Published online Sep 28, 2019. doi: 10.3748/wjg.v25.i36.5515
Peer-review started: July 25, 2019
First decision: August 17, 2019
Revised: August 25, 2019
Accepted: September 9, 2019
Article in press: September 9, 2019
Published online: September 28, 2019
Processing time: 64 Days and 21.2 Hours
Researchers have investigated the diagnostic value of protein induced by vitamin K absence or antagonist II (PIVKA-II) and alpha-fetoprotein (AFP) in hepatitis B virus (HBV)-related hepatocellular carcinoma (HCC), and obtained abundant clinical diagnostic data. However, PIVKA-II and AFP have unsatisfactory specificity and sensitivity in the diagnosis of early-stage HBV-related HCC. Gamma-glutamyltransferase (γ-GT) and aspartate aminotransferase (AST) are common biomarkers for evaluating liver function, and we hypothesized that the γ-GT/AST ratio in combination with PIVKA-II and AFP would improve the diagnosis of early-stage HBV-related HCC.
To evaluate the diagnostic value of γ-GT/AST ratio alone or in combination with PIVKA-II and AFP in HBV-related HCC.
Serum levels of γ-GT, AST, PIVKA-II, and AFP were detected and analysed in 176 patients with HBV-related HCC and in 359 patients with chronic hepatitis B. According to tumour size and serum level of HBV DNA, HBV-related HCC patients were divided into the following categories: Early-stage HCC patients, HCC patients, HBV DNA positive (HBV DNA+) HCC patients, and HBV DNA negative (HBV DNA-) HCC patients. Receiver-operating characteristic (ROC) curves were used to analyse and compare the diagnostic value of the single and combined detection of various biomarkers in different types of HBV-related HCC.
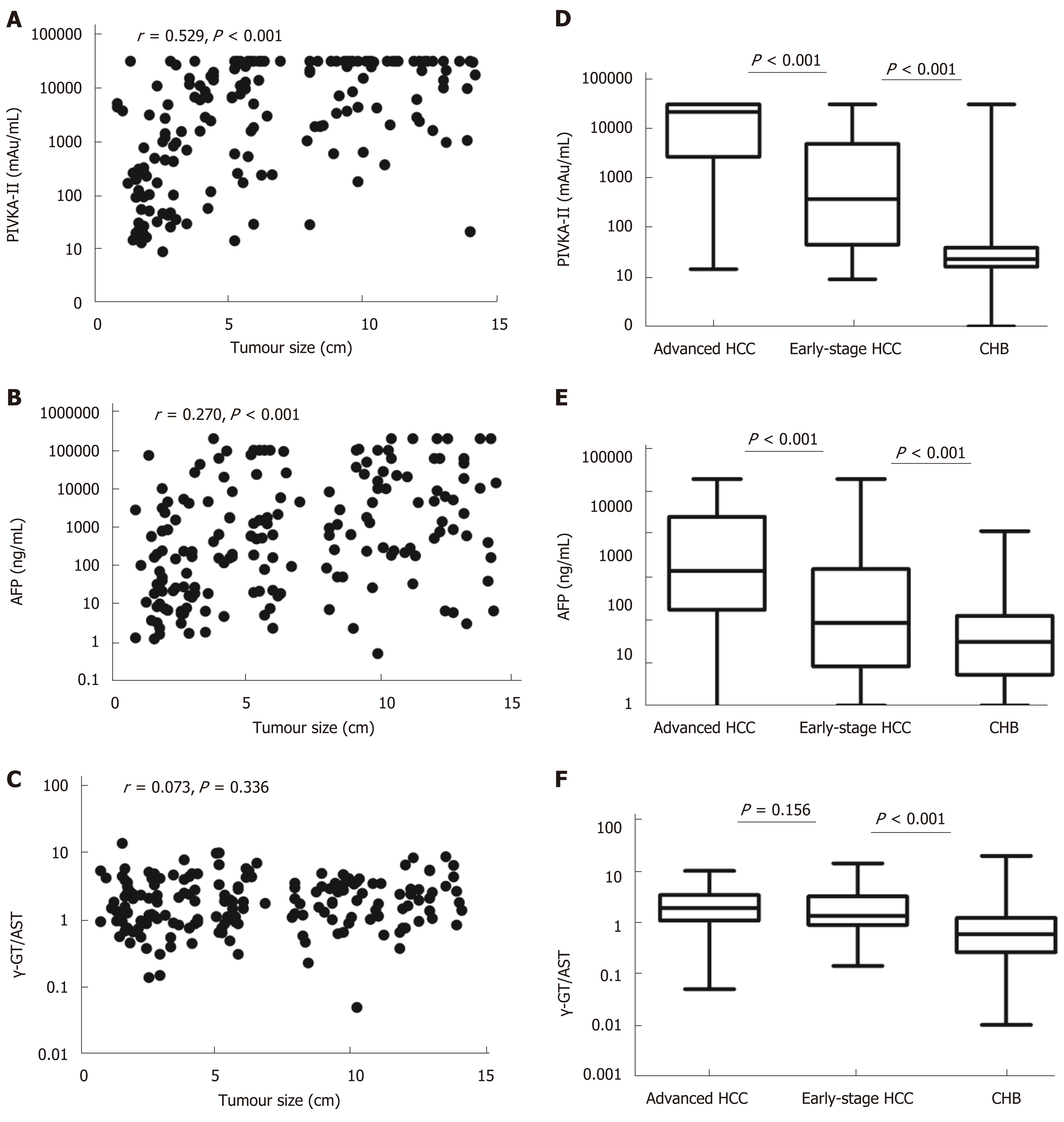
Tumour size was positively correlated with serum levels of PIVKA-II and AFP in HCC patients (r = 0.529, aP < 0.001 and r = 0.270, bP < 0.001, respectively), but there was no correlation between tumour size and the γ-GT/AST ratio (r = 0.073, P = 0.336). The areas under the receiver-operating characteristic curves (AUROCs) of the γ-GT/AST ratio in early-stage HCC patients, HBV DNA+ HCC patients and HBV DNA- HCC patients were not significantly different from that in the total HCC patients (0.754, 0.802, and 0.705 vs 0.779, respectively; P > 0.05). When PIVKA-II was combined with the γ-GT/AST ratio in the diagnosis of early-stage HCC, HCC, and HBV DNA+ HCC, the AUROCs of PIVKA-II increased, with values of 0.857 vs 0.835, 0.925 vs 0.913, and 0.958 vs 0.954, respectively. When AFP was combined with the γ-GT/AST ratio in the diagnosis of early-stage HCC, HCC, HBV DNA+ HCC, and HBV DNA- HCC, the AUROCs of AFP increased, with values of 0.757 vs 0.621, 0.837 vs 0.744, 0.868 vs 0.757, and 0.840 vs 0.828, respectively.
The γ-GT/AST ratio may be better than PIVKA-II and AFP in the diagnosis of early-stage HBV-related HCC, and its combination with PIVKA-II and AFP can improve the diagnostic value for HBV-related HCC.
Core tip: This study showed that the ratio of gamma-glutamyltransferase to aspartate aminotransferase (γ-GT/AST ratio) was not correlated with tumour size in hepatitis B virus (HBV)-related hepatocellular carcinoma (HCC), and there was no difference in the AUROC between early-stage HBV-related HCC and HBV-related HCC, indicating that the γ-GT/AST ratio may be more useful than protein induced by vitamin K absence or antagonist II (PIVKA-II) and alpha-fetoprotein (AFP) in the diagnosis of early-stage HBV-related HCC. PIVKA-II and AFP combined with the γ-GT/AST ratio can enhance the diagnostic value of these biomarkers in different categories of HBV-related HCC.
- Citation: Wang Q, Chen Q, Zhang X, Lu XL, Du Q, Zhu T, Zhang GY, Wang DS, Fan QM. Diagnostic value of gamma-glutamyltransferase/aspartate aminotransferase ratio, protein induced by vitamin K absence or antagonist II, and alpha-fetoprotein in hepatitis B virus-related hepatocellular carcinoma. World J Gastroenterol 2019; 25(36): 5515-5529
- URL: https://www.wjgnet.com/1007-9327/full/v25/i36/5515.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i36.5515
Hepatitis B virus (HBV) infection is the most common viral infection worldwide. It is estimated that more than 2 billion people worldwide have been infected with HBV, and more than 350 million people have developed chronic hepatitis B (CHB)[1]. HBV infection is associated with an increased risk of end-stage liver disease, cirrhosis, and hepatocellular carcinoma (HCC)[2-4]. Prospective population-based studies have shown that 15% of females and 20%-40% of males who are infected with HBV in the early stages of life will develop HCC[5]. It is estimated that approximately 300000 to 500000 people worldwide die from HCC every year[6,7]. In China, HCC is the 4th most common cancer and the 3rd leading cause of cancer death[8], and approximately 50% of the new cases of HCC each year worldwide occur in China[9].
Protein induced by vitamin K absence or antagonist II (PIVKA-II) and alpha-fetoprotein (AFP) are widely used biomarkers in HCC diagnosis[10-13], and have been written into the guidelines for HCC diagnosis published by the National Society of Hepatology of different countries[14-16]. During clinical use, the sensitivity of AFP in the diagnosis of HCC is only 40%-60%, and the sensitivity of AFP in the diagnosis of early-stage HCC is only 10%-20%[17,18]. PIVKA-II is a kind of prothrombin that does not use vitamin K in the process of hepatocyte synthesis, leading to a synthesis deficiency. When HCC occurs due to the abnormal synthesis of prothrombin precursors by cancer cells, prothrombin precursor carboxylation is insufficient, resulting in a large amount of PIVKA-II[19]. As a newly discovered tumour marker for the diagnosis of HCC, PIVKA-II has higher diagnostic value than AFP[20,21]. Some studies have shown that PIVKA-II has a sensitivity of more than 90% in HCC diagnosis, while its sensitivity for early-stage HBV-related HCC detection is more than 50%[12,22,23]. When PIVKA-II and AFP are combined in HCC and early-stage HCC diagnosis, the diagnostic value is significantly improved compared to that of the detection of either individual protein[23-26].
Gamma-glutamyltransferase (γ-GT) is a membrane-binding enzyme that regulates the metabolism of glutathione, catalyses the degradation of extracellular glutathione, and then promotes the recovery of amino acids for intracellular glutathione synthesis. γ-GT has been recognized as being capable of enhancing the cellular antioxidant defence[27]. The level of γ-GT is abnormally elevated in patients with HCC. However, in other liver diseases, such as viral hepatitis, alcoholic hepatitis, and cirrhosis, the level of γ-GT is also abnormally elevated[28-32]. Therefore, for a long time, γ-GT could not be used as an effective index to diagnose HCC, and its specificity is only approximately 30%[33]. Aspartate aminotransferase (AST) is a biomarker commonly used to evaluate hepatocyte damage. The level of AST is elevated in acute and chronic viral hepatitis, alcoholic hepatitis, and cirrhosis. In HCC patients, AST is not elevated or is only slightly elevated[34,35]. Based on these observations, we believe that the ratio of γ-GT to AST (γ-GT/AST ratio) may be able to differentiate HCC from other liver diseases, aiding in the diagnosis and evaluation of HCC. At the same time, we also wanted to know whether the combination of the γ-GT/AST ratio and PIVKA-II or AFP, which are recognized biomarkers for the diagnosis of HCC, can improve the diagnostic value of PIVKA-II or AFP for HCC.
A total of 535 patients with CHB, including 176 HCC patients and 359 CHB patients with other liver diseases, were retrospectively enrolled at the Affiliated Hospital of Northern Sichuan Medical College from January 2017 to March 2019. Among the 176 HCC patients, 74 early-stage HCC patients, 88 HBV DNA+ HCC patients, and 88 HBV DNA- HCC patients were included. The diagnosis of HCC was made in accordance with the standards of the guidelines for the diagnosis and treatment of primary HCC issued by the Chinese Society of Clinical Oncology (2018.V1). Early-stage HCC was defined as the presence of only a single tumour in the liver that was ≤ 5.0 cm, with no vascular invasion or extrahepatic metastasis[13,14]. A total of 359 CHB patients, including 186 with cirrhosis, 53 with cholecystitis, 37 with bile duct stones, 21 with drug-induced hepatitis, 51 with alcoholic hepatitis, 8 with hepatitis E infection, and 3 with hepatitis C infection, were included (258 HBV DNA+ patients and 101 HBV DNA- patients). The diagnosis of CHB and cirrhosis was in accordance with the revised guidelines for the prevention and treatment of CHB infection from the Chinese Society of Hepatology[36].
The study protocol was approved by the Ethics Committee of the Affiliated Hospital of North Sichuan Medical College, Nanchong, China.
Serum levels of γ-GT and AST were determined by biochemical rate-assay (AU5800, Beckman Coulter, Inc., America). Serum levels of PIVKA-II were determined by chemiluminescent microparticle immunoassay (CMIA) (Architect i1000, Abbott Laboratories, America). Serum levels of AFP were measured by electro-chemiluminescence immunoassay (Cobas E602, Roche, Inc., Germany). Serum levels of HBV DNA were determined by real-time polymerase chain reaction (LightCycler 480II, Roche, Inc., Germany). The results were interpreted as follows: HBV DNA ≥ 500 IU/mL, positive (HBV DNA+); and HBV DNA ˂ 500 IU/mL, negative (HBV DNA-).
The receiver-operating characteristic (ROC) curves were used to determine the diagnostic cut-off values of the γ-GT/AST ratio, PIVKA-II, and AFP for HCC. The fold change in the γ-GT/AST ratio, PIVKA-II, and AFP relative to the corresponding diagnostic cut-off values are expressed by the Mcut-off. This study evaluated the diagnostic value of the combined detection of biomarkers in HBV-related HCC by analysing the sums of the Mcut-off of the corresponding biomarkers.
Data are expressed as the median (interquartile range) or number (%). Comparisons between groups were performed by the Mann-Whitney U test. Pearson’s chi-square test was used for gender comparisons. Pearson correlation analysis was used for two-factor correlation analysis. ROC curves were used to determine the diagnostic cut-off values, the areas under the ROC curves (AUROCs), diagnostic sensitivities, and specificities. Statistical analyses were performed using SPSS, version 19.0 (SPSS Inc., United States) and Medcalc, version 12.3 (MedCalc Software bvba, Ostend, Belgium). The statistical significance of all tests was defined as P < 0.05 by two-tailed tests.
The clinical characteristics of 176 patients with HCC and 359 patients with CHB (control group) are expressed as medians (interquartile range) or numbers (%). The data showed that the sex of the patients in the two groups was predominately male, and the median age of HCC patients was 53 (range, 47-62) years old, which was higher than that of the CHB group [49 (39-59) years] (P < 0.001). Serum levels of PIVKA-II, AFP, γ-GT, and γ-GT /AST ratio in HCC patients were significantly higher than those in CHB patients (P < 0.001), while serum levels of AST were significantly lower in HCC patients than in CHB patients (P < 0.001). The clinical characteristics of the 535 study patients are shown in Table 1.
| Characteristic | CHB (n = 359) | HCC (n = 176) | P-value |
| Age (yr) | 49 (39-59) | 53 (47-62) | < 0.001 |
| Gender (male: female) | 281:78 | 152:24 | 0.025 |
| PIVKA-II (mAU/mL) | 22.88 (16.00-37.70) | 4882.77 (274.98-30000) | < 0.001 |
| AFP (ng/mL) | 31.00 (5.30-126.00) | 510.05 (25.91-9583.93) | < 0.001 |
| γ-GT (U/L) | 92.00 (47.50-160.70) | 151.55 (68.10-346.20) | < 0.001 |
| AST (U/L) | 138.40 (56.00-367.00) | 76.25 (44.25-131.75) | < 0.001 |
| γ-GT/AST | 0.58 (0.26-1.22) | 1.84 (0.95-3.38) | < 0.001 |
| HBV DNA+, n (%) | 258 (71.87%) | 88 (50.00%) | NA |
| Early-stage HCC, n (%) | NA | 74 (42.05%) | NA |
Tumour size was positively correlated with serum levels of PIVKA-II and AFP in HCC patients (r = 0.529, aP < 0.001 and r = 0.270, bP < 0.001, respectively), but there was no correlation between tumour size and the γ-GT/AST ratio (r = 0.073, P = 0.336) (Figure 1). Serum levels of PIVKA-II and AFP in early-stage HCC patients were significantly lower than those in advanced HCC patients (P < 0.001) and were higher than those in CHB patients (P < 0.001). The γ-GT/AST ratio was not significantly different between early-stage HCC patients and advanced HCC patients (P = 0.156), but higher than that in CHB patients (P < 0.001) (Figure 1).
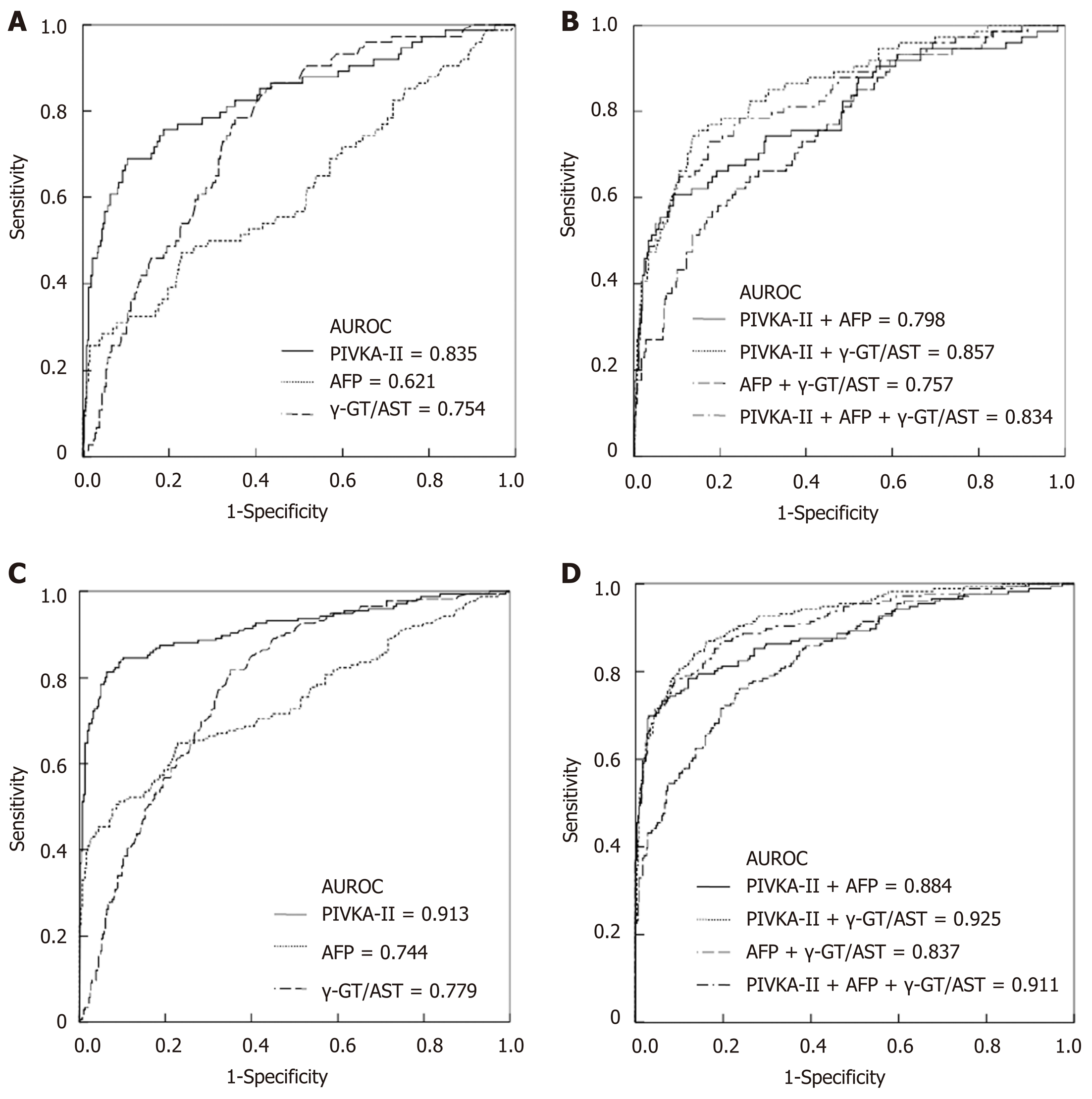
When the cut-off values of the γ-GT/AST ratio, PIVKA-II, and AFP were set as 0.845, 84.44 mAu/mL, and 145.65 ng/mL, respectively, for the diagnosis of early-stage HCC, the AUROC of the γ-GT/AST ratio was 0.754 [95% confidence interval (CI): 0.700-0.809], which was lower than that of PIVKA-II [0.835 (95%CI: 0.776-0.893)] (P = 0.048) and was higher than that of AFP [0.621 (95%CI: 0.545-0.698)] (P = 0.006) (Figure 2A). When the cut-off values of the γ-GT/AST ratio, PIVKA-II, and AFP were set as 0.845, 162.22 mAu/mL, and 145.65 ng/mL, respectively, for HCC diagnosis, there was no difference in the AUROC between the γ-GT/AST ratio [0.779 (95%CI: 0.0.740-0.819)] and AFP [0.744 (95%CI: 0.695-0.792)] (P = 0.162), but the AUROCs of both γ-GT/AST and AFP were significantly lower than that of PIVKA-II [0.913 (95%CI: 0.884-0.943)] (P < 0.001) (Figure 2C). The AUROCs of PIVKA-II and AFP for the diagnosis of early-stage HCC were significantly lower than those for the diagnosis of HCC (cP = 0.020 and dP = 0.008, respectively), but there was no significant difference in the γ-GT/AST ratio between early-stage HCC and HCC (P = 0.468).
When combined with AFP for the diagnosis of early-stage HCC and HCC, the AUROCs of PIVKA-II decreased, with values of 0.798 (95%CI: 0.735-0.861) vs 0.835 (95%CI: 0.776-0.893) and 0.884 (95%CI: 0.850-0.919) vs 0.913 (95%CI: 0.884-0.943), respectively. When combined with the γ-GT/AST ratio for the diagnosis of early-stage HCC and HCC, the AUROCs of PIVKA-II increased, with values of 0.857 (95%CI: 0.807-0.906) vs 0.835 (95%CI: 0.776-0.893) and 0.925 (95%CI: 0.900-0.949) vs 0.913 (95%CI: 0.884-0.943), respectively. When combined with the γ-GT/AST ratio for the diagnosis of early-stage HCC and HCC, the AUROCs of AFP increased, with values of 0.757 (95%CI: 0.696-0.817) vs 0.621 (95%CI: 0.545-0.698) and 0.837 (95%CI: 0.801-0.873) vs 0.744 (95%CI: 0.695-0.792), respectively. When PIVKA-II was combined with the γ-GT/AST ratio and AFP for the diagnosis of early-stage HCC and HCC, there was almost no difference between the combined and single diagnostic abilities, with values of 0.834 (95%CI: 0.778-0.890) vs 0.835 (95%CI: 0.776-0.893) and 0.911 (95%CI: 0.884-0.939) vs 0.913 (95%CI: 0.884-0.943) (Figure 2B and 2D). The diagnostic performance of PIVKA-II, AFP, and the γ-GT/AST ratio for early-stage HCC and HCC is shown in Table 2.
| Early-stage HCC | HCC | |||||||||
| Cut-off | SEN (%) | SPE (%) | PPV (%) | NPV (%) | Cut-off | SEN (%) | SPE (%) | PPV (%) | NPV (%) | |
| PIVKA-II (mAu/mL) | 84.44 | 68.90 | 89.70 | 57.96 | 93.33 | 162.22 | 81.30 | 93.60 | 86.16 | 91.08 |
| AFP (ng/mL) | 145.65 | 47.30 | 77.20 | 29.95 | 87.66 | 145.65 | 64.80 | 77.20 | 58.22 | 81.73 |
| γ-GT/AST | 0.845 | 78.40 | 64.90 | 31.53 | 93.58 | 0.845 | 81.80 | 64.90 | 53.33 | 87.91 |
| PIVKA-II + AFP | NA | 60.80 | 90.80 | 57.67 | 91.83 | NA | 73.30 | 93.90 | 85.49 | 87.77 |
| PIVKA-II + γ-GT/AST | NA | 74.30 | 86.60 | 53.34 | 94.24 | NA | 86.90 | 83.80 | 72.45 | 92.88 |
| AFP + γ-GT/AST | NA | 56.80 | 81.90 | 39.28 | 90.19 | NA | 76.10 | 76.30 | 61.15 | 86.69 |
| PIVKA-II + AFP + γ-GT/AST | NA | 73.00 | 82.50 | 46.23 | 93.68 | NA | 78.40 | 91.10 | 81.20 | 89.59 |
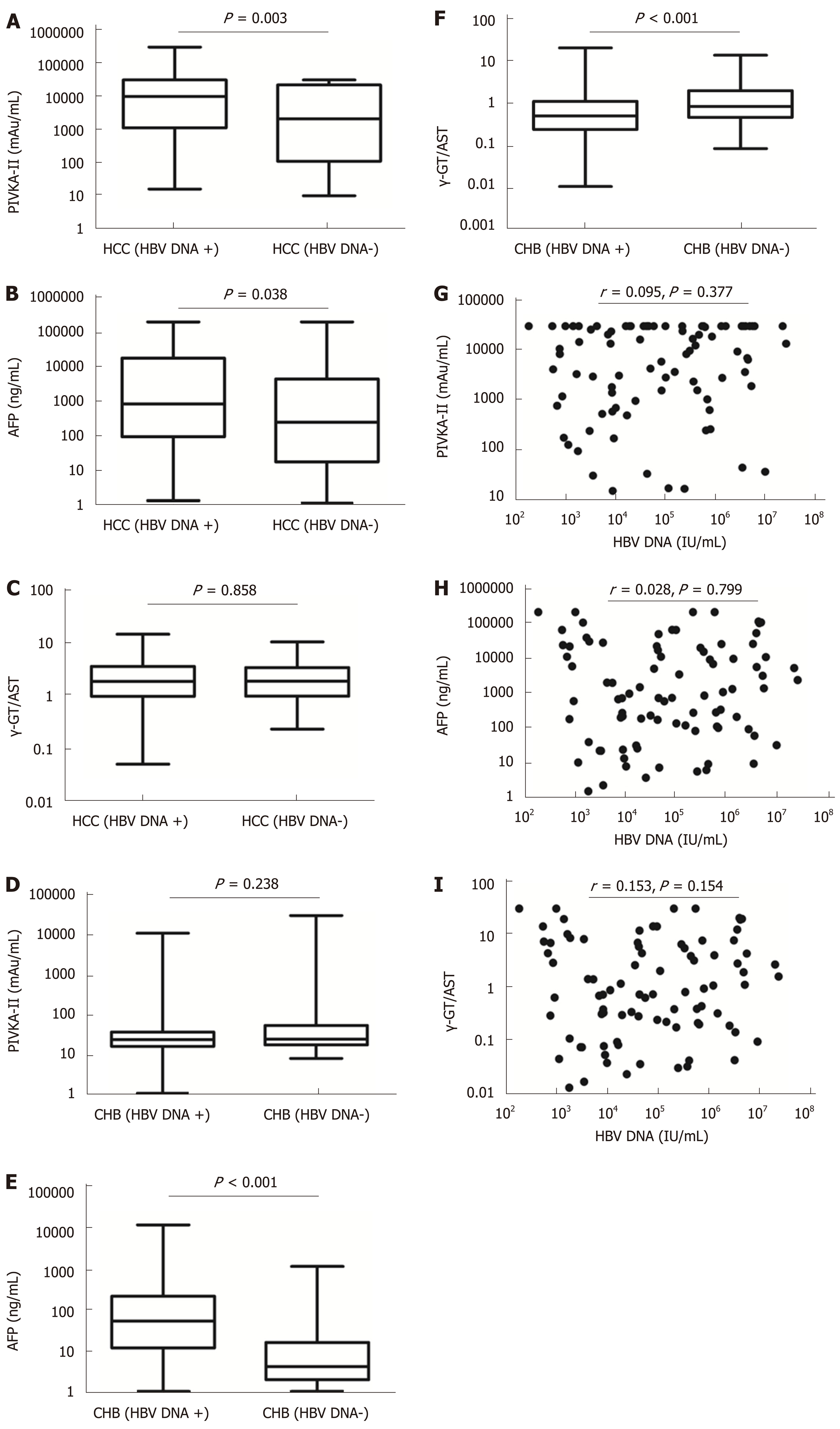
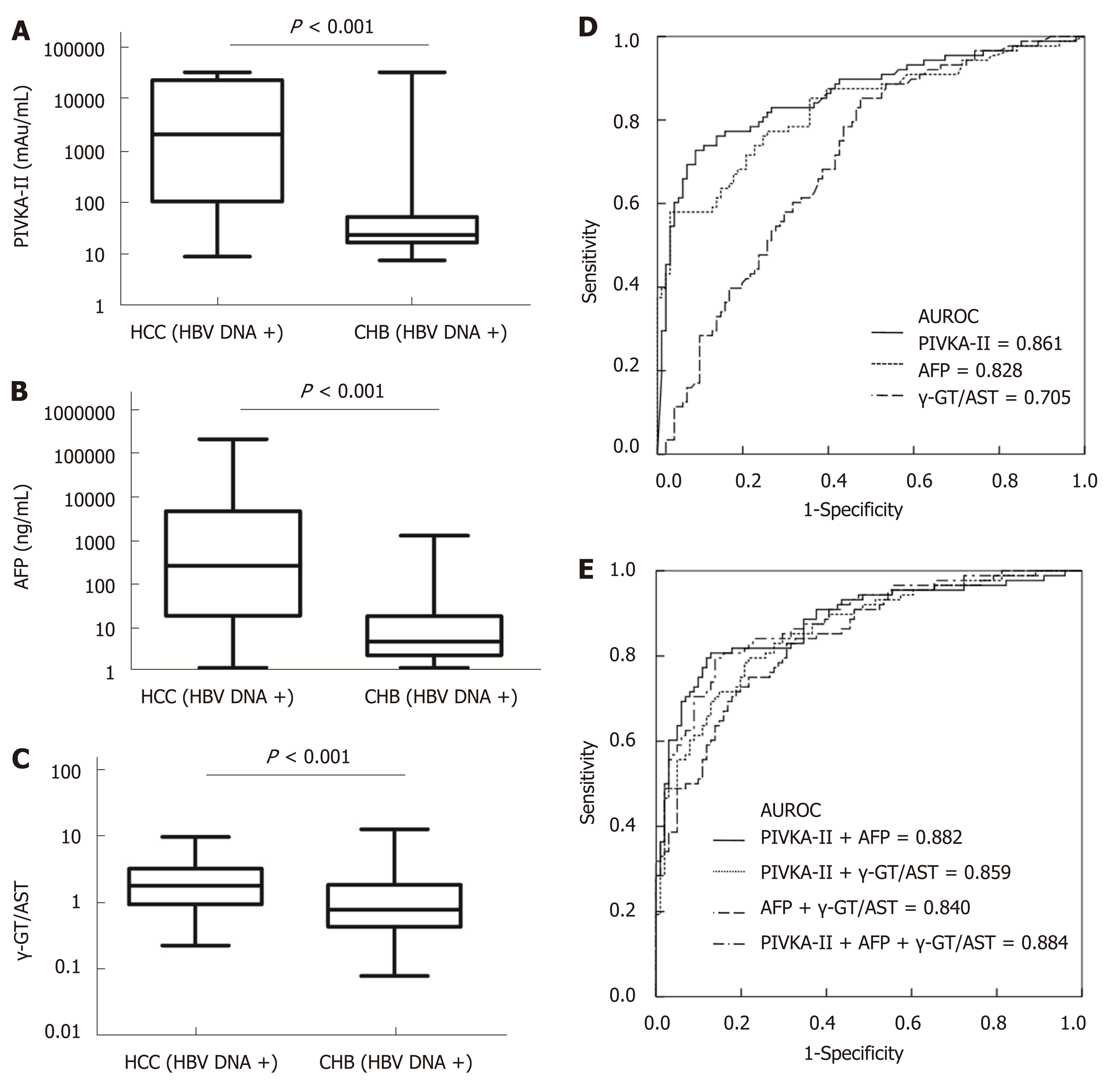
The mean tumour size of HBV DNA+ HCC patients was 7.40 (range, 4.10-10.38) cm, which was larger than that of HBV DNA- HCC patients [4.25 (2.00-8.80) cm] (P = 0.001). Serum levels of PIVKA-II and AFP in HBV DNA+ HCC patients were significantly higher than those in HBV DNA- HCC patients (aP = 0.003 and bP = 0.038, respectively), although there was no significant difference in the γ-GT/AST ratio between the two groups (P = 0.858). Serum levels of AFP and γ-GT/AST in HBV DNA+ CHB patients were significantly higher than those of HBV DNA- CHB patients (P < 0.001), but there was no significant difference in the levels of PIVKA-II between the two groups (P = 0.238). Serum levels of PIVKA-II, AFP, and γ-GT/AST had no correlation with serum levels of HBV DNA in HBV DNA+ HCC patients (cP = 0.377, dP = 0.799, and eP = 0.154, respectively) (Figure 3).
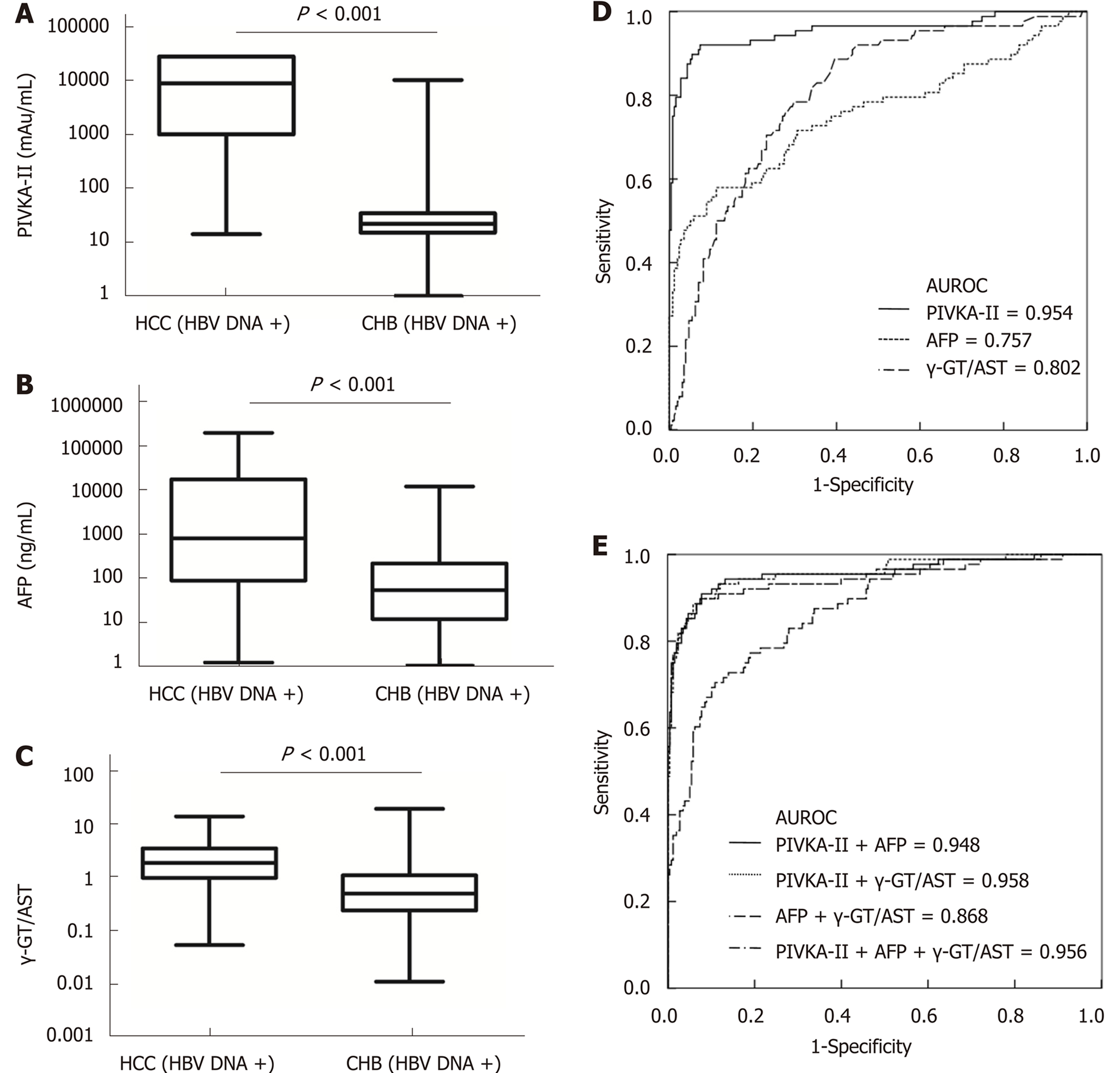
When the cut-off values of the γ-GT/AST ratio, PIVKA-II, and AFP were set as 0.640, 84.44 mAu/mL, and 497.70 ng/mL, respectively, for HBV DNA+ HCC patients, there was no significant difference in the AUROC between the γ-GT/AST ratio [0.802 (95%CI: 0.751-0.854)] and AFP [0.757 (95%CI: 0.689-0.825)] (P = 0.302), but the AUROCs of both γ-GT/AST and AFP were significantly lower than that of PIVKA-II [0.954 (95%CI: 0.923-0.985)] (P < 0.001) (Figure 4). When the cut-off value of the γ-GT/AST ratio, PIVKA-II, and AFP were set as 0.830, 154.92 mAu/mL, and 146.15 ng/mL, respectively, for the diagnosis of HBV DNA- HCC, the AUROCs of PIVKA-II and AFP were 0.861 (95%CI: 0.806-0.916) and 0.828 (95%CI: 0.768-0.888), respectively, and both of these AUROC values were significantly higher than that of the γ-GT/AST ratio [0.705 (95%CI: 0.632-0.779)] (fP < 0.001 and gP = 0.012, respectively), although there was no significant difference between the AUROCs of PIVKA-II and AFP (P = 0.430) (Figure 5). Compared with HBV DNA+ HCC patients, the AUROCs of PIVKA-II and the γ-GT/AST ratio decreased and the AUROC of AFP increased in HBV DNA- HCC patients.
When PIVKA-II was combined with AFP and the γ-GT/AST ratio in both HBV DNA+ and HBV DNA- HCC patients, there were no significant differences in the AUROCs between combined diagnosis and single diagnosis (AFP: hP = 0.791 and iP = 0.583; γ-GT/AST: jP = 0.851 and iP = 0.959). The AUROC of AFP combined with the γ-GT/AST ratio in the diagnosis of HBV DNA+ HCC patients was 0.868 (95%CI: 0.824-0.913), which was higher than that of AFP (P = 0.008). The highest AUROC for the combined diagnosis of HBV DNA- HCC was 0.884 (95%CI: 0.837-0.932), but there was no significant difference between this AUROC and the AUROC of PIVKA-II (P = 0.533) (Figure 5). The diagnostic performance of PIVKA-II, AFP, and the γ-GT/AST ratio for HBV DNA + and HBV DNA- HCC patients is shown in Table 3.
| HBV DNA+ HCC | HBV DNA- HCC | |||||||||
| Cut-off | SEN (%) | SPE (%) | PPV (%) | NPV (%) | Cut-off | SEN (%) | SPE (%) | PPV (%) | NPV (%) | |
| PIVKA-II (mAu/mL) | 84.44 | 92.00 | 92.60 | 80.92 | 97.14 | 154.92 | 72.70 | 91.10 | 87.68 | 79.30 |
| AFP (ng/mL) | 497.70 | 58.00 | 88.80 | 63.85 | 86.11 | 146.15 | 58.00 | 97.00 | 94.40 | 72.61 |
| γ-GT/AST | 0.640 | 88.60 | 60.50 | 43.34 | 93.96 | 0.830 | 85.20 | 52.50 | 60.98 | 80.28 |
| PIVKA-II + AFP | NA | 89.80 | 92.20 | 79.70 | 96.36 | NA | 80.70 | 87.10 | 84.50 | 83.82 |
| PIVKA-II + γ-GT/AST | NA | 89.80 | 92.60 | 80.54 | 96.38 | NA | 79.50 | 78.20 | 76.06 | 81.41 |
| AFP + γ-GT/AST | NA | 70.50 | 89.10 | 68.81 | 89.85 | NA | 75.00 | 78.20 | 74.98 | 78.21 |
| PIVKA-II + AFP + γ-GT/AST | NA | 90.90 | 92.20 | 79.90 | 96.74 | NA | 79.50 | 86.10 | 83.29 | 82.82 |
This study assessed the diagnostic value of the γ-GT/AST ratio, PIVKA-II, and AFP in HBV-related early-stage HCC patients, HBV DNA+ HCC patients, HBV DNA- HCC patients, and HCC patients. It was found that PIVKA-II was superior to the γ-GT/AST ratio and AFP in the diagnosis of the above mentioned categories of HCC, and the γ-GT/AST ratio was superior to AFP in the diagnosis of early-stage HCC, HBV DNA+ HCC, and HCC. AFP was superior for the diagnosis of HBV DNA- HCC compared to the diagnosis of early-stage HCC, HBV DNA+ HCC, and HCC. The γ-GT/AST ratio may be superior to PIVKA-II and AFP for the detection of early-stage HCC. At the same time, the combination of PIVKA-II and/or AFP with the γ-GT/AST ratio can improve the diagnostic value of these biomarkers for early-stage HCC, DNA+ HCC, HBV DNA- HCC, and HCC.
According to the statistical analysis of HCC at our hospital over the past five years, the proportion of HCC patients infected with HBV was 88.28% (610/691), indicating that HBV was the main cause of HCC in China. The HCC patients and control patients who were chosen for this study had suffered from HBV infection for more than 6 mo, and it was very important to evaluate the serum levels of PIVKA-II, AFP, and γ-GT/AST and their diagnostic value in this subset of HCC patients.
In this study, we evaluated the diagnostic value of the combined detection of PIVKA-II, AFP, and the γ-GT/AST ratio in HCC by analysing the Mcut-off results, which were mainly based on the following two considerations: First, in the diagnosis of cancer, a higher serum level of tumour markers indicates a higher risk that patients are suffering from tumours; second, the positivity of a single tumour marker does not necessarily indicate that the probability of cancer is high. However, the simultaneous positive expression of multiple tumour markers, even at low titres, should be taken seriously. Therefore, the use of the Mcut-off to evaluate the diagnostic value of PIVKA-II, AFP, and the γ-GT/AST ratio can effectively improve the diagnosis of HCC, and the data in this study also confirmed the hypothesis stated above.
γ-GT is considered a biomarker of alcoholic hepatitis[29-32], and the γ-GT/AST ratio has been used to differentiate the types of jaundice[37]. The data in this paper showed that serum level of γ-GT increased in CHB patients, but the level was lower than that in HCC patients. Additionally, serum level of AST also increased in CHB patients and was higher than that in HCC patients (Table 1). Therefore, the ratio of γ-GT and AST could help to differentiate HCC and CHB patients and to perform a differential diagnosis. At the same time, the statistical analysis also confirmed our previous assumptions.
When PIVKA-II, AFP, and the γ-GT/AST ratio were individually used for the diagnosis of early-stage HCC, HBV DNA+ HCC, HBV DNA- HCC, and HCC, the diagnostic value of PIVKA-II was significantly higher than that of AFP and the γ-GT/AST ratio, especially in regard to the diagnostic sensitivity, which is consistent with the view of Yu R; additionally, PIVKA-II was superior to AFP in the diagnosis of early-stage HCC[13]. However, we should also note that PIVKA-II and AFP were significantly positively correlated with the tumour size in HCC (Figure 1A and 1B), which is an important reason why PIVKA-II and AFP had significantly lower diagnostic value in early-stage HCC than in HCC (AUROC: 0.839 vs 0.914 and 0.628 vs 0.744, respectively). The γ-GT/AST ratio was not correlated with the tumour size in HCC (Figure 1C), and there was no difference in the AUROC between early-stage HCC and HCC (AUROC: 0.755 vs 0.779). Therefore, we inferred that the γ-GT/AST ratio may be more useful than PIVKA-II and AFP in the diagnosis of early-stage HCC. It may be that intrahepatic obstruction in HCC induces hepatocytes to produce abundant γ-GT, while cancer cells also synthesize γ-GT, making the serum level of γ-GT elevated higher than that of benign liver disease, while AST was elevated lower than that of benign liver disease. Thus, the γ-GT/AST ratio is conducive to early-stage HCC diagnosis. This observation will be validated in our follow-up study. Serum levels of AFP in HBV DNA+ HCC patients and HBV DNA+ CHB patients were significantly higher than those in HBV DNA- HCC patients and HBV DNA- CHB patients, respectively, indicating that HBV replication may promote the expression of AFP in abnormal hepatocytes. It is more of a stretch to assume that the continuous replication of HBV causes HCC in CHB patients. This observation may be the main reason that the cut-off value of AFP in HBV DNA+ HCC patients was significantly higher than that in HBV DNA- HCC patients (497.70 ng/mL vs 146.15 ng/mL). Additionally, AFP had the largest AUROC in HBV DNA- HCC patients, even though the tumour size of HBV DNA- HCC patients was smaller than that of HBV DNA+ HCC patients; thus, AFP has the greatest diagnostic ability for HBV DNA- HCC. Serum levels of PIVKA-II in HBV DNA+ HCC patients were higher than those in HBV DNA- HCC patients, but there was no significant difference between HBV DNA+ CHB patients and HBV DNA- CHB patients, which indicated that the reason for the decrease in the AUROC of PIVKA-II in HBV DNA- HCC have been that the tumour size of HBV DNA- HCC was significantly smaller than that of HBV DNA+ HCC. A higher AUROC value indicates that there is greater diagnostic value[38]. When PIVKA-II and AFP were combined with the γ-GT/AST ratio in the diagnosis of early-stage HCC, HBV DNA+ HCC, and HBV DNA- HCC, the AUROCs of PIVKA-II and AFP increased, and the increase for AFP was especially larger. Therefore, PIVKA-II and AFP combined with the γ-GT/AST ratio will be more useful for the differential diagnosis of HCC and other benign liver diseases than these biomarkers individually.
In conclusion, the γ-GT/AST ratio may be more useful than PIVKA-II and AFP in the diagnosis of early-stage HCC, and AFP has the greatest diagnostic ability for HBV DNA- HCC. PIVKA-II and AFP combined with the γ-GT/AST ratio can increase the diagnostic value of these biomarkers in different types of HCC. Therefore, clinicians need to select the most advantageous biomarkers for HCC according to clinical and laboratory test results.
Protein induced by vitamin K absence or antagonist II (PIVKA-II) and alpha-fetoprotein (AFP) are widely used biomarkers in the diagnosis of hepatocellular carcinoma (HCC), and have been written into the guidelines for HCC diagnosis published by the National Society of Hepatology of different countries. However, PIVKA-II and AFP have unsatisfactory specificity and sensitivity in the diagnosis of hepatitis B virus (HBV)-related HCC, especially in early-stage HBV-related HCC.
Researchers have evaluated the diagnostic value of biomarkers in HCC. However, it is difficult to obtain ideal biomarkers for early-stage HCC, even if combined detection of existing biomarkers. This study evaluated the diagnostic value of the ratio of gamma-glutamyltransferase to aspartate aminotransferase (γ-GT/AST ratio) in different classifications of HBV-related HCC, which can effectively improve the diagnostic efficiency for HBV-related HCC.
The objective of this study was to evaluate the diagnostic value of the γ-GT/AST ratio, PIVKA-II, and AFP, alone or in combination, in different categories of HBV-related HCC.
We retrospectively analyzed the clinical and laboratory data of HBV-related HCC and chronic hepatitis B (CHB) patients, and classified those data according to the tumour size and serum level of HBV DNA. The diagnostic value of the γ-GT/AST ratio, PIVKA-II, and AFP in different categories of HBV-related HCC was evaluated comprehensively and systematically.
Tumour size was positively correlated with serum levels of PIVKA-II and AFP in HCC patients, but there was no correlation between tumour size and the γ-GT/AST ratio. The areas under receiver-operating characteristic curves (AUROCs) of the γ-GT/AST ratio were not significantly different between early-stage HBV-related HCC patients and HBV-related HCC patients. When PIVKA-II and AFP were combined with the γ-GT/AST ratio in the diagnosis of HBV-related HCC patients, early-stage HBV-related HCC patients, HBV DNA+ HCC patients, and HBV DNA- HCC patients, the AUROCs of those biomarkers were increased.
The γ-GT/AST ratio is a useful biomarker for the diagnosis of HBV-related HCC and may be more useful than PIVKA-II and AFP in the diagnosis of early-stage HBV-related HCC. The combination of PIVKA-II and AFP with the γ-GT/AST ratio can enhance the diagnostic value of these biomarkers in different categories of HBV-related HCC.
This study assessed the diagnostic value of the γ-GT/AST ratio, PIVKA-II, and AFP in different categories of HBV-related HCC. According to the correlation between tumour size of HBV-related HCC patients and the γ-GT/AST ratio, we inferred that the γ-GT/AST ratio may be more useful than PIVKA-II and AFP in the diagnosis of early-stage HCC. This hypothesis should be validated in future studies.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kai K, Pompella A S-Editor: Tang JZ L-Editor: Wang TQ E-Editor: Zhang YL
| 1. | Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat. 2004;11:97-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1734] [Cited by in RCA: 1750] [Article Influence: 83.3] [Reference Citation Analysis (0)] |
| 2. | Lee WM. Hepatitis B virus infection. N Engl J Med. 1997;337:1733-1745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1728] [Cited by in RCA: 1712] [Article Influence: 61.1] [Reference Citation Analysis (0)] |
| 3. | Kanda T, Goto T, Hirotsu Y, Moriyama M, Omata M. Molecular Mechanisms Driving Progression of Liver Cirrhosis towards Hepatocellular Carcinoma in Chronic Hepatitis B and C Infections: A Review. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 208] [Cited by in RCA: 197] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 4. | Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HL, Chen CJ, Chen DS, Chen HL, Chen PJ, Chien RN, Dokmeci AK, Gane E, Hou JL, Jafri W, Jia J, Kim JH, Lai CL, Lee HC, Lim SG, Liu CJ, Locarnini S, Al Mahtab M, Mohamed R, Omata M, Park J, Piratvisuth T, Sharma BC, Sollano J, Wang FS, Wei L, Yuen MF, Zheng SS, Kao JH. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10:1-98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1985] [Cited by in RCA: 1959] [Article Influence: 217.7] [Reference Citation Analysis (0)] |
| 5. | McMahon BJ, Holck P, Bulkow L, Snowball M. Serologic and clinical outcomes of 1536 Alaska Natives chronically infected with hepatitis B virus. Ann Intern Med. 2001;135:759-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 292] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 6. | Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker-Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Bin Abdulhak A, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahodwala N, De Leo D, Degenhardt L, Delossantos A, Denenberg J, Des Jarlais DC, Dharmaratne SD, Dorsey ER, Driscoll T, Duber H, Ebel B, Erwin PJ, Espindola P, Ezzati M, Feigin V, Flaxman AD, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabriel SE, Gakidou E, Gaspari F, Gillum RF, Gonzalez-Medina D, Halasa YA, Haring D, Harrison JE, Havmoeller R, Hay RJ, Hoen B, Hotez PJ, Hoy D, Jacobsen KH, James SL, Jasrasaria R, Jayaraman S, Johns N, Karthikeyan G, Kassebaum N, Keren A, Khoo JP, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lipnick M, Lipshultz SE, Ohno SL, Mabweijano J, MacIntyre MF, Mallinger L, March L, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGrath J, Mensah GA, Merriman TR, Michaud C, Miller M, Miller TR, Mock C, Mocumbi AO, Mokdad AA, Moran A, Mulholland K, Nair MN, Naldi L, Narayan KM, Nasseri K, Norman P, O'Donnell M, Omer SB, Ortblad K, Osborne R, Ozgediz D, Pahari B, Pandian JD, Rivero AP, Padilla RP, Perez-Ruiz F, Perico N, Phillips D, Pierce K, Pope CA, Porrini E, Pourmalek F, Raju M, Ranganathan D, Rehm JT, Rein DB, Remuzzi G, Rivara FP, Roberts T, De León FR, Rosenfeld LC, Rushton L, Sacco RL, Salomon JA, Sampson U, Sanman E, Schwebel DC, Segui-Gomez M, Shepard DS, Singh D, Singleton J, Sliwa K, Smith E, Steer A, Taylor JA, Thomas B, Tleyjeh IM, Towbin JA, Truelsen T, Undurraga EA, Venketasubramanian N, Vijayakumar L, Vos T, Wagner GR, Wang M, Wang W, Watt K, Weinstock MA, Weintraub R, Wilkinson JD, Woolf AD, Wulf S, Yeh PH, Yip P, Zabetian A, Zheng ZJ, Lopez AD, Murray CJ, AlMazroa MA, Memish ZA. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095-2128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9500] [Cited by in RCA: 9582] [Article Influence: 737.1] [Reference Citation Analysis (0)] |
| 7. | Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94:153-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2651] [Cited by in RCA: 2598] [Article Influence: 108.3] [Reference Citation Analysis (0)] |
| 8. | Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11444] [Cited by in RCA: 13211] [Article Influence: 1467.9] [Reference Citation Analysis (3)] |
| 9. | Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18694] [Cited by in RCA: 21367] [Article Influence: 2136.7] [Reference Citation Analysis (3)] |
| 10. | Jun T, Hsu YC, Ogawa S, Huang YT, Yeh ML, Tseng CH, Huang CF, Tai CM, Dai CY, Huang JF, Chuang WL, Yu ML, Tanaka Y, Nguyen MH. Mac-2 Binding Protein Glycosylation Isomer as a Hepatocellular Carcinoma Marker in Patients With Chronic Hepatitis B or C Infection. Hepatol Commun. 2019;3:493-503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 11. | Kim SH, Moon DB, Kim WJ, Kang WH, Kwon JH, Jwa EK, Cho HD, Ha SM, Chung YK, Lee SG. Preoperative prognostic values of α-fetoprotein (AFP) and protein induced by vitamin K absence or antagonist-II (PIVKA-II) in patients with hepatocellular carcinoma for living donor liver transplantation. Hepatobiliary Surg Nutr. 2016;5:461-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (25)] |
| 12. | Wang X, Zhang W, Liu Y, Gong W, Sun P, Kong X, Yang M, Wang Z. Diagnostic value of prothrombin induced by the absence of vitamin K or antagonist-II (PIVKA-II) for early stage HBV related hepatocellular carcinoma. Infect Agent Cancer. 2017;12:47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Yu R, Tan Z, Xiang X, Dan Y, Deng G. Effectiveness of PIVKA-II in the detection of hepatocellular carcinoma based on real-world clinical data. BMC Cancer. 2017;17:608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 14. | Chinese Society of Clinical Oncology. Guidelines of Chinese Society of Clinical Oncology (CSCO) Hepatocellular Carcinoma. 1th ed. People's Medical Publishing House. 2018;3-21. |
| 15. | Kudo M, Izumi N, Kokudo N, Matsui O, Sakamoto M, Nakashima O, Kojiro M, Makuuchi M; HCC Expert Panel of Japan Society of Hepatology. Management of hepatocellular carcinoma in Japan: Consensus-Based Clinical Practice Guidelines proposed by the Japan Society of Hepatology (JSH) 2010 updated version. Dig Dis. 2011;29:339-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 664] [Article Influence: 47.4] [Reference Citation Analysis (0)] |
| 16. | Izumi N. Diagnostic and treatment algorithm of the Japanese society of hepatology: a consensus-based practice guideline. Oncology. 2010;78 Suppl 1:78-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Gao J, Song P. Combination of triple biomarkers AFP, AFP-L3, and PIVAKII for early detection of hepatocellular carcinoma in China: Expectation. Drug Discov Ther. 2017;11:168-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 18. | Ertle JM, Heider D, Wichert M, Keller B, Kueper R, Hilgard P, Gerken G, Schlaak JF. A combination of α-fetoprotein and des-γ-carboxy prothrombin is superior in detection of hepatocellular carcinoma. Digestion. 2013;87:121-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 83] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 19. | Zhang YS, Chu JH, Cui SX, Song ZY, Qu XJ. Des-γ-carboxy prothrombin (DCP) as a potential autologous growth factor for the development of hepatocellular carcinoma. Cell Physiol Biochem. 2014;34:903-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 20. | Hirokawa F, Hayashi M, Miyamoto Y, Asakuma M, Shimizu T, Komeda K, Inoue Y, Uchiyama K. Outcomes and predictors of microvascular invasion of solitary hepatocellular carcinoma. Hepatol Res. 2014;44:846-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 103] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 21. | Poté N, Cauchy F, Albuquerque M, Voitot H, Belghiti J, Castera L, Puy H, Bedossa P, Paradis V. Performance of PIVKA-II for early hepatocellular carcinoma diagnosis and prediction of microvascular invasion. J Hepatol. 2015;62:848-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 242] [Article Influence: 24.2] [Reference Citation Analysis (4)] |
| 22. | Svobodova S, Karlikova M, Topolcan O, Pecen L, Pestova M, Kott O, Treska V, Slouka D, Kucera R. PIVKA-II as a Potential New Biomarker for Hepatocellular Carcinoma - A Pilot Study. In Vivo. 2018;32:1551-1554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Wu J, Xiang Z, Bai L, He L, Tan L, Hu M, Ren Y. Diagnostic value of serum PIVKA-II levels for BCLC early hepatocellular carcinoma and correlation with HBV DNA. Cancer Biomark. 2018;23:235-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 24. | Yoshida M, Ogino H, Iwata H, Hattori Y, Hashimoto S, Nakajima K, Sasaki S, Hara M, Sekido Y, Mizoe JE, Shibamoto Y. Transient increases in serum α fetoprotein and protein induced by vitamin K antagonist II levels following proton therapy does not necessarily indicate progression of hepatocellular carcinoma. Oncol Lett. 2019;17:3026-3034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Seo SI, Kim HS, Kim WJ, Shin WG, Kim DJ, Kim KH, Jang MK, Lee JH, Kim JS, Kim HY, Kim DJ, Lee MS, Park CK. Diagnostic value of PIVKA-II and alpha-fetoprotein in hepatitis B virus-associated hepatocellular carcinoma. World J Gastroenterol. 2015;21:3928-3935. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 70] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (16)] |
| 26. | Hemken PM, Sokoll LJ, Yang X, Dai J, Elliott D, Gawel SH, Lucht M, Feng Z, Marrero JA, Srivastava S, Chan DW, Davis GJ. Validation of a novel model for the early detection of hepatocellular carcinoma. Clin Proteomics. 2019;16:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 27. | Whitfield JB. Gamma glutamyl transferase. Crit Rev Clin Lab Sci. 2001;38:263-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 735] [Cited by in RCA: 807] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 28. | Tsutsumi M, Sakamuro D, Takada A, Zang SC, Furukawa T, Taniguchi N. Detection of a unique gamma-glutamyl transpeptidase messenger RNA species closely related to the development of hepatocellular carcinoma in humans: a new candidate for early diagnosis of hepatocellular carcinoma. Hepatology. 1996;23:1093-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 23] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Goldberg DM, Martin JV. Role of gamma-glutamyl transpeptidase activity in the diagnosis of hepatobiliary disease. Digestion. 1975;12:232-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 64] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Braun JP, Bardies J, Thouvenot JP, Benard P, Rico AG. Serum gamma-glutamyltransferase in equids: reference physiologic values. Am J Vet Res. 1982;43:339-340. [PubMed] |
| 31. | Ruppin DC, Frydman MI, Lunzer MR. Value of serum gamma-glutamyltransferase activity in the diagnosis of hepatobiliary disease. Med J Aust. 1982;1:421-424. [PubMed] |
| 32. | Xia J, Song P, Sun Z, Sawakami T, Jia M, Wang Z. Advances of diagnostic and mechanistic studies of γ-glutamyl transpeptidase in hepatocellular carcinoma. Drug Discov Ther. 2016;10:181-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 33. | Faber W, Sharafi S, Stockmann M, Denecke T, Sinn B, Puhl G, Bahra M, Malinowski MB, Neuhaus P, Seehofer D. Long-term results of liver resection for hepatocellular carcinoma in noncirrhotic liver. Surgery. 2013;153:510-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 34. | Dufour DR, Lott JA, Nolte FS, Gretch DR, Koff RS, Seeff LB. Diagnosis and monitoring of hepatic injury. II. Recommendations for use of laboratory tests in screening, diagnosis, and monitoring. Clin Chem. 2000;46:2050-2068. [PubMed] |
| 35. | Park SJ, Jang JY, Jeong SW, Cho YK, Lee SH, Kim SG, Cha SW, Kim YS, Cho YD, Kim HS, Kim BS, Park S, Bang HI. Usefulness of AFP, AFP-L3, and PIVKA-II, and their combinations in diagnosing hepatocellular carcinoma. Medicine (Baltimore). 2017;96:e5811. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 107] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 36. | Chinese Society of Hepatology and Chinese Society of Infectious Diseases; Chinese Medical Association. The guideline of prevention and treatment for chronic hepatitis B: a 2015 update. Zhongguo Ganzangbing Zazhi. 2015;7:1–18. [DOI] [Full Text] |
| 37. | Tamarkina AD, Dement'eva ES, Krylova NI. [Enzyme diagnosis of mechanical jaundice]. Vopr Med Khim. 1985;31:101-104. [PubMed] |
| 38. | Cook NR. Statistical evaluation of prognostic versus diagnostic models: beyond the ROC curve. Clin Chem. 2008;54:17-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 470] [Cited by in RCA: 516] [Article Influence: 28.7] [Reference Citation Analysis (0)] |