Published online Aug 14, 2025. doi: 10.3748/wjg.v31.i30.109256
Revised: June 5, 2025
Accepted: July 16, 2025
Published online: August 14, 2025
Processing time: 93 Days and 15.1 Hours
Cardiopulmonary changes in noncirrhotic portal hypertension (NCPH) are poorly understood.
To investigate cardiopulmonary changes using transthoracic echocardiography (TTE) in NCPH and their correlation with clinical features.
Prospective cohort including 10 preclinical NCPH [without portal hypertension (PH)] and 32 NCPH subjects who underwent TTE with agitated saline injection and comprehensive clinical evaluation were assessed. PH was defined by presence of either varices, ascites or portosystemic shunting. Intrapulmonary vascular dilatation (IPVD) is defined as appearance of microbubbles in the left atrium after three heartbeats. Right ventricular systolic pressure (RVSP) > 38 mmHg was used to identify possible porto-pulmonary hypertension. Cardiomyopathy is defined using cirrhotic cardiomyopathy consortium criteria.
Among 42 subjects, 17 (40%) had IPVD, 4 (9.5%) had RVSP > 38 mmHg, and 6 (14%) had cardiomyopathy. Aspartate aminotransferase to alanine aminotransferase (AST/ALT) (1.3 vs 1, P = 0.04) and liver stiffness measurement (LSM) (12.4 kPa vs 7.1 kPa, P = 0.03) were higher in those with IPVD. Presence of either LSM > 10 or AST/ALT > 1.2 aided in identifying subjects with IPVD-sensitivity, specificity, and accuracy of 76%. RVSP correlated with oxygen saturation (r = -0.33), and free right hepatic vein pressure (r = 0.43). Those with PH had higher left atrial volume (LAV) (62 mL vs 48 mL, P < 0.01), and LAV index (LAVI) (35 m2vs 23 m2, P < 0.01) compared to those without PH. Total bile acids, especially primary bile acids positively correlated with LAV (r = 0.36), and LAVI (r = 0.41).
Similar to cirrhotic patients, cardiopulmonary changes are prevalent in NCPH, especially among those with PH. In NCPH, cardiopulmonary changes occur despite preserved synthetic function, suggesting the NCPH model's value in understanding cardiopulmonary dysfunction in liver disease.
Core Tip: In this study, cardiopulmonary changes in noncirrhotic portal hypertension (NCPH) were investigated using echocardiography and its association with clinical features was explored. Cardiopulmonary changes are prevalent in NCPH patients as in those with cirrhosis. Unlike cirrhosis, cardiopulmonary complications occur despite preserved synthetic function of liver. Liver stiffness measurement and aspartate aminotransferase to alanine aminotransferase ratio help identify patients at risk of hepatopulmonary syndrome.
- Citation: Gopalakrishna H, Nguyen ML, Mironova M, Viana Rodriguez GM, Afruza R, Chakraborty M, Menkart MG, Oringher JL, Scott S, Nair GB, Kleiner DE, Koh C, Fallon M, Sachdev V, Heller T. Cardiopulmonary changes and its association with clinical features in noncirrhotic portal hypertension. World J Gastroenterol 2025; 31(30): 109256
- URL: https://www.wjgnet.com/1007-9327/full/v31/i30/109256.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i30.109256
Noncirrhotic portal hypertension (NCPH) is caused by a diverse group of disorders that primarily affect hepatic vasculature resulting in portal hypertension (PH) in the absence of cirrhosis[1,2]. NCPH can be classified based on the location of resistance to blood flow, as pre-hepatic, hepatic, and post-hepatic causes. Hepatic causes in turn can be divided into presinusoidal, sinusoidal, and post-sinusoidal causes[1]. Porto-sinusoidal vascular disease (PSVD), one of the presinusoidal causes of NCPH is the most common cause of NCPH in the Western world[3]. Individuals with NCPH often maintain normal liver synthetic function and lack hepatic fibrosis, yet they remain susceptible to complications associated with PH, similar to cirrhotic patients[4]. One such group of complications is cardiopulmonary dysfunction, including hepatopulmonary syndrome (HPS), porto-pulmonary hypertension (PoPH), and cardiomyopathy, which are well-described in cirrhosis. In cirrhotic patients, these entities are attributed to a combination of hepatic dysfunction, immune dysregulation, and PH including a hyperdynamic circulatory system[5]. However, cardiopulmonary changes in NCPH patients remains poorly understood.
Among patients with cirrhosis, HPS, PoPH, and cardiomyopathy are relatively common and are initially assessed by transthoracic echocardiography (TTE)[6]. HPS, which results from microvascular remodeling in the alveolar circulation, occurs in 10-30% of patients and is detected by contrast injection and delayed shunting. PoPH occurs in 5% to 6% of patients and is screened by calculating right ventricular systolic pressure (RVSP) using tricuspid regurgitant velocity (TRV)[7,8]. Cardiomyopathy appears to be present in about 30%-35% of patients and is detected by evaluating for the presence of diastolic or systolic dysfunction[9]. Despite the substantial impact of these disorders on prognosis, they are underdiagnosed as most patients remain asymptomatic in the early stages[5]. Although sporadic case series and studies have described the occurrence of cardiopulmonary dysfunction in NCPH, our understanding of frequency, and associations with clinical features remains limited[10-13].
We aimed to explore the frequency of echocardiography findings of HPS, PoPH, and cardiomyopathy in a prospective cohort of NCPH patients and their potential association with PH. An additional exploratory focus was to analyze associations of cardiopulmonary changes with hepatic factors such as bile acids and vascular factors such as those related to angiogenesis.
A cross-sectional analysis of consecutive subjects with biopsy proven NCPH followed in a single center prospective cohort from July 2015 through July 2023. Prospective natural history study included both preclinical NCPH (without PH) and NCPH subjects (NCT02417740)[14]. PH was defined by the presence of features, namely gastric, esophageal, or ectopic varices, portal hypertensive gastropathy, or the presence of ascites or portosystemic shunting in imaging. Subjects were classified as preclinical NCPH if they had histological features of early nodular regenerative hyperplasia or obliterative portal venopathy[15] and had underlying etiology known to cause NCPH[1], but did not have any above mentioned features of PH. PSVD, one of hepatic causes of NCPH, was defined based on the previously published criteria[16]. Preclinical NCPH subjects were included in this study, since this is an exploratory study and also as it remains unclear whether cardiopulmonary changes can be observed prior to development of features of PH. Exclusion criteria included all known causes of chronic liver disease, known coronary artery disease, heart failure, valvular heart disease, intrinsic lung disease, chronic kidney disease, active infection, malignancy, human immunodeficiency virus, and sarcoidosis. All participants with varices seen after the publication of the Baveno VII guidelines in 2022 were given beta blockers after discussing with participants about potential risks and benefits[16]. Use of beta blockers and diuretics were documented but not suspended during study procedures as this was considered unethical.
All participants underwent evaluation including TTE (Supplementary material), detailed clinical history, anthropometric measurements, vital signs (recorded after sitting in an upright posture for 5 minutes), liver magnetic resonance imaging, laboratory testing, liver stiffness measurement (LSM), esophago-gastroduodenoscopy and liver biopsy. Liver biopsy was considered adequate if it was ≥ 2 cm length or was otherwise considered adequate for interpretation by the expert pathologist (DEK)[16]. LSM examinations using FibroScan® (Echosens, Paris, France) were performed by trained operators after a minimum 4-hour fasting period. Results, measured in kilopascals (kPa), required at least 10 valid individual measurements interquartile range/median ≤ 30%. Trans-jugular liver biopsy with portal pressure measure
The presence of intrapulmonary vascular dilatation (IPVD) or shunting was diagnosed by the appearance of microbubbles in the left atrium (LA) 3 or more cardiac cycles after right atrial opacification[17]. RVSP > 38 mmHg was used to identify possible PoPH[18].
Left ventricular diastolic dysfunction (LVDD) in individuals with normal ejection fraction (EF) was diagnosed using the 2020 cirrhotic cardiomyopathy consortium (CCC) criteria which used abnormality in 3 out of 4 parameters - septal early diastolic mitral annular flow velocity (e′) < 7 cm/s, early diastolic transmitral flow to early diastolic mitral annular velocity (E/e′) ≥ 15, left atrial volume index (LAVI) > 34 mL/m2, and TRV > 2.8 m/s. Left ventricular systolic dysfunction (LVSD) was defined by an EF ≤ 50% at rest, or an absolute global longitudinal strain by speckle-tracking echocardiography < 18% at rest[9].
Statistical analysis was performed using R statistical software (version 4.1.2) and PRISM Data are presented as mean (standard deviation), or median (interquartile range) respectively. Categorical variables were expressed as frequencies and percentages. Quantitative variables were compared using the independent student’s t-test or the Mann-Whitney U test for normally and non-normally distributed variables, respectively. Qualitative variables were compared using the corrected χ2 test or a 2-sided Fisher’s exact test, as appropriate. Based on variables with statistical significance a multivariable model was constructed using binary logistic regression. Test characteristics-sensitivity, specificity, negative predictive value, positive predictive value, and accuracy, were calculated. The relationship between parameters was estimated using Spearman correlation. All tests were 2-sided and P-values < 0.05 were considered to be significant.
This prospective study was approved by the Institutional Review Board of the National Institutes of Health (NCT02417740). Written informed consent was obtained from all participants.
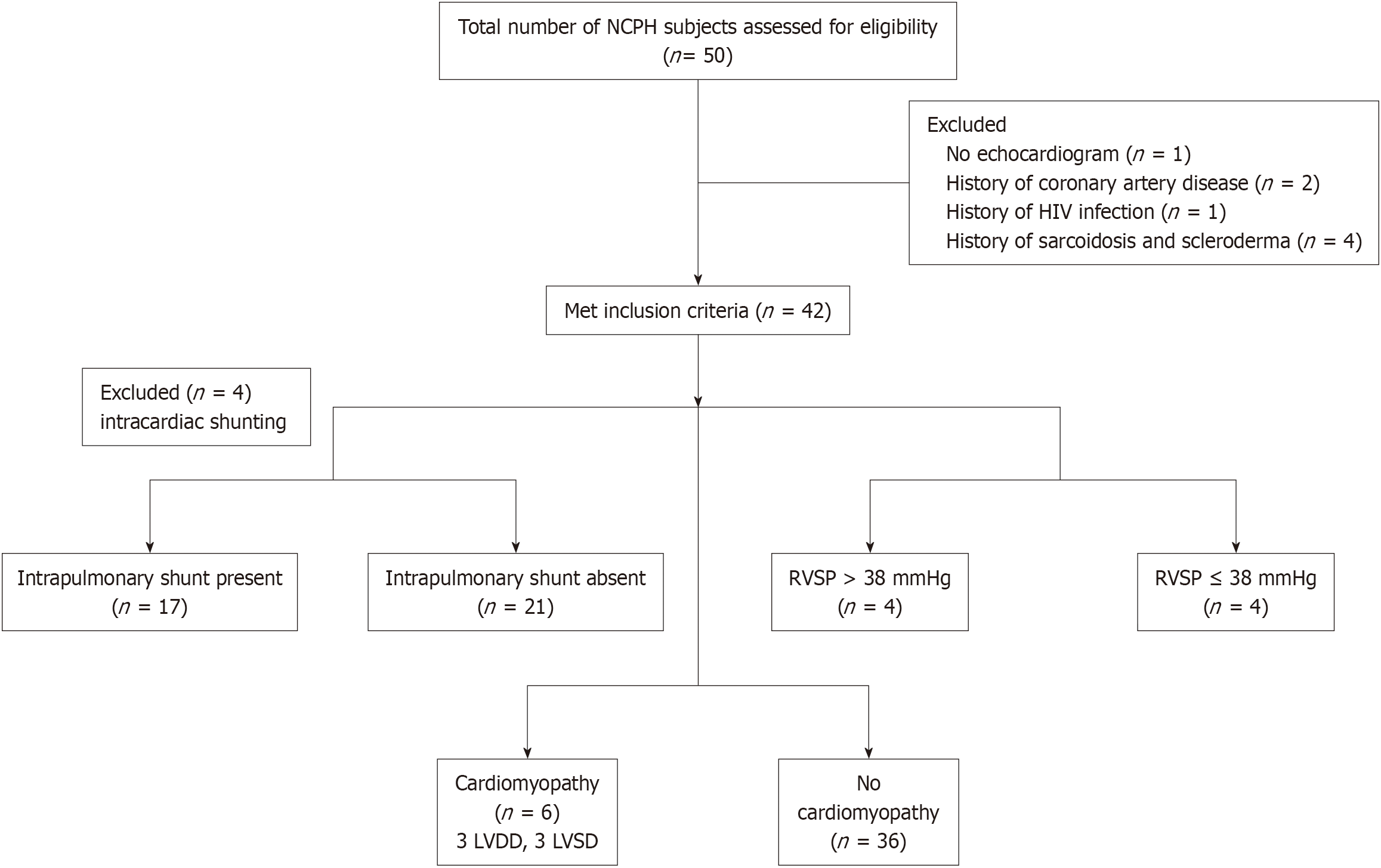
Among 50 subjects enrolled, 8 were excluded (one did not undergo TTE, and seven had extrahepatic factors that could affect the cardiopulmonary function, Figure 1). Baseline characteristics of the overall cohort, and comparison among those with preclinical NCPH and NCPH are summarized in Table 1. The median age of the cohort was 52 years, with a slight male (62%) predominance. A detailed description of the cohort was recently published[14]. Vital signs, including heart rate, blood pressure, and oxygen saturation, were similar between the groups. Only participants with NCPH were on nonselective beta-blockers for variceal prophylaxis. In the overall cohort, median fibrosis 4 (FIB-4) score, LSM, and HVPG were 3.4, 8.6 kPa, and 6 mmHg respectively. These were significantly higher in the group with NCPH compared to preclinical NCPH. The cause of NCPH is mentioned in Supplementary Table 1.
| Baseline characteristics | Overall cohort (n = 42) | NCPH (n = 32) | Preclinical NCPH (n = 10) | P value1 |
| Age (years) | 52 (41-61) | 52 (43 – 62) | 49 (40-59) | 0.5 |
| Gender, male (%) | 26 (62) | 20 (63) | 6 (60) | 0.9 |
| Body mass index (kg/m2) | 24.1 (22.1-26.8) | 24.0 (22.6-26.8) | 25.3 (19.0-26.6) | 0.2 |
| Heart rate (bpm) | 69 (62-74) | 69 (63-74) | 69 (60-74) | 0.5 |
| Systolic blood pressure (mmHg) | 116 (104-127) | 114 (103-125) | 126 (115-132) | 0.2 |
| Diastolic blood pressure (mmHg) | 67 (60-71) | 62 (59-72) | 68 (68-70) | 0.3 |
| Oxygen saturation (%) | 97 (96-98) | 98 (96-99) | 97 (96-98) | 0.6 |
| Smoking status | 0.05 | |||
| Current or previous smoker, n (%) | 15 (36) | 14 (44) | 1 (10) | |
| Non-smoker, n (%) | 27 (64) | 18 (56) | 9 (90) | |
| Underlying disorder | < 0.01 | |||
| Immunodeficiency, n (%) | 17 (40) | 9 (28) | 8 (80) | |
| Autoimmune disorders, n (%) | 2 (5) | 2 (6) | ||
| Hematological disorders, n (%) | 4 (10) | 3 (9) | 1 (10) | |
| Exposure to medication, n (%) | 4 (10) | 4 (13) | ||
| Genetic disorders, n (%) | 3 (7) | 2 (6) | 1 (10) | |
| CVID and exposure to drugs, n (%) | 1 (2) | 1 (3) | ||
| Undiagnosed, n (%) | 11 (26) | 11 (35) | ||
| Medication use | ||||
| Beta blockers, n (%) | 17 (40) | 17 (53) | ||
| Diuretics, n (%) | 13 (31) | 11 (34) | 2 (20) | 0.4 |
| Antihypertensives, n (%) | 6 (14) | 5 (16) | 1 (10) | 0.4 |
| Antidiabetics including insulin, n (%) | 6 (14) | 5 (16) | 1 (10) | 0.4 |
| Immunomodulators, n (%) | 12 (29) | 7 (22) | 5 (50) | 0.08 |
| Haemoglobin (g/dL) | 12.9 (11.1-14.4) | 12.7 (11.0-14.1) | 13.3 (12.8-15.4) | 0.06 |
| Sodium (mEq/L) | 138 (138-140) | 138 (138-141) | 139 (138-139) | 0.6 |
| Creatinine (mg/dL) | 0.8 (0.7-0.9) | 0.8 (0.7-0.9) | 0.8 (0.7 – 1.0) | 0.9 |
| Albumin (g/dL) | 3.9 (3.7-4.2) | 3.9 (3.7-4.2) | 4.1 (3.6-4.2) | 0.7 |
| C-reactive protein (mg/dL) | 2.2 (1.0-4.5) | 2.3 (1.2-4.9) | 1.7 (1.0 – 3.0) | 0.09 |
| Prothrombin time (seconds) | 14.2 (13.1-15.0) | 14.4 (13.6-15.3) | 12.9 (12.7-13.8) | < 0.01 |
| International normalized ratio | 1.1 (0.9-1.2) | 1.1 (1.0-1.2) | 1.0 (0.9-1.0) | < 0.01 |
| Platelet count (109/L) | 94 (62-153) | 77.5 (53.8-134) | 171 (128-246) | 0.02 |
| Alanine aminotransferase, AST (U/L) | 45 (25-60) | 40 (26-59) | 45 (25-77) | 0.6 |
| Aspartate aminotransferase, ALT (U/L) | 46 (31-62) | 44 (31-60) | 46.5 (31.3-75.3) | 0.6 |
| AST/ALT ratio | 1.1 (0.9-1.3) | 1.1 (0.9-1.3) | 1.1 (1.0-1.2) | 0.9 |
| Fibrosis-4 index | 3.4 (1.9-6.3) | 4.1 (2.7-7.4) | 1.5 (1.4-2.6) | < 0.01 |
| MELD-Na | 9 (8-10) | 9 (8-10) | 8 (7-10) | 0.06 |
| Liver stiffness measurement (kPa) | 8.6 (6.0-13.5) | 11.6 (7.7-14.5) | 5.3 (4.3-6.1) | < 0.01 |
| Hepatic venous pressure gradient (mmHg)2 | 6 (4-11) | 10 (5-15) | 4 (3-5) | < 0.01 |
Among 42 subjects, four subjects had intracardiac shunting. Among 38 subjects without intracardiac shunting, 17 (45%) had IPVD, of which 15 had PH and 2 did not. Four (13%) subjects with PH had RVSP > 38 mmHg, suggesting the possible PoPH. Using the CCC criteria, 6 (14%) had cardiomyopathy, 3 subjects each with LVDD and LVSD (Figure 1). Supplementary Figure 1 shows presence of cardiopulmonary changes based on their PH status.
Among those with shunting, only 4 (24%) subjects complained of dyspnea at rest or with exertion, and none had a history of smoking. Only 2 (12%) subjects with shunting had oxygen saturation < 96%, but neither required supplemental oxygen. Interestingly, oxygen saturation among those with or without shunting did not differ. Smoking status and medication use were similar between the groups. The liver related factors aspartate aminotransferase to alanine aminotransferase (AST/ALT) ratio (1.3 vs 1.1, P = 0.04) and LSM (12.4 kPa vs 7.1 kPa, P = 0.03) were significantly higher in the group with shunting compared to those without (Table 2). TTE parameters were not different among those with and without shunting (Supplementary Table 2).
| Characteristics | Shunt present (n = 17) | Shunt absent (n = 21) | P value1 |
| Age (years) | 51 (39-61) | 49 (41-57) | 0.7 |
| Male gender, n (%) | 8 (47) | 16 (76) | 0.09 |
| Body mass index (kg/m2) | 24.1 (23.4-28.7) | 23.5 (21.9-25.7) | 0.04 |
| Heart rate (bpm) | 70 (63-78) | 68 (60-70) | 0.1 |
| Systolic blood pressure (mmHg) | 116 (101-124) | 116 (105-127) | 0.6 |
| Diastolic blood pressure (mmHg) | 61 (60-69) | 68 (62-70) | 0.2 |
| Oxygen saturation (%) | 97 (96-98) | 98 (97-99) | 0.08 |
| Had features of portal hypertension (%)2 | 15 (88) | 13 (62) | 0.1 |
| Smoking status | 0.6 | ||
| Current or previous smoker, n (%) | 5 (29) | 8 (38) | |
| Non-smoker, n (%) | 12 (71) | 13 (62) | |
| Underlying disorder | < 0.01 | ||
| Immunodeficiency, n (%) | 3 (18) | 13 (62) | |
| Autoimmune disorders, n (%) | 1 (5) | 1 (5) | |
| Hematological disorders, n (%) | 2 (12) | 1 (5) | |
| Exposure to medication, n (%) | 2 (12) | 2 (9) | |
| Genetic disorders, n (%) | 3 (18) | ||
| CVID and exposure to drugs, n (%) | 1 (5) | ||
| Undiagnosed, n (%) | 6 (35) | 3 (14) | |
| Medication use | |||
| Beta blockers, n (%) | 8 (47) | 8 (38) | 0.7 |
| Diuretics, n (%) | 5 (29) | 5 (24) | 0.7 |
| Antihypertensives, n (%) | 2 (12) | 3 (14) | 0.9 |
| Antidiabetics including insulin, n (%) | 4 (24) | 1 (5) | 0.2 |
| Immunomodulators, n (%) | 3 (18) | 9 (43) | 0.2 |
| Sodium (mEq/L) | 138 (138-140) | 138 (138-139) | 0.3 |
| Creatinine (mg/dL) | 0.8 (0.7-0.9) | 0.8 (0.7-0.9) | 0.6 |
| Albumin (g/dL) | 3.9 (3.8-4.2) | 3.9 (3.6-4.2) | 0.6 |
| Haemoglobin (g/dL) | 13.5 (10.8-14.2) | 12.9 (11.8-14.4) | 0.6 |
| C-reactive protein (mg/dL) | 2.3 (1.4-3.6) | 1.9 (1.0-5.2) | 0.1 |
| Prothrombin time (seconds) | 14.2 (13.4-15.4) | 14.1 (13.0-14.5) | 0.2 |
| International normalized ratio | 1.1 (1.0-1.2) | 1.1 (0.9-1.1) | 0.2 |
| Platelet count (109/L) | 85 (65-146) | 97 (60-144) | 0.8 |
| Alanine aminotransferase, ALT (U/L) | 32 (25-53) | 45 (26-76) | 0.2 |
| Aspartate aminotransferase, AST (U/L) | 42 (32-49) | 46 (30-71) | 0.7 |
| Chenodeoxycholic acid (nmol/mL) | 6.1 (2.0-14.5) | 4.1 (1.2-1.0) | 0.1 |
| Cholic acid (nmol/mL) | 4.8 (1.8-11.1) | 1.3 (0.7-10.4) | 0.7 |
| Deoxycholic acid (nmol/mL) | 1.7 (0.8-9.1) | 0.9 (0.1-2.4) | 0.09 |
| Ursodeoxycholic acid (nmol/mL) | 0.4 (0.2-1.7) | 0.3 (0.1-0.7) | 0.2 |
| Primary bile acid (nmol/mL) | 14.2 (3.8-26.0) | 5.1 (2.0-18.7) | 0.3 |
| Secondary bile acid (nmol/mL) | 1.7 (1.2-11.6) | 1.3 (0.3-2.4) | 0.08 |
| Total Bile acid (nmol/mL) | 15.9 (5.2-33.0) | 7.5 (4.4-22.2) | 0.2 |
| Spleen length (cm) | 16.2 (12.7-20.0) | 15.8 (12.6-18.7) | 0.8 |
| Wedged hepatic venous pressure (mmHg)3 | 23 (14-27) | 19 (14-26) | 0.6 |
| Free hepatic vein pressure (mmHg)3 | 13 (10-15) | 11 (9-15) | 0.4 |
| Hepatic venous pressure gradient (mmHg)3 | 8 (5-11) | 6 (3-14) | 0.6 |
| AST/ALT ratio | 1.3 (1.0-1.4) | 1.0 (0.8-1.1) | 0.04 |
| FIB-4 index | 3.7 (2.0-5.0) | 3.3 (1.6-6.6) | 0.9 |
| MELD-Na | 10.0 (8.0-10.0) | 8.0 (7.0-10.0) | 0.7 |
| Liver stiffness measurement (kPa) | 12.4 (7.2-16.8) | 7.1 (5.4-10.3) | 0.03 |
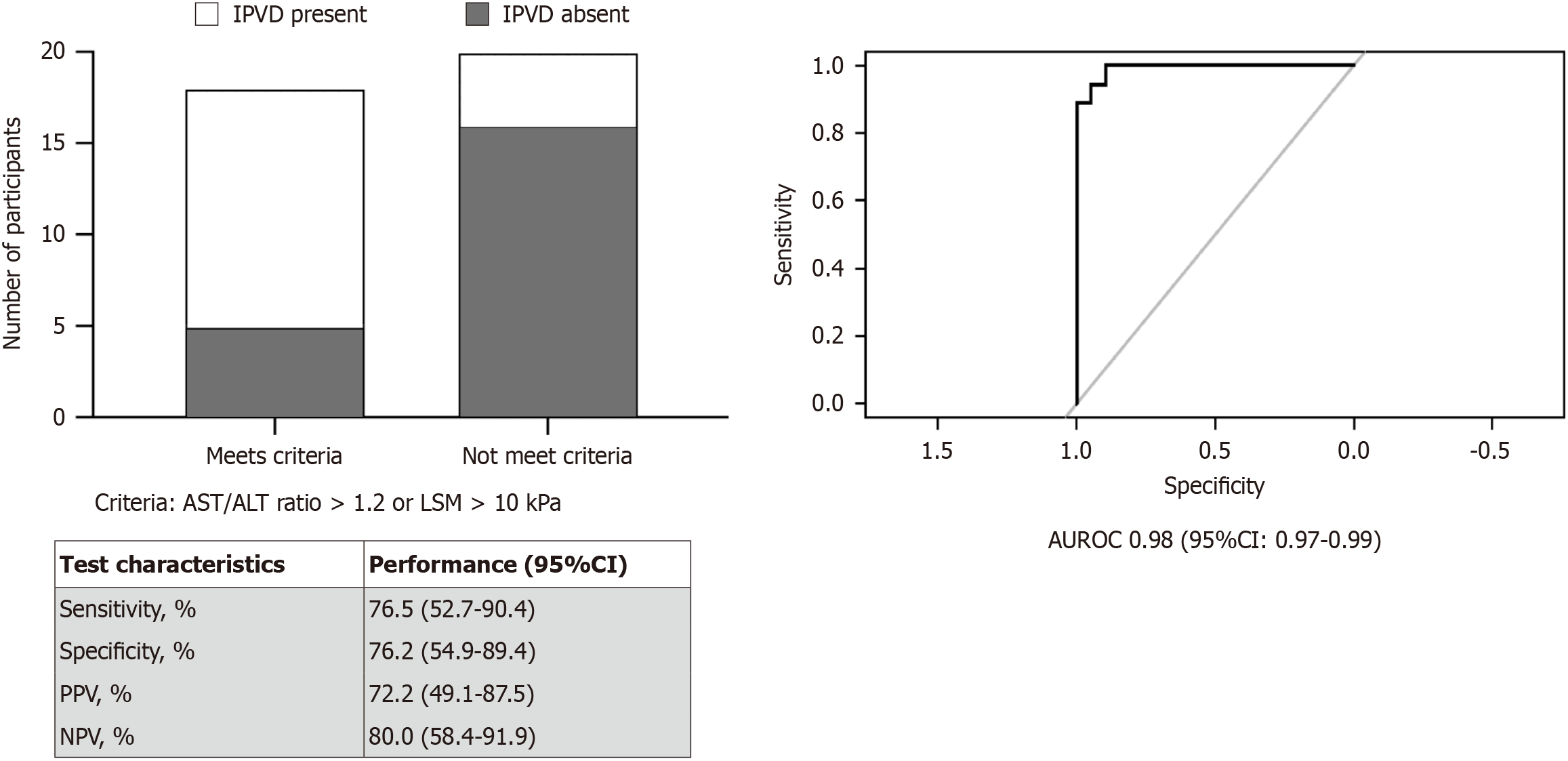
To aid in deciding the need to screen for shunting, a criterion was developed using liver related factors, which were significantly different among the groups in univariate analysis. On multivariable logistic regression analysis, a combination of AST/ALT ratio and LSM performed with an area under receiver operating curve (area under receiver operating characteristic = 0.75, 95%CI: 0.59-0.91, P < 0.01) were used to identify shunting. To simplify the screening criteria, presence of either LSM > 10 kPa or AST/ALT ratio > 1.2 were identified as cut off in deciding the need for TTE shunt screening. This criterion had more than 70% performance in all test characteristics (Figure 2). Patients who met the criteria had 10.4 (95%CI: 2.3-38.9, P < 0.01) times higher odds of having shunting.
Four patients had RVSP > 38 mmHg. There was no difference in oxygen saturation (97% vs 97%, P = 0.09), LSM (11.9 kPa vs 8.4 kPa, P = 0.5), and AST/ALT ratio (1.13 vs 1.07, P = 0.4) among those with and without elevated RVSP. However, the FIB-4 index was significantly higher in the group with possible PoPH (8.9 vs 3.2, P = 0.03). Multiple factors either positively or negatively correlated with RVSP as shown in Table 3. Notably, despite oxygen saturation being within the normal range, it negatively correlated with elevation in RVSP (r = -0.33, P = 0.04). Interestingly, AST/ALT ratio positively correlated with RVSP, but LSM did not. Free right hepatic vein pressure and IVC diameter measured by TTE positively correlated with RVSP (Supplementary Figure 2). Similar correlation findings were observed when the analysis was limited to the PH group alone (Supplementary Table 3).
| Variables | Correlation1, r | P value |
| Age | 0.31 | 0.04 |
| Oxygen saturation (%) | -0.33 | 0.03 |
| Cardiac output (L/min) | 0.33 | 0.04 |
| Cardiac index (L/min/m2) | 0.29 | 0.07 |
| LV mass index (gm/m2) | 0.31 | 0.05 |
| LA volume (mL) | 0.51 | < 0.01 |
| LA volume index (mL/m2) | 0.5 | 0.01 |
| RA volume (mL) | 0.37 | 0.02 |
| RA volume index (mL/m2) | 0.42 | < 0.01 |
| Septal MV E/e’ | 0.28 | 0.08 |
| Lateral MV E/e’ | 0.39 | 0.01 |
| TAPSE (mm) | 0.35 | 0.04 |
| IVC diameter (mm) | 0.32 | 0.04 |
| AST/ALT ratio | 0.33 | 0.03 |
| Liver stiffness measurement (kPa) | 0.04 | 0.7 |
| Wedged hepatic venous pressure (mmHg)2 | 0.09 | 0.6 |
| Free hepatic vein pressure (mmHg)2 | 0.43 | 0.02 |
| Hepatic venous pressure gradient (mmHg)2 | -0.08 | 0.6 |
All six subjects with cardiomyopathy had PH and 5 had esophageal varices. There was no difference in oxygen saturation (97% vs 97%, P = 0.3), FIB-4 index (3.3 vs 3.5, P = 0.5), LSM (14.5 kPa vs 8.3 kPa, P = 0.1) and AST/ALT ratio (1.2 vs 1.1, P = 0.3) among those with and without cardiomyopathy.
A comparison of echocardiographic findings between the groups is shown in Supplementary Table 4. As expected, cardiac chamber volumes were significantly increased in those with PH: Left ventricle (LV) diastolic volume index (66 mL/m2vs 56 mL/m2, P < 0.01), LA volume index (35 mL/m2vs 23 mL/m2, P < 0.01). Since smoking status and beta blocker use were significantly different between the groups, TTE parameters were compared after removing participants with exposure to beta- blockers and a history of smoking. In patients with no history of beta-blocker use, chamber volumes remained increased in the group with PH (Table 4).
| Baseline characteristics | NCPH (n = 7) | Preclinical NCPH (n = 9) | P value1 |
| Age (years) | 51 (42-63) | 46 (39-57) | 0.6 |
| Male gender, n (%) | 4 (57) | 5 (56) | 0.8 |
| Body mass index (kg/m2) | 25.8 (23.3-31.6) | 25.1 (18.6-25.7) | 0.09 |
| Heart rate (bpm) | 70 (67-80) | 70 (65-75) | 0.2 |
| Systolic blood pressure (mmHg) | 116 (113-128) | 124 (114-129) | 0.7 |
| Diastolic blood pressure (mmHg) | 67 (55-71) | 68 (68-69) | 0.5 |
| Oxygen saturation (%) | 96 (96-98) | 97 (96-97) | 0.7 |
| Sodium (mEq/L) | 139 (138-140) | 139 (138-139) | 0.9 |
| Creatinine (mg/dL) | 0.8 (0.7-0.8) | 0.8 (0.7-1.0) | 0.7 |
| Albumin (g/dL) | 3.9 (3.8-3.9) | 4.1 (3.5-4.2) | 0.9 |
| Haemoglobin (g/dL) | 12.7 (11.9-14.1) | 13 (12.8-15.7) | 0.3 |
| C-reactive protein (mg/dL) | 1.2 (0.6-3.9) | 1.5 (1.0-2.6) | 0.4 |
| Left ventricular ejection fraction (%) | 60 (59-62) | 62 (62-65) | 0.1 |
| Cardiac output (L/min) | 5.6 (5.2-7.8) | 5.2 (4.4-6.0) | 0.3 |
| Cardiac index (L/min/m2) | 2.9 (2.7-3.8) | 2.8 (2.7-3.0) | 0.5 |
| LV diastolic volume (mL) | 146 (133-164) | 97 (89-121) | 0.02 |
| LV diastolic volume index (mL/m2) | 69 (64-86) | 57 (48-59) | 0.04 |
| LV mass index (gm/m2) | 84.9 (80.5-98.3) | 68.4 (64.1-86.5) | 0.3 |
| LV global longitudinal strain (%) | -22.7 (-24.4--19.0) | -21.6 (-22.3--19.9) | 0.9 |
| RV free wall strain (%) | -24.6 (-26.9--23.5) | -25.9 (-28.9--24.3) | 0.6 |
| RVSP (mmHg) | 26 (24-38) | 27 (25-30) | 0.3 |
| LA volume (ml) | 80 (73-85) | 46 (34-59) | 0.03 |
| LA volume index (mL/m2) | 37 (35-44) | 23 (22-29) | 0.04 |
| RA volume (mL) | 57 (52-64) | 28 (26-43) | 0.09 |
| RA volume index (mL/m2) | 26 (26-30) | 16 (13-20) | 0.1 |
| Septal MV E/e’ | 10.0 (8.1-11.7) | 7.4 (6.5-8.8) | 0.1 |
| Lateral MV E/e’ | 7.8 (6.5-9.8) | 4.9 (4.5-6.9) | 0.1 |
| Septal e’ (cm/s) | 9 (8-11) | 11 (10-11) | 0.4 |
| Lateral e’ (cm/s) | 12 (12-15) | 15 (10-15) | 0.8 |
| TAPSE (mm) | 25 (22-26) | 24 (23-25) | 0.9 |
| Mitral E to A ratio | 1.2 (0.8-1.2) | 1.4 (1.1-1.5) | 0.9 |
| IVC diameter (mm) | 23.0 (16.8-30.8) | 20.0 (12.0-21.0) | 0.1 |
There was no difference in bile acids levels among those with and without shunting (Table 2). Bile acids also did not correlate with RVSP. Bile acids levels were not different among those with/without cardiomyopathy and elevated RVSP (data not shown). However, both total and primary bile acid levels positively correlated with left atrial and left ventricular volume and index (Supplementary Table 5).
Factors associated with angiogenesis, notably ANG2 and VCAM1 are shown to be elevated in patients with HPS due to cirrhosis[19]. In this NCPH cohort, ANG2 (3.5 ng/mL vs 1.5 ng/mL, P = 0.3), and VCAM1 (6929 ng/mL vs 6308 ng/mL, P = 0.7) were not different among those with and without shunting (Supplementary Figure 3). Also, vWF levels were not different (24939 ng/mL vs 19659 ng/mL, P = 0.2) among those with and without shunting. Angiogenesis factors were not different among those with elevated RVSP and cardiomyopathy.
This exploratory analysis demonstrates that subjects with NCPH, despite their preserved synthetic function experience a spectrum of cardiopulmonary changes like IPVD, and elevated RVSP similar to what is seen in cirrhotic patients. In this NCPH cohort, cardiomyopathy and features suggestive of possible HPS and PoPH are prevalent at rates comparable to those in patients with cirrhosis. Furthermore, a noninvasive assessment combining LSM and AST/ALT ratio can aid in identifying which subjects could be considered for HPS screening.
In this study, the prevalence of IPVD in PH group was 47%, higher than prior reports, which was approximately 12%[11,12]. This rate was also higher than what is seen in cirrhotics[20]. This difference might be due to relatively older age of our cohort, the difference in underlying disorders that led to NCPH, and variations in the severity of PH. However, similar to what has been observed previously, pulse oximetry has proven to be a less effective screening test for HPS[21]. Among NCPH participants with IPVD, only 12% had oxygen saturation below 96%. A study by DuBrock et al[22] found significant differences in CI and CO among patients with HPS compared to those who do not. However, in this cohort, there was no difference in CI and CO among those with and without IPVD. Among liver related factors, AST/ALT ratio and LSM were higher in the group with shunting compared to those who did not, indicating a potential association between advanced liver disease and the development of IPVD. Current recommendation advice screening for cardiopulmonary dysfunction in individuals with symptoms or advanced liver disease undergoing liver transplant evaluation[17]. However, symptoms and pulse oximetry are not sensitive enough to identify those with underlying HPS. Instead, using a non-invasive test which reflect disease severity, either AST/ALT ratio > 1.2 or LSM > 10 kPa can guide in deciding the need for IPVD screening in non-transplant candidates. In our cohort, these criteria performed with more than 70% test performance for all the test characteristics. However, further validation in an independent cohort is needed.
Echocardiography is a valuable screening tool for identifying PoPH when other potential etiologies for pulmonary hypertension have been excluded[18]. In this cohort, 4 subjects with PH were identified to possibly have PoPH. However, lack of cardiac catheterization to confirm PoPH diagnosis is a limitation. Among factors associated with RVSP, it was observed that oxygen saturation was negatively correlated with RVSP. All patients with RVSP > 38 mmHg had oxygen saturation < 98% in sitting position. The pathophysiology of PoPH remains incompletely understood, but one widely accepted theory suggests that PH facilitates the formation of portosystemic shunts. This, in turn, leads to the shunting of vasoactive metabolites into the pulmonary arteries, resulting in the obliteration of pulmonary arteries and an increase in pulmonary vascular resistance[23]. In our NCPH cohort, there was a positive correlation between RVSP and free hepatic pressure, which could either reflect the increased downstream effect of PoPH or increased portal pressure leading to the development of PoPH. However, there was no correlation between HVPG and RVSP. This could be because HVPG is underestimated in presinusoidal/prehepatic condition like NCPH and hence not reliably corelate with severity of PH in these patients[2].
In the overall cohort, 14% had cardiomyopathy. In cirrhosis, the prevalence of cardiomyopathy varies depending on the population being studied. Most prevalence reports focus on patients being assessed for liver transplantation[5,24]. However, a European study that examined the prevalence of cardiomyopathy in all patients with cirrhosis, following the 2020 CCM guidelines, reported that diastolic dysfunction was observed in 7.4% of cases, while systolic dysfunction was identified in 12.3%[25,26]. These results are comparable to what was seen in consecutive NCPH participants enrolled in this study. Detecting cardiomyopathy in patients with liver disease has shown to be important as it is associated with poor outcomes[27].
Studies involving cirrhotic patients have shown that higher LAVI are associated with poor outcomes[27,28]. A study by Merli et al[28] showed among cirrhotic patients, during a median follow up of 2 years, a LAVI > 35.23 mL/m2 was associated with decreased survival compared to those with lower LAVI. In our cohort, those with PH had higher LAVI compared to those without. Prognostic importance of these findings in NCPH need to be explored in future studies.
Excess bile acids are known to be cardiotoxic. One of the possible mechanisms suggested is that an increase in bile acid levels causes activation of stress kinases, inhibiting fatty acid oxidation, which is the primary energy source for the heart, resulting in metabolic derangement. Elevated bile acids levels have been shown to be associated with enlarged left atrial volume[29]. In a study of pediatric patients with biliary atresia, it was shown that bile acids levels correlated with left ventricular diameter[30]. In this NCPH cohort, a positive correlation with total bile acids and LAVI as well as LV diastolic volume index were found. These associations were mostly with primary bile acids, suggesting a potential role of bile acids in cardiac changes, but future studies are required to establish causality.
Dysregulation in angiogenesis is thought to be one of the factors associated with HPS. A study by Kawut et al[19] found significantly higher levels of ANG2 in those with HPS compared to those without. They also found VCAM1, which plays an important role in immune response and inflammation, and vWF, which has a primary role in blood clotting and platelet function, are higher in cirrhotic participants with HPS. In this study, there was no difference in any of these factors among those with and without IPVD. This could be because the underlying disorder that led to the development of NCPH, including immune deficiency and hematological disorders, potentially affects the levels of these factors.
This study has some limitations. The relatively small cohort size restricted our capacity to perform multivariate analyses, underscoring the need for caution when generalizing the findings. Echocardiography has some inherent limitations in tomographic assessments of the left ventricular outflow tract and often underestimates these values and subsequent stroke volume and cardiac output calculations. It is also unclear whether use of beta blockers during the TTE evaluation could have influenced some of the findings. Since betablockers reduce heart rate, decrease contractility, and alter chamber filling, their use may have influenced some of our echocardiographic findings. In addition, some essential features critical for diagnosing HPS and risk stratification, such as arterial blood gas and shunt quantification, were not available. Similarly, the lack of confirmatory right heart catheterization made it difficult to fully substantiate the diagnosis of PoPH. Lastly, given that the study was conducted in a referral centre, there is the possibility of selection bias, as patients referred to such centres may have unique characteristics compared to the broader population. Hence these findings need to be validated in an independent cohort.
In summary, patients with NCPH despite preserved hepatic synthetic function can develop cardiopulmonary changes, especially those with PH. Future studies are needed to explore the underlying pathophysiology, importance of cardiopulmonary changes and prognostic implications in these patients.
We acknowledge the assistance of Stanislav Sidenko, Wen Li, Lisbet Odou-Lorenzo for their assistance with data collection.
| 1. | Khanna R, Sarin SK. Non-cirrhotic portal hypertension - diagnosis and management. J Hepatol. 2014;60:421-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 264] [Article Influence: 24.0] [Reference Citation Analysis (2)] |
| 2. | Da BL, Surana P, Kapuria D, Vittal A, Levy E, Kleiner DE, Koh C, Heller T. Portal Pressure in Noncirrhotic Portal Hypertension: To Measure or Not to Measure. Hepatology. 2019;70:2228-2230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 3. | Gioia S, Nardelli S, Ridola L, Riggio O. Causes and Management of Non-cirrhotic Portal Hypertension. Curr Gastroenterol Rep. 2020;22:56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 4. | Gioia S, Nardelli S, Pasquale C, Pentassuglio I, Nicoletti V, Aprile F, Merli M, Riggio O. Natural history of patients with non cirrhotic portal hypertension: Comparison with patients with compensated cirrhosis. Dig Liver Dis. 2018;50:839-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 5. | Kaur H, Premkumar M. Diagnosis and Management of Cirrhotic Cardiomyopathy. J Clin Exp Hepatol. 2022;12:186-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 37] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 6. | Iaconi M, Maritti M, Ettorre GM, Tritapepe L. Echocardiographic evaluation in patient candidate for liver transplant: from pathophysiology to hemodynamic optimization. J Anesth Analg Crit Care. 2024;4:75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Krowka MJ, Swanson KL, Frantz RP, McGoon MD, Wiesner RH. Portopulmonary hypertension: Results from a 10-year screening algorithm. Hepatology. 2006;44:1502-1510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 231] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 8. | Surani SR, Mendez Y, Anjum H, Varon J. Pulmonary complications of hepatic diseases. World J Gastroenterol. 2016;22:6008-6015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Izzy M, Soldatova A, Sun X, Angirekula M, Mara K, Lin G, Watt KD. Cirrhotic Cardiomyopathy Predicts Posttransplant Cardiovascular Disease: Revelations of the New Diagnostic Criteria. Liver Transpl. 2021;27:876-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 10. | Hercun J, Parikh E, Kleiner DE, Fuss I, Uzel G, Strober W, Koh C, Holland SM, Heller T. Recurrent Nodular Regenerative Hyperplasia Following Liver Transplantation in Common Variable Immunodeficiency. Hepatology. 2021;74:1698-1701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Kaymakoglu S, Kahraman T, Kudat H, Demir K, Cakaloglu Y, Adalet I, Dincer D, Besisik F, Boztas G, Sözen AB, Mungan Z, Okten A. Hepatopulmonary syndrome in noncirrhotic portal hypertensive patients. Dig Dis Sci. 2003;48:556-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 57] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | De BK, Sen S, Sanyal R. Hepatopulmonary syndrome in noncirrhotic portal hypertension. Ann Intern Med. 2000;132:924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Isaak A, Chang J, Mesropyan N, Kravchenko D, Endler C, Bischoff L, Böhling N, Pieper CC, Kuetting D, Strassburg CP, Attenberger U, Jansen C, Praktiknjo M, Luetkens JA. Cardiac involvement in non-cirrhotic portal hypertension: MRI detects myocardial fibrosis and oedema similar to compensated cirrhosis. Eur Heart J Cardiovasc Imaging. 2023;24:949-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Mironova M, Gopalakrishna H, Heller T. Letter: Prospective evaluation of patients with non-cirrhotic portal hypertension-A single centre study. Authors' reply. Aliment Pharmacol Ther. 2024;60:1489-1490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Liang J, Shi C, Dupont WD, Salaria SN, Huh WJ, Correa H, Roland JT, Perri RE, Washington MK. Key histopathologic features in idiopathic noncirrhotic portal hypertension: an interobserver agreement study and proposal for diagnostic criteria. Mod Pathol. 2021;34:592-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 16. | de Franchis R, Bosch J, Garcia-Tsao G, Reiberger T, Ripoll C; Baveno VII Faculty. Baveno VII - Renewing consensus in portal hypertension. J Hepatol. 2022;76:959-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1537] [Cited by in RCA: 1482] [Article Influence: 494.0] [Reference Citation Analysis (2)] |
| 17. | Raevens S, Boret M, Fallon MB. Hepatopulmonary syndrome. JHEP Rep. 2022;4:100527. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 29] [Reference Citation Analysis (0)] |
| 18. | DuBrock HM. Portopulmonary Hypertension: Management and Liver Transplantation Evaluation. Chest. 2023;164:206-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 19. | Kawut SM, Krowka MJ, Forde KA, Al-Naamani N, Krok KL, Patel M, Bartoli CR, Doyle M, Moutchia J, Lin G, Oh JK, Mottram CD, Scanlon PD, Fallon MB; Pulmonary Vascular Complications of Liver Disease Study Group. Impact of hepatopulmonary syndrome in liver transplantation candidates and the role of angiogenesis. Eur Respir J. 2022;60:2102304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 20. | Schenk P, Fuhrmann V, Madl C, Funk G, Lehr S, Kandel O, Müller C. Hepatopulmonary syndrome: prevalence and predictive value of various cut offs for arterial oxygenation and their clinical consequences. Gut. 2002;51:853-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 209] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 21. | Forde KA, Fallon MB, Krowka MJ, Sprys M, Goldberg DS, Krok KL, Patel M, Lin G, Oh JK, Mottram CD, Scanlon PD, Kawut SM; Pulmonary Vascular Complications of Liver Disease 2 Study Group. Pulse Oximetry Is Insensitive for Detection of Hepatopulmonary Syndrome in Patients Evaluated for Liver Transplantation. Hepatology. 2019;69:270-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 22. | DuBrock HM, Forde K, Krok K, Patel M, Al-Naamani N, Lin G, Oh JK, Fallon MB, Kawut SM, Krowka MJ. Cardiac index and hepatopulmonary syndrome in liver transplantation candidates: The pulmonary vascular complications of liver disease study. Liver Transpl. 2023;29:467-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 23. | Lai YK, Kwo PY. Portopulmonary Hypertension. Clin Liver Dis. 2023;27:71-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 24. | Wiese S, Hove JD, Bendtsen F, Møller S. Cirrhotic cardiomyopathy: pathogenesis and clinical relevance. Nat Rev Gastroenterol Hepatol. 2014;11:177-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 174] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 25. | Razpotnik M, Bota S, Wimmer P, Hackl M, Lesnik G, Alber H, Peck-Radosavljevic M. The prevalence of cirrhotic cardiomyopathy according to different diagnostic criteria. Liver Int. 2021;41:1058-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 26. | Liu H, Lee SS. Diagnostic Criteria of Cirrhotic Cardiomyopathy: Out With the Old, in With the New? Hepatology. 2021;74:3523-3525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 27. | Ruíz-del-Árbol L, Achécar L, Serradilla R, Rodríguez-Gandía MÁ, Rivero M, Garrido E, Natcher JJ. Diastolic dysfunction is a predictor of poor outcomes in patients with cirrhosis, portal hypertension, and a normal creatinine. Hepatology. 2013;58:1732-1741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 114] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 28. | Merli M, Torromeo C, Giusto M, Iacovone G, Riggio O, Puddu PE. Survival at 2 years among liver cirrhotic patients is influenced by left atrial volume and left ventricular mass. Liver Int. 2017;37:700-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 29. | Desai MS, Mathur B, Eblimit Z, Vasquez H, Taegtmeyer H, Karpen SJ, Penny DJ, Moore DD, Anakk S. Bile acid excess induces cardiomyopathy and metabolic dysfunctions in the heart. Hepatology. 2017;65:189-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 96] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 30. | Virk MK, Mian MUM, Bashir DA, Wilkes JK, Schlingman T, Flores S, Kennedy C, Lam F, Arikan AA, Nguyen T, Mysore K, Galvan NTN, Coss-Bu J, Karpen SJ, Harpavat S, Desai MS. Elevated bile acids are associated with left ventricular structural changes in biliary atresia. Hepatol Commun. 2023;7:e0109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |