Published online Aug 14, 2019. doi: 10.3748/wjg.v25.i30.4213
Peer-review started: April 8, 2019
First decision: May 30, 2019
Revised: July 4, 2019
Accepted: July 5, 2019
Article in press: July 5, 2019
Published online: August 14, 2019
Processing time: 129 Days and 22.9 Hours
Clinically, tracheoesophageal fistula (TEF) is lack of effective surgical strategies. One reason is due to the lack of appropriate animal models of acquired TEF, which is usually complex and difficult. Recently, the magnetic compression technique has been applied for digestive tract anastomosis or vascular anastomosis in animals. In this study, an animal model of TEF in dogs was developed by using the magnetic compression technique, hoping to provide a new method for mimicking TEF.
To establish a TEF model in dogs by using the magnetic compression technique.
Six male beagles were used as models with two Nd-Fe-B permanent magnets for TEF. The parent magnet and the daughter magnet were placed in the cervical esophagus and trachea, respectively. The anterior wall of the esophagus and the posterior wall of the trachea were compressed when the two magnets coupled. After 4-6 d, the necrotic tissue between the two magnets fell off and the parent and daughter magnets disengaged from the target location, leaving a fistula. Gastroscopy/bronchoscopy, upper gastrointestinal contrast study, and histological analysis were performed.
The establishment of the TEF model in all six beagles was successful. The average time of magnet placement was 4.33 ± 1.11 min (range, 3-7 min). Mean time for the magnets to disengage from the target location was 4.67 ± 0.75 d (range, 4-6 d). TEFs were observed by gastroscopy/bronchoscopy and esophageal angiography. The gross anatomical structure of the esophagus and the trachea was in good condition. There was no esophageal mucosa or pseudostratified ciliated columnar epithelium at the site of the fistula according to histological analysis.
It is simple, feasible, and minimally invasive to use the magnetic compression technique for the establishment of the TEF model in dogs.
Core tip: The magnetic compression technique (MCT) is usually used clinically for anastomosis. We propose a novel application for the MCT in establishing a canine model of tracheoesophageal fistula (TEF). This is the first study to apply the MCT in the development of a disease model. Findings showed that it is simple, feasible, and minimally invasive to use magnetic compression technique for the establishment of the TEF model in dogs. It can be used to study the pathophysiological changes of TEF and to provide a good and stable model for exploring the advantages and disadvantages of various surgical methods.
- Citation: Gao Y, Wu RQ, Lv Y, Yan XP. Novel magnetic compression technique for establishment of a canine model of tracheoesophageal fistula. World J Gastroenterol 2019; 25(30): 4213-4221
- URL: https://www.wjgnet.com/1007-9327/full/v25/i30/4213.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i30.4213
Tracheoesophageal fistula (TEF) is the pathological channel between the trachea and esophagus caused by various pathogenic factors. According to the etiology, tracheoesophageal fistula can be divided into congenital and acquired. The congenital anomaly occurs in an estimated 1 in 2000-4000 live births[1], and congenital tra-cheoesophageal fistula is often combined with esophageal atresia. Acquired TEFs are rare, occurring in 0.5% of patients undergoing tracheotomy[2], 0.16%, 4.5%, and 14.75% of patients[2] with primary lung, esophageal, or tracheal malignancies, respectively, and secondary to esophageal carcinoma in 4.3%-8.1% of patients. TEF results in substantial morbidity and mortality as it is associated with coughing while swallowing, purulent bronchitis, pneumonia[1,3], sepsis, dysphagia, and malnutrition[4].
Surgery is first line treatment for TEF[5], but the techniques are diverse, and the long-term outcomes are disparate[6]. Therefore, a suitable animal model is required to explore the therapeutic effects of various surgical procedures.
Studies have shown that magnetic compression is a simple, minimally invasive, and reliable technique for surgical anastomosis[7,8]. Currently, the magnetic com-pression technique (MCT) is applied therapeutically for repair and reconstruction in esophageal atresia[9], biliary stenosis[10-14], vascular anastomosis[15-22], and gastro-intestinal anastomosis[23-30]. Conversely, the MCT can be used for destructive purposes, and a recent report described magnetic compression gastrostomy in rats[31]. In the current study, we proposed a novel application for the MCT in establishing a canine model of TEF.
This study was carried out in strict accordance with the recommendations in the Xi’an Jiaotong University Medical Center Guide for the Care and Use of Laboratory Animals. The protocol was approved by the Committee for Ethics of Animal Experiments at Xi’an Jiaotong University (Permit Number: 2017-773).
Six male beagles > 1 year old and weighing 8-10 kg were obtained from the Experimental Animal Center, College of Medicine, Xi’an Jiaotong University (Xi’an, China). The animal protocol was designed to minimize pain or discomfort to the animals. The animals were acclimatized to laboratory conditions (23 °C, 12 h/12 h light/dark, 50% humidity, and ad libitum access to food and water) for 2 wk prior to the experiments.
This was an exploratory study; therefore, all animals were included in the ex-perimental investigations, and there was no control group. At the end of the study, all animals were euthanized by a barbiturate overdose (intravenous injection, 150 mg/kg pentobarbital sodium) for tissue collection.
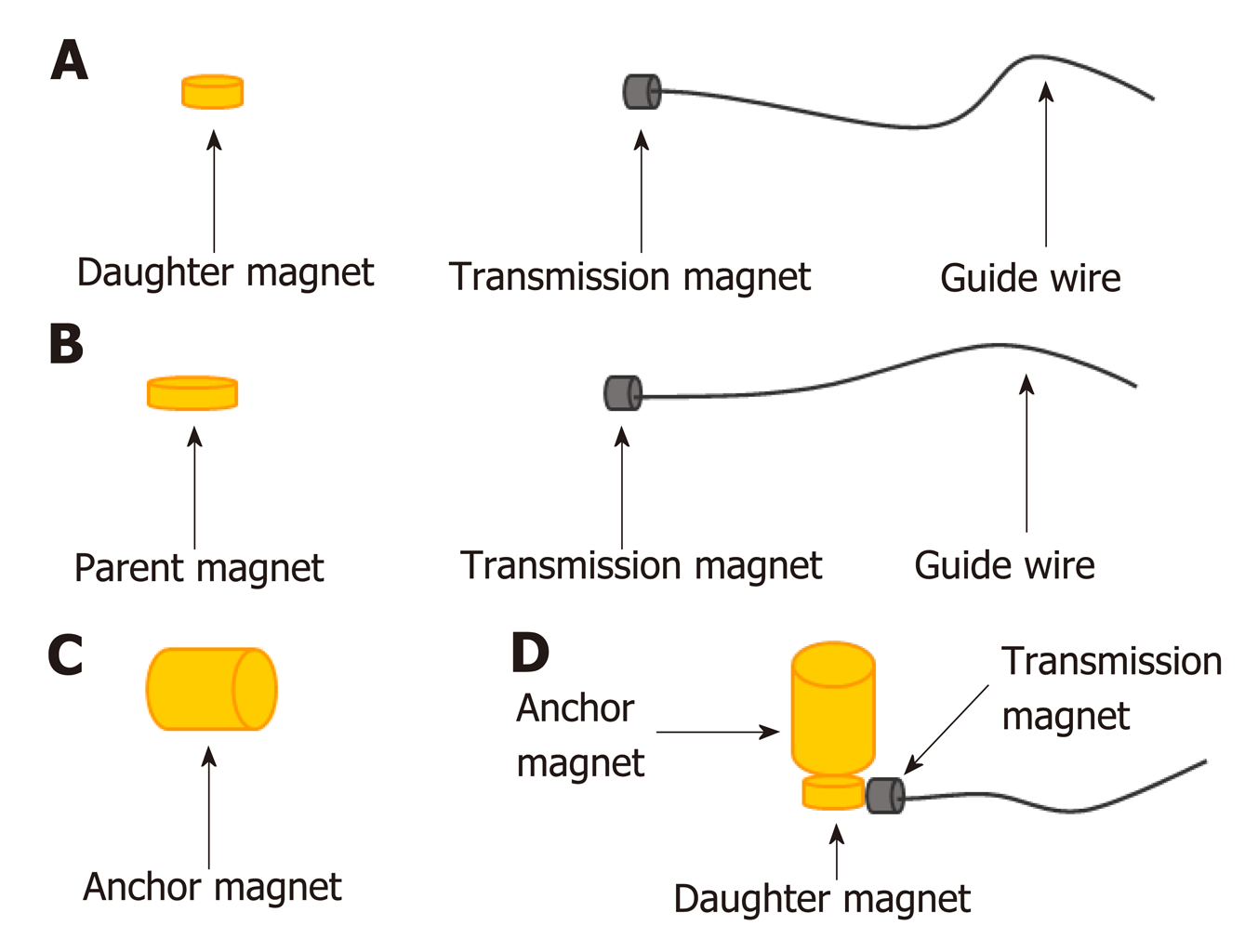
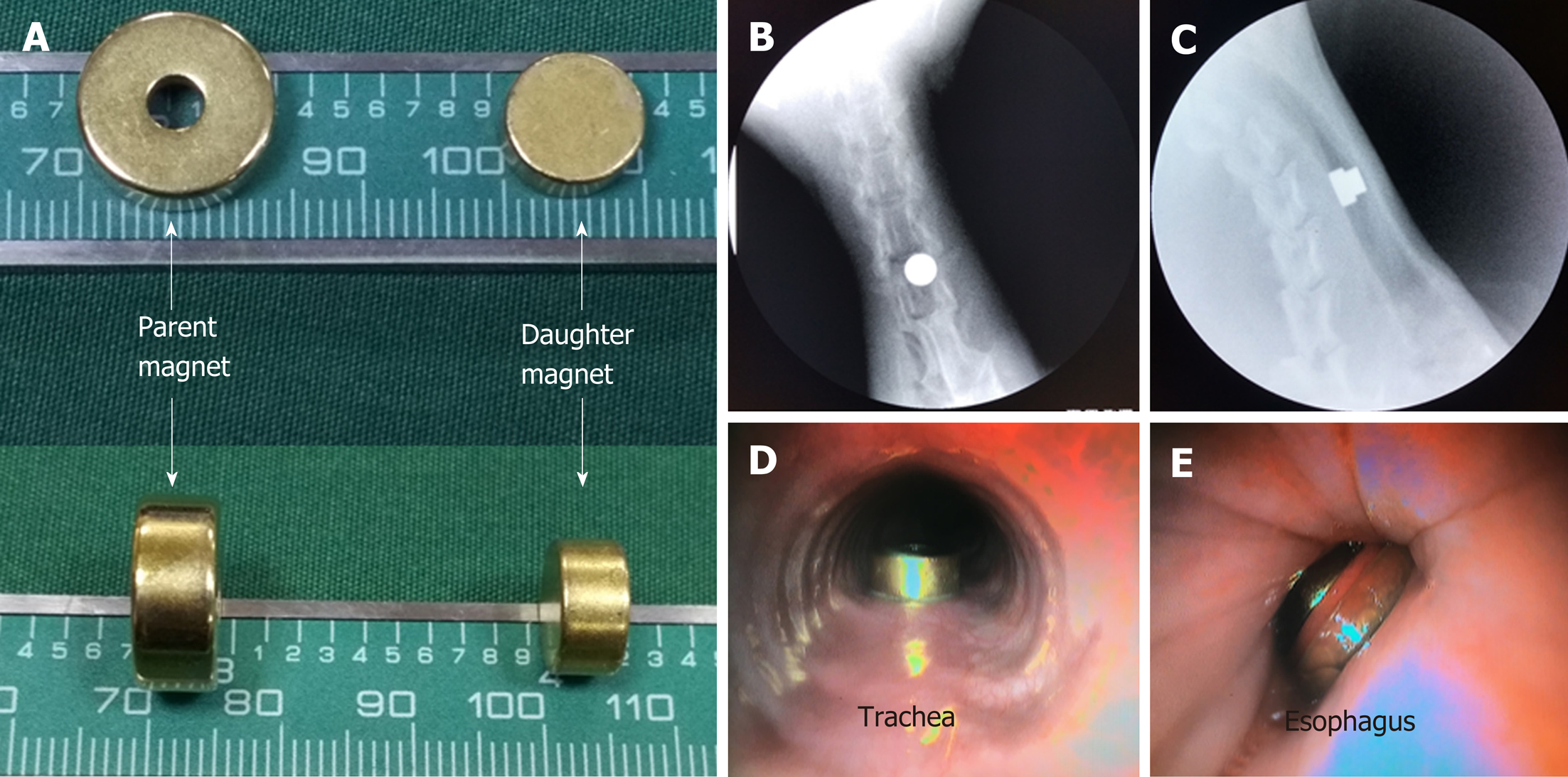
Both the parent magnet (diameter, 14 mm; thickness, 5 mm) and daughter magnet (diameter, 10 mm; thickness, 5 mm) were cylinders. An anchor magnet (cylinder; diameter, 14 mm; thickness, 20 mm) and a transmission magnet with a guide wire attached were also used (Figure 1). The magnets were constructed of neodymium-iron-boron (NdFeB, N45). The surface of the magnets was coated with titanium nitride to improve erosion resistance. The magnetic force between the daughter magnet and the parent magnet is 21 N. The magnetic density of the daughter and parent magnets is 3200 GS and 3600 GS, respectively.
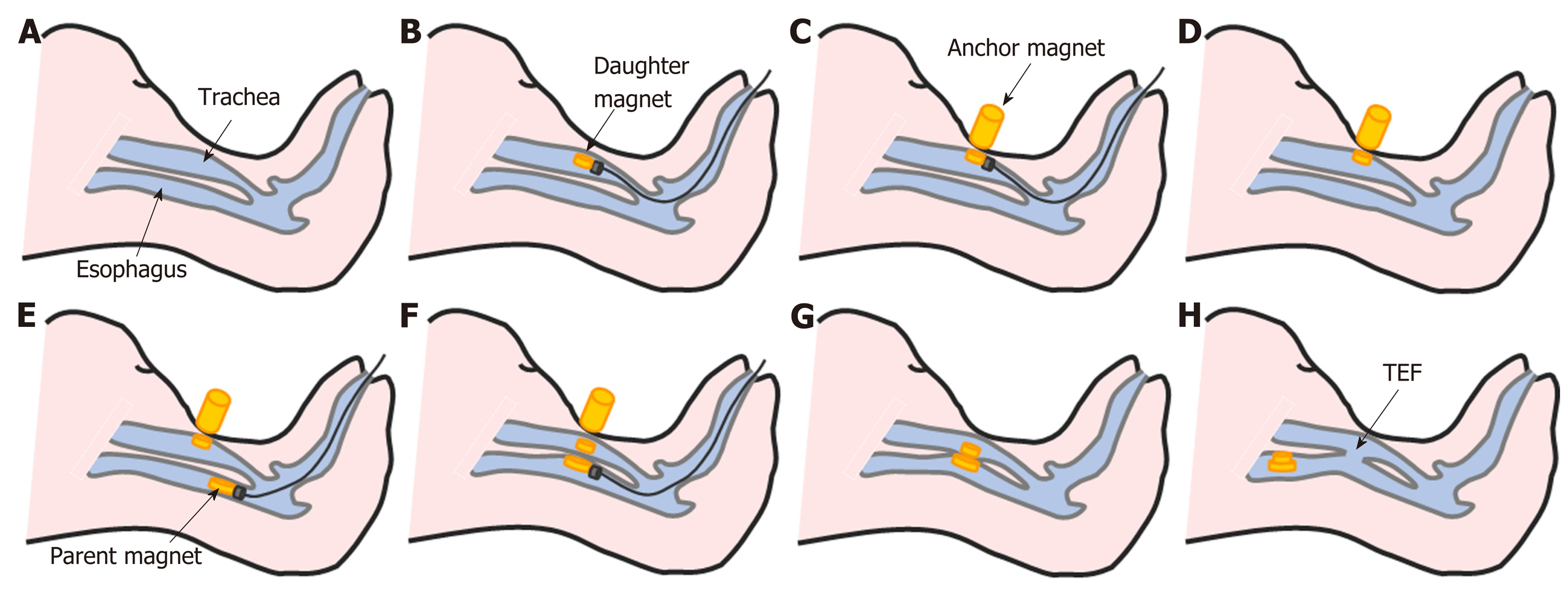
After fasting for 24 h, the beagles were weighed and anesthetized with intravenous propofol (10 mg/kg). When loss of the paw withdrawal reflex had been confirmed, the animals were placed on an operating table in the supine position. The glottis was sufficiently exposed, and the daughter magnet was inserted into the trachea and advanced to the target location using the transmission magnet and the guide wire (Figure 2B). The anchor magnet was placed on the neck of the animal over the trachea to fix the daughter magnet in its target location. The guide wire and transmission magnet were withdrawn from the trachea (Figure 2C and D). Next, the parent magnet was inserted into the cervical esophagus and advanced to the target location using the transmission magnet and the guide wire. The parent magnet and daughter magnet were automatically attracted to each other by magnetic force, the guide wire and transmission magnet were withdrawn from the esophagus, and the anchor magnet was removed (Figure 2E-G). Neck X-ray and gastroscopy were used to confirm the target location and accurate coupling of the parent and daughter magnets.
Routine X-rays were performed daily to verify the position of the magnets. X-ray gastroscopy at 5-7 d confirmed that necrotic tissue between the two magnets had been shed and the parent and daughter magnets had detached from the target location and entered the animal’s digestive tract (Figure 2H).
The tissue sandwiched between the two magnets become ischemic, necrotic, and was shed, resulting in the formation of a fistula, which was observed using esophageal intubation, gastroscopy/bronchoscopy, and esophageal angiography. Following X-ray confirmation of fistula formation, the beagles were weighed and anesthetized with intravenous propofol (10 mg/kg) and placed on an operating table in the lateral position. For gastroscopy/bronchoscopy, methylene blue was instilled into the esophagus, above the fistula, using a gastric tube. A bronchoscope was inserted into the trachea to observe the flow of methelyene blue from the esophagus to the trachea and confirm the formation of a TEF. For esophageal angiography, the contrast agent iohexol was instilled into the esophagus, above the fistula, using the gastric tube. The fistula was visualized by esophageal angiography.
Subsequently, the beagles were euthanized with high dose barbiturate. All fistulas were harvested. After gross observation, all samples were fixed in 10% formalin. Sections (4-6 µm) were stained with haematoxylin and eosin (HE) and Masson dye for light microscopy.
SPSS statistics software v17.0 was used for data analyses. Quantitative data are expressed as the mean ± standard deviation (SD).
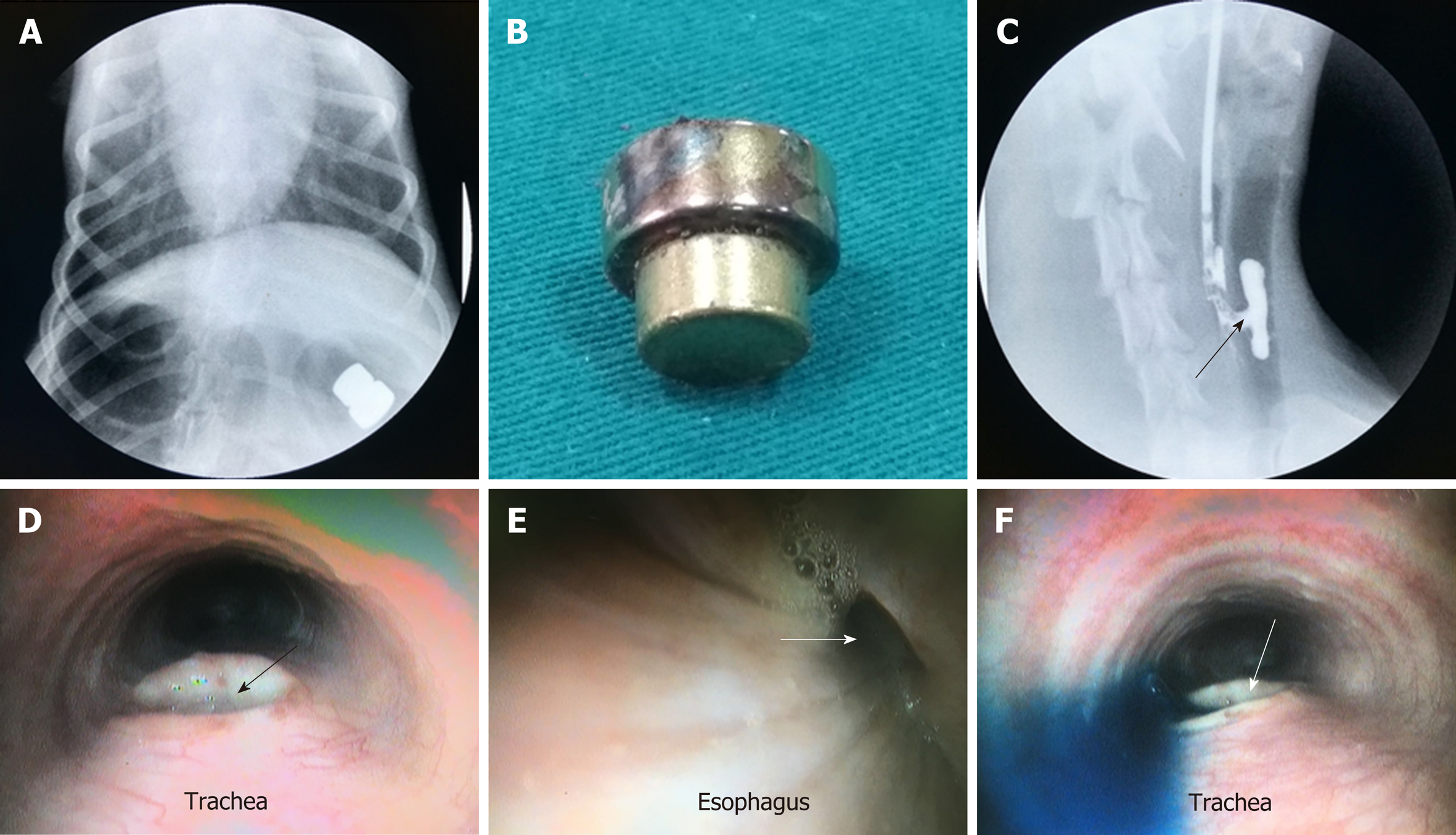
The study procedures were performed successfully in all six beagles. Mean operating time was 4.33 ± 1.11 min (range, 3-7 min). X-ray confirmed that the magnetic devices were coupled together in their target locations (Figure 3B and C). Mean time for the magnets to disengage from the target location was 4.67 ± 0.75 d (range, 4-6 d) after implantation (Figure 4A). Mean time for the magnets to be expelled from the animals’ bodies was 6.50 ± 0.96 d (range, 5-8 d) after implantation (Figure 4A and B). There was no early magnet separation, trachea blockage, digestive obstruction, or other complications resulting from implantation of the magnets.
Gastroscopy confirmed that the daughter magnet was located in the posterior wall of the trachea, and the parent magnet was located in the anterior wall of the esophagus (Figure 3D and E). When the magnets had separated from the target location, gastroscopy revealed a TEF (Figure 4D and E). Gastroscopy/bronchoscopy and esophageal angiography confirmed the formation of a TEF, as methylene blue and contrast agent, respectively, were observed to flow from the esophagus to the trachea (Figure 4C and F).
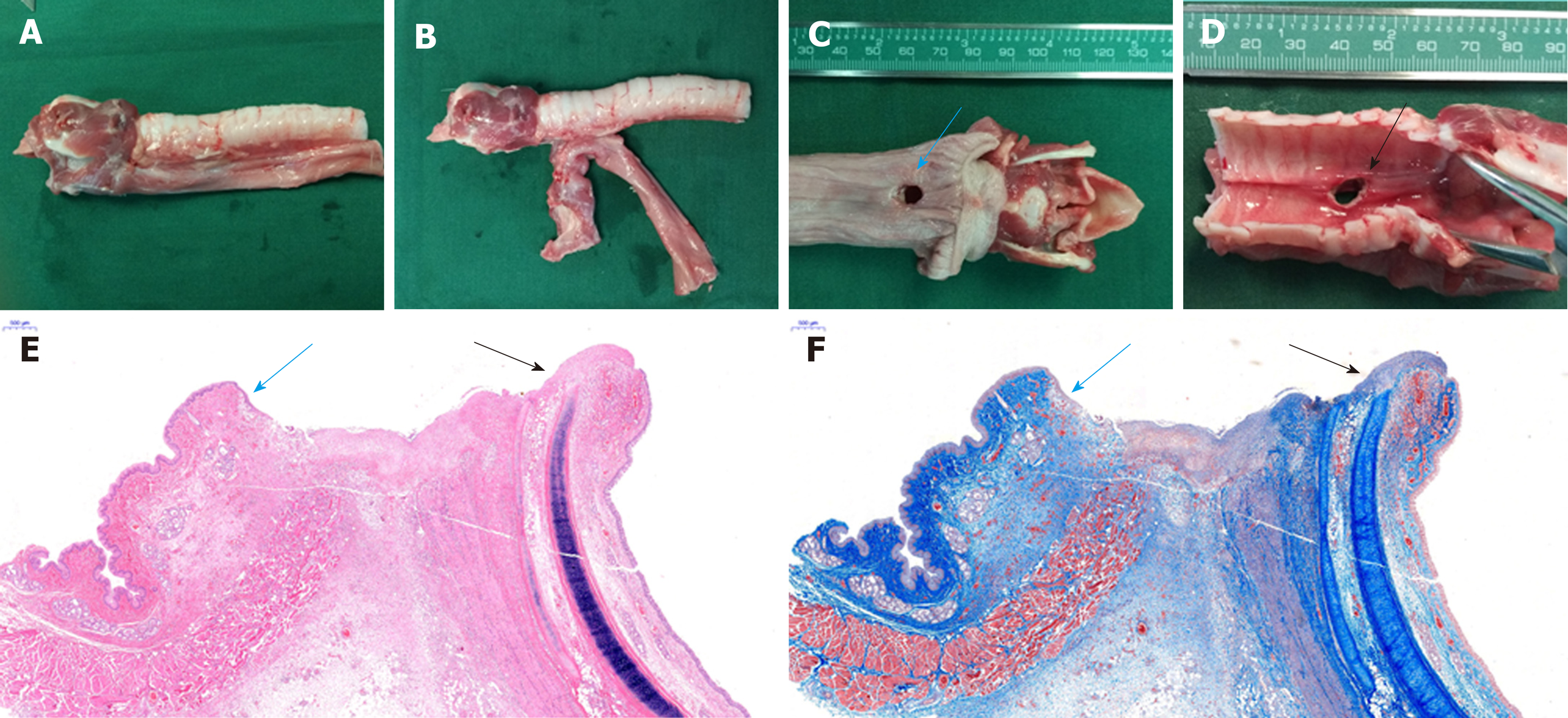
The gross anatomical structure of the esophagus and the trachea was in good condition. There was no tissue adhesion or scar formation between the trachea and esophagus, except around the complete fistula that was seen between the trachea and the esophagus. There was no hyperplasia of the tracheal mucosa, and the esophageal mucosa around the fistula was smooth (Figure 5A-D).
There was no esophageal mucosa or pseudostratified ciliated columnar epithelium at the site of the fistula according to histological analysis (Figure 5E and F).
The incidence of TEF is low, but the condition is associated with substantial morbidity and mortality. If treatment is delayed, severe pulmonary infection and even death may occur. Currently, non-surgical treatment is preferred for TEF, including endoscopic stent implantation to seal the fistula, local injection of a biological glue to close the fistula, or laser and argon plasma coagulation for thermal ablation[32]. According to the location and size of TEF, different surgical methods can also be used. However, long-term outcomes of surgery for TEF are variable, and a suitable animal model is required to explore the efficacy and safety of surgery in TEF. At present, published reports describing the establishment of animal models of TEF are scarce. A rat model of TEF was developed using an adriamycin injection administered to pregnant rats[33] and surgical procedures were used in the pig[34]. However, it is complex and traumatic to establish an animal model of TEF by surgery, and outcomes are variable. Specifically, severe adhesion of the trachea and esophagus occurs after the initial surgery, which is unfavorable when testing the effects of a second corrective surgical procedure.
The MCT is based on non-contact magnetic force and has been used successfully in vascular, gastrointestinal, and biliary tract reconstruction. To the authors’ knowledge, the MCT has not been applied to the establishment of an animal model of disease.
In the current study, the MCT was used to establish a canine model of TEF. The parent and daughter magnets were placed in the cervical esophagus and trachea, respectively. The posterior wall of the trachea and the anterior wall of the esophagus were compressed when the parent and daughter magnet were coupled together. A TEF resulted when the tissue sandwiched between the two magnets become ischemic and necrotic. Following fistula formation, when the necrotic tissue between the two magnets had been shed, the magnets detached from the target location. To avoid entering the trachea, the magnets were designed with different sizes. The parent magnet was located in the esophagus and was larger than the daughter magnet in the trachea. The larger magnet could not enter the trachea; therefore, when the magnets detached from the target location, they entered the esophagus and were spontaneously discharged through the digestive tract. This method was feasible due to the anatomical structure of the canine trachea and esophagus. There is minimal tissue between the trachea and esophagus, which allows the parent and daughter magnets to couple together.
Using the MCT to establish a canine model of TEF has several strengths: (1) The procedure is very simple and non-invasive; after the magnets are placed in the target location and coupled, no further intervention is needed, and the animals can eat and drink normally; (2) The position and size of the fistula can be precisely controlled by the anchor magnet and the diameter of the daughter magnet, respectively; therefore, the fistula can be accurately replicated in multiple animals; (3) The absence of the magnets at the target location is indicative of the presence of a fistula; this is easily determined using X-ray examination of the neck; (4) A TEF was created in 100% of the animals used in this study, indicating a high success rate; and (5) There was no tissue adhesion or scar formation between the trachea and esophagus, except around the fistula, which facilitates investigating the efficacy and safety of a corrective surgical procedure.
This method has the advantages of less additional trauma and closer to clinical reality. It can be used to study the pathophysiological changes of tracheoesophageal fistula and to provide a good and stable model for exploring the advantages and disadvantages of various surgical methods.
This study also had some limitations: (1) The corrective surgical procedure must be performed as soon as the TEF has formed, otherwise severe lung infections and even death may occur; and (2) The number of animals used in this study was small; complications and defects may be found after increasing the number of experimental animals.
In conclusion, it is simple, feasible, and minimally invasive to use the magnetic compression technique for the establishment of the TEF model in dogs.
Tracheoesophageal fistula in children is a rare human disease worldwide. To date, there is no ideal surgical strategy for the disease. The main reason is due to the lack of ideal animal models to mimic this disease.
The magnetic compression technique has been used to prepare the animal model of digestive tract anastomosis or vascular anastomosis. Therefore, we tried to use this new technique to establish a novel model mimicking tracheoesophageal fistula in children, hoping to provide a new surgical strategy for this disease.
To establish a canine model of tracheoesophageal fistula using the magnetic compression technique.
Using a magnetic device consisting of daughter and parent Nd-Fe-B permanent magnets, a tracheoesophageal fistula model was established in male beagles through placing the device in the cervical esophagus. After 4~6 days of operation, gastroscopy/bronchoscopy, esophageal angiography, and histological analysis were carried out to identify the model.
After the operation, the esophageal mucosa and pseudostratified ciliated columnar epithelium were absent at the site of the fistula in the beagle model of tracheoesophageal fistula. Mean operating time was 4.33 ± 1.11 min, and mean time for successful establishment of the tracheoesophageal fistula model was 4.67 ± 0.75 d.
A simple, minimally invasive, and feasible model of tracheoesophageal fistula in canine was first established by using the novel magnetic compression technique.
Although providing a suitable model similar to tracheoesophageal fistula in children, the feasibility, applicability, safety, and efficacy of new surgical treatment for tracheoesophageal fistula using the specific model need to be explored in future studies.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gallo G, Jana K, Yasuhiro F S-Editor: Ma RY L-Editor: Wang TQ E-Editor: Zhang YL
| 1. | Elumalai G. Tracheoesophageal fistula: embryological basis and its clinical significance. Int J Dev Biol. 2016;100:43414-43419. |
| 2. | Martini N, Goodner JT, D'Angio GJ, Beattie EJ. Tracheoesophageal fistula due to cancer. J Thorac Cardiovasc Surg. 1970;59:319-324. [PubMed] |
| 3. | Zhou C, Hu Y, Xiao Y, Yin W. Current treatment of tracheoesophageal fistula. Ther Adv Respir Dis. 2017;11:173-180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 4. | Shichinohe T, Okushiba S, Morikawa T, Kitashiro S, Manase H, Kawarada Y, Sekido M, Yamamoto Y, Kondo S. Salvage of a massive esophago-tracheal fistula resulting from a stenting treatment. Dis Esophagus. 2006;19:299-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Shen KR. Management of acquired nonmalignant tracheoesophageal fistula: Surgical pearls. J Thorac Cardiovasc Surg. 2017;154:e123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Muniappan A, Wain JC, Wright CD, Donahue DM, Gaissert H, Lanuti M, Mathisen DJ. Surgical treatment of nonmalignant tracheoesophageal fistula: a thirty-five year experience. Ann Thorac Surg. 2013;95:1141-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 7. | Yan XP, Liu WY, Ma J, Li JP, Lv Y. Extrahepatic portacaval shunt via a magnetic compression technique: A cadaveric feasibility study. World J Gastroenterol. 2015;21:8073-8080. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | She ZF, Yan XP, Ma F, Wang HH, Yang H, Shi AH, Wang L, Qi X, Xiao B, Zou YL, Lv Y. Treatment of rectovaginal fistula by magnetic compression. Int Urogynecol J. 2017;28:241-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Zaritzky M, Ben R, Zylberg GI, Yampolsky B. Magnetic compression anastomosis as a nonsurgical treatment for esophageal atresia. Pediatr Radiol. 2009;39:945-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 10. | Muraoka N, Uematsu H, Yamanouchi E, Kinoshita K, Takeda T, Ihara N, Matsunami H, Itoh H. Yamanouchi magnetic compression anastomosis for bilioenteric anastomotic stricture after living-donor liver transplantation. J Vasc Interv Radiol. 2005;16:1263-1267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 11. | Itoi T, Yamanouchi E, Ikeda T, Sofuni A, Kurihara T, Tsuchiya T, Tsuchida A, Kasuya K, Moriyasu F. Magnetic compression anastomosis: a novel technique for canalization of severe hilar bile duct strictures. Endoscopy. 2005;37:1248-1251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Okajima H, Kotera A, Takeichi T, Ueno M, Ishiko T, Hirota M, Asonuma K, Yamauchi E, Inomata Y. Magnet compression anastomosis for bile duct stenosis after duct-to-duct biliary reconstruction in living donor liver transplantation. Liver Transpl. 2005;11:473-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Avaliani M, Chigogidze N, Nechipai A, Dolgushin B. Magnetic compression biliary-enteric anastomosis for palliation of obstructive jaundice: initial clinical results. J Vasc Interv Radiol. 2009;20:614-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Jang SI, Kim JH, Won JY, Lee KH, Kim HW, You JW, Itoi T, Lee D. Magnetic compression anastomosis is useful in biliary anastomotic strictures after living donor liver transplantation. Gastrointest Endosc. 2011;74:1040-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 15. | Klima U, Falk V, Maringka M, Bargenda S, Badack S, Moritz A, Mohr F, Haverich A, Wimmer-Greinecker G. Magnetic vascular coupling for distal anastomosis in coronary artery bypass grafting: a multicenter trial. J Thorac Cardiovasc Surg. 2003;126:1568-1574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 38] [Reference Citation Analysis (0)] |
| 16. | Klima U, Maringka M, Bagaev E, Kirschner S, Haverich A. Total magnetic vascular coupling for arterial revascularization. J Thorac Cardiovasc Surg. 2004;127:602-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Casselman FP, Meco M, Dom H, Foubert L, Van Praet F, Vanermen H. Multivessel distal sutureless off-pump coronary artery bypass grafting procedure using magnetic connectors. Ann Thorac Surg. 2004;78:e38-e40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Athanasiou T, Ashrafian H, Glenville B, Casula R. Coronary artery bypass with the use of a magnetic distal anastomotic device: surgical technique and preliminary experience. Heart Surg Forum. 2004;7:356-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Klima U, MacVaugh H, Bagaev E, Maringka M, Kirschner S, Beilner J, Haverich A. Magnetic Vascular Port in minimally invasive direct coronary artery bypass grafting. Circulation. 2004;110:II55-II60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Vicol C, Eifert S, Oberhoffer M, Boekstegers P, Knez A, Christ F, Reichart B. Early clinical results with a magnetic connector for distal coronary artery anastomoses. Ann Thorac Surg. 2005;79:1738-1742; discussion 1742-1743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Shi Y, Lv Y, Wang B, Zhang Y, Jiang A, Li JH, Zhang XF, Li QY, Meng KW, Liu C, Yu L, Pan CE. Novel magnetic rings for rapid vascular reconstruction in canine liver transplantation model. Transplant Proc. 2006;38:3070-3074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Yan X, Fan C, Ma J, Li J, Dong D, Wang H, Ma F, Zheng X, Lv Y. Portacaval shunt established in six dogs using magnetic compression technique. PLoS One. 2013;8:e76873. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 23. | Cope C. Evaluation of compression cholecystogastric and cholecystojejunal anastomoses in swine after peroral and surgical introduction of magnets. J Vasc Interv Radiol. 1995;6:546-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Cope C, Clark TW, Ginsberg G, Habecker P. Stent placement of gastroenteric anastomoses formed by magnetic compression. J Vasc Interv Radiol. 1999;10:1379-1386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Cope C, Ginsberg GG. Long-term patency of experimental magnetic compression gastroenteric anastomoses achieved with covered stents. Gastrointest Endosc. 2001;53:780-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 38] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 26. | Chopita N, Vaillaverde A, Cope C, Bernedo A, Martinez H, Landoni N, Jmelnitzky A, Burgos H. Endoscopic gastroenteric anastomosis using magnets. Endoscopy. 2005;37:313-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 27. | van Hooft JE, Vleggaar FP, Le Moine O, Bizzotto A, Voermans RP, Costamagna G, Devière J, Siersema PD, Fockens P. Endoscopic magnetic gastroenteric anastomosis for palliation of malignant gastric outlet obstruction: a prospective multicenter study. Gastrointest Endosc. 2010;72:530-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 28. | Myers C, Yellen B, Evans J, DeMaria E, Pryor A. Using external magnet guidance and endoscopically placed magnets to create suture-free gastro-enteral anastomoses. Surg Endosc. 2010;24:1104-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 29. | Pichakron KO, Jelin EB, Hirose S, Curran PF, Jamshidi R, Stephenson JT, Fechter R, Strange M, Harrison MR. Magnamosis II: Magnetic compression anastomosis for minimally invasive gastrojejunostomy and jejunojejunostomy. J Am Coll Surg. 2011;212:42-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 30. | Ryou M, Cantillon-Murphy P, Azagury D, Shaikh SN, Ha G, Greenwalt I, Ryan MB, Lang JH, Thompson CC. Smart Self-Assembling MagnetS for ENdoscopy (SAMSEN) for transoral endoscopic creation of immediate gastrojejunostomy (with video). Gastrointest Endosc. 2011;73:353-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 31. | Uygun I, Okur MH, Cimen H, Keles A, Yalcin O, Ozturk H, Otcu S. Magnetic compression gastrostomy in the rat. Pediatr Surg Int. 2012;28:529-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 32. | Ke M, Wu X, Zeng J. The treatment strategy for tracheoesophageal fistula. J Thorac Dis. 2015;7:S389-S397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 33] [Reference Citation Analysis (0)] |
| 33. | Diez-Pardo JA, Baoquan Q, Navarro C, Tovar JA. A new rodent experimental model of esophageal atresia and tracheoesophageal fistula: preliminary report. J Pediatr Surg. 1996;31:498-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 94] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 34. | Wagner HJ, Stinner B, Barth P, Klose KJ. Are covered stents really effective at closing esophagotracheal fistulas? Results of an animal study. Cardiovasc Intervent Radiol. 2000;23:291-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |