Published online Oct 28, 2021. doi: 10.3748/wjg.v27.i40.6888
Peer-review started: April 17, 2021
First decision: June 23, 2021
Revised: June 28, 2021
Accepted: August 25, 2021
Article in press: August 25, 2021
Published online: October 28, 2021
Processing time: 193 Days and 4.2 Hours
Fuzi (Radix aconiti lateralis)-Gancao (Radix glycyrrhizae) is one of the most classical drug pairs of traditional Chinese medicine. In clinical practice, decoctions containing Fuzi-Gancao (F-G) are often used in the treatment of liver diseases such as hepatitis and liver failure.
To investigate the metabolomics of F-G in CCl4 induced acute liver injury in rats and its regulatory effect on the bile acid profile.
The pharmacodynamic effect of F-G on CCl4 induced acute liver injury in rats was evaluated, and an ultra performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) method for the simultaneous determination of 92 metabolites from multiple pathways was established to explore the protective metabolic mechanism of F-G in serum on the liver.
Twenty-four differential metabolites were identified in serum samples. The primary bile acid biosynthetic metabolic pathway was the major common pathway in the model group and F-G group. Subsequently, a UPLC-MS/MS method for simultaneous determination of 11 bile acids, including cholic acid, ursodeoxycholic acid, glycochenodeoxycholic acid, glycochenodeoxycholic acid, taurocholic acid, glycocholic acid, chenodeoxycholic acid, deoxycholic acid, taurochenodeoxycholic acid, taurocholic acid, and glycinic acid, was established to analyze the regulatory mechanism of F-G in serum. F-G decreased the contents of these 11 bile acids in serum in a dose-dependent manner compared with those in the model control group.
F-G could protect hepatocytes by promoting the binding of free bile acids to glycine and taurine, and reducing the accumulation of free bile acids in the liver. F-G could also regulate the compensatory degree of taurine, decreasing the content of taurine-conjugated bile acids to protect hepatocytes.
Core Tip: Fuzi-Gancao (F-G) could protect hepatocytes by promoting the binding of free bile acids to glycine and taurine, and reducing the accumulation of free bile acids in the liver. F-G could also regulate the compensatory degree of taurine, decreasing the content of taurine-conjugated bile acids to protect hepatocytes.
- Citation: Wang MF, Zhao SS, Thapa DM, Song YL, Xiang Z. Metabolomics of Fuzi-Gancao in CCl4 induced acute liver injury and its regulatory effect on bile acid profile in rats. World J Gastroenterol 2021; 27(40): 6888-6907
- URL: https://www.wjgnet.com/1007-9327/full/v27/i40/6888.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i40.6888
The liver is an important metabolic organ of the human body that has the physiological functions of detoxification, phagocytosis, and defense[1]. With the development of modern industry, liver injury caused by environmental pollution is gradually increasing. Liver injury is an important pathophysiological process in the development of hepatitis, liver fibrosis, liver cirrhosis, and liver cancer. Therefore, the treatment of liver injury is important for the prevention, treatment, and recovery of a variety of liver diseases[2,3].
Bile acids are one of the most sensitive indicators for the clinical diagnosis of liver diseases[4,5]. When the liver system is diseased, it can cause a disturbance in the metabolism of bile acids in the body, thus causing a significant change in the amount of bile acids in the blood. Therefore, biomarkers of the bile acid metabolism pathway are very important diagnostic and therapeutic indicators for liver disease[4-6]. Liver diseases such as liver injury, hepatitis, cirrhosis, and hepatocellular carcinoma have significantly increased serum total bile acid levels, and changes in total bile acid levels are usually analyzed in clinical tests but are not highly specific[6,7]. Therefore, the changes in the bile acid profile based on the metabolic pathway of bile acids have important significance for the prevention and treatment of liver diseases. Bile acids can be divided into primary and secondary bile acids[8,9]. Primary bile acids are bile acids synthesized directly from cholesterol by hepatocytes and mainly include cholic acid (CA) and chenodeoxycholic acid (CDCA). Secondary bile acids are free bile acids produced by the primary bile acids deoxycholic acid (DCA) and lithocholic acid (LCA) after 7α-hydroxy deoxygenation of the intestinal flora. These free bile acids bind with glycine and taurine to form conjugated secondary bile acids such as glycocholic acid (GCA), glycochenodeoxycholic acid (GCDCA), glycochenodeoxycholic acid (GDCA), taurocholic acid (TCA), taurochenodeoxycholic acid (TCDCA), ursodeoxycholic acid (UDCA), glycinic acid (GLCA), and taurocholic acid (TUDCA).
Chinese herbal compounds have been used clinically for thousands of years in the treatment of liver system diseases, among which Fuzi (Radix aconiti Lateralis)-Gancao (Radix glycyrrhizae) is one of the most common drug pairs in Chinese herbal compounds, and Chinese herbal compounds containing Fuzi-Gancao (F-G), such as Yinchen Sifu decoction, Sini decoction, and Fuzi Lizhong decoction, are often used in the treatment of liver diseases such as hepatitis and liver failure[10-13]. Modern pharmacological studies have shown that herbal compounds containing Fuzi and Gancao can reduce the biochemical indexes of liver injury model animals by inhibiting inflammatory stress, lipid peroxidation, and apoptosis and have significant pharmacological activity to prevent liver injury[14-16].
Fuzi and Gancao are important medicinal materials in classic prescriptions, such as Sini decoction and Fuzi Lizhong decoction. Aconite alkaloids in Fuzi and flavonoids in Gancao are the main active ingredients in F-G drug pairs. Alkaloids are the main active ingredients in aconite, and thus far, nearly 200 alkaloids have been isolated and identified. These alkaloids are mainly classified into nonester alkaloids, monoester diterpene alkaloids (MDAs), diester diterpene alkaloids (DDAs), and lipoalkaloids according to their structural properties[17]. MDAs and DDAs have very well-defined pharmacological activities, such as cardiotonic[18-20], anti-inflammatory and analgesic[21], antitumor[22], antioxidation[23], and hepatoprotective[24,25] activities. Flavonoids are the main active ingredients of Gancao and have well-defined pharmacological anti-inflammatory[26-28], antibacterial[29], antioxidant[30], hepatoprotective, and antitumor[31] activities. The Chinese Pharmacopoeia (2015 edition) prescribes the compatibility ratio of Fuzi and Gancao as 1:1, which could decrease the toxins caused by aconite alkaloids and enhance the treatment effect compared with other ratios[32-34].
In a previous study, a rapid, convenient, and stable method for the simultaneous quantitative determination of six alkaloids and three flavonoids in Radix aconiti lateralis and Radix glycyrrhizae by ultra performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) was established[17]. In this study, the effects of the F-G water extract on the prevention of liver injury induced by tetrachloride (CCl4) were evaluated. Then, the metabolic mechanism was studied by UPLC-MS/MS. Finally, a method of simultaneous quantitative detection of 11 bile acids was established, and the mechanism of F-G in the regulation of the serum bile acid profile was analyzed by UPLC-MS/MS.
Radix aconiti lateralis (Fuzi) pieces were purchased from Huang Gang Jingui Traditional Chinese Medicine Industry Development Co., Ltd. (Sichuan, China), and Radix glycyrrhizae (Gancao) was purchased from Bozhou Jinshaotang Herbal Decoction Co., Ltd. (Anhui, China). The medicinal materials were identified by Professor Fu Xiao at the First Affiliated Hospital of Jinzhou Medical University.
The aspartate aminotransferase (AST) and alanine aminotransferase (ALT) assay kits were purchased from Nanjing Jiancheng Bioengineering Co., Ltd.; silybin was purchased from Solarbio Biotech Co., Ltd.; L-leucine, L-tryptophan, L-kynurenine, 5-Hydroxytryptamine (5-HT), 5-hydroxytryptophan (5-HTP), cholic acid, N-pheny
Male Sprague-Dawley rats (200 g ± 20 g) were purchased from the Laboratory Animal Center of Chinese Medical University (animal license No.: SCXK (Liao) 2008-0005). The rats were housed under SPF laboratory conditions at a temperature of 25 ± 2 °C, a relative humidity of 40 ± 5%, and a 12-h circadian cycle for 1 wk. All procedures were performed according to the National Institute of Health guidelines regarding the principles of animal care.
Fuzi (1.3 kg) and Gancao (1.3 kg) were mixed and soaked in water (26 L) for 0.5 h, and then the mixture was refluxed for 2 h. The supernatant was then concentrated under vacuum to obtain the F-G extract. The extract was diluted with water to a final concentration of 1.5 g/mL (crude drug), and all the extracts were stored at 4 °C before use.
Forty male rats were randomly divided into five groups (n = 8): Normal control group, model control group, positive control group, F-G group with 15 g/kg crude drug, and F-G group with 30 g/kg crude drug. The positive control group was orally administered with silybin at 50 mg/kg daily, and the normal control group and model control group were given the same volume of normal saline daily by gavage once a day for 8 d.
On the 7th day, 40% CCl4 (v/v, olive oil) was intraperitoneally injected in the positive control group, model control group, and F-G groups at a dose of 2 mL/kg. The rats were anesthetized using sodium pentobarbital 2 h after administration on the 8th day. Blood samples were collected from the abdominal aorta and centrifuged at 3500 rpm for 10 min (4 °C) to obtain serum samples for transaminase (ALT and AST) and metabolic analysis. Liver tissue samples were taken for pathological analysis.
To elucidate the mechanism of F-G in the treatment of liver injury, three groups of serum samples, including that from the normal control group, model control group, and F-G group (30 g/kg), were used for metabolic analysis by UPLC-MS according to a method published by the authors with minor modifications[35].
Preparation of “stripped” serum: One hundred milliliters of rat serum including activated charcoal powder (6 g) was incubated at room temperature for 2 h. After centrifugation at 13000 rpm for 20 min (4 °C), the supernatant was filtered successively through microporous membranes with pore sizes of 5 μm, 1.2 μm, and 0.45 μm to obtain “stripped” serum.
Samples for metabolic analysis: Two hundred microliters of the serum sample was pipetted into a 1.5 mL centrifuge tube, followed by adding 200 μL of PBS solution containing NEM (10 mmol/L) and 1000 μL of methanol solution with L-phenylalanine (Ring-D5; 10 ng/mL, internal standard) in sequence, vortexing for 30 s, and incubating the sample at -20 °C for 15 min after mixing. After incubation, 1000 μL of supernatant was transferred into a 2 mL centrifuge tube by centrifugation at 12000 rpm for 10 min at 4 °C, and the samples were dried with nitrogen and stored at -80 °C. The dried residue was reconstituted with 100 μL of 5% ACN-H2O solution prior to analysis, vortexed for 1 min, and centrifuged at 12000 rpm for 10 min at 4 °C. Sixty microliters of the supernatant was aspirated accurately into an injection vial, and 10 μL of each sample was injected into UPLC-MS/MS for the analysis of metabolic biomarkers.
Preparation of standard solution: L-Leucine, 5-HT, N-phenylacetylglycine, L-tryptophan, L-kynurenine, 5-HTP, cholic acid, and GSSG were accurately weighed and dissolved in methanol to make stock solutions at a concentration of 1 mg/mL. GSH was accurately weighed and dissolved in methanol containing NEM (10 mmol/L) to make a solution at a concentration of 0.5 mg/mL. Each standard solution was mixed proportionally and serially diluted with 50% methanol-water to obtain a series of mixed standard solutions.
Method validation: Calibrators of the nine metabolites (L-Leu, 5-HT, N-Phe, L-Try, L-Kyn, 5-HTP, CA, GSH, and GSSG) were generated from the mixed standard working solutions in “stripped” serum. The linear regression standard curve was calculated with 1/x weighting and plotted with the concentrations and the peak area ratio of each analyte to the internal standard.
Pooled standard solutions were added to “stripped” serum to prepare QC samples of three different concentrations, which were then processed as described in “Samples for metabolic analysis”. Intraday precision was calculated according to the content of nine metabolites from the QC sample in six consecutive analyses. The interday precision was obtained after continuous analysis of the QC sample for 3 d. Precision is expressed as the relative standard deviation (RSD, %).
Three concentrations of the mixed standard solutions were added to the samples of a definite content and processed for analysis six times to obtain recovery. Recovery was calculated according to the formula:
Recovery (%) = (C-A)/B × 100% (A is the measured content of the test sample, B is the amount of standard content, and C is the measured content).
UPLC-MS/MS analysis: Samples were separated using a Waters ACQUITY ultra-performance liquid chromatographer with a Waters BEH column (1.7 μm, 2.1 × 50 mm, C-18) and a mobile phase consisting of 0.1% formic acid in water (A) and acetonitrile (B) using a gradient system with the following elution procedures: 0-2.0 min, 5% B; 2.0-5.0 min, 5-50% B; 5.0-6.0 min, 50% B; 6.0-17.0 min, 50-95% B; 18.0-22.0 min, 95-5% B; and 22.0-25.0 min, 5% B. The flow rate was 0.3 mL/min, and the column temperature was set at 25 °C. Mass spectral analysis was achieved using an AB 4000 Q-TRAP system with ESI in positive and negative ionization modes and multiple reaction monitoring (MRM) mode. The MS parameters were as follows: Ion spray voltage, ± 4500 V; curtain gas, 20 psi; temperature, 450 °C; and ion source gas, 40 psi. All the metabolites were analyzed using precursor ions (Q1), product ions (Q3), declustering energy, entrance pressure, collision energy, and collision cell exit potential as detection parameters.
Thirty-six male SD rats were randomly divided into six groups (n = 6): Normal control group, model control group, positive control group, and F-G groups including FGL (10 g/kg), FGM (20 g/kg), and FGH (30 g/kg). The positive control group was given 50 mg/kg silybin, the normal group and model group were given the same volume of normal saline, and all the groups were administered for 8 d.
On the 7th day, 40% CCl4 (v/v, olive oil) solution was injected intraperitoneally (2 mL/kg), and the same dose of olive oil was injected intraperitoneally in the control group. The rats were anesthetized 2 h after administration on the 8th day. Blood samples were collected from the abdominal aorta and centrifuged at 4000 rpm for 10 min (4 °C) to obtain serum samples for the analysis of bile acids.
Preparation of standard solutions: CDCA, DCA, UDCA, GLCA, GCDCA, GDCA, GCA, TCDCA, TCDCA, TUDCA, and TCA were accurately weighed and dissolved in methanol to prepare stock solutions at a concentration of 1 mg/mL and stored at -80 °C. Solutions were serially diluted with 50% methanol-water prior to testing. d4-GCDCA was accurately weighed and dissolved in methanol to make an internal standard solution. All the solutions were stored at -80 °C prior to use.
Sample preparation: Two hundred microliters of serum was added to 1000 μL of methanol containing internal standard (d4-GCDCA, 20 ng/mL). The samples were centrifuged at 12000 rpm for 15 min (4 °C) after mixing, and then 1000 μL of the supernatant was dried with nitrogen and stored at -80 °C. The dried residue was reconstituted with 50 μL of 80% methanol-water solution prior to analysis, vortexed for 1 min, and centrifuged at 12000 rpm for 15 min (4 °C). Then, 30 μL of supernatant was aspirated accurately into an injection vial, and 10 μL of each sample was injected into the UPLC-MS/MS system for bile acid quantification.
UPLC-MS analysis: Samples were separated using a Waters Acquity Uplc instrument and an Agilent ZORBAX SB-C18 column (3.5 μm, 2.1 mm × 100 mm) and a mobile phase consisting of 0.1% formic acid in water (A) and methanol (B) using a gradient system with the following elution procedure: 0-2.0 min, 10% B; 2.0-9.0 min, 10%-80% B; 9.0-11.0 min, 80% B; 11.0-20.0 min, 80%-90% B; and 20.0-25.0 min, 90%-10% B. Ten microliters of the sample was injected at a flow rate of 0.4 mL/min and column temperature of 25 °C. Mass spectral analysis was achieved using a Thermo Scientific TSQ Quantum with ESI in negative ionization mode and MRM mode. The ion spray voltage was -3200 v and the temperature was 380 °C.
Method validation: Eleven bile acids (CDCA, DCA, UDCA, CA, GLCA, GCDCA, GDCA, GCA, TCDCA, TCDCA, TUDCA, and TCA) were diluted with 50% methanol solution to prepare a series of solutions and mixed with “stripped” serum to generate calibrators for validation of the UPLC-MS/MS method. The linear regression standard curve was calculated with 1/x weighting and plotted with the concentrations and the peak area ratio of each analyte to the internal standard.
Pooled standard solutions were added to “stripped” serum to prepare QC samples of three different concentrations for analysis. Intraday precision was calculated according to the contents of 11 bile acids from QC samples for six consecutive analyses. The interday precision was obtained after continuous analysis of QC samples for 3 d. Interday precision and intraday precision are expressed as the relative standard deviation (RSD, %). Three concentrations of mixed standard solutions were added to the samples of a definite content and processed as described above for bile acid analysis six times to obtain recovery.
Three concentrations of 11 mixed bile acid standard solutions were used to investigate the stability of QC samples under different storage conditions and processing procedures. Short-term stability was assessed by analyzing the QC samples that were kept at room temperature for 12 h. Long-term stability was assessed by storing the QC samples at -20 °C for 20 d. Freeze-thaw stability was assessed after the QC samples were frozen at -80 °C and thawed at 4 °C in 1 d for 3 consecutive days. Postpreparation stability was assessed by analyzing the QC samples that were kept at 4 °C for 12 h.
Metabolic analysis was calibrated and integrated using AB Analyst (version 1.6.2, AB Applied Biosystems). Quantitative analyses of bile acids were performed using Thermo Scientific TSQ Quantum Workstation analysis software. Pathway analyses were achieved using SIMCA-P, Metaboanalyst, and KEGG software. The experimental data are expressed as the mean ± SD. The data were analyzed with SPSS 19.0 statistical software (P < 0.05 and P < 0.01).
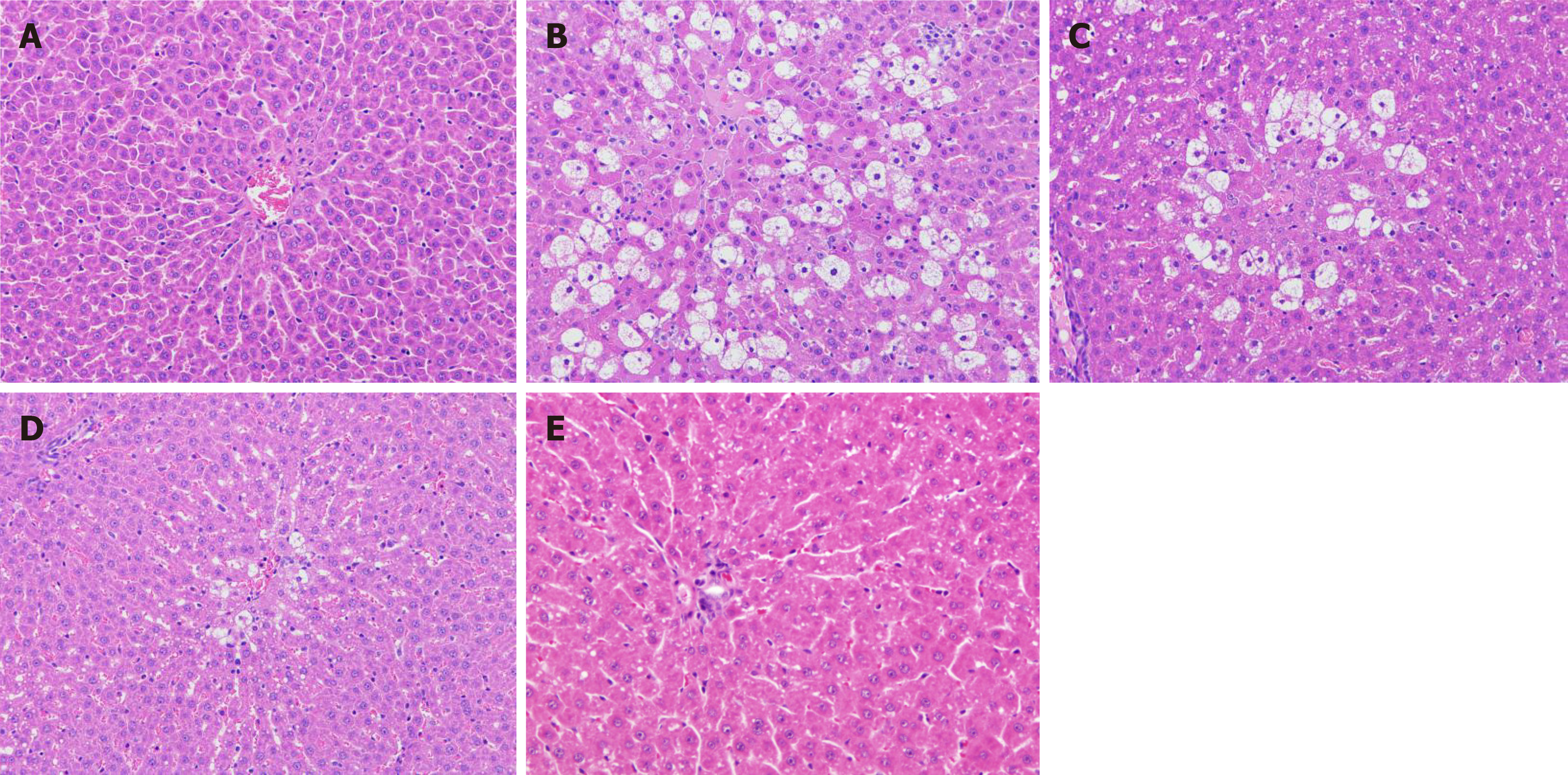
Histological analysis: The liver tissue of rats in each group was macroscopically observed. The livers of the normal control group rats had a normal morphology, a red color, no adhesion between the lobules, a smooth surface, and no bleeding spots. In the model group, the surface of the liver was milky white, and there was obvious adhesion between the lobules of the liver and the mucous membranes. In the F-G group, the adhesion degree between the lobules and mucous membrane was significantly decreased, and the color was rosier than that of the model rats. The morphological results showed that F-G extract could significantly relieve liver injury induced by CCl4 (Figure 1).
Histological analysis indicated that the structure of liver tissue in the normal control group was normal, the liver cells were neatly arranged, and there were no pathological changes, such as hepatocyte swelling, inflammatory cell infiltration, blood stasis, or steatosis of hepatocytes. Compared with the normal control group, the model control group showed a significant increase in the number of hepatocytes with extensive hydrodegeneration, steatosis, hepatocyte necrosis, and inflammatory cell infiltration. Compared with the model control group, F-G groups (15 g/kg and 30 g/kg) indicated a decrease in the amount of water-like degeneration in the liver, steatotic cells, and swelling hepatocytes with a significant dose-effect relationship.
Assay of ALT and AST: ALT and AST in the serum of rats were detected by ELISA. The results indicated that the contents of ALT and AST in the model control group were significantly higher than those in the normal control group (P < 0.01). Both transaminase levels were significantly reduced in the F-G groups compared to those in the model control group, but the magnitude of the reduction was still different from that of the positive control group (Table 1).
Linearity: The calibration curve of each standard was plotted with the concentrations of nine biomarkers and the ratio of the peak area of each component to that of the internal standard with weighted (1/x) least-square linear regression. The calibration curves of the nine analytes exhibited a good linearity with coefficient of correlation (
| Compound | Standard curve | R2 | Range (ng/mL) |
| L-Leu | Y = 0.0627X - 4.9797 | 0.9952 | 100-10000 |
| 5-HT | Y = 0.0385X - 0.0269 | 0.9939 | 0.8-80 |
| N-phe | Y = 0.0229X - 4.9120 | 0.9958 | 250-25000 |
| L-Try | Y = 0.0048X - 2.4673 | 0.9926 | 600-60000 |
| L-Kyn | Y = 0.0331X + 0.0123 | 0.9960 | 5-500 |
| 5-HTP | Y = 0.0492X - 0.0001 | 0.9983 | 0.2-20 |
| CA | Y = 0.00004X - 0.0010 | 0.9959 | 40-4000 |
| GSH | Y = 0.0032X - 0.2362 | 0.9975 | 150-20000 |
| GSSG | Y = 0.0001X + 0.0010 | 0.9949 | 50-10000 |
Accuracy, precision, and recovery: The accuracy and precision of analytes in the QC samples were less than 9.24% and 8.10%, respectively (Table 3), and the recovery of analytes is shown in Table 4. All the analytical values of the nine analytes had satisfactory results within the acceptable criteria according to the bioanalytical method validation guidance of the United States FDA.
| Ingredient | Concentration (ng/mL) | Accuracy % | Precision % | Repeatability % | Stability % |
| L-Leu | 750 | 100.35 ± 3.20 | 3.19 | 3.69 | 0.41 |
| 1000 | 105.20 ± 6.45 | 6.13 | 6.91 | 1.69 | |
| 5000 | 95.53 ± 4.55 | 4.76 | 4.98 | 1.85 | |
| 5-HT | 6 | 99.78 ± 8.61 | 8.63 | 8.10 | 2.78 |
| 8 | 101.78 ± 4.32 | 4.24 | 2.87 | 3.80 | |
| 40 | 101.76 ± 4.50 | 4.42 | 4.24 | 2.14 | |
| N-phe | 1800 | 100.29 ± 4.32 | 4.31 | 4.72 | 3.07 |
| 2500 | 98.97 ± 4.13 | 4.17 | 3.71 | 3.18 | |
| 12500 | 92.24 ± 5.12 | 5.55 | 5.95 | 1.09 | |
| L-Try | 4500 | 97.33 ± 3.25 | 3.34 | 3.75 | 0.71 |
| 6000 | 95.45 ± 4.12 | 4.32 | 4.22 | 3.35 | |
| 30000 | 94.89 ± 4.77 | 5.03 | 4.22 | 4.00 | |
| L-Kyn | 37 | 99.61 ± 4.66 | 4.68 | 5.00 | 2.98 |
| 50 | 102.43 ± 4.15 | 4.05 | 4.41 | 1.73 | |
| 250 | 99.76 ± 4.11 | 4.12 | 4.15 | 2.09 | |
| 5-HTP | 300 | 102.45 ± 4.51 | 4.40 | 3.98 | 3.65 |
| 400 | 95.20 ± 8.80 | 9.24 | 7.50 | 6.16 | |
| 2000 | 101.77 ± 8.11 | 7.97 | 7.89 | 7.36 | |
| CA | 1.5 | 99.88 ± 3.70 | 3.70 | 3.83 | 1.47 |
| 2 | 97.00 ± 3.19 | 3.29 | 2.97 | 2.47 | |
| 10 | 96.76 ± 5.30 | 5.48 | 4.17 | 5.22 | |
| GSH | 1500 | 101.91 ± 4.65 | 4.56 | 5.19 | 1.45 |
| 2000 | 96.05 ± 3.98 | 4.14 | 4.63 | 0.89 | |
| 10000 | 97.51 ± 5.88 | 6.03 | 4.45 | 2.45 | |
| GSSG | 750 | 99.64 ± 3.97 | 3.98 | 4.43 | 1.56 |
| 1000 | 100.8 ± 6.45 | 6.40 | 7.23 | 4.89 | |
| 5000 | 98.39 ± 4.54 | 4.61 | 3.90 | 3.80 |
| Ingredient | Baseline (ng/mL) | Spiked (ng/mL) | Recovery % |
| L-Leu | 1000 | 250 | 92.53 ± 7.13 |
| 500 | 93.42 ± 11.52 | ||
| 750 | 87.53 ± 9.59 | ||
| 5-HT | 8 | 2 | 85.37 ± 9.79 |
| 4 | 90.52 ± 13.47 | ||
| 6 | 93.05 ± 10.16 | ||
| N-phe | 2500 | 625 | 94.54 ± 12.15 |
| 1250 | 89.46 ± 6.54 | ||
| 1875 | 86.72 ± 8.19 | ||
| L-Try | 6000 | 1500 | 93.04 ± 13.23 |
| 3000 | 85.42 ± 7.12 | ||
| 4500 | 91.16 ± 12.25 | ||
| L-Kyn | 50 | 12.5 | 90.48 ± 6.18 |
| 25 | 85.30 ± 10.42 | ||
| 37.5 | 86.20 ± 6.13 | ||
| 5-HTP | 2 | 0.5 | 91.49 ± 5.36 |
| 1 | 94.64 ± 7.46 | ||
| 1.5 | 93.58 ± 5.15 | ||
| CA | 400 | 100 | 91.83 ± 12.12 |
| 200 | 93.29 ± 8.62 | ||
| 300 | 90.22 ± 7.28 | ||
| GSH | 1000 | 250 | 88.17 ± 6.33 |
| 500 | 91.16 ± 6.15 | ||
| 750 | 90.85 ± 9.81 | ||
| GSSG | 2000 | 500 | 91.15 ± 10.08 |
| 1000 | 93.92 ± 12.15 | ||
| 1500 | 89.27 ± 10.92 |
Metabolic analysis: Analysis of 92 metabolites was achieved by UPLC-QTRAP-LC-MS/MS. NEM was used to improve the stability of GSH. Twenty-four differential metabolites in the serum (normal control group vs model control group and F-G groups vs model control group) were confirmed according to statistical analysis (Table 5).
| Metabolite | Model group | Control group | FG group |
| L-leucine | 431.1 ± 54.32 | 331.4 ± 48.78d | 393.5 ± 53.83 |
| N-phenylacetylglycine | 5.350 ± 0.938 | 1.417 ± 0.351d | 2.915 ± 0.496b,d |
| L-tryptophan | 17.96 ± 3.014 | 14.63 ± 2.724c | 17.05 ± 2.679a |
| L-kynurenine | 6.270 ± 0.729 | 3.948 ± 0.450d | 6.646 ± 1.214b |
| Cholic acid | 1.342 ± 0.230 | 0.047 ± 0.004d | 1.001 ± 0.124b,d |
| GSH | 2.562 ± 0.433 | 0.991 ± 0.285d | 2.317 ± 0.632b |
| GSSG | 0.108 ± 0.016 | 0.021 ± 0.002d | 0.051 ± 0.009b,d |
| lactate | 257.8 ± 32.57 | 230.8 ± 37.30 | 185.3 ± 31.52b,d |
| Choline | 3.548 ± 0.539 | 2.639 ± 0.451 | 2.802 ± 0.413 |
| (E)-butenedioic acid | 163.6 ± 18.57 | 79.48 ± 8.625d | 87.82 ± 10.55d |
| Hypoxanthine | 0.052 ± 0.003 | 0.021 ± 0.002d | 0.022 ± 0.003d |
| Carnitine | 23.55 ± 5.024 | 13.52 ± 1.370d | 20.05 ± 1.323b |
| Phenylalanine | 289.5 ± 37.51 | 165.6 ± 23.76d | 261.4 ± 32.33b |
| Uric acid | 4.523 ± 0.863 | 2.769 ± 0.474d | 2.927 ± 0.488d |
| Hippuric acid | 95.58 ± 16.47 | 65.78 ± 13.92d | 76.53 ± 16.92d |
| Citric acid | 11.86 ± 1.531 | 15.62 ± 2.437d | 13.51 ± 2.002 |
| Pantothenic acid | 7.852 ± 0.953 | 4.602 ± 0.560d | 4.831 ± 0.693d |
| Uridine | 19.37 ± 2.772 | 6.816 ± 0.829d | 17.53 ± 2.503b |
| Glucosamine-1-phosphate | 0.025 ± 0.005 | 0.017 ± 0.004c | 0.020 ± 0.004 |
| 8-OH-dG | 0.008 ± 0.001 | 0.007 ± 0.001c | 0.007 ± 0.001c |
| C16:1 Lyso PC | 3.215 ± 0.491 | 4.813 ± 0.503d | 6.293 ± 1.337b,d |
| C16:0 Lyso PC | 13.37 ± 2.434 | 16.62 ± 3.027 | 18.39 ± 3.316c |
| C18:1 Lyso PC | 16.837 ± 2.392 | 23.699 ± 4.427d | 26.647 ± 4.339a,d |
| C18:0 LysoPC | 11.42 ± 1.130 | 18.332 ± 1.833d | 16.66 ± 2.603a,d |
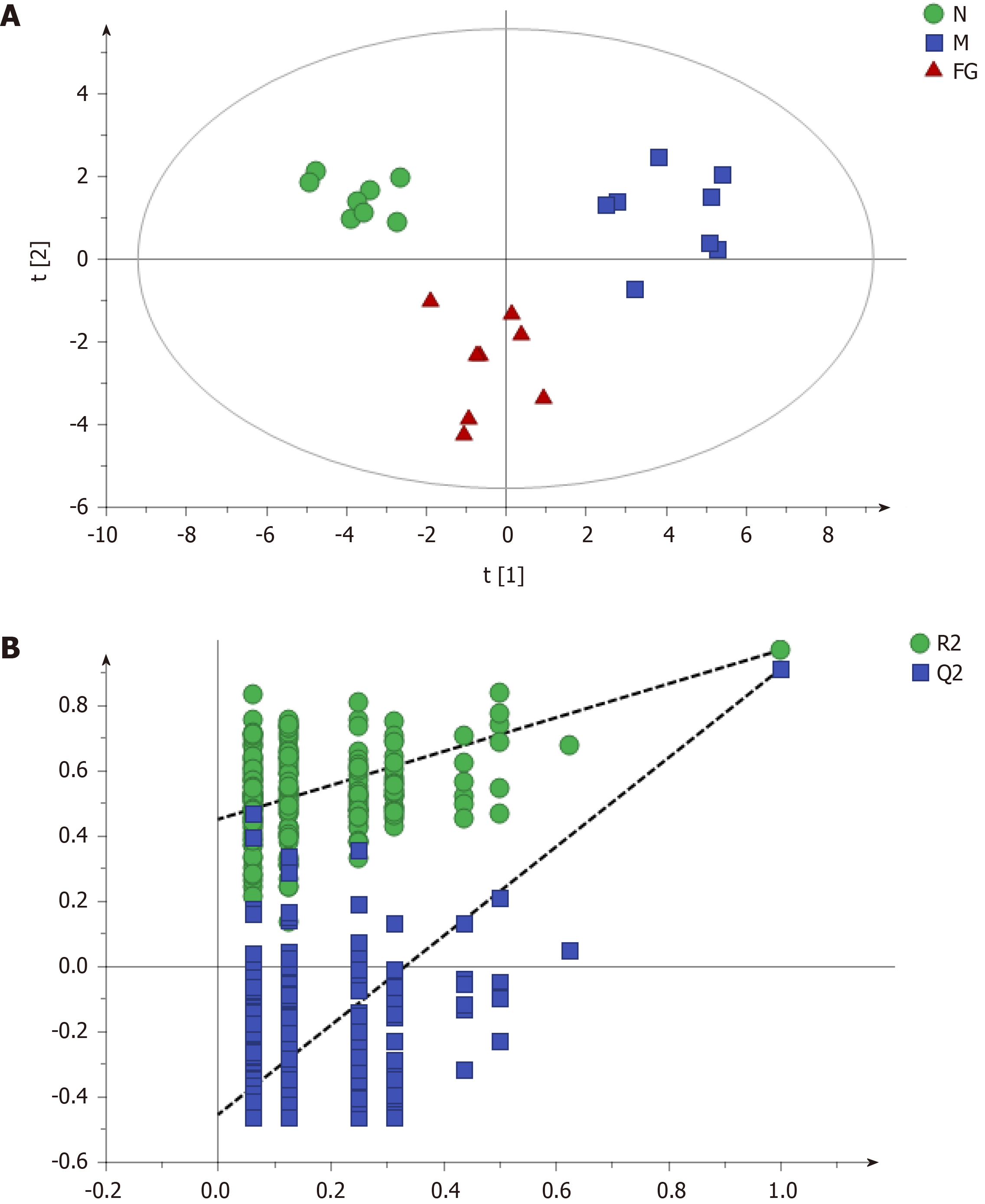
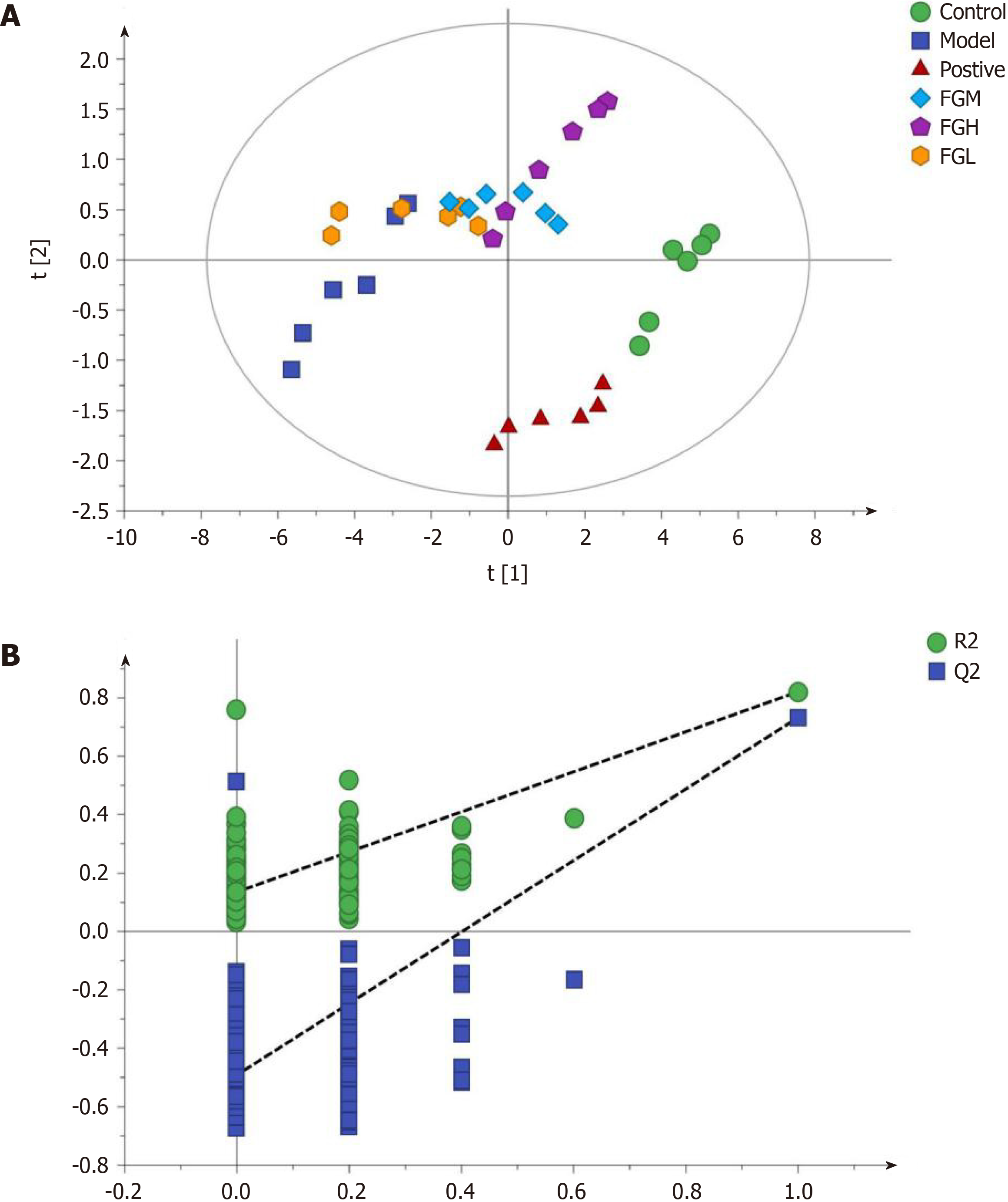
Partial Least-Squares Discriminant Analysis (PLS-DA) (Figure 2) was performed on the data matrix of the variation in individual metabolites in Table 4. The results showed that the samples of the negative control group, model control group, and F-G groups were distributed in different areas and could be separated completely, but samples of the same group had the tendency to aggregate (Figure 2A). The results showed that the difference in all metabolites between the negative control group and the model control group was significant, indicating that the contents of these 24 metabolites were significantly changed in acute liver injury induced by CCl4. The difference in all metabolites between the F-G groups and the model control group was also significant, indicating that the administration of the F-G extract could cause a significant reduction in the metabolite contents in the serum of rats with acute liver injury induced by CCl4, which could cause a significant change in the metabolic network. In the PLS-DA plot, samples of the F-G groups were closer to those of the negative control group than those of the model control group, indicating a significant difference in the metabolite contents in rats with liver injury compared to those in the negative control group after treatment with F-G. P-test analysis (Figure 2B) showed that there was no overfitting of the separation model between groups.
Combined with the variable importance in the projection (VIP) value based on PLS-DA, it was shown that the metabolites with a VIP value greater than 1.0 contributed greatly to the difference before and after treatment with the F-G extract and were the key metabolites. There were 14 key metabolites in the model control group vs the negative control group (Table 6) and 13 key metabolites in the F-G groups vs the model control group (Table 7). Ten common metabolites, namely, GSSG, cholic acid, N-phenylacetylglycine, hippuric acid, uric acid, hypoxanthine, (E)-butenedioic acid, pantothenic acid, C16:1 LPC, and C18:0 LPC, were observed.
| Var ID (primary) | M1-VIP | Variation trend |
| GSSG | 1.19631 | ↑↑ |
| Cholic acid | 1.18964 | ↑↑ |
| Uridine | 1.18609 | ↑↑ |
| N-phenylacetylglycine | 1.16986 | ↑↑ |
| Hypoxanthine | 1.13469 | ↑↑ |
| (E)-butenedioic acid | 1.1299 | ↑↑ |
| GSH | 1.12862 | ↑↑ |
| C18:0LPC | 1.12325 | ↓↓ |
| Pantothenic acid | 1.08663 | ↑↑ |
| Carnitine | 1.08264 | ↑↑ |
| Phenylalanine | 1.07794 | ↑↑ |
| Hippuric acid | 1.06083 | ↑↑ |
| C16:1LPC | 1.05866 | ↓↓ |
| Uric acid | 1.01621 | ↑↑ |
| C18:1LPC | 0.943612 | ↓↓ |
| L-kynurenine | 0.919692 | ↑ |
| L-leucine | 0.90164 | ↑↑ |
| Glucosamine-1-phosphate | 0.893372 | ↑ |
| Citric acid | 0.866932 | ↓↓ |
| L-tryptophan | 0.842517 | ↑ |
| 8-OH-dG | 0.711595 | ↑ |
| Lactate | 0.704198 | NS |
| Choline | 0.639509 | NS |
| C16:0LPC | 0.514772 | NS |
| Var ID (primary) | M1-VIP | Variation trend |
| Hypoxanthine | 1.37706 | ↓↓ |
| GSSG | 1.36516 | ↓↓ |
| Pantothenic acid | 1.28548 | ↓↓ |
| C18:1LPC | 1.27880 | ↑↑ |
| C16:1LPC | 1.25881 | ↑↑ |
| N-phenylacetylglycine | 1.22022 | ↓↓ |
| Lactate | 1.21835 | ↓↓ |
| Hippuric acid | 1.21598 | ↓↓ |
| Uric acid | 1.21291 | ↓↓ |
| (E)-butenedioic acid | 1.21107 | ↓↓ |
| Cholic acid | 1.17091 | ↓↓ |
| C18:0LPC | 1.15078 | ↑↑ |
| C16:0LPC | 1.00088 | ↑ |
| Choline | 0.906949 | NS |
| L-kynurenine | 0.893225 | ↑↑ |
| Uridine | 0.810356 | NS |
| 8-OH-dG | 0.777736 | NS |
| Citric acid | 0.633654 | NS |
| GSH | 0.512313 | NS |
| L-leucine | 0.497685 | NS |
| Carnitine | 0.447434 | NS |
| Phenylalanine | 0.427897 | NS |
| Glucosamine-1-phosphate | 0.312245 | NS |
| L-tryptophan | 0.0800812 | NS |
Pathway analysis: Metaboanalyst software was used to analyze the metabolic mechanism. Metabolic pathway differences between the negative control group and model control group and between the model control group and F-G group were analyzed by topological and enrichment analyses to confirm the important metabolic pathway effects of F-G in acute liver injury induced by CCl4.
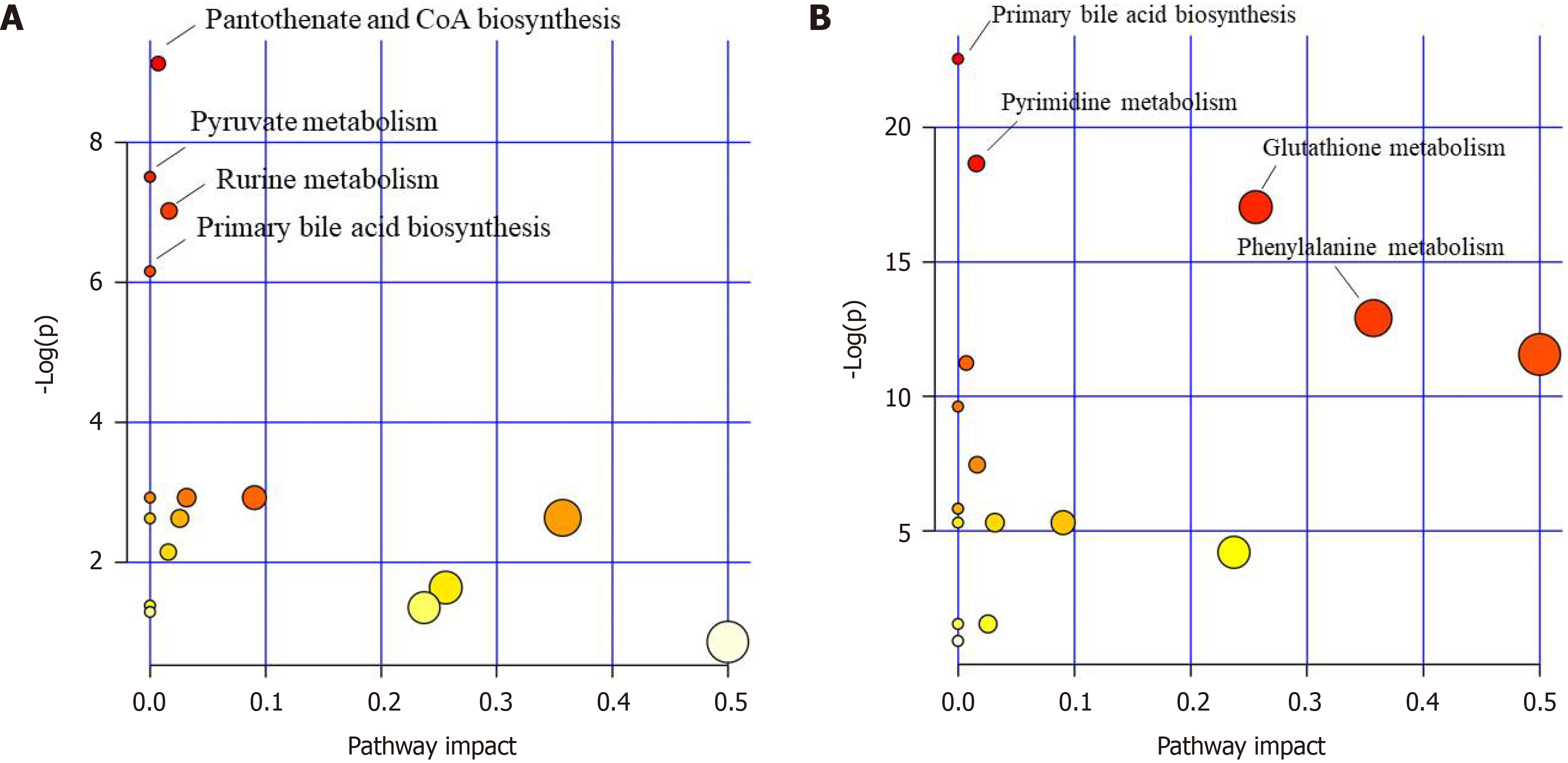
The results indicated that the primary bile acid biosynthesis pathway was the most important metabolic pathway affected in the model control group vs the negative control group (Figure 3A). Other pathways, such as pyrimidine metabolism, glutathione metabolism, glutamate metabolism, and phenylalanine metabolism, are also affected by CCl4. The pantothenate and CoA biosynthesis, primary bile acid biosynthesis, and pyruvate metabolism pathways were significantly affected by the administration of the F-G extract (Figure 3B).
Method validation: The calibration curve of each standard was plotted on the concentrations of 11 bile acids and the ratio of the peak area of each component to that of the internal standard with weighted (1/x) least square linear regression. The calibration curves of the 11 analytes exhibited a good linearity (Table 8).
| Compound | Standard curve | R² | Range (ng/mL) |
| CDCA | Y = 0.0235X - 0.0056 | 0.9981 | 2.5-1000 |
| UDCA | Y = 0.0013X + 0.2156 | 0.9972 | 1.25-500 |
| GCA | Y = 0.0164X + 1.1577 | 0.9956 | 1.25-500 |
| GDCA | Y = 0.0025X + 0.833 | 0.9982 | 1.25-500 |
| TCA | Y = 0.0198X - 0.0113 | 0.9953 | 1.25-500 |
| TUDCA | Y = 0.0332X + 0.0023 | 0.9985 | 2.5-1000 |
| TCDCA | Y = 0.0306X + 0.00422 | 0.9923 | 2.5-1000 |
| GCDCA | Y = 0.0133X - 0.00324 | 0.9915 | 2.5-1000 |
| GLCA | Y = 0.0018X + 0.00242 | 0.9931 | 2.5-1000 |
| DCA | Y = 0.0076X + 0.00614 | 0.9956 | 2.5-1000 |
| CA | Y = 0.0916X - 0.00465 | 0.9987 | 2.5-1000 |
The intraday precision of QC samples with three concentrations of bile acids ranged from 1.20% to 6.57%, the interday precision ranged from 2.10% to 6.35%, and the recovery of each analyte was greater than 80% (Table 9). Eleven bile acid analytes exhibited good stability (short-term stability, long-term stability, freeze-thaw stability, and postpreparation stability) (Table 10). All the analytical values of the 11 analytes had satisfactory results within the acceptable criteria.
| Compound | Concentration (ng/mL) | Recovery (%) | Intraday | Interday | ||
| Accuracy (%) | Precision (%) | Accuracy (%) | Precision (%) | |||
| CA | 2.5 | 90.12 ± 2.16 | 96.44 ± 2.05 | 4.26 | 91.83 ± 3.32 | 5.03 |
| 500 | 88.21 ± 5.34 | 91.65 ± 4.14 | 3.60 | 94.42 ± 4.55 | 4.36 | |
| 2000 | 85.60 ± 7.22 | 92.11 ± 4.11 | 5.42 | 94.54 ± 8.35 | 6.00 | |
| GLCA | 1.25 | 91.52 ± 5.51 | 92.13 ± 2.97 | 5.71 | 98.48 ± 2.22 | 3.75 |
| 250 | 86.74 ± 7.12 | 87.31 ± 1.92 | 4.23 | 93.42 ± 3.67 | 5.40 | |
| 1000 | 90.48 ± 3.31 | 91.55 ± 5.31 | 6.13 | 89.24 ± 4.63 | 4.57 | |
| GDCA | 1.25 | 88.54 ± 2.93 | 90.67 ± 6.87 | 6.22 | 88.55 ± 3.87 | 3.28 |
| 250 | 98.43 ± 1.26 | 97.24 ± 7.86 | 6.38 | 101.53 ± 2.54 | 2.76 | |
| 1000 | 95.56 ± 4.01 | 89.34 ± 2.65 | 2.81 | 89.23 ± 6.25 | 6.16 | |
| GCDCA | 1.25 | 96.02 ± 3.11 | 93.24 ± 3.78 | 6.30 | 89.32 ± 4.32 | 3.19 |
| 250 | 90.13 ± 3.42 | 97.43 ± 2.82 | 5.03 | 88.54 ± 4.75 | 2.10 | |
| 1000 | 89.32 ± 3.13 | 86.53 ± 4.27 | 2.96 | 89.52 ± 3.63 | 3.28 | |
| GCA | 1.25 | 80.87 ± 3.83 | 99.31 ± 5.19 | 4.18 | 89.42 ± 1.71 | 2.32 |
| 250 | 91.43 ± 6.38 | 101.33 ± 2.23 | 2.59 | 112.53 ± 5.76 | 2.29 | |
| 1000 | 92.43 ± 2.14 | 88.76 ± 1.46 | 1.20 | 89.16 ± 3.46 | 3.09 | |
| TCA | 2.5 | 84.77 ± 2.38 | 103.55 ± 1.27 | 1.50 | 98.24 ± 2.14 | 5.51 |
| 500 | 86.92 ± 4.96 | 89.04 ± 6.42 | 5.53 | 95.33 ± 2.86 | 3.82 | |
| 2000 | 81.34 ± 1.84 | 88.57 ± 4.85 | 4.77 | 97.81 ± 3.08 | 4.50 | |
| CDCA | 1.25 | 80.33 ± 3.23 | 94.33 ± 4.62 | 4.39 | 90.49 ± 4.83 | 5.18 |
| 250 | 86.34 ± 2.45 | 92.33 ± 4.93 | 3.70 | 91.49 ± 7.39 | 4.49 | |
| 1000 | 84.62 ± 3.21 | 92.33 ± 6.03 | 5.58 | 92.77 ± 4.83 | 6.18 | |
| DCA | 1.25 | 95.54 ± 2.17 | 94.67 ± 4.93 | 5.88 | 104.21 ± 7.22 | 3.87 |
| 250 | 82.56 ± 4.32 | 106.33 ± 7.37 | 4.36 | 99.31 ± 5.66 | 5.57 | |
| 1000 | 94.45 ± 5.34 | 101.33 ± 5.77 | 6.31 | 89.83 ± 4.53 | 4.71 | |
| UDCA | 1.25 | 83.32 ± 1.43 | 91.67 ± 4.62 | 6.41 | 93.43 ± 4.63 | 3.38 |
| 250 | 88.13 ± 5.94 | 95.33 ± 4.73 | 6.57 | 92.12 ± 4.49 | 2.84 | |
| 1000 | 85.26 ± 3.01 | 94 ± 4.58 | 2.90 | 91.79 ± 5.74 | 6.35 | |
| TCDCA | 1.25 | 86.04 ± 3.36 | 93.67 ± 5.86 | 6.49 | 92.77 ± 4.83 | 3.28 |
| 250 | 87.98 ± 3.11 | 94.67 ± 4.93 | 5.18 | 89.83 ± 4.53 | 2.17 | |
| 1000 | 83.72 ± 2.19 | 91.67 ± 4.62 | 3.05 | 92.77 ± 7.36 | 3.38 | |
| TUDCA | 1.25 | 87.17 ± 4.61 | 94.67 ± 7.51 | 4.31 | 96.69 ± 9.82 | 2.39 |
| 250 | 87.41 ± 4.09 | 98.67 ± 10.02 | 2.66 | 98.33 ± 8.33 | 2.36 | |
| 1000 | 99.06 ± 3.56 | 100.33 ± 8.5 | 1.23 | 92.45 ± 4.53 | 3.18 | |
| Compound | Concentration (ng/mL) | Concentration (mean ± SD) | ||||
| Initial | Freeze-thaw | Short-term | Long-term | Post-preparation | ||
| CA | 2.5 | 2.35 ± 0.17 | 2.3 ± 0.16 | 2.56 ± 0.18 | 2.33 ± 0.17 | 2.52 ± 0.08 |
| 500 | 495.43 ± 12.15 | 485.52 ± 11.91 | 538.93 ± 13.22 | 511.98 ± 12.56 | 505.09 ± 5.54 | |
| 2000 | 2080.38 ± 101 | 2038.77 ± 98.98 | 2263.03 ± 109.87 | 2149.88 ± 104.38 | 2194.52 ± 99.16 | |
| GLCA | 1.25 | 1.24 ± 0.14 | 1.22 ± 0.13 | 1.17 ± 0.15 | 1.21 ± 0.14 | 1.25 ± 0.14 |
| 250 | 247.1 ± 10.76 | 242.15 ± 10.55 | 258.79 ± 11.71 | 255.35 ± 11.12 | 250.24 ± 10.9 | |
| 1000 | 979.04 ± 40.32 | 959.46 ± 39.51 | 985 ± 43.86 | 1011.75 ± 41.66 | 991.52 ± 40.83 | |
| GDCA | 1.25 | 1.27 ± 0.05 | 1.24 ± 0.05 | 1.24 ± 0.06 | 1.19 ± 0.06 | 1.26 ± 0.08 |
| 250 | 240.43 ± 3.98 | 235.62 ± 3.9 | 261.54 ± 4.33 | 248.46 ± 4.12 | 249.32 ± 5.42 | |
| 1000 | 975.71 ± 45.93 | 956.19 ± 45.01 | 1061.38 ± 49.96 | 1008.31 ± 47.46 | 1035.03 ± 14.83 | |
| GCDCA | 1.25 | 1.27 ± 0.06 | 1.23 ± 0.08 | 1.36 ± 0.08 | 1.21 ± 0.09 | 1.21 ± 0.08 |
| 250 | 243.49 ± 4.03 | 242.1 ± 19.01 | 237.25 ± 18.63 | 263.35 ± 20.68 | 250.18 ± 19.64 | |
| 1000 | 988.14 ± 46.51 | 1047.04 ± 49.29 | 1026.1 ± 48.31 | 1108.97 ± 53.62 | 1082.02 ± 50.94 | |
| GCA | 1.25 | 1.19 ± 0.03 | 1.27 ± 0.03 | 1.27 ± 0.03 | 1.22 ± 0.03 | 1.21 ± 0.04 |
| 250 | 237.1 ± 1.93 | 232.35 ± 1.9 | 257.91 ± 2.1 | 245.02 ± 2 | 244.16 ± 1.88 | |
| 1000 | 982.38 ± 30.18 | 962.73 ± 29.58 | 1068.63 ± 32.83 | 1015.2 ± 31.19 | 1014.36 ± 44.06 | |
| TCA | 2.5 | 2.25 ± 0.17 | 2.21 ± 0.16 | 2.45 ± 0.18 | 2.33 ± 0.17 | 2.42 ± 0.08 |
| 500 | 492.1 ± 28.22 | 482.25 ± 27.65 | 535.3 ± 30.69 | 508.54 ± 29.16 | 494.76 ± 23.69 | |
| 2000 | 2013.71 ± 105.21 | 1973.43 ± 103.1 | 2190.51 ± 114.44 | 2080.99 ± 108.72 | 2142.85 ± 26.09 | |
| CDCA | 1.25 | 1.19 ± 0.1 | 1.28 ± 0.1 | 1.23 ± 0.11 | 1.24 ± 0.1 | 1.22 ± 0.08 |
| 250 | 232.1 ± 6.9 | 227.45 ± 6.77 | 252.47 ± 7.51 | 239.85 ± 7.14 | 236.41 ± 5.54 | |
| 1000 | 1010.38 ± 39.44 | 990.17 ± 38.65 | 1099.09 ± 42.91 | 1044.13 ± 40.76 | 1057.77 ± 46.98 | |
| DCA | 1.25 | 1.13 ± 0.03 | 1.15 ± 0.06 | 1.18 ± 0.13 | 1.23 ± 0.13 | 1.24 ± 0.14 |
| 250 | 236.33 ± 13.65 | 229.67 ± 21.36 | 233 ± 10.15 | 244.65 ± 10.66 | 247.1 ± 10.76 | |
| 1000 | 967.67 ± 15.82 | 947.67 ± 106.82 | 1031 ± 115.66 | 982.55 ± 53.41 | 979.04 ± 40.32 | |
| UDCA | 1.25 | 1.22 ± 0.06 | 1.19 ± 0.06 | 1.23 ± 0.07 | 1.26 ± 0.07 | 1.28 ± 0.07 |
| 250 | 252.1 ± 6.9 | 247.05 ± 6.77 | 244.23 ± 7.51 | 250.52 ± 7.14 | 257.07 ± 5.54 | |
| 1000 | 931.71 ± 85.92 | 971.43 ± 120.15 | 958.15 ± 108.46 | 940.89 ± 109.04 | 1061.11 ± 99.16 | |
| TCDCA | 1.25 | 1.22 ± 0.06 | 1.25 ± 0.06 | 1.28 ± 0.07 | 1.22 ± 0.06 | 1.19 ± 0.06 |
| 250 | 251.76 ± 3.11 | 246.73 ± 3.05 | 273.87 ± 3.39 | 260.17 ± 3.22 | 261.73 ± 2.5 | |
| 1000 | 1013.71 ± 69.97 | 993.43 ± 68.57 | 1102.71 ± 76.11 | 1047.58 ± 72.31 | 1057.77 ± 99.16 | |
| TUDCA | 1.25 | 1.22 ± 0.02 | 1.2 ± 0.02 | 1.23 ± 0.03 | 1.26 ± 0.02 | 1.27 ± 0.03 |
| 250 | 249.1 ± 6.47 | 244.11 ± 6.34 | 270.97 ± 7.04 | 257.42 ± 6.68 | 259.14 ± 8.46 | |
| 1000 | 977.04 ± 18.84 | 957.5 ± 18.46 | 1062.83 ± 20.49 | 1009.69 ± 19.47 | 1006.1 ± 26.09 | |
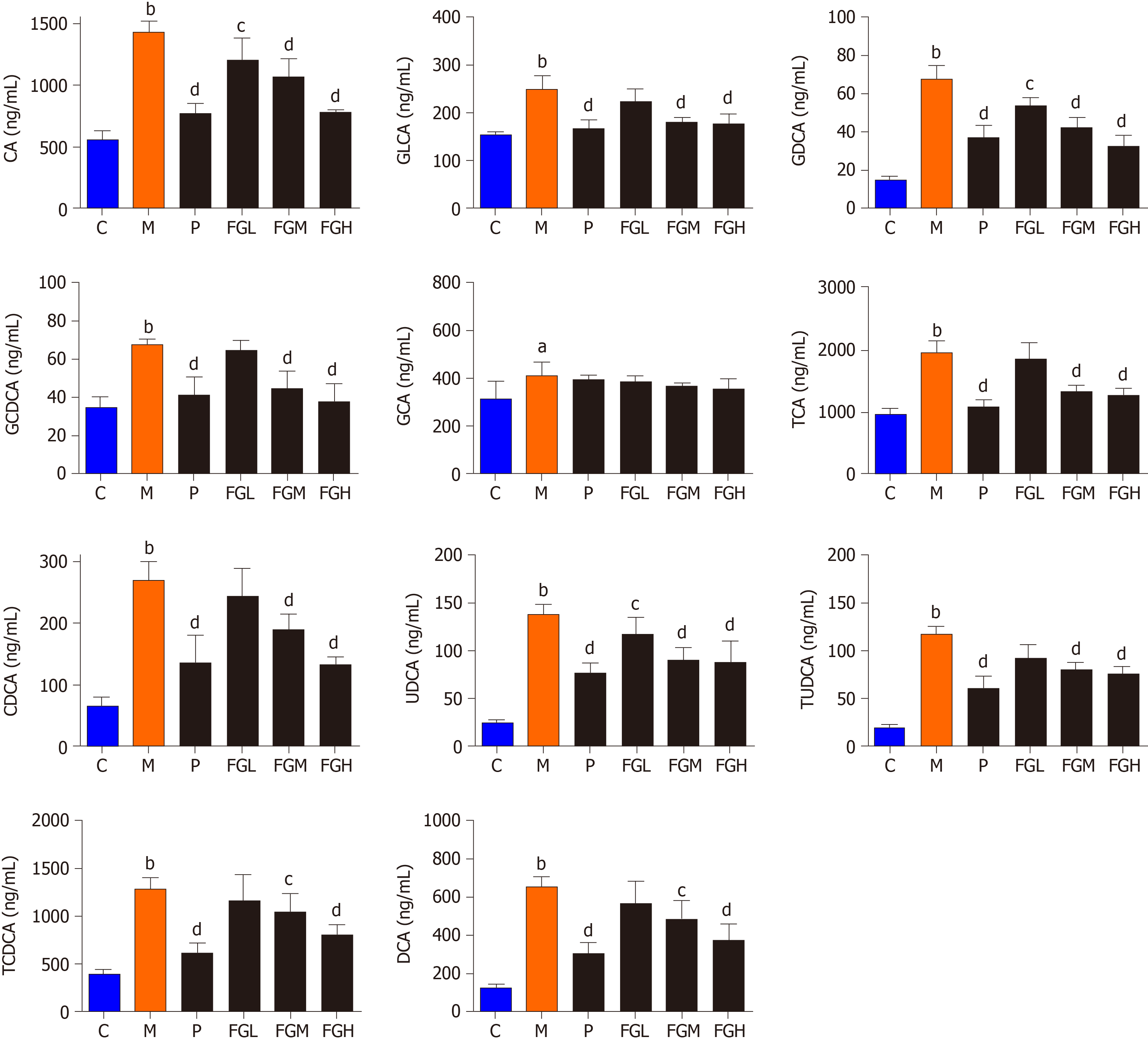
Bile acids analysis: Liver injury induced by CCl4 in rats could reduce bile secretion and rapidly alter the distribution of bile acid stores, resulting in a significant increase in serum bile acid concentrations. The results indicated that the contents of 11 analytes (CDCA, DCA, UDCA, GLCA, GLCA, GCDCA, GDCA, GCA, TCDCA, TUDCA, and TCA) in the serum of the model control group, compared with the negative control group, were significantly increased (P < 0.01), and the contents of the 11 bile acids in the serum of the F-G groups were decreased in a dose-dependent manner (Figure 4).
PLS-DA analysis was performed using a data matrix on the effect of F-G on serum bile acid levels in rats with CCl4 induced acute liver injury. As seen from the PLS-DA analysis (Figure 5A), the samples of the negative control group and model control group are distributed in different areas and far away from each other and can be completely separated from each other. The content differences of bile acids between the negative control group and model control group were significant. At the same time, the samples of the F-G group (10 g/kg) were partially overlapping those of the model control group, but with increasing dose, the samples of the mid-dose and high-dose F-G groups (20 g/kg and 30 g/kg) were clearly separated from those of the model control group, indicating that the dose-dependent relationship between the bile acid level and the dose of F-G extract was apparent in the overall analysis. P-test analysis showed that there was no overfitting of the between-group separation model (Figure 5B).
Analysis of 24 differential metabolites by UPLC-QTRAP-LC-MS/MS showed that CCl4 could affect a variety of metabolic pathways, such as primary bile acid biosynthesis, glutathione metabolism, pyrimidine metabolism, and phenylalanine metabolism. The F-G extract could affect the metabolic pathways of pantothenic acid and CoA biosynthesis, primary bile acid biosynthesis, and pyruvate biosynthesis. The primary bile acid synthesis pathway was the most important common pathway affected by CCl4 and the F-G extract. Subsequently, a UPLC-QQQ-LC-MS/MS method was established for the simultaneous quantitative detection of 11 bile acids, and the regulatory mechanism of the F-G extract on bile acids in the prevention of acute liver injury induced by CCl4 was obtained.
Since the content of different bile acids varied greatly in the serum, we first used the relative variation in each bile acid for analysis. This analysis evaluated the relative changes in bile acids for each group based on their primary contents (1.0) in the negative control group (Table 11). Free and conjugated bile acids are the two bile acid types in the liver and blood. CA, CDCA, and DCA are the main free bile acids, and bile acids conjugated with glycine or taurine compose the combined bile acids. In this study, a relatively higher increase in taurine-conjugated bile acids, except TCA, was observed in the model control group than in the negative control group (TUDCA, 6.00; TCDCA, 3.24), whereas the increase in glycine conjugated bile acids, except GDCA, was relatively low (GLCA, 1.61; GCDCA, 1.74; GCA, 1.30). Taurine possessed protective effects on hepatocellular injury and apoptosis. When hepatocellular injury occurs, the body can regulate the compensatory increase in taurine and further increase the content of taurine-conjugated bile acids[36]. The content of taurine conjugated bile acids was significantly decreased after treatment with the F-G extract in a dose-dependent manner.
| Bile acid | Negative | Model | Positive | FGL | FGM | FGH |
| CA | 1.00 | 2.57 | 1.38 | 2.16 | 1.92 | 1.40 |
| GLCA | 1.00 | 1.61 | 1.08 | 1.45 | 1.17 | 1.15 |
| GDCA | 1.00 | 4.45 | 2.43 | 3.53 | 2.78 | 2.14 |
| GCDCA | 1.00 | 1.74 | 1.18 | 1.85 | 1.28 | 1.09 |
| GCA | 1.00 | 1.30 | 1.25 | 1.22 | 1.16 | 1.12 |
| TCA | 1.00 | 1.99 | 1.11 | 1.90 | 1.37 | 1.31 |
| CDCA | 1.00 | 4.12 | 2.08 | 3.76 | 2.90 | 2.03 |
| UDCA | 1.00 | 5.68 | 3.17 | 4.87 | 3.72 | 3.62 |
| TUDCA | 1.00 | 6.00 | 3.08 | 4.73 | 4.13 | 3.87 |
| TCDCA | 1.00 | 3.24 | 1.57 | 2.92 | 2.63 | 2.03 |
| DCA | 1.00 | 5.16 | 2.40 | 4.48 | 3.84 | 2.95 |
Bile acids exhibit different hydrophilic and hydrophobic properties according to their chemical structure. Hydrophobic bile acids can lyse cell membrane lipids, exhibiting “decontamination”, resulting in hepatocellular necrosis. Therefore, the accumulation of hydrophobic bile acids in the liver is a major and important cause of liver injury. UDCA is a nontoxic hydrophilic bile acid. Taurine conjugated bile acids (TCA, TCDCA, and TDCA) and glycine conjugated bile acids (GLCA, GDCA, GCDCA, and GCA) exhibit low toxicity due to some hydrophobicity, while CA, CDCA, and DCA are free and hydrophobic bile acids that show strong hepatotoxicity. DCA is one of the most toxic bile acids. The accumulation of DCA in the liver or blood leads to mitochondrial destruction, cell membrane rupture, and the production of reactive oxygen species in hepatocytes, leading to apoptosis and necrosis[36,37]. In this study, three hydrophobic components, CA, CDCA, and DCA, were significantly increased in the model control group, showing a positive correlation with the toxic effects reported[38]. The F-G extract can promote the binding of CA, CDCA, and DCA to taurine and glycine to reduce the accumulation of free CA, CDCA, and DCA in vivo, thus preventing acute liver injury induced by CCl4.
When acute liver injury was caused by CCl4 in rats, the hepatocytes were damaged, and the activities of cholesterol 7α-hydroxylase and cholesterol 12α-hydroxylase in hepatocytes were decreased, resulting in decreased CA and CDCA produced by cholesterol synthesis. However, due to impaired hepatic uptake of bile acids, the uptake of these two bile acids in the enterohepatic circulation is greatly reduced, resulting in a significant increase in the concentrations of bile acids in the serum. In this study, CA and CDCA were significantly increased in the model control group, showing liver damage and leading to bile acid malabsorption in the hepatoenteric circulation. The F-G extract decreased the contents of CA and CDCA in serum in a dose-dependent manner, indicating that the F-G extract could repair liver injury, improve enterohepatic circulation, and promote the absorption of CA and CDCA, exhibiting almost the same pharmacological effect as silybin.
In this study, the effects of F-G extract in preventing acute liver injury induced by CCl4 have been assayed. The F-G extract decreases the adhesion between the lobules and the mucosa, reduces the bleeding point on the surface of the liver, effectively decreases ALT and AST in a rat model of acute liver injury in a dose-dependent manner, and reduces hepatocellular swelling, inflammatory cell infiltration, blood stasis, hepatic steatosis, and other pathological changes in rats.
Fuzi (Radix aconiti lateralis)-Gancao (Radix glycyrrhizae) (F-G) is often used in the treatment of liver diseases such as hepatitis and liver failure.
This study can clarify the bile acid mechanism of F-G in the treatment of liver injury, and establish a complete bile acid spectrum research method, so as to provide reference for future research.
To study the molecular mechanism and action mechanism of F-G in the treatment of liver injury, and to provide a theoretical basis for the clinical research of F-G.
An ultra performance liquid chromatography tandem mass spectrometry (UPLC-MS/MS) method for the simultaneous determination of 92 metabolites from multiple pathways was established to explore the protective metabolic mechanism of F-G in serum on the liver.
A UPLC-MS/MS method for simultaneous determination of 11 bile acids was established to analyze the regulatory mechanism of F-G in serum. F-G decreased the contents of 11 bile acids in the serum in a dose-dependent manner.
F-G could promote the conjugation of free bile acids to glycine and taurine, reduce the accumulation of free bile acids in the liver, regulate the compensatory degree of taurine, and decrease the content of taurine conjugated bile acids.
The research group will continue to study the effect of bile acid metabolism regulation on molecular regulation in the body.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Saraiva MM S-Editor: Wu YXJ L-Editor: Wang TQ P-Editor: Li JH
| 1. | Wu H, Zhang G, Huang L, Pang H, Zhang N, Chen Y, Wang G. Hepatoprotective Effect of Polyphenol-Enriched Fraction from Folium Microcos on Oxidative Stress and Apoptosis in Acetaminophen-Induced Liver Injury in Mice. Oxid Med Cell Longev. 2017;2017:3631565. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 2. | Yang Y, He Q, Wang H, Hu X, Luo Y, Liang G, Kuang S, Mai S, Ma J, Tian X, Chen Q, Yang J. The protection of meloxicam against chronic aluminium overload-induced liver injury in rats. Oncotarget. 2017;8:23448-23458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Navarro VJ, Khan I, Björnsson E, Seeff LB, Serrano J, Hoofnagle JH. Liver injury from herbal and dietary supplements. Hepatology. 2017;65:363-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 265] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 4. | Russell DW, Setchell KD. Bile acid biosynthesis. Biochemistry. 1992;31:4737-4749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 602] [Cited by in RCA: 602] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 5. | Chiang JY. Bile acids: regulation of synthesis. J Lipid Res. 2009;50:1955-1966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1034] [Cited by in RCA: 1244] [Article Influence: 77.8] [Reference Citation Analysis (0)] |
| 6. | Li T, Chiang JY. Bile acid signaling in metabolic disease and drug therapy. Pharmacol Rev. 2014;66:948-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 728] [Cited by in RCA: 694] [Article Influence: 63.1] [Reference Citation Analysis (0)] |
| 7. | Trauner M, Boyer JL. Bile salt transporters: molecular characterization, function, and regulation. Physiol Rev. 2003;83:633-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 737] [Cited by in RCA: 700] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 8. | Maillette de Buy Wenniger L, Beuers U. Bile salts and cholestasis. Dig Liver Dis. 2010;42:409-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 132] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 9. | Russell DW. The enzymes, regulation, and genetics of bile acid synthesis. Annu Rev Biochem. 2003;72:137-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1372] [Cited by in RCA: 1488] [Article Influence: 67.6] [Reference Citation Analysis (0)] |
| 10. | Zhou W. Clinical observation on treatment of acute-on-chronic liver failure with Traditional Chinese Medicine. Zhongguo Xiandai Yaowu Yingyong Zazhi. 2016;10:263-264. [DOI] [Full Text] |
| 11. | Liu QL, Sun YW. Clinical observation of Fuzi Lizhong decoction combined with western medicine in the treatment of chemotherapy-induced liver injury. Zhongguo Laonian Baojian Yixue. 13:73-74. [DOI] [Full Text] |
| 12. | Luo JX, Zhang Y, Hu XY, Yu C. The Effect of Modified Sini Decoction on Survival Rates of Patients with Hepatitis B Virus Related Acute-on-Chronic Liver Failure. Evid Based Complement Alternat Med. 2019;2019:2501847. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Li RX, Chen ZY, Liu X, Yang CS. Effect of modified Sini decoction combined with magnesium isoglycyrrhizinate on acute drug-induced liver injury and its influence on oxidative stress and humoral immune function. Xiandai Zhongxiyi Jiehe Zazhi. 2018;27:25-29. [DOI] [Full Text] |
| 14. | Chen L, Yang H, Zhang FX, Liu N, Li XY, Zhao F. Protective effects of modified Sini decoction on hepatocyte apopto-sis induced by Con A. Yunnan Daxue Zhongyiyao Zazhi. 2014;37:5-7. |
| 15. | Yang JH, Shi SH, Ma W, Tao DQ, Liu S, Chen L, Zhou XL. Effect of Fuzi Lizhong decoction in reducing fiver injury of rats with non-alcoholic fatty liver via activating AMPK and suppressing NF-κBp65 pathway. Zhongguo Zhongyao Zazhi. 2018;43:3176-3183. |
| 16. | Luo J, Zhang Y, Hu X, Zhong S, Chen G, Wang Y, Lin W, Yi C, Zhu H. The effects of modified sini decoction on liver injury and regeneration in acute liver failure induced by D-galactosamine in rats. J Ethnopharmacol. 2015;161:53-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Fu X, Lu R, Zhao S. Simultaneous quantitation of six aconitum alkaloids and three flavonoids in the herb couple of radix aconiti lateralis-radix glycyrrhizae (Fuzi-Gancao) by UHPLC-ESI-MS/MS. Pharmacogn Mag. 2017;13:425-429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Liu XX, Jian XX, Cai XF, Chao RB, Chen QH, Chen DL, Wang XL, Wang FP. Cardioactive C₁₉-diterpenoid alkaloids from the lateral roots of Aconitum carmichaeli "Fu Zi". Chem Pharm Bull (Tokyo). 2012;60:144-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 19. | Wang L, Ding JY, Liu XX, Tang MH, Chao RB, Wang FP. [Identification of aminoalcohol-diterpenoid alkaloids in Aconiti Lateralis Radix Praeparata and study of their cardiac effects]. Yao Xue Xue Bao. 2014;49:1699-1704. [PubMed] |
| 20. | Lu ZQ, Zhang YJ, Zhuang PW, Zhang JB, Zhou HF, Chen Z, Sun M, Xu LM. Effect of Aconiti Lateralis Radix Preparata on hemodynamics of rats with acute heart failure and its mechanism. Zhong Cao Yao. 2015;46:3223-3234. |
| 21. | Shao F, Li SL, Liu RH, Huang HL, Ren G, Yao YX, Hao XC. Analgesic and Anti-inflammatory Effects of Different Processed Products of Aconiti lateralis radix praeparata. Shizhen Guoyi Guoyao. 2011;22:2329-2330. [DOI] [Full Text] |
| 22. | Gao F, Li YY, Wang D, Huang X, Liu Q. Diterpenoid alkaloids from the Chinese traditional herbal "Fuzi" and their cytotoxic activity. Molecules. 2012;17:5187-5194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 23. | Zhang T, Wang GJ, Bai SG. Effect of Aconiti Lateralis Radix Preparata on antioxidant system in aged rats. Zhongguo Laonianxue Zazhi. 2001;21:135-136. [DOI] [Full Text] |
| 24. | Luo JX, Zhang Y, Hu XY, Chen G, Liu XY, Nie HM, Liu JL, Wen DC. Aqueous extract from Aconitum carmichaelii Debeaux reduces liver injury in rats via regulation of HMGB1/TLR4/NF-ΚB/caspase-3 and PCNA signaling pathways. J Ethnopharmacol. 2016;183:187-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 25. | Konda VGR, Eerike M, Prabhu L. Evaluation of hepatoprotective activity of ethanolic extract of aconitum heterophyllum root in paracetamol induced liver toxicity. Int J Pharma Bio Sci. 2013;4:714-721. |
| 26. | Yang XX, Liu D, Bian K, Zhang DD. Study on in vitro anti-inflammatory activity of total flavonoids from Glycyrrhizae Radix et Rhizoma and its ingredients. Zhongguo Zhongyao Zazhi. 2013;38:99-104. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | Furusawa J, Funakoshi-Tago M, Tago K, Mashino T, Inoue H, Sonoda Y, Kasahara T. Licochalcone A significantly suppresses LPS signaling pathway through the inhibition of NF-kappaB p65 phosphorylation at serine 276. Cell Signal. 2009;21:778-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 28. | Fu Y, Chen J, Li YJ, Zheng YF, Li P. Antioxidant and anti-inflammatory activities of six flavonoids separated from licorice. Food Chem. 2013;141:1063-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 229] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 29. | Ahn SJ, Cho EJ, Kim HJ, Park SN, Lim YK, Kook JK. The antimicrobial effects of deglycyrrhizinated licorice root extract on Streptococcus mutans UA159 in both planktonic and biofilm cultures. Anaerobe. 2012;18:590-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 30. | Dong Y, Zhao M, Zhao T, Feng M, Chen H, Zhuang M, Lin L. Bioactive profiles, antioxidant activities, nitrite scavenging capacities and protective effects on H2O2-injured PC12 cells of Glycyrrhiza glabra L. leaf and root extracts. Molecules. 2014;19:9101-9113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 31. | Kim YW, Park SM, Seo HL, Jang MH, Jung JY, Lee JH, Jang EJ, Cho IJ, Byun SH, Kim SC. Licorice and its flavonoids inhibit oxidative damage in the liver. Integr Med Res. 2015;4:77. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 32. | Zhang GP, Xie SH, Zhu XG, Zhang SY, Zhai JY, Meng H, Ye ZG. Experimental Study on Effects of Radix Glycyr-rhizae on Toxicity and Efficacy of Radix Aconiti Carmichaeli in Compatibility. Zhongguo Zhongyiyao Xinxi Zazhi. 2012;19:31-34. [DOI] [Full Text] |
| 33. | Ma LN, Ye ZG, Zhang GP. Detoxification mechanism of Aconiti lateralis radix praeparata with Glycyrrhizae Radix et Rhizoma compatibility based on the change of components in vitro-metabolism in vivo-antagonism of biological effects. Zhongguo Zhongyao Zazhi. 2019;44:99-104. [RCA] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 34. | Li H, Zhang GP, Ma M, Su P, Yang YF, Chen TF, Ye ZG. Attenuation Mechanism of Aconti Lateralis Radix Praeparata Combined with Glycyrrhizae Radix et Rhizoma Based on Heart CYP450 Isozymes. Zhongguo Shiyan Fangjixue Zazhi. 2020;26:59-64. [DOI] [Full Text] |
| 35. | Song H, Li W, Liu B, Sun X, Ding J, Chen N, Ji Y, Xiang Z. Study of the estrogenic-like mechanism of glycosides of cistanche using metabolomics. RSC Adv. 2017;7:39403-39410. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 36. | Xu Y, Chen CC, Yang L, Wang JM, Ji LL, Wang ZT, Hu ZB. Evaluation on hepatotoxicity caused by Dioscorea bulbifera based on analysis of bile acids. Acta Pharm Sin. 2011;46:39-44. [RCA] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 37. | He XL, Guan H, Li PF. Research progress of taurochodeoxycholic acid. Anim Husb Feed Sci. 2008;29:57-60. |