Published online Jun 26, 2025. doi: 10.4252/wjsc.v17.i6.105491
Revised: March 7, 2025
Accepted: June 10, 2025
Published online: June 26, 2025
Processing time: 151 Days and 18.5 Hours
Fibro-adipogenic progenitors (FAPs) are a group of mesenchymal stem cells that cause fibro-fatty degeneration in skeletal muscle in various chronic disease mode
To clarify the changes in the number of FAPs and their impact on skeletal muscle soon after tendon rupture to facilitate future studies targeting FAPs to treat muscle degeneration.
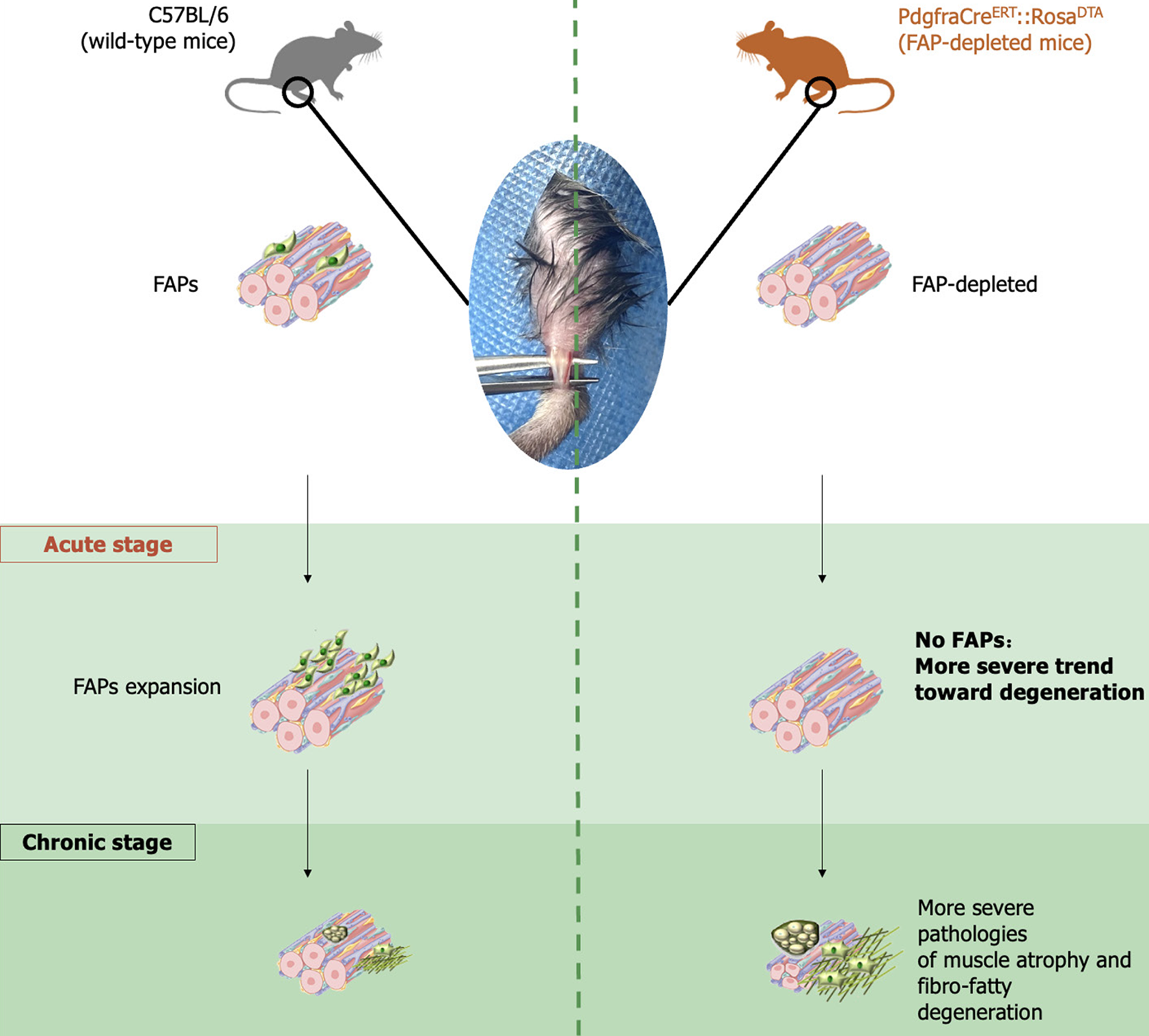
We utilized Pdgfra-H2B::eGFP mice to trace and quantify FAPs in a tibialis anterior tenotomy (TAT) model at 0 and 3 days, 1 week, 2 weeks, 3 weeks, 4 weeks, 5 weeks, and 6 weeks post-injury, and the results were further validated using fluorescence-activated cell sorting analysis with C57BL/6 mice at the same post-injury timepoints. We subsequently used PdgfraCreERT::RosaDTA mice, and evaluated the severity of post-TAT skeletal muscle degeneration with or without FAP-depletion.
The number of FAPs peaked at 1 week post-TAT before gradually declining to a level comparable to that pre-TAT. The change in the number of FAPs was potentially temporally correlated with the progression of skeletal muscle degeneration after TAT. FAP-depletion led to more severe degeneration early after TAT, indicating that FAPs potentially alleviate muscle degeneration after tendon rupture in the early post-injury phase.
FAPs potentially alleviate the degeneration of skeletal muscle in the acute stage after tendon rupture.
Core Tip: In this study, we demonstrate that fibro-adipogenic progenitors (FAPs) may actually attenuate early-stage dege
- Citation: Ding ZC, He JJ, Shi LZ, Qian J, Mei SH, Kang X, Chen JW. Fibro-adipogenic progenitors prevent skeletal muscle degeneration at acute phase upon tendon rupture in a murine tibialis anterior tenotomy model. World J Stem Cells 2025; 17(6): 105491
- URL: https://www.wjgnet.com/1948-0210/full/v17/i6/105491.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v17.i6.105491
After tendon rupture, fibro-fatty degeneration within the muscle, including muscle atrophy, fibrosis and fatty infiltration[1], progresses and can become a key factor hindering functional recovery of the muscle-tendon unit, even if the tendon is repaired[2,3]. Recently, fibro-adipogenic progenitors (FAPs), a group of mesenchymal stem cells, were identified in skeletal muscle[4,5] and were found to function as a ‘double-edged sword’ in skeletal muscle regeneration after injury[6-9].
There were only a few quiescent FAPs in situ among the intact skeletal muscle fibers. In acute injuries such as cardiotoxin injection, as well as acute phase in disease progression such as aging, FAPs proliferate, facilitating muscle regeneration and delaying muscle degeneration through the secretion of Bmp3b, interleukin-33, etc[5,7,10,11]. In contrast, in the chronic pathologies such as denervation, FAPs differentiate and lead to fibro-fatty degeneration of the muscle upon the activation of signal transducer and activator of transcription 3/interleukin-6 signaling pathways, etc[6,7,9,12,13].
Although FAPs are a popular research topic, few studies have focused on FAPs in the context of tendon rupture, which is one of the most prevalent musculotendinous conditions. It has been reported that FAPs cause fibro-fatty degeneration of the muscle in the chronic stage after tendon rupture[12,14]; therefore, researchers have suggested targeting FAPs to prevent or treat muscle degeneration after tendon rupture. However, how FAPs change during the muscle degeneration that occurs after tendon rupture remains unknown. In addition, the role FAPs play in the acute phase after tendon rupture has not been clarified. This study was performed with the aim of investigating how the number of FAPs in the muscle is altered after tendon rupture, and determining the influence of FAPs on muscle degeneration in the acute phase after tendon rupture.
All of the procedures performed on mice in this study were approved by the Institutional Animal Care and Use Committee of Shanghai General Hospital. We adhered to the ARRIVE guidelines and used the ARRIVE Checklist. Enhanced green fluorescence protein-labeled Pdgfra+ cells were used to trace FAPs in Pdgfra-H2B::eGFP mice (Jackson Laboratory, Shanghai, China). Eight-week-old male Pdgfra-H2B::eGFP mice were sacrificed, muscle samples were collected and sectioned, and then were observed via microscopy for pre-tibialis anterior tenotomy (TAT) 0 day results. Eight-week-old male Pdgfra-H2B::eGFP mice were subjected to TAT before being sacrificed for muscle collection and their samples sectioned for observation via microscopy at 3 days, 1 week, 2 weeks, 3 weeks, 4 weeks, 5 weeks, and 6 weeks after TAT (n = 3). Additionally, 8-week-old male C57BL/6 (wild-type, WT) mice underwent TAT before being sacrificed for fluorescence-activated cell sorting (FACS), and histological tests of the muscle at 0 day and 1 week, 2 weeks, 4 weeks, and 6 weeks after TAT (n = 5).
To generate mice with depleted FAPs, PdgfraCreERT::RosaDTA mice were generated by breeding PdgfraCreERT mice with RosaDTA mice (both from Cyagen Biosciences, CA, United States). Six-week-old male PdgfraCreERT::RosaDTA mice were injected intraperitoneal with either tamoxifen (n = 3) dissolved in corn oil or plain corn oil (n = 3) every 24 hours for 7 consecutive days to generate FAP-depleted mice and control mice. Then, the mice were subjected to TAT before being sacrificed for further histological tests of the muscle at 0 day, 1 week, and 6 weeks after TAT.
The mice were anesthetized, and the skin and hair of the lower limbs were prepared before an incision was made directly beneath the lateral ankle. Then, the tibialis anterior (TA) and insertion into the ankle were exposed. The TA tendon was clamped adjacent to the insertion and transected. After complete transection of the TA tendon, the incision was sterilized and closed. In both Pdgfra-H2B::eGFP mice and PdgfraCreERT::RosaDTA mice, TAT was performed both hind limbs. WT mice underwent TAT on the left hind limb and sham surgery (SS) on the right hind limb as a control treatment; in the SS, the same procedures for TAT were performed, except the TA tendon was not transected.
The TA muscle was collected and minced before being digested with 0.2% type II collagenase (Biofroxx, Germany) for 1 hour at 37 °C. Muscle slurries were filtered through 100 μm and 40 μm cell strainers (BD Bioscience, NJ, United States) in sequence. After erythrocytes were removed, the cells were resuspended in phosphate buffered saline containing 2% foetal bovine serum. The cells were subsequently stained with the following antibodies: Alexa Fluor 488-conjugated anti-mouse CD31 antibody (Biolegend, CA, United States), Alexa Fluor 488-conjugated anti-mouse CD45 antibody (Biolegend, CA, United States), APC-conjugated anti-mouse integrin α7 antibody (R&D, MN, United States), and APC-conjugated/cyanine7-conjugated anti-mouse Sca1 antibody (Biolegend, CA, United States) for 30 minutes at 4 °C in the dark. The gating strategy used was CD31-CD45-integrinα7-Sca1+. The stained cells were then subjected to FACS with a FACSAria III (BD Biosciences, NJ, United States).
TA muscle samples were collected and immediately fixed in 10% formalin for 24 hours before being dehydrated in a 30% sucrose solution for 48 hours. The samples were then flash frozen or embedded in paraffin embedded and cryo-sectioned at a thickness of 10 mm. For samples from Pdgfra-H2B::eGFP mice, the slices were then directly observed under a luminescence microscope.
For samples from WT mice and PdgfraCreERT::RosaDTA mice, hematoxylin and eosin (H&E) staining (Solarbio, Beijing, China), modified Masson’s trichrome (TRI) staining (Solarbio, Beijing, China) and oil red O (ORO) staining (Solarbio, Beijing, China) were performed before observation. H&E staining was used to facilitate the quantification of the cross-sectional area (CSA) of the TA muscle fibers to assess the degree of atrophy in the sample. TRI staining was used to determine the collagen content in order to evaluate fibrosis in the TA muscle. ORO staining was performed to visualize the lipid content to evaluate fatty infiltration of the TA muscle. FBXO32 (Affinity, OH, United States) and alpha-smooth muscle actin (α-SMA) (Affinity, OH, United States) immunohistochemistry (IHC) were performed to detect the pro-atrophic muscle fibers that had been labeled with FBXO32, as well as the pro-fibrotic content labeled with α-SMA within the TA muscle. Perilipin-1 (Abcam, MA, United States) immunofluorescence (IF) was performed to detect adipogenesis within the TA muscle. The procedures for staining, IHC and IF followed the corresponding product protocols.
For the histological tests, cross sections were chosen randomly from the mid-bellies of the TA muscle, and further assessments were made on 3 different sections, with 5 randomly selected areas on each section. All images were assessed by 2 blinded researchers using ImageJ, and the average value was calculated for each sample. CSA was measured for muscle fibers in H&E-stained sections. The severity of fibrosis was evaluated on the basis of the fraction of the entire sample area with aniline blue on TRI-stained sections. Similarly, the severity of fatty infiltration was evaluated by dividing the area of fat stained with ORO by the entire sample area on ORO-stained sections.
The fraction of the sample area positive for FBXO32 on IHC, α-SMA on IHC and perilipin 1 on IF were also determined by dividing the area of positive expression by the whole area to show the changes over time of muscle atrophy, fibrosis and adipogenesis, respectively. The collected FACS data were further analyzed with FlowJo v10. The change in the population of FAPs was determined by calculating the proportion of CD45-CD31- cells accounted for by FAPs, the proportion of live cells accounted for by FAPs, and the number of FAPs per gram of TA muscle. All the data were analyzed using two-way ANOVA with SPSS. Significance was determined at a P value < 0.05.
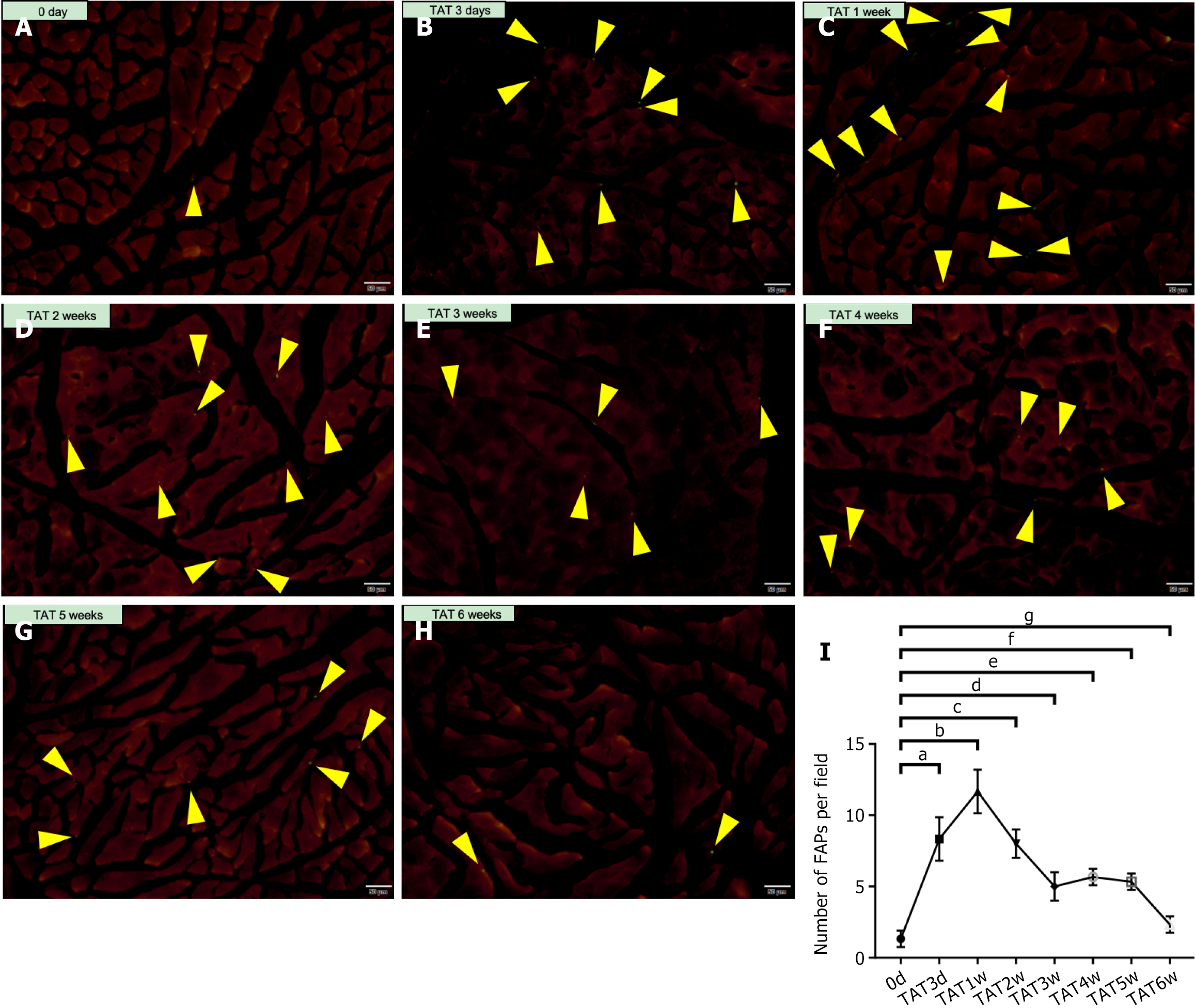
A mouse model (pdgfra-H2B::eGFP) using platelet-derived growth factor alpha, a classical surface marker for FAPs, was constructed. The TA muscles of Pdgfra-H2B::eGFP mice that underwent TAT at 0-3 days, and 1 week, 2 weeks, 3 weeks, 4 weeks, 5 weeks, and 6 weeks post-operation were collected and sectioned, and the number of FAPs at each time point were then quantified via luminescence microscopy. The results (Figure 1) revealed that the FAPs were located mainly amongst the myofibers, both before and after TAT. In addition, there were only 1.3 ± 0.5 FAPs per field of view in the muscle before TAT; however, this number increased significantly to 8.3 ± 1.2 per field at 3 days post-TAT (0 day vs 3 days, P < 0.001) and further reached 11.7 ± 1.2 per field at 1 week post-TAT (0 day vs 1 week, P < 0.001). After that time point, the number of FAPs decreased, and there was no significant difference in the number of FAPs per field between 0 day and 6 weeks post-TAT (2.3 ± 0.5 vs 1.3 ± 0.5, P > 0.05).
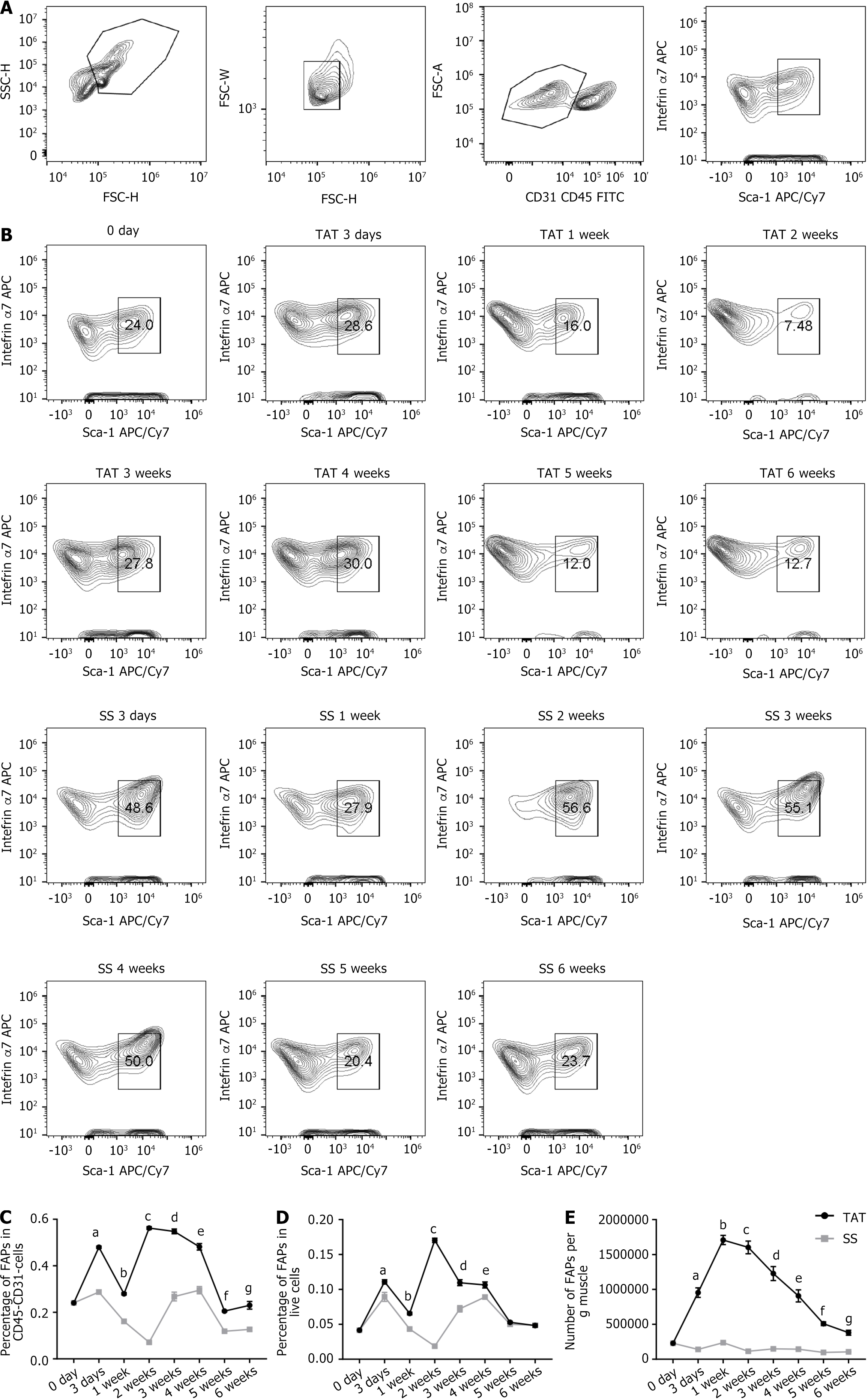
In addition, TAT was also performed in WT mice, and corresponding SSs were conducted on the contralateral side (TAT/SS model). The muscles from both sides at 0-3 days, and 1 week, 2 weeks, 3 weeks, 4 weeks, 5 weeks, and 6 weeks after injury were then processed and analyzed using FACS (gating strategies showed in Figure 2A). The results showed that the proportion of FAPs among CD45-CD31- cells in the muscle began to increase at 3 days post-TAT and subsequently remained significantly greater than that of the SS side (primary results showed in Figure 2B, further analysis shown in Figure 2C). However, this proportion demonstrated a random pattern of changes from 1 week to 6 weeks post-TAT (Figure 2C). Therefore, we further analyzed the proportion of FAPs among all live cells in the muscle (Figure 2D) and detected no obvious pattern of change. Considering that the alteration in the proportion of FAPs among CD45-CD31- cells and live cells demonstrated with FACS could be largely influenced by the significant change in the quantity of other cells, such as immune cells, it is reasonable to assume that the changes in those proportions could be biased, rather than reflecting the actual changes in the absolute numbers of FAPs themselves. Therefore, the results from FACS were analyzed again, and the number of FAPs per gram of muscle was calculated. The results (Figure 2E) revealed that the number of FAPs per gram of muscle began to increase at 3 days post-TAT, reached (17.1 ± 0.6) × 105 per gram of muscle at 1 week post-TAT, and was significantly greater than that of the SS side (P < 0.001). The number of FAPs per gram then declined and was close to, but still greater, than that on the SS side at 6 weeks post-operation. This result demonstrated a similar trend to the change pattern of FAPs obtained in Pdgfra-H2B::eGFP mice. On the basis of the above evidence, it was determined that 1 week post-TAT is a crucial timepoint, as that is when the number of FAPs was highest after TAT.
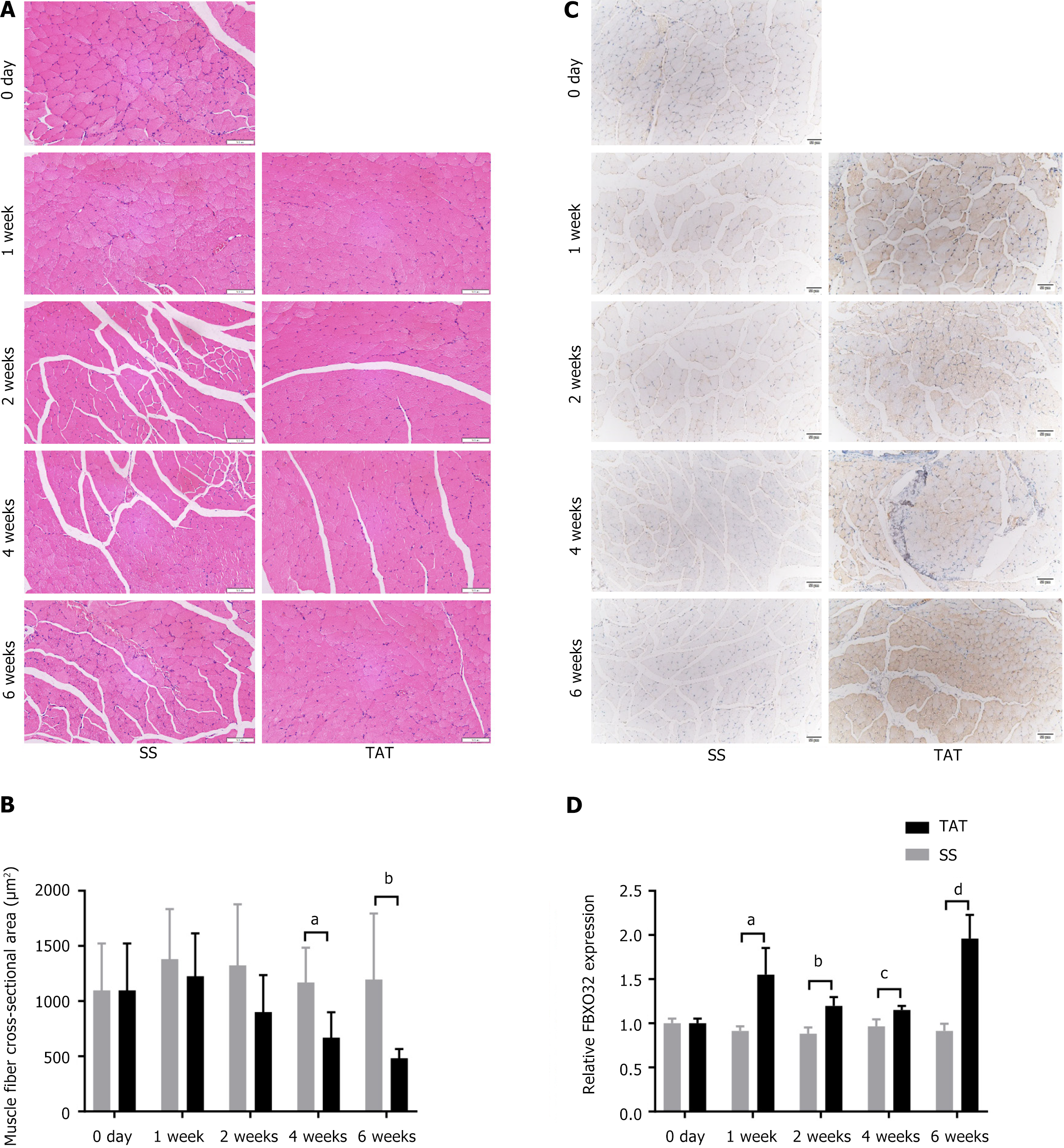
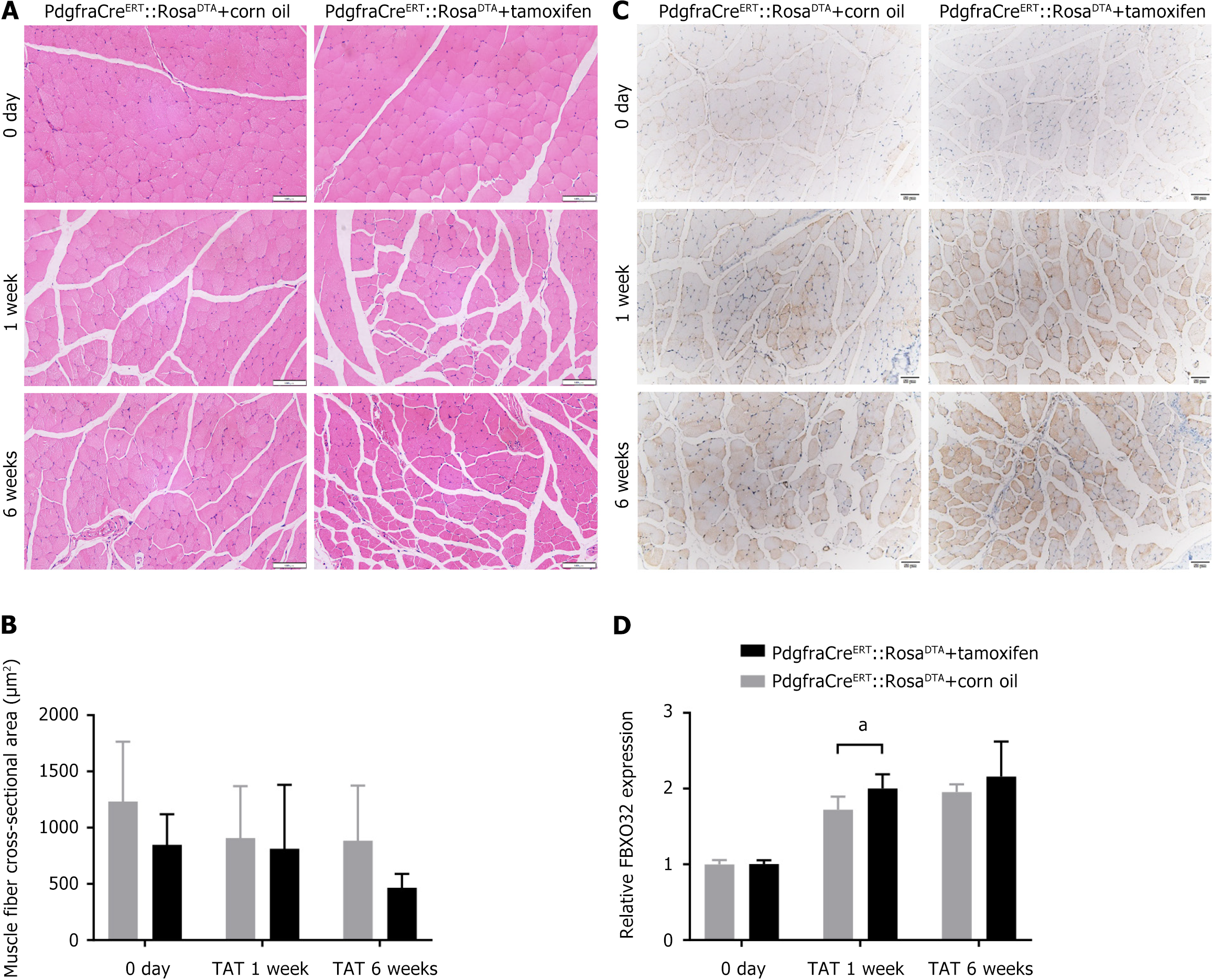
The TAT/SS model was again established in WT mice, and the muscles at 0 day, and 1 week, 2 weeks, 4 weeks, and 6 weeks post-operation were collected for further histological evaluation. The results of H&E staining (Figure 3A and B) reveal that the CSA of post-TAT muscle fibers was lower than that of post-SS muscle fibers, especially at 4 weeks and 6 weeks after TAT (both P < 0.05). Furthermore, FBXO32 IHC staining (Figure 3C and D) revealed that at 1 week after TAT, the muscle was more inclined to atrophy at all time points compared with that of the post-SS side (all P < 0.001).
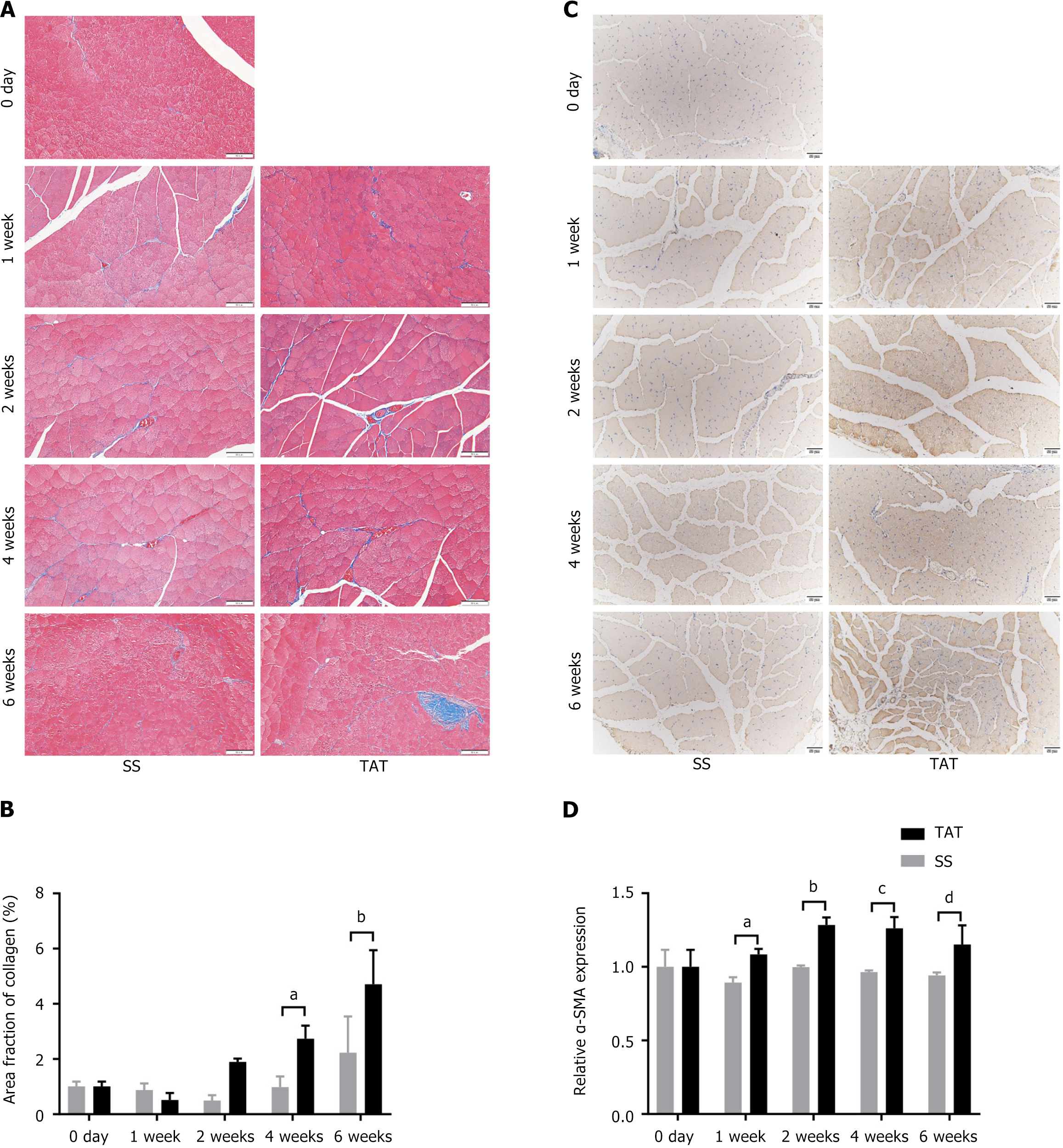
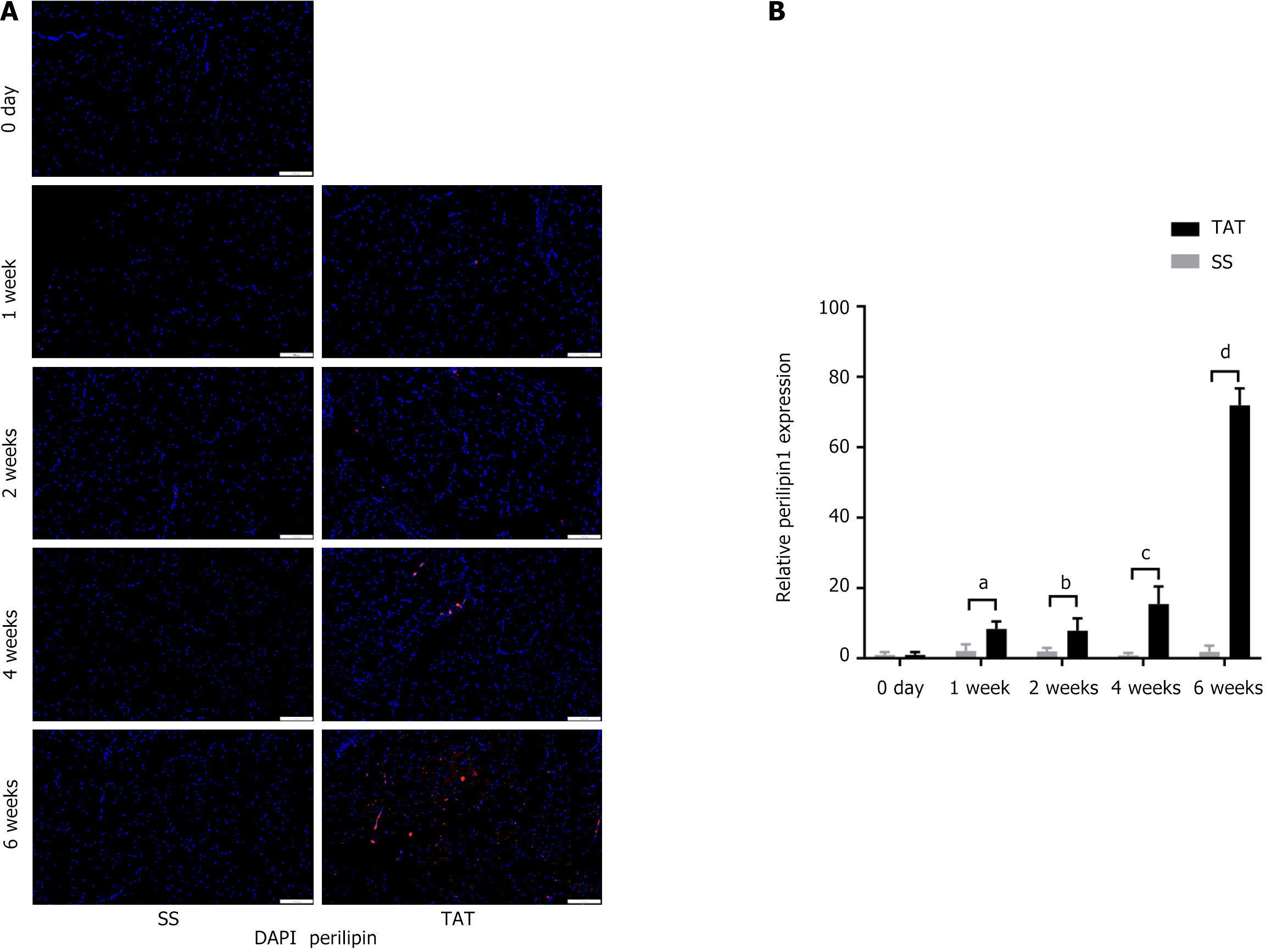
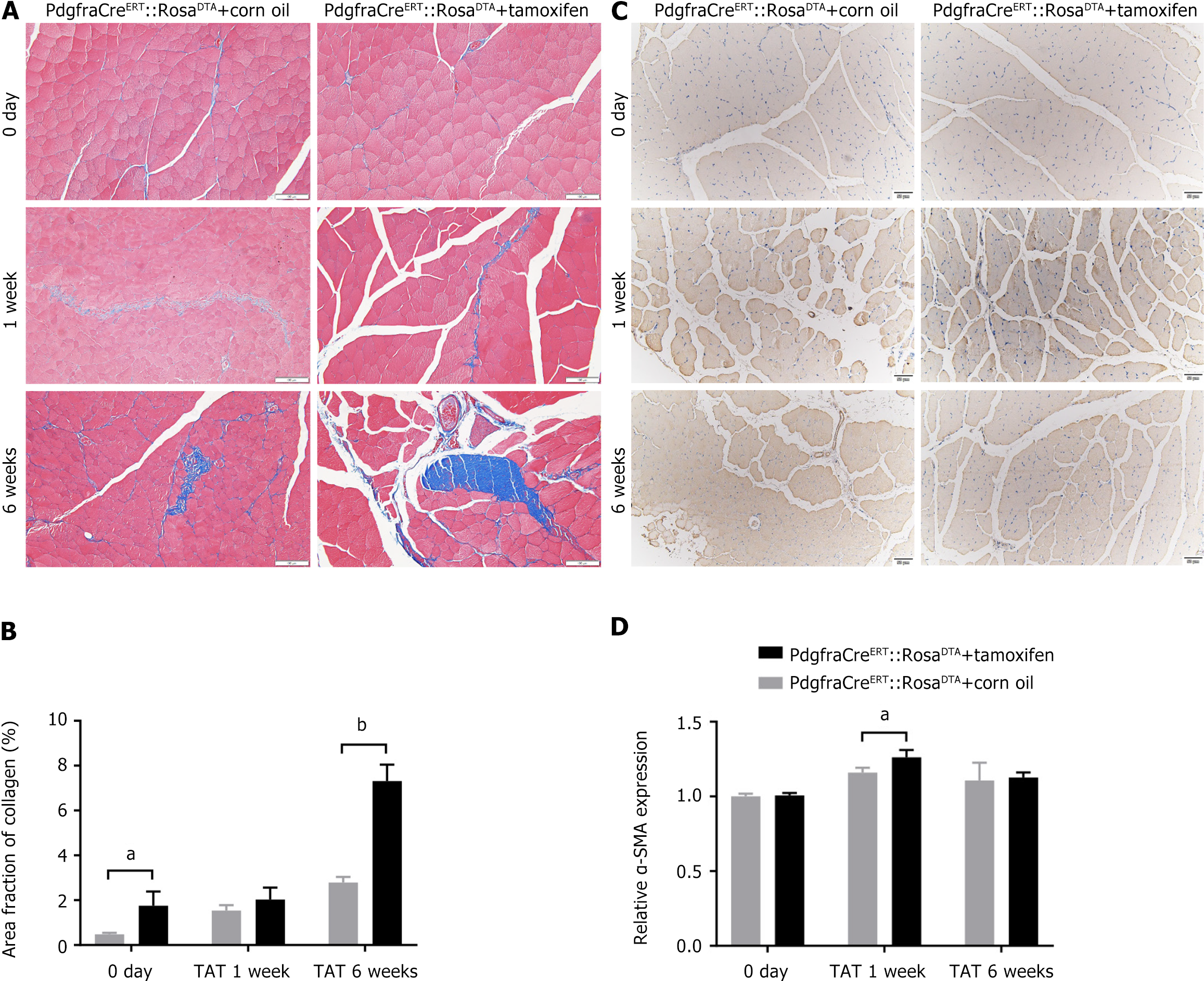
In addition, TRI staining (Figure 4A and B) and α-SMA IHC staining (Figure 4C and D) of the tissue sections reveal that fibrosis at 1 week after TAT was significantly more severe in the post-TAT muscle than in the post-SS side (all P < 0.01). In addition, ORO staining (Supplementary Figure 1) and perilipin 1 IF staining were also used to evaluate fatty degeneration of the muscle (Figure 5A and B). Although the results of ORO staining revealed no lipid droplets in any of the muscle samples, IF staining revealed that the expression of perilipin 1, a marker indicating the early phase of adipogenesis, was significantly greater at 1 week post-TAT, than at 1 week post-SS (all P < 0.05).
To explore the role of FAPs in muscle degeneration after TAT, PdgfraCreERT::RosaDTA mice were injected with tamoxifen (dissolved in corn oil) to deplete FAPs (FAP-depleted mice), and the control group was injected with an equal volume of corn oil only. The efficacy of depletion was validated using FACS (Supplementary Figure 2). Then, both the FAP-depleted mice and their control group underwent TAT, and the muscles at 0 day, and 1-6 weeks post-TAT was collected. The aforementioned histological tests were performed to compare the severity of muscle degeneration between the two groups.
The results of H&E staining (Figure 6A and B) showed that the CSA of myofibers in the muscle of the FAP-depleted mice was lower than that of the control group at 0 day, and 1-6 weeks post-TAT, although the difference was not significant (all P > 0.05). The results of FBXO32 IHC staining (Figure 6C and D) revealed that, compared with those in the control group, the muscle in the experimental group was significantly more prone to atrophy at 1 week post-TAT (1.96 ± 0.16 times vs 1.72 ± 0.17 times, P < 0.05), whereas the difference was not significant at 0 day and 6 weeks post-TAT (P > 0.05).
TRI staining revealed that neither the FAP-depleted mice nor the control mice formed obvious fibrotic scars in the muscle before TAT, but the muscle of the FAP-depleted mice demonstrated significantly more severe fibrosis than did that of the control mice at 1-6 weeks post-TAT (Figure 7A and B). Moreover, the results of α-SMA IHC staining (Figure 7C and D) reveal that, compared with that in the control group, the trend toward fibrosis in the muscle was significantly greater at 1 week post-TAT in FAP-depleted mice (1.26 ± 0.04 times vs 1.16 ± 0.03 times, P < 0.01), but the difference was not significant at 0-6 weeks after TAT (P > 0.05).
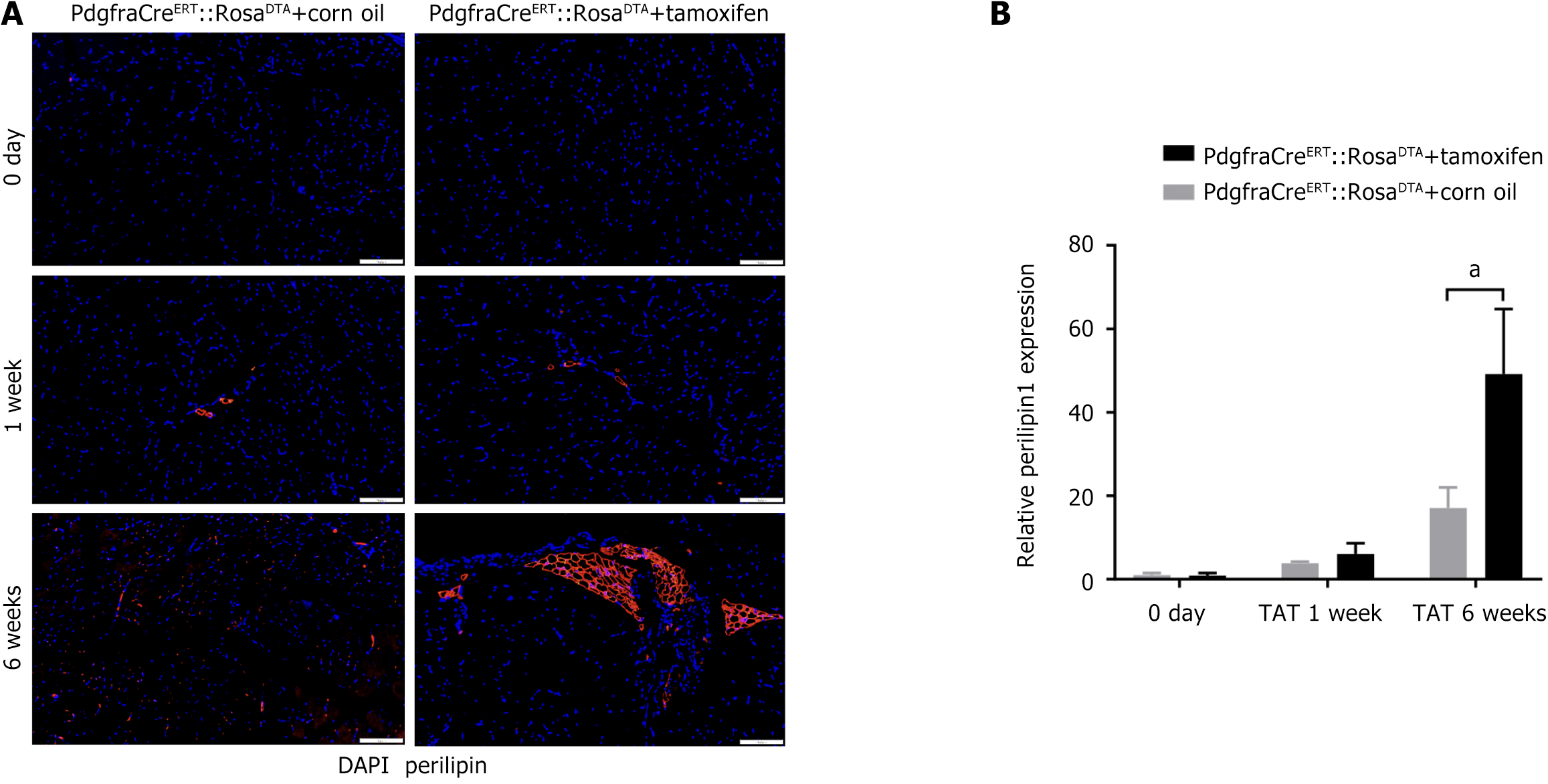
The results of ORO staining (Supplementary Figure 3) showed that no muscle among the two groups exhibited interfibrous lipid accumulation. However, the results of perilipin 1 IHC staining (Figure 8A and B) showed that the expression of perilipin 1 was significantly greater at 6 weeks post-TAT in FAP-depleted mice than in control mice (49.16 ± 12.72 times vs 17.06 ± 4.04 times, P < 0.05). However, no significant difference was found between the groups at 0-1 week after TAT.
In this study, we confirmed the change in the number of FAPs after tendon rupture, indicating that the number of FAPs primarily expands shortly after injury, before gradually declining to the preinjury level. The expanded population of FAPs at the acute stage after tendon rupture might alleviate muscle degeneration, as FAP-depletion was found to result in a more severe trend toward postinjury muscle degeneration.
Tendon ruptures, such as rotator cuff and Achilles tendon tears, are among the most common diseases presented in orthopedic sports medicine clinics. Muscle degeneration after tendon rupture was found to be the key factor hindering functional muscle recovery, even if the torn tendon was repaired successfully. Inspired by the difficulties encountered in clinical practice, we chose to study the changes in and function of FAPs in skeletal muscle degeneration after tendon rupture. The mouse model of TAT is onesome of the most classical models to study tendon tearing in animals[15]. In addition, numerous studies focusing on FAPs and muscle degeneration were performed on the TA[16-19], including the first studies that identified FAPs[4,5]. Therefore, we also chose to utilize a mouse model of TAT.
PDGFRa-H2B::eGFP mice were used to characterize FAP distribution[20-22], and TA sections from PDGFRa-H2B::eGFP mice revealed that the number of FAPs increased significantly after TAT, peaking at 1 week, and then gradually declined. To further validate these results, we established a TAT/SS model in WT mice, and subsequent FACS demonstrated results with a similar trend. One previous study also tried to clarify the change pattern of FAP quantity after multi-regional clinical trial[23]. However, their FACS results revealed that the number of FAPs plus endothelial cells was greater at 28 days than at 5 days post trauma, which was neither precise nor direct enough to address this important question. Our study, however, provided evidence at multiple timepoints obtained from both histological tests in PDGFRa-H2B::eGFP mice and FACS data from WT mice, and this data focused exclusively on FAPs.
FAPs cannot exert their function without massive proliferation, as there are only a few FAPs in situ among the myofibers. Therefore, the number of FAPs could be an important factor affecting their function. Our results indicated that FAPs proliferate in the early stage of tendon rupture, before returning to quiescence at a later stage. This pattern is similar to that of FAPs in various musculotendinous injury models reported in the literature[4,5,18,22]. Therefore, our results suggest that FAPs might also act as a ‘double-edged sword’ toward the muscle in the tendon rupture model. In addition, these results could facilitate a better understanding of the alteration of FAPs in the muscle upon tendon rupture.
Next, we conducted histological tests to visualize the progression of muscle degeneration in WT mice after TAT. Compared with that in the SS group, there was obvious fibrotic deposition within the muscle at 1 week, 4 weeks, and 6 weeks post-TAT, and the CSA at 4-6 weeks post-TAT was significantly smaller than that in the SS group. This finding is not surprising, as previous studies have reported that skeletal muscle fibro-fatty degeneration occurs at 6-8 weeks post-tendon rupture[12,13]. However, interestingly, at 1 week, 2 weeks, 4 weeks, and 6 weeks after TAT, the FBXO32+, α-SMA+ or perilipin 1+ area fractions within the muscle, respectively representing the tendency toward skeletal muscle atrophy and fibro-fatty degeneration, were significantly greater than those in the SS group. These results confirmed that a trend toward muscle degeneration could be observed even at 1 week after TAT. Considering that the peak population of FAPs also occurred at 1 week post-TAT, these results suggest that FAPs could have a potential impact on muscle degeneration in the early phase after tendon rupture.
To verify this hypothesis, we utilized FAPs-depleted mice, as in previous studies[11,20], and performed histological tests to determine whether the absence of FAPs would affect skeletal muscle degeneration after TAT. The results demonstrated that the muscle of FAPs-depleted mice presented significantly aggravated atrophy and fibrosis at 1 week after TAT and a significantly aggravated trend toward adipogenesis at 6 week after TAT. These results showed that FAP-depletion could exacerbate the degeneration of skeletal muscle after TAT, indicating that FAPs could alleviate the trend of skeletal muscle degeneration early after tendon rupture.
To our knowledge, no prior studies have reported the positive effects of FAPs on skeletal muscle after tendon rupture. Our findings support the notion that FAPs promote skeletal muscle regeneration and reduce skeletal muscle degeneration in other musculotendinous disease models[5,7,10,16,24-26]. Circulating factors derived from FAPs have been demon
There were certain limitations to the present study. First, in the current study, FAP-depletion was achieved prior to TAT, so the mitigating effect of degeneration after FAPs depletion may only apply to the early phase after TAT. It still remains unknown whether FAPs would exert the same function if they were depleted after TAT. In our study design, we intended to add a group in which the FAPs were depleted at 6 weeks post-TAT; however, the number of FAPs at 6 weeks post-TAT was already low, making depletion impossible. This may be because the surface markers of FAPs can change during their differentiation process in the late stages after TAT, making it difficult to detect FAPs with the same gating strategy.
In addition, the results of ORO staining in the present study did not agree with the results of prior studies. In our study, typical fat droplets could not be observed, even at late stages after TAT, whereas skeletal muscle fatty infiltration was observed at 6-8 weeks after tendon rupture in previous studies[12,14]. It is possible that this could be due to the content of FAPs, as well as their tendency toward adipogenesis differentiation in the skeletal muscle of mice hind limbs, like the TA, being lower than that of the upper limbs, such as the supraspinatus and infraspinatus muscles[28]. However, the TAT model is more mature and more convenient for subsequent intramuscular administration, which might be needed for further research; furthermore, with the use of the TAT model, fewer mice would need to be sacrificed, as the muscle mass of the TA is greater than that of the supraspinatus and infraspinatus muscles. Thus, we used the TAT model in favor of other tendon tear models.
Based on our findings demonstrating the beneficial role of FAPs in muscle preservation during the acute phase following tendon rupture, therapeutic strategies aimed at delaying or preventing muscle degeneration progression warrant further investigation. Preclinical studies in various pathological models, including age-related muscle atrophy and massive rotator cuff injuries, have explored the transplantation of genetically modified FAPs as a potential intervention for muscle degeneration in murine models[27,29,30]. However, the translation of FAP-based stem cell therapies into clinical applications remains challenging and requires substantial additional research. An alternative therapeutic approach involves pharmacological modulation of FAP-related pathways. Previous investigations have identified promising candidates, including the transforming growth factor-beta inhibitor decorin and the histone deacetylase inhibitor Trichostatin A, which have demonstrated efficacy in mitigating muscle degeneration through FAP modulation[31,32]. Further mechanistic studies elucidating the anti-degenerative properties of FAPs are expected to reveal novel molecular targets for pharmacological intervention. The current findings provide a foundation for future research directions and may contribute to the development of therapeutic strategies for post-tendon rupture muscle degeneration (Figure 9).
FAP population expansion at the acute stage after tendon rupture can potentially alleviate the degeneration of skeletal muscle.
We thank Prof. Kang-Lai Tang (MD, PhD) from Department of Sports Medicine, Southwest Hospital, Army Medical University for his kind support to this research.
| 1. | Chen W, You W, Valencak TG, Shan T. Bidirectional roles of skeletal muscle fibro-adipogenic progenitors in homeostasis and disease. Ageing Res Rev. 2022;80:101682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 47] [Reference Citation Analysis (0)] |
| 2. | Shin YK, Ryu KN, Park JS, Jin W, Park SY, Yoon YC. Predictive Factors of Retear in Patients With Repaired Rotator Cuff Tear on Shoulder MRI. AJR Am J Roentgenol. 2018;210:134-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 3. | Farup J, Just J, de Paoli F, Lin L, Jensen JB, Billeskov T, Roman IS, Cömert C, Møller AB, Madaro L, Groppa E, Fred RG, Kampmann U, Gormsen LC, Pedersen SB, Bross P, Stevnsner T, Eldrup N, Pers TH, Rossi FMV, Puri PL, Jessen N. Human skeletal muscle CD90(+) fibro-adipogenic progenitors are associated with muscle degeneration in type 2 diabetic patients. Cell Metab. 2021;33:2201-2214.e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 78] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 4. | Uezumi A, Fukada S, Yamamoto N, Takeda S, Tsuchida K. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat Cell Biol. 2010;12:143-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 810] [Cited by in RCA: 967] [Article Influence: 64.5] [Reference Citation Analysis (0)] |
| 5. | Joe AW, Yi L, Natarajan A, Le Grand F, So L, Wang J, Rudnicki MA, Rossi FM. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol. 2010;12:153-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1021] [Cited by in RCA: 1238] [Article Influence: 82.5] [Reference Citation Analysis (0)] |
| 6. | Uezumi A, Ito T, Morikawa D, Shimizu N, Yoneda T, Segawa M, Yamaguchi M, Ogawa R, Matev MM, Miyagoe-Suzuki Y, Takeda S, Tsujikawa K, Tsuchida K, Yamamoto H, Fukada S. Fibrosis and adipogenesis originate from a common mesenchymal progenitor in skeletal muscle. J Cell Sci. 2011;124:3654-3664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 490] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 7. | Lemos DR, Paylor B, Chang C, Sampaio A, Underhill TM, Rossi FM. Functionally convergent white adipogenic progenitors of different lineages participate in a diffused system supporting tissue regeneration. Stem Cells. 2012;30:1152-1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 8. | Natarajan A, Lemos DR, Rossi FM. Fibro/adipogenic progenitors: a double-edged sword in skeletal muscle regeneration. Cell Cycle. 2010;9:2045-2046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 9. | Hogarth MW, Defour A, Lazarski C, Gallardo E, Diaz Manera J, Partridge TA, Nagaraju K, Jaiswal JK. Fibroadipogenic progenitors are responsible for muscle loss in limb girdle muscular dystrophy 2B. Nat Commun. 2019;10:2430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 105] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 10. | Wosczyna MN, Konishi CT, Perez Carbajal EE, Wang TT, Walsh RA, Gan Q, Wagner MW, Rando TA. Mesenchymal Stromal Cells Are Required for Regeneration and Homeostatic Maintenance of Skeletal Muscle. Cell Rep. 2019;27:2029-2035.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 266] [Article Influence: 53.2] [Reference Citation Analysis (0)] |
| 11. | Uezumi A, Ikemoto-Uezumi M, Zhou H, Kurosawa T, Yoshimoto Y, Nakatani M, Hitachi K, Yamaguchi H, Wakatsuki S, Araki T, Morita M, Yamada H, Toyoda M, Kanazawa N, Nakazawa T, Hino J, Fukada SI, Tsuchida K. Mesenchymal Bmp3b expression maintains skeletal muscle integrity and decreases in age-related sarcopenia. J Clin Invest. 2021;131:e139617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 89] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 12. | Liu X, Ning AY, Chang NC, Kim H, Nissenson R, Wang L, Feeley BT. Investigating the cellular origin of rotator cuff muscle fatty infiltration and fibrosis after injury. Muscles Ligaments Tendons J. 2016;6:6-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Davies MR, Liu X, Lee L, Laron D, Ning AY, Kim HT, Feeley BT. TGF-β Small Molecule Inhibitor SB431542 Reduces Rotator Cuff Muscle Fibrosis and Fatty Infiltration By Promoting Fibro/Adipogenic Progenitor Apoptosis. PLoS One. 2016;11:e0155486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 97] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 14. | Kang X, Yang MY, Shi YX, Xie MM, Zhu M, Zheng XL, Zhang CK, Ge ZL, Bian XT, Lv JT, Wang YJ, Zhou BH, Tang KL. Interleukin-15 facilitates muscle regeneration through modulation of fibro/adipogenic progenitors. Cell Commun Signal. 2018;16:42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 73] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 15. | McGeachie J, Allbrook D. Cell proliferation in skeletal muscle following denervation or tenotomy. A series of autoradiographic studies. Cell Tissue Res. 1978;193:259-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Lemos DR, Babaeijandaghi F, Low M, Chang CK, Lee ST, Fiore D, Zhang RH, Natarajan A, Nedospasov SA, Rossi FM. Nilotinib reduces muscle fibrosis in chronic muscle injury by promoting TNF-mediated apoptosis of fibro/adipogenic progenitors. Nat Med. 2015;21:786-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 520] [Article Influence: 52.0] [Reference Citation Analysis (0)] |
| 17. | Ito T, Ogawa R, Uezumi A, Ohtani T, Watanabe Y, Tsujikawa K, Miyagoe-Suzuki Y, Takeda S, Yamamoto H, Fukada S. Imatinib attenuates severe mouse dystrophy and inhibits proliferation and fibrosis-marker expression in muscle mesenchymal progenitors. Neuromuscul Disord. 2013;23:349-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 18. | Kopinke D, Roberson EC, Reiter JF. Ciliary Hedgehog Signaling Restricts Injury-Induced Adipogenesis. Cell. 2017;170:340-351.e12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 171] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 19. | Madaro L, Passafaro M, Sala D, Etxaniz U, Lugarini F, Proietti D, Alfonsi MV, Nicoletti C, Gatto S, De Bardi M, Rojas-García R, Giordani L, Marinelli S, Pagliarini V, Sette C, Sacco A, Puri PL. Denervation-activated STAT3-IL-6 signalling in fibro-adipogenic progenitors promotes myofibres atrophy and fibrosis. Nat Cell Biol. 2018;20:917-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 214] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 20. | Kaneshige A, Kaji T, Zhang L, Saito H, Nakamura A, Kurosawa T, Ikemoto-Uezumi M, Tsujikawa K, Seno S, Hori M, Saito Y, Matozaki T, Maehara K, Ohkawa Y, Potente M, Watanabe S, Braun T, Uezumi A, Fukada SI. Relayed signaling between mesenchymal progenitors and muscle stem cells ensures adaptive stem cell response to increased mechanical load. Cell Stem Cell. 2022;29:265-280.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 21. | Heredia JE, Mukundan L, Chen FM, Mueller AA, Deo RC, Locksley RM, Rando TA, Chawla A. Type 2 innate signals stimulate fibro/adipogenic progenitors to facilitate muscle regeneration. Cell. 2013;153:376-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 530] [Cited by in RCA: 596] [Article Influence: 49.7] [Reference Citation Analysis (0)] |
| 22. | Fiore D, Judson RN, Low M, Lee S, Zhang E, Hopkins C, Xu P, Lenzi A, Rossi FM, Lemos DR. Pharmacological blockage of fibro/adipogenic progenitor expansion and suppression of regenerative fibrogenesis is associated with impaired skeletal muscle regeneration. Stem Cell Res. 2016;17:161-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 112] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 23. | Stengaard K, Hejbøl EK, Jensen PT, Degn M, Ta TML, Stensballe A, Andersen DC, Schrøder HD, Lambertsen KL, Frich LH. Early-stage inflammation changes in supraspinatus muscle after rotator cuff tear. J Shoulder Elbow Surg. 2022;31:1344-1356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 24. | Kuswanto W, Burzyn D, Panduro M, Wang KK, Jang YC, Wagers AJ, Benoist C, Mathis D. Poor Repair of Skeletal Muscle in Aging Mice Reflects a Defect in Local, Interleukin-33-Dependent Accumulation of Regulatory T Cells. Immunity. 2016;44:355-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 384] [Article Influence: 42.7] [Reference Citation Analysis (0)] |
| 25. | Biferali B, Proietti D, Mozzetta C, Madaro L. Fibro-Adipogenic Progenitors Cross-Talk in Skeletal Muscle: The Social Network. Front Physiol. 2019;10:1074. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 164] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 26. | Villalta SA, Rosenthal W, Martinez L, Kaur A, Sparwasser T, Tidball JG, Margeta M, Spencer MJ, Bluestone JA. Regulatory T cells suppress muscle inflammation and injury in muscular dystrophy. Sci Transl Med. 2014;6:258ra142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 197] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 27. | Lukjanenko L, Karaz S, Stuelsatz P, Gurriaran-Rodriguez U, Michaud J, Dammone G, Sizzano F, Mashinchian O, Ancel S, Migliavacca E, Liot S, Jacot G, Metairon S, Raymond F, Descombes P, Palini A, Chazaud B, Rudnicki MA, Bentzinger CF, Feige JN. Aging Disrupts Muscle Stem Cell Function by Impairing Matricellular WISP1 Secretion from Fibro-Adipogenic Progenitors. Cell Stem Cell. 2019;24:433-446.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 211] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 28. | Lee C, Agha O, Liu M, Davies M, Bertoy L, Kim HT, Liu X, Feeley BT. Rotator Cuff Fibro-Adipogenic Progenitors Demonstrate Highest Concentration, Proliferative Capacity, and Adipogenic Potential Across Muscle Groups. J Orthop Res. 2020;38:1113-1121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 29. | Mozzetta C, Consalvi S, Saccone V, Tierney M, Diamantini A, Mitchell KJ, Marazzi G, Borsellino G, Battistini L, Sassoon D, Sacco A, Puri PL. Fibroadipogenic progenitors mediate the ability of HDAC inhibitors to promote regeneration in dystrophic muscles of young, but not old Mdx mice. EMBO Mol Med. 2013;5:626-639. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 180] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 30. | Lee C, Liu M, Agha O, Kim HT, Feeley BT, Liu X. Beige FAPs Transplantation Improves Muscle Quality and Shoulder Function After Massive Rotator Cuff Tears. J Orthop Res. 2020;38:1159-1166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 31. | Pagano AF, Arc-Chagnaud C, Brioche T, Chopard A, Py G. Muscle Resting and TGF-β Inhibitor Treatment Prevent Fatty Infiltration Following Skeletal Muscle Injury. Cell Physiol Biochem. 2019;53:62-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 32. | Liu X, Liu M, Lee L, Davies M, Wang Z, Kim H, Feeley BT. Trichostatin A regulates fibro/adipogenic progenitor adipogenesis epigenetically and reduces rotator cuff muscle fatty infiltration. J Orthop Res. 2021;39:1452-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |