Copyright
©The Author(s) 2025.
World J Stem Cells. May 26, 2025; 17(5): 101675
Published online May 26, 2025. doi: 10.4252/wjsc.v17.i5.101675
Published online May 26, 2025. doi: 10.4252/wjsc.v17.i5.101675
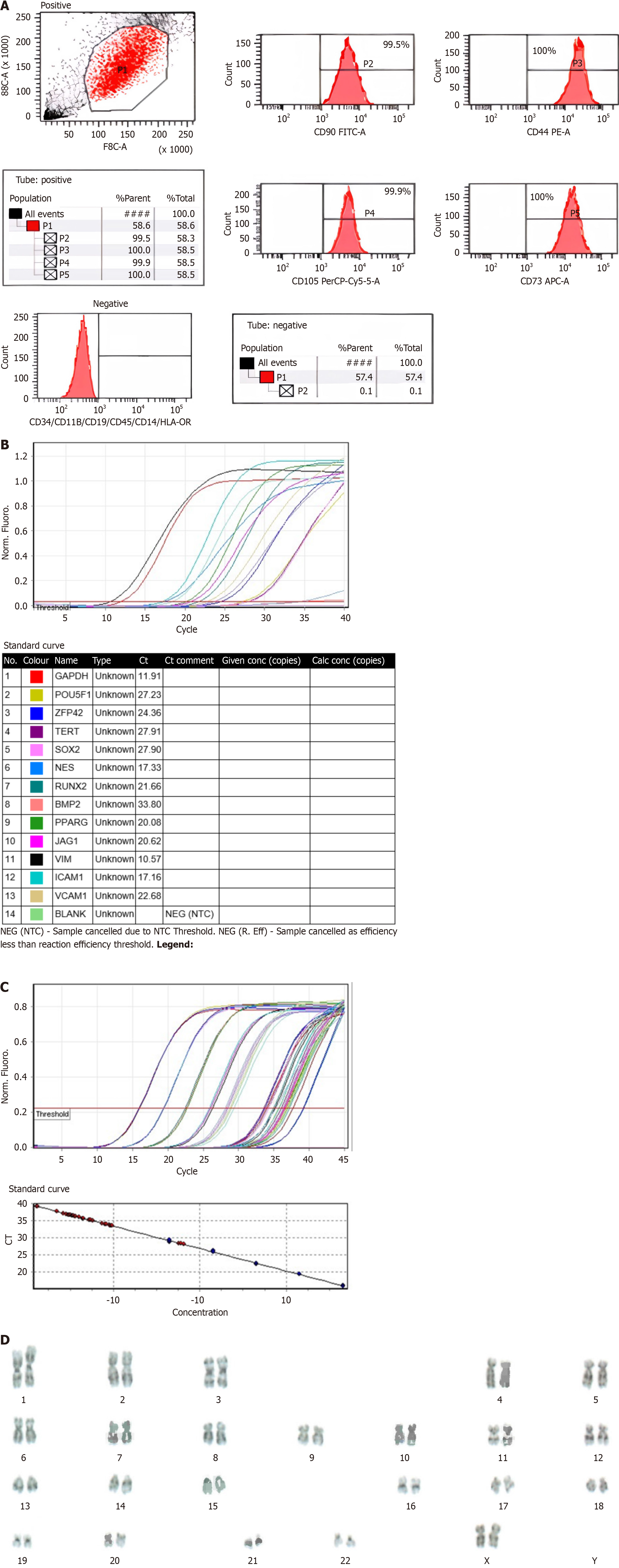
Figure 1 Wharton’s jelly derived mesenchymal stem cell flow cytometry, gene expression, telomerase activity, and karyotyping.
A: Wharton’s jelly-derived mesenchymal stem cell (WJ-MSC) flow cytometry. Positive marker values (CD90, CD105, CD73, and CD44) are above 95%. Negative marker values (CD45, CD34, CD19, CD11B, human leukocyte antigen DR, and CD14) are below 2%; B: WJ-MSC gene expression. In the gene expression test for MSCs, SOX2, OCT4, TERT, REX1, NES, RUNX2, PPARG, JAG1, ICAM1, VCAM1, and VIM had Ct values below 29 and were strongly positive. BMP2 had a Ct between 30-37 and was positive; C: WJ-MSC telomerase activity. In the telomerase activity assay of WJ-MSCs, the telomerase activity of the cell was not as high as the positive control; D: WJ-MSC karyotyping. Chromosomal analysis of metaphases obtained from WJ-MSCs after short-term cell culture using GPL banding revealed no numerical or gross structural chromosomal abnormalities. HLA: Human leukocyte antigen.
- Citation: Kaplan N, Kabatas S, Civelek E, Savrunlu EC, Akkoc T, Boyalı O, Öztürk E, Can H, Genc A, Karaöz E. Multiroute administration of Wharton’s jelly mesenchymal stem cells in chronic complete spinal cord injury: A phase I safety and feasibility study. World J Stem Cells 2025; 17(5): 101675
- URL: https://www.wjgnet.com/1948-0210/full/v17/i5/101675.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v17.i5.101675