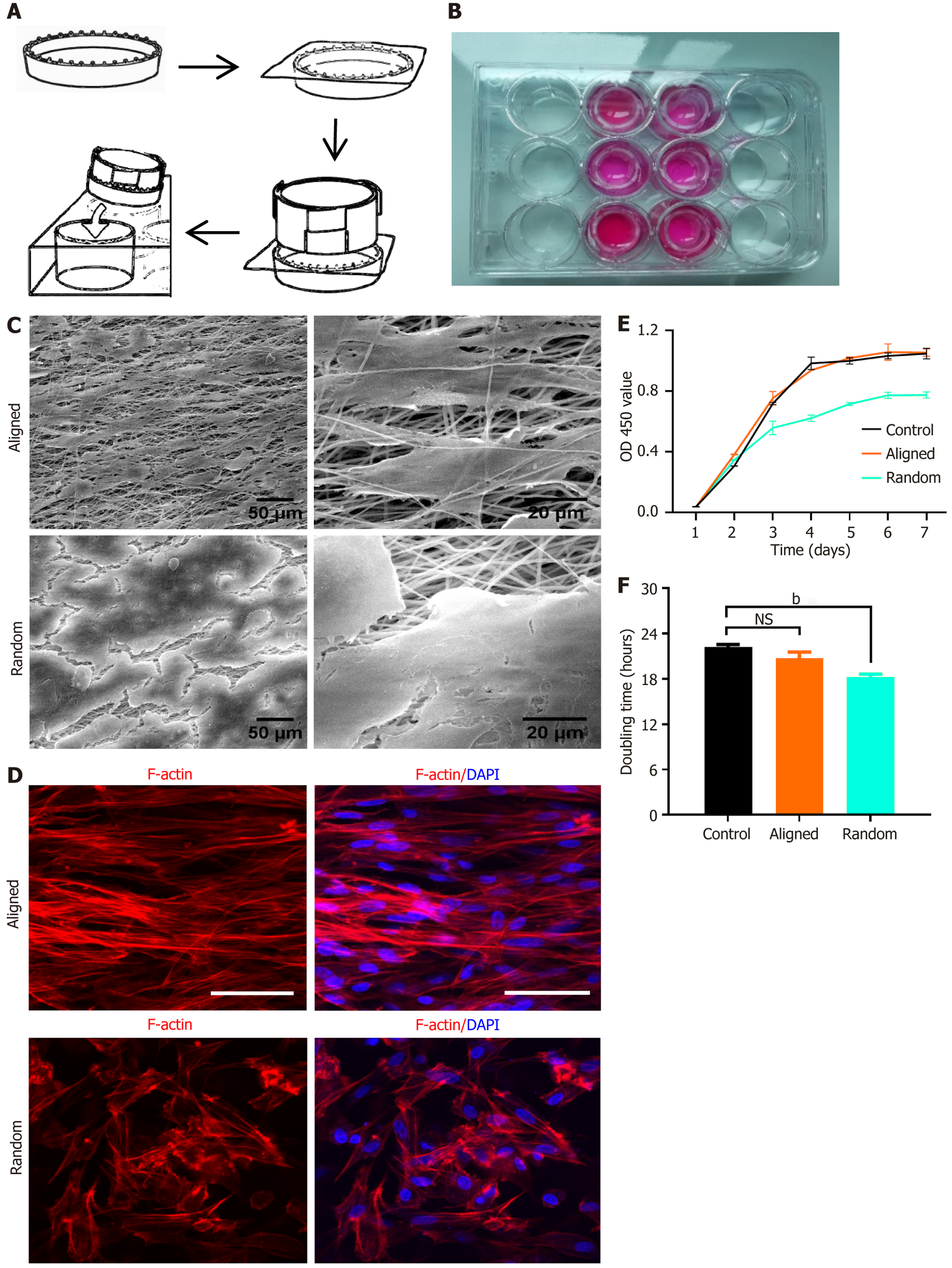
BMSCs were consistently seeded onto aligned and random nanofiber scaffolds (Figure 3A and B). After 7 days of culture, SEM images showed that cells on aligned scaffolds grew along the fiber direction, adopting an elongated, spindle-shaped morphology with clear polarity. In contrast, cells on random scaffolds exhibited a polygonal shape, growing in various directions without apparent polarity, and the extracellular matrix secreted by BMSCs covered the entire scaffold surface for both scaffold types (Figure 3C).
We stained for F-actin and nuclei and performed confocal laser scanning microscopy, which revealed the cytoskeletal arrangement and morphology of BMSCs. On aligned scaffolds, BMSCs displayed a parallel alignment along the fiber orientation, with elongated, spindle-shaped cells showing pronounced polarity. Conversely, BMSCs on random scaffolds exhibited a more disorganized, multidirectional growth pattern, appearing polygonal and lacking polarity (Figure 3D).
BMSC viability and metabolic activity on both scaffolds was assessed over 7 days using a CCK-8 assay, which quantified mitochondrial enzymatic activity via optical density at 450 nm. BMSCs exhibited increased metabolic activity over time on both scaffold types, suggesting good biocompatibility and scaffold support (Figure 3E). The control group, consisting of BMSCs cultured on standard tissue culture plates without scaffolds, was included for comparison. On day 4, we observed a significant increase in absorbance on aligned scaffolds compared to the random group (P < 0.05), while there was no significant difference between the aligned and control groups. By day 7, BMSCs on aligned scaffolds maintained higher metabolic activity than those on random scaffolds, with a similar trend observed compared to the control group, though without statistical significance. Figure 3F shows doubling time analysis, where BMSCs on aligned scaffolds exhibited a shorter doubling time compared to those on random and control scaffolds, indicating a higher viability rate on aligned nanofibers. These results highlight the influence of fiber alignment on BMSC morphology, ori
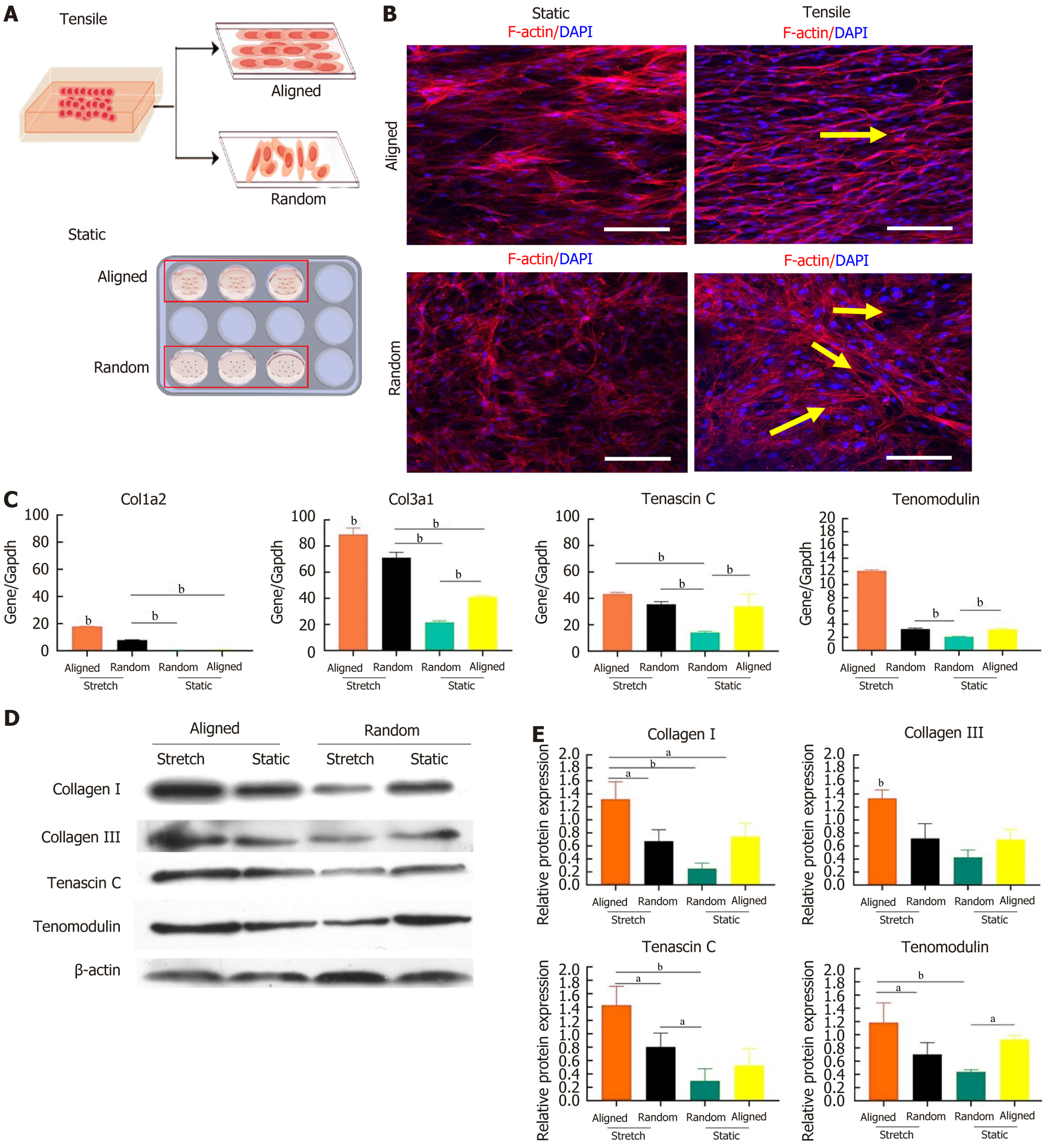
To investigate how scaffold alignment and mechanical loading affect BMSC morphology and ligament differentiation, BMSCs were cultured on aligned and random nanofiber scaffolds under both static and tensile conditions (Figure 4A). After 7 days, confocal microscopy revealed distinct morphological responses (Figure 4B). Under static conditions, BMSCs on aligned scaffolds aligned along the nanofiber direction, whereas cells on random scaffolds exhibited random growth without clear orientation. When tensile strain was applied, BMSCs on aligned scaffolds displayed pronounced elongation along the stretch direction, adopting a fibroblast-like morphology with clear polarity, while cells on random scaffolds showed partial alignment along the tensile direction, with some cells appearing elongated.
Ligament-specific gene expression in BMSCs was also influenced by scaffold alignment and tensile strain. Quantitative polymerase chain reaction analysis indicated upregulation of ligament-specific markers (COL1A2, COL3A1, teno
Protein expression results further confirmed these findings, showing that ligament-related proteins (collagen I, collagen III, tenascin C, and tenomodulin) were elevated in BMSCs on aligned scaffolds under tensile strain compared to both the static condition and random scaffolds (Figure 4D and E). These results suggested that aligned nanofiber scaffolds combined with tensile strain created an optimal environment for ligamentous differentiation of BMSCs, as indicated by the highest expression of ligament-related genes and proteins. In contrast, random scaffolds under tensile strain displayed a differentiation effect like that of aligned scaffolds under static conditions, indicating that scaffold alignment and mechanical strain synergistically enhanced BMSC differentiation toward a ligamentous phenotype.
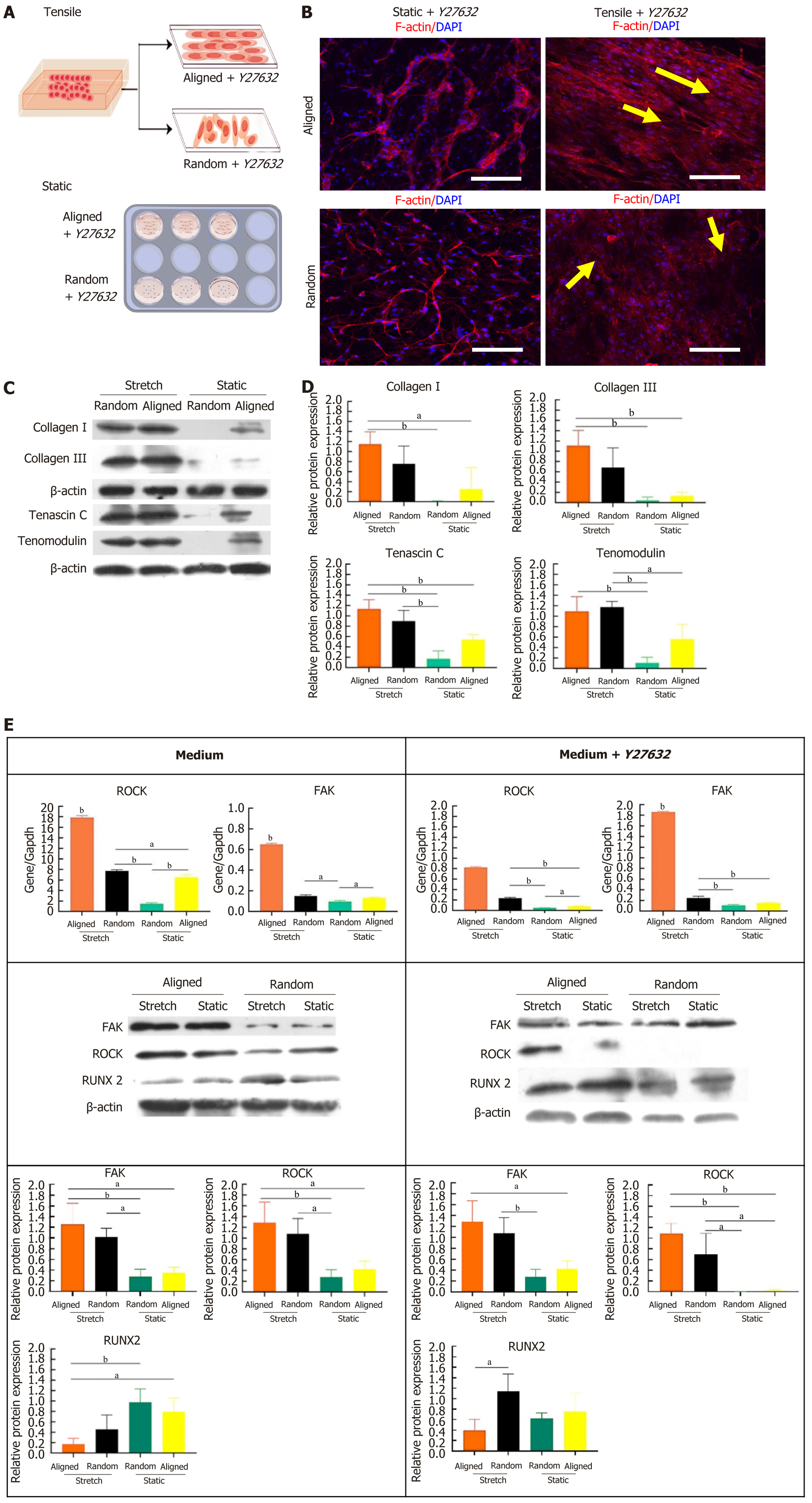
To test the influence of ROCK pathway inhibition on BMSC differentiation and morphology, we cultured BMSCs on aligned and random nanofiber scaffolds under both static and tensile conditions, with or without the ROCK inhibitor Y27632 (Figure 5A). Confocal microscopy analysis of F-actin and DAPI staining revealed that, even with ROCK inhi
Western blot analysis revealed that Y72632 significantly altered the expression of tenogenic proteins (collagen I, collagen III, tenascin C, and tenomodulin) under static and tensile conditions. As shown in Figure 5C and quantified in Figure 5D, BMSCs on aligned scaffolds under tensile conditions exhibited the highest levels of these proteins, even with ROCK inhibition. These levels were significantly higher compared to static conditions. Random scaffolds under tensile strain also showed increased protein expression compared to those under static conditions, although the increase was less pronounced than in aligned scaffolds. These results suggest that ROCK inhibition does not significantly impair tensile strain-induced tenogenic differentiation, especially on aligned scaffolds.
Further analysis of gene and protein expression of signaling molecules related to tenogenic differentiation, including ROCK, FAK, and RUNX2, was performed with and without Y27632 treatment (Figure 5E). In the presence of Y27632, aligned scaffolds under tensile strain exhibited increased FAK and RUNX2 expression, with statistically significant differences compared to static conditions and random scaffolds. As expected, ROCK expression was reduced due to the inhibitory effect of Y27632. These findings suggest that ROCK inhibition promotes tenogenic differentiation via alternative signaling pathways, such as FAK and RUNX2, particularly in the context of aligned scaffolds and tensile strain. This provides a favorable environment for BMSC ligamentous differentiation, even under ROCK inhibition.
Our study highlights that combining aligned PLA nanofiber scaffolds with cyclic uniaxial stretch provides an optimal environment to promote BMSC differentiation toward a ligament-like lineage. The major findings reveal that cyclic tensile strain on aligned nanofibers significantly upregulates tenogenic marker expression, such as COL1A2, COL3A1, tenascin C, and tenomodulin, while concurrently downregulating osteogenic markers. We found that ROCK inhibition via Y27632 did not impede this tenogenic differentiation, suggesting that alternative pathways may compensate under mechanical strain. These results underscore the potential of aligned nanofiber scaffolds combined with cyclic tensile loading to facilitate functional engineered ligament tissue formation.
In ligament tissue engineering, the objective is to recreate functional replacement tissue, mimicking the mechanical properties of natural tendons and ligaments[2,11,15]. Multiple studies have emphasized the crucial role of mechanical forces in directing MSCs toward tenogenic differentiation, especially through cyclic mechanical stimuli that replicate in vivo conditions[2,3]. In our study, BMSCs cultured on aligned nanofiber scaffolds under cyclic uniaxial stretch showed enhanced tenogenic marker expression and a decrease in osteogenic differentiation markers, further corroborating that mechanical stimuli promote tenogenesis and suppress alternative differentiation pathways, which agrees with Maharam et al[15]. Our application of low-frequency, small-amplitude cyclic stretch (2% strain at 0.5 Hz) proved effective in promoting tenogenesis while maintaining cell viability. Prior research demonstrated that low-frequency mechanical loading leads to intracellular cytoskeletal reorganization, such as actin filament thickening and the formation of brush structures[4,10,16,17]. Importantly, high-frequency mechanical loading (≥ 1.5 Hz) induced cell contraction and structural deformation due to an inability to cope with rapid changes, inhibiting mechanotransduction and stem cell differentiation[18]. By maintaining a physiological strain range (2%-4%), we avoided micro-tearing, which is seen at higher strains (≥ 6%), allowing BMSCs to differentiate effectively without overstress[6,13].
Our results show that mechanical strain promotes cell viability on both scaffold types. BMSCs on aligned scaffolds under cyclic strain demonstrated greater viability and directional elongation compared to static conditions. Consistent with prior research, mechanical cues induce cytoskeletal reorganization and align cells along the strain axis, enhancing extracellular matrix deposition and promoting tenocyte-like morphology[17,19]. The directional elongation we observed in BMSCs on aligned scaffolds mimics the spindle shape of native ligament cells, underscoring the critical role of scaffold alignment and cyclic strain in achieving native-like cell morphology and ECM organization[20]. In contrast, BMSCs on random scaffolds under similar conditions failed to orient in a consistent pattern, indicating that scaffold alignment synergizes with uniaxial stretching to induce organized cellular architecture.
Contrary to previous studies that suggest that ROCK pathway inhibition can prevent MSC differentiation into tenogenic lineages[11,15], we demonstrated that ROCK inhibition with Y27632 did not significantly suppress tenogenic differentiation when combined with cyclic uniaxial stretching. ROCK signaling is typically associated with cytoskeletal dynamics and cell morphology, and it is suggested to regulate tenogenesis via the RhoA and FAK pathways[14]. However, our findings suggest that mechanical strain may activate compensatory pathways, possibly through FAK and RUNX2, which facilitate tenogenesis independent of ROCK activation[10,15]. This observation aligns with other studies indicating that Y27632-induced ROCK inhibition leads to alternative pathways in MSCs under certain conditions, allowing cells to bypass ROCK-dependent mechanisms[21].
Mechanical stimuli have long been recognized to enhance MSC viability and tenogenesis by activating surface receptors and calcium ion channels, improving ATP availability, and facilitating nutrient transport[22]. Our study dem
While our study provides valuable insights into the effects of scaffold alignment and cyclic tensile strain on BMSC tenogenic differentiation, it is important to recognize that the in vitro environment used here does not fully replicate the intricate conditions found in vivo, where additional factors such as biochemical signaling from surrounding tissues and dynamic physiological forces play essential roles. Future research could explore these findings in animal models to better understand how these in vitro optimizations translate to practical applications in tendon and ligament repair. Addi
This study demonstrates that combining aligned nanofiber scaffolds with cyclic uniaxial stretch fosters tenogenic differentiation of BMSCs, demonstrated by enhanced expression of ligament-related markers and morphology similar to native ligament cells. The use of mechanical strain with scaffold alignment supports the development of a functional tissue-engineered ligament, promoting BMSC alignment, viability, and ligament-like ECM deposition without triggering osteogenic differentiation. Contrary to previous studies, ROCK inhibition did not suppress tenogenic differentiation under these conditions, suggesting that mechanical cues may activate alternative signaling pathways to drive teno
Future studies should explore the mechanistic roles of alternative pathways, such as FAK and RUNX2, in facilitating ligament differentiation under ROCK inhibition. Additionally, optimizing mechanical parameters like strain magnitude and duration could further improve differentiation efficiency. Translational research using animal models would also provide insights into how these in vitro strategies perform within a physiological context. Understanding the interactions between scaffold design, mechanical cues, and signaling pathways will aid in refining tissue engineering approaches for ligament regeneration, ultimately bringing these methods closer to clinical applications in musculoskeletal repair.
| 1. | Shi J, Yao H, Chong H, Hu X, Yang J, Dai X, Liu D, Wu Z, Dang M, Fei W, Wang DA. Tissue-engineered collagen matrix loaded with rat adipose-derived stem cells/human amniotic mesenchymal stem cells for rotator cuff tendon-bone repair. Int J Biol Macromol. 2024;282:137144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 2. | Tan SL, Chan CK, Ahmad TS, Teo SH, Ng WM, Selvaratnam L, Kamarul T. Growth Differentiation Factor 5-Induced Mesenchymal Stromal Cells Enhance Tendon Healing. Tissue Eng Part C Methods. 2024;30:431-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 3. | Roets B, Abrahamse H, Crous A. The Application of Photobiomodulation on Mesenchymal Stem Cells and its Potential Use for Tenocyte Differentiation. Curr Stem Cell Res Ther. 2025;20:232-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Burk J, Plenge A, Brehm W, Heller S, Pfeiffer B, Kasper C. Induction of Tenogenic Differentiation Mediated by Extracellular Tendon Matrix and Short-Term Cyclic Stretching. Stem Cells Int. 2016;2016:7342379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 5. | Caliari SR, Harley BA. Structural and biochemical modification of a collagen scaffold to selectively enhance MSC tenogenic, chondrogenic, and osteogenic differentiation. Adv Healthc Mater. 2014;3:1086-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 82] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 6. | Park H, Nazhat SN, Rosenzweig DH. Mechanical activation drives tenogenic differentiation of human mesenchymal stem cells in aligned dense collagen hydrogels. Biomaterials. 2022;286:121606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 7. | Caliari SR, Weisgerber DW, Grier WK, Mahmassani Z, Boppart MD, Harley BA. Collagen Scaffolds Incorporating Coincident Gradations of Instructive Structural and Biochemical Cues for Osteotendinous Junction Engineering. Adv Healthc Mater. 2015;4:831-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 8. | Czaplewski SK, Tsai TL, Duenwald-Kuehl SE, Vanderby R Jr, Li WJ. Tenogenic differentiation of human induced pluripotent stem cell-derived mesenchymal stem cells dictated by properties of braided submicron fibrous scaffolds. Biomaterials. 2014;35:6907-6917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 9. | Melzer M, Niebert S, Heimann M, Ullm F, Pompe T, Scheiner-Bobis G, Burk J. Differential Smad2/3 linker phosphorylation is a crosstalk mechanism of Rho/ROCK and canonical TGF-β3 signaling in tenogenic differentiation. Sci Rep. 2024;14:10393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 10. | Wang W, Deng D, Li J, Liu W. Elongated cell morphology and uniaxial mechanical stretch contribute to physical attributes of niche environment for MSC tenogenic differentiation. Cell Biol Int. 2013;37:755-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Melzer M, Schubert S, Müller SF, Geyer J, Hagen A, Niebert S, Burk J. Rho/ROCK Inhibition Promotes TGF-β3-Induced Tenogenic Differentiation in Mesenchymal Stromal Cells. Stem Cells Int. 2021;2021:8284690. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 12. | Donderwinkel I, Tuan RS, Cameron NR, Frith JE. A systematic investigation of the effects of TGF-β3 and mechanical stimulation on tenogenic differentiation of mesenchymal stromal cells in a poly(ethylene glycol)/gelatin-based hydrogel. J Orthop Translat. 2023;43:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 13. | Nam HY, Balaji Raghavendran HR, Pingguan-Murphy B, Abbas AA, Merican AM, Kamarul T. Fate of tenogenic differentiation potential of human bone marrow stromal cells by uniaxial stretching affected by stretch-activated calcium channel agonist gadolinium. PLoS One. 2017;12:e0178117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Yang G, Rothrauff BB, Lin H, Gottardi R, Alexander PG, Tuan RS. Enhancement of tenogenic differentiation of human adipose stem cells by tendon-derived extracellular matrix. Biomaterials. 2013;34:9295-9306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 132] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 15. | Maharam E, Yaport M, Villanueva NL, Akinyibi T, Laudier D, He Z, Leong DJ, Sun HB. Rho/Rock signal transduction pathway is required for MSC tenogenic differentiation. Bone Res. 2015;3:15015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 16. | Kuo CK, Tuan RS. Mechanoactive tenogenic differentiation of human mesenchymal stem cells. Tissue Eng Part A. 2008;14:1615-1627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 226] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 17. | Zhao T, Qi Y, Xiao S, Ran J, Wang J, Ghamor-Amegavi EP, Zhou X, Li H, He T, Gou Z, Chen Q, Xu K. Integration of mesenchymal stem cell sheet and bFGF-loaded fibrin gel in knitted PLGA scaffolds favorable for tendon repair. J Mater Chem B. 2019;7:2201-2211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 18. | Shi Y, Zhou K, Zhang W, Zhang Z, Zhou G, Cao Y, Liu W. Microgrooved topographical surface directs tenogenic lineage specific differentiation of mouse tendon derived stem cells. Biomed Mater. 2017;12:015013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 19. | Chen G, Zhang W, Zhang K, Wang S, Gao Y, Gu J, He L, Li W, Zhang C, Zhang W, Li M, Hao Q, Zhang Y. Hypoxia-Induced Mesenchymal Stem Cells Exhibit Stronger Tenogenic Differentiation Capacities and Promote Patellar Tendon Repair in Rabbits. Stem Cells Int. 2020;2020:8822609. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Tong WY, Shen W, Yeung CW, Zhao Y, Cheng SH, Chu PK, Chan D, Chan GC, Cheung KM, Yeung KW, Lam YW. Functional replication of the tendon tissue microenvironment by a bioimprinted substrate and the support of tenocytic differentiation of mesenchymal stem cells. Biomaterials. 2012;33:7686-7698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 21. | Yea JH, Kim Y, Jo CH. Comparison of mesenchymal stem cells from bone marrow, umbilical cord blood, and umbilical cord tissue in regeneration of a full-thickness tendon defect in vitro and in vivo. Biochem Biophys Rep. 2023;34:101486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 22. | Karimi E, Vahedi N, Sarbandi RR, Parandakh A, Ganjoury C, Sigaroodi F, Najmoddin N, Tabatabaei M, Tafazzoli-Shadpour M, Ardeshirylajimi A, Khani MM. Nanoscale vibration could promote tenogenic differentiation of umbilical cord mesenchymal stem cells. In Vitro Cell Dev Biol Anim. 2023;59:401-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 23. | Baudequin T, Gaut L, Mueller M, Huepkes A, Glasmacher B, Duprez D, Bedoui F, Legallais C. The Osteogenic and Tenogenic Differentiation Potential of C3H10T1/2 (Mesenchymal Stem Cell Model) Cultured on PCL/PLA Electrospun Scaffolds in the Absence of Specific Differentiation Medium. Materials (Basel). 2017;10:1387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |