Published online May 26, 2025. doi: 10.4252/wjsc.v17.i5.101638
Revised: November 24, 2024
Accepted: April 14, 2025
Published online: May 26, 2025
Processing time: 246 Days and 16.5 Hours
Circular RNAs (circRNAs) are a distinct type of nonlinear and noncoding RNAs endogenously expressed by pre-mRNA back-splicing and crucial in transcriptional and posttranscriptional regulation. CircRNAs can regulate cellular and molecular pathways through various mechanisms, such as microRNA sponging. Numerous studies have indicated the regulatory roles of circRNAs in the osteo
Core Tip: Several circular RNAs were found to induce osteogenesis in dental mesenchymal stem cells through acting as microRNA sponges leading to eliminating the inhibitory impacts of microRNAs on downstream target genes. These circular RNAs can help identify new molecular elements that provide diagnostic biomarkers and/or therapeutic targets for treating bone-associated dental disorders.
- Citation: Jiang YS, Wei WS, Xie DT, Guo G. Circular RNAs inducing the osteogenic differentiation of dental mesenchymal stem cells via microRNA sponging. World J Stem Cells 2025; 17(5): 101638
- URL: https://www.wjgnet.com/1948-0210/full/v17/i5/101638.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v17.i5.101638
Circular RNAs (circRNAs) are a distinct type of endogenous nonlinear noncoding RNAs formed by the back-splicing of pre-mRNAs, which play a crucial role in transcriptional and posttranscriptional regulation. They were first detected in 1991 and considered useless by-products with incorrect splicing[1-3]. CircRNAs were not found to be significant until reports of their widespread existence in mammals, particularly humans, in 2013[3-6]. They are expressed in various human cells[4] and show predominant cell/tissue-specific expression in the cytoplasm[5]. A high-throughput sequencing study showed that circRNAs are widely expressed by numerous human genes and exhibited higher expression than their corresponding homologous linear isoforms[7].
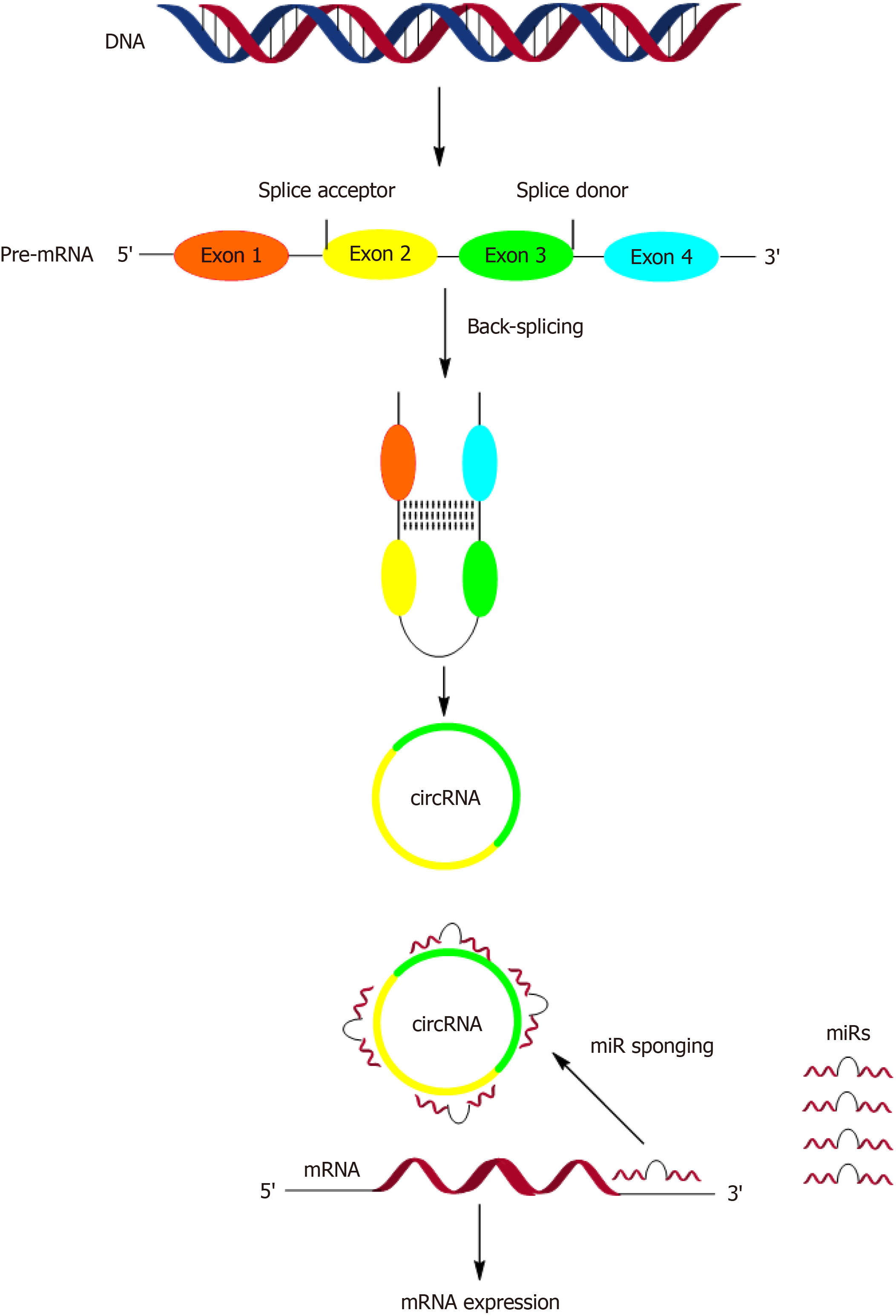
Compared with conventional linear noncoding RNAs, circRNAs have a stable head-to-tail closed circular structure created by the covalent binding of the 3’ and 5’ ends without a 5’ cap structure and 3’ polyadenylation tail[8]. Such a structure renders them resistant to exonuclease degradation and more stable than linear RNAs[9-13]. A circular loop is created by a particular alternative splicing event called back-splicing, by which an upstream splice acceptor is bound to a downstream splice donor[14] (Figure 1).
CircRNAs can influence various cellular and molecular functions through several mechanisms[15-18]. These including: (1) Acting as competitive endogenous RNAs, where circRNAs, among other transcripts, can competitively bind microRNAs (miRs) in the cytoplasm at conserved miR target sites, thereby counteracting the binding and inhibitory effects of mIR on downstream mRNA target genes (Figure 1); (2) Interacting with RNA-binding proteins; (3) Activating polymerase II machinery and U1 small nuclear ribonucleoproteins to regulate the expression of target genes; (4) Serving as scaffolding proteins that facilitate protein-protein interactions; and (5) Attaching to target proteins in the nucleus, which help stabilize these proteins so that they are not easily digested. Among the aforementioned mechanisms, circRNAs normally function as miRNA sponges[3,19,20] (Figure 1). For example, circRNA CDRlas contains several binding sites for miR-7, acting such a sponge for it and competing with its downstream target genes for binding, thereby negatively regulating the function of miR-7[21]. They have various biological functions and participate in cellular events, such as cell proliferation and differentiation[22-26]. Emerging studies have demonstrated the contribution of circRNAs to physiological and pathological events associated with cellular metabolism, inflammation, and apoptosis[27-29]. CircRNAs regulate gene expression in cancers and cardiovascular diseases. They are also employed as diagnostic biomarkers in cardiovascular and neuropsychiatric diseases[30,31].
Although most recent studies on circRNAs have focused on cancer, numerous studies have shown that they can regulate stem cell (SC) differentiation and tissue regeneration events[32-34]. Of note, circRNAs were overexpressed during the differentiation of induced pluripotent SCs in humans[32], and many studies have shown their participation in the proliferation and osteogenic differentiation of SCs by acting as miR sponges[35,36].
SCs are undifferentiated cells with high self-renewal and differentiation abilities for generating one or more types of specific cells. They serve as a primary resource in regenerative medicine, particularly in artificial bone engineering in which functional bone tissues are created as a scaffold or artificial environment for repairing bone defects. In recent years, SC therapy, particularly mesenchymal SCs (MSCs), has received considerable attention for the treatment of bone defects[37-41].
The teeth are the most natural, noninvasive source of SCs. Dental-derived MSCs (DMSCs) show great potential in regenerative medicine. These cells possess several important advantages, such as the absence of ethical concerns, easy accessibility, and ready availability, which make them of great interest for research. SCs self-renew to maintain a pool of cells that can be activated to replace terminally differentiated cells or enable wound healing, for example, the healing of periodontal tissues after surgery. DMSCs can differentiate into functional blood vessels and nerves. Several clinical trials have reported that transplanting DMSCs into disinfected necrotic teeth has allowed for the recovery of tooth vitality and vertical and horizontal root growth in immature teeth with incomplete root formation[42].
In recent years, numerous DMSCs have been isolated and characterized, such as MSCs from the gingiva, SCs from the apical papilla (SCAPs), dental follicle progenitor cells (DFPCs), SCs from exfoliated deciduous teeth, periodontal ligament SCs, and dental pulp SCs (DPSCs). Once exposed to special inducer conditions, similar to other SCs, DMSCs can differentiate into various tissue-like cells. Recently, several studies have reported the regulatory roles of circRNAs in the osteogenic differentiation of some DMSCs, including DPSCs, SCAPs, and DFPCs.
Osteogenesis is a biological process for the regeneration of lost bone volume. Tooth loss results in alveolar bone resorption. To suppress the stimulus generated by the periodontal ligament, the vestibular cortical bone undergoes resorption, resulting in the gradual disappearance of the marrow component of the alveolus. Consequently, the morphology of the alveolar ridge is altered, that is, when a few teeth are lost, the extent of the alveolar defect is affected; however, when more teeth are lost, a more noticeable atrophy occurs. In these conditions, the bone must be regenerated, taking advantage of the osteogenesis[43]. This review aimed to discuss the findings of various studies evaluating the effect of circRNAs on the osteogenic differentiation of DPSCs, SCAPs, and DFPCs (Table 1).
| CircRNA | Target miRs | Underlying mechanisms | Experimental methods | Ref. |
| CircRNAs inducing osteogenic differentiation of human dental pulp stem cells | ||||
| Hsa-circ-036872 | MiR-143-3p | IGF2 signaling activation | In vitro culture of hDPSCs isolated from orthodontic patients. In vivo experiments in BALB/c nude mice for heterotopic implantation of hDPSCs for bone formation assay | [52] |
| CircFKBP5 | MiR-708-5p | GIT2 signaling activation | In vitro culture of hDPSCs isolated from human dental pulp tissue | [34] |
| CircSIPA1L1 | MiR-617 | Smad3 signaling activation | In vitro culture of hDPSCs isolated from human dental pulp tissue. In vivo experiments in BALB/c nude mice for heterotopic implantation of hDPSCs for bone formation assay | [61] |
| CircLPAR1 | Hsa-miR-31 | Increased expression of SATB2 | In vitro culture of hDPSCs isolated from human dental pulp tissue | [66] |
| Hsa-circ-0026827 | MiR-188-3p | Enhancing Beclin-1-mediated autophagy | In vitro culture of hDPSCs isolated from human dental pulp tissue. In vivo experiments in BALB/c nude mice for heterotopic implantation of hDPSCs for bone formation assay | [68] |
| CircRNA124534 | MiR-496 | β-catenin signaling activation | In vitro culture of hDPSCs isolated from healthy pulp tissues derived from caries-free teeth of patients. In vivo experiments in BALB/c nude mice for heterotopic implantation of hDPSCs for bone formation assay | [67,73] |
| CircAKT3 | MiR-206 | Increased expression of CX43 | In vitro culture of hDPSCs isolated from human dental pulp tissue. In vivo experiments in BALB/c nude mice for heterotopic implantation of hDPSCs for bone formation assay | [77] |
| CircRNAs inducing osteogenic differentiation of apical papilla stem cells | ||||
| Hsa-circ-0008016 | MiR-337-3p | FGF/FGFR signaling activation | In vitro culture of SCAPs derived from human apical papilla tissues isolated from orthodontic patients | [93] |
| Circ-ZNF236 | MiR-218-5p | LGR4-mediated Wnt/β-catenin signaling activation | In vitro culture of SCAPs derived from human apical papilla tissues isolated from teeth with immature roots from healthy donors. In vivo experiments in a rat skull-impaired model for heterotopic implantation of SCAPs for bone formation assay | [94] |
| CircRNAs inducing osteogenic differentiation of dental follicle stem cells | ||||
| CircFgfr2 | MiR-133 | Increased expression of DLX3, RUNX2, and BMP6 in TGF-β and MAPK signaling pathways | In vitro culture of dental follicles isolated from the tooth germs of the mandibular molars of rats | [120] |
A systematic search was conducted in the electronic databases of Web of Science, Scopus, and PubMed to extract relevant articles from database inception to July 2024. The following keywords were used as part of the search strategy in each database: [“Circular RNAs” OR “circRNAs”] AND “dental stem cell” AND “osteogenesis”, and all articles were entered into the EndNote X9 reference manager software for screening. After removing duplicates, the remaining articles were assessed for selection. Articles published in English and in vitro, in vivo, and clinical studies evaluating circRNAs in the osteogenesis differentiation of DMSCs were included. Conversely, studies that did not provide sufficient data, review articles, and conference abstracts were excluded.
Human DPSCs (hDPSCs) are a class of MSCs that can differentiate into specific cell types, such as odontoblasts, osteoblasts, adipocytes, chondrocytes, and neural-like cells[44-46]. hDPSCs are frequently utilized in regenerative medicine because of their high proliferation rate, plasticity in multilineage differentiation, and ease of acquisition. Compared with MSCs, hDPSCs also show higher osteogenic potential but lower immunogenicity[47]. During osteoblast differentiation, hDPSCs express osteoblast differentiation-associated markers such as osterix (OSX), alkaline phosphatase (ALP), osteocalcin (OCN), and runt-related transcription factors (RUNX)[48,49]. RUNX2 is a transcriptional regulator of early-stage osteogenesis that directly affects the expression of genes responsible for bone tissue enrichment and intracranial secretion[50]. The OSX gene, which participates in osteoblast differentiation and bone formation, is one of the downstream targets of RUNX2[51]. ALP, as an early marker of calcification, is associated with osteogenesis specificity and osteoblast activity[52]. Emerging evidence demonstrates that circRNAs are crucial in the osteogenic differentiation of hDPSCs by sponging miRs.
The detection of differentially expressed circRNAs by next-generation sequencing technology after osteogenic promotion indicated that hsa-circ-0036872, arising from an exon of the FURIN gene, was upregulated during osteogenic differentiation of hDPSCs[53]. Notably, reduced expression of hsa-circ-0036872 suppressed the osteogenic differentiation of hDPSCs[53]. A dual-luciferase reporter assay revealed miR-143-3p as a downstream target of hsa-circ-0036872[53]. MiR-143-3p was found to inhibit osteogenesis in periodontal ligament cells by regulating kruppel-like factor 5 and suppressing Wnt/β-catenin signaling[54]. During the osteogenic differentiation of hDPSCs, the expression of miR-143-3p was reduced, and hsa-circ-0036872 upregulation inhibited the miR-143-3p expression, whereas the upregulation of miR-143-3p suppressed the osteogenic differentiation of hDPSCs[53]. Further assays demonstrated that miR-143-3p could bind to the 3’-untranslated regions (3’UTRs) of insulin-like growth factor 2 (IGF2) and inhibit its expression[53]. IGF2, which regulates different cellular processes[55], is upregulated during the osteogenic differentiation of MSCs[56,57] and promotes osteogenesis by regulating the expression of Col1 and Runx2[58]. In hDPSCs, IGF2 overexpression could reverse the suppression of osteogenic differentiation resulting from hsa-circ-0036872 silencing or miR-143-3p upregulation[53]. Of note, hsa-circ-0036872 knockout inhibits heterotopic bone formation in vivo, further supporting its promotional effect on improving hDPSC osteogenesis[53]. These findings indicate that hsa-circ-0036872 can facilitate hDPSC osteogenesis by inhibiting miR-143-3p and subsequently activating IGF2 signaling.
CircFKBP5, another circRNA generated by the back-splicing of exons 3-6 of FKBP5, was found to promote the osteogenic differentiation of hDPSCs and suppress inflammation and apoptosis[35]. Pulpitis, clinically classified as irreversible and reversible, refers to the inflammatory responses of the pulp to opportunistic infections induced by commensal oral bacteria[59]. During the pulpitis event, proinflammatory cytokines induced via microbial infection could dysregulate hDPSC viability and differentiation[60]. As a model mimicking the inflammatory condition of pulpitis, lipopolysaccharide (LPS)-induced hDPSC inflammation caused a reduction in the circFKBP5 expression by decreasing its cy
CircSIPA1 L1, expressed by a transcript encoding circSIPA1 L1, is upregulated under mineralization-promoting con
Evaluating the expression profiles of circRNAs in exosomes from osteogenic-induced DPSCs indicated increased expression of circular lysophosphatidic acid receptor 1 (circLPAR1) along with the osteogenic differentiation of DPSCs[67]. Furthermore, exosomes containing high levels of circLPAR1 exert an osteogenic effect on the recipient DPSCs[67]. Mechanistically, the dual-luciferase reporter assay revealed that circLPAR1 sponged hsa-miR-31 and eliminated its inhibitory effect on SATB2 and osteogenic differentiation of DPSCs, inducing osteogenesis of the recipient DPSCs[67]. Notably, the expression of SATB2 was upregulated in response to circLPAR1-mediated hsa-miR-31 inhibition, which caused the upregulation of its downstream genes associated with osteogenic differentiation, such as RUNX2, leading to the onset and progression of osteogenic differentiation of DPSCs[67].
Primary studies have indicated that hsa-circ-0026827 expression stimulated osteogenic differentiation[68]. Further studies have revealed that hsa-circ-0026827 expression was markedly increased during the osteoblast differentiation of DPSCs, whereas hsa-circ-0026827 knockdown inhibited the osteoblast differentiation of DPSCs[69]. This was further supported by experiments using heterotopic bone models, which demonstrated that hsa-circ-0026827 overexpression could induce heterotopic bone formation[69]. In mechanistic studies, dual-luciferase reporter assay demonstrated that hsa-circ-0026827 could induce the osteogenic differentiation of DPSCs by upregulating RUNX1 and Beclin-1-mediated autophagy by targeting and inhibiting miR-188-3p[69]. Interestingly, miR-188-3p overexpression could inhibit DPSC osteogenesis by directly inhibiting the expression of RUNX1 and Beclin-1, whereas miR-188-3p downregulation restored the osteogenic differentiation of DPSC[69]. RUNX1 is a crucial transcription factor that regulates osteogenic differentiation by affecting Smad1/5/8 and mitogen-activated protein kinase (MAPK) signaling[70]. Moreover, autophagy is a natural self-cannibalization process that not only has variety of biological effects but may also induce osteogenesis and suppress bone loss[71-73], further supporting hsa-circ-0026827-induced osteogenic differentiation of DPSCs through the enhancement of Beclin-1-mediated autophagy via the sponging of miR-188-3p.
CircRNA124534 has demonstrated a pivotal role in DPSC osteogenesis, showing significant upregulation in the process[68]. In vitro and in vivo experimental studies have uncovered that circRNA124534 overexpression could enhance the osteogenic differentiation of DPSCs, as evidenced by increased levels of the osteogenic-related genes OCN and RUNX2[74]. Mechanistically, circRNA124534, acting as an miR sponge, directly targets miR-496 and consequently upregulates β-catenin expression, eventually inducing DPSC osteogenesis[74]. The Wnt/β-catenin signaling is known to induce the osteogenic differentiation of DPSCs[75]. The Wnt/β-catenin signaling activation leads to the transcriptional activation of several downstream genes, such as cyclinDl and Runx2, which can regulate cell proliferation and osteogenic differentiation[76,77]. MiR-496 directly inhibited β-catenin expression, whereas miR-496 knockdown restored the β-catenin level and stimulated the osteogenic differentiation of DPSCs[74]. Notably, circRNA124534 and the β-catenin 3’UTR shared the same miR-496 response elements and competitively interacted with miR-496[74]. Therefore, overexpressed circ
Microarray analysis of the expression profiles of circRNAs during the osteogenic differentiation of hDPSCs indicated significant upregulation of circAKT3[78]. An in vitro investigation showed that circAKT3 knockdown led to the downregulation of osteogenic marker genes and inhibited osteogenic differentiation of hDPSCs[78]. Prediction and validation studies using a dual-luciferase reporter assay have indicated that circAKT3 could directly bind and inhibit miR-206 expression, and the latter was found to target connexin 43 (CX43) mRNA and inhibit hDPSC osteogenesis[78]. MiR-206 knockdown could reverse the suppressive effect of circAKT3 knockdown on osteogenic differentiation[78]. CX43, a positive regulator of SC osteogenesis, was predicted as a miR-206 target, and miR-206 upregulation and silencing could reduce and increase the CX43 expression, respectively[78]. Bone formation in vivo indicated that circAKT3 knockdown inhibited the expression of the osteogenic proteins OCN and COL1 and the formation of mineralized nodules[78]. Similarly, several studies have indicated that miR-206 upregulation suppressed osteoblast differentiation of osteoblasts and MSCs by targeting the 3’UTR sequence of CX43 mRNA and subsequently reducing the protein expression of CX43[79-82]. In conclusion, during hDPSC osteogenesis, circAKT3 can act as a positive regulator by directly sponging miR-206 and abolishing the inhibitory effects of miR-206 on CX43 expression.
SCAPs, a class of ectomesenchyme-derived cells in the apical tissue of immature teeth, have the abilities of multilineage differentiation and self-renewal[83]. They contribute to root development and pulp regeneration. SCAPs can induce the regeneration of tooth root-like tissues and bone/dentin-like structures[84-87] and differentiate into osteoblasts and odontoblasts[88]. They are more proliferative than DPSCs[89]. Given their advantages such as easy culture, differentiation capacity, and high proliferative rate and stable biological properties, SCAPs are suitable seed cells for pulp regeneration[90,91].
Several studies have shown the regulatory roles of circRNAs on SCAP osteogenesis. High-throughput sequencing detecting changes in circRNA expression profiles revealed 301 differentially expressed circRNAs during the osteogenic differentiation of SCAPs[92]. Validating the high-throughput sequencing data revealed that among the differentially expressed circRNAs, the expression levels of four circRNAs, including hsa-circ-0002538, has-circ-0003549, hsa-circ-0006618, and hsa-circ-0040809, were significantly increased, whereas the expression levels of three circRNAs, including hsa-circ-0002381, has-circ-0009031, and hsa-circ-0068958, were significantly downregulated[92]. The function of these circRNAs was predicted for miRs that circRNAs may bind and the target genes that miRs may regulate[92]. Subse
A study reported that hsa-circ-0008016 could actively induce osteogenic differentiation while diminishing odontogenic differentiation of SCAPs[94]. Further analysis using the dual-luciferase reporter assay revealed that hsa-circ-0008016 could sponge the miR-337-3p molecule. Fibroblast growth factor receptor 1 (FGFR1), which has a critical role in osteoblast differentiation by maintaining the balance between bone formation and remodeling, was the target gene of miRNA-337-3p and hsa-circ-0008016[94]. Therefore, hsa-circ-0008016 can abolish the suppressive effect of miR-337-3p on FGF/FGFR signaling, thereby enhancing the osteogenic differentiation of SCAPs.
A subsequent study unveiled that hsa-circ-0000857 (circ-ZNF236) was the most significantly upregulated circRNA during SCAP osteogenesis[95]. Gain/loss-of-function studies on circ-ZNF236 indicated significant upregulation of the protein expressions of various osteogenesis-related genes, such as OSX, RUNX2, ALP, and DSPP, in SCAPs overexpressing circ-ZNF236 and significant downregulation in circ-ZNF236 knockdown ones, suggesting circ-ZNF236 as a positive regulator in SCAP osteogenesis[95]. These results were further supported by in vivo studies revealing that SCAPs overexpressing circ-ZNF236 induced bone formation in a rat skull-impaired model[95]. Mechanistic studies using a dual-luciferase reporter assay showed that the high circ-ZNF236 expression could enhance SCAP osteogenesis via miR-218-5p/leucine-rich repeat-containing GPCR4 (LGR4) signaling. Specifically, circ-ZNF236 was identified to sponge miR-218-5p to promote the osteogenic differentiation of SCAPs. MiR-218-5p negatively affected SCAP osteogenesis by directly targeting the mRNA of LGR4 at its 3’-UTR[95]. LGR4, also called GPR48, is a member of the largest cell surface molecule family of GPCRs contributing to the transmission of extracellular signals to the cytoplasm[96]. LGR4 knockout could reduce the osteogenic differentiation of SCAPs[95,97], whereas miR-218-5p inhibition blocks the effect of LGR4 knockout on SCAP osteogenesis[95]. Of note, circ-ZNF236 could indirectly increase LGR4 expression by absorbing miR-218-5p, thus inducing the osteogenic differentiation of SCAPs[95]. Other studies have shown that LGR4 contributes to the committed differentiation of DMSCs and osteogenic differentiation of SCAPs by inducing Wnt/β-catenin-mediated autophagy[97-103]. Autophagy induces the osteogenic differentiation of DPSCs[104-106] and MSCs[107]. Notably, the excessive expression of circ-ZNF236 could induce autophagosome formation, and circ-ZNF236-mediated autophagy activation could strengthen the committed differentiation ability of SCAPs[95]. Therefore, these findings indicate that circ-ZNF236 could promote autophagy by abolishing the suppressive effects of miR-218-5p on the LGR4-mediated Wnt/β-catenin pathway and stimulate osteogenic differentiation of SCAPs.
Periodontitis, as a chronic progressive inflammatory status, is identified through alveolar bone resorption and pe
The potential of the aforementioned circRNAs in inducing osteogenic differentiation of DMSCs can be an efficient tool for cell-free regenerative endodontic treatment of dental diseases where bone restoration is challenging and tooth loss occurs, such as periodontal disorders, osteogenesis imperfecta, caries, and trauma. Periodontitis, also known as gum disease, is an inflammatory condition arising from a serious gum infection that damages the alveolar bone, periodontal ligament, and cementum, destroying the bone that supports teeth[130-133]. Currently, no periodontal treatment facilitates the regeneration of the modified region and the lost periodontal tissue into a normal and functional structure. Notably, with the development of SC-based regenerative medicine, tooth regeneration has become an ideal and promising method in the treatment of endodontic diseases[134-136]. Compared with SC therapy, cell-free regenerative endodontic treatment is mainly based on inducing the redevelopment of endogenous SCs at the lesion site, avoiding both technical and ethical problems that SC transplantation needs to solve[137]. Therefore, the induction of the osteogenic differentiation of dental-derived SCs using the here-reported circRNAs may be a promising and efficient approach for new tooth formation and treatment of dental diseases related to limited bone restoration, such as periodontitis.
CircRNAs take on critical roles in various cancers, and research into their functions and mechanisms suggests promising clinical applications. Based on the current research, circRNAs may serve as potential diagnostic markers (such as has-circ-0006401 in colorectal cancer and circAXIN1 in gastric cancer), prognostic markers (such as circFNDC3B, circPLCE1, and circMAPK14 in colon cancer; circDIDO1 and circMAPK1 in gastric cancer; circ-FBXW7 in glioblastoma; and circASK1 in lung adenocarcinoma), or therapeutic targets[138]. More studies using patient-derived xenograft mouse models and other tumor-bearing mouse models have demonstrated that several circRNAs might be potential therapeutic targets for cancer. These include circPLCE1, which can be a potential therapeutic target for colorectal cancer, circGprc5a in bladder cancer, circAXIN1 in gastric cancer, and circAXIN1 in the lung metastasis of gastric cancer[138]. Despite their considerable potential as biomarkers or therapeutic targets, their potential for use in clinical practice has not been established. In the development of circRNA therapeutics, multiple challenges must be addressed, mainly the design and optimization of circRNAs, circularization efficiency of circRNAs, and chemical manufacturing and control process development of circRNAs[139].
Despite recent advances in understanding circRNA biogenesis and functions, technological obstacles remain in determining the physicochemical properties and mechanisms of action of circRNAs occurring at multiple levels. The main concern is that the circRNA-miRNA-mRNA stoichiometry required for efficiency competition in miR binding is rarely observed under physiological conditions. Furthermore, because circRNA sequences fully overlap with their cognate linear RNA isoforms processed from the same pre-mRNAs, dissecting the functional significance of circRNAs has been challenging. Resolving the contribution of a circRNA from its residing gene into an observable effect remains difficult. Improvements in methods to study these RNA circles without affecting their residing genes are key to understanding what they do in cells. Further challenges exist in assays to identify circRNA-binding proteins[140,141].
This review summarized the activities and underlying mechanisms of circRNAs in regulating the osteogenic differentiation of DMSCs, such as DPSCs, SCAPs, and DFPCs. Generally, all reported circRNAs induce osteogenesis in DMSCs by acting as miR sponges, eliminating the inhibitory effects of miRs on downstream target genes. In DPSCs, these circRNAs and corresponding molecular targets include hsa-circ-0036872 and the miR-143-3p/IGF2 axis, circFKBP5 and the miR-708-5p/GIT2 axis, circSIPA1 L1 and the miR-617/Smad3 axis, circLPAR1 and the has-miR-31/RUNX2, hsa-circ-0026827 and the miR-188-3p/Beclin-1 axis, circRNA124534 and the miR-496/β-catenin axis, and circAKT3 and the miR-206/CX43 axis. In SCAPs, they are hsa-circ-0008016 and the miR-337-3p/FGFR axis and circ-ZNF236 and the miR-218-5p/LGR4 axis. Moreover, DFPC osteogenesis was found to be induced by circFgfr2 and the miR-133a-3p/DLX3-RUNX2-BMP6 axis.
These findings can help identify new molecular elements that provide diagnostic biomarkers and/or therapeutic targets for the treatment of bone-associated dental disorders. However, more evidence is needed to interpret this phenomenon and explore the application of the results of laboratory studies to the clinic. Currently, research has focused on investigating the sponge function of circRNAs in dental bone regeneration, whereas the other completely different but equally important functions have rarely been mentioned. Well-designed in vivo experiments are warranted to further explore the effects of circRNAs on the living body and realize their early clinical application in disease diagnosis and treatment. A comprehensive understanding of circRNA functions and regulation in dental bone regeneration processes could reveal novel molecular pathways crucial for maintaining dental integrity and homeostasis. This knowledge could lead to the development of targeted therapeutic interventions for various dental disorders.
| 1. | Qu S, Zhong Y, Shang R, Zhang X, Song W, Kjems J, Li H. The emerging landscape of circular RNA in life processes. RNA Biol. 2017;14:992-999. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 233] [Cited by in RCA: 325] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 2. | Yang Y, Fan X, Mao M, Song X, Wu P, Zhang Y, Jin Y, Yang Y, Chen LL, Wang Y, Wong CC, Xiao X, Wang Z. Extensive translation of circular RNAs driven by N(6)-methyladenosine. Cell Res. 2017;27:626-641. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 960] [Cited by in RCA: 1403] [Article Influence: 175.4] [Reference Citation Analysis (0)] |
| 3. | Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK, Kjems J. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495:384-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4631] [Cited by in RCA: 6046] [Article Influence: 503.8] [Reference Citation Analysis (0)] |
| 4. | Jeck WR, Sorrentino JA, Wang K, Slevin MK, Burd CE, Liu J, Marzluff WF, Sharpless NE. Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA. 2013;19:141-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3481] [Cited by in RCA: 3420] [Article Influence: 285.0] [Reference Citation Analysis (0)] |
| 5. | Salzman J, Chen RE, Olsen MN, Wang PL, Brown PO. Cell-type specific features of circular RNA expression. PLoS Genet. 2013;9:e1003777. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1046] [Cited by in RCA: 1419] [Article Influence: 118.3] [Reference Citation Analysis (0)] |
| 6. | Memczak S, Jens M, Elefsinioti A, Torti F, Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer M, Loewer A, Ziebold U, Landthaler M, Kocks C, le Noble F, Rajewsky N. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013;495:333-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6253] [Cited by in RCA: 6026] [Article Influence: 502.2] [Reference Citation Analysis (0)] |
| 7. | Barrett SP, Salzman J. Circular RNAs: analysis, expression and potential functions. Development. 2016;143:1838-1847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 505] [Cited by in RCA: 664] [Article Influence: 83.0] [Reference Citation Analysis (0)] |
| 8. | Barrett SP, Wang PL, Salzman J. Circular RNA biogenesis can proceed through an exon-containing lariat precursor. Elife. 2015;4:e07540. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 270] [Cited by in RCA: 356] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 9. | Chen LL, Yang L. Regulation of circRNA biogenesis. RNA Biol. 2015;12:381-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1045] [Cited by in RCA: 1509] [Article Influence: 167.7] [Reference Citation Analysis (0)] |
| 10. | Salzman J, Gawad C, Wang PL, Lacayo N, Brown PO. Circular RNAs are the predominant transcript isoform from hundreds of human genes in diverse cell types. PLoS One. 2012;7:e30733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1589] [Cited by in RCA: 1958] [Article Influence: 150.6] [Reference Citation Analysis (0)] |
| 11. | Qu S, Yang X, Li X, Wang J, Gao Y, Shang R, Sun W, Dou K, Li H. Circular RNA: A new star of noncoding RNAs. Cancer Lett. 2015;365:141-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1024] [Cited by in RCA: 1352] [Article Influence: 135.2] [Reference Citation Analysis (0)] |
| 12. | Misir S, Wu N, Yang BB. Specific expression and functions of circular RNAs. Cell Death Differ. 2022;29:481-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 254] [Article Influence: 84.7] [Reference Citation Analysis (0)] |
| 13. | Iravani Saadi M, Moayedi J, Hosseini F, Rostamipour HA, Karimi Z, Rahimian Z, Ahmadyan M, Ghahramani Z, Dehghani M, Yousefi K, Kheradmand N, Ramzi M, Fooladivanda N. The Effects of Resveratrol, Gallic Acid, and Piperine on the Expression of miR-17, miR-92b, miR-181a, miR-222, BAX, BCL-2, MCL-1, WT1, c-Kit, and CEBPA in Human Acute Myeloid Leukemia Cells and Their Roles in Apoptosis. Biochem Genet. 2024;62:2958-2974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 14. | Daniel C, Behm M, Öhman M. The role of Alu elements in the cis-regulation of RNA processing. Cell Mol Life Sci. 2015;72:4063-4076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 15. | Li R, Jiang J, Shi H, Qian H, Zhang X, Xu W. CircRNA: a rising star in gastric cancer. Cell Mol Life Sci. 2020;77:1661-1680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 279] [Article Influence: 55.8] [Reference Citation Analysis (0)] |
| 16. | Patop IL, Wüst S, Kadener S. Past, present, and future of circRNAs. EMBO J. 2019;38:e100836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 818] [Cited by in RCA: 883] [Article Influence: 147.2] [Reference Citation Analysis (0)] |
| 17. | Rong L, Chen B, Liu K, Liu B, He X, Liu J, Li J, He M, Zhu L, Liu K, Shi X, Shuai Y, Jin L. CircZDBF2 up-regulates RNF145 by ceRNA model and recruits CEBPB to accelerate oral squamous cell carcinoma progression via NFκB signaling pathway. J Transl Med. 2022;20:148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 18. | Wang J, Niu Y, Luo L, Lu Z, Chen Q, Zhang S, Guo Q, Li L, Gou D. Decoding ceRNA regulatory network in the pulmonary artery of hypoxia-induced pulmonary hypertension (HPH) rat model. Cell Biosci. 2022;12:27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 19. | Parsa-Kondelaji M, Musavi M, Barzegar F, Abbasian N, Rostami M, R Seyedtaghia M, S Hashemi S, Modi M, Nikfar B, A Momtazi-Borojeni A. Dysregulation of miRNA expression in patients with chronic myelogenous leukemia at diagnosis: a systematic review. Biomark Med. 2023;17:1021-1029. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 20. | Saadi MI, Nikandish M, Ghahramani Z, Valandani FM, Ahmadyan M, Hosseini F, Rahimian Z, Jalali H, Tavasolian F, Abdolyousefi EN, Kheradmand N, Ramzi M. miR-155 and miR-92 levels in ALL, post-transplant aGVHD, and CMV: possible new treatment options. J Egypt Natl Canc Inst. 2023;35:18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 21. | Hansen TB, Kjems J, Damgaard CK. Circular RNA and miR-7 in cancer. Cancer Res. 2013;73:5609-5612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 629] [Cited by in RCA: 758] [Article Influence: 63.2] [Reference Citation Analysis (0)] |
| 22. | Fan Y, Zhang Z, Deng K, Kang Z, Guo J, Zhang G, Zhang Y, Wang F. CircUBE3A promotes myoblasts proliferation and differentiation by sponging miR-28-5p to enhance expression. Int J Biol Macromol. 2023;226:730-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 23. | Geng Z, Wang W, Chen H, Mao J, Li Z, Zhou J. Circ_0001667 promotes breast cancer cell proliferation and survival via Hippo signal pathway by regulating TAZ. Cell Biosci. 2019;9:104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 24. | Krishnan RH, Sadu L, Akshaya RL, Gomathi K, Saranya I, Das UR, Satishkumar S, Selvamurugan N. Circ_CUX1/miR-130b-5p/p300 axis for parathyroid hormone-stimulation of Runx2 activity in rat osteoblasts: A combined bioinformatic and experimental approach. Int J Biol Macromol. 2023;225:1152-1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 25. | Wang M, Wu J, Wu P, Li Y. Emerging roles of circular RNAs in stem cells. Genes Dis. 2023;10:1920-1936. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 26. | Zhang Z, Zhao M, Wang G. Hsa_circ_0051079 functions as an oncogene by regulating miR-26a-5p/TGF-β1 in osteosarcoma. Cell Biosci. 2019;9:94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 27. | Kristensen LS, Andersen MS, Stagsted LVW, Ebbesen KK, Hansen TB, Kjems J. The biogenesis, biology and characterization of circular RNAs. Nat Rev Genet. 2019;20:675-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3273] [Cited by in RCA: 3151] [Article Influence: 525.2] [Reference Citation Analysis (0)] |
| 28. | Wu J, Qi X, Liu L, Hu X, Liu J, Yang J, Yang J, Lu L, Zhang Z, Ma S, Li H, Yun X, Sun T, Wang Y, Wang Z, Liu Z, Zhao W. Emerging Epigenetic Regulation of Circular RNAs in Human Cancer. Mol Ther Nucleic Acids. 2019;16:589-596. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 190] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 29. | Marques-Rocha JL, Samblas M, Milagro FI, Bressan J, Martínez JA, Marti A. Noncoding RNAs, cytokines, and inflammation-related diseases. FASEB J. 2015;29:3595-3611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 376] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 30. | Ebbesen KK, Kjems J, Hansen TB. Circular RNAs: Identification, biogenesis and function. Biochim Biophys Acta. 2016;1859:163-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 406] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 31. | Lei W, Feng T, Fang X, Yu Y, Yang J, Zhao ZA, Liu J, Shen Z, Deng W, Hu S. Signature of circular RNAs in human induced pluripotent stem cells and derived cardiomyocytes. Stem Cell Res Ther. 2018;9:56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 32. | Li L, Guo J, Chen Y, Chang C, Xu C. Comprehensive CircRNA expression profile and selection of key CircRNAs during priming phase of rat liver regeneration. BMC Genomics. 2017;18:80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 72] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 33. | Peng W, Zhu S, Chen J, Wang J, Rong Q, Chen S. Hsa_circRNA_33287 promotes the osteogenic differentiation of maxillary sinus membrane stem cells via miR-214-3p/Runx3. Biomed Pharmacother. 2019;109:1709-1717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 68] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 34. | Musavi M, Kohram F, Abasi M, Bolandi Z, Ajoudanian M, Mohammadi-Yeganeh S, Hashemi SM, Sharifi K, Fathi HR, Ghanbarian H. Rn7SK small nuclear RNA is involved in cellular senescence. J Cell Physiol. 2019;234:14234-14245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 35. | Liang C, Li W, Huang Q, Wen Q. CircFKBP5 Suppresses Apoptosis and Inflammation and Promotes Osteogenic Differentiation. Int Dent J. 2023;73:377-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 36. | Tarhriz V, Eyvazi S, Musavi M, Abasi M, Sharifi K, Ghanbarian H, Hejazi MS. Transient induction of Cdk9 in the early stage of differentiation is critical for myogenesis. J Cell Biochem. 2019;120:18854-18861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 37. | Ballini A, Scacco S, Coletti D, Pluchino S, Tatullo M. Mesenchymal Stem Cells as Promoters, Enhancers, and Playmakers of the Translational Regenerative Medicine. Stem Cells Int. 2017;2017:3292810. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 38. | Abbaszadegan MR, Bagheri V, Razavi MS, Momtazi AA, Sahebkar A, Gholamin M. Isolation, identification, and characterization of cancer stem cells: A review. J Cell Physiol. 2017;232:2008-2018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 144] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 39. | Bolandi Z, Hashemi SM, Abasi M, Musavi M, Aghamiri S, Miyanmahaleh N, Ghanbarian H. In vitro naive CD4(+) T cell differentiation upon treatment with miR-29b-loaded exosomes from mesenchymal stem cells. Mol Biol Rep. 2023;50:9037-9046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 40. | Salehi M, Darroudi M, Musavi M, Momtazi-Borojeni AA. Prediction of Age-Related MicroRNA Signature in Mesenchymal Stem Cells by using Computational Methods. Curr Stem Cell Res Ther. 2024;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 41. | Tavasolian F, Hosseini AZ, Mirzaei A, Abdollahi E, Jandaghi P, Soudi S, Naderi M, Saburi E, Momtazi-Borojeni AA, Johnston TP, Sahebkar A. Unfolded protein response-mediated modulation of mesenchymal stem cells. IUBMB Life. 2020;72:187-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 42. | Mantesso A, Nör JE. Stem cells in clinical dentistry. J Am Dent Assoc. 2023;154:1048-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 43. | Tonelli P, Duvina M, Barbato L, Biondi E, Nuti N, Brancato L, Rose GD. Bone regeneration in dentistry. Clin Cases Miner Bone Metab. 2011;8:24-28. [PubMed] |
| 44. | Mangano C, De Rosa A, Desiderio V, d'Aquino R, Piattelli A, De Francesco F, Tirino V, Mangano F, Papaccio G. The osteoblastic differentiation of dental pulp stem cells and bone formation on different titanium surface textures. Biomaterials. 2010;31:3543-3551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 96] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 45. | Spath L, Rotilio V, Alessandrini M, Gambara G, De Angelis L, Mancini M, Mitsiadis TA, Vivarelli E, Naro F, Filippini A, Papaccio G. Explant-derived human dental pulp stem cells enhance differentiation and proliferation potentials. J Cell Mol Med. 2010;14:1635-1644. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 85] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 46. | Wang L, Chen K, Wan X, Wang F, Guo Z, Mo Z. NLRP3 inflammasome activation in mesenchymal stem cells inhibits osteogenic differentiation and enhances adipogenic differentiation. Biochem Biophys Res Commun. 2017;484:871-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 77] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 47. | Ching HS, Luddin N, Rahman IA, Ponnuraj KT. Expression of Odontogenic and Osteogenic Markers in DPSCs and SHED: A Review. Curr Stem Cell Res Ther. 2017;12:71-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 136] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 48. | Victor AK, Reiter LT. Dental pulp stem cells for the study of neurogenetic disorders. Hum Mol Genet. 2017;26:R166-R171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 49. | Parsa-Kondelaji M, Ayatollahi H, Rostami M, Sheikhi M, Barzegar F, Afzalaghaee M, Moradi E, Sadeghian MH, Momtazi-Borojeni AA. Evaluating the frequency, prognosis and survival of RUNX1 and ASXL1 mutations in patients with acute myeloid leukaemia in northeastern Iran. J Cell Mol Med. 2022;26:3797-3801. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 50. | Phillips JE, Gersbach CA, Wojtowicz AM, García AJ. Glucocorticoid-induced osteogenesis is negatively regulated by Runx2/Cbfa1 serine phosphorylation. J Cell Sci. 2006;119:581-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 102] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 51. | Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, de Crombrugghe B. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108:17-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2600] [Cited by in RCA: 2680] [Article Influence: 116.5] [Reference Citation Analysis (0)] |
| 52. | Halling Linder C, Ek-Rylander B, Krumpel M, Norgård M, Narisawa S, Millán JL, Andersson G, Magnusson P. Bone Alkaline Phosphatase and Tartrate-Resistant Acid Phosphatase: Potential Co-regulators of Bone Mineralization. Calcif Tissue Int. 2017;101:92-101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 86] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 53. | Zhou J, Sui M, Ji F, Shen S, Lin Y, Jin M, Tao J. Hsa_circ_0036872 has an important promotional effect in enhancing osteogenesis of dental pulp stem cells by regulating the miR-143-3p/IGF2 axis. Int Immunopharmacol. 2024;130:111744. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 54. | Wangzhou K, Lai Z, Lu Z, Fu W, Liu C, Liang Z, Tan Y, Li C, Hao C. MiR-143-3p Inhibits Osteogenic Differentiation of Human Periodontal Ligament Cells by Targeting KLF5 and Inactivating the Wnt/β-Catenin Pathway. Front Physiol. 2020;11:606967. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 55. | Dawczynski K, Kauf E, Zintl F. Changes of serum growth factors (IGF-I,-II and IGFBP-2,-3) prior to and after stem cell transplantation in children with acute leukemia. Bone Marrow Transplant. 2003;32:411-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 56. | Al-Khafaji H, Noer PR, Alkharobi H, Alhodhodi A, Meade J, El-Gendy R, Oxvig C, Beattie J. A characteristic signature of insulin-like growth factor (IGF) axis expression during osteogenic differentiation of human dental pulp cells (hDPCs): Potential co-ordinated regulation of IGF action. Growth Horm IGF Res. 2018;42-43:14-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 57. | Zhang C, Wu S, Chen E, Yu L, Wang J, Wu M. ALX1-transcribed LncRNA AC132217.4 promotes osteogenesis and bone healing via IGF-AKT signaling in mesenchymal stem cells. Cell Mol Life Sci. 2022;79:328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 58. | Min SK, Kim M, Park JB. Insulin-like growth factor 2-enhanced osteogenic differentiation of stem cell spheroids by regulation of Runx2 and Col1 expression. Exp Ther Med. 2021;21:383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 59. | Wang X, Liao S, Nelson ER, Schmalzigaug R, Spurney RF, Guilak F, Premont RT, Gesty-Palmer D. The cytoskeletal regulatory scaffold protein GIT2 modulates mesenchymal stem cell differentiation and osteoblastogenesis. Biochem Biophys Res Commun. 2012;425:407-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 60. | Yuan H, Zhang H, Hong L, Zhao H, Wang J, Li H, Che H, Zhang Z. MicroRNA let-7c-5p Suppressed Lipopolysaccharide-Induced Dental Pulp Inflammation by Inhibiting Dentin Matrix Protein-1-Mediated Nuclear Factor kappa B (NF-κB) Pathway In Vitro and In Vivo. Med Sci Monit. 2018;24:6656-6665. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 61. | Shen Y, Jiang B, Luo B, Jiang X, Zhang Y, Wang Q. Circular RNA-FK501 binding protein 51 boosts bone marrow mesenchymal stem cell proliferation and osteogenic differentiation via modulating microRNA-205-5p/Runt-associated transcription factor 2 axis. J Orthop Surg Res. 2023;18:782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 62. | Ge X, Li Z, Zhou Z, Xia Y, Bian M, Yu J. Circular RNA SIPA1L1 promotes osteogenesis via regulating the miR-617/Smad3 axis in dental pulp stem cells. Stem Cell Res Ther. 2020;11:364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 63. | Huang X, Liu F, Hou J, Chen K. Inflammation-induced overexpression of microRNA-223-3p regulates odontoblastic differentiation of human dental pulp stem cells by targeting SMAD3. Int Endod J. 2019;52:491-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 64. | Zou Y, Kong M. Tetrahydroxy stilbene glucoside alleviates palmitic acid-induced inflammation and apoptosis in cardiomyocytes by regulating miR-129-3p/Smad3 signaling. Cell Mol Biol Lett. 2019;24:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 65. | de Kroon LM, Narcisi R, van den Akker GG, Vitters EL, Blaney Davidson EN, van Osch GJ, van der Kraan PM. SMAD3 and SMAD4 have a more dominant role than SMAD2 in TGFβ-induced chondrogenic differentiation of bone marrow-derived mesenchymal stem cells. Sci Rep. 2017;7:43164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 66. | Xu X, Jeong L, Han J, Ito Y, Bringas P Jr, Chai Y. Developmental expression of Smad1-7 suggests critical function of TGF-beta/BMP signaling in regulating epithelial-mesenchymal interaction during tooth morphogenesis. Int J Dev Biol. 2003;47:31-39. [PubMed] |
| 67. | Xie L, Guan Z, Zhang M, Lyu S, Thuaksuban N, Kamolmattayakul S, Nuntanaranont T. Exosomal circLPAR1 Promoted Osteogenic Differentiation of Homotypic Dental Pulp Stem Cells by Competitively Binding to hsa-miR-31. Biomed Res Int. 2020;2020:6319395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 68. | Zhang M, Jia L, Zheng Y. circRNA Expression Profiles in Human Bone Marrow Stem Cells Undergoing Osteoblast Differentiation. Stem Cell Rev Rep. 2019;15:126-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 75] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 69. | Ji F, Zhu L, Pan J, Shen Z, Yang Z, Wang J, Bai X, Lin Y, Tao J. hsa_circ_0026827 Promotes Osteoblast Differentiation of Human Dental Pulp Stem Cells Through the Beclin1 and RUNX1 Signaling Pathways by Sponging miR-188-3p. Front Cell Dev Biol. 2020;8:470. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 70. | Rahman MS, Akhtar N, Jamil HM, Banik RS, Asaduzzaman SM. TGF-β/BMP signaling and other molecular events: regulation of osteoblastogenesis and bone formation. Bone Res. 2015;3:15005. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 342] [Cited by in RCA: 444] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 71. | Gómez-Puerto MC, Verhagen LP, Braat AK, Lam EW, Coffer PJ, Lorenowicz MJ. Activation of autophagy by FOXO3 regulates redox homeostasis during osteogenic differentiation. Autophagy. 2016;12:1804-1816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 84] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 72. | Wan Y, Zhuo N, Li Y, Zhao W, Jiang D. Autophagy promotes osteogenic differentiation of human bone marrow mesenchymal stem cell derived from osteoporotic vertebrae. Biochem Biophys Res Commun. 2017;488:46-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 73. | Liang X, Hou Z, Xie Y, Yan F, Li S, Zhu X, Cai L. Icariin promotes osteogenic differentiation of bone marrow stromal cells and prevents bone loss in OVX mice via activating autophagy. J Cell Biochem. 2019;120:13121-13132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 74. | Ji F, Pan J, Shen Z, Yang Z, Wang J, Bai X, Tao J. The Circular RNA circRNA124534 Promotes Osteogenic Differentiation of Human Dental Pulp Stem Cells Through Modulation of the miR-496/β-Catenin Pathway. Front Cell Dev Biol. 2020;8:230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 75. | Lu X, Chen X, Xing J, Lian M, Huang D, Lu Y, Feng G, Feng X. miR-140-5p regulates the odontoblastic differentiation of dental pulp stem cells via the Wnt1/β-catenin signaling pathway. Stem Cell Res Ther. 2019;10:226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 76. | Kléber M, Sommer L. Wnt signaling and the regulation of stem cell function. Curr Opin Cell Biol. 2004;16:681-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 149] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 77. | Wilusz M, Majka M. Role of the Wnt/beta-catenin network in regulating hematopoiesis. Arch Immunol Ther Exp (Warsz). 2008;56:257-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 78. | Zhang B, Huo S, Cen X, Pan X, Huang X, Zhao Z. circAKT3 positively regulates osteogenic differentiation of human dental pulp stromal cells via miR-206/CX43 axis. Stem Cell Res Ther. 2020;11:531. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 79. | Inose H, Ochi H, Kimura A, Fujita K, Xu R, Sato S, Iwasaki M, Sunamura S, Takeuchi Y, Fukumoto S, Saito K, Nakamura T, Siomi H, Ito H, Arai Y, Shinomiya K, Takeda S. A microRNA regulatory mechanism of osteoblast differentiation. Proc Natl Acad Sci U S A. 2009;106:20794-20799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 243] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 80. | Chen Y, Yang YR, Fan XL, Lin P, Yang H, Chen XZ, Xu XD. miR-206 inhibits osteogenic differentiation of bone marrow mesenchymal stem cells by targetting glutaminase. Biosci Rep. 2019;39:BSR20181108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 81. | Fu Y, Shao ZM, He QZ, Jiang BQ, Wu Y, Zhuang ZG. Hsa-miR-206 represses the proliferation and invasion of breast cancer cells by targeting Cx43. Eur Rev Med Pharmacol Sci. 2015;19:2091-2104. [PubMed] |
| 82. | Li H, Xiang Y, Fan LJ, Zhang XY, Li JP, Yu CX, Bao LY, Cao DS, Xing WB, Liao XH, Zhang TC. Myocardin inhibited the gap protein connexin 43 via promoted miR-206 to regulate vascular smooth muscle cell phenotypic switch. Gene. 2017;616:22-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 83. | Sonoyama W, Liu Y, Yamaza T, Tuan RS, Wang S, Shi S, Huang GT. Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: a pilot study. J Endod. 2008;34:166-171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 868] [Cited by in RCA: 800] [Article Influence: 47.1] [Reference Citation Analysis (0)] |
| 84. | Chrepa V, Henry MA, Daniel BJ, Diogenes A. Delivery of Apical Mesenchymal Stem Cells into Root Canals of Mature Teeth. J Dent Res. 2015;94:1653-1659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 92] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 85. | Wang J, Liu B, Gu S, Liang J. Effects of Wnt/β-catenin signalling on proliferation and differentiation of apical papilla stem cells. Cell Prolif. 2012;45:121-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 86. | Li N, Li Z, Fu L, Yan M, Wang Y, Yu J, Wu J. PD-1 Suppresses the Osteogenic and Odontogenic Differentiation of Stem Cells from Dental Apical Papilla via Targeting SHP2/NF-κB Axis. Stem Cells. 2022;40:763-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 87. | Li Z, Yan M, Yu Y, Wang Y, Lei G, Pan Y, Li N, Gobin R, Yu J. LncRNA H19 promotes the committed differentiation of stem cells from apical papilla via miR-141/SPAG9 pathway. Cell Death Dis. 2019;10:130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 88. | Kang J, Fan W, Deng Q, He H, Huang F. Stem Cells from the Apical Papilla: A Promising Source for Stem Cell-Based Therapy. Biomed Res Int. 2019;2019:6104738. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 98] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 89. | Patil R, Kumar BM, Lee WJ, Jeon RH, Jang SJ, Lee YM, Park BW, Byun JH, Ahn CS, Kim JW, Rho GJ. Multilineage potential and proteomic profiling of human dental stem cells derived from a single donor. Exp Cell Res. 2014;320:92-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 90. | Ma S, Liu G, Jin L, Pang X, Wang Y, Wang Z, Yu Y, Yu J. IGF-1/IGF-1R/hsa-let-7c axis regulates the committed differentiation of stem cells from apical papilla. Sci Rep. 2016;6:36922. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 91. | Wongwatanasanti N, Jantarat J, Sritanaudomchai H, Hargreaves KM. Effect of Bioceramic Materials on Proliferation and Odontoblast Differentiation of Human Stem Cells from the Apical Papilla. J Endod. 2018;44:1270-1275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 92. | Li J, Wang S, Ren Y, Li H, Zhou Y, Lan X, Wang Y. Differential expression of circRNAs during osteogenic/odontogenic differentiation of stem cells from apical papilla promoted by blue light-emitting diode. Mol Biol Rep. 2024;51:710. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 93. | Li Z, Li N, Ge X, Pan Y, Lu J, Gobin R, Yan M, Yu J. Differential circular RNA expression profiling during osteogenic differentiation of stem cells from apical papilla. Epigenomics. 2019;11:1057-1073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 94. | Xu J, Han X, Yang H, He L, Wang Y, Tian J. Effect of circular RNA hsa_circ_0008016 on the Osteogenic and Odontogenic Ability of Stem Cells from Apical Papilla. J Hard Tissue Biol. 2024;33:105-112. [DOI] [Full Text] |
| 95. | Xiao Y, Chen L, Xu Y, Yu R, Lu J, Ke Y, Guo R, Gu T, Yu H, Fang Y, Li Z, Yu J. Circ-ZNF236 mediates stem cells from apical papilla differentiation by regulating LGR4-induced autophagy. Int Endod J. 2024;57:431-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 96. | Nakata S, Phillips E, Goidts V. Emerging role for leucine-rich repeat-containing G-protein-coupled receptors LGR5 and LGR4 in cancer stem cells. Cancer Manag Res. 2014;6:171-180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 97. | Zhou M, Guo S, Yuan L, Zhang Y, Zhang M, Chen H, Lu M, Yang J, Ma J. Blockade of LGR4 inhibits proliferation and odonto/osteogenic differentiation of stem cells from apical papillae. J Mol Histol. 2017;48:389-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 98. | Chen X, Chen L, Tan J, Zhang L, Xia J, Cheng B, Zhang W. Rspo1-LGR4 axis in BMSCs protects bone against radiation-induced injury through the mTOR-dependent autophagy pathway. J Cell Physiol. 2021;236:4273-4289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 99. | Liu Y, Song JW, Lin JY, Miao R, Zhong JC. Roles of MicroRNA-122 in Cardiovascular Fibrosis and Related Diseases. Cardiovasc Toxicol. 2020;20:463-473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 100. | Maity J, Barthels D, Sarkar J, Prateeksha P, Deb M, Rolph D, Das H. Ferutinin induces osteoblast differentiation of DPSCs via induction of KLF2 and autophagy/mitophagy. Cell Death Dis. 2022;13:452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 101. | Maity J, Deb M, Greene C, Das H. KLF2 regulates dental pulp-derived stem cell differentiation through the induction of mitophagy and altering mitochondrial metabolism. Redox Biol. 2020;36:101622. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 102. | Chu CW, Ko HJ, Chou CH, Cheng TS, Cheng HW, Liang YH, Lai YL, Lin CY, Wang C, Loh JK, Cheng JT, Chiou SJ, Su CL, Huang CF, Hong YR. Thioridazine Enhances P62-Mediated Autophagy and Apoptosis Through Wnt/β-Catenin Signaling Pathway in Glioma Cells. Int J Mol Sci. 2019;20:473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 101] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 103. | Vidoni C, Ferraresi A, Secomandi E, Vallino L, Gardin C, Zavan B, Mortellaro C, Isidoro C. Autophagy drives osteogenic differentiation of human gingival mesenchymal stem cells. Cell Commun Signal. 2019;17:98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 104. | He M, Lei H, He X, Liu Y, Wang A, Ren Z, Liu X, Yan G, Wang W, Wang Y, Li G, Wang T, Pu J, Shen Z, Wang Y, Xie J, Du W, Yuan Y, Yang L. METTL14 Regulates Osteogenesis of Bone Marrow Mesenchymal Stem Cells via Inducing Autophagy Through m6A/IGF2BPs/Beclin-1 Signal Axis. Stem Cells Transl Med. 2022;11:987-1001. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 62] [Reference Citation Analysis (0)] |
| 105. | Pei F, Wang HS, Chen Z, Zhang L. Autophagy regulates odontoblast differentiation by suppressing NF-κB activation in an inflammatory environment. Cell Death Dis. 2016;7:e2122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 106. | Zhang L, Qi M, Chen J, Zhao J, Li L, Hu J, Jin Y, Liu W. Impaired autophagy triggered by HDAC9 in mesenchymal stem cells accelerates bone mass loss. Stem Cell Res Ther. 2020;11:269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 107. | Li N, Yan M, Chen Y, Wang Y, Wu J, Fu L, Yu J. Extracellular IL-37 promotes osteogenic and odontogenic differentiation of human dental pulp stem cells via autophagy. Exp Cell Res. 2021;407:112780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 108. | Slots J. Periodontitis: facts, fallacies and the future. Periodontol 2000. 2017;75:7-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 697] [Article Influence: 99.6] [Reference Citation Analysis (0)] |
| 109. | Kwon T, Lamster IB, Levin L. Current Concepts in the Management of Periodontitis. Int Dent J. 2021;71:462-476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 502] [Article Influence: 125.5] [Reference Citation Analysis (0)] |
| 110. | Bee SL, Hamid ZAA. Asymmetric resorbable-based dental barrier membrane for periodontal guided tissue regeneration and guided bone regeneration: A review. J Biomed Mater Res B Appl Biomater. 2022;110:2157-2182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 44] [Reference Citation Analysis (0)] |
| 111. | Ten Cate AR. The development of the periodontium--a largely ectomesenchymally derived unit. Periodontol 2000. 1997;13:9-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 114] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 112. | Wise GE, Frazier-Bowers S, D'Souza RN. Cellular, molecular, and genetic determinants of tooth eruption. Crit Rev Oral Biol Med. 2002;13:323-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 225] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 113. | Guo S, Kang J, Ji B, Guo W, Ding Y, Wu Y, Tian W. Periodontal-Derived Mesenchymal Cell Sheets Promote Periodontal Regeneration in Inflammatory Microenvironment. Tissue Eng Part A. 2017;23:585-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 114. | Jung HS, Lee DS, Lee JH, Park SJ, Lee G, Seo BM, Ko JS, Park JC. Directing the differentiation of human dental follicle cells into cementoblasts and/or osteoblasts by a combination of HERS and pulp cells. J Mol Histol. 2011;42:227-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 115. | Li R, Guo W, Yang B, Guo L, Sheng L, Chen G, Li Y, Zou Q, Xie D, An X, Chen Y, Tian W. Human treated dentin matrix as a natural scaffold for complete human dentin tissue regeneration. Biomaterials. 2011;32:4525-4538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 170] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 116. | Morsczeck C, Götz W, Schierholz J, Zeilhofer F, Kühn U, Möhl C, Sippel C, Hoffmann KH. Isolation of precursor cells (PCs) from human dental follicle of wisdom teeth. Matrix Biol. 2005;24:155-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 609] [Cited by in RCA: 624] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 117. | Xu LL, Liu HC, Wang DS, E LL, Xu L, Jin ZL, Duan YZ. Effects of BMP-2 and dexamethasone on osteogenic differentiation of rat dental follicle progenitor cells seeded on three-dimensional beta-TCP. Biomed Mater. 2009;4:065010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 118. | Yang B, Chen G, Li J, Zou Q, Xie D, Chen Y, Wang H, Zheng X, Long J, Tang W, Guo W, Tian W. Tooth root regeneration using dental follicle cell sheets in combination with a dentin matrix - based scaffold. Biomaterials. 2012;33:2449-2461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 133] [Article Influence: 10.2] [Reference Citation Analysis (1)] |
| 119. | Yao S, Pan F, Prpic V, Wise GE. Differentiation of stem cells in the dental follicle. J Dent Res. 2008;87:767-771. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 191] [Cited by in RCA: 167] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 120. | Du Y, Li J, Hou Y, Chen C, Long W, Jiang H. Alteration of circular RNA expression in rat dental follicle cells during osteogenic differentiation. J Cell Biochem. 2019;120:13289-13301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 121. | Xu C, Xu Z, Li G, Li J, Ye L, Ning Y, Du Y. CircFgfr2 promotes osteogenic differentiation of rat dental follicle cells by targeting the miR-133a-3p/DLX3 signaling pathway. Heliyon. 2024;10:e32498. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 122. | Li J, Lin Q, Lin Y, Lai R, Zhang W. Effects of DLX3 on the osteogenic differentiation of induced pluripotent stem cellderived mesenchymal stem cells. Mol Med Rep. 2021;23:232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 123. | Liao XB, Zhang ZY, Yuan K, Liu Y, Feng X, Cui RR, Hu YR, Yuan ZS, Gu L, Li SJ, Mao DA, Lu Q, Zhou XM, de Jesus Perez VA, Yuan LQ. MiR-133a modulates osteogenic differentiation of vascular smooth muscle cells. Endocrinology. 2013;154:3344-3352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 109] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 124. | Duverger O, Isaac J, Zah A, Hwang J, Berdal A, Lian JB, Morasso MI. In vivo impact of Dlx3 conditional inactivation in neural crest-derived craniofacial bones. J Cell Physiol. 2013;228:654-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 125. | Zhang Y, Xie RL, Croce CM, Stein JL, Lian JB, van Wijnen AJ, Stein GS. A program of microRNAs controls osteogenic lineage progression by targeting transcription factor Runx2. Proc Natl Acad Sci U S A. 2011;108:9863-9868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 352] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 126. | Zhou Q, Zhao ZN, Cheng JT, Zhang B, Xu J, Huang F, Zhao RN, Chen YJ. Ibandronate promotes osteogenic differentiation of periodontal ligament stem cells by regulating the expression of microRNAs. Biochem Biophys Res Commun. 2011;404:127-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 127. | Qadir AS, Lee J, Lee YS, Woo KM, Ryoo HM, Baek JH. Distal-less homeobox 3, a negative regulator of myogenesis, is downregulated by microRNA-133. J Cell Biochem. 2019;120:2226-2235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 128. | Isaac J, Erthal J, Gordon J, Duverger O, Sun HW, Lichtler AC, Stein GS, Lian JB, Morasso MI. DLX3 regulates bone mass by targeting genes supporting osteoblast differentiation and mineral homeostasis in vivo. Cell Death Differ. 2014;21:1365-1376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 129. | Li Z, Hassan MQ, Volinia S, van Wijnen AJ, Stein JL, Croce CM, Lian JB, Stein GS. A microRNA signature for a BMP2-induced osteoblast lineage commitment program. Proc Natl Acad Sci U S A. 2008;105:13906-13911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 453] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 130. | Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, Young M, Robey PG, Wang CY, Shi S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2371] [Cited by in RCA: 2505] [Article Influence: 119.3] [Reference Citation Analysis (0)] |
| 131. | Musavi M, Shahraeen N, Azizi A, Salari N. Serological and molecular properties of isolates of Bean common mosaic virus-BCMV from Khorasan Razavi province. Arch Phytopath Plant. 2014;47:830-841. [DOI] [Full Text] |
| 132. | Haftcheshmeh SM, Mohammadi A, Soltani A, Momtazi-Borojeni AA, Sattari M. Evaluation of STAT1 and Wnt5a gene expression in gingival tissues of patients with periodontal disease. J Cell Biochem. 2019;120:1827-1834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 133. | Sattari M, Masoudnia M, Mashayekhi K, Hashemi SM, Khannazer N, Sattari S, Mohammadian Haftcheshmeh S, Momtazi-Borojeni AA. Evaluating the effect of LPS from periodontal pathogenic bacteria on the expression of senescence-related genes in human dental pulp stem cells. J Cell Mol Med. 2022;26:5647-5656. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 134. | Zha K, Tian Y, Panayi AC, Mi B, Liu G. Recent Advances in Enhancement Strategies for Osteogenic Differentiation of Mesenchymal Stem Cells in Bone Tissue Engineering. Front Cell Dev Biol. 2022;10:824812. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 35] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 135. | Zheng C, Chen J, Liu S, Jin Y. Stem cell-based bone and dental regeneration: a view of microenvironmental modulation. Int J Oral Sci. 2019;11:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 148] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 136. | Ivanovski S, Han P, Peters OA, Sanz M, Bartold PM. The Therapeutic Use of Dental Mesenchymal Stem Cells in Human Clinical Trials. J Dent Res. 2024;103:1173-1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 137. | Lin LM, Huang GT, Sigurdsson A, Kahler B. Clinical cell-based versus cell-free regenerative endodontics: clarification of concept and term. Int Endod J. 2021;54:887-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 52] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 138. | Liu H, Hao W, Yang J, Zhang Y, Wang X, Zhang C. Emerging roles and potential clinical applications of translatable circular RNAs in cancer and other human diseases. Genes Dis. 2023;10:1994-2012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 139. | Zhao X, Zhong Y, Wang X, Shen J, An W. Advances in Circular RNA and Its Applications. Int J Med Sci. 2022;19:975-985. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 115] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 140. | Li X, Yang L, Chen LL. The Biogenesis, Functions, and Challenges of Circular RNAs. Mol Cell. 2018;71:428-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 988] [Cited by in RCA: 1485] [Article Influence: 212.1] [Reference Citation Analysis (0)] |
| 141. | Nielsen AF, Bindereif A, Bozzoni I, Hanan M, Hansen TB, Irimia M, Kadener S, Kristensen LS, Legnini I, Morlando M, Jarlstad Olesen MT, Pasterkamp RJ, Preibisch S, Rajewsky N, Suenkel C, Kjems J. Best practice standards for circular RNA research. Nat Methods. 2022;19:1208-1220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 105] [Article Influence: 35.0] [Reference Citation Analysis (0)] |