Copyright
©The Author(s) 2025.
World J Stem Cells. May 26, 2025; 17(5): 101675
Published online May 26, 2025. doi: 10.4252/wjsc.v17.i5.101675
Published online May 26, 2025. doi: 10.4252/wjsc.v17.i5.101675
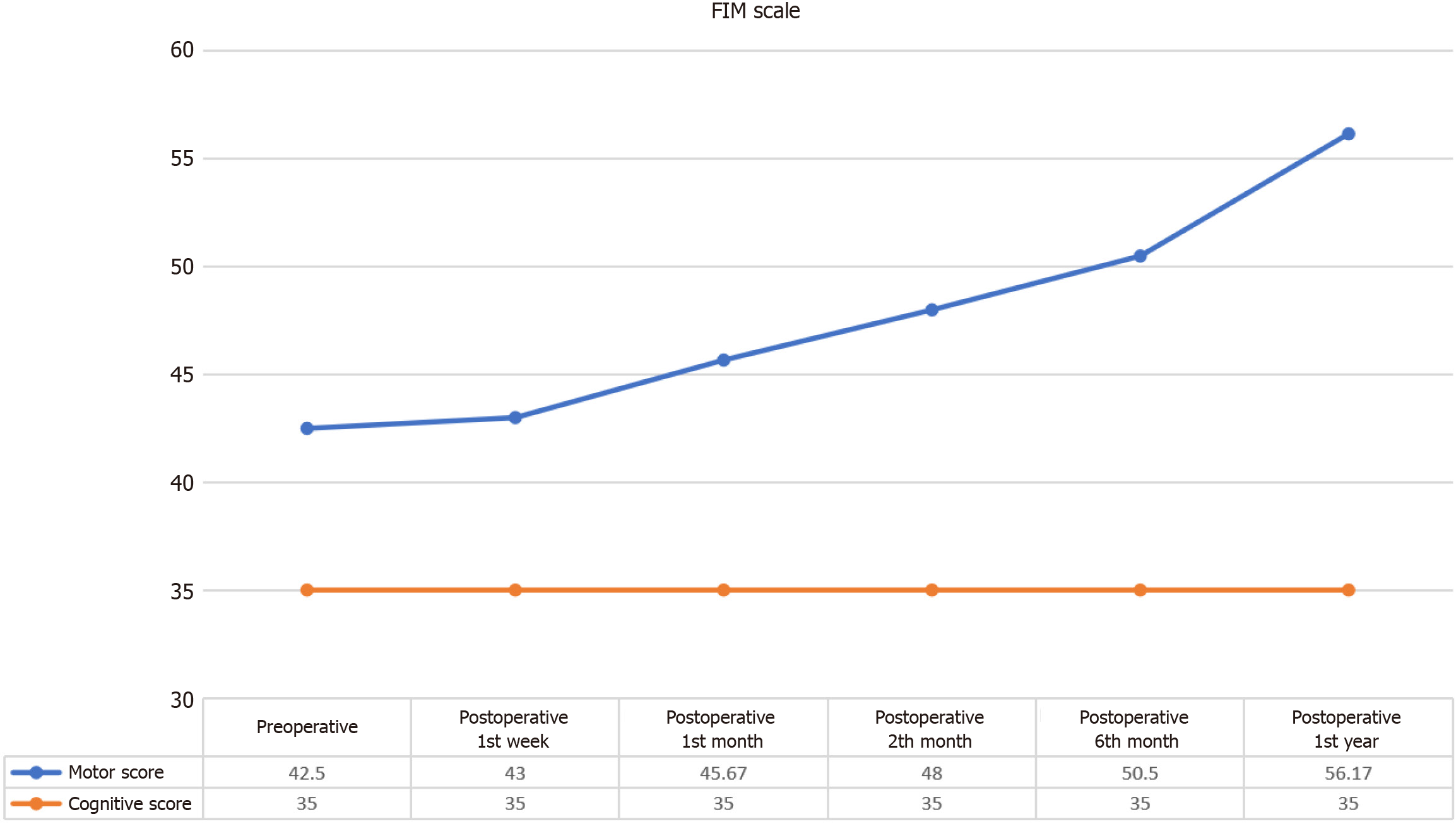
Figure 2 Changes in the Functional Independence Measure Motor scores following intervention.
This figure shows significant improvements in the Functional Independence Measure Motor scores at 1 month, 2 months, 4 months, and 1 year following intervention (χ² = 29.505, P < 0.001). Notable improvements were observed at 1 month (P = 0.039), 2 months (P = 0.026), and 4 months (P = 0.027), with sustained gains through 1 year (P = 0.027). FIM: Functional Independence Measure.
- Citation: Kaplan N, Kabatas S, Civelek E, Savrunlu EC, Akkoc T, Boyalı O, Öztürk E, Can H, Genc A, Karaöz E. Multiroute administration of Wharton’s jelly mesenchymal stem cells in chronic complete spinal cord injury: A phase I safety and feasibility study. World J Stem Cells 2025; 17(5): 101675
- URL: https://www.wjgnet.com/1948-0210/full/v17/i5/101675.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v17.i5.101675