Published online Aug 14, 2021. doi: 10.3748/wjg.v27.i30.5019
Peer-review started: April 12, 2021
First decision: May 24, 2021
Revised: June 4, 2021
Accepted: June 23, 2021
Article in press: June 23, 2021
Published online: August 14, 2021
Processing time: 120 Days and 1.6 Hours
The gut microbiome is a complex microbial community, recognized for its potential role in physiology, health, and disease. The available evidence supports the role of gut dysbiosis in pancreatic disorders, including acute pancreatitis (AP). In AP, the presence of gut barrier damage resulting in increased mucosal permeability may lead to translocation of intestinal bacteria, necrosis of pancreatic and peripancreatic tissue, and infection, often accompanied by multiple organ dysfunction syndrome. Preserving gut microbial homeostasis may reduce the systemic effects of AP. A growing body of evidence suggests the possible invo
Core Tip: We live in a world of microbes. There is a distinct microbiome sighted in every niche of our body. This review is based on current knowledge to define an overview of how the gut microbiota has accelerated the frontiers of understanding recently and empowered its importance in influencing human physiology through its potential role in various diseases. It further explores the possible application of microbiota-targeted therapeutics in routine clinical practice, meaning manipulating gut microbiota into the current therapeutics to minimize the potential risk of various diseases, including acute pancreatitis.
- Citation: Patel BK, Patel KH, Bhatia M, Iyer SG, Madhavan K, Moochhala SM. Gut microbiome in acute pancreatitis: A review based on current literature. World J Gastroenterol 2021; 27(30): 5019-5036
- URL: https://www.wjgnet.com/1007-9327/full/v27/i30/5019.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i30.5019
Recent research has confirmed the importance of the human gut microbiota in maintaining health and its involvement in disease. The human gastrointestinal (GI) tract harbors a diverse microbial population of more than 1014 microorganisms, comprising more bacterial cells than human body cells[1] and more than 3.3 million unique genes[2]. The predominant commensal bacteria in the human GI tract are members of the phyla Firmicutes and Bacteroidetes, constituting 80%–90% of the total gut microbiota[3]. Other phyla include Proteobacteria, Actinomyces, Actinobacteria, Fusobacteria, and Verrucomicrobia[4]. The composition of the gut microbiome plays a key role in modulating human immune responses to invasive pathogens, and it also prevents the pathogens from crossing the intestinal barrier[5].
Recent improvement of advanced sequencing methods, such as next-generation sequencing and metagenomics, have added to our understanding of the involvement of gut microbiome in human health and disease[6] and the potential therapeutic value of interventions that target the composition of the gut microbiome. Such interventions would manipulate the host-microbiome community by eliminating harmful taxa or reconstituting missing beneficial taxa[6,7]. Recent evidence shows that, under ideal conditions, symbiotic relationships among the microbial species in the host GI tract can function to prevent opportunistic and nosocomial infections that have become more frequent because of the widespread use of antibiotics to treat various diseases[8-11].
Research performed over the past two decades has revealed the significance of gut dysbiosis in the pathogenesis of many diseases[12], including inflammatory bowel disease[13], irritable bowel syndrome[14], colon cancer[15], Alzheimer's disease[16], coronary heart disease[17], obesity[18], and diabetes mellitus[19]. Changes in the diversity, proportions, and dominant species of the gut microbiome are probably associated with intestinal barrier dysfunction that influences onset and clinical course of multiple diseases, including pancreatic disorders[20]. In a healthy individual, no gut microbes are present in the pancreas, but changes of the gut microbiota may be involved in the pathogenesis of pancreatic disease, including acute pancreatitis (AP)[21]. AP is a common disorder of the digestive system with high morbidity and mortality worldwide. Managing AP is challenging and has a heavy financial burden on the patient and society[22,23]. Therefore, it is important to understand the primary causes and mechanism of the pathogenesis and progression of AP to facilitate early diagnosis and treatment, avoid a course leading to severe disease, and reduce AP-associated fatality[23]. Ongoing studies of AP in humans have found that premature activation of trypsinogen, dysfunctional calcium signaling, impaired endoplasmic reticulum stress-related autophagy, unfolded protein response, and mitochondrial dysfunction all promote AP. However, the cause of multiorgan dysfunction in AP is poorly understood[24]. The potential role of the intestine in promoting systemic inflammation and organ dysfunction is of interest.
In AP, hypovolemia, reflex splanchnic vasoconstriction, intestinal ischemia, and reperfusion injury due to fluid resuscitation may result in bacterial translocation [25,26]. Systemic inflammatory response syndrome accompanied by intestinal bacterial translocation is associated with high AP mortality[26]. A change in gut permeability/motility that causes bacterial translocation and leads to the activation of gut-associated lymphoid tissues may result in systemic complications in AP[27]. This review summarizes relevant human and animal studies that provide insights into the potential role of the gut microbiome in AP pathogenesis. It also summarizes treatment perspectives that target the gut microbiome.
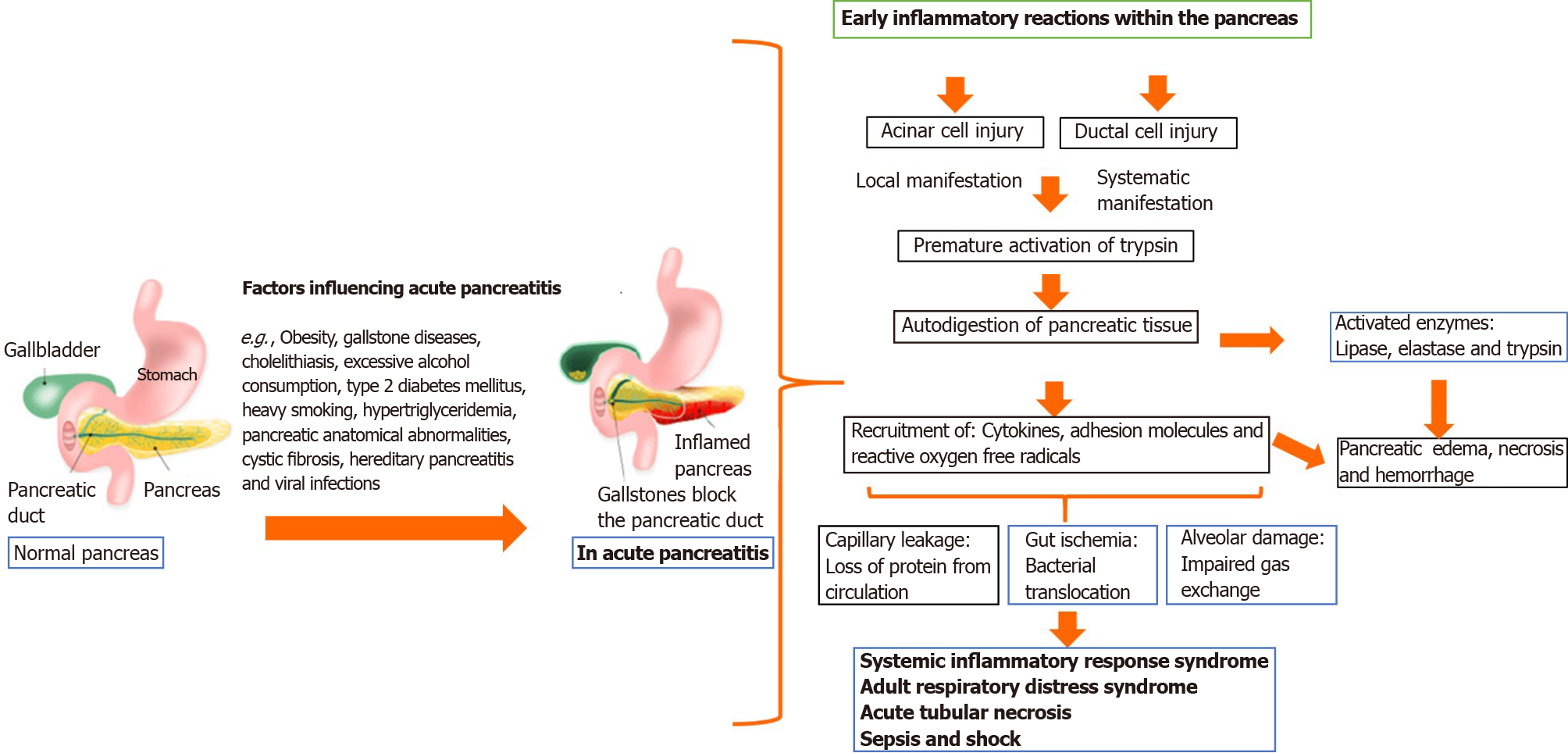
Previous studies of microbiome involvement in AP described a complex cascade of events with significant involvement of pancreatic acinar cells, but the mechanisms involved in the initiation of AP are still poorly understood[28]. Development of a well-defined clinical management protocol is challenging. Most investigations of AP pathophysiology have documented injury or disruption of the pancreatic acini that triggered the activation of pancreatic enzymes (trypsin, chymotrypsin, and elastase) in pancreatic tissue. Activated proteases (trypsin and elastase) and lipase breakdown tissue and cell membranes, leading to edema, vascular damage, hemorrhage, and necrosis[28].
During the initial phase of pancreatic injury, acinar cells release proinflammatory cytokines, like tumor necrosis factor (commonly referred to as TNF)-α, interleukin (IL)-1 and IL-6, and anti-inflammatory mediators, such as IL-10 and IL-1 receptor antagonist[29]. These mediators recruit neutrophils and macrophages to enter the pancreatic parenchyma, to propagate both local and systemic responses. Reactive oxygen metabolites, prostaglandins, platelet-activating factor, and leukotriene may also be involved[29].
Recent epidemiological studies have shown that a local inflammatory response aggravates pancreatitis by increasing permeability, which damages the microcirculation and results in local hemorrhage and pancreatic necrosis in cases of severe AP. Some of the inflammatory mediators released by neutrophils aggravate pancreatic injury by activating pancreatic enzymes (see Figure 1)[26,30]. Figure 1 is a schematic description of AP pathogenesis. The acinar cells of the pancreas cause trypsin activation followed by impairment of cell membrane trafficking and activation of the zymogen cascade mediated by trypsin. Attraction and activation of leukocytes occur with the release of pro- and anti-inflammatory cytokines and chemokines. Overt, sustained activation of proinflammatory mediators leads to systemic inflammatory response syndrome (SIRS) and may progress to multiorgan failure, infection, pancreatic necrosis, and sepsis as late complications of AP.
Approximately 15%–25% of patients diagnosed with AP may progress to severe AP. The 2012 revised Atlanta classification and definitions by international consensus include three degrees of AP severity (i.e. mild, moderately severe, and severe)[31]. The three degrees primarily manifest as transient organ failure, persistent organ dysfunction, and local or systemic AP[31]. The determinant-based classification of AP is highly dependent on clinical data, and also on the available feedback from patients (to a lesser extent)[32]. Table 1 summarizes both the Atlanta and determinant-based classifications.
| Revised Atlanta classification of disease severity | |
| Mild AP | No organ failure |
| No local or systemic complications | |
| Moderately severe AP | Organ failure that resolved within 48 h (transient organ failure) and /or |
| Local or systemic complications without persistent organ failure | |
| Severe AP | Persistent organ failure > 48 h |
| Single organ failure | |
| Multiple organ failure | |
| A modified Marshal score defines a persistent organ failure | |
| Determinant-based classification of disease severity | |
| RACAP | DBCAPS |
| Mild AP | Mild AP |
| Absence of organ failure | Absence of organ failure |
| Absence of local complications | Absence of (peri-) pancreatic necrosis |
| Moderately severe AP | Moderate AP |
| Local complications and/or | Sterile (peri-) pancreatic necrosis and/or |
| Transient organ failure | Transient organ failure |
| Severe AP | Severe AP |
| Persistent organ failure | Persistent organ failure or |
| Infected (peri-) pancreatic necrosis | |
| Critical AP persistent organ failure | |
| Infected (peri-) pancreatic necrosis | |
The initial diagnosis of AP made on arrival at a clinic or hospital relies on known medical history, comprehensive physical examination, and increased serum amylase or lipase, with or without additional imaging evaluation. Currently, there are no specific laboratory tests with consistent accuracy and reliability for predicting AP severity. However, several scoring systems are available and are routinely used by hospital physicians to predict AP severity and prognosis (Table 2)[33-41].
| No. | Multifactorial scoring system | Timeline | Threshold | Area under the curve | Ref. |
| 1 | Ranson score | 48 h | ≥ 3 | 0.81–0.88 | [33-35] |
| 2 | Glasgow score | 48 h | 2 | 0.73–0.784 | [36,37] |
| 3 | Acute Physiology and Chronic Health Evaluation-II score (APACHE-II) | 24 h | 7 | 0.80–0.895 | [33,38,39] |
| 4 | Acute Physiology and Chronic Health Evaluation II score-Obesity (APACHE:-O) | 24 h | 7 | 0.893 | [40] |
| 5 | Bedside Index of Severity score (BISAP) | 24 h | ≥ 3 | 0.79–0.875 | [33-35,41] |
| 6 | Pancreatitis Activity Scoring System (PASS) | 24 h | > 160 | 0.71 | [36] |
| 7 | Systemic inflammatory response syndrome (SIRS) | 24 h | ≥ 2 | 0.73 | [34,39] |
In addition to the various clinical scoring systems, several biomarkers have also been applied as predictors of AP severity and are shown in Table 3[42-47].
| No. | Blood biomarkers | Timeline | Threshold | Area under the curve | Ref. |
| 1 | Interleukin 8 | Preoperative | 196 pg/mL | 0.778 | [42] |
| 2 | Interleukin 6 | 24 h | 50 pg/mL | 0.9 | [39] |
| 3 | Hepcidin | 24 h | 234.4 ng/mL | 0.82 | [43] |
| 4 | Red blood cell distribution width | 24 h | 13.35% | 0.787 | [44] |
| 5 | Procalcitonin | 24 h | 1.77 ng/mL | 0.797 | [45] |
| 6 | Blood urea nitrogen | 24 h | 5.945 mg/dL | 0.677 | [44] |
| 7 | Oleic acid chlorohydrin | 24 h | 32.40 nM | 1 | [46] |
| 8 | C-reactive protein | 24 h | 150 mg/L | 0.61 | [47] |
| 9 | C-reactive protein | 48 h | 150 mg/L | 0.73–0.91 | [33,39,47] |
Recently, circular microRNAs have been studied as potential diagnostic biomarkers in AP because of their specific properties, such as stability in biological fluids, simple identification, and sequence conservation among different species (Table 4)[48-60].
| No. | miRNAs | Patients | Sample | Expression change | Reference gene | Ref. |
| 1 | miR-216a | AP | Plasma | Up | None | [49] |
| 2 | miR-551b-5p | AP | Plasma | Up | miR-16 | [50] |
| 3 | miR-216a-5p, miR-375, and miR-551b-5p | AP | Serum | Up | miR-103a-3p | [51] |
| 4 | miR-7, miR-9, miR-122, and miR-141 | AP | Serum | Up | Exogenous reference genes | [52] |
| 5 | miR-216a | AP | Plasma | Up | U6 | [53] |
| 6 | miR-551-5p | AP | PBMC- | Up | U6 | [54] |
| 7 | miR-155 | AP | Serum | Up | U6 | [55] |
| 8 | miR-29a | AP | Plasma | Up | U6 | [56] |
| 9 | miR-24-3p, miR-222-3p, miR-361-5p, and miR-1246 | HTG-AP | Serum | Up | U6 | [57] |
| 10 | miR-1260b, miR-762, miR-22-3p, miR-23b, and miR-23a | AP-associated ALI | Serum | Up | U6 | [58] |
| 11 | miR-92b, miR-10a, and miR-7 | AP | Plasma | Down | miR-16 | [50] |
| 12 | miR-155 | AP | Serum | Down | Not mentioned | [59] |
| 13 | miR-181a-5p | HTG-AP | Serum | Down | U6 | [57] |
| 14 | miR-550a, miR-324-5p, miR-484, miR-331-3p, miR-140-3p, miR-342-3p, and miR-150 | AP-associated ALI | Serum | Down | U6 | [58] |
| 15 | miR-127 | AP-associated ALI | Plasma | Down | miR-16 | [60] |
The human GI tract is home to a diverse and complex microbial community of bacteria, viruses, and fungi that help to maintain health and are involved in the pathogenesis of various diseases. The gut contains at least 1000 bacterial species and 100-fold more genes than have been identified in the human genome[4,61]. The microbiome is considered a hidden “metabolic organ”, and it has a significant impact on well-being because of its influence on our metabolism, physiology, nutrition, and immune function[62]. It has been shown that the gut microbiome co-evolves with us; hence, any changes to the microbial community can have significant consequences, both beneficial and harmful[63]. Disruption of the gut microbiota, or dysbiosis, has been associated with diverse systematic conditions, such as obesity[64,65], mal
Researchers have continued to study the microbiome at an accelerated pace over the past two decades, revealing the myriad ways these microorganisms affect our day-to-day lives. The microbiome in our gut is now understood to be a significant contributor to the development of chronic disease. Gut microbiota is now known to play a critical role in human health and disease. With the advances in microbiome research over time, more and more data has become available showing the gut microbes' overall composition and functional potential. Additionally, the number of diseases associated with changes in our gut microbial community has increased simultaneously[4,6]. The human gastrointestinal tract is a habitat crowded with microorganisms contributing to the host's immunity and pathogenesis of several diseases, including acute pancreatitis[69]. Human gastrointestinal microflora is divided into three different types by the way they present themselves in the body and perform multiple functions: e.g., There are three major categories of bacteria: Physiological bacteria, that hold over 90% and are nourishing and immune-modulating; opportunistic bacteria, which are pathogenic in situations of lower immune resistance or antibiotic abuse; and pathogenic bacteria, which have lower numbers and invade difficultly[70]. Progression in AP has been more complicated by gastrointestinal motility dysfunction, which is probably related to the neuroendocrine system, hypoxia-ischemia, ischemia-reperfusion injury (IRI), inflammatory mediators, and cajal cells[71].
Since many discoveries have postulated that commensal intestinal microbiomes play a crucial role in humans' health, immune system, and homeostasis recently, there has been a surge of interest in this area of study. The overall function of the intestine in the entire mechanism of AP pathogenesis (such as in acute and critical illnesses) is essential to understand, but often it is overlooked. This pathogenesis mechanism involves several factors contributing to the loss of gut barrier function, allowing bacteria and endotoxins to translocate into the bloodstream, which is critical for generating the second inflammatory hit of AP[72]. The data of Johnson et al[73] suggests gut bacteria translocation (via hematogenous, lymphatic, and reflux) is involved in AP infection progression, which indicates the presence of a possible correlation between gut microbiota and AP infection progression. An abnormality of the gastrointestinal microbiota (dysbiosis) is associated with the systematic inflammatory response syndrome (SIRS) and a broad range of diseases[74].
When the mucosal barrier of the intestine is damaged, intestinal bacteria may migrate into the blood or to other tissues and organs, further accelerating AP[75]. In recent years, several studies have been conducted investigating changes in intestinal flora associated with AP severity. AP progression involves the abnormal release of trypsin and destruction of pancreatic tissue due to abnormal cells. Several recent studies examined the changes in intestinal flora during AP development concerning disease severity. It is observed that abnormal trypsin secretion has occurred due to AP progression and that pancreatic structure destruction leads to an abnormal pancreatic secretion, resulting in the intestinal flora and homeostasis changes[76,77].
Numerous studies have now demonstrated the function of normal gut microbes to promote healthy gut mucosa. Gut mucosal ischemia and reperfusion during AP progression can compromise the integrity of the gut barrier, causing bacterial reabsorption from the gut to other parts of the body and causing local and systemic infections[78]. Some further research findings have also revealed that intestinal mucosal barrier injury is a significant complication in many AP patients. The intestinal mucosal barrier can be destroyed by affecting intestinal inflammation and the immune response[75]. Many studies are supporting now to demonstrate that normal gut microbes play a primary role in maintaining gut mucosal integrity. However, gut mucosal ischemia and reperfusion during AP progression can damage the overall integrity of the gut barrier and lead to gut bacterial translocation to other locations, causing local and systemic infections[78]. Thus, a significant complication of an AP patient's condition involves intestinal mucosal barrier damage. This is caused by intestinal inflammation and immune response defects. Other research has also found injuries of the intestinal mucosal barrier to patients with AP[75].
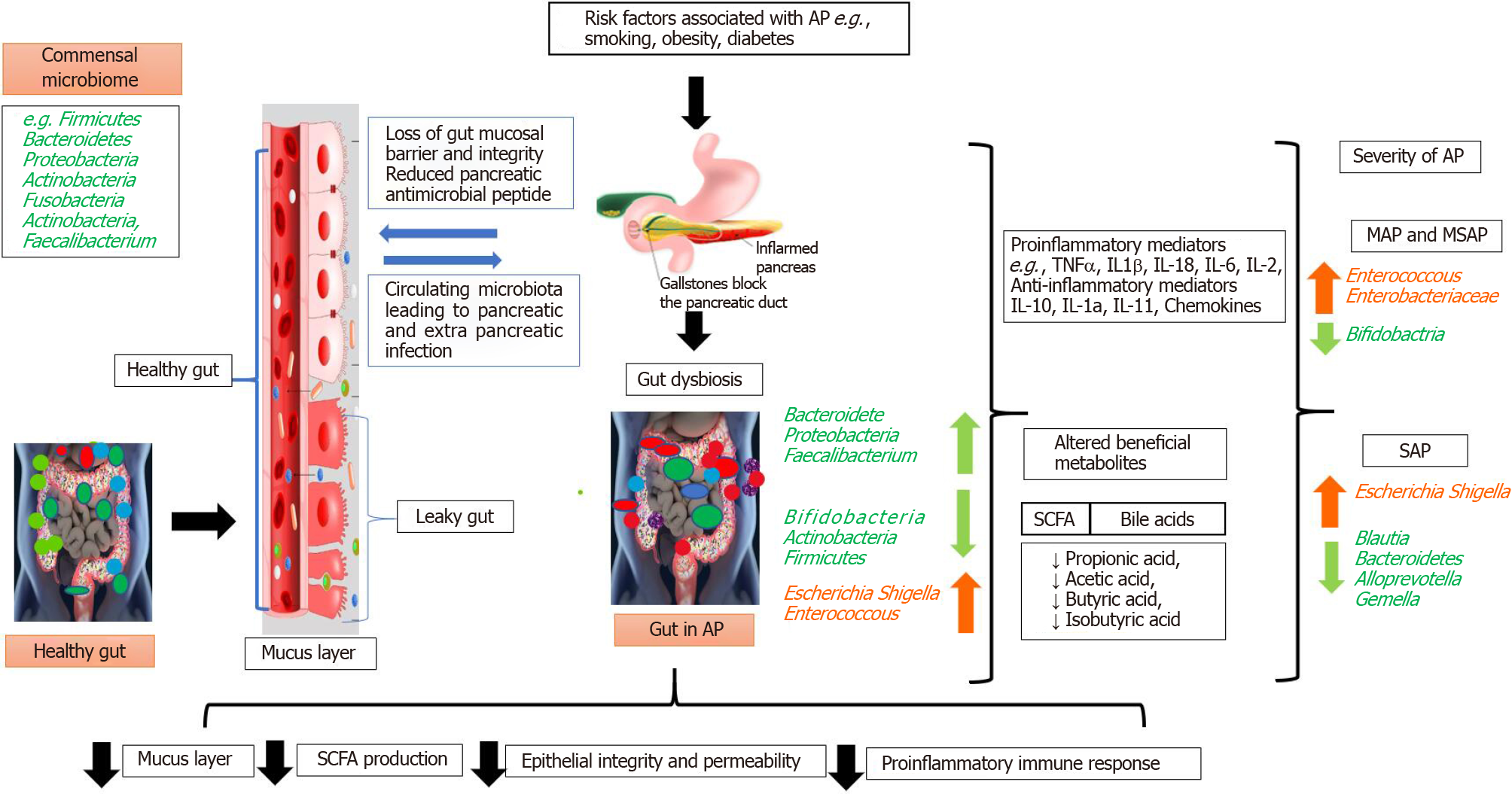
In brief, the pancreas-gut communication has been described as being in AP, with bacterial translocation as a possible consequence and the homeostatic host response noted. Translocation of bacteria from the lower gastrointestinal tract occurs via the portal circulation - the oral course and/or the mesenteric lymph nodes. The acinar cells of the pancreas secrete pancreatic antimicrobial peptides (AMPs). AMPs have homeostatic bidirectional communication with the gastrointestinal tract[79]. The lower level of the microbiome in the gastrointestinal tract may increase pancreatic antimicrobial peptide production by short-chain fatty acid metabolites. Consequently, it induces a pancreatic immunoregulatory environment which decreases proinflammatory immune cells. Conversely, decreased antimicrobial peptide production facilitates the overgrowth of the gastrointestinal microbiota leading to the induction of proinflammatory immune cells. Thus, it subsequently alters the gut microbiome and the intestinal immune system[79].
Metagenomics and next-generation sequencing have facilitated the investigation of the involvement of the gut microbiome in human physiology and various diseases. The findings have allowed for consideration of the gut microbiome as a hidden organ[80]. The close interaction of the gut microbiome with host physiology can account for the harmful effects of disruptions in the former caused by various internal or external events that initiate inflammatory conditions and some types of cancer. Therefore, it is essential that specific microbial signals maintain the host immune response and other physiological functions that protect against pathogens[81].
Injury of the microcirculation and hypovolemia that occur during AP can lead to gut mucosal ischemia and reperfusion injury that result in loss of gut barrier function. Subsequent translocation of gut bacteria can result in local pancreatic and systemic infections[82]. Leading causes of AP mortality include pancreatic infection and peripancreatic necrosis[78]. The initial onset of cerulean-driven AP depends on NOD1 activation in acinar cells by commensal microbiota that have translocated from the gut. Following activation, NOD1 induces the expression of inflammatory mediators[83]. The role of the gut in neutrophil priming and release of proinflammatory cytokines is important for the initiation and propagation of inflammation and sepsis[84]. The loss of gut barrier function has been implicated in the pathogenesis of AP-related infections. Ahuja et al[76] reported that secretion of antimicrobials from pancreatic acinar cells regulated gut microbiota composition and innate immunity[76]. Blocking acinar cell exocytosis in mice has been found to lead to gut dysbiosis, inflammation, systemic bacterial translocation, and ultimately, death. Additional evidence has revealed additional examples of crosstalk between pancreas acinar cells and the gut microbiome[76,77].
An ongoing investigation of the microbiota and AP has shown that microcirculatory disturbances associated with the loss of fluid into the “third space,” lead to hypovolemia, ischemia, and reperfusion injury in AP patients. The gut is affected by AP, but it is not a passive victim because it plays an active role in the worsening of the illness[85]. In addition to bacterial translocation, the translocation of inflammatory compounds produced in the intestinal wall and the gut's toxic products might also be responsible for initiating SIRS and distant organ injury in AP patients[86]. The contribution of the gut microbiome for protection against pathogens in AP patients has not been clearly elucidated. An increase of such pathogenic bacteria as Enterobacteriaceae and Firmicutes, and a decrease in beneficial bacteria like Bacteroidetes and Lactobacillus has been observed in AP patients[87]. Furthermore, increased serum IL-6 has been found to be positively related to increased Enterobacteriaceae and Enterococcus and inversely related to Bifidobacterium and Clostridium cluster number. Tan et al[87] reported that the extent of gut microbiota modification predicted pancreatitis severity and the occurrence of systemic complications. Gerritsen et al[88] found that "AP-associated microbiota” replaced the normal intestinal flora in a study performed in a mouse model. In AP, changes in the populations of specific commensal bacteria have been associated with reduced levels of the inflammatory cytokines IL-1b, TNF-a, CXCL1, and IL-18, and inversely correlated with pancreatitis severity and the occurrence of systemic infectious complications. The evidence highlights the resto
The 16S rRNA gene is highly conserved in bacteria, and it is highly species-specific. Consequently, 16S rRNA gene sequencing is widely used to study the gut microbiota in various disease states[90]. Zhu et al[69] reported that the relative abundance of commensal microbiota in AP patients differed from that in a healthy individual. Members of the Bacteroidetes phylum decreased significantly, but Proteobacteria were over-represented in AP. AP patients also had a relative overabundance of Escherichia/ Shigella compared to healthy control[69]. Increased abundance of two common opportunistic pathogens, including Enterococcus and an unknown genus in the Enterobacteriaceae family, were also observed in AP patients. Linear discrimination and effect size analysis revealed significant increases in Acinetobacter, Stenotrophomonas, and Geobacillus with decreased Bacteroides, Alloprevotella, Blautia and Gemella in patients with severe AP, as compared to those with mild and moderately severe AP[69]. Table 5[21,69,75,91] and Table 6[69,75,87,90,92] summarize the significant changes in the microbiome composition of healthy controls and in patients with mild, moderately severe, and severe AP.
| No. | Techniques used for microbiome profiling | Healthy control | Acute pancreatitis | Ref. |
| 1 | qPCR (Fecal samples) | Firmicutes↑ | Firmicutes↓ | [84] |
| Bacteroidetes↓ | Bacteroidetes↑ | |||
| Proteobacteria↓ | Proteobacteria↑ | |||
| Actinobacteria↑ | Actinobacteria↓ | |||
| Tenericutes↓ | Tenericutes↑ | |||
| 2 | 16S rRNA gene sequencing (Fecal samples) | Proteobacteria↓ | Bacteroidetes↓ | [83] |
| Proteobacteria↑ | ||||
| Escherichia/Shigella↑ | ||||
| Enterococcus↑ | ||||
| Enterobacteriaceae↑ | ||||
| Prevotella↓ | ||||
| Faecalibacterium↓ | ||||
| Bifidobacterium↓ | ||||
| 3 | 16S rRNA gene sequencing (Fecal samples) | NA | Bacteroidetes↑ | [85] |
| Proteobacteria↑ | ||||
| Firmicutes↓ | ||||
| Actinobacteria↓ | ||||
| 4 | 16S rRNA gene sequencing (Fecal samples) | NA | Enterobacteriaceae↑ | [21] |
| Enterococcus↑ | ||||
| Bifidobacteria↓ |
| No. | Techniques used for microbiome profiling | MAP | MSAP | SAP | Ref. |
| 1 | qPCR (Fecal samples), performed only on MAP and SAP patients | Enterococcus↑ | NA | Enterococcus↑ | [79] |
| Enterobacteriaceae↑ | Enterobacteriaceae↑ | ||||
| Bifidobacterium↓ | Bifidobacterium↓ | ||||
| 2 | 16S rRNA gene sequencing (Fecal samples) | Finegoldia↑ | NA | Acinetobacter↑ | [83] |
| Stentrophomonas↑ | |||||
| Geobacillus↑ | |||||
| Bacteroides↓ | |||||
| Alloprevotella↓ | |||||
| Blautia↓ | |||||
| Gemella↓ | |||||
| 3 | 16S rRNA sequencing (Fecal sample) | Enterobacteriaceae↑ | NA | Enterobacteriaceae↑ | [85] |
| Enterococcus↑ | Enterococcus↑ | ||||
| Bifidobacterium↓ | Bifidobacterium↓ | ||||
| Blautia↓ | |||||
| 4 | 16S rRNA gene sequencing (Rectal swab) | Bacteroides↑ | Bacteroides↑ | Bacteroides↑ | [82] |
| Escherichia/Shigella↑ | Escherichia/Shigella↑ | Escherichia/Shigella↑ | |||
| Enterococcus↑ | Enterococcus↑ | Enterococcus↑ | |||
| Eubacterium hallii↓ | |||||
| Finegoldia↑ | Anaerococcus↑ | Acinetobacter↓ | |||
| Stenotrophomonas↓ | |||||
| Blautia↓ | Eubacterium hallii↓ | Bacteroides↓ | |||
| Blautia↓ | |||||
| 5 | Shotgun metagenomics (Fecal sample) | Thermoprotei↑ | Sulfolobus↑ | Sulfolobus↑ | [86] |
| Crenarchaeota↑ | Methanobrevibacter ruminantium↑ | Methanomicrobiales - archaeon 53_19↑ | |||
| Streptococcus↑ | Methanosarcina - Thermophila↑ | Enterococcus↑ | |||
| Anaerostipes hadrus↓ | Anaerostipes hadrus↓ | Blautia↓ | |||
| Escherichia coli↑ |
Changes in the gut microbiota in AP include overexpression of opportunistic pathogens, such as Escherichia/Shigella, and reduced abundance of beneficial genera, such as Bifidobacterium[69]. It has been hypothesized that a reduction of beneficial bacteria might facilitate microbial translocation across a damaged gut barrier, thereby promoting the progression of AP. Studies performed by Li et al[75] and other investigators[93,94] found that dysbiosis that included the depletion of short-chain fatty acid-producing bacteria was associated with an impaired gut barrier and worsening of AP. Changes in the gut microbiome may thus serve as a diagnostic tool in AP. Restoring gut microbiota homeostasis and stabilizing the gut barrier might have therapeutic value in AP patients, as shown in Figure 2[21,30,69,75,87,93,94]. The overall literature suggests that there is an association between the gut microbiome and the severity of AP[95]. Additionally, in experimental acute pancreatitis, changes to the gut microbiome, e.g., administration of Clostridium butyricum can suppress AP[27] pointing to the therapeutic potential of this approach is promising.
In the past decade, substantial advancements have been made not only in under
The use of antibiotics, starting with carbapenems, quinolones, and metronidazole, has been advised in patients with AP and concomitant cholangitis symptoms, infected necrosis, or necrotizing pancreatitis accompanying a deteriorating clinical status[97]. Delaying surgical interventions decreases morbidity and mortality[100]. In some instances of AP, where infection is clinically suspected or confirmed, the use of antibiotics is recommended to avoid development of antimicrobial resistance. The predictive value of fine-needle aspiration for sampling and determination of bacterial sensitivities in diagnosing peri-pancreatic infection is comparable to that of clinical signs and imaging. The routine use of fine-needle aspiration is not recommended[101].
Early enteral nutrition is recommended in AP because it protects mucosal nutrition, the gut mucosal barrier, and gut-pancreas homeostasis[102]. In a previous randomized trial, decontamination of the gut with norfloxacin, colistin, amphotericin and standard AP therapy did not reduce mortality[103]. At present, selective gut decontamination cannot be recommended for AP patients.
Despite constant improvement in targeted therapeutics, a third of adult AP patients develop moderately severe AP and/or severe AP with SIRS, organ failure, and an increased risk of infection[104]. The gut mucosal barrier reduces the risk of infected pancreatic necrosis and thus helps to decrease mortality risk[85,105]. Microbiome-targeted therapies, such as genetic engineering of modified strains to outcompete pathogens, selective nutrient or prebiotic supplementation, or engineered bacteriophages, could steer the altered microbiome toward a healthy phenotype or change the course of critical illness[81]. Probiotics offer substantial health benefits and support the homeostasis of gut flora[81]. The most widely used probiotic bacteria in clinical trials are Lactobacillus and Bifidobacterium, which are easy to isolate from human feces or the intestinal mucosa. Prebiotics are nondigestible foods required to propagate probiotics, and they stimulate the growth and activity of the healthy gut flora. Synbiotics are nutritional supplements that include both probiotics and prebiotics[106].
A randomized controlled trial by Pan et al[107] evaluated the ability of synbiotics to restore intestinal barrier damage and reduce the infection rate in early AP. A group of 45 patients were given either live or heat-inactivated Lactobacillus plantarum 299 with an oat fiber supplement as early enteral nutrition. Supplementation plus the symbiotic significantly reduced both pancreatic necrosis and surgical interventions[107]. A subsequent clinical trial included 62 severe AP patients treated with early enteral nutrition with four different prebiotics (inulin, beta-glucan, resistant starch, and pectin) together with four Lactobacillus probiotic preparations. The treatment resulted in a reduced incidence of SIRS and organ failure, supporting the use of early enteral symbiotic nutrition in severe AP[108]. Olah et al[109] randomized 45 AP patients to receive either a freeze-dried preparation containing 109 live L. plantarum 299 in each dose together with oat fiber or a heat-inactivated Lactobacillus controlled by nasojejunal tube for one week. Infected pancreatic necrosis and abscesses were significantly lower in the treatment group than in the control group.
Other experimental pancreatitis studies in rat models confirmed the efficacy of L. plantarum spp. in reducing microbial translocation and as a possible alternative to antimicrobials[110]. Several studies have shown that probiotics containing Faecalibacterium and Bifidobacterium species had beneficial effects, including stabilizing the gut barrier, increasing anti-inflammatory responses, and attenuating bacterial translocation[111,112]. Some studies have not found any significant benefit or adverse effect of probiotics in severe AP. Still, it is essential to note the considerable patient and probiotic regimen heterogeneity in the published clinical trials of probiotics. The PROPATRIA Probiotics[113] in Pancreatitis Trial randomized 298 patients with predicted severe AP to either a multispecies probiotic mixture containing two different Bifidobacterium, three Lactobacillus, and one Lactococcus species or a placebo. The infectious complications in the two groups were similar, but the probiotic group had higher mortality (16% vs 6%) and incidence of bowel ischemia (6% vs 0%) compared with the placebo group. The high load of the probiotic mixture used in the study was thought to have been responsible for the increased mortality[114]. The findings highlight the challenges of supplementing the gut microbiome with beneficial microbial species in the setting of AP. Nevertheless, eight years later, the PROPATRIA trial was reevaluated by Bongaerts et al[115]. The team of researchers analyzed and addressed all shortcomings identified in the trial. PROPATRIA researchers contend that a lethal combination of predominantly proteolytic pancreatic enzymes and probiotic therapy was responsible for the high mortality rate, and that elevated levels of lactic acid produced by bacterial fermentation of carbohydrates significantly contributed to the high death rate. Additionally, one of them was the latency time in the first administration of probiotics; indeed, some patients were treated 24 h after onset of symptoms. Furthermore, there were errors in randomization; in fact, the onset of multi-organ failure was already present during admission in more patients in the first group than in the placebo group (41 patients vs 23 patients). Finally, last but not least, the team of researchers suggested that in future studies, when considering substituting probiotics in AP, it is necessary to assess the appropriate, effective doses of probiotics. However, caution should be mandatory to prevent bacterial overgrowth while conducting clinical trials in AP patients.
Previous studies have shown that L. plantarum decreased the occurrence of infective necrosis in AP patients[110] and that Saccharomyces boulardi spp. administered concomitantly with antibiotics such as ciprofloxacin decreased histopathologic scores in acute necrotizing pancreatitis[111]. Animal studies have provided strong evidence in support of probiotic benefits in animal models of AP. A mixture of Lactobacillus acidophilus, Streptococcus thermophilus, and Bifidobacterium lactis given by oral gavage reduced pancreatitis, bacterial translocation to extra-intestinal sites, and mortality in male albino rats because of reduced duodenal bacterial overgrowth[112].
Injury of the GI barrier is a key event in the development of AP. Few studies have reported prevention of disruption of the intestinal barrier with modulation of gut microbiome balance. In one such study conducted in a mouse model of AP, Clostridium butyricum, a producer of small-chain fatty acids, which have immunomodulatory properties, reduced infiltration of neutrophils and dendritic cells in the pancreas and inhibited inflammatory responses mediated by NLRP3 and TLR4 signaling pathways in the pancreas and colon[27]. In summary, probiotics help to maintain gut homeostasis. Research should improve the designs of future studies, for example, by detecting a peculiar strain of microorganisms (i.e., their type), standardizing the dose and duration of treatment, or standardizing the state of disease progression when considering to use in current therapy scenarios.
The gut microbiome plays a significant role in health and diseases. The resident microbiota in the human GI tract influences host metabolism, physiology, and immune system development. Disruption of this bacterial community results in GI disease. Ongoing medical and clinical research has produced a substantial body of evidence of a clear correlation between changes in the commensal microbiota and the occurrence of pancreatic disease. Application of biochemical, microbiological, and molecular biological methods have provided a description of the constituents of the gut microbiome in health and disease, their niches, and their physiological roles. Additional study is needed to explain whether microbial dysbiosis is a cause or an effect of diverse pathologies. The microbiome profile and changes in dysbiosis may influence an increase in AP severity during its clinical course.
Damage of the intestinal mucosal barrier allows migration of intestinal microbes to the blood or other tissues and organs, which enhances or aggravates AP. Changes in the resident species and abundance of the intestinal flora during AP are closely related to damage of the intestinal mucosal barrier system. Regulating the intestinal flora to repair the intestinal mucosal barrier and restore its function may be useful in AP treatment. Changes in the gut microbiota composition in AP include over-representation of opportunistic pathogens such as Escherichia/Shigella species and a significant decrease in the beneficial Bifidobacterium genus. Early dysbiosis of the gut microbiota, especially the depletion of small-chain fatty acid-producing bacteria, is probably associated with impairment of the gut barrier and increased AP severity.
The mechanisms of gut dysbiosis and the etiology of AP are not yet fully understood. The relationship of GI microbial symbiosis and AP are avenues for further research. The concomitant use of probiotics and antibiotics together with conventional treatment, such as surgery, radiotherapy, chemotherapy and targeted therapies, are further areas for research. In summary, the clinical significance of GI homeostasis during AP is emerging step by step. Thus, restoring the homeostasis of gut microbiota and stabilizing the gut barrier could be a promising therapeutic target in preventing AP progression. Challenges and specific problems that stand in the way to developing a research platform for understanding AP and its interaction with the microbiome need to be overcome. This review has explored the role of the gut microbiome in AP and the targeted use of probiotics, prebiotics, and synbiotics to maintain or restore GI microbial balance.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Singapore
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sikiric P, Song B S-Editor: Ma YJ L-Editor: A P-Editor: Liu JH
| 1. | Bäckhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915-1920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3394] [Cited by in RCA: 3546] [Article Influence: 177.3] [Reference Citation Analysis (5)] |
| 2. | Zhu B, Wang X, Li L. Human gut microbiome: the second genome of human body. Protein Cell. 2010;1:718-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 308] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 3. | Lucas López R, Grande Burgos MJ, Gálvez A, Pérez Pulido R. The human gastrointestinal tract and oral microbiota in inflammatory bowel disease: a state of the science review. APMIS. 2017;125:3-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 83] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 4. | Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, Mende DR, Li J, Xu J, Li S, Li D, Cao J, Wang B, Liang H, Zheng H, Xie Y, Tap J, Lepage P, Bertalan M, Batto JM, Hansen T, Le Paslier D, Linneberg A, Nielsen HB, Pelletier E, Renault P, Sicheritz-Ponten T, Turner K, Zhu H, Yu C, Jian M, Zhou Y, Li Y, Zhang X, Qin N, Yang H, Wang J, Brunak S, Doré J, Guarner F, Kristiansen K, Pedersen O, Parkhill J, Weissenbach J; MetaHIT Consortium; Bork P, Ehrlich SD. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59-65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9101] [Cited by in RCA: 7844] [Article Influence: 522.9] [Reference Citation Analysis (4)] |
| 5. | Schmitt FCF, Brenner T, Uhle F, Loesch S, Hackert T, Ulrich A, Hofer S, Dalpke AH, Weigand MA, Boutin S. Gut microbiome patterns correlate with higher postoperative complication rates after pancreatic surgery. BMC Microbiol. 2019;19:42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 6. | Malla MA, Dubey A, Kumar A, Yadav S, Hashem A, Abd Allah EF. Exploring the Human Microbiome: The Potential Future Role of Next-Generation Sequencing in Disease Diagnosis and Treatment. Front Immunol. 2018;9:2868. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 175] [Cited by in RCA: 178] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 7. | Sanger F, Coulson AR. A rapid method for determining sequences in DNA by primed synthesis with DNA polymerase. J Mol Biol. 1975;94:441-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1551] [Cited by in RCA: 1326] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 8. | Fraher MH, O'Toole PW, Quigley EM. Techniques used to characterize the gut microbiota: a guide for the clinician. Nat Rev Gastroenterol Hepatol. 2012;9:312-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 235] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 9. | Ianiro G, Tilg H, Gasbarrini A. Antibiotics as deep modulators of gut microbiota: between good and evil. Gut. 2016;65:1906-1915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 443] [Article Influence: 49.2] [Reference Citation Analysis (0)] |
| 10. | Blaser MJ. Antibiotic use and its consequences for the normal microbiome. Science. 2016;352:544-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 470] [Cited by in RCA: 563] [Article Influence: 62.6] [Reference Citation Analysis (0)] |
| 11. | Pamer EG. Resurrecting the intestinal microbiota to combat antibiotic-resistant pathogens. Science. 2016;352:535-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 278] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 12. | Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207-214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9292] [Cited by in RCA: 8068] [Article Influence: 620.6] [Reference Citation Analysis (2)] |
| 13. | Fischer M. Recent Research on Fecal Microbiota Transplantation in Inflammatory Bowel Disease Patients. Gastroenterol Hepatol (NY). 2019;15:44-47. [PubMed] |
| 14. | Wang Z, Xu CM, Liu YX, Wang XQ, Zhang L, Li M, Zhu SW, Xie ZJ, Wang PH, Duan LP, Zhu HQ. Characteristic dysbiosis of gut microbiota of Chinese patients with diarrhea-predominant irritable bowel syndrome by an insight into the pan-microbiome. Chin Med J (Engl). 2019;132:889-904. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 15. | Kwong TNY, Wang X, Nakatsu G, Chow TC, Tipoe T, Dai RZW, Tsoi KKK, Wong MCS, Tse G, Chan MTV, Chan FKL, Ng SC, Wu JCY, Wu WKK, Yu J, Sung JJY, Wong SH. Association Between Bacteremia From Specific Microbes and Subsequent Diagnosis of Colorectal Cancer. Gastroenterology 2018; 155: 383-390. e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 201] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 16. | Agahi A, Hamidi GA, Daneshvar R, Hamdieh M, Soheili M, Alinaghipour A, Esmaeili Taba SM, Salami M. Does Severity of Alzheimer's Disease Contribute to Its Responsiveness to Modifying Gut Microbiota? Front Neurol. 2018;9:662. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 161] [Article Influence: 23.0] [Reference Citation Analysis (1)] |
| 17. | Tang WH, Kitai T, Hazen SL. Gut Microbiota in Cardiovascular Health and Disease. Circ Res. 2017;120:1183-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1046] [Cited by in RCA: 1102] [Article Influence: 137.8] [Reference Citation Analysis (0)] |
| 18. | Liu Z, Wang N, Ma Y, Wen D. Hydroxytyrosol Improves Obesity and Insulin Resistance by Modulating Gut Microbiota in High-Fat Diet-Induced Obese Mice. Front Microbiol. 2019;10:390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 68] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 19. | Zhang B, Yue R, Chen Y, Yang M, Huang X, Shui J, Peng Y, Chin J. Gut Microbiota, a Potential New Target for Chinese Herbal Medicines in Treating Diabetes Mellitus. Evid Based Complement Alternat Med. 2019;2019:2634898. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 20. | Wang Y, Yang G, You L, Yang J, Feng M, Qiu J, Zhao F, Liu Y, Cao Z, Zheng L, Zhang T, Zhao Y. Role of the microbiome in occurrence, development and treatment of pancreatic cancer. Mol Cancer. 2019;18:173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 77] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 21. | Akshintala VS, Talukdar R, Singh VK, Goggins M. The Gut Microbiome in Pancreatic Disease. Clin Gastroenterol Hepatol. 2019;17:290-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 79] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 22. | Peery AF, Dellon ES, Lund J, Crockett SD, McGowan CE, Bulsiewicz WJ, Gangarosa LM, Thiny MT, Stizenberg K, Morgan DR, Ringel Y, Kim HP, DiBonaventura MD, Carroll CF, Allen JK, Cook SF, Sandler RS, Kappelman MD, Shaheen NJ. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology 2012; 143: 1179-1187. e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1355] [Cited by in RCA: 1467] [Article Influence: 112.8] [Reference Citation Analysis (1)] |
| 23. | Silva-Vaz P, Abrantes AM, Castelo-Branco M, Gouveia A, Botelho MF, Tralhão JG. Murine Models of Acute Pancreatitis: A Critical Appraisal of Clinical Relevance. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 24. | Lee PJ, Papachristou GI. New insights into acute pancreatitis. Nat Rev Gastroenterol Hepatol. 2019;16:479-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 516] [Article Influence: 86.0] [Reference Citation Analysis (0)] |
| 25. | Afghani E, Pandol SJ, Shimosegawa T, Sutton R, Wu BU, Vege SS, Gorelick F, Hirota M, Windsor J, Lo SK, Freeman ML, Lerch MM, Tsuji Y, Melmed GY, Wassef W, Mayerle J. Acute Pancreatitis-Progress and Challenges: A Report on an International Symposium. Pancreas. 2015;44:1195-1210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 26. | Roberts SE, Akbari A, Thorne K, Atkinson M, Evans PA. The incidence of acute pancreatitis: impact of social deprivation, alcohol consumption, seasonal and demographic factors. Aliment Pharmacol Ther. 2013;38:539-548. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 143] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 27. | Pan LL, Niu W, Fang X, Liang W, Li H, Chen W, Zhang H, Bhatia M, Sun J. Clostridium butyricum Strains Suppress Experimental Acute Pancreatitis by Maintaining Intestinal Homeostasis. Mol Nutr Food Res. 2019;e1801419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 28. | Bhatia M, Kumar R. Cells and Mediators of Inflammation in Acute Pancreatitis. Clin Anti-Inflammatory Anti-Allergy Drugs. 2014;1:11-23. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 29. | Vitali F, Ikeura T, Amodio A, Benini L, Vantini I, Frulloni L. Pathophysiology of acute damage. In: Acute and Chronic Pancreatitis: New concepts and evidence-based approaches. Testoni PA, Mariani A, Arcidiacono PG (Eds). Turin, Italy: Edizioni Minerva Medica, 2013: 1-10. [DOI] [Full Text] |
| 30. | Silva-Vaz P, Abrantes AM, Castelo-Branco M, Gouveia A, Botelho MF, Tralhão JG. Multifactorial Scores and Biomarkers of Prognosis of Acute Pancreatitis: Applications to Research and Practice. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 82] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 31. | Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4932] [Cited by in RCA: 4342] [Article Influence: 361.8] [Reference Citation Analysis (45)] |
| 32. | Xu XD, Wang ZY, Zhang LY, Ni R, Wei FX, Han W, Zhang HH, Zhang YW, Wei ZG, Guo XH, Guo LQ, Ma JZ, Zhang YC. Acute Pancreatitis Classifications: Basis and Key Goals. Medicine (Baltimore). 2015;94:e2182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 33. | Mikó A, Vigh É, Mátrai P, Soós A, Garami A, Balaskó M, Czakó L, Mosdósi B, Sarlós P, Erőss B, Tenk J, Rostás I, Hegyi P. Computed Tomography Severity Index vs. Other Indices in the Prediction of Severity and Mortality in Acute Pancreatitis: A Predictive Accuracy Meta-analysis. Front Physiol. 2019;10:1002. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 64] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 34. | Li M, Xing XK, Lu ZH, Guo F, Su W, Lin YJ, Wang DH. Comparison of Scoring Systems in Predicting Severity and Prognosis of Hypertriglyceridemia-Induced Acute Pancreatitis. Dig Dis Sci. 2020;65:1206-1211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 35. | Hagjer S, Kumar N. Evaluation of the BISAP scoring system in prognostication of acute pancreatitis - A prospective observational study. Int J Surg. 2018;54:76-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 81] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 36. | Buxbaum J, Quezada M, Chong B, Gupta N, Yu CY, Lane C, Da B, Leung K, Shulman I, Pandol S, Wu B. The Pancreatitis Activity Scoring System predicts clinical outcomes in acute pancreatitis: findings from a prospective cohort study. Am J Gastroenterol. 2018;113:755-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 37. | Kiat TTJ, Gunasekaran SK, Junnarkar SP, Low JK, Woon W, Shelat VG. Are traditional scoring systems for severity stratification of acute pancreatitis sufficient? Ann Hepatobiliary Pancreat Surg. 2018;22:105-115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 38. | Harshit Kumar A, Singh Griwan M. A comparison of APACHE II, BISAP, Ranson's score and modified CTSI in predicting the severity of acute pancreatitis based on the 2012 revised Atlanta Classification. Gastroenterol Rep (Oxf). 2018;6:127-131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 104] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 39. | Khanna AK, Meher S, Prakash S, Tiwary SK, Singh U, Srivastava A, Dixit VK. Comparison of Ranson, Glasgow, MOSS, SIRS, BISAP, APACHE-II, CTSI Scores, IL-6, CRP, and Procalcitonin in Predicting Severity, Organ Failure, Pancreatic Necrosis, and Mortality in Acute Pancreatitis. HPB Surg. 2013;2013:367581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 163] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 40. | Papachristou GI, Papachristou DJ, Avula H, Slivka A, Whitcomb DC. Obesity increases the severity of acute pancreatitis: performance of APACHE-O score and correlation with the inflammatory response. Pancreatology. 2006;6:279-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 160] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 41. | Wu BU, Johannes RS, Sun X, Tabak Y, Conwell DL, Banks PA. The early prediction of mortality in acute pancreatitis: a large population-based study. Gut. 2008;57:1698-1703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 520] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 42. | Rau B, Steinbach G, Gansauge F, Mayer JM, Grünert A, Beger HG. The potential role of procalcitonin and interleukin 8 in the prediction of infected necrosis in acute pancreatitis. Gut. 1997;41:832-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 233] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 43. | Arabul M, Celik M, Aslan O, Torun S, Beyazit Y, Alper E, Kandemir A, Ünsal B. Hepcidin as a predictor of disease severity in acute pancreatitis: a single center prospective study. Hepatogastroenterology. 2013;60:595-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 44. | Zhou H, Mei X, He X, Lan T, Guo S. Severity stratification and prognostic prediction of patients with acute pancreatitis at early phase: A retrospective study. Medicine (Baltimore). 2019;98:e15275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 88] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 45. | Woo SM, Noh MH, Kim BG, Hsing CT, Han JS, Ryu SH, Seo JM, Yoon HA, Jang JS, Choi SR, Cho JH. Comparison of serum procalcitonin with Ranson, APACHE-II, Glasgow and Balthazar CT severity index scores in predicting severity of acute pancreatitis. Korean J Gastroenterol. 2011;58:31-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 46. | de-Madaria E, Molero X, Bonjoch L, Casas J, Cárdenas-Jaén K, Montenegro A, Closa D. Oleic acid chlorohydrin, a new early biomarker for the prediction of acute pancreatitis severity in humans. Ann Intensive Care. 2018;8:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 47. | Cardoso FS, Ricardo LB, Oliveira AM, Canena JM, Horta DV, Papoila AL, Deus JR. C-reactive protein prognostic accuracy in acute pancreatitis: timing of measurement and cutoff points. Eur J Gastroenterol Hepatol. 2013;25:784-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 48. | Yang Y, Huang Q, Luo C, Wen Y, Liu R, Sun H, Tang L. MicroRNAs in acute pancreatitis: From pathogenesis to novel diagnosis and therapy. J Cell Physiol. 2020;235:1948-1961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 49. | Blenkiron C, Askelund KJ, Shanbhag ST, Chakraborty M, Petrov MS, Delahunt B, Windsor JA, Phillips AR. MicroRNAs in mesenteric lymph and plasma during acute pancreatitis. Ann Surg. 2014;260:341-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 50. | Liu P, Xia L, Zhang WL, Ke HJ, Su T, Deng LB, Chen YX, Lv NH. Identification of serum microRNAs as diagnostic and prognostic biomarkers for acute pancreatitis. Pancreatology. 2014;14:159-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 51. | Kuśnierz-Cabala B, Nowak E, Sporek M, Kowalik A, Kuźniewski M, Enguita FJ, Stępień E. Serum levels of unique miR-551-5p and endothelial-specific miR-126a-5p allow discrimination of patients in the early phase of acute pancreatitis. Pancreatology. 2015;15:344-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 52. | Lu P, Wang F, Wu J, Wang C, Yan J, Li ZL, Song JX, Wang JJ. Elevated Serum miR-7, miR-9, miR-122, and miR-141 Are Noninvasive Biomarkers of Acute Pancreatitis. Dis Markers. 2017;2017:7293459. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 53. | Zhang XX, Deng LH, Chen WW, Shi N, Jin T, Lin ZQ, Ma Y, Jiang K, Yang XN, Xia Q. Circulating microRNA 216 as a Marker for the Early Identification of Severe Acute Pancreatitis. Am J Med Sci. 2017;353:178-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 54. | Zhang Y, Yan L, Han W. Elevated Level of miR-551b-5p is Associated With Inflammation and Disease Progression in Patients With Severe Acute Pancreatitis. Ther Apher Dial. 2018;22:649-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 55. | Wang D, Tang M, Zong P, Liu H, Zhang T, Liu Y, Zhao Y. MiRNA-155 Regulates the Th17/Treg Ratio by Targeting SOCS1 in Severe Acute Pancreatitis. Front Physiol. 2018;9:686. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 73] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 56. | Gao M, Bian E, Li H, Ge W, Yin C, Wang H, Sun Y. [Up-regulation of circulating miR-29a in patients with acute pancreatitis and is positively correlated with disease severity and poor prognosis]. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2018;34:931-936. [PubMed] |
| 57. | An F, Zhan Q, Xia M, Jiang L, Lu G, Huang M, Guo J, Liu S. From moderately severe to severe hypertriglyceridemia induced acute pancreatitis: circulating miRNAs play role as potential biomarkers. PLoS One. 2014;9:e111058. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 58. | Lu XG, Kang X, Zhan LB, Kang LM, Fan ZW, Bai LZ. Circulating miRNAs as biomarkers for severe acute pancreatitis associated with acute lung injury. World J Gastroenterol. 2017;23:7440-7449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 59. | Liu S, Zou H, Wang Y, Duan X, Chen C, Cheng W, Wang L, Ning N, Tang H, Chen M, Mao X, Peng C, Li H, Jiang Y, Jiang B. miR-155-5p is Negatively Associated with Acute Pancreatitis and Inversely Regulates Pancreatic Acinar Cell Progression by Targeting Rela and Traf3. Cell Physiol Biochem. 2018;51:1584-1599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 60. | Shi N, Deng L, Chen W, Zhang X, Luo R, Jin T, Ma Y, Du C, Lin Z, Jiang K, Guo J, Yang X, Xia Q. Is MicroRNA-127 a Novel Biomarker for Acute Pancreatitis with Lung Injury? Dis Markers. 2017;2017:1204295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 61. | Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006;124:837-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2162] [Cited by in RCA: 2281] [Article Influence: 120.1] [Reference Citation Analysis (0)] |
| 62. | Guinane CM, Cotter PD. Role of the gut microbiota in health and chronic gastrointestinal disease: understanding a hidden metabolic organ. Therap Adv Gastroenterol. 2013;6:295-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 499] [Cited by in RCA: 571] [Article Influence: 47.6] [Reference Citation Analysis (0)] |
| 63. | Ley RE, Hamady M, Lozupone C, Turnbaugh PJ, Ramey RR, Bircher JS, Schlegel ML, Tucker TA, Schrenzel MD, Knight R, Gordon JI. Evolution of mammals and their gut microbes. Science. 2008;320:1647-1651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2974] [Cited by in RCA: 2554] [Article Influence: 150.2] [Reference Citation Analysis (0)] |
| 64. | Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7222] [Cited by in RCA: 6411] [Article Influence: 337.4] [Reference Citation Analysis (0)] |
| 65. | Zhang H, DiBaise JK, Zuccolo A, Kudrna D, Braidotti M, Yu Y, Parameswaran P, Crowell MD, Wing R, Rittmann BE, Krajmalnik-Brown R. Human gut microbiota in obesity and after gastric bypass. Proc Natl Acad Sci USA. 2009;106:2365-2370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1323] [Cited by in RCA: 1402] [Article Influence: 87.6] [Reference Citation Analysis (1)] |
| 66. | Kau AL, Ahern PP, Griffin NW, Goodman AL, Gordon JI. Human nutrition, the gut microbiome and the immune system. Nature. 2011;474:327-336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2116] [Cited by in RCA: 1814] [Article Influence: 129.6] [Reference Citation Analysis (0)] |
| 67. | Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, Liang S, Zhang W, Guan Y, Shen D, Peng Y, Zhang D, Jie Z, Wu W, Qin Y, Xue W, Li J, Han L, Lu D, Wu P, Dai Y, Sun X, Li Z, Tang A, Zhong S, Li X, Chen W, Xu R, Wang M, Feng Q, Gong M, Yu J, Zhang Y, Zhang M, Hansen T, Sanchez G, Raes J, Falony G, Okuda S, Almeida M, LeChatelier E, Renault P, Pons N, Batto JM, Zhang Z, Chen H, Yang R, Zheng W, Yang H, Wang J, Ehrlich SD, Nielsen R, Pedersen O, Kristiansen K. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490:55-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3971] [Cited by in RCA: 4832] [Article Influence: 371.7] [Reference Citation Analysis (1)] |
| 68. | Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci USA. 2007;104:13780-13785. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3731] [Cited by in RCA: 3432] [Article Influence: 190.7] [Reference Citation Analysis (1)] |
| 69. | Zhu Y, He C, Li X, Cai Y, Hu J, Liao Y, Zhao J, Xia L, He W, Liu L, Luo C, Shu X, Cai Q, Chen Y, Lu N. Gut microbiota dysbiosis worsens the severity of acute pancreatitis in patients and mice. J Gastroenterol. 2019;54:347-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 161] [Article Influence: 26.8] [Reference Citation Analysis (3)] |
| 70. | Cen ME, Wang F, Su Y, Zhang WJ, Sun B, Wang G. Gastrointestinal microecology: a crucial and potential target in acute pancreatitis. Apoptosis. 2018;23:377-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 71. | Eshraghian A, Eshraghian H. Interstitial cells of Cajal: a novel hypothesis for the pathophysiology of irritable bowel syndrome. Can J Gastroenterol. 2011;25:277-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 72. | Clemente JC, Manasson J, Scher JU. The role of the gut microbiome in systemic inflammatory disease. BMJ. 2018;360:j5145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 371] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 73. | Johnson CD. Antibiotic prophylaxis in severe acute pancreatitis. Br J Surg. 1996;83:883-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 74. | Swann JR, Tuohy KM, Lindfors P, Brown DT, Gibson GR, Wilson ID, Sidaway J, Nicholson JK, Holmes E. Variation in antibiotic-induced microbial recolonization impacts on the host metabolic phenotypes of rats. J Proteome Res. 2011;10:3590-3603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 107] [Article Influence: 7.6] [Reference Citation Analysis (1)] |
| 75. | Li XY, He C, Zhu Y, Lu NH. Role of gut microbiota on intestinal barrier function in acute pancreatitis. World J Gastroenterol. 2020;26:2187-2193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 69] [Cited by in RCA: 133] [Article Influence: 26.6] [Reference Citation Analysis (3)] |
| 76. | Ahuja M, Schwartz DM, Tandon M, Son A, Zeng M, Swaim W, Eckhaus M, Hoffman V, Cui Y, Xiao B, Worley PF, Muallem S. Orai1-Mediated Antimicrobial Secretion from Pancreatic Acini Shapes the Gut Microbiome and Regulates Gut Innate Immunity. Cell Metab. 2017;25:635-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 139] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 77. | Tilg H, Adolph TE. Beyond Digestion: The Pancreas Shapes Intestinal Microbiota and Immunity. Cell Metab. 2017;25:495-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 78. | Frossard JL, Steer ML, Pastor CM. Acute pancreatitis. Lancet. 2008;371:143-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 524] [Cited by in RCA: 512] [Article Influence: 30.1] [Reference Citation Analysis (1)] |
| 79. | Thomas RM, Jobin C. Microbiota in pancreatic health and disease: the next frontier in microbiome research. Nat Rev Gastroenterol Hepatol. 2020;17:53-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 218] [Article Influence: 43.6] [Reference Citation Analysis (0)] |
| 80. | O'Hara AM, Shanahan F. The gut flora as a forgotten organ. EMBO Rep. 2006;7:688-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1690] [Cited by in RCA: 1788] [Article Influence: 94.1] [Reference Citation Analysis (2)] |
| 81. | Kitsios GD, Morowitz MJ, Dickson RP, Huffnagle GB, McVerry BJ, Morris A. Dysbiosis in the intensive care unit: Microbiome science coming to the bedside. J Crit Care. 2017;38:84-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 82. | Ammori BJ, Leeder PC, King RF, Barclay GR, Martin IG, Larvin M, McMahon MJ. Early increase in intestinal permeability in patients with severe acute pancreatitis: correlation with endotoxemia, organ failure, and mortality. J Gastrointest Surg. 1999;3:252-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 235] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 83. | Tsuji Y, Watanabe T, Kudo M, Arai H, Strober W, Chiba T. Sensing of commensal organisms by the intracellular sensor NOD1 mediates experimental pancreatitis. Immunity. 2012;37:326-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 84. | Ammori BJ. Role of the gut in the course of severe acute pancreatitis. Pancreas. 2003;26:122-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 137] [Article Influence: 6.2] [Reference Citation Analysis (1)] |
| 85. | Capurso G, Zerboni G, Signoretti M, Valente R, Stigliano S, Piciucchi M, Delle Fave G. Role of the gut barrier in acute pancreatitis. J Clin Gastroenterol. 2012;46 Suppl:S46-S51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 115] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 86. | Petrov MS, Shanbhag S, Chakraborty M, Phillips AR, Windsor JA. Organ failure and infection of pancreatic necrosis as determinants of mortality in patients with acute pancreatitis. Gastroenterology. 2010;139:813-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 517] [Cited by in RCA: 564] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 87. | Tan C, Ling Z, Huang Y, Cao Y, Liu Q, Cai T, Yuan H, Liu C, Li Y, Xu K. Dysbiosis of Intestinal Microbiota Associated With Inflammation Involved in the Progression of Acute Pancreatitis. Pancreas. 2015;44:868-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 139] [Article Influence: 13.9] [Reference Citation Analysis (1)] |
| 88. | Gerritsen J, Timmerman HM, Fuentes S, van Minnen LP, Panneman H, Konstantinov SR, Rombouts FM, Gooszen HG, Akkermans LM, Smidt H, Rijkers GT. Correlation between protection against sepsis by probiotic therapy and stimulation of a novel bacterial phylotype. Appl Environ Microbiol. 2011;77:7749-7756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 89. | Rychter JW, van Minnen LP, Verheem A, Timmerman HM, Rijkers GT, Schipper ME, Gooszen HG, Akkermans LM, Kroese AB. Pretreatment but not treatment with probiotics abolishes mouse intestinal barrier dysfunction in acute pancreatitis. Surgery. 2009;145:157-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 90. | Yu S, Xiong Y, Xu J, Liang X, Fu Y, Liu D, Yu X, Wu D. Identification of Dysfunctional Gut Microbiota Through Rectal Swab in Patients with Different Severity of Acute Pancreatitis. Dig Dis Sci. 2020;65:3223-3237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 91. | Zhang XM, Zhang ZY, Zhang CH, Wu J, Wang YX, Zhang GX. Intestinal Microbial Community Differs between Acute Pancreatitis Patients and Healthy Volunteers. Biomed Environ Sci. 2018;31:81-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 29] [Reference Citation Analysis (0)] |
| 92. | Yu S, Xiong Y, Fu Y, Chen G, Zhu H, Mo X, Wu D, Xu J. Shotgun metagenomics reveals significant gut microbiome features in different grades of acute pancreatitis. Microb Pathog. 2021;154:104849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 93. | Tedelind S, Westberg F, Kjerrulf M, Vidal A. Anti-inflammatory properties of the short-chain fatty acids acetate and propionate: a study with relevance to inflammatory bowel disease. World J Gastroenterol. 2007;13:2826-2832. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 543] [Cited by in RCA: 623] [Article Influence: 34.6] [Reference Citation Analysis (5)] |
| 94. | Kelly CJ, Zheng L, Campbell EL, Saeedi B, Scholz CC, Bayless AJ, Wilson KE, Glover LE, Kominsky DJ, Magnuson A, Weir TL, Ehrentraut SF, Pickel C, Kuhn KA, Lanis JM, Nguyen V, Taylor CT, Colgan SP. Crosstalk between Microbiota-Derived Short-Chain Fatty Acids and Intestinal Epithelial HIF Augments Tissue Barrier Function. Cell Host Microbe. 2015;17:662-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 807] [Cited by in RCA: 1193] [Article Influence: 119.3] [Reference Citation Analysis (0)] |
| 95. | Memba R, Duggan SN, Ni Chonchubhair HM, Griffin OM, Bashir Y, O'Connor DB, Murphy A, McMahon J, Volcov Y, Ryan BM, Conlon KC. The potential role of gut microbiota in pancreatic disease: A systematic review. Pancreatology. 2017;17:867-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 96. | Dambrauskas Z, Gulbinas A, Pundzius J, Barauskas G. Meta-analysis of prophylactic parenteral antibiotic use in acute necrotizing pancreatitis. Medicina (Kaunas). 2007;43:291-300. [PubMed] |
| 97. | Working Group IAP/APA Acute Pancreatitis Guidelines. IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology. 2013;13:e1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1080] [Cited by in RCA: 1041] [Article Influence: 86.8] [Reference Citation Analysis (6)] |
| 98. | Yao L, Huang X, Li Y, Shi R, Zhang G. Prophylactic antibiotics reduce pancreatic necrosis in acute necrotizing pancreatitis: a meta-analysis of randomized trials. Dig Surg. 2010;27:442-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 99. | Arlt A, Erhart W, Schafmayer C, Held HC, Hampe J. Antibiosis of Necrotizing Pancreatitis. Viszeralmedizin. 2014;30:318-324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 100. | Lankisch PG, Burchard-Reckert S, Lehnick D. Underestimation of acute pancreatitis: patients with only a small increase in amylase/Lipase levels can also have or develop severe acute pancreatitis. Gut. 1999;44:542-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 92] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 102. | Oláh A, Romics L Jr. Enteral nutrition in acute pancreatitis: a review of the current evidence. World J Gastroenterol. 2014;20:16123-16131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 76] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (1)] |
| 103. | Luiten EJ, Hop WC, Lange JF, Bruining HA. Controlled clinical trial of selective decontamination for the treatment of severe acute pancreatitis. Ann Surg. 1995;222:57-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 253] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 104. | Hegazi RA, DeWitt T. Enteral nutrition and immune modulation of acute pancreatitis. World J Gastroenterol. 2014;20:16101-16105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 105. | Liu H, Li W, Wang X, Li J, Yu W. Early gut mucosal dysfunction in patients with acute pancreatitis. Pancreas. 2008;36:192-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 84] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 106. | Oláh A, Romics L Jr. Early enteral nutrition in acute pancreatitis--benefits and limitations. Langenbecks Arch Surg. 2008;393:261-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 107. | Pan LL, Li J, Shamoon M, Bhatia M, Sun J. Recent Advances on Nutrition in Treatment of Acute Pancreatitis. Front Immunol. 2017;8:762. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 108. | Oláh A, Belágyi T, Pótó L, Romics L Jr, Bengmark S. Synbiotic control of inflammation and infection in severe acute pancreatitis: a prospective, randomized, double blind study. Hepatogastroenterology. 2007;54:590-594. [PubMed] |
| 109. | Oláh A, Belágyi T, Issekutz A, Gamal ME, Bengmark S. Randomized clinical trial of specific lactobacillus and fibre supplement to early enteral nutrition in patients with acute pancreatitis. Br J Surg. 2002;89:1103-1107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 289] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 110. | Mangiante G, Colucci G, Canepari P, Bassi C, Nicoli N, Casaril A, Marinello P, Signoretto C, Bengmark S. Lactobacillus plantarum reduces infection of pancreatic necrosis in experimental acute pancreatitis. Dig Surg. 2001;18:47-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (1)] |
| 111. | Akyol S, Mas MR, Comert B, Ateskan U, Yasar M, Aydogan H, Deveci S, Akay C, Mas N, Yener N, Kocar IH. The effect of antibiotic and probiotic combination therapy on secondary pancreatic infections and oxidative stress parameters in experimental acute necrotizing pancreatitis. Pancreas. 2003;26:363-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 112. | Muftuoglu MA, Isikgor S, Tosun S, Saglam A. Effects of probiotics on the severity of experimental acute pancreatitis. Eur J Clin Nutr. 2006;60:464-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 113. | Besselink MG, van Santvoort HC, Buskens E, Boermeester MA, van Goor H, Timmerman HM, Nieuwenhuijs VB, Bollen TL, van Ramshorst B, Witteman BJ, Rosman C, Ploeg RJ, Brink MA, Schaapherder AF, Dejong CH, Wahab PJ, van Laarhoven CJ, van der Harst E, van Eijck CH, Cuesta MA, Akkermans LM, Gooszen HG; Dutch Acute Pancreatitis Study Group. Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;371:651-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 944] [Cited by in RCA: 862] [Article Influence: 50.7] [Reference Citation Analysis (0)] |
| 114. | Morrow LE, Gogineni V, Malesker MA. Synbiotics and probiotics in the critically ill after the PROPATRIA trial. Curr Opin Clin Nutr Metab Care. 2012;15:147-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 115. | Bongaerts GP, Severijnen RS. A reassessment of the PROPATRIA study and its implications for probiotic therapy. Nat Biotechnol. 2016;34:55-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |