Published online Jul 26, 2025. doi: 10.4252/wjsc.v17.i7.107212
Revised: April 15, 2025
Accepted: June 17, 2025
Published online: July 26, 2025
Processing time: 128 Days and 18.2 Hours
Intervertebral disc degeneration (IDD) results from an imbalance within the intervertebral disc, leading to alterations in extracellular matrix composition, loss of nucleus pulposus cells, increased oxidative stress, and inflammatory cascade. While IDD naturally progresses with age, some factors such as mechanical trauma, lifestyle choices, and genetic abnormalities can elevate the risk of symp
Core Tip: Intervertebral disc degeneration (IDD) has been focused on the condition that is linked to several pathological condition, but no effective treatment has been reported so far. Due to the limitations of mesenchymal stem cell-based trials for IDD regeneration, extracellular vesicle or microRNAs derived from mesenchymal stem cell have been evaluated as the next generation of therapeutic option for IDD. Thus, this review aims to provide a comprehensive overview regarding the IDD including anatomical considerations and therapeutic trials with a particular focus on extracellular vesicles and microRNAs. The authors also discuss the challenges and future directions in translating these innovative therapies into clinical practice.
- Citation: Lim YJ, Seo MS, Park S, Lee GW. Therapeutic strategies for intervertebral disc degeneration: Extracellular vesicles and microRNAs derived from mesenchymal stem cells. World J Stem Cells 2025; 17(7): 107212
- URL: https://www.wjgnet.com/1948-0210/full/v17/i7/107212.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v17.i7.107212
Intervertebral disc degeneration (IDD) is a leading cause of chronic low back pain, a condition that significantly affects global health and economic productivity[1,2]. Characterized by the progressive deterioration of the extracellular matrix (ECM) and the loss of disc cell function, IDD is influenced by a combination of mechanical, genetic, and biochemical factors. Conventional treatments, such as pain management and physical therapy, primarily focus on symptom relief rather than addressing the underlying pathophysiology[3]. Consequently, there is a growing interest in regenerative therapies aimed at restoring disc structure and function, with emerging strategies involving stem cells, extracellular vesicles (EVs), microRNAs (miRNAs), and other approaches.
Stem cell-based therapies have shown promise for IDD regeneration. In particular, mesenchymal stem cells (MSCs) have the ability to differentiate into nucleus pulposus (NP)-like cells and secrete bioactive factors that promote tissue repair and modulate inflammation[4]. Additionally, EVs, including exosomes derived from stem cells, have garnered attention as cell-free therapeutic agents[5]. These nanosized vesicles play a crucial role in intercellular communication, carrying proteins, lipids, and nucleic acids, such as miRNAs, that influence key cellular processes[6,7]. Notably, miRNAs, small noncoding RNAs that regulate gene expression post-transcriptionally, serve as critical modulators in IDD. They are implicated in pathways governing cell proliferation, apoptosis, ECM metabolism, and inflammatory responses. Harnessing miRNAs delivered through stem cells or their derived EVs and exosomes presents a promising avenue for enhancing the effectiveness of regenerative therapies.
This review aims to examine the anatomical aspects of the intervertebral disc and its surrounding structure in the context of degeneration and regeneration. Additionally, it provides a comprehensive overview of current advancements in regenerative strategies for IDD, with a particular focus on stem cell therapies, EVs, and miRNA-based approaches. We will discuss the underlying mechanisms, preclinical and clinical evidence, as well as the challenges and future directions in translating these innovative therapies into clinical practice.
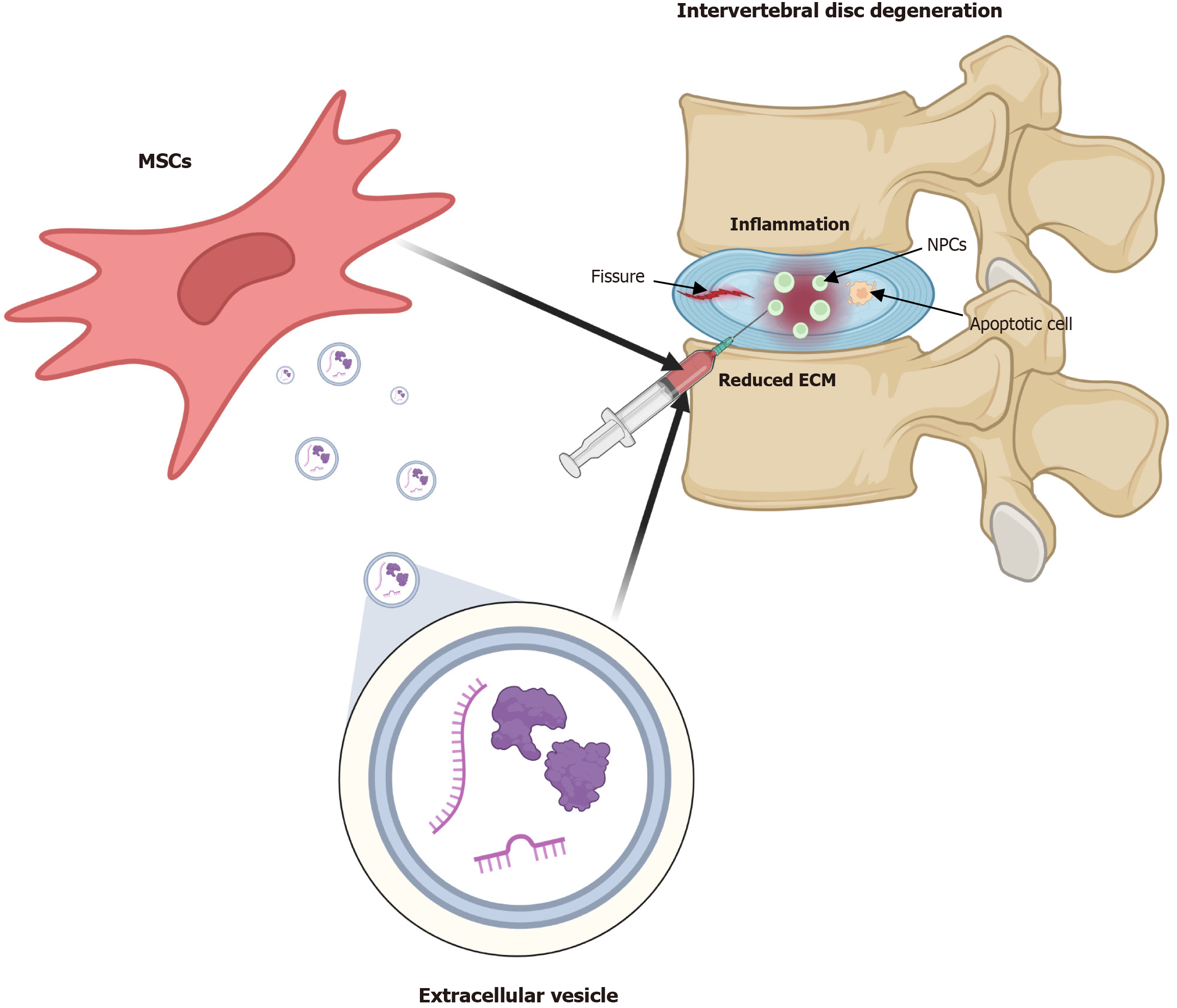
The intervertebral disc is a crucial component of the spinal column, serving as a mechanical buffer that absorbs compressive loads while enabling spinal flexibility. It consists of three primary structures: The NP, the annulus fibrosus (AF), and the cartilaginous endplates (CEPs). The NP, a gelatinous core rich in proteoglycans, collagen type II, and water, provides the disc with viscoelastic properties essential for withstanding compressive forces. Surrounding the NP, the AF comprises concentric lamellae of type I and type II collagen fibers arranged at oblique angles to resist tensile and shear stresses. The CEPs, thin layers of hyaline cartilage, interface the disc with adjacent vertebral bodies and facilitate nutrient exchange through diffusion, compensating for the disc’s avascular nature. With aging and mechanical stress, the intervertebral disc undergoes degenerative changes characterized by a decline in proteoglycan content, reduced NP hydration, and structural disruption of the AF. These changes contribute to a loss of disc height and biomechanical dysfunction, making the disc susceptible to IDD, which is often associated with low back pain and other specific disorders[8].
Surrounding structures play a crucial role in maintaining intervertebral disc health and influencing its susceptibility to degeneration. The vertebral bodies and endplates are essential for disc integrity, with bony endplates acting as conduits for nutrient and metabolite exchange. Degeneration calcification of the endplates impairs this diffusion, exacerbating cell death and ECM degradation within the disc. Ligaments such as the anterior and posterior longitudinal ligaments provide spinal stability, protecting the disc from excessive motion. However, stiffness due to ossification or degeneration changes in these ligaments disrupt spinal biomechanics and increases stress on the discs. Additionally, paraspinal muscles contribute to spinal stabilization and reduce axial loads on the intervertebral disc. Muscle atrophy or fatty degeneration, commonly observed in individuals with chronic back pain, disrupts this equilibrium, further amplifying degeneration. The limited innervation of the outer AF and the restricted vascularization of the outermost layers of the AF and CEPs make the disc highly dependent on diffusion for nutrient delivery. This unique anatomical feature renders the disc vulnerable to nutrient deprivation and mechanical overload, key factors in degeneration[9].
IDD is a multifactorial process involving mechanical, biochemical, and cellular alterations that collectively impair disc function. The pathogenesis begins with alterations in the ECM of the NP, primarily the depletion of proteoglycans and collagen degradation. This loss diminishes the disc’s water-binding capacity, leading to decreased hydrostatic pressure and disc height. Concurrently, microtears in the AF weaken its structural integrity, facilitating the extrusion of NP material. These structural changes initiate an inflammatory response characterized by the release of pro-inflammatory cytokines such as interleukin (IL)-1β, tumor necrosis factor-alpha, and matrix metalloproteinases, which further degrade ECM components. The resulting acidic and hypoxic microenvironment accelerates cellular apoptosis and senescence, further impairing matrix synthesis. Additionally, calcification of the CEPs restricts nutrient and oxygen diffusion, creating a vicious cycle that worsens degeneration[10,11].
The cellular mechanisms underlying IDD are complex, with significant contributions from oxidative stress and mitochondrial dysfunction. Reactive oxygen species accumulate due to mechanical overload and impaired antioxidant defenses, leading to DNA damage and mitochondrial dysfunction in disc cells. These alterations activate catabolic pathways, including the nuclear factor kappa B signaling pathway, which promotes inflammation and matrix degradation. Dysregulation of signaling pathways, such as transforming growth factor-beta (TGF-β) and Wnt/β-catenin further impairs matrix repair. Recent studies suggest that certain signaling pathways, including the protein kinase RNA-like endoplasmic reticulum kinase/activating transcription factor 4/IL-10 pathway and Kelch-like ECH-associated protein 1/NF-E2-related factor 2/heme oxygenase-1 pathway, play pivotal role on initiating and exacerbating disc degeneration[12,13]. The chronic inflammatory state also sensitizes nerve endings in the outer AF, contributing to pain. Understanding these pathways provides a foundation for developing targeted interventions, such as antioxidants, cytokine inhibitors, and gene therapies, to mitigate disc degeneration and promote regeneration[14].
A comprehensive understanding of the intervertebral disc and its surrounding anatomy is essential for developing effective regenerative strategies aimed at restoring disc structure and function. Cell-based approaches, particularly MSCs, seek to repopulate the degenerated disc with cells capable of producing a functional ECM. Growth factors such as TGF-β and bone morphogenetic proteins (BMPs), along with cytokines and genetic factors like miRNAs, are often incorporated to reduce the inflammation and enhance matrix synthesis and cellular proliferation[15]. In addition, biomaterial-based therapies as being explored to provide structural stability and support for regeneration. Hydrogels and 3D-printed bio-mimic structures are designed to replicate the biomechanical properties of the NP, promoting cell growth and ECM synthesis. However, significant challenges remain, particularly in overcoming the harsh microenvironment of the degenerated disc, characterized by low oxygen tension, acidic pH, and inflammation.
In disc degeneration, inflammatory cytokines are upregulated, triggering regulated cell death[16-18]. This leads to apoptosis and cellular transformation into chondrocytes, contributing to ECM fibrosis[19-22]. Cellular aging plays a pivotal role in disc degeneration[23-25] by inducing cell proliferation arrest, chronic inflammation, and ECM degradation. Inflammation also exerts nociceptive effects, with increased levels of nerve growth factor and brain-derived neurotrophic factor observed in degenerated discs[26]. MSC therapy for disc degeneration is influenced by multiple factors, including its anti-inflammatory, anti-apoptotic, anti-pyretic effects, and increased cell numbers and promote ECM production through MSC differentiation[27]. Studies have demonstrated that bone marrow-derived MSCs (BMSCs) promote the proliferation of notochordal cells in the NP[28]. Additionally, umbilical cord-derived MSCs restore stem cell capacity by upregulating octamer-binding transcription factor 4, Nanog, and TIE2 while increasing CD29 and CD105 expression in NP-derived MSCs[29]. These effects have been evaluated using conditioned media from umbilical cord-derived MSCs, demonstrating the role of EVs in enhancing cell proliferation and chondrocyte differentiation. Furthermore, extracellular secretion and endocytosis facilitate the transfer of intracellular components from MSCs to NP cells (NPCs), leading to functional changes in NPCs[30] (Figure 1).
Several factors contribute to the therapeutic efficacy of MSCs in disc degeneration. The TGF-β family is crucial in MSC differentiation, with TGF-β3 included in chondrogenic differentiation media[31,32]. Research has shown that BMSCs cultured with TGF-β3, dexamethasone, and ascorbic acid form spheroids that positively express type II collagen and genes such as ACAN, DCD, FMOD, and COMP, expression levels comparable to those in NP tissues[33]. The effects of TGF-β3 are further enhanced by growth factors like BMP2 and insulin-like growth factor (IGF)-1. When cultured with TGF-β3, BMP2, and IGF-1 BMSCs undergoes chondrocyte differentiation and activates the mitogen-activated protein kinases/extracellular signal-regulated kinase signaling pathway, leading to differentiation into NPCs[34,35]. Addi
Members of the TGF-β superfamily play essential roles in the MSC into NP or chondrocytes[42]. BMP2, when incorporated into chondrocyte differentiation media, has demonstrated a positive effect on NPMSC differentiation[32], significantly enhancing the chondrogenic differentiation of BMSCs when combined with TGF-β3[34]. BMP3 has been shown to influence MSC proliferation and chondrogenic differentiation[43]. Additionally, BMP2 and BMP7 induce osteogenic and chondrogenic differentiation, respectively, via the Smad pathway[44,45]. BMP family members increase the expression of Runx2, SPP1, and ACAN[44,46,47], suggesting that BMP2 promotes osteogenic differentiation. Beyond BMPs, other growth factors also contribute to MSC differentiation. IGF1 has been used to induce NPC differentiation[48,49], while fibroblast growth factor 2 has been shown to support NPC differentiation through TGF-β1[50]. Table 1 summarizes the above[31,32,34,35,38,44,48-57].
| Biological therapies | Effects | Ref. |
| TGF-β1 | MSC differentiation toward NP/chondrogenic phenotype and upregulation of ERK1/2 activity | [38,51] |
| TGF-β3 | MSC differentiation to NPC and AFC in combination with BMP-2 and IGF-1 | [31,32,34,35,52,53] |
| BMP2 | Differentiation into NPCs with TGF-β3 and PRP | [32,34,44,54] |
| BMP7 | MSC differentiation into NP-like cells via the Smad pathway | [55] |
| IGF1 | MSC differentiation into NPC-like cells | [48,49,56] |
| FGF2 | MSC differentiation into NPC-like cells | [48,50,57] |
Although stem cells hold great potential for regenerating damaged tissue and modulating immunological responses, significant limitations and challenges remain in research and therapeutic development. One major obstacle is the difficulty in MSC survival after transplantation. The condition, cell niche, and communication between cells determine the survival of stem cells. Second, MSCs exhibit heterogeneity due to donor variability, cell type differences, differentiation potential, and intercellular factors. Their sensitivity to environmental conditions can negatively impact disease control, particularly in cases of severe inflammation or active osteoarthritis. They are also unstable, which limits their storage duration even at ultra-low temperatures. Furthermore, the entire manufacturing process of MSCs, including ex vivo expansion, extraction technology, and culture method, has not yet been standardized. To address these limitations, EVs have recently gained attention as an alternative therapeutic strategy in regenerative medicine, with active research being conducted across various fields.
EVs are lipid bilayer-enclosed nanoparticles originating from multivesicular endosomes[58], typically ranging in diameter from 50 to 150 nm, with an average diameter of 100 nm[59,60]. Although discovered in the late 1980s, EVs have only recently been recognized as crucial mediators of cell-to-cell communication[61]. Their lipid bilayers withstand freeze-drying and extreme conditions while maintaining biological activity, immunotolerance, and therapeutic efficacy[62]. Secreted by various cells, EVs can be isolated from small amounts of biological fluids, including blood, semen, urine, breast milk, and bile, and they facilitate intercellular communication by delivering biologically active molecules, miRNA, and proteins[63,64].
EVs exhibit significant therapeutic potential in tissue repair by inhibiting apoptosis, enhancing regenerative pheno
| Biological therapies | Effects | Ref. |
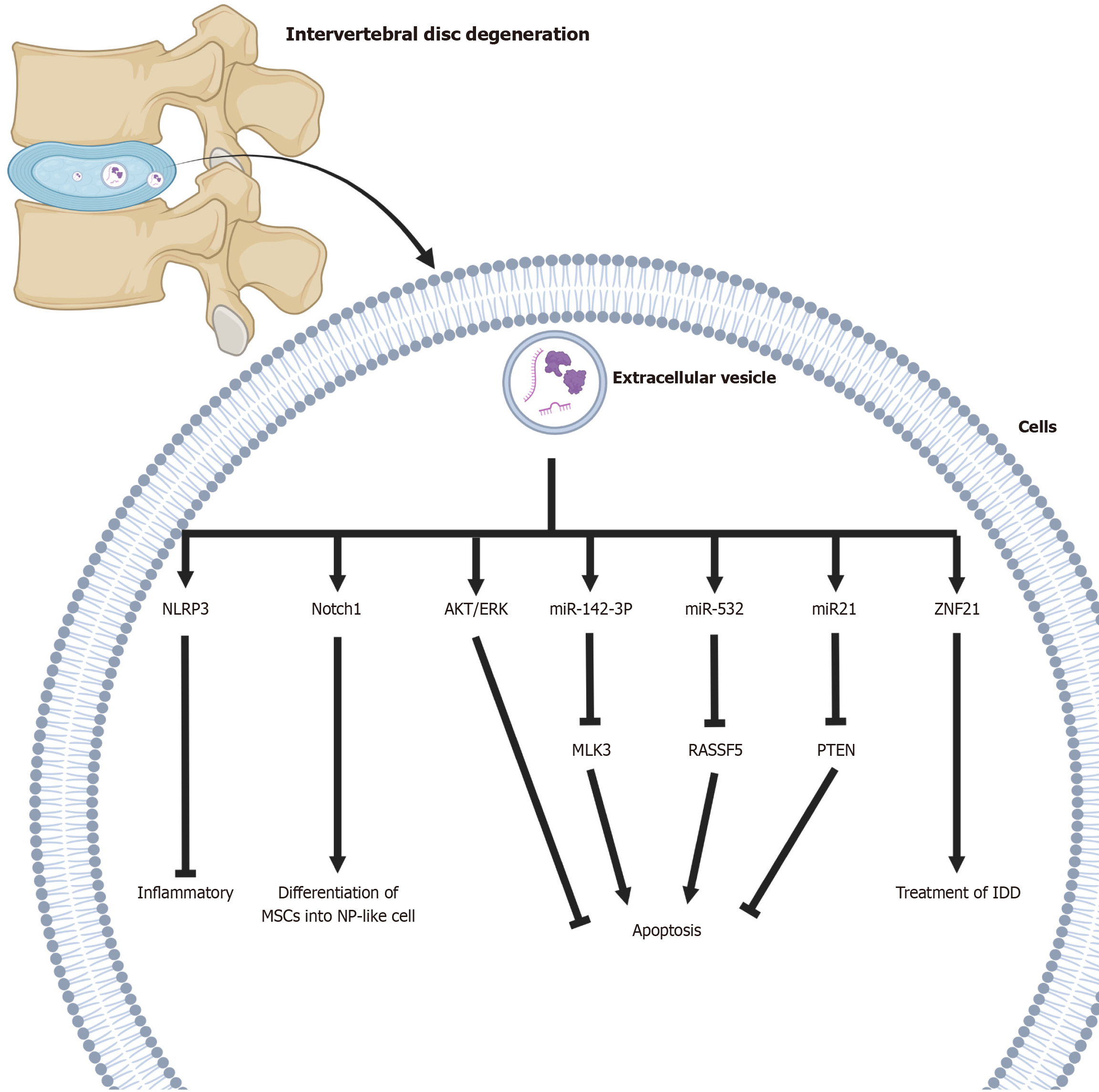
| NLRP3 | Exosomes play an anti-inflammatory role in pathological NPCs by suppressing inflammatory mediators and NLRP3 inflammasome activation | [78] |
| MLK3 | Bone marrow MSCs-derived exosomes-packaged miR-142-3p alleviates NPCs injury through suppressing MAPK signaling by targeting MLK3 | [72] |
| RASSF5 | MSCs may suppress TNF-α-induced apoptosis, ECM degradation, and fibrosis deposition in NPCs through the delivery of miR-532-5p via targeting RASSF5 | [73] |
| Notch1 | Inhibition of the Notch1 pathway facilitates NPC exosome-induced differentiation of MSCs into NP-like cells in vitro | [74] |
| PI3K and AKT | Exosomal miR-21 restrains PTEN and thus activates PI3K/AKT pathway in apoptotic NPCs | [80] |
| AKT and ERK | MSC-exosomes could attenuate ER stress-induced apoptosis by activating AKT and ERK signaling | [81] |
| ZNF121 | Exo-antagomiR-4450 retarded damage of NPCs in vitro, alleviated IDD damage, and ameliorated gait abnormality in vivo | [82] |
| ATF6 | MSC-exosomes reduced apoptosis and calcification of EPCs via regulation of the miR-31-5p/ATF6/ER stress pathway | [83] |
In a recent in vivo study on stem cell-derived EVs, it has shown that EVs prevented the progression of degenerative diseases in a rabbit model, suggesting that injected EVs may be a viable therapeutic strategy targeting the Nod-like receptor protein 3 inflammasome[78,79]. EVs could supply mitochondrial proteins to NPCs and recover damaged mitochondria in degenerative discs[79] (Figure 1). In a rat model, intradiscal injection of BMSC-EVs alleviated NPC apoptosis and slowed disc degeneration, with miR-21 playing a key role by activating the phosphatidylinositol-3-kinase/protein kinase B pathway through the inhibition of phosphatase and tensin homolog[80]. MSC-EVs may further reduce apoptosis by modulating the protein kinase B and extracellular signal-regulated kinase signaling pathways[81]. Human placental MSC-derived EVs have also demonstrated therapeutic effects in in vivo and in vitro models of NPC dege
Unsurprisingly, MSCs have emerged as a promising therapeutic approach at the forefront of neurodegenerative disease research[84]. In fact, MSCs have the ability to migrate to damaged tissues, differentiate into new cell types even in vitro over long periods of time, and promote anti-inflammation and/or regeneration[85-88]. Although there are many therapeutic indications and evidence for using MSCs[89-96], there is a consensus that MSCs are safe to use, despite the relatively limited number of human studies conducted so far. However, a recent meta-analysis on this topic found that MSC administration would lead to some side effects such as short-term fever, inflammatory effects at the injection site, constipation, fatigue, and insomnia in groups suffering from various diseases[97]. Aging of autologous and transplanted MSCs may contribute to unsatisfactory therapeutic outcomes[98], as one example is that transplantation of adipose-derived MSCs from aged mice causes physical dysfunction in recipients[99], and adverse effects of aged MSCs have also been demonstrated in vitro[100]. The proliferation of BMSCs decreases their osteogenic potential during aging[101,102], which may contribute to age-related diseases. Specifically, aged MSCs exhibit decreased cell proliferation and osteogenic activity measured by alkaline phosphatase activity, ECM mineralization, and osteogenesis-related genes, and increased adipocyte differentiation capacity in accordance with adipocyte protein 2, resistin, and lipid accumulation[103]. These mechanisms by senescent MSCs lead to decreased osteogenesis, resulting in osteoporosis and poor osseointegration capacity[104-108].
Cellular senescence affects the composition of the EVs. EVs released from aged cells contain unpredictable growth factors and cytokines. These factors will cause a “domino effect” that will cause negative effects from cellular to tissue levels[109]. Especially in the context of IDD, the senescent EVs are secreted extracellularly, including inflammatory cytokines, degradative enzymes, and senescence-related miRNAs, which can accelerate the degeneration of disc tissue by inducing inflammatory responses and cellular senescence in surrounding cells. Therefore, when designing or using therapeutic EVs, it is very important whether the mother cell is senescent or not, and it could expect a potential side effect from the use of aged EVs that may neither affect the tissue at all nor rather worsen tissue degeneration. Hao et al[110] in 2022 reported that MSC-derived EVs can regulate the senescence of intervertebral disc cells through miR-217, suggesting that EVs may have opposing biological effects depending on their molecular composition. In this regard, EV-based therapeutic approaches need to be combined with strategies that closely analyze the biological properties of EVs and preemptively remove or inhibit senescence-related signals[110].
For several decades, extensive efforts have been made to effectively restore degenerated intervertebral discs to their normal state through regeneration processes, various sources and materials, ranging from stem cells to engineered acellular vesicles and miRNAs. Despite numerous attempts to regenerate the intervertebral disc, the complexity of its anatomical structure and the mechanism involved in maintaining homeostasis continue to pose significant challenges in fully understanding the disc and identifying the ideal regeneration method. With developments in 3D-printing-based scaffolds and the discovery of novel sources, the prospect of achieving normal disc regeneration is now closer than ever. Furthermore, in the near future, these developments may lead to the creation of artificial disc structures that mimic natural discs. In addition to technological advancements, it is crucial to unravel the underlying mechanisms governing both degeneration and regeneration processes in the intervertebral disc.
| 1. | Canseco JA, Levy HA, Karamian BA, Blaber O, Chang M, Patel N, Curran J, Hilibrand AS, Schroeder GD, Vaccaro AR, Markova DZ, Surrey DE, Kepler CK. Inhibition of Neurogenic Inflammatory Pathways Associated with the Reduction in Discogenic Back Pain. Asian Spine J. 2023;17:1043-1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 2. | Wei X, Yamato Y, Hasegawa T, Yoshida G, Banno T, Oe S, Arima H, Ide K, Yamada T, Kurosu K, Matsuyama Y. Adjacent segment degeneration at a minimum 2-year follow-up after posterior lumbar interbody fusion: the impact of sagittal spinal proportion: a retrospective case series. Asian Spine J. 2024;18:681-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Trefilova VV, Shnayder NA, Petrova MM, Kaskaeva DS, Tutynina OV, Petrov KV, Popova TE, Balberova OV, Medvedev GV, Nasyrova RF. The Role of Polymorphisms in Collagen-Encoding Genes in Intervertebral Disc Degeneration. Biomolecules. 2021;11:1279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 4. | Sakai D, Andersson GB. Stem cell therapy for intervertebral disc regeneration: obstacles and solutions. Nat Rev Rheumatol. 2015;11:243-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 340] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 5. | Sung SE, Seo MS, Kang KK, Choi JH, Lee SJ, Lim JH, Yang SY, Kim SK, Lee GW. Isolation and Characterization of Extracellular Vesicle from Mesenchymal Stem Cells of the Epidural Fat of the Spine. Asian Spine J. 2022;16:153-161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 6. | DiStefano TJ, Vaso K, Danias G, Chionuma HN, Weiser JR, Iatridis JC. Extracellular Vesicles as an Emerging Treatment Option for Intervertebral Disc Degeneration: Therapeutic Potential, Translational Pathways, and Regulatory Considerations. Adv Healthc Mater. 2022;11:e2100596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 81] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 7. | Hingert D, Ekström K, Aldridge J, Crescitelli R, Brisby H. Extracellular vesicles from human mesenchymal stem cells expedite chondrogenesis in 3D human degenerative disc cell cultures. Stem Cell Res Ther. 2020;11:323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 8. | Urban JP, Roberts S. Degeneration of the intervertebral disc. Arthritis Res Ther. 2003;5:120-130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 869] [Cited by in RCA: 884] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 9. | Battié MC, Videman T, Parent E. Lumbar disc degeneration: epidemiology and genetic influences. Spine (Phila Pa 1976). 2004;29:2679-2690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 320] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 10. | Roberts S, Evans H, Trivedi J, Menage J. Histology and pathology of the human intervertebral disc. J Bone Joint Surg Am. 2006;88 Suppl 2:10-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 408] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 11. | Taylor W, Erwin WM. Intervertebral Disc Degeneration and Regeneration: New Molecular Mechanisms and Therapeutics: Obstacles and Potential Breakthrough Technologies. Cells. 2024;13:2103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 12. | Yu XJ, Zou P, Li TQ, Bai XF, Wang SX, Guan JB, Zhao YT, Li MW, Wang X, Wang YG, Hao DJ. Deciphering SPP1-related macrophage signaling in the pathogenesis of intervertebral disc degeneration. Cell Biol Toxicol. 2025;41:33. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 13. | Chen S, Huang Y, Lei L, Yang C, Ran D, Zhou E, Wang H, Ning X. Daphnetin ameliorates intervertebral disc degeneration via the Keap1/Nrf2/NF-κB axis in vitro and in vivo. Int Immunopharmacol. 2025;145:113785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 14. | Wang F, Cai F, Shi R, Wang XH, Wu XT. Aging and age related stresses: a senescence mechanism of intervertebral disc degeneration. Osteoarthritis Cartilage. 2016;24:398-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 374] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 15. | Clouet J, Fusellier M, Camus A, Le Visage C, Guicheux J. Intervertebral disc regeneration: From cell therapy to the development of novel bioinspired endogenous repair strategies. Adv Drug Deliv Rev. 2019;146:306-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 151] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 16. | Yang W, Yu XH, Wang C, He WS, Zhang SJ, Yan YG, Zhang J, Xiang YX, Wang WJ. Interleukin-1β in intervertebral disk degeneration. Clin Chim Acta. 2015;450:262-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 105] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 17. | Suzuki S, Fujita N, Fujii T, Watanabe K, Yagi M, Tsuji T, Ishii K, Miyamoto T, Horiuchi K, Nakamura M, Matsumoto M. Potential Involvement of the IL-6/JAK/STAT3 Pathway in the Pathogenesis of Intervertebral Disc Degeneration. Spine (Phila Pa 1976). 2017;42:E817-E824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 18. | Wang C, Yu X, Yan Y, Yang W, Zhang S, Xiang Y, Zhang J, Wang W. Tumor necrosis factor-α: a key contributor to intervertebral disc degeneration. Acta Biochim Biophys Sin (Shanghai). 2017;49:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 90] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 19. | Ohnishi T, Yamada K, Iwasaki K, Tsujimoto T, Higashi H, Kimura T, Iwasaki N, Sudo H. Caspase-3 knockout inhibits intervertebral disc degeneration related to injury but accelerates degeneration related to aging. Sci Rep. 2019;9:19324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 20. | Mohanty S, Pinelli R, Pricop P, Albert TJ, Dahia CL. Chondrocyte-like nested cells in the aged intervertebral disc are late-stage nucleus pulposus cells. Aging Cell. 2019;18:e13006. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 21. | Choi H, Tessier S, Silagi ES, Kyada R, Yousefi F, Pleshko N, Shapiro IM, Risbud MV. A novel mouse model of intervertebral disc degeneration shows altered cell fate and matrix homeostasis. Matrix Biol. 2018;70:102-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 98] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 22. | Ohnishi T, Iwasaki N, Sudo H. Causes of and Molecular Targets for the Treatment of Intervertebral Disc Degeneration: A Review. Cells. 2022;11:394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 77] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 23. | Novais EJ, Diekman BO, Shapiro IM, Risbud MV. p16(Ink4a) deletion in cells of the intervertebral disc affects their matrix homeostasis and senescence associated secretory phenotype without altering onset of senescence. Matrix Biol. 2019;82:54-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 81] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 24. | Cherif H, Bisson DG, Mannarino M, Rabau O, Ouellet JA, Haglund L. Senotherapeutic drugs for human intervertebral disc degeneration and low back pain. Elife. 2020;9:e54693. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 76] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 25. | Shi PZ, Wang JW, Wang PC, Han B, Lu XH, Ren YX, Feng XM, Cheng XF, Zhang L. Urolithin a alleviates oxidative stress-induced senescence in nucleus pulposus-derived mesenchymal stem cells through SIRT1/PGC-1α pathway. World J Stem Cells. 2021;13:1928-1946. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (1)] |
| 26. | Krock E, Rosenzweig DH, Chabot-Doré AJ, Jarzem P, Weber MH, Ouellet JA, Stone LS, Haglund L. Painful, degenerating intervertebral discs up-regulate neurite sprouting and CGRP through nociceptive factors. J Cell Mol Med. 2014;18:1213-1225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 122] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 27. | Esquijarosa Hechavarria M, Richard SA. Edifying the Focal Factors Influencing Mesenchymal Stem Cells by the Microenvironment of Intervertebral Disc Degeneration in Low Back Pain. Pain Res Manag. 2022;2022:6235400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 28. | Yang F, Leung VY, Luk KD, Chan D, Cheung KM. Mesenchymal stem cells arrest intervertebral disc degeneration through chondrocytic differentiation and stimulation of endogenous cells. Mol Ther. 2009;17:1959-1966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 107] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 29. | Zeng X, Lin J, Wu H, Yu J, Tu M, Cheang LH, Zhang J. Effect of Conditioned Medium from Human Umbilical Cord-Derived Mesenchymal Stromal Cells on Rejuvenation of Nucleus Pulposus Derived Stem/Progenitor Cells from Degenerated Intervertebral Disc. Int J Stem Cells. 2020;13:257-267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 30. | Lehmann TP, Filipiak K, Juzwa W, Sujka-Kordowska P, Jagodziński PP, Zabel M, Głowacki J, Misterska E, Walczak M, Głowacki M. Coculture of human nucleus pulposus cells with multipotent mesenchymal stromal cells from human bone marrow reveals formation of tunnelling nanotubes. Mol Med Rep. 2014;9:574-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Ekram S, Khalid S, Bashir I, Salim A, Khan I. Human umbilical cord-derived mesenchymal stem cells and their chondroprogenitor derivatives reduced pain and inflammation signaling and promote regeneration in a rat intervertebral disc degeneration model. Mol Cell Biochem. 2021;476:3191-3205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 32. | Liu Y, Gao GM, Yang KY, Nong LM. Construction of tissue-engineered nucleus pulposus by stimulation with periodic mechanical stress and BMP-2. iScience. 2022;25:104405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 33. | Steck E, Bertram H, Abel R, Chen B, Winter A, Richter W. Induction of intervertebral disc-like cells from adult mesenchymal stem cells. Stem Cells. 2005;23:403-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 182] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 34. | Shen B, Wei A, Tao H, Diwan AD, Ma DD. BMP-2 enhances TGF-beta3-mediated chondrogenic differentiation of human bone marrow multipotent mesenchymal stromal cells in alginate bead culture. Tissue Eng Part A. 2009;15:1311-1320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 98] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 35. | Tao Y, Zhou X, Liang C, Li H, Han B, Li F, Chen Q. TGF-β3 and IGF-1 synergy ameliorates nucleus pulposus mesenchymal stem cell differentiation towards the nucleus pulposus cell type through MAPK/ERK signaling. Growth Factors. 2015;33:326-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 36. | Colombier P, Clouet J, Boyer C, Ruel M, Bonin G, Lesoeur J, Moreau A, Fellah BH, Weiss P, Lescaudron L, Camus A, Guicheux J. TGF-β1 and GDF5 Act Synergistically to Drive the Differentiation of Human Adipose Stromal Cells toward Nucleus Pulposus-like Cells. Stem Cells. 2016;34:653-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 37. | Duan J, Li Z, Liu E, Long H, Chen L, Yang S. BSHXF-medicated serum combined with ADSCs regulates the TGF-β1/Smad pathway to repair oxidatively damaged NPCs and its component analysis. J Ethnopharmacol. 2023;316:116692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 38. | Risbud MV, Albert TJ, Guttapalli A, Vresilovic EJ, Hillibrand AS, Vaccaro AR, Shapiro IM. Differentiation of mesenchymal stem cells towards a nucleus pulposus-like phenotype in vitro: implications for cell-based transplantation therapy. Spine (Phila Pa 1976). 2004;29:2627-2632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 235] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 39. | Mietsch A, Neidlinger-Wilke C, Schrezenmeier H, Mauer UM, Friemert B, Wilke HJ, Ignatius A. Evaluation of platelet-rich plasma and hydrostatic pressure regarding cell differentiation in nucleus pulposus tissue engineering. J Tissue Eng Regen Med. 2013;7:244-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 40. | Jia J, Wang SZ, Ma LY, Yu JB, Guo YD, Wang C. The Differential Effects of Leukocyte-Containing and Pure Platelet-Rich Plasma on Nucleus Pulposus-Derived Mesenchymal Stem Cells: Implications for the Clinical Treatment of Intervertebral Disc Degeneration. Stem Cells Int. 2018;2018:7162084. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 41. | Akeda K, An HS, Pichika R, Attawia M, Thonar EJ, Lenz ME, Uchida A, Masuda K. Platelet-rich plasma (PRP) stimulates the extracellular matrix metabolism of porcine nucleus pulposus and anulus fibrosus cells cultured in alginate beads. Spine (Phila Pa 1976). 2006;31:959-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 93] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 42. | Chen G, Deng C, Li YP. TGF-β and BMP signaling in osteoblast differentiation and bone formation. Int J Biol Sci. 2012;8:272-288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1052] [Cited by in RCA: 1342] [Article Influence: 103.2] [Reference Citation Analysis (0)] |
| 43. | Hingert D, Barreto Henriksson H, Brisby H. Human Mesenchymal Stem Cells Pretreated with Interleukin-1β and Stimulated with Bone Morphogenetic Growth Factor-3 Enhance Chondrogenesis. Tissue Eng Part A. 2018;24:775-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 44. | Knippenberg M, Helder MN, Zandieh Doulabi B, Wuisman PI, Klein-Nulend J. Osteogenesis versus chondrogenesis by BMP-2 and BMP-7 in adipose stem cells. Biochem Biophys Res Commun. 2006;342:902-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 192] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 45. | Kim KM, Kim DY, Lee DS, Kim JW, Koh JT, Kim EJ, Jang WG. Peroxiredoxin II negatively regulates BMP2-induced osteoblast differentiation and bone formation via PP2A Cα-mediated Smad1/5/9 dephosphorylation. Exp Mol Med. 2019;51:1-11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 46. | Min HY, Kim KM, Wee G, Kim EJ, Jang WG. Bmal1 induces osteoblast differentiation via regulation of BMP2 expression in MC3T3-E1 cells. Life Sci. 2016;162:41-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 47. | Son HE, Kim KM, Kim EJ, Jang WG. Kisspeptin-10 (KP-10) stimulates osteoblast differentiation through GPR54-mediated regulation of BMP2 expression and activation. Sci Rep. 2018;8:2134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 48. | Ehlicke F, Freimark D, Heil B, Dorresteijn A, Czermak P. Intervertebral disc regeneration: influence of growth factors on differentiation of human mesenchymal stem cells (hMSC). Int J Artif Organs. 2010;33:244-252. [PubMed] |
| 49. | Longobardi L, O'Rear L, Aakula S, Johnstone B, Shimer K, Chytil A, Horton WA, Moses HL, Spagnoli A. Effect of IGF-I in the chondrogenesis of bone marrow mesenchymal stem cells in the presence or absence of TGF-beta signaling. J Bone Miner Res. 2006;21:626-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 260] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 50. | Tsai TT, Guttapalli A, Oguz E, Chen LH, Vaccaro AR, Albert TJ, Shapiro IM, Risbud MV. Fibroblast growth factor-2 maintains the differentiation potential of nucleus pulposus cells in vitro: implications for cell-based transplantation therapy. Spine (Phila Pa 1976). 2007;32:495-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 51. | Han C, Jiang C, Yu C, Shen H. Differentiation of transforming growth factor β1-induced mesenchymal stem cells into nucleus pulposus-like cells under simulated microgravity conditions. Cell Mol Biol (Noisy-le-grand). 2015;61:50-55. [PubMed] |
| 52. | Gruber HE, Deepe R, Hoelscher GL, Ingram JA, Norton HJ, Scannell B, Loeffler BJ, Zinchenko N, Hanley EN, Tapp H. Human adipose-derived mesenchymal stem cells: direction to a phenotype sharing similarities with the disc, gene expression profiling, and coculture with human annulus cells. Tissue Eng Part A. 2010;16:2843-2860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 53. | Jin ES, Min J, Jeon SR, Choi KH, Jeong JH. Analysis of molecular expression in adipose tissue-derived mesenchymal stem cells : prospects for use in the treatment of intervertebral disc degeneration. J Korean Neurosurg Soc. 2013;53:207-212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 54. | Hou Y, Shi G, Shi J, Xu G, Guo Y, Xu P. Study design: in vitro and in vivo assessment of bone morphogenic protein 2 combined with platelet-rich plasma on treatment of disc degeneration. Int Orthop. 2016;40:1143-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 55. | Xu J, E XQ, Wang NX, Wang MN, Xie HX, Cao YH, Sun LH, Tian J, Chen HJ, Yan JL. BMP7 enhances the effect of BMSCs on extracellular matrix remodeling in a rabbit model of intervertebral disc degeneration. FEBS J. 2016;283:1689-1700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 56. | Chon BH, Lee EJ, Jing L, Setton LA, Chen J. Human umbilical cord mesenchymal stromal cells exhibit immature nucleus pulposus cell phenotype in a laminin-rich pseudo-three-dimensional culture system. Stem Cell Res Ther. 2013;4:120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 57. | Chiou M, Xu Y, Longaker MT. Mitogenic and chondrogenic effects of fibroblast growth factor-2 in adipose-derived mesenchymal cells. Biochem Biophys Res Commun. 2006;343:644-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 96] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 58. | Zomer A, Vendrig T, Hopmans ES, van Eijndhoven M, Middeldorp JM, Pegtel DM. Exosomes: Fit to deliver small RNA. Commun Integr Biol. 2010;3:447-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 280] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 59. | Dai J, Su Y, Zhong S, Cong L, Liu B, Yang J, Tao Y, He Z, Chen C, Jiang Y. Exosomes: key players in cancer and potential therapeutic strategy. Signal Transduct Target Ther. 2020;5:145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 256] [Cited by in RCA: 792] [Article Influence: 158.4] [Reference Citation Analysis (0)] |
| 60. | Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367:eaau6977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6920] [Cited by in RCA: 6572] [Article Influence: 1314.4] [Reference Citation Analysis (0)] |
| 61. | Isola AL, Chen S. Exosomes: The Messengers of Health and Disease. Curr Neuropharmacol. 2017;15:157-165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 168] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 62. | Marbán E. The Secret Life of Exosomes: What Bees Can Teach Us About Next-Generation Therapeutics. J Am Coll Cardiol. 2018;71:193-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 105] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 63. | Gao M, Gao W, Papadimitriou JM, Zhang C, Gao J, Zheng M. Exosomes-the enigmatic regulators of bone homeostasis. Bone Res. 2018;6:36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 96] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 64. | Patel GK, Khan MA, Zubair H, Srivastava SK, Khushman M, Singh S, Singh AP. Comparative analysis of exosome isolation methods using culture supernatant for optimum yield, purity and downstream applications. Sci Rep. 2019;9:5335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 252] [Cited by in RCA: 436] [Article Influence: 72.7] [Reference Citation Analysis (0)] |
| 65. | Bobrie A, Colombo M, Raposo G, Théry C. Exosome secretion: molecular mechanisms and roles in immune responses. Traffic. 2011;12:1659-1668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 686] [Cited by in RCA: 837] [Article Influence: 59.8] [Reference Citation Analysis (0)] |
| 66. | Eldh M, Ekström K, Valadi H, Sjöstrand M, Olsson B, Jernås M, Lötvall J. Exosomes communicate protective messages during oxidative stress; possible role of exosomal shuttle RNA. PLoS One. 2010;5:e15353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 349] [Cited by in RCA: 363] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 67. | Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8246] [Cited by in RCA: 9800] [Article Influence: 544.4] [Reference Citation Analysis (0)] |
| 68. | Ratajczak J, Miekus K, Kucia M, Zhang J, Reca R, Dvorak P, Ratajczak MZ. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia. 2006;20:847-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1312] [Cited by in RCA: 1247] [Article Influence: 65.6] [Reference Citation Analysis (0)] |
| 69. | Harting MT, Srivastava AK, Zhaorigetu S, Bair H, Prabhakara KS, Toledano Furman NE, Vykoukal JV, Ruppert KA, Cox CS Jr, Olson SD. Inflammation-Stimulated Mesenchymal Stromal Cell-Derived Extracellular Vesicles Attenuate Inflammation. Stem Cells. 2018;36:79-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 187] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 70. | Qi X, Zhang J, Yuan H, Xu Z, Li Q, Niu X, Hu B, Wang Y, Li X. Exosomes Secreted by Human-Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells Repair Critical-Sized Bone Defects through Enhanced Angiogenesis and Osteogenesis in Osteoporotic Rats. Int J Biol Sci. 2016;12:836-849. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 267] [Cited by in RCA: 411] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 71. | Liao FL, Tan L, Liu H, Wang JJ, Ma XT, Zhao B, Chen Y, Bihl J, Yang Y, Chen RL. Hematopoietic stem cell-derived exosomes promote hematopoietic differentiation of mouse embryonic stem cells in vitro via inhibiting the miR126/Notch1 pathway. Acta Pharmacol Sin. 2018;39:552-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 72. | Zhu L, Shi Y, Liu L, Wang H, Shen P, Yang H. Mesenchymal stem cells-derived exosomes ameliorate nucleus pulposus cells apoptosis via delivering miR-142-3p: therapeutic potential for intervertebral disc degenerative diseases. Cell Cycle. 2020;19:1727-1739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 71] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 73. | Zhu G, Yang X, Peng C, Yu L, Hao Y. Exosomal miR-532-5p from bone marrow mesenchymal stem cells reduce intervertebral disc degeneration by targeting RASSF5. Exp Cell Res. 2020;393:112109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 74. | Lan WR, Pan S, Li HY, Sun C, Chang X, Lu K, Jiang CQ, Zuo R, Zhou Y, Li CQ. Inhibition of the Notch1 Pathway Promotes the Effects of Nucleus Pulposus Cell-Derived Exosomes on the Differentiation of Mesenchymal Stem Cells into Nucleus Pulposus-Like Cells in Rats. Stem Cells Int. 2019;2019:8404168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 75. | Lu K, Li HY, Yang K, Wu JL, Cai XW, Zhou Y, Li CQ. Exosomes as potential alternatives to stem cell therapy for intervertebral disc degeneration: in-vitro study on exosomes in interaction of nucleus pulposus cells and bone marrow mesenchymal stem cells. Stem Cell Res Ther. 2017;8:108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 168] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 76. | Bach F, Libregts S, Creemers L, Meij B, Ito K, Wauben M, Tryfonidou M. Notochordal-cell derived extracellular vesicles exert regenerative effects on canine and human nucleus pulposus cells. Oncotarget. 2017;8:88845-88856. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 77. | Rodrigues-Pinto R, Richardson SM, Hoyland JA. An understanding of intervertebral disc development, maturation and cell phenotype provides clues to direct cell-based tissue regeneration therapies for disc degeneration. Eur Spine J. 2014;23:1803-1814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 78. | Li Y, Jin D, Xie W, Wen L, Chen W, Xu J, Ding J, Ren D, Xiao Z. Mesenchymal Stem Cells-Derived Exosomes: A Possible Therapeutic Strategy for Osteoporosis. Curr Stem Cell Res Ther. 2018;13:362-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 79. | Xia C, Zeng Z, Fang B, Tao M, Gu C, Zheng L, Wang Y, Shi Y, Fang C, Mei S, Chen Q, Zhao J, Lin X, Fan S, Jin Y, Chen P. Mesenchymal stem cell-derived exosomes ameliorate intervertebral disc degeneration via anti-oxidant and anti-inflammatory effects. Free Radic Biol Med. 2019;143:1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 222] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 80. | Cheng X, Zhang G, Zhang L, Hu Y, Zhang K, Sun X, Zhao C, Li H, Li YM, Zhao J. Mesenchymal stem cells deliver exogenous miR-21 via exosomes to inhibit nucleus pulposus cell apoptosis and reduce intervertebral disc degeneration. J Cell Mol Med. 2018;22:261-276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 250] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 81. | Liao Z, Luo R, Li G, Song Y, Zhan S, Zhao K, Hua W, Zhang Y, Wu X, Yang C. Exosomes from mesenchymal stem cells modulate endoplasmic reticulum stress to protect against nucleus pulposus cell death and ameliorate intervertebral disc degeneration in vivo. Theranostics. 2019;9:4084-4100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 301] [Article Influence: 50.2] [Reference Citation Analysis (0)] |
| 82. | Yuan Q, Wang X, Liu L, Cai Y, Zhao X, Ma H, Zhang Y. Exosomes Derived from Human Placental Mesenchymal Stromal Cells Carrying AntagomiR-4450 Alleviate Intervertebral Disc Degeneration Through Upregulation of ZNF121. Stem Cells Dev. 2020;29:1038-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 83. | Xie L, Chen Z, Liu M, Huang W, Zou F, Ma X, Tao J, Guo J, Xia X, Lyu F, Wang H, Zheng C, Jiang J. MSC-Derived Exosomes Protect Vertebral Endplate Chondrocytes against Apoptosis and Calcification via the miR-31-5p/ATF6 Axis. Mol Ther Nucleic Acids. 2020;22:601-614. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 78] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 84. | Staff NP, Jones DT, Singer W. Mesenchymal Stromal Cell Therapies for Neurodegenerative Diseases. Mayo Clin Proc. 2019;94:892-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 110] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 85. | Rizvi M. Novel treatment strategies for intervertebral disc degeneration. Saudi J Med Health Sci. 2015;4:5-15. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 86. | Sampara P, Banala RR, Vemuri SK, Av GR, Gpv S. Understanding the molecular biology of intervertebral disc degeneration and potential gene therapy strategies for regeneration: a review. Gene Ther. 2018;25:67-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 136] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 87. | Zhang S, Hu B, Liu W, Wang P, Lv X, Chen S, Shao Z. The role of structure and function changes of sensory nervous system in intervertebral disc-related low back pain. Osteoarthritis Cartilage. 2021;29:17-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 68] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 88. | Zhang F, Zhao X, Shen H, Zhang C. Molecular mechanisms of cell death in intervertebral disc degeneration (Review). Int J Mol Med. 2016;37:1439-1448. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 181] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 89. | Ahsan MK, Hossain MR, Khan MSI, Zaman N, Ahmed N, Montemurro N, Chaurasia B. Lumbar revision microdiscectomy in patients with recurrent lumbar disc herniation: A single-center prospective series. Surg Neurol Int. 2020;11:404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 90. | Ju DG, Kanim LE, Bae HW. Intervertebral Disc Repair: Current Concepts. Global Spine J. 2020;10:130S-136S. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 91. | Perrini P, Gambacciani C, Martini C, Montemurro N, Lepori P. Anterior cervical corpectomy for cervical spondylotic myelopathy: Reconstruction with expandable cylindrical cage versus iliac crest autograft. A retrospective study. Clin Neurol Neurosurg. 2015;139:258-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 92. | Imai Y, Okuma M, An HS, Nakagawa K, Yamada M, Muehleman C, Thonar E, Masuda K. Restoration of disc height loss by recombinant human osteogenic protein-1 injection into intervertebral discs undergoing degeneration induced by an intradiscal injection of chondroitinase ABC. Spine (Phila Pa 1976). 2007;32:1197-1205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 79] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 93. | Masuda K. Biological repair of the degenerated intervertebral disc by the injection of growth factors. Eur Spine J. 2008;17 Suppl 4:441-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 155] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 94. | Cho H, Lee S, Park SH, Huang J, Hasty KA, Kim SJ. Synergistic effect of combined growth factors in porcine intervertebral disc degeneration. Connect Tissue Res. 2013;54:181-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 95. | Vo NV, Hartman RA, Yurube T, Jacobs LJ, Sowa GA, Kang JD. Expression and regulation of metalloproteinases and their inhibitors in intervertebral disc aging and degeneration. Spine J. 2013;13:331-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 334] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 96. | Lu L, Xu A, Gao F, Tian C, Wang H, Zhang J, Xie Y, Liu P, Liu S, Yang C, Ye Z, Wu X. Mesenchymal Stem Cell-Derived Exosomes as a Novel Strategy for the Treatment of Intervertebral Disc Degeneration. Front Cell Dev Biol. 2021;9:770510. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 97. | Wang Y, Yi H, Song Y. The safety of MSC therapy over the past 15 years: a meta-analysis. Stem Cell Res Ther. 2021;12:545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 109] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 98. | Yamaguchi S, Horie N, Satoh K, Ishikawa T, Mori T, Maeda H, Fukuda Y, Ishizaka S, Hiu T, Morofuji Y, Izumo T, Nishida N, Matsuo T. Age of donor of human mesenchymal stem cells affects structural and functional recovery after cell therapy following ischaemic stroke. J Cereb Blood Flow Metab. 2018;38:1199-1212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 99. | Wang B, Liu Z, Chen VP, Wang L, Inman CL, Zhou Y, Guo C, Tchkonia T, Rowe DW, Kuchel GA, Robson P, Kirkland JL, Xu M. Transplanting cells from old but not young donors causes physical dysfunction in older recipients. Aging Cell. 2020;19:e13106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 100. | Lavasani M, Robinson AR, Lu A, Song M, Feduska JM, Ahani B, Tilstra JS, Feldman CH, Robbins PD, Niedernhofer LJ, Huard J. Muscle-derived stem/progenitor cell dysfunction limits healthspan and lifespan in a murine progeria model. Nat Commun. 2012;3:608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 148] [Cited by in RCA: 164] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 101. | Baker N, Boyette LB, Tuan RS. Characterization of bone marrow-derived mesenchymal stem cells in aging. Bone. 2015;70:37-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 221] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 102. | D'Ippolito G, Schiller PC, Ricordi C, Roos BA, Howard GA. Age-related osteogenic potential of mesenchymal stromal stem cells from human vertebral bone marrow. J Bone Miner Res. 1999;14:1115-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 664] [Cited by in RCA: 633] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 103. | Abuna RP, Stringhetta-Garcia CT, Fiori LP, Dornelles RC, Rosa AL, Beloti MM. Aging impairs osteoblast differentiation of mesenchymal stem cells grown on titanium by favoring adipogenesis. J Appl Oral Sci. 2016;24:376-382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 104. | Marędziak M, Marycz K, Tomaszewski KA, Kornicka K, Henry BM. The Influence of Aging on the Regenerative Potential of Human Adipose Derived Mesenchymal Stem Cells. Stem Cells Int. 2016;2016:2152435. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 169] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 105. | Bonyadi M, Waldman SD, Liu D, Aubin JE, Grynpas MD, Stanford WL. Mesenchymal progenitor self-renewal deficiency leads to age-dependent osteoporosis in Sca-1/Ly-6A null mice. Proc Natl Acad Sci U S A. 2003;100:5840-5845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 183] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 106. | Moerman EJ, Teng K, Lipschitz DA, Lecka-Czernik B. Aging activates adipogenic and suppresses osteogenic programs in mesenchymal marrow stroma/stem cells: the role of PPAR-gamma2 transcription factor and TGF-beta/BMP signaling pathways. Aging Cell. 2004;3:379-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 574] [Cited by in RCA: 646] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 107. | Miura Y, Miura M, Gronthos S, Allen MR, Cao C, Uveges TE, Bi Y, Ehirchiou D, Kortesidis A, Shi S, Zhang L. Defective osteogenesis of the stromal stem cells predisposes CD18-null mice to osteoporosis. Proc Natl Acad Sci U S A. 2005;102:14022-14027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 108. | Infante A, Rodríguez CI. Osteogenesis and aging: lessons from mesenchymal stem cells. Stem Cell Res Ther. 2018;9:244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 224] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 109. | Smirnova A, Yatsenko E, Baranovskii D, Klabukov I. Mesenchymal stem cell-derived extracellular vesicles in skin wound healing: the risk of senescent drift induction in secretome-based therapeutics. Mil Med Res. 2023;10:60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 110. | Hao Y, Zhu G, Yu L, Ren Z, Zhang P, Zhu J, Cao S. Extracellular vesicles derived from mesenchymal stem cells confer protection against intervertebral disc degeneration through a microRNA-217-dependent mechanism. Osteoarthritis Cartilage. 2022;30:1455-1467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |