Published online Feb 15, 2002. doi: 10.3748/wjg.v8.i1.69
Revised: September 9, 2001
Accepted: October 24, 2001
Published online: February 15, 2002
AIM: To label anti-hepatoma monoclonal antibody (mAb) fragment HAb18 F(ab’)2 was labeled with 188Re for the pharmacokinetic model of 188Re-HAb18 F(ab’)2 and to evaluate its pharmacokinetic parameters in hepatoma-bearing nude mice.
METHODS: HAb18 F(ab’)2 was directly labeled with 188Re using 2-mercaptoethanol (2-ME) as reducing agents. Labeling efficiency and immunoreactivity of 188Re-HAb18 F(ab’)2 were evaluated by Whatman 3MM paper chromatography and live cell assay, respectively. Biodistribution analysis was also conducted in nude mice bearing human hepatoma in which animals were sacrificed at different time points (1, 4, 18, 24 and 24 h) after 188Re-HAb18 F (ab’)2 was injected through tail-vein into hepatoma-bearing nude mice. The blood and radioactivity of organs and mass were measured. The concentrations of 188Re-HAb18 F(ab’)2 were evaluated with apharmacokinetic 3P97 software.
RESULTS: The optimum labeling efficiency and immunoreactive fraction were 91.7% and 0.78% respectively. The parameters of 188Re-HAb18 F(ab’)2 were: T1/2, 2.29 h; Vd,1.49 × 10-9 L·Bq-1; AUC, 20. 49 × 109 Bq·h·L-1;CL, 0.45 × 10-3 L·h-1. 188Re-HAb18 F(ab’)2 could locate specially in hepatoma with high selective reactivity of HAb18 F(ab’)2. 188Re-HAb18 F(ab’)2 was mainly eliminated by kidney. The maximal tumor to blood ratio was at 48 h, and maximal tumor to liver ratio was at 18 h.
CONCLUTION: The pharmacokinetics of 188Re-HAb18 F (ab’)2 fital-compartment model.188Re-HAb18 F(ab’)2 can be uptaken selectively at the hepatoma site.
- Citation: Lou C, Chen ZN, Bian HJ, Li J, Zhou SB. Pharmacokinetics of radioimmunotherapeutic agent of direct labeling mAb 188Re-HAb18. World J Gastroenterol 2002; 8(1): 69-73
- URL: https://www.wjgnet.com/1007-9327/full/v8/i1/69.htm
- DOI: https://dx.doi.org/10.3748/wjg.v8.i1.69
188Re is a new radioisotope[1-16]. In the past,131I was used as the main radioisotope for radioimmunotherapy(RAIT).131I has its favour such as simple labeling, appropriate partical energy and path length,but the high energy of γ-ray produced harmness to the whole body, and β-energy(Emax,0.6MeV) was low[17-22]. So scientists have searched for more effective radioisotope. Rhenium-188 is of particular interest to this study as the 188Re may be obtained from the 188W/188Re generator, and 188Re decays by β- emission with energies (Emax = 2.12MeV) similar to 90Y and γphotons (Eγ = 155keV; aboundance = 15%) that are useful for dosimetry calculations and radioimmunoimaging, with a half-time of 17h. Furthermore, 188Re has chemical properties similar to 99Tc m, thus it can be conjugated to antibodies modeling on 99Tc m labeling methods using direct or indirect method[23-29]. Direct methods require attaching the reduced form of Re to the endogenous thiols of antibodies, whereas indirect methods require the reduced Re to be complexed by a bifunctional chelator that is conjugated to the antibody[30-32]. There has been considerable interest in the direct labeling of mAb, which would result in the formation of an instant kit formulation for imaging or therapy.
188Re can be provided at reasonable costs for routine preparation of radiopharmaceuticals for cancer treatment. 188Re is an important therapeutic radioisotope which is obtained on demand as carrier-free sodium perrhentate by saline elution of the tungsten-188/rhenium-188 generator system.Because of its prominent physical characters, 188 Re will become a new therapeutic isotope.188Re is a radioisotope currently under evaluation for a variety of therapeutic application, including that for metastatic bone pain and therapy in oncology.
The HAb18 antibody is a murine IgG1 anti-hepatoma monoclonal antibody under investigation in our laboratory. It does not cross react with normal liver cells, and only rarely with other malignant tissues. Due to the smaller size, easier penetration into tumor tissues, rapid clearance from circulation, and less human anti-mouse antibody (HAMA) reaction, F(ab’)2 fragments showed that tumor localization is faster and better than the intact antibody. Previous studies of 99Tcm labeled with HAb18 F(ab’)2 indicated that the conjugate is effective to detect hepatoma in the nude mice model[33]. The results encourage us to continue the radioimmunotherapy for hepatoma using 188Re labeled with HAb18 F(ab’)2. we have studied the pharmacokinetics of 188Re-HAb18 F(ab’)2 in hepatoma-bearing nude mice in order to prove if 188Re-HAb18 F(ab’)2 was located specially in hepatoma, to establish the pharmacokinetical model and get the parameters of pharmacokinetics.
Five-week Balb/c nude mice( derived from Experimental Animals Center of our university) were implated with 1 × 107 (0.2 mL) human hepatocellular carcinoma (HCC) cells in the right thigh. When the diameter of the tumors reached 1cm, the tumor bearing mice would be investigated further.
HAb18 F(ab’)2 fragment was generated by pepsin digestion and phenyl-sepharose HP column purification with a relative molecular mass of 110000. The solution containing the antibody fragment was concentrated by lypholization and reconstituted with distilled water.
A 7.4GBq 188W/188Re generator was eluted with normal saline.
The antibody concentrated at 5 g·L-1 was reduced by reaction with a molar excess of 2-ME at 4 °C for 20-30 min. The reduced antibody was isolated from reductant through a PD-10 column (Pharmacia) equilibrated with 0.05 mol·L-1 acetate-buffered saline.
For labeling, the reduced HAb18 F(ab’)2 was mixed with glucoheptonate (GH) solution, SnCl2 solution, and 50-100 μL perihenium solution for 2-3 h at 37 °C before it was analyzed by Whatman 3MM paper chromatography which was then developed in 100 g·L- 1 trichloroacetic acid (TCA). Rª-f (distance of some composition moved/distance of extended reagent moved) values for 100 g·L-1 TCA are: mAb 0.0, 188Re-GH 0.7, and 188ReO-4 0.7. Labeled mAb was differentiated from 188Re colloid by the method of Thrall et al[33]. The same strips impregnated with 10-20 g·L-1 human serum albumin before development with 5V:2V:1V; water: ethanol: 5 mol·L-1 NH4OH(volum ratio). Colloid remained on the bottom of the strip while mAb-bound isotope migrated with the solvent front.
The in vitro immunoreactivity of the radiolabeled HAb18 F (ab’)2 was evaluated by a live cell assay[9]. Briefly, 5 × 109·L-1 HCC cells were centrifuged at 1000 r·min-1 for 5 min and washed twice with 1 g·L-1 bovine serum albumin (BSA) in PBS, then 5 serial 1: 2 dilutions were made up in 10 g·L-1 BSA in Eppendorf tubes precoated with BSA. Radiolabeled HAb18 F(ab’)2 at a mass concentration of 40 μg·L-1 in 10 g·L-1 BSA was added using a volume equal to half the volume of cell suspension. The total volume of cell-binding assay solution was 0.3 mL. After incubation for 2 h at 37 °C, the total as well as the cell-bound radioactivity were counted in a gamma counter.
Fifteen hepatoma-bearing nude mice were divided into 5 groups randomly, the mice were tail-vein injected via tail vein with 1.85MBq188Re-HAb18 F(ab’)2 in a volume of 0.1 mL and then they were sacrificed at 1, 4, 18, 24 and 48 h (3 mice at each time). Samples of tumor, heart, liver, spleen, lung, kidney, large intestine, small intestine, muscle,bone were taken and weighed carefully. In addition, the blood sample was drawn from the heart. The radioactive concentrations in tissues were calculated and expressed as percent injected dose per gram(% ID·g-1). The radioactivity of tumor/no tumor(T/NT) was also calculated.
The concentrations of blood and other organs were mounted by 3P97 software to get the parameters of pharmacokinetics and established the mode of pharmacokinetics was established.
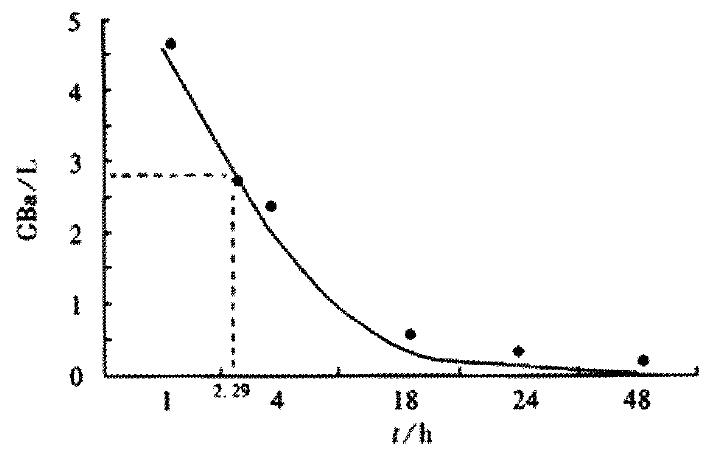
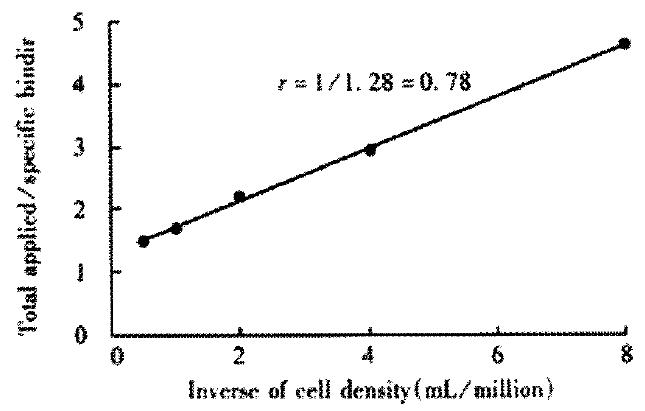
Table 1 shows the biodistribution of 188Re-HAb18 F(ab’)2. The blood concentration was measured by 3P97 software, which fits the 1-compartment model(Table 2). Figure 1 shows the curve of concentration-time in nude mice, and Table 3 shows the parameters of pharmacokinitics. The half-time(h) of each tissue was: tumor (32.99), blood (2.99), lung (5.67), bone (11.76), muscle (9.22), small intestine (7.47), large intestine (15.08), heart (2.29), liver (5.67), spleen (19.76),and kidney (11.53). Table 4 illustrates the influence of various concentrations of SnCl2 and GH on the free 188ReO-4 amounts, colloid amounts and labeling efficiency. Optimal complexation with labeling efficiency of 91.7% was achieved in 0.8 mol·L-1 GH and 2 g·L-1 SnCl2 solution. As shown in Figure 2, the immunoreactive fraction, 0.78 was determined by plotting the inverse of the bound fraction as compared with the inverse of the cell concentration, which is based on the assumption that the total antigen concentration (i.e., cell density) is a good approximation for the free antigen concentration.
| Tissue | T(post-inj)/h | 188Re-HAb18 F(ab’)2 | |
| %ID·g-1(-x±s) | T/NT ratio | ||
| Tumor | 1 | 3.01 ± 0.89 | ND |
| 4 | 3.94 ± 0.82 | ND | |
| 18 | 3.43 ± 0.28 | ND | |
| 24 | 1.96 ± 0.43 | ND | |
| 48 | 0.99 ± 0.32 | ND | |
| Blood | 1 | 4.58 ± 0.63 | 0.66 |
| 4 | 1.83 ± 0.10 | 2.15 | |
| 18 | 0.21 ± 0.04 | 16.30 | |
| 24 | 0.18 ± 0.03 | 10.90 | |
| 48 | 0.05 ± 0.01 | 19.80 | |
| Heart | 1 | 1.60 ± 0.38 | 1.88 |
| 4 | 0.80 ± 0.10 | 4.92 | |
| 18 | 0.36 ± 0.03 | 9.53 | |
| 24 | 0.30 ± 0.02 | 6.53 | |
| 48 | 0.21 ± 0.03 | 4.71 | |
| Liver | 1 | 2.07 ± 0.40 | 1.45 |
| 4 | 1.57 ± 0.31 | 2.51 | |
| 18 | 0.77 ± 0.12 | 4.45 | |
| 24 | 0.66 ± 0.10 | 2.97 | |
| 48 | 0.47 ± 0.13 | 2.11 | |
| Spleen | 1 | 1.22 ± 0.25 | 2.47 |
| 4 | 0.91 ± 0.22 | 4.33 | |
| 18 | 0.47 ± 0.07 | 7.30 | |
| 24 | 0.45 ± 0.08 | 4.36 | |
| 48 | 0.41 ± 0.10 | 2.40 | |
| Lung | 1 | 1.45 ± 0.23 | 2.08 |
| 4 | 0.86 ± 0.29 | 4.58 | |
| 18 | 0.19 ± 0.04 | 18.10 | |
| 24 | 0.18 ± 0.04 | 10.90 | |
| 48 | 0.14 ± 0.05 | 7.07 | |
| Kidney | 1 | 59.81 ± 14.52 | 0.05 |
| 4 | 47.83 ± 12.87 | 0.08 | |
| 18 | 18.72 ± 4.94 | 0.18 | |
| 24 | 15.80 ± 0.99 | 0.12 | |
| 48 | 7.31 ± 2.10 | 0.13 | |
| Large | 1 | 1.36 ± 0.38 | 2.21 |
| intestine | 4 | 0.93 ± 0.24 | 4.24 |
| 18 | 0.57 ± 0.06 | 6.02 | |
| 24 | 0.45 ± 0.00 | 4.36 | |
| 48 | 0.18 ± 0.03 | 5.50 | |
| Small | 1 | 1.61 ± 0.43 | 1.87 |
| intestine | 4 | 0.88 ± 0.29 | 4.24 |
| 18 | 0.33 ± 0.05 | 10.40 | |
| 24 | 0.29 ± 0.05 | 6.76 | |
| 48 | 0.13 ± 0.05 | 7.62 | |
| Muscle | 1 | 0.74 ± 0.29 | 4.07 |
| 4 | 0.44 ± 0.12 | 8.95 | |
| 18 | 0.19 ± 0.08 | 18.10 | |
| 24 | 0.16 ± 0.06 | 12.25 | |
| 48 | 0.05 ± 0.02 | 19.80 | |
| Bone | 1 | 1.03 ± 0.31 | 2.92 |
| 4 | 0.68 ± 0.12 | 5.79 | |
| 18 | 0.31 ± 0.09 | 11.06 | |
| 24 | 0.27 ± 0.02 | 7.26 | |
| 48 | 0.16 ± 0.02 | 6.19 | |
| REC No. | Mean No | WT | No. of camp | Weighted sum of squarers | R | R Squares | Goodness of fit | Max error C-CI | Max error % | AIC |
| 1 | 1 | 1 | 1 | 0.672E-01 | 0.9993 | 0.9955 | 0.150 | 0.18 | 100.00 | -9.499 |
| 2 | 1 | 1/c | 1 | 0.329E+00 | 0.9856 | 0.9781 | 0.331 | 0.52 | 99.1 | -1.560 |
| 3 | 1 | 1/cc | 1 | 0.119E+01 | 0.9200 | 0.9207 | 0.630 | 3.58 | 78.2 | 4.877 |
| Parameter | Unit | Value | Standard error |
| C0 | 1 × 109 Bq·L-1 | 6.18 | 3.14E-01 |
| Ke | h-1 | 0.30 | 2.88E-02 |
| Vd | 1 × 10-9 L·Bq-1 | 1.49 | |
| T1/2(Ke) | h | 2.29 | |
| AUC | 1 × 109 Bq·h·L-1 | 20.49 | |
| CL | 1 × 10-3 L·h-1 | 0.45 |
| Concentration | 188ReO-4 | Colloid | Labelingfficiency(%) | |
| aSnCl2 (g·L-1) | 8 | 0.3 | 3.6 | 90.9 |
| 4 | 0.4 | 2.8 | 90.1 | |
| 2 | 9.7 | 2.1 | 82.7 | |
| 1 | 21.8 | 1.2 | 71.2 | |
| bGH (mol·L-1) | 0.8 | 1.1 | 1.1 | 91.7 |
| 0.4 | 12.5 | 2.5 | 78.8 | |
| 0.2 | 16.6 | 2.8 | 72.7 | |
| 0.1 | 20.6 | 4.1 | 71.3 | |
The occurrence of hepatoma is high in Southeast Asia, East Africa and Middle Africa. In China, hepatoma is one of the most three common cancers related death, but there is no effective treatment[34-45]. The therapy of hepatoma includes surgical operation, chemotherapy and radiotherapy. Targeting diagnosis and therapy of hepatoma with anti-hepatoma Mab have been developed quickly,giving a hopeful prospect to hepatoma treatment. Our reaserch focuses on the targeting therapy of hepatoma[46-48]. 188Re is a generator-produced radioistope which can be obtained. There were some studies on the biodistribution and pharmacokinetics of 188Re-mAb. Safavy et al[49] have reported biodistribution of 188Re-labelded trisuccin-HuCC49 and tisuccin-C49deltaCh2 conjugates in athymic nude mice bearing intraperitoneal coloncer xenografts.188Re-labeled mAb was injected,and the mice were sacrificed 24 h postinjection. Biodistribution of the radiolabeled mAb at 24h after injection showed median tumor uptake values of 23.5%ID·g-1 and 17. 6%ID·g- 1 for the 188 Re-C49deltaCh2 and 188 Re-HuCC49, respectively. Yang et al[50] have prepared the conjugate of staphy-lococcal exterotoxin A(SEA) protein which is a bacterical Sag and the F(ab’)2 fragment of HAb18. The F(ab’)2 fragment of mAb HAb18 was prepared by papainic digestion method. The conjugate of mAb HAb18 F(ab’)2 fragment and SEA was prepared with chemical conjugating reagent N-succinimidy1-3-(2-pyirdyldithio) propionate (SPDP) and purified through chromatography column Superose 12 with FPLC system. The molecular mass was identified with SDS-PAGE assay,the antibody activity of in the conjugate was determined by indirect immunocytochemical ABC method. SEA is a protein, the method of labeling is indirect, SEA and antibody are conjugated by SPDP. 188Re’s labeling method is direct,it is more convienient and quicker than indirect method. In the animal experiment, 188Re -HAb18F (ab’)2 can inhibit the growth of tumor, but the pharmacokinetics of 188Re- HAb18F(ab’)2 in animal is seldom reported.
188Re- HAb18F(ab’)2 can last a long time at a high level (Table 1). The maximal ratio of tumor: bLood was at 48h, and maximal ratio of tumor: liver was at 18h. From Table l, we can also find that after 1, 4, and 24 h( iv) injection, the radio percent of tumor is 3.83%, 6. 48%, and 9.74%, the liver is 1.64%,2.59% and 3.19%, the kidney is 76.24%, 78.8% and 76.3% respectively, showing that the antibody and its fragments were eliminated from kidney[51-52]. The half-time of 188Re- HAb18 F(ab’)2 in the tumor was 32.99 h, it was longer in tumor than that in other organs, this indicated that 188Re- HAb18 F(ab’)2 was located in tumor, the rate of decay was low. It also showed that the mAb was specifically combined with tumor tissues and its harmness to normal tissues was low. Pharmacokinetic parameters (AUC, blood clearance, half-life, etc) were generated using the 3P97 software.
From 3P97 software, we can see the pharmacokinecs of conform to a 1-compartment model.Table 2 shows the criteria for goodness of fitting. we can judge the compartments from R squares, goodness of fit and AIC.1,1/C,1/C/C represented three weights.To the same weight,when the F test has marked significance (P < 0.05 or P < 0.01),we should choose the compartment of small AIC,and when the F test has not prominent significance (P > 0.05),we should choose the small compartment[53]. From Table 2, it can be seen that the 1-compartment model is the best. 188Re-HAb18 F(ab’)2 can distribute to the whole body instantly.The elimination rate was corresponded to the concentration of the drug. The higher the concentration was, the higher the speed of elimination was.
The half-time was 32.99 h in tumor, being much longer than that in any other organs.It showed that 188Re- HAb18 F(ab’)2 was located specifically in hepatoma and the elimination was low. It also showed the higher selective reactivity of HAb18 F(ab’)2 with hepatoma, the harmness to other organs was small. The half-time was 2.29 h in blood, and was 32.99 h in tumor,the radioation of blood can decrease more rapidly than that of the tumor. The half-time of 188Re was 17h, which was also lower than that in blood, so the 188Re can be eliminated through the blood. It has excellent value in the clinical therapy[54-62].
Carrier-free 188Re is one of βemitting radionuclides recommended for RAIT because of suitable decay characteristics and availability from 188W/188Re generator. Some methods are reported in the literature for labeling mAb with 188Re which imitate the labeling method of 99Tcm. 188Re eluted from generator will not bind to organic ligands without reduction to a lower oxidation state. We selected SnCl2 as reductant and GH as transfer ligand and stablizer to avoid Sn-or Re-collide formation. Table 1 shows that the concentration of SnCl2 and GH solutions is an important parameter to obtain good labeling results. The low percentage of free 188ReO-4 and radiocolloid shows that 0.8 mol·L-1 GH and 2 g·L-1 SnCl2 are the optimal values. Under these conditions, the labeled HAb18 F(ab’)2 keeps its immunoreactivity (Figure 2).
We believe that a variety of factors make 188Re a potential alternative to other β-emitting radionuclides for RAIT. They include an efficient generator system and the direct labeling of IgG at high specific activity. The enchanced clearance of 188Re-IgG from the circulation and the retention of immunoreactivity and tumortargeting of the Re-mAb conjugate are also important factors. In addition, the low-energy(155 keV,15%) γemission for imaging and the lack of accretion of metabolic products in nontarget tissues are important characteristics for further evaluation of 188Re-labeled antibodies for tumor therapy.
Edited by Ma JY
| 1. | Guhlke S, Beets AL, Oetjen K, Mirzadeh S, Biersack HJ, Knapp FF. Simple new method for effective concentration of 188Re solutions from alumina-based 188W-188Re generator. J Nucl Med. 2000;41:1271-1278. [PubMed] |
| 2. | Jeong JM, Lee YJ, Kim YJ, Chang YS, Lee DS, Chung JK, Song YW, Lee MC. Preparation of rhenium-188-tin colloid as a radiation synovectomy agent and comparison with rhenium-188-sulfur colloid. Appl Radiat Isot. 2000;52:851-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Kotzerke J, Glatting G, Seitz U, Rentschler M, Neumaier B, Bunjes D, Duncker C, Dohr D, Bergmann L, Reske SN. Radioimmunotherapy for the intensification of conditioning before stem cell transplantation: differences in dosimetry and biokinetics of 188Re- and 99mTc-labeled anti-NCA-95 MAbs. J Nucl Med. 2000;41:531-537. [PubMed] |
| 4. | Sykes TR, Somayaji VV, Bier S, Woo TK, Kwok CS, Snieckus V, Noujaim AA. Radiolabeling of monoclonal antibody B43.13 with rhenium-188 for immunoradiotherapy. Appl Radiat Isot. 1997;48:899-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Sharkey RM, Blumenthal RD, Behr TM, Wong GY, Haywood L, Forman D, Griffiths GL, Goldenberg DM. Selection of radioimmunoconjugates for the therapy of well-established or micrometastatic colon carcinoma. Int J Cancer. 1997;72:477-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Róka R, Séra T, Pajor L, Thurzó L, Láng J, Csernay L, Pávics L. [Clinical experience with rhenium-188 HEDP therapy for metastatic bone pain]. Orv Hetil. 2000;141:1019-1023. [PubMed] |
| 7. | Wunderlich G, Pinkert J, Andreeff M, Stintz M, Knapp FF, Kropp J, Franke WG. Preparation and biodistribution of rhenium-188 labeled albumin microspheres B 20: a promising new agent for radiotherapy. Appl Radiat Isot. 2000;52:63-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Zimmerman BE, Cessna JT, Unterweger MP, Li AN, Whiting JS, Knapp FF. A new experimental determination of the dose calibrator setting for 188Re. J Nucl Med. 1999;40:1508-1516. [PubMed] |
| 9. | Knapp FF. Rhenium-188--a generator-derived radioisotope for cancer therapy. Cancer Biother Radiopharm. 1998;13:337-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 73] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Häfeli UO, Roberts WK, Meier DS, Ciezki JP, Pauer GJ, Lee EJ, Weinhous MS. Dosimetry of a W-188/Re-188 beta line source for endovascular brachytherapy. Med Phys. 2000;27:668-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Juweid M, Sharkey RM, Swayne LC, Griffiths GL, Dunn R, Goldenberg DM. Pharmacokinetics, dosimetry and toxicity of rhenium-188-labeled anti-carcinoembryonic antigen monoclonal antibody, MN-14, in gastrointestinal cancer. J Nucl Med. 1998;39:34-42. [PubMed] |
| 12. | Junfeng Y, Duanzhi Y, Xiaofeng M, Zili G, Jiong Z, Yongxian W, Knapp FF. [188Re]Rhenium sulfide suspension: a potential radiopharmaceutical for tumor treatment following intra-tumor injection. Nucl Med Biol. 1999;26:573-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Chen J, Giblin MF, Wang N, Jurisson SS, Quinn TP. In vivo evaluation of 99mTc/188Re-labeled linear alpha-melanocyte stimulating hormone analogs for specific melanoma targeting. Nucl Med Biol. 1999;26:687-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Iznaga-Escobar N. 188Re-direct labeling of monoclonal antibodies for radioimmunotherapy of solid tumors: biodistribution, normal organ dosimetry, and toxicology. Nucl Med Biol. 1998;25:441-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Palmedo H, Guhlke S, Bender H, Sartor J, Schoeneich G, Risse J, Grünwald F, Knapp FF, Biersack HJ. Dose escalation study with rhenium-188 hydroxyethylidene diphosphonate in prostate cancer patients with osseous metastases. Eur J Nucl Med. 2000;27:123-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 102] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 16. | Meléndez-Alafort L, Ferro-Flores G, Arteaga-Murphy C, Pedraza-López M, González-Zavala MA, Tendilla JI, García-Salinas L. Labeling peptides with rhenium-188. Int J Pharm. 1999;182:165-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | van Zanten-Przybysz I, Molthoff CF, Roos JC, Plaizier MA, Visser GW, Pijpers R, Kenemans P, Verheijen RH. Radioimmunotherapy with intravenously administered 131I-labeled chimeric monoclonal antibody MOv18 in patients with ovarian cancer. J Nucl Med. 2000;41:1168-1176. [PubMed] |
| 18. | Behr TM, Béhé M, Löhr M, Sgouros G, Angerstein C, Wehrmann E, Nebendahl K, Becker W. Therapeutic advantages of Auger electron- over beta-emitting radiometals or radioiodine when conjugated to internalizing antibodies. Eur J Nucl Med. 2000;27:753-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 71] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Clarke K, Lee FT, Brechbiel MW, Smyth FE, Old LJ, Scott AM. Therapeutic efficacy of anti-Lewis(y) humanized 3S193 radioimmunotherapy in a breast cancer model: enhanced activity when combined with taxol chemotherapy. Clin Cancer Res. 2000;6:3621-3628. [PubMed] |
| 20. | Barendswaard EC, O'Donoghue JA, Larson SM, Tschmelitsch J, Welt S, Finn RD, Humm JL. 131I radioimmunotherapy and fractionated external beam radiotherapy: comparative effectiveness in a human tumor xenograft. J Nucl Med. 1999;40:1764-1768. [PubMed] |
| 21. | Smith-Jones PM, Vallabahajosula S, Goldsmith SJ, Navarro V, Hunter CJ, Bastidas D, Bander NH. In vitro characterization of radiolabeled monoclonal antibodies specific for the extracellular domain of prostate-specific membrane antigen. Cancer Res. 2000;60:5237-5243. [PubMed] |
| 22. | Juweid ME, Zhang CH, Blumenthal RD, Hajjar G, Sharkey RM, Goldenberg DM. Prediction of hematologic toxicity after radioimmunotherapy with (131)I-labeled anticarcinoembryonic antigen monoclonal antibodies. J Nucl Med. 1999;40:1609-1616. [PubMed] |
| 23. | Hnatowich DJ, Mardirossian G, Rusckowski M, Fogarasi M, Virzi F, Winnard P. Directly and indirectly technetium-99m-labeled antibodies--a comparison of in vitro and animal in vivo properties. J Nucl Med. 1993;34:109-119. [PubMed] |
| 24. | Colnot DR, Quak JJ, Roos JC, van Lingen A, Wilhelm AJ, van Kamp GJ, Huijgens PC, Snow GB, van Dongen GA. Phase I therapy study of 186Re-labeled chimeric monoclonal antibody U36 in patients with squamous cell carcinoma of the head and neck. J Nucl Med. 2000;41:1999-2010. [PubMed] |
| 25. | Lechner P, Lind P, Snyder M, Haushofer H. Probe-guided surgery for colorectal cancer. Recent Results Cancer Res. 2000;157:273-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Willkomm P, Bender H, Bangard M, Decker P, Grünwald F, Biersack HJ. FDG PET and immunoscintigraphy with 99mTc-labeled antibody fragments for detection of the recurrence of colorectal carcinoma. J Nucl Med. 2000;41:1657-1663. [PubMed] |
| 27. | Line BR, Weber PB, Lukasiewicz R, Dansereau RN. Reduction of background activity through radiolabeling of antifibrin Fab' with 99mTc-dextran. J Nucl Med. 2000;41:1264-1270. [PubMed] |
| 28. | Amato R, Kim EE, Prow D, Andreopoulos D, Kasi LP. Radioimmunodetection of residual, recurrent or metastatic germ cell tumors using technetium-99 anti-(alpha-fetoprotein) Fab' fragment. J Cancer Res Clin Oncol. 2000;126:161-167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 29. | Bian HJ, Chen ZN, Deng JL. Direct technetium-99m labeling of anti-hepatoma monoclonal antibody fragment: a radioimmunoconjugate for hepatocellular carcinoma imaging. World J Gastroenterol. 2000;6:348-352. [PubMed] |
| 30. | Dadachova E, Chapman J. 188Re(V)-DMSA revisited: preparation and biodistribution of a potential radiotherapeutic agent with low kidney uptake. Nucl Med Commun. 1998;19:173-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 31. | Schmidt PF, Smith SV, Bundesen PG. 188Re DD-3B6/22 Fab' for use in therapy of ovarian cancer: labelling and animal studies. Nucl Med Biol. 1998;25:639-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 32. | Ferro-Flores G, Pimentel-González G, González-Zavala MA, Arteaga de Murphy C, Meléndez-Alafort L, Tendilla JI, Croft BY. Preparation, biodistribution, and dosimetry of 188Re-labeled MoAb ior cea1 and its F(ab')2 fragments by avidin-biotin strategy. Nucl Med Biol. 1999;26:57-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 33. | Thrall JH, Freitas JE, Swanson D, Rogers WL, Clare JM, Brown ML, Pitt B. Clinical comparison of cardiac blood pool visualization with technetium-99m red blood cells labeled in vivo and with technetium-99m human serum albumin. J Nucl Med. 1978;19:796-803. [PubMed] |
| 34. | Qian SB, Li Y, Qian GX, Chen SS. Efficient tumor regression induced by genetically engineered tumor cells secreting interleukin-2 and membrane-expressing allogeneic MHC class I antigen. J Cancer Res Clin Oncol. 2001;127:27-33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 35. | Dai WJ, Jiang HC. Advances in gene therapy of liver cirrhosis: a review. World J Gastroenterol. 2001;7:1-8. [PubMed] |
| 36. | Cheng ML, Wu YY, Huang KF, Luo TY, Ding YS, Lu YY, Liu RC, Wu J. Clinical study on the treatment of liver fibrosis due to hepatitis B by IFN-alpha(1) and traditional medicine preparation. World J Gastroenterol. 1999;5:267-269. [PubMed] |
| 37. | Kessel D, Caruso JA, Reiners JJ. Potentiation of photodynamic therapy by ursodeoxycholic acid. Cancer Res. 2000;60:6985-6988. [PubMed] |
| 38. | Yang SS, Wu CH, Chen TH, Huang YY, Huang CS. TT viral infection through blood transfusion: retrospective investigation on patients in a prospective study of post-transfusion hepatitis. World J Gastroenterol. 2000;6:70-73. [PubMed] |
| 39. | Kang MA, Kim KY, Seol JY, Kim KC, Nam MJ. The growth inhibition of hepatoma by gene transfer of antisense vascular endothelial growth factor. J Gene Med. 2000;2:289-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 40. | Ma XD, Sui YF, Wang WL. Expression of gap junction genes connexin 32, connexin 43 and their proteins in hepatocellular carcinoma and normal liver tissues. World J Gastroenterol. 2000;6:66-69. [PubMed] |
| 41. | Huang ZH, Zhuang H, Lu S, Guo RH, Xu GM, Cai J, Zhu WF. Humoral and cellular immunogenecity of DNA vaccine based on hepatitis B core gene in rhesus monkeys. World J Gastroenterol. 2001;7:102-106. [PubMed] |
| 42. | Fang JN, Jin CJ, Cui LH, Quan ZY, Choi BY, Ki M, Park HB. A comparative study on serologic profiles of virus hepatitis B. World J Gastroenterol. 2001;7:107-110. [PubMed] |
| 43. | Tietze MK, Wuestefeld T, Paul Y, Zender L, Trautwein C, Manns MP, Kubicka S. IkappaBalpha gene therapy in tumor necrosis factor-alpha- and chemotherapy-mediated apoptosis of hepatocellular carcinomas. Cancer Gene Ther. 2000;7:1315-1323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 44. | Xu J, Mei MH, Zeng SE, Shi QF, Liu YM, Qin LL. Expressions of ICAM-1 and its mRNA in sera and tissues of patients with hepatocellular carcinoma. World J Gastroenterol. 2001;7:120-125. [PubMed] |
| 45. | Hu YP, Hu WJ, Zheng WC, Li JX, Dai DS, Wang XM, Zhang SZ, Yu HY, Sun W, Hao GR. Establishment of transgenic mouse harboring hepatitis B virus (adr subtype) genomes. World J Gastroenterol. 2001;7:111-114. [PubMed] |
| 46. | Qiu K, Wang BC, Chen ZN, Fang P, Liu CG, Wan WX, Liu YF. 99mTc-labeled HAb18 McAb Fab fragment for radioimmunoimaging in nude mice bearing human hepatocellular carcinoma. World J Gastroenterol. 1998;4:117-120. [PubMed] |
| 47. | Chen ZN, Bian HJ, Jiang JL. Kecent progress inanti-hepatoma mono-clonal antibody and its application. Huaren Xiaohua Zazhi. 1998;6:461-462. |
| 48. | Dickman S. Antibodies stage a comeback in cancer treatment. Science. 1998;280:1196-1197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 49. | Safavy A, Khazaeli MB, Safavy K, Mayo MS, Buchsbaum DJ. Biodistribution study of 188Re-labeled trisuccin-HuCC49 and trisuccin-HuCC49deltaCh2 conjugates in athymic nude mice bearing intraperitoneal colon cancer xenografts. Clin Cancer Res. 1999;5:2994s-3000s. [PubMed] |
| 50. | Yang LJ, Sui YF, Chen ZN. Preparation and activity of conjugate of monoclonal antibody HAb18 against hepatoma F(ab')(2) fragment and staphylococcal enterotoxin A. World J Gastroenterol. 2001;7:216-221. [PubMed] |
| 51. | Duan XD, Chen ZN, Bian HJ, Wen AD, Feng Q. Pharmacokinetics of technetium-99m labeled anti-hepatoma monoclonal antibody HAb18 and it's F(ab')2 fragment in mice. Zhongguo Yaoxue Zazhi. 2000;35:465-467. |
| 52. | Chen H, Gu SF, Xiao Zh, Zeng FD, Wu WZ, Zhang QH. Pharmaco-kinetics and bioavailability of sustained release capsules of nicardipine hydrochloride in healthy volunteers. Z. hongguo Yaolixue Tongbao. 2000;16:107-110. |
| 53. | Lan Q, Huang Q, Zhuang DL, Wu YF, Sun ZF. Study on the pharma-cokinetics of immunoradiotherapeutic agent S-MAb SZ39 in glioma-bearing nude mice. Zhongguo Yaoxue Zazhi. 1999;34:683-686. |
| 54. | Hosono MN, Hosono M, Zamora PO, Guhlke S, Haberberger T, Bender H, Knapp FF, Biersack HJ. Localization of colorectal carcinoma by rhenium-188-labeled B72.3 antibody in xenografted mice. Ann Nucl Med. 1998;12:83-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 55. | Gestin JF, Loussouarn A, Bardiès M, Gautherot E, Gruaz-Guyon A, Saï-Maurel C, Barbet J, Curtet C, Chatal JF, Faivre-Chauvet A. Two-step targeting of xenografted colon carcinoma using a bispecific antibody and 188Re-labeled bivalent hapten: biodistribution and dosimetry studies. J Nucl Med. 2001;42:146-153. [PubMed] |
| 56. | Hosono MN, Hosono M, Mishra AK, Faivre-Chauvet A, Gautherot E, Barbet J, Knapp FF, Chatal JF. Rhenium-188-labeled anti-neural cell adhesion molecule antibodies with 2-iminothiolane modification for targeting small-cell lung cancer. Ann Nucl Med. 2000;14:173-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 57. | Bian HJ, Chen ZN, Lou C, Mi L, Wang J, Yue XL. 188 Re-labeled HAb18 F(ab')2 of hepatoma radioimmunoimaging. Zhongliu. 2000;20:181-183. |
| 58. | Qingnuan L, Xiaodong Z, Rong S, Wenxin L. Preparation of (188Re) Re-AEDP and its biodistribution studies. Appl Radiat Isot. 2000;53:993-997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 59. | Kostarelos K, Emfietzoglou D. Tissue dosimetry of liposome-radionuclide complexes for internal radiotherapy: toward liposome-targeted therapeutic radiopharmaceuticals. Anticancer Res. 2000;20:3339-3345. [PubMed] |
| 60. | Iznaga-Escobar N, Torres LA, Morales A, Ramos M, Alvarez I, Pérez N, Fraxedas R, Rodríguez O, Rodríguez N, Pérez R. Technetium-99m-labeled anti-EGF-receptor antibody in patients with tumor of epithelial origin: I. Biodistribution and dosimetry for radioimmunotherapy. J Nucl Med. 1998;39:15-23. [PubMed] |
| 61. | Blower PJ, Kettle AG, O'Doherty MJ, Coakley AJ, Knapp FF. (99m)Tc(V)DMSA quantitatively predicts (188)Re(V)DMSA distribution in patients with prostate cancer metastatic to bone. Eur J Nucl Med. 2000;27:1405-1409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 62. | Heppeler A, Froidevaux S, Eberle AN, Maecke HR. Receptor targeting for tumor localisation and therapy with radiopeptides. Curr Med Chem. 2000;7:971-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 172] [Article Influence: 6.9] [Reference Citation Analysis (0)] |