Published online Sep 7, 2025. doi: 10.3748/wjg.v31.i33.109562
Revised: June 26, 2025
Accepted: August 7, 2025
Published online: September 7, 2025
Processing time: 109 Days and 2.7 Hours
Portal hypertension (PH), a major complication of cirrhosis, arises from increased intrahepatic resistance and splanchnic vasodilation. Epoxyeicosatrienoic acids (EETs) improve hepatic microcirculation, but their effects are rapidly inactivated by soluble epoxide hydrolase (sEH), an enzyme upregulated in the cirrhotic liver. Inhibiting sEH increases EET levels, reducing portal pressure and fibrosis. Dipe
To study the effects of vildagliptin, a DPP4-I and sacubitril/valsartan, an ARNI on PH and liver fibrosis in cirrhotic rats.
Two rodent models of liver cirrhosis: (1) Choline-deficient, L-amino acid-defined, high-fat diet (CDAHFD) diet-fed rats; and (2) Bile duct ligation-induced rats were treated with vildagliptin (10 mg/kg/day), sacubitril/valsartan (30 mg/kg/day), or a combination of both drugs. Hemodynamic parameters, sEH activity, EET levels, vascular remodeling, and fibrosis were assessed using enzyme-linked im
In CDAHFD-fed models, both DPP4-I and ARNI significantly reduced portal pressure in cirrhotic rats by decreasing intrahepatic vascular resistance without affecting systemic hemodynamics. These agents downregulated sEH expression and activity, increasing EET levels, and improved endothelial function via nitric oxide signaling enhancement. They also suppressed sinusoidal capillarization, pathological angiogenesis, and Hedgehog signaling, while restoring sinusoidal endothelial markers. Additionally, DPP4-I and ARNI attenuated liver fibrosis and stellate cell activation, reducing profibrotic gene expression. These effects were additive by the combination of both drugs. Similar effects were observed in bile duct ligation-induced PH, confirming their therapeutic potential in managing both PH and liver fibrosis through modulation of the sEH-EET pathway.
Combined DPP4-I with ARNI therapy ameliorates PH and fibrosis via sEH suppression and EET restoration, offering a promising treatment strategy for cirrhosis-related PH.
Core Tip: Portal hypertension (PH) in cirrhosis is driven by increased intrahepatic resistance and loss of epoxyeicosatrienoic acids (EETs) activity due to soluble epoxide hydrolase (sEH) upregulation. While dipeptidyl peptidase-4 inhibitors (DPP4-Is) and angiotensin receptor-neprilysin inhibitors (ARNIs) may suppress sEH, their combined effect remains unclear. This study assessed the combination effect of vildagliptin and sacubitril/valsartan on PH and fibrosis in two cirrhotic rat models, choline-deficient, L-amino acid-defined, high-fat diet- and bile duct ligation-induced cirrhotic rats. Combination therapy reduced portal pressure without affecting systemic hemodynamics, improved endothelial function and sinusoidal capillarization, suppressed sEH, restored EETs, and attenuated fibrosis. These findings suggest that DPP4-I and ARNI combination therapy may represent a novel strategy for treating cirrhosis-associated PH.
- Citation: Oyama M, Kaji K, Nishimura N, Hanatani J, Nakatani T, Nishimura N, Shibamoto A, Asada S, Tsuji Y, Kitagawa K, Sato S, Namisaki T, Yoshiji H. Dual therapy with vildagliptin and sacubitril/valsartan alleviates portal hypertension and inhibits soluble epoxide hydrolase in cirrhotic rats. World J Gastroenterol 2025; 31(33): 109562
- URL: https://www.wjgnet.com/1007-9327/full/v31/i33/109562.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i33.109562
Portal hypertension (PH), a major complication of cirrhosis, results from increased resistance to blood flow through the fibrotic liver and increased splanchnic blood flow[1-3]. It is a key driver of severe clinical outcomes, including variceal bleeding, ascites, spontaneous bacterial peritonitis, hepatic encephalopathy, and hepatorenal syndrome[1-3]. Extensive evidence indicates that portal pressure reduction improves clinical outcomes and is associated with decreased mortality in cirrhotic patients[4].
Despite therapeutic advances, the management of PH remains challenging. Non-selective beta-blockers (NSBBs) are the first-line treatment to prevent variceal bleeding; however, variable patient response and adverse events can limit their utility[2,4-6]. Endoscopic treatment options such as band ligation are effective but necessitate repeated procedures and are plagued by procedural complications[7]. In refractory cases, a transjugular intrahepatic portosystemic shunt may reduce portal pressure; however, its use is limited by the risk of hepatic encephalopathy and its unsuitability in patients with advanced liver dysfunction[8,9]. These limitations underscore the need for novel pharmacological targets capable of effectively modulating PH.
Epoxyeicosatrienoic acids (EETs), which are synthesized from arachidonic acid via cytochrome P450 epoxygenases, exhibit vasodilatory, anti-inflammatory, and anti-fibrotic properties[10,11]. EETs have been reported to play a protective role by promoting hepatic microcirculation and reducing vascular resistance in cirrhosis-based PH[12]. However, their bioactivity is curtailed by rapid hydrolysis into less active dihydroxyeicosatrienoic acids via soluble epoxide hydrolase (sEH)[10,11]. Recent studies suggest that sEH inhibition elevates endogenous EET levels, leading to reduced intrahepatic vascular resistance and portal pressure[13-15]. Experimental models of cirrhosis have demonstrated that sEH inhibitors improve endothelial function, attenuate hepatic inflammation and fibrosis, and attenuate PH[13-15]. Moreover, sEH inhibition may enhance nitric oxide (NO) bioavailability and reduce oxidative stress in the cirrhotic liver[13-15].
Dipeptidyl peptidase-4 inhibitors (DPP4-Is), commonly used for glycemic control in type 2 diabetes, have recently been implicated in the modulation of EET levels and downregulation of sEH expression or activity[16]. This mechanism may underlie their observed vascular protective effects and potential anti-fibrotic benefits, which are particularly relevant in metabolic dysfunction-associated steatohepatitis (MASH) and PH, where inflammation and vascular dysfunction are key pathogenic features. In parallel, angiotensin II (AT-II), a key effector of the renin-angiotensin-aldosterone system (RAAS), promotes vasoconstriction, inflammation, and fibrosis[17,18]. It has also been shown to upregulate sEH, thereby reducing EET levels and enhancing vascular dysfunction and organ damage[19,20]. Thus, AT-II blockade counteracts AT-II-mediated damage and may preserve EET bioactivity through sEH suppression, offering broader therapeutic benefits in PH. Angiotensin receptor-neprilysin inhibitor (ARNI) is a novel heart failure drug that simultaneously regulates RAAS and natriuretic peptide[21]. In addition, neprilysin inhibitor has been shown to reduce portal pressure via a decrease in intrahepatic vascular resistance[22]. Thus, it is suggested that ARNI efficiently reduces PH, and indeed, preclinical studies have shown its efficacy[23,24]. However, it has not been reported whether ARNI in combination with DPP4-I contributes to the suppression of PH and liver fibrosis via efficient regulation of the sEH-EETs pathway.
In this study, we aimed to examine the combined effects of vildagliptin as a DPP4-I and sacubitril/valsartan as an ARNI on PH and liver fibrosis in cirrhotic rats.
Male F344/NSlc rats, aged 10 weeks and weighing between 200-250 g, were purchased from Japan SLC (Hamamatsu, Japan). These rats were housed in stainless steel cages under sterile conditions, in an environment maintained at 23 °C, 50% humidity, and a 12-hour light-dark cycle. Animal experiments were conducted by the Guide for Care and Use of Laboratory Animals of the National Research Council. The in vivo experimental protocols were approved by the ethics committee of Nara Medical University (No. 13502-1). Vildagliptin and Carvedilol were obtained from FUJIFILM Wako Pure Chemical Corporation (Osaka, Japan). Sacubitril/valsartan, a combination of sacubitril and valsartan in a 1:1 molar ratio, was obtained from Selleck Chemicals (Houston, TX, United States).
Animal experiment 1: Thirty-six rats were randomly assigned to six experimental groups (n = 6) and underwent a 12-week study. Oral treatments were administered daily for six weeks, beginning six weeks after the initiation of dietary feeding, as follows: The rats in group 1 were fed a choline-supplemented L-amino acid-defined, normal-fat diet (CSANFD) and administered saline as a vehicle, those in groups 2-6 were fed a choline-deficient, L-amino acid-defined, high-fat diet (CDAHFD) and received the following treatments: Group 2, saline; Group 3, vildagliptin (10 mg/kg/day); Group 4, sacubitril/valsartan (30 mg/kg/day); Group 5, vildagliptin and sacubitril/valsartan; Group 6, carvedilol (10 mg/kg/day)[24-26]. Both diets were purchased from Research Diets Inc. (New Brunswick, NJ, United States).
Animal experiment 2: Twenty rats were randomly assigned to five groups (n = 4) and underwent a 4-week study protocol. Oral treatment was administered for two weeks, starting two weeks after surgery, using the same dose as in animal experiment 1. The groups were as follows: The rats in group 1 received sham operation administration of saline as a vehicle, those in groups 2-5 received bile duct ligation (BDL) and were administered saline in group 2, vildagliptin in group 3, sacubitril/valsartan in group 4, and vildagliptin and sacubitril/valsartan in group 5.
The measurement of hemodynamic parameters was described in our previous studies[27]. Briefly, all the rats were given inhalation anesthesia with isoflurane (4%-5% for induction and 1%-2% for maintenance) (Merck & Co., Rahway, NJ, United States). A transonic science pressure catheter (No. FTH-1211B-0018; Transonic Science, London, ON, Canada) was inserted into the right femoral artery to measure the mean arterial pressure (mmHg) and heart rate (bpm). Subsequently, the catheter was inserted into the portal vein to determine portal pressure (mmHg). The catheter was connected to a transducer, and the pressure signals were recorded using the transonic science pressure system (FP095B; Transonic Science). The zero-pressure point was set at the level of the right atrium. The portal blood flow (mL/minute) was calculated by the portal vein diameter and portal blood flow rate, which were measured using blood flow measurement probes (MA2PSB; Transonic Science). Intrahepatic vascular resistance was the quotient of portal pressure and portal blood flow (mmHg/mL/minute-1). Moreover, the superior mesenteric artery (SMA) blood flow was measured using the probe and SMA resistance was calculated as mean arterial pressure/SMA blood flow.
The cardiac index was calculated as the cardiac output measured by thermodilution, as previously described[28] per 100 g body weight. The systemic vascular resistance was the quotient of the mean arterial pressure and the cardiac index.
Hepatic endothelin-1 (ET-1), NO, cyclic guanosine monophosphate (cGMP), AT-II receptor type 1 (AT1R), and nicotinamide adenine dinucleotide phosphate hydrogen oxidase 4 (NOX4) levels were quantified using the following enzyme-linked immunosorbent assay (ELISA) kits, according to the manufacturers’ instructions: ET-1 ELISA kit (RD Systems, Minneapolis, MN, United States), NO assay kit (Oxford Biomedical Research, Rochester Hills, MI, United States), and cGMP complete ELISA kit (Abcam, Cambridge, United Kingdom), rat AGTR1/AT1 receptor ELISA kit (LSBio, Seattle, WA, United States) and rat NOX4 ELISA kit (Novus Biologicals, Centennial, CO, United States).
Hepatic sEH activity was assessed using the sEH activity assay kit (LSBio), following the manufacturer’s instructions. However, hepatic concentrations of the arachidonic acid metabolites 11,12-EET and 14,15-EET were measured using specific ELISA kits (Detroit RD, Detroit, MI, United States) according to the manufacturer’s instructions.
The RNeasy mini kit (Qiagen, Tokyo, Japan) was used to extract total RNA from liver tissues, and RNA concentrations were quantified with a spectrophotometer. Subsequently, 1 μg of each RNA sample was reverse transcribed into complementary DNA (cDNA) using the high-capacity RNA-to-cDNA™ kit (Thermo Fisher Scientific, Waltham, MA, United States). The quantitative real-time polymerase chain reaction analysis was conducted using the SYBR™-Green polymerase chain reaction master mix (Thermo Fisher Scientific). Each sample was assessed in triplicate, and the mean values were used for subsequent data analyses. The primer sequences employed for amplifying these genes are provided in Supplementary Table 1.
Liver tissue samples were homogenized in radio immunoprecipitation assay lysis buffer (Santa Cruz Biotechnology, Dallas, TX, United States) supplemented with 1 mmol/L phenylmethanesulfonyl fluoride. Subsequently, total protein concentrations were determined using a bicinchoninic acid assay kit (Thermo Fisher Scientific). Equal amounts of protein (50 μg per lane) were subjected to electrophoresis on 4%-12% NuPAGE® Bis-Tris gels (Thermo Fisher Scientific) and subsequently transferred onto Invitrolon™ polyvinylidene difluoride membranes (Thermo Fisher Scientific).
Thereafter, membranes were blocked in 5% (w/v) non-fat dry milk in Tris-buffered saline with 0.1% Tween-20 (TBST) for 1 hour at room temperature, followed by overnight incubation at 4 °C with primary antibodies: Phospho-endothelial NO synthase (eNOS) (Ser1177) (1:1000), eNOS (1:1000), and β-actin (1:10000) from Cell Signaling Technology (Danvers, MA, United States); And phospho-inducible NO synthase (iNOS) (Tyr151) (1:500), iNOS (1:500), phospho-moesin (1:2000), and moesin (1:1000) from Thermo Fisher Scientific. After three washes with TBST, membranes were incubated for 1 hour at room temperature with horseradish peroxidase-conjugated secondary antibodies (1:5000, Thermo Fisher Scientific). Protein bands were detected using the enhanced chemiluminescence reagent (Thermo Fisher Scientific) and quantified using ImageJ software (NIH, Bethesda, MD, United States). β-actin served as a loading control. All Western blot analyses were independently performed in triplicate using liver samples from three randomly selected rats in each group.
Liver tissues were excised from rats, fixed in 4% paraformaldehyde at 4 °C overnight, and subsequently embedded in paraffin. These paraffin-embedded samples were then sectioned at 5 μm and stained with hematoxylin and eosin and Sirius red or histopathological evaluation (performed at Narabyouri Research Co., Nara, Japan). The extent of steatohepatitis was assessed using the nonalcoholic fatty liver disease activity score (NAS), which is the sum of three histological components severity of steatosis (0-3), lobular inflammation (0-2), and hepatocyte ballooning (0-3). NAS and scores for fibrosis (0-4) were determined according to a previously described method[29]. The NAS and fibrosis scores were independently assessed by three experienced pathologists in a blinded manner.
Liver sections (5 μm thick) were deparaffinized with xylene and rehydrated through a graded ethanol series. However, antigen retrieval was performed by heating the sections in citrate buffer (potential of hydrogen = 6.0) for 15 minutes. After cooling, sections were rinsed with phosphate-buffered saline (PBS) and incubated for 1 hour at room temperature with a blocking solution containing 5% normal goat serum and 0.3% Triton X-100 in PBS to reduce non-specific binding. Sections were then incubated overnight at 4 °C with primary antibodies, including mouse monoclonal alpha-smooth muscle actin (α-SMA) (Abcam, 1:100), rabbit monoclonal cluster of differentiation (CD) 34 (Abcam, 1:100), and mouse monoclonal sEH (Santa Cruz Biotechnology, 1:100), diluted in a blocking solution. After washing with PBS, the sections were incubated with appropriate fluorophore-conjugated secondary antibodies (Alexa Fluor 488 or 594, Thermo Fisher Scientific) for 1 hour at room temperature in the dark. Nuclei were counterstained with 4’,6-diamidino-2-phenylindole (Vector Laboratories, Newark, CA, United States). Histologically and fluorescently stained tissues were viewed under a BX53 microscope (Olympus, Tokyo, Japan) for histology and a BZ-X700 microscope (Keyence, Osaka, Japan). All slides were photographed, and five images were randomly selected and analyzed using ImageJ 64-bit Java 1.8.0 (National Institute of Health).
Following the manufacturer’s protocol, the liver tissue hydroxyproline content was measured using the hydroxyproline assay kit (Abcam). Briefly, approximately 10 mg of liver tissue was homogenized in distilled water and hydrolyzed in 100 μL of 12 N hydrochloric acid at 120 °C for 3 hours in a pressure-sealed vial. Following hydrolysis, samples were cooled to room temperature and centrifuged to remove debris. An aliquot of the supernatant was collected, neutralized, and incubated with chloramine T solution for 20 minutes at room temperature to facilitate oxidation. This was followed by a 20-minute incubation at 65 °C with Ehrlich’s reagent to allow color development. Absorbance, as measured using a microplate reader, was 560 nm, and the hydroxyproline concentration was calculated from a standard curve generated from known hydroxyproline standards. Final values were normalized to tissue weight and expressed as micrograms of hydroxyproline per milligram of liver tissue (μg/mg).
Statistical analyses were performed using GraphPad Prism version 9.00 (GraphPad Software, La Jolla, CA, United States). Data are expressed as the mean ± SD from a minimum of three independent experiments. Differences between the two groups were assessed using Student’s t-test and some analyses with the Mann-Whitney U test. Correlations among variables were determined using Pearson’s correlation coefficient. A P value less than 0.05 was considered statistically significant.
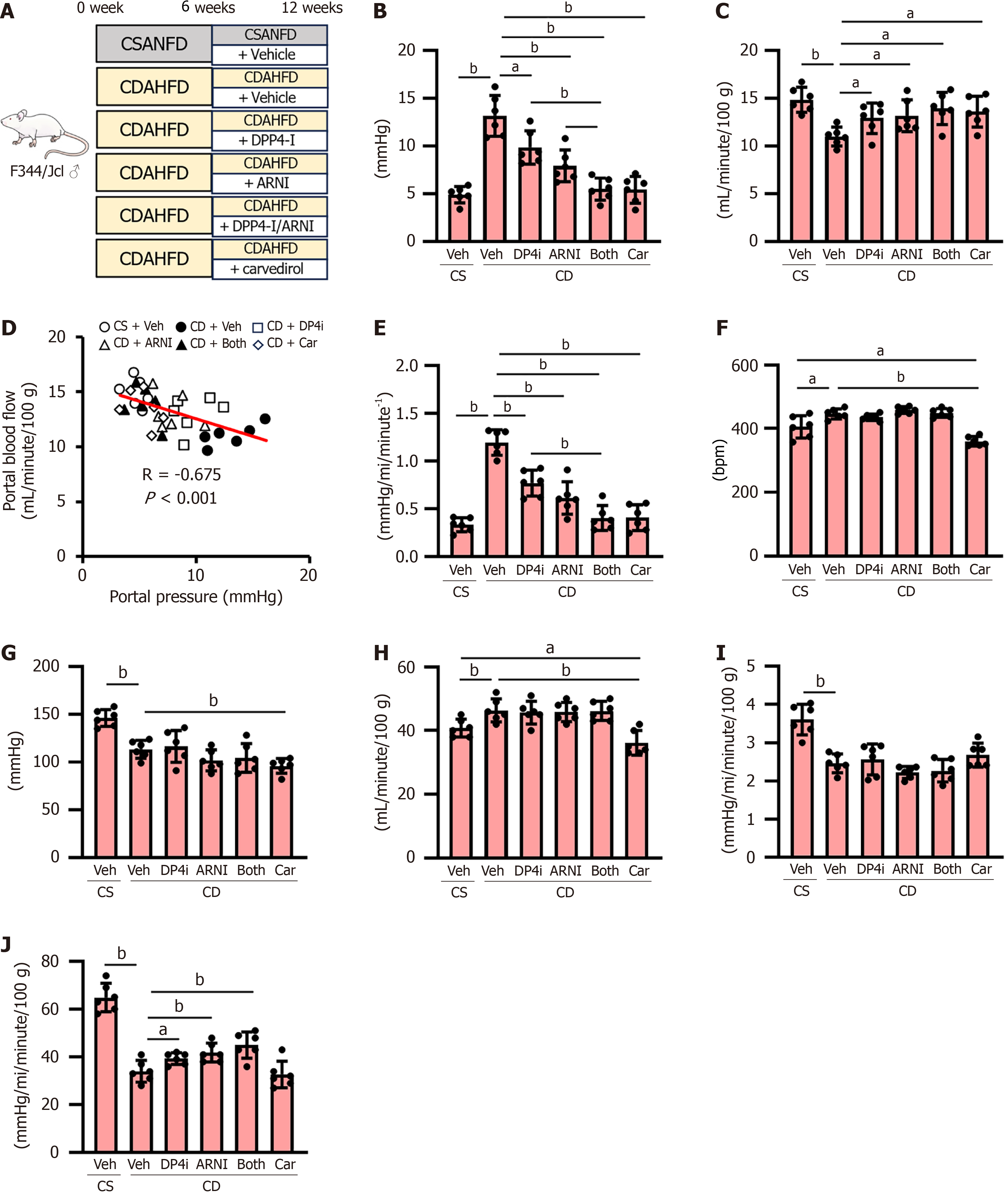
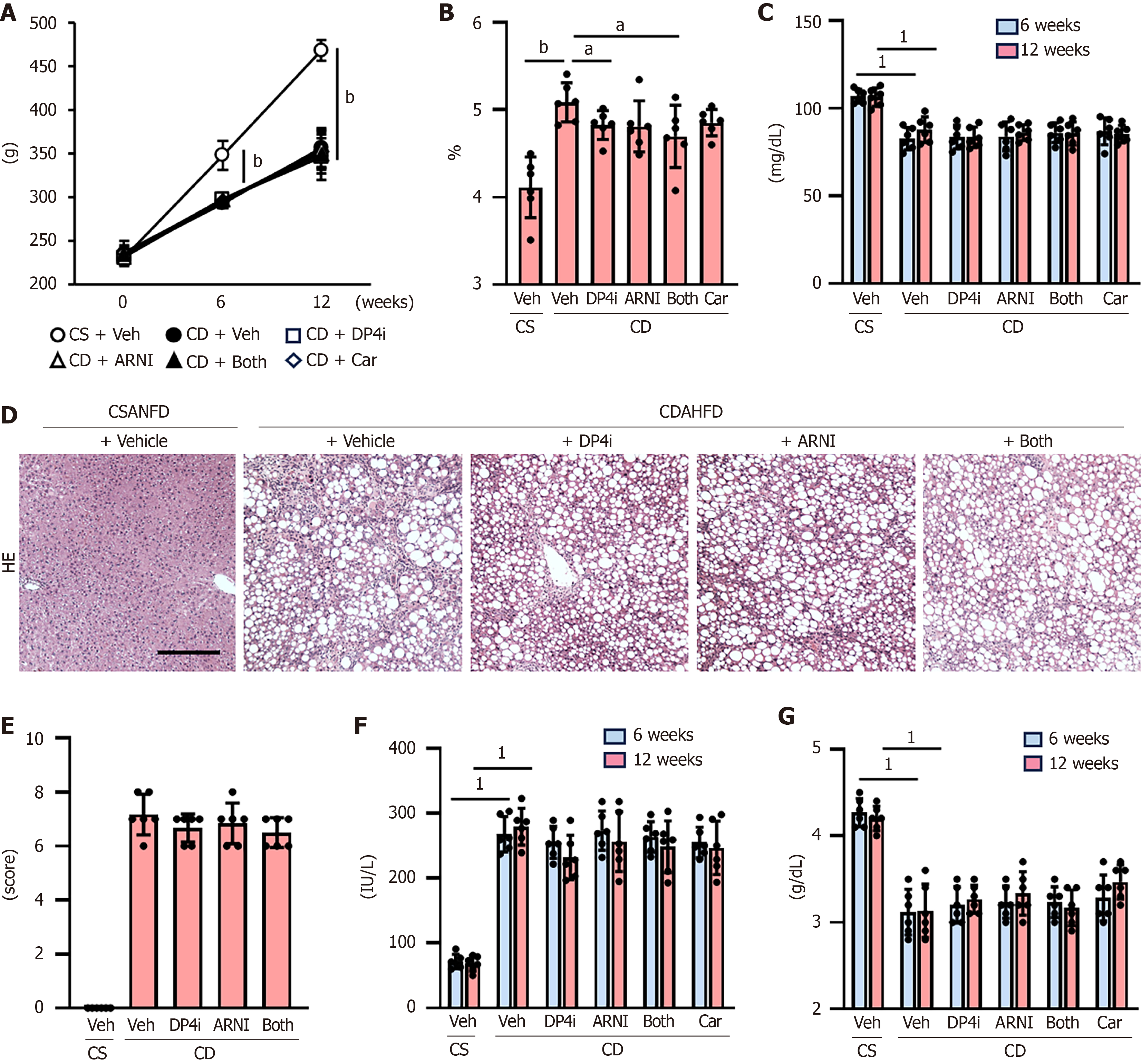
Initially, we analyzed the effect of DPP4-I and ARNI on PH in CDAHFD-fed cirrhotic rats (Figure 1A). After 12 weeks on CDAHFD, the rats exhibited significantly elevated portal pressure (13.1 ± 2.1 mmHg) compared to CSANFD-fed control rats (4.9 ± 0.9 mmHg) (Figure 1B). Monotherapy with either DPP4-I (9.9 ± 1.7 mmHg) or ARNI (7.9 ± 1.7 mmHg) reduced portal pressure, with a more pronounced effect observed when both drugs were used in combination (5.5 ± 1.2 mmHg) (Figure 1B), comparable to the reduction achieved using carvedilol (5.4 ± 1.4 mmHg) (Figure 1B).
In the DPP4-I and/or ARNI-treated groups, there was a marked increase in portal blood flow inversely correlated with a reduction in portal pressure in CDAHFD-fed rats (Figure 1C and D), which was accompanied by a reduction in Intrahepatic vascular resistance (Figure 1E). CDAHFD-fed rats showed a significant tachycardia and hypotension leading to increased cardiac output as compared to CSANFD-fed control rats (Figure 1F-H). ARNI administration tended to slightly lower mean arterial pressure in CDAHFD-fed rats, but this was not significant and had no effect on heart rate or cardiac output (Figure 1F-H). On the other hand, carvedilol administration caused significant hypotension and decreased heart rate leading to decreased cardiac output not only in CDAHFD-fed rats but also in comparison with CSANFD-fed control rats (Figure 1F-H). Moreover, systemic vascular resistance and SMA resistance were both reduced in CDAHFD-fed rats, and although DPP4-I and/or ARNI had no effect on systemic vascular resistance, it significantly increased SMA resistance (Figure 1I and J). This effect was not observed with carvedilol (Figure 1I and J).
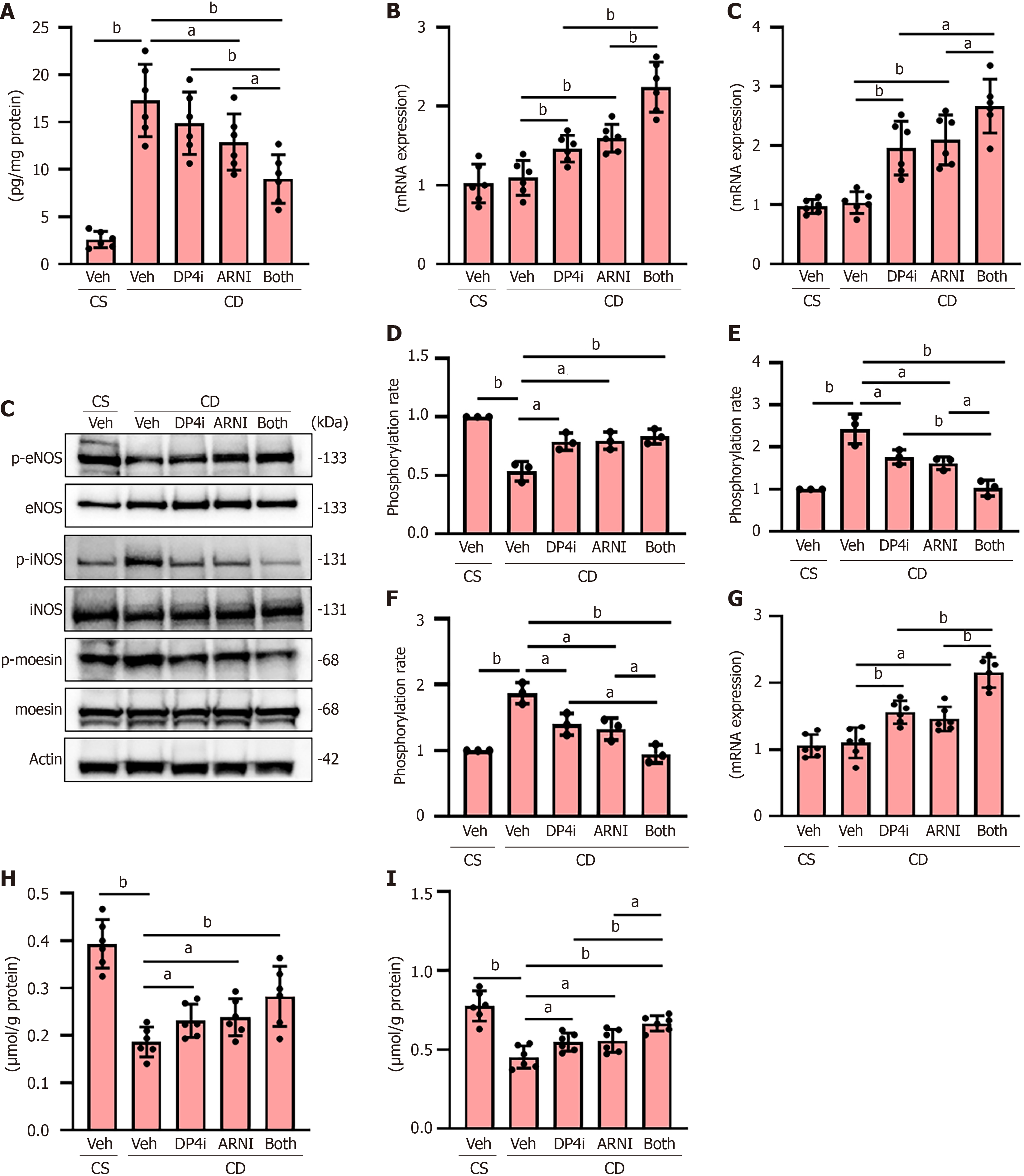
CDAHFD-fed rats exhibited elevated hepatic ET-1 Levels, indicative of increased intrahepatic vascular resistance due to sinusoidal endothelial dysfunction. Treatment with DPP4-I and ARNI reduced ET-1 Levels (Figure 2A). These treatments also upregulated the expression of vasodilatory regulators cystathionine γ-lyase and dimethylarginine dimethyl amino-hydrolase 1 (Figure 2B). We further evaluated the active state of eNOS, as reflected by their phosphorylation status, in the livers of rats in the experimental groups. As shown in Figure 2C and D, the phosphorylated ratio of eNOS was decreased in CDAHFD-fed rats, which was attenuated by treatment with DPP4-I and ARNI. Intrahepatic sinusoidal contractility, represented by the phosphorylation of iNOS and moesin, was elevated in CDAHFD-treated rats but was suppressed by the administration of both drugs (Figure 2C, E and F). Moreover, treatment with both drugs upregulated the expression of guanosine triphosphate cyclohydrolase 1 (GCH1), the rate-limiting enzyme for tetrahydrobiopterin production, an important cofactor for eNOS (Figure 2G). Consistent with these findings, hepatic levels of nitrate/nitrite and cGMP were elevated in groups of rats treated with both drugs compared to those with the vehicle, indicating improved hepatic sinusoidal relaxation (Figure 2H and I).
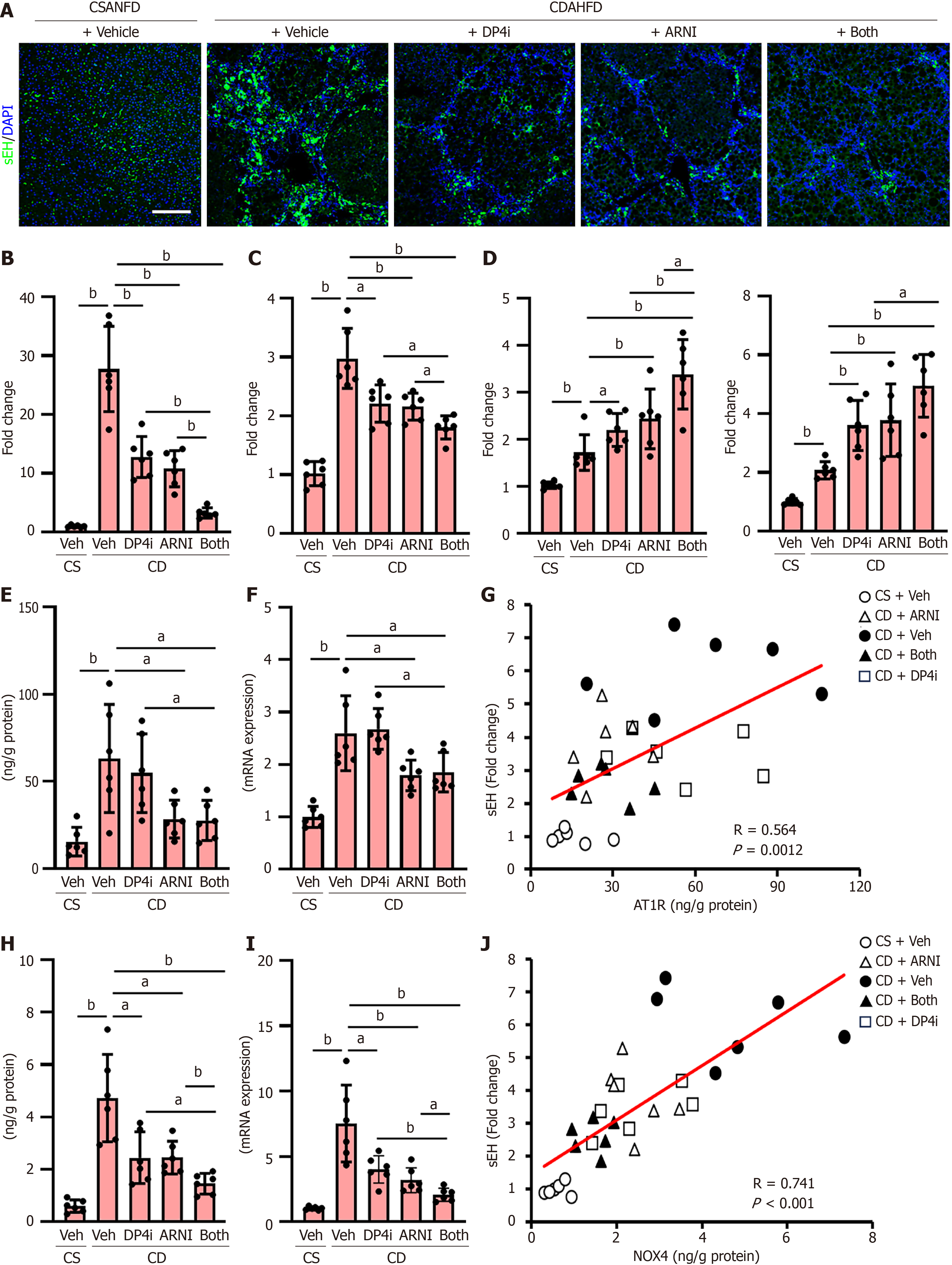
sEH has a potential role in regulating vascular tone, inflammation, and fibrosis in the liver and portal circulation[13-15]. Compared to CSANFD-fed control rats, CDAHFD-fed cirrhotic rats exhibited a significant increase in hepatic sEH expression and activity (Figure 3A-C), both of which were suppressed by DPP4-I or ARNI monotherapy and further reduced by combination therapy (Figure 3A-C). In CDAHFD-fed rats, hepatic levels of EETs (11,12-EET and 14,15-EET) increased as a compensatory response to liver fibrosis and PH, and treatment with both drugs significantly increased hepatic EET levels, in response to a decrease in sEH expression and activity (Figure 3D).
Given that AT-II can upregulate sEH, we assessed hepatic AT1R expression[19]. As shown in Figure 3E and F, hepatic protein and message RNA (mRNA) expression levels were increased in CDAHFD-fed rats, which were attenuated by treatment with ARNI. Moreover, a positive correlation between the hepatic AT1R level and sEH expression (r = 0.564, P = 0.0012) across the experimental groups supports ARNI-mediated downregulation of sEH via AT-II blockade (Figure 3G). Additionally, NOX4 and sEH have been implicated in endothelial dysfunction associated with liver cirrhosis. Increased NOX4 activity promotes reactive oxygen species production, contributing to endothelial cell injury and subsequent upregulation of sEH, thereby exacerbating vascular dysfunction[30,31]. Consistently, treatment with both DPP4-I and ARNI significantly downregulated hepatic NOX4 expression at the protein and mRNA levels (Figure 3H and I). Moreover, a strong positive correlation was observed between the hepatic NOX4 Level and sEH expression (r = 0.741, P < 0.001) (Figure 3J).
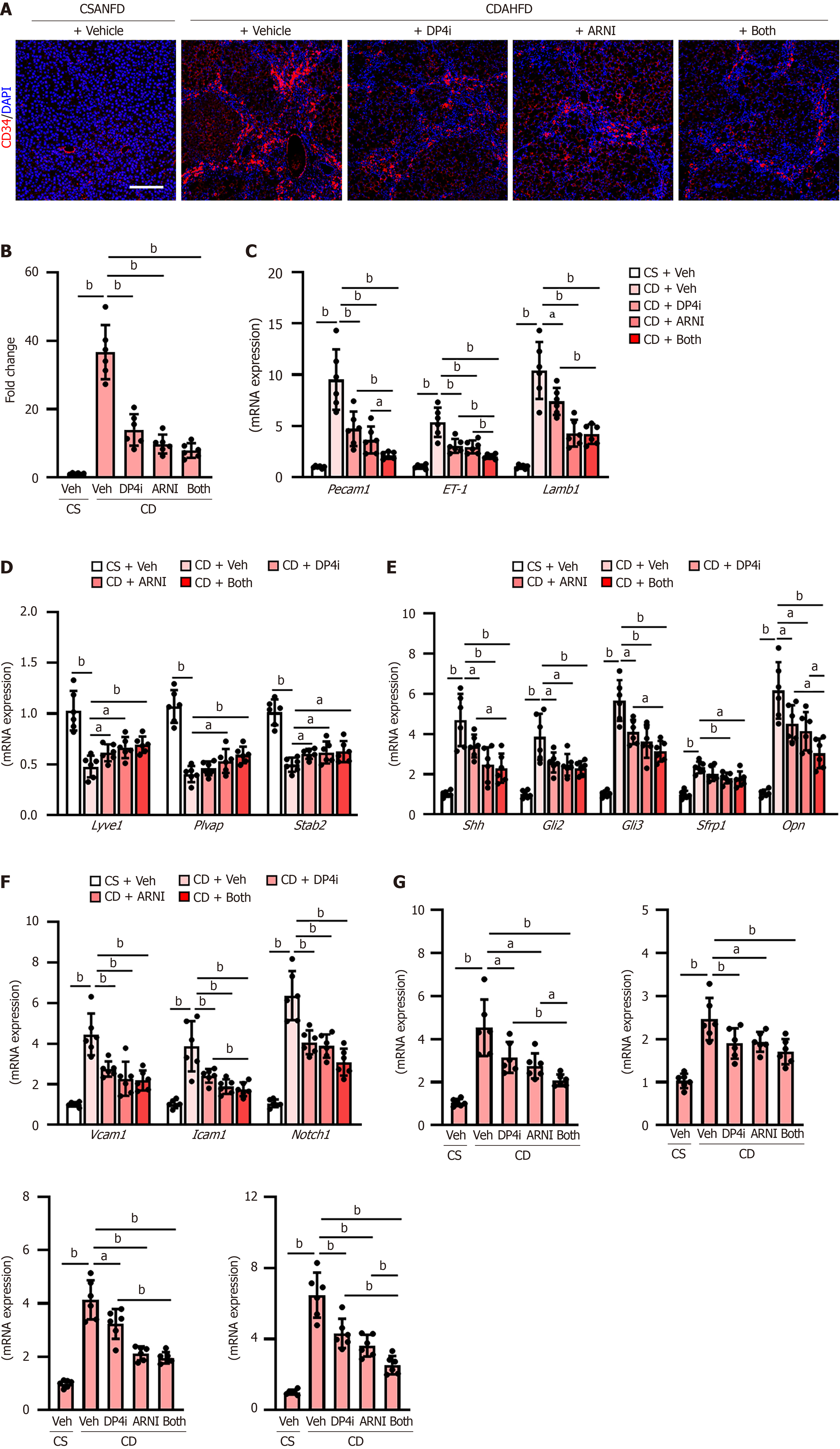
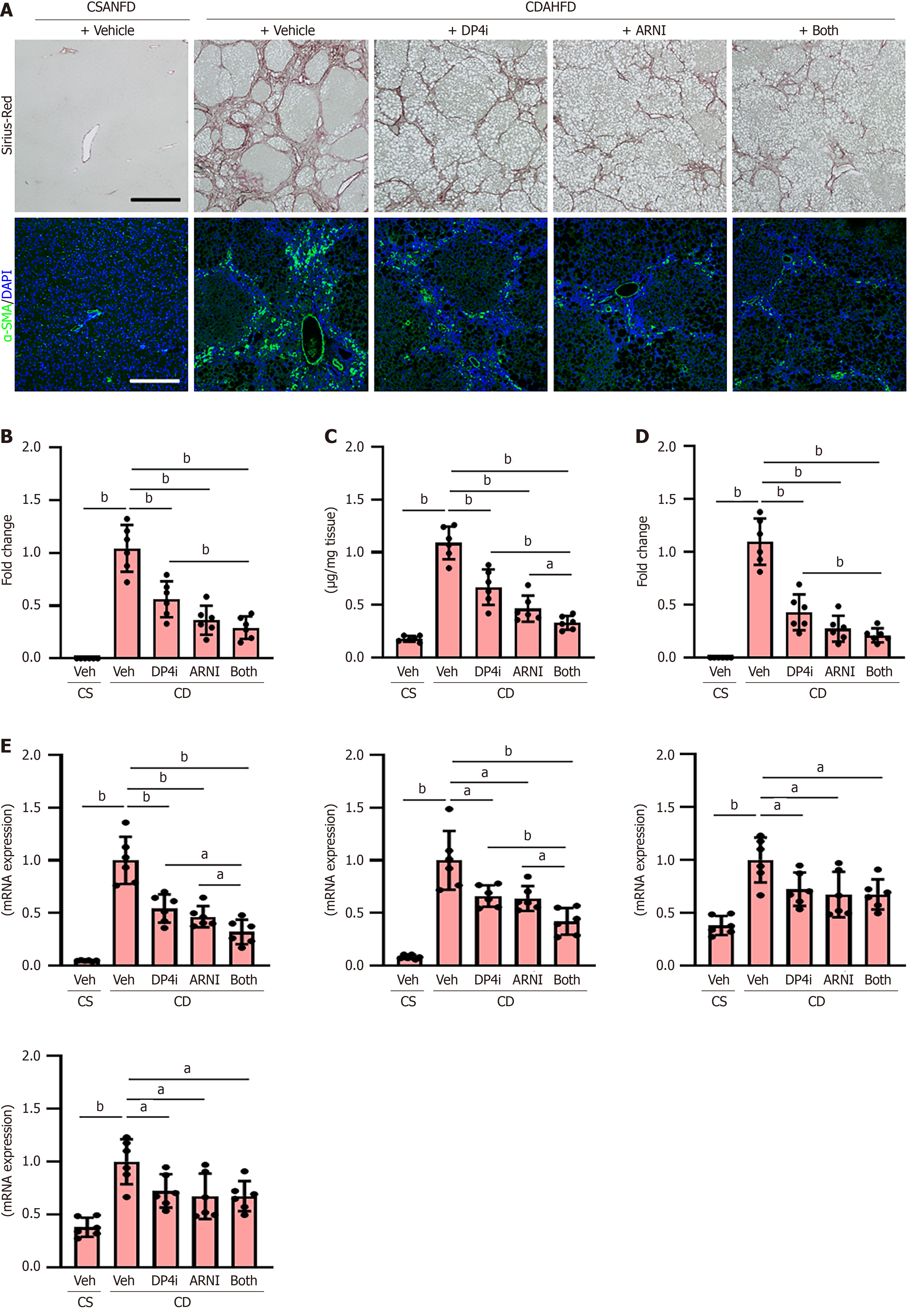
Increased intrahepatic vascular resistance is attributed to liver sinusoidal endothelial cell (LSEC) capillarization and pathological angiogenesis, leading to PH. CDAHFD-fed rats demonstrated increased intrahepatic expression of CD34, a capillary endothelial marker (Figure 4A), which was significantly reduced to approximately 25% under combination therapy (Figure 4A and B). Expression of additional capillary markers, including platelet endothelial cell adhesion molecule-1 (Pecam1), ET-1, and laminin subunit beta-1 (Lamb1), was similarly downregulated (Figure 4C). Conversely, the hepatic expression of sinusoidal endothelial markers, including lymphatic vessel endothelial hyaluronan receptor 1 (Lyve1), plasmalemma vesicle-associated protein (Plvap), and stabilin-2 (Stab2), which were lower in the CDAHFD-fed rats, was increased by treatment with both drugs (Figure 4D). Hedgehog (Hh) signaling is known to be activated during LSEC capillarization and plays a key role in regulating this process; hence, we further measured the hepatic expression of Hh signaling-related genes[32]. CDAHFD-fed rats showed a higher mRNA level of the Hh ligand (Shh), Hh-sensitive transcription factors (GLI family zinc finger 2: Gli2, Gli3), and Hh-target genes (Sfrp1, Opn) than CSANFD-fed control rats, and these gene expressions were significantly reduced by treatment with DPP4-I and ARNI (Figure 4E). In PH, the hepatic expression of vascular cell adhesion molecule 1 (Vcam1), intercellular adhesion molecule 1 (Icam1), and neurogenic locus notch homolog protein 1 (Notch1) is reportedly upregulated as markers of vascular remodeling, fibrosis, and inflammation contributing to an increase in intrahepatic resistance[33-35]. DPP4-I and ARNI also attenuated the upregulation of these gene expressions (Figure 4F). In line with this, the hepatic mRNA levels of proangiogenic genes, including Vegfa, Ptgs2, Pigf, and Vwf, were significantly decreased after treatment with a DPP4-I and ARNI (Figure 4G).
CDAHFD-fed rats showed a decrease in body weight compared to CSANFD-fed controls after 6 weeks of feeding, but no improvement was observed following the administration of each drug (Figure 5A). Increased relative liver weight in CDAHFD-fed rats was attenuated by treatment with DPP4-I but not with ARNI or carvedilol (Figure 5B). Moreover, CDAHFD-fed rats showed a lower level of serum glucose, but no treatments affected glucose or intact glucagon-like peptide-1 (GLP-1) levels (Figure 5C and Supplementary Figure 1). As shown in Figure 5D and E, CDAHFD-induced pathological changes, including steatosis, inflammation, and ballooning, did not significantly change by treatment with DPP4-I and ARNI. Similarly, elevated serum alanine aminotransferase levels or hypoalbuminemia were not observed (Figure 5F and G).
Notably, both DPP4-I and ARNI, when administered individually, significantly inhibited histological liver fibrosis development and reduced the hepatic content of hydroxyproline, a major component of the protein collagen, in CDAHFD-fed rats (Figure 6A-C). In line with the attenuation of liver fibrosis, activation of hepatic stellate cells, as indicated by α-SMA positivity, was reduced by both treatments (Figure 6A and D). Moreover, the anti-fibrotic effect was enhanced by combined treatment with DPP4-I and ARNI (Figure 6A-D). These improvements in the fibrotic phenotypes were also accompanied by reduced hepatic mRNA levels of profibrotic genes, including Acta2, Col1a1, Tgfb1, and Ctgf (Figure 6E).
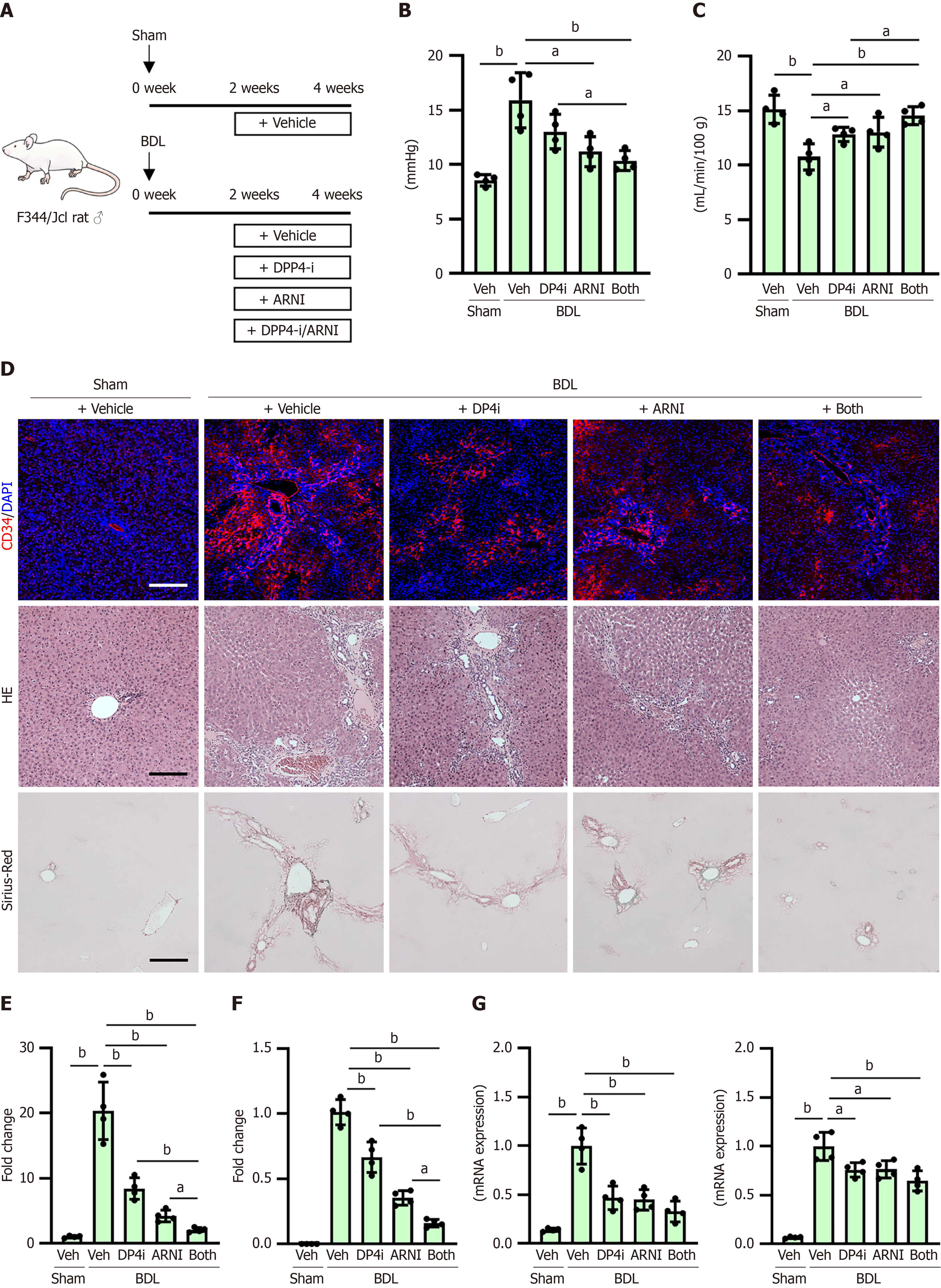
The effects of DPP4-I and ARNI on PH were also examined in BDL-treated rats, a well-known model of PH[36] (Figure 7A). Similar to the effects on CDAHFD-fed rats, DPP4-I and ARNI significantly prevented elevated portal pressure and decreased portal blood flow in the BDL-treated rats, without affecting the mean arterial pressure or heart rate (Figure 7B and C, Supplementary Figure 2). Moreover, DPP4-I and ARNI inhibited CD34 + sinusoidal capillarization in this model (Figure 7D and E). Regarding anti-fibrotic effects, both drugs suppressed histological liver fibrosis and reduced intrahepatic hydroxyproline content with downregulation of profibrotic markers in BDL-treated rats (Figure 7D, F and G, Supplementary Figure 3).
This study demonstrates that combined treatment with vildagliptin (DPP4-I) and sacubitril/valsartan (ARNI) significantly attenuates PH and liver fibrosis in two established rodent models of cirrhosis CDAHFD-fed rats and BDL-treated rats[36,37]. Our findings reveal a novel therapeutic synergy targeting key pathogenic mechanisms underlying increased intrahepatic resistance, vascular remodeling, and fibrogenesis in chronic liver disease.
The present study first confirmed the marked portal pressure elevation in CDAHFD-fed rats, a model mimicking features of MASH[37]. DPP4-I and ARNI monotherapies significantly reduced portal pressure, with an additive effect observed in the combination group. These hemodynamic improvements were achieved without significant changes in systemic blood pressure or cardiac output, differentiating this approach from conventional vasodilators like carvedilol, an NSBB with additional α1 adrenergic blocking activity[38,39]. Instead, increased portal blood flow, reduced Intrahepatic vascular resistance as well as increased SMA resistance were observed, indicating the effects on intrahepatic and mesenteric vessels rather than systemic circulatory compromise.
Mechanistic studies revealed that both drugs alleviated intrahepatic vasoconstriction by enhancing endothelial NO signaling. The treatment restored the eNOS phosphorylation, upregulated GCH1 expression, and elevated hepatic nitrate/nitrite and cGMP levels. Conversely, it reduced the phosphorylation of iNOS and moesin hallmarks of advanced PH which collectively indicate enhanced NO bioavailability[40,41]. Furthermore, the hepatic expression of ET-1, a potent vasoconstrictor whose levels are elevated in cirrhosis, was significantly suppressed[1,3,41]. These changes collectively support the restoration of endothelial function and sinusoidal relaxation.
A key finding of this study is the suppression of sEH and the concomitant elevation of hepatic EETs following treatment. Although several preclinical studies have shown that sEH inhibitor such molecule trans-4-{4-[3-(4-trifluoromethoxyphenyl)-ureido]cyclohexyloxy}benzoic acid has a potential to suppress cirrhosis-based PH, these challenges limit the clinical application in PH[13-15]. First, the short half-life and rapid metabolism of EETs may reduce sustained therapeutic effects. Second, systemic vasodilation from elevated EET levels could lead to hypotension or impair renal perfusion. Third, inter-individual variability in cytochrome P450 enzyme activity may affect EET production and response to sEH inhibition. Furthermore, long-term safety data in humans are lacking, and potential off-target effects remain a concern. We found that sEH expression and activity were upregulated in CDAHFD-fed rats, which is consistent with previous reports in cirrhotic models. Treatment with either DPP4-I or ARNI suppressed hepatic sEH levels, with the most pronounced effect seen with combination therapy, leading to increased EET concentrations. These results provide the first in vivo evidence that clinically available agents like DPP4-Is and ARNI may potentiate the EET pathway by modulating sEH expression and activity.
Furthermore, our data further suggest that ARNI-mediated effects may involve the inhibition of AT-II signaling, which is known to upregulate sEH and exacerbate vascular dysfunction[19,20]. Indeed, hepatic AT1R expression was increased in CDAHFD-fed rats and reduced by ARNI treatment. The positive correlation between AT1R and sEH expression supports a mechanistic link between AT-II blockade and sEH suppression. Similarly, both drugs suppressed the hepatic expression of NOX4, a nicotinamide adenine dinucleotide phosphate hydrogen oxidase implicated in oxidative stress-induced endothelial dysfunction and sEH upregulation[30,31]. The strong correlation between NOX4 and sEH suggests that reduced oxidative stress may improve vascular tone and EET preservation. However, the mechanisms underlying the additive effect of DPP4-I and ARNI require further clarification. In future studies to elucidate the mechanism, it will be necessary to clarify the complementary effects of both drugs by using pathway-specific inhibitors and knockout models.
Beyond hemodynamic benefits, DPP4-I and ARNI modulated key structural contributors to PH. Notably, they inhibited sinusoidal capillarization, a process in which LSECs lose their fenestrae and acquire capillary-like features, thereby increasing intrahepatic resistance[42,43]. Treatment downregulated capillarization markers (CD34, Pecam1, ET-1, and Lamb1), and restored LSEC-specific markers (Lyve1, Plvap, and Stab2)[44,45]. These changes were associated with the downregulation of Hh signaling components, a pathway implicated in LSEC dedifferentiation and fibrosis in chronic liver injury[32]. Thus, DPP4-I and ARNI appear to restore LSEC phenotype and microvascular architecture.
Angiogenesis is another key feature contributing to vascular remodeling and increased portal resistance in cirrhosis[46-48]. Proangiogenic mediators (Vegfa, Ptgs2, Pigf, and Vwf), along with inflammation-related vascular adhesion molecules (Vcam1, Icam1) and Notch1, which are involved in vascular remodeling and endothelial activation, were also downregulated[33-35]. These findings indicate a broad antiangiogenic and anti-inflammatory effect of DPP4-I and ARNI, contributing to the mitigation of PH.
Beyond vascular effects, our study demonstrated the significant anti-fibrotic activity of both agents. These results were consistent across both CDAHFD and BDL models, underscoring the robustness and translatability of the findings. Interestingly, the results showed a suppression of liver fibrosis by DPP4-I and ARNI without any effect on liver weight, hepatic steatosis, inflammation and ballooning. Our previous report demonstrated that DPP4-I suppressed the proliferation and collagen synthesis in activated hepatic stellate cells by inhibiting extracellular regulated protein kinase 1/2, p38, and Smad 2/3 signaling[49]. Moreover, we recently reported that ARNI can prevent hepatic stellate cell proliferation and profibrogenic activity by activating atrial natriuretic peptide-dependent guanylyl cyclase A/cGMP/protein kinase G signaling and inhibiting AT-II-dependent AT1R/protein kinase C signaling[50]. These findings support the possibility that both agents exert anti-fibrotic effects by directly acting on hepatic stellate cells in this study, but further analysis is needed to clarify the mechanism of action. Given that hepatic stellate cell activation contributes to increased sinusoidal resistance, the anti-fibrotic action of DPP4-I and ARNI further supports their role in attenuating PH.
Notably, despite the strong vascular and anti-fibrotic effects, the two treatments, especially DPP4-I, did not signi
Nevertheless, this study has several limitations that should be considered. First, in our study, DPP4-I treatment significantly suppressed hepatic sEH expression and activity; however, the upstream mechanisms remain to be fully elucidated. Since serum GLP-1 Levels were unchanged in our non-diabetic CDAHFD-fed rats, it is unlikely that this effect is mediated via the classical GLP-1-dependent pathway. This observation supports the notion that DPP4-I may act through GLP-1-independent mechanisms, consistent with previous reports suggesting direct anti-inflammatory and antioxidant effects of DPP4 inhibitors. In particular, DPP4-I has been shown to modulate mitogen-activated protein kinase and transforming growth factor-β/Smad signaling, and reduce oxidative stress via downregulation of NOX4 in hepatic stellate cells. Given the observed positive correlation between hepatic NOX4 and sEH levels, it is plausible that the suppression of NOX4 contributes to the downregulation of sEH expression. Further mechanistic studies, such as the use of GLP-1 receptor-deficient or NOX4-overexpressing models, are warranted to delineate the precise signaling pathways involved in DPP4-I-mediated sEH inhibition.
Second, PH results not only from increased intrahepatic vascular resistance due to liver fibrosis and architectural distortion but also from a hyperdynamic circulatory state characterized by splanchnic vasodilatation[1-3]. In the present study, we also elucidated both agents increased SMA resistance suggesting that the efficacy of both drugs in treating PH may also involve an inhibitory effect on splanchnic vasodilation. The molecular mechanism underlying this action has not been clarified in this study and remains a topic for future investigation.
In conclusion, our findings suggest that combined therapy with vildagliptin and sacubitril/valsartan offers a promising strategy to ameliorate PH and liver fibrosis through vascular and fibrotic remodeling, primarily mediated via the suppression of sEH and restoration of EET signaling. These data support further preclinical and clinical studies to explore this combination as a novel therapeutic approach in cirrhosis-associated PH.
| 1. | Gracia-Sancho J, Marrone G, Fernández-Iglesias A. Hepatic microcirculation and mechanisms of portal hypertension. Nat Rev Gastroenterol Hepatol. 2019;16:221-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 178] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 2. | de Franchis R, Bosch J, Garcia-Tsao G, Reiberger T, Ripoll C; Baveno VII Faculty. Baveno VII - Renewing consensus in portal hypertension. J Hepatol. 2022;76:959-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1537] [Cited by in RCA: 1506] [Article Influence: 502.0] [Reference Citation Analysis (2)] |
| 3. | Guixé-Muntet S, Quesada-Vázquez S, Gracia-Sancho J. Pathophysiology and therapeutic options for cirrhotic portal hypertension. Lancet Gastroenterol Hepatol. 2024;9:646-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 4. | Villanueva C, Tripathi D, Bosch J. Preventing the progression of cirrhosis to decompensation and death. Nat Rev Gastroenterol Hepatol. 2025;22:265-280. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 5. | Villanueva C, Albillos A, Genescà J, Garcia-Pagan JC, Calleja JL, Aracil C, Bañares R, Morillas RM, Poca M, Peñas B, Augustin S, Abraldes JG, Alvarado E, Torres F, Bosch J. β blockers to prevent decompensation of cirrhosis in patients with clinically significant portal hypertension (PREDESCI): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2019;393:1597-1608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 453] [Article Influence: 75.5] [Reference Citation Analysis (0)] |
| 6. | Rodrigues SG, Mendoza YP, Bosch J. Beta-blockers in cirrhosis: Evidence-based indications and limitations. JHEP Rep. 2020;2:100063. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 95] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 7. | de Brito Nunes M, Knecht M, Wiest R, Bosch J, Berzigotti A. Predictors and management of post-banding ulcer bleeding in cirrhosis: A systematic review and meta-analysis. Liver Int. 2023;43:1644-1653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | Lv Y, Wang Q, Luo B, Bai W, Li M, Li K, Wang Z, Xia D, Guo W, Li X, Yuan J, Zhang N, Wang X, Xie H, Pan Y, Nie Y, Yin Z, Fan D, Han G. Identifying the optimal measurement timing and hemodynamic targets of portal pressure gradient after TIPS in patients with cirrhosis and variceal bleeding. J Hepatol. 2025;82:245-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 9. | Xiang Y, Tie J, Wang G, Zhuge Y, Wu H, Zhu X, Xue H, Liu S, Yang L, Xu J, Zhang F, Zhang M, Wei B, Li P, Wang Z, Wu W, Chen C, Yang S, Han Y, Tang C, Qi X, Zhang C. Post-TIPS Overt Hepatic Encephalopathy Increases Long-Term but Not Short-Term Mortality in Cirrhotic Patients With Variceal Bleeding: A Large-Scale, Multicenter Real-World Study. Aliment Pharmacol Ther. 2025;61:1183-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Node K, Huo Y, Ruan X, Yang B, Spiecker M, Ley K, Zeldin DC, Liao JK. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science. 1999;285:1276-1279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 944] [Cited by in RCA: 994] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 11. | Zeldin DC. Epoxygenase pathways of arachidonic acid metabolism. J Biol Chem. 2001;276:36059-36062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 523] [Cited by in RCA: 518] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 12. | Di Pascoli M, Zampieri F, Verardo A, Pesce P, Turato C, Angeli P, Sacerdoti D, Bolognesi M. Inhibition of epoxyeicosatrienoic acid production in rats with cirrhosis has beneficial effects on portal hypertension by reducing splanchnic vasodilation. Hepatology. 2016;64:923-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Deng W, Zhu Y, Lin J, Zheng L, Zhang C, Luo M. Inhibition of soluble epoxide hydrolase lowers portal hypertension in cirrhotic rats by ameliorating endothelial dysfunction and liver fibrosis. Prostaglandins Other Lipid Mediat. 2017;131:67-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Zhang CH, Zheng L, Gui L, Lin JY, Zhu YM, Deng WS, Luo M. Soluble epoxide hydrolase inhibition with t-TUCB alleviates liver fibrosis and portal pressure in carbon tetrachloride-induced cirrhosis in rats. Clin Res Hepatol Gastroenterol. 2018;42:118-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 15. | Zhao Z, Zhang C, Lin J, Zheng L, Li H, Qi X, Huo H, Lou X, Hammock BD, Hwang SH, Bao Y, Luo M. COX-2/sEH Dual Inhibitor PTUPB Alleviates CCl (4) -Induced Liver Fibrosis and Portal Hypertension. Front Med (Lausanne). 2021;8:761517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Wang X, Gu H, Li K, Lin J, Zhu Y, Deng W. DPP4 inhibitor reduces portal hypertension in cirrhotic rats by normalizing arterial hypocontractility. Life Sci. 2021;284:119895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3381] [Cited by in RCA: 4123] [Article Influence: 206.2] [Reference Citation Analysis (3)] |
| 18. | Nguyen Dinh Cat A, Touyz RM. Cell signaling of angiotensin II on vascular tone: novel mechanisms. Curr Hypertens Rep. 2011;13:122-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 19. | Ai D, Fu Y, Guo D, Tanaka H, Wang N, Tang C, Hammock BD, Shyy JY, Zhu Y. Angiotensin II up-regulates soluble epoxide hydrolase in vascular endothelium in vitro and in vivo. Proc Natl Acad Sci U S A. 2007;104:9018-9023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 135] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 20. | Vasconez AE, Janetzko P, Oo JA, Pflüger-Müller B, Ratiu C, Gu L, Helin K, Geisslinger G, Fleming I, Schröder K, Fork C, Brandes RP, Leisegang MS. The histone demethylase Jarid1b mediates angiotensin II-induced endothelial dysfunction by controlling the 3'UTR of soluble epoxide hydrolase. Acta Physiol (Oxf). 2019;225:e13168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Hubers SA, Brown NJ. Combined Angiotensin Receptor Antagonism and Neprilysin Inhibition. Circulation. 2016;133:1115-1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 172] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 22. | Sansoè G, Aragno M, Mastrocola R, Restivo F, Mengozzi G, Smedile A, Rosina F, Danni O, Parola M, Rizzetto M. Neutral endopeptidase (EC 3.4.24.11) in cirrhotic liver: a new target to treat portal hypertension? J Hepatol. 2005;43:791-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Ortiz C, Klein S, Reul WH, Magdaleno F, Gröschl S, Dietrich P, Schierwagen R, Uschner FE, Torres S, Hieber C, Meier C, Kraus N, Tyc O, Brol M, Zeuzem S, Welsch C, Poglitsch M, Hellerbrand C, Alfonso-Prieto M, Mira F, Keller UAD, Tetzner A, Moore A, Walther T, Trebicka J. Neprilysin-dependent neuropeptide Y cleavage in the liver promotes fibrosis by blocking NPY-receptor 1. Cell Rep. 2023;42:112059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 24. | Pun CK, Chang CC, Chuang CL, Huang HC, Hsu SJ, Huang YH, Hou MC, Lee FY. Dual angiotensin receptor and neprilysin inhibitor reduced portal pressure through peripheral vasodilatation and decreasing systemic arterial pressure in cirrhotic rats. J Chin Med Assoc. 2023;86:786-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 25. | El-Demerdash E, Abdel-Sattar SA, El-Bakly WM, Mohamed EA. Antifibrotic Effects of Carvedilol and Impact of Liver Fibrosis on Carvedilol Pharmacokinetics in a Rat model. Eur J Drug Metab Pharmacokinet. 2017;42:767-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Hendawy AS, El-Lakkany NM, Mantawy EM, Hammam OA, Botros SS, El-Demerdash E. Vildagliptin alleviates liver fibrosis in NASH diabetic rats via modulation of insulin resistance, oxidative stress, and inflammatory cascades. Life Sci. 2022;304:120695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 27. | Asada S, Kaji K, Nishimura N, Koizumi A, Matsuda T, Tanaka M, Yorioka N, Sato S, Kitagawa K, Namisaki T, Akahane T, Yoshiji H. Tofogliflozin Delays Portal Hypertension and Hepatic Fibrosis by Inhibiting Sinusoidal Capillarization in Cirrhotic Rats. Cells. 2024;13:538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 28. | Albillos A, Bañares R, Barrios C, Clemente G, Rossi I, Escartin P, Bosch J. Oral administration of clonidine in patients with alcoholic cirrhosis. Hemodynamic and liver function effects. Gastroenterology. 1992;102:248-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 30] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 29. | Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ; Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6807] [Cited by in RCA: 8249] [Article Influence: 412.5] [Reference Citation Analysis (5)] |
| 30. | Hu P, Wu X, Khandelwal AR, Yu W, Xu Z, Chen L, Yang J, Weisbrod RM, Lee KSS, Seta F, Hammock BD, Cohen RA, Zeng C, Tong X. Endothelial Nox4-based NADPH oxidase regulates atherosclerosis via soluble epoxide hydrolase. Biochim Biophys Acta Mol Basis Dis. 2017;1863:1382-1391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 31. | Liu X, Qin Z, Liu C, Song M, Luo X, Zhao H, Qian D, Chen J, Huang L. Nox4 and soluble epoxide hydrolase synergistically mediate homocysteine-induced inflammation in vascular smooth muscle cells. Vascul Pharmacol. 2019;120:106544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 32. | Xie G, Choi SS, Syn WK, Michelotti GA, Swiderska M, Karaca G, Chan IS, Chen Y, Diehl AM. Hedgehog signalling regulates liver sinusoidal endothelial cell capillarisation. Gut. 2013;62:299-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 104] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 33. | Panés J, Perry MA, Anderson DC, Muzykantov VR, Carden DL, Miyasaka M, Granger DN. Portal hypertension enhances endotoxin-induced intercellular adhesion molecule 1 up-regulation in the rat. Gastroenterology. 1996;110:866-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 34. | Buck M, Garcia-Tsao G, Groszmann RJ, Stalling C, Grace ND, Burroughs AK, Patch D, Matloff DS, Clopton P, Chojkier M. Novel inflammatory biomarkers of portal pressure in compensated cirrhosis patients. Hepatology. 2014;59:1052-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 35. | Hilscher MB, Sehrawat T, Arab JP, Zeng Z, Gao J, Liu M, Kostallari E, Gao Y, Simonetto DA, Yaqoob U, Cao S, Revzin A, Beyder A, Wang RA, Kamath PS, Kubes P, Shah VH. Mechanical Stretch Increases Expression of CXCL1 in Liver Sinusoidal Endothelial Cells to Recruit Neutrophils, Generate Sinusoidal Microthombi, and Promote Portal Hypertension. Gastroenterology. 2019;157:193-209.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 170] [Article Influence: 28.3] [Reference Citation Analysis (1)] |
| 36. | Zhu CP, Liu SQ, Wang KQ, Xiong HL, Aristu-Zabalza P, Boyer-Díaz Z, Feng JF, Song SH, Luo C, Chen WS, Zhang X, Dong WH, Gracia-Sancho J, Xie WF. Targeting 5-Hydroxytryptamine Receptor 1A in the Portal Vein to Decrease Portal Hypertension. Gastroenterology. 2024;167:993-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 37. | Wei G, An P, Vaid KA, Nasser I, Huang P, Tan L, Zhao S, Schuppan D, Popov YV. Comparison of murine steatohepatitis models identifies a dietary intervention with robust fibrosis, ductular reaction, and rapid progression to cirrhosis and cancer. Am J Physiol Gastrointest Liver Physiol. 2020;318:G174-G188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 38. | Naik I, Yeo YH, Ng WH, Ma KS, Yang JD. Carvedilol is associated with lower risk of hepatic decompensation compared to other non-selective beta-blockers in cirrhosis. J Hepatol. 2025;83:e88-e90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 39. | Tripathi D, Handley K, Holden L, Abdali Z, Jowett S, Mathers J, Poyner C, Richardson P, Ferguson J, Rowe I; CALIBRE trial collaborative group. Clinical Trial: A Multicentre Randomised Controlled Trial of Carvedilol Versus Variceal Band Ligation in Primary Prevention of Variceal Bleeding in Liver Cirrhosis (CALIBRE Trial). Aliment Pharmacol Ther. 2025;61:1740-1754. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 40. | Klein S, Van Beuge MM, Granzow M, Beljaars L, Schierwagen R, Kilic S, Heidari I, Huss S, Sauerbruch T, Poelstra K, Trebicka J. HSC-specific inhibition of Rho-kinase reduces portal pressure in cirrhotic rats without major systemic effects. J Hepatol. 2012;57:1220-1227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 41. | Ezhilarasan D. Endothelin-1 in portal hypertension: The intricate role of hepatic stellate cells. Exp Biol Med (Maywood). 2020;245:1504-1512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 42. | Li H. Intercellular crosstalk of liver sinusoidal endothelial cells in liver fibrosis, cirrhosis and hepatocellular carcinoma. Dig Liver Dis. 2022;54:598-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 43. | Dai Q, Ain Q, Seth N, Rooney M, Zipprich A. Liver sinusoidal endothelial cells: Friend or foe in metabolic dysfunction- associated steatotic liver disease/metabolic dysfunction-associated steatohepatitis. Dig Liver Dis. 2025;57:493-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 44. | Yao Q, Lin Y, Li X, Shen X, Wang J, Tu C. Curcumin ameliorates intrahepatic angiogenesis and capillarization of the sinusoids in carbon tetrachloride-induced rat liver fibrosis. Toxicol Lett. 2013;222:72-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 45. | Gao J, Lan T, Kostallari E, Guo Y, Lai E, Guillot A, Ding B, Tacke F, Tang C, Shah VH. Angiocrine signaling in sinusoidal homeostasis and liver diseases. J Hepatol. 2024;81:543-561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Reference Citation Analysis (0)] |
| 46. | Corpechot C, Barbu V, Wendum D, Kinnman N, Rey C, Poupon R, Housset C, Rosmorduc O. Hypoxia-induced VEGF and collagen I expressions are associated with angiogenesis and fibrogenesis in experimental cirrhosis. Hepatology. 2002;35:1010-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 370] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 47. | Ehling J, Bartneck M, Wei X, Gremse F, Fech V, Möckel D, Baeck C, Hittatiya K, Eulberg D, Luedde T, Kiessling F, Trautwein C, Lammers T, Tacke F. CCL2-dependent infiltrating macrophages promote angiogenesis in progressive liver fibrosis. Gut. 2014;63:1960-1971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 225] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 48. | Thabut D, Shah V. Intrahepatic angiogenesis and sinusoidal remodeling in chronic liver disease: new targets for the treatment of portal hypertension? J Hepatol. 2010;53:976-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 219] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 49. | Kaji K, Yoshiji H, Ikenaka Y, Noguchi R, Aihara Y, Douhara A, Moriya K, Kawaratani H, Shirai Y, Yoshii J, Yanase K, Kitade M, Namisaki T, Fukui H. Dipeptidyl peptidase-4 inhibitor attenuates hepatic fibrosis via suppression of activated hepatic stellate cell in rats. J Gastroenterol. 2014;49:481-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 50. | Suzuki J, Kaji K, Nishimura N, Kubo T, Tomooka F, Shibamoto A, Iwai S, Tsuji Y, Fujinaga Y, Kitagawa K, Namisaki T, Akahane T, Yoshiji H. A Combination of an Angiotensin II Receptor and a Neprilysin Inhibitor Attenuates Liver Fibrosis by Preventing Hepatic Stellate Cell Activation. Biomedicines. 2023;11:1295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |