Published online Jun 15, 2001. doi: 10.3748/wjg.v7.i3.357
Revised: April 3, 2001
Accepted: April 12, 2001
Published online: June 15, 2001
AIM: To evaluate if the administration of an enteral diet supplemented with glutamine, arginine and ω-3 fatty acids modulates inflammatory and immune responses after surgery.
METHODS: A prospective randomized double-blind, clinical trial was performed. Forty-eight patients with gastrointestinal cancer were randomized into two groups, one group was given an isocaloric and isonitrogenous standard diet and the other was fed with the supplemented diet with glutamine, arginine and ω-3 fatty acids. Feedings were started within 48 h after operation, and continued until day 8. All variables were measured before operation and on postoperative day 1 and 8. Immune responses were determined by phagocytosis ability, respiratory burst of polymorphonuclear cells, total lymphocytes lymphocyte subsets, nitric oxide, cytokines concentration, and inflammatory responses by plasma levels of C-reactive protein, prostaglandin E2 level.
RESULTS: Tolerance of both formula diets was excellent. There were significant differences in the immunological and inflammatory responses between the two groups. In supplemented group, phagocytosis and respiratory burst after surgery was higher and C-reactive protein level was lower (P < 0.01) than in the standard group. The supplemented group had higher levels of nitric oxide, total lymphocytes, T lymphocytes, T-helper cells, and NK cells. Postoperative levels of IL-6 and TNF-α were lower in the supplemented group (P < 0.05).
CONCLUSION: It was clearly established in this trial that early postoperative enteral feeding is safe in patients who have undergone major operations for gastrointestinal cancer. Supplementation of enteral nutrition with glutamine, arginine, and ω-3 fatty acids positively modulated postsurgical immunosuppressive and inflammatory responses.
- Citation: Wu GH, Zhang YW, Wu ZH. Modulation of postoperative immune and inflammatory response by immune-enhancing enteral diet in gastrointestinal cancer patients. World J Gastroenterol 2001; 7(3): 357-362
- URL: https://www.wjgnet.com/1007-9327/full/v7/i3/357.htm
- DOI: https://dx.doi.org/10.3748/wjg.v7.i3.357
Patients with gastrointestinal cancer frequently suffer from protein-calorie malnutrition. This is due, largely to a systemic anorexia in conjunction with varying degrees of local anorexia in response to obstructive symptoms. In addition, the metabolic response to injury that is seen in these patients postoperatively produces a redistribution of endogenous macronutrients, causing further depletion of body fat stores and lean body mass. If the injury response persists and nutritional support is not instituted, pre-existing malnutrition will be exacerbated and the risk of postoperative complications as well as morbidity and mortality will be increased. It is well known that major surgery and other types of trauma are associated with severe alterations of the host defense mechanisms, making the patients highly susceptible to septic and inflammatory complications. Nutritional support after injury may modulate the immune, inflammatory, and metabolic responses, and the clinical outcome of critically ill subjects. In different experimental and clinical settings, enteral vs parenteral route, early vs delayed enteral feeding, and immune-enhancing enteral vs standard diets are related to better results[1-3]. Supplementation of enteral diets with glutamine, arginine and ω-3 fatty acids has been reported to improve clinical outcome after major operations and to reduce the risk of septic complications above and beyond the role of standard enteral formula[4].
The purpose of this study is to compare the effect of early enteral nutrition with immune enhancing enteral diet with standard diets on postoperative immune, inflammatory and metabolic response in a group of cancer patients. The endpoints of the study were the evaluation of the immune response by polymorphonucler (PMN) cell respiratory burst and phagocytosis ability, total lymphocytes, lymphocyte subsets, nitric oxide (NO), and cytokines concentrations, the inflammatory response by C-reactive protein (CRP), α1-antitrypsin, fibrinogen levels, and prostaglandin E2 level.
In this prospective, randomized, and blinded study, 48 adult patients, aged between 34 and 75 years, with gastrointestinal malignancies, who were scheduled for major abdominal surgery due to their cancer, were included in the study. Exclusion criteria were clinical relevant alterations of the pulmonary, cardiovascular, renal, intestinal or hepatic function; history of recent immunosuppressive therapy (including preoperative radiochemotherapy) or immunological disease; ongoing infection; intestinal obstruction; or emergency surgery. The comparability of the groups is shown in Table 1.
| Characteristics | Supplemented (n = 25) | Control (n = 23) |
| Age/y | 55.2 ± 12.1 | 52.6 ± 9.8 |
| M/F | 16/9 | 15/8 |
| Site of cancer | ||
| Stomach | 14 | 13 |
| Colorectum | 8 | 7 |
| Pancreas | 3 | 3 |
| Type of operation | ||
| Gastrectomy (subtotal or total) | 14 | 13 |
| Miles or Dixon procedure | 8 | 7 |
| Pancreatoduodenectomy | 3 | 3 |
| Time of surgery/min | 220 ± 45 | 245 ± 60 |
| Operative blood loss/mL | 400 ± 325 | 450 ± 400 |
| Transfused patients | 22 | 20 |
Patients were randomized to receive either an immune-enhancing enteral diets, Stresson (Nutricia; n = 25) or a standard diets, Nutrison (Nutricia; n = 23) supplemented with protein power to make the diets isonitrogenous. Table 2 summarizes the nutrient content of these diets. Enteral feeding was started within 48 h of operation via a needle catheter jejunostomy, or a nasoenteric tube, and was delivered by continuous pump infusion. The diets were started at half strength 50 mL·h-1, and all patients reached their nutritional goals by 72 h after the initiation of enteral nutrition. The goals of nutritional delivery in the study included 146 kJ·kg-1·d-1 and protein 2.2 g·kg-1·d-1. Patients had to receive enteral nutrition for a minimum of 7 days to be included in the study. All patients received intravenous fluid (50 g·L-1 dextrose and normal saline solution) and other electrolytes as indicated.
| Variables | Composition of formula (per 100 mL) | |
| Supplemented | Control | |
| Calories (kcal) | 100 | 125 |
| Protein (g) | 4.00 | 7.50 |
| Glutamine | 0.40 | 1.30 |
| Arginine | 0.16 | 0.89 |
| Fat (g) | 3.89 | 4.17 |
| LCT | 3.89 | 2.45 |
| MCT | 0.00 | 1.72 |
| EPA | 0.00 | 0.079 |
| DHA | 0.00 | 0.030 |
| n6:n3 | 5:1 | 3.45:1 |
| β carotene (mg) | - | 0.40 (66.70 μg RE) |
| Vit. E (mg TE) | 0.81 | 4.92 |
| Vit. C (mg) | 5.00 | 13.30 |
| Osmolarity (mOsm/L) | 250 | 380 |
During the postoperative period, patients were evaluated prospectively for a number of symptoms: nausea, vomiting, abdominal cramping, abdominal distention, and diarrhea. Advanced the tube feeds or adjust the rates as the patients tolerate.
On admission, the following baseline variables were determined in all patients: plasma levels of glutamine, arginine, CRP, α1-antitrypsin, and fibrinogen. polymorphyonuclear (PMN) cell function, nitric oxide (NO), lymphocyte subsets (CD3, CD4 and CD8), natural killer cells, circulating cytokine level (IL-1, IL-2, IL-6 and TNF-α) and prostaglandin E-2 (PGE-2) level were also determined. Baseline variables were reassessed 1 day and 8 days after surgery.
PMNs were separated on a sodium metrizoate and methylecellulose column by the method of Boyum and suspended in pH-adjusted (7.2-7.4) Eagle's medium at a density of 5 × 109 cells·L-1. The oxidative metabolism of PMNs was assayed by measuring the superoxide production with nitroblue tetrazolium (NBT) red uction test[5]. The PMNs ability to phagocytose zymosan particle was detected as described by Brain. The degree of phagocytosis was expressed as percentage of PMNs in which zymosan particles were detected over a hundred PMNs. The production of NO by macrophages was measured by the Greiss reaction. This is an indirect colorimetric assay of NO. Greiss reaction consists of measurement of stable end breakdown products of NO such as nitrite which are considered to be reliable markers for NO formation.
The cytokines (IL-1, IL-2, IL-6, TNF-α) concentration in the supernatant were measured using commercially available ELISA kits. Total lymphocyte and lymphocyte subset were assayed by flow cytometry.
Prostaglandin E2 (PGE2) level in the supernatants were measured using the PGE2125I-scintillation proximity assay system. Plasma levels of CRP, α-1 antitrypsin, and fibrinogen were measured with standard techniques.
Data were presented as the -x±s and analyzed by analysis of variance (ANOVA) using Sigmastat software (Jandel Scientific, San Rafael, CA, USA). Statistical significance was predetermined as P < 0.05.
The preoperative diagnoses and operations were similar between the two groups. At the time of entry into the study, there were no significant differences in nutritional state or type of disease, the age ranges were similar in both groups. There were no significant differences between groups in mean age, usual body weight, mean duration of operation, or the number of patients who received transfusions during the procedure or postoperatively (Table 1). Tolerance of both formula diets was excellent and there were no differences between the groups. No patient was withdrawn because of intolerance. Three patients in immune enhancing enteral diet group and two patients in the standard diet group developed diarrhoea.
Plasma arginine and glutamine levels are shown in Table 3, at baseline, there were no significant differences between the groups, but decreased 1 d after operation in both groups. After 7 d feeding, the study group had a higher plasma levels of arginine and glutamine, but, in the control group, no variation was observed. As expected, plasma levels of PGE2, CRP, α1-antitrypsin, and fibrinogen were increased after operation in both groups. In patients receiving immune enhancing enteral diet, the levels of CRP were significantly lower than in the standard diet group over the 7 d feeding. However, the levels of PGE2, α1-antitrypsin and fibrinogen had no significant differences between the groups at the end of study (Table 3).
| Variables | Period | Supplemented | Control | P |
| GLN (nm/mL) | Baseline | 558.7 ± 62.5 | 584.1 ± 51.5 | NS |
| D 1 | 386.0 ± 74.6 | 412.4 ± 65.3 | NS | |
| D 8 | 722.4 ± 116.5 | 555.0 ± 72.2 | < 0.05 | |
| ARG (nm/mL) | Baseline | 62.7 ± 21.0 | 58.5 ± 23.1 | NS |
| D 1 | 56.2 ± 18.6 | 50.9 ± 20.0 | NS | |
| D 8 | 115.4 ± 47.2 | 66.7 ± 31.1 | < 0.001 | |
| PGE2 (pg/mL) | Baseline | 62.2 ± 26.4 | 73.7 ± 42.1 | NS |
| D 1 | 167.7 ± 73.8 | 184.4 ± 91.3 | NS | |
| D 8 | 85.5 ± 53.0 | 112.6 ± 84.0 | NS | |
| CRP (g/L) | Baseline | 0.4 ± 0.1 | 0.3 ± 0.1 | NS |
| D 1 | 4.8 ± 2.3 | 4.6 ± 1.8 | NS | |
| D 8 | 2.0 ± 1.1 | 3.9 ± 2.3 | < 0.05 | |
| α1-antitrypsin (g/L) | Baseline | 15.5 ± 0.2 | 13.2 ± 0.2 | NS |
| D 1 | 42.0 ± 3.4 | 40.9 ± 4.7 | NS | |
| D 8 | 28.2 ± 2.8 | 30.0 ± 3.1 | NS | |
| Fibrinogen | Baseline | 32.4 ± 3.3 | 30.6 ± 3.1 | NS |
| D 1 | 36.8 ± 4.2 | 35.5 ± 4.4 | NS | |
| D 8 | 34.5 ± 3.5 | 35.0 ± 3.9 | NS |
Total lymphocyte and lymphocyte subset analyses are depicted in Table 4. At baseline, the study and control groups did not have significant numbers of total lymphocytes, T lymphocytes (CD3), T helper (CD4), T suppressors (CD8) or natural killer cells. A significant postoperative decrease in total lymphocyte, CD3, CD4 and natural killer cells were observed in both groups, but they recovered after 7 days feeding in the supplemented group. No significant difference was noted in CD8 cell numbers.
| Variables | Period | Supplemented | Control | P |
| Total lymphocytes No. cells × 109/cm3 | Baseline | 1.62 ± 0.41 | 1.73 ± 0.38 | NS |
| D 1 | 1.12 ± 0.39 | 1.55 ± 0.33 | NS | |
| D 8 | 1.55 ± 0.33 | 1.18 ± 0.35 | < 0.05 | |
| CD3 (%) | Baseline | 72.2 ± 11.1 | 69.0 ± 9.8 | NS |
| D 1 | 60.1 ± 8.8 | 57.7 ± 10.3 | NS | |
| D 8 | 76.4 ± 14.5 | 60.3 ± 9.3 | < 0.05 | |
| CD4 (%) | Baseline | 45.0 ± 8.5 | 42.5 ± 9.2 | NS |
| D 1 | 38.2 ± 9.4 | 37.7 ± 9.0 | NS | |
| D 8 | 57.5 ± 7.8 | 40.0 ± 8.6 | < 0.05 | |
| CD8 (%) | Baseline | 23.3 ± 7.4 | 24.5 ± 7.7 | NS |
| D 1 | 22.7 ± 8.0 | 22.2 ± 8.0 | NS | |
| D 8 | 19.6 ± 6.6 | 23.1 ± 5.9 | < 0.05 | |
| CD4/CD8 | Baseline | 1.9 ± 0.8 | 1.7 ± 0.7 | NS |
| D 1 | 1.7 ± 1.2 | 1.7 ± 0.9 | NS | |
| D 8 | 2.9 ± 1.4 | 1.7 ± 0.8 | < 0.01 | |
| NK cells (%) | Baseline | 17.7 ± 7.1 | 18.3 ± 6.9 | NS |
| D 1 | 11.1 ± 8.2 | 10.2 ± 7.2 | NS | |
| D 8 | 16.6 ± 5.4 | 11.1 ± 4.3 | < 0.05 |
Circulating cytokine levels of IL-1, IL-2, IL-6 and TNF-α are displayed in Table 5. At baseline, there were no significant differences between groups. There was a significant postoperative decrease in all cytokines. After 7 d of feeding, the absolute levels of IL-1 and IL-2 as well as the changes from baseline were not different. However, IL-6 and TNF-α concentrations were significantly lower in the group given immune enhancing enteral diet on postoperative d8.
| Variables | Period | Supplemented | Control | P |
| IL-1 | Baseline | 2520 ± 566 | 2480 ± 502 | NS |
| D 1 | 1375 ± 304 | 1528 ± 312 | NS | |
| D 8 | 2020 ± 385 | 2155 ± 450 | NS | |
| IL-2 | Baseline | 1255 ± 226 | 1310 ± 252 | NS |
| D 1 | 892 ± 173 | 900 ± 186 | NS | |
| D 8 | 990 ± 201 | 1156 ± 192 | NS | |
| IL-6 | Baseline | 405 ± 141 | 432 ± 128 | NS |
| D 1 | 365 ± 110 | 389 ± 143 | NS | |
| D 8 | 277 ± 106 | 762 ± 199 | < 0.001 | |
| TNF-α | Baseline | 260 ± 96 | 236 ± 101 | NS |
| D 1 | 178 ± 85 | 159 ± 93 | NS | |
| D 8 | 202 ± 104 | 345 ± 133 | < 0.050 |
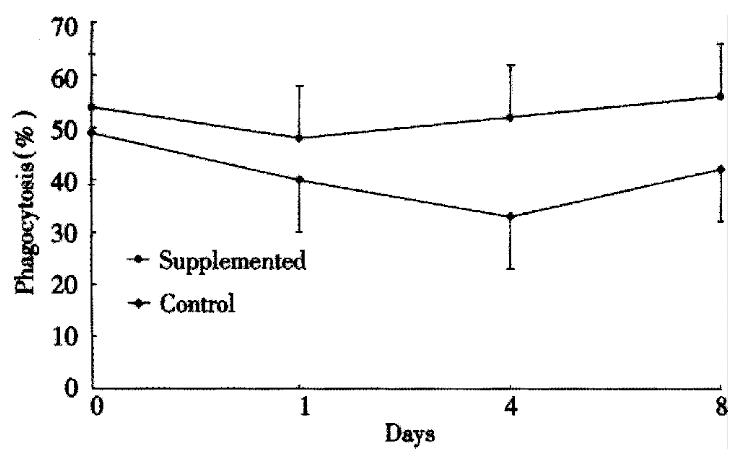
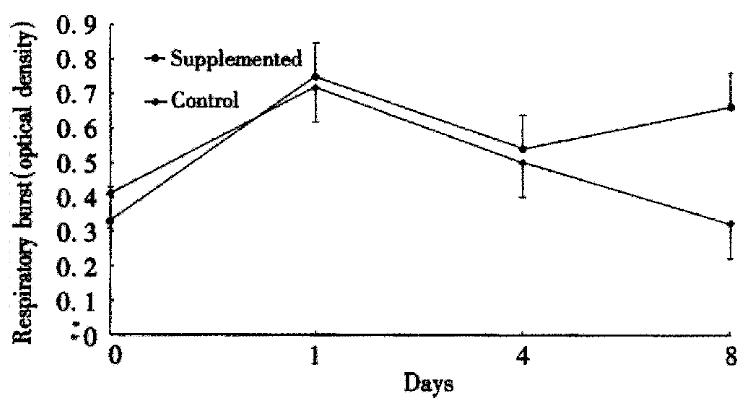
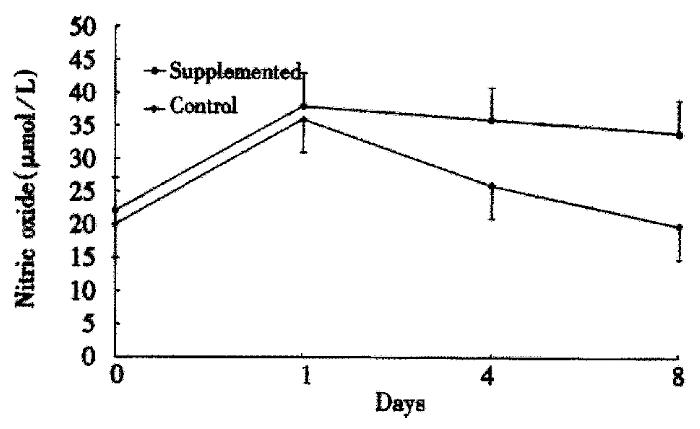
The ability of PMNs to phagocytose zymosan particles is shown in Figure 1. Both groups had a similar baseline phagocytosis ability. A significant decrease in phagocytosis was observed after surgery in the control group. Conversely, phagocytosis ability remained similar to the preoperative values in the study group. The PMN oxidative metabolism is shown in Figure 2. At baseline, there are no significant differences between the groups. A sharp postoperative increase of the oxidative metabolism compared with preoperative values was observed in both groups, the levels still remained higher in the study group than the control group (P < 0.05). Figure 3 gives the variations of NO. The circulating levels of NO increased after operation in both groups, but, the study group had significantly higher NO levels than control group postoper atively (P < 0.05).
Malnutrition in patients with cancer has been shown to have increased risk of postoperative complications. Effects to reduce the impact of malnutrition initially focused on the use of parenteral and enteral nutrition for perioperative nutritional support. Enteral feeding has gained popularity for the nutritional support of surgical patients. Biologically, there have been several reasons reported including better substrate utilization, prevention of mucosal atrophy, preservation of normal gut flora, gut integrity, and immune competence. Early enteral vs parenteral feeding in traumatized and surgical patients is gaining wide consensus after the promising results showing good tolerance and notable reduction of septic morbidity[1,6-9]. Our data confirm that early postoprative enteral feeding may be carried out safely in patients who have undergone major operations for cancer. Gastrointestinal side effects were minimal and were similar in both groups. Though diarrhoea, bloating, and abdominal cramps occurred, there were no major gastrointestinal complications in either group.
Current investigations in enteral nutrition have focused on the ability to modulate the metabolic and immune response to injury via specially formulated enteral diets. Specific nutrients shown to have the potential to modulate immune function include glutamine, arginine, and ω-3 fatty acids as well as nucleotides. These specialized diets have generally been termed "immune-enhancing diets" (IEDs). A number of recent studies have compa red IEDs with standard enteral diet in the management of patients with trauma, critically illness and cancer[3,10,11]. Although evidence appears to be accumulating in support of some clinical benefits for IEDs, the specialized diets used in most of these studies generally delivered more nitrogen and often more calories than the control "standard" diet, thus making it difficult to conclude that the beneficial effects noted were due to the special nutrient supplements. The current study was intentionally designed such that both groups of patients received isocaloric and isonitrogenous diets, and differed only in their glutamine and arginine content, fatty acid compositi on, and the levels of the antioxidant micronutrients vitamin C, E and carotene. Our purpose is to examine the influence exerted by the dietary supplements on postoperative immune, inflammatory and metabolic response in a group of cancer patients.
Both cellular and humoral immune function are compromised in patients undergoing operations for cancer. The immune suppression is a common phenomenon and seems to be related to both postoperative outcome and to disease-free survival. There are many reports about the use of enteral nutrition during the early posto perative period to ameliorate immune dysfunction induced by the tumor and operation[12]. Certain nutrients such as glutamine, arginine, and ω-3 fatty acids as well as nucleotides, may act pharmacologically on the immune system. It has been suggested that these nutrients may improve host immune defences[13-16]. Our data show that plasma glutamine and arginine levels were decreased 1 d after operation in both groups. It is likely that glutamine uptake in certain tissues increase, and glutamine utilization may exceed endogenous glutamine production after injury. It is indicated that glutamine supplementation upregulates human immune cell number and function. Glutamine is critical for human lymphocyte proliferation in in vitro system. Our study demonstrated that enteral glutamine supplement ation could increase plasma glutamine concentrations, and might improve immune function.
Arginine is a semiessential amino acid which may become essential in catabolic states. Supplementation with arginine improved wound healing and enhanced immune function in animals by decreasing the T-cell dysfunction associated with injury. Additionally, arginine is a precursor for nitrates, nitrites, and nitric oxide. Nitric oxide is particularly important as a vasodilator but also participates in immunologic reactions, including the ability of macrophages to kill tumor cells and bacteria, and it may inhibit the development of precursor cytoloytic T-cells. In the present study, the increased NO levels observed 1 and 8 days after operation in the supplemented group may be a mechanism involved in the enhanced immune response early after surgery as shown by the improved phagocytosis ability and respiratory burst of PMNs.
Cytokines play a vital and integral role in both cellular and humoral immunity. The different mechanisms of action of the cytokines can be summarised as a coord inated immune response of the organism to exogenous or endogenous stimuli such as cancer, infection, injury, and operation. Measurement of cytokine concentrations may, therefore, give information about the immune response in patients after major operations. In this study, the mean IL-1 and IL-2 concentrations were only a slight but not significant difference between the two groups until the postoperative d8. The IL-6 and TNF-α concentrations were similar before operation and the first postoperative day in both groups, and they both showed a decline in IL-6 and TNF-α concentrations on the first postoperative day. Subsequently, IL-6 and TNF-α concentrations exceeded preoperative levels in the group that received the standard diet and kept rising as compared with the group given the supplemented diet, whose IL-6 and TNF-α concentrations remained below the preoperative values. As both IL-6 and TNF-α are important mediators of the acute phase reaction, it seems that systemic inflammatory responses were reduced in the group given the supplemented diet.
The acute phase reaction is a systemic inflammatory reaction to injury, including fever, tachycardia, leucocytosis, and changes in circulating protein concentrations. It has been proposed that injury promotes the switching of protein synthesis from constitutive to acute phase proteins through the release of proinflammatory cytokines such as IL-6 and TNF-α. In the present study, CRP levels significantly increased 1 d after operation in both groups, but, this increase in patients receiving the supplemented diet was significantly lower than in the standard diet group after 7 d feeding. A reduction in CRP concentrations may therefore be at least partly responsible for the lower postoperative levels of IL-6 and TNF-α in the group given the supplemented diet. ω-3 fatty acids, e.g., present in fish oils or canola oil, can replace arachidonic acid, which is derived from ω-6 fatty acids, in cell membranes and have modulating effects on immune function. Because the arachidonic acid metabolite PGE2 is known to inhibit the function of many immune cells, decreasing its generation by supplementing diets with ω-3 fatty acids would theoretically result in a relative enhancement of the immune response. Our data demonstrated that macrophage PGE2 production increased after operation in both groups. However, there was no trend toward significance between the supplemented and standard diet groups on the d8 after operation, and it was an unexpected finding. Many of the immunosuppressive effects of the ω-6 fatty acids are found to be related to the immunosupp ressive prostaglandins, particularly PGE2. It has been shown in previous studies that increasing the ratio ω-3 fatty acids in the diet effectively alters the monocyte/macrophage lipid membrane, decreasing the production of PGE2. This decrease in immune inhibition thus should potentiate immune function. The normal to increased level of PGE2 seen in the monocytes from supplemented diet group represents several alternatives. It is likely that the nutritional fat source used in the present study was not an effective source of ω-3 fatty acids. The duration of feeding might be insufficient to induce the changes in cell membrane fatty acids necessary to alter PGE2 production. Alternatively, arginine or other contents of the supplemented diet may differentially affect PGE2 production. Higher PGE2 levels may be a counter-regulatory phenomenon that results from an autocrine effect of the higher levels of cytokines produced by these monocytes.
The heterogeneity of T lymphocytes responsible for cell-mediated im munity is well known. There are two major types of T-cells; CD4 cells recognize antigens associated with major histocompatibility complex (MHC) class II molecules, whereas CD8 suppressor cells recognize antigens associated with MHC class I molecules. CD4 T-cells play an important role in immunoregulation, and CD8 cells provide an inhibitory function, suppressing cell-mediated immune responses. In the present study, supplemented diet improved immune response in surgical patients as demonstrated by increase in peripheral mature total lymphoc ytes, T lymphocytes, T-helper cells, and NK cells. Alterations in the number and ratio of the various T-cell subsets may subsequently influence the effectiveness of the overall immune response.
Edited by Ma JY
| 1. | Moore FA, Feliciano DV, Andrassy RJ, Mcardle AH, Booth FVM, Morgenstein-Wagner TB, Kellum JM, Welling RE, Moore EE. Early enteral feeding, compared with parenteral, reduces postoperative septic complications. Ann Surg. 1992;216:172-183. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1015] [Cited by in RCA: 913] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 2. | Kenler AS, Swails WS, Driscoll DF, DeMichele SJ, Daley B, Babineau TJ, Peterson MB, Bistrian BR. Early enteral feeding in postsurgical cancer patients. Ann Surg. 1996;223:316-333. [RCA] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 99] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 3. | Kudsk KA, Minard G, Croce MA, Brown RO, Lowrey TS, Pritchard FE, Dickerson RN, Fabian TC. A randomized trial of isonitrogenous enteral diets after severe trauma. An immune-enhancing diet reduces septic complications. Ann Surg. 1996;224:531-540; discussion 540-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 270] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 4. | Moore FA, Moore EE, Kudsk KA, Brown RO, Bower RH, Koruda MJ, Baker CC, Barbul A. Clinical benefits of an immune-enhancing diet for early postinjury enteral feeding. J Trauma. 1994;37:607-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 189] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 5. | Wu GH, Jarstrand C, Nordenström J. Phagocyte-induced lipid peroxidation of different intravenous fat emulsions and counteractive effect of vitamin E. Nutrition. 1999;15:359-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Harrison LE, Hochwald SN, Heslin MJ, Berman R, Burt M, Brennan MF. Early postoperative enteral nutrition improves peripheral protein kinetics in upper gastrointestinal cancer patients undergoing complete resection: a randomized trial. JPEN J Parenter Enteral Nutr. 1997;21:202-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 33] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Heyland D, Cook DJ, Winder B, Brylowski L, Van deMark H, Guyatt G. Enteral nutrition in the critically ill patient: a prospective survey. Crit Care Med. 1995;23:1055-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 203] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 8. | Heslin MJ, Latkany L, Leung D, Brooks AD, Hochwald SN, Pisters PW, Shike M, Brennan MF. A prospective, randomized trial of early enteral feeding after resection of upper gastrointestinal malignancy. Ann Surg. 1997;226:567-577; discussion 577-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 238] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 9. | Hochwald SN, Harrison LE, Heslin MJ, Burt ME, Brennan MF. Early postoperative enteral feeding improves whole body protein kinetics in upper gastrointestinal cancer patients. Am J Surg. 1997;174:325-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 41] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Mendez C, Jurkovich GJ, Garcia I, Davis D, Parker A, Maier RV. Effects of an immune-enhancing diet in critically injured patients. J Trauma. 1997;42:933-940; discussion 940-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 79] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Beale RJ, Bryg DJ, Bihari DJ. Immunonutrition in the critically ill: a systematic review of clinical outcome. Crit Care Med. 1999;27:2799-2805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 296] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 12. | Cunningham-Rundles S. Analytical methods for evaluation of immune response in nutrient intervention. Nutr Rev. 1998;56:S27-S37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Senkal M, Kemen M, Homann HH, Eickhoff U, Baier J, Zumtobel V. Modulation of postoperative immune response by enteral nutrition with a diet enriched with arginine, RNA, and omega-3 fatty acids in patients with upper gastrointestinal cancer. Eur J Surg. 1995;161:115-122. [PubMed] |
| 14. | Saffle JR, Wiebke G, Jennings K, Morris SE, Barton RG. Randomized trial of immune-enhancing enteral nutrition in burn patients. J Trauma. 1997;42:793-800; discussion 800-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 69] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Senkal M, Zumtobel V, Bauer KH, Marpe B, Wolfram G, Frei A, Eickhoff U, Kemen M. Outcome and cost-effectiveness of perioperative enteral immunonutrition in patients undergoing elective upper gastrointestinal tract surgery: a prospective randomized study. Arch Surg. 1999;134:1309-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 208] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 16. | Gianotti L, Braga M, Fortis C, Soldini L, Vignali A, Colombo S, Radaelli G, Di Carlo V. A prospective, randomized clinical trial on perioperative feeding with an arginine-, omega-3 fatty acid-, and RNA-enriched enteral diet: effect on host response and nutritional status. JPEN J Parenter Enteral Nutr. 1999;23:314-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 106] [Article Influence: 4.1] [Reference Citation Analysis (0)] |