Copyright
©The Author(s) 2025.
World J Stem Cells. May 26, 2025; 17(5): 101675
Published online May 26, 2025. doi: 10.4252/wjsc.v17.i5.101675
Published online May 26, 2025. doi: 10.4252/wjsc.v17.i5.101675
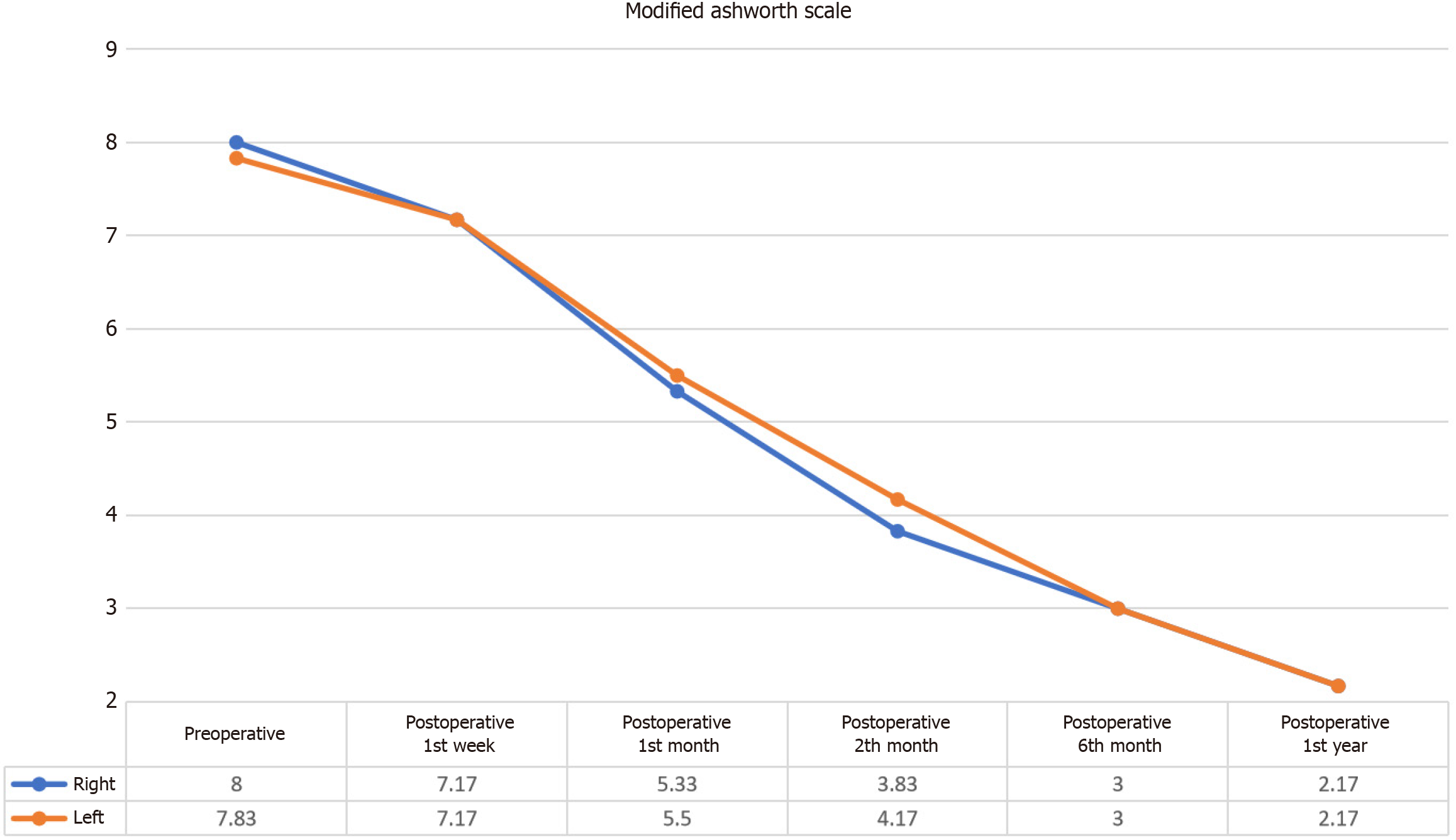
Figure 3 Changes in spasticity as measured by the Modified Ashworth scale.
This figure illustrates the significant reduction in spasticity in both the right and left lower extremities following intervention. Statistically significant decreases in the Modified Ashworth scale scores were observed at 1 month, 2 months, 4 months, and 1 year for both extremities (right: χ² = 28.452, P < 0.001; left: χ² = 29.030, P < 0.001).
- Citation: Kaplan N, Kabatas S, Civelek E, Savrunlu EC, Akkoc T, Boyalı O, Öztürk E, Can H, Genc A, Karaöz E. Multiroute administration of Wharton’s jelly mesenchymal stem cells in chronic complete spinal cord injury: A phase I safety and feasibility study. World J Stem Cells 2025; 17(5): 101675
- URL: https://www.wjgnet.com/1948-0210/full/v17/i5/101675.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v17.i5.101675