Published online May 26, 2025. doi: 10.4252/wjsc.v17.i5.101675
Revised: January 9, 2025
Accepted: March 27, 2025
Published online: May 26, 2025
Processing time: 241 Days and 22 Hours
Traumatic spinal cord injury (SCI) is a life-altering condition that results in long-term complications, including progressive neurodegeneration and cord atrophy. It presents a significant unmet medical need with extensive social and economic burdens.
To evaluate the safety and preliminary efficacy of allogeneic mesenchymal stem cells derived from Wharton’s jelly (WJ-MSCs) in patients with chronic complete SCI. The primary objective was to assess whether WJ-MSCs could facilitate neurological recovery and improve the quality of life in this patient population.
This open-label, multicenter phase I study investigated the effects of administering WJ-MSCs via three delivery routes: Intrathecal (for localized spinal targeting); intramuscular (for targeting end organ); and intravenous (for systemic immunomodulation). While all three routes were used concurrently to enhance therapeutic synergy, neurological, sensory, and functional scales were used to assess overall efficacy. Participants with chronic SCI (duration of at least 6 months) who had significant impairment and disability were eligible for inclusion. WJ-MSCs were administered twice monthly for 2 months, with each route receiving a dose of 1 × 106 cells/kg. Patients were closely monitored for 1 year following treatment.
At baseline, participants displayed considerable functional deficits, as indicated by the following scores: Functional independence measure of 77.5 ± 2.26; Modified Ashworth Scale of 15.83 ± 4.83; American Spinal Injury Association (ASIA) Motor score of 1.67 ± 2.66; ASIA Light Touch and Pin-Prick scores of 62 ± 18.42 each; Wexner Incontinence Score of 20; and Qualiveen Short Form, a validated questionnaire specifically designed to assess the impact of urinary dysfunction on quality of life in individuals with SCI, score of 32. Following WJ-MSC therapy, significant improvements were observed in all neurological functions over the 1-year follow-up. Notably, the ASIA Motor score improved significantly (χ2 = 23.938, P < 0.001), and Qualiveen Short Form scores demonstrated a substantial enhancement in quality of life (z = -2.214, P < 0.05).
This phase I study, conducted without a control group, suggests that the administration of WJ-MSCs through multiple routes is both safe and potentially effective in patients with chronic complete SCI. However, the observed neurological improvements cannot be solely attributed to WJ-MSC therapy, as concurrent pharmacological and rehabilitative interventions were not controlled. These findings indicated that WJ-MSC therapy may offer a promising approach for enhancing neurological function and quality of life in this challenging patient population. Further research with larger cohorts and extended follow-up is necessary to validate these preliminary results.
Core Tip: Spinal cord injury causes severe disability with significant impairments in sensory and motor functions and dysfunction in multiple organs. We investigated the therapeutic potential of Wharton’s jelly-derived mesenchymal stem cells administered through a triple-route delivery system (intrathecal, intramuscular, and intravenous). The treatment resulted in significant improvements in motor and sensory functions. Patients experienced an improved quality of life. The procedure was well-tolerated with no significant side effects or safety concerns. No adverse events were reported highlighting the potential of Wharton's jelly-derived mesenchymal stem cell therapy as a safe and effective treatment option for individuals with chronic spinal cord injury.
- Citation: Kaplan N, Kabatas S, Civelek E, Savrunlu EC, Akkoc T, Boyalı O, Öztürk E, Can H, Genc A, Karaöz E. Multiroute administration of Wharton’s jelly mesenchymal stem cells in chronic complete spinal cord injury: A phase I safety and feasibility study. World J Stem Cells 2025; 17(5): 101675
- URL: https://www.wjgnet.com/1948-0210/full/v17/i5/101675.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v17.i5.101675
Traumatic spinal cord injury (SCI) remains one of the most devastating conditions, primarily affecting young adults and resulting in life-altering neurological deficits. The incidence of SCI is estimated at approximately 12500 new cases annually in North America, with a substantial burden on both individuals and healthcare systems[1]. SCI disrupts sensory and motor pathways, leading to permanent disability and secondary complications that compromise the function of multiple organ systems[2]. The global incidence varies significantly, ranging from 20.7 to 83.0 cases per million in North America and from 8.0 to 130.6 cases per million in Europe[3]. Despite significant advances in acute management, including early surgical decompression and intensive rehabilitation, these interventions have largely focused on enhancing survival rather than restoring meaningful neurological function[4,5].
The pathophysiology of SCI is complex and occurs in two distinct but interrelated phases: The primary phase, characterized by mechanical trauma leading to immediate axonal and neuronal injury; and the secondary phase, marked by a cascade of events including excitotoxicity, oxidative stress, inflammation, and apoptosis that exacerbate the initial damage[6-9]. Current therapeutic strategies, such as the administration of high-dose methylprednisolone, aim to mitigate secondary injury processes but have not consistently demonstrated long-term efficacy in functional recovery[10]. Given these limitations, there is an urgent need for novel therapeutic approaches that address both the acute and chronic phases of SCI.
Recent advances in regenerative medicine, particularly in the application of mesenchymal stem cells (MSCs), offer a promising avenue for the treatment of SCI. MSCs have shown the potential to modulate the immune response, promote neuroprotection, and enhance tissue regeneration in preclinical models of SCI. For example, studies by Aras et al[11] and Kabatas et al[12] have reported that MSCs derived from adipose tissue and dental pulp, respectively, can improve motor function and reduce lesion size in rodent models of SCI, suggesting a potential role for MSCs in promoting spinal cord repair. Additionally, our previous studies have demonstrated the safety and feasibility of Wharton’s jelly-derived MSCs (WJ-MSCs) for treating various neurological disorders, including SCI[13,14].
These findings have set the stage for the present phase I clinical trial, which aimed to evaluate the safety and preliminary efficacy of WJ-MSCs administered via multiple routes in patients with chronic, complete SCI[14]. This study represents a pivotal step toward translating the regenerative potential of WJ-MSCs into a clinically viable therapy for SCI, with the ultimate goal of improving neurological outcomes and quality of life for affected patients.
This open-label, multicenter phase I clinical trial was designed to evaluate the safety and preliminary efficacy of WJ-MSC transplantations using a triple-route administration strategy. The study cohort, as detailed in Table 1, included adults with radiologically confirmed chronic SCI accompanied by substantial functional deficits. Participants were monitored for a duration of 1 year following WJ-MSC infusion. During the follow-up period, no restrictions were placed on concurrent pharmacological or rehabilitative interventions. Informed consent was obtained in accordance with the Declaration of Helsinki, and the study protocol was approved by the Ministry of Health. Patient demographics and clinical characteristics are provided in Table 2.
| Criteria | Details |
| Inclusion criteria | Age ≥ 18 years |
| Radiologically confirmed chronic SCI | |
| No chronic illnesses (e.g., cancer, organ failure) | |
| Life expectancy > 12 months | |
| Severe disability (wheelchair-bound, assistance required) | |
| No significant functional improvement for at least 3 months | |
| Commitment to standard care and follow-up | |
| Provide written informed consent | |
| Exclusion criteria | Significant medical/psychiatric conditions affecting safety |
| Recent severe infections (meningitis, sepsis, etc.) | |
| Uncontrolled seizure disorder | |
| CNS neoplasm or cancer history (last 5 years) | |
| Abnormal blood cell counts (WBC ≥ 15000/μL, platelets ≤ 100000/μL) | |
| Abnormal liver/kidney function (AST/ALT > 3 × ULN, creatinine > 1.5 × ULN) | |
| Prior participation in other investigational stem cell trials | |
| Patient decision to withdraw or death during study | |
| Treatment timeline | Baseline assessment (week 0): Neurological and functional evaluations |
| MSC therapy initiation (week 1-8): 4 rounds of triple-route administration | |
| Short-term follow-up (weeks 1-12): Safety and early functional outcomes | |
| Mid-term assessment (month 6): Progress evaluation | |
| Long-term follow-up (month 12): Final outcome assessment |
| Characteristic | n | % | |
| Age, years | 19 | 1 | 16.7 |
| 20 | 1 | 16.7 | |
| 29 | 3 | 50.0 | |
| 39 | 1 | 16.7 | |
| Sex | Female | 1 | 16.7 |
| Male | 5 | 83.3 | |
| Cause of SCI | Fell from tree | 1 | 16.7 |
| Firearm injury | 2 | 33.3 | |
| Ski accident | 1 | 16.7 | |
| Traffic accident | 2 | 33.3 | |
| Level of injury | T11 burst fracture | 1 | 16.7 |
| T12 burst fracture with T11-T12 and T12-L1 traumatic disc herniations | 1 | 16.7 | |
| T12 burst fracture with dislocation | 1 | 16.7 | |
| T5 burst fracture | 1 | 16.7 | |
| T7-8 burst fracture | 1 | 16.7 | |
| T8-9 burst fracture | 1 | 16.7 | |
| Pre-transplantation treatments | Only physiotherapy | 3 | 50.0 |
| T11 laminectomy + physiotherapy | 1 | 16.7 | |
| T12 corpectomy + posterior instrumentation + laminectomy + physiotherapy | 1 | 16.7 | |
| T12 corpectomy + posterior instrumentation + laminectomy + physiotherapy | 1 | 16.7 | |
| Comorbidity | None | 6 | 100.0 |
| Duration of SCI and first transplantation | 6 months | 1 | 16.7 |
| 9 months | 2 | 33.3 | |
| 12 months | 1 | 16.7 | |
| 4 years | 1 | 16.7 | |
| 12 years | 1 | 16.7 | |
Ethics and consent: Umbilical cords were procured from a facility adhering to Good Manufacturing Practice standards. Prior to collection, ethical protocols were observed, including obtaining informed consent from the donors and approval from an institutional review board (protocol number: 56733164-203-E.2659). The umbilical cords were donated by individuals who had undergone full-term pregnancies. Written informed consent was secured from all study participants[13,14].
Processing and quality control of umbilical cords: The cord was washed with PBS to ensure cleanliness. Following the removal of blood vessels, the cord tissues were cut into small pieces (5-10 mm3) known as explants. These tissue explants were then placed in culture dishes and incubated under humanized culture conditions (5% CO2, 37 °C, and high humidity) until the cells migrated from the tissue pieces. When the cells reached approximately 70% to 80% confluency in passage 3, they were harvested, and characterization assays were conducted[13,14].
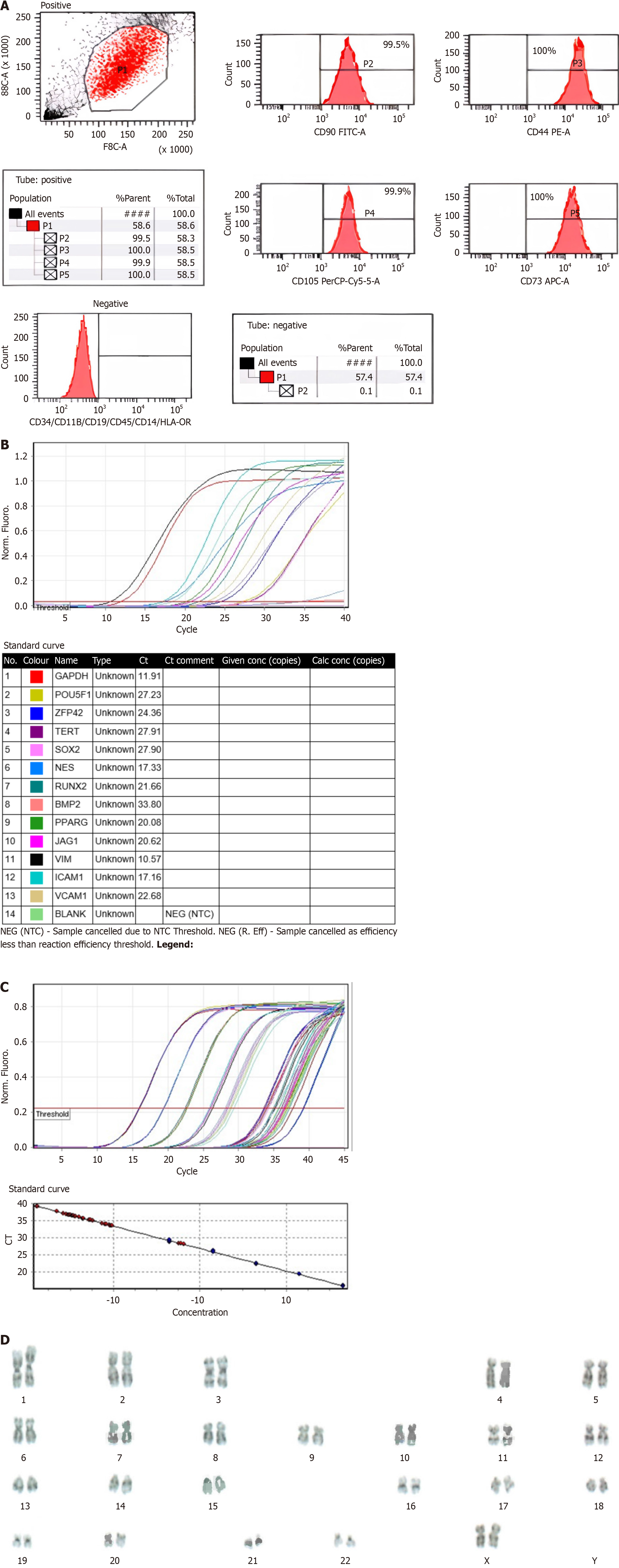
Characterization of WJ-MSCs by flow cytometry: Flow cytometry analysis revealed that WJ-MSCs were positive for surface markers CD44, CD73, CD105, and CD90 and negative for hematopoietic lineage markers CD34, CD45, and human leukocyte antigen DR. Telomerase activity was maintained throughout cell culture, and cells exhibited a characteristic flattened morphology[13,14]. The flow cytometry assay was performed using the human MSC Analysis Kit (BD, NJ, United States, Cat no: 562245) on the BD FACS CANTO II device. Telomerase activity assay was performed using TRAPEZE® RT Telomerase Detection Kit (Sigma-Aldrich, MO, United States, Cat No: S7710). The protocol stipulated by the kit was followed meticulously without any deviations.
Cell differentiation and karyotyping: Expression of key stem cell markers (TERT, POU5F1, SOX2, ZFP42) and differentiation markers (CD44, VCAM1, THY1, BMP2, RUNX-1) was assessed to confirm the pluripotency of WJ-MSCs. Differentiation assays demonstrated that WJ-MSCs could differentiate into osteoblasts, adipocytes, and chondrocytes. Karyotype analysis confirmed that the cells exhibited a normal chromosomal complement without any structural or numerical abnormalities[13,14]. In the gene expression test, RNA isolation was performed using the RNeasy® Plus Mini Kit (QIAGEN, Netherlands, Cat No: 74104). Subsequently, the RT2 First Strand Kit (QIAGEN, Netherlands, Cat No: 330401) was utilized for the translation of the RNA into cDNA. The protocol stipulated by the kit was followed meticulously without any deviations (Figure 1 and Table 3).
| QC parameters | |
| Cell count and viability | Cell viability > 90. Cell count: Varies according to the patient |
| Immunophenotyping | CD90, CD44, CD105, CD73 ≥ 95%. CD45, CD34, CD19, CD11B, HLA-DR, CD14 ≤ 2% (Figure 1A) |
| Gene expression | SOX2, OCT4, TERT, REX1, NES, BMP2, RUNX2, PPARG, JAG1, ICAM1, VCAM1, VIM. It was concluded that there was strong positivity in genes with Ct values lower than 29, positive in genes with values between 30-37, and poor expression in samples with Ct values between 38-40 or there may be environmental contamination (Figure 1B) |
| Telomerase | The result should be lower than that of the positive control (Figure 1C) |
| Mycoplasma | Negative |
| Endotoxin | < 0.0625 EU/mL |
Pre-transplantation process: Final preparations for WJ-MSC infusion were derived from passage 3 cultures. The cell preparations were resuspended in normal saline at concentrations of 1 × 106 cells in 3 mL, 1 × 106 cells in 20 mL, and 1 × 106 cells in 30 mL[13,14].
Surgical procedure and WJ-MSC transplantation procedure: Prior to treatment, comprehensive evaluations were performed by anesthesiology, neurosurgery, physical therapy, and rehabilitation specialists. The WJ-MSC implantation was carried out under conditions of stable patient health, with no contraindications from internal medicine or cardiology that would preclude the use of sedation or general anesthesia. The absence of severe infections, such as sepsis, was also confirmed.
The standard protocol for MSC administration involved allogeneic WJ-MSCs being administered via intrathecal, intramuscular, and intravenous routes. The triple application route was applied together in each treatment round for each patient. Intrathecal injections were performed using lumbar puncture, guided by established protocols. Intramuscular injections were conducted with ultrasonographic guidance, and intravenous infusion was administered over 30 min. Post-procedure, patients were monitored in the intensive care unit for 1 day and then transferred to the Department of Neurosurgery for ongoing physical therapy and rehabilitation. Exercise programs were suspended on administration days, and the protocol was repeated before and after each infusion, as outlined in Table 4.
| Rounds | Route | WJ-MSC |
| Round 1 | IT | 1 × 106 cells/kg in 3 mL |
| IV | 1 × 106 cells/kg in 30 mL | |
| IM | 1 × 106 cells/kg in 20 mL | |
| Round 2 (week 1) | IT | 1 × 106 cells/kg in 3 mL |
| IV | 1 × 106 cells/kg in 30 mL | |
| IM | 1 × 106 cells/kg in 20 mL | |
| Round 3 (week 4) | IT | 1 × 106 cells/kg in 3 mL |
| IV | 1 × 106 cells/kg in 30 mL | |
| IM | 1 × 106 cells/kg in 20 mL | |
| Round 4 (week 6) | IT | 1 × 106 cells/kg in 3 mL |
| IV | 1 × 106 cells/kg in 30 mL | |
| IM | 1 × 106 cells/kg in 20 mL |
Pre-treatment neurological examination: A multidisciplinary team performed a thorough pre-treatment assessment using the American Spinal Injury Association (ASIA) impairment scale to evaluate neurological and functional status. Spasticity was quantified using the Modified Ashworth (MA) scale. The impact of incontinence on quality of life was assessed with the Wexner Incontinence Score (WIS) and the Qualiveen Short Form (SFQ) score. Overall functional independence was evaluated using the Functional Independence Measure (FIM)[15,16].
Safety evaluation criteria: During the transplantation procedure, safety was monitored rigorously. Criteria included surveillance for infection (fever, elevated C-reactive protein levels, leukocytosis), complications related to anesthesia and analgesia, wound infections, and potential allergic reactions or shock. Immediate safety was assessed for 1-2 weeks post-procedure, with long-term follow-up extending 1 year to monitor for infection, cancer development, neuropathic pain, and neurological deterioration[13,14].
Follow-up assessment of treatment success: Follow-up evaluations included comprehensive assessments of neurological and functional outcomes. Spasticity was measured using the MA scale, and the impact of incontinence was evaluated with the WIS and SFQ score. Functional recovery was assessed using the FIM score. Additionally, the incidence of neuropathic pain, urinary tract infections, secondary infections, and pressure ulcers was monitored. These assessments provided a detailed understanding of patient progress and treatment efficacy.
Non-parametric statistical methods, including the Friedman test and the Wilcoxon signed-rank test, were employed to analyze changes in FIM scores, MA scale, ASIA Motor and Sensory scores, WIS, and SFQ values before and after treatment. Non-parametric tests were utilized due to the small sample size and non-normal distribution of the data, making parametric tests unsuitable for analysis. While non-parametric tests were chosen based on the characteristics of the data, we acknowledge that mixed-effects models could provide additional insights by accounting for repeated measures and potential variability between subjects. However, given the sample size and the exploratory nature of this phase I study, the use of non-parametric methods was deemed most appropriate.
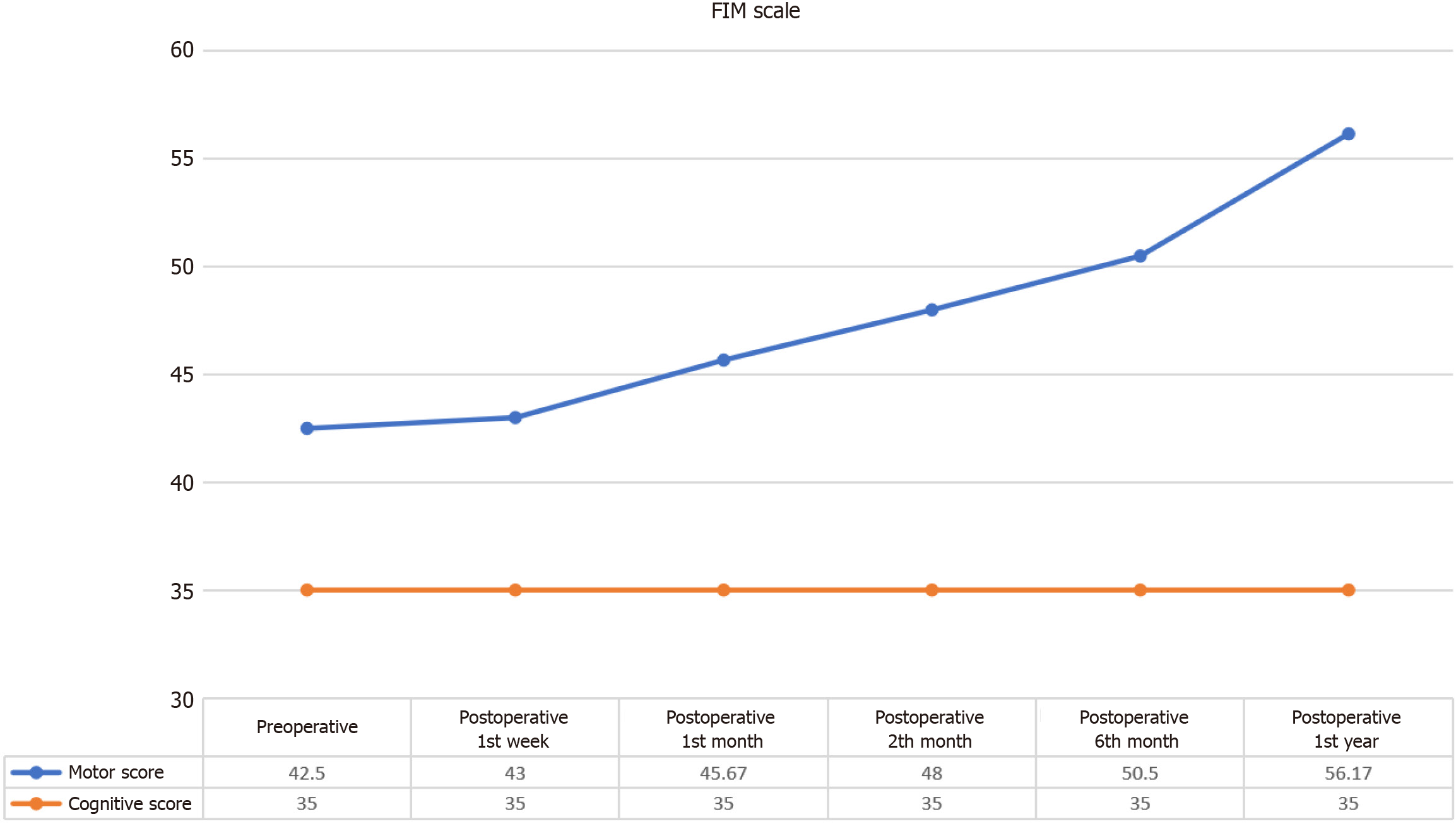
The FIM Motor scores demonstrated a statistically significant progressive improvement following the intervention, indicating enhanced motor function and independence over time. In contrast, the FIM Cognitive scores exhibited stability, showing no substantial changes post-intervention, as expected. A detailed statistical analysis revealed a significant difference between pre-intervention and post-intervention FIM Motor Scores (χ² = 29.505, P < 0.001), confirming the effectiveness of the intervention in improving motor function. To further elucidate the temporal effects of the intervention, Wilcoxon signed-rank tests were conducted at various time points (Table 5 and Figure 2).
| n | Mean | SD | Mean rank | χ2 | df | P value | |
| Preoperative | 6 | 42.50 | 2.26 | 1.50 | |||
| Postoperative week 1 | 6 | 43.00 | 1.90 | 1.67 | |||
| Postoperative month 1 | 6 | 45.67 | 1.97 | 2.83 | 29.505 | 5 | 0.000 |
| Postoperative month 2 | 6 | 48.00 | 1.90 | 4.00 | |||
| Postoperative month 6 | 6 | 50.50 | 2.17 | 5.00 | |||
| Postoperative month 12 | 6 | 56.17 | 2.99 | 6.00 |
First week: No significant change was observed in FIM Motor scores during the first week post-intervention (z = -1.00,
One month: Significant improvement was observed at 1 month (z = -2.060, P = 0.039), indicating an initial positive response to the intervention.
Two months: The improvement continued with significant gains observed at 2 months (z = -2.226, P = 0.026), further supporting the efficacy of the intervention in enhancing motor function.
Four months: At 4 months, the significant improvement in FIM Motor scores was sustained (z = -2.207, P = 0.027), highlighting the ongoing positive effects of the intervention.
One year: The significant improvement persisted through the 1-year follow-up (z = -2.207, P = 0.027), demonstrating the long-term benefits of the intervention in motor function recovery. While the mean FIM Motor score before the application was 42.50 ± 2.26, this value increased to 56.17 ± 2.99 in the first year after the application. Overall, these findings underscore the potential of the intervention in significantly improving motor function over time.
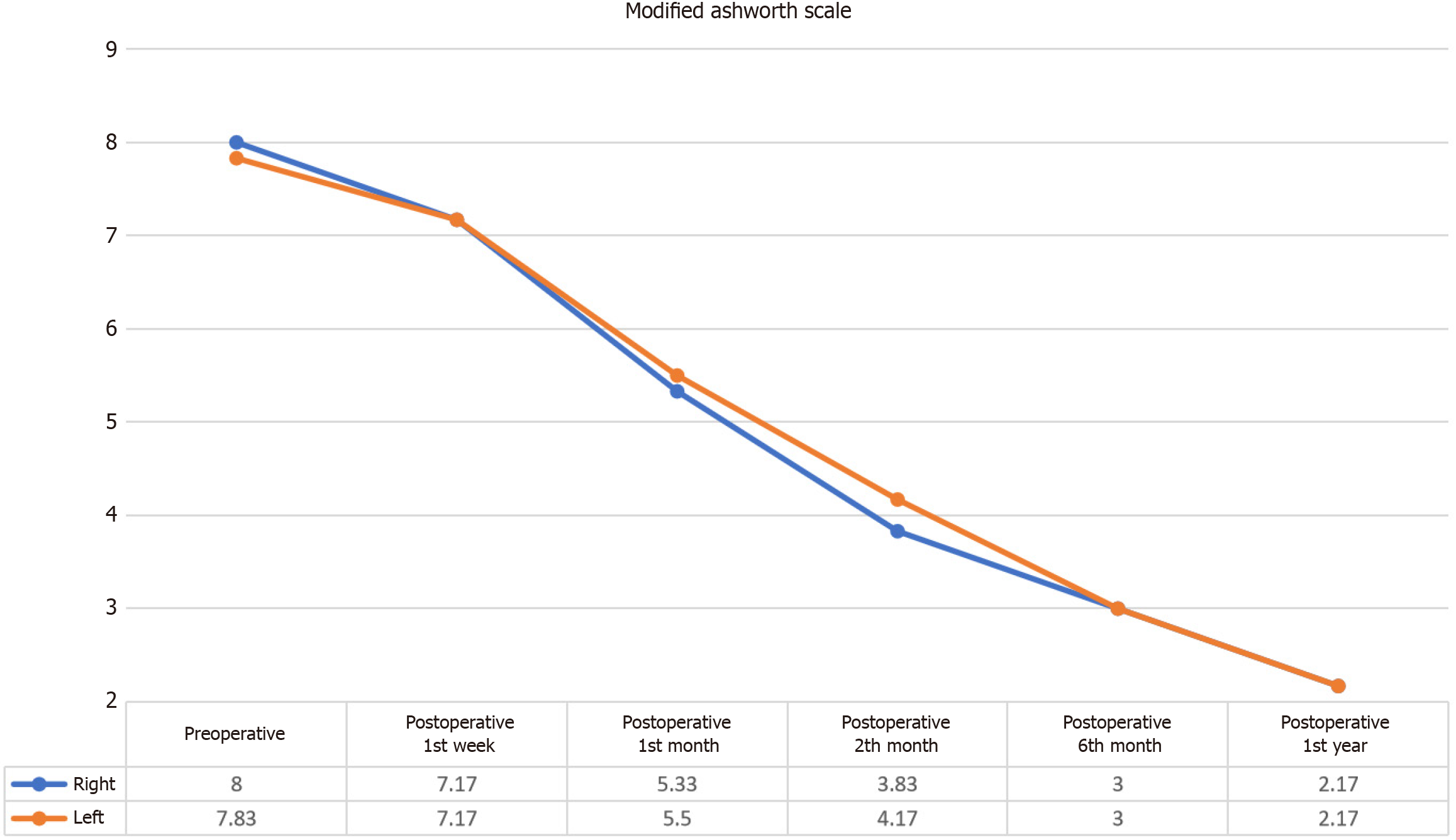
The MA scale assessments indicated a statistically significant reduction in spasticity following the intervention, as evidenced by decreased scores for both the right and left lower extremities (Figure 3).
Right lower extremity spasticity: Statistical analysis demonstrated a significant difference in the MA scale right values between pre-intervention and post-intervention assessments (χ² = 28.452, P < 0.001). Wilcoxon signed-rank tests revealed no significant differences in the right MA Scale scores during the first week post-intervention (z = -1.890, P = 0.059). However, significant reductions in spasticity were observed at 1 month (z = -2.271, P = 0.023), 2 months (z = -2.207, P = 0.027), 4 months (z = -2.214, P = 0.027), and 1 year (z = -2.207, P = 0.027). These findings suggest that while initial changes were not apparent within the first week, substantial and sustained improvements in spasticity were achieved over the subsequent months (Table 6). While the mean MA scale for the right lower extremity before the application was 8.00 ± 2.45, this value decreased to 2.17 ± 1.94 in the first year after the application.
| n | Mean | SD | Mean rank | χ2 | df | P value | |
| Preoperative | 6 | 8.00 | 2.45 | 5.83 | |||
| Postoperative week 1 | 6 | 7.17 | 3.06 | 5.17 | |||
| Postoperative month 1 | 6 | 5.33 | 2.66 | 3.67 | 28.452 | 5 | 0.000 |
| Postoperative month 2 | 6 | 3.83 | 2.48 | 3.00 | |||
| Postoperative month 6 | 6 | 3.00 | 2.19 | 2.08 | |||
| Postoperative month 12 | 6 | 2.17 | 1.94 | 1.25 |
Left lower extremity spasticity: Similarly, the MA scale left values demonstrated a significant difference between pre-intervention and post-intervention scores (χ² = 29.030, P < 0.001). The Wilcoxon signed-rank test showed no significant differences in left MA scale scores during the first week post-intervention (z = -1.633, P = 0.102). Significant decreases in spasticity were recorded at 1 month (z = -2.226, P = 0.026), 2 months (z = -2.207, P = 0.027), 4 months (z = -2.207, P = 0.027), and 1 year (z = -2.214, P = 0.027). This indicates that while the reduction in spasticity was not immediately detectable, there was a notable and progressive decrease in spasticity over time (Table 7). While the mean MA scale score for the left lower extremity before the application was 7.83 ± 2.40, this value decreased to 2.17 ± 1.84 in the first year after the application.
| n | Mean | SD | Mean rank | χ2 | df | P value | |
| Preoperative | 6 | 7.83 | 2.40 | 5.75 | |||
| Postoperative week 1 | 6 | 7.17 | 2.64 | 5.25 | |||
| Postoperative month 1 | 6 | 5.50 | 2.43 | 3.92 | 29.030 | 5 | 0.000 |
| Postoperative month 2 | 6 | 4.17 | 2.64 | 2.92 | |||
| Postoperative month 6 | 6 | 3.00 | 2.28 | 1.92 | |||
| Postoperative month 12 | 6 | 2.17 | 1.84 | 1.25 |
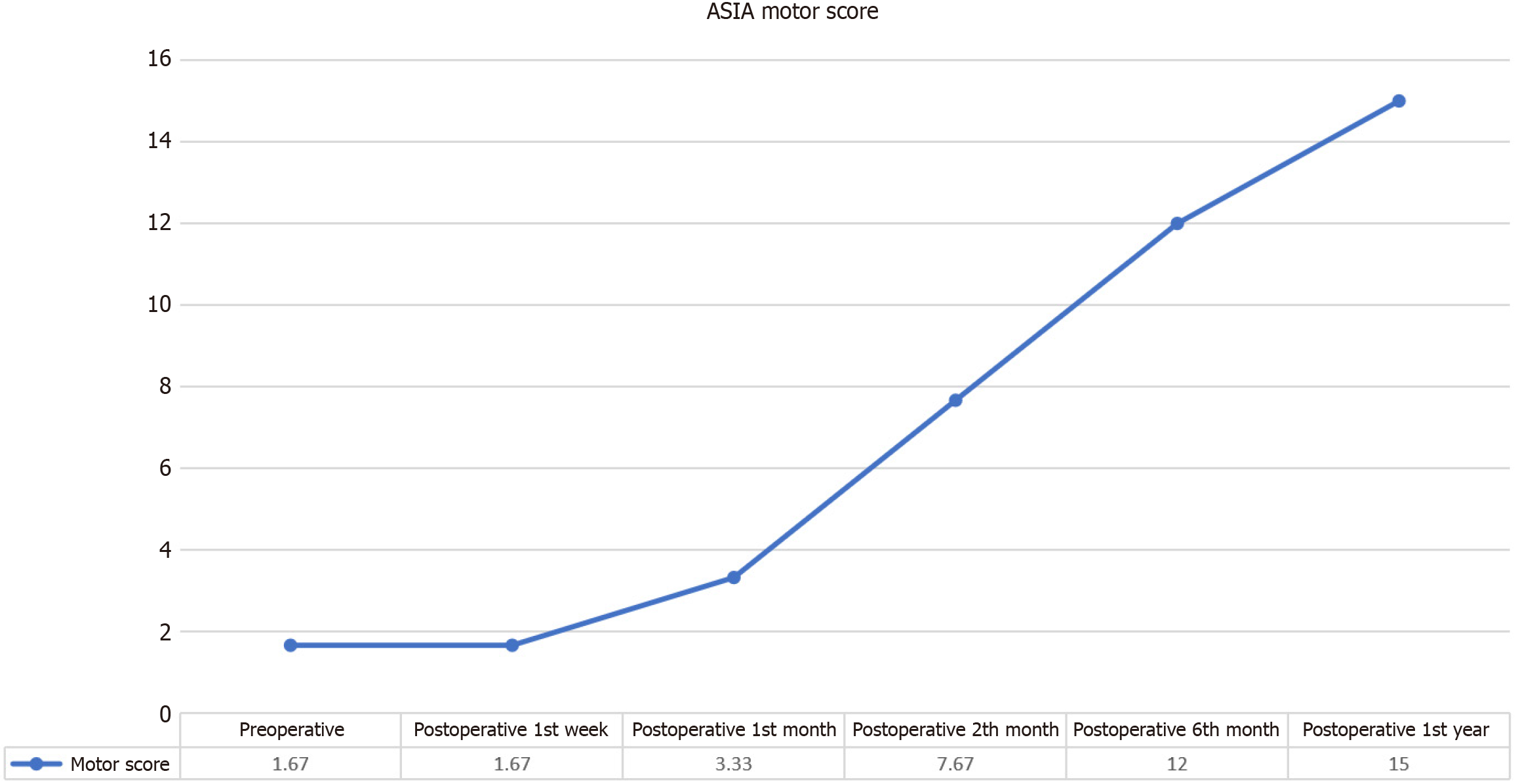
The ASIA Motor scores demonstrated a statistically significant and progressive improvement following the intervention (Figure 4). While the mean ASIA Motor score for lower extremities before the application was 1.67 ± 2.66, this value increased to 15.00 ± 11.23 in the first year after the application. Initial analyses revealed a notable difference in ASIA Motor scores between pre-intervention and post-intervention evaluations, with a χ2 statistic of χ² = 23.938 (P < 0.001), indicating a robust effect of the intervention on motor function (Table 8).
| n | Mean | SD | Mean rank | χ2 | df | P value | |
| Preoperative | 6 | 1.67 | 2.66 | 2.00 | |||
| Postoperative week 1 | 6 | 1.67 | 2.66 | 2.00 | |||
| Postoperative month 1 | 6 | 3.33 | 3.93 | 2.75 | 23.938 | 5 | 0.000 |
| Postoperative month 2 | 6 | 7.67 | 5.28 | 4.08 | |||
| Postoperative month 6 | 6 | 12.00 | 8.10 | 4.75 | |||
| Postoperative month 12 | 6 | 15.00 | 11.23 | 5.42 |
Initial observations: During the first week post-intervention, the Wilcoxon signed-rank tests showed no statistically significant changes in ASIA Motor scores (z = 0.00, P = 1.00), suggesting that immediate post-intervention assessments did not reflect the potential benefits of the treatment. Similarly, no significant changes were observed in scores during the first month (z = -1.633, P = 0.102), which may be attributed to the time required for the intervention to manifest its effects.
Progressive improvements: Significant enhancements in motor function were first detected at the 2-month follow-up, with a Wilcoxon signed-rank test statistic of z = -2.060 (P = 0.039). This was followed by further significant improvements at the 4-month mark (z = -2.023, P = 0.043) and sustained progress up to the 1-year follow-up (z = -2.023, P = 0.043). These results highlight a delayed but substantial improvement in motor function, suggesting that the benefits of the intervention accrue progressively over time.
Long-term effects: The observed increase in ASIA Motor scores underscores the potential of the intervention to induce meaningful and lasting improvements in motor function. The delayed onset of significant changes may reflect the time required for cellular or physiological adaptations to the intervention, leading to improved motor outcomes in the longer term. Overall, the data indicate that while immediate effects were not apparent, the intervention resulted in a marked and sustained enhancement in motor function, with notable improvements emerging within the first 2 months and continuing through the 1-year follow-up period.
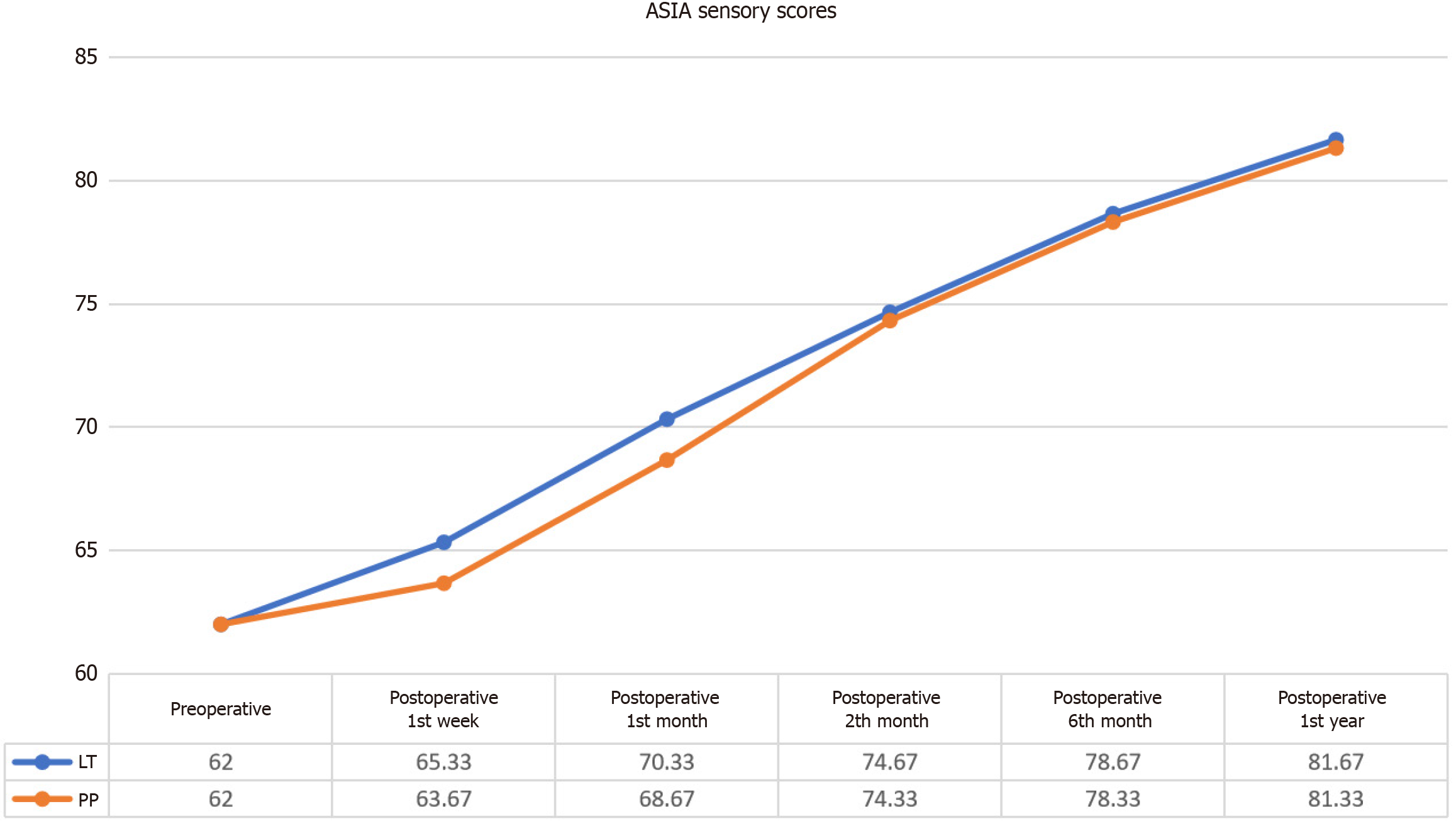
Post-intervention assessments revealed significant improvements in ASIA Sensory scores for both Light Touch (LT) and Pin-Prick (PP) modalities (Figure 5).
LT: Statistical analysis showed significant differences between pre-intervention and post-intervention ASIA Sensory LT scores (χ² = 28.299, P < 0.001). While the mean ASIA Sensory LT score before the application was 62.00 ± 18.42, this value increased to 81.67 ± 22.50 in the first year after the application. Wilcoxon signed-rank tests indicated no significant differences in LT scores during the first week (z = -1.342, P = 0.180) and the first month (z = -1.826, P = 0.068). Significant improvements were noted at 2 months (z = -2.207, P = 0.027), 4 months (z = -2.201, P = 0.028), and 1 year (z = -2.201, P = 0.028), reflecting an enhancement in sensory function over time (Table 9).
| n | Mean | SD | Mean rank | χ2 | df | P value | |
| Preoperative | 6 | 62.00 | 18.42 | 1.50 | |||
| Postoperative week 1 | 6 | 65.33 | 18.23 | 1.83 | |||
| Postoperative month 1 | 6 | 70.33 | 18.26 | 2.75 | 28.299 | 5 | 0.000 |
| Postoperative month 2 | 6 | 74.67 | 19.04 | 4.08 | |||
| Postoperative month 6 | 6 | 78.67 | 20.73 | 5.08 | |||
| Postoperative month 12 | 6 | 81.67 | 22.50 | 5.75 |
PP: Statistical analysis revealed significant differences in ASIA Sensory PP scores between pre-intervention and post-intervention assessments (χ² = 28.838, P < 0.001). While the mean ASIA Sensory PP score before the application was 62.00 ± 18.42, this value increased to 81.33 ± 22.26 in the first year after the application. Wilcoxon signed-rank tests demonstrated no significant differences in PP scores during the first week (z = -1.342, P = 0.180) and the first month (z =
| n | Mean | SD | Mean rank | χ2 | df | P value | |
| Preoperative | 6 | 62.00 | 18.42 | 1.50 | |||
| Postoperative week 1 | 6 | 63.67 | 18.13 | 1.83 | 28.838 | 5 | 0.000 |
| Postoperative month 1 | 6 | 68.67 | 17.47 | 2.75 | |||
| Postoperative month 2 | 6 | 74.33 | 19.82 | 3.92 | |||
| Postoperative month 6 | 6 | 78.33 | 20.92 | 5.08 | |||
| Postoperative month 12 | 6 | 81.33 | 22.26 | 5.92 |
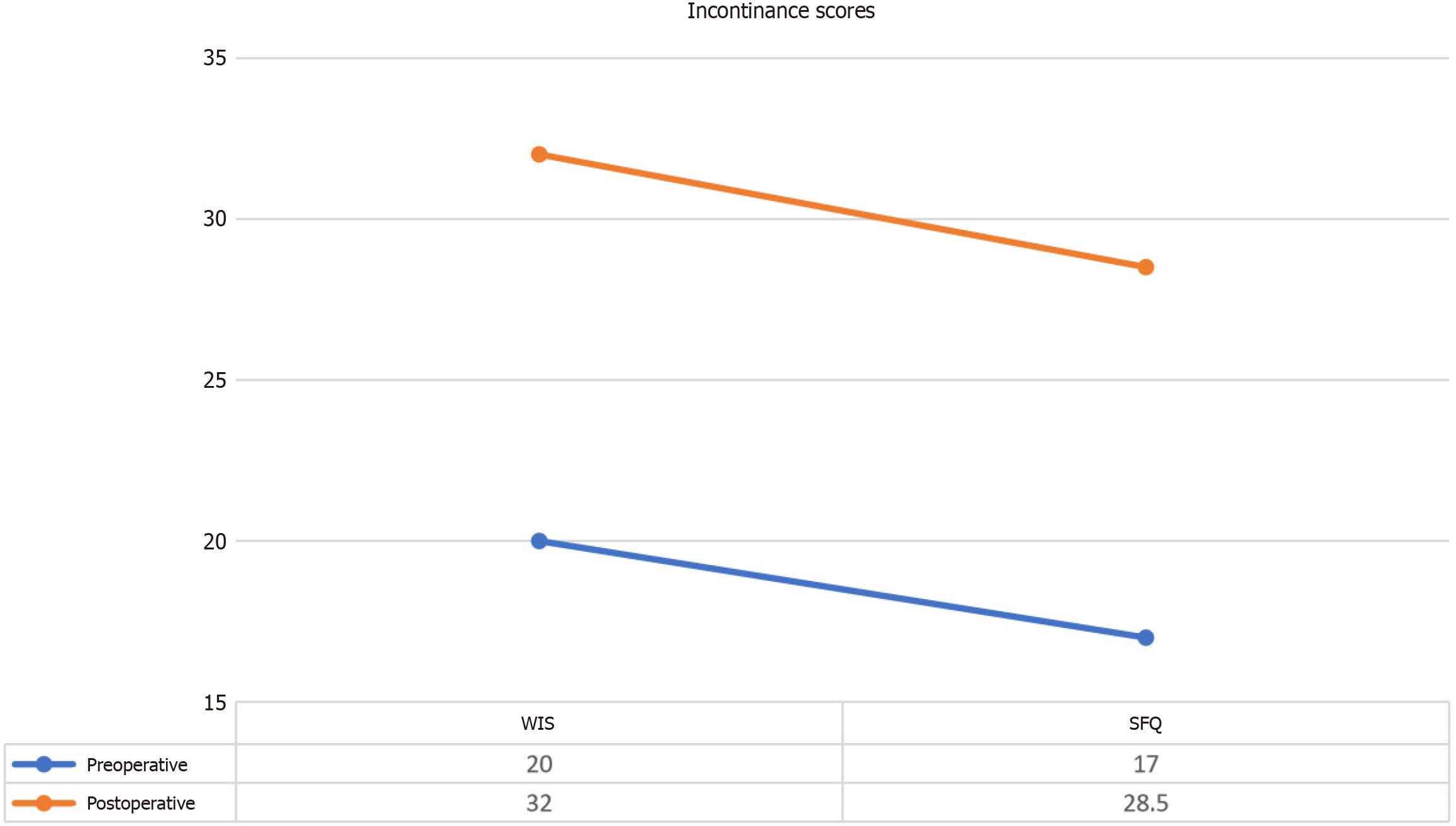
The evaluation of incontinence using the WIS and the SFQ values revealed notable improvements following the intervention (Figure 6). These metrics were used to assess both gastrointestinal (GI) and urinary incontinence as well as overall quality of life related to these conditions.
WIS: The WIS is a validated instrument that quantifies the severity of GI incontinence, encompassing symptoms such as frequency, urgency, and leakage. Our data indicated a significant reduction in WIS scores post-intervention, reflecting an improvement in GI control and a subsequent enhancement in the patients’ quality of life. The statistical analysis demonstrated a significant decrease in WIS scores from baseline to follow-up, with a calculated z value of -2.226 and a P value < 0.05 (Table 11). This suggests that the intervention had a meaningful impact on alleviating symptoms of GI incontinence.
| Wexner Incontinence Score | Ranks | n | Mean rank | Sum of ranks | Z score | P value |
| Preoperative | Negative ranks | 0 | 3.50 | 21.00 | -2.226 | 0.026 |
| Positive ranks | 6 | 0.00 | 0.00 | |||
| Postoperative | Ties | 0 | ||||
| Total | 6 |
SFQ: The SFQ is a widely used tool for assessing the impact of urinary incontinence on quality of life. It provides a comprehensive measure of how urinary incontinence affects daily functioning and psychological well-being. The results indicated a significant improvement in SFQ scores following the intervention. The statistical analysis yielded a z value of
| SFQ | Ranks | n | Mean rank | Sum of ranks | Z score | P value |
| Preoperative | Negative ranks | 0 | 3.50 | 21.00 | -2.214 | 0.027 |
| Positive ranks | 6 | 0.00 | 0.00 | |||
| Postoperative | Ties | 0 | ||||
| Total | 6 |
Overall impact on quality of life: Both WIS and SFQ score improvements underscore the potential benefits of the WJ-MSC intervention in managing incontinence. These results indicate that the intervention not only addressed neurological deficits but also contributed to a reduction in the adverse effects of incontinence on daily life. The significant decreases in both GI and urinary incontinence scores highlight the comprehensive nature of the therapeutic benefits observed, supporting the notion that MSC-based treatments may offer substantial improvements in various aspects of patient well-being beyond motor and sensory function.
The WJ-MSC transplantation procedures were generally well-tolerated across all participants, with no serious adverse effects linked to the WJ-MSC injections. The majority of patients exhibited a favorable response to the intervention, without experiencing any major complications. However, 4 patients reported transient, minor complications post-intervention (Table 13): (1) Subfebrile episodes: A total of 4 patients experienced mild, transient fever, categorized as subfebrile. This response was effectively managed with standard antipyretics and resolved without further medical intervention; (2) Moderate headaches: Two patients reported moderate headaches following intramuscular injections. These headaches were managed with standard analgesics and were resolved within a short period; and (3) Localized muscle soreness: Mild muscle soreness at the injection sites was noted in 2 patients. This discomfort was self-limiting and was alleviated with minimal symptomatic treatment.
| Complications | Patient No. 11 | Patient No. 21 | Patient No. 31 | Patient No. 41 | Patient No. 51 | Patient No. 61 | |||||||||||||||||||
| 1st | 2nd | 3rd | 4th | 1st | 2nd | 3rd | 4th | 1st | 2nd | 3rd | 4th | 1st | 2nd | 3rd | 4th | 1st | 2nd | 3rd | 4th | 1st | 2nd | 3rd | 4th | ||
| Early | Infection | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Fever | - | + | + | - | - | - | - | - | + | + | - | - | - | - | - | - | - | + | - | - | + | - | + | - | |
| Pain | - | - | - | - | - | - | - | - | + | + | + | - | - | - | - | - | + | - | - | - | - | - | - | - | |
| Headache | - | - | - | - | - | - | - | - | - | - | + | - | - | - | - | - | - | - | - | - | + | + | + | - | |
| Increased C-reactive protein levels | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| Leukocytosis | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| Allergic reaction or shock | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| Perioperative complications | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| Late | Secondary infections | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Urinary tract infections | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| Deterioration of neurological status | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| Neuropathic pain | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| Carcinogenesis | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
Throughout the 1-year follow-up period, no significant safety concerns or adverse events were reported. There were no instances of severe infections, complications related to anesthesia, allergic reactions, or episodes of shock. Additionally, no evidence of neurological deterioration or progression of SCI was observed. The absence of serious adverse effects and the manageable nature of the minor complications underscore the safety and feasibility of the WJ-MSC transplantation procedure for SCI treatment.
SCI is a life-altering event that leads to profound and multifaceted challenges. The neurological deficits resulting from SCI, ranging from complete paralysis to partial loss of function, significantly impact motor and sensory abilities. These deficits often give rise to secondary complications such as chronic pain, pressure ulcers, and autonomic dysfunction, which further exacerbate the patient’s condition. The psychological burden of SCI cannot be understated, as patients frequently grapple with heightened levels of depression, anxiety, and post-traumatic stress disorder. The ripple effects extend to family members and caregivers, who face emotional distress and substantial financial costs associated with long-term care and rehabilitation. Societally, SCI contributes to increased healthcare expenditures and a greater need for specialized care services. Although it was once thought that the central nervous system had limited regenerative capacity, contemporary research indicates that targeted modulation of the injury microenvironment can foster neuroprotection and stimulate repair mechanisms[17].
Historically, SCI treatments included the transplantation of fetal spinal cord tissue, peripheral nerves, Schwann cells, and fibroblasts, aiming to overcome inhibitory factors and create a supportive environment for nerve regeneration[18,19]. More recent strategies have shifted towards the use of MSCs, which exhibit remarkable self-renewal and differentiation abilities. MSCs can give rise to various mesodermal lineages such as bone, cartilage, and muscle[20,21]. The substantial increase in MSC-based clinical trials (over 1138 registered by June 2020) highlights their emerging role as a promising therapeutic approach for SCI and other complex disorders[22]. This rising interest underscores the potential of MSCs to address the challenges associated with traditional treatment modalities.
The dramatic increase in MSC-related clinical trials reflects an expanding exploration of their therapeutic potential. The number of registered trials surged from 220 in 2012 to over 1138 by mid-2020[22]. This escalation indicates a growing recognition of MSCs as a viable treatment for SCI, suggesting that ongoing research may reveal further benefits and optimize therapeutic strategies. Bone marrow (BM) has traditionally been the primary source of MSCs, but its use is limited by the invasive nature of BM aspiration and a decline in cell proliferation and differentiation potential with donor age. Recent research has identified alternative sources, such as fetal tissues including Wharton’s jelly from the umbilical cord, which offer distinct advantages. WJ-MSCs are characterized by their primitive state and reduced immunogenicity, which may enhance their therapeutic efficacy compared to BM-derived MSCs[23]. Wharton’s jelly, a unique connective tissue within the umbilical cord, provides a non-invasive, abundant source of MSCs, facilitating their application in both research and clinical settings[24-26].
Stem cell therapy (SCT) for SCI is designed to promote neuronal regeneration and neurotrophic factor production. This approach involves either endogenous recruitment of stem cells to the injury site or ex vivo manipulation and subsequent transplantation[27]. Research involving MSC-derived neurospheres in animal models has demonstrated improvements in motor function, suggesting that SCT can aid recovery through mechanisms such as neuroprotection, modulation of inflammation, and promotion of axonal growth[28]. These mechanisms highlight the potential for SCT to address the complex pathophysiology of SCI.
The therapeutic efficacy of MSCs is primarily attributed to their paracrine effects, which involve the secretion of a diverse array of growth factors, cytokines, and exosomes. These factors modulate the local microenvironment, promoting tissue repair and reducing inflammation. MSCs secrete anti-inflammatory cytokines and neurotrophic factors that mitigate inflammation and support neuronal survival[29]. Research has demonstrated that MSCs can reduce immune cell activation and lower proinflammatory cytokines such as interleukin-6, which are critical markers of spinal cord inflammation. Additionally, MSC treatment has been associated with increased levels of anti-inflammatory mediators like interleukin-1 receptor antagonist, which further supports the therapeutic potential of MSCs[30].
In this study, patients with SCI received MSC injections via intrathecal, intravenous, and intramuscular routes approximately 38.00 ± 54.25 months post-injury. The combination of SCT and intensive neurorehabilitation led to moderate improvements in FIM scores across both motor and cognitive domains (77.50 ± 2.26). Notably, FIM Motor scores increased significantly, while FIM Cognitive scores showed minimal change. It is crucial to differentiate between treatment-induced improvements and spontaneous recovery in such studies[31]. The data indicate that SCT, when administered between 6 months and 12 years post-injury, can enhance neurological and functional outcomes, reinforcing the importance of integrating SCT with comprehensive physiotherapy for optimal results.
This study represents the largest trial to date evaluating the use of allogeneic WJ-MSCs through multiple administration routes and doses in SCI patients. It builds upon earlier pilot studies[13,14]. The treatment was generally well-tolerated, with adverse events being infrequent, mild, and transient. The target dose of 1 × 106 cells/kg for each administration route was deemed safe, with only minor complications such as subfebrile fever, headache, and localized muscle pain, all resolving within 24 h.
The study demonstrated a 13.67-point improvement in the FIM Motor score and a 13.33-point improvement in the ASIA Motor score (Tables 5 and 8) among individuals with chronic SCI. These results are promising, but further validation through larger, controlled studies is necessary. Detailed physical examinations revealed a gradual enhancement in motor function, with ASIA Motor scores increasing from 1.67 ± 2.66 to 12.00 ± 8.10 at the 1-year endpoint. The percentage of patients achieving significant functional outcomes (total FIM Motor score ≥ 91) increased from 46.7% at baseline to 61.7% at 1 year (Table 5). Additionally, significant improvements were observed in sensory scores, with ASIA LT and PP scores showing notable increases.
Significant reductions in spasticity were observed, as indicated by the MA scale. The combined right and left scores improved from 15.83 ± 4.85 to 4.34 ± 3.78, with reductions of 5.83 points in the right values and 5.66 points in the left values. These improvements were sustained at the 1-year follow-up, suggesting that MSCs have a substantial impact on managing spasticity and enhancing spinal cord function. These findings warrant further validation through larger, controlled trials to confirm their robustness.
Chronic SCI often leads to GI dysfunction, which significantly impacts patients’ quality of life. Bowel disorders, which affect approximately 30% of SCI patients, often present greater challenges than bladder or sexual dysfunction[32]. The study utilized the WIS to assess GI function, revealing a 15% improvement (scores decreased from 20 to 17). Urinary dysfunction, prevalent in 70%-84% of SCI patients, was evaluated using the SFQ score, which indicated a 10.9% improvement in bladder function. These findings underscore the broader therapeutic impact of MSC therapy on various aspects of quality of life affected by SCI.
The therapeutic effects of WJ-MSCs in chronic SCI may be attributed to their unique biological properties and multifaceted mechanisms of action. WJ-MSCs are known to secrete a variety of trophic factors, including brain-derived neurotrophic factor, vascular endothelial growth factor, and transforming growth factor beta, which collectively promote neuroprotection, angiogenesis, and modulation of the inflammatory microenvironment[33,34]. These cells can also enhance the recruitment and differentiation of endogenous progenitor cells, potentially facilitating neuronal regeneration[35]. Furthermore, WJ-MSCs possess immunomodulatory capabilities, mitigating the chronic inflammation often observed in SCI and thus reducing secondary tissue damage[35]. Additionally, extracellular vesicles derived from WJ-MSCs play a significant role in intercellular communication, delivering bioactive molecules such as microRNAs and proteins that promote neural plasticity and repair processes[36]. These mechanisms, individually or synergistically, may contribute to the observed improvements in neurological function and quality of life, as evidenced in this study.
The study’s strengths include its focus on a large cohort of patients with chronic SCI, the use of allogeneic MSCs, and the application of multiple administration routes with restricted cell culture passages. Comprehensive safety assessments conducted over a 1-year period further enhance the credibility of the study. However, the absence of a control group limits the ability to accurately assess the observed benefits. Additionally, the study did not investigate the specific mechanisms underlying MSC therapy, such as growth factor release, anti-inflammatory effects, and exosome-mediated signaling. Future research should address these limitations by incorporating control groups and exploring the underlying mechanisms of MSC therapy. Effective restorative therapies are often enhanced by appropriate training, although this aspect was not fully implemented in the current safety-focused study.
Future research should focus on elucidating the mechanisms underlying MSC therapy, including the identification of key paracrine mediators and optimization of treatment protocols. Multicenter clinical trials are essential to validate the safety and efficacy of MSC therapies and refine treatment strategies for SCI. Further exploration of the mechanisms of action, such as the roles of growth factors, anti-inflammatory effects, and exosomes, will deepen understanding and potentially improve clinical outcomes for SCI patients. Enhanced understanding of these mechanisms will be critical for developing targeted therapies and improving patient care in the context of SCI.
This study underscored the potential of WJ-MSCs in treating chronic SCI. Notable improvements in motor and sensory functions, alongside reductions in spasticity and enhancements in quality of life, were observed following WJ-MSC treatment. These findings support WJ-MSC therapy as a promising option for SCI. However, given the absence of a control group and the influence of concurrent pharmacological and rehabilitative interventions, the observed improvements cannot be definitively attributed to WJ-MSCs alone. Further research with controlled trials is necessary to confirm efficacy and elucidate mechanisms of action.
| 1. | Hachem LD, Ahuja CS, Fehlings MG. Assessment and management of acute spinal cord injury: From point of injury to rehabilitation. J Spinal Cord Med. 2017;40:665-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 212] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 2. | Kang Y, Ding H, Zhou H, Wei Z, Liu L, Pan D, Feng S. Epidemiology of worldwide spinal cord injury: a literature review. J Neurorestoratology. 2018;6:1-9. [RCA] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 95] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 3. | Cripps RA, Lee BB, Wing P, Weerts E, Mackay J, Brown D. A global map for traumatic spinal cord injury epidemiology: towards a living data repository for injury prevention. Spinal Cord. 2011;49:493-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 205] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 4. | Devivo MJ. Epidemiology of traumatic spinal cord injury: trends and future implications. Spinal Cord. 2012;50:365-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 542] [Cited by in RCA: 581] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 5. | Alizadeh A, Dyck SM, Karimi-Abdolrezaee S. Traumatic Spinal Cord Injury: An Overview of Pathophysiology, Models and Acute Injury Mechanisms. Front Neurol. 2019;10:282. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 342] [Cited by in RCA: 808] [Article Influence: 134.7] [Reference Citation Analysis (0)] |
| 6. | Kim YH, Ha KY, Kim SI. Spinal Cord Injury and Related Clinical Trials. Clin Orthop Surg. 2017;9:1-9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 108] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 7. | Sekhon LH, Fehlings MG. Epidemiology, demographics, and pathophysiology of acute spinal cord injury. Spine (Phila Pa 1976). 2001;26:S2-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 909] [Cited by in RCA: 909] [Article Influence: 37.9] [Reference Citation Analysis (0)] |
| 8. | Bramlett HM, Dietrich WD. Progressive damage after brain and spinal cord injury: pathomechanisms and treatment strategies. Prog Brain Res. 2007;161:125-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 247] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 9. | Park E, Velumian AA, Fehlings MG. The role of excitotoxicity in secondary mechanisms of spinal cord injury: a review with an emphasis on the implications for white matter degeneration. J Neurotrauma. 2004;21:754-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 411] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 10. | Bracken MB. Steroids for acute spinal cord injury. Cochrane Database Syst Rev. 2012;1:CD001046. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 155] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 11. | Aras Y, Sabanci PA, Kabatas S, Duruksu G, Subasi C, Erguven M, Karaoz E. The Effects of Adipose Tissue-Derived Mesenchymal Stem Cell Transplantation During the Acute and Subacute Phases Following Spinal Cord Injury. Turk Neurosurg. 2016;26:127-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Kabatas S, Demir CS, Civelek E, Yilmaz I, Kircelli A, Yilmaz C, Akyuva Y, Karaoz E. Neuronal regeneration in injured rat spinal cord after human dental pulp derived neural crest stem cell transplantation. Bratisl Lek Listy. 2018;119:143-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Kabatas S, Civelek E, Savrunlu EC, Kaplan N, Boyalı O, Diren F, Can H, Genç A, Akkoç T, Karaöz E. Feasibility of allogeneic mesenchymal stem cells in pediatric hypoxic-ischemic encephalopathy: Phase I study. World J Stem Cells. 2021;13:470-484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 14. | Kabataş S, Civelek E, Savrunlu EC, Kaplan N, Çetin E, Diren F, Boyalı O, Güven G, Karaöz E. Functional recovery after Wharton’s jelly–derived mesenchymal stem cell administration in a patient with traumatic spinal cord injury: A pilot study. J Turk Spinal Surg. 2021;32:38-46. [DOI] [Full Text] |
| 15. | Huang H, Young W, Chen L, Feng S, Zoubi ZMA, Sharma HS, Saberi H, Moviglia GA, He X, Muresanu DF, Sharma A, Otom A, Andrews RJ, Al-Zoubi A, Bryukhovetskiy AS, Chernykh ER, Domańska-Janik K, Jafar E, Johnson WE, Li Y, Li D, Luan Z, Mao G, Shetty AK, Siniscalco D, Skaper S, Sun T, Wang Y, Wiklund L, Xue Q, You SW, Zheng Z, Dimitrijevic MR, Masri WSE, Sanberg PR, Xu Q, Luan G, Chopp M, Cho KS, Zhou XF, Wu P, Liu K, Mobasheri H, Ohtori S, Tanaka H, Han F, Feng Y, Zhang S, Lu Y, Zhang Z, Rao Y, Tang Z, Xi H, Wu L, Shen S, Xue M, Xiang G, Guo X, Yang X, Hao Y, Hu Y, Li J, Ao Q, Wang B, Zhang Z, Lu M, Li T. Clinical Cell Therapy Guidelines for Neurorestoration (IANR/CANR 2017). Cell Transplant. 2018;27:310-324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 16. | Osunronbi T, Sharma H. International Standards for Neurological Classification of Spinal Cord Injury: factors influencing the frequency, completion and accuracy of documentation of neurology for patients with traumatic spinal cord injuries. Eur J Orthop Surg Traumatol. 2019;29:1639-1648. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Blesch A, Lu P, Tuszynski MH. Neurotrophic factors, gene therapy, and neural stem cells for spinal cord repair. Brain Res Bull. 2002;57:833-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 120] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 18. | Girard C, Bemelmans AP, Dufour N, Mallet J, Bachelin C, Nait-Oumesmar B, Baron-Van Evercooren A, Lachapelle F. Grafts of brain-derived neurotrophic factor and neurotrophin 3-transduced primate Schwann cells lead to functional recovery of the demyelinated mouse spinal cord. J Neurosci. 2005;25:7924-7933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 93] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 19. | Kuh SU, Cho YE, Yoon DH, Kim KN, Ha Y. Functional recovery after human umbilical cord blood cells transplantation with brain-derived neutrophic factor into the spinal cord injured rat. Acta Neurochir (Wien). 2005;147:985-92; discussion 992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 86] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 20. | Zhao ZM, Li HJ, Liu HY, Lu SH, Yang RC, Zhang QJ, Han ZC. Intraspinal transplantation of CD34+ human umbilical cord blood cells after spinal cord hemisection injury improves functional recovery in adult rats. Cell Transplant. 2004;13:113-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 69] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 21. | Malgieri A, Kantzari E, Patrizi MP, Gambardella S. Bone marrow and umbilical cord blood human mesenchymal stem cells: state of the art. Int J Clin Exp Med. 2010;3:248-269. [PubMed] |
| 22. | Rodríguez-Fuentes DE, Fernández-Garza LE, Samia-Meza JA, Barrera-Barrera SA, Caplan AI, Barrera-Saldaña HA. Mesenchymal Stem Cells Current Clinical Applications: A Systematic Review. Arch Med Res. 2021;52:93-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 221] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 23. | Li J, Lepski G. Cell transplantation for spinal cord injury: a systematic review. Biomed Res Int. 2013;2013:786475. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 24. | Witkowska-Zimny M, Wrobel E. Perinatal sources of mesenchymal stem cells: Wharton's jelly, amnion and chorion. Cell Mol Biol Lett. 2011;16:493-514. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 25. | In 't Anker PS, Scherjon SA, Kleijburg-van der Keur C, de Groot-Swings GM, Claas FH, Fibbe WE, Kanhai HH. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells. 2004;22:1338-1345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 824] [Cited by in RCA: 818] [Article Influence: 40.9] [Reference Citation Analysis (0)] |
| 26. | Wang XY, Lan Y, He WY, Zhang L, Yao HY, Hou CM, Tong Y, Liu YL, Yang G, Liu XD, Yang X, Liu B, Mao N. Identification of mesenchymal stem cells in aorta-gonad-mesonephros and yolk sac of human embryos. Blood. 2008;111:2436-2443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 64] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 27. | Pearse DD, Bunge MB. Designing cell- and gene-based regeneration strategies to repair the injured spinal cord. J Neurotrauma. 2006;23:438-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 66] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 28. | Zhang L, Zhang HT, Hong SQ, Ma X, Jiang XD, Xu RX. Cografted Wharton's jelly cells-derived neurospheres and BDNF promote functional recovery after rat spinal cord transection. Neurochem Res. 2009;34:2030-2039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 29. | Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815-1822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3271] [Cited by in RCA: 3279] [Article Influence: 156.1] [Reference Citation Analysis (0)] |
| 30. | Németh K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, Robey PG, Leelahavanichkul K, Koller BH, Brown JM, Hu X, Jelinek I, Star RA, Mezey E. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15:42-49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1919] [Cited by in RCA: 1820] [Article Influence: 113.8] [Reference Citation Analysis (1)] |
| 31. | Bydon M, Dietz AB, Goncalves S, Moinuddin FM, Alvi MA, Goyal A, Yolcu Y, Hunt CL, Garlanger KL, Del Fabro AS, Reeves RK, Terzic A, Windebank AJ, Qu W. CELLTOP Clinical Trial: First Report From a Phase 1 Trial of Autologous Adipose Tissue-Derived Mesenchymal Stem Cells in the Treatment of Paralysis Due to Traumatic Spinal Cord Injury. Mayo Clin Proc. 2020;95:406-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 32. | Kabatas S, Yu D, He XD, Thatte HS, Benedict D, Hepgul KT, Black PM, Sabharwal S, Teng YD. Neural and anatomical abnormalities of the gastrointestinal system resulting from contusion spinal cord injury. Neuroscience. 2008;154:1627-1638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 33. | Galipeau J, Sensébé L. Mesenchymal Stromal Cells: Clinical Challenges and Therapeutic Opportunities. Cell Stem Cell. 2018;22:824-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1009] [Cited by in RCA: 1208] [Article Influence: 172.6] [Reference Citation Analysis (0)] |
| 34. | Nakajima H, Uchida K, Guerrero AR, Watanabe S, Sugita D, Takeura N, Yoshida A, Long G, Wright KT, Johnson WE, Baba H. Transplantation of mesenchymal stem cells promotes an alternative pathway of macrophage activation and functional recovery after spinal cord injury. J Neurotrauma. 2012;29:1614-1625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 313] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 35. | Montoto-Meijide R, Meijide-Faílde R, Díaz-Prado SM, Montoto-Marqués A. Mesenchymal Stem Cell Therapy in Traumatic Spinal Cord Injury: A Systematic Review. Int J Mol Sci. 2023;24:11719. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 23] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 36. | Willis GR, Reis M, Gheinani AH, Fernandez-Gonzalez A, Taglauer ES, Yeung V, Liu X, Ericsson M, Haas E, Mitsialis SA, Kourembanas S. Extracellular Vesicles Protect the Neonatal Lung from Hyperoxic Injury through the Epigenetic and Transcriptomic Reprogramming of Myeloid Cells. Am J Respir Crit Care Med. 2021;204:1418-1432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |