Published online Jun 28, 2025. doi: 10.3748/wjg.v31.i24.107661
Revised: April 26, 2025
Accepted: June 9, 2025
Published online: June 28, 2025
Processing time: 91 Days and 3.3 Hours
Metabolic syndrome-comprising central adiposity, dyslipidaemia, insulin resis
Core Tip: Emerging evidence supports a biological link between chronic gastrointestinal inflammation and the development of cardiometabolic diseases later in life. This is particularly relevant for inflammatory bowel diseases (IBD), such as Crohn’s disease and ulcerative colitis, which are chronic immune-mediated disorders of the gastrointestinal tract. Observational studies indicate that IBD may increase the risk of cardiometabolic diseases. However, the role of uncontrolled intestinal inflammation in exacerbating this risk-and whether treat-to-target strategies aimed at controlling inflammation in IBD could mitigate these comorbidities-remains unclear. This review provides clinicians with an evidence-based summary of the associations between IBD and cardiometabolic diseases.
- Citation: Dutt K, Vasudevan A, Hodge A, Nguyen TL, Srinivasan AR. Cardiometabolic diseases in patients with inflammatory bowel disease: An evidence-based review. World J Gastroenterol 2025; 31(24): 107661
- URL: https://www.wjgnet.com/1007-9327/full/v31/i24/107661.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i24.107661
The metabolic syndrome, characterised by a cluster of disorders including dyslipidaemia, insulin resistance, hyperten
Emerging evidence suggests a potential biological link between chronic gastrointestinal inflammation and the later development of cardiometabolic diseases. This connection is particularly relevant for patients with Crohn’s disease and ulcerative colitis, which are chronic immune-mediated disorders of the gastrointestinal tract. Observational studies have indicated that IBD may increase the risk of cardiometabolic comorbidities[15-17]. Furthermore, associations between active IBD, clinical flares, the need for surgical interventions, and progressive disease complications are well-established[18,19]. However, the impact of uncontrolled intestinal inflammation on the development of cardiometabolic disorders in IBD patients and whether targeted treatment strategies aimed at controlling inflammation can reduce these risks remains unclear[20,21].
This review aims to provide an evidence-based summary of the associations between IBD and cardiometabolic diseases, emphasising the need for further research and clinical awareness of the interconnected nature of these con
In 2005, the International Diabetes Federation (IDF)[22,23] defined metabolic syndrome as the presence of central adiposity, identified by a body mass index (BMI) of more than 30 kg/m2, or increased ethnicity-based waist circumference, in addition to two or more of the following criterion: Fasting glucose > 100 mg/dL (5.55 mmol/L) or on anti-hyperglycaemic therapy, triglycerides > 150 mg/dL (1.69 mmol/L) or on lipid lowering therapy or high density lipo
| NCEP ATP III (2005)[199] | IDF (2005)[22] | |
| Criteria required | Three of five criteria below | Central obesity required plus two other required criteria |
| Obesity | Waist circumference: > 40 inches (101 cm) in men; > 35 inches (88 cm) in women | Waist circumference based on ethnicity1 |
| Insulin resistance-serum glucose | Fasting serum glucose ≥ 100 mg/dL (≥ 5.6 mmol/L); or medical therapy for hyperglycaemia | Fasting serum glucose ≥ 100 mg/dL (≥ 5.6 mmol/L); or medical therapy for hyperglycaemia |
| Dyslipidaemia-serum triglycerides | Serum triglyceride level ≥ 150 mg/dL (> 1.7 mmol/L); or medical therapy for dyslipidaemia | Triglyceride level ≥ 150 mg/dL (> 1.7 mmol/L); or medical therapy for dyslipidaemia |
| Dyslipidaemia-serum HDL-C | Serum HDL-C: < 40 mg/dL (< 1.03 mmol/L) in men; < 50 mg/dL (< 1.29 mmol/L) in women; or medical therapy for dyslipidaemia | Serum HDL-C: < 40 mg/dL (< 1.03 mmol/L) in men; < 50 mg/dL (< 1.29 mmol/L) in women; medical therapy for dyslipidaemia |
| Hypertension | Systolic blood pressure ≥ 130 mmHg; or diastolic blood pressure ≥ 80 mmHg; or medical therapy for hypertension | Systolic blood pressure ≥ 130 mmHg; or diastolic blood pressure ≥ 80 mmHg; or medical therapy for hypertension |
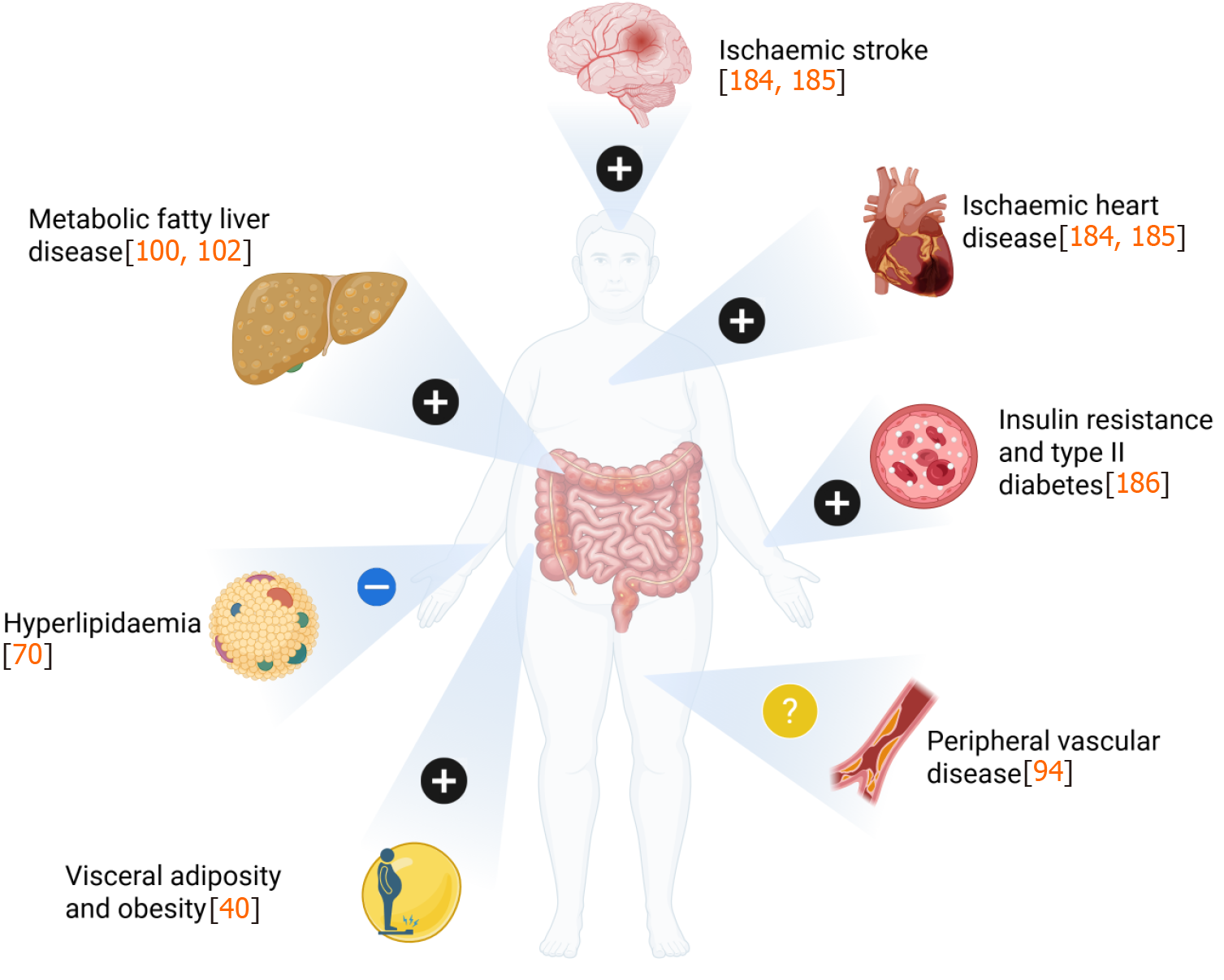
The metabolic syndrome has been associated with unfavourable cardiometabolic sequalae such as ischaemic heart disease (IHD), ischaemic stroke, peripheral vascular disease (PVD), cirrhosis, and cancer. Several of these cardiometabolic disorders have been shown to occur more frequently in patients with IBD compared to the general population (Figure 1 and Table 2)[15,24-29]. These findings have also been replicated across other immune mediated inflammatory disorders (IMID), implying that a pro-inflammatory state may play a role in the development of metabolic syndrome[30].
| Ref. | Country | Study type | Reference criteria | Total number of IBD patients (CD/UC/IBD-U) | Prevalence in IBD (%) | Prevalence in CD (%) | Prevalence in UC (%) |
| Metabolic syndrome | |||||||
| Nagahori et al[32] | Japan | Prospective, cross-sectional cohort | NCEP-ATP III[188] | 102 (28/74/-) | 18.6 | 7.1 | 23.0 |
| Yorulmaz et al[38] | Turkey | Prospective cross-sectional cohort | IDF[22] | 177 (62/115/-) | 25.4 | 17.7 | 29.5 |
| Sourianarayanane et al[34] | United States | Retrospective cohort | NCEP-ATP III[188] | 217 (110/107/-) | 16.6 | ||
| Fitzmorris et al[200] | United States | Retrospective cohort | IDF[22] | 868 (868/-/-) | 4.3 | ||
| Carr et al[35] | United States | Retrospective cohort | NCEP-ATP III[188] | 84 (60/24/-) | 23.0 | 20.0 | 29.0 |
| Sartini et al[201] | Italy | Retrospective cohort | NCEP-ATP III[188] | 78 (42/36/-) | 23.1 | ||
| Dragasevic et al[37] | Serbia | Prospective, cross-sectional cohort | NCEP-ATP III[188] and IDF[22] | 104 (50/54/-) | 32.7 | 36.0 | 29.6 |
| Magrì et al[36] | Italy | Prospective cohort | NCEP-ATP III[188] | 178 (83/95/-) | 19.1 | ||
| Jovanovic et al[202] | Serbia | Retrospective cohort | NCEP-ATP III[188] | 89 (-/89/-) | 81.0 | ||
| Kang et al[33] | Korea | Retrospective cohort | NCEP-ATP III[188] | 443 (274/169/-) | 10.6 | ||
| Obesity | |||||||
| Long et al[44] | United States | Prospective cross-sectional cohort | BMI ≥ 30 kg/m2 | 1598 (paediatric population) (1027/571/-) | 23.6 | 20.0 | 30.1 |
| Nic Suibhne et al[50] | Ireland | Prospective case-control study | BMI ≥ 30 kg/m2 | 100 (100/-/-) | 17.0 | ||
| Flores et al[49] | United States | Retrospective cohort | BMI ≥ 30 kg/m2 | 581 (297/284/-) | 32.7 | 30.3 | 35.2 |
| Seminerio et al[47] | United States | Retrospective cohort | BMI ≥ 30 kg/m2 | 1494 (860/634/-) | 31.5 | 27.0 | 32.0 |
| Elangovan et al[40] | United States | Retrospective cohort | BMI ≥ 30 kg/m2 | 286, 760 (-/-/-) | 37.3 | 36.9 | 38.5 |
| Metabolic fatty liver disease | |||||||
| Martínez-Domínguez et al[117] | Spain | Prospective cross-sectional cohort | MASLD[197] | 646 (-/-/-) | 45.7 | ||
| Zhang et al[122] | United Kingdom | Prospective cross-sectional cohort | MASLD[197] | 4203 (1217/2642/344) | 36.4 | ||
| Oliveira et al[203] | Brazil | Prospective cross-sectional cohort | MASLD[197] | 140 (89/51/-) | 50.7 | 44.9 | 43.1 |
| McHenry et al[118] | United States | Prospective cross-sectional cohort | NAFLD[195] | 363 (363/-/-) | 39.7 | ||
| Hyun et al[204] | Korea | Prospective cross-sectional cohort | NAFLD[195] | 3356 (1129/2227/-) | 16.7 | 11.8 | 19.2 |
| Zhang et al[123] | United Kingdom | Prospective cross-sectional cohort | MASLD[197] | 4622 (1313/2914/-) | 38.1 | ||
A 2024 systematic review and meta-analysis by Shen et al[31] estimated the pooled prevalence of metabolic syndrome in patients with IBD patient to be 21.9% [95% confidence interval (CI): 18.0% to 25.8%] on the basis of 273 cases of metabolic syndrome among 1544 patients with IBD derived from nine studies. This suggests that 1 in 5 patients with IBD had features of metabolic syndrome in this study[31]. These data were derived from nine studies, comprising IBD patients across North America, Europe and Asia, with lower rates of metabolic syndrome described across Asian [Japan (18.6%); Korea (10.6%)][32,33] compared to North American (16.6%-23%)[34,35] and European (19.1%-32.7%)[36,37] cohorts. This implies that factors beyond IBD, perhaps related to environmental, lifestyle and patient factors, may contribute to the development and sequelae of metabolic syndrome. Although the majority of studies applied the NCEP ATP III definition of metabolic syndrome, heterogeneity in how metabolic syndrome was defined, in conjunction with the relatively modest number of IBD patients included in the calculation of pooled prevalence, reflect obvious limitations. Nevertheless, the pooled prevalence reported by Shen et al[31] remains comparable with geographical variability data across non-IBD cohorts which have also documented lower rates of metabolic syndrome across Asian (ranging between 8.4% to 19.4%) relative to North American and European (ranging between 9.7% to 41.9%) cohorts[6].
Several studies have also sought to examine whether the risk of developing metabolic syndrome differs between patients with Crohn’s disease and ulcerative colitis. Carr et al[35] observed no differences (20.00% vs 29.16%, P = 0.36) in the prevalence of metabolic syndrome between patients with Crohn’s disease (n = 60) and ulcerative colitis (n = 24), respectively, with co-existing non-alcoholic fatty liver disease (NAFLD). Nagahori et al[32], examined 107 patients with quiescent IBD and demonstrated a non-significant difference between the frequencies of metabolic syndrome between patients with ulcerative colitis compared to Crohn’s disease, respectively 23.0% vs 7.1% (P = 0.089). These findings con
A 2022 retrospective analysis of United States of America population-level data between 2010 to 2019 documented that the proportion of adult patients with IBD who had co-existing metabolic co-morbidities was reported to have increased between 2010 to 2019[40]. This was characterised by proportional increases in obesity (19.7%-30.1%), sleep apnoea (4.1%-10.8%), hypertension (25.1%-33.9%), type II diabetes (8.3%-12.5%), and hyperlipidaemia (22.1%-32.2%, all P < 0.0001)[40]. This emphasises the need to evaluate the impact of individual cardiometabolic disorders in patients with IBD which will be the focus of the following section.
Obesity represents a key component of metabolic syndrome and is typically characterised by central adiposity, defined on the basis of either waist circumference or BMI. The World Health Organization defines obesity as a BMI above 30 kg/m2 which does not account for body composition[41]. This contrasts with both the IDF and NCEP ATP III definitions of metabolic syndrome which include assessments of waist circumference (Table 3), which provides a better account of visceral adiposity than BMI alone, in their definitions of central adiposity[42]. This is of particular importance given that visceral adiposity has been shown to be key driver of morbidity and mortality, including cardiometabolic complications[42,43].
| Reference population | Reference criteria | Waist circumference threshold for men | Waist circumference threshold for women |
| Europoid | IDF[199] | ≥ 94 cm | ≥ 80 cm |
| Caucasian | WHO[205] | ≥ 94 cm = increased risk; ≥ 102 cm = higher risk | ≥ 80 cm = increased risk; ≥ 88 cm = higher risk |
| United States of America | ATP III[206] | ≥ 102 cm | ≥ 88 cm |
| Canada | Health Canada[207,208] | ≥ 102 cm | ≥ 88 cm |
| European | European Cardiovascular Societies[209] | ≥ 102 cm | ≥ 88 cm |
| Asian | IDF[199] and WHO[205] | ≥ 90 cm | ≥ 80 cm |
| Japanese | Japanese Obesity Society[210] | ≥ 85 cm | ≥ 90 cm |
| China | Cooperative Task Force | ≥ 85 cm | ≥ 90 cm |
| Middle East/Mediterranean | IDF[199] | ≥ 94 cm | ≥ 80 cm |
| Sub-Saharan Africa | IDF[199] | ≥ 94 cm | ≥ 80 cm |
| Ethnic Central and South American | IDF[199] | ≥ 90 cm | ≥ 80 cm |
Population-level data which examined temporal trends in the diagnosis of obesity in patients with IBD, documented an increase in rates of obesity between 2010 (19.7%) and 2019 (37.3%) amongst patients with IBD [ulcerative colitis (38.5%) vs Crohn’s disease (36.9%), P < 0001][40]. These findings are corroborated by cross-sectional studies which have reported that between 15%-40% of IBD patients are obese, with an additional 20%-40% considered overweight[44-50]. Collectively, these data dispel the premise that IBD is a malabsorptive disorder that cannot summarily be associated with obesity.
Obesity itself has been described as a pro-inflammatory state, and has thus been suggested as a possible co-factor in the pathogenesis of IBD, albeit data remains conflicting[14]. When examining studies using BMI as a measure of obesity, a pooled analysis of five prospective cohorts encompassing 601009 individuals, isolated an increased risk of Crohn’s disease, but not ulcerative colitis, amongst obese (BMI ≥ 30 kg/m2) individuals [adjusted hazard ratio (aHR) = 1.34, 95%CI: 1.05-1.71] compared to those with a BMI ≥ 18 kg/m2 and < 30 kg/m2[51]. These findings were reproduced in a large 2019 meta-analysis that included one million pooled individuals, primarily from European and North American cohorts, reported an increased risk of Crohn’s disease but not ulcerative colitis in those with a BMI above 30 kg/m2 (aHR = 1.42, 95%CI: 1.18-1.71)[52]. Similarly, a United States based study of 111489 women within the general population found a similar association between obesity and Crohn’s disease incidence (aHR = 2.33, 95%CI: 1.15-4.69) irrespective of age (aHR = 1.58, 95%CI: 1.01-2.47)[53]. A more recent study across a United Kingdom population reported statistically significant increased risk of both Crohn’s disease [increased BMI hazard ratio (HR) = 1.16, P < 0.001, increased waist circumference HR = 1.17, P < 0.001] and ulcerative colitis (increased BMI HR = 1.09, P < 0.001, increased waist circumference = 1.09, P < 0.001) in patients with increased BMI and waist circumference[54]. Conversely, other smaller studies have found no association, including a Danish study of 75008 women from the general population, identifying no increased risk of Crohn’s disease with obesity (HR = 1.00, 95%CI: 0.96 to 1.04, Ptrend = 0.89)[55]. Similarly, a Norwegian study, encompassing 334 IBD cases from a population 55896 participants, documented that there was no increased risk of either Crohn’s disease or ulcerative colitis associated with increases in BMI over a 10 year period[56]. On the basis of these studies, it remains difficult to attribute a direct relationship between obesity and the subsequent development of IBD.
Nevertheless, the presence of obesity has been shown to have deleterious effects on IBD outcomes. A 2023 systematic review, which evaluated associations between radiological body composition measurements in patients with Crohn’s disease and disease related outcomes, described a higher incidence of unfavourable outcomes, including disease recurrence, need for surgical treatment, and higher rates of post-operative complications, in 4219 patients with Crohn’s disease[57]. These findings were replicated in a retrospective study, conducted between 2010 and 2022, with patients included if they had a disease flare, with subsequent computed tomography imaging and colonoscopy performed within 30 days of this index flare. The primary outcome was the time to next disease flare, with a significantly higher risk of flare in those with higher visceral to subcutaneous fat; visceral fat to subcutaneous fat ratio > 1, [HR = 4.8 (95%CI: 1.7-13, P < 0.01)][58]. The limited data available does not suggest a higher risk of worsening perianal disease with obesity, with a cross-sectional study of 704 Crohn’s disease patients, finding no association between obesity, defined by BMI ≥ 30 kg/m2, and the risk of perianal fistulas (20.6% of obese, 19.4% of non-obese, P = 0.719), complex fistulising disease (88.9% in obese, 78.7% in non-obese, P = 0.144), or need for perianal surgery[59]. Though this study is limited by several factors including its retrospective nature, and the use of BMI as a measure of obesity.
Obesity appears to be associated with a higher rate of post-operative complications in IBD[60]. A 2023 study identified that visceral fat, evaluated on the basis of body composition imaging, increased the risk of post-operative venous thromboembolism [odds ratio (OR) = 1.02, P = 0.028] in patients with Crohn’s disease[61]. Similarly, a large retrospective study of 6601 non-obese and 671 obese (defined by BMI ≥ 30 kg/m2) IBD patients, reported increased rates of post-operative complications following bowel resection in obese patients with IBD. This included higher rates of acute kidney injury (6.7% in obese vs 3.0% in non-obese individual, P = 0.006) and higher rates of post operative intensive care unit admission (6.1% in obese vs 2.7% in non-obese)[62]. However, another study failed to document an increased risk of post-operative complications, anastomotic recurrence or anastomotic leak associated with increased visceral fat across 223 patients with Crohn’s disease following ileocaecal resection over a 10 year period of follow-up[63].
Though a direct correlation between obesity and IBD is unfounded, there is sufficient data to observe that patients with IBD are not immune to the increased rates of obesity as seen among the general population with the current obesity epidemic. Undoubtably, this will lead to poorer general health outcomes for IBD patients, but may also lead to significant IBD related complications.
Data pertaining to the prevalence of insulin resistance in patients with IBD remains limited. In fact, the largest study to-date, included 151 patients with IBD, and noted no differences in characteristics of insulin resistance, defined on the basis of elevated C-peptide and glucose levels, between IBD patients and non-IBD patients[64]. However, among IBD patients that did have evidence of insulin resistance, traditional metabolic risk factors such as elevated waist circumference, hypertension and elevated serum triglycerides were associated with an increased risk of insulin resistance[64]. Though not shown in an IBD population, studies using similar methodologies have demonstrated higher rates of insulin resistance in patients with other autoimmune conditions such as systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA)[65,66]. Both SLE and RA demonstrated independent disease specific associations with insulin resistance, even after traditional metabolic risk factors and steroid exposure were accounted for, so it is plausible that a similar association between IBD and insulin resistance exists although more studies are needed. This is under the guise that chronic inflammation can stimulate insulin resistance, indirectly via inflammatory cytokines that modulate insulin receptors[67].
Despite studies having not demonstrated associations between IBD and insulin resistance, a greater incidence of type 2 diabetes mellitus has been reported among IBD patients compared to the general population. A Danish IBD population-based study, that applied coding data from the entire population (n = 6028844), with 65180 IBD patients, documented a higher incidence rate of type 2 diabetes mellitus in IBD (4.67 per 1000 years) compared to the population incidence rate of 3.02 per 1000 years[68]. The study reported a higher incidence of type 2 diabetes in IBD patients who were diagnosed with IBD over the age of 40, but did not document any differences in the incidence of type 2 diabetes between IBD-subtypes and between corticosteroid naïve and experienced patients[68]. However, the study’s design limited identification of metabolic risk factors such as increased BMI and waist circumference, which may have contributed to the development of diabetes. A Taiwanese population-based study, applied a similar approach to identify patients with co-existing type 2 diabetes mellitus and IBD, found no differences between the incidence rates of diabetes among IBD and non-IBD (1.34 vs 1.30 per 100 person-years; 95%CI: 0.94-1.14; P = 0.531) populations, respectively[69].
A 2023 United Kingdom based biobank study, consisting of prospectively collected data of over 500000 United Kingdom citizens aged between 37 and 73 years of age between 2006 and 2010, documented an association between type 2 diabetes and both IBD subtypes, specifically Crohn’s disease (HR = 2.11, 95%CI: 1.67-2.67, P < 0.001) and ulcerative colitis (HR = 1.76, 95%CI: 1.47-2.11, P < 0.01)[16]. This study also illuminated a possible bidirectional relationship between type II diabetes and IBD, implying that the risk of developing IBD may be increased in patients with type 2 diabetes (HR = 1.61, 95%CI: 1.26-2.06, P < 0.001), and vice versa[16].
A meta-analysis of observational studies documented that co-existing type 2 diabetes was not associated with an increased risk of IBD related complications (OR = 1.12, 95%CI: 0.52-2.45) or all-cause mortality (OR = 1.52, 95%CI: 0.61-3.81), though was associated with a higher frequency of IBD related hospitalisation (OR = 2.54, 95%CI: 2.17-2.98)[70]. However, infectious complications such as pneumonia (OR = 1.71, 95%CI: 1.38-2.14), urinary tract infections (OR = 1.93, 95%CI: 1.51-2.47) and sepsis (OR = 1.56, 95%CI: 1.06-2.29) were noted to be higher amongst IBD patients with co-existing diabetes relative to those who did not[70].
Based on the above literature, it is unclear if IBD predilects the development of insulin resistance and type 2 diabetes. Overall, the data is scant in this regard, and at present clinicians should be aware of the potential development of insulin resistance and diabetes among the IBD subpopulation, due to many being exposed to medications that may contribute to diabetes development such as corticosteroids.
A 2023 systematic review identified 36 studies that evaluated lipid profiles in patients with IBD[71]. These studies documented significantly lower total cholesterol [weighted mean difference (WMD) = -0.506, P < 0.001] in patients with IBD compared to healthy controls[71]. HDL-C (WMD = -0.122, P < 0.004) and low density lipoprotein cholesterol (LDL-C) (WMD = -0.371 P < 0.001) levels were also significantly lower in IBD patients across 29 studies, while there were no significant differences in triglyceride (WMD = -0.077, P = 0.161) levels between IBD and non-IBD patients across 33 studies[71]. Though both IBD and healthy control patients appeared to have relatively normal lipid profiles, subgroup analysis, undertaken on the basis of IBD-subtype, was suggestive of lower total cholesterol levels in patients with Crohn’s disease compared to ulcerative colitis (WMD = -0.349, P < 0.0001)[71]. No significant differences were observed across other lipid parameters such as HDL-C, LDL-C and triglyceride levels[71]. The activity of IBD, through cycles of activity and remission, may also impact the lipid profile of patients with IBD. Studies have documented reductions in total cholesterol (WMD = -0.454, P = 0.001) and LDL-C (WMD = -0.225 P = 0.045) levels during periods of clinically active IBD relative to periods of remission, although similar differences were not observed in HDL-C (WMD = -0.248, P = 0.099) and triglycerides (WMD = -0.129, P = 0.08)[71]. However, dyslipidaemia may also be associated with more severe IBD, defined on the basis of clinical and endoscopic disease activity, and elevated high sensitivity C-reactive protein[72]. The pathological mechanisms behind alterations in lipid profiles in IBD, and chronic inflammation, is poorly understood. Hence associations between dyslipidaemia and disease activity in patients with IBD still remain to be clarified.
Despite largely favourable lipid profiles, patients with IBD have been documented to have a higher risk of cardio
A higher incidence of cardiovascular events has been reported in patients with autoimmune disorders, including IBD (Table 4). A United Kingdom based study, encompassing 22 million individuals, reported a higher incidence of cardio
| Ref. | Country | Study type | Total number of IBD patients (CD/UC/IBD-U) | Risk in IBD vs non-IBD HR (95%CI) | Risk in CD vs non-IBD HR (95%CI) | Risk in UC vs non-IBD HR (95%CI) |
| Ischaemic heart disease | ||||||
| Zhu et al[16] | United Kingdom | Prospective cross-sectional cohort | 5496 (1861/3635) | HR = 0.93 (0.64-1.36) | HR = 1.29 (1.03-1.62) | |
| Heart failure | ||||||
| Aniwan et al[99] | United States | Retrospective cohort | 736 (339/397/-) | aHR = 2.03 (1.36-3.03) | aHR = 1.73 (0.98-3.07) | aHR = 2.06 (1.18-3.65) |
| Zhu et al[16] | United Kingdom | Prospective cross-sectional cohort | 5496 (1861/3635/-) | HR = 1.72 (1.22-2.42) | HR = 1.38 (1.07-1.80) | |
| Sun et al[98] | Sweden | Prospective cross-sectional cohort | 81749 (24303/45709/11737) | aHR = 1.19 (1.15-1.23) | aHR = 1.28 (1.20-1.36) | aHR = 1.14 (1.09-1.19) |
| Ischaemic stroke | ||||||
| Zhu et al[16] | United Kingdom | Prospective cross-sectional cohort | 5496 (1861/3635/-) | HR = 1.32 (0.82-2.12) | HR = 1.59 (1.16-2.14) | |
Whether atherosclerotic-related inflammation may confer a higher risk of subsequent IBD has been studied. A large Swedish nationwide case-control study that included 56212 individuals with IBD and 531014 controls, sought to determine whether patients who experienced an atherosclerotic-related condition, defined as a myocardial infarction, thromboembolic stroke, or atherosclerosis itself, were more likely to develop IBD over a 19 year follow-up period[78]. This study documented that patients who were exposed to an atherosclerotic-related condition were more likely to develop IBD (OR = 1.30, 95%CI: 1.24-1.37), including Crohn’s disease (OR = 1.37, 95%CI: 1.26-1.48) and ulcerative colitis (OR = 1.27, 95%CI: 1.20-1.35), particularly among older adults.
The following sections will evaluate associations between IBD and common cardiovascular complications such as IHD, congestive cardiac failure, ischaemic stroke, and PVD.
IHD: The general mechanism of IHD is the formation of atherosclerotic plaques, which occur in the context of traditional cardiovascular risk factors including hypertension, diabetes, and hyperlipidaemia[79]. In IBD, additional risk factors are proposed to increase the burden of IHD including; activation of prothrombotic protein plasminogen activator inhibitor-1, increased production of radical oxygen species and nitric oxide leading to endothelin dysfunction, and increases in circulating lipopolysaccharides due to gut mucosal barrier breakdown promoting atherosclerosis, and overexpression of matrix metalloproteinases causing coronary vessel stiffening[80-84].
Although the prevalence of IHD in patients with IBD remains unclear, several studies support the notion that IBD patients remain at higher risk of atherosclerotic complications such as acute coronary syndromes. This is supported by a 2023 meta-analysis which observed rates of acute coronary syndrome, defined as either unstable angina or myocardial infarction, to be slightly increased in patients with IBD (HR = 1.23, 95%CI: 1.08-1.41)[85]. In the same year, another meta-analysis evaluated the risk of myocardial infarction in patients with IBD, reporting that there was an increased risk of myocardial infarction across both IBD subtypes [Crohn’s disease (HR = 1.36, 95%CI: 1.12-1.64); ulcerative colitis (HR = 1.24, 95%CI: 1.05-1.46)][86]. In 2024, Takagi et al[87] studied the incidence rates of major adverse cardiac events (MACE) in Japanese patients with ulcerative colitis using Japanese administrative claims data, reporting a cumulative incidence rate of MACE of 2.86% (95%CI: 1.89-4.32) over 120 months.
Traditional risk factors are associated with the development of IHD in patients with IBD. A Japanese study identified older age (> 65 years) (HR = 4.56, 95%CI: 2.79-7.45), presence of diabetes (HR = 1.71, 95%CI: 1.03-2.84) and atrial fibrillation (HR = 2.76, 95%CI: 1.19-6.41) to be associated with increased cumulative incidence rate of MACE[87]. Similar findings were also described in a retrospective multi-centre Chinese cohort study of 1435 patients with IBD, which documented that the risk of IHD in patients with IBD was higher in Crohn’s disease (OR = 2.69, 95%CI: 2.02-3.57) and ulcerative colitis (OR = 1.73, 95%CI: 1.20-2.51) across all ages compared to matched non-IBD patients[88]. Notably, the risk of IHD was observed to peak in IBD patients between the ages of 18-35 years across both Crohn’s disease (OR = 6.33, 95%CI: 3.29-12.16) and ulcerative colitis (OR = 3.00, 95%CI: 1.18-7.60) relative to matched non-IBD subjects[88]. Contrary to general population data, smoking was not associated with an increased risk of IHD, and female patients with Crohn’s disease (OR = 3.15, 95%CI: 1.77-5.61) and ulcerative colitis (OR = 7.35, 95%CI: 4.52-11.96) were noted to have a higher risk of IHD, in this study[88]. Similarly, IBD appears to increase the risk of atherosclerosis even at younger ages. This observation has also been identified in a large registry study of patients with IBD, with a higher prevalence of premature (age ≤ 55) or extremely premature (age < 40) ischaemic atherosclerotic events in patients with IBD compared to healthy controls; premature (OR = 1.14, 95%CI: 1.08-1.21) and extremely premature (OR = 1.82, 95%CI: 1.52-2.17)[89].
Beyond traditional risk factors, IBD disease activity may also be associated with the development of IHD. This was described by a Danish study in which IBD flares (risk ratio = 1.49, 95%CI: 1.16-1.93) and periods of persistent disease activity (risk ratio = 2.05, 95%CI: 1.58-2.56) were observed to be associated with an increased risk of myocardial infarction (risk ratio = 1.17, 95%CI: 1.05-1.31) in patients with IBD[90]. Clinically active IBD was also described to increase the frequency of acute arterial events, defined as myocardial infarction or ischaemic stroke, among a cohort of 30 French patients with IBD (OR = 12.3, 95%CI: 2.8-53.6)[91]. The study also suggested that an elevated C-reactive protein (> 5 mg/L) was associated with an increased risk of ischaemic stroke or myocardial infarction (OR = 3.2, 95%CI: 1.2-8.5). The post-event course of IBD patients who encounter an ischaemic cardiac event does not appear to differ from that of non-IBD patients. This was demonstrated by a French case-control study, with 246 IBD patients and 1470 age-sex matched controls, which reported all-cause mortality (9% vs 8.3%, P = 0.729) and frequency of rehospitalisation with heart failure (0.3% vs 3.5%, P = 0.846) following an ischaemic cardiac event to be similar between IBD and non-IBD patients[92]. However, a 2021 study of 70 patients with IBD who underwent percutaneous coronary intervention for IHD, indicates that patients with IBD may be subject to more frequent readmission for acute coronary syndrome within 12 months compared to non-IBD individuals (34.3% vs 25.5% P < 0.0001)[93]. This may indicate that the severity and complexity of IHD may differ between IBD and non-IBD patients.
The literature is forthcoming in suggesting a higher risk of IHD among patients with IBD. Notably, beyond traditional cardiovascular risk factors, IBD disease activity correlates with higher rate of IHD events. This is supported by studies in the cardiovascular space that have suggested that systemic and local inflammation may play a significant role in the development of IHD[94,95]. This may suggest that a treat-to-target approach focused on improving IBD outcomes may also dampen the risk cardiovascular complications.
Heart failure: Heart failure can occur as a complication of IHD as well as via other pathogenic mechanisms. There is an association between dysregulation of immune and inflammatory responses in the pathogenesis of heart failure which may place individuals with IBD at higher risk of this condition. This is mediated via inflammatory cytokines that can subjugate cardiac remodelling[96,97]. Data regarding the prevalence of heart failure in patients with IBD remains limited, however several studies have evaluated whether an association between IBD and heart failure exists. A large Swedish nationwide cohort of 81 749 patients with IBD reported that IBD was associated with a moderately increased risk of heart failure compared to patients without IBD (aHR = 1.19, 95%CI: 1.15-1.23)[98]. The study calculated an incident rate of 50.3 per 10000 person years for patients with IBD, compared to 37.9 per 10000 person years among non-IBD controls over 12.4 years of median follow-up[98]. This increased risk was observed across all IBD subtypes, including Crohn’s disease [incidence rate = 46.9 vs 34.4; aHR = 1.28 (95%CI: 1.20-1.36)], ulcerative colitis [incidence rate = 50.1 vs 39.7; aHR = 1.14 (95%CI: 1.09-1.19)], and IBD-unclassified [incidence rate = 60.9 vs 39.0; aHR = 1.28 (95%CI: 1.16-1.42)][98]. A smaller study that included 736 IBD patients documented that the relative risk of heart failure was increased among patients with ulcerative colitis (aHR = 2.06; 95%CI: 1.18-3.65) but not Crohn’s disease, as well as IBD patients exposed to systemic corticosteroids (aHR = 2.51; 95%CI: 1.93-4.57)[99]. The cumulative incidence of heart failure in IBD patients across this study was higher than age and sex-matched controls, and similar to a Swedish study, in which IBD was identified as an independent risk factor for heart failure development with an aHR = 2.03 (95%CI: 1.36-3.03)[99].
Few studies have evaluated whether the presence of heart failure is associated with less favourable IBD outcomes. A United States based study that utilised a large institutional database, consisting of 2449 patients with IBD and an additional 271 with IBD and concomitant heart failure, sought to evaluate the impact of heart failure on IBD outcomes and disease course[100]. This study indicated that IBD patients with co-existing heart failure had higher risk of requiring an IBD-related hospitalisation [HR = 1.42 (95%CI: 1.2-1.69)], experiencing an IBD flare [HR = 1.32 (95%CI: 1.09-1.58)] and complications [HR = 1.7 (95%CI: 1.33-2.17)], compared to IBD patients without heart failure[100]. These findings reflect that IBD patients with co-existing heart failure are likely to experience more unfavourable IBD outcomes than those without heart failure. Moreover, the mortality risk of IBD patients with co-existing heart failure was shown to be higher than IBD patients without heart failure, but similar to those of patients with heart failure without IBD[100]. This may imply that the increased mortality is more likely to be conferred by heart failure rather than co-existing IBD.
It is difficult to determine whether the higher incidence of heart failure in patients with IBD is due to the disease itself. Indeed, a plausible pathophysiological mechanism exists that may underpin the higher incidence, though these findings may also occur in the context of higher rates of IHD amongst individuals with IBD.
Ischaemic stroke: In view of the shared pathophysiology with IHD, patients with IBD have also been suggested to have a higher risk of atherosclerotic and thromboembolic ischaemic stroke than the general population. This was demonstrated in a 2025 meta-analysis, that included pooled outcomes from 2.8 million patients across 13 studies, which reported that patients with IBD had an increased risk of ischaemic stroke [pooled HR = 1.3 (95%CI: 1.21-1.39, P < 0.001)] compared to non-IBD patients, with no differences observed between sexes[101]. This risk was also observed across IBD subtypes of Crohn’s disease [HR = 1.35 (95%CI: 1.22-1.49 P < 0.001)] and ulcerative colitis [HR = 1.15 (95%CI: 1.09-1.22, P < 0.001)][101]. These findings reflect that IBD patients are at increased risk of stroke, particularly ischaemic stroke, irrespective of IBD-subtype. The risk of ischaemic stroke has been shown to be highest immediately following the diagnosis of IBD, and plateaus after several years; perhaps implying that early active disease around the time of IBD diagnosis may potentiate the risk of thromboembolic ischaemic stroke rather than disease duration[102]. This is supported by data from two studies included in a recent meta-analysis which suggested that there may be higher risk of ischaemic stroke during IBD disease flares [incidence rate ratio (IRR) = 1.70 (95%CI: 1.36-2.12, P < 0.0001)], and also during periods of chronic persistent disease activity [IRR = 2.20 (95%CI: 1.38-3.52, P = 0.001)][103]. Nevertheless, the increased risk of ischaemic stroke in patients with IBD was shown to persist for up to 25 years following IBD diagnosis, highlighting the need for ongoing clinical vigilance.
PVD: There is limited data evaluating associations between PVD in patients with IBD. A large retrospective observational study of French insurance data, consisting of more than 170000 patients, sought to evaluate the impact that exposure to thiopurines and anti-tumour necrosis factor (TNF) therapies had on the risk of acute arterial events, comprising IHD, stroke, and PVD, in patients with IBD[104]. This study reported an incidence rate of 5.4, 2.8, 1.7 and 0.9 per 1000 person-years across all arterial events, IHD, stroke, and PVD, respectively, although comparisons with a non-IBD cohort were not reported[104]. Another retrospective observational study of a large United States based claims database, comprising 17487 IBD patients and 69948 controls, documented no significant differences in the adjusted risks of PVD events in IBD patients compared to controls (HR = 1.0), including across IBD subtypes of Crohn’s disease (HR = 0.9) and ulcerative colitis (HR = 1.1)[105]. Similar findings were reported in a large prospective study that used data from the United Kingdom Biobank to evaluate the risk of acute arterial events in patients with IBD[106]. This study documented no significant difference in the incidence of PVD in patients with IBD compared to those without IBD (55.1 vs 39.8 per 1000 years, P = 0.098), respectively, including premature PVD [HR = 1.05, (P = 0.889)], defined as that occurring before the age of 55 years in men and 65 years in women[106].
Though similar pathological mechanisms to coronary vessel disease may potentiate the development of PVD, data in this area is lacking, leading to uncertainty regarding its significance among patients with IBD.
Metabolic fatty liver disease consists of multiple disorders; NAFLD, metabolic dysfunction associated fatty liver disease (MAFLD) and metabolic dysfunction associated steatotic liver disease (MASLD) (Table 5). Despite varied nomenclature and definitions, these conditions represent similar patient populations that collectively represent metabolic fatty liver disease[107].
| NAFLD[211] | MAFLD[212] | MASLD[213] | |
| Required criteria | Radiological or Histological evidence of hepatic steatosis | ||
| Cardiometabolic risks required | At least 1 of the following 3 criteria required: (1) Obesity: BMI ≥ 25 kg/m2 in Caucasians; or BMI ≥ 23 kg/m2 in Asians; (2) Fasting glucose ≥ 100 mg/dL (≥ 5.6 mmol/L), or on treatment for hyperglycaemia; and (3) Two or more of the following: Elevated waist circumference (Table 2); Systolic blood pressure ≥ 130 mmHg; Diastolic blood pressure ≥ 85 mmHg; Serum triglycerides ≥ 150 mg/dL (> 1.7 mmol/L); HDL-C < 40 mg/dL (< 1.03 mmol/L); hsCRP > 2 mg/L; Insulin resistance-HOMA-IR ≥ 2.5; or On treatments for diabetes | At least 1 of the following 5 criteria required: (1) Obesity defined by BMI ≥ 25 kg/m2 in Caucasians; or BMI ≥ 23 kg/m2 in Asians; or elevated waist circumference: > 94 cm (males), > 80 cm (females); (2) Fasting glucose ≥ 100 mg/dL (≥ 5.6 mmol/L), or glucose level post glucose tolerance test ≥ 140 mg/dL (7.8 mmol/L), or HbA1c ≥ 5.7% (39 mmol/L) or on treatment for hyperglycaemia; (3) Systolic blood pressure ≥ 130 mmHg or diastolic blood pressure ≥ 85 mmHg, or on anti-hypertensive treatment; (4) Serum triglycerides ≥ 150 mg/dL (> 1.7 mmol/L) or on lipid lowering therapy; and (5) HDL-C < 40 mg/dL (< 1.03 mmol/L) in men of < 50 mg/dL (< 1.29 mmol/L) in women or on treatment for dyslipidaemia | |
| Additional criteria | Other causes of hepatic steatosis excluded | ||
The global burden of metabolic fatty liver diseases are significant, affecting approximately 30% of the global popu
Given that metabolic syndrome can be a pathogenic catalyst in the development of metabolic fatty liver disease, and the high prevalence of this syndrome among IBD patients, it can be postulated that metabolic liver disease may also be prevalent among this subpopulation. A 2023 metanalysis looking at NAFLD within the IBD population showed similar rates to the general population, with geographical variation[112]. Prevalences reported using any measure to define fatty liver revealed rates of 31.8% (95%CI: 22.1-42.4, 32 studies) among European nations, 27.7% (95%CI: 19.5-36.8, 10 studies) in East Mediterranean countries, 14.2% (95%CI: 12.5-16, 32 studies) in the Americas, 19.7% (95%CI: 10.8-30.4, 8 studies) in South East Asia, and 18.7% (95%CI: 12.2-26.2, 5 studies) in Western Pacific region[112]. Analysis of outcomes using transient elastography and controlled attenuation parameter, which are clinically recognised measures of liver fibrosis and steatosis, have also been undertaken[113]. These analysis identified a higher prevalence of liver steatosis in all geographical regions; 43.1% in Europe, 35.7% in the Americas and 28.6% in South East Asia[112]. Within a Spanish cohort of patients, MAFLD prevalence was significantly higher in patients with IBD than those without (349/831, 42.00% vs 563/1718, 32.77%; P < 0.001)[114].
IBD may hasten the development of metabolic fatty liver disease. Multitudinous factors may play a role, including systemic inflammation disrupting leptin signalling pathways causing alterations in fat metabolism and insulin resistance, and direct hepatoxic effects via reactive oxygen species[115,116]. A recent study of patients with MASLD and concomitant IBD showed a higher incidence of MASLD in lean IBD patients compared to lean non-IBD patients, 21.3% in the IBD group vs 10.0% in the non-IBD group (P = 0.022), with IBD independently being associated as a risk factor for MASLD in lean IBD patients; OR = 2.71 (95%CI: 1.05-7.01, P = 0.04)[117]. Another study similarly showed IBD as an independent risk factor for the development of MAFLD; OR = 2.00 (95%CI: 1.59-2.51, P < 0.001)[114]. The same study also identified IBD as an independent risk factor for the development of fibrosis diagnosed on liver biopsy; OR = 5.55 (95%CI: 2.69-11.47, P < 0.001)[114]. Despite this, associations between the development of fatty liver disease and IBD disease activity have been explored by few studies. A 2023 study found patients with quiescent Crohn’s disease had higher rates of liver steatosis based on magnetic resonance imaging compared to those with active disease[118]. In patients with Crohn’s disease a 2024 study revealed that disease duration was an independent predictor of fatty liver development, with an OR of 1.02 (95%CI: 1.001-1.04)[119], suggesting potential longer systemic inflammation, even low-grade, may have an influence on fatty liver disease. Older age and traditional metabolic risk factors such as obesity have also been identified as risk factors across multiple studies[114,118,120].
Conversely, liver steatosis may also be associated with the development of IBD. In a large Chinese cohort of 418721 participants, 160807 had a diagnosis of NAFLD. Within the population of NAFLD participants there was significantly higher risk of incident IBD (HR = 1.13, 95%CI: 1.04-1.24, P = 0.005), particularly a higher risk of incident Crohn’s disease (HR = 1.36, 95%CI: 1.17-1.58, Ptrend < 0.001)[121].
Beyond hepatic complications, IBD patients with metabolic fatty liver disease may have increased risk of other metabolic disorders. One study found that patients with a combination of MASLD and IBD may be at a higher risk of cardiometabolic complications; higher risk of incident cardiovascular disease (HR = 1.77, 95%CI: 1.26-2.49, P = 0.001), increased risk of IHD (HR = 2.54, 95%CI: 1.62-3.98, P < 0.001) and heart failure (HR = 3.36, 95%CI: 1.53-7.38, P = 0.003) compared to those with IBD alone[122]. There may also be an increased risk of all-cause mortality, with a 2024 United Kingdom study independently associating the combination of IBD and MASLD, with higher rates of all-cause mortality compared to IBD patients without MASLD; HR = 1.58, 95%CI: 1.07-2.32[123]. Risk of mortality also increased with the presence of metabolic risk factors; 3 risk factors with (HR = 1.85, 95%CI: 1.20-2.85), 4 or more risk factors (HR = 1.83, 95%CI: 1.16-2.89)[123].
The rising global burden of metabolic fatty liver disease cannot be ignored among IBD patients. Often considered a disorder of obese individuals, the literature suggests a higher burden of metabolic fatty liver disease, even among lean IBD patients, potentially implicating non-traditional pathogenic mechanisms, such as systemic inflammation. Overall, clinicians should be aware and vigilant of the potential of metabolic fatty liver disease among patients with IBD; particu
Beyond the risks associated with IBD and the metabolic syndrome, the effect of treatments on the risk of metabolic complications is another important consideration for clinicians managing IBD. Corticosteroids are frequently used to induce clinical remission, however, a large proportion of patients with IBD require ongoing corticosteroid sparing therapies to maintain remission and achieve more stringent treatment targets as espoused by the STRIDE-II guidelines[20,124]. Maintenance IBD therapies include small molecule and biological therapies, and once initiated, patients with IBD frequently remain these therapies for extended periods. The following section will examine the cardiometabolic risk associated with commonly used IBD medications.
Corticosteroids have multiple adverse metabolic effects that are related to dose and duration of exposure[125]. Short term metabolic effects include alterations in hepatic glucose metabolism, while medium to longer term effects can include diabetogenic complications, alterations in lipid metabolism, onset of central adiposity and increased cardiovascular risk[126].
From a metabolic perspective, corticosteroids have been associated with alterations in hepatic glucose metabolism and have been demonstrated to invoke insulin resistance through multiple mechanism, both of which can potentiating diabetogenic complications[127-129]. This risk is driven by the dose and length of exposure to corticosteroid therapy. This was illuminated in a 2020 meta-analysis that included 100722 patients with IMID, 28.2% of whom had IBD, which documented strong dose-related associations with diabetic events in those exposed to corticosteroids compared to those without exposure[130]. This was exemplified by corticosteroid doses of 25 mg daily or above (HR = 4.34, 95%CI: 3.83-4.91) having stronger associations with hyperglycaemic complications compared to doses of 5 mg or less (HR = 1.90, 95%CI: 1.73-2.09)[130].
Corticosteroids have also been shown to have a negative impact on lipid metabolism in a dose dependent manner that remains independent of duration of exposure[131-133]. Three studies have explored these associations within an IBD context, with a meta-analysis reporting a pooled mean increase in total cholesterol across 73 IBD patients exposed to corticosteroids of 1.25 mmol/L (95%CI: 0.31-2.39) during periods of steroid exposure during induction, and 1.29 mmol/L (95%CI: 0.40-2.38) when corticosteroids were used for maintenance[134]. A non-significant increase in triglycerides was observed during induction, with a pooled change of 0.08 (95%CI: -0.13 to 0.34), however, serum HDL-C and LDL-C were not evaluated[134].
Corticosteroid exposure has also been associated with increased cardiovascular risk in patients with IBD. This was evident in a large United Kingdom based population cohort study, that included 27739 patients with IBD, which docu
Anti-TNF-adalimumab, certolizumab, golimumab, infliximab: Patients with heart failure and myocardial infarction have been documented to have higher circulating levels of TNF alpha, with reductions in TNF levels shown to improve heart failure in mouse models[138-140]. This provided the basis to evaluate the clinical utility of anti-TNF therapy to treat heart failure and myocardial infarction. However, owing to an increased risk of death, and worsening of heart failure of moderate to severe severity (New York Heart Association class 3 to 4) with very high doses of infliximab, anti-TNF therapy is generally avoided in this patient subset[141]. Notably, studies subsequent to this have not have not docu
Several studies have sought to evaluate associations between anti-TNF therapy and cardiovascular risk. A large French cohort of 177827 IBD patients exposed to anti-TNF agents reported that exposure to this class of therapy was associated with a decrease in acute arterial events such as cerebrovascular disease, IHD, and PVD (HR = 0.79, 95%CI: 0.66-0.95)[104]. Exposure to anti-TNF therapy has also been associated with improved mortality in patients with Crohn’s disease (21.4 vs 30.1 per 1000 person-years, OR = 0.78, 95%CI: 0.65-0.93), albeit in comparison to prolonged corticosteroid exposure, with benefits attributed to reductions in major adverse cardiovascular events (OR = 0.68, 95%CI: 0.55-0.85)[146]. Anti-TNF therapy has also been associated with a decrease in arterial stiffness that closely approximates to controls after 4 years of exposure[147]. A 2023 meta-analysis by Shehab et al[148] indicated that biologic therapies, including anti-TNF, had no significant impact on the risk of MACE during periods of induction and maintenance in IBD randomised controlled trials. However, a 2024 network meta-analysis, that included 40 studies encompassing anti-TNF exposure across IBD (6 studies consisting of a cohort of 7463 IBD patients), dermatology, and rheumatology indications, demon
Anti-TNF agents have also been associated with dyslipidaemia, albeit in patients with RA[150]. Although short-term changes in lipid profiles have been reported in patients with IMID, the impact of these changes on future cardiovascular events remains to be clarified. A study that sought to evaluate risk factors for developing NAFLD among IBD patients indicated that patients not receiving anti-TNF therapy exhibited a higher occurrence of NAFLD (29.7% vs 17.1%, P = 0.048)[34]. These data indicate that exposure to anti-TNF therapy may have favourable associations with overall mortality and cardiometabolic risk; however whether these benefits are related to the drug’s mechanism, or simply as a con
Anti-interleukin-12/interleukin-23-ustekinumab, risankizumab, mirikizumab: Interleukin (IL)-12 has been implicated in the pathogenesis of atherosclerosis, with inhibition of IL-12 identified as a potential novel mechanism to address atherosclerosis on the basis of murine models[151,152]. However, studies of briakinumab, an IL-12/23 inhibitor, in patients with chronic plaque psoriasis documented an increased association with MACE events[153]. Similarly, a French cohort study suggested that patients with high cardiovascular risk (OR = 4.17, 95%CI: 1.19-14.59), were at increased risk of developing significant ischaemic events, unstable angina and myocardial infarction, within the first 6 months of commencing ustekinumab, with no comparable increase observed in patients with low cardiovascular risk (OR = 0.30, 95%CI: 0.03-3.13)[154]. This remains in keeping with current mechanistic models for atherosclerotic disease in which the period following initiation of anti-IL 12/23p40 may be associated with destabilisation of atherosclerotic plaque via inhibition of helper Th-17 cells[155-157]. A 2024 network meta-analysis, which examined cardiovascular events in patients with IMIDs, also demonstrated that exposure to ustekinumab for the management of IMIDs may be associated with an increased risk of major adverse cardiovascular events compared to placebo (OR = 3.15, 95%CI: 1.01 to 13.35)[149]. However, these findings have not been replicated in other studies, including pooled analysis of 3117 patients exposed to ustekinumab for the treatment of psoriasis or PsA which was suggestive of a trend toward decreased MACE events over at least 4 years of follow-up[153,158,159]. Similarly, data from IBD trials have not demonstrated any material change in the risk of cardiovascular events following up to two-years of ustekinumab exposure[160,161]. More recent IBD therapeutics such as risankizumab and mirikizumab, both of which selectively inhibit IL-23, have not demonstrated any significant signal for MACE[162,163]. Collectively, these data reflect that controversies regarding the association between exposure to anti–IL-12/23p40 therapies and cardiovascular atherosclerotic disease remain to be resolved[153,158,159]. However, no intrinsic effect of IL-23 has been reported on plaque vulnerability to-date.
Several case reports have documented potential temporal associations between the administration of ustekinumab and heart failure, with documented reversibility following discontinuation of ustekinumab in some cases[164-166]. However, unfavourable associations between ustekinumab and heart failure have not been widely reported across clinical trials and larger ‘real-world’ cohorts.
Anti-integrin-vedolizumab: Overall, vedolizumab does not appear to increase the risk of cardiometabolic complications, although data is limited. Real world data and registration studies for vedolizumab did not reveal any negative cardiometabolic outcomes[167-169]. A 2022 study examining the safety of vedolizumab in a matched cohort of elderly and non-elderly patients with IBD did not document any significant association between vedolizumab exposure and cardiovascular disease in the elderly cohort over the age of 65[170].
5-aminosalicylic acid: The impact that 5-aminosalicylic acid (5-ASA) therapies have on cardiovascular disease is inconsistent. A Danish study found that IBD patients exposed to 5-ASA therapy had lower risk of IHD compared to unexposed patients (IRR = 1.16 vs 1.36, P = 0.02)[136]. Conversely, a United Kingdom based study reported a negative association between 5-ASA exposure and the development of cardiovascular disease. This study examined a cohort of 18000 patients with IBD who had 1400 exposures to 5-ASA therapies, and reported exposure to 5-ASA was associated with an increased risk of cardiovascular events (unadjusted HR = 1.2, 95%CI: 1.1-1.3)[171]. It is important for clinicians to be aware of non-traditional cardiac manifestations of 5-ASA therapy such as the development of myocarditis and pericarditis[172]. There is no evidence that exposure to 5-ASA therapy has any negative metabolic effects in patients with IBD.
Thiopurines: Thiopurines, which inhibit purine synthesis, may provide benefit in patients with metabolic syndrome. A recent French cohort study in IBD patients with a prior history arterial thrombosis (cardiovascular event, cerebrovascular event or PVD) had lower rates of recurrence if treated with both anti-TNF therapy and thiopurines[173]. Though it is difficult to discern if the effect is due to thiopurine or anti-TNF exposure, or simply related to better IBD control. Another small study comprising of 111 patients with IBD and concomitant CVD, documented a statistically insignificant reduction in cardiovascular events; OR = 0.45 (95%CI: 0.19-1.06, P = 0.07) in patients exposed to thiopurines, though was potentially underpowered for this outcome[174].
Methotrexate: Data pertaining to the cardiometabolic impact that methotrexate may have in patients with IBD remains unclear. However, two meta-analyses of patients with rheumatic conditions have demonstrated that exposure to methotrexate may be associated with a reduced risk of cardiovascular events; relative risk = 0.72 (95%CI: 0.57-0.91) and relative risk = 0.79 (95%CI: 0.71-0.88)[145,175]. This has been attributed to methotrexate’s ability to reduce oxidative stress and reduce the burden of inflammatory cytokines. Methotrexate has also demonstrated associations with reductions in arterial blood pressure, improvements in lipid profile, and a decreased risk of type II diabetes[176].
Janus kinase inhibitors-tofacitinib, upadacitinib: Janus kinase inhibitors may have deleterious effects on the metabolic syndrome through increased lipid concentrations with proportionate, dose-related, increases in both HDL and LDL, with no significant alterations in lipid ratios[177-180]. These agents have been used in inflammatory arthropathies for a longer duration than IBD, with safety data suggesting a higher risk of all form cardiovascular disease in these cohorts, particularly if individuals had a pre-existing cardiovascular risk factor[181]. Though in patients without discernible cardiac risk factors, several cohort studies, including meta-analysis have shown no preponderance to develop IHD, including in those with IBD[182-184]. In light of these findings, clinicians should consider pre-existing cardiovascular risk at the time of prescribing JAK inhibitors.
Sphingosine-1-phosphate modulators-ozanimod, etrasimod: Sphingosine-1-phosphate (S1P) modulators have been shown to be relatively safe, with the main concern being the risk of significant hypertension[185]. S1P modulators can also interact with other medications including monoamine oxidase (MAO) inhibitors, and serotonin and norepinephrine modulators[186]. Concomitant use may increase the risk of hypertension and hypertensive crisis, particularly with MAO inhibitors[186]. These therapies are contraindicated in heart failure, and those with recent myocardial infarction, though data suggest that there is no increased risk of MACE developing de novo with exposure to S1P modulators[185,187].
Obesity has been suggested to impact the therapeutic effectiveness of several advanced IBD therapies, particularly monoclonal antibodies. A 2020 meta-analysis, identified a higher risk of treatment failure and loss of response (LOR) in obese patients treated with anti-TNF therapies such as adalimumab, certolizumab and infliximab, with a combined OR = 1.20, P = 0.015[188]. Similar findings were also observed in the personalised anti-TNF therapy in Crohn’s disease personalised anti-TNF therapy in Crohn’s disease study cohort study, which reported an increased risk of LOR (HR = 1.62, 95%CI: 1.08-2.42) in patients defined as obese on the basis of a BMI ≥ 30 kg/m2[189]. However, data from a paediatric cohort of IBD patients showed no significant differences in terms of anti-TNF treatment response between obese and non-obese patients; although obese patients were more likely to require dose escalation of either infliximab or adalimumab therapy within 6-months (30% vs 19.5%, P = 0.04)[190].
Other biologic agents, including vedolizumab and ustekinumab, have not demonstrated reduced clinical efficacy in obese individuals. This is exemplified by similar dose escalation requirements between obese and non-obese (48.9% and 42.0%, P = 0.4) IBD patients exposed to vedolizumab, and lower rate of vedolizumab discontinuation among obese patients (31.9% vs 53.2%, P = 0.01)[191]. Post-hoc analysis of the IM-UNITI trial did not identify differences in clinical response between Crohn’s disease patients categorised as normal, overweight and obese on the basis of BMI, who were exposed to ustekinumab[192]. However, ustekinumab trough levels were noted to be lower in patients classified as obese compared to those categorised as overweight and normal (obese: 2.98 mcg/mL vs overweight: 4.84 mcg/mL vs normal: 4.43 mcg/mL, P = 0.01). Risankizumab, an anti-IL-23 agent recently approved for use in IBD, has shown no differences in treatment responses in obese psoriasis patients derived from long term observational study data[193,194].
The relationship between cardiometabolic diseases and IBD is multifaceted. Cardiometabolic diseases are more prevalent in IBD patients compared to the general population; however, identifying which IBD patients are at the highest risk of developing these conditions later in life remains challenging. The European Society for Clinical Nutrition and Metabolism and United European Gastroenterology guidelines recommend measuring waist circumference and conducting non-invasive assessments of hepatic steatosis for patients with a BMI between 25-30 kg/m², and screening for insulin resistance in those with a BMI > 30 kg/m²[195]. However, IBD-specific guidance that incorporates non-traditional risk factors are yet to be defined. Emerging evidence suggests a biological link between chronic gastrointestinal inflammation and the development of cardiometabolic disorders, a concern for IBD patients given the typical early age of onset. Although associations between IBD disease activity and cardiometabolic risk have been observed, it remains unclear whether a treat-to-target approach aimed at eliminating bowel inflammation can reduce the risk of these disorders in the longer term. Current evidence linking IBD activity to cardiometabolic risk remains limited, primarily due to the reliance on national registry-based data, which often lacks the granularity necessary to establish robust associations. These data sources typically fail to capture key variables such as disease severity, treatment regimens, and lifestyle factors that may influence cardiometabolic outcomes in IBD patients. To better understand the medium- and longer-term effects of gastrointestinal inflammation on the development of cardiometabolic diseases, large-scale, prospective studies with extended follow-up are needed. Additionally, regional variability in the prevalence of cardiometabolic comorbidities among IBD patients underscores the necessity of conducting studies across diverse geographical populations. Such research is essential to elucidate the factors contributing to cardiometabolic risk in IBD and to develop more targeted prevention and management strategies. The growing number of IBD therapies that help achieve better disease control offers promising opportunities for reducing cardiometabolic risk. However, clinicians must remain cautious about the potential negative cardiometabolic effects of some therapies, particularly in patients with pre-existing cardiometabolic risk factors. This review highlights the clinically significant relationship between IBD and cardiometabolic disorders and emphasizes the importance of considering non-traditional risk factors, such as disease activity, in this context. While observational studies suggest a link, further research is needed to clarify the biological and pathogenic mechanisms underlying this relationship. This research is crucial for identifying which IBD patients are at highest risk with a view towards developing strategies to mitigate this risk. In the meantime, IBD clinicians should consider incorporating cardiometabolic risk assessments into clinical practice, particularly for patients with pre-existing cardiometabolic risk factors and in the context of therapy-related decisions. Future research should focus on whether treat-to-target strategies that control intestinal inflammation can simultaneously improve both long-term IBD and cardiometabolic outcomes.
| 1. | Huang PL. A comprehensive definition for metabolic syndrome. Dis Model Mech. 2009;2:231-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1429] [Cited by in RCA: 1436] [Article Influence: 89.8] [Reference Citation Analysis (0)] |
| 2. | Kassi E, Pervanidou P, Kaltsas G, Chrousos G. Metabolic syndrome: definitions and controversies. BMC Med. 2011;9:48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 993] [Cited by in RCA: 960] [Article Influence: 68.6] [Reference Citation Analysis (0)] |
| 3. | Isomaa B, Almgren P, Tuomi T, Forsén B, Lahti K, Nissén M, Taskinen MR, Groop L. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001;24:683-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3238] [Cited by in RCA: 3131] [Article Influence: 130.5] [Reference Citation Analysis (0)] |
| 4. | Noubiap JJ, Nansseu JR, Lontchi-Yimagou E, Nkeck JR, Nyaga UF, Ngouo AT, Tounouga DN, Tianyi FL, Foka AJ, Ndoadoumgue AL, Bigna JJ. Geographic distribution of metabolic syndrome and its components in the general adult population: A meta-analysis of global data from 28 million individuals. Diabetes Res Clin Pract. 2022;188:109924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 257] [Article Influence: 85.7] [Reference Citation Analysis (0)] |
| 5. | Chew N, Ng CH, Tan D, Kong G, Lin CX, Chin YH, Foo R, Chan M, Muthiah M. Global burden of metabolic diseases: data from Global Burden of Disease 2000-2019. A cosortium of metabolic disease. Eur Heart J. 2023;44. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 6. | Neeland IJ, Lim S, Tchernof A, Gastaldelli A, Rangaswami J, Ndumele CE, Powell-Wiley TM, Després JP. Metabolic syndrome. Nat Rev Dis Primers. 2024;10:77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 73] [Article Influence: 73.0] [Reference Citation Analysis (0)] |
| 7. | Hoveling LA, Liefbroer AC, Bültmann U, Smidt N. Understanding socioeconomic differences in metabolic syndrome remission among adults: what is the mediating role of health behaviors? Int J Behav Nutr Phys Act. 2021;18:147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 8. | Puolakka E, Pahkala K, Laitinen TT, Magnussen CG, Hutri-Kähönen N, Tossavainen P, Jokinen E, Sabin MA, Laitinen T, Elovainio M, Pulkki-Råback L, Viikari JS, Raitakari OT, Juonala M. Childhood Socioeconomic Status in Predicting Metabolic Syndrome and Glucose Abnormalities in Adulthood: The Cardiovascular Risk in Young Finns Study. Diabetes Care. 2016;39:2311-2317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 9. | Matthews KA, Räikkönen K, Gallo L, Kuller LH. Association between socioeconomic status and metabolic syndrome in women: testing the reserve capacity model. Health Psychol. 2008;27:576-583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 88] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 10. | Bankoski A, Harris TB, McClain JJ, Brychta RJ, Caserotti P, Chen KY, Berrigan D, Troiano RP, Koster A. Sedentary activity associated with metabolic syndrome independent of physical activity. Diabetes Care. 2011;34:497-503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 312] [Cited by in RCA: 325] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 11. | Nsabimana P, Sombié OO, Pauwels NS, Boynito WG, Tariku EZ, Vasanthakaalam H, De Henauw S, Abbeddou S. Association between urbanization and metabolic syndrome in low- and middle-income countries: A systematic review and meta-analysis. Nutr Metab Cardiovasc Dis. 2024;34:235-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 12. | Li Y, Sun Y, Wu H, Yang P, Huang X, Zhang L, Yin L. Metabolic syndromes increase significantly with the accumulation of bad dietary habits. J Nutr Health Aging. 2024;28:100017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 13. | Ng SC, Zeng Z, Niewiadomski O, Tang W, Bell S, Kamm MA, Hu P, de Silva HJ, Niriella MA, Udara WS, Ong D, Ling KL, Ooi CJ, Hilmi I, Lee Goh K, Ouyang Q, Wang YF, Wu K, Wang X, Pisespongsa P, Manatsathit S, Aniwan S, Limsrivilai J, Gunawan J, Simadibrata M, Abdullah M, Tsang SW, Lo FH, Hui AJ, Chow CM, Yu HH, Li MF, Ng KK, Ching JY, Chan V, Wu JC, Chan FK, Chen M, Sung JJ; Asia-Pacific Crohn’s and Colitis Epidemiology Study (ACCESS) Group. Early Course of Inflammatory Bowel Disease in a Population-Based Inception Cohort Study From 8 Countries in Asia and Australia. Gastroenterology. 2016;150:86-95.e3; quiz e13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 90] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 14. | Singh S, Dulai PS, Zarrinpar A, Ramamoorthy S, Sandborn WJ. Obesity in IBD: epidemiology, pathogenesis, disease course and treatment outcomes. Nat Rev Gastroenterol Hepatol. 2017;14:110-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 302] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 15. | Adolph TE, Meyer M, Jukic A, Tilg H. Heavy arch: from inflammatory bowel diseases to metabolic disorders. Gut. 2024;73:1376-1387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 16. | Zhu Z, Jia Y, Li FR, Li Y, Chen LH, Yang HH, Guo D, Sun L, Shi M, Wang T, Rohan TE, Qi Q, Qin LQ, Zhang Y, Chen GC. Inflammatory Bowel Disease and Risk of Global Cardiovascular Diseases and Type 2 Diabetes. Inflamm Bowel Dis. 2024;30:1130-1137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 17. | Wu H, Hu T, Hao H, Hill MA, Xu C, Liu Z. Inflammatory bowel disease and cardiovascular diseases: a concise review. Eur Heart J Open. 2022;2:oeab029. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 18. | Neurath MF, Travis SP. Mucosal healing in inflammatory bowel diseases: a systematic review. Gut. 2012;61:1619-1635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 676] [Cited by in RCA: 662] [Article Influence: 50.9] [Reference Citation Analysis (0)] |
| 19. | Ungaro RC, Yzet C, Bossuyt P, Baert FJ, Vanasek T, D'Haens GR, Joustra VW, Panaccione R, Novacek G, Reinisch W, Armuzzi A, Golovchenko O, Prymak O, Goldis A, Travis SP, Hébuterne X, Ferrante M, Rogler G, Fumery M, Danese S, Rydzewska G, Pariente B, Hertervig E, Stanciu C, Serrero M, Diculescu M, Peyrin-Biroulet L, Laharie D, Wright JP, Gomollón F, Gubonina I, Schreiber S, Motoya S, Hellström PM, Halfvarson J, Butler JW, Petersson J, Petralia F, Colombel JF. Deep Remission at 1 Year Prevents Progression of Early Crohn's Disease. Gastroenterology. 2020;159:139-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 169] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 20. | Turner D, Ricciuto A, Lewis A, D'Amico F, Dhaliwal J, Griffiths AM, Bettenworth D, Sandborn WJ, Sands BE, Reinisch W, Schölmerich J, Bemelman W, Danese S, Mary JY, Rubin D, Colombel JF, Peyrin-Biroulet L, Dotan I, Abreu MT, Dignass A; International Organization for the Study of IBD. STRIDE-II: An Update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): Determining Therapeutic Goals for Treat-to-Target strategies in IBD. Gastroenterology. 2021;160:1570-1583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 473] [Cited by in RCA: 1632] [Article Influence: 408.0] [Reference Citation Analysis (1)] |
| 21. | Srinivasan AR. Treat to target in Crohn's disease: A practical guide for clinicians. World J Gastroenterol. 2024;30:50-69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 14] [Article Influence: 14.0] [Reference Citation Analysis (8)] |
| 22. | Alberti KG, Zimmet P, Shaw J. Metabolic syndrome--a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med. 2006;23:469-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3852] [Cited by in RCA: 4244] [Article Influence: 223.4] [Reference Citation Analysis (0)] |
| 23. | Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC Jr; International Diabetes Federation Task Force on Epidemiology and Prevention; Hational Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; International Association for the Study of Obesity. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640-1645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8720] [Cited by in RCA: 10555] [Article Influence: 659.7] [Reference Citation Analysis (0)] |
| 24. | Tune JD, Goodwill AG, Sassoon DJ, Mather KJ. Cardiovascular consequences of metabolic syndrome. Transl Res. 2017;183:57-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 356] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 25. | Perrone-Filardi P, Paolillo S, Costanzo P, Savarese G, Trimarco B, Bonow RO. The role of metabolic syndrome in heart failure. Eur Heart J. 2015;36:2630-2634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 92] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 26. | Mottillo S, Filion KB, Genest J, Joseph L, Pilote L, Poirier P, Rinfret S, Schiffrin EL, Eisenberg MJ. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol. 2010;56:1113-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2169] [Cited by in RCA: 1962] [Article Influence: 130.8] [Reference Citation Analysis (0)] |
| 27. | Yki-Järvinen H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2014;2:901-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 764] [Cited by in RCA: 937] [Article Influence: 85.2] [Reference Citation Analysis (0)] |
| 28. | Godoy-Matos AF, Silva Júnior WS, Valerio CM. NAFLD as a continuum: from obesity to metabolic syndrome and diabetes. Diabetol Metab Syndr. 2020;12:60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 184] [Cited by in RCA: 398] [Article Influence: 79.6] [Reference Citation Analysis (0)] |
| 29. | Koren-Morag N, Goldbourt U, Tanne D. Relation between the metabolic syndrome and ischemic stroke or transient ischemic attack: a prospective cohort study in patients with atherosclerotic cardiovascular disease. Stroke. 2005;36:1366-1371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 116] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 30. | Pereira RM, de Carvalho JF, Bonfá E. Metabolic syndrome in rheumatological diseases. Autoimmun Rev. 2009;8:415-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 75] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 31. | Shen Z, Zhang M, Liu Y, Ge C, Lu Y, Shen H, Zhu L. Prevalence of metabolic syndrome in patients with inflammatory bowel disease: a systematic review and meta-analysis. BMJ Open. 2024;14:e074659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 32. | Nagahori M, Hyun SB, Totsuka T, Okamoto R, Kuwahara E, Takebayashi T, Naganuma M, Watanabe M. Prevalence of metabolic syndrome is comparable between inflammatory bowel disease patients and the general population. J Gastroenterol. 2010;45:1008-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 33. | Kang MK, Kim KO, Kim MC, Park JG, Jang BI. Sarcopenia Is a New Risk Factor of Nonalcoholic Fatty Liver Disease in Patients with Inflammatory Bowel Disease. Dig Dis. 2020;38:507-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 34. | Sourianarayanane A, Garg G, Smith TH, Butt MI, McCullough AJ, Shen B. Risk factors of non-alcoholic fatty liver disease in patients with inflammatory bowel disease. J Crohns Colitis. 2013;7:e279-e285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 101] [Article Influence: 8.4] [Reference Citation Analysis (2)] |
| 35. | Carr RM, Patel A, Bownik H, Oranu A, Kerner C, Praestgaard A, Forde KA, Reddy KR, Lichtenstein GR. Intestinal Inflammation Does Not Predict Nonalcoholic Fatty Liver Disease Severity in Inflammatory Bowel Disease Patients. Dig Dis Sci. 2017;62:1354-1361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 36. | Magrì S, Paduano D, Chicco F, Cingolani A, Farris C, Delogu G, Tumbarello F, Lai M, Melis A, Casula L, Fantini MC, Usai P. Nonalcoholic fatty liver disease in patients with inflammatory bowel disease: Beyond the natural history. World J Gastroenterol. 2019;25:5676-5686. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 36] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 37. | Dragasevic S, Stankovic B, Kotur N, Sokic-Milutinovic A, Milovanovic T, Lukic S, Milosavljevic T, Srzentic Drazilov S, Klaassen K, Pavlovic S, Popovic D. Metabolic Syndrome in Inflammatory Bowel Disease: Association with Genetic Markers of Obesity and Inflammation. Metab Syndr Relat Disord. 2020;18:31-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 38. | Yorulmaz E, Adali G, Yorulmaz H, Ulasoglu C, Tasan G, Tuncer I. Metabolic syndrome frequency in inflammatory bowel diseases. Saudi J Gastroenterol. 2011;17:376-382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (1)] |
| 39. | Hildrum B, Mykletun A, Hole T, Midthjell K, Dahl AA. Age-specific prevalence of the metabolic syndrome defined by the International Diabetes Federation and the National Cholesterol Education Program: the Norwegian HUNT 2 study. BMC Public Health. 2007;7:220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 213] [Cited by in RCA: 248] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 40. | Elangovan A, Shah R, Ali SMJ, Katz J, Cooper GS. High Burden of Obesity and Low Rates of Weight Loss Pharmacotherapy in Inflammatory Bowel Disease: 10-Year Trend. Crohns Colitis 360. 2023;5:otad007. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 41. | WHO. A healthy lifestyle - WHO recommendations. [cited May 20, 2025]. Available from: https://www.who.int/europe/news-room/fact-sheets/item/a-healthy-lifestyle---who-recommendations#:~:text=BMI%2C%20formerly%20called%20the%20Quetelet,have%20a%20BMI%20of%2022.9.,%202010. |
| 42. | Ross R, Neeland IJ, Yamashita S, Shai I, Seidell J, Magni P, Santos RD, Arsenault B, Cuevas A, Hu FB, Griffin BA, Zambon A, Barter P, Fruchart JC, Eckel RH, Matsuzawa Y, Després JP. Waist circumference as a vital sign in clinical practice: a Consensus Statement from the IAS and ICCR Working Group on Visceral Obesity. Nat Rev Endocrinol. 2020;16:177-189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 483] [Cited by in RCA: 1025] [Article Influence: 205.0] [Reference Citation Analysis (0)] |
| 43. | Després JP, Lemieux I, Bergeron J, Pibarot P, Mathieu P, Larose E, Rodés-Cabau J, Bertrand OF, Poirier P. Abdominal obesity and the metabolic syndrome: contribution to global cardiometabolic risk. Arterioscler Thromb Vasc Biol. 2008;28:1039-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 970] [Cited by in RCA: 1040] [Article Influence: 61.2] [Reference Citation Analysis (0)] |
| 44. | Long MD, Crandall WV, Leibowitz IH, Duffy L, del Rosario F, Kim SC, Integlia MJ, Berman J, Grunow J, Colletti RB, Schoen BT, Patel AS, Baron H, Israel E, Russell G, Ali S, Herfarth HH, Martin C, Kappelman MD; ImproveCareNow Collaborative for Pediatric IBD. Prevalence and epidemiology of overweight and obesity in children with inflammatory bowel disease. Inflamm Bowel Dis. 2011;17:2162-2168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 172] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 45. | Pringle PL, Stewart KO, Peloquin JM, Sturgeon HC, Nguyen D, Sauk J, Garber JJ, Yajnik V, Ananthakrishnan AN, Chan AT, Xavier RJ, Khalili H. Body Mass Index, Genetic Susceptibility, and Risk of Complications Among Individuals with Crohn's Disease. Inflamm Bowel Dis. 2015;21:2304-2310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 46. | Mendall MA, Gunasekera AV, John BJ, Kumar D. Is obesity a risk factor for Crohn's disease? Dig Dis Sci. 2011;56:837-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 47. | Seminerio JL, Koutroubakis IE, Ramos-Rivers C, Hashash JG, Dudekula A, Regueiro M, Baidoo L, Barrie A, Swoger J, Schwartz M, Weyant K, Dunn MA, Binion DG. Impact of Obesity on the Management and Clinical Course of Patients with Inflammatory Bowel Disease. Inflamm Bowel Dis. 2015;21:2857-2863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 134] [Article Influence: 13.4] [Reference Citation Analysis (1)] |
| 48. | Stabroth-Akil D, Leifeld L, Pfützer R, Morgenstern J, Kruis W. The effect of body weight on the severity and clinical course of ulcerative colitis. Int J Colorectal Dis. 2015;30:237-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 49. | Flores A, Burstein E, Cipher DJ, Feagins LA. Obesity in Inflammatory Bowel Disease: A Marker of Less Severe Disease. Dig Dis Sci. 2015;60:2436-2445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 132] [Article Influence: 13.2] [Reference Citation Analysis (4)] |
| 50. | Nic Suibhne T, Raftery TC, McMahon O, Walsh C, O'Morain C, O'Sullivan M. High prevalence of overweight and obesity in adults with Crohn's disease: associations with disease and lifestyle factors. J Crohns Colitis. 2013;7:e241-e248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 120] [Article Influence: 10.0] [Reference Citation Analysis (1)] |
| 51. | Chan SSM, Chen Y, Casey K, Olen O, Ludvigsson JF, Carbonnel F, Oldenburg B, Gunter MJ, Tjønneland A, Grip O; DEFINe-IBD Investigators, Lochhead P, Chan AT, Wolk A, Khalili H. Obesity is Associated With Increased Risk of Crohn's disease, but not Ulcerative Colitis: A Pooled Analysis of Five Prospective Cohort Studies. Clin Gastroenterol Hepatol. 2022;20:1048-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 52. | Rahmani J, Kord-Varkaneh H, Hekmatdoost A, Thompson J, Clark C, Salehisahlabadi A, Day AS, Jacobson K. Body mass index and risk of inflammatory bowel disease: A systematic review and dose-response meta-analysis of cohort studies of over a million participants. Obes Rev. 2019;20:1312-1320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 53. | Khalili H, Ananthakrishnan AN, Konijeti GG, Higuchi LM, Fuchs CS, Richter JM, Chan AT. Measures of obesity and risk of Crohn's disease and ulcerative colitis. Inflamm Bowel Dis. 2015;21:361-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 124] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 54. | He Z, Fu T, Lu S, Sun Y, Zhang Y, Shi W, Li Z, Deng M, Chen J, Bai Y. Adiposity as a risk factor for inflammatory bowel disease and the mediating effect of metabolic and inflammatory status: A population-based cohort study. United European Gastroenterol J. 2023;11:973-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 55. | Harpsøe MC, Basit S, Andersson M, Nielsen NM, Frisch M, Wohlfahrt J, Nohr EA, Linneberg A, Jess T. Body mass index and risk of autoimmune diseases: a study within the Danish National Birth Cohort. Int J Epidemiol. 2014;43:843-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 172] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 56. | Campo M, Hjelle H, Granlund AVB, Sandvik AK, Ness-Jensen E. The relationship between weight change and inflammatory bowel disease. A population-based cohort study, the HUNT study. Scand J Gastroenterol. 2024;59:830-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 57. | Tang W, Xie G, Wang D, Li T, Ren Y, Li J, Deng J, Li K. Imaging-based assessment of body composition in patients with Crohn's disease: a systematic review. Int J Colorectal Dis. 2023;38:126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 58. | Sehgal P, Su S, Zech J, Nobel Y, Luk L, Economou I, Shen B, Lewis JD, Freedberg DE. Visceral Adiposity Independently Predicts Time to Flare in Inflammatory Bowel Disease but Body Mass Index Does Not. Inflamm Bowel Dis. 2024;30:594-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 22] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 59. | Youn J, Hsia K, Khadilkar S, Zeina T, Rai P, Rastogi A, Hussani S, Spence S, Adavelly P, Yanes J, Kotlier J, Sweigart B, Levy AN, Friedman S. The Impact of Obesity on the Prevalence and Complications of Perianal Fistulas of Crohn's Disease. Dig Dis Sci. 2025;70:323-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 60. | Jiang K, Chen B, Lou D, Zhang M, Shi Y, Dai W, Shen J, Zhou B, Hu J. Systematic review and meta-analysis: association between obesity/overweight and surgical complications in IBD. Int J Colorectal Dis. 2022;37:1485-1496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 61. | Donnelly M, Driever D, Ryan ÉJ, Elliott JA, Finnegan J, McNamara D, Murphy I, Conlon KC, Neary PC, Kavanagh DO, O'Riordan JM. Obesity, Sarcopenia and Myosteatosis: Impact on Clinical Outcomes in the Operative Management of Crohn's Disease. Inflamm Bowel Dis. 2024;30:1517-1528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 62. | Park L, McKechnie T, Lee Y, Tessier L, Passos E, Doumouras A, Hong D, Eskicioglu C. Short-term postoperative outcomes for obese versus non-obese inflammatory bowel disease patients undergoing bowel resection: a propensity score matched analysis. Int J Colorectal Dis. 2024;39:17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 63. | Schineis CHW, Pozios I, Boubaris K, Weixler B, Kamphues C, Margonis GA, Kreis ME, Strobel RM, Beyer K, Seifarth C, Luitjens J, Kaufmann D, Lauscher JC. Role of visceral fat on postoperative complications and relapse in patients with Crohn's disease after ileocecal resection: Is it overrated? Int J Colorectal Dis. 2024;39:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 64. | Carrillo-Palau M, Hernández-Camba A, Hernández Alvarez-Buylla N, Ramos L, Alonso-Abreu I, Hernández-Pérez A, Vela M, Arranz L, Hernández-Guerra M, González-Gay MÁ, Ferraz-Amaro I. Insulin Resistance Is Not Increased in Inflammatory Bowel Disease Patients but Is Related to Non-Alcoholic Fatty Liver Disease. J Clin Med. 2021;10:3062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 65. | Sánchez-Pérez H, Tejera-Segura B, de Vera-González A, González-Delgado A, Olmos JM, Hernández JL, Corrales A, López-Mejías R, González-Gay MA, Ferraz-Amaro I. Insulin resistance in systemic lupus erythematosus patients: contributing factors and relationship with subclinical atherosclerosis. Clin Exp Rheumatol. 2017;35:885-892. [PubMed] |
| 66. | Ferraz-Amaro I, García-Dopico JA, Medina-Vega L, González-Gay MA, Díaz-González F. Impaired beta cell function is present in nondiabetic rheumatoid arthritis patients. Arthritis Res Ther. 2013;15:R17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 67. | Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793-1801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2726] [Cited by in RCA: 3083] [Article Influence: 162.3] [Reference Citation Analysis (0)] |
| 68. | Jess T, Jensen BW, Andersson M, Villumsen M, Allin KH. Inflammatory Bowel Diseases Increase Risk of Type 2 Diabetes in a Nationwide Cohort Study. Clin Gastroenterol Hepatol. 2020;18:881-888.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 76] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 69. | Lai SW, Kuo YH, Liao KF. Association Between Inflammatory Bowel Disease and Diabetes Mellitus. Clin Gastroenterol Hepatol. 2020;18:1002-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 70. | Fuschillo G, Celentano V, Rottoli M, Sciaudone G, Gravina AG, Pellegrino R, Marfella R, Romano M, Selvaggi F, Pellino G. Influence of diabetes mellitus on inflammatory bowel disease course and treatment outcomes. A systematic review with meta-analysis. Dig Liver Dis. 2023;55:580-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 11.5] [Reference Citation Analysis (1)] |
| 71. | Chen H, Li W, Hu J, Xu F, Lu Y, Zhu L, Shen H. Association of serum lipids with inflammatory bowel disease: a systematic review and meta-analysis. Front Med (Lausanne). 2023;10:1198988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 72. | Liu Z, Tang H, Liang H, Bai X, Zhang H, Yang H, Wang H, Wang L, Qian J. Dyslipidaemia Is Associated with Severe Disease Activity and Poor Prognosis in Ulcerative Colitis: A Retrospective Cohort Study in China. Nutrients. 2022;14:3040. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 73. | Singh S, Kullo IJ, Pardi DS, Loftus EV Jr. Epidemiology, risk factors and management of cardiovascular diseases in IBD. Nat Rev Gastroenterol Hepatol. 2015;12:26-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 89] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 74. | Tews HC, Schmelter F, Kandulski A, Büchler C, Schmid S, Schlosser S, Elger T, Loibl J, Sommersberger S, Fererberger T, Gunawan S, Kunst C, Gülow K, Bettenworth D, Föh B, Maaß C, Solbach P, Günther UL, Derer S, Marquardt JU, Sina C, Müller M. Unique Metabolomic and Lipidomic Profile in Serum From Patients With Crohn's Disease and Ulcerative Colitis Compared With Healthy Control Individuals. Inflamm Bowel Dis. 2024;30:2405-2417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 75. | Pan Z, Guo H, Wang Q, Tian S, Zhang X, Li C, Ma Z. Relationship between subclasses low-density lipoprotein and carotid plaque. Transl Neurosci. 2022;13:30-37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 76. | Conrad N, Verbeke G, Molenberghs G, Goetschalckx L, Callender T, Cambridge G, Mason JC, Rahimi K, McMurray JJV, Verbakel JY. Autoimmune diseases and cardiovascular risk: a population-based study on 19 autoimmune diseases and 12 cardiovascular diseases in 22 million individuals in the UK. Lancet. 2022;400:733-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 224] [Article Influence: 74.7] [Reference Citation Analysis (0)] |
| 77. | Sun HH, Tian F. Inflammatory bowel disease and cardiovascular disease incidence and mortality: A meta-analysis. Eur J Prev Cardiol. 2018;25:1623-1631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 79] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 78. | Faye AS, Axelrad JE, Sun J, Halfvarson J, Söderling J, Olén O, Ludvigsson JF. Atherosclerosis as a Risk Factor of Inflammatory Bowel Disease: A Population-Based Case-Control Study. Am J Gastroenterol. 2024;119:313-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 79. | Roberts WC. Atherosclerotic risk factors — are there ten, or is there only one? Atherosclerosis. 1992;97:S5-S9. [RCA] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 80. | Srikanthan K, Feyh A, Visweshwar H, Shapiro JI, Sodhi K. Systematic Review of Metabolic Syndrome Biomarkers: A Panel for Early Detection, Management, and Risk Stratification in the West Virginian Population. Int J Med Sci. 2016;13:25-38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 324] [Cited by in RCA: 292] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 81. | Roifman I, Sun YC, Fedwick JP, Panaccione R, Buret AG, Liu H, Rostom A, Anderson TJ, Beck PL. Evidence of endothelial dysfunction in patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2009;7:175-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 99] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 82. | Xiao Y, Powell DW, Liu X, Li Q. Cardiovascular manifestations of inflammatory bowel diseases and the underlying pathogenic mechanisms. Am J Physiol Regul Integr Comp Physiol. 2023;325:R193-R211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 83. | Maziere C, Conte MA, Dantin F, Maziere JC. Lipopolysaccharide enhances oxidative modification of low density lipoprotein by copper ions, endothelial and smooth muscle cells. Atherosclerosis. 1999;143:75-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 84. | Shamseya AM, Hussein WM, Elnely DA, Adel F, Header DA. Serum matrix metalloproteinase-9 concentration as a marker of disease activity in patients with inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2021;33:e803-e809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 85. | Zaka A, Mridha N, Subhaharan D, Jones M, Niranjan S, Mohsen W, Ramaswamy PK. Inflammatory bowel disease patients have an increased risk of acute coronary syndrome: a systematic review and meta-analysis. Open Heart. 2023;10:e002483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 86. | D'Ascenzo F, Bruno F, Iannaccone M, Testa G, De Filippo O, Giannino G, Caviglia GP, Bernstein CN, De Ferrari GM, Bugianesi E, Armandi A, Ribaldone DG. Patients with inflammatory bowel disease are at increased risk of atherothrombotic disease: A systematic review with meta-analysis. Int J Cardiol. 2023;378:96-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 87. | Takagi T, Xu L, Hoshi M, Arai S. Identifying Risk Factors of Major Adverse Cardiac Events in Patients With Ulcerative Colitis: A Retrospective Japanese Claims Data Analysis. J Gastroenterol Hepatol. 2025;40:421-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 88. | Fang L, Gao H, Gao X, Wu W, Miao Y, Zhang H, Guleng B, Zhang H, Wang Y, Li M, Yang H, Gao X, Liang J, Cao Q, Shen J, Ran Z, Wu K, Qian J, Chen M, Liu Z. Risks of Cardiovascular Events in Patients With Inflammatory Bowel Disease in China: A Retrospective Multicenter Cohort Study. Inflamm Bowel Dis. 2022;28:S52-S58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 89. | Lee MT, Mahtta D, Chen L, Hussain A, Al Rifai M, Sinh P, Khalid U, Nasir K, Ballantyne CM, Petersen LA, Virani SS. Premature Atherosclerotic Cardiovascular Disease Risk Among Patients with Inflammatory Bowel Disease. Am J Med. 2021;134:1047-1051.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 90. | Kristensen SL, Ahlehoff O, Lindhardsen J, Erichsen R, Jensen GV, Torp-Pedersen C, Nielsen OH, Gislason GH, Hansen PR. Disease activity in inflammatory bowel disease is associated with increased risk of myocardial infarction, stroke and cardiovascular death--a Danish nationwide cohort study. PLoS One. 2013;8:e56944. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 178] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 91. | Le Gall G, Kirchgesner J, Bejaoui M, Landman C, Nion-Larmurier I, Bourrier A, Sokol H, Seksik P, Beaugerie L. Clinical activity is an independent risk factor of ischemic heart and cerebrovascular arterial disease in patients with inflammatory bowel disease. PLoS One. 2018;13:e0201991. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 92. | Popovic B, Varlot J, Hennequin J, Metzdorf PA, Jay N, Camenzind E, Bannay A. Outcomes after acute coronary syndrome in patients with inflammatory bowel disease. Heart Vessels. 2022;37:1604-1610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 93. | Marcusohn E, Zukermann R, Kerner A, Roguin A, Kobo O. Long-term outcomes of patients with chronic inflammatory diseases after percutaneous coronary intervention. Catheter Cardiovasc Interv. 2021;98:E655-E660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 94. | Henein MY, Vancheri S, Longo G, Vancheri F. The Role of Inflammation in Cardiovascular Disease. Int J Mol Sci. 2022;23:12906. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 310] [Article Influence: 103.3] [Reference Citation Analysis (0)] |
| 95. | Prati F, Mastroianni F, Paoletti G, Marco V, Biccirè FG, Gatto L. Seeking and treating inflammation in ischaemic heart disease: are we ready? Eur Heart J Suppl. 2025;27:iii122-iii125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 96. | Yokoyama T, Vaca L, Rossen RD, Durante W, Hazarika P, Mann DL. Cellular basis for the negative inotropic effects of tumor necrosis factor-alpha in the adult mammalian heart. J Clin Invest. 1993;92:2303-2312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 513] [Cited by in RCA: 522] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 97. | Al-Roub A, Akhter N, Al-Rashed F, Wilson A, Alzaid F, Al-Mulla F, Sindhu S, Ahmad R. TNFα induces matrix metalloproteinase-9 expression in monocytic cells through ACSL1/JNK/ERK/NF-kB signaling pathways. Sci Rep. 2023;13:14351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |
| 98. | Sun J, Yao J, Olén O, Halfvarson J, Bergman D, Ebrahimi F, Rosengren A, Sundström J, Ludvigsson JF. Risk of heart failure in inflammatory bowel disease: a Swedish population-based study. Eur Heart J. 2024;45:2493-2504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 99. | Aniwan S, Pardi DS, Tremaine WJ, Loftus EV Jr. Increased Risk of Acute Myocardial Infarction and Heart Failure in Patients With Inflammatory Bowel Diseases. Clin Gastroenterol Hepatol. 2018;16:1607-1615.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 101] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 100. | Kumar A, Lukin DJ. Incident heart failure is a predictor of adverse outcomes in inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2020;32:205-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 101. | Luo C, Liu L, Zhu D, Ge Z, Chen Y, Chen F. Risk of stroke in patients with inflammatory bowel disease: a systematic review and meta-analysis. BMC Gastroenterol. 2025;25:114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 102. | Sun J, Halfvarson J, Appelros P, Bergman D, Ebrahimi F, Roelstraete B, Olén O, Ludvigsson JF. Long-term Risk of Stroke in Patients With Inflammatory Bowel Disease: A Population-Based, Sibling-Controlled Cohort Study, 1969-2019. Neurology. 2023;101:e653-e664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 103. | Wan J, Wang X, Zhang YJ, Yin Y, Wang Z, Che X, Chen M, Liang J, Wu KC. Incidence and disease-related risk factors for cerebrovascular accidents in patients with inflammatory bowel diseases: A systematic review and meta-analysis. J Dig Dis. 2023;24:504-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 104. | Kirchgesner J, Nyboe Andersen N, Carrat F, Jess T, Beaugerie L; BERENICE study group. Risk of acute arterial events associated with treatment of inflammatory bowel diseases: nationwide French cohort study. Gut. 2020;69:852-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 105. | Ha C, Magowan S, Accortt NA, Chen J, Stone CD. Risk of arterial thrombotic events in inflammatory bowel disease. Am J Gastroenterol. 2009;104:1445-1451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 173] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 106. | Alayo QA, Loftus EV Jr, Yarur A, Alvarado D, Ciorba MA, de las Fuentes L, Deepak P. Inflammatory Bowel Disease Is Associated With an Increased Risk of Incident Acute Arterial Events: Analysis of the United Kingdom Biobank. Clin Gastroenterol Hepatol. 2023;21:761-770.e13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 26] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 107. | Song R, Li Z, Zhang Y, Tan J, Chen Z. Comparison of NAFLD, MAFLD and MASLD characteristics and mortality outcomes in United States adults. Liver Int. 2024;44:1051-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 55] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 108. | Younossi ZM, Golabi P, Paik JM, Henry A, Van Dongen C, Henry L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): a systematic review. Hepatology. 2023;77:1335-1347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 270] [Cited by in RCA: 1425] [Article Influence: 712.5] [Reference Citation Analysis (2)] |
| 109. | Amini-Salehi E, Letafatkar N, Norouzi N, Joukar F, Habibi A, Javid M, Sattari N, Khorasani M, Farahmand A, Tavakoli S, Masoumzadeh B, Abbaspour E, Karimzad S, Ghadiri A, Maddineni G, Khosousi MJ, Faraji N, Keivanlou MH, Mahapatro A, Gaskarei MAK, Okhovat P, Bahrampourian A, Aleali MS, Mirdamadi A, Eslami N, Javid M, Javaheri N, Pra SV, Bakhsi A, Shafipour M, Vakilpour A, Ansar MM, Kanagala SG, Hashemi M, Ghazalgoo A, Kheirandish M, Porteghali P, Heidarzad F, Zeinali T, Ghanaei FM, Hassanipour S, Ulrich MT, Melson JE, Patel D, Nayak SS. Global Prevalence of Nonalcoholic Fatty Liver Disease: An Updated Review Meta-Analysis comprising a Population of 78 million from 38 Countries. Arch Med Res. 2024;55:103043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 110. | Allen AM, Therneau TM, Ahmed OT, Gidener T, Mara KC, Larson JJ, Canning RE, Benson JT, Kamath PS. Clinical course of non-alcoholic fatty liver disease and the implications for clinical trial design. J Hepatol. 2022;77:1237-1245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 81] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 111. | Huang DQ, El-Serag HB, Loomba R. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2021;18:223-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 840] [Cited by in RCA: 1206] [Article Influence: 301.5] [Reference Citation Analysis (0)] |
| 112. | Navarro P, Gutiérrez-Ramírez L, Tejera-Muñoz A, Arias Á, Lucendo AJ. Systematic Review and Meta-Analysis: Prevalence of Non-Alcoholic Fatty Liver Disease and Liver Fibrosis in Patients with Inflammatory Bowel Disease. Nutrients. 2023;15:4507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 113. | Cao YT, Xiang LL, Qi F, Zhang YJ, Chen Y, Zhou XQ. Accuracy of controlled attenuation parameter (CAP) and liver stiffness measurement (LSM) for assessing steatosis and fibrosis in non-alcoholic fatty liver disease: A systematic review and meta-analysis. EClinicalMedicine. 2022;51:101547. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 73] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 114. | Rodriguez-Duque JC, Calleja JL, Iruzubieta P, Hernández-Conde M, Rivas-Rivas C, Vera MI, Garcia MJ, Pascual M, Castro B, García-Blanco A, García-Nieto E, Olmo SC, Cagigal ML, Lopez-Montejo L, Fernández-Lamas T, Rasines L, Fortea JI, Vaque JP, Frias Y, Rivero M, Arias-Loste MT, Crespo J. Increased risk of MAFLD and Liver Fibrosis in Inflammatory Bowel Disease Independent of Classic Metabolic Risk Factors. Clin Gastroenterol Hepatol. 2023;21:406-414.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 40] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 115. | Ding Z, Wei Y, Peng J, Wang S, Chen G, Sun J. The Potential Role of C-Reactive Protein in Metabolic-Dysfunction-Associated Fatty Liver Disease and Aging. Biomedicines. 2023;11:2711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 116. | Bian F, Yang XY, Xu G, Zheng T, Jin S. CRP-Induced NLRP3 Inflammasome Activation Increases LDL Transcytosis Across Endothelial Cells. Front Pharmacol. 2019;10:40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 117. | Martínez-Domínguez SJ, García-Mateo S, Gargallo-Puyuelo CJ, Gallego-Llera B, Callau P, Mendi C, Arroyo-Villarino MT, Simón-Marco MÁ, Ampuero J, Gomollón F. Inflammatory Bowel Disease Is an Independent Risk Factor for Metabolic Dysfunction-Associated Steatotic Liver Disease in Lean Individuals. Inflamm Bowel Dis. 2024;30:1274-1283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 118. | McHenry S, Glover M, Ahmed A, Alayo Q, Zulfiqar M, Ludwig DR, Ciorba MA, Davidson NO, Deepak P. NAFLD Is Associated With Quiescent Rather Than Active Crohn's Disease. Inflamm Bowel Dis. 2024;30:757-767. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 119. | Martínez-Domínguez SJ, García-Mateo S, Gargallo-Puyuelo CJ, Gallego Llera B, Refaie E, Callau P, Mendi C, Baptista PM, Hernández Ainsa M, Arroyo-Villarino MT, López de la Cruz J, Martínez-García J, Alfambra E, Simón Marco MÁ, Ampuero J, Gomollón F. Crohn´s disease is an independent risk factor for liver fibrosis in patients with inflammatory bowel disease and non-alcoholic fatty liver disease. Eur J Intern Med. 2024;120:99-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 120. | Capela TL, Silva VM, Freitas M, Arieira C, Gonçalves TC, de Castro FD, Magalhães J, Cotter J. Identifying inflammatory bowel disease patients at risk of metabolic dysfunction-associated fatty liver disease: usefulness of non-invasive steatosis predictive scores. BMC Gastroenterol. 2023;23:437. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 121. | Zhang Q, Liu S, Wu J, Zhu S, Wu Y, Wu S, Zhang S. Non-alcoholic fatty liver degree and long-term risk of incident inflammatory bowel disease: A large-scale prospective cohort study. Chin Med J (Engl). 2024;137:1705-1714. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 122. | Zhang Q, Xu F, Liu S, Zhu S, Zhang S, Wu J, Wu S. Long-term risk of cardiovascular disease associated with MASLD and different cardiometabolic risk factors in IBD patients: A prospective cohort study. Liver Int. 2024;44:2315-2328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 123. | Zhang Q, Wang Y, Liu S, Zhu S, Li P, Wu S. Mortality risk associated with MASLD, MASLD type and different cardiometabolic risk factors in IBD patients: A long-term prospective cohort study. Dig Liver Dis. 2025;57:744-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 124. | Dorrington AM, Selinger CP, Parkes GC, Smith M, Pollok RC, Raine T. The Historical Role and Contemporary Use of Corticosteroids in Inflammatory Bowel Disease. J Crohns Colitis. 2020;14:1316-1329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 125. | Huscher D, Thiele K, Gromnica-Ihle E, Hein G, Demary W, Dreher R, Zink A, Buttgereit F. Dose-related patterns of glucocorticoid-induced side effects. Ann Rheum Dis. 2009;68:1119-1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 382] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 126. | Yasir M, Goyal A, Sonthalia S. Corticosteroid Adverse Effects. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing, 2025. [PubMed] |
| 127. | Vegiopoulos A, Herzig S. Glucocorticoids, metabolism and metabolic diseases. Mol Cell Endocrinol. 2007;275:43-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 352] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 128. | Qi D, Rodrigues B. Glucocorticoids produce whole body insulin resistance with changes in cardiac metabolism. Am J Physiol Endocrinol Metab. 2007;292:E654-E667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 110] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 129. | Bonaventura A, Montecucco F. Steroid-induced hyperglycemia: An underdiagnosed problem or clinical inertia? A narrative review. Diabetes Res Clin Pract. 2018;139:203-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 130. | Wu J, Mackie SL, Pujades-Rodriguez M. Glucocorticoid dose-dependent risk of type 2 diabetes in six immune-mediated inflammatory diseases: a population-based cohort analysis. BMJ Open Diabetes Res Care. 2020;8:e001220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 131. | Ettinger WH, Klinefelter HF, Kwiterovitch PO. Effect of short-term, low-dose corticosteroids on plasma lipoprotein lipids. Atherosclerosis. 1987;63:167-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 78] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 132. | Chung CP, Giles JT, Petri M, Szklo M, Post W, Blumenthal RS, Gelber AC, Ouyang P, Jenny NS, Bathon JM. Prevalence of traditional modifiable cardiovascular risk factors in patients with rheumatoid arthritis: comparison with control subjects from the multi-ethnic study of atherosclerosis. Semin Arthritis Rheum. 2012;41:535-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 98] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 133. | Hafström I, Rohani M, Deneberg S, Wörnert M, Jogestrand T, Frostegård J. Effects of low-dose prednisolone on endothelial function, atherosclerosis, and traditional risk factors for atherosclerosis in patients with rheumatoid arthritis--a randomized study. J Rheumatol. 2007;34:1810-1816. [PubMed] |
| 134. | Sleutjes JAM, Roeters van Lennep JE, Boersma E, Menchen LA, Laudes M, Farkas K, Molnár T, Kennedy NA, Pierik MJ, van der Woude CJ, de Vries AC. Systematic review with meta-analysis: effect of inflammatory bowel disease therapy on lipid levels. Aliment Pharmacol Ther. 2021;54:999-1012. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 135. | Pujades-Rodriguez M, Morgan AW, Cubbon RM, Wu J. Dose-dependent oral glucocorticoid cardiovascular risks in people with immune-mediated inflammatory diseases: A population-based cohort study. PLoS Med. 2020;17:e1003432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 161] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 136. | Rungoe C, Basit S, Ranthe MF, Wohlfahrt J, Langholz E, Jess T. Risk of ischaemic heart disease in patients with inflammatory bowel disease: a nationwide Danish cohort study. Gut. 2013;62:689-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 169] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 137. | Yarur AJ, Deshpande AR, Pechman DM, Tamariz L, Abreu MT, Sussman DA. Inflammatory bowel disease is associated with an increased incidence of cardiovascular events. Am J Gastroenterol. 2011;106:741-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 206] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 138. | Morton AC, Rothman AM, Greenwood JP, Gunn J, Chase A, Clarke B, Hall AS, Fox K, Foley C, Banya W, Wang D, Flather MD, Crossman DC. The effect of interleukin-1 receptor antagonist therapy on markers of inflammation in non-ST elevation acute coronary syndromes: the MRC-ILA Heart Study. Eur Heart J. 2015;36:377-384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 257] [Cited by in RCA: 271] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 139. | Levine B, Kalman J, Mayer L, Fillit HM, Packer M. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N Engl J Med. 1990;323:236-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1845] [Cited by in RCA: 1816] [Article Influence: 51.9] [Reference Citation Analysis (0)] |
| 140. | Kadokami T, Frye C, Lemster B, Wagner CL, Feldman AM, McTiernan CF. Anti-tumor necrosis factor-alpha antibody limits heart failure in a transgenic model. Circulation. 2001;104:1094-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 68] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 141. | Chung ES, Packer M, Lo KH, Fasanmade AA, Willerson JT; Anti-TNF Therapy Against Congestive Heart Failure Investigators. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: results of the anti-TNF Therapy Against Congestive Heart Failure (ATTACH) trial. Circulation. 2003;107:3133-3140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1110] [Cited by in RCA: 1175] [Article Influence: 53.4] [Reference Citation Analysis (0)] |
| 142. | Singh S, Fumery M, Singh AG, Singh N, Prokop LJ, Dulai PS, Sandborn WJ, Curtis JR. Comparative Risk of Cardiovascular Events With Biologic and Synthetic Disease-Modifying Antirheumatic Drugs in Patients With Rheumatoid Arthritis: A Systematic Review and Meta-Analysis. Arthritis Care Res (Hoboken). 2020;72:561-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 110] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 143. | Solomon DH, Rassen JA, Kuriya B, Chen L, Harrold LR, Graham DJ, Lewis JD, Lii J, Liu L, Griffin MR, Curtis JR. Heart failure risk among patients with rheumatoid arthritis starting a TNF antagonist. Ann Rheum Dis. 2013;72:1813-1818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 144. | Ljung L, Rantapää-Dahlqvist S, Jacobsson LT, Askling J. Response to biological treatment and subsequent risk of coronary events in rheumatoid arthritis. Ann Rheum Dis. 2016;75:2087-2094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 145. | Roubille C, Richer V, Starnino T, McCourt C, McFarlane A, Fleming P, Siu S, Kraft J, Lynde C, Pope J, Gulliver W, Keeling S, Dutz J, Bessette L, Bissonnette R, Haraoui B. The effects of tumour necrosis factor inhibitors, methotrexate, non-steroidal anti-inflammatory drugs and corticosteroids on cardiovascular events in rheumatoid arthritis, psoriasis and psoriatic arthritis: a systematic review and meta-analysis. Ann Rheum Dis. 2015;74:480-489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 692] [Cited by in RCA: 655] [Article Influence: 65.5] [Reference Citation Analysis (0)] |
| 146. | Lewis JD, Scott FI, Brensinger CM, Roy JA, Osterman MT, Mamtani R, Bewtra M, Chen L, Yun H, Xie F, Curtis JR. Increased Mortality Rates With Prolonged Corticosteroid Therapy When Compared With Antitumor Necrosis Factor-α-Directed Therapy for Inflammatory Bowel Disease. Am J Gastroenterol. 2018;113:405-417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 118] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 147. | Zanoli L, Rastelli S, Inserra G, Castellino P. Arterial structure and function in inflammatory bowel disease. World J Gastroenterol. 2015;21:11304-11311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 41] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 148. | Shehab M, Alrashed F, Alkazemi A, Lakatos PL, Bessissow T. Impact of biologic therapies and small molecules on the risk of major adverse cardiovascular events in patients with inflammatory bowel diseases: systematic review and meta-analysis of randomized controlled trials. Expert Rev Gastroenterol Hepatol. 2023;17:469-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 149. | Mattay SS, Zamani M, Saturno D, Loftus EV Jr, Ciorba MA, Yarur A, Singh S, Deepak P. Risk of Major Adverse Cardiovascular Events in Immune-Mediated Inflammatory Disorders on Biologics and Small Molecules: Network Meta-Analysis. Clin Gastroenterol Hepatol. 2024;22:961-970.e12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 24] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 150. | Gabay C, McInnes IB, Kavanaugh A, Tuckwell K, Klearman M, Pulley J, Sattar N. Comparison of lipid and lipid-associated cardiovascular risk marker changes after treatment with tocilizumab or adalimumab in patients with rheumatoid arthritis. Ann Rheum Dis. 2016;75:1806-1812. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 114] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 151. | Ruparelia N, Chai JT, Fisher EA, Choudhury RP. Inflammatory processes in cardiovascular disease: a route to targeted therapies. Nat Rev Cardiol. 2017;14:133-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 355] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 152. | Hauer AD, Uyttenhove C, de Vos P, Stroobant V, Renauld JC, van Berkel TJ, van Snick J, Kuiper J. Blockade of interleukin-12 function by protein vaccination attenuates atherosclerosis. Circulation. 2005;112:1054-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 136] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 153. | Hugh J, Van Voorhees AS, Nijhawan RI, Bagel J, Lebwohl M, Blauvelt A, Hsu S, Weinberg JM. From the Medical Board of the National Psoriasis Foundation: The risk of cardiovascular disease in individuals with psoriasis and the potential impact of current therapies. J Am Acad Dermatol. 2014;70:168-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 112] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 154. | Poizeau F, Nowak E, Kerbrat S, Le Nautout B, Droitcourt C, Drici MD, Sbidian E, Guillot B, Bachelez H, Ait-Oufella H, Happe A, Oger E, Dupuy A. Association Between Early Severe Cardiovascular Events and the Initiation of Treatment With the Anti-Interleukin 12/23p40 Antibody Ustekinumab. JAMA Dermatol. 2020;156:1208-1215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 72] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 155. | Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol. 2011;12:204-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1445] [Cited by in RCA: 1680] [Article Influence: 120.0] [Reference Citation Analysis (0)] |
| 156. | Taleb S, Tedgui A, Mallat Z. IL-17 and Th17 cells in atherosclerosis: subtle and contextual roles. Arterioscler Thromb Vasc Biol. 2015;35:258-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 203] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 157. | Caiazzo G, Fabbrocini G, Di Caprio R, Raimondo A, Scala E, Balato N, Balato A. Psoriasis, Cardiovascular Events, and Biologics: Lights and Shadows. Front Immunol. 2018;9:1668. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 81] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 158. | Agca R, Heslinga SC, Rollefstad S, Heslinga M, McInnes IB, Peters MJ, Kvien TK, Dougados M, Radner H, Atzeni F, Primdahl J, Södergren A, Wallberg Jonsson S, van Rompay J, Zabalan C, Pedersen TR, Jacobsson L, de Vlam K, Gonzalez-Gay MA, Semb AG, Kitas GD, Smulders YM, Szekanecz Z, Sattar N, Symmons DP, Nurmohamed MT. EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann Rheum Dis. 2017;76:17-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 957] [Cited by in RCA: 877] [Article Influence: 109.6] [Reference Citation Analysis (0)] |
| 159. | Papp KA, Griffiths CE, Gordon K, Lebwohl M, Szapary PO, Wasfi Y, Chan D, Hsu MC, Ho V, Ghislain PD, Strober B, Reich K; PHOENIX 1 Investigators; PHOENIX 2 Investigators; ACCEPT Investigators. Long-term safety of ustekinumab in patients with moderate-to-severe psoriasis: final results from 5 years of follow-up. Br J Dermatol. 2013;168:844-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 306] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 160. | Adedokun OJ, Xu Z, Marano C, O'Brien C, Szapary P, Zhang H, Johanns J, Leong RW, Hisamatsu T, Van Assche G, Danese S, Abreu MT, Sands BE, Sandborn WJ. Ustekinumab Pharmacokinetics and Exposure Response in a Phase 3 Randomized Trial of Patients With Ulcerative Colitis. Clin Gastroenterol Hepatol. 2020;18:2244-2255.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 79] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 161. | Sandborn WJ, Rutgeerts P, Gasink C, Jacobstein D, Zou B, Johanns J, Sands BE, Hanauer SB, Targan S, Ghosh S, de Villiers WJS, Colombel JF, Feagan BG. Long-term efficacy and safety of ustekinumab for Crohn's disease through the second year of therapy. Aliment Pharmacol Ther. 2018;48:65-77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 133] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 162. | Ferrante M, Panaccione R, Baert F, Bossuyt P, Colombel JF, Danese S, Dubinsky M, Feagan BG, Hisamatsu T, Lim A, Lindsay JO, Loftus EV Jr, Panés J, Peyrin-Biroulet L, Ran Z, Rubin DT, Sandborn WJ, Schreiber S, Neimark E, Song A, Kligys K, Pang Y, Pivorunas V, Berg S, Duan WR, Huang B, Kalabic J, Liao X, Robinson A, Wallace K, D'Haens G. Risankizumab as maintenance therapy for moderately to severely active Crohn's disease: results from the multicentre, randomised, double-blind, placebo-controlled, withdrawal phase 3 FORTIFY maintenance trial. Lancet. 2022;399:2031-2046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 189] [Article Influence: 63.0] [Reference Citation Analysis (0)] |
| 163. | Sands BE, D'Haens G, Clemow DB, Irving PM, Johns JT, Hunter Gibble T, Abreu MT, Lee S, Hisamatsu T, Kobayashi T, Dubinsky MC, Vermeire S, Siegel CA, Peyrin-Biroulet L, Moses RE, Milata J, Arora V, Panaccione R, Dignass A. Two-Year Efficacy and Safety of Mirikizumab Following 104 Weeks of Continuous Treatment for Ulcerative Colitis: Results From the LUCENT-3 Open-Label Extension Study. Inflamm Bowel Dis. 2024;30:2245-2258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 20] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 164. | Abdelnabi M, ElNawaa S, Benjanuwattra J, Elmassry M, Nair N. Ustekinumab-Induced Fatal Acute Heart Failure in a Young Female: A Case Report. Methodist Debakey Cardiovasc J. 2022;18:54-58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 165. | Beroukhim K, Danesh M, Nguyen C, Koo J, Leon A. A case report of heart failure after therapy with ustekinumab. J Dermatol Dermatol Surg. 2015;19:117-119. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 166. | Morgenweck E, Park B, Bower R. Heart failure associated with ustekinumab therapy for treatment of Crohn's Disease. BMJ Case Rep. 2022;15:e250376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 167. | Chaparro M, Garre A, Ricart E, Iborra M, Mesonero F, Vera I, Riestra S, García-Sánchez V, Luisa De Castro M, Martin-Cardona A, Aldeguer X, Mínguez M, de-Acosta MB, Rivero M, Muñoz F, Andreu M, Bargalló A, González-Muñoza C, Pérez Calle JL, García-Sepulcre MF, Bermejo F, Huguet JM, Cabriada JL, Gutiérrez A, Mañosa M, Villoria A, Carbajo AY, Lorente R, García-López S, Piqueras M, Hinojosa E, Arajol C, Sicilia B, Conesa AM, Sainz E, Almela P, Llaó J, Roncero O, Camo P, Taxonera C, Domselaar MV, Pajares R, Legido J, Madrigal R, Lucendo AJ, Alcaín G, Doménech E, Gisbert JP; GETECCU study group. Short and long-term effectiveness and safety of vedolizumab in inflammatory bowel disease: results from the ENEIDA registry. Aliment Pharmacol Ther. 2018;48:839-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 80] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 168. | Colombel JF, Sands BE, Rutgeerts P, Sandborn W, Danese S, D'Haens G, Panaccione R, Loftus EV Jr, Sankoh S, Fox I, Parikh A, Milch C, Abhyankar B, Feagan BG. The safety of vedolizumab for ulcerative colitis and Crohn's disease. Gut. 2017;66:839-851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 509] [Cited by in RCA: 610] [Article Influence: 76.3] [Reference Citation Analysis (0)] |
| 169. | Loftus EV Jr, Colombel JF, Feagan BG, Vermeire S, Sandborn WJ, Sands BE, Danese S, D'Haens GR, Kaser A, Panaccione R, Rubin DT, Shafran I, McAuliffe M, Kaviya A, Sankoh S, Mody R, Abhyankar B, Smyth M. Long-term Efficacy of Vedolizumab for Ulcerative Colitis. J Crohns Colitis. 2017;11:400-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 170. | Pugliese D, Privitera G, Crispino F, Mezzina N, Castiglione F, Fiorino G, Laterza L, Viola A, Bertani L, Caprioli F, Cappello M, Barberio B, Ricci C, Balestrieri P, Daperno M, Pluchino D, Rizzello F, Scribano ML, Sablich R, Pastorelli L, Manguso F, Variola A, Di Sario A, Grossi L, Armuzzi A; IG‐IBD LIVE Study Group. Effectiveness and safety of vedolizumab in a matched cohort of elderly and nonelderly patients with inflammatory bowel disease: the IG-IBD LIVE study. Aliment Pharmacol Ther. 2022;56:95-109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 171. | Close H, Mason JM, Wilson DW, Hungin AP, Jones R, Rubin G. Risk of Ischaemic Heart Disease in Patients with Inflammatory Bowel Disease: Cohort Study Using the General Practice Research Database. PLoS One. 2015;10:e0139745. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 172. | Brown G. 5-Aminosalicylic Acid-Associated Myocarditis and Pericarditis: A Narrative Review. Can J Hosp Pharm. 2016;69:466-472. [PubMed] |
| 173. | Dheyriat L, Ward D, Beaugerie L, Jess T, Kirchgesner J. Risk of Recurrent Acute Arterial Events Associated With Thiopurines and Anti-Tumor Necrosis Factor in Inflammatory Bowel Diseases. Clin Gastroenterol Hepatol. 2023;21:164-172.e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 174. | Thapa SD, Hadid H, Schairer J, Imam W, Jafri SM. Effect of Inflammatory Bowel Disease-Related Characteristics and Treatment Interventions on Cardiovascular Disease Incidence. Am J Med Sci. 2015;350:175-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 175. | Micha R, Imamura F, Wyler von Ballmoos M, Solomon DH, Hernán MA, Ridker PM, Mozaffarian D. Systematic review and meta-analysis of methotrexate use and risk of cardiovascular disease. Am J Cardiol. 2011;108:1362-1370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 433] [Cited by in RCA: 395] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 176. | Mangoni AA, Sotgia S, Zinellu A, Carru C, Pintus G, Damiani G, Erre GL, Tommasi S. Methotrexate and cardiovascular prevention: an appraisal of the current evidence. Ther Adv Cardiovasc Dis. 2023;17:17539447231215213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 177. | Sands BE, Taub PR, Armuzzi A, Friedman GS, Moscariello M, Lawendy N, Pedersen RD, Chan G, Nduaka CI, Quirk D, Salese L, Su C, Feagan BG. Tofacitinib Treatment Is Associated With Modest and Reversible Increases in Serum Lipids in Patients With Ulcerative Colitis. Clin Gastroenterol Hepatol. 2020;18:123-132.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 78] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 178. | Li N, Gou ZP, Du SQ, Zhu XH, Lin H, Liang XF, Wang YS, Feng P. Effect of JAK inhibitors on high- and low-density lipoprotein in patients with rheumatoid arthritis: a systematic review and network meta-analysis. Clin Rheumatol. 2022;41:677-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 179. | Paolino G, Valenti M, Carugno A, Bianco M, Didona D, Di Nicola MR, Acutis PL, Cantisani C, Bianchi VG, Zerbinati N, Narcisi A, Costanzo A, Mercuri SR. Serum Lipids Alterations in Patients Under Systemic JAK Inhibitors Treatments in Dermatology: Clinical Aspects and Management. Medicina (Kaunas). 2025;61:54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 180. | Xie W, Huang Y, Xiao S, Sun X, Fan Y, Zhang Z. Impact of Janus kinase inhibitors on risk of cardiovascular events in patients with rheumatoid arthritis: systematic review and meta-analysis of randomised controlled trials. Ann Rheum Dis. 2019;78:1048-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 130] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 181. | Ytterberg SR, Bhatt DL, Mikuls TR, Koch GG, Fleischmann R, Rivas JL, Germino R, Menon S, Sun Y, Wang C, Shapiro AB, Kanik KS, Connell CA; ORAL Surveillance Investigators. Cardiovascular and Cancer Risk with Tofacitinib in Rheumatoid Arthritis. N Engl J Med. 2022;386:316-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 970] [Article Influence: 323.3] [Reference Citation Analysis (0)] |
| 182. | Ingrassia JP, Maqsood MH, Gelfand JM, Weber BN, Bangalore S, Lo Sicco KI, Garshick MS. Cardiovascular and Venous Thromboembolic Risk With JAK Inhibitors in Immune-Mediated Inflammatory Skin Diseases: A Systematic Review and Meta-Analysis. JAMA Dermatol. 2024;160:28-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 30] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 183. | Meissner Y, Schäfer M, Albrecht K, Kekow J, Zinke S, Tony HP, Strangfeld A. Risk of major adverse cardiovascular events in patients with rheumatoid arthritis treated with conventional synthetic, biologic and targeted synthetic disease-modifying antirheumatic drugs: observational data from the German RABBIT register. RMD Open. 2023;9:e003489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 184. | Olivera PA, Lasa JS, Peretto G, Zuily S, Danese S, Peyrin-Biroulet L. Review article: Risk of cardiovascular events in patients with inflammatory bowel disease receiving small molecule drugs. Aliment Pharmacol Ther. 2023;57:1231-1248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 185. | Vermeire S, Rubin DT, Peyrin-Biroulet L, Dubinsky MC, Regueiro M, Irving PM, Goetsch M, Lazin K, Gu G, Wu J, Modesto I, McDonnell A, Guo X, Green J, Dalam AB, Yarur AJ. Cardiovascular events observed among patients in the etrasimod clinical programme: an integrated safety analysis of patients with moderately to severely active ulcerative colitis. BMJ Open Gastroenterol. 2025;12:e001516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 186. | Sands BE, Schreiber S, Blumenstein I, Chiorean MV, Ungaro RC, Rubin DT. Clinician's Guide to Using Ozanimod for the Treatment of Ulcerative Colitis. J Crohns Colitis. 2023;17:2012-2025. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 21] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 187. | Long-Term Cardiac Safety of Ozanimod in a Phase 3 Clinical Program of Ulcerative Colitis and Relapsing Multiple Sclerosis. Gastroenterol Hepatol (N Y). 2022;18:8-9. [PubMed] |
| 188. | Dai ZH, Xu XT, Ran ZH. Associations Between Obesity and the Effectiveness of Anti-Tumor Necrosis Factor-α Agents in Inflammatory Bowel Disease Patients: A Literature Review and Meta-analysis. Ann Pharmacother. 2020;54:729-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 189. | Chanchlani N, Lin S, Bewshea C, Hamilton B, Thomas A, Smith R, Roberts C, Bishara M, Nice R, Lees CW, Sebastian S, Irving PM, Russell RK, McDonald TJ, Goodhand JR, Ahmad T, Kennedy NA; PANTS Consortium. Mechanisms and management of loss of response to anti-TNF therapy for patients with Crohn's disease: 3-year data from the prospective, multicentre PANTS cohort study. Lancet Gastroenterol Hepatol. 2024;9:521-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 42] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 190. | Sila S, Aloi M, Cucinotta U, Gianolio L, Granot M, Hradsky O, Hussey S, Kang B, Karoliny A, Kolho KL, de Laffolie J, Lega S, Matar M, Norsa L, Omiwole S, Orlanski-Meyer E, Palomino L, Rohani P, Scarallo L, Sladek M, Sohouli MH, Urlep D, Yerushalmy-Feler A, Zifman E, Hojsak I. Effect of Overweight and Obesity on the Response to Anti-TNF Therapy and Disease Course in Children With IBD. Inflamm Bowel Dis. 2025;31:1263-1271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 191. | Levine LJ, Gaidos JKJ, Proctor DD, Viana AV, Al-Bawardy B. Effect of obesity on vedolizumab response in inflammatory bowel disease. Ann Gastroenterol. 2022;35:275-280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 192. | Wong ECL, Marshall JK, Reinisch W, Narula N. Body Mass Index Does Not Impact Clinical Efficacy of Ustekinumab in Crohn's Disease: A Post Hoc Analysis of the IM-UNITI Trial. Inflamm Bowel Dis. 2021;27:848-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 193. | Rompoti N, Koumprentziotis IA, Stefanaki I, Vavouli C, Papoutsaki M, Politou M, Panagakis P, Befon A, Kousta F, Lazou E, Chasapi V, Stratigos A, Nicolaidou E. Risankizumab in obese patients with moderate-to-severe plaque psoriasis: a 104-week, real-world, retrospective study. Arch Dermatol Res. 2024;316:667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 194. | Ricceri F, Chiricozzi A, Peris K, Prignano F. Successful use of anti-IL-23 molecules in overweight-to-obese psoriatic patients: A multicentric retrospective study. Dermatol Ther. 2022;35:e15793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 195. | Bischoff SC, Ockenga J, Eshraghian A, Barazzoni R, Busetto L, Campmans-Kuijpers M, Cardinale V, Chermesh I, Kani HT, Khannoussi W, Lacaze L, Léon-Sanz M, Mendive JM, Müller MW, Tacke F, Thorell A, Vranesic Bender D, Weimann A, Cuerda C. Practical guideline on obesity care in patients with gastrointestinal and liver diseases - Joint ESPEN/UEG guideline. Clin Nutr. 2023;42:987-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 196. | Bunu DM, Timofte CE, Ciocoiu M, Floria M, Tarniceriu CC, Barboi OB, Tanase DM. Cardiovascular Manifestations of Inflammatory Bowel Disease: Pathogenesis, Diagnosis, and Preventive Strategies. Gastroenterol Res Pract. 2019;2019:3012509. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 197. | Chen B, Collen LV, Mowat C, Isaacs KL, Singh S, Kane SV, Farraye FA, Snapper S, Jneid H, Lavie CJ, Krittanawong C. Inflammatory Bowel Disease and Cardiovascular Diseases. Am J Med. 2022;135:1453-1460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 48] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 198. | Michalak A, Mosińska P, Fichna J. Common links between metabolic syndrome and inflammatory bowel disease: Current overview and future perspectives. Pharmacol Rep. 2016;68:837-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 199. | Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC Jr, Spertus JA, Costa F; American Heart Association; National Heart, Lung, and Blood Institute. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735-2752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7515] [Cited by in RCA: 8293] [Article Influence: 414.7] [Reference Citation Analysis (0)] |
| 200. | Fitzmorris PS, Colantonio LD, Torrazza Perez E, Smith I, Kakati DD, Malik TA. Impact of metabolic syndrome on the hospitalization rate of Crohn's disease patients seen at a tertiary care center: a retrospective cohort study. Digestion. 2015;91:257-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 201. | Sartini A, Gitto S, Bianchini M, Verga MC, Di Girolamo M, Bertani A, Del Buono M, Schepis F, Lei B, De Maria N, Villa E. Non-alcoholic fatty liver disease phenotypes in patients with inflammatory bowel disease. Cell Death Dis. 2018;9:87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 70] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 202. | Jovanovic M, Simovic Markovic B, Gajovic N, Jurisevic M, Djukic A, Jovanovic I, Arsenijevic N, Lukic A, Zdravkovic N. Metabolic syndrome attenuates ulcerative colitis: Correlation with interleukin-10 and galectin-3 expression. World J Gastroenterol. 2019;25:6465-6482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 203. | Oliveira LDRP, Ribeiro TCDR, Mourao Junior CA, Barra MAL, Silva MHG, Shafee LP, Zacarias SM, Campos LDC, Valério HMG, Chebli JMF. Metabolic dysfunction-associated steatotic liver disease prevalence and risk factors in inflammatory bowel disease in tertiary center. Rev Assoc Med Bras (1992). 2024;70:e20231321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 204. | Hyun HK, Lee HW, Park J, Park SJ, Park JJ, Kim TI, Lee JS, Kim BK, Park JY, Kim DY, Ahn SH, Kim SU, Cheon JH. Hepatic Steatosis but Not Fibrosis Is Independently Associated with Poor Outcomes in Patients with Inflammatory Bowel Disease. Gut Liver. 2024;18:294-304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 205. | Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894: i-xii, 1. [PubMed] |
| 206. | Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults--The Evidence Report. National Institutes of Health. Obes Res. 1998;6 Suppl 2:51S-209S. [PubMed] |
| 207. | Khan NA, McAlister FA, Rabkin SW, Padwal R, Feldman RD, Campbell NR, Leiter LA, Lewanczuk RZ, Schiffrin EL, Hill MD, Arnold M, Moe G, Campbell TS, Herbert C, Milot A, Stone JA, Burgess E, Hemmelgarn B, Jones C, Larochelle P, Ogilvie RI, Houlden R, Herman RJ, Hamet P, Fodor G, Carruthers G, Culleton B, Dechamplain J, Pylypchuk G, Logan AG, Gledhill N, Petrella R, Tobe S, Touyz RM; Canadian Hypertension Education Program. The 2006 Canadian Hypertension Education Program recommendations for the management of hypertension: Part II - Therapy. Can J Cardiol. 2006;22:583-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 84] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 208. | Health Canada. Canadian Guidelines for Body Weight Classification in Adults. [cited May 20, 2025]. Available from: https://publications.gc.ca/collections/Collection/H49-179-2003E.pdf. |
| 209. | Graham I, Atar D, Borch-Johnsen K, Boysen G, Burell G, Cifkova R, Dallongeville J, De Backer G, Ebrahim S, Gjelsvik B, Herrmann-Lingen C, Hoes A, Humphries S, Knapton M, Perk J, Priori SG, Pyorala K, Reiner Z, Ruilope L, Sans-Menendez S, Op Reimer WS, Weissberg P, Wood D, Yarnell J, Zamorano JL; ESC Committee for Practice Guidelines. European guidelines on cardiovascular disease prevention in clinical practice: executive summary. Atherosclerosis. 2007;194:1-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 156] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 210. | Oka R, Kobayashi J, Yagi K, Tanii H, Miyamoto S, Asano A, Hagishita T, Mori M, Moriuchi T, Kobayashi M, Katsuda S, Kawashiri MA, Nohara A, Takeda Y, Mabuchi H, Yamagishi M. Reassessment of the cutoff values of waist circumference and visceral fat area for identifying Japanese subjects at risk for the metabolic syndrome. Diabetes Res Clin Pract. 2008;79:474-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 119] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 211. | Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55 2005-2023 [PMID:22488764 DOI: 10.1002/hep.25762. |
| 212. | Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, Zelber-Sagi S, Wai-Sun Wong V, Dufour JF, Schattenberg JM, Kawaguchi T, Arrese M, Valenti L, Shiha G, Tiribelli C, Yki-Järvinen H, Fan JG, Grønbæk H, Yilmaz Y, Cortez-Pinto H, Oliveira CP, Bedossa P, Adams LA, Zheng MH, Fouad Y, Chan WK, Mendez-Sanchez N, Ahn SH, Castera L, Bugianesi E, Ratziu V, George J. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J Hepatol. 2020;73:202-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2883] [Cited by in RCA: 2827] [Article Influence: 565.4] [Reference Citation Analysis (1)] |
| 213. | Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal AJ, Kanwal F, Romero D, Abdelmalek MF, Anstee QM, Arab JP, Arrese M, Bataller R, Beuers U, Boursier J, Bugianesi E, Byrne CD, Castro Narro GE, Chowdhury A, Cortez-Pinto H, Cryer DR, Cusi K, El-Kassas M, Klein S, Eskridge W, Fan J, Gawrieh S, Guy CD, Harrison SA, Kim SU, Koot BG, Korenjak M, Kowdley KV, Lacaille F, Loomba R, Mitchell-Thain R, Morgan TR, Powell EE, Roden M, Romero-Gómez M, Silva M, Singh SP, Sookoian SC, Spearman CW, Tiniakos D, Valenti L, Vos MB, Wong VW, Xanthakos S, Yilmaz Y, Younossi Z, Hobbs A, Villota-Rivas M, Newsome PN; NAFLD Nomenclature consensus group. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology. 2023;78:1966-1986. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1212] [Cited by in RCA: 1312] [Article Influence: 656.0] [Reference Citation Analysis (0)] |