Published online Aug 14, 2025. doi: 10.3748/wjg.v31.i30.109585
Revised: June 8, 2025
Accepted: July 2, 2025
Published online: August 14, 2025
Processing time: 84 Days and 1.4 Hours
Celiac disease (CeD), an autoimmune disorder triggered by gluten ingestion, is characterized by non-specific clinical manifestations such as fatigue, abdominal pain, and nutritional deficiencies, often leading to substantial diagnostic delays. Prolonged delays (≥ 2 years from symptom onset) are associated with increased risks of complications like osteoporosis, small intestinal lymphoma, and reduced quality of life.
To estimate diagnostic delay prevalence and identify risk factors in Chinese CeD patients.
We reviewed clinical records of 166 patients diagnosed with CeD from 2017 onward. Patient-attributed delays were measured from symptom onset to first consultation, while physician-related delays were measured from initial visit to diagnosis/treatment. Data on demographics, symptoms, time from onset to diagnosis, and laboratory results were analyzed. Logistic regression models were used to identify associations, while restricted cubic splines explored nonlinearities. Mediation analysis assessed the roles of intermediate factors in delayed diagnosis.
Delayed diagnosis (over 2 years from symptom onset) was observed in 42.2% of patients. Patients with diagnostic delay exceeding 5 years accounted for 18.7%. The mean interval from symptom onset to the first medical visit was 12.32 months, with an average of 20.57 months from the first visit to diagnosis. The time from first consultation to diagnosis significantly increased with prolonged delay (P < 0.001). Multivariate analysis showed that blood urea nitrogen (BUN) was an independent risk factor (OR = 1.29, 95%CI: 1.01–1.65, P = 0.038). A nonlinear association was observed between BUN and delayed diagnosis, with a threshold of 4.3 mmol/L; the risk significantly increased above this threshold (OR = 1.39, P = 0.04). Subgroup analyses indicated that the risk effect of BUN was stronger in females, non-classical CeD patients, Kazak ethnic group members, individuals without vitamin D deficiency/anemia, and those with Marsh III pathology (all P < 0.05). Mediation analysis revealed that folic acid deficiency and anemia mediated 11.9% (P = 0.028) and 13.0% (P = 0.044) of the effect of BUN on diagnostic delay, respectively.
Elevated BUN levels are independent predictors of diagnostic delay in CeD, with heterogeneity observed across gender, disease subtype, ethnicity, and pathological type. Clinicians should prioritize high-risk populations with BUN ≥ 4.3 mmol/L, particularly female patients with non-classical CeD and Kazak individuals, to reduce diagno
Core Tip: This study presents the largest single-center cohort of Chinese celiac disease (CeD) patients (Marsh ≥ II histopathology) leveraging Xinjiang’s regional referral advantage. Among 166 CeD patients, 42.2% experienced diagnostic delay (> 2 years). Blood urea nitrogen (BUN) was identified as an independent risk factor (OR = 1.29), with risk significantly increasing at ≥ 4.3 mmol/L. Subgroup analyses showed stronger associations of BUN with diagnostic delay in females, patients with non-classical CeD phenotypes, Kazak individuals, and other subgroups. These findings highlight BUN as a novel predictor and provide evidence to prioritize high-risk subgroups for timely screening.
- Citation: Li T, Feng Y, Wang M, Wang C, Gao F. Factors influencing diagnostic delays in celiac disease. World J Gastroenterol 2025; 31(30): 109585
- URL: https://www.wjgnet.com/1007-9327/full/v31/i30/109585.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i30.109585
Celiac disease (CeD) is an immune-mediated disorder triggered by gluten in genetically predisposed individuals, affecting about 1% of the global population[1]. Early diagnosis primarily depends on clinical symptoms, while serological testing and histological examination serve as key diagnostic tools, with the latter being the gold standard for diagnosis. Despite established diagnostic algorithms, many cases remain undiagnosed or misdiagnosed due to silent, asymptomatic, or atypical presentations. In developed countries, there are an estimated average of five undiagnosed cases for every diagnosed case[2]. In developing countries, diagnosis often depends on symptomatic presentation. The diagnostic delay for CeD ranges from 9.7 to 13.3 years[3], highlighting the significant time many patients endure symptoms before receiving a diagnosis.
In many cases, the atypical nature of symptoms affects a significant proportion of patients with undiagnosed CeD. The wide spectrum of CeD symptoms complicates timely diagnosis. Many patients present with atypical or non-gastrointes
Delayed diagnosis prolongs patient suffering, reduces quality of life, and increases the risk of complications. Delayed diagnoses correlate with poorer mental health, greater anxiety, and worse self-perceived health outcomes[4]. In pediatric and adolescent patients, delays can severely impair growth and development[5]. Additionally, prolonged diagnostic delays result in increased healthcare utilization, higher medical costs, and elevated risks of autoimmune diseases and certain cancers, including intestinal lymphoma[6].
Thus, timely diagnosis and treatment of CeD are essential to improve patients' quality of life, reduce their risk of health complications, and improve their overall health. This study aimed to explore factors associated with diagnostic delays in CeD by analyzing clinical data from patients with previously diagnosed CeD.
This retrospective study included patients aged 18 years or older with a primary diagnosis of CeD who were admitted to the Department of Gastroenterology, People’s Hospital of Xinjiang Uygur Autonomous Region from March 2016 to October 2024. Patients without a pathological diagnosis, relevant laboratory data, or complete clinical records were excluded. A diagnostic delay was defined as a period exceeding 2 years between symptom onset and CeD diagnosis.
CeD was diagnosed based on the World Gastroenterology Organization’s 2013 guidelines[7]. Patients with a positive serum anti-tissue transglutaminase antibody-IgA test and a small intestinal biopsy showing Marsh ≥ II pathological changes were confirmed to have CeD. For individuals with total IgA deficiency, deaminated gliadin peptide antigen-IgG was used for diagnosis. Patients with a lack of pathological diagnosis and laboratory examination indexes and incomplete clinical medical records were excluded from the analysis.
Patient-attributed delays were determined by analyzing the time from the onset of symptoms reported by patients to the first consultation with a healthcare provider. In contrast, physician-related delays were calculated from the initial consultation to the definitive diagnosis or treatment initiation. We utilized patient interviews and medical records to gather these data points.
Demographic and clinical data, including age, gender, ethnicity, height, weight, body mass index (BMI), disease duration, clinical symptoms, complications, gastroscopy findings, pathology results, complete blood count, anemia-related markers, and bone mineral density, were retrieved from medical records. Additional details were supplemented through follow-up assessments.
Biopsies were performed with at least one specimen from the duodenal bulb (at 9 or 12 o’clock positions) and four specimens from the descending duodenum. Specimens were fixed in 3.7% neutral formaldehyde solution and examined for pathological changes based on the Marsh-Oberhuber classification system[8].
Clinical classification of CeD: Based on the Oslo definition, there are three common types: (1) Classical CeD, which is characterized by symptoms of malabsorption, such as diarrhea, weight loss, or growth delay; (2) Non-classical CeD, characterized by symptoms other than classical features, excluding significant malabsorption; and (3) Subclinical CeD, characterized by symptomatic cases below the clinical detection threshold.
Anemia: Defined by the World Health Organization as hemoglobin (Hb) < 130 g/L for males and < 120 g/L for females at sea level.
Frequent hospitalizations: Defined as three or more hospital admissions.
Complications: Included thyroid disease, elevated serum aminotransferase levels, ulcerative colitis, nephropathy, connective tissue disease, IgA deficiency, osteomalacia, epilepsy, tumors, and lymphoma.
Generalized linear regression models were used to evaluate relationships between clinical and biochemical indicators and diagnostic timing. Multiple logistic regression estimated ORs for these indicators. Restricted cubic splines (RCS) were employed to assess nonlinear relationships, with a two-stage analysis conducted to identify turning points in RCS curves. Subgroup analyses examined stratified relationships, and mediation analysis was performed to evaluate the role of specific factors in the association between diagnostic delay and clinical outcomes. All statistical analyses were conducted using R version 4.2.2, with two-sided P values < 0.05 considered statistically significant.
Clinical records of 166 patients with newly diagnosed CeD were analyzed. Of these, 116 patients (69.9%) were female, and 64 (38.6%) were of Kazakh ethnicity. Ninety-three patients (56.02%) received a diagnosis within 2 years of symptom onset, while 43.98% of the patients had delayed diagnosis, among which 31 patients (18.7%) were diagnosed 5 years later. The average time from symptom onset to the first clinical visit was 12.32 months, whereas the mean duration from the first visit to diagnosis was 20.57 months, reflecting a significantly longer delay attributable to physicians compared to patients. Notably, delayed diagnosis was associated with significantly longer time from first visit to diagnosis. Among the patients, 144 (86.7%) had at least one complication, and 138 (83.1%) presented with classic CeD. The frequency of hospitalizations was higher among those with delayed diagnoses. Most patients (63, or 38.0%) experienced disease onset between the ages of 45 and 55, whereas 81 patients (48.8%) received their diagnosis between 45 and 60 years of age. There were no statistically significant differences across groups in terms of the anti-tissue transglutaminase levels, BMI, education level, occupation, or pathological Marsh classification (Table 1).
| Measures | Diagnosed within 2 years | Delayed diagnosis group | ||
| Diagnosed within 2-5 years (n = 42) | Diagnosed over 5 years | P value | ||
| Gender | 0.485 | |||
| Female | 66 (71.0) | 31 (73.8) | 19 (61.3) | |
| Male | 27 (29.0) | 11 (26.2) | 12 (38.7) | |
| Ethnicity | 0.241 | |||
| Han | 17 (18.3) | 12 (28.6) | 5 (16.1) | |
| Uygur | 30 (32.3) | 17 (40.5) | 13 (41.9) | |
| Kazak | 41 (44.1) | 10 (23.8) | 13 (41.9) | |
| Other ethnic | 5 (5.4) | 3 (7.1) | 0 (0.0) | |
| tTG (CU), M (P25, P75) | 928.1 (45.4, 2279.2) | 243.4 (32.1, 1254.6) | 1082.5 (82.7, 1988.5) | 0.745 |
| BMI (kg/m2), M (P25, P75) | 20.6 (18.7, 24.7) | 22.3 (20.2, 25.1) | 22.7 (19.1, 25.3) | 0.874 |
| First onset of symptoms to visit (months), | 1 (0.6, 15) | 12.3 (2, 36) | 12 (1, 21) | < 0.001 |
| First visit to diagnosis (months), M (P25, P75) | 0 (0, 0) | 20.6 (0, 24) | 96 (60, 114) | < 0.001 |
| Patients with comorbidities | 15 (16.1) | 5 (11.9) | 2 (6.5) | 0.371 |
| Patients without comorbidities | 78 (83.9) | 37 (88.1) | 29 (93.5) | |
| Non-classical CeD | 15 (16.1) | 9 (21.4) | 4 (12.9) | 0.605 |
| Classical CeD | 78 (83.9) | 33 (78.6) | 27 (87.1) | |
| Number of hospitalizations ≤ 3 | 85 (91.4) | 33 (78.6) | 19 (61.3) | < 0.001 |
| Number of hospitalizations > 3 | 8 (8.6) | 9 (21.4) | 12 (38.7) | |
| Age at diagnosis | 0.046 | |||
| ≤ 45 years old | 48 (51.6) | 14 (33.3) | 9 (29.0) | |
| 45-60 years old | 37 (39.8) | 23 (54.8) | 21 (67.7) | |
| > 60 years old | 8 (8.6) | 5 (11.9) | 1 (3.2) | |
| Age of onset | 0.308 | |||
| ≤ 45 years old | 33 (35.5) | 11 (26.2) | 9 (29.0) | |
| 45-55 | 37 (39.8) | 13 (31.0) | 13 (41.9) | |
| > 55 years old | 23 (24.7) | 18 (42.9) | 9 (29.0) | |
| Education | 31 (33.3) | 13 (31.0) | 10 (32.3) | 0.826 |
| Below junior high school | ||||
| High school | 37 (39.8) | 21 (50.0) | 13 (41.9) | |
| Bachelor's degree or above | 25 (26.9) | 8 (19.0) | 8 (25.8) | |
| Job | 44 (47.3) | 18 (42.9) | 14 (45.2) | 0.888 |
| Freelance | ||||
| Contract | 49 (52.7) | 24 (57.1) | 17 (54.8) | |
| Pathology | 51 (54.8) | 25 (59.5) | 18 (58.1) | 0.865 |
| Marsh II | ||||
| Marsh III | 42 (45.2) | 17 (40.5) | 13 (41.9) | |
Univariate regression analysis showed that diagnostic age (OR = 1.02, 95%CI: 1.00-1.05, P = 0.063), white blood cell count (OR = 0.83, 95%CI: 0.70-0.99, P = 0.042), blood urea nitrogen (BUN) level (OR = 1.37, 95%CI: 1.10-1.71, P = 0.005), and serum iron (OR = 1.10, 95%CI: 1.02-1.19, P = 0.016) were associated with delayed diagnosis. Multivariate regression analysis revealed that after adjusting for confounders, only BUN level remained an independent risk factor (OR = 1.29, 95%CI: 1.01-1.65, P = 0.038; Table 2).
| Measures | Diagnosed within 2 years | Delayed diagnosis group | Univariate regression analysis, OR (95%CI), P value | Multivariate regression analysis, OR (95%CI), P value | |
| Diagnosed within 2-5 years | Diagnosed over 5 years | ||||
| Age at diagnosis | 46.8 ± 13.1 | 51.3 ± 14.2 | 49.6 ± 10.3 | 1.02 (1.00-1.05), P = 0.063 | 1.01 (0.98-1.04), P = 0.554 |
| Leukocytes | 6.4 ± 1.9 | 5.6 ± 1.6 | 6.1 ± 1.8 | 0.83 (0.70-0.99), P = 0.042 | 0.83 (0.68-1.00), P = 0.051 |
| Hemoglobin | 119.6 ± 23.6 | 121.8 ± 23.1 | 118.2 ± 28.4 | 1.00 (0.99-1.01), P = 0.856 | |
| Total protein | 68.7 ± 10.5 | 68.1 ± 9.9 | 65.8 ± 9.5 | 0.98 (0.95-1.01), P = 0.307 | |
| Serum calcium | 2.1 ± 0.2 | 2.2 ± 0.2 | 2.2 ± 0.2 | 3.85 (0.76-19.45), P = 0.103 | 1.44 (0.23-8.80), P = 0.696 |
| Thyroid stimulating hormone | 4.0 ± 5.0 | 3.1 ± 2.4 | 3.5 ± 4.6 | 0.96 (0.89-1.04), P = 0.295 | |
| Blood urea nitrogen | 4.0 ± 1.4 | 4.7 ± 1.7 | 4.9 ± 2.1 | 1.37 (1.10-1.71), P = 0.005 | 1.29 (1.01-1.65), P = 0.038 |
| Serum iron | 8.2 ± 3.1 | 10.5 ± 5.5 | 10.4 ± 9.3 | 1.10 (1.02-1.19), P = 0.016 | 1.07 (1.00-1.15), P = 0.063 |
A significant dose-response relationship was observed between BUN levels and diagnostic delay. Without covariate adjustment, each 1 mmol/L increase in BUN was associated with a 37% increased risk of delayed diagnosis (OR = 1.37, 95%CI: 1.12-1.73, P = 0.005). After stepwise adjustment for age, sex, BMI, comorbidities (vitamin D deficiency, anemia, thyroid disease), and hematologic parameters (Table 3), Model 4 showed a persistent significant independent effect of BUN (OR = 1.42, 95%CI: 1.09-1.91, P = 0.015), suggesting that BUN may influence diagnostic delay by reflecting systemic metabolic status or underlying comorbidities (Figure 1).
| Model | OR | 95%CI | P value |
| 1 | 1.37 | 1.12-1.73 | 0.005 |
| 2 | 1.34 | 1.07-1.72 | 0.015 |
| 3 | 1.37 | 1.09-1.78 | 0.013 |
| 4 | 1.42 | 1.09-1.91 | 0.015 |
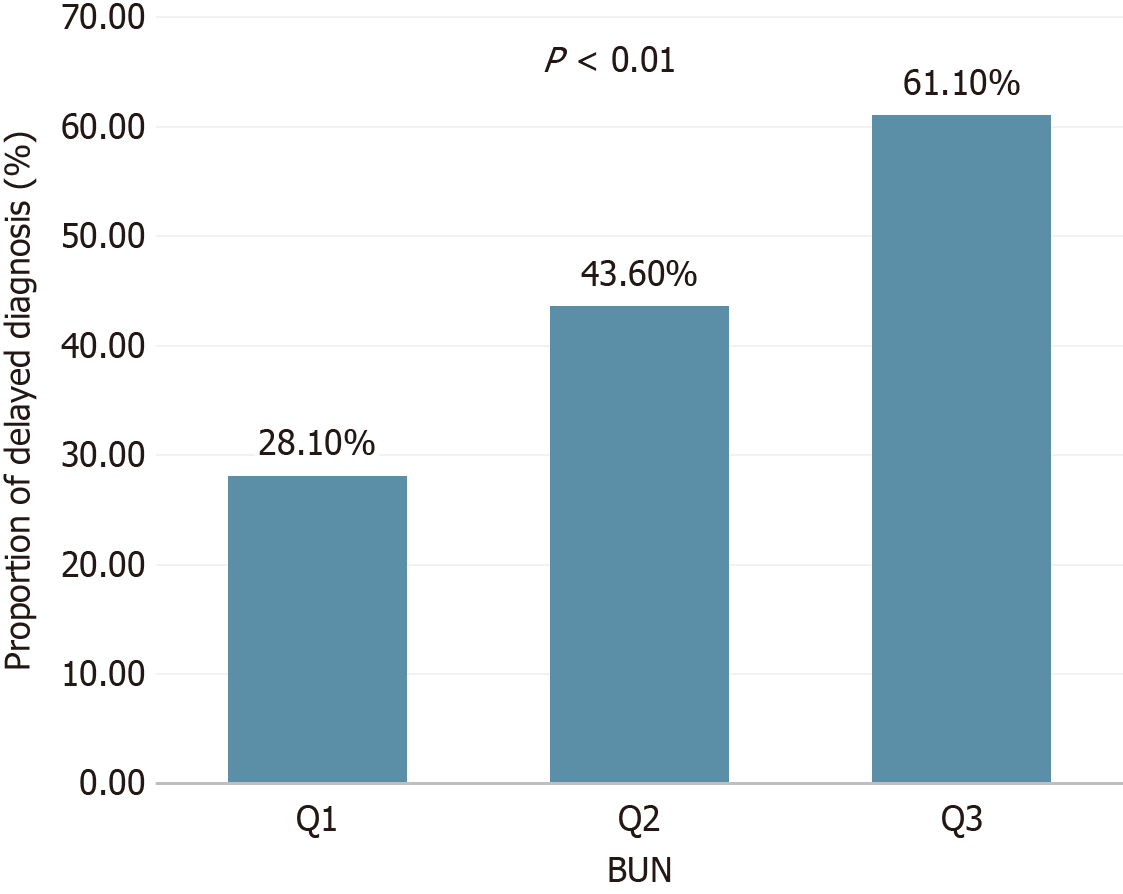
Population characteristic analysis based on BUN tertiles demonstrated that the high BUN group (Q3) had a significantly older diagnostic age (P < 0.001), a longer time from first visit to diagnosis (P = 0.002), and higher proportions of male sex (44.4% vs Q1 15.8%, P = 0.004) and Uyghur ethnicity (46.3% vs Q1 29.8%, P = 0.001; Table 4). These findings suggest potential interactions between BUN and factors such as age and ethnicity.
| Indicators | Q1 | Q2 | Q3 | P value |
| Gender | 0.004 | |||
| Female | 48 (84.2) | 38 (69.1) | 30 (55.6) | |
| Male | 9 (15.8) | 17 (30.9) | 24 (44.4) | |
| Ethnicity | 0.001 | |||
| Han | 5 (8.8) | 13 (23.6) | 16 (29.6) | |
| Uygur | 17 (29.8) | 18 (32.7) | 25 (46.3) | |
| Kazak | 30 (52.6) | 24 (43.6) | 10 (18.5) | |
| Other ethnic | 5 (8.8) | 0 (0.0) | 3 (5.6) | |
| tTG (CU) | 769.3 (29, 1896.5) | 967.4 (71.7, 2673.4) | 263.2 (38.8, 1660) | 0.639 |
| BMI (kg/m2) | 20.1 (18.4, 24.2) | 21.9 (19, 24.1) | 22.5 (20, 25.9) | 0.705 |
| First onset of symptoms to visit (months) | 1 (0.5, 12) | 3 (1, 12) | 6 (1, 12.3) | 0.514 |
| First visit to diagnosis (months) | 0 (0, 1) | 0 (0, 18) | 14 (0, 48) | 0.002 |
| Patients with comorbidities (%) | 11 (19.3) | 7 (12.7) | 4 (7.4) | 0.18 |
| Patients without comorbidities | 46 (80.7) | 48 (87.3) | 50 (92.6) | |
| Non-classical CeD | 5 (8.8) | 10 (18.2) | 13 (24.1) | 0.094 |
| Classical CeD | 52 (91.2) | 45 (81.8) | 41 (75.9) | |
| Number of hospitalizations ≤ 3 | 48 (84.2) | 49 (89.1) | 40 (74.1) | 0.109 |
| Number of hospitalizations > 3 | 9 (15.8) | 6 (10.9) | 14 (25.9) | |
| Age at diagnosis | 43.89 ± 11.78 | 47.67 ± 12.79 | 54.02 ± 12.67 | < 0.001 |
| Age of onset | 42.85 ± 12.74 | 46.95 ± 12.87 | 52.70 ± 13.52 | 0.001 |
| Education | 0.636 | |||
| Below junior high school | 19 (33.3) | 15 (27.3) | 20 (37.0) | |
| High school | 22 (38.6) | 28 (50.9) | 21 (38.9) | |
| Bachelor's degree or above | 16 (28.1) | 12 (21.8) | 13 (24.1) | |
| Job | 0.031 | |||
| Freelance | 19 (33.3) | 32 (58.2) | 25 (46.3) | |
| Contract | 38 (66.7) | 23 (41.8) | 29 (53.7) | |
| Pathology | 0.438 | |||
| Marsh II | 29 (50.9) | 31 (56.4) | 34 (63.0) | |
| Marsh III | 28 (49.1) | 24 (43.6) | 20 (37.0) |
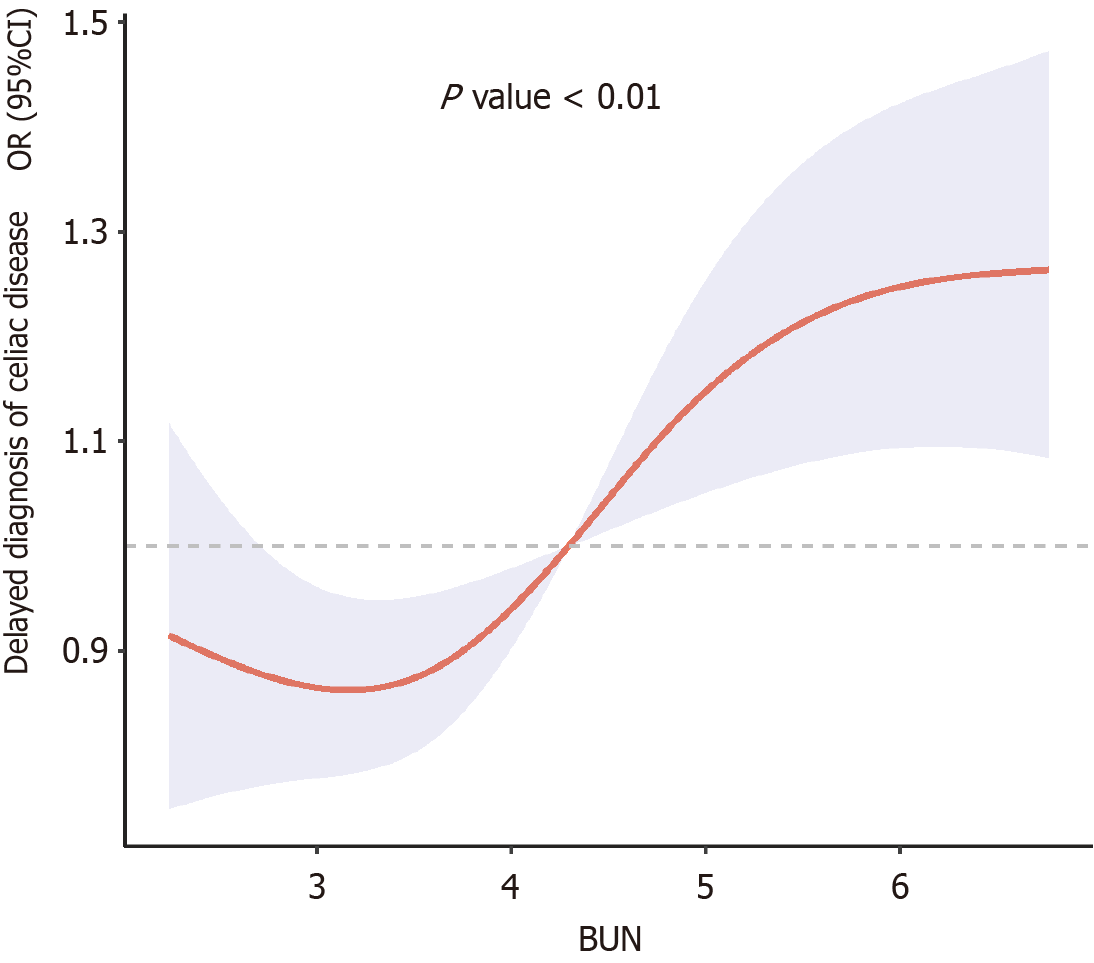
RCS analysis revealed a nonlinear dose-response relationship between BUN levels and diagnostic delay (Figure 2). Two-stage piecewise linear regression showed that there was a significant inflection point when the BUN threshold was 4.3 mmol/L (P = 0.04). When BUN was ≤ 4.3 mmol/L, the risk of delayed diagnosis did not increase significantly (OR = 0.67, 95%CI: 0.23-1.93, P = 0.44); When BUN > 4.3 mmol/L, the risk significantly increased (OR = 1.39, 95%CI: 1.04-1.99, P = 0.04; Table 5). In the two-stage analysis, patients with BUN ≤ 4.3 mmol/L exhibited a significantly reduced risk of diagnostic delay (OR = 0.67, 95%CI: 0.23–1.93, P = 0.44) compared to those with BUN > 4.3 mmol/L. However, this effect was not statistically significant (P = 0.44), whereas BUN > 4.3 mmol/L independently increased the risk (OR = 1.39, P = 0.04). This result may reflect that mild abnormalities in renal function can serve as a marker of the severity of underlying diseases, leading to delayed clinical diagnosis.
| BUN | OR | 95%CI | P value |
| BUN or less tendency for 4.3 mmol/L | 0.67 | 0.23-1.93 | 0.44 |
| BUN > tendency for 4.3 mmol/L | 1.39 | 1.04-1.99 | 0.04 |
Subgroup analyses (Table 6) showed heterogeneous effects of BUN across patient characteristics. The association was stronger in females (OR = 1.49, P = 0.008) and non-classical CeD patients (OR = 3.71, P = 0.009). Among ethnic groups, Kazak patients exhibited a significant association (OR = 1.64, P = 0.040). Patients without anemia (OR = 1.76, P = 0.001) or vitamin D deficiency (OR = 1.57, P = 0.002) had stronger BUN-delay relationships. Hospitalization history (≥ 3 times, OR = 1.31, P = 0.032) and lower education level (below junior high, OR = 1.74, P = 0.012) further modified the association, suggesting complex interactions between renal function, socioeconomic factors, and diagnostic processes in CeD.
| Variables | OR | 95%CI | P value |
| Gender | |||
| Female | 1.49 | 1.11-1.99 | 0.008 |
| Male | 1.26 | 0.88-1.8 | 0.206 |
| Age (year) | |||
| < 45 | 1.26 | 0.89-1.69 | 0.197 |
| 45-65 | 1.67 | 0.91-3.06 | 0.099 |
| > 65 | 1.24 | 0.88-1.75 | 0.215 |
| Typing | |||
| Non-classical celiac disease | 3.71 | 1.38-9.95 | 0.009 |
| Classic celiac disease | 1.26 | 1.01-1.57 | 0.042 |
| Ethnic groups | |||
| Han nationality | 1.45 | 0.8-2.65 | 0.224 |
| Uygur | 1.17 | 0.9-1.53 | 0.245 |
| Kazak | 1.64 | 1.02-2.64 | 0.040 |
| Other ethnic | 3.24 | 0.6-17.35 | 0.171 |
| Combined vitamin D deficiency | |||
| No vitamin D deficiency | 1.57 | 1.19-2.09 | 0.002 |
| Have a vitamin D deficiency | 0.86 | 0.53-1.38 | 0.528 |
| Presence of anemia | |||
| No anemia | 1.76 | 1.25-2.48 | 0.001 |
| Having anemia | 1.07 | 0.82-1.4 | 0.613 |
| Number of hospitalizations | |||
| ≤ 3 | 1.65 | 0.9-3.05 | 0.107 |
| > 3 | 1.31 | 1.02-1.67 | 0.032 |
| Level of education | |||
| Below junior high school | 1.74 | 1.13-2.68 | 0.012 |
| High school | 1.2 | 0.83-1.75 | 0.339 |
| Bachelor's degree or above | 1.26 | 0.88-1.82 | 0.208 |
| Job | |||
| Freelance | 1.49 | 1.03-2.15 | 0.032 |
| Contract | 1.32 | 1-1.75 | 0.052 |
| Pathology | |||
| Marsh II | 1.29 | 0.98-1.7 | 0.073 |
| Marsh III | 1.5 | 1.05-2.16 | 0.027 |
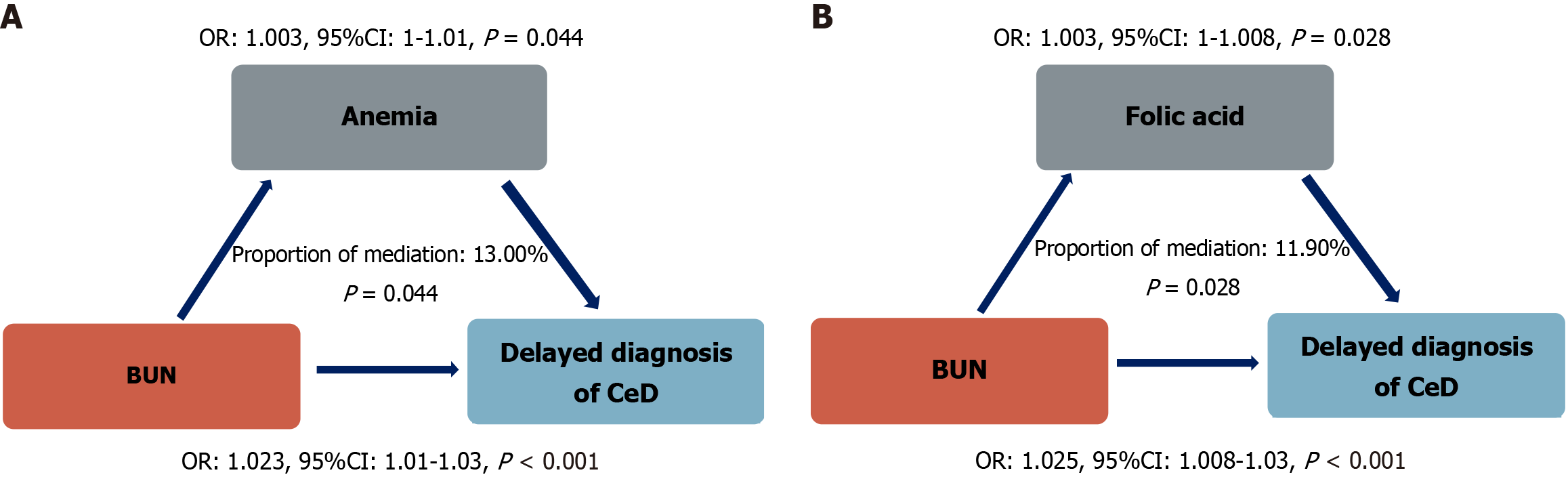
Analysis of the mediating role of folic acid deficiency and anemia in the association between BUN levels and the delayed diagnosis of CeD showed that the mediating effect of folic acid deficiency is significant. The total effect of BUN level on the delayed diagnosis of CeD is OR = 1.025 (95%CI: 1.008-1.03, P < 0.001), among which the mediating effect of folic acid deficiency accounted for 11.90% of the total effect (P = 0.028), and the effect of folic acid level itself on the delayed diagnosis of CeD was OR = 1.003 (95%CI: 1-1.008, P = 0.028). The mediating effect of anemia was also statistically significant. The total effect of BUN level on the delayed diagnosis of CeD was OR = 1.023 (95%CI: 1.01-1.03, P = 0.01), the mediating effect of anemia accounted for 13.00% of the total effect (P = 0.044), and the effect of anemia on the delayed diagnosis of CeD was OR = 1.003 (95%CI: 1-1.01, P = 0.044; Figure 3).
Despite the increasing prevalence of CeD, diagnostic delays remain a significant issue, adversely affecting patient quality of life and contributing to complications. This study provides the first detailed analysis of diagnostic delays and contributing factors among patients with CeD in China. Our findings show that 43.98% of patients experienced diagnostic delays, with 18.67% diagnosed more than 5 years after symptom onset. The average patient-attributed delay was 12.32 months, while physician-related delays averaged 20.57 months. These delays highlight gaps in both patient awareness and healthcare delivery. Contributing factors include non-specific symptoms, limited physician expertise, cultural and socioeconomic barriers, and healthcare system inefficiencies.
CeD symptoms vary widely, complicating diagnosis. While some patients present with classic gastrointestinal (GI) symptoms such as abdominal pain, diarrhea, and weight loss, others exhibit non-digestive manifestations like fatigue, anemia, skin rashes, or bone pain. Our study revealed that asymptomatic cases were typically diagnosed within 2 to 5 years, yet 16.3% of patients with GI symptoms experienced delays exceeding 5 years. This variation often leads clinicians to explore alternative diagnoses, such as infections or irritable bowel syndrome, before considering CeD. Early symptoms may be mild and non-specific, evolving over time, which further obscures diagnosis and delays appropriate interventions. Our finding that non-classical CeD (OR = 3.71) has a stronger association with diagnostic delay than classical CeD (OR = 1.26) accords with atypical presentations hindering clinical recognition. Classical CeD overt GI symptoms prompt earlier referrals, while non-classical CeD extraintestinal/subtle symptoms are often misattributed, delaying diagnosis. This underscores the need to raise awareness of non-classical symptoms for early detection.
Comorbidities present another diagnostic challenge. Conditions like thyroid disease and type 1 diabetes often coexist with CeD, masking symptoms or producing overlapping signs such as weight changes, fatigue, or GI discomfort. These diseases may cause an abnormal immune response in the patient, which can mask the symptoms of CeD[9]. Singh et al[10] recently highlighted this interconnection, advocating for targeted CeD screening in patients with autoimmune disease. Our findings indicate higher rates of diagnostic delay among patients with comorbidities, likely due to the increased complexity of their clinical presentations. Mental health conditions, including depression and anxiety, further complicate diagnosis by affecting patients' symptom reporting and physicians' clinical judgment[11]. The stronger link between BUN elevation and diagnostic delay in patients without vitamin D deficiency or anemia fits “silent” CeD. Common comorbidities like vitamin D deficiency and anemia often alert clinicians to CeD, but without these markers, mild symptoms may be dismissed as functional. This “masking effect” means those with BUN elevation but normal Hb and vitamin D might get renal/cardiac workups instead of GI assessment, highlighting the need for CeD screening in unexplained metabolic issues.
Age and gender also play roles in diagnostic delays. Older patients, particularly those aged 45-60, often present with atypical or vague symptoms, making diagnosis more challenging. Our study also showed that the incidence of delayed diagnosis was higher in patients aged 45-60 years, and patients over 60 years of age showed fewer delays, possibly due to increased health awareness and proactive healthcare-seeking behavior. However, we observed that age lost its significance in the multivariable model, a finding that may be attributed to the following mechanisms: First, BUN retained its unique independent predictive value after adjustment, which diminished the contribution of weaker correlates—including age and white blood cell count—as key drivers of diagnostic delay. Age, in this context, may represent a secondary phenomenon or downstream effect of underlying metabolic dysregulation rather than a primary driver of delayed diagnosis. Second, the nominal significance of age in univariate analysis (OR = 1.02, P = 0.063) reflects limited discriminative power, as the marginal association may not translate to clinical relevance. This, combined with reduced statistical power due to the moderate sample size and potential collinearity between age and BUN, age-related changes in renal function could influence both age itself and BUN levels, ultimately led to its loss of significance in the multivariable framework. Gender differences were also evident, with female patients experiencing longer diagnostic delays. Women often report non-GI symptoms, such as anemia or bone mineral density issues, which may divert attention from CeD. Subgroup analysis revealed that elevated BUN levels were strongly associated with delayed diagnosis in women, consistent with findings from studies conducted in other countries[12].
Psychological factors further contribute to diagnostic delays. Persistent symptoms can lead to depression or anxiety, discouraging patients from seeking timely medical care. Mild or episodic symptoms may prompt patients to pursue symptomatic treatment rather than definitive diagnoses, delaying further investigations[13]. Conversely, frequent healthcare visits may create mistrust in physicians' recommendations, leading to delays in essential diagnostic tests. Our findings showed that patients with frequent hospitalizations and elevated BUN levels faced higher risks of delayed diagnoses, often due to misdirected attention toward nephrology-related conditions.
Socioeconomic status and healthcare-seeking behavior are critical determinants of diagnostic delays. Patients with lower socioeconomic status are more likely to present with atypical symptoms, such as fatigue or anemia, rather than classic CeD symptoms[14]. Financial constraints, lack of health insurance, limited education, and restricted access to healthcare information exacerbate these delays. Low-income patients may be reluctant to seek medical care because of concerns about healthcare costs or even delay in seeking care after symptoms develop, which significantly increases diagnostic delay. Our subgroup analysis indicated that patients with lower education levels or unemployment experienced greater diagnostic delays, particularly when BUN levels were elevated.
From a healthcare system perspective, CeD remains underrecognized in certain regions, leading to misinterpretation of symptoms, especially in atypical cases. Physicians may fail to consider CeD or delay serological testing and biopsies. Effective communication between patients and physicians is also critical. Patients may inadequately describe their symptoms, while physicians may neglect detailed history-taking, resulting in diagnostic oversight[15]. The physician-related delay in this study (20.57 months) was shorter than delays reported in some countries, where diagnosis can take 6 to 14 years[16-19]. In contrast, our data are similar to those reported in a Danish study[20].
A key finding of this study is the association between elevated BUN levels and diagnostic delays. Dose-response and two-stage analyses revealed that higher BUN levels were significantly correlated with longer delays and increased diagnostic risk. Mediation analysis identified anemia and folate deficiency as contributing factors to this association, emphasizing the role of nutritional deficiencies in CeD pathophysiology[21].
Elevated BUN levels are multifactorial in origin, commonly linked to impaired renal function, hypovolemia, or excessive protein intake. Critically, these etiological factors may collectively disrupt folate absorption and metabolism. Folate deficiency not only predisposes individuals to megaloblastic anemia but also manifests as a constellation of non-specific clinical features—including profound fatigue and myasthenia—that overlap significantly with the manifestations of CeD. Reduced enterocyte turnover prolongs the exposure of the intestinal lumen to undigested nutrients, increasing urea production from bacterial fermentation and elevating BUN levels[22]. This pathophysiological convergence creates diagnostic ambiguity, potentially delaying recognition of underlying CeD in comorbid presentations. Patients with CeD may face an increased risk of kidney disease. Elevated BUN levels may exert a diagnostic masking effect on CeD, predisposing clinicians to prioritize investigations for renal or metabolic etiologies during initial evaluations, while potentially overlooking underlying CeD. This diagnostic overshadowing effect, compounded by symptom overlap, frequently delays CeD recognition, particularly in patients with concomitant BUN elevation and hematologic abnormalities. Anemia may elevate BUN indirectly: Reduced erythropoietin production in chronic disease states can impair renal function, slowing urea clearance[23]. Therefore, for patients with elevated BUN and anemia, especially those with related symptoms or high-risk factors, screening for CeD should be considered to ensure early diagnosis and timely intervention. This not only helps improve the nutritional status of patients, but also prevents long-term complications related to CeD. Future studies should further explore the interactions of BUN, folic acid, and anemia in the diagnosis of CeD to optimize the diagnostic process and improve diagnostic efficiency.
This study, while representing the largest single-center CeD cohort in China and leveraging our institution's role as the Xinjiang Celiac Disease Alliance hub (receiving all regional suspected CeD referrals), has some key limitations. First, sample size constraints persist. Though 166 cases is the largest Marsh ≥ II cohort in China, this remains modest compared to international multicenter studies, potentially limiting statistical power to detect weak but clinically meaningful associations—particularly in subgroup analyses with smaller strata. Second, selection bias affects generalizability. Our cohort comprises consecutive referred patients confirmed by Marsh ≥ II pathology, ensuring diagnostic accuracy but excluding two critical groups: (1) “Potential CeD” patients (seropositive but Marsh < II, possibly with longer delays due to lower clinical vigilance); and (2) Mild/asymptomatic CeD cases, which are under-referred. Thus, findings may not fully reflect the full CeD spectrum. Third, focus on Marsh ≥ II-confirmed CeD enhances result robustness by minimizing serological false positives but narrows scope. Excluding “potential CeD” individuals limits inference to advanced/symptomatic disease, potentially missing distinct delay patterns in earlier stages. Fourth, instability in subgroup analyses arises from small sample sizes in certain strata. Small samples are prone to random variation, risking over/underestimation of effect sizes; these findings are exploratory and require validation. Finally, the retrospective design introduces information bias. Data rely on historical records, which may be incomplete or subject to recall bias. Subtle/intermittent symptoms may be under-documented.
Diagnostic delays in CeD stem from a complex interplay of non-specific symptoms, patient behavior, healthcare system challenges, and comorbidities. Elevated BUN levels are independent predictors of diagnostic delay in CeD, with heterogeneity observed across gender, disease subtype, ethnicity, and pathological type. Clinicians should prioritize high-risk populations with BUN ≥ 4.3 mmol/L, particularly female patients with non-classical CeD and Kazak individuals, to reduce diagnostic delay.
| 1. | Catassi C, Verdu EF, Bai JC, Lionetti E. Coeliac disease. Lancet. 2022;399:2413-2426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 234] [Article Influence: 78.0] [Reference Citation Analysis (1)] |
| 2. | Lionetti E, Catassi C. New clues in celiac disease epidemiology, pathogenesis, clinical manifestations, and treatment. Int Rev Immunol. 2011;30:219-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 145] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 3. | Norström F, Lindholm L, Sandström O, Nordyke K, Ivarsson A. Delay to celiac disease diagnosis and its implications for health-related quality of life. BMC Gastroenterol. 2011;11:118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 121] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 4. | Fuchs V, Kurppa K, Huhtala H, Mäki M, Kekkonen L, Kaukinen K. Delayed celiac disease diagnosis predisposes to reduced quality of life and incremental use of health care services and medicines: A prospective nationwide study. United European Gastroenterol J. 2018;6:567-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 5. | Mehta S, Agarwal A, Pachisia AV, Singh A, Dang S, Vignesh D, Ahmed A, Chaudhari BR, Prasad S, Goyal RM, Chavan A, Singh A, Kumar S, Sharma D, Chauhan A, Rajput MS, Rajput S, Das P, Falodia S, Sinha SK, Kochhar R, Ahuja V, Makharia GK. Impact of delay in the diagnosis on the severity of celiac disease. J Gastroenterol Hepatol. 2024;39:256-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 6. | Fjorback SSO, Eskildsen FR, Kårhus LL, Linneberg A, Lund AF, Schiøtz ML, Grew J. 'It was hell on earth': perspectives of people living with celiac disease on diagnostic delay. J Hum Nutr Diet. 2024;37:1486-1495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 7. | World Gastroenterology Organisation Global Guidelines - Celiac Disease, February 2017: Erratum. J Clin Gastroenterol. 2019;53:313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 8. | Oberhuber G, Granditsch G, Vogelsang H. The histopathology of coeliac disease: time for a standardized report scheme for pathologists. Eur J Gastroenterol Hepatol. 1999;11:1185-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1142] [Cited by in RCA: 1205] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 9. | Arigo D, Anskis AM, Smyth JM. Psychiatric comorbidities in women with celiac disease. Chronic Illn. 2012;8:45-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 10. | Singh P, Singh AD, Ahuja V, Makharia GK. Who to screen and how to screen for celiac disease. World J Gastroenterol. 2022;28:4493-4507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (3)] |
| 11. | Ciaccio EJ, Lee AR, Lebovits J, Wolf RL, Lewis SK, Ciacci C, Green PHR. Psychological, Psychiatric, and Organic Brain Manifestations of Celiac Disease. Dig Dis. 2024;42:419-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 12. | Dhar J, Samanta J, Sharma M, Kumar S, Sinha SK, Kochhar R. Impact of delay in diagnosis in patients with celiac disease: A study of 570 patients at a tertiary care center. Indian J Gastroenterol. 2022;41:30-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Elli L, Leffler D, Cellier C, Lebwohl B, Ciacci C, Schumann M, Lundin KEA, Chetcuti Zammit S, Sidhu R, Roncoroni L, Bai JC, Lee AR, Dennis M, Robert ME, Rostami K, Khater S, Comino I, Cebolla A, Branchi F, Verdu EF, Stefanolo JP, Wolf R, Bergman-Golden S, Trott N, Scudeller L, Zingone F, Scaramella L, Sanders DS. Guidelines for best practices in monitoring established coeliac disease in adult patients. Nat Rev Gastroenterol Hepatol. 2024;21:198-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 14. | Roy A, Mehra S, Kelly CP, Tariq S, Pallav K, Dennis M, Peer A, Lebwohl B, Green PH, Leffler DA. The association between socioeconomic status and the symptoms at diagnosis of celiac disease: a retrospective cohort study. Therap Adv Gastroenterol. 2016;9:495-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Khan AS, Albaqshi BM, Alismael AM, Bohamad AH, Almutawah AA, Alabdellah AH, Almajed AS, Almajed AS, Almajed AS. The Role of Physicians' Factors in Underdiagnosis of Celiac Disease in the Eastern Province of Saudi Arabia. Cureus. 2023;15:e44690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 16. | Vavricka SR, Vadasz N, Stotz M, Lehmann R, Studerus D, Greuter T, Frei P, Zeitz J, Scharl M, Misselwitz B, Pohl D, Fried M, Tutuian R, Fasano A, Schoepfer AM, Rogler G, Biedermann L. Celiac disease diagnosis still significantly delayed - Doctor's but not patients' delay responsive for the increased total delay in women. Dig Liver Dis. 2016;48:1148-1154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Fuchs V, Kurppa K, Huhtala H, Collin P, Mäki M, Kaukinen K. Factors associated with long diagnostic delay in celiac disease. Scand J Gastroenterol. 2014;49:1304-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 18. | Tan IL, Withoff S, Kolkman JJ, Wijmenga C, Weersma RK, Visschedijk MC. Non-classical clinical presentation at diagnosis by male celiac disease patients of older age. Eur J Intern Med. 2021;83:28-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 19. | Green PHR, Stavropoulos SN, Panagi SG, Goldstein SL, Mcmahon DJ, Absan H, Neugut AI. Characteristics of adult celiac disease in the USA: results of a national survey. Am J Gastroenterol. 2001;96:126-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 335] [Article Influence: 14.0] [Reference Citation Analysis (1)] |
| 20. | Mouslih A, El Rhazi K, Bahra N, Lakhdar Idrissi M, Hida M. Celiac Disease in Moroccan Children: Diagnostic Characteristics and Determinants of Diagnosis Delay. Cureus. 2023;15:e50800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 21. | Nurmi R, Pasternack C, Salmi T, Hervonen K, Koskinen I, Järvelin J, Huhtala H, Collin P, Mustonen J, Kaukinen K, Mäkelä S. The risk of renal comorbidities in celiac disease patients depends on the phenotype of celiac disease. J Intern Med. 2022;292:779-787. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 22. | Wang L, Tan X, Wang H, Wang Q, Huang P, Li Y, Li J, Huang J, Yang H, Yin Y. Effects of varying dietary folic acid during weaning stress of piglets. Anim Nutr. 2021;7:101-110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | Rahman A, Yamazaki D, Sufiun A, Kitada K, Hitomi H, Nakano D, Nishiyama A. A novel approach to adenine-induced chronic kidney disease associated anemia in rodents. PLoS One. 2018;13:e0192531. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 66] [Article Influence: 9.4] [Reference Citation Analysis (0)] |